Abstract
Cytochrome P460s are heme enzymes that oxidize hydroxylamine to nitrous oxide. They bear specialized “heme P460” cofactors that are cross-linked to their host polypeptides by a post-translationally modified lysine residue. Wild-type N. europaea cytochrome P460 may be isolated as a cross-link deficient proenzyme following anaerobic overexpression in E. coli. When treated with peroxide, this proenzyme undergoes maturation to active enzyme with spectroscopic and catalytic properties that match wild-type cyt P460. This maturation reactivity requires no chaperones—it is intrinsic to the protein. This behavior extends to the broader cytochrome c′β superfamily. Accumulated data reveal key contributions from the secondary coordination sphere that enable selective, complete maturation. Spectroscopic data support the intermediacy of a ferryl species along the maturation pathway.
Graphical Abstract
Introduction
Post-translational modifications (PTMs)—covalent modification of amino acids following protein translation—are an important means by which nature extends the vast proteomic complexity available through assemblies of the canonical amino acids, cofactors, and prosthetic groups.1,2 Elucidation of both the functional roles and origins of PTMs is a critical goal for understanding natural biological systems. Moreover, insights from such studies should afford valuable strategies for the design of non-natural enzymes with tailored functions. One class of PTM that has received substantial investigation are heme-protein cross-links, which are ubiquitous and are encountered in both eukaryotes and prokaryotes.3 Key examples of these cross-links occur in c-heme proteins, where cysteine residues are attached to heme vinyl groups to form covalent thioether linkages (Figure 1).4 Additional, non-canonical cross-links are known, and relatively current understanding of the origins and roles of these cross-links has been reviewed by Lin.3
Figure 1.
Known heme-protein cross-links. The purple arrows represent ester bonds to the heme methyl groups, the blue arrows represent cross-links to the vinyl group of the heme, the red arrows represent the tyrosine-heme cross-link in HAO, and the green arrow represents the lysine-heme cross-link in cyt P460. Adapted with permission from Reference 3. Copyright 2015, Elsevier.
Of the known, natural heme-protein cross-link PTMs, almost all comprise covalent attachments to the heme substituents—specifically the β-pyrollic methyl and vinyl groups. Deviations from this trend, where the cross-link involves the porphyrin macrocycle itself, are thus far unique to the heme P460 cofactors employed in the primary metabolisms of nitrifying and anaerobic ammonia oxidizing (anammox) bacteria for hydroxylamine (NH2OH) redox catalysis.5 Heme P460 proteins comprise hydroxylamine oxidoreductases (HAOs) and cytochromes (cyts) P460. HAOs are soluble, homotrimeric assemblies of octaheme subunits that support one heme P460 catalytic center per monomer. These sites are differentiated from the other cofactors by an open coordination site at the Fe and the presence (or absence) of a heme-Tyr double cross-link to the meso-carbon and β-pyrollic positions of the c-heme (Figure 2). The presence of the cross-link alters the electronics of this cofactor, giving rise to the characteristic 460 nm Soret absorption maximum it displays in its FeII form. HAOs featuring heme-Tyr cross-linked cofactors oxidize NH2OH to nitric oxide (NO). HAO variants that do not possess the heme-Tyr cross-link, are ineffective at NH2OH oxidation.6,7 Rather, they are proposed to serve as catalysts for reduction of nitrite (NO2−).8 Thus, the heme-Tyr cross-links in HAO are proposed to bias HAOs towards oxidative chemistry. However, the ability to test this hypothesis is limited by the lack of a viable recombinant expression platform for oxidative HAOs, which precludes site-directed mutagenesis experiments. Meanwhile, no successful attempts at installing cross-links in recombinantly expressed “reductive” HAOs have been reported.
Figure 2.
The heme P460 active site in N. europaea HAO (PDB 4N4N) and cyt P460 (PDB 2JE3). Both are c-type hemes that feature additional covalent attachments. In HAO, a tyrosine residue forms cross-links from the phenolate O to the pyrrole α-carbon and from Cε to the α-meso-carbon. The heme in cyt P460 contains a cross-link from a lysine N to the γ-meso-carbon.
Cyt P460 enzymes, however, can be recombinantly expressed.9,10 These soluble, homodimeric enzymes commonly feature a single heme P460 center per subunit whose γ-meso-carbon is bound to the amine-terminated sidechain of a nearby Lys residue. These cofactors also exhibit a 460 nm Soret absorption maximum in their reduced form. Cyt P460s have been shown to carry out the oxidation of two equivalents of NH2OH to generate nitrous oxide (N2O).10 We have previously speculated that cyt P460 may play a role in preventing accumulation of cytotoxic cellular quantities of NH2OH and NO.10 Substitution of Lys with Tyr11 or Leu12 via site-directed mutagenesis generates c-type hemoproteins that only contain the standard Cys cross-links. These variants are catalytically inactive toward NH2OH: They can form all intermediates of the NH2OH oxidation catalytic cycle via shunts, but the initial NH2OH oxidation events do not occur.11 Complementary investigations of cyt P460 variants with substituted residues in their distal pockets, combined with a 2.25 Å structure of a cross-link deficient (CLD) cyt P460 variant strongly suggest that the role of the heme-Lys cross-link is to position the cofactor sufficiently close to the carboxylate group of a distal glutamate to enable proton transfer during the initial step(s) of NH2OH oxidation.12,13
While a clear picture is emerging of the functional role of the heme-Lys cross-link, its origins have remained elusive. The apparent non-necessity of molecular chaperones apart from standard c-heme maturation proteins to successfully express functional, cross-linked cyt P460 has led to speculation that the heme-Lys PTM forms via an autocatalytic process.9 To date, no further progress had been reported. However, an early crystal structure of Nitrosomonas europaea cyt P460 provides some clues. This structure featured a second modification to the c-heme: It is hydroxylated at its α-meso carbon, opposite to the heme-Lys cross-link on the γ-meso-carbon.14 Such susceptibility to oxidative damage is not unexpected due to the highly ruffled nature of the P460 cofactor. If one assumes a fully symmetric, planar heme cofactor to have D4h symmetry, ruffling is a B1u distortion away from planarity.15 This type of distortion of the macrocycle away from planarity has been investigated at length and has been implicated in tuning heme FeII/III reduction potentials and promoting directed, oxidative heme degradation.16,17 Importantly, the aforementioned CLD structure shows that ruffling in cyt P460 is not a consequence of the cross-link, but persists even without the cross-link.12
Given that cyt P460s are produced by obligate aerobic organisms and that the cross-link forms even with recombinant expression, we hypothesized that the heme-Lys cross-link forms in an oxygen-dependent process—similar to other non-canonical cross-links.18 To this end, we now report key discoveries concerning the origin of the heme-Lys cross-link in cyt P460. We have identified conditions for expression and purification of CLD proenzyme, as well as for maturation of this material into functional, cross-linked cyt P460. The emergent picture exhibits considerable parallels to the reactivity of heme oxygenases,19 although with a key divergence in outcome—productive enzyme maturation, rather than cofactor destruction.
Materials and Methods
Anaerobic expression and purification of N. europaea cyt P460
The pET22b(+) vector containing the gene for N. europaea cyt P460 gene was co-transformed with a pEC86 vector encoding for cyt c maturation genes ccmABCDEFGH into E. coli strain BL21(DE3) and selected for on lysogeny broth plates with 100 μg mL−1 ampicillin and chloramphenicol as previously described.10,20 A single colony was used to inoculate a 5 mL lysogeny broth (LB) starter culture containing 100 μg mL−1 ampicillin and 37 μg mL−1 chloramphenicol. After growing the starter culture overnight at 30 °C and 180 rpm, it was spun down and the cell pellet was brought into an anaerobic glove box. Four septum-sealed, 1L Schott bottles containing 750 mL of Terrific Broth (TB) medium supplemented with 0.5% glycerol, ampicillin, and chloramphenicol were degassed by 2 cycles of evacuation for 12 minutes followed by sparging with N2 for 12 minutes. The media was brought into the glove box and used to resuspend the cell pellet. The suspension was used to inoculate the four bottles of degassed media. Cultures brought outside the glovebox and were grown overnight at 37 °C and 60 rpm in a floor shaker. They were induced with 0.4 M isopropyl β-D-1-thiogalactopyranoside (IPTG) at 30 °C and 60 rpm for 8 hours. Alternatively, the starter culture was used to inoculate two 6L flasks containing 2L of TB medium with ampicillin and chloramphenicol aerobically and were grown at 30 °C overnight. Cells were pelleted at an OD600 between 0.7–1.0 to prevent auto-induction. The brown cell pellet was brought into the box and distributed between four bottles of degassed TB medium prepared as described above. The cultures were induced with 0.4 M IPTG at 30 °C and 60 rpm for 8 hours.
