ABSTRACT
Introduction: Pituitary adenomas [PAs] constitute the third most common primary intracranial tumours, with a wide prevalence rate of 1% to 40%. Histologic (H & E) classification into acidophilic, basophilic and chromophobic adenomas have little clinical relevance but WHO recommended immunohistochemical subclassification has both therapeutic and prognostic significance. This immunohistochemical subclassification has not been done in our environment, making it imperative for us to evaluate the patterns in our environment.
Aim: To determine the immunohistochemical patterns of PAs in Southeastern Nigeria.
Materials and Methods: This was a 10-year retrospective review of all PA biopsies received at University of Nigeria Teaching Hospital Enugu, Memphys Hospital for Neurosurgery Enugu and Grace Pathology Consults Enugu, Nigeria. The age, sex, histologic, immunohistochemical subtypes and biopsy size of all diagnosed PAs were analyzed using Statistical Package for Social Sciences (SPSS) version 20 (New York: IBM Inc.) and the results were expressed in descriptive statistics.
Results: One hundred cases of PAs were identified in this study constituting 19.6% of all primary intracranial tumors received at our study centers during the period under review. There were 45 (45.0%) females and 55 (55.0%) males giving a female to male ratio of 1:1.2, and a mean age of 45.3 years. The commonest histologic type was acidophilic adenoma (49.0%), followed by basophilic (40.0%) and chromophobic (11.0%) adenomas. Null cell adenomas were the most common immunohistochemical subtype (44.0%), followed by PRL-secreting adenomas (27.0%). Others were Luteinizing hormone (LH) − 13 (13.0%), follicle stimulating hormone (FSH) − 7 (7.0%), growth hormone (GH) − 3(3.0%), TSH − 2(2.0%) and ACTH − 1(1.0%) adenomas.
Conclusion: PAs predominate amongst males, occurring mostly in the middle age groups in Southeastern Nigeria. Null cell adenoma is the commonest immunohistochemical subtype followed by PRL-secreting adenomas. Routine immunohistochemical characterization is required for accurate diagnosis and optimal patient care.
KEYWORDS: Histology, immunohistochemistry, pituitary, adenoma, tumour
1. Introduction
Pituitary adenomas (PAs) are the third most common primary intracranial tumors globally and in Nigeria [1,2]. They constitute 10.0% to 15.0% of intracranial neoplasms globally, with a wide variation in prevalence, ranging from 1.0% to 40.0% [3–5]. In Nigeria, pituitary adenomas constitute 16.8% to 21.0% of primary intracranial tumours as reported in previous studies [6,7].
The pituitary gland is mainly made up of anterior (adenohypophysis) and posterior (neurohypophysis) lobes and weighs 0.5 g to 1.0 g, yet the organ plays a critical role in the effective function of many organs in the body, through the various hormones produced by the adenohypophysis [8,9]. The adenohypophysis produces and secretes distinctive hormones, namely: growth hormone (GH), adrenocorticotrophic hormone (ACTH), thyroid stimulating hormone (TSH), prolactin (PRL), luteinizing hormone (LH) and follicle stimulating hormone (FSH) [8,10]. The neurohypophysis only stores and releases oxytocin and anti-diuretic hormone (ADH) which are synthesized in the supra-optic and paraventricular nuclei of the hypothalamus, respectively [8,10].
Pituitary adenomas are benign tumors of the adenohypophysis [11]. Morphologically, pituitary adenomas are classified based on their cellular cytoplasmic staining characteristics on Hematoxylin and eosin (H&E) stains into acidophilic, basophilic and chromophobic adenomas [12]. However, characterization based only on H&E features is no longer sufficient as it does not properly identify the hormone specific adenoma types [1,13].
Thus, in 2004, the WHO classified pituitary neuroendocrine tumours into three categories, reflecting the potential for malignancy of pituitary adenomas (typical and atypical) and pituitary carcinoma [14–16]. The atypical adenoma was defined as a tumour with a Ki-67 index above 3% and diffuse p53 immunoreactivity, which predicted potential malignancy [16]. The typical pituitary adenomas were subclassified based on their immunohistochemical staining characteristics (immunoreactivity) to various anterior pituitary hormones [17] and include; PRL-secreting, GH-secreting, ACTH-secreting, TSH-secreting, FSH-secreting, LH-secreting and null-cell (those with negative immunostaining for hormones) adenomas [12].
However, in the 2017 WHO classification, pituitary adenomas were re-classified based on cell lineages and routine immunohistochemistry (IHC) for transcription factors (TFs) that regulate cell differentiation (PIT-1, SF-1 and T-PIT [18–20]) and those for anterior pituitary hormones (anterior pituitary GH, PRL, TSH-beta, ACTH, FSH-beta, LH-beta and alpha subunit) [16]. The term ‘atypical adenoma’ (replaced by ‘aggressive adenoma’) was removed, due to the lack of sufficient evidence to predict poor prognosis with pathological markers alone [14]. Prognostic prediction using just one cell proliferation index (Ki-67) was considered inadequate [15,21] and mitotic count was added to assess cell proliferation [16]. The three tumor types of the neurohypophysis (pituicytoma, spindle cell oncocytoma, granular cell tumor) were defined by their common expression of TTF‑1 [22].
The classification of PAs was again revisited in 2022, with a new WHO classification (5th Edition) of Endocrine and Neuroendocrine Tumors [23]. This classification distinguished anterior lobe (adenohypophyseal) from posterior lobe (neurohypophyseal) and hypothalamic tumors as well as other tumors arising in the sellar region [23]. The anterior lobe tumors include: (i) well-differentiated adenohypophyseal tumors that are now classified as pituitary neuroendocrine tumors (PitNETs); formerly known as pituitary adenomas), (ii) pituitary blastoma, and (iii) the two types of craniopharyngioma [23].
