Abstract
Background:
Previous studies have reported that ambient temperature may affect perinatal outcomes. However, whether extreme temperature affects the risk of preterm birth (PTB) remains controversial. Studies on the associations of extreme temperature with PTB subtypes are lacking.
Objectives:
We aimed to investigate the associations of extreme climate events with the risks of PTB and its subtypes, discerning possible modifiers.
Methods:
Data on all singleton deliveries were obtained from the China Labor and Delivery Survey (CLDS), a nationwide investigation implemented in 2015 and 2016. PTB was defined as gestational weeks and then categorized as early (24–34 wk) and late PTBs (35–36 wk), and clinical subtypes [spontaneous PTB, preterm premature rupture of the fetal membranes (PPROM), iatrogenic PTB]. Ambient temperature data were provided by the China National Weather Data Sharing System. Five heat indexes and five cold indexes were used to define heat waves and cold spells. Generalized linear mixed models with a random term by hospital unit were used to assess the associations of short-term prenatal extreme temperature exposure. The Cox proportional hazard regression model was applied to assess the nonlinear associations of low- or high-temperature exposure at the whole and different trimesters of pregnancy with the risk of PTB. Stratified analyses were conducted to assess the possible modification by geographic region and fetal sex.
Results:
A total of 70,818 singleton births from 96 hospitals in China were included, among which 4,965 (7.01%) were PTBs. Exposure to extreme cold events 1 wk before delivery was associated with an increased PTB risk, with an adjusted odds ratio (aOR) [95% confidence intervals (CIs)] of 1.07 (95% CI: 1.04, 1.10) and 1.06 (1.04, 1.09) for the total days when the daily average temperature below the fifth percentile (fifth-days) and the 10th percentile (10th-days), 1.18 (1.04, 1.34) for the cold spells when the daily average temperature below the fifth percentile for two consecutive days (fifth-2D), 1.09 (1.03, 1.16) and 1.12 (1.06, 1.19) for the cold spells when the daily average temperature below the 10th percentile for three and two consecutive days (10th-3D and 10th-2D), respectively. Results of extreme temperature exposure during 2 weeks before delivery showed similarly significant associations. The association between cold spells and PTB tended to be stronger for late PTB than for early PTB. Cold spells were mainly associated with spontaneous PTB and late PPROM. A stratified analysis indicated that pregnant women in western and northern regions tended to be more sensitive to cold spells, and pregnant women with a female fetus appeared to be at a higher risk of PTB when exposed to cold spells. Pregnant women in late pregnancy were more susceptible to extreme temperatures. No significant or stable association was found between heat waves and preterm birth.
Discussion:
Exposure to cold spells was associated with an increased risk of PTB, especially late, spontaneous PTB and PPROM. The associations appeared to be more pronounced in the north and west regions and in pregnancies with female fetuses. https://doi.org/10.1289/EHP10831
Introduction
Preterm birth (PTB), defined as delivery before 37 wk of gestation, is a major cause of neonatal mortality and child health.1 It can also increase the risk of metabolic disorders and immune and respiratory diseases in later life.2,3 Every year, 10.6% (more than ) of all births are preterm worldwide, of which were contributed by China.4 The etiology of PTB is complex and varies by region. Although some factors, such as previous PTB history, multiple pregnancies, pregnancy complications, and infections, have been well established, specific causes of most PTBs remain ambiguous.5
Associations of prenatal exposure to environmental factors, such as extreme climate, air pollution, and environmental endocrine disruptors with adverse birth outcomes, especially PTB, have received increasing attention.6,7 In the context of global warming and growing concerns about extreme weather events, the impacts of these factors on health might have universal public health interest.8 Recent studies suggest that maternal exposure to extremely high temperatures may pose a potential risk of PTB.9 For example, studies in the United States and Australia have found that increases in ambient temperature and heat waves could increase the PTB risk and decrease the gestational length.10,11 In comparison with the extensive studies on the impacts of maternal heat exposure on PTB, studies on the associations of extremely low temperature with PTB are limited. Ha et al. and He et al. found that extremely cold temperature exposure may induce PTB.12,13 One possible explanation is that stress induced by maternal extreme temperature exposure might trigger early labor.14
However, the results were far from consistent.15,16 Two multicenter studies in the United States also showed the regional heterogeneity of the association between extreme temperature and PTB.12,17 This discrepancy may be because of the spatial heterogeneity of the climate and some uncontrolled confounding. Besides, various definitions of heat and cold waves constitute another source of heterogeneity.18 Taking into account the sensitivity of different extreme temperature indicators, using multiple definitions to explore the impact of extreme temperature on PTB may help to discern the possible association in different regions, which can also avoid the limitations of the generalizability of single-city studies. Exploring the associations of temperature exposure with PTB across different pregnancy stages can help determine the critical window and formulate intervention policies. More large-scale, multicenter studies covering different climatic and geographic conditions are warranted.
Further, PTB has a diverse etiology. It can be divided into different etiological subtypes, such as spontaneous PTB, preterm premature rupture of the fetal membranes (PPROM), and iatrogenic PTB.19 Growing evidence suggests that maternal environmental exposure during pregnancy (e.g., phthalate, phenols, and per- and polyfluoroalkyl substances) may be related to spontaneous PTB.20–22 But to our knowledge, the associations of extreme temperature exposure with spontaneous PTB have not been fully explored. PPROM can be caused by a variety of maternal factors, such as amniotic membrane infection, increased pressure in the amniotic cavity, and nutritional deficiencies.23 Maternal environmental exposure such as extreme temperature exposure may also trigger PPROM.24 Unfortunately, most of the previous studies on the current topic examined the total PTB without excluding iatrogenic PTB, which is less relevant to ambient temperature. The inclusion of such PTB may underestimate the true effect size because it may dilute the association strength of extreme temperatures.25,26
Our study aimed to explore the associations of cold and hot temperature exposure on the risk of PTB and its subtypes at different time windows using data from a multicenter survey. We further explored whether the associations of the extreme climate might be modified by the maternal sociodemographic status, region, and fetal sex.
Data and Methods
Study Population
The China Labor and Delivery Survey (CLDS) was a large-scale multicenter study conducted in the period 2015–2017, aiming to describe the current status of obstetric practice in China and to identify the key determinants for cesarean section. Hospitals with at least 1,000 deliveries a year were potentially eligible to participate. They included secondary and tertiary, general, and maternity hospitals. All hospitals were asked to provide basic information on their obstetric practice if they expressed interest in participating in the CLDS project. Because the annual delivery volume varied widely from hospital to hospital, a random sample of births was selected in each hospital. For large hospitals with births annually, 6 wk within a consecutive 12-month period were randomly selected for each hospital, whereas for smaller hospitals with births a year, 10 wk were randomly selected for each hospital. This strategy was to ensure that every hospital had sufficient statistical power to provide a set of basic obstetric and perinatal statistics. This methodology and questionnaire used were adopted from previous large surveys.27
All births with gestational age wk or in the selected weeks were eligible. Medical records were retrieved and extracted by trained research nurses. Included was information on maternal demographic characteristics, reproductive history, disease history, pregnancy complications, assessment at labor and delivery admission, delivery summary, and neonatal conditions. Logic checks were built into the database to detect data entry errors and missing values for quality control during data collection. More details on this project have been described elsewhere.28 Given that only anonymous information was recorded from medical records for research, no individual written consent by the patients was required. Ethical approval was obtained from all participating hospitals.
For the CLDS project, we calculated the average number of deliveries per hospital and our surveyed sample size based on the total population, the number of births, and the number of different kinds of hospitals in each province as recorded in the China Health Statistics Yearbook 2015, 2016, and 2017 (Table S1).29 A total of 191 hospitals across different levels participated, and 82,221 deliveries from 28 provinces nationwide were submitted to our data management system. We then excluded the ineligible hospitals, such as hospitals with low completion rates and dropout hospitals, and finally, a total of 75,132 births in 96 hospitals from 24 provinces were selected, covering most regions of China (Figure S1).30 Gestational age was estimated based on the date of the last menstrual period, and 940 cases were excluded because the gestational age, date of the last menstrual period, or birth date was missing. We excluded births whose maternal age was or (), the gestational week was or wk (), and birth weight was (). We further restricted to singleton births (excluding 1,876 records). Stillbirths and births with unknown outcomes () were also excluded. Finally, 70,818 pregnancies remained in our analysis (Figure S2).
Outcome Definition
We defined PTB as births with gestational age of completed weeks, and further categorized PTB into different subtypes: early (early-PTB, 24–34 wk) and late PTBs (late-PTB, 35–36 gestational weeks), and spontaneous PTB, PPROM, and iatrogenic PTB.31,32 A list of medical or obstetric complications, including chronic/pregnancy–induced hypertension, preeclampsia, diabetes, placenta previa, placental abruption, etc., was considered to define iatrogenic PTB.28 PPROM was defined as the cases with the fetal membrane rupture before the onset of labor and without medical indications. The remaining PTB were classified as spontaneous PTB.
Exposure Assessment
Meteorological data during the study period was accessed freely from the National Meteorological Data Center,29 and the daily average, maximum and minimum temperature, relative humidity, and wind speed were included for analysis. The geographic coordinate was used to calculate the distance between the hospital and each neighboring meteorological station, and the nearest station was selected as the source of meteorological data. We further matched the meteorological data to each day of pregnancy. In this study, the fifth, 10th, 90th, and 95th percentiles of the daily mean temperature of the hospital-located regions during the study period were selected as the cutoff values to assess the extreme temperature. A total of 10 temperature indices (5 cold indices and 5 heat indices) were created to represent extreme cold and hot weather scenarios per previous studies.33,34 In addition, fifth-days and 10th-days represent the total number of days when the daily mean temperature was lower than the fifth and 10th percentiles, respectively, within a certain exposure window. The cold spell event in this study was defined as the daily average temperature below the fifth percentile for 2 consecutive days or the daily average temperature below the 10th percentile for 2 or 3 consecutive days. The variables “fifth-2D,” “10th-2D,” and “10th-3D” represented the number of cold wave events experienced within a certain period. Accordingly, 90th-days and 95th-days represented the total number of days when the daily mean temperature was higher than the 90th and 95th percentiles, respectively. A heat wave event was defined as the daily average temperature above the 95th percentile for 2 consecutive days or the 90th percentile for 2 or 3 consecutive days, expressed as 95th-2D, 90th-2D, and 90th-3D, respectively. We further calculated the cold and heat indexes and average daily mean temperature for different time windows (the entire pregnancy; the first, second, and third trimesters; and 1, 2, 3, and 4 wk before delivery) to evaluate their associations of temperature exposure with PTB. Because the home address of each participant was not obtained by the survey for confidentiality, the average exposure estimates were made by geocoded matching based on the hospital location to represent the exposure level of pregnant women who gave birth in that hospital.
