Abstract
Background:
Anastomotic leakage (AL) and stenosis (AS) are two of the most severe postoperative complications after total gastrectomy with esophagojejunostomy. The stapler diameter can be chosen by the surgeon. Therefore, this study aims to assess the correlation between the stapler size as main independent variable as well as other different risk factors and AL and AS.
Methods:
We conducted a retrospective analysis of data from 356 patients who underwent open total gastrectomy between 2000 and 2018, mostly due to gastric cancer (96.9%). After propensity score matching the outcome parameters AL and AS were compared between the two stapler size groups. We also assessed different risk factors for AL and AS in cancer patients using multivariate analysis.
Results:
Small circular stapler diameter (21/25 mm; n = 147 vs 28/29/31 mm; n = 209) was identified as a significant risk factor for the occurrence of AL (10% vs 4% for smaller vs larger staplers; P = 0.042). In multivariate analysis for the occurrence of AL an ASA score ≥ 3 could be identified as a risk factor (OR 2.85; 95% CI = 1.13–7.15; P = 0.026). Additionally, smaller stapler size could be identified as a risk factor for AS (OR small 1.00, OR large 0.24; 95% CI: 0.06–0.97; P = 0.045). AL was associated with lower survival (18.1 vs 38.16 months; P = 0.0119).
Conclusion:
The application of a larger circular stapler for esophagojejunostomy in open total gastrectomy shows significantly lower rates of AL and stenosis. Therefore, the largest possible stapler diameter should be applied.
Keywords: anastomosis, anastomotic leakage, esophagojejunostomy, gastrectomy, anastomotic stenosis, surgical stapler
Mini-abstract: This retrospective propensity score matched study aims to assess the correlation between different risk factors and anastomotic leakage (AL) and stenosis after open total gastrectomy. Three hundred fifty-six patients who underwent open total gastrectomy between 2000 and 2018 were included. Small circular stapler diameter (P = 0.042) and ASA score ≥ 3 (P = 0.026) were identified as significant risk factors for the occurrence of AL. Additionally, smaller stapler size could be identified as a risk factor for anastomotic stenosis (P = 0.045). AL was significantly associated with lower survival (P = 0.0119). Therefore, the largest possible stapler diameter should be applied.
INTRODUCTION
Circular staplers are commonly used tools in surgical procedures involving anastomotic suturing of gastrointestinal organs, including esophagojejunostomy. However, serious postoperative complications in the form of anastomotic leakage (AL) and stenosis (AS) can occur at the sutured sites. They are associated with increased morbidity and mortality, a negative impact on functional outcomes, quality of life, and a burden on hospital resources and the health system.1,2 Although the use of circular staplers may reduce the risk of AL compared with other suturing techniques,3,4 additional improvements are required to further reduce the risk of these complications and to improve surgical outcomes.
Several factors have been shown to increase the risk of AL. These include higher age (≥65 years), anemia, malnutrition, BMI > 25, weight loss and steroid administration5 as well as proximal located lesions and higher Eastern Cooperative Oncology Group (ECOG) Performance Status 2 or 3.6 For the development of AS, the diameter of the circular stapler has been identified as a potential risk factor, with smaller staplers generally being associated with a higher risk.2 However, the impact of each of these risk factors varies with the anatomical location of the anastomosis as well as with the used suturing technique. It also remains unclear whether stapler diameter also affects the risk of AL in addition to the risk of AS.
In the present retrospective analysis, we thus investigated the impact of circular stapler diameter and other potential risk factors on the rate of AL and AS, as well as the impact of these two complications on patient survival after open total gastrectomy and esophagojejunostomy. Additionally, we looked for other risk factors than stapler size for the occurrence of AL and AS.
