Abstract
PURPOSE
Metastatic castration-resistant prostate cancer (mCRPC) remains a lethal disease with current standard-of-care therapies. Homologous recombination repair (HRR) gene alterations, including BRCA1/2 alterations, can sensitize cancer cells to poly (ADP-ribose) polymerase inhibition, which may improve outcomes in treatment-naïve mCRPC when combined with androgen receptor signaling inhibition.
METHODS
MAGNITUDE (ClinicalTrials.gov identifier: NCT03748641) is a phase III, randomized, double-blinded study that evaluates niraparib and abiraterone acetate plus prednisone (niraparib + AAP) in patients with (HRR+, n = 423) or without (HRR−, n = 247) HRR-associated gene alterations, as prospectively determined by tissue/plasma-based assays. Patients were assigned 1:1 to receive niraparib + AAP or placebo + AAP. The primary end point, radiographic progression-free survival (rPFS) assessed by central review, was evaluated first in the BRCA1/2 subgroup and then in the full HRR+ cohort, with secondary end points analyzed for the full HRR+ cohort if rPFS was statistically significant. A futility analysis was preplanned in the HRR− cohort.
RESULTS
Median rPFS in the BRCA1/2 subgroup was significantly longer in the niraparib + AAP group compared with the placebo + AAP group (16.6 v 10.9 months; hazard ratio [HR], 0.53; 95% CI, 0.36 to 0.79; P = .001). In the overall HRR+ cohort, rPFS was significantly longer in the niraparib + AAP group compared with the placebo + AAP group (16.5 v 13.7 months; HR, 0.73; 95% CI, 0.56 to 0.96; P = .022). These findings were supported by improvement in the secondary end points of time to symptomatic progression and time to initiation of cytotoxic chemotherapy. In the HRR− cohort, futility was declared per the prespecified criteria. Treatment with niraparib + AAP was tolerable, with anemia and hypertension as the most reported grade ≥ 3 adverse events.
CONCLUSION
Combination treatment with niraparib + AAP significantly lengthened rPFS in patients with HRR+ mCRPC compared with standard-of-care AAP.
Efficacy and safety of niraparib + AAP for first-line treatment in patients with HRR+ mCRPC.
INTRODUCTION
Metastatic castration-resistant prostate cancer (mCRPC) remains a lethal disease, highlighting a need for new therapies.1-3 Alterations in homologous recombination repair (HRR)–associated genes, present in up to approximately 30% of mCRPC, have been associated with poor prognosis and resistance to current systemic therapies.4-8 Deleterious gene alterations, particularly in BRCA1/2, can sensitize prostate cancer cells to inhibition of poly (ADP-ribose) polymerase (PARP) through a mechanism of synthetic lethality.4,9 Inhibition of androgen receptor signaling can also result in downregulated expression of DNA repair genes and sensitize prostate cancer cells to PARP inhibition.10,11 Therefore, we hypothesized that targeting both oncogenic pathways concurrently may improve outcomes in patients with and without HRR alterations.10,12
CONTEXT
Key Objective
Metastatic castration-resistant prostate cancer (mCRPC) with alterations in homologous recombination repair (HRR)–associated genes has been associated with poor prognosis and resistance to current systemic therapies. The phase III MAGNITUDE study prospectively enrolled patients into two cohorts on the basis of HRR biomarker status and compared the efficacy and safety of niraparib and abiraterone acetate plus prednisone (niraparib + AAP) versus placebo + AAP as first-line treatment for patients with mCRPC.
Knowledge Generated
Patients with mCRPC and HRR alterations experienced substantial and clinically meaningful benefit from the combination of niraparib + AAP, with no new safety signals that affected the benefit-risk profile. Such results underscore the need for HRR gene testing for metastatic prostate cancer and support the use of niraparib + AAP as first-line combination therapy for these patients with particularly poor prognoses.
Relevance (M.A. Carducci)
-
These results underscore the need for HRR gene testing for metastatic prostate cancer and support the use of niraparib + AAP as first-line combination therapy for these patients with particularly poor prognoses.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
Niraparib, a potent and highly selective inhibitor of PARP-1 and PARP-2, is approved in the United States, Canada, Europe, and China in select patients for several indications, including ovarian, fallopian tube, and primary peritoneal cancers.13-17 In the phase II GALAHAD trial (ClinicalTrials.gov identifier: NCT02854436), an objective response rate (ORR) of 34.2% with niraparib monotherapy as third-line or higher therapy was achieved in patients with mCRPC, measurable metastases, and BRCA1/2 pathogenic alterations.18 Abiraterone acetate (AA) plus prednisone (AAP) is a standard first-line therapy that improves progression-free survival (PFS) and overall survival (OS) in patients with mCRPC.1 The daily combination dose of niraparib in combination with AA was established as 200/1,000 mg, respectively, plus prednisone on the basis of achieving maximum concentration and area under the curve values within the efficacious target combination ranges, with no dose-limiting toxicities (DLTs) observed in the phase Ib BEDIVERE study. When the 300-mg dose of niraparib was combined with AAP, three of eight patients experienced DLT. Given the pharmacokinetic results and safety profile, 200-mg niraparib was chosen to be combined with AAP for future studies.19
The phase III MAGNITUDE study was designed to compare the efficacy and safety of niraparib + AAP versus placebo + AAP for first-line mCRPC. Clinical data have shown the efficacy of combining androgen receptor–targeted therapy and a PARP inhibitor in metastatic prostate cancer, but the patient population who could have an optimal benefit with this combination was not yet defined at study initiation. Hence, patients in MAGNITUDE were prospectively enrolled into two cohorts on the basis of HRR biomarker status.
METHODS
Patients
Patients ≥18 years of age with mCRPC and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 were eligible for prescreening to determine HRR biomarker status using a required assay on tissue and/or blood samples (FoundationOne tissue test [FoundationOneCDx], Resolution Bioscience homologous recombination deficiency plasma test, AmoyDx blood and tissue assays, or accredited local laboratory biomarker tests with central review demonstrating a pathogenic germline or somatic alteration listed in the study biomarker gene panel). Patients must have been tested by both tissue and plasma to be randomly assigned in the HRR− cohort. Patients had to have a gene alteration detected by ≥1 assay to be eligible for the HRR+ cohort.
Patients could not have received prior PARP inhibitors. Systemic therapy (eg, apalutamide, enzalutamide, darolutamide, and docetaxel) for mCRPC was exclusionary; systemic therapies for metastatic castration-sensitive prostate cancer (mCSPC) or nonmetastatic castration-resistant prostate cancer (nmCRPC) were allowed. Up to 4 months of AAP for first-line mCRPC before random assignment was allowed while completing HRR testing. Patients who received >2 months of AAP underwent prostate-specific antigen (PSA) testing to document a lack of PSA progression before random assignment. Full eligibility criteria are described in the trial protocol. The study protocol and amendments were reviewed by an independent ethics committee or institutional review board; the study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements. All patients provided written informed consent.
Trial Design and Interventions
This is a phase III, randomized, double-blind, placebo-controlled, multicenter study. The HRR+ cohort consisted of patients with either monoallelic or biallelic pathogenic gene alterations in ≥1 of the following: ATM, BRCA1, BRCA2, BRIP1, CDK12, CHEK2, FANCA, HDAC2, or PALB2; the HRR− cohort included patients who had no detectable alterations in any of these genes. This nine-gene panel was chosen on the basis of previous clinical data of PARP inhibitor activity in this disease setting.20-22 A third, open-label cohort to evaluate a dual-action tablet of niraparib + AA (given with prednisone) was enrolled; the results will be reported later.
