Abstract
Background
In this study, we explored the commutability of reference materials (RMs) for carcinoembryonic antigen (CEA), selected the appropriate diluent matrix of the first International Reference Preparation (IRP) 73/601 of the World Health Organization (WHO 73/601) for CEA, and improved the comparability of CEA measurement results among different assay systems.
Methods
Forty serum samples were divided into five aliquots. WHO 73/601 was diluted into nine concentrations using five diluents with different components, and the candidate RMs for CEA at five concentrations (C1–C5) were prepared by the Beijing Clinical Laboratory Center (BCCL). The samples were analyzed via five automated CEA immunoassays.
Results
Carcinoembryonic antigen candidate RMs were commutable among all immunoassays based on the CLSI approach and among 7 of 10 assay combinations based on the IFCC approach. WHO 73/601 diluted in phosphate‐buffered saline (PBS) was commutable among all assays based on the CLSI approach and among 5 of 10 pairwise comparisons based on the IFCC approach with correction of bias at diluted concentrations, except for the lowest concentration, which had the smallest variation among systems. The median percentage biases among assays were decreased after calibration.
Conclusion
The BCCL candidate RMs (C2–C5) for CEA were commutable among all immunoassays. WHO 73/601 RMs diluted in a PBS buffer matrix were selected as common calibrators for five immunoassays, which reduced bias, thereby effectively improving the harmonization of CEA detection; therefore, they could be used to assign values to CEA candidate RMs developed by BCCL. Our findings promote the harmonization of CEA detection in immunoassays.
Keywords: carcinoembryonic antigen, commutability, comparability, reference material
The bias for the logarithmic transformation of concentration [ln(concentration)] between two measuring systems. The error bars indicate the uncertainty of the difference in bias between each BCCL RM and diluted WHO matrix and the average bias for the clinical samples. The solid black line (BCS line) represents the mean bias for all the clinical samples. The red dashed lines (C line) indicate the maximum allowable commutability‐related bias.
1. INTRODUCTION
Carcinoembryonic antigen (CEA) is a rich polysaccharide–protein complex present on the cell surface, with a molecular weight of approximately 150,000–200,000 Da. CEA was first isolated in 1965 by Gold and Freedman from the liver metastasis of colonic adenocarcinoma and the normal fetal digestive tract. 1 , 2 CEA is frequently expressed in digestive system cancers of endodermal origin, in the digestive tract tissues of normal embryos, and trace amounts in normal human serum. Therefore, CEA is commonly used to monitor patients with cancer post‐operatively. 3 , 4 , 5
Harmonization of test results is widely achieved by standardizing test methods, which is key to ensuring test result traceability. 6 Currently, there is no internationally recognized reference method for CEA. However, there is an international reference material (RM), WHO 73/601, 7 which plays an important role in ensuring consistency. Various analyzers and methods have been used to measure CEA in clinical laboratories; however, the working standards of most of these assays are not calibrated to WHO 73/601, 8 resulting in variable results. In addition, commutable RMs play a crucial role in the standardization and harmonization plan; RMs that are not commutable in the calibration traceability chain produce inconsistent results. 9 Owing to the lack of commutability, the application of WHO 73/601, which is a high‐purity international RM, 7 may be limited to certain measurement methods. In addition, although commutable external quality assessment (EQA) materials have been provided by the 2021 EQA program of the Beijing Center for Clinical Laboratories (BCCL), the coefficients of variation (CVs) for CEA measurements among laboratories were large, and the comparability between different assays was poor.
The present study aimed to explore the commutability of CEA RMs and select the appropriate WHO 73/601 diluent matrix to improve the comparability of CEA measurement results of different assays. This study could provide a method for assigning the value of CEA candidate RMs and promote the harmonization of CEA determination in immunoassays.
2. MATERIALS AND METHODS
2.1. Instruments and reagents
The five immunoassays and reagents used in this study were as follows: Abbott Architect i2000 (Abbott Diagnostics), Beckman DxC 800 (Beckman Coulter Inc.), Roche Cobas E601 (Roche Diagnostics GmbH), Diasorin Liaison XL (DiaSorin S.p.A), and Maccura IS1200 (Maccura Biology Co., Ltd.). The quantity values reported by these immunoassays were in mass concentration units ng/mL.
The commercial diluents were phosphate‐buffered saline (PBS) (Na2HPO4, KH2PO4, NaCl, and KCl; pH 7.2–7.4), Dulbecco's modified Eagle medium (DMEM) Sugar‐Free (without glutamine, sodium Pyruvatea, and phenol Red; 14430‐01; Gibco), minimal essential medium (MEM, with Earle's Salts, without L‐glutamine and phenol Red; 51200‐038; Gibco), and RPMI 1640 medium (with sodium bicarbonate, without L‐glutamine and phenol red; r7509‐500 mL; Sigma‐Aldrich). In addition, a healthy human serum pool prepared from leftover patient serum samples collected from Beijing Chaoyang Hospital was used as a diluent for WHO 73/601, with a mean native CEA concentration of 2.43 ng/mL. The preparation process was the same as that of candidate RMs (described in section 2.2.2).
The first International Reference Preparation (IRP) of the World Health Organization (CEA, human) was purchased from the National Institute for Biological Standards and Control (NIBSC) (code: 73/601).
2.2. Prepared materials
2.2.1. Individual serum samples
In total, 40 individual leftover patient serum samples with low, medium, and high CEA levels were collected at the laboratory department of Beijing Chaoyang Hospital, and the CEA concentrations were 2.41–888.57 ng/mL (as measured by the Roche Cobas E601 system). Each sample (at least 2.5 mL) stored 2–8°C refrigerator was evenly divided into five aliquots, which were stored at −80°C until use.
