Abstract
Retinitis pigmentosa (RP) is the most common inherited retinal disease, in which photoreceptor cells degenerate, leading to blindness. Mutations in the rod photoreceptor cGMP phosphodiesterase β subunit (PDEβ) gene are found in patients with autosomal recessive RP as well as in the rd mouse. We have recently shown that lentivirus vectors based on human immunodeficiency virus (HIV) type 1 achieve stable and efficient gene transfer into retinal cells. In this study, we evaluated the potential of HIV vector-mediated gene therapy for RP in the rd mouse. HIV vectors containing a gene encoding a hemagglutinin (HA)-tagged PDEβ were injected into the subretinal spaces of newborn rd mouse eyes. One to three rows of photoreceptor nuclei were observed in the eyes for at least 24 weeks postinjection, whereas no photoreceptor cells remained in the eyes of control animals at 6 weeks postinjection. Expression of HA-tagged PDEβ in the rescued photoreceptor cells was confirmed by two-color confocal immunofluorescence analysis using anti-HA and anti-opsin antibodies. HIV vector-mediated gene therapy appears to be a promising means for the treatment of recessive forms of inherited retinal degeneration.
Retinitis pigmentosa (RP) is a genetically and clinically heterogeneous group of retinal degenerative diseases, affecting approximately 1 in 3,500 people (29). Symptoms include night blindness, progressive loss of peripheral visual field, and eventual loss of central vision caused by degeneration of photoreceptor cells. There are no adequate therapies for RP at present. However, increasing numbers of genes responsible for RP have been identified (11, 33), providing an avenue for gene therapy in the treatment of RP. Most of the identified genes responsible for RP are expressed specifically in photoreceptor cells, and degeneration primarily affects photoreceptor cells. Therefore, methods for efficient gene transfer into terminally differentiated photoreceptor cells are essential for gene therapy of RP.
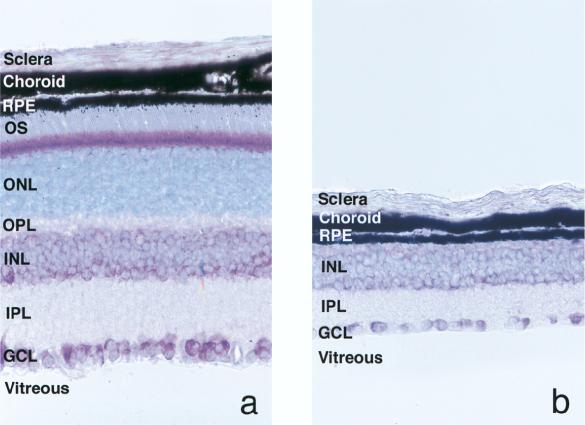
Mutations in the rod photoreceptor cGMP phosphodiesterase β subunit (PDEβ) gene are found in patients with autosomal recessive RP as well as in rd mice and rcd1 Irish setters (10, 24, 30, 32). The rd mouse is the best-studied animal model of RP and is characterized by a rapid photoreceptor degeneration, although photoreceptor cells develop normally until postnatal day 7. Degeneration first appears in the rod photoreceptor cells between postnatal days 7 and 9 and is detectable by electron microscopy, and a majority of photoreceptor cells have degenerated by 30 days (7, 8, 31). As shown in Fig. 1, the photoreceptor layer in the rd mice has degenerated completely by 6 weeks of age. Transgenic rd mice that express a functional bovine PDEβ gene showed preservation of photoreceptor cells, suggesting that somatic gene therapy to prevent degeneration is also feasible (20). However, therapeutic gene delivery to the rd mouse eye by using adenovirus or adeno-associated virus (AAV) vectors has been inefficient and could only delay the degeneration (4, 9, 14, 19, 21).
FIG. 1.
Light micrographs of retinas collected from normal (a) and rd (b) mice at 6 weeks of age. Paraffin-embedded sections of eyes were counterstained with toluidine blue. In the rd mouse, the photoreceptor layer that includes the outer segment (OS), the outer nuclear layer (ONL), and the outer plexiform layer (OPL) has degenerated completely. RPE, retinal pigment epithelium; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Original magnification, ×400.
