Abstract
Objective:
To determine the safety of hepatectomy after combined lenvatinib and anti-PD-1 preoperative systemic therapy (PST) in patients with marginally resectable hepatocellular carcinoma (HCC).
Background:
PST followed by hepatectomy (PSTH) is an emerging treatment for HCC. However, the impact of PST with lenvatinib plus anti-PD-1 antibodies on surgical safety is unknown.
Methods:
Medical records from consecutive patients with marginally resectable advanced HCC who underwent hepatectomy after PST with lenvatinib and anti-PD-1 antibodies between January 2018 and August 2021 were retrieved from a prospectively designed database. Propensity score matching (1:2) was performed with a further 2318 HCC patients who underwent upfront hepatectomy (UH) without initial antitumor treatment during the same period.
Results:
In total, 49 and 98 matched patients were included in the PSTH and UH groups, respectively. Compared to the UH group, individuals in the PSTH group experienced more intraoperative blood loss, blood transfusions, and longer postoperative hospital stays. Moreover, posthepatectomy liver failure was more common in the PSTH group, who also had worse albumin-bilirubin (ALBI) scores on postoperative days 1–7. A significantly greater amount of drainage was also required in the PSTH group. However, the 30-day morbidity and 90-day mortality were similar among the two groups. Additionally, the duration of surgery, use of hepatic inflow occlusion during surgery, and the levels of postoperative inflammation-based markers were not statistically different between the two groups.
Conclusions:
Despite more intraoperative and postoperative adverse events, PSTH had comparable 30-day morbidity and 90-day mortality as UH. Thus, PSTH appears to be a viable treatment option for marginally resectable HCC patients with careful preoperative evaluation.
Keywords: hepatocellular carcinoma, immunotherapy, lenvatinib, resection
Mini-abstract: This propensity score-matched study assessed the safety of combined lenvatinib and anti-PD-1 preoperative systemic therapy prior to hepatectomy (PSTH), compared to upfront hepatectomy (UH), in patients with hepatocellular carcinoma. Despite more adverse events in the PSTH group, the 30-day morbidity and 90-day mortality rates were similar for both groups.
INTRODUCTION
Hepatectomy represents the best treatment option for patients with hepatocellular carcinoma (HCC).1 Unfortunately, most HCC patients are diagnosed at an advanced stage of disease, often missing the opportunity for radical resection, due to declining liver function, higher tumor burden, or metastatic disease.2 However, the last 5 years have seen significant progress in the nonsurgical treatment of liver cancer. For example, systemic therapy, and especially the combination of targeted or antiangiogenic drugs and immunotherapy, can achieve an objective response rate (ORR) of around 30% in patients with advanced or unresectable liver cancer.3,4 Reducing tumor size using systemic therapy prior to hepatectomy (also known as neoadjuvant therapy) is therefore being considered as a promising treatment strategy for patients with initially unresectable HCC.5,6
Based on these premises, Ho et al7 published positive results of a phase I study (NCT03299946) investigating the neoadjuvant combination of oral cabozantinib together with nivolumab in 15 patients with borderline resectable or locally advanced HCC. Furthermore, Zhu et al8 reported that when 63 patients with initially unresectable HCC were treated with an antiprogrammed cell death receptor (PD)-1 antibody plus tyrosine kinase inhibitors (TKIs), the conversion rate for resection was 15.9%. Finally, Lu et al9 showed that ten HCC patients with major vascular invasion met the successful conversion criteria after receiving systemic combination therapy consisting of PD-1 and TK inhibitors.
Despite these promising initial findings showing that systemic therapy allows a proportion of patients with initially unresectable HCC to undergo surgery, numerous preoperative systemic treatment (PST)-associated adverse events (AEs) have been reported, potentially compromising the efficacy of this preoperative treatment approach. In the phase 3 REFLECT trial of lenvatinib versus sorafenib monotherapy in patients with advanced HCC, the most common treatment emergent adverse events (TEAEs) among patients who received lenvatinib were hypertension (42%), diarrhea (39%), decreased appetite (34%), and weight loss (31%), which led to lenvatinib drug interruption in 40% of patients, dose reduction in 37% of patients, and drug withdrawal in 9% of patients.10 Furthermore, in the phase Ib KEYNOTE-524 study of lenvatinib plus pembrolizumab in patients with advanced HCC, hypertension (36%), diarrhea (35%), fatigue (30%), decreased appetite (28%), and hypothyroidism (25%) were the most common AEs, with drug discontinuation reported for 6% of patients.11 Liver-associated AEs were also relatively common in both of the aforementioned studies, with probabilities of 15%–30%.10,11 In this study, we therefore set out to evaluate the impact of PST on the safety of subsequent hepatectomy in patients with marginally resectable HCC.
