Abstract
Endometriosis, a heterogeneous, inflammatory, and estrogen-dependent gynecological disease defined by the presence and growth of endometrial tissues outside the lining of the uterus, affects approximately 5–10% of reproductive-age women, causing chronic pelvic pain and reduced fertility. Although the etiology of endometriosis is still elusive, emerging evidence supports the idea that immune dysregulation can promote the survival and growth of retrograde endometrial debris. Peritoneal macrophages and natural killer (NK) cells exhibit deficient cytotoxicity in the endometriotic microenvironment, leading to inefficient eradication of refluxed endometrial fragments. In addition, the imbalance of T-cell subtypes results in aberrant cytokine production and chronic inflammation, which contribute to endometriosis development. Although it remains uncertain whether immune dysregulation represents an initial cause or merely a secondary enhancer of endometriosis, therapies targeting altered immune pathways exhibit satisfactory effects in preventing disease onset and progression. Here, we summarize the phenotypic and functional alterations of immune cells in the endometriotic microenvironment, focusing on their interactions with microbiota and endocrine and nervous systems, and how these interactions contribute to the etiology and symptomology of endometriosis.
Keywords: Endometriosis, Immune cells, Microenvironment, Inflammation
Introduction
Endometriosis is an enigmatic, chronic, and inflammatory gynecological condition that affects 5–10% of women of childbearing age worldwide.[1] Women with endometriosis are predisposed to experience chronic pelvic pain, dysmenorrhea, and reduced fertility, which are harmful to women's physical and mental health. Endometriosis is defined by the presence and growth of vascularized endometrial tissues outside the uterus, which typically implant onto and survive on the peritoneal surface (superficial peritoneal lesions) or ovaries (ovarian endometrioma). Nonetheless, the etiology of endometriosis is still poorly understood. Retrograde menstruation, which refers to the reflux of endometrial debris containing glandular and stromal cells into the pelvic cavity during menstruation, is believed to be the primary origin of endometriosis.[2] Although increased exposure to menstruation (early age at menarche, shorter menstrual cycles, and prolonged menstrual bleeding) contributes to a higher risk of endometriosis,[1] the theory has several explanatory difficulties. For example, this theory is unable to well explain the low incidence of endometriosis, although it is estimated that retrograde menstruation occurs in more than 90% of reproductive-age women, implying that certain underlying factors may drive the process as well.
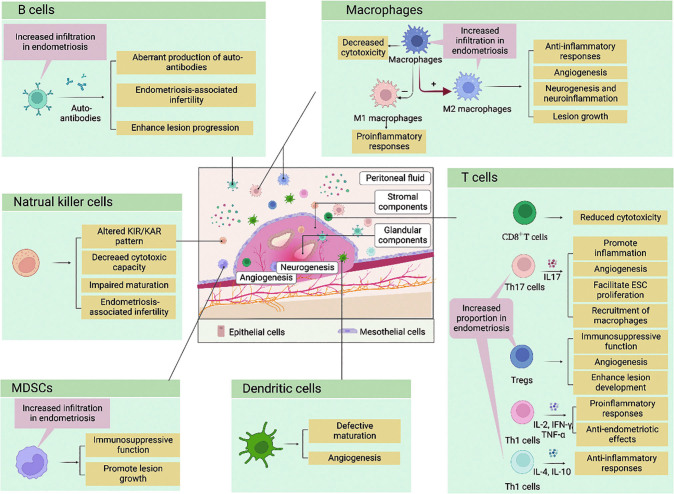
Endometriosis is a multifactorial condition in which hereditary, endocrine, inflammatory, and environmental signals converge to initiate and sustain the development of ectopic lesions. In recent years, immune dysregulation has become one of the most intriguing theories pertaining to the pathogenesis of endometriosis. Emerging evidence has reported a phenotypic and functional adaptation of diverse immune cells in response to stimuli of chronic inflammation provoked by ectopic lesions.[3-5] Based on the retrograde menstruation theory proposed by Sampson,[2] deficient cytotoxicity of various effector immune cells has been confirmed in patients with endometriosis. This may contribute to the establishment of an immunosuppressive milieu within the endometriotic lesions, which is favorable for the survival and proliferation of regurgitated endometrium fragments. Furthermore, transcriptional analysis of immunoinflammatory signatures revealed distinct molecular profiles between the ectopic endometrium from endometriosis patients and the eutopic endometrium from the control. Moreover, among the differentially expressed genes, those implicated in inflammation regulation, immune cell recruitment, and cellular adhesion were aberrantly elevated in the ectopic lesions.[6] Additionally, recent advances in multiomics and single-cell sequencing technology have enabled a more comprehensive appreciation of immunological scenarios within the endometriotic niche. The integrated analysis of single-cell RNA sequencing and bulk tissue deconvolution identified different cellular compositions of endometria from endometriosis and control, emphasizing that both immune cells and non-immune cells in endometriotic patients exerted proinflammatory effects on the eutopic endometrial environment [Figure 1].[7]
Figure 1.
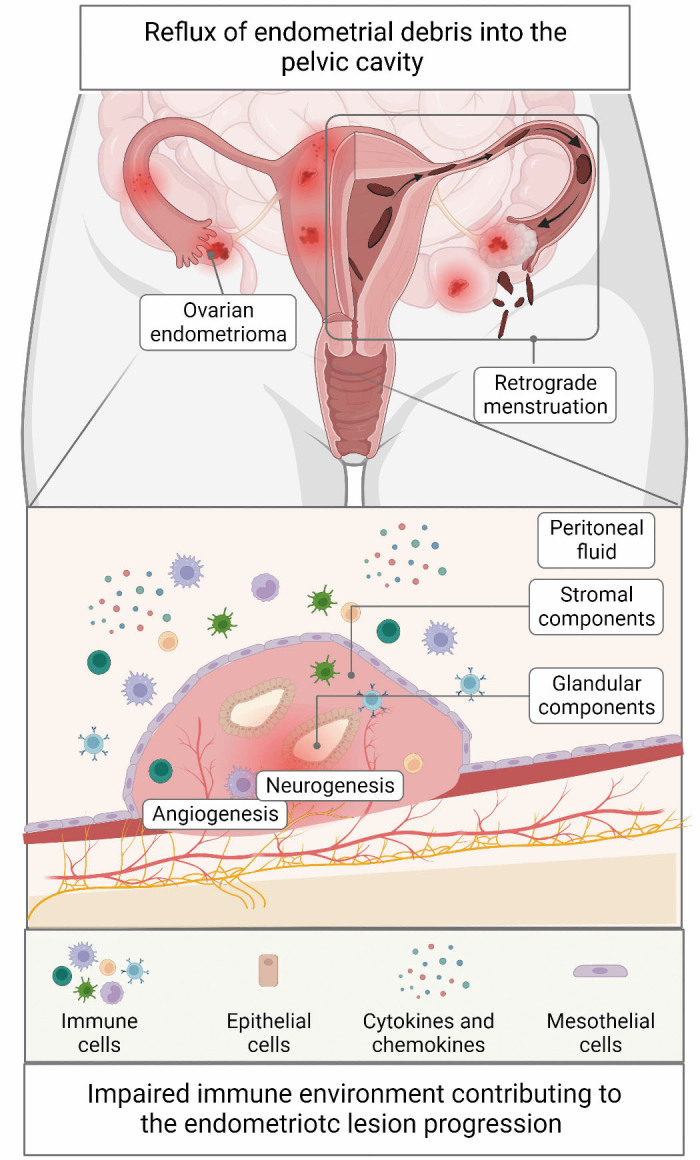
Etiology of endometriosis: retrograde menstruation and impaired immune environment. Following the retrograde menstruation theory proposed by Sampson, stromal and glandular components of shed endometrial tissue reflux into the pelvic cavity during menstruation. The regurgitated fragments trigger an aberrant infiltration of multiple immune cells into the endometriotic niche, inducing reduced cytotoxicity and aberrant cytokine secretion. The impaired microenvironment, in return, contributes to the angiogenesis, neurogenesis, and endometriotic lesion progression.
In this review, we summarized the hallmarks of the immune components of the endometriotic microenvironment with a focus on their crosstalk with microbiota and endocrine and nervous systems, highlighting the potential of immunotherapy in the management of endometriosis [Table 1].
Table 1.