Patients in the HRR+ and HRR− cohorts were randomly assigned in a 1:1 ratio to receive either niraparib 200 mg once daily with AA 1,000 mg once daily plus prednisone 5 mg twice daily (niraparib + AAP group) or placebo + AAP. Treatment was continuous in 28-day cycles until unequivocal clinical progression, unacceptable toxicity, or death. Patients with radiographic progression could continue study treatment at the investigator's discretion but had to be discontinued after unequivocal clinical progression.
Study Assessments and End Points
The primary end point was radiographic PFS (rPFS), defined as the time from random assignment to the first occurrence of radiographic progression assessed by blinded independent central review or death due to any cause. rPFS was determined by first observation of progression by bone scan (per Prostate Cancer Working Group 3) or progression of soft-tissue lesions by CT or MRI (per RECIST 1.1), with imaging done every 8 weeks for the first 6 months and every 12 weeks after.23,24 Secondary end points were time to initiation of cytotoxic chemotherapy (TCC), time to symptomatic progression (TSP), and OS (Data Supplement [online only]). Other end points included time to PSA progression, ORR, and patient-reported outcomes (Data Supplement). Treatment-emergent adverse events (AEs) were defined as AEs occurring after the first dose of study drug to 30 days after the last dose and summarized by category and preferred term. Detailed biomarker analyses were planned and will be reported in the future.
A futility analysis for the HRR− cohort was preplanned when approximately 200 patients had been enrolled and approximately 125 composite end point events (the first of either PSA progression, radiographic progression, or death; Data Supplement) had been observed.
Statistical Analysis
The primary and secondary end points in the HRR+ cohort were tested using a graphical testing approach to control the family-wise type I error rate at a two-sided level of 0.05. rPFS was tested first in the BRCA1/2 subgroup and then the HRR+ cohort. Secondary end points were analyzed for the full HRR+ cohort if rPFS was statistically significant. Two interim analyses and a final analysis were planned for the secondary end points. Approximately 220 radiographic progression events were required to provide 87% power in detecting a hazard ratio (HR) of 0.65 at a two-tailed significance level of .05. Approximately 102 radiographic progression events were to be observed to provide 93% power to detect an HR of 0.5 in the BRCA1/2 subgroup. For rPFS and OS, multivariate analyses were preplanned, adjusting for selected baseline prognostic factors. Additional details are provided in the Data Supplement.
RESULTS
Screening and Random Assignment
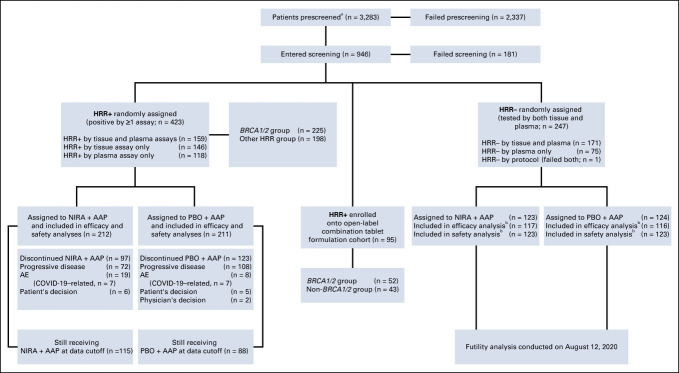
A total of 2,984 patients were prescreened for HRR status across the three cohorts using tissue and plasma tests. Of them, 742 (24.9%) tested positive for ≥1 of the nine genes (Fig 1).
FIG 1.
CONSORT diagram. a3,283 includes those 2,984 patients who entered prescreening de novo (did not have known HRR status from local testing or from the local vendor in China, AmoyDx). bFourteen patients with CDK12 alterations were prospectively included in the HRR− cohort before amendment 4. Reasons for failed screening include AE, death, progressive disease, screen failure, withdrawal by patient, and others. HRR biomarker status was determined using the required assay on tissue and/or blood samples (FoundationOne tissue test [FoundationOneCDx], Resolution Bioscience HRD plasma test, AmoyDx blood and tissue assays, or accredited local laboratory biomarker tests with central review demonstrating a pathogenic germline or somatic alteration listed in the study biomarker gene panel). AAP, abiraterone acetate plus prednisone; AE, adverse event; HRD, homologous recombination deficiency; HRR, homologous recombination repair; NIRA, niraparib; PBO, placebo.
Patient Characteristics
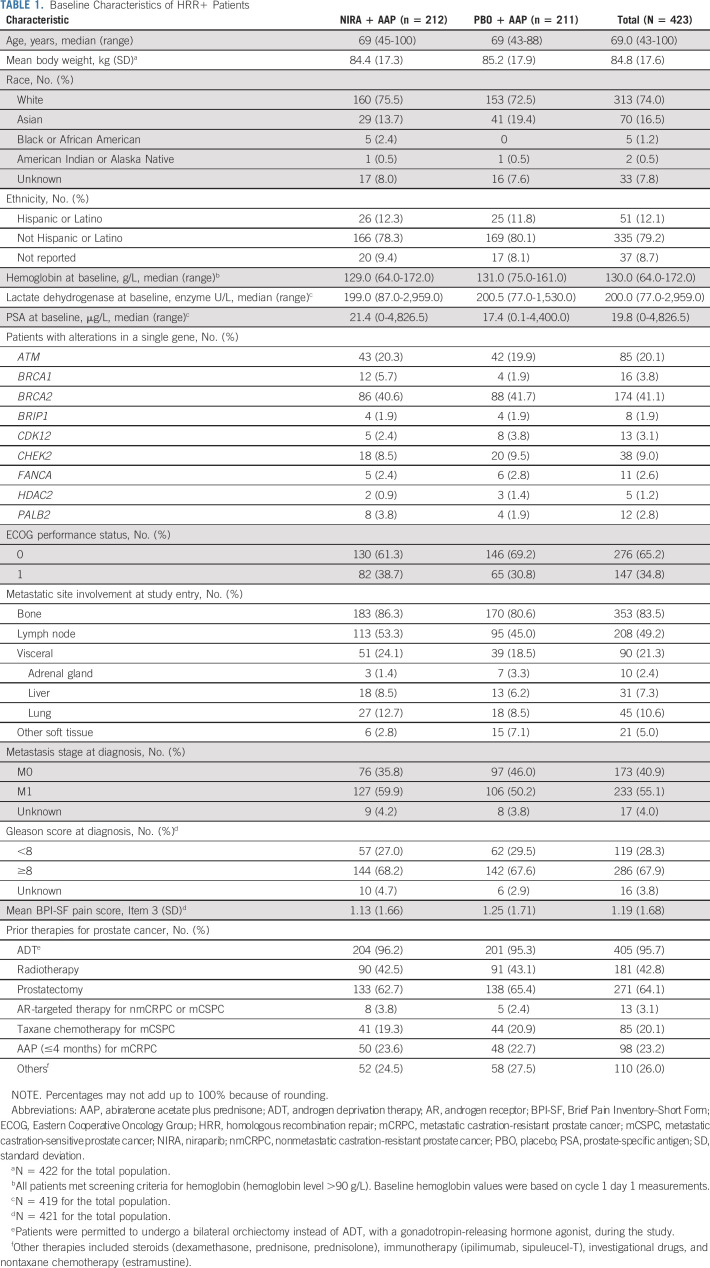
Between May 2019 and March 2021, 423 patients were enrolled, with 212 randomly assigned to the niraparib + AAP group and 211 to the placebo + AAP group. Although baseline characteristics were broadly comparable between treatment arms, rates of visceral metastases, bone metastases, and ECOG performance status of one were higher in the niraparib + AAP group (Table 1). Three percent of patients had prior exposure to novel hormonal agents in mCSPC or nmCRPC and 23% had <4 months of AAP for mCRPC before study treatment. In the HRR− cohort, between March 2019 and March 2020, 123 patients were randomly assigned to the niraparib + AAP group and 124 to the placebo + AAP group. Baseline characteristics were generally comparable; rates of lung metastases and ECOG performance status of one were higher in the niraparib + AAP group (Data Supplement).
