Abstract
Objectives:
To estimate the effects of obesity on all types of upper extremity compression neuropathies (UECN) (carpal tunnel syndrome and other median nerve, radial nerve, and ulnar nerve compression neuropathies) and to assess whether bariatric surgery modifies these effects.
Background:
UECN are increasingly prevalent and decrease the quality of life of affected individuals. Studies suggest obesity as a risk factor for carpal tunnel syndrome, the most common type of UECN.
Methods:
A retrospective cohort study was conducted using the PearlDiver Mariner Database, an all-payor claims database containing claims for over 53 million patients from 2010 to 2019 in all 50 US states. Rates and odds of all types of UECN were compared between 1:1:1 exact matched cohorts of obese patients who were medically managed, obese patients who underwent bariatric surgery, and nonobese patients (111,967 patients in each cohort).
Results:
Compared with nonobese patients, patients with obesity were significantly more likely to develop any UECN (odds ratio [OR], 1.13; 95% confidence interval [CI], 1.09–1.18), carpal tunnel syndrome (OR, 1.15; 95% CI, 1.10–1.30), and 2 or more UECN (OR, 1.34; 95% CI, 1.20–1.48). Compared with obese patients who were managed medically, obese patients who underwent bariatric surgery were significantly less likely to develop any UECN (OR, 0.87; 95% CI, 0.84–0.91) and carpal tunnel syndrome (OR, 0.85; 95% CI, 0.81–0.89).
Conclusions:
Obese patients have higher odds of both single and concomitant UECN, specifically carpal tunnel syndrome, compared with nonobese patients. Bariatric surgery decreases the odds of developing UECN compared with obese patients not undergoing surgical intervention.
Keywords: bariatric surgery, carpal tunnel syndrome, compression neuropathies, peripheral neuropathies, upper extremity neuropathies
Mini-abstract: This retrospective study investigated rates of upper extremity compression neuropathies in matched cohorts of medically managed obese patients, surgically managed obese patients, and nonobese patients. Obese patients who underwent bariatric surgery had decreased odds of developing UECN compared with medically managed obese patients.
INTRODUCTION
Obesity and upper extremity compression neuropathies (UECN) are 2 increasingly prevalent conditions carrying substantial negative consequences on an individual’s physical and mental health.1,2 Several studies have suggested obesity as a risk factor for carpal tunnel syndrome, the most common type of UECN in developed nations.3–7 The pathogenesis of excess body mass increasing the risk of carpal tunnel syndrome is not known but may be a microvascular sequala of metabolic syndrome or increased intracarpal pressure from excess adipose tissue within the carpal tunnel.6,8,9 Other compression neuropathies may similarly be more prevalent in states of obesity; however, this relationship is yet to be studied.
Bariatric surgery has been proven to be the most effective treatment to induce and maintain weight loss long-term, as well as improve morbidity and mortality.10–15 However, little is currently known about the relative contribution of bariatric surgical intervention to the overall burden of UECN in the general population. Obesity-directed treatments leading to lasting weight loss may serve to lessen the severity and disease burden of UECN.
To date, no studies have examined the relationship between obesity, bariatric surgery, and all types of UECN (carpal tunnel syndrome and other median nerve, radial nerve, and ulnar nerve compression neuropathies). The aims of this study were twofold: (1) to estimate the effects of obesity on UECN and (2) to assess whether bariatric surgery modifies this association.
METHODS
Data Source
This study followed the Strengthening and Reporting of Observational Studies in Epidemiology reporting guidelines. Data from the PearlDiver Mariner Database (PearlDiver Technologies, Colorado Springs, CO), which contains over 53 million patients from 2010 to 2019 was used for this retrospective cohort study (Supplemental Table 1, http://links.lww.com/AOSO/A105).16 Patients are from all 50 US states and may any type of insurance. Data consists of records for healthcare encounters processed by physician networks, including outpatient, inpatient, and prescription claims billed to all payers.16 Claims are adjudicated using auditing policies and internal review is conducted by independent third parties.16 Diagnoses and procedures can be identified with International Classification of Diseases (ICD)-9 and -10 diagnosis/procedure codes and Current Procedural Terminology codes. The study was provided exemption status by the Rush University Medical Center Institutional Review Board given that the database is Health Insurance Portability and Accountability Act-compliant and primary data collection was not conducted.