METHODS
Patients
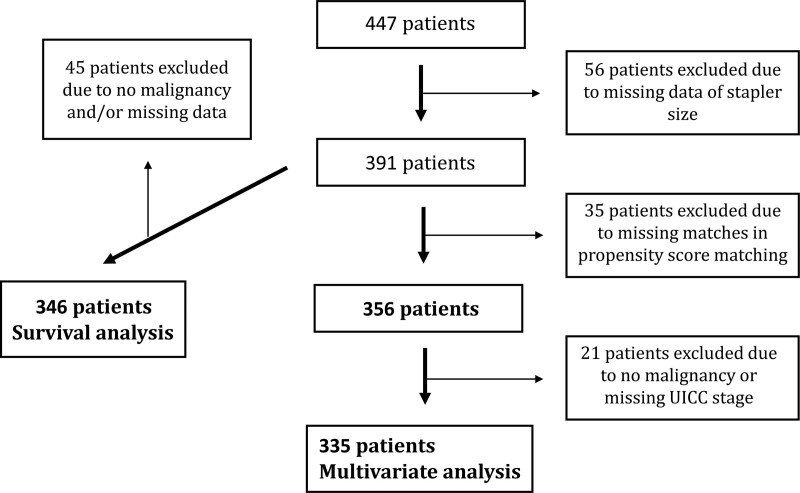
Four hundred forty-seven patients received open gastrectomy at the University Hospital Erlangen between 2000 and 2018. The study includes all patients on whom open gastrectomy was performed, for whom stapler diameter was documented (n = 391) and propensity score matching was applicable (n = 356). The majority of these patients presented with malignant gastric cancer disease (96.9%). Among others the main independent variable was stapler size and the dependent variables were AL and AS. We also looked for other risk factors for the occurrence of AL and AS such as ASA (American Society of Anesthesiologists) score, preoperative chemotherapy, preoperative radiotherapy, additional distal esophageal resection, UICC stage, stapler size and for AS additionally resection status, postoperative chemotherapy, postoperative radiotherapy, and AL. For this multivariate analysis, we only included the patients with malignancy and for whom UICC stadium was available (n = 335) (Figure 1).
FIGURE 1.
Study design.
Aside from the impact of risk factors on the occurrence of AL and AS, we also investigated the effect of these two complications on postoperative patient survival. Forty-five from 391 patients had to be excluded due to missing data. These 45 patients also included the patients without malignancy. Therefore, survival analysis was just performed for patients with gastric cancer (Figure 1). Patients were followed up for at least 5 years. In the first 2 years at 3-month intervals and thereafter at 6-month intervals. In 2004, the follow-up interval was changed according to the guidelines: every 6 months for the first 2 years and annually thereafter. Follow-up data were collected either through follow-up visits at the university hospital or through written correspondence with the patients’ treating physicians. After 5 years, at least a vital status check was carried out annually at the local registration office.
Data were collected prospectively and analyzed retrospectively.
Surgical Procedure
All surgeries were performed by at least one of altogether 9 experienced surgeons in that 18-year time period. After application of a purse string clamp, the distal esophagus was sutured and transected. The clamp was removed and the anvil of the circular stapler was introduced and fixated. The alimentary loop of the jejunum was transected and pulled retrocolically into the upper abdomen. The circular stapler was introduced into the jejunum and connected with the anvil. The stapler was then fired to generate the esophagojejunal anastomosis. The biliopancreatic limb was anastomosed with the alimentary limb in a classic Y-technique.
Definition of Anastomotic Leakage
Postoperative AL of the esophagojejunal anastomosis was defined as the presence of at least one of the following criteria: (1) Evidence of AL by endoscopy; (2) Radiological evidence of leakage by contrast-enhanced computer tomography; (3) Evidence of leakage during re-surgery.
Definition of Anastomotic Stenosis
Postoperative anastomotic stenosis (AS) of the esophagojejunal anastomosis was diagnosed by endoscopy.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 28. Propensity score matching was performed for the independent variable stapler size and included age, gender, ASA classification, height, and weight to minimize bias caused by potential confounders in this retrospective series and to homogenize the groups.7 Predefined stapler diameter groups were small diameter (21/25 mm) and large diameter (28/29/31 mm). For calculation the nearest neighbor method with a 1:2 ratio (patients with small stapler size vs patients with large stapler size) was used. Propensity score deviation width was set to a threshold of <0.2. Due to this process, 35 nonmatchable patients had to be excluded. After matching comparisons of metric and ordinal data were calculated with the Student’s t-test or Mann-Whitney U test. The Chi-square test was used for categorical data. Additionally, multivariate analysis was performed for AL and AS of cancer patients with the potential risk factors ASA, preoperative chemotherapy, preoperative radiotherapy, additional distal esophageal resection, UICC stage, stapler size and for AS additionally resection status, postoperative chemotherapy, postoperative radiotherapy, and AL. The predefined threshold for significance was P < 0.05.
