Abstract
Background:
Rubella-containing vaccines (RCV) are not yet part of the Democratic Republic of the Congo’s (DRC) vaccination program; however RCV introduction is planned before 2020. Because documentation of DRC’s historical burden of rubella virus infection and congenital rubella syndrome (CRS) has been minimal, estimates of the burden of rubella virus infection and of CRS would help inform the country’s strategy for RCV introduction.
Methods:
A rubella antibody seroprevalence assessment was conducted using serum collected during 2008–2009 from 1605 pregnant women aged 15–46 years attending 7 antenatal care sites in 3 of DRC’s provinces. Estimates of age- and site-specific rubella antibody seroprevalence, population, and fertility rates were used in catalytic models to estimate the incidence of CRS per 100,000 live births and the number of CRS cases born in 2013 in DRC.
Results:
Overall 84% (95% CI 82, 86) of the women tested were estimated to be rubella antibody seropositive. The association between age and estimated antibody seroprevalence, adjusting for study site, was not significant (p = 0.10). Differences in overall estimated seroprevalence by study site were observed indicating variation by geographical area (p ⩽ 0.03 for all). Estimated seroprevalence was similar for women declaring residence in urban (84%) versus rural (83%) settings (p = 0.67). In 2013 for DRC nationally, the estimated incidence of CRS was 69/100,000 live births (95% CI 0, 186), corresponding to 2886 infants (95% CI 342, 6395) born with CRS.
Conclusions:
In the 3 provinces, rubella virus transmission is endemic, and most viral exposure and seroconversion occurs before age 15 years. However, approximately 10–20% of the women were susceptible to rubella virus infection and thus at risk for having an infant with CRS. This analysis can guide plans for introduction of RCV in DRC. Per World Health Organization recommendations, introduction of RCV should be accompanied by a campaign targeting all children 9 months to 14 years of age as well as vaccination of women of child bearing age through routine services.
Keywords: Rubella, Rubella antibody seroprevalence, Rubella serosurvey, Rubella IgG, Rubella incidence, Rubella transmission, Africa, Democratic Republic of the Congo, Pregnant women, Antenatal, Congenital rubella syndrome
1. Introduction
Rubella is a vaccine-preventable disease with safe and effective vaccines available since 1969. In the absence of vaccination, infection with the rubella virus usually occurs in childhood and causes a mild, self-limited illness characterized by rash and fever. However, if rubella virus infection occurs in a susceptible woman during the first trimester of pregnancy, miscarriage, fetal death, or congenital rubella syndrome (CRS) in the surviving infant often occurs. CRS can result in hearing impairment, blindness, congenital heart disease, mental retardation, and/or other manifestations [1,2].
A single dose of the most common rubella vaccine, RA27/3, is highly efficacious in providing lifelong protection against disease. Prevention of congenital rubella virus infection, including CRS, is the primary goal of rubella vaccination. The preferred approach for prevention of rubella and CRS is for countries to introduce a rubella-containing vaccine (RCV) through a wide-age range campaign and then incorporate it into the national childhood vaccination schedule [2].
In recent years, several World Health Organization (WHO) regions have established rubella/CRS elimination or accelerated control goals [2–6]. In 2003 the WHO region of the Americas set a rubella/CRS elimination goal, achieved the goal in 2009, and in April 2015, was declared free of endemic rubella and CRS [2–5,7]. The WHO European region set a 2015 rubella elimination goal [3,4,8]. In October 2014, a regional rubella elimination goal for the WHO Western Pacific Region was endorsed by its Regional Committee [6]. The WHO African region has not yet established a rubella elimination goal but recommends that countries document the burden of rubella virus infection/CRS and, when feasible, introduce RCVs [9].
RCVs have not been widely administered in the Democratic Republic of the Congo (DRC) nor introduced into the country’s national vaccination program [10]. However, there are tentative plans for introduction into the childhood vaccination schedule before 2020 [10]. Documentation of DRC’s historical burden of rubella virus infection and CRS has been minimal [1,11,12]. Moreover, DRC has no surveillance system for either disease, but rubella virus transmission within the country has been documented by a rubella antibody seroprevalence assessment conducted in Kinshasa city in 1987–1988 and by measles case-based surveillance since 2005, with serological testing for rubella-specific immunoglobulin type M (IgM) when suspected measles cases are negative for measles IgM [1,11,12]. Considering the interest in rubella control/elimination in the WHO African region, estimates of the burden of rubella virus infection/CRS in DRC are urgently needed [8,9]. We describe analyses of sera from 1605 pregnant women aged 15–46 years from 3 provinces in DRC which were available from a human immunodeficiency virus (HIV) sentinel survey among women attending antenatal care (ANC) sites [13–15]. Estimates of age- and site-specific rubella antibody seroprevalence, population, and fertility rates were used in catalytic models to estimate the incidence of CRS per 100,000 live births and the number of CRS cases born in 2013 in DRC. These estimates will be valuable to DRC’s Ministry of Public Health (MOPH) in planning for RCV introduction [10].
2. Methods
2.1. Rubella antibody seroprevalence assessment
HIV sentinel surveys among pregnant women attending ANC sites are based on a convenience sample of sentinel sites chosen to capture women from a variety of geographical and socioeconomic backgrounds. Details on how sites are selected can be found here [16]. The 2008–2009 HIV sentinel surveys in DRC included 30 sentinel ANC sites [13,14]. This study focuses on a subset of 7 ANC sites in 3 provinces.
Sera prepared from venous blood collected during 2008–2009, per WHO guidelines from 6615 pregnant women aged 15–47 years from 7 ANC sites in Bandundu, Kinshasa, and Kasaï Occidental provinces for national HIV sentinel serosurveys in DRC, had been used for a polio serosurvey in adults and were available for additional testing [13–16]. Specifically, the 7 ANC sites were (1) Kikwit (urban) and (2) Vanga (rural) in Bandundu, (3) Binza-Meteo, (4) Boyambi, and 5) Kingasani (all urban) in Kinshasa, and 6) Mikalayi (rural), and (7) Tshikapa (urban) in Kasaï Occidental (Fig. 1). The Demographic and Health Survey II (DHS II) conducted in 2013–2014 in DRC reported that, nationally, 88% of women aged 15–49 years participating in the survey who had a live birth in the 5 years preceding the survey had sought antenatal care during their pregnancy for their most recent live birth; the results were 90%, 89%, and 96% for women declaring residence in Bandundu, Kasai Occidental, and Kinshasa provinces, respectively, and were 94% and 86% for those declaring residence in urban and rural areas, respectively [17]. A survey conducted in 2009 in Kinshasa province among women at least 18 years of age who had been pregnant within the prior 3 years reported that 98% of women surveyed had attended ANC during their most recent pregnancy [18].
Fig. 1.
Approximate location of antenatal care (ANC) sites where blood specimens were collected in 2008–2009 from pregnant women in the rubella antibody seroprevalence assessment population, by zone de santé (health zone) and province, Democratic Republic of the Congo.