Changes in the frequency and function of immune cell population in endometriosis.
| Immune cell | Peritoneal fluid | Eutopic endometrium | Ectopic endometrium | Function |
|---|---|---|---|---|
| Macrophages | Increased M2 macrophages[4] | Increased macrophages in global[4] | Increased M2 macrophages[4] | Deficient cytotoxicity in eliminating refluxed fragments |
| Decreased M1 macrophages[4] | Decreased M1 macrophages[4] | Neurogenesis and neuroinflammation[85,90] | ||
| Angiogenesis[3] | ||||
| Promotion of lesion growth by M2 macrophages[3] | ||||
| Inhibition of lesion growth by M1 macrophages[3] | ||||
| NK cells | – | No differences in global[18] | – | Impaired uNK cell maturation[18] |
| Increased immature NK cells[18] | Deficient cytotoxicity in eliminating refluxed fragments[5,12] | |||
| Endometriosis-associated infertility[18] | ||||
| Induction of immune escape[16] | ||||
| DCs | No differences in global[19] | Increased immature DCs[20] | Increased immature DCs[20] | Impaired DC maturation[20] |
| Increased immature DCs[20] | Angiogenesis[22] | |||
| Promotion of lesion growth[22,24] | ||||
| Inhibition of lesion growth[25] (controversies) | ||||
| MDSCs | Increased MDSCs[26] | – | Increased MDSCs[27] | Immunosuppression |
| Promotion of lesion growth[28] | ||||
| CD8+ T cells | – | – | – | Deficient cytotoxicity in eliminating refluxed fragments[31] |
| CD4+ Th17 cells | Increased Th17 cells[34] | – | – | Promotion of ESC proliferation[37] |
| Angiogenesis[38] | ||||
| Pro-inflammation responses[38] | ||||
| Recruitment of macrophages and neutrophils[39] | ||||
| Induction of M2 macrophages[39] | ||||
| CD4+ Treg cells | Increased Treg cells[41] | Increased Treg cells[42] | Increased Treg cells[61] | Angiogenesis[45] |
| Induction of pro-repair macrophages[46] | ||||
| Promotion of lesion growth[47] | ||||
| Immunosuppression[47] | ||||
| CD4+ Th1 cells | – | – | Decreased Th1/Th2 ratio[48] | Proinflammatory immune responses[49] |
| CD4+ Th2 cells | – | – | Decreased Th1/Th2 ratio[48] | Anti-inflammatory immune responses[49] |
| Promotion of lesion growth[50] | ||||
| B cells | Increased B cells[52] | Increased B cells[61] | Increased B cells[52] | Aberrant production of autoantibodies[53] |
| Deceased B cells[52] | No differences[61] (controversies) | Deceased B cells[61] | Endometriosis-associated infertility[81,82] | |
| No differences[52](controversies) | No differences[52] (controversies) | Promotion of lesion growth[60] |
DCs: Dendritic cells; ESC: Endometrial stromal cell; MDSCs: Myeloid-derived suppressor cells; M1: Classically activated phenotypes of macrophages; M2: Alternatively activated phenotypes of macrophages; NK cells: Natural killer cells; Th: T helper; Tregs: Regulatory T cells; uNK: Uterine NK.
Innate Immunity
Macrophages
Under healthy circumstances, increased infiltration of macrophages in the endometrium mainly occurs during the late secretory phase and menstrual phase,[4] indicating that macrophages play a role in the initiation of endometrial shedding, clearance of endometrial debris, and regeneration of the functional layer. However, patients with endometriosis did not have an increase in endometrial macrophages, which may lead to compromised eradication of denuded fragments, enabling the fragments to disperse to the pelvic cavity and subsequently form ectopic lesions.[4] Moreover, deep immunophenotyping analysis by Vallvé-Juanico et al[8] revealed that macrophages from endometriosis patients possessed a higher proinflammatory profile and reduced phagocytic capacity compared to macrophages from healthy women; this might result from the overexpression of CD64 (associated with inflammation) and CD172a (phagocytosis inhibitor), respectively. In addition, evidence from a mouse model indicated that depletion of macrophages by clodronate liposomes or monoclonal antibodies could inhibit the growth and vascularization of ectopic lesions, implying a critical role of macrophages in the establishment and angiogenesis of endometriosis.[3]
Macrophages are a group of heterogeneous cells exhibiting plasticity in response to environmental stimuli. Depending on membrane receptors and polarization states, macrophages are categorized as either classically activated phenotypes (M1) or alternatively activated phenotypes (M2). In recent years, the dynamic equilibrium and reciprocal interaction between M1 and M2 macrophages have attracted much interest in the pathophysiology of endometriosis. In comparison to the control, endometriotic lesions and peritoneal fluid from endometriosis patients exhibited a relative increase in the M2 macrophage abundance and a corresponding decrease in the M1 macrophage population.[3,9] This could be partially attributable to a switch from M1 to M2. According to Nie et al,[9] exposure to endometriotic homogenates could induce a switch from M1 to M2, and intraperitoneal adoptive transfer of M1 or M2 macrophages in an endometriosis mouse model could considerably either hamper or accelerate the establishment and growth of ectopic lesions.[3] Moreover, exosomes from endometrial stromal cells (ESCs) of endometriosis patients could also encourage a shift of macrophages toward M2 polarization in vitro and facilitate the implantation and growth of the ectopic endometrium by recruiting M2 macrophages in vivo.[10,11] These findings imply that the intricate signal network in the ectopic environment can modify the polarization of infiltrated macrophages. In turn, alternatively activated macrophages produce anti-inflammatory cytokines and induce immune tolerance, which ultimately generates a permissive microenvironment that is favorable for ectopic lesion growth.
Natural killer (NK) cells
NK cells are essential components of the innate immune system that play a crucial role in defending against viral infection and tumorigenesis. Numerous investigations have proven that the activities of the local and systemic NK cells of endometriosis patients are lower than those of healthy women.[5,12] The extent and state of NK cell activity are determined by the interplay of various killer cell inhibitory receptors (KIRs) and killer cell-activating receptors (KARs). Previous research has demonstrated that KIR expression in circulating and peritoneal NK cells was enhanced in endometriosis-affected women,[13] which led to a functional depletion of NK cells. The diminished cytotoxic activity may further impede the clearance of the autologous endometrium and promote its extrauterine implantation.
The deficiency in NK cell cytotoxicity appears to be the most basic explanation for endometriosis etiology. Nevertheless, it might also represent a consequence of the inflammatory microenvironment generated by the endometriotic lesions. Peritoneal fluid from endometriosis patients has been shown to suppress NK cells, implying that there should be certain molecules generated in the endometriotic environment affecting NK cell activity.[14] Of note, soluble ligands for natural killer group 2, member D (NKG2D), an essential activating receptor on NK cells, are present in higher concentrations in the peritoneal fluid of endometriosis patients. This indicates a decreased expression of NKG2D ligands (NKG2DLs) on the surface of endometriotic cells, leading to less recognition and reduced clearance from NK cells.[15] In fact, the release of soluble NKG2DLs has been interpreted as a mechanism by which neoplastic cells depress NK cell activity and escape from immunosurveillance. Additionally, Guo et al[16] illustrated that platelets, which were markedly increased in the peritoneal fluid of endometriosis-affected women, could also impair NK cell cytotoxicity through a variety of mechanisms. Since activated platelets displayed high expression of histocompatibility complex I (MHC-I), endometriotic cells were induced to pseudoexpress MHC-I by platelet coating and thus were recognized as "pseudo self" cells by NK cells, which ultimately resulted in immune escape.[17] In addition, by downregulating NKG2D, platelet-derived transforming growth factor (TGF)-β can directly inhibit the function of NK cells. Concerning uterine NK (uNK) cells, increased immature NK cells have been observed in the eutopic endometrium and peripheral circulation in women with endometriosis compared to women without this disorder.[18] Interestingly, surgical excision of endometriotic lesions could partially reverse the rise of immature NK cells in peripheral blood, suggesting that the presence of ectopic lesions may interfere with the differentiation and maturation of NK cells.[1]
The etiology of endometriosis is heavily dependent on NK cells; in endometriosis, these cells display reduced cytotoxicity in the peritoneal fluid and peripheral circulation, which may aid in the implantation and growth of endometriotic fragments. However, little research has been carried out on the function of uNK cells in endometriosis. Given the primary function of uNK cells in decidual homeostasis, placental development, and embryonic growth, investigating the mechanisms underlying the defect in the phenotype and function of uNK cells may provide new insights into the infertility of endometriosis patients and offer innovative therapeutic approaches.
Dendritic cells (DCs)
Currently, phenotypic and functional alterations in DCs have been linked to the pathogenesis of endometriosis. The absolute number of DCs in the peritoneal fluid is the same in endometriosis patients and the control.[19] Nevertheless, endometriosis patients have considerable immature DCs in their endometrium.[20] In addition, immature DCs are abundant at the endometriotic site and gradually decline from the adjacent to the distant peritoneum.[20] Moreover, peritoneal fluid from patients with advanced endometriosis was proven to associated with the differentiation of monocytes from DCs to macrophages,[21] revealing that the aberrant endometriotic microenvironment might induce defective maturation of DCs, which, in turn, may lead to the inefficient eradication of regurgitated fragments. On the flip side, increasingly infiltrated immature DCs have been regarded as a strategy for tumor cells to induce and sustain angiogenesis. In vivo data from Fainaru et al[22] support this notion by demonstrating an elevated infiltration of immature DCs at angiogenesis sites in murine endometriosis models. Moreover, immature DCs displayed increased expression of the vascular endothelial growth factor receptor (VEGFR) and were more responsive to VEGF, indicating a switch in their functions from immune modulation toward angiogenesis promotion. Findings from Suen et al[23] further proved that enhanced interleukin (IL)-10 production from DCs might partially underlie the mechanism of their proangiogenic effects in endometriosis.
However, in vivo research on the impacts of DCs on endometriosis development has yielded contradictory results. According to Fainaru et al,[22] intraperitoneal transfer of immature DCs in an endometriosis mouse model augmented lesion formation and facilitated neovascularization, indicating that these cells are critical for the onset and progression of endometriosis. Similarly, Pencovich et al[24] reported that systematic ablation of DCs in a murine endometriosis model could result in considerable lesion shrinkage, supporting the idea that the presence of DCs is required for lesion development. However, some studies have reported conflicting results. Evidence from Stanic et al[25] has shown a negative impact of DC ablation on ectopic lesion growth. They found that DCs could impair the early establishment of endometriotic lesions by activating T cells. These contradictory findings warrant more thorough investigations to determine whether DCs encourage or hinder the development of endometriosis. Given the critical roles of DC maturation in the pathogenesis of endometriosis, single-cell sequencing is required to precisely define the dynamic phenotypic and functional modification of DCs throughout their maturation, which is a tremendously promising topic for innovative therapy development.
