Abstract
Context:
Over the years, numerous treatment modalities have been researched for the management of dentinal hypersensitivity. A recent remineralizing agent containing a phase of amorphous calcium phosphate combined with fluoride has shown the ability to rapidly convert into biomimetic hydroxyapatite. This potential can be utilized in occluding the dentinal tubules for the treatment of hypersensitivity.
Aims:
The present study aims to compare the effectiveness of biomimetic hydroxyapatite-based tooth mousse and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) tooth mousse as desensitizing agents on dentinal tubule occlusion.
Materials and Methods:
The in vitro study design involved 30 prepared dentin specimens that were divided into three groups: Group A: negative control, Group B: CPP-ACP tooth mousse (GC tooth mousse™), and Group C: fluoride-doped amorphous calcium phosphate (F-ACP) tooth mousse (Curasept Biosmalto Denti Sensibili Tooth Mousse). The specimens were observed under a scanning electron microscope (SEM) and an attached energy-dispersive X-ray (EDX) spectroscopy apparatus after 1 week.
Statistical Analysis:
The data were analyzed using Kruskal–Wallis test and post hoc Mann–Whitney test for intragroup and intergroup analysis, respectively.
Results:
The SEM analysis of the F-ACP group showed statistically greater percentage of tubule occlusion (40.21%) compared to the CPP-ACP group (38.15%). EDX analysis of both F-ACP and CPP-ACP groups revealed calcium, phosphorus, carbon, oxygen, and silica with an additional fluoride element in the F-ACP group.
Conclusions:
In the present study, both remineralizing agents were able to occlude the dentinal tubules. Among the two, the F-ACP tooth mousse showed greater tubule occlusion, and therefore, appears promising as an upcoming remineralizing agent in the management of dentinal hypersensitivity.
Keywords: Dentin hypersensitivity, fluoride-doped amorphous calcium phosphate, fluoroapatite, scanning electron microscopy, tooth mousse
INTRODUCTION
With a ubiquitous prevalence of 33.5%, the chronic “symptom complex” known as “dentinal hypersensitivity” (DH) rarely resolves completely and its management poses a continual challenge.[1,2] Based on Brännström’s “hydrodynamic” theory on the mechanism of DH, the treatment of DH aims at either blocking the dentinal tubules or desensitizing the nerve.[3] Dentifrices containing strontium, fluoride, and oxalate salts are among the common dentin-occluding agents.[4] Newer biomaterials such as bioactive glass and nano-hydroxyapatite crystal for the treatment of DH have also shown the ability to occlude the open dentinal tubules and to remineralize the tooth structure.[5,6] Apart from these treatment modalities, remineralizing agents that are routinely used for primary teeth in the treatment of initial caries or enamel erosion, such as casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), have also been found to lower dentin permeability by forming precipitates on the dentin surface that reduce the diameter of dentinal tubules.[7] Recently, a bioactive and biomimetic hydroxyapatite-based tooth mousse containing fluoride-doped amorphous calcium phosphate (F-ACP) has been introduced as a remineralizing agent. It can rapidly convert into a stable hydroxyapatite called fluoroapatite.[8] Over the years, numerous agents have been introduced for DH, but none have resulted in complete resolution. Therefore, the present in vitro study aimed to compare and evaluate the efficacy of the biomimetic F-ACP tooth mousse in dentin tubule occlusion with the CPP-ACP technology.
MATERIALS AND METHODS
In vitro design
This in vitro study was conducted in the department of periodontology of a teaching institute. A sample size of 30 (10 per group) was considered, for a level of significance of 5% and 90% power. The primary objective of the study was to compare and evaluate the dentin tubule occlusion after the application of CPP-ACP tooth mousse and F-ACP tooth mousse. The secondary objective was to compare and evaluate the elemental composition of the dentin surface coating and dentinal plug formed between the groups. The null hypothesis stated that there would be no statistically significant difference between F-ACP tooth mousse and CPP-ACP tooth mousse on dentinal tubule occlusion.