We have developed a lentivirus vector based on human immunodeficiency virus type 1 (HIV-1) that can transduce nondividing cells in vitro and in vivo (6, 15, 27, 28). Recently, we have shown that HIV vectors can mediate efficient transfer and sustained long-term expression of the transgene in retinal cells, particularly in photoreceptor cells, when gene expression is controlled by the rhodopsin promoter (26). The present study was designed to evaluate the therapeutic potential of HIV vectors for treatment of RP.
HIV vector plasmids containing the murine PDEβ cDNA under the control of the cytomegalovirus (CMV) promoter (CMV-PDEβ) or the bovine rhodopsin promoter (Rho-PDEβ) were constructed by replacing the green fluorescent protein (GFP) fragments of pHR′-CMV-GFP and pHR′-Rho-GFP (26), respectively, with the PDEβ cDNA fragment of plasmid MPB-71 (2). To detect the expression of PDEβ, the hemagglutinin (HA) epitope tag was fused to the amino terminus of PDEβ. Expression of HA-tagged PDEβ in vitro was confirmed by immunoblot analysis and immunofluorescence microscopy by using anti-HA antibody in 293T cells transfected with the CMV-PDEβ vector plasmid (data not shown).
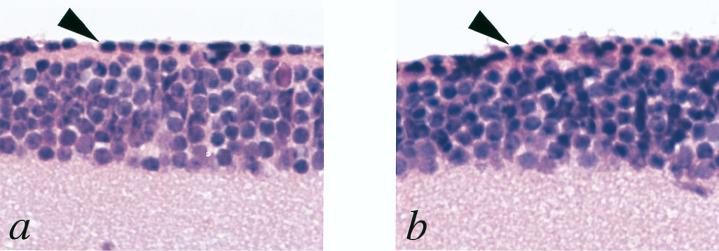
HIV vectors pseudotyped with vesicular stomatitis virus G glycoprotein were generated as described previously (27). The titer of HIV vectors was determined by measuring the amount of HIV-1 p24 antigen by using enzyme-linked immunosorbent assay. Approximately 5 × 105 infectious units of HIV vectors was injected into the subretinal space of C3H/HeJ rd/rd mouse eyes between postnatal days 2 and 5. HIV vector containing the GFP gene under the control of the rhodopsin promoter (Rho-GFP) was used as a control. Altogether, 18, 58, and 60 rd mouse eyes were injected with the Rho-GFP, the CMV-PDEβ, and the Rho-PDEβ vectors, respectively. At 6, 12, and 24 weeks postinjection, mice were sacrificed and eyes were subjected to histological and immunohistochemical analyses. In control eyes collected at 6 weeks postinjection, histological analysis of hematoxylin-and-eosin-stained paraffin-embedded sections demonstrated a single row of photoreceptor-like nuclei (Fig. 2a). However, as shown in Fig. 3d, immunohistochemistry with an anti-opsin antibody showed no opsin immunoreactivity, indicating that these retained cells were not photoreceptor cells but dislocated cells in the inner nuclear layer. It is often difficult to distinguish cell nuclei in the inner nuclear layer from remaining pyknotic photoreceptor nuclei in the degenerated rd mouse retina (Fig. 2b). Thus, we assessed rescue of photoreceptor cells from degeneration by immunohistochemistry in this study.
FIG. 2.
Light micrographs of retinas collected from rd mice 6 weeks after injection of the Rho-GFP vector (a) or the Rho-PDEβ vector (b). Paraffin-embedded sections of eyes were counterstained with hematoxylin and eosin. Arrowheads point to a photoreceptor-like nucleus in the inner nuclear layer (panel a) and a pyknotic photoreceptor nucleus (panel b). Original magnification, ×400.
FIG. 3.
