Abstract
Objective:
This cohort study investigated short- and long-term postoperative outcomes of laparoscopic procedures for rectal cancer performed with versus without certified surgeons.
Background:
In Japan, the Endoscopic Surgical Skill Qualification System (ESSQS) evaluates surgical skills deemed essential for laparoscopic surgery; however, it is unknown whether this certification contributes to procedural safety.
Methods:
Outcomes of laparoscopic rectal resections for cStage II and III rectal cancer performed from 2014 to 2016 at 56 Japanese hospitals were retrospectively reviewed. The impact of having versus not having certified surgeons on postoperative complications and other short- and long-term outcomes were assessed. In cases with ESSQS-certified surgeons, surgeons attended surgery in the capacity of an operator, assistant, scope operator, or advisor.
Results:
Overall, 3188 procedures were analyzed, with 2644 procedures performed with and 544 without ESSQS-certified surgeons. A multivariate logistic regression model showed that the adjusted odds ratio of postoperative complications after procedures performed with ESSQS-certified surgeons was 0.68 (95% confidence interval, 0.51–0.91; P = 0.009). The adjusted odds ratios for conversion and pathological R0 resection rates with ESSQS-certified surgeons were 0.20 (P < 0.001) and 2.10 (P = 0.04), respectively. Multiple linear regression analyses showed significantly shorter surgical duration and more harvested lymph nodes for operations performed with ESSQS-certified surgeons. Multivariate Cox regression showed that the adjusted hazard ratios for poor overall and recurrence-free survival after operations performed with ESSQS-certified surgeons were 0.88 (P = 0.35) and 1.04 (P = 0.71), respectively.
Conclusions:
This study showed the superiority of the short-term postoperative results for laparoscopic rectal procedures performed with ESSQS-certified surgeons.
Keywords: rectal cancer, laparoscopic, safety, technical qualification
INTRODUCTION
Laparoscopic surgery is a minimally invasive procedure; however, it takes much time and effort to obtain adequate levels of proficiency for this procedure.1–3 For standardization and efficient progression of the procedures, a feasible and clinically valid nationwide competency assessment method is required for this advanced surgical procedure. There have been reported several established technical skill assessment systems such as the National Training Programme for Laparoscopic Colorectal Surgery in England,4,5 the Global Operative Assessment of Laparoscopic Skills in Canada,6 and the University MASTERS Program provided by The Society of American Gastrointestinal and Endoscopic Surgeons.7 In Japan, the Japan Society for Endoscopic Surgery (JSES) introduced the Endoscopic Surgical Skill Qualification System (ESSQS)8–10 to maintain and improve the quality of laparoscopic surgeries in 2004. This certification program is more advanced and is positioned above the board certification by the Japan Surgical Society. Based on a review of the procedural video, ESSQS-certified surgeons have been designated not only as skilled masters but also as coaches. Every general surgeon in Japan who wants to perform laparoscopic surgery should be qualified by this certification implicitly. However, this is not necessary for performing the procedures because it is an advanced qualification for which surgeons are certified as trainers. The certification of the surgeons reflects the difference in their technical and teaching skills. Candidacy for the ESSQS requires academic achievements, laparoscopic experience, attendance at JSES official training seminars, and 2 years of general surgery experience after board certification by the Japan Surgical Society. The examination is based on an anonymized, nonedited random video review, and scoring is performed by 2 or 3 expert laparoscopic surgeons designated by the JSES. ESSQS-certified surgeons in the colorectal section are qualified to perform sigmoidectomies for colon cancer and high anterior resections for rectosigmoid cancer.
ESSQS certification is deemed essential for safe conduct of laparoscopic surgery in Japan. Among ESSQS examinees, only 20%–30% are judged adequately skilled for this qualification each year, and, currently, less than 10% of Japanese general surgeons are ESSQS certified. However, the usefulness of ESSQS certification has not yet been proven scientifically. It has been reported that laparoscopic colorectal resections performed by or with ESSQS-certified surgeons had improved postoperative results in 1428 cases of colorectal cancer at 11 regional general hospitals in Japan from 2010 to 2013.11 During that period, laparoscopic rectal surgery was a challenging procedure, with only about 30% of low anterior resections performed endoscopically in Japan.12 Since then, laparoscopic rectal resection has become more standardized, with the National Survey of Endoscopic Surgery observing that more than 75% of low anterior resections, performed in Japanese institutions belonging to the JSES, were performed laparoscopically in 2017.13 Despite this, it remains unclear whether presence of ESSQS-certified surgeons during the procedure contributes to safety of rectal resections following popularization of the technique. A recent report using Japanese National Clinical Database data involving 65,717 patients who underwent laparoscopic low anterior resections between 2014 and 2016 showed that ESSQS certification did not affect postoperative mortality but may have contributed to favorable outcomes related to anastomotic leakage.14 However, in that report, there were potential biases related to heterogeneities in the surgical quality and performance of each hospital, and oncological and long-term outcome data were not available. In the present study, data from a continuous series of rectal cancer cases were accumulated from the institutions belonging to the Japan Society of Laparoscopic Colorectal Surgery (JSLCS). The surgical quality and performance of these institutions is supposed to be higher and more uniform than the institutions in the national database, as reported by several previous studies conducted by the JSLCS indicating favorable surgical outcomes.15–18 The current study involved an investigation into not only short-term outcomes, but also long-term outcomes, which have not previously been reported in a large cohort with rectal cancer. Another novel point of this investigation was the way in which the groups were classified. The cases were classified as part of the ESSQS group if ESSQS-certified surgeons attended the surgery as an operator, assistant, or advisor. However, in the national database study, only cases in which ESSQS-certified surgeons participated as operators were included in the ESSQS group.14 This difference is important because ESSQS-certified surgeons have been designated not only as skilled masters, but also as coaches. 1,2 Thus, the present study aimed to evaluate the impact of ESSQS certification on the technical safety and long-term oncological outcomes of laparoscopic colorectal resections.