Air pollution measures were also used as potential confounders. The data were freely accessed from a 6-y air pollution estimation data set in China from 2013 to 2018.35 Developed by the Chinese Academy of Sciences, this data set assimilated information from more than 1,000 surface air quality monitoring sites and the Nested Air Quality Prediction Modeling System. This product could provide six conventional air pollutants [i.e., particulate matter (PM) with aerodynamic diameter , PM with aerodynamic diameter , , , carbon monoxide (CO), and ] in China with spatial and temporal resolutions: and 1 h, respectively,36–38 the cross-validation of which was 0.52–0.81, root mean square error (RMSE) was for CO, and RMSE were for the other pollutants on an hourly scale. The quality of this data set has also been cross-validated with other independent data sets, such as the U.S. Department of State Air Quality Monitoring Program over China with and in different cities, which indicates that this data set is reliable and has higher spatial resolutions than most other similar data sets with regarding the surface concentrations of air pollutants in China.39 More details have been described elsewhere.9 A recent review has shown that and might affect PTB, whereas the associations of other air pollutants were not stable. Therefore, in the present study, we estimated the average and concentrations of individual exposure during the first, second, and third trimesters, the entire pregnancy, and the 1 and 2 wk before delivery to control for their confounding effects.
Covariates
In this study, maternal age, ethnicity, education, insurance, child sex, parity, conception year, and season were extracted from the electronic medical record and used as covariates. Gross domestic product (GDP) per capita, geographic region, [per inter quantile range (IQR) increase, ], and (per IQR increase, ) were also controlled. Covariates except for and were included as categorical variables, the definition of which were as follows: a) maternal age was grouped in 5-y increments; b) ethnicity was categorized as Han, minority, and unknown; c) maternal education was classified as lower than high school, high school, less than college ( y), college graduate ( y), or higher, and unknown; d) maternal insurance type was grouped into three categories: health insurance, self-pay, and unknown; and e) child sex included male, female, and unknown groups. The conception season was created as four groups to control for seasonality: spring (March–May), summer (June–August), Autumn (September–November), and winter (December–February). The conception year was also included to control for long-term trends. Furthermore, we collected census district (county)-level average annual GDP per capita from the statistical yearbook published by the local government and linked it to each hospital where the woman gave birth. GDP per capita was grouped into quantiles for each year. The classification of regions was first made according to the China National Bureau of Statistics, as follows: South, North, East, Central, Southwest, Northwest, and Northeast. Considering the study sample size, we reclassified the study area into four regions according to geographical and climatic characteristics (West, including Southwest and Northwest, because both are inland; North, including North, Northeast, and part of the central region in the north of the Huai River; East; South, including South, and part of the central region in the south of the Huai River).40 Potential confounders were selected based on prior knowledge and a directed acyclic graph (DAG)9,13,17,41–45 (see Supplementary Figure S3).
Statistical Analysis
The distribution of covariates, climate, and air pollution indicators was first assessed. Categorical covariates were reported as numbers and percentages, whereas continuous variables such as climate and air pollution indicators were reported as the median and IQR, or mean and standard deviation (SD). Differences in characteristics and environmental variables between PTB and non-PTB groups were tested using the chi-squared test or Wilcoxon’s rank sum test where appropriate. A logistic regression model with the hospital as a random item using generalized linear mixed models (GLMMs) was applied. Hospitals served as second-level units to minimize the potential bias caused by the variation of the quality of medical information and the possible incomplete independence among individuals. In this model, we explored the associations of exposure to extreme weather events 1 and 2 wk before delivery with PTB subtypes, respectively. Tolerance was used to assess the collinearity among covariates. We further applied the Cox proportional hazard regression to estimate the associations of the entire pregnancy and trimester-specific temperature exposure with the risk of PTB. The exposure–response associations of each long-term exposure window (entire pregnancy, first, second, and third trimesters) were modeled while adjusting for temperature exposure from the other 2 trimesters, simultaneously, to avoid potential bias.46 Gestational age was fitted as the time scale, and PTB could be treated as a time-to-event outcome with term births censored at week 37. The average daily temperature of the whole pregnancy and different trimesters were fitted as time-independent variables, respectively. To further fit the possible nonlinear relationship between temperature and PTB, we constructed a penalized cubic spline with 5 knots (fifth, 25th, 50th, 75th, and 95th percentiles) to estimate the nonlinear exposure–response associations. Linear hypotheses test for nonlinear association was examined by Wald chi-square. All models were adjusted for maternal age, maternal ethnicity, maternal education, insurance type, child sex, parity, GDP per capita, geographic region, conception season, conception year, , and . Individuals with missing information were classified into one category and treated as dummy variables in all models. Stratified analyses were performed for different PTB subtypes (early and late PTB, spontaneous PTB, PPROM, and iatrogenic PTB) to discern the true relationship between extreme temperature and PTB and for the geographic region and child sex to identify high-risk women.
In addition, several sensitivity analyses were conducted to assess the robustness of the results. First, extreme temperature events were evaluated using daily average temperature, but we felt that it may not be an ideal indicator to reflect the environmental temperature that the human body endures. Thus, we used the average apparent temperature (AT) and daily maximal and minimal temperatures to calculate heat and cold indexes, respectively. AT is a comprehensive index that integrates average temperature, relative humidity, and wind speed and can better reflect the temperature that the human body endures.47 Second, considering the possible delayed and cumulative effects of short-term temperature exposure on health, we further extended our exposure windows to 3 and 4 wk before delivery to assess the association changes of different time windows. Third, to more accurately capture the associations of extreme temperatures on PTB and avoid confounding by seasonality, we restricted the study period to the warm (1 May–31 October) and cold (1 November–30 April) seasons based on the month of birth. Fourth, we further excluded subjects with PTB history to avoid possible confounding. Fifth, some women, especially women with high-risk pregnancies, may travel outside of their local area to attend specialist care. We, therefore, excluded subjects with severe maternal diseases (diabetes, hypertensive disorders, nephropathy, and toxemia). Sixth, we further analyzed the effects of extreme weather events after additional adjustment for background temperature, considering its potential effect on the gestational length. Background temperature was calculated from the averaged ambient temperature during each exposure window. Finally, the main analysis in this study treated the missing variable as a separate category in the regression, which might introduce bias. Thus, multiple imputations with the fully conditional specification method were used to validate the robustness of the main results. A total of 10 imputed data sets were created, and the results were pooled to get the final estimated parameters based on Rubin’s rules.48 This method can specify the type of multivariate imputation model on a variable-by-variable basis and offer a principled yet flexible method to address missing data.49 All statistical analyses were conducted in SAS (version 9.4; SAS Institute Inc.) and R (version 3.6; R Development Core Team). Two-sided was set for the confidence level of the hypothesis testing.
Results
The demographic characteristics of the study population are presented in Table 1. A total of 4,965 (7.01%) women had PTB. Among them, the number of spontaneous PTB, PPROM, and iatrogenic PTB were 2,454 (3.47%), 1,358 (1.92%), and 1,153 (1.63%), respectively. The mean maternal age was 28.7 y old. In addition, 95.3% of women were of Han ethnicity. Almost half of the women had some college ( y) or higher level of education. The majority had health insurance (58.5%), 53.4% of pregnant women had a male child, and 56.1% were nulliparous. PTB and non-PTB women were similar in conception season but differed significantly in maternal age, maternal ethnicity, maternal education, health insurance, child sex, parity, GDP per capita, and geographic region ().
Table 1.
Characteristics of the study population in China Labor and Delivery Survey, 2015–2017 ().
| Characteristics | Total births |
Term births |
Preterm births |
-Value |
|---|---|---|---|---|
| Maternal age [n (%)] | — | — | — | |
| 15–19 y | 1,021 (1.4) | 887 (1.3) | 134 (2.7) | — |
| 20–24 y | 10,496 (14.8) | 9,783 (14.9) | 713 (14.4) | — |
| 25–29 y | 33,026 (46.6) | 31,056 (47.2) | 1,970 (39.7) | — |
| 30–34 y | 18,301 (25.8) | 16,958 (25.8) | 1,343 (27.0) | — |
| 35–49 y | 7,974 (11.3) | 7,169 (10.9) | 805 (16.2) | — |
| Maternal ethnicity [n (%)] | — | — | — | 0.019 |
| Han | 67,507 (95.6) | 62,816 (95.6) | 4,691 (94.9) | — |
| Minority | 3,134 (4.4) | 2,882 (4.4) | 252 (5.1) | — |
| Unknown | 177 | 155 | 22 | — |
| Maternal education [n (%)] | — | — | — | |
| Less than middle school | 15,976 (25.1) | 14,495 (24.5) | 1,481 (33.1) | — |
| High school | 12,654 (19.9) | 11,729 (19.8) | 925 (20.7) | — |
| College ( y) | 15,639 (24.6) | 14,661 (24.8) | 978 (21.9) | — |
| College graduate or higher ( y) | 19,319 (30.4) | 18,232 (30.8) | 1,087 (24.3) | — |
| Unknown | 7,230 | 6,736 | 494 | — |
| Insurance type [n (%)] | — | — | — | |
| Health insurance | 41,422 (60.5) | 38,794 (60.9) | 2,628 (55.8) | — |
| Self-pay | 27,016 (39.5) | 24,938 (39.1) | 2,078 (44.2) | — |
| Unknown | 2,380 | 2,121 | 259 | — |
| Child sex [n (%)] | — | — | — | |
| Male | 37,826 (53.5) | 34,963 (53.2) | 2,863 (57.9) | — |
| Female | 32,872 (46.5) | 30,789 (46.8) | 2,083 (42.1) | — |
| Unknown | 120 | 101 | 19 | — |
| Parity [n (%)] | — | — | — | |
| 0 | 39,726 (56.2) | 37,148 (56.6) | 2,578 (52.2) | — |
| 30,900 (43.8) | 28,538 (43.4) | 2,362 (47.8) | — | |
| Unknown | 192 | 167 | 25 | — |
| GDP per capita [n (%)] | — | — | — | |
| Q1 ( CNY) | 12,729 (18.0) | 11,874 (18.0) | 855 (17.2) | — |
| Q2 ( CNY) | 19,826 (28.0) | 18,242 (27.7) | 1,584 (31.9) | — |
| Q3 ( CNY) | 16,837 (23.8) | 15,654 (23.8) | 1,183 (23.8) | — |
| Q4 () | 21,426 (30.3) | 20,083 (30.5) | 1,343 (27.0) | — |
| Geographic region [n (%)] | — | — | — | — |
| North | 19,927 (28.1) | 18,823 (28.6) | 1,104 (22.2) | |
| East | 23,640 (33.4) | 22,120 (33.6) | 1,520 (30.6) | — |
| South | 13,192 (18.6) | 12,152 (18.5) | 1,040 (21.0) | — |
| West | 14,059 (19.9) | 12,758 (19.4) | 1,301 (26.2) | — |
| Conception season [n (%)] | — | — | — | 0.054 |
| Spring | 15,216 (21.5) | 14,128 (21.5) | 1,088 (21.9) | — |
| Summer | 20,578 (29.1) | 19,167 (29.1) | 1,411 (28.4) | — |
| Autumn | 16,712 (23.6) | 15,475 (23.5) | 1,237 (24.9) | — |
| Winter | 18,312 (25.9) | 17,083 (25.9) | 1,229 (24.8) | — |
| Spontaneous PTB [n (%)] | 2,454 (3.47) | — | — | — |
| PPROM [n (%)] | 1,358 (1.92) | — | — | — |
| Iatrogenic PTB [n (%)] | 1,153 (1.63) | — | — | — |
Note: Term birth: gestational age wk. Preterm birth: gestational age wk. Unknown refer to the missing value of each variable, which was included in all analyses as a missing category. Differences between groups were analyzed using the chi-square test. -Value was derived from the chi-squared test and represents a significant difference between preterm and term births. —, no data; CNY, Chinese yuan; GDP, gross domestic product; PPROM, preterm premature rupture of the fetal membranes; PTB, preterm birth.