Sera from a randomly-sampled subset of the above-mentioned 6615 women were quantitatively analyzed for rubella-specific immunoglobulin type G (IgG). Prior to random sampling of the women for the rubella antibody serosurvey described in this report, HIV-positive women were excluded since HIV infection may negatively impact serum IgG levels; HIV prevalence in the 7 above-mentioned ANC sites ranged from 1.8% to 5.1% in 2008–2009 [13,14,19]. Also prior to random sampling for the rubella serosurvey, women attending the 3 ANC sites in the densely populated urban area of Kinshasa city (Binza-Meteo, Boyambi, and Kingasani) were pooled. Kinshasa was thereafter considered a single study site (referred to as the ‘‘Kinshasa” study site); thus, there were 5 study sites for the serosurvey (Table 1). From 5829 HIV-negative women from the original 6615, 1650 women (66 serum samples from each of 25 strata, i.e., 5 age groups from each of the 5 study sites) were randomly chosen. The sample size was determined based on the estimation of rubella antibody seroprevalence with a precision of assuming true prevalence of 80% and 5% unusable serum samples. Of the 1650 sera, 45 (3%) had insufficient volume for IgG assessment: 16, 7, 8, 10, and 4 from the Kikwit, Kinshasa, Mikalayi, Tshikapa, and Vanga study sites, respectively. Demographic attributes (e.g., age at blood collection, age at first pregnancy, number of pregnancies, rural or urban residence, level of education, occupation, and civil status) were analyzed for associations with rubella antibody seropositivity [13,14].
Table 1.
Demographic characteristics of the rubella antibody seroprevalence assessment population, overall and by 5 study sites in Bandundu, Kasaï Occidental, and Kinshasa Provinces, Democratic Republic of the Congo.
| Characteristic | Overall (n = 1605) No. (% of n) | Kikwit (n = 314, urban) No. (% of n) | Vanga (n = 326, rural) No. (% of n) | Kinshasa (n = 323, urban) No. (% of n) | Tshikapa (n = 320, urban) No. (% of n) | Mikalayi (n = 322, rural) No. (% of n) |
|---|---|---|---|---|---|---|
| Residence | ||||||
| Urban | 914 (57.0) | 275 (87.6) | 3 (0.9) | 322 (99.7) | 309 (96.6) | 5 (1.6) |
| Rural | 690 (43.0) | 39 (12.4) | 323 (99.1) | 0 (0) | 11 (3.4) | 317 (98.5) |
| Unknown | 1 (0.1) | 1 (0.3) | ||||
| Age at time of blood collection (years) | Range: 15–46 years | Range: 15–46 years | Range: 15–44 years | Range: 15–46 years | Range: 15–46 years | Range: 15–46 years |
| 15–19 | 321 (20.0) | 64 (20.4) | 65 (19.9) | 64 (19.8) | 62 (19.4) | 66 (20.5) |
| 20–24 | 322 (20.1) | 65 (20.7) | 65 (19.9) | 64 (19.8) | 64 (20.0) | 64 (19.9) |
| 25–29 | 318 (19.8) | 60 (19.1) | 66 (20.3) | 63 (19.5) | 66 (20.6) | 63 (19.6) |
| 30–34 | 320 (19.9) | 63 (20.1) | 65 (19.9) | 66 (20.4) | 62 (19.4) | 64 (19.9) |
| ⩾35 | 324 (20.2) | 62 (19.8) | 65 (19.9) | 66 (20.4) | 66 (20.6) | 65 (20.2) |
| Age at first pregnancy (years) | Range: 12–37 years | Range: 12–33 years | Range: 12–33 years | Range: 13–36 years | Range: 13–37 years | Range: 13–28 years |
| 12–17 | 664 (41.4) | 80 (25.5) | 117 (35.9) | 78 (24.2) | 178 (55.6) | 211 (65.5) |
| 18–23 | 769 (47.9) | 176 (56.1) | 178 (54.6) | 186 (57.6) | 120 (37.5) | 109 (33.9) |
| ⩾24 | 172 (10.7) | 58 (18.5) | 31 (9.5) | 59 (18.3) | 22 (6.9) | 2 (0.6) |
| Number of times pregnant including current | Range: 1–15 | Range: 1–10 | Range: 1–15 | Range: 1–12 | Range: 1–14 | Range: 1–12 |
| 1 time | 367 (22.9) | 95 (30.3) | 70 (21.5) | 94 (29.1) | 55 (17.2) | 53 (16.5) |
| 2 times | 254 (15.8) | 56 (17.8) | 57 (17.5) | 69 (21.4) | 34 (10.6) | 38 (11.8) |
| ⩾3 times | 984 (61.3) | 163 (51.9) | 199 (61.0) | 160 (49.5) | 231 (72.2) | 231 (71.7) |
| Civil status | ||||||
| Married at some timea | 1528 (95.2) | 289 (92.0) | 309 (94.8) | 302 (93.5) | 311 (97.2) | 317 (98.5) |
| Not-married | 77 (4.8) | 25 (8.0) | 17 (5.2) | 21 (6.5) | 9 (2.8) | 5 (1.6) |
| Educational level | ||||||
| None/1o completedb | 627 (39.1) | 40 (12.7) | 191 (58.6) | 31 (9.6) | 123 (38.4) | 242 (75.2) |
| 2o attended or higher | 978 (60.9) | 274 (87.3) | 135 (41.4) | 292 (90.4) | 197 (61.6) | 80 (24.8) |
| Occupation | ||||||
| Housekeeper | 638 (40.0) | 150 (48.1) | 38 (11.7) | 168 (53.0) | 247 (77.4) | 35 (10.9) |
| Farmer | 558 (35.0) | 43 (13.8) | 244 (74.9) | 0 (0) | 14 (4.4) | 257 (79.8) |
| Otherc,d | 400 (25.0) | 119 (38.1) | 44 (13.5) | 149 (47.0) | 58 (18.2) | 30 (9.3) |
Married at some time (refers to those married monogamous, married polygamous, in a free-union, separated, divorced, and widowed), Not-married (refers to those single).
None/1o completed (no education, primary school attended, or primary school completed), 2o attended or higher (secondary school attended, secondary school completed, graduate, or license obtained).
Other (refers to student, no occupation, government worker, business person, and other).
Nine responses were excluded from the analysis as their meaning could not be interpreted; the 9 were distributed as follows: 2 in Kikwit, 6 in Kinshasa, and 1 in Tshikapa.
Sera were shipped by air from DRC to the Centers for Disease Control and Prevention (CDC-Atlanta) on dry ice and stored at −20 °C prior to rubella IgG assessments performed at CDC-Atlanta’s Measles, Mumps, Rubella, and Herpesvirus Branch laboratory. Rubella-specific IgG antibody concentrations, expressed as International Units/millimeter (IU/ml), were determined using the Rubella IgG enzyme-linked immunosorbent assay (ELISA) II system according to the manufacturer’s instructions (Wampole Laboratories, Princeton, New Jersey). The optical density (OD) ratio was calculated by dividing the specimen OD by the cutoff value supplied by the manufacturer. Specimens with OD ratios >2.2 were diluted with kit dilution buffer, and rubella-specific IgG antibody concentrations were determined from the diluted serum. Sera with titers of 10 IU/ml were considered seropositive for rubella antibody, whereas those with an equivocal determination (8.19–9.99 IU/ml) or with titers of <8.19 IU/ml were considered seronegative [2]. The 14 women with equivocal determination were distributed among 4 study sites as follows: Mikalayi (n = 6, ages in years = 17, 19, 22, 23, 37, 38), Vanga (n = 1, age in years = 34), Tshikapa (n = 4, ages in years = 17, 29, 34, 35), and Kinshasa (n = 3, ages in years = 17, 17, 18). Immune individuals with ELISA-determined IgG < 10 IU/ml should be too small in number to affect the results presented in this report [20].