Study Population
Patients who were eligible for bariatric surgery were identified using the definition endorsed by the Centers for Medicare & Medicaid Services: body mass index (BMI) of 40 kg/m2 or more or BMI of 35 kg/m2 or more and at least 1 or more obesity-related comorbidities (type II diabetes mellitus, hypertension, obstructive sleep apnea, nonalcoholic fatty liver disease, osteoarthritis, lipid abnormalities, gastrointestinal disorders, or heart disease).17 The presence of the qualifying comorbidity had to be within 1 year of the obesity diagnosis.
Intervention and Control Groups
Among those who were eligible for bariatric surgery, patients were divided into surgical management (Roux-en-Y gastric bypass or vertical sleeve gastrectomy) or medical management (obesity cohort). Additionally, a third cohort of patients who did not have obesity, defined as no recorded BMI of 30 kg/m2 or greater over the study period, was identified (nonobesity cohort). Supplemental Table 2 (http://links.lww.com/AOSO/A105) reports the ICD-9 and -10 diagnosis/procedure and Current Procedural Terminology codes that were used to identify bariatric surgery procedures. The index date for bariatric surgery patients was the date of the operation (initial surgery for those with multiple bariatric surgeries). Since obesity controls had healthcare encounters with a diagnosis of obesity that spanned multiple dates, the midpoint was used as the index date. All patients who did not have an active insurance record for 1 year before and 5 years after their index date were excluded to ensure the ability to identify outcomes. In addition, patients 17 years or younger and patients with UECN prior to their index date were excluded.
Upper Extremity Compression Neuropathies
The primary outcome of this study was any UECN within 5 years following the index date, defined by the presence of any diagnosis for the following: carpal tunnel syndrome, other median nerve neuropathy, radial nerve neuropathy, and ulnar nerve neuropathy. The full list of ICD-9 and -10 diagnosis codes for these outcomes are provided in Supplemental Table 3 (http://links.lww.com/AOSO/A105).
Potential Confounders
ICD-9 and -10 diagnosis codes were used to identify patient comorbidities within 1 year prior to their enrollment in our study (Supplemental Table 3, http://links.lww.com/AOSO/A105). Comorbidities included hypertension, coronary artery disease, anemia deficiency, hypothyroidism, type 2 diabetes mellitus, peptic ulcer disease, gastroesophageal reflux disease, nonalcoholic fatty liver disease, rheumatoid arthritis, osteoarthritis, obstructive sleep apnea, and smoking. Descriptive statistics were calculated for our entire patient population (4,822,771 patients). Two sample t tests and chi-square test were used to compare confounders by bariatric surgery versus obesity versus nonobesity cohorts. Given the potential concern for confounding, multivariate logistic regression models were constructed to identify any associations between specific comorbidities and each outcome of interest. Any variables with a P value of 0.05 or less were deemed statistically significant and used to execute 1 to 1 to 1 exact matching between our 3 cohorts, resulting in 111,967 patients in each cohort.
Statistical Analysis
Incidence of UECN over 5 years from the index date was calculated for each cohort. Rates were compared between cohorts using chi-square tests and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Statistical analysis was performed using Mariner’s Bellwether Software that executes the queries to conduct all analyses.16
RESULTS
Descriptive Characteristics of the Unmatched Population
A total of 4,822,771 patients were eligible for our study following exclusion of patients ages 17 or less (n = 190,223) and noncontinuous insurance for 1 year before and 5 years after enrollment (n = 309,777) (Fig. 1). The unmatched population was comprised of 500,000 patients without obesity and 4,322,771 patients with obesity who underwent bariatric surgery (n = 172,813) or were medically managed (n = 4,149,958). Demographic data and descriptive characteristics for the unmatched population were collected (Supplemental Table 4, http://links.lww.com/AOSO/A105). All comorbidities included in our study were found to be statistically significant on multivariate analysis (Supplemental Table 5, http://links.lww.com/AOSO/A105).
