Abstract
Objective:
To compare the effect of different methods of bowel preparation on the incidence of surgical site infections (SSI), anastomotic leakage (AL), and mortality in patients undergoing elective colorectal surgery.
Background:
Recent guidelines advise mechanical bowel preparation with oral antibiotics (MBP-OA) for the prevention of SSI in colorectal surgery. Recent trials suggest oral antibiotics (OA) alone may be sufficient.
Methods:
PubMed, MEDLINE, and Embase were searched from inception until 10-08-2021. We included randomized controlled trials (RCTs) comparing multiple methods of bowel preparation (mechanical bowel preparation [MBP], OA, MBP-OA, or no preparation) with regards to clinical outcomes such as incidence of SSI, AL, and mortality rates. A frequentist random-effects network meta-analysis was conducted to estimate the network effects of the different treatment options.
Results:
We included 48 studies with 13,611 patients. Compared to no preparation, combined direct and indirect network estimates showed a relative risk (RR) for SSI of 0.57 (95% confidence interval [CI], 0.45–0.72) for MBP-OA, 0.68 (95% CI, 0.49–0.95) for OA, and 1.05 (95% CI, 0.87–1.26) for MBP. The RR for MBP-OA compared to OA was 0.84 (95% CI, 0.60–1.19); in sensitivity analysis of mainly laparoscopic procedures this effect of MBP-OA was more profound (RR, 0.56; 95% CI, 0.31–0.99).
Conclusions:
This network meta-analysis of RCTs finds that both mechanical bowel preparation with oral antibiotics and oral antibiotics alone are comparably effective in the prevention of SSI. The evidence is uncertain about the relative benefit of MBP-OA compared to OA alone. Therefore, it seems justified to use either of the 2 for the prevention of SSI in colorectal surgery.
Keywords: oral antibiotics, surgical site infection, colorectal surgery, bowel preparation, mechanical bowel preparation
Mini-Abstract: This network meta-analysis of 48 randomized clinical trials evaluates the best bowel preparation method before elective colorectal surgery for the prevention of surgical site infections (SSI), anastomotic leakage, and all-cause mortality. No considerable advantage of mechanical bowel preparation with oral antibiotics was found over oral antibiotics alone; both are comparably effective in the prevention of SSI. As oral antibiotics are more patient-friendly than mechanical bowel preparation combined with oral antibiotics, it seems justified to use either mechanical bowel preparation in combination with oral antibiotics or oral antibiotics alone for the prevention of SSI in colorectal surgery.
INTRODUCTION
Surgical site infections (SSI) and anastomotic leakage (AL) are serious complications after colorectal surgery and are associated with high morbidity, mortality, and costs.1 Incidence of 5% to 25%1,2 for SSI and 3% to 12%3,4 for AL have been reported. Bowel preparation may prevent a large proportion of SSI and can be performed using mechanical bowel preparation (MBP), oral antibiotics alone (OA), and a combination of both (MBP-OA).
Recent guidelines for the prevention of surgical site infection by the World Health Organization (WHO) and National Institute for Health and Care Excellence (NICE) recommend the combination of OA and MBP and advise against the use of MBP alone.5,6 Recommendations are based on multiple systematic reviews7,8 directly comparing the effect of multiple treatment modalities pairwise. Nonetheless, the recommendations are controversial.9,10 MBP is associated with possible harms, such as dehydration and great discomfort.11 Patients treated with MBP are sometimes admitted early to the hospital to complete preparation. Furthermore, few randomized controlled trials (RCTs) study the effect of OA alone (without MBP). Therefore, the added effect of MBP on OA is unclear.
Regular pairwise meta-analyses compare 1 intervention directly to another intervention (or control). Often, multiple treatments are available, but a regular pairwise meta-analysis cannot compare multiple treatments in 1 single analysis. For instance, in multiple RCTs treatment A is compared with treatment B, and treatment B is compared with treatment C. Treatment A and C are not directly investigated and cannot be compared. However, treatments A and C share a common comparator B. A network meta-analysis (NMA) uses the common comparator and allows simultaneous comparison of all treatment modalities through direct and indirect effects. In this example, an NMA is able to make a (indirect) comparison between A and C, although A and C have not been directly compared in RCTs, and quantify its effect size. Direct comparisons are pooled estimates of studies that pairwise compare the interventions (similarly to classical pairwise meta-analysis). Indirect effects are estimated using mathematical combinations of the available direct effect estimates in the network. A network meta-analysis thereby utilizes more available information than a pairwise meta-analysis can. The network estimate is the result of a weighted combination of the direct and indirect effect estimates.
Two previous network meta-analyses have been performed on this topic. One NMA12 demonstrates that the combination of MBP-OA is associated with the lowest SSI rate, while the latest review13 concludes that OA only is most beneficial. These studies, however, have included few RCTs studying the effect of OA only compared to no preparation. In recent years, multiple relevant RCTs14–16 studying the effect of OA only have been conducted, and contain crucial information not included in previous NMAs. These new RCTs permit a far more precise effect estimate. Moreover, both previous NMAs12,13 have included studies in which no standard intravenous surgical antibiotic prophylaxis (SAP) was used. Intravenous SAP is a standard, well-implemented preventive measure in the current clinical setting. Generalizability for current practice is limited if studies performed in an outdated clinical setting are included.
The aim of this systematic review and network meta-analysis is to provide an up-to-date evaluation on the effect of different methods of bowel preparation on surgical site infections rate, anastomotic leakage and mortality after elective colorectal surgery, research the effect of OA alone (without MBP) and provide a Grading of Recommendations Assessment, Development and Evaluation (GRADE) recommendation based on current available evidence.
METHODS
This network meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Network Meta-analyses statement.17 The study protocol is registered in PROSPERO (CRD42021225091).
Search Strategy
A systematic literature search was carried out using PubMed, MEDLINE, and Embase on 10-08-2021. Search terms included: surgical site infection, anastomotic leakage, colorectal surgery, preoperative period, and antibiotic prophylaxis. A clinical librarian was consulted on the search strategy. Reference lists of published reviews and included studies were examined to identify additional articles. The complete search strategy is included in Appendix S1, see http://links.lww.com/AOSO/A132.
Eligibility Criteria
We included RCTs comparing MBP and/or OA to MBP or OA or no bowel preparation in patients undergoing elective colorectal surgery that reported rates of surgical site infections and other complications.
We excluded studies investigating pediatric participants, effects of different treatments within a specific treatment modality (eg, only different kinds of mechanical bowel preparation), and studies that did not provide standard preoperative intravenous SAP. In addition, studies including emergency surgery for which bowel preparation could not have been performed and treatment arms containing probiotics were also excluded. The language was restricted to English. We applied no restrictions regarding the year of publication.
Study Selection and Data Extraction
Two authors (H.J. and N.W.) independently reviewed titles and abstracts for eligibility. These authors also reviewed full texts of potentially eligible studies. Disagreements were resolved through discussion and, if necessary, the senior author (M.B.) was consulted.
The following data were extracted using a prespecified form: author, year, country, study period, primary and secondary outcomes, number of patients in each arm, type of surgery, open or laparoscopic procedure, methods of bowel preparation (agent/medication, dose, and timing of administration), regiment of preoperative intravenous SAP, definition of SSI and AL. When data were incomplete or unclear, authors were contacted for additional information. To uniformly assess SSI and AL, authors of the original publications were contacted to clarify if AL was included in the SSI rate or if AL was reported separately.