Cells were harvested by centrifugation of bottles loaded and sealed in the glovebox. Centrifugation was carried out at 4000g for 25 minutes. Pellets were returned to the glovebox and the cells were then resuspended in 10 mL of degassed buffer containing 20 mM MOPS (pH 8.0), 300 mM NaCl, 0.1% Triton X-100, and 2 mM EDTA. To lyse the cells, they were treated 20 mg lysozyme, 10 mg DNase I, and 10 mg RNase and then added to a Hungate tube that was subsequently crimp sealed. These tubes were removed from the glovebox and were incubated at 37 °C in a water bath for one hour. The tubes were then returned to the glovebox, and lysate was then centrifuged in the glovebox in Eppendorf tubes at 14000 rpm for 60 minutes. The supernatant was then diluted in a buffer containing 20 mM MOPS (pH 8.0), 20 mM imidazole, and 150 mM NaCl. This solution was applied to HisPur Ni-NTA resin (Thermo Scientific) packed in a gravity column. Once bound to the column, the protein was washed with 40 mL of the aforementioned buffer and then was eluted a second buffer comprised of 20 mM MOPS (pH 8.0) containing 150 mM NaCl and 330 mM imidazole. The fractions containing the target protein were buffer exchanged into 20 mM Tris (pH 8.0) using centrifugal filtration columns. The resulting protein solution was then applied to a Q Sepharose Fast Flow (GE Healthcare) gravity column. The protein on the column was washed with 3 column volumes of 20 mM Tris (pH 8.0) followed by 3 column volumes of 20 mM Tris (pH 8.0) with 100 mM NaCl. The protein was eluted with 20 mM Tris (pH 8.0) containing 200 mM NaCl yielding homogeneously pure cyt P460. Purity was assessed by SDS-PAGE (Figure S1). Proenzyme concentrations were quantified using the extinction coefficient at 280 nm for WT cyt P460 (ε280 =38.9 mM−1cm−1).
Maturation Experiments
To mature cyt P460 proenzyme using oxygen, a final concentration of 8 μM proenzyme was added to an anaerobic Spectrosil quartz cuvette (Starna Cells, 10 mm) containing a final volume of 2 mL in 20 mM Tris/200 mM NaCl (pH 8.0). The cuvette was equilibrated in the UV/vis spectrometer at 25 °C with stirring for at least 3 minutes and was then opened to the atmosphere. In cases where O2 was added, a needle was inserted into the cuvette and O2 was gently passed through the protein solution. Scans from 200–800 nm were recorded every 10 minutes during experiments under air or every 0.3 minutes when O2 was bubbled.
For cross-link reactions using Li2O2, an anaerobic cuvette containing 8 μM proenzyme in 20 mM Tris/200 mM NaCl (pH 8.0) was equilibrated at 25 °C with stirring for at least 3 minutes. Full-wavelength scans were recorded every 0.3 minutes for several minutes before the addition of 3 equivalents of Li2O2. Scans were recorded until there were no longer changes in the UV/vis spectra, at which point excess sodium dithionite (Na2S2O4) was added to quench the reaction. The cuvette was returned to the glovebox, after which protein was washed with 20 mM Tris/200 mM NaCl (pH 8.0). To re-oxidize the protein, [Ru(NH3)6]Cl3 was added and subsequently removed by buffer exchange. The Arg44Ala mutant was matured in a similar fashion, but instead using 8-12 equivalents of Li2O2. To determine Li2O2 concentration dependence of the maturation reaction, the same procedure was followed maintaining proenzyme at 8 μM and adding 3, 6, or 9 equivalents of Li2O2.
Maturation of the proenzyme using glucose oxidase (GOD) to generate O22− was carried out by first adding 12 μM cyt P460 to an anaerobic cuvette containing 20 mM Tris/200 mM NaCl (pH 8.0) such that the final volume would be 2 mL. The cuvette was equilibrated at 25 °C with stirring for at least 3 minutes. Full-wavelength scans were then collected every 0.3 minutes. After 3 minutes, 50 μL of 1 mg/mL GOD was added to the cuvette using a Hamilton syringe. After another 3 minutes, the cuvette was opened to the atmosphere. The reaction was initiated after 10 minutes of equilibration with atmosphere by addition of 500 μM glucose.
For Li2O2 reactions in the presence of guaiacol, 2 mM guaiacol was added to the anaerobic cuvette with the protein solution and was equilibrated at 25 °C with stirring for at least 3 minutes. Scans were recorded every 0.2 minutes. The reactions were initiated by addition of Li2O2 via Hamilton syringe.
Results and Discussion
Analogy to Heme Oxygenase Reactivity
Two key observations led us to hypothesize that modification of the heme P460 γ-meso-carbon proceeds via a reaction similar to those reported for heme oxygenases.17,21 One is the observation by Wilmot and co-workers of hydroxylation at the cyt P460 heme α-meso-carbon that presumably arose from oxidative damage. The second is the high degree of ruffling common to both heme P460 cofactors and hemes bound by heme oxygenases.
Heme oxygenase chemistry is principally driven by reactive oxygen species.22 One outcome of heme oxygenation is meso-carbon hydroxylation, although the mechanism by which this proceeds remains a topic of investigation.22-24 In brief, possibilities include formation of a FeIII-heme peroxo or hydroperoxo intermediate that directly attacks the meso-carbon, or this intermediate could undergo homolytic cleavage to form ferryl compound II (FeIV=O) and a transient hydroxyl radical. In non-canonical heme oxygenases such as IsdG, a significant ruffling deformation of the heme was shown to allow for selective hydroxylation of the β- or δ-meso-carbons.25 The substantial, cross-link independent ruffling of the cyt P460 macrocycle could allow for a similar mechanism to hydroxylate the γ-meso-carbon which would conveniently supply a functional group that can undergo keto-enol tautomerization. Although heme oxygenases have not been shown to selectively hydroxylate a γ-meso-carbon, differences in the active site of cyt P460 may allow for control over this regioselectivity. The keto form will be susceptible to condensation with the proximal Lys amine, thus forming the C–N cross-link and the loss of a water molecule. Alternatively, the mechanism could be similar to peroxidases where a heme peroxo species undergoes heterolytic cleavage to compound I (FeIV=O porphyrin radical cation), which could participate in oxygen atom transfer reactions with the meso-C. However, compound I of heme oxygenase was shown to be incompetent for heme hydroxylation.26 These possible mechanisms are summarized in Figure 3.
Figure 3.
Hypothetical mechanism of peroxide-dependent heme-Lys cross-link formation in cyt P460. A) Possible routes for hydroxylation of the meso-carbon followed by B) condensation by lysine.
Anaerobic overexpression yields cross-link deficient cyt P460 proenzyme
Given the dependence of heme oxygenase chemistry on reactive oxygen species, we leveraged the facultative anaerobic nature of E. coli to carry out anaerobic overexpression and purification of wild-type (WT) N. europaea cyt P460. Gratifyingly, expression of the protein under these conditions led to the isolation of a red protein with spectroscopic features characteristic of cross-link deficient (CLD) cyt P460, although with yields significantly lower than those obtained following aerobic expression. Produced this way, WT N. europaea cyt P460 exhibits a Soret maximum at 404 nm (Figure 4). The presence of a shoulder at 440 nm was consistent with a minor amount of cross-linked protein (vide infra). This presumably formed from small amounts of oxygen present during protein overexpression.
Figure 4.
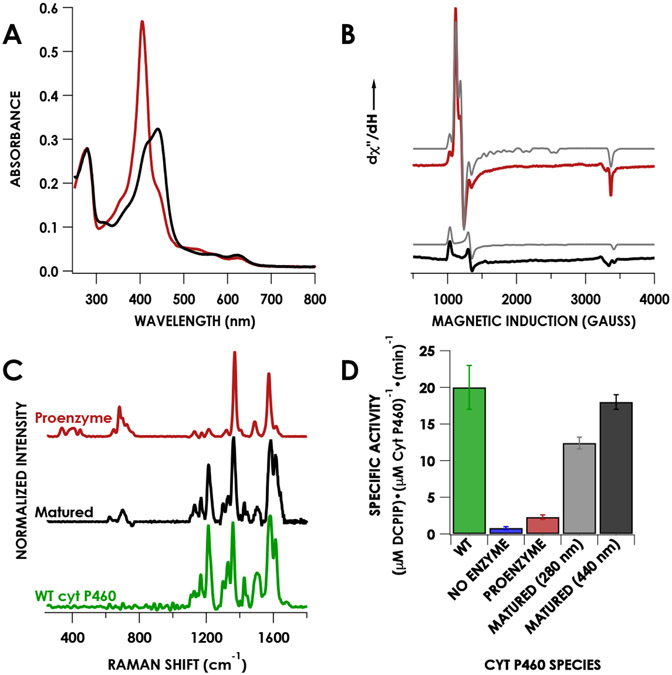
Characterization of the anaerobically purified proenzyme and matured proenzyme, which was generated by reaction of the proenzyme with 3 equivalents of Li2O2 followed by quenching with sodium dithionite, re-oxidization with [Ru(NH3)6]Cl3, and washing. A) UV/vis absorption spectra of the proenzyme (red) and matured enzyme (black). B) CW X-band EPR spectra collected at 12 K of the proenzyme (red) and matured enzyme (black). Grey traces are the corresponding simulations. C) Resonance Raman spectra obtained via excitation at 405 nm for the proenzyme (red), matured (black), and WT cyt P460 (green). D) Specific activities for WT cyt P460, the proenzyme, and matured proenzyme quantified by the 280 nm extinction coefficient (grey) or the 440 nm extinction coefficient (black). Error bars represent standard deviations of three trials.
