Abstract
Background
Delirium is a common mental disorder, which is distressing and has serious adverse outcomes in hospitalised patients. Prevention of delirium is desirable from the perspective of patients and carers, and healthcare providers. It is currently unclear, however, whether interventions for preventing delirium are effective.
Objectives
To assess the effectiveness of interventions for preventing delirium in hospitalised non‐Intensive Care Unit (ICU) patients.
Search methods
We searched ALOIS ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 4 December 2015 for all randomised studies on preventing delirium. We also searched MEDLINE (Ovid SP), EMBASE (Ovid SP), PsycINFO (Ovid SP), Central (The Cochrane Library), CINAHL (EBSCOhost), LILACS (BIREME), Web of Science core collection (ISI Web of Science), ClinicalTrials.gov and the WHO meta register of trials, ICTRP.
Selection criteria
We included randomised controlled trials (RCTs) of single and multi‐ component non‐pharmacological and pharmacological interventions for preventing delirium in hospitalised non‐ICU patients.
Data collection and analysis
Two review authors examined titles and abstracts of citations identified by the search for eligibility and extracted data independently, with any disagreements settled by consensus. The primary outcome was incidence of delirium; secondary outcomes included duration and severity of delirium, institutional care at discharge, quality of life and healthcare costs. We used risk ratios (RRs) as measures of treatment effect for dichotomous outcomes; and between group mean differences and standard deviations for continuous outcomes.
Main results
We included 39 trials that recruited 16,082 participants, assessing 22 different interventions or comparisons. Fourteen trials were placebo‐controlled, 15 evaluated a delirium prevention intervention against usual care, and 10 compared two different interventions. Thirty‐two studies were conducted in patients undergoing surgery, the majority in orthopaedic settings. Seven studies were conducted in general medical or geriatric medicine settings.
We found multi‐component interventions reduced the incidence of delirium compared to usual care (RR 0.69, 95% CI 0.59 to 0.81; seven studies; 1950 participants; moderate‐quality evidence). Effect sizes were similar in medical (RR 0.63, 95% CI 0.43 to 0.92; four studies; 1365 participants) and surgical settings (RR 0.71, 95% CI 0.59 to 0.85; three studies; 585 participants). In the subgroup of patients with pre‐existing dementia, the effect of multi‐component interventions remains uncertain (RR 0.90, 95% CI 0.59 to 1.36; one study, 50 participants; low‐quality evidence).
There is no clear evidence that cholinesterase inhibitors are effective in preventing delirium compared to placebo (RR 0.68, 95% CI, 0.17 to 2.62; two studies, 113 participants; very low‐quality evidence).
Three trials provide no clear evidence of an effect of antipsychotic medications as a group on the incidence of delirium (RR 0.73, 95% CI, 0.33 to 1.59; 916 participants; very low‐quality evidence). In a pre‐planned subgroup analysis there was no evidence for effectiveness of a typical antipsychotic (haloperidol) (RR 1.05, 95% CI 0.69 to 1.60; two studies; 516 participants, low‐quality evidence). However, delirium incidence was lower (RR 0.36, 95% CI 0.24 to 0.52; one study; 400 participants, moderate‐quality evidence) for patients treated with an atypical antipsychotic (olanzapine) compared to placebo (moderate‐quality evidence).
There is no clear evidence that melatonin or melatonin agonists reduce delirium incidence compared to placebo (RR 0.41, 95% CI 0.09 to 1.89; three studies, 529 participants; low‐quality evidence).
There is moderate‐quality evidence that Bispectral Index (BIS)‐guided anaesthesia reduces the incidence of delirium compared to BIS‐blinded anaesthesia or clinical judgement (RR 0.71, 95% CI 0.60 to 0.85; two studies; 2057 participants).
It is not possible to generate robust evidence statements for a range of additional pharmacological and anaesthetic interventions due to small numbers of trials, of variable methodological quality.
Authors' conclusions
There is strong evidence supporting multi‐component interventions to prevent delirium in hospitalised patients. There is no clear evidence that cholinesterase inhibitors, antipsychotic medication or melatonin reduce the incidence of delirium. Using the Bispectral Index to monitor and control depth of anaesthesia reduces the incidence of postoperative delirium. The role of drugs and other anaesthetic techniques to prevent delirium remains uncertain.
Plain language summary
Interventions to prevent delirium in hospitalised patients, not including those on intensive care units
Review question
We reviewed the evidence for the effectiveness of interventions for preventing delirium in hospitalised patients, not including those on intensive care units (ICU) (specialised wards for the care of critically ill patients).
Background
Delirium is a common and serious illness for people admitted to hospital. It can be distressing for patients and their families. It also increases the chances of developing other complications in hospital, being admitted to a care home or dying in hospital. Delirium is a very expensive condition for health services. Prevention of delirium is therefore desirable for patients, families and health services.
There are many risk factors for developing delirium (e.g. infection, dehydration, certain medications). Therefore, one approach (called ‘multi‐component interventions’) to preventing delirium is to target these multiple risk factors. Some medications have effects on the brain chemicals implicated in developing delirium, and may, therefore, have a role in prevention. There are also a number of other interventions that target delirium risk factors related to anaesthesia and medical treatment around the time of surgery.
Study characteristics
This evidence is current to 4 December 2015. We found 39 trials that recruited 16,082 participants testing 22 different multi‐component interventions, medications or anaesthetic interventions, compared to usual care, placebo, or different interventions.
Key findings
We found strong evidence that multi‐component interventions can prevent delirium in both medical and surgical settings and less robust evidence that they reduce the severity of delirium. Evidence about their effect on the duration of delirium is inconclusive.
There is evidence that monitoring the depth of anaesthesia can reduce the occurrence of delirium after general anaesthetic.
We found no clear evidence that a range of medications or other anaesthetic techniques or procedures are effective in preventing delirium.
Quality of the evidence
There is moderate‐quality evidence to indicate that multi‐component interventions reduce the incidence of delirium. The evidence supports implementing multi‐component delirium prevention interventions into routine care for patients in hospital.
There is moderate‐quality evidence that monitoring depth of general anaesthesia can be used to prevent delirium postoperatively.
The quality of the evidence for a range of medications or other anaesthetic techniques or procedures for preventing delirium is poor (because of the small number of trials and the variable quality of trial methods), and cannot be used to inform changes to practice.
External funding
None.