METHODS
Patients
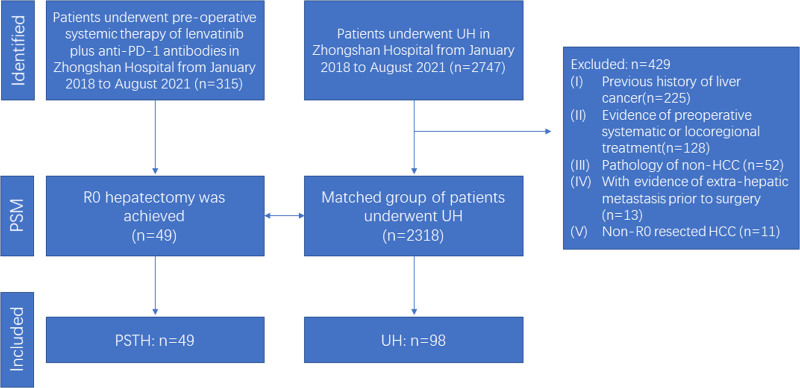
This retrospective study included consecutive HCC patients who underwent PST with lenvatinib and an anti-PD-1 antibody, followed by hepatectomy, at the Zhongshan Hospital (Xuhui District, Shanghai, China) between January 2018 and August 2021. The PST information and dosing regimens were described in detail in our previous research.8 HCC was diagnosed using standard imaging techniques either in the presence or absence of elevated serum tumor markers. Patients were considered inoperable before the initiation of PST if they had advanced stage HCC or insufficient remnant liver volume (<40% of standard liver volume for patients with liver cirrhosis or <30% of standard liver volume for patients without liver cirrhosis) or outside up-to-seven criteria.12 Patients who received preoperative locoregional treatment, including transarterial chemoembolization (TACE) or hepatic artery infusion chemotherapy (HAIC) were excluded. Patients receiving other types of monotherapy or combination regimens were also excluded. A control cohort was comprised 2318 patients who underwent upfront hepatectomy (UH) for pathologically confirmed HCC during the same time period. The exclusion criteria for the UH patient group were as follows: (1) previous history of liver cancer; (2) evidence of preoperative systematic or locoregional treatment; (3) non-HCC pathology; or (4) evidence of extrahepatic metastasis prior to surgery; (5) non-R0 resected HCC (Fig. 1). Following propensity score matching (PSM; 1:2), a total of 49 and 98 HCC patients were included in the PSTH and UH groups, respectively. All patients included in this study provided written informed consent before undergoing PSTH or UH at Zhongshan Hospital. This study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1975 and was approved by Zhongshan Hospital Research Ethics Committee.
FIGURE 1.
Flowchart of patient selection. .
Hepatectomy
Patients receiving PST were evaluated every 2 months for AEs, tumor response, and resectability. Hepatectomy was considered possible if (1) R0 resection could be achieved with sufficient remnant liver volume (≥40% of standard liver volume for patients with liver cirrhosis or ≥30% of standard liver volume for patients without liver cirrhosis); (2) the intrahepatic tumor was evaluated as a partial response (PR) or stable disease for at least 2 months according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (3) no severe AEs occurred following lenvatinib and anti-PD-1 antibody combination PST, according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0; and (4) there was no contraindication for hepatectomy.
Patients who fulfilled the liver resection criteria had to discontinue anti-PD-1 antibody therapy for at least 1 month and lenvatinib treatment for at least 1 week before surgery. The surgical procedures for PSTH and UH patient groups were similar, according to the Brisbane 2000 terminology13 and guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition).14 Hepatectomy was performed as previously described,15,16 and was defined as either major (more than 3 segments were resected) or minor (no more than 3 segments were resected), according to the number of resected segments.