FIGURE 1.
Patient selection flow chart.
Descriptive Characteristics of the Matched Population
The exact-matched population analyzed in this study contained 335,901 patients that were equally represented in the bariatric surgery cohort (n = 111,967, 33.33%), obesity controls cohort (n = 111,967, 33.33%), and nonobesity cohort (n = 111,967, 33.33%). Each cohort was predominantly between 35 and 54 years old (n = 57,491, 51.34%) and female gender (n = 85,327, 76.21%). Hypertension (n = 38,161, 34.08%), gastroesophageal reflux disease (n = 32,397, 28.93%), and obstructive sleep apnea (n = 21,280, 19.01%) were the most common comorbidities (Table 1).
TABLE 1.
Descriptive Characteristics for the Matched Population
| Total Matched Population, n = 335,901 | Bariatric Surgery Cohort, n = 111,967 | Obesity Cohort, n = 111,967 | Nonobesity Cohort, n = 111,967 | P | |
|---|---|---|---|---|---|
| Age, n (%) | |||||
| 34 or less | 80,436 (23.95) | 26,812 (23.95) | >0.999 | ||
| 35–44 | 85,128 (25.34) | 28,376 (25.34) | |||
| 45–54 | 87,345 (26.00) | 29,115 (26.00) | |||
| 55–64 | 61,644 (18.35) | 20,548 (18.35) | |||
| 65–74 | 20,910 (6.23) | 6970 (6.23) | |||
| 75 or more | 438 (0.13) | 146 (0.13) | |||
| Gender, n (%) | |||||
| Female | 255,981 (76.21) | 85,327 (76.21) | >0.999 | ||
| Male | 79,920 (23.79) | 26,640 (23.79) | |||
| Comorbidities, n (%) | |||||
| Hypertension | 114,483 (34.08) | 38,161 (34.08) | >0.999 | ||
| Coronary artery disease | 426 (0.13) | 142 (0.13) | >0.999 | ||
| Anemia deficiency | 34,038 (10.13) | 11,346 (10.13) | >0.999 | ||
| Hypothyroidism | 43,923 (13.08) | 14,641 (13.08) | >0.999 | ||
| Diabetes mellitus, type II | 61,896 (18.43) | 20,632 (18.43) | >0.999 | ||
| Peptic ulcer disease | 9534 (2.84) | 3178 (2.84) | >0.999 | ||
| Gastroesophageal reflux disease | 97,191 (28.93) | 32,397 (28.93) | >0.999 | ||
| Nonalcoholic fatty liver disease | 10,416 (3.10) | 3472 (3.10) | >0.999 | ||
| Rheumatoid arthritis | 4413 (1.31) | 1471 (1.31) | >0.999 | ||
| Osteoarthritis | 56,178 (16.72) | 18,726 (16.72) | >0.999 | ||
| Obstructive sleep apnea | 63,840 (19.01) | 21,280 (19.01) | >0.999 | ||
| Smoking | 35,622 (10.60) | 11,874 (10.60) | >0.999 | ||
| 5-y UE neuropathy outcomes, n (%) | |||||
| Any neuropathy | 16,225 (4.83) | 5153 (4.60) | 5860 (5.23) | 5212 (4.65) | <0.0005* |
| Carpal tunnel syndrome | 12,863 (3.83) | 4022 (3.59) | 4713 (4.21) | 4128 (3.69) | <0.0005* |
| Other median nerve neuropathy | 515 (0.15) | 160 (0.14) | 190 (0.17) | 165 (0.15) | 0.0881 |
| Radial nerve neuropathy | 229 (0.07) | 83 (0.07) | 66 (0.06) | 80 (0.07) | 0.3398 |
| Ulnar nerve neuropathy | 2618 (0.78) | 888 (0.79) | 891 (0.80) | 839 (0.75) | 0.3737 |
| 2 or more neuropathies | 2231 (0.66) | 763 (0.68) | 839 (0.75) | 629 (0.56) | <0.0005* |
*Statistically significant value (P < 0.05).