Survival analysis was performed using the Log-rank test with the program Graph Pad Prism 9.
RESULTS
Demographics
Propensity score matching of the 391 patients (mean age: 65 years, 29% female) meeting inclusion criteria revealed 356 matched patients. Of these 356 patients, the smaller stapler was applied in 147 patients and the larger stapler in 209 patients. In the smaller stapler group were significantly more female patients compared with the larger stapler group (38% vs 22%, P = 0.001). All other demographic parameters including age, BMI, ASA, preoperative chemotherapy, preoperative radiotherapy, additional distal esophageal resection, malignancy, resection status, UICC stage, postoperative chemotherapy, postoperative radiotherapy did not significantly differ between the groups (Table 1).
TABLE 1.
Patients Characteristics
| All Patients | Patients With Small Stapler Size | Patients With Large Stapler Size | P | |
|---|---|---|---|---|
| Number | 356 | 147 | 209 | |
| Age (yrs)* | 65 ± 12 | 65 ± 13 | 65 ± 12 | 0.602 |
| Gender* | 0.001 | |||
| Female | 102 (29) | 56 (38) | 46 (22) | |
| Male | 254 (71) | 91 (62) | 163 (78) | |
| BMI (kg/m2)† | 26.0 ± 4.3 | 25.8 ± 4.3 | 26.1 ± 4.3 | 0.522 |
| ASA* | 0.558 | |||
| 1 | 15 (4) | 5 (3) | 10 (5) | |
| 2 | 211 (59) | 83 (57) | 128 (61) | |
| 3 | 125 (35) | 56 (38) | 69 (33) | |
| 4 | 5 (1) | 3 (2) | 2 (1) | |
| Preoperative chemotherapy | 139 (39) | 50 (34) | 89 (43) | 0.122 |
| Preoperative radiotherapy | 44 (12) | 14 (10) | 30 (14) | 0.193 |
| Additional distal esophageal resection | 142 (40) | 57 (39) | 85 (41) | 0.743 |
| Malignancy | 0.375 | |||
| Yes | 345 (97) | 144 (98) | 201 (96) | |
| No | 11 (3) | 4 (2) | 8 (4) | |
| Resection status | 0.371 | |||
| R0 | 288 (86) | 117 (83) | 171 (87) | |
| R1/2 | 49 (14) | 24 (17) | 25 (13) | |
| UICC stage‡ | 0.768 | |||
| 0 | 14 (4) | 6 (4) | 8 (4) | |
| I | 74 (22) | 36 (26) | 38 (20) | |
| II | 75 (22) | 30 (21) | 45 (23) | |
| III | 92 (28) | 38 (27) | 54 (28) | |
| IV | 80 (24) | 31 (22) | 49 (25) | |
| Postoperative chemotherapy | 103 (29) | 41 (28) | 62 (30) | 0.724 |
| Postoperative radiotherapy | 10 (3) | 5 (3) | 5 (2) | 0.747 |
Data are presented as mean ± standard deviation or n (%).
*Matched parameter.
†Matched for weight and height.
‡n = 335 (patients without malignancy [n = 11] and patients with unknown UICC stage [n = 10] were excluded).
Outcome Parameter
The overall AL rate equals 6% (22/356 patients) and the AS rate 3% (6/356 patients). AL occurred more often in the smaller stapler group compared to the larger stapler group (10% vs 4%, P = 0.042). There were no differences for Clavien-Dindo classification for AL nor for AS between the groups (Table 2).
TABLE 2.