A resonance Raman (rR) measurement using 405 nm laser excitation yielded a spectrum consistent with a ruffled c-heme, as encountered in previously reported CLD cyt P460 variants (Table 1). The oxidation state marker band (ν4) occurs at 1369 cm−1, consistent with a ferric assignment for the heme. The spin state marker band (ν3) occurs at 1488 cm−1.27 While the energy of the ν3 band is lower than that reported for the cross-link deficient N. europaea Lys70Tyr at 1501 cm−1, it is consistent with energies expected for high-spin ferric hemes.11 The ν10 band, known to be sensitive to out-of-plane distortions of the heme, was assigned to the peak at 1616 cm−1 based on previous assignments for c-type hemes.28,29 In WT cyt P460 this band appears at 1614 cm−1. These similar frequencies suggest that the CLD proenzyme remains ruffled, in accord with the structural data obtained for CLD Lys106Leu/Ala131Glu N. sp AL212 cyt P460.12 A spin-counted X-band EPR spectrum obtained at 12 K revealed a mixture of signals that were consistent with both mature and CLD cyt P460 (Table 2). The CLD signal was the major component, comprising 80% of the mixture, while mature enzyme constituted the remaining 20%.
Table 1.
Resonance Raman shifts of porphyrin marker bands from N. europaea cyt P460 variants.a
| Cyt P460 Variant |
ν3 (cm−1) |
ν4 (cm−1) |
ν10 (cm−1) |
Reference |
|---|---|---|---|---|
| Lys70Tyr cyt P460 (CLD) | 1501 | 1372 | NR | 11 |
| WT cyt P460, aerobic preparation | 1504 | 1359 | 1614 | This Work |
| WT cyt P460 proenzyme (CLD) | 1488 | 1369 | 1616 | This Work |
| WT cyt P460 matured enzyme | 1504 | 1364 | 1615 | This Work |
| Arg44Ala (CLD) | 1487 | 1367 | 1618 | This Work |
Obtained using 405 nm laser excitation.
Table 2.
Spin Hamiltonian parameters obtained from fitting X-band EPR spectra of cyt P460 variants.
| Cyt P460 Variant | Species (FeIII, S = 5/2) | ||
|---|---|---|---|
| geff | E/D | Reference | |
| Lys70Tyr cyt P460 (CLD) | 5.78, 1.98 | 0.00 | 11 |
| WT cyt P460, aerobic preparation | 6.57, 5.09, 1.97 | 0.03 | 10 |
| WT cyt P460 proenzyme, minor component (CL) | 6.52, 5.06, 1.97 | 0.03 | This Work |
| WT cyt P460 proenzyme, major component (CLD) | 6.02, 5.54, 1.99 | 0.01 | This Work |
| WT cyt P460 matured enzyme | 6.50, 5.06, 1.97 | 0.02 | This Work |
| Arg44Ala (CLD) | 5.69, 1.99 | 0.00 | This Work |
| Arg44Ala matured, major component | 5.76, 1.99 | 0.00 | This Work |
| Arg44Ala matured, minor component | 5.38, 2.00 | 0.00 | This Work |
| NpAv Leu105Lys | 6.63, 5.07, 1.96 | 0.03 | This Work |
Cyt P460 maturation is driven by peroxide
Given that the formation of the cross-link appeared dependent on oxygen during expression, anaerobically purified proenzyme was exposed to ambient air to determine whether O2 is sufficient to drive enzyme maturation. Upon opening an anaerobic cuvette containing proenzyme to air, the 404 nm proenzyme Soret absorption decayed slowly, and a new feature at 413 nm grew in intensity. The appearance of the 413 nm feature coincided with an increase in absorbance at 440 nm assigned to matured cyt P460 (Figure S2). After 16 hours, the reaction remained incomplete. After two days, the heme absorbance was almost entirely diminished, consistent with cofactor degradation rather than maturation. In summary, exposing the proenzyme to O2 in air led to only a slow (kobs(440) = 0.0018 min−1) conversion to cross-linked enzyme and, ultimately, heme degradation.
We next considered the participation of reactive oxygen species in driving cofactor maturation. To this end, we treated the proenzyme with 3 equivalents of lithium peroxide (Li2O2). Initial attempts using H2O2 yielded inconsistent results, likely owing to the difficulty in quantifying H2O2 and the sensitivity of the protein to degradation if excess peroxide is added. Using Li2O2 instead allowed for better control over the concentration by dissolving a known amount in buffer. Regardless, considering the pKa of H2O2 is 11.6, dissolution of Li2O2 in a pH 8 buffer would generate H2O2.
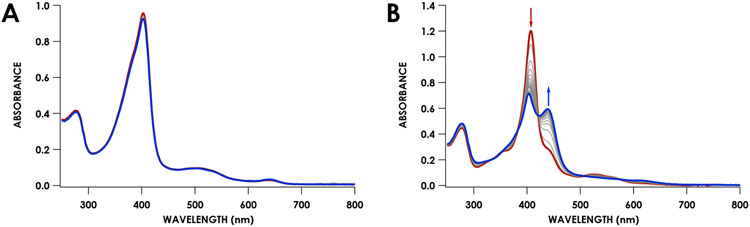
The previously observed 413 nm intermediate formed immediately after addition of 21 μM Li2O2 to 7 μM proenzyme by manual mixing (Figure 5). This intermediate subsequently decayed, yielding a final spectrum with a broad Soret absorption with a maximum near 445 nm. After ca. 4 minutes there were no further spectral changes. The reaction was then quenched by addition of Na2S2O4. UV/vis absorption spectroscopy of the resulting solution exhibited the 460 nm Soret band characteristic of FeII cyt P460. Following removal of Na2S2O4, the protein was treated with the oxidant [Ru(NH3)6]Cl3. The resulting UV/vis absorption spectrum was identical to that of WT FeIII cyt P460 (Figure 4A). As an alternative to adding Li2O2, peroxide could be generated within the reaction mixture using GOD, which oxidizes glucose to H2O2 and D-glucono-δ-lactone. Addition of 500 μM glucose to a cuvette containing 12 μM proenzyme and 156 nM GOD resulted in formation of the 413 nm intermediate, which decayed to the same broad spectrum observed for the reaction with Li2O2 (Figure S3).
Figure 5.
Reaction of 7 μM proenzyme (red) with 3 equivalents (21 μM) of Li2O2 at 25 °C. Scans were recorded every 0.3 minutes. The scan immediately following Li2O2 addition is shown in black. The final scan is shown in blue, after which the reaction was quenched with Na2S2O4. Time courses of the absorbances corresponding to the proenzyme (404 nm) and product (460 nm) are shown in the inset.
The final spectrum obtained following the maturation reaction before quenching with Na2S2O4 differs from matured WT P460. We speculated that this could occur for one of two reasons. One is that the matured P460 subsequently reacts with the remaining excess peroxide. Alternatively, the observed species could be the product following activation of peroxide to modify the macrocycle, e.g. a ferryl species such as compound I or II. These high-valent FeIV-oxo species are used in the catalytic cycles of several heme enzymes including cyt P450 and peroxidase. Compound I refers to an FeIV-oxo heme with a porphyrin radical cation, which can be reduced by one electron to form compound II. To test whether CL cyt P460 could react with peroxide to form a ferryl species, 4 μM aerobically grown, fully mature WT cyt P460 was allowed to react with 3 equivalents (12 μM) of Li2O2. The 440 nm Soret evolved to the same final spectrum observed during the proenzyme maturation (Figure S4). X-band EPR at 10 K for this species contained a new feature with g = [6.25, 5.68, 2.00], a small S = ½ component with g = [2.01, 2.02, 2.01], and a third feature at g = 4.28 (Figure S5). The new species can plausibly be assigned to a hydroxylated heme that would eventually proceed toward degradation. The S = ½, meanwhile, is not consistent with that of any characterized compound I species. The reaction of proenzyme with 3 equivalents of Li2O2 frozen after 4 minutes contained the same three species in its EPR spectrum. To further characterize the product of the reaction of WT cyt P460 with Li2O2, an 57Fe Mössbauer spectrum was obtained for 57Fe-enriched WT protein that had been treated with 3 equivalents of Li2O2 and frozen after 4 minutes (Figure S6). This spectrum contained two Fe-containing components. The minor component, accounting for 29% of the signal, had an isomer shift (δ of 0.59 mm/s and quadrupole splitting of 0.72 mm/s, which is consistent with the new signal observed in the EPR spectrum. The major component had an isomer shift of 0.09 mm/s and quadrupole splitting of 1.60 mm/s, which are characteristic of FeIV=O-containing species such as compound I or compound II.30 Given that this species was EPR silent, it can plausibly be assigned as compound II.