The new 2022 WHO classification also provides detailed histological subtyping of PitNET based on tumor cell lineage, cell type, and related characteristics [23]. The routine use of immunohistochemistry for pituitary transcription factors (PIT1, TPIT, SF1, GATA3, and ERα) was endorsed by this classification. IHC examination of pituitary-specific transcription factors (TFs), including PIT1, TPIT, SF-1, GATA2/3, and ERα, is endorsed to determine the tumor cell lineage and to facilitate the classification of PitNET/PA subgroups [24]. The major PIT1, TPIT, and SF1 lineage-defined PitNET types and subtypes feature distinct morphologic, molecular, and clinical differences. The term ‘null cell adenoma’, which is a diagnosis of exclusion, is now reserved for PitNETs with no evidence of adenohypophyseal lineage differentiation i.e. those negative to transcription factors T-Pit, Pit-1, SF-1 & GATA3 [23].
As a result of the introduction of IHC for transcription factors, null cell adenoma is now defined as an adenoma that does not present immunoreactivity for either anterior pituitary transcription factors or hormone production [15]. It previously accounted for about 10% of all pituitary adenomas but with the introduction of immunoreactivity for anterior pituitary transcription factors, it now accounts for just 1% of all pituitary adenomas [15].
The 2017 and 2022 WHO classifications of PAs require immunohistochemical markers for TFs as well as for anterior pituitary hormones. Thus, the WHO guidelines for the histological classification of endocrine tumours (2004, 2017 & 2022), recommends detailed immunohistochemical assessments for further subclassification of pituitary adenomas [3,4,15,18,19,22–24]. This comes with additional costs and problems of unavailability of immunohistochemistry kits and antibodies, making their use in routine practice very challenging especially in low resource setting like ours.
The new WHO classification of PitNET/PA has incorporated tremendous advances in the understanding of the cytogenesis and pathogenesis of pituitary tumors [24]. However, due to the shortcomings of the technology used in the diagnosis of PitNET/PA and the limited understanding of the tumorigenesis of PitNET/PA, the application of this new classification system in practice need to be further evaluated and validated [24].
Thus, most centers still rely on the 2014 classification in the reporting of pituitary adenomas. This classification subclassified PAs based on their immunohistochemical staining characteristics into; PRL-secreting, GH-secreting, ACTH-secreting, TSH-secreting, FSH-secreting, LH-secreting and null-cell (those with negative immunostaining for hormones) adenomas [12].
The subclassification of these tumors based on immunohistochemical staining features of the hormones they produce is still being used in most centers in Nigeria today. This enables accurate diagnosis and optimal management, while identifying features that may determine tumor behavior and prognosis [1,12,25], in low resource setting like ours.
Although pituitary adenomas have been found to be among the most common primary intracranial tumors, there is paucity of data on the histologic and immunohistochemical patterns of pituitary adenomas in southeastern Nigeria, with only a few studies carried out so far [3,4].
In Southeastern Nigeria, a careful search of literature showed lack of data on the histologic and immunohistochemical patterns of pituitary adenomas. This study, therefore, aims to evaluate the immunohistochemical patterns of pituitary adenomas in southeastern Nigeria over a retrospective ten-year period, 2008 to 2017.
This study in addition to broadening the body of knowledge and providing baseline data on the immunohistochemical patterns of pituitary adenomas especially in southeastern Nigeria, will also provide clinicians with significant information necessary for optimal patient care and management.
2. Materials and methods
This was a 10-year retrospective review of all pituitary adenoma biopsies received at University of Nigeria Teaching Hospital (UNTH) Enugu, Memphys Hospital for Neurosurgery (MHN) Enugu and Grace Pathology Consults (GPC) Enugu, Nigeria from the year 2008 to 2017. The study was conducted in University of Nigeria Teaching Hospital (UNTH), Ituku-Ozalla, Enugu, Memfys Hospital for Neurosurgery (MHN), Enugu and Grace Pathology Consults (GPC), Enugu. UNTH Enugu and MHN Enugu are the core neurosurgery referral centers, offering neurosurgery services to all the five states of the southeastern region of Nigeria. All the biopsies from these two centers are routinely processed and analyzed in the histopathology laboratories of University of Nigeria Teaching Hospital (UNTH), Ituku-Ozalla, Enugu and Grace Pathology Consults (GPC), Enugu.
The ethical clearance for this study was obtained from the Health Research Ethical Committee of University of Nigeria Teaching Hospital. Relevant clinical data of the patients such as histology number, age, sex, biopsy size and other relevant clinical information of the patients were obtained from the case notes in the hospital medical record archives as well as duplicate copies of the request forms in the histopathology laboratories of University of Nigeria Teaching Hospital (UNTH), Ituku-Ozalla, Enugu, Memfys Hospital for Neurosurgery (MHN), Enugu and Grace Pathology Consults (GPC), Enugu.
The Hematoxylin and Eosin (H&E) stained slides and corresponding archived paraffin-embedded tissue blocks of all pituitary biopsies received within the study period were retrieved and reviewed. The tissue blocks of all cases with missing or damaged slides were also retrieved, re-cut, stained with H&E and subsequently reviewed. All histologically confirmed pituitary adenoma cases were used for the study. These histologically diagnosed pituitary adenoma cases were classified into histologic variants based on their H&E staining characteristics.
Six fresh serial sections cut at 3-–5 microns each were obtained from the archived paraffin embedded tissue blocks for each case. Immunohistochemical staining with antibodies against PRL, GH, ACTH, TSH, FSH and LH was done to determine the specific immunohistochemical subtypes of all the histologically diagnosed pituitary adenoma cases, based on hormone staining characteristics.
The antibody staining pattern for the respective hormones were scored as positive when a group or majority of the pituitary adenoma cells showed brown cytoplasmic staining, and negative when there was no cytoplasmic staining of the pituitary adenoma cells, only isolated nuclear staining or staining of inflammatory cells and connective tissue.