2.2.2. BCCL candidate RMs
Human serum pools without hemolysis, lipemia, and icterus were prepared using serum samples from the laboratory department of the Beijing Chaoyang Hospital as leftover serum samples for CEA measurement. The leftover samples with different CEA concentrations were directly collected into tubes and frozen at −80°C daily. During a period of approximately 3 months, 5 levels of serum pools with a total serum volume of approximately 600 mL (each level was approximately 120 mL) comprising 260 patient samples were obtained. The frozen serum aliquots were thawed at room temperature, pooled, and analyzed using a Beckman DxC 800 analyzer in 1 day. The five levels of serum pools with CEA values were approximately 4, 20, 40, 60, and 100 ng/mL. These pools were thoroughly mixed, filtered through 0.22‐mm membranes, aliquoted at 1 mL into 2‐mL cryogenic vials, and stored at −80°C to be used as trueness controls or for EQA (proficiency testing).
2.3. Study methods
2.3.1. WHO 73/601 standards
WHO 73/601 calibrators for the immunoassays were reconstituted with deionized water (0.5 mL of deionized water by weight to the concentration of 200 IU/mL). Thereafter, the sample was then diluted in five different matrices, including PBS (P), a healthy human serum pool (XQ), DMEM (D), MEM (M), and RPMI 1640 (RP) approximately to 0.02, 0.0625, 0.125, 0.25, 0.5, 1, 2, 4, and 8 IU/mL, labeled P1–P9, XQ1–XQ9, D1–D9, M1–M9, and RP1–RP9, respectively. Each diluted WHO 73/601 sample was divided into five equal parts, which were measured in the same batch via five different immunoassays on the same day: Roche Cobas E601, Diasorin liaison XL, Beckman DxC 800, Abbott Architect i2000, and Maccura IS1200. All samples were analyzed in triplicate. The mean values and CVs were calculated and compared.
2.3.2. Commutability study
According to the Clinical and Laboratory Standards Institute (CLSI) guideline EP30A, 10 nine levels of WHO 73/601 standards diluted by five different diluent components and five levels of Beijing Clinical Laboratory Center (BCCL) candidate RMs were randomly allocated among the 40 individual serum samples, which were all measured in triplicate by the five assays in 1 day. For commutability evaluation, the measurement results were logarithm‐transformed. The transformed data were analyzed using Deming regression, and 95% prediction intervals were calculated for each pair of assays. The commutability of the CEA RMs was evaluated based on whether the RM value of the logarithm was within the prediction interval for clinical samples measured by the pairs of assays. 10 , 11 , 12 The commutability assessment was also performed according to the difference in bias analysis based on the recommendations of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Working Group on Commutability. 9 , 13 The medical requirements were used as criteria. Biological variation was used as our bias limitation. The available biological variation data for CEA used in this research is 21.39% (Bias). The difference between the bias for RM and the average bias for clinical samples is denoted as dRM, and the standard uncertainty U(dRM) was calculated according to the distribution of the mean of bias for patient samples in the whole concentration interval. 9 A maximum value of |dRM| for the RM needs to be specified, which is called the commutability criterion (C). The RM is commutable when the uncertainty interval dRM ± U(dRM) is within 0 ± C. 13
2.3.3. Comparison before and after calibration
WHO 73/601 was diluted with PBS (P), which is commonly used to dilute calibrators, into eight different concentrations (0.0625, 0.125, 0.25, 0.5, 1, 2, 4, and 8 IU/mL) to calibrate the five CEA immunoassays conducted using the Roche Cobas E601, Diasorin liaison XL, Beckman DxC 800, Abbott Architect i2000, and Maccura IS1200 systems. The measurement results of the 40 individual patient samples were compared before (with manufacturer calibrators) and after calibration (with WHO‐derived calibrators) using the five assays.
2.3.4. Value assignment for BCCL candidate RMs
WHO 73/601 diluted standards in PBS (P1–P9) and BCCL candidate RMs (C1–C5) were measured in the same analytical sequence in triplicate for two consecutive days using the five assays. For each assay, the linear regression analysis of the theoretical concentration (IU/mL) of WHO 73/601 diluted standards and the actual measured concentrations expressed in mass concentration units (ng/mL) were pairwise plotted.
2.4. Statistical processing
Data analysis was performed using Microsoft Excel 2010 (Microsoft).
3. RESULTS
3.1. Comparability of WHO73/601 diluted standards in different CEA immunoassays
Diluted WHO 73/601 RMs labeled P1–P9, XQ1–XQ9, D1–D9, M1–M9, and RP1–RP9 were assessed using five immunoassays. The values obtained by diluting WHO 73/601 RMS at the lowest concentration (0.02 IU/mL) exhibit a large bias compared to the certificate values, which were not included in the calculation results. The five assays for WHO 73/601 prepared in the PBS buffer matrix (P) had a CV range of 13.02–18.00% for P2–P9. The CV ranges of WHO 73/601 diluted in XQ, RP, D, and M were 13.91–19.23% (XQ2–XQ9), 14.45–18.50% (RP2–RP9), 12.89–18.20% (D2–D9), and 14.12–22.06% (M2–M9), respectively (Table 1).
TABLE 1.