Outcome Parameter
| All Patients (n = 356) | Patients With Small Stapler Size (n = 147) | Patients With Large Stapler Size (n = 209) | P | |
|---|---|---|---|---|
| Anastomotic leakage (AL) | 22 (6) | 14 (10) | 8 (4) | 0.042 |
| Clavien-Dindo-classification for AL | 0.201 | |||
| I | 0 (0) | 0 (0) | 0 (0) | |
| II | 2 (9) | 2 (14) | 0 (0) | |
| III | 10 (45) | 6 (43) | 4 (50) | |
| IV | 3 (14) | 1 (7) | 2 (25) | |
| V | 7 (32) | 5 (36) | 2 (25) | |
| Anastomotic stenosis (AS) | 12 (3) | 8 (5) | 4 (2) | 0.080 |
Data are presented as mean ± standard deviation or n (%).
AS indicates anastomotic stenosis.
Risk Factors for Anastomotic Leakage
In the multivariate analysis for the occurrence of AL a higher ASA score (3 and 4) could be identified as an independent risk factor (OR 2.85; 95% CI: 1.13–7.15; P = 0.026). Additionally, a larger stapler diameter protects from AL (OR 0.37; 95% CI: 0.15-0.93; P = 0.034).
Preoperative chemotherapy, preoperative radiotherapy, additional esophageal resection, and UICC stage did not have any effect (Table 3).
TABLE 3.
Multivariate Analysis for Occurrence of AL in Cancer Patients (n = 335)
| Multivariate Analysis for AL | |||
|---|---|---|---|
| OR | 95% CI | P | |
| ASA | 0.026 | ||
| 1/2 | 1.00 | ||
| 3/4 | 2.85 | 1.13–7.15 | |
| Preoperative chemotherapy | 0.989 | ||
| No | 1.00 | ||
| Yes | 0.99 | 0.34–2.86 | |
| Preoperative radiotherapy | 0.662 | ||
| No | 1.00 | ||
| Yes | 1.36 | 0.34–5.50 | |
| Additional distal esophageal resection | 0.108 | ||
| No | 1.00 | ||
| Yes | 2.15 | 0.85–5.48 | |
| UICC stage | 0.112 | ||
| UICC 0/I/II | 1.00 | ||
| UICC III/IV | 2.12 | 0.70–6.42 | |
| Stapler size | 0.034 | ||
| Small | 1.00 | ||
| Large | 0.37 | 0.15–0.93 | |
AL indicates anastomotic leakage; CI, confidence intervals; OR, odds ratio.
Risk Factors for Anastomotic Stenosis
Interestingly, in the multivariate analysis for the occurrence of AS a smaller stapler size could be identified as an independent risk factor for AS in comparison to a larger stapler size (OR small 1.00, OR large 0.24; 95% CI: 0.06–0.97; P = 0.045). All other calculated risk factors could not show any significant differences (Table 4).
TABLE 4.
Multivariate Analysis for Occurrence of AS in Cancer Patients (n = 335)
| Multivariate analysis for AS | |||
|---|---|---|---|
| OR | 95% CI | P | |
| ASA | 0.346 | ||
| 1/2 | 1.00 | ||
| 3/4 | 0.49 | 0.11–2.17 | |
| Preoperative chemotherapy | 0.185 | ||
| No | 1.00 | ||
| Yes | 2.80 | 0.61–12.78 | |
| Preoperative radiotherapy | 0.667 | ||
| No | 1.00 | ||
| Yes | 0.58 | 0.05–7.06 | |
| Additional distal esophageal resection | 0.086 | ||
| No | 1.00 | ||
| Yes | 0.22 | 0.04–1.24 | |
| UICC stage | 0.245 | ||
| UICC 0/I/II | 1.00 | ||
| UICC III/IV | 2.48 | 0.54–11.50 | |
| Stapler size | 0.045 | ||
| Small | 1.00 | ||
| Large | 0.24 | 0.06–0.97 | |
| Resection status | 0.703 | ||
| R0 | 1.00 | ||
| R1/2 | 0.72 | 0.13–3.91 | |
| Postoperative chemotherapy | 0.797 | ||
| No | 1.00 | ||
| Yes | 0.81 | 0.17–3.89 | |
| Postoperative radiotherapy | 0.435 | ||
| No | 1.00 | ||
| Yes | 2.76 | 0.22–35.19 | |
| Anastomotic leakage | 0.181 | ||
| No | 1.00 | ||
| Yes | 3.55 | 0.56–22.73 | |
AS indicates anastomotic stenosis; CI, confidence intervals; OR, odds ratio.
