Abstract
Background:
Minimally invasive liver surgery (MILS) has a high variance in the type of resection and complexity, which has been underestimated in learning curve studies in the past. The aim of this work was to evaluate complexity-adjusted learning curves over time for laparoscopic liver resection (LLR) and robotic liver resection (RLR).
Methods:
Cumulative sum analysis (CUSUM) and complexity adjustment were performed using the Iwate score for LLR and RLR (n = 647). Lowest point of smoothed data was used to capture the cutoff of the increase in complexity. Data were collected retrospectively at the Department of Surgery of the Charité-Universitätsmedizin Berlin.
Results:
A total of 132 RLR and 514 LLR were performed. According to the complexity-adjusted CUSUM analysis, the initial learning phase was reached after 117 for LLR and 93 procedures for RLR, respectively. With increasing experience, the rate of (extended) right hemihepatectomy multiplied from 8.4% to 18.9% for LLR (P = 0.031) and from 21.6% to 58.3% for RLR (P < 0.001). Complication rates remained comparable between both episodes for LLR and RLR (T1 vs T2, P > 0.05). The complexity-adjusted CUSUM analysis demonstrated for blood transfusion, conversion, and operative time an increase during the learning phase (T1), while a steady state was reached in the following (T2).
Conclusions:
The learning phase for MILS after adjusting for complexity is about 4 times longer than assumed in previous studies, which should urge caution.
Mini-abstract: Minimally invasive liver surgery has a high variance in the type of resection and complexity, which has been underestimated in learning curve studies in the past. Interestingly, the learning phase after adjusting for complexity is about four times longer than assumed in previous studies, which should urge caution.
Supplemental Digital Content is available in the text.
INTRODUCTION
The introduction of minimally invasive liver surgery (MILS) has brought many benefits and improvements for patients. In fact, MILS shows better postoperative recovery and comparable oncologic outcomes to the open approach1 recommended in expert consensus statements as the safe preferred procedure.2 MILS shows a great degree of difficulty from wedge to extended resection. The International Society for Laparoscopic Liver Surgery (ILLS) recently published a training concept for laparoscopic liver surgery.3 Nevertheless, such training programs can only be implemented when surgical techniques are established.
The learning curve for establishing new techniques can be used to define this process descriptively. Skills usually increase with experience, that is, the more often an operation is performed, the better the surgeon becomes4. In a recent meta-analysis, Chua et al analyzed learning curves in MILS (40 studies in total) and unraveled a low median number of 20 procedures for robotic liver resections (RLR) and 34 procedures for laparoscopic liver resections (LLR) needed to surmount the learning curve.5 This seems to be at odds with clinical experience, as the complexity in MILS is challenging over years in clinical practice. Interestingly, the most commonly used learning curve parameters were operative time (OT), blood loss, and length of stay.5 This makes sense when analyzing similar cases like right hemihepatectomies. However, learning curve studies in the past subsumed resection from atypical segment III resections to extended right resections and analyzed surgery characteristics within these groups.5 This is contradictory, since these are different types of procedures which are, obviously, associated with different OT and complications shaping the learning curve. Therefore, it seems necessary in the analysis of learning curves to normalize liver resection according to its complexity. Based on this evidence, the present study aims to analyze complexity-adjusted learning curves of MILS (LLR and RLR) considering the type of resection, tumor location, tumor size, liver function, use of hand-assisted laparoscopy, proximity to major vessels (Iwate score).
METHODS
All consecutive patients who underwent MILS (n = 647) at the Department of Surgery, Campus Charité—Mitte and Campus Virchow Klinikum, Charité—Universitätsmedizin Berlin were retrospectively analyzed. This study was approved by the local ethics committee (EA2/006/16). LLR were analyzed between 2008 and 2020; the RLR were analyzed between 2018 and 2020. In all cases of malignant disease, a pre- and postoperative discussion was held in our tumor conference. RLR and LLR were performed according to our previous reports.1,6 Iwate score were determined for each procedure, calculated from a score based on tumor location, tumor size, extent of liver resection, liver function, proximity to major vessels, and use of hybrid approach/hand-assisted laparoscopic liver resection.7
Statistics
Cumulative sum analysis (CUSUM) of the Iwate score was performed for both, LLR and RLR. First, the patients were sorted chronologically according to the date of their procedure and data for each patient in the series were plotted on a chart from left to right. The Y value was the cumulative difference of the Iwate score for each liver resection in chronological order (1-n) from the mean of the entire series. The lowest point of the smoothed data according to the method of Savistsky and Golay (second order of the polynomial) was used to capture the turning point for complexity and to divide the curves into a initial phase (T1) and steady state phase (T2).8 Next, a complexity-adjusted CUSUM analysis (CA-CUSUM) was then performed for OT, conversion rate, and blood transfusion normalized by the Iwate score (n1-n/Iwate score). For example, for the conversion rate, this means that each slope of the curve represents the predicted conversion probability according to the Iwate score for each liver resection multiplied by the actual conversion performed (–1 = no conversion; 1 = conversion). Thus, the depth of the curve corresponds to the predicted conversion probability combined with the actual conversion rate. For a procedure without conversion, the curve decreases with the predicted conversion probability. Pearson’s chi-squared test or exact Fisher-Test were used for categorical variables; Mann–Whitney U test were used for continuous variables. A heatmap with no clustering was used for the Iwate criteria. The selected significance level was 0.05 (α level).