Mean and coefficients of variation (CVs) of carcinoembryonic antigen (CEA) measurements (ng/mL) from five routine systems.
| CEA (IU/mL) | System | PB | XQ | RP | D | M | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (ng/mL) | CV (%) | Mean (ng/mL) | CV (%) | Mean (ng/mL) | CV (%) | Mean (ng/mL) | CV (%) | Mean (ng/mL) | CV (%) | |
| 0.0625 | 5 | 4.02 | 13.14 | 4.83 | 13.91 | 4.20 | 15.42 | 4.34 | 14.60 | 4.16 | 14.12 |
| 0.0625 | 5 | 4.02 | 13.14 | 4.83 | 13.91 | 4.20 | 15.42 | 4.34 | 14.60 | 4.16 | 14.12 |
| 0.125 | 5 | 7.64 | 14.65 | 8.62 | 15.09 | 8.25 | 17.56 | 8.23 | 12.89 | 8.06 | 15.51 |
| 0.25 | 5 | 15.34 | 14.99 | 17.17 | 14.68 | 16.66 | 17.03 | 17.16 | 17.26 | 16.25 | 15.16 |
| 0.5 | 5 | 32.90 | 14.95 | 34.17 | 14.59 | 32.92 | 14.45 | 35.06 | 16.53 | 34.40 | 16.48 |
| 1 | 5 | 63.20 | 13.02 | 66.91 | 16.07 | 64.39 | 14.61 | 67.47 | 13.02 | 64.78 | 15.24 |
| 2 | 5 | 120.91 | 15.02 | 126.81 | 16.08 | 125.86 | 16.82 | 130.49 | 16.61 | 127.46 | 18.05 |
| 4 | 5 | 243.32 | 17.19 | 250.73 | 19.23 | 250.49 | 18.50 | 253.19 | 16.67 | 244.30 | 22.06 |
| 8 | 5 | 488.48 | 18.00 | 503.09 | 18.90 | 484.45 | 17.31 | 522.41 | 18.20 | 503.71 | 21.23 |
Abbreviations: D, Dulbecco's modified Eagle medium; M, minimal essential medium; PB, phosphate‐buffered saline buffer; RP, RPMI 1640 medium; XQ, healthy human serum.
3.2. Commutability of RMs for CEA measurement
Figure 1 shows the regression curves for the CEA measurements of 40 individual serum samples determined using the five assays according to the CLSI method. The BCCL candidate RMs for CEA developed by BCCL were commutable across all five assays in ten pairwise comparisons. Commutability was observed for all WHO 73/601 RMs diluted using all buffers, except for the lowest concentration, only in comparisons involving Roche versus Diasorin, Roche versus Beckman, Roche versus Maccura, and Diasorin versus Beckman systems Figure 2. shows the commutability of the relevant RMs among the five assays for CEA measurement based on the IFCC method. The BCCL candidate RMs were commutable across all five assays in ten pairwise comparisons; however, C1 was indeterminate for the Roche/Abbott/Maccura versus Beckman system comparisons. The WHO 73/601RMs diluted to 8 different concentrations were commutable among 2/10, 3/10, 1/10, and 1/10 pairwise comparisons in the PBS, serum, RPMI, DMEM, and MEM matrices, respectively. The commutability status of each material is provided in Tables S1 and S2.
FIGURE 1.
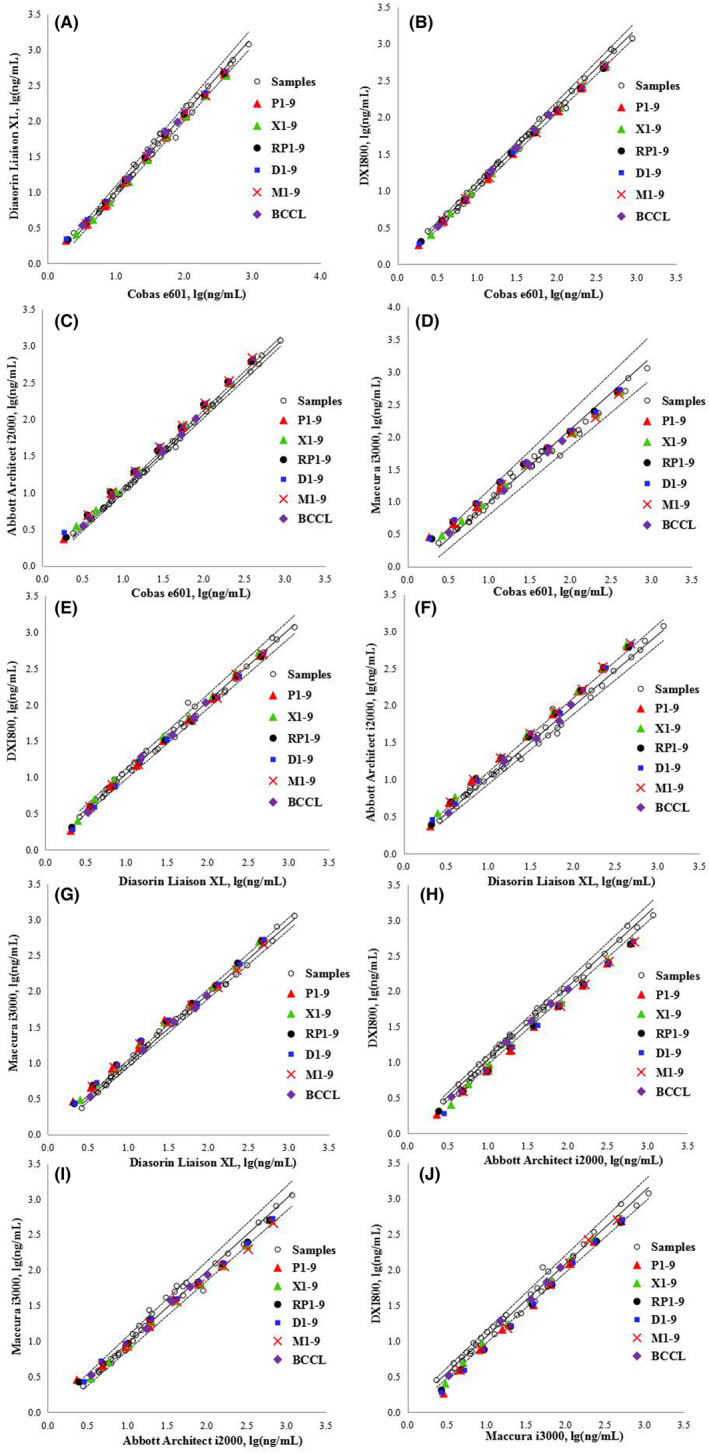
Commutability of the relevant reference materials among the five analytical systems for determining carcinoembryonic antigen (CEA) based on the CLSI method. The solid black lines are regression curves, and the dashed lines are the two‐tailed 95% prediction lines. P1–9, XQ1–9, D–9, M1–9, and RP1–9 represent the nine levels of WHO 73/601 diluted in PBS (P), a healthy human serum pool (XQ), DMEM (D), MEM (M), and RPMI 1640 (RP), respectively. BCCL represents the five levels of candidate RMs.