Matured protein matches properties of the aerobically-expressed cyt P460
To further characterize the protein resulting from the maturation of proenzyme with peroxide, we obtained an X-band EPR spectrum at 12 K of matured protein that was treated with Na2S2O4 and re-oxidized (vide supra). The signal corresponding to the proenzyme was completely absent. The only signal present was that of a rhombic system with g-values of 6.50, 5.06, and 1.97 (E/D = 0.02). These values are consistent with those of native, cross-linked WT cyt P460 (Table 2). Additionally, rR spectrum obtained via excitation at 405 nm exhibited an increased number of bands in accord with the descent in cofactor symmetry attending cross-link formation (Figure 4). This spectrum of Li2O2-matured cyt P460 is indistinguishable from WT cyt P460 purified from aerobic growths.
To establish whether in vitro maturation imbues cyt P460 with NH2OH-oxidation activity, we carried out steady-state activity assays (Figure 4). Under anaerobic conditions, 2 mM NH2OH was added to a cuvette containing 50 μM DCPIP and 6 μM PMS, followed by addition of 1 μM protein. The specific activity obtained by monitoring consumption of DCPIP for the proenzyme was 4.5 ± 0.7 μM DCPIP μM cyt P460−1 min−1, significantly less than that of WT cyt P460 (20 ± 3 μM DCPIP μM cyt P460−1 min−1). This lowered activity of the proenzyme was expected due to the predominantly CLD character of proenzyme preparations. We attributed the minimal activity observed to the presence of a minor amount of cross-linked protein (vide supra). After maturing the proenzyme with Li2O2 and removing all reagents by washing in a spin column, the specific activity was restored to 18 ± 1 μM DCPIP • μM cyt P460−1 min−1 with protein quantified using the heme Soret maximum. This value is within error of values obtained previously for WT cyt P460. There was, however, some evidence of heme loss by the protein, as quantification on the basis of total protein yielded a specific activity of 12.4 ± 0.8 μM DCPIP μM cyt P460−1 min−1. While we do not observe formation of a peptide-linked biliverdin, we cannot rule it out on the basis of UV/vis and would need HPLC and MS analysis of the products, which is beyond the scope of this work.31 Nevertheless, the restoration of WT cyt P460 spectroscopic properties as well as NH2OH oxidation activity to inactive proenzyme produced during anaerobic expression strongly evidences the peroxide dependence of cyt P460 cofactor maturation.
Identity of the 413 nm intermediate
A key, common observation from the maturation experiments described above is that of the 413 nm intermediate. This intermediate species was observed in reactions employing either air (O2) or peroxide for maturation. It is the only intermediate observed by UV/vis during the maturation reaction, and it is isosbestically converted to the final product. Thus, its consumption is the rate-determining step of the reaction. Plausible identities for the 413 nm intermediate are [Fe–O2]1+ (e.g. FeIII–O22− or FeII–O2), FeIII–OOH−, compound I, or compound II. Treatment of the proenzyme with meta-chloroperoxybenzoic acid (m-CPBA) showed no reactivity (Figure S7), suggesting that compound I is not formed by the proenzyme.
Stopped-flow UV/vis absorption spectroscopy was carried out to scrutinize the reactivity of the 413 nm intermediate on the ms–s timescale. Rapidly mixing 10 μM CLD protein with 2 equivalents of Li2O2 (20 μM) and recording spectra every 8 ms revealed a decay of the 404 nm peak and shift to 413 nm within 2 seconds (Figure S8). A linear correlation between the observed rate of the 404 nm decay (after addition of Li2O2) and Li2O2 concentration suggests the kobs for the conversion of CLD cyt P460 to the 413 nm intermediate exhibits a first-order dependence on [Li2O2], with a rate constant of 0.13 s−1 μM−1 (Figure S9). Meanwhile, the rate of decay of the 413 nm intermediate to mature enzyme exhibits no dependence on [Li2O2] (Figure S10). This 413 nm intermediate ultimately leads to the final species that features a broad Soret and Q-bands.
The 413 nm species was trapped by RFQ of the reaction of 200 μM proenzyme with 400 μM Li2O2 at 0.5 second and 1 second time points. Based on the UV/vis absorption time-course of this reaction, the 413 nm intermediate should be mostly formed at 0.5 second and completely formed after 1 second. The EPR spectra for both the 0.5 second and 1 second sample are absent of any S = 5/2 signals and show formation of a new S = ½ species (Figure S11). The absence of a high-spin feature strongly suggests that the 413 nm intermediate is not the meso-hydroxylated heme but rather is an Fe-bound peroxo or superoxo adduct.32 Simulation of the low-spin signal gave g-values of 2.08, 2.01, and 1.98 (Figure 6). Interestingly the g-value of 2.08 is lower than those typically observed for ferric-peroxo or hydroperoxo intermediates, which have a higher g1 and larger spread of g-values (g1 > g2 > ge > g3). 33,34 For example, a ferric-hydroperoxo intermediate of heme-oxygenase was reported to have g-values of 2.37, 2.187, and 1.924.19 Instead, the EPR spectrum of the 413 nm intermediate is more consistent with a ferrous-superoxo species, which are characterized by g1 ≫ g2 ≳ g3 ≈ ge. A key example of such a species is cryoreduced oxy-hemoglobin, which exhibits g1 = 2.11 and g2,3 ≈ ge.33 It is unlikely, however, that the observed EPR spectrum corresponds to the 413 nm intermediate. Given the low intensity of the signal, we conclude that this is a minor species. Another EPR silent species is likely present that could be FeII or FeIV. Thus, the EPR plausibly corresponds to minor accumulation of a precursor to the 413 nm intermediate, which is itself silent under our EPR conditions.
Figure 6.
Continuous-wave X-band EPR spectrum collected at 10 K of the rapid freeze-quench reaction of 200 μM proenzyme with 400 μM Li2O2 frozen at 1 second (black) with the simulated spectrum (red). The fit gave g-values of 2.08, 2.01, and 1.98. Oxygen background was removed from the raw experimental spectrum and the cavity was subtracted.
To obtain further support for ferryl formation during maturation, the proenzyme was allowed to react with Li2O2 in the presence of guaiacol. Guaiacol is commonly used in peroxidase assays, during which heme ferryl species oxidize guaiacol to tetraguaiacol, which exhibits a characteristic absorption at 470 nm.35 The high-valent ferryl species compound I and compound II are strong oxidants that are able to activate relatively inert bonds. Peroxidases use these ferryl intermediates formed from peroxide to oxidize a variety of substrates, including guaiacol. Guaiacol oxidation has thus been a marker of peroxidase activity, where the oxidation power of compound I or compound II are required. When Li2O2 was added to the proenzyme with excess (2 mM) guaiacol, there was an immediate formation of a broad absorbance at 470 nm (Figure 7). The Soret band of the proenzyme did not change, and after 30 minutes the mixture was reduced to reveal completely unreacted proenzyme. The peak at 460 nm is attributed to cross-linked protein in the as-isolated sample that formed as a result of some oxygen present during growth. Oxidation of guaiacol thus intercepted the intermediate responsible for cross-link formation. While concerted hydroxylation of the meso-carbon would not be affected by the presence of guaiacol, a compound I or compound II intermediate would be competent for guaiacol oxidation.26,36 A non-canonical heme oxygenase IsdI that hydroxylates heme via a concerted mechanism was shown to proceed even in the presence of guaiacol and did not result in guaiacol oxidation, while generation of the ferryl species by the O-atom donor m-CPBA was competent for guaiacol oxidation.37 In the case of cyt P460, the cross-link mechanism does not proceed in the presence of guaiacol and instead only guaiacol oxidation is observed, indicating that a ferryl species is responsible for cross-link formation.
Figure 7.
A) UV/vis spectral time-course for the reaction of the proenzyme with 8 equivalents of Li2O2 in the presence of excess (2 mM) guaiacol. The red trace is before Li2O2 was added, the black trace was immediately after addition, and the blue trace was 30 minutes after addition. Grey traces represent scans every 0.2 minutes. B) UV/vis spectrum from the reaction after addition of Na2S2O2.