Data Collection
Preoperative characteristics of the patients with HCC in the PSTH and UH groups were collected from medical records stored in Zhongshan Hospital. The PSTH tumor response was evaluated by abdominal contrast-enhanced magnetic resonance imaging (MRI)/computed tomography (CT) according to RECIST version 1.1.17 Posthepatectomy liver failure (PHLF) was assessed according to the criteria established by the International Study Group of Liver Surgery (ISGLS);18 ALBI scores were calculated as (0.66 × log10 bilirubin) + (−0.085 × albumin) on postoperative day (POD)s 1, 3, 5, and 7, where bilirubin was measured in μmol/L and albumin in g/L19; postoperative complications were classified using the Clavien-Dindo classification20; and the systemic immune-inflammation index (SII = platelet count × neutrophil count/lymphocyte count), platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and monocyte-to-lymphocyte ratio (MLR), were measured on PODs 1, 3, 5, and 7.21
PSM Analysis
PSM analysis was employed to reduce bias in patient selection to investigate the differences between the UH and PSTH groups. 1:2 PSM was performed based on the “nearest neighbor” method with a caliper width of 0.02. Variables including age, gender, α-fetoprotein, hemoglobin (HB), total bilirubin, albumin, alanine aminotransferase (ALT), aspartate aminotransferase, gamma-glutamyl transferase, prothrombin time, international normalized ratio, HBsAg, HCV-RNA, presence of ascites, severity of hepatic cirrhosis, the number of tumors and maximal tumor size, were comprehensively included in the calculation of the propensity score. All the matched indicators were measurements at the time of 3–5 days before hepatectomy.
Statistical Analysis
Statistical analyses were performed using SPSS software version 25.0 (IBM Corp., Armonk, NY), R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria), and GraphPad Prism 8 (LLC, San Diego, CA). Continuous variables were presented as medians (range). Mann–Whitney U-test (Wilcoxon rank-sum test), Student’s t-test, Pearson χ2 test, and Fisher’s exact test were used to assess statistical significance as appropriate. A repeated measures ANalysis Of VAriance (ANOVA) was used for indicator analysis of multiple measurements postoperatively. P < 0.05 was used as a threshold of statistical significance.
RESULTS
Patient Characteristics
A total of 49 HCC patients who underwent PSTH were eligible for this study; the patients’ characteristics are shown in Table 1 and Supplemental Table 1, http://links.lww.com/AOSO/A120. Of the 49 patients in the PSTH group, 2 had Barcelona Clinic Liver Cancer stage A disease with insufficient future remnant liver volume if resected, and 8 with Barcelona Clinic Liver Cancer stage B HCC did not receive TACE before surgery due to exceeding the up-to-seven criteria. 31 and 18 were classified as Eastern Cooperative Oncology Group 0 and 1, respectively, and all patients achieved a Child-Pugh class A score before undergoing surgery. With the exception of one HCC patient, who was classed as having progressive disease (PD) due to the development of adrenal metastases during systemic therapy (despite his intrahepatic lesion being classed as PR), all patients were classed as having either PR or stable disease for at least 2 months, according to RECIST v1.1. All 49 patients met the hepatectomy indication criteria.
TABLE 1.
Characteristics of the 49 HCC Patients in the PSTH Group, Before Hepatectomy
| Characteristic | PSTH-receiving HCC Patients (n) |
|---|---|
| BCLC stage (A/B/C) (Before PST) | 2/8/39 |
| China liver cancer stage (Ib/IIa/IIb/IIIa/IIIb) (Before PST) | 2/3/5/38/1 |
| ECOG performance status (0/1/2) | 31/18/0 |
| Child-Pugh class (A/B) | 49/0 |
| Anti-PD-1 antibody use (Niv/Pem/Sin/Cam) | 13/22/4/10 |
| Tumor response, according to RECIST v1.1 (CR/PR/SD/PD) | 0/33/15/1 |
| Tumor response, according to mRECIST (CR/PR/SD/PD) | 4/34/10/1 |
BCLC, Barcelona Clinic Liver Cancer; Cam, camrelizumab; CR, complete response; ECOG, Eastern Cooperative Oncology Group; mRECIST, modified RECIST; Niv, nivolumab; PD, progressive disease; Pem, pembrolizumab; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; Sin, sintilimab.