FIGURE 2.
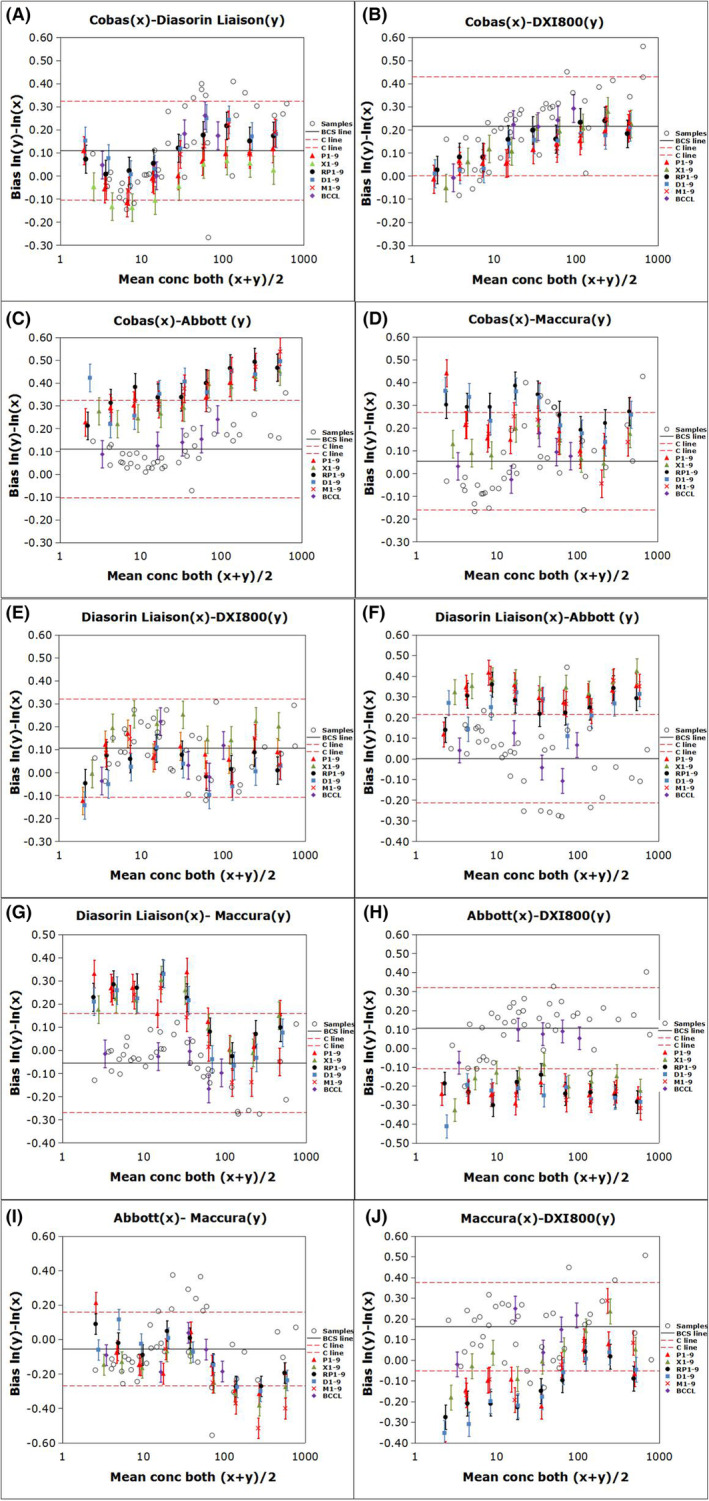
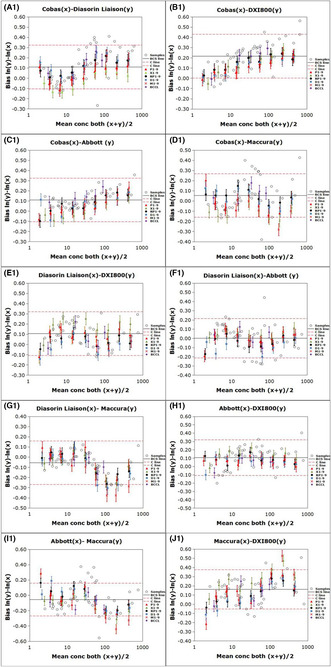
Commutability of the relevant reference materials among the five analytical systems for determining carcinoembryonic antigen (CEA) based on the IFCC method. The bias of the logarithmic transformation of concentration [ln(concentration)] between two measuring systems. The error bars indicate the uncertainty of the difference in bias between each BCCL RM and diluted WHO matrix and the average bias for the clinical samples. The solid black line (BCS line) represents the mean bias for all the clinical samples, and the red dashed lines (C line) indicate the maximum allowable commutability‐related bias.