Myeloid-derived suppressor cells (MDSCs)
MDSCs represent a highly heterogeneous group of immature myeloid cells with the capacity to repress immune responses. Since impaired immunosurveillance has been identified as a major contributor to endometriosis, MDSCs, which elicit substantial immunosuppressive effects in multiple inflammatory diseases, have received much attention in the pathophysiology of endometriosis in recent years. Accumulating data suggest that individuals with endometriosis have increased MDSC infiltration in their peritoneal fluid compared with those without this disease.[26,27] This increase could be partially reversed following surgical excision of ectopic lesions, indicating that certain environmental cues should be responsible for the activation and expansion of MDSCs.[28] In fact, multiple chemokines have been shown to have vital roles in directing circulating MDSCs to a specific local niche in a tumor or inflammatory milieu. In accordance with these findings, chemokines, such as chemokine (C-X-C motif) ligand (CXCL)1, CXCL2, chemokine C-C motif ligand (CCL)3, CCL5, and CCL25, were increased in the peritoneal fluid of endometriosis patients. Meanwhile, the receptors of these chemokines, C-X-C motif chemokine receptor (CXCR)2, C-C motif chemokine receptor (CCR)5, and CCR9, are abundantly expressed on MDSCs, boosting the recruitment of MDSCs.[26-28] Indeed, the presence of MDSCs is proven to be critical for the development of ectopic lesions since MDSC depletion with anti-Gr-1 monoclonal antibodies dramatically suppressed the growth of endometrium implants, whereas adoptive transfer of exogenous MDSCs could reverse the suppressive effect.[28] Jiang et al[29] discovered that endometriosis-induced MDSCs were correlated with aberrant activation of the Notch pathway and that blocking Notch signals could not only slow the development of ectopic lesions but also reduce the infiltration of MDSCs. This implies that MDSCs play a fundamental role in the progression of endometriosis in a Notch-dependent manner. Notably, He et al[30] demonstrated that sunitinib, a receptor tyrosine kinase inhibitor, could significantly weaken the formation and progression of ectopic lesions in an endometriosis mouse model by transforming immature mononuclear MDSCs into a mature polynuclear phenotype. This alteration in morphology may also reflect a shift in their functional activities from pro-endometriotic toward anti-endometriotic as the RNA-sequencing data revealed a substantial elevation of immune activation genes in the sunitinib group.
These findings imply that MDSCs are crucial in establishing the immunosuppressive microenvironment in endometriosis. The fundamental process of their differentiation, recruitment, and activation throughout this disease is not yet fully understood. Since MDSCs consist of a range of immature myeloid cells and lack unique phenotypic molecules, it is challenging to isolate and modify MDSCs without interfering with normal monocytes and neutrophils. Further studies, potentially with the application of single-cell RNA sequencing and multiomics technology, are needed to determine whether MDSCs can be divided into smaller subgroups with distinct functional properties. Additionally, proper demonstration of each group's unique molecules is necessary to better understand their pathobiological impacts on endometriosis and ensure more precise targets for ectopic lesions.
Adaptive Immunity
T cells
CD8+ T cells
CD8+ T cells, also named cytotoxic T cells, serve as the primary effector cells to eliminate infected cells and tumor cells through cytokine secretion or FasL-induced apoptosis, providing persistent immunosurveillance to maintain homeostasis. However, impaired CD8+ T-cell immune responses have been reported in endometriosis patients, given their decreased perforin expression and deficient cellular cytotoxicity against autologous endometrial cells.[31] Moreover, the number of CD8+ T cells in peripheral circulation from healthy individuals is more abundant in the luteal phase than in the follicular phase. This fluctuation throughout the menstrual cycle was not observed in endometriosis patients, reflecting aberrant regulation of CD8+ T-cell distribution and activity in endometriosis.[32] Melioli et al[33] reported that the impaired cytotoxicity of CD8+ T cells could be restored with recombinant IL-2, providing an intriguing therapeutic strategy to overcome the restrained anti-endometriotic immune responses.
CD4+ Th17 cells
In endometriosis, the number of Th17 cells in the peritoneal fluid is significantly elevated compared with that in healthy women, and the percentage of these cells coincides with the severity of this disease.[34] The increased abundance of Th17 cells is proposed to result from the interaction of CCL20 and its receptor CCR6. Hirata et al[35] discovered that the predominant Th17 cells in the peritoneal fluid from endometriosis patients expressed a high level of CCR6. Additionally, these CCR6+ Th17 cells could be selectively recruited to the lesion site in response to the CCL20 secreted by the endometrial stromal and epithelial cells, suggesting that the endometriotic microenvironment has a role in regulating Th17 cell activity. In return, IL-17, the signature cytokine derived from Th17 cells, is dramatically elevated in the peritoneal fluid of endometriosis patients and plays a crucial role in the initiation, development, and progression of endometriosis via multiple mechanisms.[36] Hirata et al[37] substantiated that IL-17 could induce the proliferation and IL-8 secretion of endometriotic stromal cells, which could facilitate the development of ectopic lesions. Additionally, IL-17 could provoke the secretion of a wide range of angiogenetic factors and proinflammatory cytokines, hence favoring the development of new capillaries and the growth of the implanted endometrium.[38] Furthermore, given its chemotactic capacity, IL-17 is involved in the recruitment of macrophages and neutrophils to endometriotic lesions and is responsible for triggering M2 polarization.[36,39] These findings emphasize the critical role of Th17 cells in orchestrating complex interactions among a variety of immune cells in endometriosis. Markedly, current research has characterized distinct gene expression profiles of Th17 cells in individuals with and without endometriosis, providing a novel understanding and potential therapeutic targets of endometriosis.[40]
CD4+ Treg cells
Treg cells are potent immunosuppressive cells that exert inhibitory effects on the immune responses of cytotoxic T cells, macrophages, and NK cells. The number of Treg cells is elevated in the peritoneal fluid of individuals with endometriosis compared to those without.[41] Similar findings were also observed in the eutopic endometrium.[42] Consistent with these findings, Khan et al[43] found that the increase in Treg cells was relevant to the stage of endometriosis, implying an important role of Treg cells in the progression of this condition. Furthermore, evidence from Olkowska-Truchanowicz et al[44] suggested that the elevation of the Treg cell population might be due to the chemotactic activity of CCL20 in the endometriotic microenvironment as the elevation could be restrained with the administration of anti-CCL20 antibodies. Moreover, Wang et al[45] reported that coculture of ESCs and macrophages resulted in dramatically increased levels of CCL22 and CCL17, which could stimulate the recruitment and TGF-β secretion of Treg cells. Furthermore, Treg-derived TGF-β was reported to function synergistically with IL-1β and tumor necrosis factor (TNF)-α to promote angiogenesis of ectopic lesions, potentially accelerating the progression of endometriosis. Treg cells can also trigger pro-repair polarization of macrophages via the secretion of soluble fibrinogen-like protein 2 (sFGL2). The sFGL2-induced prorepair macrophages could reciprocally facilitate the differentiation of Treg cells, forming a positive feedback loop to promote endometriosis.[46] In line with these results, in vivo data revealed that depletion of Treg cells significantly decelerated the growth of ectopic lesions and led to a decreased accumulation of Th2 cells, Th17 cells, and macrophages but an elevated accumulation of Th1 cells.[47] These findings revealed an intricate microenvironment within the ectopic lesions, where Treg cells, macrophages, T helper cells, and endometrial cells work synergically to initiate endometriosis.
CD4+ Th1 and Th2 cells
Th1 cells are characterized by the capacity to produce IL-2, interferon (IFN)-γ, and TNF-α, which are involved in proinflammatory immune responses and cellular immunity. By secreting IL-4 and IL-10, Th2 cells, by contrast, exhibit anti-inflammatory properties and have a role in modulating B-cell proliferation and differentiation. In endometriosis patients, the percentage of Th1 cells in ectopic lesions was relatively reduced compared with that in the eutopic endometrium, while the proportion of Th2 cells remained consistent, showing a skew of the T helper cell balance toward Th2-mediated anti-inflammatory responses within the endometriotic niche.[48] Moreover, Olkowska-Truchanowicz et al[49] demonstrated that the peritoneal fluid of endometriosis patients could dramatically trigger the secretion of IL-4 and IL-10 while inhibiting the production of IL-2, IFN-γ, and TNF. These findings strongly suggested that the endometriotic milieu was responsible for inducing a shift in the Th1/Th2 balance toward Th2-skewed immunity. The Th1/Th2 imbalance might, in turn, facilitate the establishment of an immunosuppressive environment, which is favorable for the survival and development of ectopic lesions. Furthermore, Mier-Cabrera et al[50] evaluated the effects of Th1-derived and Th2-derived cytokines on the growth of the implanted endometrium in an endometriosis mouse model. They demonstrated that the lesions significantly shrank with the administration of IFN-γ and IL-2, rather than IL-4 and IL-10, implying an inhibitory role of Th1-related responses in the progression of endometriosis. Additionally, evidence from Li et al[51] reported that IL-37 could inhibit the development of mouse ectopic lesions by repressing Th2 differentiation. These findings provide a possible therapeutic strategy for restraining the progression of endometriosis by remodeling the Th/Th2 balance.