Site-specific rubella antibody seroprevalence was estimated overall and for each 5-year age group, accounting for the sampling probability in each stratum and treating the equivocals as seronegative. The rubella antibody seroprevalence estimates and associated confidence intervals (CIs) are representative of the study site assessment populations only and not of any DRC populations at large. The Pearson Chi-square test was used to assess differences in rubella antibody seroprevalence overall for the 5 study sites, across 5 age strata (overall and within each site), and across the other demographic attributes (Table 1); when statistically significant differences were observed, pairwise analyses were conducted using the Pearson Chi-square test. The Cochran-Mantel-Haenszel (CMH) Chi-square was used to test for statistically significant associations between rubella antibody seroprevalence and site controlling for age and between rubella antibody seroprevalence and age controlling for site. Tests were considered statistically significant at p < 0.05.
2.2. Estimating CRS incidence and the number of CRS cases born in 2013
The age- and site-specific rubella antibody seroprevalence estimates were used in catalytic models to estimate the rate at which susceptible women were infected with rubella virus (i.e., force of rubella virus infection). The force of infection estimates were then used with estimated populations and fertility rates to obtain the CRS incidence/100,000 live births in 2013 and numbers of CRS cases born in 2013. Details follow.
2.2.1. Demographic data
The total number of women of child-bearing age (WCBA) for 2013 in the zones de santé (health zones) in which the ANC sites were situated were extracted from DRC’s Expanded Programme on Immunization (EPI)-MOPH population projections based on the 1984 census (the only official census ever conducted at the zone de santé level); health zones in DRC are the equivalent of districts in other countries [17]. Based upon those EPI-MOPH projections, in 2013 DRC’s estimated total population was 86,508,633, and the estimated total population of Bandundu, Kinshasa, and Kasaï Occidental provinces was 8,350,279, 8,103,633, and 8,252,695, respectively; estimates indicate that in 2013 the population of WCBA in each province was 21% of the province’s estimated total population. The 2013 estimated population of WCBA for the health zones in which the 7 ANC sites were located were as follows: Boyambi (32,523), Binza Meteo (85,987), Kikwit (42,713), Kingasani (47,452), Mikalayi (44,715), Tshikapa (73,249), and Vanga (57,273).
The age distribution of women in urban and rural areas was extracted from the 2013–2014 DRC DHS II [17]. To calculate site-specific numbers of women in a given age group, the total number of WCBA in the corresponding health zone was multiplied by the proportion of WCBA in the age of interest according to whether or not the site was considered urban or rural. In these analyses, the Kinshasa, Kikwit and Tshikapa study sites were considered urban, and the Mikalayi and Vanga sites were considered rural [13,14,17]. The total female population size (33,976,774 for 2013) and the proportion of DRC’s population living in urban and rural settings (35.4% and 64.6%, respectively, for 2013) were extracted from United Nations (UN) population sources, and the two were multiplied together to obtain the number of females living in urban and rural areas in DRC [21,22]. These numbers were then scaled up by 28%, to account for a 28% difference between the total population size according to UN sources and that in the DRC EPI-MOPH projections for 2013 (67,513,677 vs 86,508,633, respectively). The number of women in each five year age group (15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years) in urban and rural areas was calculated by multiplying the female population size in urban or rural areas by the proportion of the female population in the given area that was in the age group of interest. Age-specific fertility rates for 2013–2014 for urban and rural settings were extracted from the 2013–2014 DRC DHS II [17]. The number of live births in each site or setting in DRC among mothers in each 5-year age group was calculated by multiplying the corresponding age-specific fertility rates and numbers of women in the site or area of interest.
2.2.2. CRS incidence and CRS case estimations by site
Following previous methods, four age-structured catalytic models were fitted to the observed age-stratified rubella antibody seroprevalence estimates from the different study sites using maximum likelihood to estimate the force of rubella virus infection [23–25]. This was assumed to differ (models A and B) or be identical (models C and D) for the ages <15 and 15 years [23,26]. The sensitivity of the rubella serological (antibody) assay was either estimated (models A and C) or assumed to be 100% (models B and D) [23,26]. Models A–D are described in the article supplement (Table S1). The following equation gives the proportion of individuals of age that are seronegative, where is the sensitivity of the serological assay, and and are the average force of infection among younger and older individuals respectively.
Subsequent estimates of the CRS incidence were based on models that were selected according to biological plausibility using criteria described elsewhere, with the additional criterion that model B was selected in preference to model A if all the other criteria were satisfied and the estimated sensitivity of the assay was 100% for model A, and the lower limit of the 95% confidence interval was implausibly low (less than 95%) [24]. If no model provided biologically-plausible estimates or if the model fitted the data from a given site poorly (passed through the confidence intervals of one or fewer datapoints), we excluded those data from estimates for urban or rural areas and from the whole of DRC. The article supplement provides details on the fitting.
For all sites, the best fitting value for the force of rubella virus infection was used to estimate the CRS incidence per 100,000 live births among women in 5 year age groups between 15 and 44 years using the following expression, where s(A) is the proportion of women in age group A that are susceptible.
As in previous analyses, the risk of a child being born with CRS was assumed to be 65% if the mother was infected during the first 16 weeks of pregnancy and zero thereafter [23,24]. The weighted CRS incidence per 100,000 live births among women aged 15–44 years for each site was calculated as the average of the CRS incidence per 100,000 live births in each 5 year maternal age group, weighted by the site-specific number of live births in each maternal age group in 2013. The number of CRS cases born in each site was calculated by multiplying the site-specific number of live births occurring in each 5 year maternal age group by the estimated CRS incidence for each site. CIs (95%) for the force of rubella virus infection and CRS incidence for each site and catalytic model were obtained by bootstrapping using 1000 bootstrap datasets generated using the approach of Shkedy et al. [27]. These bootstrap-derived estimates were then used to compile the force of rubella virus infection, weighted CRS incidence per 100,000 live births in urban and rural areas and for the whole of DRC. Additional details are provided in the article supplement (Table S2). In sensitivity analyses and for consistency with previous analyses, we repeated the analyses, treating the equivocals as seropositive [24]. In addition, given the discrepancy between the population size according to UN sources and that of DRC’s EPI-MOPH projections for 2013, we calculated the number of CRS cases in urban and rural areas, and overall in DRC obtained by using female population size, as calculated according to UN sources [21,22]. The site-specific number of CRS cases consistent with the population size based on UN population sources were calculated by scaling down the estimates obtained using population data from DRC’s EPI-MOPH projections for 2013 by 28%.
2.3. Data analyses
Data analyses were conducted using SAS version 9.2 (SAS Institute, Cary, North Carolina), EPI-INFO version 7 (CDC, Atlanta, Georgia), and EXCEL version 2010 (Microsoft Corporation, Redmond, Washington). Fig. 1 was created using ArcGIS version 10.1 (Environmental Systems Research Institute, Redlands, California). The catalytic modeling analyses were carried out using a program written in the ‘‘C” programming language [28]. The fitting used an algorithm based on the simplex method of Nelder and Mead [29].
2.4. Ethical approval
The Human Subjects Research Coordinator of the Center for Global Health, CDC-Atlanta reviewed the protocol for the work described in this report. The work was determined to be research not involving human subjects, because it involved using unlinked/anonymous specimens collected for another purpose, and was therefore exempt from institutional review board approval. The protocol was reviewed and approved by DRC’s MOPH.