Both the low-signal EPR spectrum and oxidation of guaiacol suggest maturation of the proenzyme involves a step that forms a ferryl intermediate. The sharp UV/vis Soret band and absence of a radical in the EPR spectra are consistent with this intermediate being a compound II species, whereas compound I typically has broad UV/vis features and a porphyrin radical signal in the EPR spectrum.30 Fe K-edge X-ray absorption spectroscopy of the intermediate, trapped by reaction of 500 μM proenzyme with 500 μM Li2O2 after 1 second, showed a shift in the pre-edge to 7114.2 eV compared to that of WT at 7112.4 eV consistent with oxidation to a formally FeIV center (Figure 8). The pre-edge was also more intense, which has been observed for other compound II species and is consistent with a descent in symmetry at the Fe center promoting 3d/4p mixing. Fitting of the extended X-ray absorption fine structure (EXAFS) region (Figure S12) over a k-range of 2–13 Å revealed an Fe–O scatter with a distance of 1.83 Å (Table S2). This distance is consistent with a protonated FeIV–OH species, i.e. protonated compound II. However, the increased intensity of the pre-edge is more consistent with an unprotonated FeIV–oxo. Typically compound II species captured for EXAFS exhibit distances closer to 1.65 Å that are consistent with the unprotonated Fe(IV)–oxo.38,39
Figure 8.
Fe K-edge XAS XANES region for WT cyt P460 (green) and the reaction of ca. 500 μM proenzyme with 1 equivalent of Li2O2 frozen at 1 second (black).
The data in sum are consistent with formation of compound II. However, more work will be needed to clarify the protonation state. Homolytic cleavage to compound II would produce a hydroxyl radical that could hydroxylate the γ-meso-carbon, allowing Lys to condense and form the cross-link. Outer coordination sphere effects likely control the regioselectivity of such a reactive radical, which would otherwise indiscriminately attack the porphyrin. The mechanism in cyt P460 may be similar to that of heme oxygenases, where a distal water cluster H-bonded with an Arg-Asp pair was suggested to play an important role in both stabilizing a hydroxyl radical and directing it towards the α-meso-carbon.40 Tautomerization of a hydroxylated meso-carbon would then provide an electrophilic carboxyl group for the nucleophilic lysine to attack and form a covalent attachment. The nucleophilic nature of the lysine sidechain allows for a large diversity of covalent modifications including methylation, acetylation, and carbonylation, among others.41,42 Carbonylation has been shown to correlate with oxidative stress, and recently lysine carbonylation was observed in cyt c induced by reactivity with peroxide.43 While many PTMs require additional enzymes, both cyt c and cyt P460 catalyze their own active-site modifications using peroxide as a substrate, allowing them to gain new functions.
Influence of hydrogen bonding networks on cofactor maturation
Heme ruffling is known to control function, like that of nitrophorins44,45 or heme oxygenase25. Cytochrome P460 shares in this ruffling deformation even in cross-link deficient variants. If the Lys is not responsible for ruffling, there must be other structural factors that are responsible. Inspection of the N. europaea cyt P460 active site reveals a distal Arg residue, Arg44, that is involved in a hydrogen-bonding (H-bonding) network that engages the heme 6-β-pyrrolic propionate (Figure 9). We suspected that this H-bond network contributes to ruffling the heme P460 cofactor. To test whether this Arg is indeed promoting ruffling, and by extension to test whether the ruffling deformation is required for cross-link formation, we generated the Arg44Ala cyt P460 mutant. Purification of this mutant yielded a completely CLD protein despite being grown under aerobic conditions (Figure S13A). The EPR spectrum obtained at X-band and at 10 K was axial with g = [5.69, 1.99] (Figure S13B). The rR spectrum obtained via excitation at 405 nm resembled that of the WT proenzyme (Figure S13C) with little deviation in the porphyrin marker bands (Table 1).
Figure 9.
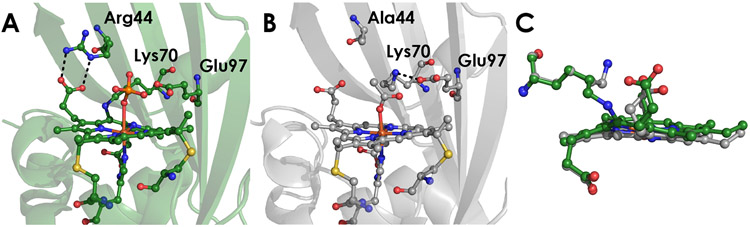
A) Structure of WT N. europaea cyt P460 (PDB 2JE3) B) Structure of the N. europaea cyt P460 Arg44Ala mutant (PDB 8GAR). C) Overlay of the heme groups from the WT (green) and Arg44Ala (grey) cyt P460 structures, highlighting the change in position of the 6-β-pyrrolic propionate and γ-meso-carbon.
Having established that Arg44 influences cross-link formation, we crystallized CLD Arg44Ala cyt P460 to probe the role of the Arg44 H-bond on the active site structure. Red diamond-shaped crystals of N. europaea Arg44Ala cyt P460 were grown in 38-42 % PEG 3000, 0.2 M calcium acetate hydrate, and 0.1 M sodium cacodylate (pH 6.5) using the sitting drop method. The largest crystals were obtained using 9 μL of 350 μM protein in 50 mM MOPS (pH 8.0) with 1 μL crystallization buffer and grew in 5 days. These crystals diffracted to 1.55 Å (Table 3). Density for an active-site capping loop region was absent in our data as it was with Wilmot’s WT structure.14 Previously this was attributed to oxidative damage due to the protracted crystallization period. Given our briefer crystallization period, this could instead be due to this loop being highly flexible in the N. europaea protein. The impact of the Arg44Ala mutation on the heme architecture was apparent: the 6-β-pyrrolic propionate that interacts with Arg44 in WT cyt P460 was significantly perturbed. The propionate’s carboxyl carbon shifts by 1.1 Å compared to WT. Removing the Arg-propionate interaction effectively eliminated any heme ruffling (Figure 9). Normal coordinate structural decomposition showed that the ruffling deformation was only 0.1 Å, about 7-fold less than that of WT (Figure S14). Notably, the γ-meso-carbon was shifted by 0.9 Å relative to WT. While the ruffling deformation was significantly altered, the heme maintained its saddling deformation of 0.8 Å. The cross-linking lysine residue—which has previously never been observed in a crystal structure of cyt P460 without being covalently attached—to the porphyrin, formed a salt-bridge with Glu96 in our structure. Prior to covalent attachment, the lysine sidechain is free to interact within the active site and could play an important outer-sphere role during cross-link formation.
Table 3.
Data Collection and Refinement Statistics for N. europaea Arg44Ala cyt P460
| Wavelength (Å) | 0.979 |
|---|---|
| Temperature (K) | 80 |
| Space Group | P 31 2 1 |
| a (Å) | 53.111 |
| b (Å) | 53.111 |
| c (Å) | 125.894 |
| α(deg) | 90 |
| β(deg) | 90 |
| γ(deg) | 120 |
| No. of reflections | 606675 (60977) |
| No. of reflections in the Rwork set | 30604 (2999) |
| No. of reflections in the Rfree set | 1454 (150) |
| Resolution (Å) | 43.2 – 1.55 (1.605 – 1.55) |
| Rmerge (%) | 0.05202 (1.518) |
| Rmeas (%) | 0.05344 (1.557) |
| CC1/2 | 1 (0.916) |
| Completeness (%) | 1.00 (1.00) |
| Redundancy | 19.8 (20.2) |
| I/σ(I) | 32.17 (2.11) |
| Rwork | 0.1635 (0.2603) |
| Rfree | 0.1968 (0.3169) |
| Root-mean-square deviation from ideality | |
| Bonds (Å) | 0.009 |
| Angles (deg) | 0.98 |
| Average B factor (Å2) | 34.37 |
| Ramachandran plot | |
| Favored Regions (%) | 97 |
| Allowed regions (%) | 2.6 |
| Disallowed regions (%) | 0 |
| PDBID | 8GAR |
Reaction of as-purified Arg44Ala cyt P460 with 8 equivalents of Li2O2 resulted in formation of a 410 nm intermediate that converted to a broad Soret maximum centered near 438 nm (Figure S15). When the reaction was quenched with Na2S2O4 the product featured a new Soret at 457 nm that resembled FeII cross-linked cyt P460. After re-oxidation using [Ru(NH3)6]Cl3 the UV/vis spectrum had a broad Soret at 432 nm and a new feature at 413 nm (Fig. S13A). The EPR spectrum of this FeIII product could be fit with two axial, high-spin components. The first component accounted for 34% of the total spin and had g = [5.76, 1.99] while the second component accounted for 58% of the spin and had g = [5.38, 2.00]. The rest of the spin was accounted for by a g = 4.24 signal that was likely a result of heme degradation. Maturation of the Arg44Ala mutant restored hydroxylamine oxidation activity (Fig. S13D), giving strong evidence that the cross-link was formed. However, the presence of a second species identified by the UV/vis and EPR spectra of the product suggests non-specific reactivity. Although the ruffling deformation was not required for reactivity with peroxide, it can explain the apparent unselective product formation. Heme ruffling is expected to localize spin density onto the meso-carbons making them more susceptible to hydroxylation.46 Without this influence of ruffling on the meso-carbons, in particular the γ-meso-carbon of cyt P460, the reactive intermediates such as the FeIII-peroxo or compound II could indiscriminately attack the porphyrin ring leading to non-specific heme hydroxylation.