Disease-free positively stained pituitary tissue obtained at autopsy was used as positive control for the respective antibody stains and negatively stained pituitary adenoma cases in the study were used as negative controls.
The slides were examined using binocular light microscope (Leica Microsystems-Wetzlar, Germany), and diagnosis and subtyping of PA were made based on histopathologic and immunochemical features.
3. Statistical analysis
The data obtained were analyzed using the Statistical Package for Social Sciences (SPSS) version 20 (New York: IBM Inc.) and the results obtained are presented as tables, figures and charts using descriptive statistics.
4. Results
One hundred (100) cases of PAs were identified in this study constituting 19.6% of all primary intracranial tumors that were surgically resected and immunohistochemically diagnosed in the study area during the study period (Table 1).
Table 1.
Distribution of Intracranial Tumours in Enugu [2008–2017].
| Intracranial Tumours | Frequency | Proportion (%) |
|---|---|---|
| Meningioma | 199 | 38.9 |
| Gliomas | 158 | 30.9 |
| Pituitary adenomas | 100 | 19.6 |
| Craniopharyngioma | 18 | 3.5 |
| Medulloblastoma | 13 | 2.5 |
| Haemangioblastoma | 8 | 1.6 |
| Choroid plexus papilloma | 3 | 0.6 |
| Pineoblastoma | 3 | 0.6 |
| Neurocytoma | 2 | 0.4 |
| High Grade Lymphoma | 1 | 0.2 |
| Neuroblastoma | 1 | 0.2 |
| Pineocytoma | 1 | 0.2 |
| Mixed pineocytoma and pineoblastoma | 1 | 0.2 |
| Atypical teratoid/rhabdoid tumour | 1 | 0.2 |
| Intracranial mature cystic teratoma | 1 | 0.2 |
| Pineal parencymal tumour of intermediate differentiation (WHO grade III) | 1 | 0.2 |
| Metastatic brain tumours | - | - |
| Total | 511 | 100 |
The age range was from 23 years to 75 years, with a median age of 43.0 years and a mean age of 45.3 years. The age group of 30–39 years had the highest number of 29 (29.0%) cases, followed by 40–49 years and 50–59 years which accounted for 28 (28.0%) and 18 (18.0%) cases (Table 2).
Table 2.
Age and sex distribution of pituitary adenomas in Enugu [2008–2017].
| Variable | Frequency (N = 100) |
Proportion (%) |
|---|---|---|
| Age Groups | ||
| <20 years | 0 | 0.0 |
| 20–29 years | 11 | 11.0 |
| 30–39 yrs | 29 | 29.0 |
| 40–49 years | 28 | 28.0 |
| 50–59 years | 18 | 18.0 |
| 60–69 years | 12 | 12.0 |
| ≥70 | 2 | 2.0 |
| Sex | ||
| Male | 55 | 55.0 |
| Female | 45 | 45.0 |
| Total 100 100.0 | ||
Most of the cases occurred between the 3rd and 7th decades, with a slight male preponderance. There were 45 (45.0%) females and 55 (55.0%) males giving a female to male ratio of 1:1.2. (Table 2).
The commonest histologic type was acidophilic adenoma 49 (49.0%), followed by basophilic 40 (40.0%) and chromophobic 11 (11.0%) adenomas respectively (Figures 1-3).
Figure 1.
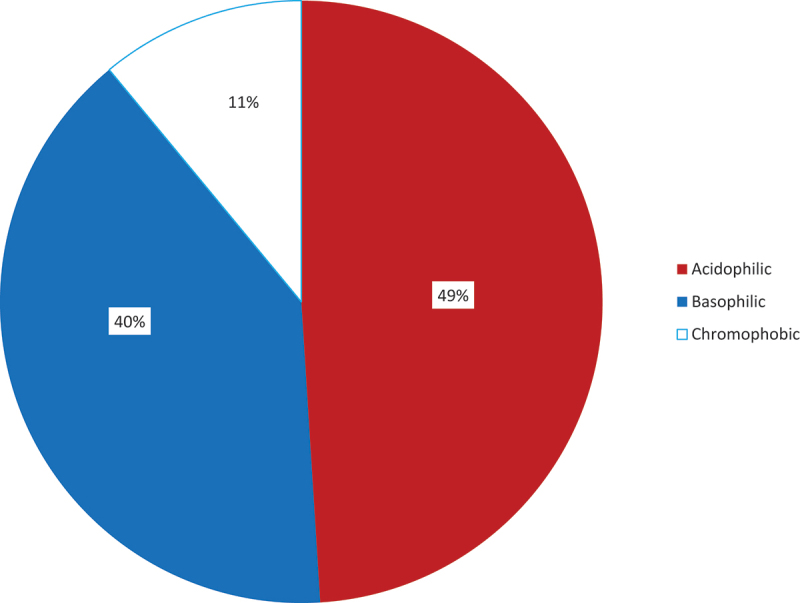
Histologic patterns of pituitary adenomas in Enugu (2008–2017)..
Figure 2.