Because the results of the 40 individual serum samples assessed using the Abbott and Maccura systems were well‐correlated with those of the Roche, Diasorin, and Beckman systems (Table S3), we applied a correction factor to the assigned value of WHO 73/601 as a calibrator within the calibration hierarchy for the Abbott and Maccura systems. This was necessary because the diluted WHO RMs for these systems were not commutable with the human samples. The correction factor was derived from the measured values of the diluted WHO RMs and clinical samples from each of the two comparison measurement procedures. The quadratic relationship (mathematical transformation) was used for WHO RMs at multiple levels. An approach to correct for the bias caused by the non‐commutability of an RM is described by Miller et al. 14 Figures 3 and 4 show the commutability results of RMs of all five assays after bias correction. WHO 73/601 in PBS was commutable among all assays according to the CLSI approach and among 5/10 pairwise comparisons according to the IFCC approach with corrected bias at 8 diluted concentrations, except for the lowest concentration. Therefore, WHO/73/601 diluted with PBS was selected as the standard calibrator. The commutability status of each material is presented in Tables S4 and S5.
FIGURE 3.
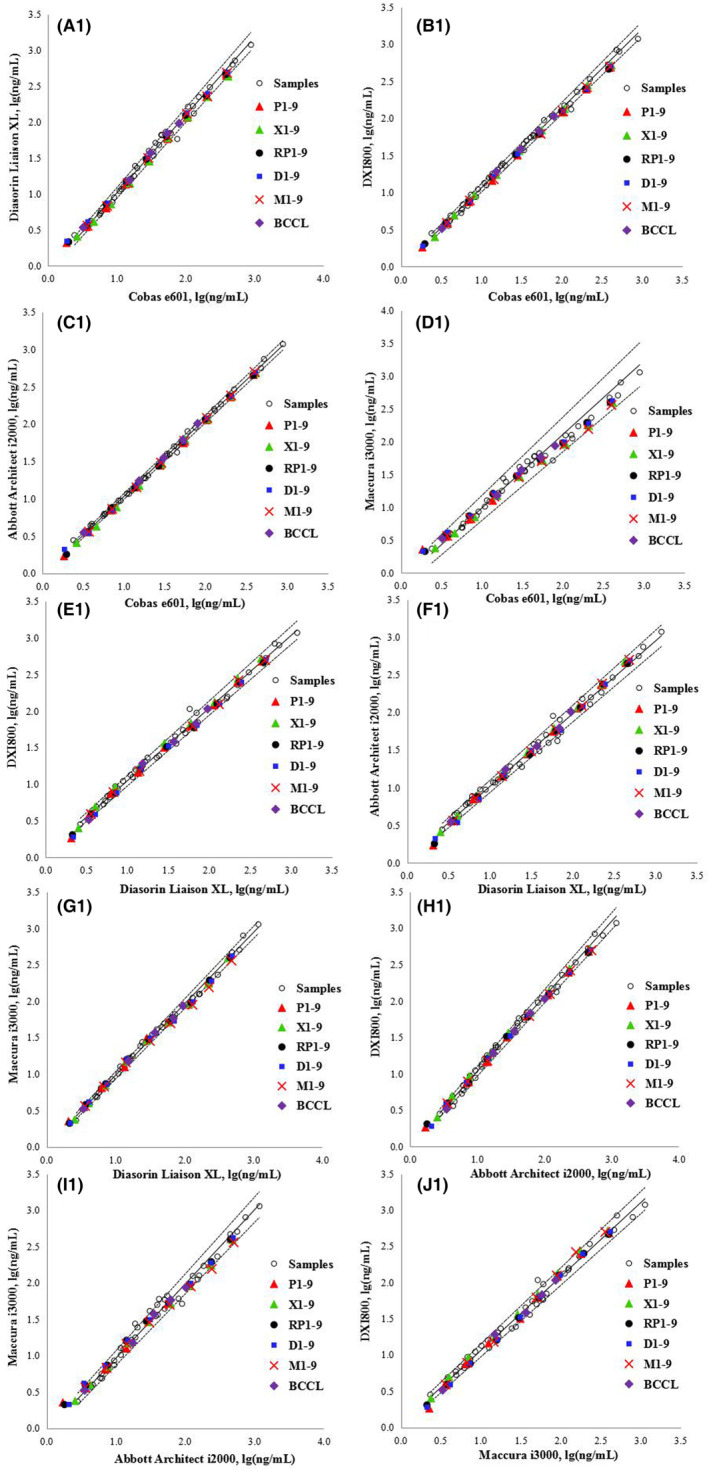
Commutability assessment of RMs among the five analytical systems after correcting for biases according to the CLSI method. The solid black lines are regression curves, and the dashed lines are the two‐tailed 95% prediction lines. P1–9, XQ1–9, D–9, M1–9, and RP1–9 represent the nine levels of WHO 73/601 diluted in PBS (P), a healthy human serum pool (XQ), DMEM (D), MEM (M), and RPMI 1640 (RP), respectively. BCCL represents the five levels of candidate RMs.
FIGURE 4.