Influence of Arg44 on the mechanism of cross-link formation
It is evident that Arg44 plays an important but non-essential role in cross-link formation. To determine whether the mechanism was the same as the WT proenzyme, the reaction was studied by stopped-flow kinetics. Stopped-flow reactions with varying concentrations of Li2O2 showed formation of an intermediate with a Soret band at 410 nm and Q-bands at 524 nm and 557 nm (Figure 10). This species decayed rapidly to the final product, in contrast to the WT proenzyme which did not convert to the final product on this timescale. Decay of the absorbance at 403 nm could not be fit to a single exponential and was better fit by a double exponential. The two components of this fit correspond to the formation of the 410 nm intermediate which still absorbs at 403 nm, followed by the decay of the intermediate to give the final species. Plotting kobs of the first step against the concentration of Li2O2 ranging from 60 μM – 160 μM showed saturation (Figure S16). The observed rate at 160 μM was 1.89 s−1, lower than that obtained for the WT proenzyme at only 30 μM Li2O2 (3.67 s−1). The requirement for higher concentrations of Li2O2 to react indicates that the Arg44Ala mutant has a lower binding affinity for peroxide. The slower rate of formation of the intermediate is consistent with the Arg H-bond network facilitating O─O bond cleavage. These data together suggest that Arg44 could play a role in interacting with the bound peroxo ligand. In peroxidases, a distal Arg aids in O─O cleavage by providing a H–bond, but it is not required for cleavage to occur.47,48
Figure 10.
A) UV/vis spectral time-course for the stopped-flow reaction of 10 μM Arg44Ala with 8 equivalents (80 μM) Li2O2. The red trace is the first spectrum after mixing and the blue trace is the final spectrum at 30 seconds. The inset shows the absorbance at 403 nm over time fit to a double exponential. B) UV/vis spectrum comparing as-isolated Arg44Ala (red) and the intermediate formed during the stopped-flow reaction (black).
Based on the stopped-flow kinetics, samples of 200 μM R44A mixed with 1600 μM Li2O2 were subjected to RFQ at 100 ms, 400 ms, 600 ms, and 4 s. Only the signal of the as-isolated species was observed for the 100 ms and 400 ms samples, and after 600 ms there was no observable signal (Figure S17). This EPR silent intermediate is consistent with the mechanism of the WT proenzyme, which we posit proceeds through EPR-silent compound II. To determine whether the compound II intermediate is responsible for cross-link formation in the Arg44Ala mutant, the reaction was repeated in the presence of guaiacol (Figure S18). Upon addition of Li2O2, the 470 nm peak of oxidized guaiacol formed immediately. The Soret band of CLD Arg44Ala remained unchanged during the reaction. Reduction of the product with Na2S2O4 yielded a UV/vis lacking the 457 nm Soret corresponding to cross-linked protein, in contrast to the reaction without guaiacol. Guaiacol therefore intercepts compound II formation and prevents cross-link formation. Compound II is responsible for cross-link formation in both the WT proenzyme and the Arg44Ala mutant, but removal of the H-bond network supported by Arg impacts the ability to selectively form the cross-link. Without the H-bond network, the reactivity of compound II cannot be tightly controlled for hydroxylation of the γ-meso-carbon, leading to only partial conversion to cross-linked protein.
Heme-lysine cross-link formation in cyt P460 is intrinsic to the c′β protein fold
The cross-link-forming Lys is highly conserved across the cytochrome P460 protein family. A sequence logo generated from a multiple sequence alignment of 236 annotated cyt P460 species reveals that the predominant residue at the cross-link position is Lys (Figure S19), appearing in approximately 73% of the sequences. The next most frequent is Leu in about 10% of the sequences. The sequences lacking the Lys cross-link residue are better classified in the cyt c′β subfamily, which is closely related to cyt P460.49 Cyt c′β proteins have been implicated in NO binding and are proposed to play a role in NO detoxification, although their function in vivo remains unknown.50 Crystal structures of cyt c′β from both N. europaea and Methylococcus capsulatus have shown that these proteins have the same overall fold as cyt P460 and overlay well with the N. europaea cyt P460 structure.51,52 In place of the cross-linking lysine, these proteins contain a methionine (Met79) or phenylalanine (Phe61), respectively, that do not form cross-links. We identified a cyt c′β gene from Nitrosospira sp. NpAV that contains a Leu (Leu105) residue in the cross-linking position. To test whether the conserved protein fold is sufficient to promote cross-link formation given the presence of an appropriate residue, we expressed and purified WT cyt c′β from Nitrosospira sp. NpAv along with a Leu105Lys mutant.
Purified WT Nitrosospira sp. NpAv cyt c′β is a red protein with a Soret maximum at 400 nm and shoulder at ca. 378 nm (Figure S20), which is consistent with cyt c′β from both N. europaea and Methylococcus capsulatus.51,53 When reacted with Li2O2 at concentrations comparable to the cyt P460 reactions above, Nitrosospira sp. NpAv cyt c′β showed no reactivity. This contrasts with cyt c′β from N. europaea, although the concentrations used in that study were 10-fold higher. It is possible that the residue in the cross-linking position plays a critical role in peroxide reactivity. Substitution of Lys in the cross-linking position yielded a green protein with a Soret maximum at 442 nm. The overall shape of the UV/vis absorption spectrum was similar in appearance to WT N. europaea cyt P460. When the Leu105Lys Nitrosospira sp. NpAv cyt P460 variant is reduced, the Soret maximum shifts to 460 nm. The X-band EPR spectrum of the Leu105Lys variant collected at 12K had a gmax, gmid, and gmin of 6.63, 5.07, and 1.96 with an E/D of 0.02, which is similar to the WT N. europaea g-values of 6.57, 5.09, and 1.97 and E/D of 0.03.10 Altogether, these data are consistent with a cross-link containing protein species.
To extend our studies on cyt c′β we expressed and purified WT cyt c′β from Methylococcus capsulatus as well as its Phe61Lys variant. The WT protein did not react with peroxide. The purified Phe61Lys variant exhibited a UV/vis absorption spectrum with a sharp Soret band at 407 nm and q-bands at 528 nm and 558 nm (Figure S21), in contrast to the broad Soret of the WT cyt c′β and more similar to spectra of cross-link deficient cyt P460 variants. Although the Lys variant was not purified as cross-linked protein like the Nitrosospira sp. NpAv variant was, it did contain a small shoulder at 440 nm. Upon reaction with peroxide, the peak at 440 nm grew in while the Soret at 407 nm decayed (Figure 11). The product was a mixture of a 403 nm species and the 440 nm species. This reactivity is reminiscent of the Arg44Ala mutant, which could form the cross-link but did not go to completion. Cyt c′β has minimal ruffing distortion of the heme comparable to the Arg44Ala mutant, so it is possible that the ruffling deformation aids in cross-link formation but is not essential. However, the presence of a Lys in the cross-linking position is required for reactivity with peroxide and subsequent cross-link formation in these proteins. This suggests a role of Lys in either a hydrogen-bond network or proton donation to the Fe-peroxo species to aid in the homolytic cleavage of the O─O bond. The ability to install a heme-Lys cross-link in cyt c′β shows that the cross-link formation is intrinsic to the fold and points to a further evolutionary relationship between the cyt c′β and cyt P460 families.
Figure 11.
UV/vis spectral time-course for the reaction of 8 μM Methylococcus capsulatus WT cyt c′β (A) or F61K cyt c′β (B) with 8 equivalents (64 μM) Li2O2. The red trace is before the addition of Li2O2 and the blue trace is the final spectrum after Li2O2 additions. Grey traces represent scans every 0.2 minutes.