Outcomes
The primary outcome was the rate of SSI (anastomotic leakage included in the SSI rate), as defined by the authors of the original publication. Secondary outcomes included anastomotic leakage, as defined by the authors of the original publication, and mortality rates.
Risk of Bias and GRADE Assessment
The risk of bias within individual studies was assessed using the Cochrane Risk of Bias-2 tool.18 Assessment was performed by 2 authors (H.J. and N.W.). Publication bias was assessed using a comparison-adjusted funnel plot.19 The GRADE methodology was used to judge the certainty of evidence.20
Statistical Analysis
Relative risks (RRs), corresponding 95% confidence intervals (CIs) and standard errors were calculated for the individual studies. Studies with no events in both arms were excluded from quantitative analysis.21
We performed a network meta-analysis using the frequentist method22 and chose a subsequent random-effects model. A P < 0.05 was considered statistically significant. Heterogeneity was assessed by the I2 statistic. An I2 up to 40% might not be important, between 30% and 60% may represent moderate heterogeneity, between 50% and 90% may represent substantial heterogeneity, and 75% to 100% is considerable heterogeneity.21
We assessed the transitivity of the treatment modalities, which recalls that studies are similar regarding methodological aspects and clinical settings. Theoretically, the interventions in different studies are exchangeable and effect modifiers should not differ. Methodological aspects and clinical aspects that may cause inconsistencies were evaluated. We assessed inconsistency using the node splitting method23 and the effect estimates of the direct and indirect comparisons were examined to check agreement. If inconsistency was detected, the network evidence was either downgraded when direct and indirect estimates had overlapping CIs, or focus was put on either the direct or indirect estimate, whichever shows a narrower CI.20
Results of the NMA were expressed in pooled RRs with 95% CI. We calculated P-scores to rank the different treatment modalities.24 A high P-score (nearing 1) means that the treatment modality is preferable, a score nearing 0 means the treatment is least preferable.
Additional subgroup analysis was carried out based on the use of aminoglycosides (yes/no). Furthermore, sensitivity analyses were conducted with studies published after the year 2000 and based on surgical approach (studies in which all patients had open surgery versus studies with a combination of open and laparoscopic surgery). Quantitative analysis was performed using R version 4.0.3 (R Core Team [2016] R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria), using the packages “netmeta,” “metafor,” “meta,” “dmetar,” “devtools,” and “tidyverse”.
RESULTS
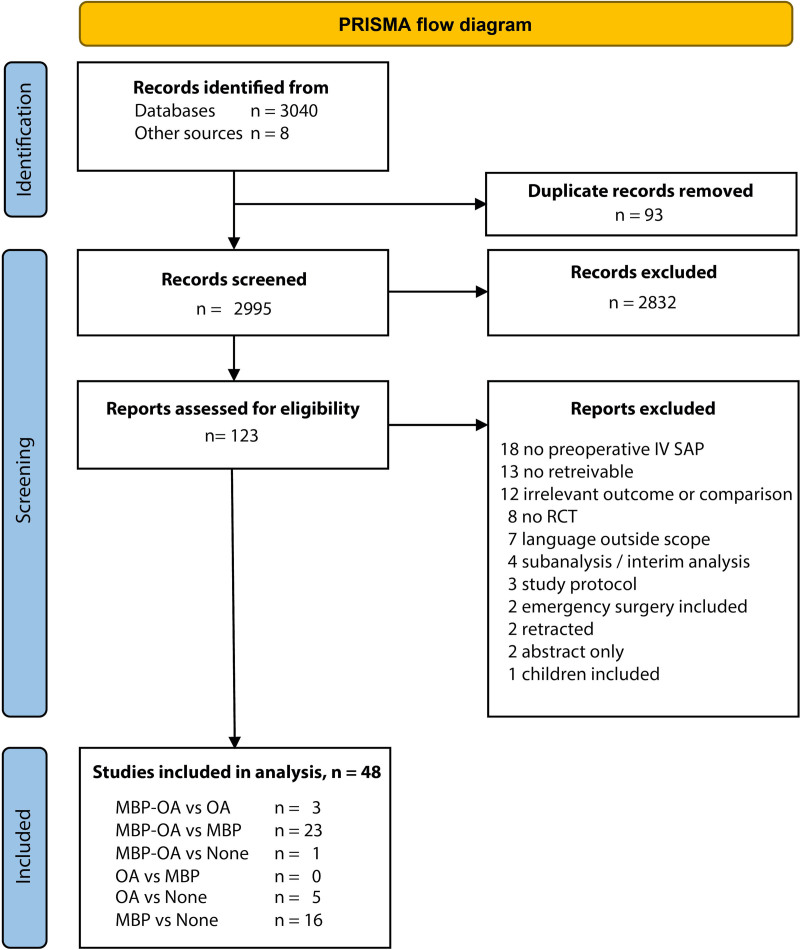
Our search resulted in 3040 potential studies. Eight additional studies were found through forward and backward citation tracking. We reviewed 123 full texts. A total of 48 studies, with 13,611 patients, were included in the quantitative analyses. All but 9 articles were published after the year 2000. The selection process is summarized in Fig. 1. Reasons for exclusion of full texts are listed in Appendix S2, see http://links.lww.com/AOSO/A132.
FIGURE 1.
Study selection process. Reasons for exclusion after full text review can be found in Supplementary Appendix 2
Study Characteristics
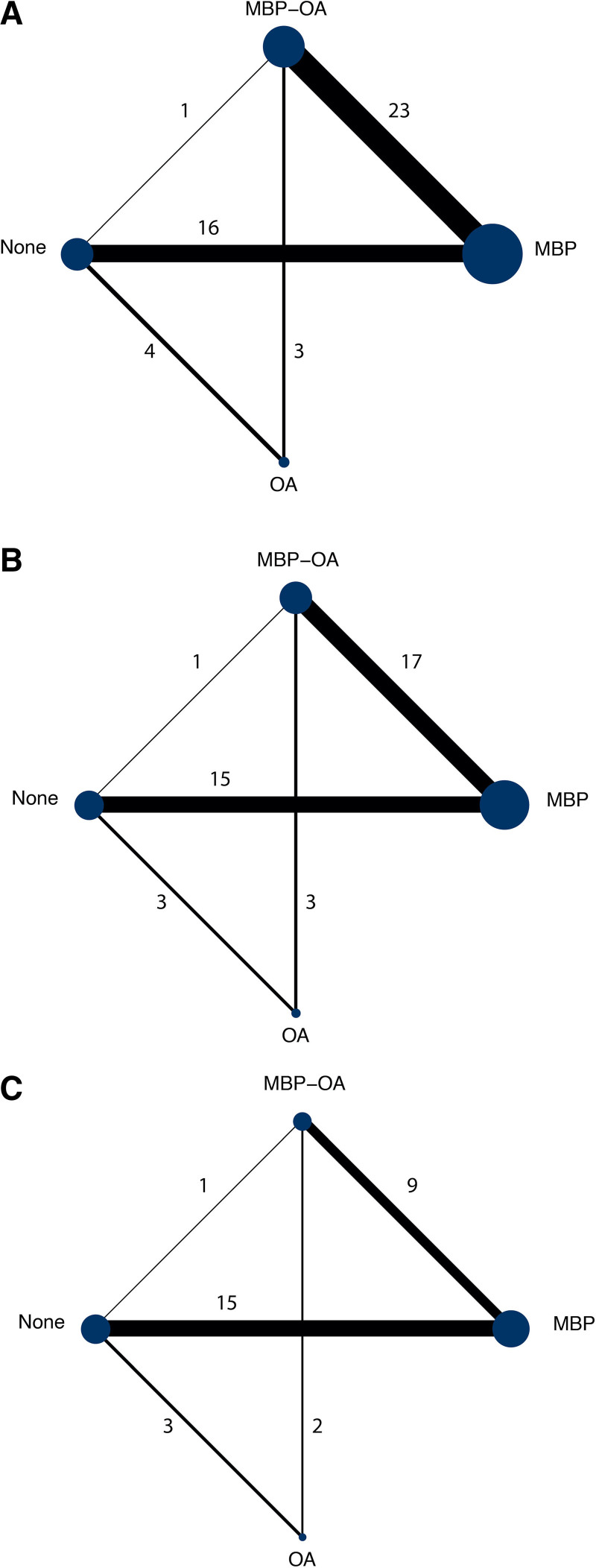
The study characteristics of the 48 included RCTs are presented in Appendix S3, see http://links.lww.com/AOSO/A132. Twenty-three RCTs compared MBP-OA and MBP, 16 RCTs compared MBP and no preparation, 5 compared OA with no preparation, 3 compared OA and MBP-OA, and 1 RCT compared MBP-OA and no preparation. Figure 2 shows the network plot of direct comparisons between included RCTs.