The basic exposure characteristics of average temperature and air pollution by outcome and time windows are shown in Table S2. The average exposure temperature during pregnancy was 15.9°C, and the average temperature in different trimesters was similar. The average concentrations in the whole pregnancy and different trimesters were , which was much higher than the World Health Organization (WHO) standard of (24 h) for . On the other hand, the median level was slightly lower than of the WHO standard for (peak season). Average temperature, , and levels were similar in the preterm and nonpreterm groups during the 1 and 2 wk before delivery.
The temperature distribution by the hospitals and geographic regions is shown in Table S3. Overall, our study population covered most parts of China (Figure S1), and the variation in average temperature was large. The average temperature ranged from 3°C to 26.1°C for the whole pregnancy, 1.3°C–26.1°C for the first trimester, for second trimester, for third trimester, and for 1 and 2 wk before delivery, respectively. The characteristics of extreme climate indices are shown in Table S4. Extreme cold indexes such as fifth-days, fifth-2D, 10th-days, 10th-2D, 10th-3D, and extreme heat indexes such as 95th-2D were significantly higher in the PTB women for 1 and 2 wk before delivery ().
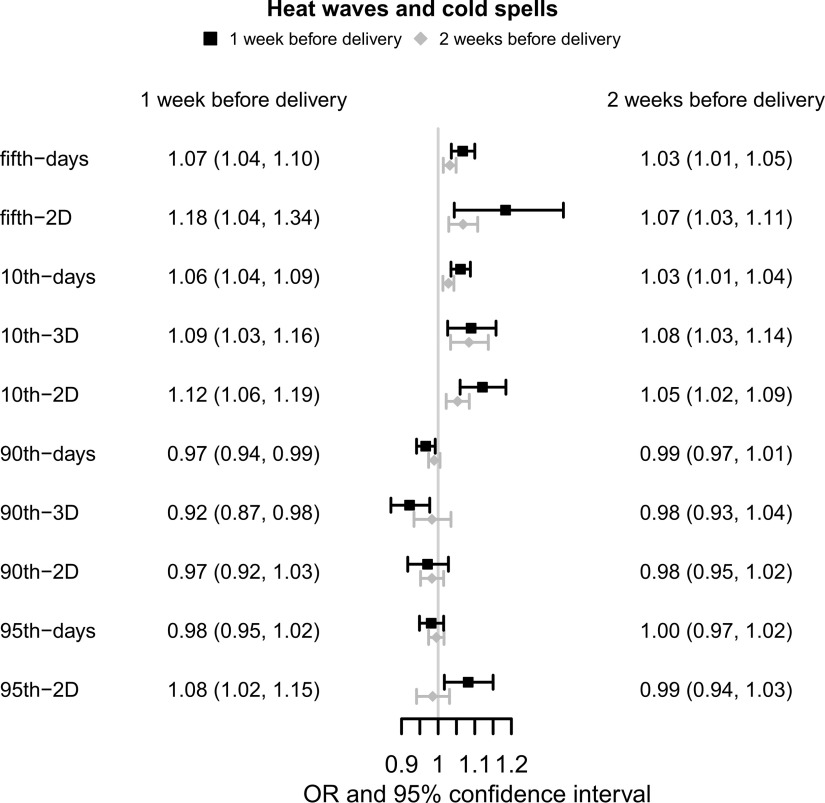
The associations between prenatal extreme temperature and the risk of PTB are illustrated in Figure 1. Cold spell was significantly associated with an increased risk of PTB after adjustment for covariates. For fifth-days and 10th-days during 1 wk before delivery, the number of days when the temperature was below the fifth percentile and 10th percentile was positively associated with PTB, with (95% CI: 1.04, 1.10) and 1.06 (95% CI: 1.04, 1.09), respectively. For fifth-2D, 10th-3D, and 10th-2D during 1 wk before delivery, the number of cold spell events was associated with increased PTB risk, with (95% CI: 1.04, 1.34), 1.09 (95% CI: 1.03, 1.16) and 1.12 (95% CI: 1.06, 1.19), respectively. Similarly, exposure to cold spell events 2 wk before delivery was also significantly associated with an increased risk of PTB, but the association intensity appeared smaller than that of exposure 1 wk before delivery. The association of heat waves with PTB was unstable. The 95th-2D had a positive association with PTB whereas 90th-days and 90th-3D showed inverse associations. Associations of 90th-2D and 95th-days with PTB during 1 and 2 wk before delivery were nonsignificant.
Figure 1.
Associations of extreme climate events with the preterm birth in China Labor and Delivery Survey (). Note: Generalized linear mixed model was used to examine the associations. ORs represent a 1-unit increase in extreme weather events and are presented with a 95% CI. All models were adjusted for maternal age, maternal ethnicity, maternal education, insurance type, child sex, parity, GDP per capita, geographic region, conception season, conception year, , and . CI, confidence interval; GDP, gross domestic product; OR, odds ratio; , particulate matter with aerodynamic diameter .
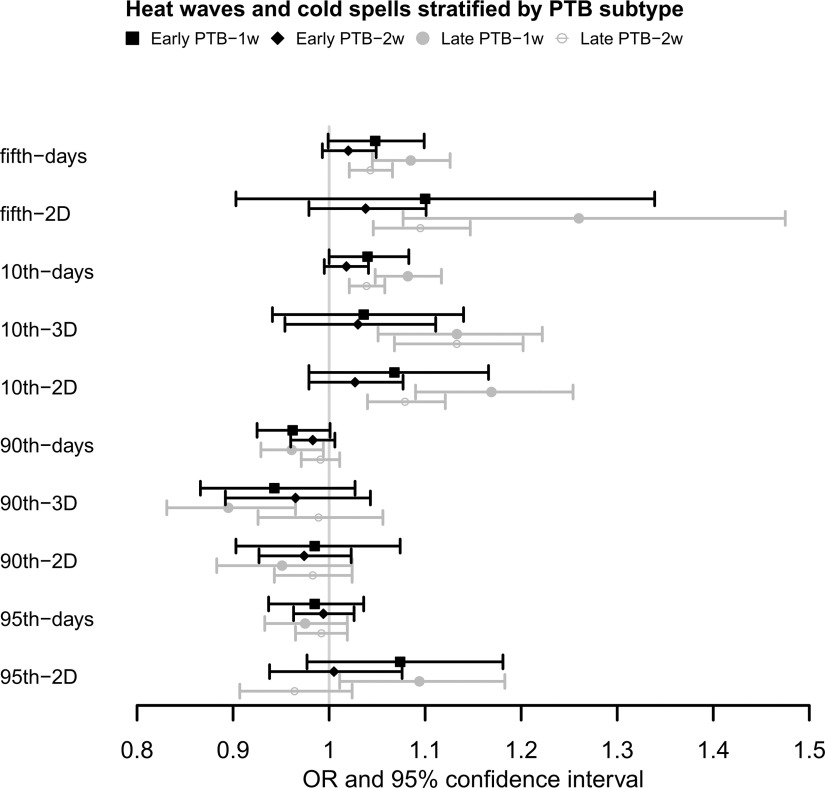
Associations of extreme temperature with PTB subtypes are shown in Figure 2. The impact of a cold spell on late PTB tended to be stronger than early PTB during 1 wk before delivery, with (95% CI: 1.05, 1.13) and 1.08 (95% CI: 1.05, 1.12) for fifth-days and 10th-days, and 1.26 (95% CI: 1.08, 1.48), 1.13 (95% CI: 1.05, 1.22), and 1.17 (95% CI: 1.09, 1.25) for fifth-2D, 10th-3D, and 10th-2D, respectively (See Table S5 for the corresponding numeric data). The pattern of the associations for 2 wk before delivery was similar, but the association intensity appeared smaller. In early PTB, only 10th-days were found to be positively associated with early PTB, with (95% CI: 1.00, 1.08) for 1 wk before delivery. The associations of extreme heat exposure with PTB subtypes were inconsistent. The 95th-2D exposure during 1 wk before delivery had a positive impact on late PTB, but 90th-days and 90th-3D showed inverse associations.
Figure 2.
Associations of extreme climate events with the early and late preterm births in 1 and 2 wk before delivery in China Labor and Delivery Survey. (See Table S5 for corresponding numeric data). Note: Generalized linear mixed model was used to examine the associations. ORs represent a 1-unit increase in extreme weather events and are presented with a 95% CI. All models were adjusted for maternal age, maternal ethnicity, maternal education, insurance type, child sex, parity, GDP per capita, geographic region, conception season, conception year, , and . CI, confidence interval; GDP, gross domestic product; OR, odds ratio; , particulate matter with aerodynamic diameter .
The associations of extreme temperature with PTB subtypes are shown in Table 2. Cold spell was positively associated with spontaneous PTB and PPROM but had no association with iatrogenic PTB. Fifth-days and 10th-days were associated with increased risks of spontaneous PTB, with (95% CI: 1.03, 1.12) and 1.06 (95% CI: 1.03, 1.10), and increased PPROM risk, with (95% CI: 1.04, 1.16) and 1.10 (95% CI: 1.05, 1.15), respectively. Cold spell event in 10th-3D and 10th-2D was associated with increased spontaneous PTB risks, with (95% CI: 1.06, 1.24) and 1.13 (95% CI: 1.05, 1.22), respectively. Fifth-2D and 10th-2D were also positively associated with PPROM, with (95% CI: 1.12, 1.75) and 1.16 (95% CI: 1.05, 1.29), respectively. Cold spells during 2 wk before delivery had a similar association with spontaneous PTB and PPROM and no significant association was found with iatrogenic PTB. A similar difference in the associations of cold spells on early and late PTB was found for different PTB subtypes (Table S6). The cold spell could increase the risk of late spontaneous PTB but had a stronger association with late PPROM. A 95th-2D exposure during 1 wk before delivery could increase the risk of spontaneous PTB, with (95% CI: 1.06, 1.26), but 90th-days and 90th-3D during 1 and 2 wk before delivery showed inverse associations with PPROM. The associations of 90th-2D during 1 wk before delivery, 95th-2D during 2 wk before delivery, and 95th-days during 1 and 2 wk before delivery with PTB were not significant.