METHODS
Study Design
All elective laparoscopic rectal resections performed from January 2014 to December 2016 at 56 Japanese hospitals belonging to the JSLCS were retrospectively reviewed. The inclusion criteria for this study specified that patients have a histologically confirmed rectal adenocarcinoma, with Stage II or III identified during a clinical preoperative assessment.
Patients who had synchronous or metachronous cancers (excluding in situ cancer) within 5 years and who had undergone multiple intra-abdominal procedures during a single operation, or robotic surgery, were excluded. Patients with ulcerative colitis-associated cancers were also excluded.
The internal review committees of Hokkaido University Hospital (No. 019-0328) and all participating hospitals approved this study as exempt human subject research, and informed consent was obtained by the opt-out method, in accordance with the guidelines of the Japanese Ministry of Health, Labor, and Welfare. This study was registered in the UMIN Clinical Trials Registry System on June 3rd, 2020 (UMIN 000040645).
Outcome Measures
For each patient, the records analyzed included the clinical features, institution, surgical data, postoperative outcomes, and follow-up. The procedures were categorized according to whether they were performed by/with or without a surgeon with ESSQS qualification, and the short- and long-term outcomes in both groups were compared. Short-term results included the operative time, blood loss, conversion rate, intraoperative complication rate (defined as injury to major vessels, the intestinal tract, or other surrounding organs; trouble with anastomoses; or other intraoperative accidental events), overall postoperative complication rate, postoperative bleeding rate, surgical site infection rate, postoperative ileus rate, anastomotic leak rate, reoperation rate, length of postoperative hospital stay, number of harvested lymph nodes, and R0 resection rate. Postoperative complications were assessed according to the Clavien-Dindo classification.19 Postoperative complications and reoperation were defined as those that occurred within 30 days after the surgery. Follow-up was conducted at day-clinic visits. The 3-year overall survival (defined as the time from operation to the last visit or death), recurrence-free survival (defined as the time from operation to recurrence or any cause of death, where patients alive at the end of follow-up period were censored), and cumulative local recurrence (defined as the time from operation to local recurrence, where patients, dead or alive, without local recurrence at the end of follow-up period were censored) were compared in patients with cancers of clinical stage II or III. Additionally, the associations between overall and local recurrences and an intervention by an ESSQS-certified surgeon were examined.
Endpoints
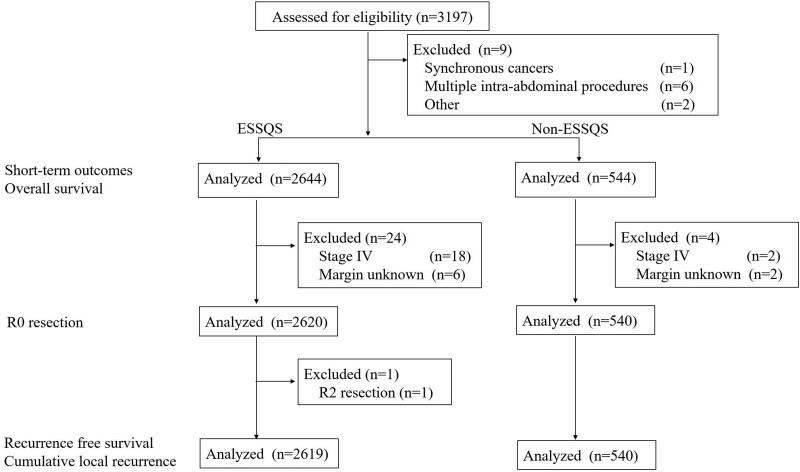
The primary endpoint of this study was the proportion of major postoperative complications (Clavien-Dindo classification grade III or over). Other short- and long-term outcomes described above were assessed as secondary endpoints. A patient flow diagram is shown in Figure 1.
FIGURE 1.
Patient flow diagram.
Statistical Analysis
Continuous data are reported as means and standard deviation. All statistical tests were performed using an alpha level of 0.05 (2-sided). Chi-squared and Student’s t-tests were used for categorical and normal continuous data, respectively. Clinical and surgical features, including age; sex; body mass index (BMI); American Society of Anesthesiologists (ASA) class; obstruction; cT, cN, and clinical stage; location of the tumor; preoperative therapy; procedures; combined resections of surrounding organs; lymph node dissection (D1, D2, or D3); lateral pelvic node dissection; high ligations of the inferior mesenteric artery (IMA); mobilization of the splenic flexure; a diverting stoma; and the type and volume of the institution, were selected as factors for multivariate logistic regression models and for multiple regression analyses assessing the relationship between the attendance of ESSQS-certified surgeons at surgery and short-term outcomes, including postoperative complications. All the collected covariates were fully incorporated in the regression model. For the analysis assessing the relationship between attendance of ESSQS-certified surgeons and R0 resection rates, stage IV cases and cases with unknown histological margins were excluded. Survival curves among the cases for the studied groups were estimated using the Kaplan-Meier method and were statistically tested using log-rank test with 2-sided alpha of 0.05. Additionally, clinical, surgical, and pathological features, including age, sex, BMI, ASA class, cT, cN, location of the tumor, preoperative therapy, lymph node dissection [D1, D2, or D3], lateral pelvic node dissection, combined resection of surrounding organs, pT, pN, R status, tumor differentiation, primary tumor size, adjuvant chemotherapy, lymph and venous vessel invasion in primary lesion, and types and volumes of institutions were selected as factors for Cox multiple regression analyses to assess the relationship between the attendance of ESSQS-certified surgeons at surgery and long-term outcomes. All the collected covariates were fully incorporated in the regression model. In the analysis where the relationship between attendance of ESSQS-certified surgeons and recurrence-free survival and cumulative local recurrences were assessed, cases with Stage IV disease, R2 resections, and unknown histological margins were accordingly excluded. Postoperative complications and long-term outcomes were assessed by multivariate logistic regression and Cox regression analysis respectively between cases with the sole ESSQS-certified surgeon as the operator and cases without an ESSQS-certified surgeon, and between cases with the sole ESSQS-certified surgeon as the assistant and cases without an ESSQS-certified surgeon, in the same manner as that described above. All statistical analyses were performed using JMP Pro version 16.0.0 (SAS Institute, Inc., Cary, NC) and R (R Core Team, 2021).