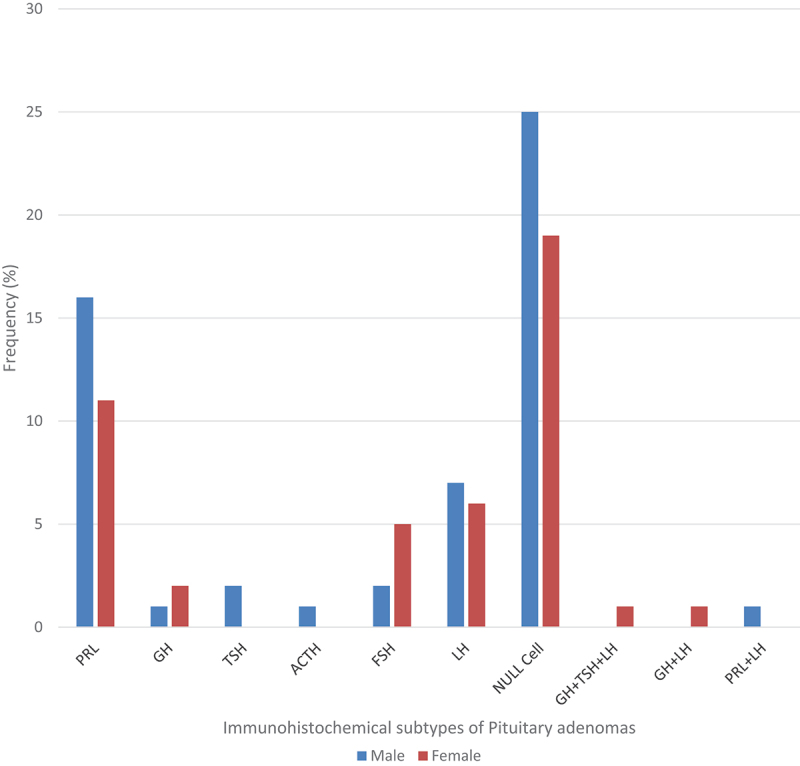
Distribution of immunohistochemical subtypes of pituitary adenomas by sex in Enugu (2008–2017).
Figure 3.
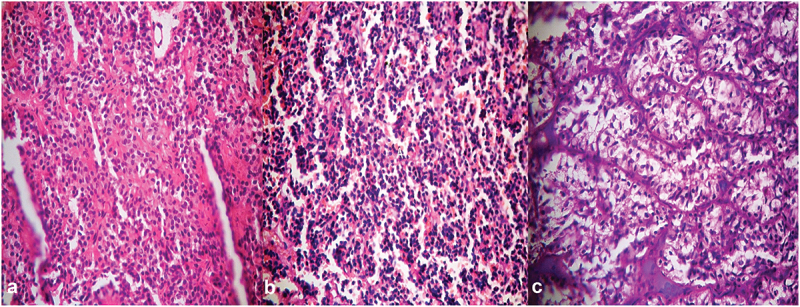
Photomicrographs of pituitary adenomas showing (a) acidophilic, (b) basophilic and (c) chromophobic adenomas [H&E stain x 400].
The most common immunohistochemical subtype in this study was null cell adenoma which accounted for 44 (44.0%) of all the pituitary adenomas (Table 3), (Figure 4). This was followed by PRL-only adenomas which constituted 27 (27.0%) of the total cases, Luteinizing hormone (LH) – only adenomas 13 (13.0%) and follicle stimulating hormone (FSH)-only adenomas 7 (7.0%) of all the cases. Growth hormone (GH)-only, TSH-only and ACTH-only adenomas were the least common accounting for 3 (3.0%), 2 (2.0%) and 1 (1.0%) of the total cases (Figures 4, 5). Three (3) Plurihormonal adenomas consisting of a case each of the following combinations: GH, TSH and LH, GH and LH, and PRL and LH (Table 3).
Table 3.
Immunohistochemical subtypes of Pituitary Adenomas in Enugu, 2008–2017.
| Variable | Frequency N = 100 |
Proportion (%) |
|---|---|---|
| Monohormonal | ||
| Null cell | 44 | 44.0 |
| PRL | 27 | 27.0 |
| LH | 13 | 13.0 |
| FSH | 7 | 7.0 |
| GH | 3 | 3.0 |
| TSH | 2 | 2.0 |
| ACTH | 1 | 1.0 |
| Plurihormonal | ||
| GH+TSH+LH | 1 | 1.0 |
| GH+LH | 1 | 1.0 |
| PRL+LH | 1 | 1.0 |
| Total | 100 | 100.0 |
NB: PRL – prolactin, LH – luteinizing hormone, FSH – follicle stimulating hormone, GH – growth hormone, TSH – thyroid stimulating hormone, ACTH – adrenocorticotrophic hormone.
Figure 4.
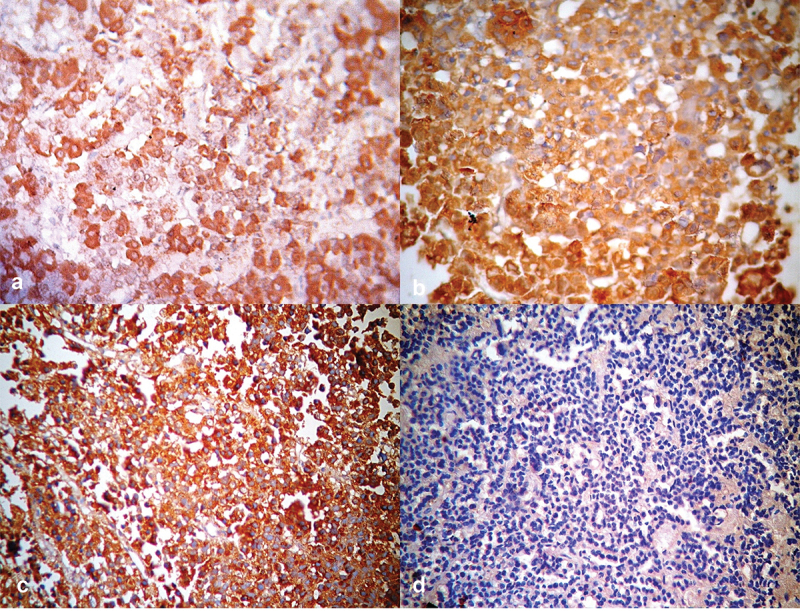
Photomicrographs of pituitary tissue showing (a) positive cytoplasmic staining with growth hormone monoclonal antibody in normal pituitary gland [Positive GH Immunostain x 400], (b) positive cytoplasmic staining with growth hormone monoclonal antibody in pituitary adenoma [Positive GH Immunostain x 400], (c) positive cytoplasmic staining with prolactin hormone monoclonal antibody in pituitary adenoma [Prolactin Immunostain x 400] and (d) negative cytoplasmic staining in pituitary adenoma [Negative immunostain for all six antibodies x 400].