Table 2.
Associations of extreme climate events with preterm birth, stratified by clinical subtypes in China Labor and Delivery Survey ().
| Variables | Spontaneous PTB [OR (95% CI)] |
PPROM [OR (95% CI)] |
Iatrogenic PTB [OR (95% CI)] |
|---|---|---|---|
| 1 wk before delivery | |||
| 5th-days | 1.07 (1.03, 1.12) | 1.10 (1.04, 1.16) | 1.02 (0.95, 1.08) |
| 5th-2D | 1.13 (0.95, 1.35) | 1.40 (1.12, 1.75) | 1.04 (0.80, 1.36) |
| 10th-days | 1.06 (1.03, 1.10) | 1.10 (1.05, 1.15) | 1.02 (0.96, 1.07) |
| 10th-3D | 1.15 (1.06, 1.24) | 1.10 (0.98, 1.23) | 0.94 (0.82, 1.08) |
| 10th-2D | 1.13 (1.05, 1.22) | 1.16 (1.05, 1.29) | 1.06 (0.94, 1.19) |
| 90th-days | 0.99 (0.95, 1.03) | 0.92 (0.87, 0.96) | 0.96 (0.91, 1.01) |
| 90th-3D | 0.94 (0.87, 1.02) | 0.86 (0.77, 0.96) | 0.94 (0.84, 1.05) |
| 90th-2D | 1.00 (0.92, 1.08) | 0.90 (0.81, 1.01) | 0.98 (0.87, 1.10) |
| 95th-days | 1.00 (0.95, 1.04) | 0.97 (0.91, 1.03) | 0.96 (0.90, 1.03) |
| 95th-2D | 1.16 (1.06, 1.26) | 1.07 (0.95, 1.20) | 0.94 (0.83, 1.08) |
| 2 wk before delivery | |||
| 5th-days | 1.04 (1.01, 1.06) | 1.05 (1.02, 1.08) | 1.00 (0.97, 1.04) |
| 5th-2D | 1.08 (1.02, 1.13) | 1.09 (1.02, 1.17) | 1.02 (0.94, 1.11) |
| 10th-days | 1.03 (1.01, 1.05) | 1.05 (1.02, 1.08) | 1.00 (0.97, 1.03) |
| 10th-3D | 1.09 (1.02, 1.16) | 1.17 (1.07, 1.28) | 0.99 (0.89, 1.10) |
| 10th-2D | 1.06 (1.02, 1.10) | 1.09 (1.03, 1.15) | 1.01 (0.95, 1.08) |
| 90th-days | 1.00 (0.98, 1.03) | 0.96 (0.93, 0.98) | 0.99 (0.96, 1.03) |
| 90th-3D | 1.02 (0.95, 1.10) | 0.88 (0.80, 0.98) | 1.02 (0.92, 1.12) |
| 90th-2D | 1.01 (0.96, 1.05) | 0.93 (0.87, 0.99) | 0.99 (0.92, 1.05) |
| 95th-days | 1.01 (0.99, 1.04) | 0.97 (0.93, 1.01) | 0.98 (0.94, 1.03) |
| 95th-2D | 1.03 (0.96, 1.09) | 0.94 (0.86, 1.03) | 0.95 (0.86, 1.04) |
Note: Generalized linear mixed models were used to examine the associations. All models were adjusted for maternal age, maternal ethnicity, maternal education, insurance type, child sex, parity, GDP per capita, geographic region, conception season, conception year, , and . Fifth-days, 10th-days, 95th-days, and 90th-days represent the total number of days when the daily mean temperature was lower than the 5th or the 10th percentile or higher than the 95th or the 90th percentile, respectively. Fifth-2D, 10th-3D, 10th-2D, 95th-2D, 90th-3D and 90th-2D represent the total number of extreme weather events. An extreme weather event can be defined as the daily mean temperature below the fifth percentile or higher than the 95th percentile for 2 consecutive days or the daily mean temperature below the 10th percentile or higher than the 90th percentile for 2 or 3 consecutive days. GDP, gross domestic product; , particulate matter with aerodynamic diameter ; PPROM, preterm premature rupture of the fetal membranes; PTB, preterm birth.
Stratified analyses show that the impact of cold spells on PTB had substantial regional heterogeneity. Pregnant women in the West tended to be the most affected population by cold spells and heat waves, followed by people in the North (Figure 3 and Table S7). As a whole, both extreme cold and extreme hot might increase the risk of PTB in the West. However, for pregnant women in the northern regions, extreme cold significantly increased the risk of PTB, but heat waves had an inverse association. We also found that pregnancies with female fetuses tended to be at a higher risk than those with male fetuses for both cold spells and heat waves (Figure 3; Table S8). Almost all indicators of extreme cold and heat exposure could increase the risk of PTB in female children, but only the fifth-days, 10th-days, and 10th-2D during 1 wk before delivery increased the risk of PTB in male children.
Figure 3.
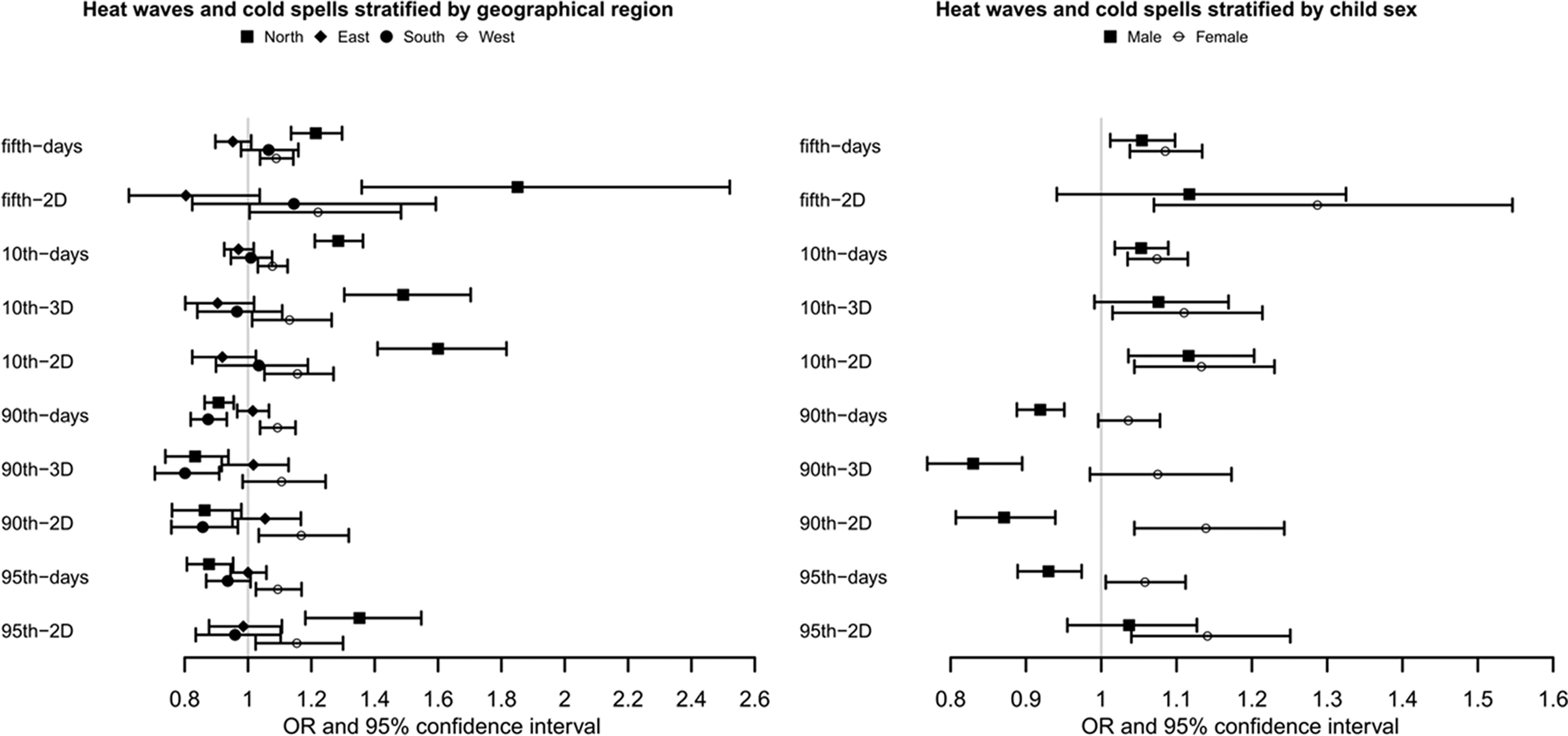
Associations of extreme climate events with preterm births in 1 wk before delivery in China Labor and Delivery Survey, stratified by geographical region and child sex. (See Tables S7 and S8 for corresponding numeric data.) Note: Generalized linear mixed model was used to examine the associations. Numbers of cases/observations in each stratum were: North (1,104/19,927), East (1,520/23,640), South (1,040/13,192), and West (1,301/14,059) for the geographical region and male (2,863/37,826), and female (2,083/32,872) for child sex. ORs represent a 1-unit increase in extreme weather events and are presented with a 95% CI. All models were adjusted for maternal age, maternal ethnicity, maternal education, insurance type, parity, GDP per capita, geographic region, conception season/child sex, conception year, , and . CI, confidence interval; GDP, gross domestic product; OR, odds ratio; , particulate matter with aerodynamic diameter .
We fitted the exposure–response relationships between the daily mean temperature and PTB risk during the entire pregnancy using Cox proportional hazard model (see Figure 4; Table S9). Overall, there was an inverted J-shaped relationship between ambient temperature and the risk of PTB. The exposure–response relationship was nonlinear (). Both relatively low and high temperatures may increase the PTB risk, and the association of low-temperature exposure was more pronounced. The exposure–response relationship by trimester shows that the association of extreme temperature with PTB was mainly manifested in the third trimester (Figure S4). Despite the significance of the nonlinear relationship test during first and second trimesters, the exposure–response relationship appears to be unstable and biologically less plausible.
Figure 4.