3. Results
Sera from 1605 HIV-negative, pregnant women, aged 15–46 years, who attended ANC in Bandundu, Kasaï Occidental, and Kinshasa provinces in DRC during 2008–2009, were analyzed for rubella-specific IgG. Relevant demographic attributes of these women are included in Table 1. Overall and at all sites, >80% of women had their first pregnancy before age 24 years, 50% of women had been pregnant 3 times, and >90% had been married at some time. The Kinshasa and Kikwit sites had the highest proportion of women having attended secondary school or higher education. In the rural sites (Mikalayi and Vanga), farming was the most common profession, as compared with housekeeping in the urban sites (Kikwit, Kinshasa, and Tshikapa).
Among the 1605 women overall, 84% (95% CI 82, 86) were estimated to be seropositive for rubella IgG (Fig. 2). Within the Kinshasa site, estimated rubella antibody seroprevalence was higher in the 20–24 year age group (89%) than the 15–19 (78%, p value = 0.01) and 25–29 (75%, p value < 0.001) year age groups. In contrast, no statistically significant trends or differences in estimated rubella antibody seroprevalence among age groups were found in the overall assessment population or within the Kikwit, Vanga, Mikalayi, or Tshikapa sites (Fig. 2). The association between age and antibody seroprevalence, adjusting for study site, was not significant (CMH Chi-square p value = 0.10).
Fig. 2.
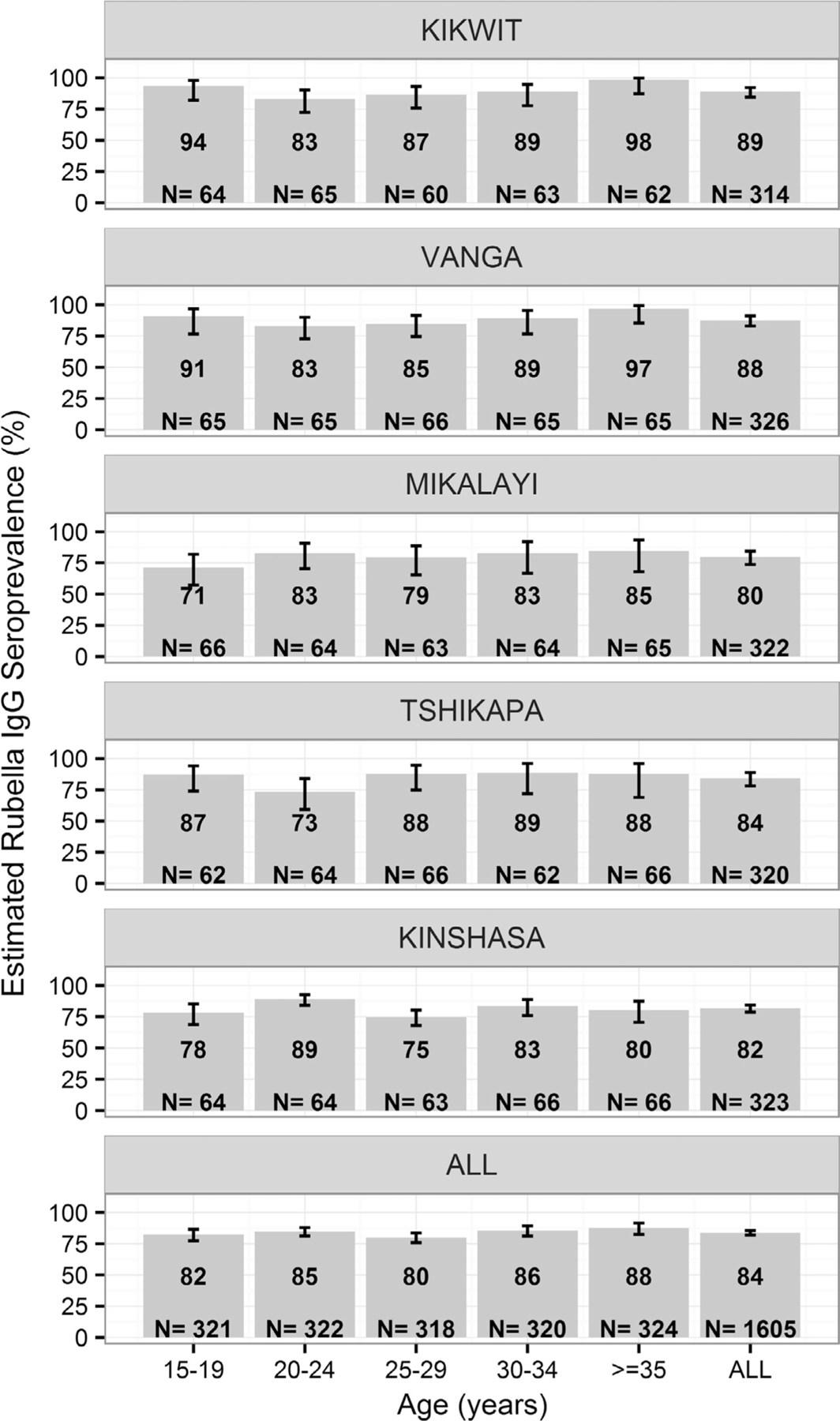
Estimated seroprevalence (%) of rubella IgG in the assessment population, by age group and study site in Bandundu, Kasaï Occidental, and Kinshasa provinces, Democratic Republic of the Congo. Equivocals were classified as seronegative in these analyses. Footnote: For each age group and overall, the number of serum samples analyzed by enzyme-linked immunosorbent assay (ELISA) is noted. Within the Kinshasa site, the 20–24 year age group had a higher antibody seroprevalence than the 15–19 (p value = 0.01) and 25–29 (p value < 0.001) year age groups. Kikwit (89%) and Vanga (88%) study sites had higher overall rubella antibody seroprevalence than the Mikalayi (80%) and Kinshasa (82%) sites (all p values ⩽0.03).
The association between site and antibody seroprevalence, controlling for age, was statistically significant (CMH Chi-square p value = 0.01). In pairwise comparisons, Kikwit (89%) and Vanga (88%) study sites (both in Bandundu province) each had higher estimated overall rubella antibody seroprevalence than the Mikalayi (80%) and Kinshasa (82%) sites (all p values ⩽0.03).
In the overall assessment population, estimated rubella antibody seroprevalence was similar for women declaring residence in urban (84%) versus rural (83%) settings (p value = 0.67). Moreover, no statistically significant associations between estimated rubella antibody seroprevalence and the other demographic variable categories described in Table 1 were observed.
Table 2 summarizes estimates from the selected models for the force of rubella virus infection per 1000 susceptible individuals per year aged <15 and 15 years, CRS incidence per 100,000 live births for women aged 15–44 years in 2013, and the number of CRS cases born in 2013; all estimates are shown by study site, by rural and urban settings, and for DRC overall. The estimates based upon UN population and DRC EPI-MOPH projections total population estimates are presented separately. The best-fitting estimates of the annual force of rubella virus infection, the serological assay sensitivity, and the CRS incidence estimated from each of the 4 catalytic models are described in the article supplement. For Kikwit, Vanga, Mikalayi, and Tshikapa, the best-fitting model (Model B) assumed that the force of rubella virus infection was different for persons aged <15 years and those aged 15 years and was estimated, and the sensitivity of the assay was fixed at 100% (Supplement Tables S1 and S2). The best-fitting model for Kinshasa fit the data poorly (Supplement Fig. S1 and Table S2); thus Kinshasa’s antibody seroprevalence data were excluded when generating estimates for urban areas and the whole of DRC.