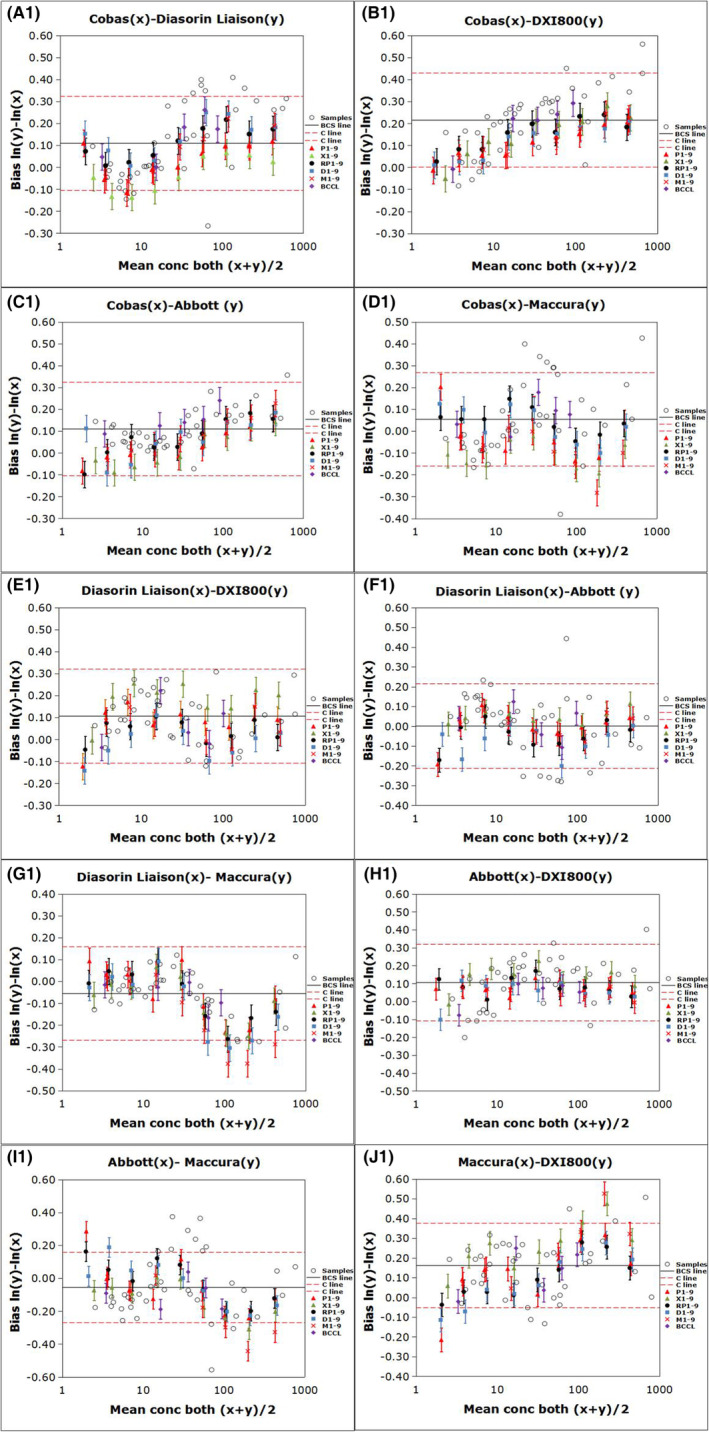
Commutability assessment of RMs among the five analytical systems after correcting for biases according to the IFCC method. The bias for the logarithmic transformation of concentration [ln(concentration)] between two measuring systems. The error bars indicate the uncertainty of the difference in bias between each BCCL RM and diluted WHO matrix and the average bias for the clinical samples. The solid black line (BCS line) represents the mean bias for all the clinical samples. The red dashed lines (C line) indicate the maximum allowable commutability‐related bias.
3.3. Comparison of CEA results of the five immunoassays before and after calibration
The trimmed mean target of the five measurement procedure results and the percent difference from the trimmed mean target for individual patient samples were calculated. 15 The results indicated that the median percentage biases across all 40 individual patient samples for each measurement procedure after calibration using WHO 73/601 diluted in PBS on the Roche, Diasorin, Beckman, Abbott, and Maccura immunoassay platforms decreased from −1.22–10.09% to 0.25–−6.49%. For each assay, 10 pairs of comparisons were performed, and the average correlation coefficient (r), slope (b), and intercept (a) values were calculated (Table 2 and Table S6).
TABLE 2.
Comparability of the immunoassays for carcinoembryonic antigen.
| Before calibration (ng/mL) | After calibration (IU/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roche | Diasorin | Beckman | Abbott | Maccura | Roche | Diasorin | Beckman | Abbott | Maccura | ||
| n | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| Median | 18.39 | 23.53 | 23.84 | 19.72 | 25.89 | 0.328 | 0.418 | 0.383 | 0.418 | 0.428 | |
| Min | 2.41 | 2.65 | 2.82 | 2.78 | 2.33 | 0.044 | 0.056 | 0.054 | 0.024 | 0.063 | |
| Max | 888.57 | 1180 | 1180 | 1178 | 1134 | 18.413 | 21.592 | 19.724 | 18.975 | 22.422 | |
|
|
0.994 | 0.991 | 0.990 | 0.992 | 0.987 | 0.992 | 0.991 | 0.987 | 0.990 | 0.983 | |
|
|
1.34 | 0.93 | 0.84 | 0.96 | 0.97 | 1.15 | 0.93 | 0.94 | 1.00 | 0.95 | |
|
|
−4.41 | 0.28 | −1.24 | 3.07 | 6.82 | −0.01 | 0.00 | −0.02 | 0.02 | 0.13 | |
|
|
−7.31 | −1.22 | 10.09 | 1.44 | −3.52 | −6.49 | 1.34 | 3.06 | 0.25 | 2.28 | |
Note: , , , and denote the mean value of the correlation coefficient, slope, intercept between one analytical system and others, and median percentage biases across the 40 individual samples for each assay, respectively.
3.4. Final certified values of BCCL candidate RMs
The certified concentrations of BCCL candidate RMs for CEA on individual assay platforms were converted into IU/mL based on the calibration curves of low‐ (0.0625–0.5 IU/mL) and high‐value (1–8 IU/mL) samples established by linear fitting, as shown in Figures S1 and S2 (also see Table S7).