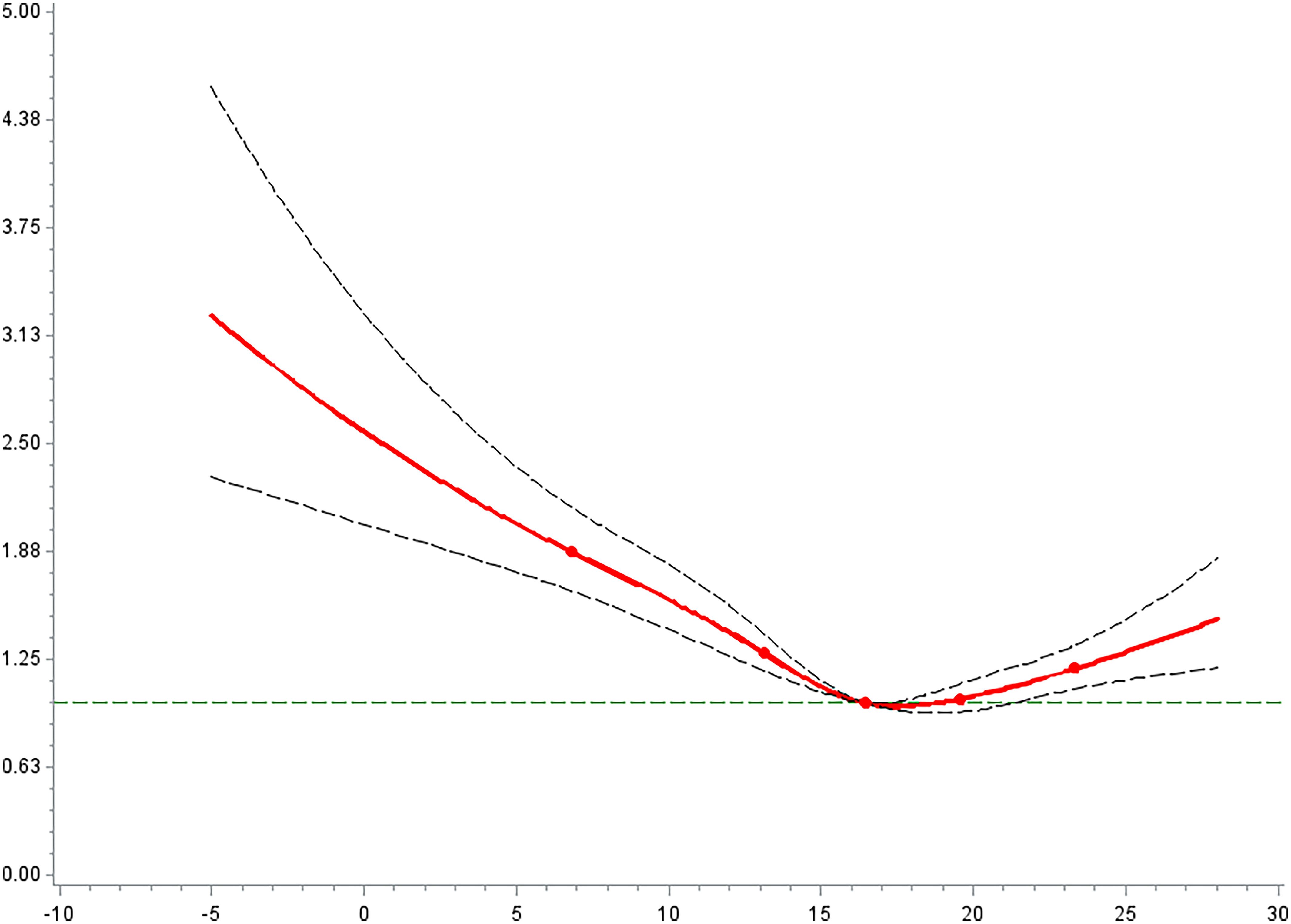
The relationship between the average temperature exposed during the whole pregnancy and the preterm birth risk in China Labor and Delivery Survey (). (See Tables S9 for corresponding numeric data.) Note: Cox proportional hazard regression incorporated with penalized cubic spline was used to examine exposure-response associations. HRs represent a 1-unit increase in extreme weather events and are presented with a 95% CI. All models were adjusted for maternal age, maternal ethnicity, maternal education, insurance type, child sex, parity, GDP per capita, geographic region, conception season, conception year, , and . CI, confidence interval; GDO, gross domestic product; HR, hazard ratio; , particulate matter with aerodynamic diameter .
In the sensitivity analyses, we used apparent temperature and daily maximal and minimal temperature to define extreme temperature events. The results remained the same (Table S10). When extending the exposure window to 3 and 4 wk before delivery, the results showed that extreme cold exposure still significantly increased the risk of PTB, although the association was attenuated compared with that during 1 and 2 wk before delivery (Table S11). The associations of the cold spell were more pronounced for late PTB. When restricting the study period to the cold and warm seasons, we found a similar pattern, with cold spells having a stronger impact (Table S12). The association tended to be stronger for late PTB. But the impact of heat waves during the 2 wk before delivery on PTB was statistically significant.
Tables S13 and S14 show the associations of extreme temperature exposure with PTB and PTB subtypes after excluding cases with a history of PTB and cases with severe maternal disease. Overall, the results were highly stable, showing that cold spells significantly increased the risk of PTB, whereas the associations of heat exposure with PTB tended to be null. These associations differed between early and late PTB. The results of the association analysis with the additional inclusion of background temperature were consistent with the results in Figure 1, Table 2, and Table S4, indicating that the results without controlling background temperature were robust (Table S15). The results with multiple imputations for missing values were consistent with those in which the missing information was treated as a separate category (Table S16). Similarly, the extremely cold exposure imposed a higher risk for PTB, especially for late PTB.
Discussion
In this study, we estimated the associations of extreme temperature exposure with PTB and PTB subtypes at different time windows by analyzing nationwide survey data in China. These results add to the growing literature linking climate change and PTB. We found that exposure to extreme cold temperatures during pregnancy increased the PTB risk, particularly for late PTB, spontaneous PTB, and PPROM. Further, the associations were more pronounced in the western and northern regions, and women with female fetuses.
An increasing number of studies have linked extreme temperature with PTB. A national retrospective study in mainland China found that gestational exposure to relatively low or high temperatures could significantly increase the PTB risk, but the association with heat waves was more prominent.43 The association with high temperature was mainly concentrated in the first and second trimesters, whereas the association with low temperature was in the third trimester. This finding partly supports the results of our study, especially the association with low temperature, but that study focused on the association of long-term temperature exposure with PTB. Considering the adaptability of the human body to temperature change, our study focused on the associations of extreme temperature exposure with PTB and further shortened the exposure period from the third trimester to 1–4 wk before delivery, which is helpful to discover the sensitivity window. A retrospective cohort study in Guangzhou, China, found that the relationship between temperature and PTB was U-shaped or inverted J-shaped, which is consistent with our results.13 In comparison with single-city studies, we included delivery data from 24 provinces with different temperature ranges across the country, and the results are more representative and generalizable. Similarly, a multicenter birth cohort of the U.S. Consortium on Safe Labor (U.S. CSL) found that cold temperature during gestational weeks 1–7 may increase the early and late PTB risks.12
However, some studies failed to show any association between extreme cold temperatures with PTB. For example, a study in Israel found that gestational high-temperature exposure was associated with a higher risk of PTB and early PTB, but the association of low temperature with PTB was not statistically significant.50 Geographical differences may explain the inconsistency of the findings. Israel has a typical Mediterranean climate, characterized by long, hot, drier summers and relatively short, cool, rainy winters, making the country vulnerable to heat waves.51 A retrospective study including U.S. singleton births found that cold temperature had a negative association with PTB.17 Widespread use of heating might be an important confounding factor. Thus, more evidence is needed.
Previous studies paid more attention to the heat effects in the context of global warming. Studies in Guangzhou (China), Brisbane (Australia), and Alabama (United States) found that heat waves and high temperatures could significantly increase the PTB risk.10,45,52 A recent systematic review and meta-analysis showed that 40 of 47 studies on PTB demonstrated a significant association between high temperature and PTB, despite considerable heterogeneity in the magnitude of the associations.6 A U-shaped association was observed when using temperature as a continuous exposure metric, which can be supported by most previous studies.13,53 But the associations of heat waves were inconsistent. This heterogeneity may be attributed to the usage of heating and air-conditioning, climatic zones, and socioeconomic status. We assume that people may prefer to stay indoors and use indoor air conditioning more frequently during heat waves, and housing factors could affect heat vulnerability and heat-related illness, which partly explains why we found a positive association of high temperatures with PTB, but this effect size was attenuated.54,55 One study in Shenzhen, China, reported an apparent negative association of heat exposure with PTB, which was considered to be related to the high air conditioning use in the study area.56 Shenzhen is the most economically developed city in southern China. Similarly, our study also found that the association of the heat waves with PTB was weaker, or even became protective in southern and eastern China because these two regions have better economic conditions and well-established infrastructure. Another similar study in northern California also noted that the effect sizes of heat exposure were the smallest in the areas that had the highest use of air conditioning.57 All these lend credence to our conjecture that the use of air conditioning might distort the associations between high temperatures and PTB.