Table 2.
Estimated force of rubella virus infection among susceptible individuals <15 and 15 years of age/year, CRS incidence/100,000 live births among women aged 15–44 years in 2013, and estimated number of CRS cases born in 2013 by 5 study sites in Bandundu, Kasaï Occidental, and Kinshasa provinces, rural and urban settings, and overall in the Democratic Republic of the Congo. Equivocals were classified as seronegative in these analyses.
| Study site or setting | Force of Rubella Virus Infection per 1000 susceptible individuals/year (95% Confidence Intervals)a |
Estimated weighted CRS incidence/100,000 live births among women aged 15–44 years in 2013b | Estimated number of CRS cases born in 2013 (95% confidence intervals),a with the population size obtained from: |
||
|---|---|---|---|---|---|
| <15 years of age | ⩾15 years of age | (95% confidence intervals)a | Democratic Republic of the Congo Expanded Programme on Immunization-Ministry of Public Health Population projections based on the 1984 census | UN population sources [21] | |
| Kikwit | 129 (98, 164) | 27 (0, 69) | 61 (0, 151) | 5 (0, 11) | 3 (0, 8) |
| Vanga | 118 (88, 155) | 32 (0, 72) | 81 (0, 184) | 11 (0, 24) | 8 (0, 18) |
| Mikalayi | 92 (64, 120) | 23 (0, 68) | 92 (0, 246) | 10 (0, 26) | 7 (0, 18) |
| Tshikapa | 113 (80, 146) | 20 (0, 73) | 61 (0, 202) | 8 (0, 26) | 6 (0, 18) |
| Kinshasa | 66 (59, 72) | 66 (59, 72) | 252 (238, 264) | 73 (68, 76) | 52 (49, 55) |
| Urbanc | 120 (83, 159) | 24 (0, 73) | 61 (0, 186) | 724 (0, 2211) | 565 (0, 1725) |
| Rurald | 104 (70, 149) | 28 (0, 71) | 82 (0, 218) | 2037 (0, 5397) | 1590 (0, 4212) |
| Overalle | 110 (71, 152) | 27 (0, 72) | 69 (0, 186) | 2886 (342, 6395) | 2253 (267, 4991) |
CRS, Congenital Rubella Syndrome.
Confidence intervals were obtained by bootstrapping.
Weighted by the number of live births occurring among women in different maternal age groups.
Compiled using estimates derived from rubella antibody seroprevalence data from Kikwit and Tshikapa. As explained in Sections 2 and 3, antibody seroprevalence data from Kinshasa were excluded from these estimations.
Compiled using estimates derived from rubella antibody seroprevalence data from Mikalayi and Vanga.
Compiled using estimates from the urban and rural settings.
With the exception of the Kinshasa site, where the selected best-fit model assumed that the force of rubella virus infection was identical for all age groups, the estimated force of rubella virus infection was higher for <15 year olds compared to 15 year olds for each site, setting, and the whole of DRC, e.g. 120 per 1000 per year (95% CI 83, 159) vs 24 (95% CI 0, 73), respectively, for urban areas (Table 2). Moreover except for Kinshasa, the forces of rubella virus infection for a given age group did not differ significantly between sites or for rural and urban settings and overall for DRC (Table 2).
The estimated CRS incidence (CRS cases/100,000 live births) for 2013 ranged from 61 in both Kikwit (95% CI 0, 151) and Tshikapa (95% CI 0, 202), to 92 (95% CI 0, 246) in Mikalayi. In urban settings, the estimated CRS incidence was 61 (95% CI 0, 186) per 100,000 live births, and the estimated number of CRS cases was 724 (95% CI 0, 2211). In rural settings, the estimated CRS incidence was 82 (95% CI 0, 218) per 100,000 live births, and the estimated number of CRS cases was 2037 (95% CI 0, 5397). The overall estimated CRS incidence for DRC for 2013 was 69 (95% CI 0, 186) per 100,000 live births, and the estimated number of CRS cases was 2886 (95% CI 342, 6395). When the population size was based on UN population sources, the estimated number of CRS cases in 2013 in urban areas, rural areas, and overall DRC decreased to 565 (95% CI 0, 1725), 1590 (95% CI 0, 4212) and 2253 (95% CI 267,4991) respectively. In general, including equivocals as seropositive did not greatly affect the estimates, with the confidence intervals overlapping with those obtained by treating equivocals as seronegative (Table S3 in the supplement).
4. Discussion
This is the first documentation of rubella antibody seroprevalence among WCBA in geographic areas outside of DRC’s capital, Kinshasa [11]. Availability of sera previously obtained from pregnant women attending ANC in three provinces made the study feasible [13–15]. The results indicate an overall estimated rubella antibody seroprevalence of 84% in the assessment population with a range of 80–89% among the 5 study sites. Rubella virus transmission is endemic in DRC, and the results in this report suggest that the majority of women are exposed to rubella virus and subsequently seroconvert before age 15 years. No trends or differences in the estimated rubella antibody seroprevalence were observed between the age groups in the overall assessment population.
A previous serosurvey in Kinshasa, conducted in 1987 among 106 women aged 16–45 years having just given birth reported high, age-independent rubella antibody seroprevalence (93%), suggesting a high level of viral transmission [11]. Other publications confirm more recent rubella virus transmission in DRC and report the majority of cases being aged <15 years with some cases among WCBA [1,12]. Our finding of lower antibody seroprevalence in Kinshasa and lower overall antibody seroprevalence than previously documented may be explained by conducting the studies at different points in the epidemic cycle of rubella, differences in laboratory methodologies (e.g., haemagglutination inhibition versus ELISA), or differences in the age distribution of the populations [11,30].
Our observations are generally consistent with trends in overall rubella IgG seroprevalence described among pregnant women from other countries in the WHO African region before the introduction of RCV [1,31–52]. Our finding of no statistically significant increases in rubella antibody seroprevalence with increasing age (after approximately 15 years of age) has been observed in a number of the above-mentioned serosurveys and others [34,35,39,42–46,48, 49,53,54]. Moreover, in agreement with published observations from other African countries, for the assessment population in DRC overall, rubella antibody seroprevalence was similar among women declaring residence in urban versus rural settings; however, differences in antibody seroprevalence were observed between different geographic areas in the country [31,34,38,41,43,44,46,51]. Last and consistent with reports from other African countries, age at first pregnancy, number of pregnancies, civil status, educational level, and occupation were not associated with rubella antibody seroprevalence in DRC [34,38,41,44–46,53].
The estimate for CRS incidence of 69 per 100,000 live births for 2013 in DRC overall is consistent with estimates for other African countries and the African region as a whole; more specifically, estimates of CRS incidence in 2010 for 13 African countries ranged from 19 to 283 per 100,000 live births [24]. Additionally, for the African region overall in 2010, CRS incidence was estimated to be 116 per 100,000 live births (95% CI 56, 235) [24]. DRC was among 7 African countries estimated to have >1000 CRS cases born in 2010 [24].