Preparation of the dentin discs
Thirty molars (extracted due to periodontal conditions and impacted third molars), devoid of caries, were collected, and an ultrasonic scaler was used to remove any soft-tissue remnant and calculus. Before further use, the teeth were stored in 10% formalin at room temperature. The dentin discs were prepared based on a model described by Shah et al., in 2017.[6] A diamond disc mounted on a slow-speed handpiece was used to section the teeth. They were first cut perpendicular to their long axis above the cemento-enamel junction. Another cut was made parallel to the first to remove all the coronal enamel. Three millimeters thick dentinal blocks with exposed flat dentin surfaces were obtained. The surrounding enamel of the blocks was removed creating specimens measuring 5 mm × 5 mm × 3 mm. Following this, the dentin specimens were embedded in acrylic resin blocks [Figure 1].
Figure 1.
Dentin specimens embedded in acrylic resin blocks
The prepared dentin specimens were randomly allocated into three study groups:
Group A: no tooth mousse (negative control)
Group B: samples treated with CPP-ACP tooth mousse GC Tooth Mousse™, GC India Dental Private Limited, India
Group C: samples treated with F-ACP tooth mousse Curasept Biosmalto Denti Sensibili Tooth Mousse, Curaden Healthcare SpA, Italy.
Once the acrylic blocks were set, the prepared specimens were polished with 600 grit silicon carbide polishing paper for 30 s to create a standard smear layer on the dentin surface. They were dipped in 17% ethylene diamine tetra-acetic acid for 2 min to remove the smear layer. They were then thoroughly rinsed in distilled water.[6,9]
Application of desensitizing agents
The two varieties of tooth mousses were topically applied onto the specimens using a cotton tip in the respective groups, i.e., Groups B and C, for 3 min each, and then wiped off [Figure 2]. To simulate the clinical condition where the patient must avoid consumption of any food or liquid for 30 min after the application of the tooth mousses, the samples were left undisturbed for 30 min, followed by rinsing with distilled water. The tooth mousses were applied twice daily (12-h intervals) for 1 week. The specimens were kept in distilled water after each treatment.[9]
Figure 2.

Application of F-ACP tooth mousse using a cotton tip applicator. F-ACP – Fluoride doped amorphous calcium phosphate
Scanning electron microscopy
After 1 week, all the samples from the three groups were thoroughly washed in distilled water. They were dried in a vacuum chamber for 24 h, and sputter coated with a thin layer of gold in the sputter coating machine. The coated specimens were then placed on aluminum stubs, within the scanning electron microscope (SEM) machine. Photomicrographs were taken using SEM (ZEISS EVO MA18 with Oxford EDS-X-act) at 3000X magnification and 5 kV voltage. The elemental composition on the dentin surfaces of all groups was identified using an energy-dispersive X-ray (EDX) spectroscopy apparatus [Figure 3].
Figure 3.
Specimens examined under a scanning electron microscope with attached energy dispersive X-ray spectroscopy apparatus
Study outcome measures
Percentage of occluded tubules
This is calculated by dividing the total number of occluded tubules by the total number of tubules in the SEM photomicrographs. Each dentinal tubule was evaluated quantitatively by the criteria proposed by Chen et al., in 2015.[10]
Occluded (100% of tubule occluded)
Mostly occluded (50% to <100% of tubule occluded)
Partially occluded (25% to <50% of tubule occluded)
Mostly unoccluded (<25% of tubule occluded)
Unoccluded (0%, no tubule occlusion).
The purpose of this measure is to quantify the effectiveness of the tooth mousses to block the patent dentinal tubules.
Quantitative and qualitative evaluation of the chemical composition of the dentinal plugs
This is reported as mean weight percentage using the EDX apparatus. Through this parameter, we can determine the chemical nature of the surface coating and dentinal plug formed occluding the tubule.
Statistical analysis
Data were entered into an Excel spreadsheet and SPSS (Statistical Package for Social Sciences) version 20 [IBM SPASS statistics (IBM corp. Armonk, NY, USA released 2011)] was used to perform the statistical analysis. Descriptive statistics of the explanatory and outcome variables were calculated by median and interquartile range for quantitative variables as the data showed skewness based on the normalcy test (Shapiro–Wilk test). Inferential statistics like the Kruskal–Wallis test were applied to compare the statistical difference of elements and dentinal tubule occlusion among the three groups. The post hoc Mann–Whitney test was used for intergroup comparison. The level of significance was set at 5%.
