Abstract
Some individuals infected with only R5 strains of human immunodeficiency virus type 1 progress to AIDS as quickly as individuals harboring X4 strains. We determined that three R5 viruses were much less pathogenic than an X4 virus in SCID-hu Thy/Liv mice, suggesting that R5 virus-mediated rapid disease progression is associated with host, not viral, factors.
Human immunodeficiency virus type 1 (HIV-1) infects cells expressing CD4 and chemokine receptors (coreceptors), such as CXCR4 or CCR5. R5 strains (2) utilize CCR5 and replicate throughout the course of infection in all individuals, while X4 strains (2) utilize CXCR4 and are usually found in abundance during later stages of disease in some (about half) individuals with HIV-1 disease. The appearance of X4 strains nearly always coincides with the onset of clinical symptoms, and CD4+-T-cell counts usually drop more quickly in individuals with X4 strains than in those with only R5 strains (18, 24). X4 strains have accordingly been considered more pathogenic than R5 strains (18, 24) and to be, in fact, the proximal cause of rapid disease progression. This association may be linked, at least in part, to the expression of CXCR4 and CCR5 during successive stages of T-cell differentiation. High levels of CXCR4 are found on key intrathymic (CD3− CD4+ CD8−) T-progenitor cells (5), CD4+ CD8+ cortical thymocytes (5, 17), and nearly every CD4+ T cell in the periphery (5, 22). In contrast, CCR5 is expressed only at low levels on CD4+ CD8+ thymocytes (5, 12) and on a subset of circulating memory or activated CD4+ T cells (5, 9, 22, 30). Given this differential display of coreceptors, it is more likely that X4 isolates (as opposed to R5 isolates) rapidly infect and destroy both immature and mature (8) cells within the T-lymphoid system, resulting in immune system collapse.
Notwithstanding the apparent enhanced pathogenicity of X4 isolates, many individuals with only R5 isolates (termed here “R5-AIDS” viruses) develop progressive HIV-1 disease. Such individuals may be asymptomatic for long periods of time prior to the onset of AIDS, while others progress rapidly with kinetics similar to those in individuals containing X4 viruses. Rapid disease progression caused by certain R5 viruses must be due either to enhanced pathogenic determinants in the viruses or to susceptibility determinants in the infected hosts. Evidence for the former exists; R5 isolates in some individuals have been shown to evolve into variants which replicate faster during the asymptomatic period (7, 11), and these fast-replicating viruses have been associated with decreasing peripheral CD4+-T-cell levels and with disease progression (7, 27). Whether these changes are sufficient to overcome the relatively infrequent expression of CCR5 and to make the viruses as pathogenic as X4 viruses is not known. Alternatively, R5-AIDS viruses associated with rapid disease progression may not be especially pathogenic; instead, the rapid progression may be due to host-related factors.
To better relate viral phenotypes to pathogenicity in vivo, we have chosen to evaluate R5-AIDS isolates from rapid progressors in the context of an experimentally manipulable animal model, the SCID-hu Thy/Liv mouse. In this model, the implanted human thymus organ has been well characterized and shown to be similar to the normal human fetal thymus with respect to its composition of thymocyte subpopulations and to their expression of such coreceptors as CXCR4 and CCR5 (5, 21, 23). The tropism and pathogenicity of various HIV-1 isolates have also been studied extensively and shown to correlate well with X4 and R5 tropism as defined for humans and for other model systems in vitro. Thus, X4 strains like NL4-3 (1) infect CD3− CD4+ CD8− intrathymic T-progenitor cells and deplete thymocytes much more quickly (3, 10, 15, 16, 26) than do R5 viruses like Ba-L (13) and JR-CSF (20). When paired non-syncytium-inducing (NSI) and SI isolates from individuals with HIV-1 disease were tested, the latter behaved like NL4-3 and the former behaved like the Ba-L (16). When NL4-3 was changed from X4 to R5 status by insertion of the Ba-L V3 loop, the chimeric virus exhibited the Ba-L phenotype in the thymus organ (4). This result indicates that the increased replicative and pathogenic character of NL4-3 is due solely to the utilization of the more abundant CXCR4 protein and not to any intrinsic replicative or cytopathic determinants within the X4 virus. Indeed, in T-cell lines (6) and lymph node explants (14), X4 and R5 viruses appear to be equally cytopathic to their target cells. Unlike the X4 isolate NL4-3, R5 isolates like Ba-L were observed to move through a two-phase infection pattern in the SCID-hu model, first replicating slowly within stromal cells of the medulla, and then infecting CD4+ CD8+ cortical thymocytes and occasionally causing modest thymocyte depletion (3).