UE indicates upper extremity.
Rates of Upper Extremity Compression Neuropathies—Matched Population
A total of 16,225 patients, or 4.83%, developed a single UECN within 5 years of enrollment in our study. Carpal tunnel syndrome was the most commonly occurring neuropathy (n = 12,863, 3.83%). Additionally, 0.63% of patients developed 2 or more UECN with the most frequent combination being carpal tunnel syndrome and ulnar nerve neuropathy (n = 1474, 66.07%) (Supplemental Table 6, http://links.lww.com/AOSO/A105).
Odds of Upper Extremity Compression Neuropathies—Obesity Versus Nonobesity Cohorts
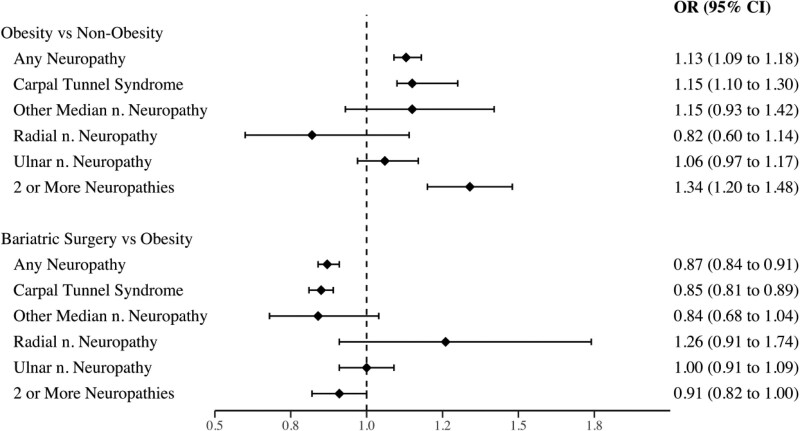
Patients with obesity who were managed medically were significantly more likely to develop any UECN (OR, 1.13; 95% CI, 1.09–1.18), carpal tunnel syndrome (OR, 1.15; 95% CI, 1.10–1.30), and 2 or more UECN (OR, 1.34; 95% CI, 1.20–1.48) compared with the nonobesity cohort. The odds of other median nerve, radial nerve, and ulnar nerve neuropathies did not significantly differ between these cohorts (Fig. 2).
FIGURE 2.
ORs comparing upper extremity compression neuropathies in obesity versus nonobesity cohorts and bariatric surgery versus obesity cohorts. CI indicates confidence interval; n., nerve; OR = odds ratio.
Odds of Upper Extremity Compression Neuropathies—Bariatric Surgery Versus Obesity Cohorts
Patients with obesity undergoing bariatric surgery had decreased odds of developing any UECN (OR, 0.87; 95% CI, 0.84–0.91) and carpal tunnel syndrome (OR, 0.85; 95% CI, 0.81–0.89) compared with obesity controls. There was no significant difference in the odds of other median nerve, radial nerve, ulnar nerve, or 2 or more UECN between bariatric surgery and obesity controls (Fig. 2).
DISCUSSION
Obesity and UECN are common conditions encountered by physicians. Prior literature has solely focused on obesity’s influence on carpal tunnel syndrome and has not yet examined its relationship to other UECN. Additionally, bariatric surgery is well-known to improve metabolic dysfunction and other obesity-related comorbidities, but no study has compared rates of UECN in patients with obesity who underwent bariatric surgery to those who did not. This novel study attempts to fill these voids by conducting a national cohort study of patients without obesity and bariatric surgery candidates who did and did not undergo surgical intervention.
In this matched analysis, patients with obesity had higher rates and odds of developing any UECN, carpal tunnel syndrome, and 2 or more concomitant UECN compared with patients without obesity. Additionally, our study found patients undergoing bariatric surgery had reduced rates and odds of developing any UECN overall and carpal tunnel syndrome compared with patients with obesity not undergoing surgical intervention. As carpal tunnel syndrome accounted for nearly 80% of all UECN in our study, we suspect it is likely the cause of the significantly different rates seen with any single and combination neuropathies.