Survival Analysis
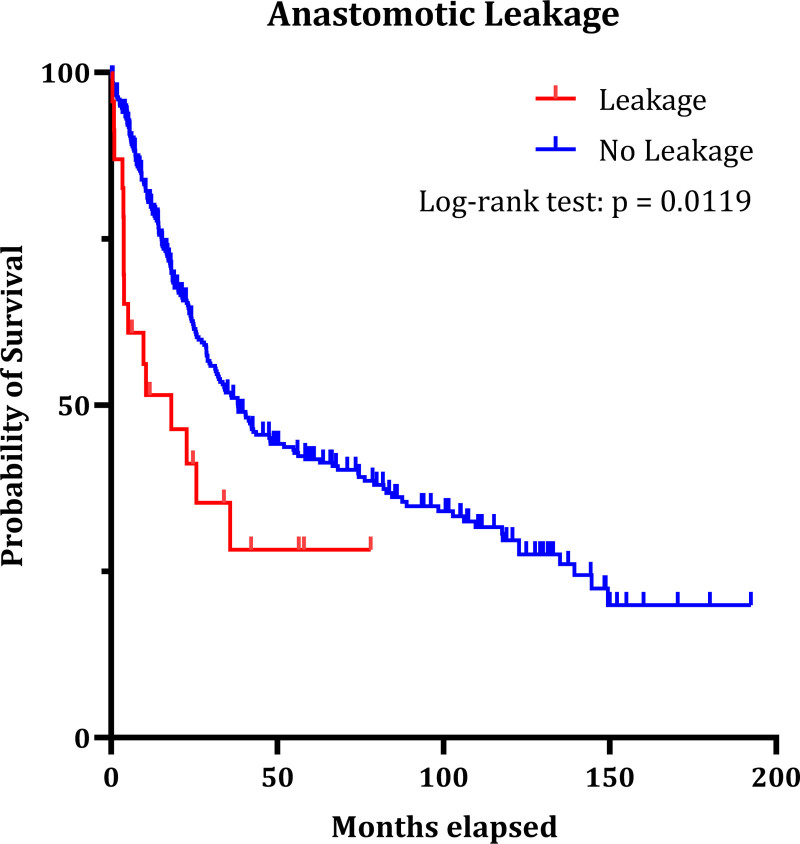
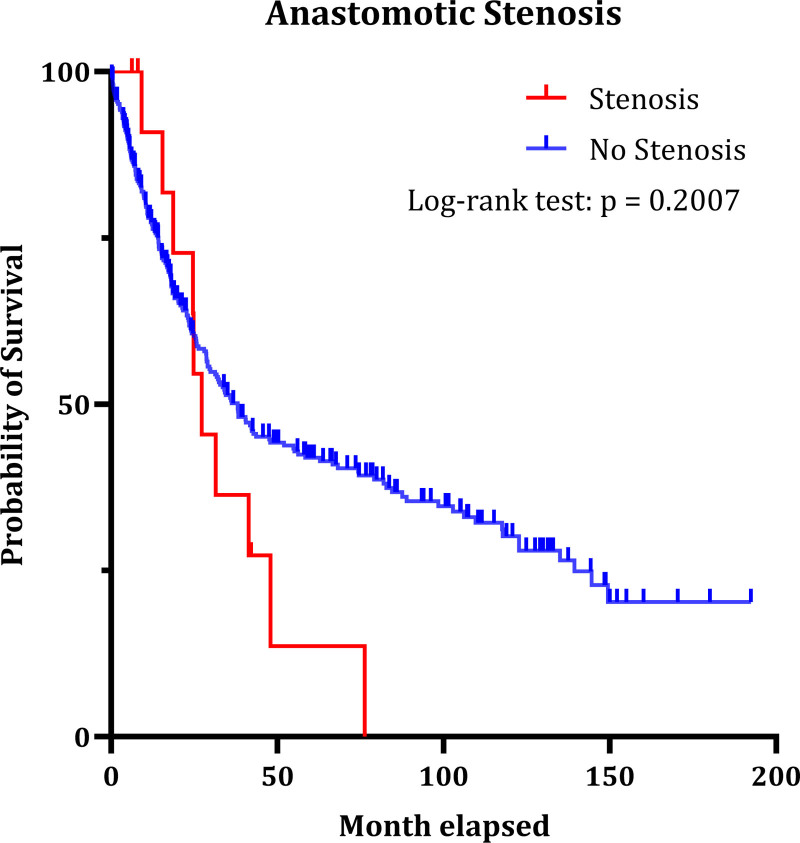
As is shown in Figure 2, the occurrence of AL is significantly associated with lower survival (median overall survival 18.1 vs 38.16 months; P = 0.0119). We did not observe significant correlation between the occurrence of AS and decreased survival (Figure 3) (27.28 vs 38.03 months; P = 0.2007).