B lymphocytes
Most studies have identified an elevated amount of B cells in endometriosis patients, although some have reported no differences or even a decrease in B-cell infiltration in endometriosis patients compared with control.[52] Despite the conflicting observations on the numbers of B cells, there is a consensus that B cells in the endometriotic milieu display excessive activity, manifested by the heightened production of autoantibodies. Although endometriosis has not yet been characterized as a specific autoimmune disease, it shares certain characteristics with autoimmune diseases, such as familial prevalence, increased cytokine production, and aberrant immune responses.[53] Moreover, several anti-endometrium antibodies were correlated with the severity of this disease and identified as promising biomarkers for the diagnosis of minimal to mild endometriosis.[54] It is speculated that the aberrant production of autoantibodies may be caused by the exposure of endometrial antigens to the extrauterine environment during menstrual retrograde.[53] Moreover, several studies found deposits of autoantibodies in the eutopic endometrium of endometriosis patients with infertility,[55] suggesting that autoimmune responses provoked by the regurgitated endometrium may, in turn, impair the fecundity of the eutopic endometrium. Notably, the decreased pregnancy rate of autoantibody-positive patients could be restored with the administration of corticosteroids,[56] providing a novel therapeutic approach to overcome autoantibody-induced infertility.
Although the exact mechanism through which B cells are activated in ectopic foci remains mostly unknown, several factors have been proposed to specifically trigger B-cell responses.[52] In particular, B lymphocyte stimulator (BLyS), which is essential for the proliferation and differentiation of B cells, was dramatically elevated in the serum of endometriosis patients compared with healthy women.[57] Notably, the aberrant expression of BLyS was identified as a critical driver of autoimmune responses in a variety of diseases. Therefore, it is plausible that the elevated BLyS in the endometriotic milieu may contribute to the overactivation of B cells and excessive production of autoantibodies, both of which are favorable for the progression of endometriosis. In addition, B-cell lymphoma 6 (BCL6), a critical regulator of humoral immunity, was overexpressed in endometriosis patients at both protein and messenger RNA (mRNA) levels.[58] Furthermore, BCL6 was validated to have favorable diagnostic value for endometriosis, and its excessive accumulation was related to individuals' reduced fertility.[59] In vivo findings from Riccio et al[60] demonstrated that CD20 antibody-mediated B-cell depletion had no impact on the size and weight of ectopic lesions in mice. However, ibrutinib, a specific inhibitor of Bruton's tyrosine kinase (Btk), which is important for B-cell differentiation, significantly restrained the growth of ectopic lesions by skewing activated B cells to regulatory B cells (Bregs). Given that the shift toward Bregs, rather than the depletion of total B cells, impaired the development of the implanted endometrium,[60] it is apparent that various B-cell subtypes may exhibit distinct effects on the pathophysiology of endometriosis. Thus, single-cell sequencing holds excellent promise in clarifying the molecular states of each B-cell subpopulation in endometriosis, which could deepen our understanding of the relationship between endometriosis and B cells. Moreover, recent advances in omics technology have enabled the discovery of novel autoantibodies, which may offer valuable diagnostic biomarkers and therapeutic targets for endometriosis [Figure 2].
Figure 2.
Immune landscape of the endometriotic lesions. The endometriotic immune cell repertoire is remodeled during endometriosis and takes part in the chronic inflammatory microenvironment, which facilitates neuro-angiogenesis and immune escape, further exaggerating the lesion progression. The pathological roles of each immune cell population are summarized. ESC: Endometrial stromal cell; IFN: Interferon; IL: Interleukin; KAR: Killer cell-activating receptor; KIR: Killer cell inhibitory receptor; MDSCs: Myeloid-derived suppressor cells; Th: T helper; TNF: Tumor necrosis factor; Treg cells: Regulatory T cells.
Immune–endocrine Interplay in Endometriosis
Endometriosis exhibits both dysregulated immune responses and inadequate hormonal control. However, the complex interactions between these two distinct, but correlated, systems and their synergistic impacts on the pathogenesis of endometriosis are not yet fully understood. Multiple immune populations fluctuate across the menstrual cycle in healthy women,[61] but the fluctuation is disturbed in women with endometriosis, reflecting the dysregulated modulatory effect of sex hormones on immune cells.
Estrogen receptor α (ERα) and estrogen receptor β (ERβ), nuclear cognate receptors of estrogen, are increasingly expressed and activated in the ectopic lesions of endometriosis patients compared with the normal endometrium of healthy controls.[62,63] Moreover, using ERα-/- mice with endometriosis, Burns et al[62] demonstrated that ERα-mediated signaling was essential for the proliferation, angiogenesis, and growth of ectopic lesions. Their follow-up study further explored the underlying ERα-mediated mechanism favoring the establishment of endometriosis. In ERα-knockout conditions, they revealed that the development of endometriosis in the initial 48 h was an immune-dependent phase, as evidenced by a substantial influx of macrophages and neutrophils.[64] However, the estradiol–ERα-IL-6 axis was shown to govern the development of endometriosis 48 h after disease initiation. These findings suggested that the early initiation of endometriosis was precisely regulated by the dynamic cooperation of innate immunity and ERα-mediated signaling.[64]
Concerning the role of ERβ in endometriosis, Han et al[63] reported that inhibition of ERβ with the administration of its selective antagonist significantly decelerated lesion formation in mice with endometriosis. Conversely, overexpression of ERβ promoted endometriosis growth both in vitro and in vivo. Mechanically, ERβ could interact with the cytoplasmic apoptosome to repress TNF-β-mediated apoptosis, thus favoring the immune escape of the regurgitated endometrium.[63] In line with these findings, Gou et al[65] revealed that enhanced ERβ expression in endometriotic stromal cells contributed to the secretion of CCL2, which was responsible for the recruitment of M2 macrophages to the endometriotic niche. In turn, aberrant infiltration of M2 macrophages was reported to accelerate the proliferation of endometriotic stromal cells,[9] thereby forming a positive feedback loop conducive to the progression of endometriosis. Recently, Zhao et al[66] developed two ER-selective ligands, oxabicycloheptene sulfonate (OBHS) and chloroindazole (CLI), with ERβ-dependent efficiency and ERα-predominant activity, respectively. Both ligands exhibited negative effects on macrophage infiltration and lesion growth in a mouse endometriosis model, providing a potential treatment for endometriosis.
Taken together, enhanced estrogen signaling and dysregulated immune responses cooperate closely to promote the development of endometriosis. Despite the great challenge it presents, simultaneously targeting these pathways is a promising direction for creating innovative therapies.
Intricate Crosstalk between Microbiota and Endometriosis
Microbiota, which are composed of bacteria, fungi, viruses, and protists living in the human body, have evolved to maintain and regulate human physiology during their long symbiotic relationship with their hosts. Impairment or imbalance of microbiota, also known as dysbiosis, is implicated in the perturbed immune responses and chronic inflammation of the hosts and, as a result, has a fundamental role in a broad spectrum of diseases.[67-69] As endometriosis is characterized by defective immunosurveillance and perpetuating inflammation, it is speculated that there is an inextricable link between microbiota and endometriosis. Indeed, microbiota alterations in the endometrium, cervix, vagina, and gut were detected in individuals with endometriosis compared to those without this disease, which were manifested with decreased probiotics and elevated opportunistic bacteria.[70-72] Specifically, the abundance of Lactobacillus, which is dominant in the cervicovaginal microenvironment under healthy conditions, was significantly reduced in the genital tract of endometriosis patients.[70,71] As Lactobacillus is critical for maintaining vaginal homeostasis by secreting lactic acid and other antimicrobial factors, it is reasonable to deduce that the reduction in Lactobacillus may alter the local immune cell repertoire and trigger a proinflammatory environment that favors the development of ectopic lesions. Supporting this proposition, oral administration of Lactobacillus gasseri OLL2809 could both significantly suppress the progression of endometriotic lesions in a mouse model and effectively relieve dysmenorrhea and menstrual pain in endometriosis patients.[73,74] Intriguingly, Atopobium and Gardnerella,[70,75] two significant pathogenic microorganisms of bacterial vaginosis (BV), are enriched in the cervix and vagina of endometriosis patients, revealing a possible correlation of endometriosis with reproductive tract infections. Given the significant differences in microbiota compositions between women with and without endometriosis, there is an expectation that microbiota profiles could be employed as a non-invasive diagnostic tool for endometriosis. Two studies have demonstrated the diagnostic value of vaginal and gut microbiome profiles in predicting the presence and stages of endometriosis.[72,76]
Recently, attempts have been made to elucidate the causal relationship between microbiota and endometriosis. In vivo evidence from a mouse model has shown an alteration in the gut microbiota composition between the control mice and endometriosis mice,[77] suggesting that endometriosis may induce alterations in the gut microbiota. Several studies have also suggested a regulatory role of the microbiota in the course of endometriosis. Depleting gut microbiota with broad-spectrum antibiotics, Chadchan et al[75] reported a significantly reduced size of ectopic lesions in an endometriosis mouse model, while the anti-endometriotic effects of antibiotics could be reversed by oral supplementation of feces from endometriosis mice. Likewise, eliminating vaginal microbiota with the vaginal administration of antibiotics has also shown an inhibitory impact on the growth of the implanted endometrium.[78] Although the exact mechanisms through which microbiota trigger the initiation of endometriosis are still speculative, the bacterial contamination theory has gained much attention in recent years. It was postulated that bacterial lipopolysaccharides (LPSs) carried by retrograde menstruation may initiate endometriosis via the TLR4 pathway.[79]
Overall, alterations in microbiota profiles were heavily implicated in the pathophysiology of endometriosis. Manipulating the microbiota through antibiotics or microbiota transplantation may lead to the development of novel therapeutic avenues for endometriosis. Further studies are needed to identify the core microbiota driving the pathogenesis of endometriosis and to comprehensively elucidate the underlying mechanism that mediates the intricate interplay between endometriosis and microbiota.