TABLE 1.
Baseline Characteristics of HRR+ Patients
Efficacy
HRR+ cohort.
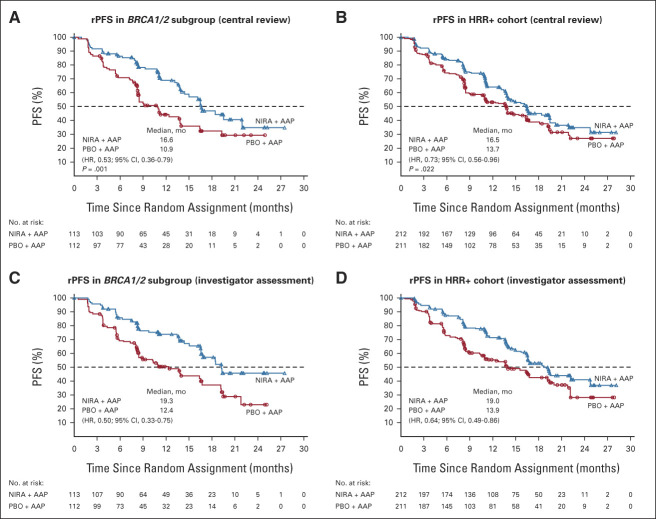
At clinical cutoff (October 8, 2021), the median duration of follow-up in the HRR+ cohort was 18.6 months (range, 0.3-29.0 months). In the BRCA1/2 subgroup, median rPFS by central review was significantly longer in the niraparib + AAP group than in the placebo + AAP group (16.6 v 10.9 months; HR, 0.53; 95% CI, 0.36 to 0.79; P = .001; Fig 2A). Similarly, HRR+ patients in the niraparib + AAP group experienced a significantly longer rPFS (16.5 v 13.7 months; HR, 0.73; 95% CI, 0.56 to 0.96; P = .022; Fig 2B). Findings were consistent in the prespecified sensitivity analyses of investigator-assessed rPFS (Figs 2C and 2D). An HR of 0.99 (95% CI, 0.68 to 1.44) was observed for rPFS in the subgroup of patients with HRR alterations other than BRCA1/2; in a preplanned sensitivity analysis of patients with HRR alterations excluding BRCA1/2, single ATM or CDK12 alterations and co-occurring ATM/CDK12 alterations, a trend toward benefit was observed (HR, 0.87; 95% CI, 0.51 to 1.49). Additional gene-by-gene analysis showed that when combined into functional groups, patients with an alteration in the HRR-Fanconi pathway (BRIP1, FANCA, and PALB2) or an HRR-associated alteration (CHEK2 or HDAC2) showed improvement in all end points.
FIG 2.
Kaplan-Meier estimates of rPFS. (A) rPFS of the subgroup of patients with BRCA1/2 alterations as assessed by blinded independent central review. (B) rPFS of the overall HRR+ cohort as assessed by blinded independent central review. (C) Prespecified sensitivity analysis of rPFS of patients with BRCA1/2 alterations by investigator assessment. (D) rPFS of the overall HRR+ cohort by investigator assessment. AAP, abiraterone acetate plus prednisone; HR, hazard ratio; HRR, homologous recombination repair; NIRA, niraparib; PBO, placebo; PFS, progression-free survival; rPFS, radiographic progression-free survival.
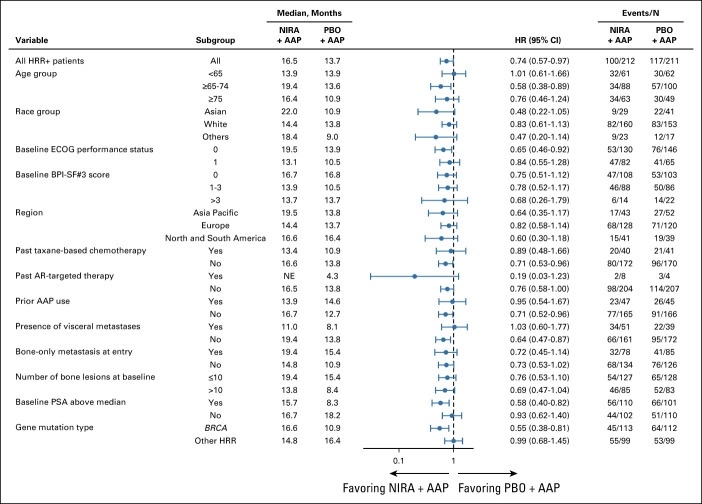
rPFS in prespecified subgroups defined by baseline characteristics showed no heterogeneity of effect across subgroups (Fig 3). Adjustment for imbalances in baseline characteristics (PSA value, lactate dehydrogenase, alkaline phosphatase, and presence of visceral disease at baseline) in the multivariate model underscores the benefit of niraparib + AAP (Data Supplement).
FIG 3.
Forest plot of rPFS for subgroups defined by baseline clinical disease characteristics in the HRR+ cohort. AAP, abiraterone acetate plus prednisone; AR, androgen receptor; BPI-SF#3, Brief Pain Inventory–Short Form, Item 3; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; HRR, homologous recombination repair; NE, not evaluable; NIRA, niraparib; PBO, placebo; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival.