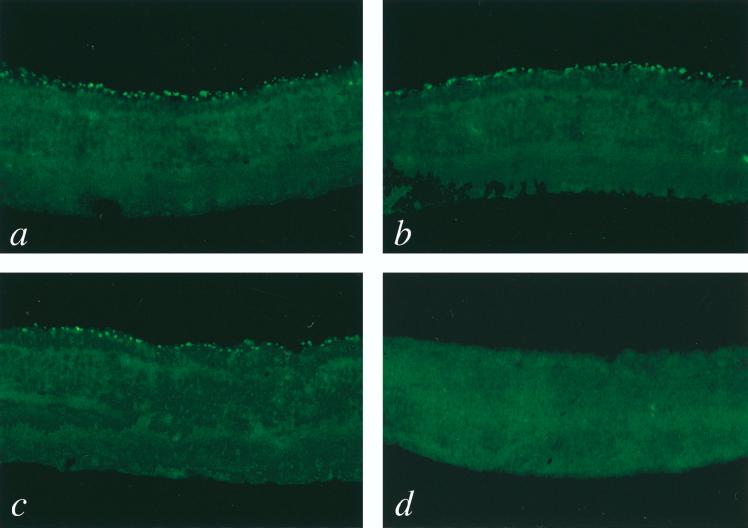
Immunohistochemical detection of opsin-positive photoreceptor cells in rd mice following injection of HIV vectors. Mice injected with the Rho-PDEβ vector (a, b, and c) or the Rho-GFP vector (d) were sacrificed at 6 (panels a and d), 12 (panel b), and 24 (panel c) weeks postinjection, and eye sections were prepared as described previously (26). Sections were stained with mouse anti-opsin antibody (kindly provided by C. J. Barnstable) and then with fluorescein isothiocyanate-conjugated donkey anti-mouse immunoglobulin G (Jackson Immunochemicals). Immunofluorescence was detected with a confocal laser scanning microscope (Bio-Rad). Representative confocal microscope images of sections are shown. Opsin-expressing photoreceptor cells (bright green) were seen only in eyes injected with the Rho-PDEβ vector. Original magnification, ×200.
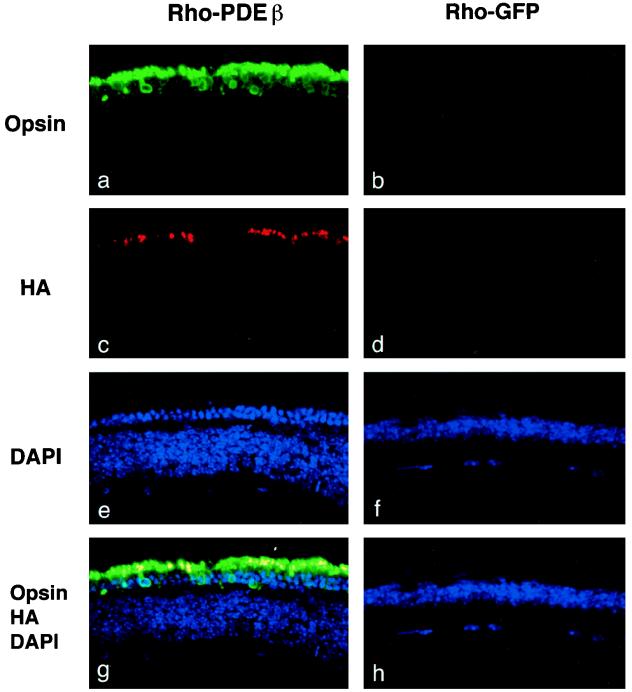
In eyes injected with either the Rho-PDEβ vector or the CMV-PDEβ vector, numerous opsin-positive photoreceptor cells were observed at 6, 12, and 24 weeks postinjection. In contrast, all control eyes injected with the Rho-GFP vector showed no opsin-positive photoreceptor cells. Representative results are shown in Fig. 3. Two-color confocal immunofluorescence analysis using anti-opsin and anti-HA antibodies indicated that most of the rescued photoreceptor cells expressed HA-tagged PDEβ (Fig. 4). As expected, HA-tagged PDEβ as well as opsin was found localized exclusively in the outer segments of photoreceptor cells, where it normally exists. Taken together, these results indicate that HA-tagged PDEβ was functional and that its expression was able to rescue photoreceptor cells from degeneration. Up to three rows of photoreceptor nuclei were observed with 4′,6-diamidino-2-phenylindole (DAPI) staining (Fig. 4e). Rescued photoreceptor cells expressing PDEβ were seen not only around the injection site but also over the entire retina, suggesting that virus particles diffused in the subretinal space. This phenomenon has also been observed in our previous study of rat pups (26).