FIGURE 2.
Impact of anastomotic leakage on patient survival after total gastrectomy (P = 0.0119). Forty-five of 391 patients had to be excluded due to no malignancy and or missing data for survival.
FIGURE 3.
Impact of anastomotic stenosis on patient survival after total gastrectomy (P = 0.2007). Forty-five of 391 patients had to be excluded due to no malignancy and or missing data for survival.
DISCUSSION
Anastomotic leakage remains a potentially life-threatening complication in patients who underwent total gastrectomy. This is reaffirmed by the data of the present substantive retrospective series, which show that AL after total gastrectomy and esophagojejunostomy is associated with a significant decrease in postoperative survival. AS is an uncomfortable morbidity which can lead to dysphagia, reflux, malnutrition, and lung infections due to regurgitation and aspiration.2 To improve patients’ survival and to reduce complications such as AL or AS, it is important to identify and minimize the risk factors that may contribute to an increased rate of AL or AS. We identified two important risk factors: (1) A smaller surgical stapler diameter was associated with a significant increase in the rate of AL and AS. (2) An ASA Score ≥ 3 was correlated with a higher risk for AL.
The potential impact of circular stapler diameter on the rate of AS has long been in contention, with some studies suggesting that a smaller diameter is associated with a higher rate of stenosis8 and other studies finding no such correlation.9 A recent meta-analysis of 21 studies confirmed that larger stapler diameter is associated with a lower rate of stenosis in the upper gastrointestinal tract while there was insufficient data on the effect in the lower gastrointestinal tract.2 In this context, it is worth noting that both the site and the type of anastomosis are likely to affect the rate of stenosis. Out of the 21 studies included in the meta-analysis, only two investigated the effect of stapler diameter in esophagojejunostomy. The first of these two studies (25 mm vs 28/31 mm, n = 45) concluded that larger stapler diameter is associated with a lower rate of stenosis,10 the other study (25 mm vs 28 mm, n = 60) found no significant correlation between stapler diameter and stenosis of the anastomotic site.11 In the present study (21/25 mm vs 28/29/31 mm, n = 335), in multivariate analysis, we identified a significant correlation between a smaller stapler diameter and rate of AS.
Aside from AS, AL is another complication of anastomosis that may be affected by the site and type of anastomosis and possibly by stapler diameter. Although a previous study (25 mm vs 28 mm, n = 60) observed no significant correlation between stapler diameter and leakage rate,11 the present study (21/25 mm vs 28/29/31 mm, n = 356) found a significant association between larger stapler diameter and a lower rate of AL. The reason for this could be that a larger stapler diameter allows for more of the mucosa and subserosa to be placed in the space between the stapler and anvil, that is, inside the staple line. This increases the surface of the anastomosis and might thus reduce the tension and therefore the risk of leakage. It must be mentioned that for patients with a small esophagus there might be no option for inserting a larger anvil. Nevertheless, the largest possible stapler diameter should be used and where applicable a larger size at least tried. Still there is further need to investigate the role of circular stapler diameter on the risk of complications after esophagojejunostomy.