The exposure window and extreme temperature indices used may be other sources of heterogeneity on heat effect across studies. Most previous studies focused on an acute exposure period in the week prior to delivery, particularly the last 0–3 d, and the findings could support the short-term effects shown in this study.6 We found a stronger association using the 95th rather than the 90th as the cutoff for the heat-wave definition, which was consistent with a study in Guangzhou, suggesting that a higher cutoff temperature may be more sensitive.13 Besides, green space and urbanization levels also had significant interactions with associations of heat waves and air pollution with PTB.58 Race/ethnicity in different studies may be another source for discrepant results. Four studies in the United States indicated that Black and Hispanic women had higher effect sizes than other races.59–62
The precise mechanisms by which extreme temperature exposure may influence the PTB risk remain unclear. Maternal physiological changes may alter the thermoregulation efficiency of pregnant women. The increasing body surface area of pregnant women may increase the ratio of body surface area to body weight, which may reduce pregnant women’s capacity for heat loss and increase the contact area of temperature exposure.17 A few studies suggested that exposure to low temperature may lead to increased blood viscosity, peripheral vasoconstriction, and hypertension, which might alter uteroplacental perfusion and adversely affect the developing fetus and, thus, induce PTB.63 The low-temperature living environment might also bring about a series of unhealthy behaviors and lifestyles, such as indoor passive smoking, lack of exercise, and respiratory pathogen infections, which could further exacerbate PTB.64,65
In contrast, heat exposure may disturb hormone homeostasis of the hypothalamic-pituitary-adrenal axis and increase the release of cortisol and adrenocorticotropic hormone. These hormones may activate myometrial function and increase uterine contractions, potentially leading to an early delivery.66 In addition, dehydration caused by heat stress can reduce a pregnant woman’s body fluid and decrease uterine blood flow, induce uterine contraction, and, consequently, contribute to the onset of labor.60 In addition, heat stress may result in the release of cytokines such as prostaglandin, oxytocin, and heat-shock proteins, which may disrupt the delicate balance of cytokines at the maternal–fetal interface and then activate the parturition mechanism prematurely.67,68
The majority of previous studies on this topic did not separate the subtypes of PTB, which may have masked the underlying mechanisms of this association. We found that a cold spell may increase the risk of late PTB, mainly through PPROM and spontaneous PTB. A multicenter study in the United States also showed that both low and high temperatures could increase the risk of PROM.24 Another population-based study showed that high temperature was an independent risk factor for PPROM, and PPROM was more subject to ambient temperature fluctuations than was preterm labor.69 The human body can adapt to small environmental changes through proper thermoregulation, but PPROM may be triggered when the temperature changes exceed a certain threshold. Both animal and human studies have shown that high- and low-temperature exposures are associated with increased oxidative stress and systemic inflammation.70–72 Programmed cell death, activation of stress and inflammation-induced catabolic enzymes, maternal inflection, and/or mechanical forces may trigger the membrane rupture.73 Nonetheless, the pathogenesis of spontaneous PTB and PPROM remains poorly understood. Furthermore, we cannot exclude the potential impact of differences in sample size between PTB subgroups on the estimation of temperature effects. More research is warranted.74,75
Our stratified analysis revealed that associations of temperature with PTB might be sex dimorphic. Pregnant women with female fetuses appeared to be more sensitive, which was consistent with studies from the United States, Korea, and Israel.50,57,76 Several previous studies reported that exposure to prenatal stress such as extreme ambient temperature may lead to a decrease in the male-to-female live birth ratio, suggesting that pregnant women may tend to selectively terminate male fetuses that exhibit signs of weakness or vulnerability when experiencing significant stressors.77–79 Expressed X chromosome genes are more abundant in female placentas, which may explain the varied responses to stress in the maternal environment.80 The placenta of the female fetus may exhibit a higher sensitivity to maternal stress, which can be indicated by elevated levels of glucocorticoids in the mother.81
Assessing temperature-related health effects and identifying vulnerable populations and regions are necessary for the development of targeted policies to improve public health. In this study, we found that women living in northern and western China seem to be more susceptible to cold exposure. The population susceptibility in different regions is not only affected by genetic factors but also closely related to local climatic conditions and socioeconomic factors. A county-level modeling study across China characterized several key factors (such as health status, aging rate, agricultural population rate, education, ethnic structure, economic status, air conditioner ownership rate, and the number of hospitals) of heat vulnerability; each of the factors had different levels of importance to the heat vulnerability of the counties with different urbanization levels.82 The average temperature in northern China is low, and cold waves are frequent. Although some areas provide heating in winter, there are still some economically underdeveloped areas that use traditional coal-fired heating or no heating equipment, which makes this part of the population more vulnerable to extreme cold weather events.83,84 In addition, solid fuel combustion produces a large amount of volatile organic compounds, which will further affect maternal and fetal health.85 Furthermore, the economy in western China is less developed and the distribution of medical resources is uneven and limited, leading to inaccessible heating equipment and poor baseline health,86,87 which may reduce the adaptability of pregnant women to extreme temperatures.
Our study found that the association of temperature with PTB was mainly concentrated in the third trimester, which is supported by other studies.50,58 Although late PTB may be considered a less clinical problem, ample evidence has demonstrated in recent years that even late PTB may have a long-term impact on the child as well as adult health.88,89 The associations of extreme cold temperature in the third trimester, especially with late PTB, suggest that it may be an important time window for preventive intervention. Thus, our findings may have important public health implications.
The present study has several strengths. It was based on a nationwide survey and is so far one of the largest multicenter studies conducted in developing countries. Wide coverage and good representation can provide a good basis for assessing spatial heterogeneity. Identifying the association difference of extreme temperatures on different clinical subtypes of PTB makes this study unique. The impact of extreme temperature at different time scales during pregnancy on PTB and the modification of fetal sex and geographic area were also considered. In addition, we analyzed the associations using a survival approach and GLMMs, which helps compare fetuses of the same gestational age, avoid biases caused by different probabilities of giving birth at different gestational ages, and address the internal correlation of multicenter data.30,90
Still, our study has some limitations. First, the usage of air conditioning and heating may mitigate the associations of extreme temperature exposure with PTB. Previous studies have shown that pregnant women spend most of their time indoors (more than an average of 15 h/day), especially during the late stage of pregnancy, and the time–activity patterns might affect the actual exposure level.91–93 Thus, using ambient temperature as a proxy may be biased. Unfortunately, we did not have information on the time length of staying indoors and outdoors for pregnant women. Hence, our findings may be an underestimation of the association of extreme temperature exposure with PTB. Second, although we assumed that most Chinese pregnant women chose the nearest hospital for childbirth, measurement errors may arise when assigning temperature based on the study hospital location. However, our previous study based on two representative birth cohorts with detailed home addresses showed that 75% of pregnant women lived within of the delivery hospital.30,94 A buffer analysis for exposure assessment at different radii showed that spatial variability had no significant association with the association between air pollutants and gestational diabetes based on hospital locations. The spatial variability of ambient temperature is actually smaller when compared with air pollutants, and this study included a larger sample. Thus, the bias introduced by exposure assessment based on hospital address is likely to be limited.95,96 Therefore, we would assume that using hospital location as a proxy for home address may be reasonable, and the spatial variability may not significantly impact the association in this study.
Third, errors in the gestational age estimation may have influenced the incidence of PTB and led to inaccurate effect estimates. Because the estimation of gestational age was unlikely to be influenced by extreme temperature events, the error in gestational age was mostly nondifferential, which may have drawn the results toward the null. Fourth, the influence of live birth bias on the associations cannot be ruled out. Previous studies have found that heat waves might cause stillbirths.9 Environmental exposure causing miscarriage or stillbirth might lead to a downward bias of the exposure–preterm birth association, or even an inverse association.97,98 It is also possible that cold spell exposure induced miscarriage or stillbirth in low gestational age fetuses, resulting in unobserved outcomes. Thus, the effects of the heat waves on PTB and the effects of cold spells on early PTB in this study might have been underestimated and need further evaluation. Finally, our study is a retrospective cohort study, and fixed cohort bias may be a potential issue. However, unlike a traditional cohort study, we randomly selected our study population in each participating hospital for 6–10 wk each year; i.e., our cohort had multiple starting and ending time points in each hospital. The missed population could be distributed at any time in a year. Thus, the potential seasonal bias, if any, was spread across a year, and its impact on the effect estimation would likely be limited.
Conclusions
Our study found that a cold spell during pregnancy was associated with an increased risk of PTB, particularly spontaneous PTB, and PPROM. The associations of cold spells tended to be more prominent in late pregnancy, in the North and West of China, and in pregnancies with female fetuses. Therefore, pregnant women living in a cold climate and having a female fetus in late gestation may be particularly vulnerable to PTB when exposed to the cold spell. This information has important public health implications in PTB prevention. Pregnant women should be more aware of the potential adverse effects of extreme temperatures and try their best to protect themselves from exposure to extreme temperatures to prevent PTB. Early temperature warning and surveillance systems should be developed and tailored according to the local climate to provide a guide to clinicians and other health care providers on how to counsel women and benefit the prevention of extreme temperature-related disease. Meanwhile, local governments could consider improving building heating or cooling systems, especially for low-income senior residents and vulnerable populations, e.g., pregnant women.
Supplementary Material
Acknowledgments
G-Q.Y., L.Y., and M.L. did the background research, analyzed and interpreted the data, and drafted the manuscript. G-Q.Y., C-P.W., and X-L.S. helped collect and matched the data and take responsibility for the integrity and accuracy of the climate data. J.Z. is the designer of the CLDS project, and J.Z. and L-C.F. carefully revised the manuscript and had full access to all the delivery data in the study.
This work was supported by the Shanghai Municipal Health Commission (2021CXJQ01), the Shanghai Municipal Science and Technology Commission (21410713500), and the Hainan Provincial Medical Center Program.
The funders had no role in the study design, data collection and analysis, publication decisions, or manuscript preparation.