A total of 2318 HCC patients who underwent UH were eligible for this study. Before PSM, statistically significant differences were found in baseline HB, albumin, α-fetoprotein, ALT, prothrombin time, international normalized ratio, presence of ascites, and single or multiple tumors, between UH (n = 2318) and PSTH groups (n = 49). Ninety-eight patients were identified in the UH group after PSM. There were no significant differences in the baseline characteristics of individuals in the post-PSM PSTH (n = 49) and UH (n = 98) groups (Supplemental Table 1, http://links.lww.com/AOSO/A120), who were included in all subsequent analyses.
Intraoperative findings
In total, 25 of 49 patients in the PSTH group and 46 of 98 patients of the UH group received major hepatectomy (removal of ≥3 liver segments; 52.1% vs 46.9%; P = 0.641). The average blood loss (545 vs 230 ml; P < 0.001) and rate of blood transfusion (16.3% vs 5.1%; P = 0.032) were significantly higher in the PSTH group, compared to the UH group. However, there were no significant differences between the two groups in either the duration (27 vs 25 minutes; P = 0.165) or incidence (95.9 vs 98.0; P = 0.147) of Pringle maneuvers, or surgery duration (269 vs 260 minutes; P = 0.543) (Table 2).
TABLE 2.
Surgical Outcomes After PSM
| Outcomes | PSTH Group (n = 49) | UH Group (n = 98) | P |
|---|---|---|---|
| Surgery duration (minutes) | 269 | 260 | 0.543 |
| Major hepatectomy, n (%) | 25 (52.1) | 46 (46.9) | 0.641 |
| Blood loss (ml) | 545 | 230 | 0.001† |
| Blood transfusion, n (%) | 8 (16.3) | 5 (5.1) | 0.032* |
| Pringle maneuver time (minutes) | 27 | 25 | 0.165 |
| Pringle maneuver rate(0/1/≥2) | 2/14/33 | 2/44/52 | 0.147 |
| Hospital stay duration (days) | 12 | 8 | 0.001† |
| PHLF (0/A/B/C) | 35/12/1/1 | 87/10/1/0 | 0.028† |
| 30-day morbidity (0/1/2/≥3) | 31/4/7/7 | 75/8/10/5 | 0.203 |
| 90-day mortality | 1 | 0 | 0.333 |
Values are presented as n (%). Pearson χ2, Fisher’s exact, and Mann–Whitney U (Wilcoxon rank-sum test) tests were used, as appropriate;
*P < 0.05.
†P < 0.01.
Postoperative Outcomes
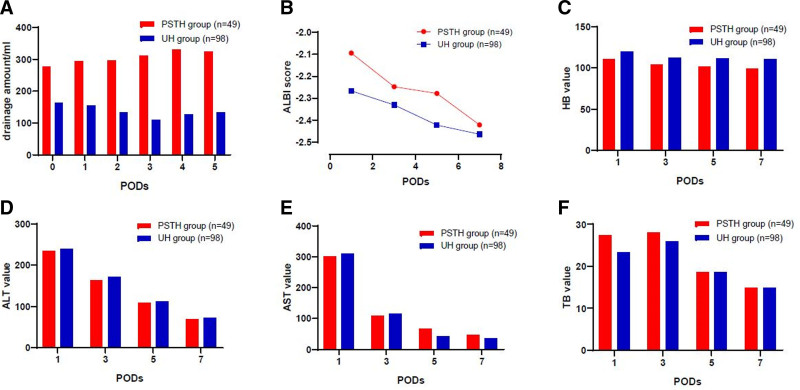
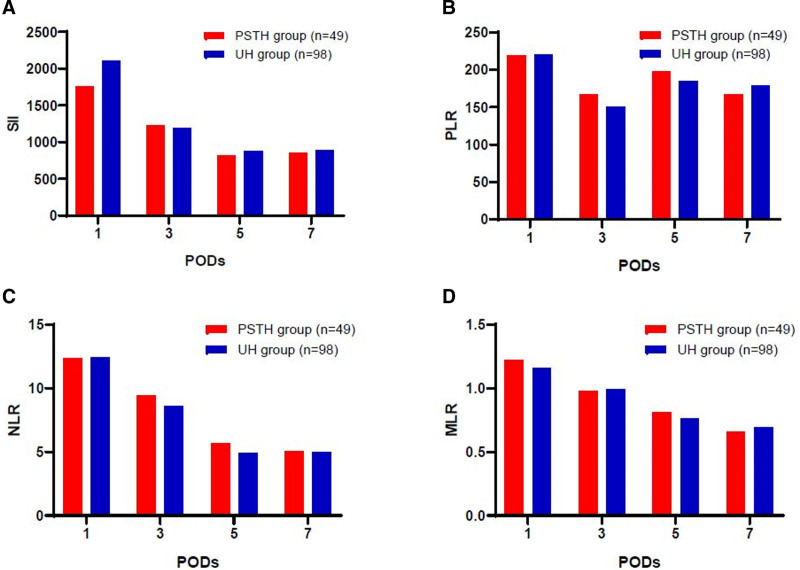