FIGURE 2.
Network Plots. Network plots of included studies. The thickness of the lines and circles correspond with the number of studies. The squares and lines represent relative risks with corresponding 95% confidence intervals. (A) Total SSI. (B) Anastomotic leakage. (C) Mortality.
For MBP, studies used the following solutions, alone or in combination with others: polyethylene glycol solution (24 studies), sodium picosulfate (10 studies), sodium phosphate (8 studies), magnesium citrate (5 studies), bisacodyl (2 studies), mannitol (1 study), and senna (1 study). The solutions (2–4 liters) were mostly given the day before surgery.
The protocols regarding OA in the 32 studies studying OA varied greatly. Aminoglycosides (eg, kanamycin, neomycin, and erythromycin) were used in 27 out of 32 studies, of which in 14 studies in combination with metronidazole. OA were usually started the day before surgery. Alternative protocols span from 3 days preoperative until postoperative day 7, ranging from 2 to 4 times a day.
Cephalosporins (1–2 g) alone or in combination with metronidazole (0.5–1 g), or flomoxef (1 g) were often used for preoperative SAP. Redosing of SAP during surgery was performed in 15 out of 48 studies if surgery lasted longer than 2–4 hours depending on the half-life of antibiotics used.
Network Model
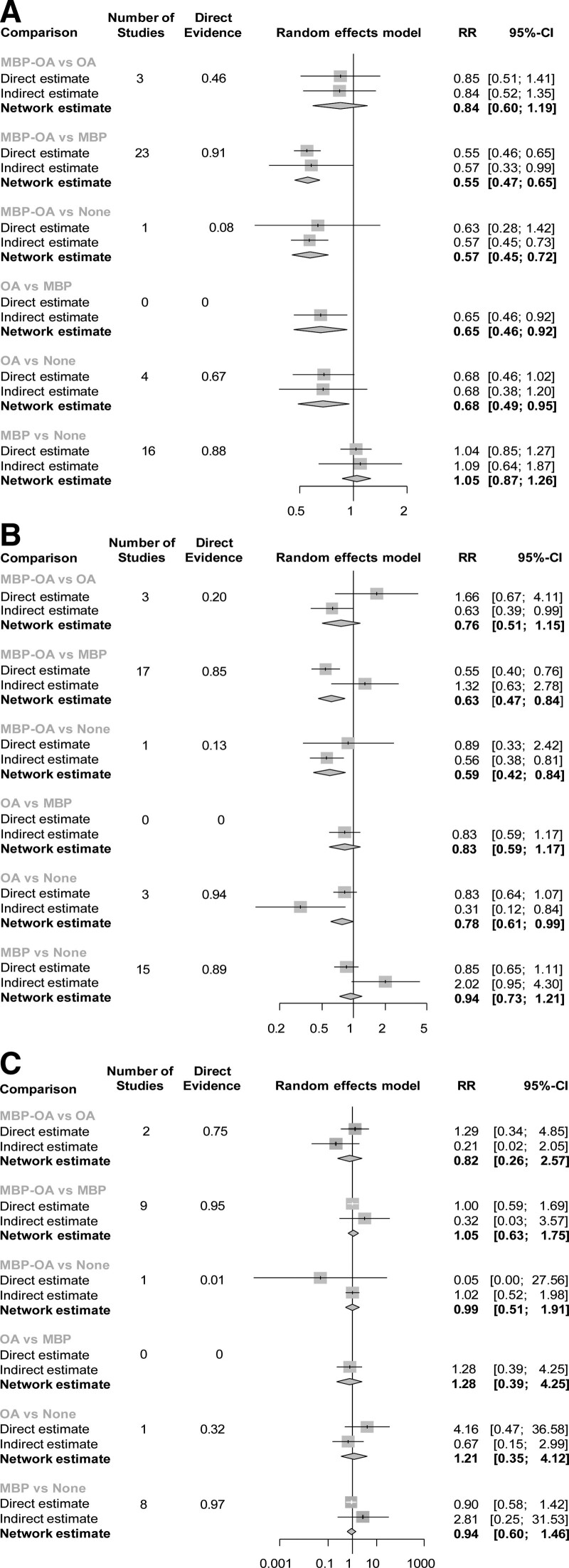
We evaluated methodological and clinical aspects that may cause inconsistencies and deemed these aspects comparable to allow the construction of a network and quantitative analysis. Overall, the network showed moderate inconsistency for SSI (heterogeneity between studies: I2 = 37.3%; P = 0.88), and low inconsistency for AL (I2 = 0%; P = 0.10) and mortality (I2 = 0%; P = 0.27). Inconsistency was also evaluated through node splitting and checking the agreement between the direct and indirect comparisons (Fig. 4). For SSI and mortality, no inconsistency was detected in any comparison. Two comparisons of AL (MBP vs MBP-OA and MBP vs no preparation) showed significant inconsistency (P <0.05).
FIGURE 4.
Forest plots – direct, indirect and network estimate. Node splitting separates the network estimates into the evidence of direct and indirect evidence. (A) (SSI). * One study did not have any events in both arms and was thus excluded from analysis. Direct evidence: Weight of the direct estimate in the network estimate. Direct estimate: Estimate of pairwise comparison of the interventions [similarly to classical pairwise meta-analysis]. Indirect estimate: estimate using mathematical combinations of the available direct effect estimates in the network. Network estimate: Combined estimate of direct and indirect estimate. (B) Anastomotic leakage. (C) Mortality.
Primary Outcome and Network Meta-Analysis
All 48 RCTs report on SSI rates. Twelve out of 23 RCTs25–47 comparing MBP-OA and MBP reported a significant reduction of SSI when using MBP-OA. Two48,49 out of three15 RCTs comparing MBP-OA and OA report a significant benefit favoring OA. Only one50 investigation compared MBP-OA to no preparation and found no significant difference in SSI. Two RCTs16,51 comparing OA to no preparation reported a significant lower SSI rate in the OA group, 2 found no difference,14,52 and 1 reported no events in both arms.53 In the 16 RCTs11, 54–68 comparing MBP and no preparation, 15 out of 16 did not find a significant difference.