FIG. 4.
Two-color confocal immunofluorescence analysis of rd mouse eyes injected with HIV vectors. Sections of rd mouse eyes obtained 6 weeks after injection of the Rho-PDEβ vector (a, c, e, and g) or the Rho-GFP vector (b, d, f, and h) were stained with mouse anti-opsin antibody and rabbit anti-HA antibody (MBL, Nagoya, Japan). Primary antibodies were detected with fluorescein isothiocyanate-conjugated donkey anti-mouse immunoglobulin G (IgG) (green) (panels a and b) and cyan red-conjugated donkey anti-rabbit IgG (red) (panels c and d) and visualized by confocal laser scanning microscopy. Cell nuclei were counterstained with DAPI (blue) (panels e and f). Double-labeled cells (yellow) demonstrate HA-tagged PDEβ expression in the outer segments of photoreceptor cells (panel g). Original magnification, ×400.
Rescued photoreceptor cells were found in 8 of 22, 10 of 28, and 3 of 8 eyes injected with the CMV-PDEβ vector and in 20 of 34, 8 of 18, and 4 of 8 eyes injected with the Rho-PDEβ vector at 6, 12, and 24 weeks postinjection, respectively. Furthermore, the total number of rescued photoreceptor cells varied greatly from eye to eye. These results were presumably due to technical variations, since the lens opacity of newborn mice made it difficult to confirm the successful subretinal injection by fundus examination. In the most-successfully rescued eyes, however, there was no significant difference in the numbers of rescued photoreceptor cells in each group at all the periods evaluated (Fig. 5), although we previously observed that the rhodopsin promoter was more effective than the CMV promoter in rat photoreceptor cells (26).
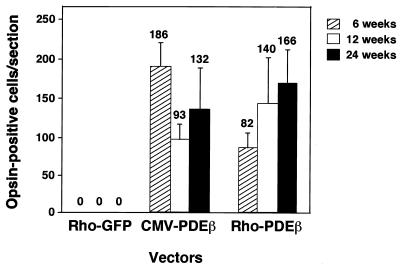
FIG. 5.
The number of opsin-positive photoreceptor cells per section at each time point. Four sections near the equator were selected from the most-successfully rescued eyes (n = 3) in each group, and opsin-positive cells were counted under a fluorescence microscope. The mean numbers of opsin-positive cells per section were calculated and are indicated above the standard deviation bars.
The outer segments are continuously shed from the end of each photoreceptor cell and phagocytosed by cells of the retinal pigment epithelium. Therefore, constitutive expression of PDEβ is required to prevent photoreceptor degeneration and maintain the outer segments. Others have shown that adenovirus and AAV vectors can efficiently transfer a reporter gene to retinal cells (1, 5, 13, 22). However, when these vectors were applied for gene therapy of RP in the rd mouse, the therapeutic effect was inefficient and resulted in only a delay of photoreceptor degeneration until up to 6 weeks after birth (4, 14). A major problem with the adenovirus vectors is the transient expression of the transgene due to a cellular immune response(s) against the transduced cells (16), limiting the use of this vector for long-term gene therapy. Unsuccessful results obtained with AAV vectors may be due to a slow onset of transgene expression since it takes more than 3 weeks to obtain maximum expression (1, 3, 13). In contrast, our results clearly show that HIV vector-mediated PDEβ gene expression persisted for at least 24 weeks, resulting in significant rescue of authentic photoreceptor cells detected by anti-opsin antibody. It is unclear whether more photoreceptor cells survived at earlier time points as others have reported (4, 9, 14, 19, 21). However, our goal in the present study was to examine long-term rescue from photoreceptor degeneration. This is the first study to establish the therapeutic ability of HIV vectors by using an animal model. Long-term rescue from photoreceptor degeneration was achieved, although only one to three rows of photoreceptor nuclei remained in the HIV vector-injected mice, in contrast with eight to ten rows in normal mice. However, clinicopathologic studies in humans with RP have suggested that a single layer of photoreceptor cells is sufficient to maintain minimal visual function, even though electroretinogram response is undetectable (23, 34). One possible explanation for partial rescue from photoreceptor degeneration is that the time of injection (postnatal days 2 to 5) was too late to rescue all photoreceptor cells. Although photoreceptor development appears morphologically normal until postnatal day 7 (7, 31), degeneration at the molecular level may already be ongoing in the majority of photoreceptor cells at the time of injection or earlier. Another possible explanation is that the amount of PDEβ provided by HIV vectors was insufficient to rescue all photoreceptor cells. Half of the normal expression level of PDEβ is sufficient to maintain normal photoreceptor morphology and visual function, since the RP phenotype in the rd mouse is inherited as a recessive trait. In any case, it will be important to ascertain when and how much PDEβ is required to rescue all photoreceptor cells.