Figure 5.
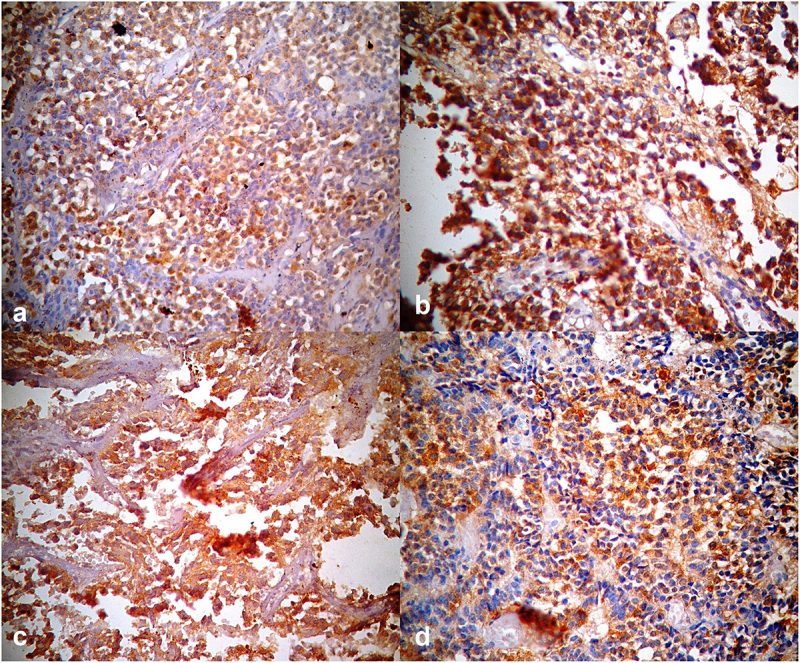
Photomicrographs of pituitary tissue showing (a) positive cytoplasmic staining with Thyroid Stimulating Hormone Monoclonal Antibody [TSH Immunostain x 400] (b) positive cytoplasmic staining with Adrenocorticotrophic Hormone Monoclonal Antibody [ACTH Immunostain x 400], (c) positive cytoplasmic staining with Follicle Stimulating Hormone Monoclonal Antibody [FSH Immunostain x 400] and (d) positive cytoplasmic staining with Luteinizing Hormone Monoclonal Antibody [LH Immunostain x 400].
The frequency and distribution of the respective immunohistochemical subtypes of surgically resected and immunohistochemically diagnosed pituitary adenomas by sex is outlined in Figure 2 while sex, age and biopsy size are outlined in (Table 4).
Table 4.
Distribution of Immunohistochemical Subtypes of Pituitary Adenomas by Age, Sex and Biopsy Size in Enugu [2008–2017].
| Variable | Null cell n = 44%) |
PRL only n = 27%) |
GH only n = 3%) |
TSH only n = 2%) |
ACTH only n = 1%) |
FSH only n = 7%) |
LH only n = 13%) |
Plurihormonal n = 3%) |
Total |
|---|---|---|---|---|---|---|---|---|---|
| Age groups (Years) | |||||||||
| <20 | - | - | - | - | - | - | - | - | 0.0 |
| 20–29 | 6 (13.6) | 5 (18.5) | - | - | - | - | - | - | 11.0 |
| 30–39 | 13 (29.6) | 5 (18.5) | 2 (66.7) | - | 1(100.0) | 3(42.8) | 4 (30.8) | 1(33.3) | 29.0 |
| 40–49 | 13(29.6) | 9 (33.3) | 1 (33.3) | 1 (50.0) | - | 1(14.3) | 3(23.0) | - | 28.0 |
| 50–59 | 5(11.3) | 6 (22.2) | - | 1 (50.0) | - | 1(14.3) | 4(30.8) | 1(33.3) | 18.0 |
| 60–69 | 6(13.6) | 2 (7.4) | - | - | - | 1(14.3) | 2(15.4) | 1(33.3) | 12.0 |
| ≥70 | 1 (2.3) | - | - | - | - | 1(14.3) | - | - | 2.0 |
| Sex | |||||||||
| Male | 25(56.8) | 16 (59.3) | 1 (33.3) | 2 (100.0) | 1(100.0) | 2(28.6) | 7 (53.8) | 1(33.3) | 55.0 |
| Female | 19(43.2) | 11 (40.7) | 2 (66.7) | - | - | 5(71.4) | 6(46.2) | 2(66.6) | 45.0 |
| Biopsy size (cm) | |||||||||
| <1 | 4 (9.1) | 5 (18.5) | - | - | - | - | 2 (15.4) | - | 11 |
| 1–4 | 39(88.6) | 21 (77.8) | 3 (100.0) | 2 (100.0) | 1(100.0) | 7(100.0) | 11(84.6) | 3(100.0) | |
| >4 | 1(2.3) | 1 (3.7) | - | - | - | - | - | - | 2 |
Note: NB: PRL – prolactin, LH – luteinizing hormone, FSH – follicle stimulating hormone, GH – growth hormone, TSH – thyroid stimulating hormone, ACTH – adrenocorticotrophic hormone.