RESULTS
Scanning electron microscopy results
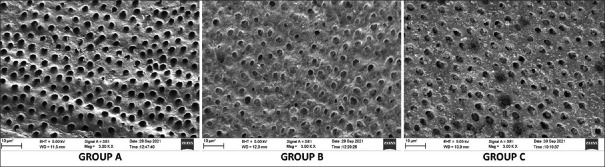
The scanning electron microscopic examination provided photomicrographs that were obtained at 3000X magnification and 5 kV voltage [Figure 4].
Figure 4.
SEM photomicrograph of Group A (negative control), Group B (CPP-ACP tooth mousse), and Group C (F-ACP tooth mousse) specimens showing open dentinal tubules and closed dentinal tubules respectively. SEM – Scanning electron microscope; F-ACP – Fluoride doped amorphous calcium phosphate; CPP-ACP – Casein phosphopeptide-amorphous calcium phosphate
Negative control (Group A)
The photomicrographs of almost all these specimens presented a smooth dentin surface with no smear layer. Almost all the dentinal tubules were open and regular. Some of the tubules were partially occluded (0.49%) and mostly unoccluded (5.37%).
Casein phosphopeptide-amorphous calcium phosphate tooth mousse (Group B)
A regular dentin surface with visible dentin tubules was observed. A thin homogeneous layer coating the dentin surface was seen that appeared to surround each dentinal tubule, therefore, narrowing its diameter. The majority of the dentinal tubules were mostly occluded (38.15%), some of them were partially occluded (14.51%), and a few of the tubules were completely occluded (0.67%) with crystal-like deposits. Almost half of the dentinal tubules were still completely unoccluded (41.7%).
Fluoride-doped amorphous calcium phosphate tooth mousse (Group C)
The photomicrographs showed an irregular dentin surface with visible dentin tubules. An irregular granular layer covered the dentinal surface with irregularly scattered crystal-like deposits occluding the openings. Only a few of the tubules appeared completely occluded (0.66%) and 40.21% were mostly occluded. About 17.53% were partially occluded and 40.11% were completely unoccluded in all the dentin samples.
Both CPP-ACP and F-ACP groups showed statistically higher percentages of partially occluded, mostly occluded, and completely occluded dentinal tubules compared to the negative control. The F-ACP group had significantly higher percentage of mostly occluded and partially occluded dentinal tubules compared to the CPP-ACP group, but had significantly lower percentage of mostly unoccluded and completely unoccluded tubules. No statistically significant difference was seen between the groups in terms of completely occluded dentinal tubule percentages [Table 1].
Table 1.
Comparison of the dentinal tubule occlusion among the groups (in percentage) using Kruskal-Wallis test
| Elements | Groups | Minimum | Maximum | Median | IQR | p value |
|---|---|---|---|---|---|---|
| Completely occluded | Group 1 | 0 | 0 | 0 | 0 | 0.00* |
| Group 2 | 0.51 | 1.55 | 0.67 | 0.48 | ||
| Group 3 | 0.51 | 1.2 | 0.66 | 0.51 | ||
| Mostly occluded | Group 1 | 0 | 0 | 0 | 0 | 0.00* |
| Group 2 | 37.27 | 39.64 | 38.15 | 1.44 | ||
| Group 3 | 39.42 | 41.02 | 40.21 | 0.92 | ||
| Partially occluded | Group 1 | 0.15 | 0.65 | 0.49 | 0.23 | 0.00* |
| Group 2 | 12.67 | 15.71 | 14.51 | 1.25 | ||
| Group 3 | 15.83 | 17.9 | 17.53 | 0.63 | ||
| Mostly unoccluded | Group 1 | 4.23 | 6.42 | 5.37 | 1.27 | 0.00* |
| Group 2 | 3.94 | 5.87 | 4.97 | 0.92 | ||
| Group 3 | 1.14 | 2.17 | 1.49 | 0.38 | ||
| Completely unoccluded | Group 1 | 94.05 | 95.21 | 94.14 | 0.32 | 0.00* |
| Group 2 | 39.52 | 43.29 | 41.7 | 1.62 | ||
| Group 3 | 39.17 | 41.36 | 40.11 | 1.03 |
Significant P value < 0.05. IQR – Inter Quartile Range
Energy-dispersive X-ray spectroscopic results
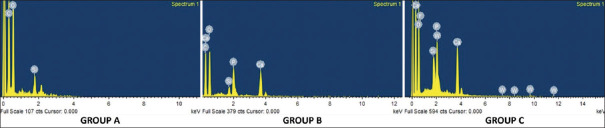
The chemical composition of the surface coating and occluding deposit was analyzed using EDX spectroscopy [Figure 5]. The negative control group revealed high percentages of carbon (45.29%), oxygen (42.91%), and silica (10.20%). The elements identified in the CPP-ACP group were calcium (12.11%), phosphorous (8.75%), oxygen (48.67%), carbon (29.06%), and silica (0.96%). The F-ACP group contained calcium (17.48%), phosphorus (10.41%), fluoride (1.64%), carbon (22.46%), oxygen (42%), and silica (3.48%). Other elements like strontium were found in only one specimen (7.78%) in the F-ACP group, and chloride was found in 8 of the 10 specimens in the range of 0.59%–1.48%.