We next examined postoperative outcomes and found a significant difference in the average postoperative hospital stay for HCC patients in the PSTH and UH groups (12 vs 8 days, P = 0.001). The amount of postsurgical drainage was also significantly greater for individuals in the PSTH group than in UH group (P = 0.001). Six of 49 patients in PSTH group and one of 98 patient of the UH group underwent ultrasound-guided puncture and catheterization due to effusion at the surgical site or thoracic cavity (P = 0.006). Although the levels of ALT, aspartate aminotransferase, and total bilirubin were comparable on PODs 1–7 in the 2 groups (P = 0.783, P = 0.852, and P = 0.954, respectively, repeated measures ANOVA were adopted), patients in the PSTH group had a higher ALBI score during the postoperative hospital stay period (P = 0.008), and lower HB levels on PODs 1–7 (P = 0.001) (Fig. 2). The rate of PHLF was 28.6% in the PSTH group (14/49 patients), with class A failure experienced by 12 patients, class B by one patient, and class C by one patient. In comparison, the rate of PHLF was significantly lower in the UH group (11.2%, 11/98 patients; P = 0.028), with class A failure experienced by 10 patients, class B by one patient, and no class C events reported. Furthermore, there were no differences in the following inflammation marker values: SII, PLR, NLR, and MLR (P = 0.376; P = 0.519; P = 0.456; P = 0.642, respectively; repeated measures ANOVA were adopted), between the PSTH and UH groups during the postoperative hospital stay (Fig. 3).
FIGURE 2.
Postoperative outcomes after PSM analysis of the PSTH and UH patients. Changes in the: (A) amount of drainage; (B) ALBI score; (C) HB value; (D) ALT value; (E) AST value; and (F) TB value, on PODs 1, 3, 5, and 7. Repeated measures ANOVA was used for the statistical analysis. AST, aspartate aminotransferase; TB, total bilirubin.
FIGURE 3.
Comparison of postoperative inflammation-associated markers between the PSTH and UH groups. Changes in the amount of (A) SII; (B) PLR; (C) NLR; and (D) MLR, on PODs 1, 3, 5, and 7. Repeated measures ANOVA was used for the statistical analysis.
The 30-day postoperative complications rates, classified according to the Clavien-Dindo criteria, were not significantly different between the PSTH and UH groups (36.7% vs 23.5%, respectively; P = 0.203). The 90-day mortality rate for the PSTH group was 2.0% (1/49); the patient died 2.4 months after their hepatectomy, most likely from a fulminant immunoreaction in his liver, lung, pancreas, and skin. Meanwhile, no deaths were reported within 90 days of hepatectomy in the UH group. However, the difference in the 90-day mortality rates for the 2 study groups was not significant (P = 0.333).