RP is an excellent candidate for human gene therapy. Direct access to the target cells, photoreceptor cells, is possible by means of subretinal injection. In comparison with the rd mouse, humans suffering from RP show later onset and slower progression, i.e., typically onset of night blindness in childhood and progression to legal blindness by 60 years of age. In addition, only a small area of the retina, for example, a 500-μm-diameter area of macula, is sufficient to preserve minimal visual function. Thus more successful therapeutic effects could be expected in humans. HIV vector-mediated gene transfer appeared to be safe, as there were no adverse effects on the animals at any time point after injection. However, safety is still a potential concern for clinical application of HIV vectors. In this regard, recent improvements, including self-inactivating vectors (25, 35), packaging constructs eliminating all accessory genes (12, 18, 36), and inducible packaging cell lines (17), could further minimize the risk. Although further studies in animal models are required to determine the efficacy and safety of HIV vectors before human clinical trials are conducted, our results substantiate the feasibility of gene therapy for recessive forms of retinal degenerative disease.
Acknowledgments
M.T. and H.M. contributed equally to this work.
We thank H. Okuda for hematoxylin and eosin staining, S. Forbes for care of the mice, and N. Somia for critical reading of the manuscript. We also thank W. Baehr for providing murine PDEβ cDNA plasmid and C. J. Barnstable for providing mouse anti-opsin antibody.
M.T. was supported by a fellowship from the Nippon Eye Bank Association. H.M. was supported by a fellowship from the Uehara Memorial Foundation. This work was supported by grants from NIH, NIA, NINDS, March of Dimes Foundation, Frances Berger Foundation, and Valley Foundation. I.M.V. is an American Cancer Society Professor of Molecular Biology.
REFERENCES
- 1.Ali R R, Reichel M B, De Alwis M, Kanuga N, Kinnon C, Levinsky R J, Hunt D M, Bhattacharya S S, Thrasher A J. Adeno-associated virus gene transfer to mouse retina. Hum Gene Ther. 1998;9:81–86. doi: 10.1089/hum.1998.9.1-81. [DOI] [PubMed] [Google Scholar]
- 2.Baehr W, Champagne M S, Lee A K, Pittler S J. Complete cDNA sequences of mouse rod photoreceptor cGMP phosphodiesterase α- and β-subunits, and identification of β′-, a putative β-subunit isozyme produced by alternative splicing of the β-subunit gene. FEBS Lett. 1991;278:107–114. doi: 10.1016/0014-5793(91)80095-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett J, Duan D, Engelhardt J F, Maguire A M. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Investig Ophthalmol Vis Sci. 1997;38:2857–2863. [PubMed] [Google Scholar]
- 4.Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire A M. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J, Wilson J, Sun D, Forbes B, Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Investig Ophthalmol Vis Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- 6.Blomer U, Naldini L, Kafri T, Trono D, Verma I M, Gage F H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caley D W, Johnson C, Liebelt R A. The postnatal development of the retina in the normal and rodless CBA mouse: a light and electron microscopic study. Am J Anat. 1972;133:179–212. doi: 10.1002/aja.1001330205. [DOI] [PubMed] [Google Scholar]
- 8.Carter-Dawson L D, LaVail M M, Sidman R L. Differential effect of the rd mutation on rods and cones in the mouse retina. Investig Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 9.Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther. 1997;8:423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- 10.Danciger M, Blaney J, Gao Y Q, Zhao D Y, Heckenlively J R, Jacobson S G, Farber D B. Mutations in the PDE6B gene in autosomal recessive retinitis pigmentosa. Genomics. 1995;30:1–7. doi: 10.1006/geno.1995.0001. [DOI] [PubMed] [Google Scholar]