RESULTS
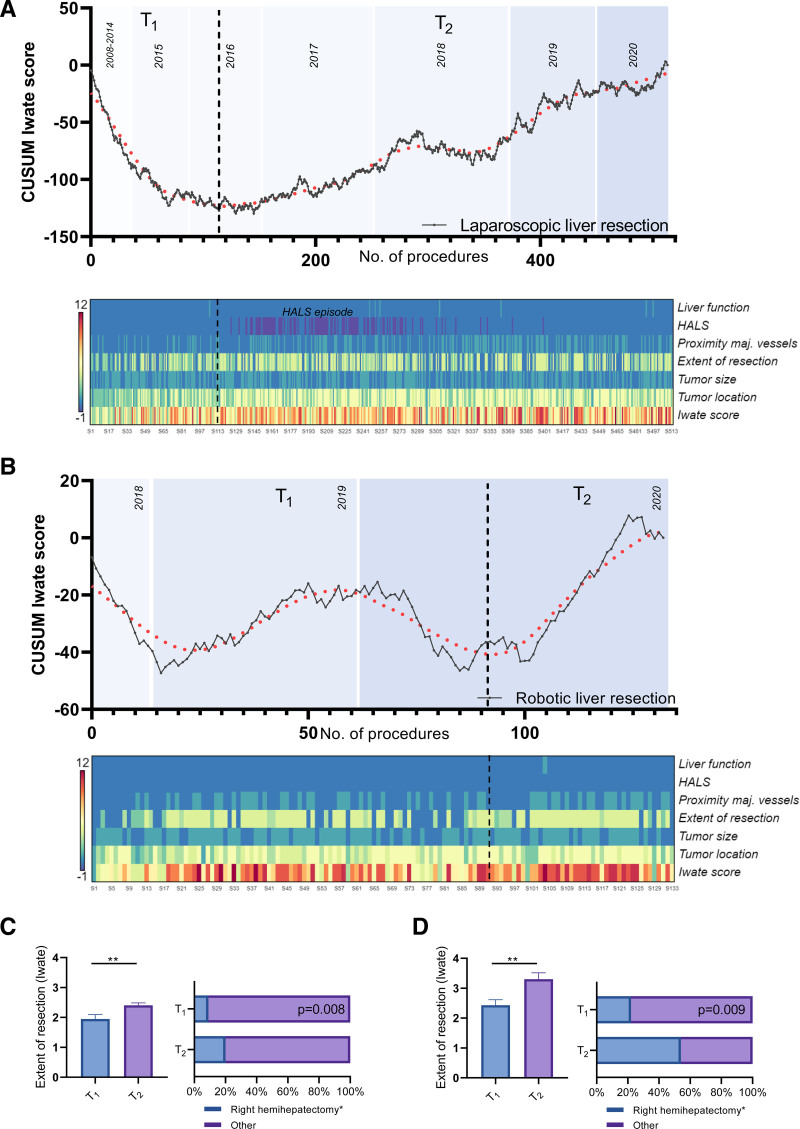
A total of 514 LLRs were performed with a turning point of complexity after 117 procedures (Fig. 1A). A total of 133 RLRs were performed, and the learning phase was reached after 93 procedures, according to the statistical analysis (Fig. 1B). In contrast, the learning curve not adjusted for complexity (CUSUMOT) demonstrated a consecutive decrease of OT for LLR and RLR in T1 indicating that the operations were faster than average (Figure S1, see http://links.lww.com/AOSO/A98). Note, when adjusted for complexity, the operations took longer on average during T1. Interestingly, a hand-assisted laparoscopic liver resection episode could be identified for LLR (heatmap, Fig. 1A), which was initially used when complexity increased, but then abandoned. In addition, an increase of Iwate criteria was observed between T1 and T2 for both technical variations of MILS (Table 1). For example, the rate of right hemihepatectomies (including extended resection) multiplied from 9.4% to 21.1% (P = 0.008) for LLR and from 22.5% to 52.5% (P = 0.009) for RLR (Figs. 1C, D). Complication rates remained comparable between both episodes for laparoscopic and robotic procedures (Table 1, T1 vs T2, P > 0.05). In addition, a complexity-adjusted CUSUM (CA-CUSUM) analysis was performed for blood transfusion, conversion, and OT (Figure S2, see http://links.lww.com/AOSO/A98). It was observed for LLR and RLR that after a learning phase (T1) a steady state was reached in the following (T2).