RESULTS
Overall, 3188 laparoscopic rectal procedures were performed between 2014 and 2016, where 2644 procedures were performed with ESSQS-certified surgeons and 544 procedures were performed without them. ESSQS-certified surgeon contributed as either an operator (n = 1843), assistant (n = 1357), scope operator (n = 159), or advisor (n = 33). Multiple ESSQS-certified surgeons were in attendance during surgery in 34% of the procedures (Table S1, http://links.lww.com/AOSO/A117). We defined the ESSQS group as cases in which the surgery was performed with at least one ESSQS-certified surgeon, regardless of their role.
Patient characteristics are summarized in Table 1. A total of 1684 males and 960 females with a mean age of 64.1 years were in the ESSQS group. In the non-ESSQS group, 337 males and 207 females with a mean age of 65.9 years underwent surgery. No differences were observed between the groups in terms of the BMI, frequency of preoperative comorbidities with an ASA class greater than 3, or frequency of patients with preoperative obstructions requiring decompression due to tumors. The ESSQS group had more patients at high-volume centers (41% vs. 12%, P < 0.001) and academic institutions (50% vs. 35%, P < 0.001) and a higher frequency of low rectal cancers (70% vs. 64%, P < 0.001). The distributions of clinical T and N stages were also different between the 2 groups, although the clinical stage was similar. More patients had undergone preoperative treatment in the ESSQS group (25% vs. 15%, P < 0.001; Table 2). The distributions of the types of procedures were different between the 2 groups; however, the frequency of lymph node dissections and combined resections of surrounding organs were similar between the groups. High ligations of the IMA (77% vs. 57%, P < 0.001; Table 2), mobilizations of the splenic flexure (10% vs. 2%, P < 0.001; Table 2), lateral pelvic node dissections (20% vs. 8%, P < 0.001; Table 2), and the creation of diverting stomas (34% vs. 27%, P = 0.001; Table 2) were performed more frequently in the ESSQS group.
TABLE 1.
Patient Characteristics
| With ESSQS(n = 2644) | Without ESSQS(n = 544) | P | ||
|---|---|---|---|---|
| Age (y) | 64.1 (12.0) | 65.9 (11.9) | 0.002 | |
| Sex | Male | 1684 (64%) | 337 (62%) | 0.44 |
| Female | 960 (36%) | 207 (38%) | ||
| BMI (kg/m2) | 22.6 (3.4) | 22.6 (3.7) | 0.86 | |
| ASA class 3 or 4* | 183 (7%) | 52 (10%) | 0.06 | |
| Obstruction | 128 (5%) | 28 (5%) | 0.76 | |
| Institution (volume) | Cases ≥100 | 1085 (41%) | 67 (12%) | <0.001 |
| Cases <100 | 1559 (59%) | 477 (88%) | ||
| Institution (academic) | Medical school | 1314 (50%) | 188 (35%) | <0.001 |
| Others | 1330 (50%) | 356 (65%) | ||
| Location* | RS | 803 (30%) | 197 (36%) | <0.001 |
| Ra | 838 (32%) | 196 (36%) | ||
| Rb | 998 (38%) | 151 (28%) | ||
| Clinical T stage* | 1 | 34 (1%) | 2 (0.4%) | <0.001 |
| 2 | 135 (5%) | 26 (5%) | ||
| 3 | 1957 (74%) | 388 (71%) | ||
| 4a | 355 (13%) | 112 (21%) | ||
| 4b | 161 (6%) | 16 (3%) | ||
| Clinical N stage | 0 | 1171 (44%) | 260 (48%) | 0.04 |
| 1 | 1012 (38%) | 193 (35%) | ||
| 2 | 297 (11%) | 70 (13%) | ||
| 3 | 164 (6%) | 21 (4%) | ||
| Clinical stage | II | 1171 (44%) | 260 (48%) | 0.13 |
| III | 1473 (66%) | 284 (52%) |
Continuous data are described as mean (standard deviation). The values with
* include missing data.
ASA, American Society of Anesthesiologists Physical Status Classification; BMI, body mass index; ESSQS, intervention by surgeons qualified by the Endoscopic Surgical Skill Qualification System.
TABLE 2.
Procedural Characteristics
| With ESSQS(n = 2644) | Without ESSQS(n = 544) | P | ||
|---|---|---|---|---|
| Preoperative therapy | None | 1979 (75%) | 461 (85%) | <0.001 |
| Chemotherapy | 263 (10%) | 24 (4%) | ||
| Chemoradiotherapy | 288 (11%) | 47 (9%) | ||
| TNT | 113 (4%) | 11 (2%) | ||
| Other | 1 (0.04%) | 1 (0.18%) | ||
| Procedures | High anterior resection | 507 (19%) | 116 (21%) | 0.002 |
| Low anterior resection | 1487 (56%) | 322 (59%) | ||
| APR | 375 (14%) | 57 (10%) | ||
| Hartmann’s | 79 (3%) | 24 (4%) | ||
| ISR | 176 (7%) | 25 (5%) | ||
| TPE | 17 (0.6%) | 0 | ||
| Others | 3 (0.1%) | 0 | ||
| Combined resection | Surrounding organ | 134 (5%) | 18 (3%) | 0.06 |
| IMA high ligation | 2047 (77%) | 313 (57%) | <0.001 | |
| Mobilization of SF* | 274 (10%) | 13 (2%) | <0.001 | |
| LND | D0/1 | 10 (0.4%) | 2 (0.4%) | 0.33 |
| D2 | 228 (9%) | 40 (7%) | ||
| D3 | 2406 (91%) | 502 (92%) | ||
| LPND | None | 2109 (80%) | 500 (92%) | <0.001 |
| Unilateral | 162 (6%) | 19 (3%) | ||
| Bilateral | 373 (14%) | 25 (5%) | ||
| Diverting Stoma | 891 (34%) | 146 (27%) | 0.001 |
The values with * include missing data.