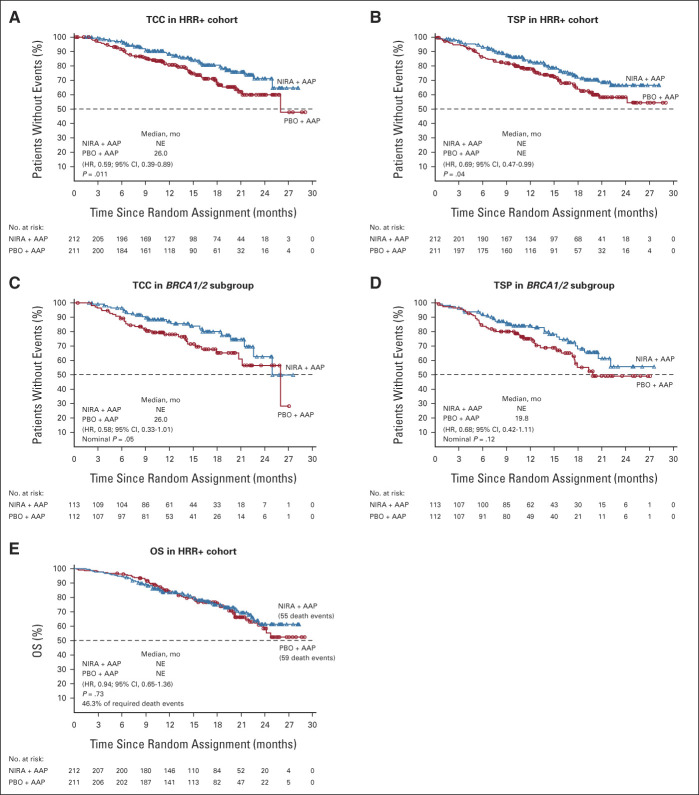
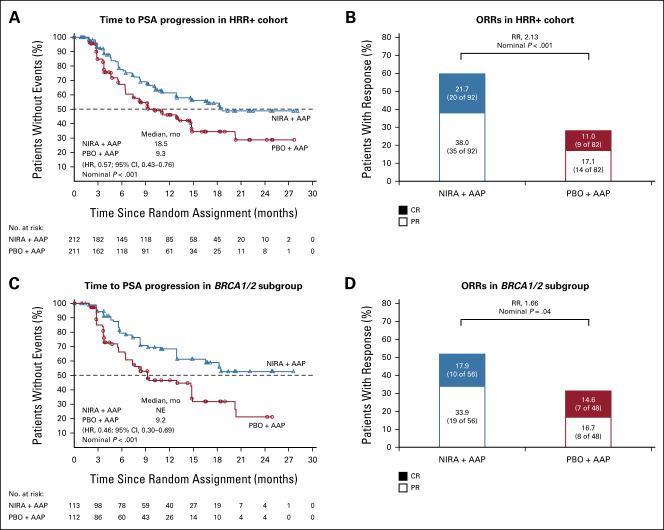
Niraparib + AAP showed consistent benefits in the patient-relevant secondary and exploratory end points across HRR alterations. In the HRR+ cohort, niraparib + AAP delayed TCC (HR, 0.59; 95% CI, 0.39 to 0.89; P = .011; Fig 4A) and TSP (HR, 0.69; 95% CI, 0.47 to 0.99; P = .04; Fig 4B), which was also observed in the BRCA1/2 subgroup (Figs 4C and 4D). At this preplanned interim analysis, the significance boundary of .0001 (O'Brien-Fleming method) was not met by either end point. OS data are immature at this first interim analysis, with 46.3% of the required events for final analysis (Fig 4E; Data Supplement). The prespecified multivariate analysis of OS, adjusting for baseline variables, favored the niraparib + AAP arm in the HRR+ cohort (0.77; 95% CI, 0.53 to 1.12; Data Supplement). Additionally, niraparib + AAP prolonged time to PSA progression and led to higher ORR in the HRR+ and BRCA1/2 groups (Fig 5). Time to PSA progression and rPFS were strongly correlated, with an overall r = 0.67 (95% CI, 0.56 to 0.75). In the HRR+ cohort, patient-reported quality of life (QoL) changes over time between treatment arms were similar as determined by FACT-P total score (Data Supplement).
FIG 4.
Kaplan-Meier estimates of secondary end points. (A and B) Kaplan-Meier estimates of TCC and TSP in the HRR+ cohort, respectively. (C and D) Kaplan-Meier estimates of TCC and TSP in the BRCA1/2 subgroup, respectively. (E) Kaplan-Meier estimate of OS in the HRR+ cohort. AAP, abiraterone acetate plus prednisone; HR, hazard ratio; HRR, homologous recombination repair; NE, not evaluable; NIRA, niraparib; OS, overall survival; PBO, placebo; TCC, time to initiation of cytotoxic chemotherapy; TSP, time to symptomatic progression.
FIG 5.
Kaplan-Meier estimates of time to PSA progression and ORRs. (A and B) Kaplan-Meier estimates of time to PSA progression and ORRs in the HRR+ cohort, respectively. (C and D) Kaplan-Meier estimates of time to PSA progression and ORRs in the BRCA1/2 subgroup, respectively. AAP, abiraterone acetate plus prednisone; CR, complete response; HR, hazard ratio; HRR, homologous recombination repair; NE, not evaluable; NIRA, niraparib; ORR, objective response rate; PBO, placebo; PR, partial response; PSA, prostate-specific antigen; RR, relative risk.
HRR− cohort.
In the futility analysis, 233 HRR− patients (niraparib + AAP, n = 117; placebo + AAP, n = 116) were evaluated for the composite end point of time to PSA progression and/or rPFS (HR, 1.09; 95% CI, 0.75 to 1.57; P = .66; Data Supplement) and the individual end points of rPFS and time to PSA progression (Data Supplement). On the basis of the prespecified criteria, futility was declared for the HRR− cohort in August 2020, which was closed to further enrollment on the basis of Independent Data Monitoring Committee recommendations. All patients in the HRR− cohort were unblinded, and patients randomly assigned to niraparib + AAP were allowed to continue niraparib + AAP or AAP alone per the investigator's discretion. Additional efficacy assessments were not performed once patients entered a safety data collection phase.
Safety
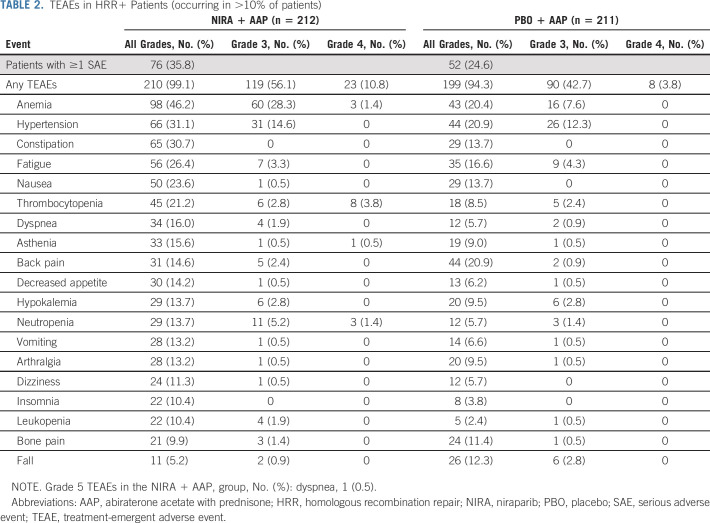
The median total duration of assigned treatment for the HRR+ cohort was 13.8 months (range, 0-29.0) in the niraparib + AAP group and 12.1 months (range, 0-29.0) in the placebo + AAP group. The estimated relative dose intensity was generally high and comparable in the groups (Data Supplement). The incidence of grade ≥ 3 AEs was 67.0% with niraparib + AAP and 46.4% with placebo + AAP. The most common grade 3 AEs were anemia (28.3% v 7.6%) and hypertension (14.6% v 12.3%) with niraparib + AAP versus placebo + AAP, respectively. Other grade 3/4 AEs of note include thrombocytopenia (6.6% v 2.4%) and neutropenia (6.6% v 1.4%) with niraparib + AAP versus placebo + AAP, respectively (Table 2). AEs leading to dose reductions and treatment discontinuations are presented in the Data Supplement. A total of 38 patients died during study treatment, with 19 in each group (Data Supplement). In patients who died due to AEs, infections (eg, COVID-19 and pneumonia) were the leading cause of death in the niraparib + AAP group; cardiac disorders were the leading cause of death in the placebo + AAP group. Patients with niraparib drug interruptions or dose reductions had comparable rPFS benefit from niraparib + AAP compared with the observed benefit in the overall HRR+ population (HR, 0.72; 95% CI, 0.53 to 0.97 and HR, 0.70; 95% CI, 0.46 to 1.08, respectively). In the HRR− cohort, the median duration of assigned treatment was 16.8 months (range, 0-29.0); AEs were similar to those in the HRR+ cohort (Data Supplement).
TABLE 2.