Given this background of clearly demarcated distinctions between X4 and R5 isolates in the SCID-hu Thy/Liv model, we evaluated a panel of R5-AIDS isolates from rapid progressors in SCID-hu Thy/Liv implants to determine whether they are as pathogenic as the X4 virus NL4-3. R5-AIDS viruses were isolated from three rapid progressors (Table 1); in each of these three patients, SI virus was never isolated at any point during the course of disease. Each R5-AIDS virus was confirmed to utilize CCR5, but not CXCR4, in vitro by two separate assays, both involving selective infection of CCR5+, but not CXCR4+, cell lines (data not shown and Fig. 1, data for ACH424). In addition, each virus was found to be unable to utilize CCR1, CCR2b, or CCR3 (data not shown). These viruses, as well as NL4-3 and Ba-L, an R5 control virus, were then directly injected into three SCID-hu Thy/Liv implants each, as previously described (3). Seventeen days later, the implants were removed from the mice and analyzed by p24 enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, and multiparameter flow cytometry.
TABLE 1.
Properties of R5-AIDS viruses analyzed in this study
| Virus | No. of mo to AIDSa | Mo of isolationb | Source tissuec | Culture cellsd | Clade | Growth in MDMe | References |
|---|---|---|---|---|---|---|---|
| ACH424 | 38 | 38 | PBMC | PBMC | B | Unable | 7, 28 |
| ACH172 | 25 | 63 | BAL | MDM | B | Moderate | 19, 25 |
| p198 | ∼60 | ∼84 | CSF | MDM | B | Extreme | 19, 29 |
Number of months from seroconversion to AIDS.
Number of months from seroconversion to virus isolation.
PBMC, peripheral blood mononuclear cells; BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid.
Type of cells used to grow virus in vitro. MDM, monocyte-derived macrophages.
Relative efficiency of growth.
FIG. 1.
Virus ACH424 utilizes CCR5 but not CXCR4 in vitro. HOS cell lines expressing CD4, HIV-1 Tat-inducible green fluorescent protein (GFP), and either CXCR4 or CCR5 were infected with 1,000 50% tissue culture infective doses of ACH424, the R5 control virus Ba-L, or the X4 control virus NL4-3. After 6 days, the indicator cells were analyzed by flow cytometry for GFP expression. The percentage of GFP+ indicator cells (within the gate) is indicated inside each plot. The low-level infection of the HOS-CCR5 cell line by NL4-3 was likely mediated by low levels of endogenous CXCR4, known to be expressed on HOS cells.
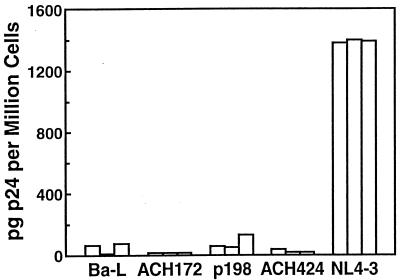
For ELISA, 106 dispersed Thy/Liv cells were diluted in 160 μl of p24 lysis buffer (containing 1% Triton X-100, 0.5% deoxycholate, 5 mM EDTA, 25 mM Tris-HCl, 250 mM NaCl, and 1% aprotinin) and rotated overnight at 4°C. The lysate was then transferred into a quantitative ELISA (DuPont) for the detection of p24 antigen. The ELISA analysis indicated that each of the R5-AIDS viruses replicated at levels similar to those observed for Ba-L but not NL4-3 (Fig. 2). The average amount of p24 per 106 dispersed Thy/Liv cells ranged from 7 (virus ACH172) to 81 (virus p198) pg, while the concentrations of Ba-L and NL4-3 were 53 and 1,378 pg per million cells, respectively.
FIG. 2.
The three R5-AIDS viruses replicated to levels similar to those of Ba-L and not NL4-3. Thy/Liv cells were isolated from cohort 1 implants 17 days postinoculation and analyzed for intracellular p24 levels by quantitative ELISA. Each bar represents an individual implant.