To date, 8 of 47 countries in the WHO African region (Burkina Faso, Cape Verde, Ghana, Mauritius, Rwanda, Senegal, Seychelles, and Tanzania) have introduced RCV into routine vaccination schedules, given at age 9 months simultaneously with measles containing vaccine [2]. In most of these countries, introduction of RCV was accompanied by a wide age range campaign targeting all children aged 9 months to 14 years (catch-up campaigns) [2,8]. Moreover, vaccination of girls not eligible for catch-up campaigns and of WCBA is recommended through routine immunization service delivery [2].
RCV introduction is planned in DRC before 2020 [10]. It is recommended that countries introducing rubella vaccination be able to maintain rubella vaccination coverage of at least 80% with at least one dose nationally either through routine immunization services or through campaigns [2,3,8]. A proxy indicator for being able to achieve this recommendation is a country’s experience and success with the delivery of routine measles vaccination. Available reports indicate that DRC has had challenges with achieving national and sub-national annual measles vaccination coverage of 80% [17,55–57]. Therefore, during the years before introducing RCV, DRC will need to (a) focus efforts on improving the delivery of measles vaccination, thereby creating a successful platform on which to introduce rubella vaccination; (b) establish an integrated nationwide measles-rubella surveillance system as well as, at least, sentinel sites for CRS surveillance; and (c) use best practices from measles vaccination campaigns to assure a high-quality rubella wide-age campaign. Moreover, as found in two Nigerian studies, awareness of rubella virus infection in DRC is probably low; therefore, increased public awareness of CRS should accompany RCV introduction [44,46]. According to this report, a significant proportion of WCBA in DRC (including adolescent girls in whom pregnancies at age 12 years are recorded in DRC’s ANC site data) are susceptible to rubella virus infection and must be considered in the country’s RCV introduction. Studies measuring rubella-specific IgM in pregnant women and measles case-based surveillance data from the WHO African region provide evidence that new rubella virus infections occur in adult women in Africa [1,12,33,42,46,50,51,54,58].
We note that the selected catalytic model used to estimate the force of infection was a poor fit to the Kinshasa rubella antibody seroprevalence data. This poor fit resulted from the fact that rubella antibody seroprevalence for older women remained similar to that for the youngest women, whereas catalytic models assume that the proportion of women that are susceptible decreases with increasing age, if the force of infection is non-zero and that the average force of infection is constant over time. The similar rubella antibody seroprevalence for younger and older women in Kinshasa could have resulted from several factors which remain unclear. For example, it could have occurred if there was much migration of either younger or older women from high or low transmission settings, respectively, into Kinshasa; if the force of infection increased disproportionately for younger people, or if, the women attending ANC were not representative of others in their age group.
The analysis had limitations. Because the ANC sites selected for the HIV sentinel serosurveys were a convenience sample of all ANC sites in the various provinces in DRC, the population of pregnant women was not designed to be representative of all pregnant women/WCBA in the health zones, provinces, or in DRC as a whole [13,14,16]. Data regarding the lifetime residential history of the women in the assessment population were unavailable; thus, it was not possible to hypothesize on why higher rubella antibody seroprevalence was observed in the Kikwit and Vanga sites versus the Kinshasa and Mikalayi sites or among the 20–24 age group at the Kinshasa site. Because the catalytic model used to estimate the force of rubella virus infection poorly fitted the Kinshasa rubella antibody seroprevalence data, the Kinshasa data were excluded when calculating the estimates for CRS incidence in urban areas, and then it was assumed that the estimated CRS incidence could be applied to Kinshasa. The latter assumption would have led to an overestimate in the overall CRS incidence in DRC if the true force of infection in Kinshasa was so high that most women had been infected in childhood. We acknowledge the wide confidence intervals associated with the CRS incidence and CRS case estimates and that many of the lower confidence intervals for the site and urban/rural estimates approach zero; nonetheless, the estimates provide information for DRC, beyond what is currently available in the absence of specific rubella or CRS surveillance. However, it should be noted that the lower 95% confidence limits do not approach zero for the national estimates of the burden of CRS.
5. Conclusions
As the WHO African Region begins discussions about rubella and CRS elimination, data are needed to document the burden of rubella virus transmission/infection and of CRS prior to introducing RCV [8,9]. In the absence of formal surveillance for rubella/CRS, the historical and current burden of both in DRC is largely unknown; however, there is evidence for rubella virus transmission [this report, 1,11,12]. The use of sera from HIV sentinel surveys among pregnant women attending ANC provided a unique opportunity to estimate the burden of rubella virus infection and CRS. The results reported here can add to other available data to guide plans for introduction of RCV in DRC and will provide a background from which the impact of vaccination can be assessed.
Supplementary Material
Acknowledgements
The work presented in this report was supported by the research program of the Global Immunization Division, CDC-Atlanta. The authors express their appreciation to Drs. Luca Flamigni, Hypolite Sadiki, and Rogers Ngalamulume, former and current staff of the Division of Global HIV/AIDS, Centers for Disease Control and Prevention (CDC), Kinshasa, DRC, for facilitating the collaboration with DRC’s Programme National de Lutte Contre les IST/SIDA (PNLS). Appreciation also goes to the technical staff of the PNLS laboratory for preparing and shipping the serum samples used in the rubella antibody seroprevalence assessment to CDC-Atlanta, to Mr. Brian Kaplan and Ms. Gina Marie Perleoni, at the Geospatial Research Analysis and Services Program at the Agency for Toxic Substances and Disease Registry of CDC-Atlanta, for preparing the map in Fig. 1, to Dr. Kim Porter and Ms. Kristin Brown, formerly of CDC-Atlanta’s Global Immunization Division, for cleaning and merging the study databases, and to Drs. James Alexander, David Bell, Allen Craig, Eric Mast, and Steve Wassilak and Ms. Clarice Conley, currently of CDC-Atlanta, for valuable input on the original version of the manuscript.
Funding
The work presented in this report was supported by the research program of the Global Immunization Division, Centers for Disease Control and Prevention.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.10.059.
References
- [1].Goodson JL, Masresha B, Dosseh A, et al. Rubella epidemiology in Africa in the prevaccine era, 2002–2009. J Infect Dis 2011;204(Suppl 1):S215–25. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. Rubella vaccines: WHO position paper Wkly Epidemiol Rec; 2011;86:301–16. [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention. Progress toward control of rubella and prevention of congenital rubella syndrome-worldwide, 2009. MMWR Morb Mortal Wkly Rep 2010;59:1307–10. [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention. Rubella and congenital rubella syndrome control and elimination-global progress, 2000–2012. MMWR Morb Mortal Wkly Rep 2013;62:983–6. [PMC free article] [PubMed] [Google Scholar]
- [5].Castillo-Solorzano C, Reef SE, Morice A, et al. Guidelines for the documentation and verification of measles, rubella, and congenital rubella syndrome elimination in the region of the Americas. J Infect Dis 2011;204(suppl 2): S683–9. [DOI] [PubMed] [Google Scholar]
- [6].World Health Organization Western Pacific Region. Report of the 24th meeting of the technical advisory group on immunization and vaccine-preventable diseases in the Western Pacific region, 9–12 June 2015. Manilla [Available from the Western Pacific Regional World Health Organization Office].