Immunological Factors Involved in the Endometriosis Symptomology
Endometriosis-associated infertility
Endometriosis-associated infertility has long been linked to dysregulated immune responses as they can interfere with sperm motility, endometrial decidualization, and embryo implantation. Utilizing NanoString transcriptomic analysis, Ahn et al[6] revealed distinct molecular profiles of immunoinflammatory genes between the ectopic lesions from infertile endometriosis patients and the eutopic endometrium from the fertile control. Notably, genes related to decidualization were significantly decreased in those with endometriosis, indicating that aberrant inflammation might be implicated in endometriosis-associated infertility. This hypothesis has been supported by the discovery of alterations in a variety of inflammatory cytokines in the serum and peritoneal fluid of infertile endometriosis patients. Specifically, IL-6, associated with recurrent implantation failure, displayed increased expression in the peritoneal fluid from infertile endometriosis patients compared to control.[80] However, most findings about the role of inflammatory cytokines in endometriosis-induced infertility remain observational, and further studies are warranted to elucidate cause-and-effect relationships.
Another important factor involved in endometriosis-associated infertility is the excessive production of autoantibodies by B cells. The serum concentrations of antinuclear antibodies (ANAs) and antiphospholipid antibodies (aPLs) were dramatically elevated in endometriosis patients compared with control.[81,82] Given that aPLs interfere with oocyte maturation, endometrium receptivity, and embryo development during pregnancy[82] and that ANAs impede embryo development in mice,[83] it is reasonable to deduce that the aberrant accumulation of ANAs and aPLs in endometriosis patients may contribute to the impairment of fertility. By contrast, autoantibodies targeting laminin-1, an integral element for basement membrane assembly during early embryogenesis, were correlated with fetal resorption in mice and recurrent first-trimester miscarriages in humans.[83] Moreover, anti-laminin-1 antibodies are overexpressed in the plasma of infertile endometriosis patients compared with control non-pregnant women, implying an involvement of these antibodies in endometriosis-associated infertility. Corticosteroids are well recognized as autoantibody-suppressive immunomodulators and are extensively utilized in the treatment of autoimmune diseases. Dmowski et al[56] and Kim et al[84] reported that autoantibodies negatively affected the pregnancy rates of endometriosis patients undergoing in vitro fertilization (IVF) and that this adverse impact could be reversed with the application of corticosteroids, providing a novel therapeutic strategy to overcome endometriosis-associated infertility. However, the exact mechanisms by which these autoantibodies are produced and how they react with the reproductive system remain unclear and thus require further investigation.
The chronic inflammation triggered by ectopic lesions has a significant impact on the local immune cell repertoire, causing aberrant cytokine secretion and defective cytotoxicity. This immune derangement appears not to be restricted to the ectopic endometrium but also extends to the eutopic endometrium. Since immune homeostasis of the fetal-maternal interface is essential for the establishment and maintenance of pregnancy, the immunological dysregulation of the eutopic endometrium in endometriosis patients may contribute to endometriosis-associated infertility. In particular, uNK cells, which are essential for embryo implantation and placental development, exhibit decreased cytotoxicity in endometriosis patients compared with healthy controls.[61] Intriguingly, compared with endometriosis patients with normal fertility, uNK cells of those with infertility displayed an enhanced capacity to secrete cytotoxic factors, with potential reactivity toward trophoblast cells.[18] Therefore, the altered activity of eutopic NK cells seems to be partially responsible for the reduced fertility of endometriosis patients, and the assessment of their activity could be a promising approach to predict endometriosis-associated infertility. Moreover, uterine Treg cells are essential for creating an immune-tolerant environment that is favorable for embryo implantation by preventing the inflammation evoked by paternally derived antigens. However, according to the findings by Chen et al[42], an abundant accumulation of Treg cells was detected in the eutopic endometrium of endometriosis patients with infertility, suggesting that these immunosuppressive cells may be relevant to endometriosis-associated infertility. However, current evidence of the relationship between immune dysregulation and endometriosis-associated infertility is still insufficient, and further explorations are needed.
Endometriosis-associated pain
Pelvic pain represents the main symptom of endometriosis. However, the process by which ectopic lesions elicit chronic pain is still unclear. Of note, surgical removal of endometriotic lesions does not relieve pain in all patients.[1] Thus, factors beyond the implanted endometrial tissue, such as dysregulated immune responses, might contribute to the induction of endometriosis-associated pain. In recent years, many investigations have proposed a strong relation between immunological dysregulation and neurogenesis, hypersensitization, and subsequent severe pelvic pain in endometriosis.[85]
Pain perception requires the existence of sensory nerve fibers to convey peripheral impulses to the central nervous system, but the refluxed endometrium has a deficient supply of sensory nerves. Therefore, neurogenesis undoubtedly occurs during the formation of ectopic lesions to transfer nociceptive signals and induce pelvic pain. Indeed, increased sensory nerve infiltration within ectopic lesions has been confirmed by many studies.[85] Moreover, recent literature has revealed the coexistence and proximity of newly formed nerves and macrophages in the endometriotic milieu,[86] implying potential immune–nervous system crosstalk in endometriosis. Consistently, macrophages in the ectopic microenvironment have been determined to be the primary cellular source of brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT-3), both of which are essential for the proliferation, differentiation, and apoptosis of neurons.[87] Moreover, insulin-like growth factor-1 (IGF1), a known sensitizing and neurotrophic element implicated in nerve sprouting, was significantly increased in the peritoneal fluid of endometriosis patients. Its concentration showed a positive correlation with pain severity. Findings from Foster et al[88] suggested that the elevated IGF1 was derived from endometriosis-modified macrophages. They found that the hyperalgesia of endometriosis mice could be relieved with either inhibition of IGF1 signaling or depletion of macrophages, implying a significant involvement of immune cells and immune cell-derived factors in the neurogenesis of endometriosis. In turn, infiltrating nerves also play a role in altering the migration and polarization status of nearby macrophages. According to Greaves et al,[87] the elevated CCL-2 secreted by infiltrating neurons in endometriotic lesions served as a chemotactic substance for macrophages and facilitated their recruitment into the ectopic environment. Remarkably, evidence from Kwon et al[89] further demonstrated that the CCL-2/CCR-mediated nerve–macrophage interaction could induce a shift of macrophages toward the M2 phenotype, which was involved in neurogenesis and endometriosis initiation.
Neuronal sensitization, a condition in which neurons have a lower activation threshold and can produce pain from modest stimuli that would not typically trigger nociception, plays a significant role in the pathophysiology of chronic pain. In endometriosis, several proinflammatory cytokines (IL-1β and TNFα) are highly expressed in patients with chronic pelvic pain and are all capable of interacting with sensory neurons and inducing sensitization.[90] Transient receptor potential subfamily V member 1 (TRPV1) and transient receptor potential cation channel, subfamily A member 1 (TRPA1), critical nociceptive receptors integrating external stimuli and regulating neuronal activation threshold, were reported to be overexpressed in ectopic lesions compared with the eutopic endometrium.[85] Although the exact mechanism remains unknown, recent findings from Zhu et al[91] suggested that this aberrant elevation of TRPV1/TRPA1 might be derived from inflammation-mediated macrophage polarization. Interestingly, inflammation-induced hypersensitized nerves could also maintain and exaggerate endometriotic inflammation by secreting proinflammatory neuropeptides such as substance P (SP) and calcitonin gene-related peptide (CGRP),[92] resulting in a positive feedback loop known as "neurogenic inflammation" to exacerbate endometriosis-associated pain. Previous studies reported a correlation of reduced pelvic pain with a history of severe gram-negative infection in endometriosis patients. In addition, adoptive transfer of macrophages pretreated with a low dose of lipopolysaccharides significantly suppressed lesion growth in an endometriosis mouse model. These findings suggested that reprograming macrophages with bacterial challenges may be effective in relieving endometriosis-associated pain.[93] Moreover, a placebo-controlled randomized clinical trial substantiated some positive benefits of oral administration of Lactobacillus, a probiotic, on pelvic pain relief in endometriosis patients.[94]
These findings provide novel mechanistic insights into how the nervous and immune systems interact to initiate pelvic pain and accelerate lesion growth within the endometriotic environment. Targeting the interplay between these two systems is anticipated to be an invaluable therapeutic strategy to reduce neurogenic inflammation and relieve pelvic pain in endometriosis patients.
Immunotherapy for Endometriosis
Although immunomodulation shows great therapeutic potential in preclinical murine and non-human primate models of endometriosis, none of its related immunotherapies are recommended in the guidelines of endometriosis treatment, and few have been studied in clinical trials.[1]
TNF-α, which is known to promote inflammation and neurogenesis, was increasingly expressed in the peritoneal fluid of endometriosis patients compared with control.[95] In a baboon model with spontaneous endometriosis, the application of etanercept, by blocking TNF-α, significantly decreased the size and number of ectopic lesions.[96] Furthermore, etanercept could improve the pregnancy outcomes of endometriosis patients receiving assisted reproductive technology.[97] A randomized controlled trial (RCT) revealed that infliximab, another TNF-antibody, showed no significantly different therapeutic effectiveness in dysmenorrhea, non-menstrual pain, or dyspareunia in comparison to a placebo control for deep infiltrating endometriosis (DIE) patients.[98] The insufficient therapeutic effects of infliximab reported in this investigation may result from the distinct pathological nature between DIE and typical endometriotic lesions (deep infiltrating lesions with more fibrosis and adhesions, resistant to conventional drugs). Therefore, further studies are needed to explore its role in other subtypes of endometriosis. Apart from anti-TNF treatment, probiotics could be another validated strategy for controlling endometriosis. According to double-blind, placebo-controlled randomized clinical trials, oral administration of Lactobacillus, important probiotics involved in the homeostasis maintenance of the female reproductive milieu, exhibited vigorous activity in reducing endometriosis-associated pain.[74,94] Certain immunomodulatory therapies, such as Bacillus Calmette–Guérin (BCG) vaccines and mammalian target of rapamycin (mTOR) inhibitors, have shown valuable efficiency in suppressing murine or baboon endometriosis. It remains a significant challenge to translate these preclinical findings into clinical applications. Thus, further strictly designed clinical trials are warranted to investigate the safety and efficiency of these immunotherapies.