TEAEs in HRR+ Patients (occurring in >10% of patients)
DISCUSSION
Prior studies have demonstrated that niraparib and other PARP inhibitors have clinical benefit as monotherapy in HRR+ mCRPC after progression on androgen receptor pathway inhibitors and chemotherapy.18,25,26 In the phase III MAGNITUDE study, rPFS was significantly longer in the niraparib + AAP group versus placebo + AAP in the HRR+ cohort, with the greatest benefit in the BRCA1/2 subgroup. The median rPFS was 10.9 months in the BRCA1/2 subgroup that received AAP alone compared with the historical rPFS of approximately 16 months in unselected patients.6,27-29 These findings are consistent with prior reports of patients with BRCA1/2 gene alterations having poor prognosis and worse treatment outcomes with standard therapies. In MAGNITUDE, treatment effect for niraparib + AAP was generally consistent across gene alteration groups, possibly mitigating the negative prognostic impact of HRR alterations. The study design allowed for a definitive evaluation of niraparib + AAP in patients with BRCA1/2 alterations; however, the rarity of some other HRR alterations precluded statistical significance testing in these individual genes. A preplanned sensitivity analysis showed the increase in HR for other HRR alterations were driven by ATM or CDK12 alterations. Additionally, the previously reported gene-by-gene analysis demonstrated that patients with alterations in the HRR-Fanconi pathway and HRR-associated alterations showed improvement in all end points, supporting the benefit of niraparib + AAP in HRR alterations beyond BRCA1/2.30
The benefit in rPFS in the current study was also supported by clinically meaningful improvements in TCC and TSP. Although the P value for both end points was <.05, the conservative boundary for statistical significance (.0001; O'Brien-Fleming method) was not crossed. These end points will be further tested at the prespecified second interim and final analysis. Both of these secondary end points are well established in mCRPC as clinically meaningful measures of patient outcome.31-33 In contrast to radiographic progression, which can have limited clinical impact on worsening of symptoms in some patients, the events that constitute symptomatic progression are significant drivers of morbidity.
OS data were immature at this first interim analysis, although results from the OS multivariate analysis favor the niraparib + AAP group. As noted, the conservative boundary for statistical significance at this first interim analysis for all secondary end points was not crossed. Follow-up of patients with HRR+ mCRPC enrolled in MAGNITUDE is ongoing. Other limitations of the MAGNITUDE study include the limited diversity in patient race and demographics. Furthermore, although the nine-gene panel selection was based on previous clinical data, comprehensive assessment of other genes implicated in HRR was not undertaken.
The incidence of grade 3/4 AEs was higher with niraparib + AAP, with approximately a 4-fold increase of grade ≥ 3 anemia with niraparib + AAP compared with 7% with placebo + AAP, similar to previously reported data for AAP.34 The safety profile of niraparib + AAP was manageable and consistent with prior studies of each therapy in prostate cancer, with no new safety signals that affected the benefit-risk profile. Outcomes for patients with dose interruptions/reductions support its use in patient management without concerns of negatively affecting outcomes. QoL was also maintained with the addition of niraparib to AAP. Additional analyses that included patient-reported outcomes have been presented, and further in-depth analyses will be reported.30,35
MAGNITUDE was designed intentionally to determine which group of patients would derive the greatest benefit from a PARP inhibitor with standard-of-care AAP therapy while limiting undue risks associated with combination therapy. HRR status was prospectively and comprehensively determined by plasma- and tissue-based assays. The study was practically designed with prior chemotherapy and use of novel hormonal agents allowed in the mCSPC or nmCRPC settings. Patients were allowed to receive up to 4 months of prior AAP for mCRPC, and approximately one-fourth of patients in the HRR+ cohort had received prior AAP treatment (median duration [range], 1.9 [0.3-4.1] months), which may have affected the disease characteristics and outcomes. As HRR alterations testing is not yet routine practice in early prostate cancer, allowing a short course of AAP before random assignment acknowledged the need to initiate therapy for advancing prostate cancer while HRR alteration status was determined. These features of a pragmatic trial design were implemented to suit the evolving standard of care in advanced prostate cancer while informing a key practice pattern.
Futility was declared, and no benefit was observed in patients without HRR alterations (HRR– cohort), who were prospectively identified before random assignment. As time to PSA progression and rPFS have demonstrated a strong correlation in prior trials and that correlation was also observed in the HRR+ cohort in MAGNITUDE, the composite end point allowed for a rapid assessment of possible futility.36 Although the more limited sample size of the HRR− cohort potentially precludes detection of a modest benefit, the individual components of rPFS and PSA progression each had an HR > 1.0, and demonstration of benefit-risk ratio in this subset would be challenging. Nevertheless, results from MAGNITUDE highlight the importance of testing for HRR status before initiating niraparib + AAP to identify who will gain the most benefit from combination therapy balanced with additional toxicity.
In the recently published phase III PROpel study, which assessed olaparib + AAP versus placebo + AAP for first-line mCRPC in patients who were unselected for HRR alterations, the primary end point of rPFS was met (24.8 v 16.6 months; HR, 0.66; 95% CI, 0.54 to 0.81; P < .0001). A retrospective analysis demonstrated rPFS improvement with olaparib + AAP in both HRR+ and HRR− populations, with lesser benefit in the latter.37 Differences, including study design, gene testing strategies, prior exposure to novel hormonal agents, and patient population, limit the comparability between PROpel and MAGNITUDE; however, together they confirm the substantial benefit of PARP inhibition with AAP for patients with HRR+ mCRPC and the need for continued development of predictive biomarkers.38
In summary, HRR+ patients with mCRPC derived significant and clinically meaningful benefit from niraparib + AAP. The safety profile of this combination was manageable, with no new safety signals that affected the benefit-risk profile. These data underscore the need to test for HRR gene alterations for metastatic prostate cancer and support the use of niraparib + AAP as first-line combination therapy for these patients with particularly poor prognoses.
ACKNOWLEDGMENT
We thank the patients who participated in this study, along with their families and carers, and the study teams who were involved at each participating institution. We also thank Namphuong Tran, MD, Xin Zhao, MD, PhD, and Nishi Kothari, MD, for overall assistance and intellectual guidance, including assistance with trial design; Kavitha Nagarajan, BC, and Susan Li, PhD, for assistance with data analysis; Angela Trinh, BS, Mardy Eckhardt, PhD, Marcin Zmarzlik, MS, Danuta Gawryluk, PhD, Lauren Boswell, BS, Susan Laabs, BA, and Eileen Kan, MS, MBA, for assistance with study conduct and data analysis; and Natalie Hutnick, PhD, and Karen Urtishak, PhD, for assistance with biomarker development and analyses. Medical writing and editorial assistance were provided by Danyang Zhou, PharmD, Lauren Fink, PhD, Michelle Kwon, PhD, and Kristen Evaul, PhD, of Lumanity Communications Inc (Yardley, PA, USA), which was provided in accordance with Good Publication Practice (GPP3) guidelines and funded by Janssen Research & Development.