- [7].Pan American Health Organization. Elimination of rubella and congenital rubella syndrome in the Americas. Fact Sheet 2015, 29 April 2015. <http://www.paho.org/hq/index.php?option=com_content&view=article&id=10798%3A2015-americas-free-of-rubella&Itemid=1926&lang=en> [accessed 26 July 2016]. [Google Scholar]
- [8].Burki T GAVI alliance to roll our rubella vaccine. Lancet Infect Dis 2012;12:15–6. [Google Scholar]
- [9].World Health Organization. Consensus points from the African regional consultation on measles and rubella/CRS elimination, 27–29 November 2013. <http://www.sabin.org/updates/events/african-regional-consultation-measles-rubellacrs-elimination> [accessed 26 July 2016].
- [10].Ministry of Public Health-Democratic Republic of the Congo. Plan Pluri Annuel Complet du PEV de la République Démocratique du Congo, 2015–2019, novembre 2014, Programme Elargi de Vaccination, Ministère de la Santé Publique, Kinshasa, République Démocratique du Congo (Comprehensive Multi-Year Plan of the EPI of the Democratic Republic of the Congo, 2015–2019, November 2014, Expanded Programme on Immunization, Ministry of Public Health, Kinshasa, Democratic Republic of the Congo) [Available from the Expanded Programme on Immunization, Ministry of Public Health, Democratic Republic of the Congo].
- [11].Omanga U, Goussard B, Kapepela K, et al. Séroprévalence de la rubéole à Kinshasa (Zaïre) [Seroprevalence of rubella in Kinshasa (Zaïre)]. Bull Soc Path Ex 1991;84:994–1001. [PubMed] [Google Scholar]
- [12].Nsambu MN, Coulibaly T, Donnen P, et al. Fréquence de la rubéole à Kinshasa de 2010–2012, République Democratic du Congo (RDC): données issues du système de surveillance de la rougeole [Incidence of rubella in 2010–2012 in Kinshasa, Democratic Republic of the Congo (DRC): data from the measles case-based surveillance system]. Santé Publique 2014;26:393–7. [PubMed] [Google Scholar]
- [13].Ministry of Public Health-Democratic Republic of the Congo. Rapport épidémiologique de surveillance du VIH chez les femmes enceintes fréquentant les structures de CPN. 2008. (Epidemiological report of HIV surveillance in pregnant women attending prenatal consultations 2008) [Available from the National HIV/AIDS Program, Ministry of Public Health-Democratic Republic of the Congo].
- [14].Ministry of Public Health-Democratic Republic of the Congo. Rapport épidémiologique de surveillance du VIH/SIDA chez les femmes enceintes fréquentant les structures de CPN. 2009. (Epidemiological report of HIV/AIDS surveillance in pregnant women attending prenatal consultations 2009) [Available from the National HIV/AIDS Program, Ministry of Public Health-Democratic Republic of the Congo.].
- [15].Alleman MM, Wannemuehler KA, Weldon WC, et al. Factors Contributing to Outbreaks of Wild Poliovirus Type 1 Infection Involving Persons Aged >= 15 Years in the Democratic Republic of the Congo, 2010–2011, Informed by a Pre-Outbreak Poliovirus Immunity Assessment. J Infect Dis 2014;210(Suppl 1): S62–73. [DOI] [PubMed] [Google Scholar]
- [16].UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. Guidelines for conducting HIV Sentinel Serosurveys among Pregnant Women and Other Groups; 2003. <http://www.who.int/hiv/pub/surveillance/anc_guidelines/en/> [accessed 26 July 2016].
- [17].Ministry of Planning and Monitoring of the Implementation of the Revolution of Modernity, Ministry of Public Health, Democratic Republic of the Congo and MEASURE DHS. Ministère du Plan et Suivi de la Mise en Œuvre de la Révolution de la Modernité, Ministère de la Santé Publique, Kinshasa, République Démocratique du Congo, et MEASURE DHS, ICF International, Rockville, Maryland, USA. République Démocratique du Congo Enquête Démographique et de Santé, 2013–14. (Ministry of Planning and Monitoring of the Implementation of the Revolution of Modernity, Ministry of Public Health, Kinshasa, Democratic Republic of the Congo and MEASURE DHS, ICF International, Rockville, Maryland, USA. Democratic Republic of the Congo Demographic and Health Survey, 2013–14) <http://dhsprogram.com/publications/publication-FR300-DHS-Final-Reports.cfm> [accessed 26 July 2016].
- [18].Feinstein L, Dimomfu B, Mupenda B, et al. Antenatal and delivery services in Kinshasa, Democratic Republic of Congo: care-seeking and experiences reported by women in household-based survey. Trop Med Int Health 2013;18:1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Singh H, Chiu Y, Wilkin T. Measles, Mumps, and Rubella Serostatus and response to MMR vaccination among HIV-infected adults. AIDS Patient Care STDS 2015;29(9):461–4. doi: 10.1089/apc.2015.0050. Epub 2015 Jul 8. [DOI] [PubMed] [Google Scholar]
- [20].Zaman K, Fleming JA, Victor JC, et al. Noninterference of rotavirus vaccine with measles-rubella vaccine at 9 months of age and improvements in antirotavirus immunity: a randomized trial. J Infect Dis 2016;213:1686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].UN Statistics Division. United Nations population division. World Population Prospects; 2012. [revision].
- [22].United Nations Department of Economic and Social Affairs. World statistics pocketbook, 2014 World Edition. New York (NY) <http://unstats.un.org/unsd/pocketbook/> [accessed 26 July 2016]. [Google Scholar]
- [23].Cutts FT, Vynnycky E. Modelling the incidence of congenital rubella syndrome in developing countries. Int J Epidemiol 1999;28:1176–84. [DOI] [PubMed] [Google Scholar]
- [24].Vynnycky E, Adams EJ, Cutts FT, et al. Using seroprevalence and immunisation coverage data to estimate the global burden of Congenital Rubella Syndrome, 1996–2010. PLoS One 2016. doi: 10.1371/journal.pone.0149160. [DOI] [PMC free article] [PubMed]
- [25].Mao B, Chheng K, Wannemuehler K, et al. Immunity to polio, measles and rubella in women of child-bearing age and estimated congenital rubella syndrome incidence, Cambodia, 2012. Epidemiol Infect 2015;143:1858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Muench H Catalytic models in epidemiology Cambridge: Harvard University Press; 1959. [Google Scholar]
- [27].Shkedy Z, Aerts M, Molenberghs G, et al. Modelling age-dependent force of infection from prevalence data using fractional polynomials. Stat Med 2006;25:1577–91. [DOI] [PubMed] [Google Scholar]
- [28].Press WH, Teukolsky SA, Vetterling WT, et al. Numerical recipes in C: the art of scientific computing 2nd ed. New York: Cambridge University Press; 1992. [Google Scholar]
- [29].Nelder JA, Mead R. A simplex method for function minimization. Comp J 1965;7:308–13. doi: 10.1093/comjnl/7.4.308. [DOI] [Google Scholar]