Conclusion and Perspectives
Recently, phenotypic and functional alterations of immune cells, impaired immunosurveillance, and immune escape have garnered massive attention in the pathogenesis of endometriosis. Among those altered immune cells, it is evident that macrophages, NK cells, and B cells have an essential role in the endometriosis etiology and symptomology. Given that the majority of endometriotic lesions form in the peritoneal cavity, macrophages are predisposed to play the most significant role in endometriosis development since they are the predominant leukocyte population in the peritoneal fluid. Indeed, macrophages are critically involved in lesion progression, vascularization, and innervation. In addition, manipulating endometriosis-associated macrophages to a "healthy" phenotype has shown beneficial effects in several in vivo studies. Additionally, by secreting cytokines or chemokines, macrophages participate in the recruitment and functional regulation of diverse immune cells. In turn, these immune cells were indicated to mediate macrophage activity and polarization. In fact, endometriosis is referred to as "the disease of macrophages." In addition, the aberrant activity of NK cells and B cells is associated with reduced recognition of regurgitated debris and persistent inflammation, both of which are permissive to lesion survival. Furthermore, deficient maturation of NK cells and excessive production of autoantibodies have been linked with compromised fertility. These discoveries position macrophages, NK cells, and B cells at the center of this enigmatic disease. Since current findings on the involvement of immune cells in endometriosis are mostly based on observational studies, it remains unclear whether dysregulated immune responses trigger the initiation and progression of endometriosis or merely represent a consequence of this mysterious disease. In addition, intricate interactions among immune, endocrine, nervous, and microbiota systems undoubtedly occur during the course of endometriosis, which contribute to the onset, maintenance, and progression of ectopic lesions. However, the mechanisms underlying these intricate communications are just beginning to be uncovered, and the majority of our current understanding is still based on investigations of individual cell populations in endometriosis pathogenesis, making it difficult to generalize these findings to all other cells found in the ectopic environment. Studies are warranted to clarify the intricacy of these interactions in future, which will certainly provide stirring mechanistic insights into the etiology of endometriosis. Advances in high-resolution sequencing and the availability of high-dimensionality multiomics data have led to a comprehensive exploration and assessment of cellular heterogeneity, molecular profiles, and spatial distribution for cells existing in the ectopic microenvironment, holding substantial promise for identifying novel, pharmaceutically targetable pathways that play central roles in the pathogenesis of endometriosis.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 82273255, 81822034, 81821002, and 81773119), National Key Research and Development Program of China (Nos. 2022YFA1106600, 2017YFA0106800, and 2018YFA0109200), Sichuan Science-Technology Project (Nos. 22ZYZYTS0070 and 2019YFH0144), and Direct Scientific Research Grants from West China Second Hospital, Sichuan University (Nos. KS021 and K1907).
Conflicts of interest
None.
Footnotes
Dian Fan, Xu Wang, and Zhixian Shi contributed equally to this study.
How to cite this article: Fan D, Wang X, Shi ZX, Jiang YT, Zheng BH, Xu L, Zhou ST. Understanding endometriosis from an immunomicroenvironmental perspective. Chin Med J 2023;136:1897–1909. doi: 10.1097/CM9.0000000000002649
References
- 1.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers 2018;4: 9. doi: 10.1038/s41572-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 2.Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol 1927;3:93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 3.Bacci M Capobianco A Monno A Cottone L Di Puppo F Camisa B, et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 2009;175: 547–556. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod 2009;24: 325–332. doi: 10.1093/humrep/den393. [DOI] [PubMed] [Google Scholar]
- 5.Wilson TJ, Hertzog PJ, Angus D, Munnery L, Wood EC, Kola I. Decreased natural killer cell activity in endometriosis patients: Relationship to disease pathogenesis. Fertil Steril 1994;62: 1086–1088. doi: 10.1016/s0015-0282(16)57082-4. [DOI] [PubMed] [Google Scholar]
- 6.Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril 2016;106: 1420–1431. doi: 10.1016/j.fertnstert.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunis DG Wang W Vallvé-Juanico J Houshdaran S Sen S Ben Soltane I, et al. Whole-Tissue deconvolution and scRNAseq analysis identify altered endometrial cellular compositions and functionality associated with endometriosis. Front Immunol 2021;12: 788315. doi: 10.3389/fimmu.2021.788315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallvé-Juanico J George AF Sen S Thomas R Shin MG Kushnoor D, et al. Deep immunophenotyping reveals endometriosis is marked by dysregulation of the mononuclear phagocytic system in endometrium and peripheral blood. BMC Med 2022;20: 158. doi: 10.1186/s12916-022-02359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nie MF Xie Q Wu YH He H Zou LJ She XL, et al. Serum and ectopic endometrium from women with endometriosis modulate macrophage M1/M2 polarization via the Smad2/Smad3 pathway. J Immunol Res 2018;2018: 6285813. doi: 10.1155/2018/6285813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun H Li D Yuan M Li Q Zhen Q Li N, et al. Macrophages alternatively activated by endometriosis-exosomes contribute to the development of lesions in mice. Mol Hum Reprod 2019;25: 5–16. doi: 10.1093/molehr/gay049. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y Zhu L Li H Ye J Lin N Chen M, et al. Endometriosis derived exosomal miR-301a-3p mediates macrophage polarization via regulating PTEN-PI3K axis. Biomed Pharmacother 2022;147: 112680. doi: 10.1016/j.biopha.2022.112680. [DOI] [PubMed] [Google Scholar]
- 12.Wang L Li L Li Y Huang C Lian R Wu T, et al. A history of endometriosis is associated with decreased peripheral NK cytotoxicity and increased infiltration of uterine CD68+ macrophages. Front Immunol 2021;12: 711231. doi: 10.3389/fimmu.2021.711231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda N, Izumiya C, Yamamoto Y, Oguri H, Kusume T, Fukaya T. Increased killer inhibitory receptor KIR2DL1 expression among natural killer cells in women with pelvic endometriosis. Fertil Steril 2002;77: 297–302. doi: 10.1016/s0015-0282(01)02964-8. [DOI] [PubMed] [Google Scholar]
- 14.Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR, Vandeputte M. Immunosuppressive activity of peritoneal fluid in women with endometriosis. Obstet Gynecol 1993;82: 206–212. [PubMed] [Google Scholar]
- 15.González-Foruria I, Santulli P, Chouzenoux S, Carmona F, Batteux F, Chapron C. Soluble ligands for the NKG2D receptor are released during endometriosis and correlate with disease severity. PLoS One 2015;10: e0119961. doi: 10.1371/journal.pone.0119961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo SW, Du Y, Liu X. Platelet-derived TGF-β1 mediates the down-modulation of NKG2D expression and may be responsible for impaired natural killer (NK) cytotoxicity in women with endometriosis. Hum Reprod 2016;31: 1462–1474. doi: 10.1093/humrep/dew057. [DOI] [PubMed] [Google Scholar]
- 17.Du Y, Liu X, Guo SW. Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms. Hum Reprod 2017;32: 794–810. doi: 10.1093/humrep/dex014. [DOI] [PubMed] [Google Scholar]
- 18.Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 2014;72: 262–269. doi: 10.1111/aji.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tariverdian N Siedentopf F Rücke M Blois SM Klapp BF Kentenich H, et al. Intraperitoneal immune cell status in infertile women with and without endometriosis. J Reprod Immunol 2009;80: 80–90. doi: 10.1016/j.jri.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod 2009;24: 1695–1703. doi: 10.1093/humrep/dep071. [DOI] [PubMed] [Google Scholar]
- 21.Na YJ, Jin JO, Lee MS, Song MG, Lee KS, Kwak JY. Peritoneal fluid from endometriosis patients switches differentiation of monocytes from dendritic cells to macrophages. J Reprod Immunol 2008;77: 63–74. doi: 10.1016/j.jri.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Fainaru O Adini A Benny O Adini I Short S Bazinet L, et al. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. FASEB J 2008;22: 522–529. doi: 10.1096/fj.07-9034com. [DOI] [PubMed] [Google Scholar]
- 23.Suen JL Chang Y Shiu YS Hsu CY Sharma P Chiu CC, et al. IL-10 from plasmacytoid dendritic cells promotes angiogenesis in the early stage of endometriosis. J Pathol 2019;249: 485–497. doi: 10.1002/path.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pencovich N, Luk J, Hantisteanu S, Hornstein MD, Fainaru O. The development of endometriosis in a murine model is dependent on the presence of dendritic cells. Reprod Biomed Online 2014;28: 515–521. doi: 10.1016/j.rbmo.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Stanic AK, Kim M, Styer AK, Rueda BR. Dendritic cells attenuate the early establishment of endometriosis-like lesions in a murine model. Reprod Sci 2014;21: 1228–1236. doi: 10.1177/1933719114525267. [DOI] [PubMed] [Google Scholar]