List of MAGNITUDE principal investigators are listed in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
List of MAGNITUDE Principal Investigators
Kim N. Chi
Honoraria: Janssen, Astellas Pharma, Bayer, AstraZeneca, Roche, Merck, POINT Biopharma
Consulting or Advisory Role: ESSA, Astellas Pharma, Janssen, Sanofi, Amgen, Bayer, AstraZeneca, Roche, POINT Biopharma, Daiichi Sankyo, Merck, Constellation Pharmaceuticals
Research Funding: Janssen (Inst), Astellas Pharma (Inst), Bayer (Inst), Sanofi (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Roche (Inst), AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), ESSA (Inst)
Expert Testimony: AstraZeneca, Novartis
Dana Rathkopf
Consulting or Advisory Role: Janssen, Genentech, AstraZeneca, Bayer, Myovant Sciences
Research Funding: Janssen Oncology (Inst), Medivation (Inst), Celgene (Inst), Takeda (Inst), Millennium (Inst), Ferring (Inst), Novartis (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), TRACON Pharma (Inst), Bayer (Inst), Phosplatin Therapeutics (Inst)
Matthew R. Smith
Consulting or Advisory Role: Bayer, Janssen Oncology, Amgen, Pfizer, Lilly, Novartis, Astellas Pharma
Research Funding: Janssen Oncology (Inst), Bayer (Inst), Lilly (Inst), ESSA (Inst), ORIC Pharmaceuticals (Inst)
Eleni Efstathiou
Honoraria: Sanofi, Janssen-Cilag, Astellas Pharma, Takeda, Merck, Pfizer
Consulting or Advisory Role: Sanofi, Janssen Oncology, Tokai Pharmaceuticals, AstraZeneca, Myovant Sciences, Bayer, AstraZeneca
Research Funding: Janssen-Cilag
Gerhardt Attard
Honoraria: Janssen, Astellas Pharma
Consulting or Advisory Role: Janssen-Cilag, Veridex, Ventana Medical Systems, Astellas Pharma, Medivation, Novartis, Millennium, Abbott Laboratories, ESSA, Bayer, Pfizer, AstraZeneca, Ferring
Speakers' Bureau: Janssen, Astellas Pharma, Takeda, Sanofi, Ventana Medical Systems, Ipsen, AstraZeneca, Ferring
Research Funding: Janssen (Inst), Arno Therapeutics (Inst), Innocrin Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I am on The ICR rewards to inventors list of Abiraterone acetate
Travel, Accommodations, Expenses: Janssen, Astellas Pharma, Medivation, Ventana Medical Systems, Abbott Laboratories, Bayer, ESSA, Janssen, Astellas Pharma, Pfizer, Ferring
Other Relationship: Institute of Cancer Research
David Olmos
Honoraria: Janssen, Bayer, Astellas Pharma (Inst)
Consulting or Advisory Role: Janssen (Inst), AstraZeneca (Inst), Clovis Oncology, Bayer (Inst), Daiichi Sankyo, MSD Oncology
Research Funding: AstraZeneca (Inst), Bayer (Inst), Janssen (Inst), Genentech/Roche (Inst), Pfizer (Inst), Astellas Medivation (Inst), Tokai Pharmaceuticals (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Bayer, Janssen, Ipsen, Astellas Pharma, Roche, AstraZeneca Spain
Eric Small
Stock and Other Ownership Interests: Fortis, Harpoon Therapeutics, Teon Therapeutics
Honoraria: Janssen, Johnson and Johnson
Consulting or Advisory Role: Janssen Oncology, Teon Therapeutics, Fortis
Open Payments Link: https://openpaymentsdata.cms.gov/physician/660367/summary
Andrea Pereira de Santana Gomes
Honoraria: Astellas Pharma, Bayer, Janssen Oncology, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Bayer
Speakers' Bureau: Janssen Oncology, Astellas Pharma, Bayer, AstraZeneca
Research Funding: Janssen Oncology, MSD Oncology, Bayer, Roche, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology
Guilhem Roubaud
Honoraria: Astellas Pharma (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst)
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen-Cilag (Inst), AstraZeneca (Inst), Ipsen (Inst), AAA/Endocyte/Novartis (Inst), Merck/Pfizer (Inst), Bayer (Inst), Bayer (Inst)
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag, Bayer
Marniza Saad
Honoraria: Astellas Pharma, Novartis, Johnson & Johnson, Ipsen, Cipla, AstraZeneca, Eisai, Pfizer, Amgen
Consulting or Advisory Role: Johnson & Johnson, MSD, AstraZeneca, Novartis, Merck, BMS, Viatris
Research Funding: Johnson & Johnson, MSD
Bogdan Zurawski
Employment: MSD Oncology, Janssen Oncology, Bristol Myers Squibb/Roche, Novartis, GlaxoSmithKline, Merck Serono
Honoraria: Roche, Astellas Pharma
Gary E. Mason
Employment: Janssen Research & Development, Merck
Stock and Other Ownership Interests: Johnson & Johnson, Merck
Travel, Accommodations, Expenses: Janssen Research & Development
Peter Francis
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Travel, Accommodations, Expenses: Johnson & Johnson
George Wang
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Daphne Wu
Employment: Johnson & Johnson/Janssen
Brooke Diorio
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Janssen Research & Development
Angela Lopez- Gitlitz
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Patents, Royalties, Other Intellectual Property: I am named as an inventor on the following patents: (1) WO 2021/224467 Treatment of Prostate Cancer with a Combination of Abiraterone Acetate and Niraparib (2) WO 2021/224469 Treatments of Prostate Cancer with Combinations of Abiraterone Acetate and Niraparib (3) WO 2021/224471 Pharmaceutical Formulations of Abiraterone Acetate and Niraparib
Shahneen Sandhu
Honoraria: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst)
Consulting or Advisory Role: AstraZeneca (Inst), Bristol Myers Squibb/Roche (Inst), Merck Sharp and Dohme (Inst), Amgen (Inst), Novartis (Inst), Genentech (Inst)
Speakers' Bureau: Bristol Myers Squibb, Merck, Roche/Genentech, AstraZeneca (Inst)
Research Funding: Amgen (Inst), AstraZeneca (Inst), Merck (Inst), Endocyte/Advanced Accelerator Applications (Inst), Genentech/Roche (Inst), Novartis (Inst), Pfizer (Inst), Senhwa Biosciences (Inst), Roche/Genentech (Inst)
Uncompensated Relationships: AAA/Endocyte/Novartis (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the ASCO 2022 Genitourinary Cancers Symposium, San Francisco, CA, February 17-19, 2022.
SUPPORT
Supported by Janssen Research & Development, LLC.
CLINICAL TRIAL INFORMATION
Contributor Information
Collaborators: Maria Alvarez, Gabriela Gatica, Martin Greco, Ernesto Korbenfeld, Esteban Metrebian, Jorge Salinas, Alejandro Salvatierra, Marcelo Tatangelo, Tom Ferguson, Howard Gurney, Elizabeth Hovey, Anthony Joshua, Matos Marco, Gavin Marx, Michelle Morris, Siobhan Ng, David Pook, Shahneen Sandhu, Ali Tafreshi, Thean Hsiang Tan, Tsvetanka Tosheva, Livia Andrade, Felipe Cruz, Luiza Faria, Jose Figueiredo, Fabio Franke, Andrea Juliana Gomes, Ariel Kann, Celio Kussumoto, Suelen Martins, Andre Murad, Helio Pinczowski, Giovani Pioner, Luis Pires, Daniel Preto, Gisele Santos, Eduardo Silva, Jamile Silva, Luciano Viana, Karina Vianna, Adriano Paula, Zhiwen Chen, Kim Chi, Urban Emmenegger, Neil Fleshner, Sunil Parimi, Fred Saad, Francisco Vera-Badillo, Hongqian Guo, Hong Luo, Lulin Ma, Mingxing Qui, Wei Xue, Guo Yonglian, Lei Li, Jinxian Pu, Song Zheng, Qing Zou, Milos Brodak, Milan Hora, Vladimir Samal, Vladimir Student, Jaroslav Vanasek, Emmanuelle Bompas, Philippe Barthelemy, Delphine Borchiellini, Aude Flechon, Hakim Mahammedi, Guilhem Roubaud, Antoine Thiery-Vuillemin, Diego Tosi, Spaeth Dominique, Carole Helissey, Martin Boegemann, Susan Feyerabend, Eva Hellmis, Martin Schostak, Gerhardt Attard, Anna Lydon, Ian Sayers, Omi Parikh, Duncan Wheatley, Peter Arkosy, Jozsef Erfan, Lajos Geczi, Peter Nyirady, Judit Olah, Istvan Papos, Bela Piko, Raanan Berger, Avivit Peer, Umberto Basso, Sergio Bracarda, Orazio Caffo, Francesco Carrozza, Gianluca Del Conte, Luca