Most of the null cell adenomas in the study occurred in the age groups of 30–39 years and 40–49 years and accounted for 13 (29.6%) cases in each age group. A total of 25 (56.8%) were males and 19 (43.2%) were females, with a male to female ratio of 1.3:1 (Figure 2). Forty (90.9%) of the 44 null cell adenoma cases were macroadenomas, one case of which was a giant macroadenoma (biopsy size ˃4 cm) (Table 4) and the mean biopsy size was 2.0 cm
PRL-secreting adenomas occurred between the 3rd and 7th decades with most 9 (33.3%) of the cases, found in the age group of 40–49 years. Sixteen (59.3%) of the cases were males and 11 (40.7%) were females. Twenty-two (81.5%) were macroadenomas (Table 4) and the mean biopsy size was 1.6 cm.
The most common frequency of these adenoma cases occurred in the age groups 30–39 years and 50–59 years and each accounted for 4 (30.8%) cases, respectively. There was a slight male preponderance with a male to female ratio of 1.2:1 (Table 4). Macroadenomas accounted for 84.6% of the cases.
There were only three (3) plurihormonal adenoma cases. The case with a combination of GH and LH occurred in the 30–39 years age group, while that with a combination of PRL and LH was found in the 50–59 years age group. The plurihormonal adenoma with a combination of GH, TSH and LH occurred in the 60–69 years age group.
5. Discussion
Pituitary adenomas constituted 19.6% of all surgically resected and histologically diagnosed intracranial tumours within the study area, during the study period, and ranked third among all intracranial tumours, behind meningiomas and gliomas. This is similar to a study by Jibrin P. et al. in Abuja which found pituitary adenomas to constitute 22.0% of all intracranial tumours, although they were the second most common intracranial tumours [26]. Also, independent studies by Ndubuisi et al. and Idowu et al. in Enugu and Lagos, respectively, found Pas to be the third most common intracranial tumours [2,6].
The age range of pituitary adenomas in our study was from 23 years to 75 years and the mean age was 45.3 years. The majority of the cases occurred between the 3rd and 7th decades. Studies by Salami et al. in Ibadan also found most pituitary adenomas to occur between the 4th and 7th decades, with a mean age of 42 years [4]. A study in Benin Republic reported an average age of 40.9 years and another study in South Africa reported a mean age of 44.9 years [27,28]. De Mello et al in a study in Brazil reported an age range of 19–80 years with a mean age of 44.8 years [29]. In another study in Northern Finland, the average age was 32.5 years [30]. This lower average age may be due to earlier presentation and intervention.
There was also a slight male preponderance in our study which found males to constitute 55% and females 45%, with a male to female ratio of 1.2:1. This is similar to the findings of Idowu et al. in Lagos which reported a slight male predilection [6]. Salami et al. in Ibadan also reported 46.8% males and 53.2% females [4]. Gandaho et al. (Benin Republic) reported a male to female ratio of 1:1.1 [27]. A study by De Mello et al. in Brazil found males to constitute 50.1% and females 49.9% [29]. In another study by Shamim et al. in Pakistan, males constituted 63% of the cases [31].
Pituitary macroadenomas constituted 89% of the cases and the overall mean biopsy size was 1.8 cm. Gandaho et al. (Benin Republic) found macroadenomas to constitute 97.4% of the cases [27]. Also Tiruneh G. et al. in Ethiopia reported 61.9% of cases to be macroadenomas [32].
In our study, null cell adenomas were the most common and constituted 44% of the cases, followed by PRL-secreting adenomas which constituted 28% of the cases. This is similar to the findings of Salami et al. in Ibadan, Nigeria that reported null cell adenomas as the most common (34.0%), followed by PRL-secreting adenomas (14.9%), although with a lower rate [4]. In another study of 150 cases by Matshana K. et al. in South Africa, null cell adenomas were the most common, constituting 77.3%, followed by PRL-secreting adenomas which constituted 28.6% in one study and 14.7% in another study [33]. The high rates of null cell adenoma (NCA) recorded in this study can explained by the classification of PA based on only the immunoreactivity of the hormones they produce. These hormone negative adenomas (NCAs) previously accounted for 10% of all PAs [15] but with the introduction of immunoreactivity for anterior pituitary transcription factors in the 2017 and 2022 WHO classification of pituitary adenomas, the null cell adenomas now account for just 1% of all pituitary adenomas [16]. This is expected to happen to our rates if we had also used immunoreactivity for anterior pituitary transcription factors to define our null cell adenoma cases. The unavailability of the immunohistochemical markers for these transcription factors was also a limitation in our study.
Our study also found GH-secreting, TSH-secreting and ACTH-secreting adenomas as the least common constituting 5.0%, 3.0% and 1.0% of all cases, respectively. This finding is in tandem with studies by Salami et al. in Ibadan and Matshana K. et al. in South Africa which also reported these adenoma subtypes as the least common [4,33]. Two other independent studies in Northern Finland and Belgium found PRL-secreting adenomas to be the most common and constituted 51% and 66% of the cases respectively, followed by null cell adenomas which constituted 37% and 14.7% respectively [30,34]. Macroadenomas accounted for 82% of the null cell adenomas in the study in Northern Finland [30]. This is similar to our study which found 90.9% of the null cell adenomas to be macroadenomas. Also, in our study, males accounted for more PRL-secreting macroadenomas (59.1%) than females. This finding is in agreement with the study by Raappana et al. in Northern Finland which reported 75% of PRL-secreting macroadenomas in males [30]. There were only 11 (11.0%) cases of microadenomas in our study, the majority of which, 8 (72.7%), were males. Daly A. et al., in their study in Belgium found 57.4% of the cases to be microadenomas, more than two-thirds of the total cases (67.6%) were females and PRL-secreting adenomas were the most common (66%) [34,35]. This contrasts with our findings and may be due to early onset of clinical symptoms such as menstrual irregularities and nipple discharge in the females with PRL-secreting adenomas which could necessitate a relatively earlier presentation and therapeutic intervention. However, in a study in Turkey, most silent pituitary adenomas were null cell adenomas, followed by FSH+LH adenomas and presented a clinically non-functional and challenging diagnostic tumour group [36]. It is important to note that while our study was on patients who had PAs and were operated on, some of the studies reported from other centers could be from patients who were not operated. This might explain the variance of some of our results when compared with such studies. Hence, inferences from our work are on only surgically resected PAs.