Figure 5.
EDX spectrum of Group A, Group B, and Group C specimens. EDX – Energy-dispersive X-ray
DISCUSSION
The hydrodynamic theory states that the changes in the fluid movement within the dentinal tubules stimulate the mechanoreceptors in the pulp which in turn excites sensory nerves to cause pain.[3]
The fluid flow and diffusion through the dentinal tubules follow certain physical laws called the Hagen–Poiseuille law for capillary flow. This is measured as hydraulic conductance and is directly proportional to the radius of the dentinal tubules (flow ∝ r4).[11] Based on this concept, if a desensitizing agent can reduce tubule radii anywhere throughout the length of the tubule, the resistance to fluid flow increases. This relationship predicts that dentin tubules need not necessarily be completely sealed to reduce DH.[12]
The main strategies to reduce DH are by targeting the dentin permeability or the interdental nerves. Dentin permeability is reduced by occluding the dentinal tubules, either by depositing a material directly within the dentinal tubules and/or forming a layer over the surface of the exposed dentin.[13]
Numerous desensitizing agents possess tubule occluding properties. One such example is tooth remineralizing agents which have shown promising results in the management of DH.[9,14]
Considering this information, this in vitro study was carried out to compare and evaluate dentin tubule occlusion between two remineralizing agents, i.e., CPP-ACP tooth mousse and an upcoming tooth mousse containing F-ACP.
CPP-ACP tooth mousse (GC tooth mousse™) is a nano-complex patented by Reynolds at the University of Melbourne, Australia.[15] This complex consists of Casein phosphopeptide (CPP) protein, which is derived from milk (RECALDENT®) and it is structured by residues of phosphorylated serine and glutamic amino acid. The CPP-ACP complex provides a calcium and phosphate reservoir by binding to the biofilm present on the tooth surface. The precipitated Ca2+ and PO43- ions bind to the phosphorylated fibrils of the collagen present in the intertubular dentin and promote the formation of apatite. These deposits block the tubules, eventually decreasing dentin hypersensitivity.[16] This is visualized, in the present study, where the SEM images of the CPP-ACP group reveal a thin homogenous layer coating the dentin surface and surrounding the dentinal tubule thereby reducing its diameter. Crystal-like deposits were also seen occluding the dentinal tubules.
In terms of dental tubule occlusion, 38.15% of mostly occluded dentinal tubules and a few completely occluded tubules (0.67%) were found in the present study. Comparable results were reported by Corneli et al. in 2020 who found 40% partially occluded tubules in the group that received CPP-ACP tooth mousse.[9] Another in vitro study reported 56.3% dentinal tubule occlusion by the CPP-ACP containing dentifrice.[12]
The chemical composition of the dentin surface and plug in the CPP-ACP group shows a high percentage of calcium and phosphorus. This is in accordance with a study by Berkathullah et al., in 2018, where the EDX spectra also revealed prevalent content of calcium and phosphorus following the application of an agent containing CPP-ACP.[17] This could be attributed to its composition and mechanism of action.