Summary of findings
Summary of findings for the main comparison. A multi‐component delirium prevention intervention compared to usual care for hospitalised non‐ICU patients.
| Multi‐component delirium prevention intervention compared to usual care for hospitalised non‐ICU patients | ||||||
| Intervention: A multi‐component delirium prevention intervention versus usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| A multi‐component delirium prevention intervention | ||||||
| Incidence of delirium validated instruments1 | 209 per 10002 | 144 per 1000 (123 to 172) | RR 0.69 (0.59 to 0.81) | 1950 (7 studies3) | ⊕⊕⊕⊝ moderate4,5,6 | |
| Duration of delirium (days) | The mean duration of delirium in the control groups ranged from 2.1 to 10.2 days | The mean duration of delirium in the intervention groups was 1.16 days shorter (2.96 shorter to 0.64 longer) | 244 (4 studies) | ⊕⊝⊝⊝ very low4,6,7,8,9 | ||
| Severity of delirium DRS‐R‐98 and CAM‐S10 | The standardised mean severity of delirium in the intervention groups was 1.04 standard deviations lower (1.65 to 0.43 lower)11 | 67 (2 studies) | ⊕⊕⊝⊝ low4,12 | |||
| Length of admission Days | The mean length of admission in the control groups ranged from 5 to 38 days | The mean length of admission in the intervention groups was 0.01 days longer (0.48 days shorter to 0.51 days longer) | 1920 (6 studies) | ⊕⊕⊕⊝ moderate4,6,7 | ||
| Return to independent living | 682 per 10002 | 648 per 1000 (580 to 723) | RR 0.95 (0.85 to 1.06) | 1116 (4 studies) | ⊕⊕⊕⊝ moderate4,6,13 | |
| Inpatient mortality | 81 per 10002 | 73 per 1000 (45 to 116) | RR 0.90 (0.56 to 1.43) | 859 (3 studies) | ⊕⊝⊝⊝ very low6,14,15 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Three validated methods for delirium detection used ‐ the CAM, OBS and DRS 2 The assumed risk is the risk in the control group 3 Four studies in medical in patients, three studies in surgical patients 4 High risk of performance bias due to the lack of blinding of participants and personal in all studies (due to the nature of the intervention). 5 Outcomes assessors unblinded 2 studies (one of which carries the largest weighting (58%) due to high event rate). Risk of bias otherwise low across studies
6 Higher baseline prevalence of dementia in the control groups of two studies compared to the intervention groups causing risk of bias 7Outcomes assessors unblinded in two studies 8 Minimal important difference (MID) of 1 day assumed. 95% confidence limits around the pooled estimate of mean difference includes both 'no difference', and the MID.
9 Downgraded because inconsistent results
10 Delirium Rating Scale‐Revised‐98 (0 to 46) and Confusion Assessment Method‐Severity (0 to 10) 11This is a difference in standard deviations. A standard deviation of > 0.8 represents a large effect. 12 Imprecise results ‐ small pooled sample size 13 Outcomes assessors unblinded in one study 14There is some inconsistency of results 15Imprecise results ‐ pooled estimate includes both no effect, appreciable benefit and appreciable harm
Summary of findings 2. Prophylactic cholinesterase inhibitor versus placebo for preventing delirium in hospitalised non‐ICU patients.
| Prophylactic cholinesterase inhibitor versus placebo for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic cholinesterase inhibitor versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic cholinesterase inhibitors | |||||
| Incidence of delirium DSM‐IV criteria, DSI, CAM, | 218 per 10001 | 148 per 1000 (37 to 572) | RR 0.68 (0.17 to 2.62) | 113 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium MDAS | The mean severity of delirium in the control groups was 1.3 points | The mean severity of delirium in the intervention groups was 0.30 points lower (4.17 lower to 3.57 higher) | 16 (1 study) | ⊕⊕⊝⊝ low5 | ||
| Length of admission Days | The mean length of admission ranged across control groups from 4‐12.1 days | The mean length of admission in the intervention groups was 0.34 days shorter (1.54 shorter to 0.86 longer) | 128 (3 studies) | ⊕⊕⊝⊝ low6,7 | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The assumed risk is the risk in the control group
2 Both studies are at high risk of attrition bias and have incomplete outcome data.
3 Downgraded because inconsistent results
4 Estimate of effect includes 'no benefit' and both appreciable benefit and appreciable harm.
5 Estimate of effect includes both 'no effect' and minimally important difference, downgraded two levels due to serious imprecision
6 Risk of bias unclear in all domains in one study (abstract only available). Remaining two studies have incomplete outcome reporting and are at risk of attrition bias
7 Downgraded due to imprecision in result
Summary of findings 3. Prophylactic antipsychotic medications for preventing delirium in hospitalised non‐ICU patients.
| Prophylactic antipsychotic medications for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic antipsychotic medications versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic antipsychotic medications | |||||
| Incidence of delirium CAM/NEECHAM Follow‐up range: 0‐8 postoperative days | 300 per 10001 | 165 per 1000 (69 to 390) | RR 0.55 (0.23 to 1.3) | 916 (3 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Duration of delirium Days Follow‐up: 3‐8 postoperative days | The mean duration of delirium in the control groups ranged from 2.2 to 5.4 days |
The mean duration of delirium in the intervention groups was 2.74 days shorter (9.59 shorter to 4.11 longer) | 178 (2 studies) | ⊕⊝⊝⊝ very low2,5 | ||
| Severity of delirium DRS. Scale from: 0 to 46. Follow‐up: 3‐8 postoperative days | The mean severity of delirium in the control groups ranged from 14.4 to 16.4 points |
The mean severity of delirium in the intervention groups was 1.02 points lower (6.8 lower to 4.76 higher) | 178 (2 studies) | ⊕⊝⊝⊝ very low2,5 | ||
| Length of admission Days | The mean length of admission in the control group was 17.1 days |
The mean length of admission in the intervention groups was 5.5 days shorter (12.17 shorter to 1.17 longer) | 68 (1 study) | ⊕⊕⊝⊝ low5 | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| Inpatient mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The assumed risk is the risk in the control group
2Downgraded because inconsistent results
3 Downgraded because of imprecision in results
4 Downgraded due to risk of bias
5 Downgraded two levels because very imprecise results
Summary of findings 4. Prophylactic melatonin for preventing delirium in hospitalised non‐ICU patients.
| Prophylactic melatonin for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Prophylactic melatonin versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic melatonin | |||||
| Incidence of delirium CAM/DSM IV/DRS‐R‐9s Follow‐up: every 24 to 48 hours until discharge or 8 days | 242 per 10001 | 128 per 1000 (22 to 788) | RR 0.53 (0.09 to 3.25) | 529 (3 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Duration of delirium Days Follow‐up: every 24 to 48 hours until discharge | The mean duration of delirium in the control group was 2 days |
The mean duration of delirium in the intervention groups was 0 days longer (0.57 shorter to 0.57 longer) | 104 (1 study) | ⊕⊕⊕⊝ moderate3 | ||
| Severity of delirium (binary severe vs. not severe) Number of patients requiring greater than 3mg of haloperidol Follow‐up: daily until discharge | 531 per 1000 | 457 per 1000 (308 to 674) | RR 0.86 (0.58 to 1.27) | 104 (1 study) | ⊕⊕⊕⊝ moderate3 | |
|
Severity of delirium DRS‐R‐98 score |
The mean severity of delirium in the control group was 6.3 points |
The mean severity of delirium in the intervention group was 4.1 points lower (19.47 points lower to 11.27 points higher) |
6 (1 study) |
⊕⊕⊝⊝ low5 | ||
| Length of admission Days | The mean length of admission in the control groups ranged from 11 to 18.5 days |
The mean length of admission in the intervention groups was 0.09 days longer (1.2 shorter to 1.39 longer) | 500 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality Mortality Follow‐up: every 24 to 48 hours until discharge or 8 days | 47 per 10001 | 39 per 1000 (17 to 88) | RR 0.84 (0.37 to 1.88) | 543 (3 studies) | ⊕⊕⊝⊝ low6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The assumed risk is the risk in the control group
2 Downgraded because inconsistent results
3 Downgraded because imprecise results
4 Downgraded due to risk of bias
5 Downgraded because imprecise results and very small number of events
Summary of findings 5. Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement for preventing delirium in hospitalised non‐ICU patients.
| Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement for preventing delirium in hospitalised non‐ICU patients | ||||||
| Intervention: Bispectral index (BIS)‐guided anaesthesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| BIS‐blinded/clinical judgement | BIS‐guided | |||||
|
Incidence of delirium CAM, DSM‐IV Follow‐up: daily after surgery until discharge; twice daily from postoperative day 1 to 7 |
226 per 10001 | 160 per 1000 (135 to 192) | RR 0.71 (0.60 to 0.85) | 2057 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| Duration of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
| Severity of delirium ‐ not measured | N/A | N/A | N/A | N/A | ||
|
Length of admission Days |
The mean length of admission in the control groups ranged from 7 to 15.7 days |
The mean length of admission in the intervention group was 0.94 days shorter (0.43 days shorter to 1.45 days shorter) | ‐ | 2057 (2 studies) | ⊕⊕⊕⊝ moderate2 | |
| Return to independent living ‐ not measured | N/A | N/A | N/A | N/A | ||
| In‐hospital mortality ‐ not measured | N/A | N/A | N/A | N/A | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The assumed risk is the risk in the control group (BIS‐blinded/clinical judgement) 2 Downgraded due to risk of bias
Background
Description of the condition
Delirium is a disturbance of consciousness and cognition, which usually has a rapid onset and a fluctuating course. It has been variously termed acute organic brain syndrome, acute organic mental disorder and toxic confusional state. Until the 19th century delirium was used to describe a disorder of thinking and later descriptions included disturbances of perception, often with overactive behaviour, or impaired consciousness. The publication of Diagnostic and Statistical Manual (DSM) III (APA 1987) in 1987 brought these ideas together, combining disturbance of consciousness with impairment of cognition. The core features of delirium (disturbance in attention, changes in cognition, and acute onset and fluctuating course) have now been clarified in the International Classification of Diseases version 10 (ICD‐10) (WHO 1992), DSM‐IV (APA 1994), and most recently DSM‐V (APA 2013). This consensus has allowed some standardisation of research, and greater comparability between studies, although differences remain, such as the requirement for evidence of an underlying cause in DSM‐IV and DSM‐V, but not in ICD‐10.
Delirium is common in hospitalised patients. Ten per cent to 30% of admissions to a general hospital develop delirium (Levkoff 1991; Trzepacz 1996) and in general medical inpatients, occurrence rates ranging from 11% to 42% have been reported (Siddiqi 2006). Delirium has a prevalence of up to 60% in frail elderly patients (Francis 1990), and 7% to 9.6% in elderly patients presenting to emergency departments (Elie 2000; Hustey 2003). Following coronary artery bypass grafting in the elderly, the incidence has been reported as 33.6% (Santos 2004), and after bilateral knee replacements 41% (Williams‐Russo 1992). Following hip fracture, the overall prevalence is 43% to 61% (Holmes 2000). Cancer also increases the risk of developing delirium; 18% of those admitted to an oncology ward, and 26% to 44% of those admitted to hospital or a hospice with a diagnosis of advanced cancer developed delirium (Centeno 2004; Ljubisavljevic 2003). In patients with AIDS who are unwell enough to be admitted, incidence of delirium is also high, being reported as 46% (Uldall 1997).
Delirium is serious, with significant short‐ and long‐term outcomes. Mortality is increased (McCusker 2002), functional abilities reduced (Moller 1998), admission to long‐term care increased (Inouye 1998a), and length of stay increased (McCusker 2003a; Stevens 1998). Impairment of cognitive function can persist for at least one year (McCusker 2001), as can the symptoms of delirium, especially inattention, disorientation and impaired memory (McCusker 2003b). Increasingly recognised is the distress an episode of delirium produces for both sufferers and their carers (Breitbart 2002).
Research in older people has identified a range of risk factors for delirium. The condition clearly has a multi‐factorial aetiology, and these risk factors interact (Inouye 1998b); the more risk factors that are present, the greater the likelihood that the patient will develop delirium. Risk factors that have so far been identified include: increased age, sensory deprivation (visual or hearing impairment), sleep deprivation, social isolation, physical restraint, use of bladder catheter, iatrogenic adverse events, poly‐pharmacy (more than three new medications added), use of psychoactive drugs, co‐morbidities, severe illness (especially infection, fracture or stroke), prior cognitive impairment, temperature abnormality (fever or hypothermia), dehydration, malnutrition and low serum albumin (Inouye 1998b; Inouye 1999c; NICE 2010).
Studies in oncology patients have also identified a range of risk factors for delirium, for example bone metastases, the presence of haematological malignancy, advanced age, cognitive impairment, and low albumin levels (Ljubisavljevic 2003).
The identification of such a varied list of aetiological factors suggests several things. First, we may be able to identify patients at high risk of developing delirium, and by modifying these risk factors could attempt to prevent it; such prevention strategies could be targeted to specific groups of patients.
Second, many of these risk factors can be seen as hospital 'quality of care' measures, e.g. malnutrition, dehydration, use of physical restraints, iatrogenic events. Occurrence of delirium can, therefore, be seen as a proxy measure of the quality of inpatient care (Inouye 1999b; Inouye 2014); and effective interventions to prevent delirium may be considered integral to quality improvement.
Quality improvement is a major issue for healthcare, particularly in services for older people (Institute for Innovation 2006). We know that healthcare systems and services, internationally, have not kept pace with demographic transitions, and often fail to meet the complex needs requiring multidisciplinary care of growing numbers of older people (Hubbard 2004). General hospitals, in fact, frequently have attributes that unintentionally stimulate or aggravate delirium (Young 2007). However, addressing this is challenging and requires wide‐ranging changes to systems of care. Focusing on delirium prevention may help develop the necessary professional skills, cultural aspects, and service design in such a way as to drive up quality of care.
Prevention of delirium is clearly desirable for both patients and carers, and can also reduce health service costs. Healthcare costs in patients who developed delirium in intensive care units (ICUs) were 31% higher ($41,836 versus $27,106) (Milbrandt 2004). A non‐randomised study of a multi‐component intervention for delirium also demonstrated overall improved cost‐effectiveness (Rizzo 2001).
Description of the intervention
This review assesses the effectiveness of non‐pharmacological and pharmacological interventions for preventing delirium in hospitalised patients, excluding the ICU setting.