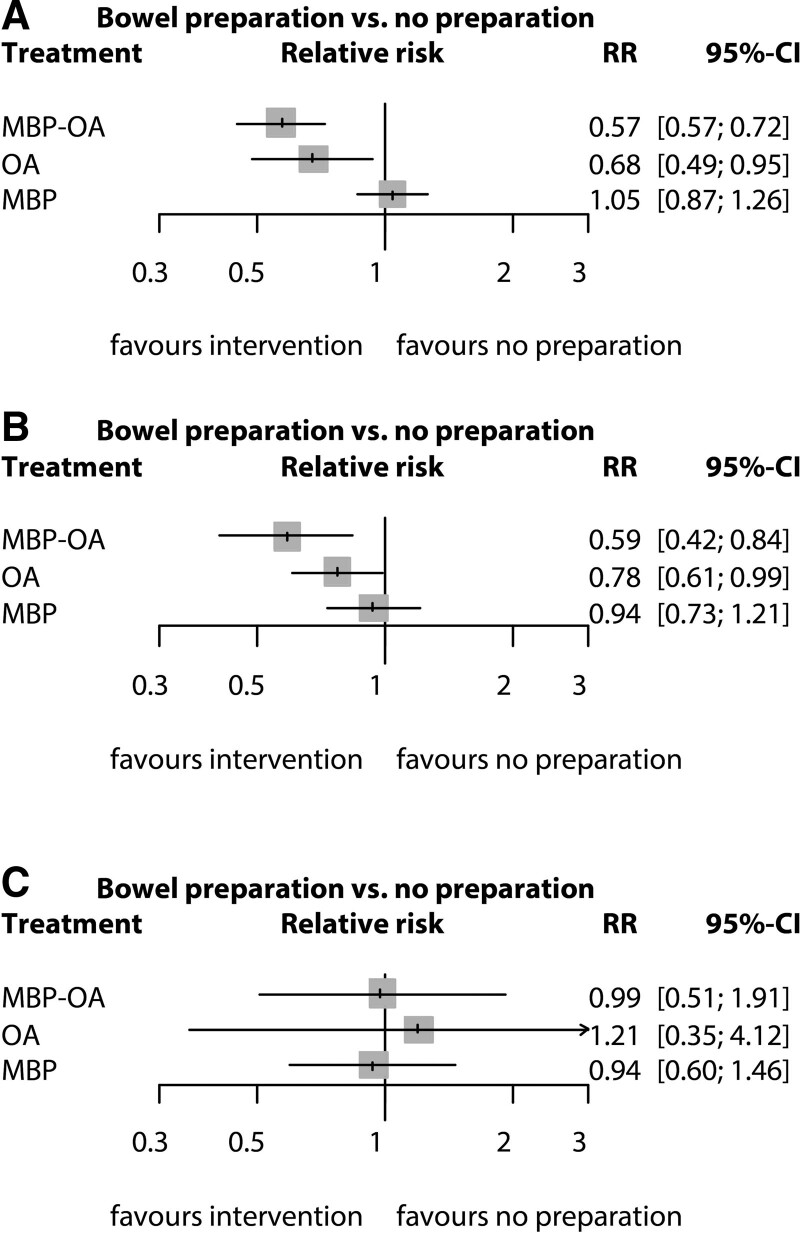
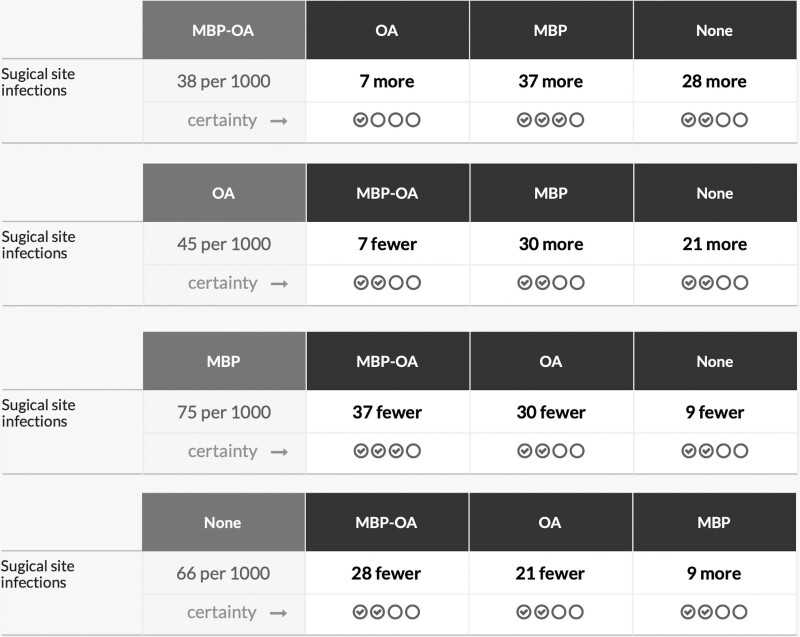
The NMA estimated RRs using direct and indirect evidence. The forest plots are shown in Fig. 3A. Compared to no preparation, RR were 0.57 (95% CI, 0.45–0.72) for MBP-OA, 0.68 (95% CI, 0.49–0.95) for OA, and 1.05 (95% CI 0.87–1.26) for MBP. The RRs of all comparisons are described in Table 1 and Fig. 4A. The effectiveness of MBP-OA and OA was comparable (RR, 0.84; 95% CI, 0.60–1.19) and higher compared to MBP (RR, 0.55; 95% CI, 0.47–0.65). OA compared with MBP only was 0.65 (95% CI, 0.46–0.92). In the network ranking, the P-score of MBP-OA was 0.944, 0.717 for OA, 0.230 for no preparation and MBP had the lowest P-score of 0.110.
FIGURE 3.
Forest plots. Figures show the pooled estimates from the included studies, comparing different bowel preparation methods with no preparation. (A) Total SSI. (B) Anastomotic leakage. (C) Mortality.
TABLE 1.