- [30].Horstmann DM. Rubella: the challenge of its control. J Infect Dis 1971;123:640–54. [DOI] [PubMed] [Google Scholar]
- [31].Gomwalk NE, Ahmad AA. Prevalence of rubella antibodies on the African Continent. Rev Infect Dis 1989;11:116–21. [DOI] [PubMed] [Google Scholar]
- [32].Rodier MH, Berthonneau J, Bourgoin A, et al. Seroprevalences of toxoplasmosis, malaria, rubella, cytomegalovirus, HIV and treponemal infections among pregnant women in Cotonou, Republic of Benin. Acta Trop 1995;59:271–7. [DOI] [PubMed] [Google Scholar]
- [33].Linguissi LS, Nagalo BM, Bisseye C, et al. Seroprevalence of toxoplasmosis and rubella in pregnant women attending antenatal private clinic at Ouagadougou, Burkina Faso. Asian Pac J Trop Med 2012;5:810–3. [DOI] [PubMed] [Google Scholar]
- [34].Tahita MC, Hübschen JM, Tarnagda Z, et al. Rubella seroprevalence among pregnant women in Burkina Faso. BMC Infect Dis 2013;13:164. doi: 10.1186/1471-2334-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fokunang CN, Chia J, Ndumbe P, et al. Clinical studies on seroprevalence of rubella virus in pregnant women of Cameroon regions. Afr J Cln Exper Microbiol 2010;11:79–94. [Google Scholar]
- [36].Ndumbe PM, Andela A, Nkemnkeng-Asong J, et al. Prevalence of infections affecting the child among pregnant women in Yaoundé, Cameroon. Med Microbiol Immunol 1992;181:127–30. [DOI] [PubMed] [Google Scholar]
- [37].Sandow D, Okubagzhi GS, Arnold U, et al. Seroepidemiological study in rubella in pregnant women in Gondar Region, northern Ethiopia. Ethiop Med J 1982;20:173–8. [PubMed] [Google Scholar]
- [38].Lawn JE, Reef S, Baffoe-Bonnie B, et al. Unseen blindness, unheard deafness, and unrecorded death and disability: congenital rubella in Kumasi, Ghana. Am J Public Health 2000;90:1555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Faye-Kette YH, Sylla-Koko DJ, Akoua-Koffi GC, et al. Séroprévalence de la rubéole chez 461 femmes enceintes à Abidjan (Côte d’Ivoire). [Seroprevalence of rubella in 461 pregnant women in Abidjan (Ivory Coast)]. Bull Soc Pathol Exot 1993;86:185–7. [PubMed] [Google Scholar]
- [40].Vrinat M, Dutertre J, Helies H, et al. Prévalence sérologique de la rubéole chez la femme enceinte à Abidjan (A serological survey of rubella among pregnant women in Abidjan). Med Trop 1978;38:53–7. [PubMed] [Google Scholar]
- [41].Kombich JJ, Muchai PC, Borus PK. Seroprevalence of natural rubella antibodies among antenatal attendees at Moi Teaching and Referral Hospital, Eldoret, Kenya. J Immunol Tech Infect Dis 2012;1:1. doi: 10.4172/2329-9541.1000102. [DOI] [Google Scholar]
- [42].Dromigny JA, Pécarrère JL, Ollivier GL, et al. Séroprévalence de la rubéole chez la femme enceinte à Antananarivo, Etude Effectuée à l’Institut Pasteur de Madagascar sur 853 sérums (Rubella seroprevalence among pregnant women at Antananarivo, study conducted by the Pasteur Institute of Madagascar on 853 sera). Arch Inst Pasteur Madagascar 1996;63:53–5. [PubMed] [Google Scholar]
- [43].Barreto J, Sacramento I, Robertson SE, et al. Antenatal rubella serosurvey in Maputo, Mozambique. Trop Med Int Health 2006;11:559–64. [DOI] [PubMed] [Google Scholar]
- [44].Amina MD, Oladapo S, Habib S, et al. Prevalence of rubella IgG antibodies among pregnant women in Zaria, Nigeria. Int Health 2010;2:156–9. doi: 10.1016/j.inhe.2010.03.004. [DOI] [PubMed] [Google Scholar]
- [45].Kolawole OM, Anjorin EO, Adekanle DA, et al. Seroprevalence of rubella IgG antibody in pregnant women in Osogbo, Nigeria. Int J Prev Med 2014;5:287–92. [PMC free article] [PubMed] [Google Scholar]
- [46].Olajide OM, Aminu M, Randawa AJ, et al. Seroprevalence of rubella-specific IgM and IgG antibodies among pregnant women seen in a tertiary hospital in Nigeria. Int J Women’s Health 2015;7:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Onyenekwe CC, Kehinde-Agbeyangi TA, Ofor US, et al. Prevalence of rubella IgG antibody in women of childbearing age in Lagos, Nigeria. West Afr J Med 2000;19:23–6. [PubMed] [Google Scholar]
- [48].Dromigny JA, Nabeth P, Perrier Gros Claude JD. Evaluation of the seroprevalence of rubella in the region of Dakar (Senegal). Trop Med Int Health 2003;8:740–3. [DOI] [PubMed] [Google Scholar]
- [49].Corcoran C, Hardie DR. Seroprevalence of rubella antibodies among antenatal patients in the Western Cape. S Afr Med J 2005;95:688–90. [PubMed] [Google Scholar]
- [50].Maselle SY, Haukenes G, Rutahindurwa A. Preliminary observations on rubella infection in Tanzania and the challenge for its control. East Afr Med J 1988;65:319–24. [PubMed] [Google Scholar]
- [51].Mwambe B, Mirambo MM, Mshana SE, et al. Sero-positivity rate of rubella and associated factors among pregnant women attending antenatal care in Mwanza, Tanzania. BMC Preg Childb 2014;14:95. doi: 10.1186/1471-2393-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bracken PM, Stanfield JP. Rubella antibodies in Uganda. East Afr Med J 1971;48:176–81. [PubMed] [Google Scholar]
- [53].Bamgboye AE, Afolabi KA, Esumeh FI, et al. Prevalence of rubella antibody in pregnant women in Ibadan, Nigeria. West Afr J Med 2004;23:245–8. [DOI] [PubMed] [Google Scholar]
- [54].Onakewhor JU, Chiwuzie J. Seroprevalence survey of rubella infection in pregnancy at the University of Benin Teaching Hospital, Benin City, Nigeria. Niger J Clin Pract 2011;14:140–5. doi: 10.4103/1119-3077.84002. [DOI] [PubMed] [Google Scholar]
- [55].World Health Organization. WHO/UNICEF estimates of measles vaccination coverage <http://www.who.int/immunization/monitoring_surveillance/data/en/> [accessed 26 July 2016].
- [56].Ministère du Plan avec la collaboration du Ministère de la Santé Kinshasa. République Démocratique du Congo and Macro International Inc. Calverton, Maryland, USA. République Démocratique du Congo Enquête Démographique de la Santé; 2007 (Ministry of Planning in collaboration with the Ministry of Health, Kinshasa, Democratic Republic of the Congo and Macro International Inc. Calverton, Maryland, USA. Democratic Republic of the Congo Demographic and Health Survey, 2007) <http://dhsprogram.com/publications/publication-FR208-DHS-Final-Reports.cfm> [accessed 26 July 2016].
- [57].United Nations Children’s Fund, National Institute of Statistics of the Democratic Republic of Congo. Democratic Republic of the Congo Multiple Indicator Cluster Survey; 2010. <http://www.agrodep.org/dataset/democratic-republic-congo-multiple-indicator-cluster-survey-mics-2010-0> [accessed 26 July 2016].
- [58].Pennap G, Amauche G, Ajoge H, et al. Serologic survey of specific rubella virus IgM in the sera of pregnant women in Makurdi, Benue State, Nigeria. Afr J Reprod Health 2009;13:69–73. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