A range of non‐pharmacological interventions for preventing delirium in hospitalised patients have been developed. Most have taken a multi‐factorial approach to delirium prevention, attempting to prevent several risk factors by protocols, education or systems redesign, (Cole 2002; Inouye 2000; Milisen 2001), although some target a single risk factor only. Examples include programmes of education for ward nursing staff (Rockwood 1999), protocols targeting specific risk factors and implemented by a trained interdisciplinary team (Inouye 1999a; Young 2015), and specialist nursing interventions to educate nursing staff, assess and change medication, encourage mobilisation and improve the environment of the patient (Wanich 1992).
Pharmacological interventions are based on an understanding of the multiple neurotransmitter pathways involved in developing delirium and substances that might potentially modify these or modify other important risk factors. These include, for example, cholinesterase inhibitors, antipsychotics and analgesics. There are also a number of other interventions that target delirium risk factors related to surgery and perioperative care, such as varying approaches to anaesthesia, optimising blood transfusion, and postoperative pain relief.
How the intervention might work
Delirium has many risk factors and precipitating factors, some of which may be modifiable. Previous work has suggested that a combination of risk factors may interact to increase vulnerability to delirium, and models to predict this risk have been developed and validated (Inouye 1993a). Measures to reduce the number or severity of these factors may help to prevent delirium and may attenuate the poor outcomes associated with it.
Single‐ and multi‐component non‐pharmacological interventions target one or more of these risk factors.
Pharmacological interventions either target the important neurotransmitter pathways that have been implicated in the complex pathophysiology of delirium ((e.g. antipsychotics, cholinesterase inhibitors) or aim to address important risk factors such as sleep and pain (e.g. melatonin and gabapentinoids).
Various anaesthetic approaches and perioperative procedures also address potential risk factors for delirium.
Why it is important to do this review
Given that delirium is associated with such poor outcomes (Witlox 2010), which do not appear to be modified with treatment (NICE 2010), interventions to prevent delirium may be particularly important. Previous reviews (Cole 1999; Milisen 2005) have suggested a role for multi‐component delirium prevention interventions, but have not been systematic or have employed less rigorous selection criteria. A previous Cochrane review of delirium prevention in hospitalised patients published in 2007 found the evidence was sparse and recommended further research was needed (Siddiqi 2007). It is currently unclear whether interventions for prevention of delirium are effective.
Objectives
To assess the effectiveness of interventions designed to prevent delirium in hospitalised non‐intensive care unit patients.
Methods
Criteria for considering studies for this review
Types of studies
We only considered randomised controlled trials for this review.
Types of participants
We included patients aged 16 years or over, admitted to acute general hospitals and at risk of developing delirium. We excluded studies conducted in ICU as both the population and interventions in this setting are likely to be very different. We also excluded community settings e.g. nursing homes. We excluded studies in mixed settings unless data could be extracted separately for hospitalised inpatients.
Types of interventions
We considered all non‐pharmacological and pharmacological interventions designed to prevent delirium. Trials including a control group receiving standard care and trials comparing two types of intervention were included. Trials of co‐ordinated multi‐strategy initiatives to prevent delirium (multi‐component interventions) were included. We defined standard care as the usual care available on that unit.
Types of outcome measures
We identified the primary, secondary and adverse outcome measures that are important for patients, carers and for health and social care systems.
Primary outcomes
Incidence of delirium, using a validated diagnostic method
Secondary outcomes
Duration of delirium
Severity of delirium, measured by validated instruments including the Memorial Delirium Assessment Scale (MDAS) (Breitbart 1997), Delirium Rating Scale (DRS) (Trzepacz 1988), and DRS‐R‐98 (Trzepacz 2001)
Length of admission
Cognitive status
Use of psychotropic medication
Behavioural disturbance
Activities of daily living
Return to independent living
Institutional care at discharge
Quality of life
Carers' psychological morbidity
Staff psychological morbidity
Cost of intervention
Cost to healthcare services
Withdrawal from protocols by patients
Adverse outcomes
Adverse events (as defined by study authors)
Postoperative complications
Falls
Pressure ulcers
Infections (specifically wound infections, urinary tract infections, pneumonia)
Cardiac adverse events (specifically myocardial infarction & cardiac failure)
Mortality
Secondary outcomes were chosen as those likely to be influenced by preventing delirium; and adverse outcomes defined as unfavourable effects that might be associated with the intervention or comparator, although for some outcomes the distinction between the two may be arbitrary.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois) ‐ the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register on 4th December 2015. The advanced search was used to retrieve all randomised studies in which delirium was the focus.
ALOIS is maintained by the Trials Search Co‐ordinator and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy. The studies were identified from the following searches.
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and LILACS
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others)
Quarterly search of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL)
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
We performed additional searches in many of the sources listed above to cover the time frame from the last searches performed for ALOIS, to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1 and results of the searches in Appendix 2.
Searches conducted between October 2008 and December 2015 retrieved a total of 542 results after initial de‐duplication and first assessment by the Cochrane Dementia and Cognitive Improvement Group's Trials Search Co‐ordinator.
Searching other resources
We reviewed bibliographies of books and review articles on delirium, and also references from retrieved articles. We contacted experts in this field for further references and to locate unpublished trials. The Internet was searched using the search engines Google and Copernic to try to find further evidence of unpublished trials using the same terms as stated above.
We did not apply any time restrictions or language constraints.
Data collection and analysis
Selection of studies
Two review authors independently examined all titles and abstracts of citations identified by the search for eligibility, and assessed full texts of potentially eligible studies for inclusion. All disagreements were resolved by consensus.
Data extraction and management
Two review authors used a piloted data extraction form to extract data on each study independently, and settled any disagreements by consensus. We created a table of 'Characteristics of included studies' using Review Manager 5 (RevMan 2012). Review authors were not blinded to study authors and institution for study selection, data extraction or quality assessment. Reports from the same study were collated under a single study reference.
For delirium incidence and severity, where results were presented for multiple time points and no summary data were available, we used the highest recorded number or peak values for the intervention and control arm. This was because we were interested in interventions that reduced the overall burden of delirium. For example, if delirium severity was ascertained on days one, three, and five of the hospital stay, then we included only the highest of those three ascertainments in our analysis of delirium severity.
For severity and duration of delirium, data were included only from patients with delirium.
To allow use of more of the reported data for syntheses, where medians and Interquartile ranges (IQR) or ranges were presented rather than means and standard deviations, we converted values as follows. We assumed the median value was equivalent to the mean. We estimated the standard deviation as 'IQR/1.35' or 'range/4' (small studies, n < 70) or 'range/6' (larger studies, n > 70).
Assessment of risk of bias in included studies
Two review authors independently assessed risks of bias for all included studies using the criteria described in the Cochrane Handbookfor Systematic Reviews of Interventions (Cochrane Handbook 2011). We assessed included trials for adequacy of sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias. For each domain, we made a judgement of low risk, high risk or unclear risk of bias and presented these in a 'Risk of bias' table for each study. We settled any disagreements by consensus.
Measures of treatment effect
We used risk ratios (RRs) as measures of treatment effect for dichotomous outcomes; and between group mean differences and standard deviations for continuous outcomes.