DISCUSSION
In the present study, we compared various surgical safety parameters in HCC patients who underwent combined lenvatinib and anti-PD-1 antibody PSTH or UH, and demonstrated that PSTH represents a feasible option for patients with initially unresectable HCC. However, we found that HCC patients in the PSTH group experienced more AEs, including intraoperative blood loss, which resulted in higher transfusion rates and lower postoperative HB levels. The reasons for this difference in blood loss between the PSTH and UH groups could be associated with lenvatinib treatment. Lenvatinib is an oral small molecule, multikinase inhibitor of vascular endothelial growth factor (VEGF) receptors 1, 2, and 3, as well as other receptor tyrosine kinases implicated in proangiogenic and oncogenic signaling pathways.22,23 Inhibiting the proangiogenic function of VEGF may raise the blood pressure within the delicate tumor microvasculature and increase vascular fragility, thus leading to more blood loss. This notion is supported by findings from a phase III trial of lenvatinib in HCC patients, which reported that hypertension occurred in 42% of patients treated with lenvatinib.10 Moreover, Uchida-Kobayashi et al24 showed that intracerebral, upper gastrointestinal, intestinal, and tumoral hemorrhaging occurred in 24.6% of HCC patients receiving lenvatinib.
The abdominal drainage volume for HCC patients in the PSTH group was higher compared to the UH group, and it was still increasing 5 days after surgery in the PSTH group. Furthermore, a higher proportion of patients in the PSTH group underwent ultrasound-guided puncture and catheterization due to effusion at the surgical site or thoracic cavity. This greater production of abdomen fluid in patients receiving PSTH may be related to the effect of lenvatinib and anti-PD-1 combination therapy. It has been reported that TKIs may increase vascular permeability,25 which may lead to increased postoperative drainage.
PHLF is a relatively rare but life-threatening complication that occurs after hepatectomy. The rate of PHLF has been reported in the literature to range from 9% to 18.6%, according to the ISGLS definition.26 Both PD-1 and TK inhibitors have been reported to cause functional liver damage.27,28 Although all patients in the PSTH group received a class A Child-Pugh score after a sufficiently long period of discontinuation of systemic therapy and evaluation, the impact of PST on liver function is likely to increase the probability of PHLF. Indeed, the PHLF rate in the PSTH group was 28.6%, which is much higher than that of the UH group (11.2%). Furthermore, PSTH group patients had a significantly higher ALBI score than those in the UH group. However, the rate of PHLF among patients in the PSTH group was comparable to that associated with hepatectomy procedures such as associated liver partition and portal vein ligation for staged hepatectomy (ALPPS), in which PHLF is reported to occur at a rate of 26.9%.29,30 Meanwhile, more basic step-down procedures, such as sequential transcatheter arterial chemoembolization (TACE) and portal vein embolization (PVE) (TACE-PVE), or portal vein ligation and PVE alone, are associated with much lower PHLF rates of 6.2%–7.5%.31,32 Furthermore, in a prior study of hepatectomy after chemotherapy for colorectal liver metastases, preoperative chemotherapy was associated with liver injury and 10.2% of patients had class ≥ B PHLF scores.33,34 In addition, a further study showed that for patients receiving bevacizumab, a monoclonal antibody that inhibits VEGF, subsequent extended hepatic resection may be encouraged due to its good tolerability and positive impact on liver regeneration.35 In summary, in the present study, we showed that, although PSTH led to a higher incidence of PHLF than UH, PSTH-associated PHLF was predominantly class A, compared to other investigation of preoperative chemotherapy that reported a higher proportion of class ≥ B PHLF scores.
We next compared the impact of combined lenvatinib and anti-PD-1 PSTH on the 90-day mortality of HCC patients. In the present study, one patient in the PSTH group died 2.4 months after undergoing hepatectomy. The cause of death was immune hepatitis, and not the typical postoperative complications, which result in relatively low postoperative mortality. We therefore concluded that anti-PD-1 treatment, followed by hepatectomy, may be associated with a risk of fulminant immunoreaction. Besides this incident, there were no differences in the duration of surgery, postoperative complications, or mortality between the PSTH and UH groups, which suggests that PSTH is safe and feasible after careful patient evaluation.