References
- 1.Crump C. 2020. Preterm birth and mortality in adulthood: a systematic review. J Perinatol 40(6):833–843, PMID: , 10.1038/s41372-019-0563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urs R, Kotecha S, Hall GL, Simpson SJ. 2018. Persistent and progressive long-term lung disease in survivors of preterm birth. Paediatr Respir Rev 28:87–94, PMID: , 10.1016/j.prrv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Luu TM, Rehman Mian MO, Nuyt AM. 2017. Long-Term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin Perinatol 44(2):305–314, PMID: , 10.1016/j.clp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, et al. 2019. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 7(1):e37–e46, PMID: , 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips C, Velji Z, Hanly C, Metcalfe A. 2017. Risk of recurrent spontaneous preterm birth: a systematic review and meta-analysis. BMJ Open 7(6):e015402, PMID: , 10.1136/bmjopen-2016-015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chersich MF, Pham MD, Areal A, Haghighi MM, Manyuchi A, Swift CP, et al. 2020. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ 371:m3811, PMID: , 10.1136/bmj.m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porpora MG, Piacenti I, Scaramuzzino S, Masciullo L, Rech F, Benedetti Panici P. 2019. Environmental contaminants exposure and preterm birth: a systematic review. Toxics 7(1):11, PMID: , 10.3390/toxics7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Beagley J, Belesova K, et al. 2021. The 2020 report of the lancet countdown on health and climate change: responding to converging crises. Lancet 397(10269):129–170, PMID: , 10.1016/S0140-6736(20)32290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekkar B, Pacheco S, Basu R, DeNicola N. 2020. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open 3(6):e208243, PMID: , 10.1001/jamanetworkopen.2020.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent ST, McClure LA, Zaitchik BF, Smith TT, Gohlke JM. 2014. Heat waves and health outcomes in Alabama (USA): the importance of heat wave definition. Environ Health Perspect 122(2):151–158. Feb, PMID: , 10.1289/ehp.1307262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward A, Clark J, McLeod J, Woodul R, Moser H, Konrad C. 2019. The impact of heat exposure on reduced gestational age in pregnant women in North Carolina, 2011–2015. Int J Biometeorol 63(12):1611–1620, PMID: , 10.1007/s00484-019-01773-3. [DOI] [PubMed] [Google Scholar]
- 12.Ha S, Liu D, Zhu Y, Kim SS, Sherman S, Mendola P. 2017. Ambient temperature and early delivery of singleton pregnancies. Environ Health Perspect 125(3):453–459, PMID: , 10.1289/EHP97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J-R, Liu Y, Xia X-Y, Ma W-J, Lin H-L, Kan H-D, et al. 2016. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001–2011). Environ Health Perspect 124(7):1100–1106, PMID: , 10.1289/ehp.1509778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang Z, Wang P, Zhao Q, Wang B-Q, Ma Y, Lin H, et al. 2018. Effect of the 2008 cold spell on preterm births in two subtropical cities of Guangdong Province, Southern China. Sci Total Environ 642:307–313, PMID: , 10.1016/j.scitotenv.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Wolf J, Armstrong B. 2012. The association of season and temperature with adverse pregnancy outcome in two German states, a time-series analysis. PLoS One 7(7):e40228, PMID: , 10.1371/journal.pone.0040228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo T, Wang Y, Zhang H, Zhang Y, Zhao J, Wang Y, et al. 2018. The association between ambient temperature and the risk of preterm birth in China. Sci Total Environ 613-614:439–446, PMID: , 10.1016/j.scitotenv.2017.09.104. [DOI] [PubMed] [Google Scholar]
- 17.Sun S, Weinberger KR, Spangler KR, Eliot MN, Braun JM, Wellenius GA. 2019. Ambient temperature and preterm birth: a retrospective study of 32 million US singleton births. Environ Int 126:7–13, PMID: , 10.1016/j.envint.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen SR, Kaczmarek M, Hundy R, Lal A. 2020. Comparison of heat-illness associations estimated with different temperature metrics in the Australian Capital Territory, 2006–2016. Int J Biometeorol 64(12):1985–1994, PMID: , 10.1007/s00484-020-01899-9. [DOI] [PubMed] [Google Scholar]
- 19.Dehaene I, Scheire E, Steen J, De Coen K, Decruyenaere J, Smets K, et al. 2020. Obstetrical characteristics and neonatal outcome according to aetiology of preterm birth: a cohort study. Arch Gynecol Obstet 302(4):861–871, PMID: , 10.1007/s00404-020-05673-5. [DOI] [PubMed] [Google Scholar]
- 20.Aung MT, Ferguson KK, Cantonwine DE, McElrath TF, Meeker JD. 2019. Preterm birth in relation to the bisphenol A replacement, bisphenol S, and other phenols and parabens. Environ Res 169:131–138, PMID: , 10.1016/j.envres.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, et al. 2019. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ Int 132:105099, PMID: , 10.1016/j.envint.2019.105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Chen D, Wang B, Xu F, Pang Y, Zhang L, et al. 2020. Does low maternal exposure to per- and polyfluoroalkyl substances elevate the risk of spontaneous preterm birth? A nested case-control study in China. Environ Sci Technol 54(13):8259–8268, PMID: , 10.1021/acs.est.0c01930. [DOI] [PubMed] [Google Scholar]
- 23.Ocviyanti D, Wahono WT. 2018. Risk factors for neonatal sepsis in pregnant women with premature rupture of the membrane. J Pregnancy 2018:4823404, PMID: , 10.1155/2018/4823404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha S, Liu D, Zhu Y, Sherman S, Mendola P. 2018. Acute associations between outdoor temperature and premature rupture of membranes. Epidemiology 29(2):175–182, PMID: , 10.1097/EDE.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spirtes P, Zhang K. 2016. Causal discovery and inference: concepts and recent methodological advances. Appl Inform (Berl) 3:3, PMID: , 10.1186/s40535-016-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderWeele TJ, Jackson JW, Li S. 2016. Causal inference and longitudinal data: a case study of religion and mental health. Soc Psychiatry Psychiatr Epidemiol 51(11):1457–1466, PMID: , 10.1007/s00127-016-1281-9. [DOI] [PubMed] [Google Scholar]
- 27.Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. 2013. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and newborn Health): a cross-sectional study. Lancet 381(9879):1747–1755, PMID: , 10.1016/S0140-6736(13)60686-8. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Zhang JW, Xia HW, Zhang HX, Betran AP, Zhang L, et al. 2019. Preterm birth in China between 2015 and 2016. Am J Public Health 109(11):1597–1604. Nov, PMID: , 10.2105/AJPH.2019.305287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.China National Health Statistics Bureau. China Health Statistics Yearbook 2015–2017. http://www.nhc.gov.cn/mohwsbwstjxxzx/tjzxtjcbw/tjsj_list.shtml?R0NMKk6uozOC=1689388328319 [accessed 13 December 2019].
- 30.Yu G, Ao J, Cai J, Luo Z, Martin R, Donkelaar AV, et al. 2020. Fine particular matter and its constituents in air pollution and gestational diabetes mellitus. Environ Int 142:105880. Sep, PMID: , 10.1016/j.envint.2020.105880. [DOI] [PubMed] [Google Scholar]
- 31.Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GDV, et al. 2009. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med 6(5):e1000061, PMID: , 10.1371/journal.pmed.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Ye R, Zhang L, Li H, Liu J, Ren A. 2014. Periconceptional folic acid supplementation and the risk of preterm births in China: a large prospective cohort study. Int J Epidemiol 43(4):1132–1139, PMID: , 10.1093/ije/dyu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Y, Gasparrini A, Armstrong BG, Tawatsupa B, Tobias A, Lavigne E, et al. 2017. Heat wave and mortality: a multicountry, multicommunity study. Environ Health Perspect 125(8):087006, PMID: , 10.1289/EHP1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morignat E, Gay E, Vinard JL, Sala C, Calavas D, Hénaux V. 2018. Impact of heat and cold waves on female cattle mortality beyond the effect of extreme temperatures. J Therm Biol 78:374–380, PMID: , 10.1016/j.jtherbio.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Kong L, Tang X, Zhu J, Wang Z, Li J, Wu H, et al. 2021. A 6-year-long (2013–2018) high-resolution air quality reanalysis dataset in China based on the assimilation of surface observations from CNEMC. Earth Syst Sci Data 13(2):529–570, 10.5194/essd-13-529-2021. [DOI] [Google Scholar]
- 36.Lin CQ, Liu G, Lau AKH, Li Y, Li CC, Fung JCH, et al. 2018. High-resolution satellite remote sensing of provincial PM2.5 trends in China from 2001 to 2015. Atmos Environ 180:110–116, 10.1016/j.atmosenv.2018.02.045. [DOI] [Google Scholar]
- 37.Liu J, Weng F, Li Z. 2019. Satellite-based PM2.5 estimation directly from reflectance at the top of the atmosphere using a machine learning algorithm. Atmos Environ 208:113–122, 10.1016/j.atmosenv.2019.04.002. [DOI] [Google Scholar]
- 38.Zhan Y, Luo Y, Deng X, Chen H, Grieneisen ML, Shen X, et al. 2017. Spatiotemporal prediction of continuous daily PM2.5 concentrations across China using a spatially explicit machine learning algorithm. Atmos Environ 155:129–139, 10.1016/j.atmosenv.2017.02.023. [DOI] [Google Scholar]
- 39.Inness A, Ades M, Agustí-Panareda A, Barré J, Benedictow A, Blechschmidt A-M, et al. 2019. The CAMS reanalysis of atmospheric composition. Atmos Chem Phys 19(6):3515–3556, 10.5194/acp-19-3515-2019. [DOI] [Google Scholar]
- 40.Zhou M, Astell-Burt T, Yin P, Feng X, Page A, Liu Y, et al. 2015. Spatiotemporal variation in diabetes mortality in China: multilevel evidence from 2006 and 2012. BMC Public Health 15:633, PMID: , 10.1186/s12889-015-1982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basagaña X, Michael Y, Lensky IM, Rubin L, Grotto I, Vadislavsky E, et al. 2021. Low and high ambient temperatures during pregnancy and birth weight among 624,940 singleton term births in Israel (2010–2014): an investigation of potential windows of susceptibility. Environ Health Perspect 129(10):107001, PMID: , 10.1289/EHP8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ’dagitty’. Int J Epidemiol 45(6):1887–1894, PMID: , 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y-Y, Li Q, Guo Y, Zhou H, Wang Q-M, Shen H-P, et al. 2020. Ambient temperature and the risk of preterm birth: a national birth cohort study in the mainland China. Environ Int 142:105851, PMID: , 10.1016/j.envint.2020.105851. [DOI] [PubMed] [Google Scholar]
- 44.Basu R, Sarovar V, Malig BJ. 2016. Association between high ambient temperature and risk of stillbirth in California. Am J Epidemiol 183(10):894–901, PMID: , 10.1093/aje/kwv295. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Li B, Benmarhnia T, Hajat S, Ren M, Liu T, et al. 2020. Independent and combined effects of heatwaves and PM2.5 on preterm birth in Guangzhou, China: a survival analysis. Environ Health Perspect 128(1):17006, PMID: , 10.1289/EHP5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson A, Chiu YM, Hsu HL, Wright RO, Wright RJ, Coull BA. 2017. Potential for bias when estimating critical windows for air pollution in children’s health. Am J Epidemiol 186(11):1281–1289, PMID: , 10.1093/aje/kwx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi W, Zhang X, Gao J, Wei Q, Pan R, Duan J, et al. 2019. Examining the association between apparent temperature and admissions for schizophrenia in Hefei, China, 2005–2014: a time-series analysis. Sci Total Environ 672:1–6, PMID: , 10.1016/j.scitotenv.2019.03.436. [DOI] [PubMed] [Google Scholar]