Dealing with missing data
Missing data and dropout rates were assessed for each of the included studies. We reported the number of participants included in the final analysis as a proportion of all participants in the study. An available case analysis was performed, including data only on those whose results were known. Incomplete outcomes assessment was reported in the 'Risk of bias' table for each study and discussed in the main text to enable consideration of the potential impact of missing data.
Data synthesis
We synthesised dichotomous outcomes for meta‐analysis and calculated pooled RRs with 95% confidence intervals (CIs) using random‐effects methods. We synthesised continuous outcomes and calculated pooled mean differences, or standardised mean differences with 95% CIs using random‐effects inverse variance methods.
Subgroup analysis and investigation of heterogeneity
We conducted a pre‐planned intervention level subgroup analysis for multi‐component delirium prevention interventions in surgical and medical settings, and for studies reporting delirium in the presence of diagnosed dementia. We carried out a further pre‐planned intervention level subgroup analysis to investigate whether typical and atypical antipsychotic medications were associated with varying levels of effectiveness.
Data presentation ‐ 'Summary of findings' tables
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess the quality of the supporting evidence behind each estimate of treatment effect (Schunemann 2011a; Schunemann 2011b) for selected key interventions and outcomes. We presented key findings of the review including a summary of the amount of data, the magnitude of the effect size and the overall quality of the evidence, in 'Summary of findings' tables, created using GRADEpro software (GRADEpro 2014). We selected the following interventions: multi‐component delirium prevention interventions; cholinesterase inhibitors; antipsychotics; melatonin and bispectral‐index guided‐anaesthesia; and the following outcomes: incidence of delirium, severity of delirium, duration of delirium, length of admission, return to independent living and in‐hospital mortality, as being most relevant for clinical practice across a range of hospital settings.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The search results are summarised in a PRISMA diagram (Figure 1). Of the 136 full‐text articles retrieved, 40 were considered eligible for inclusion; 69 were excluded (see Excluded studies); and 27 are ongoing (see Ongoing studies). Several articles identified as eligible reported outcome data for the same trial. Therefore, 33 new studies were eligible for inclusion and added to the six studies included in the original review (Siddiqi 2007), resulting in 39 included studies (see Included studies). Study authors were contacted for further information for six of these studies (Ashraf 2015; Bonaventura 2007; de Jonghe 2014; Hatta 2014; Jeffs 2013; Gauge 2014). However, unpublished data were only used for Hatta 2014, for which data for the subgroup of non‐ICU study participants were provided by the authors.
1.
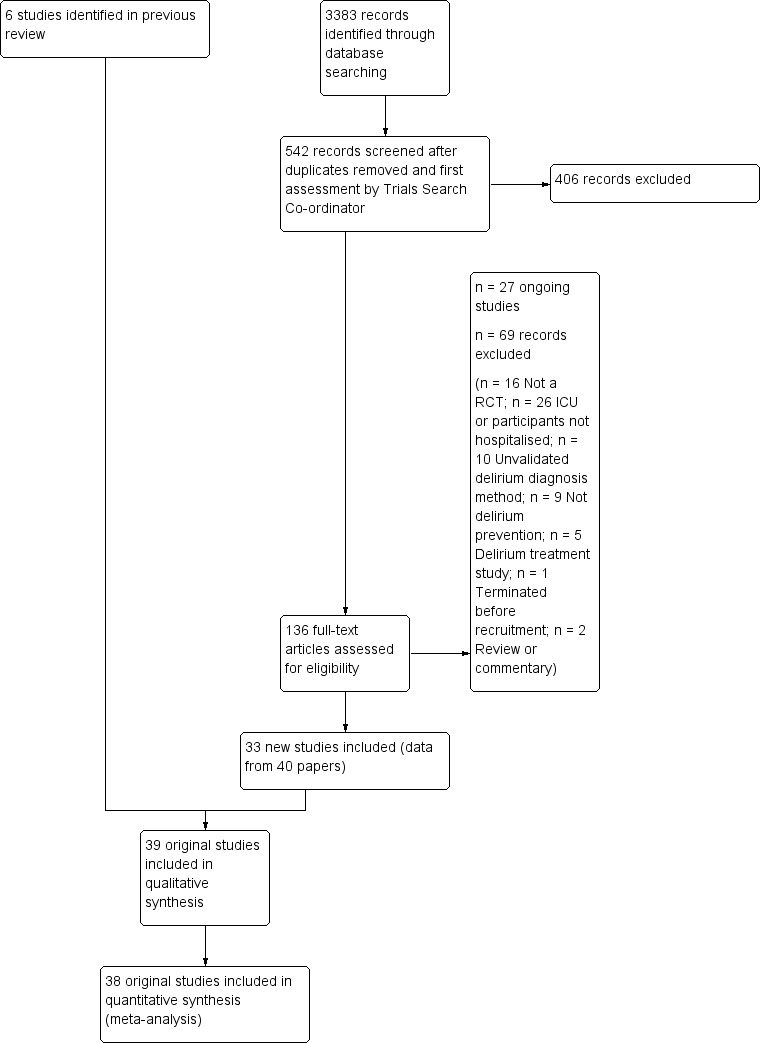
Study flow diagram
Included studies
The 39 studies included a total study population of 16,082 randomised participants, and assessed 22 different interventions or comparisons (Abizanda 2011; Aizawa 2002; Al‐Aama 2011; Ashraf 2015; Beaussier 2006; Berggren 1987; Bonaventura 2007; Boustani 2012; Chan 2013; de Jonghe 2014; Diaz 2001; Fukata 2014; Gauge 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Jia 2014; Kalisvaart 2005; Larsen 2010; Leung 2006; Li 2013; Liptzin 2005; Lundstrom 2007; Lurati 2012; Marcantonio 2001; Marcantonio 2011; Martinez 2012; Mouzopoulos 2009; Munger 2008; Papaioannou 2005; Pesonen 2011; Radtke 2013; Sampson 2007; Sieber 2010; Stoppe 2013; Urban 2008; Watne 2014; Whitlock 2015).
Study design
Fourteen studies were placebo‐controlled trials (Al‐Aama 2011; de Jonghe 2014; Diaz 2001; Hatta 2014; Kalisvaart 2005; Larsen 2010; Leung 2006; Liptzin 2005; Marcantonio 2011; Mouzopoulos 2009; Munger 2008; Pesonen 2011; Sampson 2007; Whitlock 2015). Fifteen studies evaluated a delirium prevention intervention against usual care (Abizanda 2011; Aizawa 2002; Ashraf 2015; Bonaventura 2007; Boustani 2012; Fukata 2014; Gauge 2014; Gruber‐Baldini 2013; Hempenius 2013; Jeffs 2013; Jia 2014; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Urban 2008). Ten studies compared two different interventions (Beaussier 2006; Berggren 1987; Chan 2013; Li 2013; Lurati 2012; Papaioannou 2005; Radtke 2013; Sieber 2010; Stoppe 2013;Watne 2014).
Sample Size
The sample size of included studies was highly variable, ranging from 15 to 7507 randomised participants. Eighteen studies randomised less than 100 participants, of which eight randomised less than 50 (Aizawa 2002; Ashraf 2015; Hatta 2014; Leung 2006; Marcantonio 2011; Munger 2008; Stoppe 2013; Urban 2008).