APR, abdominoperineal resection; ESSQS, intervention by surgeons qualified by the Endoscopic Surgical Skill Qualification System; IMA, inferior mesenteric artery; ISR, intersphincteric resection; LND, lymph node dissection; LPND, lateral pelvic node dissection; SF, splenic flexure; TNT, Total neoadjuvant therapy; TPE, Total pelvic exenteration.
Short-term Outcomes
The frequencies of postoperative complications in the ESSQS group and the non-ESSQS group were 12.5% and 14.1%, respectively (Table S2, http://links.lww.com/AOSO/A118). The primary endpoint, which was the adjusted odds ratio of postoperative complications of procedures with ESSQS-certified surgeons in attendance, was 0.68 (P = 0.009; Table 3). The operative time and blood loss were 301 min and 99 mL in the ESSQS group, respectively, and 304 minutes and 100 mL in the non-ESSQS group, respectively, with the adjusted difference revealing the superiority of the ESSQS group (operative time, -16.1 min with attendance of ESSQS-certified surgeon, P < 0.001; blood loss, –10.5 mL with attendance of ESSQS-certified surgeons, P = 0.08; Table 4). Moreover, the conversion rates in the ESSQS and non-ESSQS groups were 1.1% and 6.8%, respectively, with an adjusted odds ratio of 0.20 for procedures with ESSQS-certified surgeons in attendance (P < 0.001; Table 3). Regarding postoperative complications, the risk of each complication was lower in the ESSQS group, and the risks of intra-abdominal abscesses and wound infections were significantly lower in the ESSQS group (intra-abdominal abscess, adjusted odds ratio, 0.42 with attendance of ESSQS-certified surgeon, P = 0.01; wound infection, adjusted odds ratio, 0.43 with attendance of ESSQS-certified surgeon, P = 0.04; Table 3). There were no differences in the outcomes related to reoperations. Postoperative stays were 17.2 days and 17.9 days in the ESSQS and non-ESSQS groups, respectively, with the adjusted difference between them revealing the superiority of the ESSQS group (–0.91 days with attendance by ESSQS-certified surgeon, P = 0.01; Table 4). The numbers of harvested lymph nodes were 22.3 and 18.1 in the ESSQS and non-ESSQS groups, respectively, with the adjusted difference between them revealing the favorability of the ESSQS group (1.29 with attendance by ESSQS-certified surgeon, P < 0.001; Table 4). Pathological R0 resections were performed in 99% and 98% of procedures in the ESSQS and non-ESSQS groups, respectively, with the adjusted odds ratio revealing the favorability of the ESSQS group (2.10 for attendance by ESSQS-certified surgeon, P = 0.04; Table 3).
TABLE 3.
Operative Outcomes (Adjusted Odds Ratio With 95% CI)
| With ESSQS(n = 2644) | Without ESSQS(n = 544) | P | |
|---|---|---|---|
| Conversion | 0.20 (0.10, 0.39) | 1 | <0.001 |
| Intraoperative complication | 0.79 (0.39, 1.62) | 1 | 0.52 |
| Postoperative complication (G3) | 0.68 (0.51, 0.91) | 1 | 0.009 |
| Intraperitoneal bleeding (G3) | 0.72 (0.14,3.71) | 1 | 0.69 |
| Anastomotic bleeding (G3) | 0.68 (0.24,1.94) | 1 | 0.47 |
| Anastomotic leakage (G3) | 0.80 (0.53, 1.22) | 1 | 0.29 |
| Intraperitoneal abscess (G3) | 0.42 (0.21, 0.83) | 1 | 0.01 |
| Wound infection (G3) | 0.43 (0.18, 0.99) | 1 | 0.04 |
| Ileus (G3) | 0.66 (0.33, 1.32) | 1 | 0.24 |
| Other postoperative complications (G3) | 0.88 (0.40, 1.92) | 1 | 0.75 |
| Reoperation | 0.78 (0.49, 1.23) | 1 | 0.29 |
| Pathological R0 resection | 2.10 (1.01, 4.37) | 1 | 0.04 |
Each odds ratio was adjusted considering the following clinical and surgical features: sex, body mass index, American Society of Anesthesiologists class, presence of obstructive pathology, cT, cN, and clinical stage, location of the tumor, type of preoperative therapy, procedures, combined resections of surrounding organs, lymph node dissection, lateral pelvic node dissection, high ligations of the inferior mesenteric artery, mobilization of the splenic flexure, a diverting stoma, and the type and volume of the institution.
CI, confidence interval; ESSQS, intervention by surgeons qualified by the Endoscopic Surgical Skill Qualification System; G3, grade 3 according to the Clavien-Dindo classification system.
TABLE 4.
Operative Outcomes (Adjusted Difference With 95% CI)
| With ESSQS(n = 2644) | Without ESSQS(n = 544) | P | |
|---|---|---|---|
| Operative time (min) | –16.1 (–20.5, –11.7) | 0 | <0.001 |
| Blood loss (mL) | –10.0 (–21.4, 1.4) | 0 | 0.08 |
| Postoperative hospital stay (d) | –0.91 (–1.22, –0.21) | 0 | 0.01 |
| Number of harvested lymph nodes | 1.29 (0.78, 1.81) | 0 | <0.001 |
Each difference was adjusted considering the following clinical and surgical features: sex, body mass index, American Society of Anesthesiologists class, presence of obstructive pathology, cT, cN, and clinical stage, location of the tumor, type of preoperative therapy, procedures, combined resections of surrounding organs, lymph node dissection, lateral pelvic node dissection, high ligations of the inferior mesenteric artery, mobilization of the splenic flexure, a diverting stoma, and the type and volume of the institution.
CI, confidence interval; ESSQS, intervention by surgeons qualified by the Endoscopic Surgical Skill Qualification System.