League tables
| Primary analysis: all RCTs | Sensitivity analysis: RCTs with laparoscopic surgery only or with both open and laparoscopic colorectal surgery | ||||||
|---|---|---|---|---|---|---|---|
| Total SSI (RR with 95% CI) | Total SSI (RR with 95% CI) | ||||||
| MBP-OA P-score 0.944 |
MBP-OA | ||||||
| 0.84 (0.60–1.19) |
OA P-score 0.717 |
0.56(0.31–0.99) | OA | ||||
| 0.57(0.45–0.72) | 0.68(0.49–0.95) | None P-score 0.230 |
0.41(0.27–0.62) | 0.73 (0.48–1.12) |
None | ||
| 0.55(0.47–0.65) | 0.65(0.46–0.92) | 0.96 (0.79–1.15) |
MBP P-score 0.110 |
0.44(0.34–0.58) | 0.80 (0.46–1.38) |
1.09 (0.74–1.59) |
MBP |
| Anastomotic leakage (RR with 95% CI) | Anastomotic leakage (RR with 95% CI) | ||||||
| MBP-OA P-score 0.967 |
*0.55(0.40-0.70) | MBP-OA | |||||
| 0.76 (0.51–1.15) |
OA P-score 0.645 |
0.54 (0.29–1.03) |
OA | ||||
| 0.63(0.47–0.84) | 0.83 (0.59–1.17) |
MBP P-score 0.276 |
*0.85 (0.65–1.07) |
0.50(0.35–0.71) | 1.14 (0.60–2.16) |
MBP | |
| 0.59(0.42–0.84) | 0.78(0.61–0.99) | 0.94 (0.73–1.21) |
None P-score 0.111 |
0.45(0.26–0.80) | 0.83 (0.64–1.07) |
0.72 (0.40–1.30) |
None |
| Mortality (RR with 95% CI) | Mortality (RR with 95% CI) | ||||||
| MBP P-score 0.615 |
MBP | ||||||
| 0.95 (0.57–1.59) |
MBP-OA P-score 0.526 |
1.12 (0.52–2.43) |
MBP-OA | ||||
| 0.94 (0.60–1.46) |
0.99 (0.51–1.91) |
None P-score 0.496 |
0.74 (0.01–69.25) |
0.65 (0.01–61.51) |
None | ||
| 0.78 (0.24–2.58) |
0.87 (0.26–2.57) |
0.83 (0.24–2.84) |
OA P-score 0.363 |
0.18 (0.18–27.26) |
0.16 (0.00–24.22) |
0.24 (0.03–2.11) |
OA |
League tables showing the network relative risks of pairwise comparisons between different bowel preparation methods. Calculated P-scores are shown, a higher P-score (nearing 1) means that the treatment modality is preferable, a score nearing 0 means the treatment is least preferable. RR with 95% CI in bold are statistically significant.
*RR with 95% CI of direct comparisons.
Secondary Outcomes and Network Meta-Analysis
Anastomotic leakage rates were reported by 39 RCTs. Three of 17 RCTs comparing MBP-OA versus only MBP found a significant benefit with MBP-OA. All three15,48,49 RCTs comparing MBP-OA and OA alone did not find a significant difference in rates of AL. Three RCTs14,16,51 comparing OA and no preparation reporting on AL, did not find a significant difference. Only one69 of 15 RCTs comparing AL rates of MBP and no preparation found a significant reduction with the use of MBP. None of the 20 RCTs reporting on mortality found a significant difference. In nine14,32,36,54,55,58,65–67 publications no deaths occurred.
Network meta-analysis for anastomotic leakage showed a RR of 0.76 (95% CI, 0.51–1.15) for MBP-OA compared to OA and 0.59 (95% CI, 0.42–0.84) versus no preparation (Fig. 3b and 4b). The RR for OA compared with MBP is 0.83 (95% CI, 0.59–1.17) and 0.78 (95% CI, 0.61–0.99) compared with no preparation. MBP compared to no preparation and MBP-OA compared to MBP showed significant inconsistency. In both comparisons, direct estimates had a narrower CI, with a RR of 0.85 (95% CI, 0.65–1.11) for MBP vs. no preparation and RR of 0.55 (95% CI, 0.40–0.76) for MBP-OA vs MBP.
For mortality rates, the RR of MBP-OA was 0.87 (95% CI, 0.26–2.57) compared with OA, 1.05 (95% CI, 0.63–1.75) versus MBP and 0.99 (95% CI, 0.51–1.91) versus no preparation (Figs. 3c and 4c). The RR for mortality for OA compared with MBP was 1.28 (95% CI, 0.39–4.17) and 1.21 (95% CI, 0.35–4.12) compared with no preparation. RR for MBP compared with no preparation was 0.94 (95% CI, 0.24–2.58).
Sensitivity and Subgroup Analyses
The sensitivity analysis of studies that focussed on laparoscopic surgery or had a mixed cohort of both laparoscopic and open surgical approaches, included 20 RCTs with a total of 5213 patients (thereby excluding 28 studies using only an open surgical approach). The sensitivity analysis showed a more prominent effect of MBP-OA than all other bowel preparations on SSI rate and AL in studies that included also laparoscopic surgery. For MBP-OA vs OA, a RR for SSI of 0.56 (0.31–0.99) and a RR for AL of 0.54 (0.29–1.03) were found. All other comparisons of preparations in open versus mixed/laparoscopic surgery RCTs were comparable to the overall analyses, as shown in Table 1. It must be noted that this is not a true comparison between laparoscopic and open surgery, since only 60% of operations (3062 of 5213) in the laparoscopic/mixed group were laparoscopic.
Results of the sensitivity analysis for the study year (after the year 2000) and subgroup analysis for the type of antibiotics (with or without aminoglycosides) showed no major differences compared to the overall analysis. Detailed results are shown in Appendix S4, see http://links.lww.com/AOSO/A132.
Risk of Bias Assessment
Among the 48 RCTs included, 5 RCTs had a high risk of bias, 35 had some concerns regarding bias, and 7 had a low risk of bias. The overall quality of the included studies was deemed satisfactory. A detailed Risk of Bias assessment can be found in Appendix S5, see http://links.lww.com/AOSO/A132. Funnel plots did not show signs of publication bias, which can be found in Appendix S6, see http://links.lww.com/AOSO/A132.
GRADE Assessment
The GRADE assessment for SSI can be found in Fig. 5. The level of evidence for SSI comes from RCTs and therefore starts high. The level of evidence was downgraded by 2 levels because the CI crosses the minimal clinically important difference (MCID)-boundary (imprecision, −1) and because of study limitations (risk of bias −1). If there was a high level of inconsistency and the direct evidence had superior quality and confidence over the indirect evidence, we looked at the direct evidence and did not downgrade for inconsistency.20 The overall quality of evidence for all outcomes was deemed low.
FIGURE 5.
GRADE assessment for SSI.
The elaborate GRADE assessment for all comparisons including the secondary outcomes, is presented in Appendix S7, see http://links.lww.com/AOSO/A132 and on the MAGICapp (https://magicevidence.org/match-it/210519dist-json-temp/#!/sof/data-set/template).
DISCUSSION
This network meta-analysis investigated the effect of different types of bowel preparation on the rates of surgical site infections, anastomotic leakage, and mortality for patients undergoing elective colorectal surgery. We found a significant reduction of SSI when using MBP-OA or OA alone compared with MBP only or no preparation. MBP-OA and OA showed comparable effectiveness. There was no difference in effect between MBP and no preparation. Overall, the certainty of the evidence was graded as low because of imprecision of the results and the risk of bias. Furthermore, MBP-OA and OA may both be effective for the prevention of anastomotic leakage, whereas MBP was not. There was no clear association between the method of bowel preparation and the all-cause mortality rate. Only in a sensitivity analysis of studies that focussed on laparoscopic surgery or a mixed laparoscopic/open population, MBP-OA seemed more effective than other methods of bowel preparation including OA alone.
The present review is of great added value to current literature and existing guideline recommendations. The results are partly in line with current international guidelines but give an important new perspective. WHO5 and NICE6 guidelines both advise against the use of only MBP as routine preparation. The WHO advises MBP-OA in colorectal surgery. However, both guidelines did not include studies investigating the effect of OA alone. The NICE guidelines acknowledge this limitation and state that their current guideline should be updated with newly published evidence, including studies investigating the effect of OA alone. The current guideline on this topic by the Centers for Disease Control and Prevention70 does not mention bowel preparation.