Setting
Thirty‐ two studies were conducted in patients undergoing surgery or procedural interventions.
Orthopaedic practice was the most common setting (18 studies). Six of these evaluated interventions in patients undergoing elective arthroplasty or joint replacement (Kalisvaart 2005; Larsen 2010; Leung 2006; Liptzin 2005; Sampson 2007; Urban 2008); 11 included patients undergoing hip fracture repair Berggren 1987; de Jonghe 2014; Diaz 2001; Gruber‐Baldini 2013; Li 2013; Lundstrom 2007; Marcantonio 2001; Marcantonio 2011; Mouzopoulos 2009; Sieber 2010; Watne 2014), and one study was conducted in combined elective and emergency orthopaedic settings (Munger 2008).
Four studies were in patients undergoing cardiac surgery (Gauge 2014; Pesonen 2011; Stoppe 2013; Whitlock 2015); and one in patients undergoing inpatient cardiac catheterisation (Ashraf 2015).
Two studies were in patients undergoing surgery for cancer (Hempenius 2013 and Jia 2014), the latter specifically for colorectal cancer.
Two studies were in patients having general and colorectal surgery or colorectal surgery alone (Aizawa 2002; Beaussier 2006).
Five studies were in patients undergoing various other elective surgical procedures (Chan 2013; Fukata 2014; Lurati 2012; Papaioannou 2005; Radtke 2013). One of these included patients having abdominal surgery under general anaesthesia or orthopaedic surgery under general or spinal anaesthesia (Fukata 2014); and one study was in patients undergoing non‐cardiac surgery under general anaesthesia (Lurati 2012).
Only seven studies (2011 participants) evaluated interventions in a general medical or geriatric medical hospital environment (Abizanda 2011; Al‐Aama 2011; Bonaventura 2007; Boustani 2012; Hatta 2014; Jeffs 2013; Martinez 2012 ).
Participants
Age
In 29 studies, participants had a mean age in both allocation arms of more than 70 years. Six studies had a mean age of less than 70 years in one or both groups (Chan 2013; Liptzin 2005; Radtke 2013; Sampson 2007; Stoppe 2013; Whitlock 2015); and two studies had very low mean age of included participants, Urban 2008 (mean age 53 and 48 years in the intervention and control groups respectively) and Leung 2006 (overall mean age 59.6 years). Two studies did not present data on the mean age of participants (Bonaventura 2007; Papaioannou 2005).
Co‐morbidities
Eight studies used the Charlson Index (Charlson 1994) (Boustani 2012; de Jonghe 2014; Hatta 2014; Jeffs 2013; Leung 2006; Marcantonio 2001; Martinez 2012; Sieber 2010) to compare co‐morbidities between intervention and control groups. One study (Boustani 2012), reported higher Charlson Index scores in the usual care group.
Five studies presented the total number of co‐morbidities present for intervention and control groups (Abizanda 2011; Al‐Aama 2011; Bonaventura 2007; Diaz 2001; Hempenius 2013).
Nine studies presented the frequency of a range of specific co‐morbidities in both the intervention and control groups (Ashraf 2015; Berggren 1987; Chan 2013; Gruber‐Baldini 2013; Jia 2014; Lundstrom 2007; Lurati 2012; Pesonen 2011; Whitlock 2015). Lundstrom 2007 reported a difference between the intervention and control arms, with a higher rate of depression in the control group, and Ashraf 2015 had higher rates of coronary artery disease in the usual care group and higher rates of depression in the intervention group.
Seventeen studies did not report co‐morbidities at baseline (Aizawa 2002; Beaussier 2006; Fukata 2014; Gauge 2014; Kalisvaart 2005; Larsen 2010; Li 2013; Liptzin 2005; Marcantonio 2011; Mouzopoulos 2009; Munger 2008; Papaioannou 2005; Radtke 2013; Sampson 2007; Stoppe 2013; Urban 2008; Watne 2014).
Dementia
Eleven of the included studies excluded participants with dementia. This included using dementia diagnosis as an exclusion criteria (Diaz 2001; Jia 2014; Larsen 2010) or based on performance in cognitive testing (Ashraf 2015; Berggren 1987; Bonaventura 2007; Chan 2013; Li 2013; Papaioannou 2005; Radtke 2013; Stoppe 2013), most commonly using the Mini‐Mental State Examination (MMSE) score (Folstein 1975).
There were three studies where the proportion of participants with dementia differed between the intervention and control groups: in Gruber‐Baldini 2013, it was 27.3% in intervention versus 36.1% in control; in Lundstrom 2007, 27.5% in intervention versus 37.1% in control; and in Marcantonio 2001, 37% in intervention and 51% in control.
Interventions
Multi‐component interventions
Seven studies (2018 participants) evaluated non‐pharmacological multi‐component interventions (Abizanda 2011; Bonaventura 2007; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012) in comparison to usual care. Individual components of each multi‐component intervention are summarised in Table 6. The number of components varied between two (Jeffs 2013) and 13 (Hempenius 2013) (Table 6). Most included individualised care, an educational component, reorientation, and early mobilisation. Many of the delirium risk factors targeted with multi‐component interventions relate to good basic care. The nature in which interventions were implemented varied between the studies: some relied on a protocol‐driven approach (Bonaventura 2007; Jeffs 2013; Marcantonio 2001), whilst others were more pragmatic in the delivery of the intervention (e.g. the family delivered the reorientation intervention in Martinez 2012). Two studies were based on therapist interventions (Abizanda 2011; Jeffs 2013), one was multidisciplinary including a Comprehensive Geriatric Assessment (Lundstrom 2007), and two were based on proactive perioperative input from a geriatrician (Hempenius 2013; Marcantonio 2001).
1. Individual components of multi‐component interventions.
| Study | Intervention Components | |||||||||||||||||||
| Individualised care |
Checklists/ protocols |
Education/ training1 |
Re‐orientation | Attention to sensory deprivation | Familiar objects | Cognitive stimulation |
Nutrition/ hydration |
Identification of infection | Mobilisation | Sleep hygiene | MDTcare2 | CGA3 | Oxygenation | Electrolytes | Pain control | Medication review | Mood4 |
Bowel/ bladder care |
Postoperative complications | |
| Abizanda 2011 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Bonaventura 2007 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||||||
| Jeffs 2013 | ✔ | ✔ | ||||||||||||||||||
| Martinez 2012 | ✔ | ✔ | ✔ | ✔ | ||||||||||||||||
| Hempenius 2013 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| Lundstrom 2006 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| Marcantonio 2001 | ✔ | |||||||||||||||||||
1Education/training: structured education/training of staff or carers; 2MDT Multidisciplinary Team; 3CGA Comprehensive Geriatric Assessment; 4Mood: assessment for depression/anxiety
Pharmacological interventions
Thirteen studies assessed various pharmacological agents.