Our study is in line with others showing obesity increases the rates of carpal tunnel syndrome.3–6,8,9,18 There are currently 2 generally accepted pathophysiologic theories that may explain this phenomenon. The first proposes that excess adipose tissue in and around the wrist joint may cause increased intracarpal pressures and a chronic compressive force on the median nerve within the carpal tunnel.19 The second proposes that obesity’s systemic manifestations create a hyperglycemic state causing alteration of neurotrophic factors and the microvascular system with subsequent ischemic damage.19,20
These mechanisms are not mutually exclusive. A cross-sectional study suggested the bimodal age distribution of carpal tunnel syndrome is likely due to mechanical factors such as obesity in younger patients (around age 40) versus ischemic vascular disease in older patients (over age 70).21 The demographics of the matched patients in our study contained less than 7% of patients over the age of 65, which makes the neuropathic changes less likely to be due to an exclusively vascular pathology. Additionally, reversal of obesity-induced metabolic and vascular system derangements likely requires more than the 5-year period utilized in our study.22 Thus, our findings further suggest mechanical factors may influence the development of upper extremity neuropathies more so than systemic factors.
Our results further describe the negative implications associated with obesity, a condition plaguing nearly 40% of Americans.23 The trends reported in this study call for collaboration between our public health and hospital healthcare systems in hopes of combating the obesity epidemic and improving patients’ quality of life. While numerous studies conducted support the use of bariatric surgery as an effective treatment for obesity-related comorbidities, our findings suggest it may also aid in the prevention of UECN in an otherwise at-risk patient population.11,24 The decreased odds of developing UECN among patients who underwent bariatric surgery was small in magnitude (OR, 0.87). As such, our findings should not be viewed as an indication for bariatric surgery but as an added benefit of bariatric surgery. Additionally, this novel study sheds light on the etiology of compression neuropathies in patients with obesity and contributes to the rapidly growing fields of bariatric and peripheral nerve surgery.
Several limitations should be considered when interpreting our results. First, the accuracy and reliability of this data depends on the subjective interpretation of physician records by a medical reviewer and are usually meant for financial and administrative purposes. Furthermore, administrative data fails to note details such as surgeon technique/experience, disease-severity, or specificity. For example, the billing codes do not specify if ulnar nerve compression occurred at the elbow or in Guyon’s canal that limits the conclusions we can deduce. Additionally, we are unable to identify which patients experienced some degree of insufficient weight loss or weight regain, as these commonly occur after bariatric surgery patients reach their nadir weight.25–27 In addition, any patients that may have experienced UECN but did not seek care during the follow-up period would not have been captured. Last, the observation of increased rates of UECN among patients may in part be due to increased utilization of the healthcare system compared with patients without obesity with a lack of significant comorbid conditions. This was addressed by utilizing strict match criterion between our cohorts, which encompassed age, gender, and all comorbidities included in our study. However, the cohorts could not be matched based on socioeconomic status due to the data that was used. Despite these limitations, it is our hope that physicians find our analysis interesting and beneficial to the current bodies of peripheral nerve and bariatric surgery literature.
CONCLUSIONS
This study is the first to estimate the association between bariatric surgery and the development of both common and uncommon UECN. Patients with obesity were found to have higher rates and odds of both single and concomitant UECN, specifically carpal tunnel syndrome. Bariatric surgery decreases the rates and odds of developing UECN compared with obese patients not undergoing surgical intervention.