The ASA Score estimates the anesthetic risk of an intervention and mirrors the degree of a patient’s comorbidity. In several studies for colorectal cancer, a higher ASA score is associated with AL.12,13 A retrospective study including 1,192 patients with resection of the gastroesophageal junction for adenocarcinoma showed an ASA score ≥ 3 as a risk factor for operative mortality and morbidity as well as for AL.14 There are more studies which corroborate these results for anastomosis after esophagectomy.15,16 This retrospective series shows that an ASA Score ≥ 3 is an independent risk factor for AL after open total gastrectomy for gastric cancer. There are several conditions such as impairment of the kidney function, cardiovascular disorders, diabetes, hypertension, and chronic obstructive pulmonary disease which are also integrated in the ASA classification and known to compromise tissue perfusion and oxygenation with consequently impaired anastomotic healing.17
Concerning a possible role of radiotherapy or chemotherapy as risk factors for AL, previous studies on patients who underwent gastrectomy reported no increase in the AL under radiotherapy or chemotherapy.18,19 Two other studies on patients with intestinal anastomosis produced partially conflicting results: while the first study identified preoperative chemotherapy as a strong risk factor for AL,20 the second study found no increase in leakage rate to be associated with pre- or postoperative chemotherapy.21 In line with the present results, the majority of these studies found no connection between chemotherapy and AL.
The present study has some limitations. First of all, it is a retrospective analysis which is known for bias by undetected confounders. Therefore, we implemented propensity score matching to homogenize the groups for at least basic parameters such as age, gender, ASA, weight, and height. In addition to that, we used a multivariate analysis for the different risk factors for AL and AS.
Our patient collective includes some patients with UICC stage IV (n = 80). According to the current guidelines, these patients should not receive surgery since they do not benefit from resection and might have a higher risk for complications. These patients were mostly operated because of symptomatic tumors (i.e., bleeding or perforation) or because of macroscopically not identified metastasis, for example, small local peritoneal carcinomatosis. Nevertheless, neither the chi-quadrat test nor the multivariate analysis revealed any significant risk for AL or AS between the different UICC stages.
In summary, we found significant correlations between smaller stapler diameter and AL as well as AS after open total gastrectomy and esophagojejunostomy. Additionally, we discovered an ASA score ≥3 as an independent risk factor for AL in these patients. These results underline the risk of postoperative complications posed by circular staplers with small diameter (≤25 mm). If possible, a larger stapler should be used to reduce complications. Further studies on different and larger patient cohorts as well as meta-analyses will be needed to corroborate these results.
A.M. did original draft preparation, formal analysis, data curation, and visualization. H.R., M.E., J.F., M.B., A.A., C.K., S.M., and R.G. did data curation, writing—review and editing. M.L. and M.B. did formal analysis supervision. G.F.W. did conceptualization, supervision; validation; writing—review, and editing. All authors have read and agreed to this version of the article.
Footnotes
Published online 7 September 2022
Disclosure: The authors declare that they have nothing to disclose.
The principles outlined in the Declaration of Helsinki have been followed.