Galli, Donatello Gasparro, Sandro Pignata, Riccardo Ricotta, Daniele Santini, Giampaolo Tortora, Seoksoo Byun, SeolHo Choo, ByungHa Chung, Jaeyoung Joung, Wonho Jung, Taek Won Kang, Cheol Kwak, TaeGyun Kwon, Hyo Jin Lee, Ji Youl Lee, SeongIl Seo, YoungDeuk Choi, HongKoo Ha, ChoungSoo Kim, Flora Li Tze Chong, Chun Sen Lim, Vijayan Manogran, Hwoei Fen Soo Hoo, Guan Chou Teh, Marniza Saad, David Calvo Dominguez, Abraham Cardenas, Julia Saenz, Andre Bergman, Helgi Helgason, C.B. Hunting, Rik Somford, Tomasz Byrski, Tomasz Drewa, Dorota Filarska, Jolanta LuniewskaBury, Adam Marcheluk, Konrad Talasiewicz, Krzysztof Tupikowski, Renata Zaucha, Bogdan Zurawski, Pedro Madeira, Andre Mansinho, Nuno Vau, Anna Alyasova, Victoria Chistyakova, Denis Khvorostenko, Dmitry Kirtbaya, Gennady Kolesnikov, Evgeny Kopyltsov, Igor Lifirenko, Alexander Lykov, Konstantin Penkov, Andrey Semenov, Mikhail Shkolnik, Pavel Skopin, Roman Smirnov, Evgeny Usynin, Sergey Varlamov, Vladimir Vladimirov, Teresa Alonso, Sara Breijo, Elena Castro, Enrique Gallardo, Jose Gutierrez Banos, Alvaro Juarez, Rebeca Lozano, Pablo Maroto, Javier Puente, Alejo Rodriguez-Vida, Regina Girones, Begona Mellado, Enrique Castellanos, Chunde Li, Chao-Hsiang Chang, Hsiao-Jen Chung, Kuan-Hua Huang, Yen-Chuan Ou, Yu-Chieh Tsai, Shian-Shiang Wang, Wen-Jeng Wu, See Tong Pang, Cagatay Arslan, Devrim Cabuk, Hasan Coskun, Mahmut Gumus, Mustafa Ozguroglu, Berna Oksuzoglu, Berksoy Sahin, Deniz Tural, Bulent Yalcin, Irfan Cicin, Igor Bondarenko, Yaroslav Gotsuliak, Yevhen Hotko, Gennadii Khareba, Oleksandr Lychkovskyy, Iryna Lytvyn, Taron Nalbandyan, Viktor Paramonov, Valerii Sakalo, Eduard Stakhovsky, Viktor Stus, Sunil Babu, Alan Bryce, David Cahn, Herta Chao, Franklin Chu, Curtis Dunshee, Oscar Goodman, Michael Grable, Jason Hafron, Joelle Hamilton, Ralph Hauke, Joseph Maly, David Morris, Gregg Newman, Patrick Pilie, Neal Shore, Paul Sieber, Matthew Smith, Andrew Trainer, and Ronald Tutrone
DATA SHARING STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
AUTHOR CONTRIBUTIONS
Conception and design: Kim N. Chi, Matthew R. Smith, David Olmos, Eric J. Small, Valerii Sakalo, Peter Francis, George Wang, Angela Lopez-Gitlitz
Provision of study materials or patients: Matthew R. Smith, David Olmos, Ji Youl Lee, Andrea J. Pereira de Santana Gomes, Valerii Sakalo, Shahneen Sandhu
Collection and assembly of data: Kim N. Chi, Matthew R. Smith, David Olmos, Ji Youl Lee, Andrea J. Pereira de Santana Gomes, Bogdan Zurawski, Peter Francis, Angela Lopez-Gitlitz, Shahneen Sandhu
Data analysis and interpretation: Kim N. Chi, Matthew R. Smith, David Olmos, Andrea J. Pereira de Santana Gomes, Peter Francis, George Wang, Angela Lopez-Gitlitz, Shahneen Sandhu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kim N. Chi
Honoraria: Janssen, Astellas Pharma, Bayer, AstraZeneca, Roche, Merck, POINT Biopharma
Consulting or Advisory Role: ESSA, Astellas Pharma, Janssen, Sanofi, Amgen, Bayer, AstraZeneca, Roche, POINT Biopharma, Daiichi Sankyo, Merck, Constellation Pharmaceuticals
Research Funding: Janssen (Inst), Astellas Pharma (Inst), Bayer (Inst), Sanofi (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Roche (Inst), AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), ESSA (Inst)
Expert Testimony: AstraZeneca, Novartis
Dana Rathkopf
Consulting or Advisory Role: Janssen, Genentech, AstraZeneca, Bayer, Myovant Sciences
Research Funding: Janssen Oncology (Inst), Medivation (Inst), Celgene (Inst), Takeda (Inst), Millennium (Inst), Ferring (Inst), Novartis (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), TRACON Pharma (Inst), Bayer (Inst), Phosplatin Therapeutics (Inst)
Matthew R. Smith
Consulting or Advisory Role: Bayer, Janssen Oncology, Amgen, Pfizer, Lilly, Novartis, Astellas Pharma
Research Funding: Janssen Oncology (Inst), Bayer (Inst), Lilly (Inst), ESSA (Inst), ORIC Pharmaceuticals (Inst)
Eleni Efstathiou
Honoraria: Sanofi, Janssen-Cilag, Astellas Pharma, Takeda, Merck, Pfizer
Consulting or Advisory Role: Sanofi, Janssen Oncology, Tokai Pharmaceuticals, AstraZeneca, Myovant Sciences, Bayer, AstraZeneca
Research Funding: Janssen-Cilag
Gerhardt Attard
Honoraria: Janssen, Astellas Pharma
Consulting or Advisory Role: Janssen-Cilag, Veridex, Ventana Medical Systems, Astellas Pharma, Medivation, Novartis, Millennium, Abbott Laboratories, ESSA, Bayer, Pfizer, AstraZeneca, Ferring
Speakers' Bureau: Janssen, Astellas Pharma, Takeda, Sanofi, Ventana Medical Systems, Ipsen, AstraZeneca, Ferring
Research Funding: Janssen (Inst), Arno Therapeutics (Inst), Innocrin Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I am on The ICR rewards to inventors list of Abiraterone acetate
Travel, Accommodations, Expenses: Janssen, Astellas Pharma, Medivation, Ventana Medical Systems, Abbott Laboratories, Bayer, ESSA, Janssen, Astellas Pharma, Pfizer, Ferring
Other Relationship: Institute of Cancer Research
David Olmos
Honoraria: Janssen, Bayer, Astellas Pharma (Inst)
Consulting or Advisory Role: Janssen (Inst), AstraZeneca (Inst), Clovis Oncology, Bayer (Inst), Daiichi Sankyo, MSD Oncology
Research Funding: AstraZeneca (Inst), Bayer (Inst), Janssen (Inst), Genentech/Roche (Inst), Pfizer (Inst), Astellas Medivation (Inst), Tokai Pharmaceuticals (Inst), MSD Oncology (Inst)
Travel, Accommodations, Expenses: Bayer, Janssen, Ipsen, Astellas Pharma, Roche, AstraZeneca Spain
Eric Small
Stock and Other Ownership Interests: Fortis, Harpoon Therapeutics, Teon Therapeutics
Honoraria: Janssen, Johnson and Johnson
Consulting or Advisory Role: Janssen Oncology, Teon Therapeutics, Fortis
Open Payments Link: https://openpaymentsdata.cms.gov/physician/660367/summary
Andrea Pereira de Santana Gomes
Honoraria: Astellas Pharma, Bayer, Janssen Oncology, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Bayer
Speakers' Bureau: Janssen Oncology, Astellas Pharma, Bayer, AstraZeneca
Research Funding: Janssen Oncology, MSD Oncology, Bayer, Roche, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology
Guilhem Roubaud
Honoraria: Astellas Pharma (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst)
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen-Cilag (Inst), AstraZeneca (Inst), Ipsen (Inst), AAA/Endocyte/Novartis (Inst), Merck/Pfizer (Inst), Bayer (Inst), Bayer (Inst)
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag, Bayer
Marniza Saad
Honoraria: Astellas Pharma, Novartis, Johnson & Johnson, Ipsen, Cipla, AstraZeneca, Eisai, Pfizer, Amgen
Consulting or Advisory Role: Johnson & Johnson, MSD, AstraZeneca, Novartis, Merck, BMS, Viatris
Research Funding: Johnson & Johnson, MSD
Bogdan Zurawski
Employment: MSD Oncology, Janssen Oncology, Bristol Myers Squibb/Roche, Novartis, GlaxoSmithKline, Merck Serono
Honoraria: Roche, Astellas Pharma
Gary E. Mason
Employment: Janssen Research & Development, Merck
Stock and Other Ownership Interests: Johnson & Johnson, Merck
Travel, Accommodations, Expenses: Janssen Research & Development
Peter Francis
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Travel, Accommodations, Expenses: Johnson & Johnson
George Wang
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Daphne Wu
Employment: Johnson & Johnson/Janssen
Brooke Diorio
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Janssen Research & Development
Angela Lopez- Gitlitz