Supplementary Material
Footnotes
Published online 16 March 2022
Disclosure: The authors declare that they have nothing to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Tremmel M, Gerdtham UG, Nilsson PM, et al. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14:E435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker-Bone K, Palmer KT, Reading I, et al. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. 2004;51:642–651. [DOI] [PubMed] [Google Scholar]
- 3.Nathan PA, Keniston RC, Myers LD, et al. Obesity as a risk factor for slowing of sensory conduction of the median nerve in industry. A cross-sectional and longitudinal study involving 429 workers. J Occup Med. 1992;34:379–383. [PubMed] [Google Scholar]
- 4.Karpitskaya Y, Novak CB, Mackinnon SE. Prevalence of smoking, obesity, diabetes mellitus, and thyroid disease in patients with carpal tunnel syndrome. Ann Plast Surg. 2002;48:269–273. [DOI] [PubMed] [Google Scholar]
- 5.Werner RA, Albers JW, Franzblau A, et al. The relationship between body mass index and the diagnosis of carpal tunnel syndrome. Muscle Nerve. 1994;17:632–636. [DOI] [PubMed] [Google Scholar]
- 6.Shiri R, Pourmemari MH, Falah-Hassani K, et al. The effect of excess body mass on the risk of carpal tunnel syndrome: a meta-analysis of 58 studies. Obes Rev. 2015;16:1094–1104. [DOI] [PubMed] [Google Scholar]
- 7.Mackinnon S, Novak C. Compression neuropathies. In: Wolfe S, Pederson W, Kozin S, Cohen M, eds. Green’s Operative Hand Surgery. 7th ed. Elsevier; 2017:921–956. [Google Scholar]
- 8.Bland JD. Carpal tunnel syndrome. Curr Opin Neurol. 2005;18:581–585. [DOI] [PubMed] [Google Scholar]
- 9.Callaghan B, Feldman E. The metabolic syndrome and neuropathy: therapeutic challenges and opportunities. Ann Neurol. 2013;74:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Eisenberg D, Azagury D, et al. American Society for Metabolic and Bariatric Surgery position statement on long-term survival benefit after metabolic and bariatric surgery. Surg Obes Relat Dis. 2016;12:453–459. [DOI] [PubMed] [Google Scholar]
- 11.Maciejewski ML, Arterburn DE, Van Scoyoc L, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer PR, Bhatt DL, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med. 2014;371:682. [DOI] [PubMed] [Google Scholar]
- 13.Murali SB. Long-term survival following bariatric surgery in the VA health system. JAMA. 2015;313:1473–1474. [DOI] [PubMed] [Google Scholar]
- 14.Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjöström L, Lindroos AK, Peltonen M, et al. ; Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 16.PearlDiver. Healthcare Research. 2020. Available at: http://www.pearldiverinc.com/researchinfo.html. Accessed May 5, 2021.
- 17.Medicare Coverage Database. National Coverage Determination (NCD) for Bariatric Surgery for Treatment of Co-Morbid Conditions Related to Morbid Obesity (100.1). 2013. Available at: https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=57&ncdver=5&bc=AAAAIAAAAAAA&. Accessed May 5, 2021.
- 18.Mondelli M, Aretini A, Ginanneschi F, et al. Waist circumference and waist-to-hip ratio in carpal tunnel syndrome: a case-control study. J Neurol Sci. 2014;338:207–213. [DOI] [PubMed] [Google Scholar]
- 19.Mackinnon SE. Pathophysiology of nerve compression. Hand Clin. 2002;18:231–241. [DOI] [PubMed] [Google Scholar]
- 20.Bales JG, Meals R. Peripheral neuropathy of the upper extremity: medical comorbidity that confounds common orthopedic pathology. Orthopedics. 2009;32:758. [DOI] [PubMed] [Google Scholar]
- 21.Shiri R, Heliövaara M, Moilanen L, et al. Associations of cardiovascular risk factors, carotid intima-media thickness and manifest atherosclerotic vascular disease with carpal tunnel syndrome. BMC Musculoskelet Disord. 2011;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miras AD, Ravindra S, Humphreys A, et al. Metabolic changes and diabetes microvascular complications 5 years after obesity surgery. Obes Surg. 2019;29:3907–3911. [DOI] [PubMed] [Google Scholar]
- 23.Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Ansari W, Elhag W. Weight regain and insufficient weight loss after bariatric surgery: definitions, prevalence, mechanisms, predictors, prevention and management strategies, and knowledge gaps-a scoping review. Obes Surg. 2021;31:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karmali S, Brar B, Shi X, et al. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23:1922–1933. [DOI] [PubMed] [Google Scholar]
- 27.Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377:1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.