6. Limitation
The authors hereby acknowledge that this study may not be fully representative of the general population because the study was based on cases on which pituitary biopsy and histology were done to the exclusion of those for which biopsy and histology were not done. Also the classification of pituitary adenoma into subgroups was done using only immunoreactivity to hormones (WHO 2014 classification), to the exclusion of immunoreactivity to transcription factors (WHO 2017 and 2022 classifications). This was also a limitation and affected our classification of null cell adenomas which would have had lower numbers had we ruled out those that had immunoreactivity to TFs.
7. Conclusion
PAs predominate amongst males and middle aged patients operated on Southeastern Nigeria. Null cell adenoma is the commonest immunohistochemical subtype followed by PRL-secreting adenomas. Routine immunohistochemical characterization is required for accurate diagnosis and optimal patient care.
8. Recommendation
A multi-institutional and multi-regional study of the histologic and immunohistochemical patterns of pituitary adenomas in Nigeria is recommended. This will further broaden the body of knowledge and provide the much desired baseline data for optimal patient care in Nigeria. These multi-institutional and multi-regional centers can make available transcription factor IHC kits and antibodies to regional centers like ours.
Acknowledgments
The authors acknowledge the technical support provided by staff of Morbid Anatomy, University of Nigeria Teaching Hospital (UNTH), Ituku-Ozalla, Enugu, Memfys Hospital for Neurosurgery (MHN), Enugu and Grace Pathology Consults (GPC), Enugu.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
references
- [1].Bǎlinişteanu B, Ceauşu RA, Cîmpean AM, et al. Conventional examination versus immunohistochemistry in the prediction of hormone profile of pituitary adenomas. An analysis on 142 cases. Rom J Morphol Embryol. 2012;52:1041–9. [PubMed] [Google Scholar]
- [2].Ndubuisi C, Ohaegbulam S, Iroegbu L, et al. Histologically confirmed intracranial tumors managed at Enugu, Nigeria. J Neurosci Rural Pract. 2017;8(4):585. doi: 10.4103/jnrp.jnrp_155_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Salami A, Malomo A, Shokunbi T, et al. Pattern of pituitary adenomas in a West African ospital. Pathology. 2014;46(2):119–120. doi: 10.1097/01.PAT.0000454506.80556.c0 [DOI] [Google Scholar]
- [4].Salami A, Malomo A, Shokunbi T, et al. Immunohistochemical analysis of pituitary adenomas in a West African Hospital. African J Neurol Sci. 2013;32(2):72–80. [Google Scholar]
- [5].Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: A systematic review. Cancer. 2004;101(3):613–619. doi: 10.1002/cncr.20412 [DOI] [PubMed] [Google Scholar]
- [6].Idowu O, Akang E, Malomo A.. Symptomatic primary intracranial neoplasms in Nigeria, West Africa. J Neurol Sci. 2007;24(3):212–218. [Google Scholar]
- [7].Igun G. Diagnosis and management of brain tumours at Jos University teaching hospital, Nigeria. East Afr Med J. 2001;78(3):148–151. doi: 10.4314/eamj.v78i3.9082 [DOI] [PubMed] [Google Scholar]
- [8].Salami A, Akang E, Adefolarin M. Recent advances in the pathology of pituitary adenomas. J Morphol Sci. 2015;32(1):043–052. doi: 10.4322/jms.055414 [DOI] [Google Scholar]
- [9].Young B, Woodford P, O‘Dowd G. Functional histology. 6th ed. wheater’s functional histology: a Text and colour Atlas. Philadelphia, PA: Churchill Livingston, Elsevier; 2014. p. 318–322. [Google Scholar]
- [10].Guyton AC, Hall JE. Pituitary hormones and their control by the hypothalamus textbook of medical physiology. 12th ed. Philadelphia, PA: Saunders, Elsevier; 2011. p. 895–906. [Google Scholar]
- [11].Kumar V, Abbas AK, Aster JC. Robbins and cotran pathologic basis of disease. 9th ed. Philadelphia, PA: Elsevier, Saunders; 2015. p. 1073–1139. [Google Scholar]
- [12].Syro LV, Rotondo F, Ramirez A, et al. Progress in the diagnosis and classification of pituitary adenomas. Front Endocrinol. 2015;6:97. doi: 10.3389/fendo.2015.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Syro LV, Builes CE, Di Ieva A, et al. Improving differential diagnosis of pituitary adenomas. Expert Rev Endocrinol Metab. 2014;9(4):377–386. doi: 10.1586/17446651.2014.922412 [DOI] [PubMed] [Google Scholar]
- [14].Osamura RY, Lopes MBS, Grossman A, et al. WHO classification of tumours of the pituitary. In: Lloyd RV, Osamura RY, and Klöppel RJ, editors WHO classification of tumours of endocrine glands. 4th ed. Lyon, France: IARC; 2017. p. 11–63. [Google Scholar]
- [15].Inoshita N, Nishioka H. The 2017 WHO classification of pituitary adenoma: overview and comments. Brain Tumor Pathol. 2018;35(2):51–56. doi: 10.1007/s10014-018-0314-3 [DOI] [PubMed] [Google Scholar]
- [16].Pérez-López C, Zamarrón Á, Isla A, et al. ¿Es práctica la actual clasificación de la OMS para adenomas hipofisarios? Endocrinol Diabetes Nutr. 2022;69(3):234–235. doi: 10.1016/j.endien.2020.10.014 [DOI] [PubMed] [Google Scholar]