- 48.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Statist Sci 7(4):457–472, 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 49.Liu Y, De A. 2015. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res 4(3):287–295, PMID: , 10.6000/1929-6029.2015.04.03.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spolter F, Kloog I, Dorman M, Novack L, Erez O, Raz R. 2020. Prenatal exposure to ambient air temperature and risk of early delivery. Environ Int 142:105824, PMID: , 10.1016/j.envint.2020.105824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kranc H, Novack V, Shtein A, Sherman R, Novack L. 2021. Extreme temperature and out-of-hospital-cardiac-arrest. Nationwide study in a hot climate country. Environ Health 20(1):38, PMID: , 10.1186/s12940-021-00722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Williams G, Guo Y, Pan X, Tong S. 2013. Maternal exposure to heatwave and preterm birth in Brisbane, Australia. BJOG 120(13):1631–1641, PMID: , 10.1111/1471-0528.12397. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Chen G, Jaakkola JJK, Williams G, Guo Y. 2018. Temporal change in the impacts of ambient temperature on preterm birth and stillbirth: Brisbane, 1994–2013. Sci Total Environ 634:579–585, PMID: , 10.1016/j.scitotenv.2018.03.385. [DOI] [PubMed] [Google Scholar]
- 54.Samuelson H, Baniassadi A, Lin A, Izaga González P, Brawley T, Narula T. 2020. Housing as a critical determinant of heat vulnerability and health. Sci Total Environ 720:137296, PMID: , 10.1016/j.scitotenv.2020.137296. [DOI] [PubMed] [Google Scholar]
- 55.Williams AA, Spengler JD, Catalano P, Allen JG, Cedeno-Laurent JG. 2019. Building vulnerability in a changing climate: indoor temperature exposures and health outcomes in older adults living in public housing during an extreme heat event in Cambridge, MA. Int J Environ Res Public Health 16(13):2373, PMID: , 10.3390/ijerph16132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang Z, Lin Y, Ma Y, Zhang L, Zhang X, Li L, et al. 2016. The association between ambient temperature and preterm birth in Shenzhen, China: a distributed lag non-linear time series analysis. Environ Health 15(1):84, PMID: , 10.1186/s12940-016-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avalos LA, Chen H, Li DK, Basu R. 2017. The impact of high apparent temperature on spontaneous preterm delivery: a case-crossover study. Environ Health 16(1):5, PMID: , 10.1186/s12940-017-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y, Ilango SD, Schwarz L, Wang Q, Chen J-C, Lawrence JM, et al. 2020. Examining the joint effects of heatwaves, air pollution, and green space on the risk of preterm birth in California. Environ Res Lett 15(10):104099, PMID: , 10.1088/1748-9326/abb8a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmichael SL, Cullen MR, Mayo JA, Gould JB, Loftus P, Stevenson DK, et al. 2014. Population-level correlates of preterm delivery among black and white women in the U.S. PLoS One 9(4):e94153, PMID: , 10.1371/journal.pone.0094153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basu R, Malig B, Ostro B. 2010. High ambient temperature and the risk of preterm delivery. Am J Epidemiol 172(10):1108–1117, PMID: , 10.1093/aje/kwq170. [DOI] [PubMed] [Google Scholar]
- 61.Basu R, Chen H, Li DK, Avalos LA. 2017. The impact of maternal factors on the association between temperature and preterm delivery. Environ Res 154:109–114, PMID: , 10.1016/j.envres.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Porter KR, Thomas SD, Whitman S. 1999. The relation of gestation length to short-term heat stress. Am J Public Health 89(7):1090–1092, PMID: , 10.2105/ajph.89.7.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruckner TA, Modin B, Vågerö D. 2014. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915–1929. Ann Epidemiol 24(2):116–121, PMID: , 10.1016/j.annepidem.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Argacha JF, Bourdrel T, van de Borne P. 2018. Ecology of the cardiovascular system: a focus on air-related environmental factors. Trends Cardiovasc Med 28(2):112–126, PMID: , 10.1016/j.tcm.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Moriyama M, Hugentobler WJ, Iwasaki A. 2020. Seasonality of respiratory viral infections. Annu Rev Virol 7(1):83–101, PMID: , 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- 66.Wang LI, Liu F, Luo Y, Zhu L, Li G. 2015. Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats. Biomed Rep 3(3):425–429, PMID: , 10.3892/br.2015.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dadvand P, Basagaña X, Sartini C, Figueras F, Vrijheid M, de Nazelle A, et al. 2011. Climate extremes and the length of gestation. Environ Health Perspect 119(10):1449–1453, PMID: , 10.1289/ehp.1003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schifano P, Lallo A, Asta F, De Sario M, Davoli M, Michelozzi P. 2013. Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001–2010. Environ Int 61:77–87, PMID: , 10.1016/j.envint.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Gat R, Kachko E, Kloog I, Erez O, Yitshak-Sade M, Novack V, et al. 2021. Differences in environmental factors contributing to preterm labor and PPROM - Population based study. Environ Res 196:110894, PMID: , 10.1016/j.envres.2021.110894. [DOI] [PubMed] [Google Scholar]
- 70.Quindry J, Miller L, McGinnis G, Kliszczewiscz B, Slivka D, Dumke C, et al. 2013. Environmental temperature and exercise-induced blood oxidative stress. Int J Sport Nutr Exerc Metab 23(2):128–136, PMID: , 10.1123/ijsnem.23.2.128. [DOI] [PubMed] [Google Scholar]
- 71.Cheng C-H, Yang F-F, Liao S-A, Miao Y-T, Ye C-X, Wang A-L, et al. 2015. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J Therm Biol 53:172–179, PMID: , 10.1016/j.jtherbio.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 72.Hong Y-C, Kim H, Oh S-Y, Lim Y-H, Kim S-Y, Yoon H-J, et al. 2012. Association of cold ambient temperature and cardiovascular markers. Sci Total Environ 435-436:74–79, PMID: , 10.1016/j.scitotenv.2012.02.070. [DOI] [PubMed] [Google Scholar]
- 73.Lannon SM, Vanderhoeven JP, Eschenbach DA, Gravett MG, Adams Waldorf KM. 2014. Synergy and interactions among biological pathways leading to preterm premature rupture of membranes. Reprod Sci 21(10):1215–1227, PMID: , 10.1177/1933719114534535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Esplin MS. 2014. Overview of spontaneous preterm birth: a complex and multifactorial phenotype. Clin Obstet Gynecol 57(3):518–530, PMID: , 10.1097/GRF.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 75.Romero R, Dey SK, Fisher SJ. 2014. Preterm labor: one syndrome, many causes. Science 345(6198):760–765, PMID: , 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Son JY, Lee JT, Lane KJ, Bell ML. 2019. Impacts of high temperature on adverse birth outcomes in Seoul, Korea: disparities by individual- and community-level characteristics. Environ Res 168:460–466, PMID: , 10.1016/j.envres.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catalano R, Ahern J, Bruckner T, Anderson E, Saxton K. 2009. Gender-specific selection in utero among contemporary human birth cohorts. Paediatr Perinat Epidemiol 23(3):273–278, PMID: , 10.1111/j.1365-3016.2009.01028.x. [DOI] [PubMed] [Google Scholar]
- 78.Bruckner T, Catalano R. 2007. The sex ratio and age-specific male mortality: evidence for culling in utero. Am J Hum Biol 19(6):763–773, PMID: , 10.1002/ajhb.20636. [DOI] [PubMed] [Google Scholar]
- 79.Hansen D, Moller H, Olsen J. 1999. Severe periconceptional life events and the sex ratio in offspring: follow up study based on five national registers. BMJ 319(7209):548–549, PMID: , 10.1136/bmj.319.7209.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dunn GA, Morgan CP, Bale TL. 2011. Sex-specificity in transgenerational epigenetic programming. Horm Behav 59(3):290–295, PMID: , 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 81.Torche F, Kleinhaus K. 2012. Prenatal stress, gestational age and secondary sex ratio: the sex-specific effects of exposure to a natural disaster in early pregnancy. Hum Reprod 27(2):558–567, PMID: , 10.1093/humrep/der390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Q, Zhang Y, Ban J, Zhu H, Xu H, Li T. 2021. The relationship between population heat vulnerability and urbanization levels: a county-level modeling study across China. Environ Int 156:106742, PMID: , 10.1016/j.envint.2021.106742. [DOI] [PubMed] [Google Scholar]
- 83.Wang S, Luo K. 2018. Life expectancy impacts due to heating energy utilization in China: distribution, relations, and policy implications. Sci Total Environ 610-611:1047–1056, PMID: , 10.1016/j.scitotenv.2017.08.195. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Zhong H, Yang Z, Wang M, Kammen DM, Liu Z, et al. 2020. Exploring the trade-offs between electric heating policy and carbon mitigation in China. Nat Commun 11(1):6054, PMID: , 10.1038/s41467-020-19854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin L, Wang HH, Liu Y, Lu C, Chen W, Guo VY. 2021. Indoor solid fuel use for heating and cooking with blood pressure and hypertension: a cross-sectional study among middle-aged and older adults in China. Indoor Air 31(6):2158–2166, PMID: , 10.1111/ina.12872. [DOI] [PubMed] [Google Scholar]
- 86.Bai L, Woodward A, Cirendunzhu ,Liu Q. 2016. County-level heat vulnerability of urban and rural residents in Tibet, China. Environ Health 15:3, PMID: , 10.1186/s12940-015-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He C, Ma L, Zhou L, Kan H, Zhang Y, Ma W, et al. 2019. Exploring the mechanisms of heat wave vulnerability at the urban scale based on the application of big data and artificial societies. Environ Int 127:573–583, PMID: , 10.1016/j.envint.2019.01.057. [DOI] [PubMed] [Google Scholar]
- 88.Richter LL, Ting J, Muraca GM, Synnes A, Lim KI, Lisonkova S. 2019. Temporal trends in neonatal mortality and morbidity following spontaneous and clinician-initiated preterm birth in Washington state, USA: a population-based study. BMJ Open 9(1):e023004. 1, PMID: , 10.1136/bmjopen-2018-023004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Serrano-Lomelin J, Hicks A, Kumar M, Johnson DW, Chari R, Osornio-Vargas A, et al. 2021. Patterns of respiratory health services utilization from birth to 5 years of children who experienced adverse birth outcomes. PLoS One 16(2):e0247527, PMID: , 10.1371/journal.pone.0247527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strand LB, Barnett AG, Tong S. 2011. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol 11:49, PMID: , 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouidir M, Giorgis-Allemand L, Lyon-Caen S, Morelli X, Cracowski C, Pontet S, et al. 2015. Estimation of exposure to atmospheric pollutants during pregnancy integrating space-time activity and indoor air levels: does it make a difference? Environ Int 84:161–173, PMID: , 10.1016/j.envint.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nethery E, Brauer M, Janssen P. 2009. Time-activity patterns of pregnant women and changes during the course of pregnancy. J Expo Sci Environ Epidemiol 19(3):317–324, PMID: , 10.1038/jes.2008.24. [DOI] [PubMed] [Google Scholar]
- 93.Yi L, Xu Y, Eckel SP, O’Connor S, Cabison J, Rosales M, et al. 2022. Time-activity and daily mobility patterns during pregnancy and early postpartum - evidence from the MADRES cohort. Spat Spatiotemporal Epidemiol 41:100502, PMID: , 10.1016/j.sste.2022.100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai J, Zhao Y, Kan J, Chen R, Martin R, van Donkelaar A, et al. 2020. Prenatal exposure to specific PM2.5 Chemical constituents and preterm birth in China: a nationwide cohort study. Environ Sci Technol 54(22):14494–14501, PMID: , 10.1021/acs.est.0c02373. [DOI] [PubMed] [Google Scholar]
- 95.Lin G, Fu J, Jiang D, Wang J, Wang Q, Dong D. 2015. Spatial variation of the relationship between PM2.5 concentrations and meteorological parameters in China. Biomed Res Int 2015:684618, PMID: , 10.1155/2015/684618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li R, Wang Z, Cui L, Fu H, Zhang L, Kong L, et al. 2019. Air pollution characteristics in China during 2015–2016: spatiotemporal variations and key meteorological factors. Sci Total Environ 648:902–915, PMID: , 10.1016/j.scitotenv.2018.08.181. [DOI] [PubMed] [Google Scholar]
- 97.Leung M, Kioumourtzoglou MA, Raz R, Weisskopf MG. 2021. Bias due to selection on live births in studies of environmental exposures during pregnancy: a simulation study. Environ Health Perspect 129(4):47001, PMID: , 10.1289/EHP7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lenters V, Iszatt N, Forns J, Čechová E, Kočan A, Legler J, et al. 2019. Early-life exposure to persistent organic pollutants (OCPs, PBDEs, PCBs, PFASs) and attention-deficit/hyperactivity disorder: a multi-pollutant analysis of a Norwegian birth cohort. Environ Int 125:33–42, PMID: , 10.1016/j.envint.2019.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.