Although the pathophysiology of delirium remains unclear, acetylcholine is the neurotransmitter that has been most implicated in studies (Koponen 1999; Tune 1999), leading to suggestions that cholinesterase inhibitors may have a role in delirium management. Four studies tested the use of prophylactic cholinesterase inhibitors (Liptzin 2005; Marcantonio 2011; Munger 2008; Sampson 2007).
Three studies assessed antipsychotic medication (Fukata 2014; Kalisvaart 2005; Larsen 2010).
Melatonin is a hormone that has a role in sleep/wake regulation, and may be responsible for the disruption of the sleep/wake cycle seen in delirium (Figueroa‐Ramos 2009). This has led to suggestions that it could have a role in delirium prevention (Lewis 2004). Melatonin supplementation has been proposed as a treatment option for delirium (Bourne 2006), and there is case report evidence of its usefulness (Hanania 2002). Two studies investigated the use of melatonin (Al‐Aama 2011; de Jonghe 2014 ); and one used a melatonin agonist (Hatta 2014).
Citicoline (cytidine 5′‐diphosphocholine (CDP‐choline)), is a drug that has been implicated in cognitive impairment and memory, and therefore has been proposed as a treatment in traumatic brain injury, stroke, vascular dementia, Parkinson’s disease, and brain aging (Fioravanti 2006a). Citicoline has the function in the brain of stabilising cell membranes and reducing the presence of free radicals. In particular, there is some evidence that citicoline stimulates the release of dopamine neurotransmitters in the brain (Fioravanti 2005). One study tested citicoline (Diaz 2001).
Diazepam is a long‐acting benzodiazepine which is often used as an anxiolytic and has been used in the cardiac catheterisation setting with good effect (Woodhead 2007). Diphyenhydramine is an antihistamine medication which can cause sedation and has been used as an adjunct for individuals undergoing colonoscopy with good effect (Tu 2006). Evidence regarding premedication and postoperative delirium is unclear (Fines 2006) with concern that administering these medications may increase rates of post‐procedure or postoperative delirium. One study evaluated the combination of diazepam and diphenhydramine as premedication before cardiac catheterisation (Ashraf 2015).
Methylprednisolone is an intravenous steroid preparation with a wide range of clinical uses. Steroid use has been thought to be beneficial to individuals undergoing cardiopulmonary bypass, with evidence of reduction in new onset atrial fibrillation, postoperative bleeding and length of stay in the intensive care unit (ICU) (Whitlock 2008). A subsequent clinical trial failed to show benefit for the entire population undergoing cardiopulmonary bypass, but subgroup analysis suggested those at higher risk of adverse outcomes may benefit (Dieleman 2012). This formed the basis of the design of Whitlock 2015, evaluating methylprednisolone for those at high risk undergoing cardiopulmonary bypass, with incidence of delirium as a safety outcome measure.
Perioperative interventions
Postoperative delirium is a common complication of surgery in older people (Holmes 2000; Santos 2004; Williams‐Russo 1992), likely to be a consequence of the physiological and biochemical derangement induced by the underlying pathology, surgical trauma pain and anaesthesia. Perioperative care is, therefore, a potential focus for interventions to reduce postoperative delirium.
In surgical practice, there has been a move towards a concept of ‘enhanced recovery’ whereby surgical intervention, anaesthesia and postoperative care are modified in such a way as to minimise the overall impact of surgery, reducing postoperative complications and expediting recovery (Douglas 2001). Many postoperative complications (e.g. ileus, respiratory depression, chest infections, and myocardial ischaemia, all of which may predispose to delirium) could be reduced by the use of regional anaesthesia and opioid‐sparing analgesics (Bonnet 2005).
Eighteen studies tested various interventions addressing modifications to perioperative practice that might influence postoperative delirium. These are subdivided into five broad approaches; i) those that reduce opioid utilisation, ii) those that control/reduce depth of general anaesthesia, iii) those that consider alternative forms of general anaesthesia, iv) those which avoid general anaesthesia altogether and v) a miscellaneous group including studies investigating transfusion practice, fast track surgery and a 'delirium‐free protocol'.
i) Opioid‐sparing measures:
Techniques to reduce opioid utilisation include the administration of adjuvant analgesics; addition of intrathecal opioid to general anaesthesia; and peripheral local anaesthetic blockade. These were tested in six studies.
Gabapentinoids are commonly used for treatment of epilepsy, anxiety, and neuropathic pain, but also have a role as opioid‐sparing adjuncts for postoperative pain relief (Tippana 2007). Leung 2006 tested gabapentin and Pesonen 2011 tested pregabalin.
Ketamine is widely used as an adjuvant analgesic in a variety of perioperative pain settings (Bell 2006). Urban 2008 investigated the effect of adding ketamine at induction of anaesthesia as a postoperative infusion.
Parecoxib sodium is an intravenous analgesic preparation called a pro‐drug of another medication, valdecoxib, which is a selective cyclo‐oxygenase‐2 inhibitor (Cheer 2001). The use of non‐opioid adjuvant analgesia is a recognised approach to reduce the need for opiate medication and thus the associated side effects, particularly for older adults (Aubrun 2007). One study compared a regimen of regular intravenous parecoxib to a dose of morphine followed by administration of saline as postoperative analgesia, with morphine doses available to either group based on their pain scores.
The use of a ‘single shot spinal’ combined with general anaesthesia and patient controlled analgesia (PCA) is increasingly used as an alternative to continuous epidural infusions for intra and postoperative analgesia. The premise is that intrathecal opioid, with or without local anaesthetic adequately replaces an epidural regarding its intended benefits of reduced intraoperative and immediate postoperative opioid requirements, but without prolonged motor block or hypotension that would impede immediate postoperative mobilisation. Beaussier 2006 tested using a 'single shot spinal’ with general anaesthesia compared to general anaesthesia alone; and Mouzopoulos 2009 tested a fascia iliac compartment block performed every 24 hours from admission to discharge compared to treatment with paracetamol and intramuscular pethidine for patients with a fractured neck of femur.
ii) Controlling/reducing the depth of anaesthesia:
Finer titration of depth of anaesthesia could reduce delirium. Bispectral index (BIS), a number derived from analysis of the EEG, is increasingly used to monitor depth of anaesthesia. A BIS value of 100 is equivalent to full awareness and a value of 0 represents no electrical activity.
Sieber 2010 investigated light compared to deep sedation. Light sedation was represented by a BIS value of 80 and a patient responsive to vocal commands; and deep sedation by a BIS value of 50 and a patient unresponsive to noxious stimuli (i.e. equivalent to the effect of a general anaesthetic). Chan 2013 compared BIS‐guided anaesthesia to routine general anaesthesia with propofol. In the BIS‐guided group, the propofol infusion was titrated to maintain a BIS value of 40 to 60, whereas in the routine group anaesthesia was titrated according to clinical judgement. Radtke 2013 compared BIS‐guided and BIS‐blinded groups undergoing induction and maintenance of general anaesthesia and postoperative analgesia for a range of surgical interventions. Gauge 2014 compared targeted BIS and cerebral oxygenation monitoring for patients undergoing coronary bypass grafting compared to no BIS and oxygenation monitoring.
iii) Changing the mode of general anaesthesia:
Two studies explored the effect of mode of general anaesthesia, one using propofol (Stoppe 2013) and the other xenon (Lurati 2012), compared to sevoflurane.
iv) Avoiding general anaesthesia:
Two studies compared regional anaesthesia with general anaesthesia (Berggren 1987; Papaioannou 2005).
v) Miscellaneous perioperative interventions:
The remaining three studies each tested a different perioperative intervention.