Previous, non-network meta-analyses have shown results in favor of MBP-OA compared to MBP8 and no clear difference between MBP and no preparation.7 These results are still in line with the present study, but lack data on the relative effect of OA alone. One of the trials14 investigating the effect of OA alone ended prematurely due to results of a new nonrandomized study71 favoring OA. The authors no longer considered clinical equipoise.
The most recent NMA13 concludes that OA without MBP shows the greatest reduction in SSI. This is not in line with our findings. Current evidence from present NMA shows that the effectiveness of OA alone does not significantly differ from that of MBP-OA (RR, 0.84; 95% CI, 0.60–1.19). Our results support the use of OA alone, but we do not find OA to be superior to MBP-OA. The most recent NMA13 has not included some of these new RCTs,14,16,25–27,29 which explains the difference in results. Some studies were published after the search date; others were excluded for unknown reasons. An earlier NMA12 has identified a knowledge gap with respect to the effectiveness of OA as few studies compared OA alone to MBP-OA or no preparation. We included 4 additional RCTs14–16,51 investigating OA as a sole intervention without MBP; all published since 2020. One RCT compared OA alone to MBP-OA15 and 3 studies compared OA alone to no preparation.14,16,51
In recent years, minimally invasive procedures are widely performed and a distinction between the effects of the various bowel preparations in open and laparoscopic procedures could be very helpful in clinical practice. Therefore, we performed an additional sensitivity analysis excluding RCTs with only open surgical procedures. In the remaining cohort for analysis, still, 40% of the procedures were open procedures. It was not possible to attribute SSI to either laparoscopic or open surgery among these mixed studies as such details were not supplied in the original publications. This limits us to draw firm conclusions on possible differences in the efficacy of the various bowel preparation methods between open and laparoscopic procedures. However, when excluding studies investigating only open surgical procedures, the results were in favor of MBP-OA. More studies including only laparoscopic procedures are needed to draw definite conclusions.
In contrast to earlier NMAs,12,13 we excluded all studies without preoperative intravenous antibiotic prophylaxis around the time of induction, as such prophylaxis is considered standard state-of-art perioperative care.
Our study is limited by the quality and number of studies available. Especially OA alone has not been investigated extensively and the number of available RCTs is limited. By carrying out an NMA we chose a design aimed to precisely estimate the effects of treatments for which limited data exists. Nonetheless, a network estimate will provide more precise estimations when more direct comparisons are available. The reported treatment rankings (expressed in P-scores) are based on the estimated point estimates and standard errors. With limited data available effects can be overvalued, especially when relative effects are small.17 Moreover, treatment rankings take only 1 outcome into consideration and do not look at potential harms of a treatment, which makes them prone to misinterpretation when focusing only on the P-score ranking. Therefore, we have put an emphasis on the RRs with corresponding 95% CIs, depicted in the forest plots, rather than drawing conclusions only based on the P-score rankings.72
Although the overall network for AL showed low inconsistency (I2 < 0%), a node split of the results of AL showed two comparisons (MBP vs MBP-OA and MBP vs no preparation) had significant inconsistencies. The GRADE working group advises against modification or exclusion of inconsistent data without a strong rationale.20 Rather, if both direct and indirect evidence are of comparable quality, it is preferred to rate down the quality of the network estimate in the GRADE assessment. The second solution is to focus not on the network estimate but to focus on either the direct or indirect estimate, whichever shows greater confidence. Both comparisons that showed inconsistency had a higher quality of direct evidence, thus we valued the direct comparison over the indirect comparison. Additional research comparing MBP-OA to OA alone is needed. This will generate more direct comparisons, allowing a more precise estimation of treatment effect and more valid treatment ranking. Additionally, the emergence of antimicrobial resistance has not been widely investigated when looking at bowel preparation and needs further investigation. Two protocols for new RCTs including an OA study arm have been published and seem promising.73,74
Second, there is clinical heterogeneity between studies with respect to type, duration, and dosage of MBP, OA, and preoperative surgical antibiotic prophylaxis. The heterogeneity in antibiotic administration may be attributed to regional antibiotic availability and resistance. Espin Basany et al38 did compare 1 or 3 doses of oral antibiotics with no preparation and found no difference in efficacy. In the future, when large datasets are available, it would be interesting to perform additional subgroup analyses based on the type and dosage of oral antibiotics.
Third, even though most studies used Centers for Disease Control and Prevention criteria75 to define SSI and AL, there were methodological differences. Especially regarding anastomotic leakage, definitions were not always clear; for example, some studies categorized abscesses in the proximity of the anastomosis as anastomotic leakage,28 and others diagnosed AL only when extraluminal contrast was seen on imaging.65 In addition, some studies include AL as SSI, whereas others reported AL separately. Authors of the original publications were contacted to clarify if AL was included in SSI rates to unify the results, but this was not always possible.
This network meta-analysis suggests that MBP-OA and OA alone reduce SSI compared to no bowel preparation and that MBP-OA result in little to no difference in SSI and AL rates compared to OA alone. When excluding studies investigating only open surgical procedures, MBP-OA seems to have favorable effects over OA alone but more studies on laparoscopic procedures are needed to draw definitive conclusions. However, analysis of effectivity does not take the discomfort and possible harms (eg, electrolytes imbalance and dehydration) of MBP into consideration nor its practical concerns such as early hospital admission. The harms and benefits should be carefully weighed, and these results justify questioning the additional value of MBP to OA. Therefore, it seems plausible to use either MBP-OA or OA alone for the prevention of SSI in colorectal surgery. This may result in the need for current guidelines to be revisited.
ACKNOWLEDGMENT
The authors thank S. van Dusseldorp for her advice on the search strategy. We also thank Guido Schwarzer, creator of R package meta, for his helpful responses in relation to our analyses.
Design: H.J., N.W.,and M.B.. Data collection: H.J. and N.W. Data analysis and interpretation: H.J., N.W., and M.B. Drafting the article: H.J. and N.W.. Critical revision of manuscript: H.J., N.W., and M.B. Final approval: H.J., N.W. and M.B. Visualization: W.H. M.G.
Supplementary Material
Footnotes
Published online 23 June 2022
H.J. and N.W. contributed equally to this work.
Funding: This study is funded by the Dutch Association for Quality Funds Medical Specialists (SKMS).
Disclosure: M.B. reported receiving institutional grants from J&J/Ethicon, KCI/3M, Bard and New Compliance; and being a speaker and/or instructor for 3M/KCI, J&J/Ethicon, Allergan/LifeCell, BD Bard, Gore, Smith & Nephew, GDM, Medtronic. No other disclosures were reported. Other authors declare that they have nothing to disclose.
PROSPERO Registration: CRD42021225091.