REFERENCES
- 1.Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg 2016;20:2035–51. [DOI] [PubMed] [Google Scholar]
- 2.Allen W, Wells CI, Greenslade M, Bissett IP, O’Grady G. Association between circular stapler diameter and stricture rates following gastrointestinal anastomosis: systematic review and meta-analysis. World J Surg 2018;42:3097–105. [DOI] [PubMed] [Google Scholar]
- 3.Liu QX, Min JX, Deng XF, Dai JG. Is hand sewing comparable with stapling for anastomotic leakage after esophagectomy? A meta-analysis. World J Gastroenterol 2014;20:17218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda M, Kuriyama A, Noma H, Nunobe S, Furukawa TA. Hand-sewn versus mechanical esophagogastric anastomosis after esophagectomy: a systematic review and meta-analysis. Ann Surg 2013;257:238–48. [DOI] [PubMed] [Google Scholar]
- 5.Tu RH, Lin JX, Zheng CH, Li P, Xie JW, Wang JB, et al. Development of a nomogram for predicting the risk of anastomotic leakage after a gastrectomy for gastric cancer. Eur J Surg Oncol 2017;43:485–92. [DOI] [PubMed] [Google Scholar]
- 6.Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol 2011;104:734–40. [DOI] [PubMed] [Google Scholar]
- 7.Thoemmes F. Propensity score matching in SPSS. arXiv 2012;1201. [Google Scholar]
- 8.Fisher BL, Atkinson JD, Cottam D. Incidence of gastroenterostomy stenosis in laparoscopic Roux-en-Y gastric bypass using 21- or 25-mm circular stapler: a randomized prospective blinded study. Surg Obes Relat Dis 2007;3:176–9. [DOI] [PubMed] [Google Scholar]
- 9.Yendamuri S, Gutierrez L, Oni A, Mashtare T, Khushalani N, Yang G, et al. Does circular stapled esophagogastric anastomotic size affect the incidence of postoperative strictures? J Surg Res 2011;165:1–4. [DOI] [PubMed] [Google Scholar]
- 10.Tokunaga Y, Ryo J, Kitaoka A, Yagi T, Tokuka A, Ohsumi K. Jejunal pouch to avoid stricture after esophagojejunostomy with circular stapler. J Am Coll Surg 1999;189:466–9. [DOI] [PubMed] [Google Scholar]
- 11.Çakabay B, Aksel B, Ünal E, Bayar S, Kocaoğlu H, Demirci S, et al. Influence of the stapler size used in esophagojejunostomy anastomosis: anastomotic leak and strictures after total gastrectomy. Turkiye Klinikleri J Med Sci 2012;32:428–31. [Google Scholar]
- 12.Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014;101:424–32; discussion 432. [DOI] [PubMed] [Google Scholar]
- 13.Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 2008;23:265–70. [DOI] [PubMed] [Google Scholar]
- 14.Sauvanet A, Mariette C, Thomas P, Lozac’h P, Segol P, Tiret E, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005;201:253–62. [DOI] [PubMed] [Google Scholar]
- 15.Gooszen JAH, Goense L, Gisbertz SS, Ruurda JP, van Hillegersberg R, van Berge Henegouwen MI. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg 2018;105:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenmann J, Fink-Neuboeck N, Porubsky C, Fediuk M, Anegg U, Kornprat P, et al. A nomogram illustrating the probability of anastomotic leakage following cervical esophagogastrostomy. Surg Endosc 2021;35:6123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briel JW, Tamhankar AP, Hagen JA, DeMeester SR, Johansson J, Choustoulakis E, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;198:536–41; discussion 541–2. [DOI] [PubMed] [Google Scholar]
- 18.Ikoma N, Das P, Blum M, Estrella JS, Devine CE, Wang X, et al. Preoperative chemoradiation therapy does not increase risk of anastomotic leak in patients with gastric cancer. Int J Radiat Oncol Biol Phys 2017;99:660–6. [DOI] [PubMed] [Google Scholar]
- 19.Haskins IN, Kroh MD, Amdur RL, Ponksy JL, Rodriguez JH, Vaziri K. The effect of neoadjuvant chemoradiation on anastomotic leak and additional 30-day morbidity and mortality in patients undergoing total gastrectomy for gastric cancer. J Gastrointest Surg 2017;21:1577–83. [DOI] [PubMed] [Google Scholar]
- 20.Luján JJ, Németh ZH, Barratt-Stopper PA, Bustami R, Koshenkov VP, Rolandelli RH. Factors influencing the outcome of intestinal anastomosis. Am Surg 2011;77:1169–75. [PubMed] [Google Scholar]
- 21.Morse BC, Simpson JP, Jones YR, Johnson BL, Knott BM, Kotrady JA. Determination of independent predictive factors for anastomotic leak: analysis of 682 intestinal anastomoses. Am J Surg 2013;206:950–5; discussion 955–6. [DOI] [PubMed] [Google Scholar]