Pathological Outcomes and Survival
Overall, no differences in the tumor size, tumor depth, node status, pathological stage, undifferentiated features, or venous vessel invasion were observed between subgroups. However, the rates of lymph vessel invasion were 54% and 64% in the ESSQS and non-ESSQS groups, respectively (P < 0.001; Table 5). Adjuvant chemotherapy accompanied surgery in 45% and 44% of cases in the ESSQS and non-ESSQS groups, respectively (P = 0.22; Table 5).
TABLE 5.
Pathological Features
| With ESSQS(n = 2644), n (%) | Without ESSQS(n = 544), n (%) | P | ||
|---|---|---|---|---|
| Pathological T factor | 0 | 2 (0.08) | 0 | 0.88 |
| 1 | 93 (4) | 16 (3) | ||
| 2 | 439 (17) | 92 (17) | ||
| 3 | 1743 (66) | 355 (65) | ||
| 4a | 240 (9) | 55 (10) | ||
| 4b | 73 (3) | 17 (3) | ||
| pCR | 54 (2) | 9 (2) | ||
| Pathological N factor | 0 | 1402 (53) | 272 (50) | 0.1 |
| 1 | 814 (31) | 193 (35) | ||
| 2 | 324 (12) | 65 (12) | ||
| 3 | 104 (4) | 14 (3) | ||
| Pathological Stage | 0 | 2 (0.08) | 0 | 0.68 |
| I | 332 (13) | 67 (12) | ||
| II | 1015 (38) | 196 (36) | ||
| III | 1225 (46) | 270 (50) | ||
| IV | 18 (0.7) | 2 (0.4) | ||
| pCR | 52 (2) | 9 (2) | ||
| Tumor size (mm) | 44.2 (18.8) | 44.7 (17.8) | 0.61 | |
| Lymph vessel invasion* | 1421 (54) | 349 (64) | <0.001 | |
| Venous vessel invasion* | 1813 (69) | 370 (68) | 0.71 | |
| Differentiated adenocarcinoma* | 2483 (94) | 522 (96) | 0.17 | |
| Adjuvant chemotherapy* | 1195 (45) | 237 (44) | 0.22 |
The values with
* include missing data.
ESSQS, intervention by surgeons qualified by the Endoscopic Surgical Skill Qualification System.
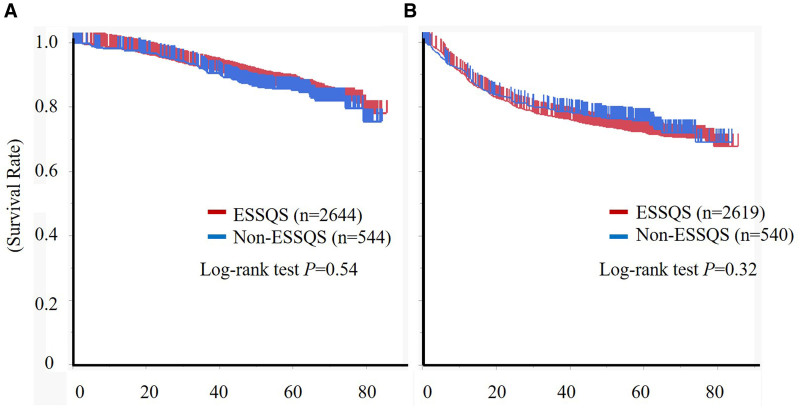
The median follow-up periods were 55.5 and 56.6 months for the ESSQS and non-ESSQS groups, respectively, with 98 (3.7%) and 27 (5.0%) patients in the ESSQS and non-ESSQS groups lost to follow-up, respectively. The 3-year overall survival rates were 92.2% and 91.3% in the ESSQS and non-ESSQS groups, respectively (log-rank test, P = 0.54; Figure 2). Upon Cox regression analysis, attendance by ESSQS-certified surgeons was not a significant factor for overall survival (hazard ratio, 0.88; 95% confidence interval [CI], 0.67–1.15; P = 0.35). The 3-year recurrence-free survival rates were 76.3% and 78.2% in the ESSQS and non-ESSQS groups, respectively (log-rank test, P = 0.32; Figure 2). In the Cox regression analysis, attendance by ESSQS-certified surgeons was not a significant factor for recurrence-free survival (hazard ratio, 1.04; 95% CI, 0.83–1.19; P = 0.71). The 3-year cumulative local recurrence rates were 4.9% and 4.6% in the ESSQS and non-ESSQS groups, respectively (P = 0.86). In the Cox regression analysis, attendance by ESSQS-certified surgeons was not a significant factor for local recurrence (hazard ratio, 0.91; 95% CI, 0.59–1.41; P = 0.67).
FIGURE 2.
Association between an intervention by an ESSQS-certified surgeon and recurrence in patients with clinical stage II or III rectal cancer. Kaplan-Meier curve representing (A) overall survival in the whole cohort and (B) recurrence-free survival in an unadjusted cohort, excluding R2 resections and stage IV disease. ESSQS, intervention by surgeons certified by the Endoscopic Surgical Skill Qualification System.
Outcomes According to the Role of an ESSQS-Certified Surgeon
The primary outcomes and long-term outcomes were additionally assessed according to the role of an ESSQS-certified surgeon (Figure S1, http://links.lww.com/AOSO/A116). The adjusted odds ratios were 0.78 (95% CI, 0.56–1.11; P = 0.18) for cases performed by ESSQS-certified surgeons (n = 1032) and 0.55 (95% CI, 0.36–0.85; P = 0.006) for cases assisted by ESSQS-certified surgeons (n = 569), when compared with non-ESSQS surgeons (n = 545) in postoperative complications. The hazard ratios in the 3-year overall survival, recurrence-free survival, and cumulative local recurrence were 0.86 (95% CI, 0.63–1.17; P = 0.35), 1.06 (95% CI, 0.84–1.33; P = 0.59), and 1.08 (95% CI, 0.66–1.78; P = 0.74), respectively, in cases where ESSQS-certified surgeons performed the procedure compared to cases without a ESSQS-certified surgeon, and 0.99 (95% CI, 0.68–1.43; P = 0.97), 1.03 (95% CI, 0.579–1.35; P = 0.78), and 1.12 (95% CI, 0.61–2.04; P = 0.70), respectively, in cases where a ESSQS-certified surgeon assisted the procedure compared to cases where a ESSQS-certified surgeon was not available.