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Patents, Royalties, Other Intellectual Property: I am named as an inventor on the following patents: (1) WO 2021/224467 Treatment of Prostate Cancer with a Combination of Abiraterone Acetate and Niraparib (2) WO 2021/224469 Treatments of Prostate Cancer with Combinations of Abiraterone Acetate and Niraparib (3) WO 2021/224471 Pharmaceutical Formulations of Abiraterone Acetate and Niraparib
Shahneen Sandhu
Honoraria: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst)
Consulting or Advisory Role: AstraZeneca (Inst), Bristol Myers Squibb/Roche (Inst), Merck Sharp and Dohme (Inst), Amgen (Inst), Novartis (Inst), Genentech (Inst)
Speakers' Bureau: Bristol Myers Squibb, Merck, Roche/Genentech, AstraZeneca (Inst)
Research Funding: Amgen (Inst), AstraZeneca (Inst), Merck (Inst), Endocyte/Advanced Accelerator Applications (Inst), Genentech/Roche (Inst), Novartis (Inst), Pfizer (Inst), Senhwa Biosciences (Inst), Roche/Genentech (Inst)
Uncompensated Relationships: AAA/Endocyte/Novartis (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ryan CJ, Smith MR, Fizazi K, et al. : Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16:152-160, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Khalaf DJ, Annala M, Taavitsainen S, et al. : Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol 20:1730-1739, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Beer TM, Armstrong AJ, Rathkopf D, et al. : Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: Extended analysis of the phase 3 PREVAIL study. Eur Urol 71:151-154, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson D, Van Allen EM, Wu YM, et al. : Integrative clinical genomics of advanced prostate cancer. Cell 161:1215-1228, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui M, Gao XS, Gu X, et al. : BRCA2 mutations should be screened early and routinely as markers of poor prognosis: Evidence from 8,988 patients with prostate cancer. Oncotarget 8:40222-40232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro E, Romero-Laorden N, Del Pozo A, et al. : PROREPAIR-B: A prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J Clin Oncol 37:490-503, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Warner E, Herberts C, Fu S, et al. : BRCA2, ATM, and CDK12 defects differentially shape prostate tumor driver genomics and clinical aggression. Clin Cancer Res 27:1650-1662, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Jayaram A, Wingate A, Wetterskog D, et al. : Plasma tumor gene conversions after one cycle abiraterone acetate for metastatic castration-resistant prostate cancer: A biomarker analysis of a multicenter international trial. Ann Oncol 32:726-735, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Schiewer MJ, Goodwin JF, Han S, et al. : Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2:1134-1149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Karanika S, Yang G, et al. : Androgen receptor inhibitor-induced "BRCAness" and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal 10:eaam7479, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polkinghorn WR, Parker JS, Lee MX, et al. : Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 3:1245-1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asim M, Tarish F, Zecchini HI, et al. : Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun 8:374, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washington CR, Moore KN: PARP inhibitors in the treatment of ovarian cancer: A review. Curr Opin Obstet Gynecol 33:1-6, 2021 [DOI] [PubMed] [Google Scholar]
- 14.GlaxoSmithKline : ZEJULA™ (Niraparib) Highlights of Prescribing Information. Research Triangle Park, NC: US Food and Drug Administration, 2020 [Google Scholar]
- 15.Canada's Drug and Health Technology Agency : Niraparib (Zejula) for First Line Ovarian Cancer - Details. https://www.cadth.ca/niraparib-zejula-first-line-ovarian-cancer-details [Google Scholar]
- 16.Rosa K: Niraparib Approved in China for Frontline Maintenance in Ovarian Cancer https://www.onclive.com/view/niraparib-approved-in-china-for-frontline-maintenance-in-ovarian-cancer [Google Scholar]
- 17.GlaxoSmithKline : European Commission Approves Zejula (Niraparib) as First-Line Monotherapy Maintenance Treatment in Advanced Ovarian Cancer. GlaxoSmithKline, London, UK, 2020 [Google Scholar]
- 18.Smith MR, Scher HI, Sandhu S, et al. : Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): A multicentre, open-label, phase 2 trial. Lancet Oncol 23:362-373, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saad F, Chi KN, Shore ND, et al. : Niraparib with androgen receptor-axis-targeted therapy in patients with metastatic castration-resistant prostate cancer: Safety and pharmacokinetic results from a phase 1b study (BEDIVERE). Cancer Chemother Pharmacol 88:25-37, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Hussain M, Mateo J, Fizazi K, et al. : Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med 383:2345-2357, 2020 [DOI] [PubMed] [Google Scholar]
- 22.LYNPARZA (olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals, 2020 [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Scher HI, Morris MJ, Stadler WM, et al. : Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 34:1402-1418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bono JS, Mehra N, Scagliotti GV, et al. : Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): An open-label, phase 2 trial. Lancet Oncol 22:1250-1264, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Abida W, Patnaik A, Campbell D, et al. : Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol 38:3763-3772, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castro E, Goh C, Olmos D, et al. : Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 31:1748-1757, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annala M, Struss WJ, Warner EW, et al. : Treatment outcomes and tumor loss of heterozygosity in germline DNA repair–deficient prostate cancer. Eur Urol 72:34-42, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Annala M, Vandekerkhove G, Khalaf D, et al. : Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov 8:444-457, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Sandhu S, Attard G, Olmos D, et al. : Gene-by-gene analysis in the MAGNITUDE study of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. J Clin Oncol 40:5020, 2022 [Google Scholar]
- 31.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith MR, Saad F, Chowdhury S, et al. : Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 378:1408-1418, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Zajaczkowska R, Kocot-Kepska M, Leppert W, et al. : Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci 20:1451, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fizazi K, Scher HI, Molina A, et al. : Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13:983-992, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Rathkopf DE, Roubaud G, Chi KN, et al. : Health-related quality of life (HRQoL) and pain in the MAGNITUDE study of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. J Clin Oncol 40:5060, 2022 [Google Scholar]
- 36.Fizazi K, Tran N, Fein L, et al. : Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 377:352-360, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. : Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid 1:1-16, 2022 [DOI] [PubMed] [Google Scholar]
- 38.Higano C: MAGNITUDE and PROpel Discussion. Presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium. February 17-19, 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.