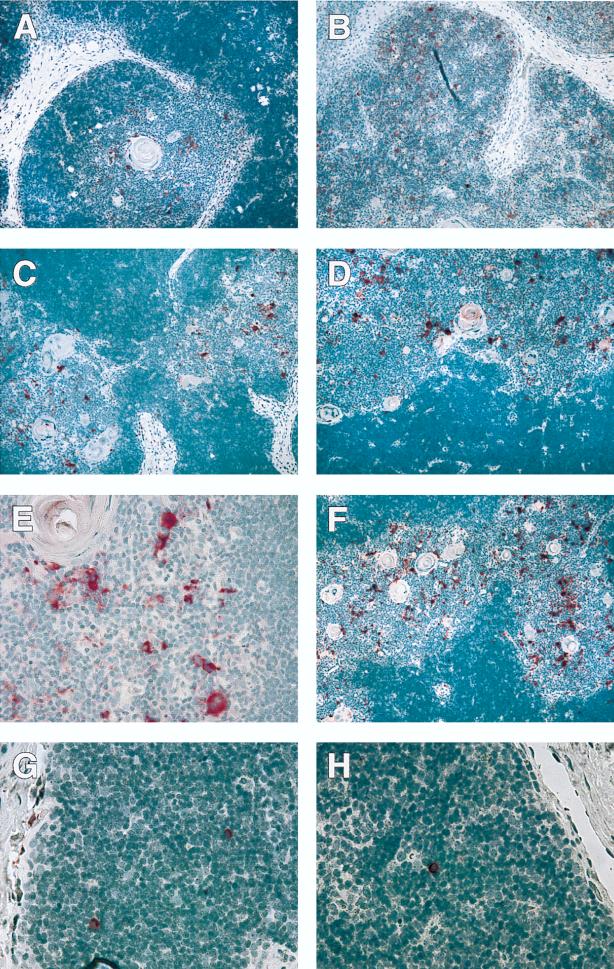
Immunohistochemistry was performed as previously described (3). Immunohistochemical detection of p24 protein in situ indicated that each of the R5-AIDS viruses replicated in medullary stromal cells in a pattern similar to the one observed for Ba-L (Fig. 3A F). Virus ACH424, a non-macrophage-tropic R5 virus, also infected small, round medullary cells of unknown identity. NL4-3 replicated primarily in cortical thymocytes.
FIG. 3.
The three R5-AIDS viruses infected cell types similar to those infected by Ba-L and not NL4-3. Infected Thy/Liv implants were analyzed in situ 17 days postinoculation for HIV-1 p24 protein by immunohistochemistry. p24 protein is indicated in red, while cell nuclei are indicated in blue-purple. (A to F) Images of cohort 1 implants infected with Ba-L (A), NL4-3 (B), ACH172 (C), p198 (D and E), or ACH424 (F) for 17 days, revealing HIV-1 infection of cortical thymocytes (NL4-3) or medullary stromal cells (Ba-L and the R5-AIDS viruses). (G and H) Images of cohort 2 implants infected with p198 (G) or ACH424 (H) for 35 days, revealing HIV-1 infection of single cortical thymocytes. Magnifications, ×10 (A to D and F) and ×40 (E, G, and H).
For flow cytometric analysis, 5 × 105 dispersed Thy/Liv cells were diluted to 50 μl with a monoclonal antibody cocktail containing CD4-fluorescein isothiocyanate, CD8-phycoerythrin, and CD3-tricolor or the relevant isotype control monoclonal antibodies. After a 20-min incubation in the dark, the cells were washed with phosphate-buffered saline containing 2% fetal calf serum and resuspended in 200 μl of 1% paraformaldehyde. Fluorescence-activated cell sorter analysis of the implants indicated that each of the R5-AIDS viruses failed to cause thymocyte depletion, similar to Ba-L (Fig. 4). Only NL4-3 caused a significant increase in the percentage of dead and dying cells (data not shown) and a decrease in the percentage of live CD4+ CD8+ thymocytes.
FIG. 4.
The three R5-AIDS viruses did not cause thymocyte depletion soon after inoculation. Thy/Liv cells isolated from representative implants 17 days postinoculation were analyzed by flow cytometry for surface expression of CD4 and CD8. The percentage of thymocytes expressing CD4 and CD8 is indicated in each plot. Uninfected Thy/Liv implants typically contain 80 to 90% CD4+ CD8+ thymocytes.