DISCUSSION
In the present study, the superiority of the attendance of ESSQS-certified surgeons versus non-ESSQS-certified surgeons at laparoscopic rectal resections was apparent in terms of postoperative complication (Clavien-Dindo grade ≥3) rates. Patient backgrounds were different between the 2 groups. More difficult cases were reported in the ESSQS group, such as those involving low rectal cancers, T4b staging, and preoperative therapy. Procedures performed by ESSQS-certified surgeons were also considered more advanced, including intersphincteric resections, total pelvic exenterations, combined resection of surrounding organs, mobilization of the splenic flexure, and lateral pelvic node dissections. The difference in the diverting stoma and high ligations of the IMA might have also influenced the postoperative results. However, following adjustment of the clinical and surgical features, including age; sex; BMI; ASA class; obstruction; cT, cN, and clinical stage; location of the tumor; preoperative therapy; procedures; combined resections of surrounding organs; lymph node dissection; lateral pelvic node dissection; high ligations of the IMA; mobilization of the splenic flexure; a diverting stoma; and the type and volume of the institution, the odds ratio revealed that the attendance of ESSQS-certified surgeons significantly reduced the risk of postoperative complications.
The complication rates of the ESSQS group (12.5%) and non-ESSQS group (14.1%) were both much more favorable than the 21%–40% that has been reported in previous clinical trials.20–22 Unlike the current results, the assessment using the national database showed that the risks of postoperative complication did not differ depending on whether or not ESSQS-certified surgeons attended the surgery.14 The first difference involved the annual experience with these procedures at each institution. An annual experience of less than 5 low anterior rectal resection cases has been shown to lead to worse outcomes for postoperative complications.14 However, in the present study, almost all institutions had managed more than 5 low anterior rectal resection cases annually. The other differing point involved the definition of “attendance” by ESSQS-certified surgeons. In the national database study, only cases in which ESSQS-certified surgeons were operators were included in the ESSQS group.14 However, in our study, all cases in which an ESSQS-certified surgeon attended the surgery as an operator, assistant, or advisor were classified into the ESSQS group. We used this classification system, which parallels a previous report,11 because ESSQS-certified surgeons are designated not only as skilled masters, but also as skilled coaches.1,2 Furthermore, we believe that ESSQS-certified surgeons are useful for improving surgical outcomes whether they are the operator or the coach, that is, the assistant or the advisor. In fact, the odds ratios for postoperative complications were lower in cases where an ESSQS-certified surgeon assisted the procedure than in cases where an ESSQS-certified surgeon performed the procedure. The result might indicate that the impact of ESSQS-certified surgeons on safe operations was more significant as a skilled coach. However, further investigations are needed to validate this finding.
The current study also showed the superiority of laparoscopic rectal resections performed with ESSQS-certified surgeons in attendance in terms of surgical time, conversion rate, number of harvested lymph nodes, duration of the postoperative stay, and R0 resection rate. In particular, there were marked differences in the operative time and conversion rate that indicated higher technical skill. Several studies have similarly shown that a shorter operative time11,23–25 and lower conversion rate25 can be achieved in colorectal procedures performed by ESSQS-certified surgeons. Additionally, the favorability of the R0 resection rate and the number of harvested lymph nodes may indicate a higher quality procedure from an oncological perspective. Our study is the first to demonstrate an oncological advantage in the R0 resection rate, although a previous study supports the finding of a higher number of harvested lymph nodes.11 Thus, the superiority of ESSQS-certified surgeons for multiple short-term outcomes reinforces the utility of the technical and coaching expertise of ESSQS-certified surgeons on short-term outcomes.
To our knowledge, there have been no previous reports that investigated the long-term usefulness of attendance by ESSQS-certified surgeons in a large cohort. Here, for the first time, we have assessed this question in over 3000 cases of rectal cancer. Unexpectedly, we did not find any differences in the rates of recurrence. Each role of an ESSQS-certified surgeon did not have impact on these results. These finding results indicate that the attendance of ESSQS-certified surgeons did not have an impact on long-term outcomes. In a previous study, a Cox regression analysis revealed that nonattendance of ESSQS-certified surgeons was independently associated with local recurrence of stage II colorectal cancer from 2010 to 2013.11 However, that study involved only 431 cases, and rectal cancer comprised only 30% of the cases. In addition, the results were controversial because the risks of local recurrence differed between ESSQS and non-ESSQS groups only in cases where the patient had stage II colorectal cancer, but not when the patient had stage III disease.11 Moreover, the investigated age range was different in the 3 years between the previous report8 and the current study. During those 3 years, the proficiency level for laparoscopic rectal resections performed in Japanese hospitals may have improved. Indeed, the proportion of ESSQS-certified surgeons was much higher in the current study than in the previous study11 (74% vs. 41%). This supports the idea that laparoscopic rectal resections have become a more popular and a more standardized procedure. There can be no doubt about the lack of difference in long-term outcomes between the ESSQS and non-ESSQS groups if we consider that the fundamental quality of laparoscopic rectal surgeries has progressed to adequate levels nationwide.