- [17].Nosé V. The 11th meeting of the international pituitary pathology society october 16–20, 2009 Awaji Island, Japan. Endocr Pathol. 2010;21(1):48–68. doi: 10.1007/s12022-010-9109-8 [DOI] [Google Scholar]
- [18].Lopes MBS. The 2017 world health organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134:521–535. doi: 10.1007/s00401-017-1769-8 [DOI] [PubMed] [Google Scholar]
- [19].Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017;28(3):228–243. doi: 10.1007/s12022-017-9498-z [DOI] [PubMed] [Google Scholar]
- [20].Laws ER, Penn DL, Repetti CS. Advances and controversies in the classification and grading of pituitary tumors. J Endocrinol Invest. 2019;42:129–135. doi: 10.1007/s40618-018-0901-5 [DOI] [PubMed] [Google Scholar]
- [21].Al-Shraim M, Asa SL. The 2004 world health organization classification of pituitary tumors: what is new? Acta Neuropathol. 2006;111(1):1–7. [DOI] [PubMed] [Google Scholar]
- [22].Saeger W. The 2017 WHO classification of pituitary tumors. Pathologe. 2021;42(3):333–351. doi: 10.1007/s00292-021-00932-x [DOI] [PubMed] [Google Scholar]
- [23].Asa SL, Mete O, Perry A, et al. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr Pathol. 2022. Mar;33(1):6–26. doi: 10.1007/s12022-022-09703-7 [DOI] [PubMed] [Google Scholar]
- [24].Wan XY, Chen J, Wang JW, et al. Overview of the 2022 WHO classification of pituitary adenomas/pituitary neuroendocrine tumors: Clinical practices, controversies, and perspectives. Curr Med Sci. 2022. Dec;42(6):1111–1118. doi: 10.1007/s11596-022-2673-6 [DOI] [PubMed] [Google Scholar]
- [25].Lim CT, Korbonits M. Update on the clinicopathology of pituitary adenomas. Endocr Pract. 2018; EP – 2018 – 0034. Available from: http://journals.aace.com/doi/10.4158/EP - 2018- 0034. [DOI] [PubMed]
- [26].Jibrin P, Ibebuike K, Ado-Wanka AN. Histo-pathological pattern of intracranial tumours in the national Hospital, Abuja. Afr Health Sci. 2018;18(2):281–286. doi: 10.4314/ahs.v18i2.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gandaho HJ, Adeleye AO, Kerekou A, et al. Constraints in the neurosurgical management of pituitary tumours in an African Developing Country: A 5-Year observational study from Benin Republic. Afr J Med Sci. 2016;45(3):261–267. [PubMed] [Google Scholar]
- [28].M KJ. ANALYSIS of PITUITARY TUMOURS: RETROSPECTIVE STUDY at CHRIS HANI BARAGWANATH and CHARLOTTE MAXEKE JOHANNESBURG ACADEMIC HOSPITALS, KENNEDY JOHN MATSHANA a research report submitted to the Faculty of Health Sciences, University of the Witwatersrand, Jo [Internet]. Johannesburg, South Africa; 2010. 1–44 p. Available from: http://hdl.handle.net/10539/8798 [Google Scholar]
- [29].Mello PA, Naves LA, Pereira Neto A, et al. Clinical and laboratorial characterization and post-surgical follow-up of 87 patients with non-functioning pituitary macroadenomas. Arq Neuropsiquiatr. 2013;71(5):307–312. doi: 10.1590/0004-282x20130026 [DOI] [PubMed] [Google Scholar]
- [30].Raappana A, Koivukangas J, Ebeling T, et al. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab. 2010;95(9):4268–4275. doi: 10.1210/jc.2010-0537 [DOI] [PubMed] [Google Scholar]
- [31].Shamim MS, Bari ME, Khursheed F, et al. Pituitary adenomas: Presentations and outcomes in a South Asian country. Can J Neurol Sci. 2008;35(2):198–203. doi: 10.1017/S0317167100008635 [DOI] [PubMed] [Google Scholar]
- [32].GG T, KD R, F Y, et al. Magnitude, Clinical presentation and outcome of patients with pituitary lesions: An experience from tikur anbessa specialized Hospital, Ethiopia. Endocrinol Metab Syndr. 2017;6(02):263. Internet Available from. www.omicsonline.org/open-access/magnitude-clinical-presentation-and-outcome-of-patients-with-pituitary-lesionsan-experience-from-tikur-anbessa-specialized-hospita-2161-1017-1000263.php?aid=87782 [Google Scholar]
- [33].Matshana K Analysis of pituitary tumours: Retrospective Study at Chris Hani Baragwanath and Charlotte Maxeke Johannesburg Academic Hospitals: a research report submitted to the faculty of health sciences, University Of The Witwatersrand, Johannesburg, South Africa; 2010. p. 1–44. [Google Scholar]
- [34].Daly AF, Rixhon M, Adam C, et al. High prevalence of pituitary adenomas: a cross-sectional study in the Province of Liege. Belgium J Clin Endocrinol Metab. 2007;91(12):4769–4775. doi: 10.1210/jc.2006-1668 [DOI] [PubMed] [Google Scholar]
- [35].Daly AF, Beckers A. The epidemiology of pituitary adenomas. Endocrinol Metab Clin North Am. 2020. Sep;49(3):347–355. doi: 10.1016/j.ecl.2020.04.002 [DOI] [PubMed] [Google Scholar]
- [36].Akkus G, Karagun B, Çetinalp NE, et al. Clinical relevance and immunohistochemical patterns of silent pituitary adenomas: 10 years of single-centre experience. Curr Med Imaging. 2021;17(2):310–317. doi: 10.2174/1573405616666201223125642 [DOI] [PubMed] [Google Scholar]