FIGURE 1.
Cumulative sum analysis of the Iwate criteria for LLR and RLR. Segmental regression was used to capture the increase of complexity for liver resection (fits one line to all data points where X is less than a given value X0 and another line to all points where X is greater than X0). An increase in complexity was obtained after 71 LLR (A) and after 97 RLR (B) (T1 vs T2). Heatmaps were created based on the Iwate criteria: tumor location, tumor size, extent of liver resection, liver function, proximity to major vessels, and use of hybrid approach/HALS. The extent of resection (0 = partial resection, 2 = left lateral resection, 3 = segmentectomy, 4 = sectionectomy, or more) and the rate of hemihepatectomy (including extended ones) are depicted for LLR (C) and RLL (D). HALS, hand-assisted laparoscopic liver resection; LLR, laparoscopic liver resection; RLR, robotic liver resection.
TABLE 1.
Study Cohort Characteristics Mean (±SD), Median (IQR), or N (%)
| Technique | LLR (n = 514) | RLR (n = 133) | ||||
|---|---|---|---|---|---|---|
| Episode | 117 T1 | 397 T2 | P | 93 T1 | 40 T2 | P |
| Age (years) | 56.3 (±17.0) | 60.6 (±14.2) | 0.036 | 61.0 (±14.4) | 59.5 (±12.8) | 0.450 |
| Gender (female) | 64 (54.7%) | 172 (43.3%) | 0.034 | 45 (48.4%) | 19 (47.5%) | >0.999 |
| BMI (kg/m2) | 26.2 (±5.7) | 26.6 (±4.7) | 0.218 | 25.6 (±4.6) | 26.7 (±5.0) | 0.378 |
| ASA-classification | ||||||
| ASA 1–2 | 84 (71.8%) | 192 (48.4%) | <0.001 | 43 (46.2%) | 23 (57.5%) | 0.260 |
| ASA 3–4 | 33 (28.2%) | 205 (51.6%) | 50 (53.8%) | 17 (42.5%) | ||
| History of surgery (abd.) | 35 (37.6%) | 227 (57.2%) | <0.001 | 59 (63.4%) | 221 (52.5%) | 0.252 |
| Pathology | ||||||
| Malignant | 68 (58.1%) | 333 (83.8%) | <0.001 | 74 (79.6%) | 35 (87.5%) | 0.333 |
| HCC | 39 (33.3%) | 100 (25.1%) | 22 (23.7%) | 9 (22.5%) | ||
| CRLM | 16 (11.9%) | 158 (39.8%) | 24 (25.8%) | 14 (35.0%) | ||
| iCC | 5 (13.7%) | 26 (6.5%) | 12 (12.9%) | 4 (10.0%) | ||
| Other | 8 (6.8%) | 49 (12.3%) | 16 (17.2%) | 8 (20.0%) | ||
| Benign | 49 (41.8%) | 64 (16.1%) | <0.001 | 19 (20.4%) | 5 (12.5%) | 0.333 |
| Adenoma | 21 (17.9%) | 15 (3.7%) | 5 (5.3%) | 2 (5.0%) | ||
| Hemangioma | 6 (5.1%) | 10 (2.5%) | 3 (3.2%) | 1 (2.5%) | ||
| Other | 22 (18.8%) | 39 (9.8%) | 11 (27.5%) | 2 (5.0%) | ||
| Length of stay (days) | 8 [7-10.5] | 8 [6-10] | 0.169 | 8 [6-10] | 7 [6.2-10.7] | 0.951 |
| Type of surgery | ||||||
| Hemihepatectomy* left | 5 (4.2%) | 32 (8.0%) | <0.001 | 16 (17.2%) | 5 (12.5%) | 0.017 |
| Hemihepatectomy* right | 11 (9.4%) | 84 (21.1%) | 21 (22.5%) | 21 (52.5%) | ||
| Left lat. sectionectomy | 35 (29.9%) | 58 (14.6%) | 8 (8.6%) | 2 (5.0%) | ||
| Sectionectomy other | 9 (7.6%) | 50 (12.6%) | 8 (8.6%) | 3 (7.5%) | ||
| Segment or wedge | 53 (45.3%) | 181 (45.6%) | 40 (43.0%) | 9 (22.5%) | ||
| Lymphadenectomy | 4 (3.4%) | 26 (6.5%) | 0.264 | 24 (25.8%) | 7 (17.5%) | 0.374 |
| Low/intermediate difficulty† | 80 (68.3%) | 189 (47.6%) | <0.001 | 40 (43.0%) | 7 (17.5%) | 0.005 |
| Clavien-Dindo 1–2 | 14 (17.5%) | 37 (19.6%) | 0.769 | 4 (10.0%) | 0 (0.0%) | >0.99 |
| Clavien-Dindo 3–5 | 2 (2.5%) | 15 (7.9%) | 0.107 | 8 (20.0%) | 1 (14.2%) | >0.99 |
| Advanced/expert difficulty‡ | 37 (31.6%) | 208 (52.4%) | <0.001 | 53 (56.9%) | 33 (82.5%) | 0.005 |
| Clavien-Dindo 1–2 | 4 (10.8%) | 42 (20.2%) | 0.252 | 8 (15.1%) | 5 (15.1%) | >0.99 |
| Clavien-Dindo 3–5 | 7 (18.9%) | 47 (22.6%) | 0.829 | 10 (18.8%) | 9 (27.3%) | 0.434 |
Chi Square Test or Exact Fisher-Test for categorical variables, Mann-Whitney U test for continuous variables.