These results indicate that each of the R5-AIDS viruses was similar to Ba-L and not NL4-3 in replicative capacity, tropism, and pathogenicity after 17 days of infection. To determine whether the R5-AIDS viruses exhibited the pathogenicity of NL4-3 at a later time, we injected a second cohort of mice with large doses (2,000 50% tissue culture infective doses) of either p198 (seven implants) or ACH424 (five implants) and analyzed the implants 35 days later. This time point was chosen because Ba-L had always started its second phase of infection (i.e., spread to cortical thymocytes) but had never caused thymocyte depletion by this time point in previous experiments (3). As before, the implants were analyzed by p24 ELISA, immunohistochemistry, and flow cytometry.
Both p198 and ACH424 exhibited replicative capacity, tropism, and pathogenicity that were similar to those of Ba-L and not NL4-3 at this later time point. As determined by ELISA, both R5-AIDS viruses were still replicating slowly; p198 and ACH424 replicated and produced averages of 41 and 43 pg of p24 per 106 cells, respectively (data not shown). Immunohistochemical analysis indicated that p198 and ACH424 had begun to spread to cortical thymocytes in some of the implants, but only to a limited extent (Fig. 3G and H). As determined by fluorescence-activated cell sorter analysis, none of the 12 implants were depleted, indicating that viruses p198 and ACH424 were much less pathogenic than NL4-3 (data not shown).
In summary, we have determined that three R5-AIDS viruses are much less pathogenic than the X4 strain NL4-3 in SCID-hu Thy/Liv mice despite originating in individuals exhibiting X4-type rapid rates of disease progression. This result suggests that R5-AIDS isolates do not contain special replicative or cytopathic determinants which overcome the infrequent expression of CCR5 relative to CXCR4.
Although our data suggest that R5-AIDS viruses are not as pathogenic as X4 viruses, it is quite possible that R5-AIDS viruses are more pathogenic than “regular” R5 viruses. In another study of R5 virus pathogenesis in the SCID-hu model, R5-AIDS viruses were found to cause more thymocyte depletion than R5 viruses isolated before disease progression when implants were analyzed at 6 to 12 weeks postinoculation (10a). Our observation that the three R5-AIDS viruses were no more pathogenic than Ba-L within 5 weeks postinoculation should be interpreted with caution since it is not known whether Ba-L is an R5-AIDS virus (i.e., it is not known whether the AIDS patient from which Ba-L was isolated also carried X4 viruses).
There are several trivial explanations that might underlie our results. First, X4 isolates may actually be present and undetectable in those AIDS patients from whom the R5-AIDS isolates were recovered and may be the proximal cause of rapid disease progression, as would be expected. Alternatively, the unique pathogenicity of R5-AIDS isolates may not be revealed in the SCID-hu animal model, even if such attributes existed. These possibilities may be addressed by a more thorough examination of AIDS patients who appear to harbor R5 isolates only and perhaps also by the use of other models, in vivo and in vitro, to assess viral pathogenicity.
At the same time, it would be of interest to address an alternative explanation for our results: R5 isolates may affect some individuals in a differential manner because of host-dependent factors. Thus, there may exist polymorphisms in the human population with respect to CCR5 expression during T-cell differentiation. If so, and if this important coreceptor is expressed in some individuals at key intermediate stages of maturation (e.g., the CD3− CD4+ CD8− intrathymic T-progenitor cell stage), then R5 isolates may be expected to be more pathogenic as a consequence of expanded tropism. Alternatively, enhanced replication of R5 isolates may be facilitated by host-dependent factors in the absence of expanded tropism. If so, and if such factors are also polymorphic in their expression, R5 virus spread may occur more rapidly in some individuals. Polymorphisms which determine differences in coreceptor expression and/or viral replication kinetics might be genetically determined. They might also be induced by exogenous agents. For example, coinfection with other viruses (e.g., herpesviruses such as cytomegalovirus or human herpesvirus 6) may result in alterations in the surface phenotype of potential target cells for HIV-1 infection and/or in the transactivation of the HIV-1 genome. Indeed, such perturbations might represent mechanisms by which these infectious agents could serve as cofactors in the development of AIDS after HIV-1 infection. Discrimination between these possibilities may provide insight into the interplay between intrinsic properties of the HIV-1 genome and host-dependent variables associated with disease progression.