The test agent in this study was an upcoming biomimetic tooth mousse (Curasept Biosmalto Denti Sensibili Tooth Mousse) whose active ingredient is amorphous calcium phosphate combined with carbonate, fluoride, and citrate (F-ACP complex). The amorphous calcium phosphate in the tooth mousse is a transient phase of natural hydroxyapatite formation and releases a high concentration of calcium and phosphate ions.[18] Doping ACP with fluoride ions stabilizes it and ensures rapid conversion of F-ACP to fluoroapatite.[18] The F-ACP complex, in contact with saliva rapidly releases the active substances, i.e., calcium, phosphate, and fluoride, selectively in the areas where the enamel and dentin are demineralized. The citrate facilitates the penetration of these ions into the tooth and favors the nucleation and stabilization of the newly formed apatite crystals (fluoroapatite) as can be seen in the SEM images of the F-ACP group in the present study.[8]
The EDX analysis also proves this concept based on the high concentration of calcium, phosphorus, and fluoride found, suggesting the possibility of fluoroapatite formation. An in vitro study by Iafisco et al., in 2018, incorporated fluoride ions into the ACP nanoparticles affecting their amorphous nature, to enhance its anticaries and remineralizing properties.[19] They reported that fluoride-doped amorphous calcium phosphate nanoparticles showed good ability to partially occlude the acid-etched dentinal tubules and to remineralize enamel, which is in accordance with the current study where the F-ACP group was able to mostly occlude 40.21% of the dentinal tubules.
On comparing both groups, the present study reports that the F-ACP group had statistically better tubule occlusion. The EDX analysis also showed a greater percentage of calcium and phosphorus, with an additional element of fluoride. The better results in terms of tubule occlusion in the F-ACP group may be due to the following reasons. (1) Fluoride in the F-ACP complex is responsible for the formation of a stable biomimetic hydroxyapatite called fluoroapatite, which is believed to be more acid resistant.[19,20] This may contribute to both anticaries and remineralization action. (2) In addition, the smaller particle size of the F-ACP complex in the tooth mousse enables it to penetrate the dentinal tubules and allowing the deposition of fluoroapatite crystals within them. This leads to dentinal tubule occlusion, thereby exerting a desensitizing effect.
Even though the CPP-ACP group lacked fluoride and had statistically lesser tubule occlusion, previous studies have shown that agents containing CPP-ACP had comparable remineralizing and desensitizing abilities to fluoride-containing agents.[9,20,21] This may be because of the interdependency of fluoride on calcium and phosphate for its remineralizing action. Fluoride requires calcium and phosphate from the saliva to get involved in as well as to enhance the remineralization process.[22] The aforesaid properties of remineralization seen in both tooth mousses could be applied in the management of DH.
A limitation of the present study is that the depth of these agents’ penetration into the dentinal tubules was not determined. Moreover, the resistance of the occluded tubules was not evaluated. Long-term clinical studies are required to corroborate the findings obtained in the present in vitro study.
CONCLUSIONS
The present study has asserted that in addition to enhancing enamel resistance, remineralizing agents such as CPP-ACP and F-ACP tooth mousses have the capacity to occlude dentinal tubules. The tooth mousse containing the F-ACP complex showed statistically greater tubule occlusion compared to the CPP-ACP group. In terms of the chemical characteristics of the dentin surface coating and occluding plugs, the upcoming F-ACP tooth mousse may have better resistance and stability due to the formation of fluoroapatite as compared to the CPP-ACP group. As this is a preliminary in vitro study, the observations made here may help to initiate further long-term clinical trials.
Financial support and sponsorship
Dr. TMA Pai University Grant.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Deepa Bullappa (Public Health Dentist, Clinical Data Consultant and Analyst) for her help regarding the statistical analysis for this work. We also thank Mr. Suketh and Mrs. Sushma (Manipal Institute of Technology) in the use of the scanning electron microscope required for this study.