WHO 73/601 was used as a calibrator for the Abbott and Maccura systems, which were assigned values based on the correction factor. The final certified value of BCCL candidate RMs was assigned based on the five assays. The assignment value of the low‐concentration samples C1–C3 was calculated using the low‐value curves (P2–P5) and that of high‐concentration samples C4–C5 was calculated using the high‐value curves (P6–P9).
After converting the units into IU/mL using WHO/73/601 RMs diluted with PBS as standard calibrators, the final certified values of BCCL candidate RMs for C1–C5 were 0.065 ± 0.016, 0.276 ± 0.040, 0.617 ± 0.084, 1.168 ± 0.201, and 1.642 ± 0.160 IU/mL, respectively (see Table S8 for the calculation of uncertainty; converted ng/mL values were also reported in the Appendix S1).
4. DISCUSSION
Chemiluminescent or electrochemiluminescence immunoassays are the most widely used assays for CEA measurements. 8 , 16 Currently, the harmonization of CEA among multiple immunoassay systems is not optimal. 8 , 16 , 17 The calibrators of most CEA reagent manufacturers are not traceable to WHO 73/601 as the protein structure and antigen epitope of WHO 73/601 may be altered during the production process. Moreover, different dilutions may affect the binding force of antigens and antibodies. Therefore, differences exist in the results obtained among the assays. A previous study has demonstrated that common standards could minimize the differences between CEA immunoassay kits. 18 Traceability is effective for determining the accuracy of measurement results; that is, the value of high‐order RMs is transmitted to the manufacturer's calibrators through a series of uninterrupted traceability chains. 6 However, ensuring RM commutability in the traceability process is imperative. 6
During calibration using WHO 73/601, an appropriate dilution matrix should be selected to ensure the accuracy of the calibrator assignment. Börmer et al. 19 reported differences between the recovery of WHO 73/601 dissolved in serum and that dissolved in bovine serum albumin (BSA). Similarly, Zhang et al. 8 compared the potency of WHO 73/601 dissolved in different buffers (BSA matrix) obtained from four different manufacturers and revealed variations. The potency of WHO 73/601 dissolved in different buffers differed even with the same system, demonstrating that the diluent matrix may affect the estimated CEA value. In the present study, we compared five common buffers (PBS, a healthy human serum pool, DMEM, MEM, and RPMI 1640) and found that WHO 73/601 diluted in the PBS buffer matrix had the smallest difference among assay systems and was the optimal choice among the five diluent matrices.
In addition, we analyzed the commutability of the BCCL candidate RMs and WHO 73/601 RMs with the five diluted matrices using five assays according to the CLSI and IFCC methods. WHO 73/601 dilutions were not commutable in the Abbott versus Roche/Diasorin/Beckman and Maccura versus Diasorin/Beckman systems based on the CLSI method and Abbott/Maccura versus Roche/Diasorin/Beckman systems based on the IFCC method. This result indicates that bias due to non‐commutability could be propagated to the results of clinical samples, thereby causing incorrect metrological traceability to the WHO 73/601 RMs and nonequivalent clinical sample results among different assays. According to ISO17511, 20 certified RM may be used as a calibrator within the calibration hierarchy for a specified in vitro diagnostic medical device for which the RM does not demonstrate commutability to human samples. However, a correction factor or function must be applied to the assigned certified RM value. Therefore, in the present study, we corrected the bias of WHO 73/601 used in the calibration hierarchy of the Abbott and Maccura measurement procedures. After bias correction, WHO 73/601 RMs diluted in PBS were selected as standard calibrators to calibrate the five assays. Consequently, the variability between assays was significantly minimized; therefore, we used commutable common calibrators 15 to calibrate the multi‐assay systems and assign values for candidate RMs. This approach can provide a valid multi‐assay system assignment method for CEA candidate RMs based on traceability.
In the present study, the frozen mixed human serum candidate RMs for CEA prepared by BCCL were evaluated by the five selected assays, which were calibrated by common WHO 73/601 RMs. The candidate RMs were assigned international conventional units (IU/mL) to maintain traceability to the WHO RMs. However, currently, most methods available for CEA determination worldwide report results in ng/mL. Unfortunately, the unit conversion formula from IU/mL to ng/mL is not provided in the WHO 73/601 instructions, representing a limitation of the guidelines. Nevertheless, Roche Diagnostics applied the traceability measurement procedure to WHO 73/601 and estimated that 1 ng/mL of CEA equals approximately 0.0169 IU/mL. The assigned RMs are expected to be further utilized in EQA schemes 21 , 22 , 23 to uncover problems related to analytical specificity and reagent traceability from manufacturers based on correctness. In addition, the developed BCCL candidate RMs were commutable, and target RMs with good commutability could be used by manufacturers to verify traceability or measurement accuracy.
The present study had some limitations. A major limitation of the study is that only five assays were examined. In addition, the specific diluent solutions recommended by the five immunoassay manufacturers were not included in this study. Therefore, future verification studies with more assays and different specific diluent matrices are required.
In conclusion, our study demonstrated that the BCCL candidate RMs for CEA have good commutability. After correcting for bias caused by non‐commutability, WHO 73/601 RMs diluted in the PBS buffer matrix were selected as common calibrators for the five immunoassays and could be used to assign values to CEA candidate RMs developed based on BCCL. This study provides important insights into promoting the harmonization of CEA detection in immunoassays.
AUTHOR CONTRIBUTIONS
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
FUNDING INFORMATION
This study was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX202137) and the Beijing Key Clinical Specialty Excellence Project (Laboratory).
CONFLICT OF INTEREST STATEMENT
The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Supporting information
Appendix S1.