Conclusions
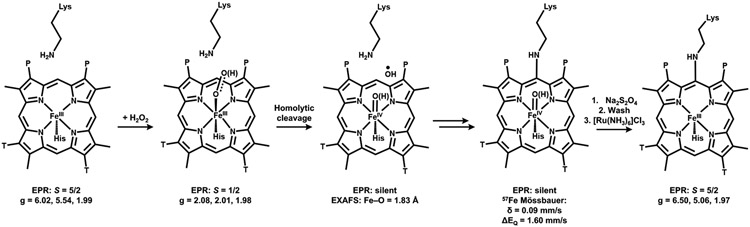
We have demonstrated the necessity for peroxide in cyt P460 maturation. This unique family of enzymes is primed for PTM by virtue of the protein fold and the influence of this fold on the structure of the bound c-heme. The assembled data strongly support a peroxide-dependent PTM is involved in the maturation of cyt P460 proenzyme (Figure 12). A single intermediate accumulates during this maturation that has a Soret absorption maximum at 413 nm. This species can be generated by direct addition of exogenous peroxide to proenzyme and will decay to form catalytically competent cross-linked cyt P460. Once peroxide binds, homolytic cleavage of the O─O bond leads to a high-valent heme consistent with compound II and a hydroxyl radical, the latter of which likely hydroxylates the γ-meso-carbon. We showed using an Arg44Ala mutation that ruffling is not required for cross-link formation, but that Arg44 plays an important role in directing reactivity to the γ-meso-carbon through a H-bond network. Additionally, the installation of a Lys to the cross-linking position in the related cytochrome c′β family, which has the same fold as cyt P460 but does not contain the Lys cross-link, imbues reactivity with peroxide and leads to cross-link formation. This revealed that Lys does not simply wait in the active site to form the covalent attachment to the porphyrin, but also plays a role in O─O bond cleavage.
Figure 12.
Summary of species observed during peroxide-driven cyt P460 maturation.
Supplementary Material
Acknowledgments
KML gratefully acknowledges the National Institutes of Health for support in the form of an Early Stage Investigator (ESI) Maximizing Investigators’ Research Award (MIRA) (R35-GM124908). MMB and SHM acknowledge the National Science Foundation Graduate Research Program (DGE-1650441) for support. EPR data were collected at ACERT, which is supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 1R24GM146107. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P30GM133894). This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Eiger 16M detector on the 24-ID-E beam line is funded by a NIH-ORIP HEI grant (S10OD021527). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. We thank Siddarth Chandrasekharan for assistance with EPR data collection. We also thank Robert W. Voland for assistance with rapid freeze-quench experiments. The table of contents graphic was created with Biorender.com.
Footnotes
Supporting Information
SDS-PAGE, additional kinetics data, EPR spectra, 57Fe Mössbauer spectra, EXAFS data and fit parameters, heme planarity analysis, cyt P460 sequence logo. The Supporting Information is available free of charge on the ACS Publications website.
References
- (1).Macek B; Forchhammer K; Hardouin J; Weber-Ban E; Grangeasse C; Mijakovic I Protein Post-Translational Modifications in Bacteria. Nat. Rev. Microbiol 2019, 17 (11), 651–664. [DOI] [PubMed] [Google Scholar]
- (2).Walsh CT; Garneau-Tsodikova S; Gatto GJ Jr. Protein Posttranslational Modifications: The Chemistry of Proteome Diversifications. Angew. Chem. Int. Ed 2005, 44 (45), 7342–7372. [DOI] [PubMed] [Google Scholar]
- (3).Lin Y-W The Broad Diversity of Heme-Protein Cross-Links: An Overview. Biochim. Biophys. Acta 2015, 1854 (8), 844–859. [DOI] [PubMed] [Google Scholar]
- (4).Bowman SEJ; Bren KL The Chemistry and Biochemistry of Hemec: Functional Bases for Covalent Attachment. Nat. Prod. Rep 2008, 25 (6), 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Coleman RE; Lancaster KM Heme P460: A (Cross) Link to Nitric Oxide. Acc. Chem. Res 2020, 53 (12), 2925–2935. 10.1021/acs.accounts.0c00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Haase D; Hermann B; Einsle O; Simon J Epsilonproteobacterial Hydroxylamine Oxidoreductase (EHao): Characterization of a ‘Missing Link’ in the Multihaem Cytochrome c Family. Mol. Microbiol 2017, 105 (1), 127–138. [DOI] [PubMed] [Google Scholar]
- (7).Ferousi C; Schmitz RA; Maalcke WJ; Lindhoud S; Versantvoort W; Jetten MSM; Reimann J; Kartal B Characterization of a Nitrite-Reducing Octaheme Hydroxylamine Oxidoreductase That Lacks the Tyrosine Cross-Link. J. Biol. Chem 2021, 296, 100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Klotz MG; Schmid MC; Strous M; op den Camp HJM; Jetten MSM; Hooper AB Evolution of an Octahaem Cytochrome c Protein Family That Is Key to Aerobic and Anaerobic Ammonia Oxidation by Bacteria. Environ. Microbiol 2008, 10 (11), 3150–3163. [DOI] [PubMed] [Google Scholar]
- (9).Bergmann DJ; Hooper AB Cytochrome P460 of Nitrosomonas Europaea. Eur. J. Biochem 2003, 270 (9), 1935–1941. [DOI] [PubMed] [Google Scholar]
- (10).Caranto JD; Vilbert AC; Lancaster KM Nitrosomonas Europaea Cytochrome P460 Is a Direct Link between Nitrification and Nitrous Oxide Emission. Proc. Natl. Acad. Sci. USA 2016, 113 (51), 14704–14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Vilbert AC; Caranto JD; Lancaster KM Influences of the Heme-Lysine Crosslink in Cytochrome P460 over Redox Catalysis and Nitric Oxide Sensitivity. Chem. Sci 2018, 9 (2), 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Coleman RE; Vilbert AC; Lancaster KM The Heme–Lys Cross-Link in Cytochrome P460 Promotes Catalysis by Enforcing Secondary Coordination Sphere Architecture. Biochemistry 2020, 59 (24), 2289–2298. [DOI] [PubMed] [Google Scholar]
- (13).Smith MA; Majer SH; Vilbert AC; Lancaster KM Controlling a Burn: Outer-Sphere Gating of Hydroxylamine Oxidation by a Distal Base in Cytochrome P460. Chem. Sci 2019, 10 (13), 3756–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Pearson AR; Elmore BO; Yang C; Ferrara JD; Hooper AB; Wilmot CM The Crystal Structure of Cytochrome P460 of Nitrosomonas Europaea Reveals a Novel Cytochrome Fold and Heme–Protein Cross-Link. Biochemistry 2007, 46 (28), 8340–8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jentzen W; Song X-Z; Shelnutt JA Structural Characterization of Synthetic and Protein-Bound Porphyrins in Terms of the Lowest-Frequency Normal Coordinates of the Macrocycle. J. Phys. Chem. B 1997, 101 (9), 1684–1699. 10.1021/jp963142h. [DOI] [Google Scholar]
- (16).Liptak MD; Wen X; Bren KL NMR and DFT Investigation of Heme Ruffling: Functional Implications for Cytochrome c. J. Am. Chem. Soc 2010, 132 (28), 9753–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Graves AB; Horak EH; Liptak MD Dynamic Ruffling Distortion of the Heme Substrate in Non-Canonical Heme Oxygenase Enzymes. Dalton Trans. 2016, 45 (24), 10058–10067. [DOI] [PubMed] [Google Scholar]
- (18).Colas C; Ortiz de Montellano PR Autocatalytic Radical Reactions in Physiological Prosthetic Heme Modification. Chem. Rev 2003, 103 (6), 2305–2332. [DOI] [PubMed] [Google Scholar]
- (19).Davydov R; Kofman V; Fujii H; Yoshida T; Ikeda-Saito M; Hoffman BM Catalytic Mechanism of Heme Oxygenase through EPR and ENDOR of Cryoreduced Oxy-Heme Oxygenase and Its Asp 140 Mutants. J. Am. Chem. Soc 2002, 124 (8), 1798–1808. [DOI] [PubMed] [Google Scholar]
- (20).Thöny-Meyer L; Fischer F; Künzler P; Ritz D; Hennecke H Escherichia Coli Genes Required for Cytochrome c Maturation. J Bacteriol 1995, 177 (15), 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Conger MA; Cornetta AR; Liptak MD Spectroscopic Evidence for Electronic Control of Heme Hydroxylation by IsdG. Inorg. Chem 2019, 58 (22), 15455–15465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Matsui T; Unno M; Ikeda-Saito M Heme Oxygenase Reveals Its Strategy for Catalyzing Three Successive Oxygenation Reactions. Acc. Chem. Res 2010, 43 (2), 240–247. [DOI] [PubMed] [Google Scholar]