REFERENCES
- 1.Idon PI, Esan TA, Bamise CT. Efficacy of three in-office dentin hypersensitivity treatments. Oral Health Prev Dent. 2017;15:207–14. doi: 10.3290/j.ohpd.a38523. [DOI] [PubMed] [Google Scholar]
- 2.Favaro Zeola L, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J Dent. 2019;81:1–6. doi: 10.1016/j.jdent.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Brannstrom M. Dentin sensitivity and aspiration of odontoblasts. J Am Dent Assoc. 1963;66:366–70. doi: 10.14219/jada.archive.1963.0104. [DOI] [PubMed] [Google Scholar]
- 4.Alrusayes AA, Assal NA, Althobity AM, Alfraih YK, Alfraih MI, Aldossary MS. Dentin hypersensitivity: A review of its treatment modalities. AIMDR. 2021;7:540–53. [Google Scholar]
- 5.Saffarpour M, Mohammadi M, Tahriri M, Zakerzadeh A. Efficacy of modified bioactive glass for dentin remineralization and obstruction of dentinal tubules. J Dent (Tehran) 2017;14:212–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Shah S, Shivakumar AT, Khot O, Patil C, Hosmani N. Efficacy of NovaMin-and Pro-Argin-containing desensitizing dentifrices on occlusion of dentinal tubules. Dent Hypotheses. 2017;8:104–9. [Google Scholar]
- 7.Kijsamanmith K, Banomyong D, Burrow MF, Kanchanasantikul P, Wipawiwat S, Srikam S, et al. Effect of conventional and acid-modified casein phosphopeptide-amorphous calcium phosphate crèmes on dentin permeability before and after acid challenge. Oper Dent. 2019;44:530–5. doi: 10.2341/17-382-L. [DOI] [PubMed] [Google Scholar]
- 8.Curasept Biosmalto Sensitive Teeth Product Information. [[Last accessed on 2023 Feb 03]]. Available from: https://www.curaseptspa.it/brand.php ?brand=curasept-biosmalto .
- 9.Corneli R, Kolakemar A, Damda A, Naik R. An in vitro evaluation of dentinal tubule occlusion using three desensitizing methods: A scanning electron microscopic study. J Conserv Dent. 2020;23:86–90. doi: 10.4103/JCD.JCD_94_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CL, Parolia A, Pau A, Celerino de Moraes Porto IC. Comparative evaluation of the effectiveness of desensitizing agents in dentine tubule occlusion using scanning electron microscopy. Aust Dent J. 2015;60:65–72. doi: 10.1111/adj.12275. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz K, Pashley D. The physiological basis of dentin hypersensitivity. In: Gillam D, editor. Dentine Hypersensitivity: Advances in Diagnosis, Management, and Treatment. Switzerland: Springer International Publishing; 2015. pp. 11–40. [Google Scholar]
- 12.Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endod. 1990;16:48–53. doi: 10.1016/S0099-2399(06)81563-3. [DOI] [PubMed] [Google Scholar]
- 13.Wainer C. How do desensitising agents in dental products work? BDJ Stud. 2020;27:43–5. [Google Scholar]
- 14.Zhao X, Wang L, Pan J, Malmstrom H, Ren YF. Effects of desensitizing dentifrices on dentin tubule occlusion and resistance to erosive challenges. BMC Oral Health. 2021;21:610. doi: 10.1186/s12903-021-01977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Mei ML, Xu J, Lo EC, Li Q, Chu CH. Biomimetic mineralisation of phosphorylated dentine by CPP-ACP. J Dent. 2013;41:818–25. doi: 10.1016/j.jdent.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Berkathullah M, Farook MS, Mahmoud O. The effectiveness of remineralizing agents on dentinal permeability. Biomed Res Int. 2018;2018:12. doi: 10.1155/2018/4072815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ionescu AC, Degli Esposti L, Iafisco M, Brambilla E. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci Rep. 2022;12:5994. doi: 10.1038/s41598-022-09787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iafisco M, Degli Esposti L, Ramírez-Rodríguez GB, Carella F, Gómez-Morales J, Ionescu AC, et al. Fluoride-doped amorphous calcium phosphate nanoparticles as a promising biomimetic material for dental remineralization. Sci Rep. 2018;8:17016. doi: 10.1038/s41598-018-35258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair MC, Patil RU, Bahutule SR. Comparative evaluation of hydroxyapatite fluoride and casein phosphopeptide amorphous calcium phosphate fluoride as remineralizing agents in primary teeth using pH cycling and single-sectioning technique. Int J Med Oral Res. 2022;7:31–5. [Google Scholar]
- 21.Ghafournia M, Tehrani MH, Nekouei A, Faghihian R, Mohammadpour M, Feiz A. In vitro evaluation of dentin tubule occlusion by three bioactive materials: A scanning electron microscopic study. Dent Res J (Isfahan) 2019;16:166–71. [PMC free article] [PubMed] [Google Scholar]
- 22.Singh T, Garg S, Dhindsa A, Jain N. Effect of remineralization potential of ACP–CPP with fluoride and ACP–CPP on enamel subsurface lesion in primary and young permanent teeth: In situ study. J South Asian Assoc Pediatr Dent. 2018;1:47–53. [Google Scholar]