- 11.Dryja T P, Li T. Molecular genetics of retinitis pigmentosa. Hum Mol Genet. 1995;4:1739–1743. doi: 10.1093/hmg/4.suppl_1.1739. [DOI] [PubMed] [Google Scholar]
- 12.Dull T, Zufferey R, Kelly M, Mandel R J, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flannery J G, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jomary C, Vincent K A, Grist J, Neal M J, Jones S E. Rescue of photoreceptor function by AAV-mediated gene transfer in a mouse model of inherited retinal degeneration. Gene Ther. 1997;4:683–690. doi: 10.1038/sj.gt.3300440. [DOI] [PubMed] [Google Scholar]
- 15.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 16.Kafri T, Morgan D, Krahl T, Sarvetnick N, Sherman L, Verma I. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kafri T, van Praag H, Ouyang L, Gage F H, Verma I M. A packaging cell line for lentivirus vectors. J Virol. 1999;73:576–584. doi: 10.1128/jvi.73.1.576-584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim V N, Mitrophanous K, Kingsman S M, Kingsman A J. Minimal requirement for a lentivirus vector based on human immunodeficiency virus type 1. J Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar-Singh R, Farber D B. Encapsidated adenovirus minichromosome-mediated delivery of genes to the retina: application to the rescue of photoreceptor degeneration. Hum Mol Genet. 1998;7:1893–1900. doi: 10.1093/hmg/7.12.1893. [DOI] [PubMed] [Google Scholar]
- 20.Lem J, Flannery J G, Li T, Applebury M L, Farber D B, Simon M I. Retinal degeneration is rescued in transgenic rd mice by expression of the cGMP phosphodiesterase beta subunit. Proc Natl Acad Sci USA. 1992;89:4422–4426. doi: 10.1073/pnas.89.10.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewin A S, Drenser K A, Hauswirth W W, Nishikawa S, Yasumura D, Flannery J G, LaVail M M. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat Med. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- 22.Li T, Adamian M, Roof D J, Berson E L, Dryja T P, Roessler B J, Davidson B L. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Investig Ophthalmol Vis Sci. 1994;35:2543–2549. [PubMed] [Google Scholar]
- 23.Li Z Y, Jacobson S G, Milam A H. Autosomal dominant retinitis pigmentosa caused by the threonine-17-methionine rhodopsin mutation: retinal histopathology and immunocytochemistry. Exp Eye Res. 1994;58:397–408. doi: 10.1006/exer.1994.1032. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin M E, Ehrhart T L, Berson E L, Dryja T P. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA. 1995;92:3249–3253. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi H, Blomer U, Takahashi M, Gage F H, Verma I M. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi H, Takahashi M, Gage F H, Verma I M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naldini L, Blomer U, Gage F H, Trono D, Verma I M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 29.Pagon R A. Retinitis pigmentosa. Surv Ophthalmol. 1988;33:137–177. doi: 10.1016/0039-6257(88)90085-9. [DOI] [PubMed] [Google Scholar]
- 30.Pittler S J, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci USA. 1991;88:8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiose Y. Electron microscopic aspects on early changes of inherited dystrophic mouse retina. Jpn J Ophthalmol. 1969;12:181–190. [Google Scholar]
- 32.Suber M L, Pittler S J, Qin N, Wright G C, Holcombe V, Lee R H, Craft C M, Lolley R N, Baehr W, Hurwitz R L. Irish setter dogs affected with rod/cone dysplasia contain a nonsense mutation in the rod cGMP phosphodiesterase beta-subunit gene. Proc Natl Acad Sci USA. 1993;90:3968–3972. doi: 10.1073/pnas.90.9.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan L S, Daiger S P. Inherited retinal degeneration: exceptional genetic and clinical heterogeneity. Mol Med Today. 1996;2:380–386. doi: 10.1016/s1357-4310(96)10037-x. [DOI] [PubMed] [Google Scholar]
- 34.Szamier R B, Berson E L. Retinal ultrastructure in advanced retinitis pigmentosa. Investig Ophthalmol Vis Sci. 1977;16:947–962. [PubMed] [Google Scholar]
- 35.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]