Physical stress and medication induce changes in organ function and, subsequently, the levels of various inflammatory markers.36 Monitoring inflammatory markers such as SII, PLR, NLR, and MLR is a rapid and cost-effective method for evaluating a patient’s risk of disease progression and death after surgery.21 Patients subjected to PSTH are likely to suffer from multiple forms of physical and biological stress, induced by surgery and/or PST. A given patient’s response to PSTH could therefore be measured via specific inflammatory indicators. A previous study from our hospital demonstrated that SII serves as an independent predictor of prognosis after hepatectomy in patients with HCC,37 and is associated with a shorter hospital stay.36 In the present study, the SII, PLR, NLR, and MLR were not significantly different between the PSTH and UH groups, which suggests that PSTH and UH may produce a similar degree of physical stress and inflammation.
This study had several limitations. First, the long-term outcomes of patients who underwent either PSTH or UH is unknown as long-term follow-up data are not available. Second, a variety of different anti-PD-1 antibodies were used. To date, there was no evidence for differences in the effects of different anti-PD-1 antibodies, either as monotherapy4,38,39 or in combination therapy11,40,41. Third, the sample size was small, meaning that some rare complications, such as immune-related problems, were not well represented in our study groups. In addition, the preoperative laboratory tests used in this study may not have accurately described the inflammatory state of the liver tissue.
In summary, we report the intraoperative and postoperative outcomes of HCC patients who received PSTH, in comparison to those undergoing UH alone. To the best of our knowledge, the present study was performed on the largest cohort of hepatectomized HCC patients in receipt of lenvatinib and anti-PD-1 PSTH reported to date. We found that, although the patients in the PSTH group experienced more AEs postsurgery, including higher average blood loss and blood transfusion rate, a larger postoperative drainage volume, a longer postoperative hospital stay, and a higher rate of PHLF, their 30-day morbidity and 90-day mortality rates were similar to those of the UH group. Our preliminary findings therefore suggest that the combined lenvatinib and anti-PD-1 preoperative treatment strategy is feasible for use in patients with unresectable HCC, and that subsequent hepatectomy is considered safe after careful patient evaluation.
ACKNOWLEDGMENTS
We thank all participants and their families for their involvement in this study. We thank our clinical investigators Miss Li M-L and Miss Zhu J-J. We thank Professor Liang F for his statistical review of this study.
Supplementary Material
Footnotes
Published online 2 May 2022
Disclosure: H.-C.S. has received speaker fees from Bayer, Eisai, Roche, MSD, Hengrui, Innovent, Beigene, TopAlliance and Zelgene. X.-D.Z. has received speaker fees from Eisai and MSD. For the remaining authors none were declared. This study was funded by the National Natural Science Foundation of China (Grant No. 82172799, 81672326, 81871928, and 81871929) and the Leading Investigator Program of the Shanghai municipal government (2019).
Y.-H.S., C.H., X.-D.Z., M.-H.X. contributed equally to this study. H.-C.S., Y.-H.S., C.H., X.-D.Z., M.-H.X., Z.-S.C., and C.-J.T. contributed in the surgery and collection of data. J.Z., J.F., H.-C.S., Y.-H.S., and M.-H.X. did the analysis and interpretation of data. H.-C.S., Y.-H.S., and M.-H.X. interpreted the results and wrote the article. All authors give final approval of the version to be published.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, Qin S, Ikeda M, et al.; IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Ryoo BY, Merle P, et al.; KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. [DOI] [PubMed] [Google Scholar]
- 5.Pinato DJ, Fessas P, Sapisochin G, et al. Perspectives on the neoadjuvant use of immunotherapy in hepatocellular carcinoma. Hepatology. 2021;74:483–490. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo A. Tyrosine kinase inhibitors plus immune checkpoint inhibitors as neoadjuvant therapy for hepatocellular carcinoma: an emerging option? Expert Opin Investig Drugs. 2021;31:1–3. [DOI] [PubMed] [Google Scholar]
- 7.Ho WJ, Zhu Q, Durham J, et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced hcc into resectable disease with enhanced antitumor immunity. Nat Cancer. 2021;2:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu XD, Huang C, Shen YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer. 2021;10:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Hu B, Han J, et al. Surgery after conversion therapy with PD-1 inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: a pilot study of ten patients. Front Oncol. 2021;11:747950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M, Arizumi T, Ueshima K, et al. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified Bolondi’s subclassification (Kinki criteria). Dig Dis. 2015;33:751–758. [DOI] [PubMed] [Google Scholar]