- (23).Kumar D; de Visser SP; Shaik S Theory Favors a Stepwise Mechanism of Porphyrin Degradation by a Ferric Hydroperoxide Model of the Active Species of Heme Oxygenase. J. Am. Chem. Soc 2005, 127 (22), 8204–8213. [DOI] [PubMed] [Google Scholar]
- (24).Unno M; Matsui T; Ikeda-Saito M Structure and Catalytic Mechanism of Heme Oxygenase. Nat. Prod. Rep 2007, 24 (3), 553–570. [DOI] [PubMed] [Google Scholar]
- (25).Schuelke-Sanchez AE; Cornetta AR; Kocian TAJ; Conger MA; Liptak MD Ruffling Is Essential for Staphylococcus Aureus IsdG-Catalyzed Degradation of Heme to Staphylobilin. J. Inorg. Biochem 2022, 230, 111775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Matsui T; Kim SH; Jin H; Hoffman BM; Ikeda-Saito M Compound I of Heme Oxygenase Cannot Hydroxylate Its Heme Meso -Carbon. J. Am. Chem. Soc 2006, 128 (4), 1090–1091. [DOI] [PubMed] [Google Scholar]
- (27).Spiro TG; Strekas TC Resonance Raman Spectra of Heme Proteins. Effects of Oxidation and Spin State. J. Am. Chem. Soc 1974, 96 (2), 338–345. [DOI] [PubMed] [Google Scholar]
- (28).Kleingardner JG; Levin BD; Zoppellaro G; Andersson KK; Elliott SJ; Bren KL Influence of Heme c Attachment on Heme Conformation and Potential. J Biol Inorg Chem 2018, 23 (7), 1073–1083. [DOI] [PubMed] [Google Scholar]
- (29).Takahashi S; Nambu S; Matsui T; Fujii H; Ishikawa H; Mizutani Y; Tsumoto K; Ikeda-Saito M Unique Electronic Structures of the Highly Ruffled Hemes in Heme-Degrading Enzymes of Staphylococcus Aureus , IsdG and IsdI, by Resonance Raman and Electron Paramagnetic Resonance Spectroscopies. Biochemistry 2020, 59 (40), 3918–3928. [DOI] [PubMed] [Google Scholar]
- (30).Huang X; Groves JT Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev 2018, 118 (5), 2491–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Li S; Isiorho EA; Owens VL; Donnan PH; Odili CL; Mansoorabadi SO A Noncanonical Heme Oxygenase Specific for the Degradation of C-Type Heme. J. Biol. Chem 2021, 296, 100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Matera KM; Takahashi S; Fujii H; Zhou H; Ishikawa K; Yoshimura T; Rousseau DL; Yoshida T; Ikeda-Saito M Oxygen and One Reducing Equivalent Are Both Required for the Conversion of α-Hydroxyhemin to Verdoheme in Heme Oxygenase. J. Biol. Chem 1996, 271 (12), 6618–6624. [DOI] [PubMed] [Google Scholar]
- (33).Davydov R; Satterlee JD; Fujii H; Sauer-Masarwa A; Busch DH; Hoffman BM A Superoxo-Ferrous State in a Reduced Oxy-Ferrous Hemoprotein and Model Compounds. J. Am. Chem. Soc 2003, 125 (52), 16340–16346. [DOI] [PubMed] [Google Scholar]
- (34).Walker FA Magnetic Spectroscopic (EPR, ESEEM, Mössbauer, MCD and NMR) Studies of Low-Spin Ferriheme Centers and Their Corresponding Heme Proteins. Coord. Chem. Rev 1999, 185–186, 471–534. [Google Scholar]
- (35).Chance B; Maehly AC [136] Assay of Catalases and Peroxidases. In Methods in Enzymology; Elsevier, 1955; Vol. 2, pp 764–775. [Google Scholar]
- (36).Lightning LK; Huang H; Moënne-Loccoz P; Loehr TM; Schuller DJ; Poulos TL; de Montellano PRO Disruption of an Active Site Hydrogen Bond Converts Human Heme Oxygenase-1 into a Peroxidase. J. Biol. Chem 2001, 276 (14), 10612–10619. [DOI] [PubMed] [Google Scholar]
- (37).Takayama SJ; Loutet SA; Mauk AG; Murphy MEP A Ferric–Peroxo Intermediate in the Oxidation of Heme by IsdI. Biochemistry 2015, 54 (16), 2613–2621. [DOI] [PubMed] [Google Scholar]
- (38).Ledray AP; Krest CM; Yosca TH; Mittra K; Green MT Ascorbate Peroxidase Compound II Is an Iron(IV) Oxo Species. J. Am. Chem. Soc 2020, 142 (48), 20419–20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Green MT Application of Badger’s Rule to Heme and Non-Heme Iron–Oxygen Bonds: An Examination of Ferryl Protonation States. J. Am. Chem. Soc 2006, 128 (6), 1902–1906. [DOI] [PubMed] [Google Scholar]
- (40).Chen H; Moreau Y; Derat E; Shaik S Quantum Mechanical/Molecular Mechanical Study of Mechanisms of Heme Degradation by the Enzyme Heme Oxygenase: The Strategic Function of the Water Cluster. J. Am. Chem. Soc 2008, 130 (6), 1953–1965. [DOI] [PubMed] [Google Scholar]
- (41).Maas MN; Hintzen JCJ; Mecinović J Probing Lysine Posttranslational Modifications by Unnatural Amino Acids. Chem. Commun 2022, 58 (52), 7216–7231. [DOI] [PubMed] [Google Scholar]
- (42).Bachi A; Dalle-Donne I; Scaloni A Redox Proteomics: Chemical Principles, Methodological Approaches and Biological/Biomedical Promises. Chem. Rev 2013, 113 (1), 596–698. [DOI] [PubMed] [Google Scholar]
- (43).Yin V; Shaw GS; Konermann L Cytochrome c as a Peroxidase: Activation of the Precatalytic Native State by H2O2-Induced Covalent Modifications. J. Am. Chem. Soc 2017, 139 (44), 15701–15709. [DOI] [PubMed] [Google Scholar]
- (44).Walker F Nitric Oxide Interaction with Insect Nitrophorins and Thoughts on the Electron Configuration of the FeNO Complex. J. Inorg. Biochem 2005, 99 (1), 216–236. [DOI] [PubMed] [Google Scholar]
- (45).He C; Ogata H; Lubitz W Elucidation of the Heme Active Site Electronic Structure Affecting the Unprecedented Nitrite Dismutase Activity of the Ferriheme b Proteins, the Nitrophorins. Chem. Sci 2016, 7 (8), 5332–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Rivera M; Caignan GA; Astashkin AV; Raitsimring AM; Shokhireva T. Kh.; Walker FA Models of the Low-Spin Iron(III) Hydroperoxide Intermediate of Heme Oxygenase: Magnetic Resonance Evidence for Thermodynamic Stabilization of the dxy Electronic State at Ambient Temperatures. J. Am. Chem. Soc 2002, 124 (21), 6077–6089. [DOI] [PubMed] [Google Scholar]
- (47).Pfanzagl V; Nys K; Bellei M; Michlits H; Mlynek G; Battistuzzi G; Djinovic-Carugo K; Van Doorslaer S; Furtmüller PG; Hofbauer S; Obinger C Roles of Distal Aspartate and Arginine of B-Class Dye-Decolorizing Peroxidase in Heterolytic Hydrogen Peroxide Cleavage. J. Biol. Chem 2018, 293 (38), 14823–14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Rodriguez-Lopez JN; Smith AT; Thorneley RNF Role of Arginine 38 in Horseradish Peroxidase. J. Biol. Chem 1996, 271 (8), 4023–4030. [DOI] [PubMed] [Google Scholar]
- (49).Elmore BO; Bergmann DJ; Klotz MG; Hooper AB Cytochromes P460 and c ′-Beta; A New Family of High-Spin Cytochromes c. FEBS Letters 2007, 581 (5), 911–916. [DOI] [PubMed] [Google Scholar]
- (50).Poret-Peterson AT; Graham JE; Gulledge J; Klotz MG Transcription of Nitrification Genes by the Methane-Oxidizing Bacterium, Methylococcus Capsulatus Strain Bath. ISME J. 2008, 2 (12), 1213–1220. [DOI] [PubMed] [Google Scholar]
- (51).Adams HR; Krewson C; Vardanega JE; Fujii S; Moreno T; Chicano C; Sambongi Y; Svistunenko D; Paps J; Andrew CR; Hough MA One Fold, Two Functions: Cytochrome P460 and Cytochrome c ′-β from the Methanotroph Methylococcus Capsulatus (Bath). Chem. Sci 2019, 10 (10), 3031–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Abendroth J; Buchko GW; Liew FN; Nguyen JN; Kim HJ Structural Characterization of Cytochrome c′β-Met from an Ammonia-Oxidizing Bacterium. Biochemistry 2022, 61 (7), 563–574. [DOI] [PubMed] [Google Scholar]
- (53).Liew FN; Brandys MA; Biswas S; Nguyen JN; Rahmawati M; Nevala M; Elmore BO; Hendrich MP; Kim HJ Cytochrome c′–-Met Is a Variant in the P460 Superfamily Lacking the Heme–Lysyl Cross-Link: A Peroxidase Mimic Generating a Ferryl Intermediate. Biochemistry 2020, 59 (5), 704–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.