Acknowledgments
This work was supported by Public Health Service grants AI-40312 and AI-65309 from the National Institute of Allergy and Infectious Diseases (to J.M.M.) and by grant R96-GI-041 from the University of California Universitywide AIDS Research Program (to R.D.B.). J.M.M. is an Elizabeth Glaser Scientist supported by the Elizabeth Glaser Pediatric AIDS Foundation.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HOS-CD4 and -CCR5 cell lines from Vineet KewalRamani and Dan Littman. U87-CCR1, -CCR2b, -CCR3, -CCR5, and -CXCR4 cell lines were donated by Dan Littman.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger E A, Doms R W, Fenyo E M, Korber B T, Littman D R, Moore J P, Sattentau O J, Schuitemaker H, Sodroski J, Weiss R A. A new classification for HIV-1. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz R D, Alexander S, Bare C, Lindquist-Stepps V, Bogan M, Moreno M E, Gibson L, Wieder E D, Kosek J, Soddart C A, McCune J M. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J Virol. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz, R. D., S. Alexander, and J. M. McCune. Causal relationships between the HIV-1 V3 loop, coreceptor utilization, tropism, and pathogenesis in vivo. Submitted for publication. [DOI] [PubMed]
- 5.Berkowitz R D, Beckerman K P, Schall T J, McCune J M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 6.Björndal Å, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaak H, Brouwer M, Ran L J, de Wolf F, Schuitemaker H. In vitro replication kinetics of human immunodeficiency virus type 1 (HIV-1) variants in relation to virus load in long-term survivors of HIV-1 infection. J Infect Dis. 1998;177:600–610. doi: 10.1086/514219. [DOI] [PubMed] [Google Scholar]
- 8.Blaak H, van ’t Wout A B, Brouwer M, Hooibrink B, Hovenkamp E, Schuitemaker H. Abstracts of the 6th Conference on Retroviruses and Opportunistic Infections. 1999. Differential infection of CD45RA+ and CD45RO+ CD4 T cells by syncytium inducing (SI) and non-syncytium inducing (NSI) HIV-1 variants, abstr. 28. [Google Scholar]
- 9.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocyte. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 10a.Camerini, D., and R. Scoggins. Personal communication.
- 11.Conner R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dairaghi D J, Franz-Bacon K, Callas E, Cupp J, Schall T J, Tamraz S A, Boehme S A, Taylor N, Bacon K B. Macrophage inflammatory protein-1 beta induces migration and activation of human thymocytes. Blood. 1998;91:2905–2913. [PubMed] [Google Scholar]
- 13.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 14.Grivel J-C, Margolis L B. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson B D, Pang S, Aldrovandi G M, Zha J, Zack J A. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J Virol. 1995;69:6259–6264. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneshima H, Su L, Bonyhadi M L, Connor R I, Ho D D, McCune J M. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koot M, Keet I P, Vos A H, de Goede R E, Roos M T, Coutinho R A, Miedema F, Schellekens P T, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kootstra N A, Schuitemaker H. Proliferation-dependent replication in primary macrophages of macrophage-tropic HIV type 1 variants. AIDS Res Hum Retroviruses. 1998;14:339–345. doi: 10.1089/aid.1998.14.339. [DOI] [PubMed] [Google Scholar]
- 20.Koyanagi Y, Miles S, Misuyasu R T, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 21.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 22.Mo H, Monard S, Pollack H, Ip J, Rochford G, Wu L, Hoxie J, Borkowsky W, Ho D D, Moore J P. Expression patterns of the HIV type 1 coreceptors CCR5 and CXCR4 on CD4+ T cells and monocytes from cord and adult blood. AIDS Res Hum Retroviruses. 1998;14:607–617. doi: 10.1089/aid.1998.14.607. [DOI] [PubMed] [Google Scholar]
- 23.Namikawa R, Weilbaecher K N, Kaneshima H, Yee E J, McCune J M. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richman D D, Bozzette S A. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 25.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E Y, van Steenwijk R P, Lange J M A, Schattenkerk J K M E, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baselar M, Adelsberger J, Bonyhadi M, Orenstein J, Fox C H, Fauci A S. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tersmette M, Gruters R A, de Wolf F, de Goede R E Y, Lange J M A, Schellekens P T A, Goudsmit J, Huisman H G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van ’t Wout A B, Blaak H, Ran L J, Brouwer M, Kuiken C, Schuitemaker H. Evolution of syncytium-inducing and non-syncytium-inducing biological virus clones in relation to replication kinetics during the course of human immunodeficiency virus type 1 infection. J Virol. 1998;72:5099–5107. doi: 10.1128/jvi.72.6.5099-5107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van’t Wout A B, Ran L J, Kuiken C L, Kootstra N A, Pals S T, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]