- 26.Guo P Bi K Lu Z Wang K Xu Y Wu H, et al. CCR5/CCR5 ligand-induced myeloid-derived suppressor cells are related to the progression of endometriosis. Reprod Biomed Online 2019;39: 704–711. doi: 10.1016/j.rbmo.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y Shao J Jiang F Wang Y Yan Q Yu N, et al. CD33+ CD14+ CD11b+ HLA-DR- monocytic myeloid-derived suppressor cells recruited and activated by CCR9/CCL25 are crucial for the pathogenic progression of endometriosis. Am J Reprod Immunol 2019;81: e13067. doi: 10.1111/aji.13067. [DOI] [PubMed] [Google Scholar]
- 28.Zhang T Zhou J Man GCW Leung KT Liang B Xiao B, et al. MDSCs drive the process of endometriosis by enhancing angiogenesis and are a new potential therapeutic target. Eur J Immunol 2018;48: 1059–1073. doi: 10.1002/eji.201747417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H Bi K Wang K Lu Z Xu Y Guo P, et al. Reduction of myeloid derived suppressor cells by inhibiting Notch pathway prevents the progression of endometriosis in mice model. Int Immunopharmacol 2020;82: 106352. doi: 10.1016/j.intimp.2020.106352. [DOI] [PubMed] [Google Scholar]
- 30.He Y Hung SW Liang B Zhang R Gao Y Chu CY, et al. Receptor tyrosine kinase inhibitor sunitinib as novel immunotherapy to inhibit myeloid-derived suppressor cells for treatment of endometriosis. Front Immunol 2021;12: 641206. doi: 10.3389/fimmu.2021.641206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitz T Hoffmann V Olliges E Bobinger A Popovici R Nößner E, et al. Reduced frequency of perforin-positive CD8+ T cells in menstrual effluent of endometriosis patients. J Reprod Immunol 2021;148: 103424. doi: 10.1016/j.jri.2021.103424. [DOI] [PubMed] [Google Scholar]
- 32.Slabe N, Meden-Vrtovec H, Verdenik I, Kosir-Pogacnik R, Ihan A. Cytotoxic T-cells in peripheral blood in women with endometriosis. Geburtshilfe Frauenheilkd 2013;73: 1042–1048. doi: 10.1055/s-0033-1350702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melioli G, Semino C, Semino A, Venturini PL, Ragni N. Recombinant interleukin-2 corrects in vitro the immunological defect of endometriosis. Am J Reprod Immunol 1993;30: 218–227. doi: 10.1111/j.1600-0897.1993.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 34.Gogacz M Winkler I Bojarska-Junak A Tabarkiewicz J Semczuk A Rechberger T, et al. Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J Reprod Immunol 2016;117: 39–44. doi: 10.1016/j.jri.2016.04.289. [DOI] [PubMed] [Google Scholar]
- 35.Hirata T Osuga Y Takamura M Kodama A Hirota Y Koga K, et al. Recruitment of CCR6-expressing Th17 cells by CCL 20 secreted from IL-1 beta-, TNF-alpha-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology 2010;151: 5468–5476. doi: 10.1210/en.2010-0398. [DOI] [PubMed] [Google Scholar]
- 36.Shi JL, Zheng ZM, Chen M, Shen HH, Li MQ, Shao J. IL-17: An important pathogenic factor in endometriosis. Int J Med Sci 2022;19: 769–778. doi: 10.7150/ijms.71972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirata T Osuga Y Hamasaki K Yoshino O Ito M Hasegawa A, et al. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology 2008;149: 1260–1267. doi: 10.1210/en.2007-0749. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A contributes to the pathogenesis of endometriosis by triggering proinflammatory cytokines and angiogenic growth factors. J Immunol 2015;195: 2591–2600. doi: 10.4049/jimmunol.1501138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JE Ahn SH Marks RM Monsanto SP Fazleabas AT Koti M, et al. IL-17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Front Immunol 2020;11: 108. doi: 10.3389/fimmu.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang YP Peng YQ Wang L Qin J Zhang Y Zhao YZ, et al. RNA-sequencing identifies differentially expressed genes in T helper 17 cells in peritoneal fluid of patients with endometriosis. J Reprod Immunol 2022;149: 103453. doi: 10.1016/j.jri.2021.103453. [DOI] [PubMed] [Google Scholar]
- 41.Hanada T Tsuji S Nakayama M Wakinoue S Kasahara K Kimura F, et al. Suppressive regulatory T cells and latent transforming growth factor-β-expressing macrophages are altered in the peritoneal fluid of patients with endometriosis. Reprod Biol Endocrinol 2018;16: 9. doi: 10.1186/s12958-018-0325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S, Zhang J, Huang C, Lu W, Liang Y, Wan X. Expression of the T regulatory cell transcription factor FoxP3 in peri-implantation phase endometrium in infertile women with endometriosis. Reprod Biol Endocrinol 2012;10: 34. doi: 10.1186/1477-7827-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan KN Yamamoto K Fujishita A Muto H Koshiba A Kuroboshi H, et al. Differential levels of regulatory T cells and T-helper-17 cells in women with early and advanced endometriosis. J Clin Endocrinol Metab 2019;104: 4715–4729. doi: 10.1210/jc.2019-00350. [DOI] [PubMed] [Google Scholar]
- 44.Olkowska-Truchanowicz J Sztokfisz-Ignasiak A Zwierzchowska A Janiuk I Dąbrowski F Korczak-Kowalska G, et al. Endometriotic peritoneal fluid stimulates recruitment of CD4+CD25highFOXP3+ Treg cells. J Clin Med 2021;10: 3789. doi: 10.3390/jcm10173789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang XQ, Zhou WJ, Luo XZ, Tao Y, Li DJ. Synergistic effect of regulatory T cells and proinflammatory cytokines in angiogenesis in the endometriotic milieu. Hum Reprod 2017;32: 1304–1317. doi: 10.1093/humrep/dex067. [DOI] [PubMed] [Google Scholar]
- 46.Hou XX, Wang XQ, Zhou WJ, Li DJ. Regulatory T cells induce polarization of pro-repair macrophages by secreting sFGL2 into the endometriotic milieu. Commun Biol 2021;4: 499. doi: 10.1038/s42003-021-02018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao F, Liu X, Guo SW. Platelets and regulatory T cells may induce a type 2 immunity that is conducive to the progression and fibrogenesis of endometriosis. Front Immunol 2020;11: 610963. doi: 10.3389/fimmu.2020.610963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takamura M Koga K Izumi G Hirata T Harada M Hirota Y, et al. Simultaneous detection and evaluation of four subsets of CD4+ T lymphocyte in lesions and peripheral blood in endometriosis. Am J Reprod Immunol 2015;74: 480–486. doi: 10.1111/aji.12426. [DOI] [PubMed] [Google Scholar]
- 49.Olkowska-Truchanowicz J Białoszewska A Zwierzchowska A Sztokfisz-Ignasiak A Janiuk I Dąbrowski F, et al. Peritoneal fluid from patients with ovarian endometriosis displays immunosuppressive potential and stimulates Th2 response. Int J Mol Sci 2021;22: 8134. doi: 10.3390/ijms22158134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mier-Cabrera J, González-Gallardo S, Hernández-Guerrero C. Effect of nitric oxide and TH1/TH2 cytokine supplementation over ectopic endometrial tissue growth in a murine model of endometriosis. Reprod Sci 2013;20: 1332–1338. doi: 10.1177/1933719113485297. [DOI] [PubMed] [Google Scholar]
- 51.Li L, Liao Z, Ye M, Jiang J. Recombinant human IL-37 inhibited endometriosis development in a mouse model through increasing Th1/Th2 ratio by inducing the maturation of dendritic cells. Reprod Biol Endocrinol 2021;19: 128. doi: 10.1186/s12958-021-00811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riccio LGC, Baracat EC, Chapron C, Batteux F, Abrão MS. The role of the B lymphocytes in endometriosis: A systematic review. J Reprod Immunol 2017;123: 29–34. doi: 10.1016/j.jri.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Greenbaum H, Galper BL, Decter DH, Eisenberg VH. Endometriosis and autoimmunity: Can autoantibodies be used as a non-invasive early diagnostic tool? Autoimmun Rev 2021;20: 102795. doi: 10.1016/j.autrev.2021.102795. [DOI] [PubMed] [Google Scholar]
- 54.Gajbhiye R Bendigeri T Ghuge A Bhusane K Begum S Warty N, et al. Panel of autoimmune markers for noninvasive diagnosis of minimal-mild endometriosis. Reprod Sci 2017;24: 413–420. doi: 10.1177/1933719116657190. [DOI] [PubMed] [Google Scholar]
- 55.Kreiner D, Fromowitz FB, Richardson DA, Kenigsberg D. Endometrial immunofluorescence associated with endometriosis and pelvic inflammatory disease. Fertil Steril 1986;46: 243–246. doi: 10.1016/S0015-0282(16)49519-1. [PubMed] [Google Scholar]
- 56.Dmowski WP, Rana N, Michalowska J, Friberg J, Papierniak C, el-Roeiy A. The effect of endometriosis, its stage and activity, and of autoantibodies on in vitro fertilization and embryo transfer success rates. Fertil Steril 1995;63: 555–562. doi: 10.1016/s0015-0282(16)57425-1. [DOI] [PubMed] [Google Scholar]
- 57.Hever A Roth RB Hevezi P Marin ME Acosta JA Acosta H, et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci U S A 2007;104: 12451–12456. doi: 10.1073/pnas.0703451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Louwen F, Kreis NN, Ritter A, Friemel A, Solbach C, Yuan J. BCL6, a key oncogene, in the placenta, pre-eclampsia and endometriosis. Hum Reprod Update 2022;28: 890–909. doi: 10.1093/humupd/dmac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans-Hoeker E Lessey BA Jeong JW Savaris RF Palomino WA Yuan L, et al. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod Sci 2016;23: 1234–1241. doi: 10.1177/1933719116649711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riccio LGC Jeljeli M Santulli P Chouzenoux S Doridot L Nicco C, et al. B lymphocytes inactivation by Ibrutinib limits endometriosis progression in mice. Hum Reprod 2019;34: 1225–1234. doi: 10.1093/humrep/dez071. [DOI] [PubMed] [Google Scholar]