The present study had some limitations. First, it was neither a prospective study nor a randomized controlled trial. We could not conduct a prospective study or a randomized controlled trial because of the difficulty in the selecting and recruiting the required number of candidate surgeons. As a retrospective study, there could be substantial selection bias that cannot be removed. We adjusted every clinically considerable bias by multivariate analysis. However, there were differences in patient numbers and several background factors between the 2 groups, and the number of confounding factors is the major weakness of this study. Second, some of the outcomes would have tested overpowered statistically. However, postoperative complications, our primary outcome, were clearly declared and defined in our study protocol and showed a statistically significant and clinically meaningful difference. Third, there were some weaknesses regarding the assessment. We did not perform analysis based on surgeons’ experience. It is obvious that number of cases performed has an impact on the learning curve. However, we focused only on the proficiency levels at the time of the procedure and classified the surgeons in terms whether they were certified because the proficiency levels are different among surgeons with the same experience with cases. Additionally, we did not assess the difference in the utilization of mechanical and oral antibiotic bowel preparation. Furthermore, the rates of cases with ESSQS-certified surgeons differed between the institutions. However, it can be assumed that the surgical skills between the institutions were similar. In a future study, we need to investigate the impact of attendance by ESSQS-certified surgeons on surgical outcomes depending on the type and volume of the institutions. One oncological aspect that might require avoiding generalizing in this investigation is that the neoadjuvant therapy was underutilized. This is because the neoadjuvant therapy is not a standard therapeutic strategy in Japan. In fact, the hazard ratio of local recurrence was lower in cases receiving conventional neoadjuvant chemoradiotherapy than in cases not receiving neoadjuvant therapy in the current study (data not shown). However, after the adjustment of the utility of neoadjuvant therapy, the R0 resection was less in the with ESSQS group than in the without ESSQS group, but local recurrence was similar between both the groups.
The present study shows that attendance by ESSQS-certified surgeons is useful for achieving technical safety during procedures for rectal cancers. ESSQS certification has certainly contributed to the maintenance and improvement of the quality of Japanese laparoscopic procedures. As a result, the number of laparoscopic surgeries has risen as a whole nationwide. However, the field of laparoscopic rectal resections is changing in terms of the prevalence of new approaches, such as transanal total mesorectal excisions and robotic surgery. Therefore, the importance of the ESSQS certification should be altered in accordance with these changes. However, conventional laparoscopic procedures are basic and essential for colorectal surgeons to master. We should make good use of this ESSQS certification, even if it may become necessary to reconsider how ESSQS certification is conducted in the future.
In conclusion, laparoscopic rectal procedures performed with ESSQS-certified surgeons showed improved postoperative results in terms of technical safety. The present study showed the utility of the ESSQS certification established by the JSES.
ACKNOWLEDGMENTS
The authors thank Dr. Masahiko Watanabe of Kitasato University Kitasato Institute Hospital for his deeply meaningful suggestions.
EnSSURE study group collaboratives: Akinobu Furutani, Akiyoshi Kanazawa, Akiyoshi Noda, Atsushi Ishibe, Chikayoshi Tani, Fumihiko Fujita, Fuminori Teraishi, Fumitaka Asahara, Heita Ozawa, Hideaki Karasawa, Hideki Osawa, Hiroaki Nagano, Hiroaki Takeshita, Hirofumi Ota, Hirokazu Suwa, Hiroki Ochiai, Hiroomi Ogawa, Hiroshi Saeki, Hirotoshi Hasegawa, Hiroyuki Bando, Hisanaga Horie, Hisashi Nagahara, Kaori Hayashibara, Kay Uehara, Kazuhiro Takehara, Ken Kojo, Ken Okamoto, Kenichiro Saito, Koji Ikeda, Koji Munakata, Koki Otsuka, Kunihiko Nagakari, Manabu Shimomura, Manabu Shiozawa, Manabu Takata, Manabu Yamamoto, Masakatsu Numata, Masashi Miguchi, Mayumi Ozawa, Mitsuhisa Takatsuki, Naoya Aisu, Naruhiko Sawada, Nobuaki Suzuki, Ryo Ikeshima, Ryo Inada, Ryuichi Oshima, Satoshi Maruyama, Shigehiro Kojima, Shiki Fujino, Shinichiro Mori, Shinobu Ohnuma, Sho Takeda, Shota Aoyama, Shuji Saito, Shusaku Takahashi, Takahiro Sasaki, Takeru Matsuda, Takuya Miura, Tatsunori Ono, Tatsuya Kinjo, Tatsuya Shonaka, Teni Godai, Tohru Funakoshi, Tomohiro Adachi, Tomohisa Furuhata, Toshimoto Kimura, Toshisada Aiba, Toshiyoshi Fujiwara, Tsukasa Shimamura, Tsunekazu Mizushima, Yasuhito Iseki, Yasuo Sumi, Yasushi Rino, Yohei Kurose, Yoshiaki Kita, Yoshihiro Kakeji, Yoshihiro Takashima, Yoshihito Ide, Yoshinori Munemoto, Yoshito Akagi, Yoshiyuki Ishii, Yuji Inoue, Yuki Kiyozumi, Yukihito Kokuba, Yukitoshi Todate, Yusuke Suwa, Yusuke Sakimura, Yusuke Shimodaira
Supplementary Material
Footnotes
Published online 22 April 2022
J.W. received grants from Medtronic, AMCO, and TERUMO; payment for lectures from Medtronic, Johnson and Johnson, and Lilly. D.Y. received payment for lectures from Johnson and Johnson, Medtronic, CHUGAI, PHARMACEUTICAL CO., Ltd, and TSUMURA and CO. H.I. received Fees for participation in statistical analysis. S.Y. received payment for lectures from Johnson and Johnson and Covidien Japan. T.N. received grants from Japan Society for the Promotion of Science, Medtronic, Chugai Pharmaceutical, and MC medical, Taiho Pharmaceutical, Kaken Pharmaceutical, Daiichi-Sankyo, and Eli Lilly Japan; payment for lectures from Johnson and Johnson, Medtronic, Olympus, TERUMO, Sumitomo Bakelite, Takeda Pharmaceutical, Chugai Pharmaceutical, Merck, Karl Storz Japan, Nikkiso, Boehringer Ingelheim, Taiho Pharmaceutical, Kaken Pharmaceutical, and Daiichi-Sankyo. The remaining authors declare that they have nothing to disclose.