- 13.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepato-biliary Pancreatic Surgery. 2005;12:351–355. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9:682–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han HS, Shehta A, Ahn S, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma: case-matched study with propensity score matching. J Hepatol. 2015;63:643–650. [DOI] [PubMed] [Google Scholar]
- 16.Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:721–727. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European J Cancer (Oxford, England: 1990). 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 18.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713–724. [DOI] [PubMed] [Google Scholar]
- 19.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273:532–541. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichikawa K, Watanabe Miyano S, Minoshima Y, et al. Activated FGF2 signaling pathway in tumor vasculature is essential for acquired resistance to anti-VEGF therapy. Sci Rep. 2020;10:2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uchida-Kobayashi S, Kageyama K, Yamamoto A, et al. Lenvatinib-induced tumor-related hemorrhages in patients with large hepatocellular carcinomas. Oncology. 2021;99:186–191. [DOI] [PubMed] [Google Scholar]
- 25.Xuan Z, Chen C, Tang W, et al. TKI-resistant renal cancer secretes low-level exosomal miR-549a to induce vascular permeability and angiogenesis to promote tumor metastasis. Front Cell Dev Biol. 2021;9:689947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skrzypczyk C, Truant S, Duhamel A, et al. Relevance of the ISGLS definition of posthepatectomy liver failure in early prediction of poor outcome after liver resection: study on 680 hepatectomies. Ann Surg. 2014;260:865–870; discussion 870. [DOI] [PubMed] [Google Scholar]
- 27.D’Avola D, Granito A, de la Torre-Aláez M, Piscaglia F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 28.Choi WM, Lee D, Shim JH, et al. Effectiveness and safety of nivolumab in Child-Pugh B Patients with hepatocellular carcinoma: a real-world cohort study. Cancers (Basel). 2020;12:E1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Björnsson B, Sparrelid E, Hasselgren K, et al. Associating liver partition and portal vein ligation for primary hepatobiliary malignancies and non-colorectal liver metastases. Scand J Surg. 2016;105:158–162. [DOI] [PubMed] [Google Scholar]
- 30.Cai X, Tong Y, Yu H, et al. The ALPPS in the treatment of hepatitis B-related hepatocellular carcinoma with cirrhosis: a single-center study and literature review. Surg Innov. 2017;24:358–364. [DOI] [PubMed] [Google Scholar]
- 31.Ji W, Li JS, Li LT, et al. Role of preoperative selective portal vein embolization in two-step curative hepatectomy for hepatocellular carcinoma. World J Gastroenterol. 2003;9:1702–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogata S, Belghiti J, Farges O, et al. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. [DOI] [PubMed] [Google Scholar]
- 33.Yoh T, Perrot A, Beaufrère A, et al. Liver surface nodularity: a novel predictor of post-hepatectomy liver failure in patients with colorectal liver metastases following chemotherapy. Eur Radiol. 2021;31:5830–5839. [DOI] [PubMed] [Google Scholar]
- 34.Robinson SM, Wilson CH, Burt AD, et al. Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:4287–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valverde A, Ciria R, Caballero-Villarraso J, et al. Bevacizumab allows preservation of liver function and its regenerative capacity after major hepatectomy. Anticancer Agents Med Chem. 2019;19:1388–1398. [DOI] [PubMed] [Google Scholar]
- 36.Chen JF, Fu XT, Gao Z, et al. Laparoscopic vs. open repeat hepatectomy for recurrent liver tumors: a propensity score-matched study and meta-analysis. Front Oncol. 2021;11:646737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. [DOI] [PubMed] [Google Scholar]
- 38.Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Annal Oncol. 2019;30. [Google Scholar]
- 39.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571–580. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, Shen J, Gu S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27:1003–1011. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, Zhu XD, Shen YH, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res. 2021;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.