- 61.Vallvé-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update 2019;25: 564–591. doi: 10.1093/humupd/dmz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burns KA, Rodriguez KF, Hewitt SC, Janardhan KS, Young SL, Korach KS. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology 2012;153: 3960–3971. doi: 10.1210/en.2012-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han SJ Jung SY Wu SP Hawkins SM Park MJ Kyo S, et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 2015;163: 960–974. doi: 10.1016/j.cell.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burns KA, Thomas SY, Hamilton KJ, Young SL, Cook DN, Korach KS. Early endometriosis in females is directed by immune-mediated estrogen receptor α and IL-6 cross-talk. Endocrinology 2018;159: 103–118. doi: 10.1210/en.2017-00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gou Y Li X Li P Zhang H Xu T Wang H, et al. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum Reprod 2019;34: 646–658. doi: 10.1093/humrep/dez019. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y Gong P Chen Y Nwachukwu JC Srinivasan S Ko C, et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med 2015;7: 271ra9. doi: 10.1126/scitranslmed.3010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Q, He W, Tang R, Ma X. Intestinal homeostasis in autoimmune liver diseases. Chin Med J 2022;135: 1642–1652. doi: 10.1097/cm9.0000000000002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J Yan L Ma X Yuan P Zhao F Han Z, et al. Alteration of gut microbiota in type 2 diabetes complicated with cholelithiasis patients. Chin Med J 2022;135: 2125–2127. doi: 10.1097/cm9.0000000000002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan R Yang L Yao G Geng S Ge Q Bo S, et al. Features of gut microbiota in patients with anorexia nervosa. Chin Med J 2022;135: 1993–2002. doi: 10.1097/cm9.0000000000002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ata B Yildiz S Turkgeldi E Brocal VP Dinleyici EC Moya A, et al. The endobiota study: Comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep 2019;9: 2204. doi: 10.1038/s41598-019-39700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernandes C Silveira P Rodrigues Sereia AF Christoff AP Mendes H Valter de Oliveira LF, et al. Microbiome profile of deep endometriosis patients: Comparison of vaginal fluid, endometrium and lesion. Diagnostics (Basel) 2020;10: 163. doi: 10.3390/diagnostics10030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang L Liu B Liu Z Feng W Liu M Wang Y, et al. Gut microbiota exceeds cervical microbiota for early diagnosis of endometriosis. Front Cell Infect Microbiol 2021;11: 788836. doi: 10.3389/fcimb.2021.788836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itoh H, Sashihara T, Hosono A, Kaminogawa S, Uchida M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 2011;63: 205–210. doi: 10.1007/s10616-011-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Itoh H Uchida M Sashihara T Ji ZS Li J Tang Q, et al. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: Randomized, double-blind, placebo-controlled study. Cytotechnology 2011;63: 153–161. doi: 10.1007/s10616-010-9326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chadchan SB Cheng M Parnell LA Yin Y Schriefer A Mysorekar IU, et al. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum Reprod 2019;34: 1106–1116. doi: 10.1093/humrep/dez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perrotta AR Borrelli GM Martins CO Kallas EG Sanabani SS Griffith LG, et al. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: A pilot study. Reprod Sci 2020;27: 1064–1073. doi: 10.1007/s43032-019-00113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan M, Li D, Zhang Z, Sun H, An M, Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod 2018;33: 607–616. doi: 10.1093/humrep/dex372. [DOI] [PubMed] [Google Scholar]
- 78.Lu F Wei J Zhong Y Feng Y Ma B Xiong Y, et al. Antibiotic therapy and vaginal microbiota transplantation reduce endometriosis disease progression in female mice via NF-κB signaling pathway. Front Med (Lausanne) 2022;9: 831115. doi: 10.3389/fmed.2022.831115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan KN Fujishita A Hiraki K Kitajima M Nakashima M Fushiki S, et al. Bacterial contamination hypothesis: A new concept in endometriosis. Reprod Med Biol 2018;17: 125–133. doi: 10.1002/rmb2.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang XM, Ma ZY, Song N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur Rev Med Pharmacol Sci 2018;22: 2513–2518. doi: 10.26355/eurrev_201805_14899. [DOI] [PubMed] [Google Scholar]
- 81.Vilas Boas L, Bezerra Sobrinho C, Rahal D, Augusto Capellari C, Skare T, Nisihara R. Antinuclear antibodies in patients with endometriosis: A cross-sectional study in 94 patients. Hum Immunol 2022;83: 70–73. doi: 10.1016/j.humimm.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Beltagy A, Trespidi L, Gerosa M, Ossola MW, Meroni PL, Chighizola CB. Anti-phospholipid antibodies and reproductive failures. Am J Reprod Immunol 2021;85: e13258. doi: 10.1111/aji.13258. [DOI] [PubMed] [Google Scholar]
- 83.Deroux A, Dumestre-Perard C, Dunand-Faure C, Bouillet L, Hoffmann P. Female infertility and serum auto-antibodies: A systematic review. Clin Rev Allergy Immunol 2017;53: 78–86. doi: 10.1007/s12016-016-8586-z. [DOI] [PubMed] [Google Scholar]
- 84.Kim CH, Chae HD, Kang BM, Chang YS, Mok JE. The immunotherapy during in vitro fertilization and embryo transfer cycles in infertile patients with endometriosis. J Obstet Gynaecol Res 1997;23: 463–470. doi: 10.1111/j.1447-0756.1997.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 85.Maddern J, Grundy L, Castro J, Brierley SM. Pain in endometriosis. Front Cell Neurosci 2020;14: 590823. doi: 10.3389/fncel.2020.590823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran LVP, Tokushige N, Berbic M, Markham R, Fraser IS. Macrophages and nerve fibres in peritoneal endometriosis. Hum Reprod 2009;24: 835–841. doi: 10.1093/humrep/den483. [DOI] [PubMed] [Google Scholar]
- 87.Greaves E, Temp J, Esnal-Zufiurre A, Mechsner S, Horne AW, Saunders PT. Estradiol is a critical mediator of macrophage-nerve cross talk in peritoneal endometriosis. Am J Pathol 2015;185: 2286–2297. doi: 10.1016/j.ajpath.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forster R Sarginson A Velichkova A Hogg C Dorning A Horne AW, et al. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J 2019;33: 11210–11222. doi: 10.1096/fj.201900797R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon MJ Shin HY Cui Y Kim H Thi AH Choi JY, et al. CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J Neurosci 2015;35: 15934–15947. doi: 10.1523/jneurosci.1924-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McKinnon BD, Bertschi D, Bersinger NA, Mueller MD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab 2015;26: 1–10. doi: 10.1016/j.tem.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Zhu H, Wang Y, He Y, Yu W. Inflammation-mediated macrophage polarization induces TRPV1/TRPA1 heteromers in endometriosis. Am J Transl Res 2022;14: 3066–3078. [PMC free article] [PubMed] [Google Scholar]
- 92.Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod 2006;21: 3001–3007. doi: 10.1093/humrep/del260. [DOI] [PubMed] [Google Scholar]
- 93.Jeljeli M Riccio LGC Chouzenoux S Moresi F Toullec L Doridot L, et al. Macrophage immune memory controls endometriosis in mice and humans. Cell Rep 2020;33: 108325. doi: 10.1016/j.celrep.2020.108325. [DOI] [PubMed] [Google Scholar]
- 94.Khodaverdi S Mohammadbeigi R Khaledi M Mesdaghinia L Sharifzadeh F Nasiripour S, et al. Beneficial effects of oral Lactobacillus on pain severity in women suffering from endometriosis: A pilot placebo-controlled randomized clinical trial. Int J Fertil Steril 2019;13: 178–183. doi: 10.22074/ijfs.2019.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bedaiwy MA Falcone T Sharma RK Goldberg JM Attaran M Nelson DR, et al. Prediction of endometriosis with serum and peritoneal fluid markers: A prospective controlled trial. Hum Reprod 2002;17: 426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- 96.Barrier BF, Bates GW, Leland MM, Leach DA, Robinson RD, Propst AM. Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertil Steril 2004;81(Suppl 1): 775–779. doi: 10.1016/j.fertnstert.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 97.Önalan G, Tohma YA, Zeyneloğlu HB. Effect of etanercept on the success of assisted reproductive technology in patients with endometrioma. Gynecol Obstet Invest 2018;83: 358–364. doi: 10.1159/000484895. [DOI] [PubMed] [Google Scholar]
- 98.Koninckx PR, Craessaerts M, Timmerman D, Cornillie F, Kennedy S. Anti-TNF-α treatment for deep endometriosis-associated pain: A randomized placebo-controlled trial. Hum Reprod 2008;23: 2017–2023. doi: 10.1093/humrep/den177. [DOI] [PMC free article] [PubMed] [Google Scholar]