Data availability statement: All data is published either in this manuscript, the included articles or the supplementary appendix.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Gantz O, Zagadailov P, Merchant AM. The cost of surgical site infections after colorectal surgery in the United States from 2001 to 2012: a longitudinal analysis. Am Surg. 2019;85:142–149. [PubMed] [Google Scholar]
- 2.Smith RL, Bohl JK, McElearney ST, et al. Wound infection after elective colorectal resection. Ann Surg. 2004;239:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker IS, Grossmann I, Henneman D, et al. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg. 2014;101:424–432. [DOI] [PubMed] [Google Scholar]
- 4.Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc. 2015;29:3608–3617. [DOI] [PubMed] [Google Scholar]
- 5.Global guidelines for the prevention of surgical site infection. 2nd ed. World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 6.NICE. 2019 Exceptional Surveillance of Surgical Site Infections: Prevention and Treatment (NICE Guideline NG125). 2019. Available at: https://www.nice.org.uk/guidance/ng125/resources/2019-exceptional-surveillance-of-surgical-site-infections-prevention-and-treatment-nice-guideline-ng125-pdf-8718507017413. Accessed December 7, 2021. [PubMed]
- 7.Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;2011:CD001544. [DOI] [PubMed] [Google Scholar]
- 8.Rollins KE, Javanmard-Emamghissi H, Acheson AG, et al. The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Ann Surg. 2019;270:43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson RL, Hassan M, Grant MD. Antibiotic prophylaxis in colorectal surgery: are oral, intravenous or both best and is mechanical bowel preparation necessary? Tech Coloproctol. 2020;24:1233–1246. [DOI] [PubMed] [Google Scholar]
- 10.Alverdy JC. Bowel preparation in colorectal surgery: the day of reckoning is here. Br J Surg. 2021;108:340–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung B, Lannerstad O, Påhlman L, et al. Preoperative mechanical preparation of the colon: the patient’s experience. BMC Surg. 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh JWT, Phan K, Hitos K, et al. Association of mechanical bowel preparation and oral antibiotics before elective colorectal surgery with surgical site infection: a network meta-analysis. JAMA Netw Open. 2018;1:e183226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodfield JC, Clifford K, Schmidt B, et al. Strategies for antibiotic administration for bowel preparation among patients undergoing elective colorectal surgery: a network meta-analysis. JAMA Surg. 2022;157:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulder T, Kluytmans-van den Bergh M, Vlaminckx B, et al. Prevention of severe infectious complications after colorectal surgery using oral non-absorbable antimicrobial prophylaxis: results of a multicenter randomized placebo-controlled clinical trial. Antimicrob Resist Infect Control. 2020;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki T, Sadahiro S, Tanaka A, et al. Usefulness of preoperative mechanical bowel preparation in patients with colon cancer who undergo elective surgery: a prospective randomized trial using oral antibiotics. Dig Surg. 2020;37:192–198. [DOI] [PubMed] [Google Scholar]
- 16.Arezzo A, Mistrangelo M, Bonino MA, et al. Oral neomycin and bacitracin are effective in preventing surgical site infections in elective colorectal surgery: a multicentre, randomized, parallel, single-blinded trial (COLORAL-1). Updates Surg. 2021;73:1775–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 21.Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds, Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available at: www.training.cochrane.org/handbook. [Google Scholar]
- 22.Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312–324. [DOI] [PubMed] [Google Scholar]
- 23.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. [DOI] [PubMed] [Google Scholar]
- 24.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp G, Saftics G, Szabó BE, et al. Systemic versus Oral and Systemic Antibiotic Prophylaxis (SOAP) study in colorectal surgery: prospective randomized multicentre trial. Br J Surg. 2021;108:271–276. [DOI] [PubMed] [Google Scholar]
- 26.Rybakov E, Nagudov M, Sukhina M, et al. Impact of oral antibiotic prophylaxis on surgical site infection after rectal surgery: results of randomized trial. Int J Colorectal Dis. 2021;36:323–330. [DOI] [PubMed] [Google Scholar]
- 27.Schardey HM, Wirth U, Strauss T, et al. Prevention of anastomotic leak in rectal cancer surgery with local antibiotic decontamination: a prospective, randomized, double-blind, placebo-controlled single center trial. Int J Colorectal Dis. 2020;35:847–857. [DOI] [PubMed] [Google Scholar]
- 28.Abis GSA, Stockmann HBAC, Bonjer HJ, et al. ; SELECT trial study group. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br J Surg. 2019;106:355–363. [DOI] [PubMed] [Google Scholar]
- 29.Uchino M, Ikeuchi H, Bando T, et al. Efficacy of preoperative oral antibiotic prophylaxis for the prevention of surgical site infections in patients with crohn disease: a randomized controlled trial. Ann Surg. 2019;269:420–426. [DOI] [PubMed] [Google Scholar]
- 30.Anjum N, Ren J, Wang G, et al. A randomized control trial of preoperative oral antibiotics as adjunct therapy to systemic antibiotics for preventing surgical site infection in clean contaminated, contaminated, and dirty type of colorectal surgeries. Dis Colon Rectum. 2017;60:1291–1298. [DOI] [PubMed] [Google Scholar]
- 31.Hata H, Yamaguchi T, Hasegawa S, et al. Oral and parenteral versus parenteral antibiotic prophylaxis in elective laparoscopic colorectal surgery (JMTO PREV 07-01): a phase 3, multicenter, open-label, randomized trial. Ann Surg. 2016;263:1085–1091. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda A, Konishi T, Ueno M, et al. Randomized clinical trial of oral and intravenous versus intravenous antibiotic prophylaxis for laparoscopic colorectal resection. Br J Surg. 2016;103:1608–1615. [DOI] [PubMed] [Google Scholar]
- 33.Sadahiro S, Suzuki T, Tanaka A, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: prospective randomized trial. Surgery. 2014;155:493–503. [DOI] [PubMed] [Google Scholar]
- 34.Oshima T, Takesue Y, Ikeuchi H, et al. Preoperative oral antibiotics and intravenous antimicrobial prophylaxis reduce the incidence of surgical site infections in patients with ulcerative colitis undergoing IPAA. Dis Colon Rectum. 2013;56:1149–1155. [DOI] [PubMed] [Google Scholar]
- 35.Roos D, Dijksman LM, Oudemans-van Straaten HM, et al. Randomized clinical trial of perioperative selective decontamination of the digestive tract versus placebo in elective gastrointestinal surgery. Br J Surg. 2011;98:1365–1372. [DOI] [PubMed] [Google Scholar]
- 36.Horie T. Randomized controlled trial on the necessity of chemical cleaning as preoperative preparation for colorectal cancer surgery. DJMS. 2007;34:205–212. [Google Scholar]
- 37.Kobayashi M, Mohri Y, Tonouchi H, et al. ; Mie Surgical Infection Research Group. Randomized clinical trial comparing intravenous antimicrobial prophylaxis alone with oral and intravenous antimicrobial prophylaxis for the prevention of a surgical site infection in colorectal cancer surgery. Surg Today. 2007;37:383–388. [DOI] [PubMed] [Google Scholar]
- 38.Espin-Basany E, Sanchez-Garcia JL, Lopez-Cano M, et al. Prospective, randomised study on antibiotic prophylaxis in colorectal surgery. Is it really necessary to use oral antibiotics? Int J Colorectal Dis. 2005;20:542–546. [DOI] [PubMed] [Google Scholar]