*Including extended hemihepatectomies.
†Iwate criteria score 1–6.
‡Iwate criteria score 7–12.
ASA indicates American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range.
DISCUSSION
In the present study, the learning curve for laparoscopic and robotic liver resection was normalized and evaluated using the Iwate criteria defining the complexity of each procedure. We found that after 117 procedures for LLR and 93 procedures for RLR a learning phase was overcome, although the first cases were “self-taught” but performed by trained hepatobiliary surgeons. The initial learning curve was prolonged compared with a review by Chua et al, which showed that the number of procedures needed to surmount the learning curve for LLR and RLR procedures were 34 (range 18–60) and 20 (17–25).5 The main reason might be that not all liver resections were considered in the analysis, leading to certain bias. There are studies that have analyzed only major resections, but they omit liver resections performed chronologically before or after major hepatectomies.9,10 At the time of the 25th major hepatectomy, we had already performed a total of 137 LLR at our center. It is important to note, LLR is composed of repetitive substeps, with some being performed at each resection, and thus a transfer of learned skills is very likely.3 For example, the location of the tumor in segment VII or VIII can be quite challenging, even if a minor resection is performed by its definition.7 Complexity has not been adequately represented in learning curve models, although it strongly correlates with major learning curve readouts such as OT, outcome, and complication rates. Taken together, the present study demonstrated that the learning curve for MILS after adjusting for complexity is about 4 times longer than assumed in previous studies, which should urge caution.
Supplementary Material
Footnotes
Published online 25 January 2022
Disclosure: The authors declare that they have nothing to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Schmelzle M, Krenzien F, Schöning W, et al. Laparoscopic liver resection: indications, limitations, and economic aspects. Langenbecks Arch Surg. 2020;405:725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. [DOI] [PubMed] [Google Scholar]
- 3.Krenzien F, Schöning W, Brunnbauer P, et al. ; study group of the International Laparoscopic Liver Society (ILLS) . The ILLS Laparoscopic Liver Surgery Fellow Skills Curriculum. Ann Surg. 2020;272:786–792. [DOI] [PubMed] [Google Scholar]
- 4.Valsamis EM, Chouari T, O’Dowd-Booth C, et al. Learning curves in surgery: variables, analysis and applications. Postgrad Med J. 2018;94:525–530. [DOI] [PubMed] [Google Scholar]
- 5.Chua D, Syn N, Koh Y-X, et al. Learning curves in minimally invasive hepatectomy: systematic review and meta-regression analysis. Br J Surg. 2021; 108:351–358. [DOI] [PubMed] [Google Scholar]
- 6.Feldbrügge L, Ortiz Galindo SA, Frisch O, et al. Safety and feasibility of robotic liver resection after previous abdominal surgeries [Epub ahead of print June 2, 2021]. Surg Endosc. doi: 10.1007/s00464-021-08572-1 https://pubmed.ncbi.nlm.nih.gov/34076760/. [DOI] [PubMed] [Google Scholar]
- 7.Krenzien F, Wabitsch S, Haber P, et al. Validity of the Iwate criteria for patients with hepatocellular carcinoma undergoing minimally invasive liver resection. J Hepatobiliary Pancreat Sci. 2018;25:403–411. [DOI] [PubMed] [Google Scholar]
- 8.Savitzky A, Golay MJE. Smoothing and differentiation of data by simplified least squares procedures. Analytical Chemistry. 1964;36:1627–1639. [Google Scholar]
- 9.van der Poel MJ, Besselink MG, Cipriani F, et al. Outcome and learning Curve in 159 consecutive patients undergoing total laparoscopic hemihepatectomy. JAMA Surg. 2016;151:923–928. [DOI] [PubMed] [Google Scholar]
- 10.Nomi T, Fuks D, Kawaguchi Y, et al. Learning curve for laparoscopic major hepatectomy. Br J Surg. 2015;102:796–804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.