Zhang R, Xu Z, Zhao R, et al. Accurate method for value assignment of carcinoembryonic antigen reference materials. J Clin Lab Anal. 2023;37:e24936. doi: 10.1002/jcla.24936
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gold P, Freedman SO. Demonstration of tumor‐specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439‐462. doi: 10.1084/jem.121.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467‐481. doi: 10.1084/jem.122.3.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozawa T, Matsuda K, Ishihara S, et al. The robust performance of carcinoembryonic antigen levels after adjuvant chemotherapy for the recurrence risk stratification in patients with colorectal cancer. J Surg Oncol. 2021;124:97‐105. doi: 10.1002/jso.26497 [DOI] [PubMed] [Google Scholar]
- 4. Iacuzzo C, Germani P, Troian M, et al. Serum carcinoembryonic antigen pre‐operative level in colorectal cancer: revisiting risk stratification. ANZ J Surg. 2021;124:97‐105. doi: 10.1002/jso.26497 [DOI] [PubMed] [Google Scholar]
- 5. Suzuki Y, Ogura A, Uehara K, et al. The carcinoembryonic antigen ratio is a potential predictor of survival in recurrent colorectal cancer. Int J Clin Oncol. 2021;26:1264‐1271. doi: 10.1007/s10147-021-01919-7 [DOI] [PubMed] [Google Scholar]
- 6. Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem. 2009;55:1067‐1075. doi: 10.1373/clinchem.2008.107052 [DOI] [PubMed] [Google Scholar]
- 7. Laurence DJ, Turberville C, Anderson SG, Neville AM. First British standard for carcinoembryonic antigen (CEA). Br J Cancer. 1975;32:295‐299. doi: 10.1038/bjc.1975.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang K, Huo H, Lin GG, Yue YH, Wang QT, Li JM. A long way to go for the harmonization of four immunoassays for carcinoembryonic antigen. Clin Chim Acta. 2016;454:15‐19. doi: 10.1016/j.cca.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 9. Miller WG, Schimmel H, Rej R, et al. IFCC working group recommendations for assessing commutability part 1: general experimental design. Clin Chem. 2018;64:447‐454. doi: 10.1373/clinchem.2017.277525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CLSI . Characterization and Qualification of Commutable Reference Materials for Laboratory Medicine; Approved Guideline. Document EP30‐A (formerly C53‐A). Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 11. Deprez L, Toussaint B, Zegers I, et al. Commutability assessment of candidate reference materials for pancreatic alpha‐amylase. Clin Chem. 2016;454:15‐19. doi: 10.1016/j.cca.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 12. Yue Y, Zhang S, Xu Z, Chen X, Wang Q. Commutability of reference materials for alpha‐fetoprotein in human serum. Arch Pathol Lab Med. 2017;141:1421‐1427. doi: 10.5858/arpa.2016-0441-OA [DOI] [PubMed] [Google Scholar]
- 13. Nilsson G, Budd JR, Greenberg N, et al. IFCC working group recommendations for assessing commutability part 2: using the difference in bias between a reference material and clinical samples. Clin Chem. 2018;64:455‐464. doi: 10.1373/clinchem.2017.277541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller WG, Budd J, Greenberg N, et al. IFCC working group recommendations for correction of bias caused by noncommutability of a certified reference material used in the calibration hierarchy of an end‐user measurement procedure. Clin Chem. 2020;66:769‐778. doi: 10.1093/clinchem/hvaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budd JR, Weykamp C, Rej R, et al. IFCC working group recommendations for assessing commutability part 3: using the calibration effectiveness of a reference material. Clin Chem. 2018;64:465‐474. doi: 10.1373/clinchem.2017.277558 [DOI] [PubMed] [Google Scholar]
- 16. Park J, Lee S, Kim Y, et al. Comparison of four automated carcinoembryonic antigen immunoassays: ADVIA centaur XP, ARCHITECT I2000sr, Elecsys E170, and Unicel Dxi800. Ann Lab Med. 2018;38:355‐361. doi: 10.3343/alm.2018.38.4.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sorensen CG, Karlsson WK, Pommergaard HC, Burcharth J, Rosenberg J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence‐a systematic review. Int J Surg. 2016;25:134‐144. doi: 10.1016/j.ijsu.2015.11.065 [DOI] [PubMed] [Google Scholar]
- 18. Zucchelli GC, Pilo A, Chiesa MR, Masini S, Baccini C. Minimizing between‐kit variability in immunoassay for carcinoembryonic antigen by use of a common standard. Clin Chem. 1986;32:1942‐1943. [PubMed] [Google Scholar]
- 19. Börmer OP. Standardization, specificity, and diagnostic sensitivity of four immunoassays for carcinoembryonic antigen. Clin Chem. 1991;37:231‐236. [PubMed] [Google Scholar]
- 20. ISO 17511:2020 (2nd edition) . In Vitro Diagnostic Medical Devices—Requirements for Establishing Metrological Traceability of Values Assigned to Calibrators, Trueness Control Materials and Human Samples. International Organization for Standardization; 2020. [Google Scholar]
- 21. Miller WG, Jones GR, Horowitz GL, Weykamp C. Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 2011;57:1670‐1680. doi: 10.1373/clinchem.2011.168641 [DOI] [PubMed] [Google Scholar]
- 22. Yi X, Wang Y, Zhang T, et al. Commutability of possible external quality assessment materials for progesterone measurement. Clin Biochem. 2021;87:39‐45. doi: 10.1016/j.clinbiochem.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 23. Braga F, Panteghini M. Commutability of reference and control materials: an essential factor for assuring the quality of measurements in laboratory medicine. Clin Chem Lab Med. 2019;57:967‐973. doi: 10.1515/cclm-2019-0154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