N.I., S.H., K.H., T.A., Y.K., H.I., S.Y., M.I., Y.S., T.N., and A.T. conceived the idea for this study. N.I., K.H., T.A., Y.K., T.Y., M.I., F.I., J.W., and D.Y. contributed to the data collection. N.I. and H.I. performed statistical analyses and wrote the article. All authors participated in interpretation and analysis of the data, revised the article, and read and approved the final version.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Ichikawa N, Homma S, Yoshida T, et al. Supervision by a technically qualified surgeon affects the proficiency and safety of laparoscopic colectomy performed by novice surgeons. Surg Endosc. 2018;32:436–442. [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa N, Homma S, Yoshida T, et al. Mentor tutoring: an efficient method for teaching laparoscopic colorectal surgical skills in a general hospital. Surg Laparosc Endosc Percutan Tech. 2017;27:479–484. [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa N, Homma S, Yoshida T, et al. Proficiency level of novice technically qualified surgeons in laparoscopic rectal resection. Surg Laparosc Endosc Percutan Tech. 2020;30:49–54. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie H, Ni M, Miskovic D, et al. Clinical validity of consultant technical skills assessment in the English National Training Programme for Laparoscopic Colorectal Surgery. Br J Surg. 2015;102:991–997. [DOI] [PubMed] [Google Scholar]
- 5.Miskovic D, Ni M, Wyles SM, et al. ; National Training Programme in Laparoscopic Colorectal Surgery in England. Is competency assessment at the specialist level achievable? A study for the national training programme in laparoscopic colorectal surgery in England. Ann Surg. 2013;257:476–482. [DOI] [PubMed] [Google Scholar]
- 6.Vassiliou MC, Feldman LS, Andrew CG, et al. A global assessment tool for evaluation of intraoperative laparoscopic skills. Am J Surg. 2005;190:107–113. [DOI] [PubMed] [Google Scholar]
- 7.Jones DB, Stefanidis D, Korndorffer JR, Jr, et al. SAGES University MASTERS Program: a structured curriculum for deliberate, lifelong learning. Surg Endosc. 2017;31:3061–3071. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, Mori T, Konishi F, Kitajima M. Endoscopic surgical skill qualification system in Japan: five years of experience in the gastrointestinal field. Asian J Endosc Surg. 2010;3:66–70. [Google Scholar]
- 9.Yamakawa T, Kimura T, Matsuda T, et al. Endoscopic Surgical Skill Qualification System (ESSQS) of the Japanese Society of Endoscopic Surgery (JSES). BH Surg. 2013;3:6–8. [Google Scholar]
- 10.Mori T, Kimura T, Kitajima M. Skill accreditation system for laparoscopic gastroenterologic surgeons in Japan. Minim Invasive Ther Allied Technol. 2010;19:18–23. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa N, Homma S, Funakoshi T, et al. Impact of technically qualified surgeons on laparoscopic colorectal resection outcomes: results of a propensity score-matching analysis. BJS Open. 2020;4:486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsubara N, Miyata H, Gotoh M, et al. Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum. 2014;57:1075–1081. [DOI] [PubMed] [Google Scholar]
- 13.Inomata M, Shiroshita H, Uchida H, et al. Current status of endoscopic surgery in Japan: The 14th National Survey of Endoscopic Surgery by the Japan Society for Endoscopic Surgery. Asian J Endosc Surg. 2020;13:7–18. [DOI] [PubMed] [Google Scholar]
- 14.Akagi T, Endo H, Inomata M, et al. Clinical impact of Endoscopic Surgical Skill Qualification System (ESSQS) by Japan Society for Endoscopic Surgery (JSES) for laparoscopic distal gastrectomy and low anterior resection based on the National Clinical Database (NCD) registry. Ann Gastroenterol Surg. 2020;4:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto S, Ito M, Okuda J, et al. ; Japan Society of Laparoscopic Colorectal Surgery. Laparoscopic surgery for stage 0/I rectal carcinoma: short-term outcomes of a single-arm phase II trial. Ann Surg. 2013;258:283–288. [DOI] [PubMed] [Google Scholar]
- 16.Hida K, Okamura R, Sakai Y, et al. ; Japan Society of Laparoscopic Colorectal Surgery. Open versus laparoscopic surgery for advanced low rectal cancer: a large, multicenter, propensity score matched cohort study in Japan. Ann Surg. 2018;268:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinoi T, Kawaguchi Y, Hattori M, et al. ; Japan Society of Laparoscopic Colorectal Surgery. Laparoscopic versus open surgery for colorectal cancer in elderly patients: a multicenter matched case-control study. Ann Surg Oncol. 2015;22:2040–2050. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi T, Konishi T, Kinugasa Y, et al. Laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer: a subgroup analysis of a large multicenter cohort study in Japan. Dis Colon Rectum. 2017;60:954–964. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, Sugito M, Kobayashi A, et al. Influence of learning curve on short-term results after laparoscopic resection for rectal cancer. Surg Endosc. 2009;23:403–408. [DOI] [PubMed] [Google Scholar]
- 21.Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–645. [DOI] [PubMed] [Google Scholar]
- 22.van der Pas MH, Haglind E, Cuesta MA, et al. ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. [DOI] [PubMed] [Google Scholar]
- 23.Miki H, Fukunaga Y, Nagasaki T, et al. Feasibility of needlescopic surgery for colorectal cancer: safety and learning curve for Japanese Endoscopic Surgical Skill Qualification System-unqualified young surgeons. Surg Endosc. 2020;34:752–757. [DOI] [PubMed] [Google Scholar]
- 24.Kazama K, Numata M, Aoyama T, et al. Does the endoscopic surgical skill qualification system improve patients’ outcome following laparoscopic surgery for colon cancer? A multicentre, retrospective analysis with propensity score matching. World J Surg Oncol. 2021;19:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoyama S, Inoue Y, Ohki T, et al. Usefulness of the endoscopic surgical skill qualification system in laparoscopic colorectal surgery: short-term outcomes: a single-center and retrospective analysis. BMC Surg. 2019;19:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.