- 39.Lewis RT. Oral versus systemic antibiotic prophylaxis in elective colon surgery: a randomized study and meta-analysis send a message from the 1990s. Can J Surg. 2002;45:173–180. [PMC free article] [PubMed] [Google Scholar]
- 40.Ishida H, Yokoyama M, Nakada H, et al. Impact of oral antimicrobial prophylaxis on surgical site infection and methicillin-resistant Staphylococcus aureus infection after elective colorectal surgery. Results of a prospective randomized trial. Surg Today. 2001;31:979–983. [DOI] [PubMed] [Google Scholar]
- 41.Takesue Y, Yokoyama T, Akagi S, et al. A brief course of colon preparation with oral antibiotics. Surg Today. 2000;30:112–116. [DOI] [PubMed] [Google Scholar]
- 42.Taylor EW, Lindsay G. Selective decontamination of the colon before elective colorectal surgery. West of Scotland Surgical Infection Study Group. World J Surg. 1994;18:926–931. [DOI] [PubMed] [Google Scholar]
- 43.Stellato TA, Danziger LH, Gordon N, et al. Antibiotics in elective colon surgery. A randomized trial of oral, systemic, and oral/systemic antibiotics for prophylaxis. Am Surg. 1990;56:251–254. [PubMed] [Google Scholar]
- 44.Reynolds J, Jones J, Evans D, et al. Do preoperative oral antibiotics influence sepsis rates following elective colorectal surgery in patients receiving perioperative intravenous prophylaxis. Surg Res Commun. 1989;7:71–77.. [Google Scholar]
- 45.Coppa GF, Eng K. Factors involved in antibiotic selection in elective colon and rectal surgery. Surgery. 1988;104:853–858. [PubMed] [Google Scholar]
- 46.Lau WY, Chu KW, Poon GP, et al. Prophylactic antibiotics in elective colorectal surgery. Br J Surg. 1988;75:782–785. [DOI] [PubMed] [Google Scholar]
- 47.Playforth MJ, Smith GM, Evans M, et al. Antimicrobial bowel preparation. Oral, parenteral, or both? Dis Colon Rectum. 1988;31:90–93. [DOI] [PubMed] [Google Scholar]
- 48.Zmora O, Mahajna A, Bar-Zakai B, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg. 2003;237:363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zmora O, Mahajna A, Bar-Zakai B, et al. Is mechanical bowel preparation mandatory for left-sided colonic anastomosis? Results of a prospective randomized trial. Tech Coloproctol. 2006;10:131–135. [DOI] [PubMed] [Google Scholar]
- 50.Koskenvuo L, Lehtonen T, Koskensalo S, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet. 2019;394:840–848. [DOI] [PubMed] [Google Scholar]
- 51.Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:729–738. [DOI] [PubMed] [Google Scholar]
- 52.Viddal KO, Semb LS. Tinidazole and doxycycline compared to doxycycline alone as prophylactic antimicrobial agents in elective colorectal surgery. Scand J Gastroenterol Suppl. 1980;59:21–24. [PubMed] [Google Scholar]
- 53.Hanel KC, King DW, McAllister ET, et al. Single-dose parenteral antibiotics as prophylaxis against wound infections in colonic operations. Dis Colon Rectum. 1980;23:98–101. [DOI] [PubMed] [Google Scholar]
- 54.Mai-Phan AT, Nguyen H, Nguyen TT, et al. Randomized controlled trial of mechanical bowel preparation for laparoscopy-assisted colectomy. Asian J Endosc Surg. 2019;12:408–411. [DOI] [PubMed] [Google Scholar]
- 55.Bhat AH, Parray FQ, Chowdri NA, et al. Mechanical bowel preparation versus no preparation in elective colorectal surgery: a prospective randomized study. Int J Surg Open. 2016;2:26–30.. [Google Scholar]
- 56.Bhattacharjee PK, Chakraborty S. An open-label prospective randomized controlled trial of mechanical bowel preparation vs nonmechanical bowel preparation in elective colorectal surgery: personal experience. Indian J Surg. 2015;77(suppl 3):1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasaki J, Matsumoto S, Kan H, et al. Objective assessment of postoperative gastrointestinal motility in elective colonic resection using a radiopaque marker provides an evidence for the abandonment of preoperative mechanical bowel preparation. J Nippon Med Sch. 2012;79:259–266. [DOI] [PubMed] [Google Scholar]
- 58.Bertani E, Chiappa A, Biffi R, et al. Comparison of oral polyethylene glycol plus a large volume glycerine enema with a large volume glycerine enema alone in patients undergoing colorectal surgery for malignancy: a randomized clinical trial. Colorectal Dis. 2011;13:e327–e334. [DOI] [PubMed] [Google Scholar]
- 59.Bretagnol F, Panis Y, Rullier E, et al. ; French Research Group of Rectal Cancer Surgery (GRECCAR). Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann Surg. 2010;252:863–868. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe M, Murakami M, Nakao K, et al. Randomized clinical trial of the influence of mechanical bowel preparation on faecal microflora in patients undergoing colonic cancer resection. Br J Surg. 2010;97:1791–1797. [DOI] [PubMed] [Google Scholar]
- 61.Pena-Soria MJ, Mayol JM, Anula R, et al. Single-blinded randomized trial of mechanical bowel preparation for colon surgery with primary intraperitoneal anastomosis. J Gastrointest Surg. 2008;12:2103–2108. [DOI] [PubMed] [Google Scholar]
- 62.Contant CM, Hop WC, van’t Sant HP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet. 2007;370:2112–2117. [DOI] [PubMed] [Google Scholar]
- 63.Platell C, Barwood N, Makin G. Randomized clinical trial of bowel preparation with a single phosphate enema or polyethylene glycol before elective colorectal surgery. Br J Surg. 2006;93:427–433. [DOI] [PubMed] [Google Scholar]
- 64.Ram E, Sherman Y, Weil R, et al. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg. 2005;140:285–288. [DOI] [PubMed] [Google Scholar]
- 65.Bucher P, Gervaz P, Soravia C, et al. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg. 2005;92:409–414. [DOI] [PubMed] [Google Scholar]
- 66.Fa-Si-Oen P, Roumen R, Buitenweg J, et al. Mechanical bowel preparation or not? Outcome of a multicenter, randomized trial in elective open colon surgery. Dis Colon Rectum. 2005;48:1509–1516.. [DOI] [PubMed] [Google Scholar]
- 67.Miettinen RP, Laitinen ST, Mäkelä JT, et al. Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery: prospective, randomized study. Dis Colon Rectum. 2000;43:669–75; discussion 675. [DOI] [PubMed] [Google Scholar]
- 68.Burke P, Mealy K, Gillen P, et al. Requirement for bowel preparation in colorectal surgery. Br J Surg. 1994;81:907–910. [DOI] [PubMed] [Google Scholar]
- 69.Platell C, Barwood N, Dorfmann G, et al. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007;9:71–79. [DOI] [PubMed] [Google Scholar]
- 70.O’Hara LM, Thom KA, Preas MA. Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Guideline for the Prevention of Surgical Site Infection (2017): a summary, review, and strategies for implementation. Am J Infect Control. 2018;46:602–609. [DOI] [PubMed] [Google Scholar]
- 71.Mulder T, Crolla RMPH, Kluytmans-van den Bergh MFQ, et al. Preoperative oral antibiotic prophylaxis reduces surgical site infections after elective colorectal surgery: results from a Before-After Study. Clin Infect Dis. 2019;69:93–99. [DOI] [PubMed] [Google Scholar]
- 72.Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Apte SS, Moloo H, Jeong A, et al. Prospective randomised controlled trial using the REthinking Clinical Trials (REaCT) platform and National Surgical Quality Improvement Program (NSQIP) to compare no preparation versus preoperative oral antibiotics alone for surgical site infection rates in elective colon surgery: a protocol. BMJ Open. 2020;10:e036866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pellino G, Solís-Peña A, Kraft M, et al. Preoperative oral antibiotics with versus without mechanical bowel preparation to reduce surgical site infections following colonic resection: Protocol for an international randomized controlled trial (ORALEV2). Colorectal Dis. 2021;23:2173–2181.. [DOI] [PubMed] [Google Scholar]
- 75.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.