Intraoperative blood transfusion has been implicated as a risk factor postoperative delirium (Carson 2011; Robinson 2009), although there are likely to be other aspects of the individual's condition or care which also influence the risk of developing delirium (Edelstein 2004). Gruber‐Baldini 2013 tested the use of liberal versus restrictive blood transfusion thresholds.
Jia 2014 tested fast‐track surgery compared to usual care; this approach as a means of reducing delirium and postoperative cognitive dysfunction has been suggested previously (Krenk 2012). The fast‐track approach tested by Jia 2014 included alterations in the preoperative preparation, anaesthesia, pain control and postoperative management compared to traditional care. This included: bowel preparation with oral purgatives rather than enemas, shorter period of fasting, avoidance of nasogastric tube, epidural rather than general anaesthesia and earlier removal of urinary catheter and mobilisation on the first postoperative day.
Aizawa 2002 tested a postoperative delirium‐free protocol (DFP), which contained benzodiazepines and pethidine compared to usual care. They administered intramuscular diazepam at 8 pm with a continuous infusion of flunitrazepam to maintain sleep and pethidine for analgesia, given for eight hours for the first three nights after surgery.
Computerised clinical decision support (CCDS)
Computerised clinical decision support software (CCDS) has been reported as an effective tool in prompting healthcare practitioners to comply with established protocols and preventive measures (Dexter 2001). It has also been trialled for improving the care of patients with delirium superimposed on dementia (Fick 2011). One study in our review (Boustani 2012), investigated the use of CCDS in medical inpatients.
Care in geriatric medicine unit versus orthopaedic unit following hip fracture
Individuals admitted following a fracture are typically placed under the care of an orthopaedic surgeon, pending operative intervention. However, the complex nature of the predominantly older adult population who experience a hip fracture has led to the emergence of orthogeriatric services, where input is also received from geriatricians. Comprehensive geriatric assessment (CGA) is an evidence‐based "multidimensional interdisciplinary diagnostic process used to determine the medical, psychological and functional capabilities of a frail older person to develop a coordinated and integrated plan for treatment and long‐term follow‐up" associated with improved outcomes, particularly when delivered in a dedicated ward (Ellis 2011). Watne 2014 designed their trial around their local service reconfiguration where older adults were admitted to their specialist geriatric medicine unit and received CGA comparing this to the care received in the orthopaedic unit.
Outcomes
Primary outcome
The incidence of delirium was recorded using several validated instruments, used singly or in combination.
In 15 studies, the Confusion Assessment Method (CAM) (Inouye 1990) alone was used to determine delirium incidence (Abizanda 2011; Ashraf 2015; Beaussier 2006; Boustani 2012; Chan 2013; Gauge 2014; Jeffs 2013; Leung 2006; Lurati 2012; Marcantonio 2001; Martinez 2012; Munger 2008; Sieber 2010; Urban 2008; Whitlock 2015). However, Munger 2008 presented data for the mean CAM score, rather than using the CAM score to determine delirium presence as a dichotomous outcome. The CAM‐ICU (Ely 2001) was used in two studies (Pesonen 2011; Stoppe 2013), although Pesonen 2011 used it as a continuous measure. Diagnostic and Statistical Manual (DSM‐III and DSM‐IV)criteria alone were used in five studies (Aizawa 2002; Li 2013; Lundstrom 2007; Papaioannou 2005; Radtke 2013). Jia 2014 used the DRS‐R‐98 (Trzepacz 2001) to diagnose incident delirium. Berggren 1987 used the Modified Organic Brain Syndrome Scale (OBS) (Gustafson 1985); Fukata 2014 used the NEECHAM confusion scale (Neelon 1996); and Sampson 2007 used the Delirium Symptom Interview (DSI) (Albert 1992).
Ten studies used multiple instruments for assessing delirium, some of which included measures to assess delirium severity. The CAM (Inouye 1990) and Memorial Delirium Assessment Scale (MDAS) (Breitbart 1997) were used by Al‐Aama 2011; Gruber‐Baldini 2013; Marcantonio 2011 and Watne 2014. However, Marcantonio 2011 only reported aggregated data for repeated CAM assessments within the same participant, which could not, therefore, be included in analysis of the primary outcome. Bonaventura 2007 used the CAM and DRS‐R‐98. DSM III‐R or IV were used in addition to the CAM by Kalisvaart 2005; to which Hatta 2014; Larsen 2010 and Mouzopoulos 2009 added the DRS‐R‐98; while Liptzin 2005 added the DSI. de Jonghe 2014 also used the Delirium Observation Screening Scale (DOSS) (Schuurmans 2003) in addition to DSM‐IV. Hempenius 2013 used the DOSS which, if positive, resulted in an assessment using DSM‐IV criteria and the DRS‐R‐98.
Frequency of primary outcome assessment
Nineteen studies assessed for delirium on a daily basis (Abizanda 2011; de Jonghe 2014; Diaz 2001; Fukata 2014; Hatta 2014; Hempenius 2013; Jia 2014; Kalisvaart 2005; Larsen 2010; Leung 2006; Liptzin 2005; Marcantonio 2001; Martinez 2012; Mouzopoulos 2009; Munger 2008; Papaioannou 2005; Pesonen 2011; Stoppe 2013;Watne 2014 ). Marcantonio 2011 assessed for delirium daily until discharge and again at two, four and six weeks after recruitment.
Three studies assessed delirium several times a day: Radtke 2013 and Aizawa 2002 conducted delirium assessments twice daily and Sampson 2007 assessed three times daily.
Delirium assessments were performed on days one, two, four and seven following admission by Bonaventura 2007, and on the first and seventh postoperative day by Berggren 1987. Al‐Aama 2011 assessed participants every 24 to 48 hours and Jeffs 2013 assessed every 48 hours. Boustani 2012 assessed participants every weekday. Urban 2008 assessed for delirium on postoperative day (POD) one; Lurati 2012 assessed on POD one, two and seven; and Sieber 2010 assessed on POD two and daily thereafter.
At the end of one study (Lundstrom 2007), a retrospective case notes review was performed by a blinded independent investigator to identify delirium according to DSM‐IV criteria for each postoperative day until discharge. A single delirium assessment with the OBS was also performed between the third and fifth postoperative day in this study. In Gauge 2014, delirium assessment was performed on day three +/‐ one day. Whitlock 2015 assessed only on postoperative day three, and Li 2013 assessed on postoperative day three and at one, three and six months. Ashraf 2015 assessed for delirium four hours post‐procedure and on the following day.
In three studies the specific frequency of delirium assessment was unclear (Beaussier 2006; Chan 2013; Gruber‐Baldini 2013), but described as 'regularly', 'throughout study period' or 'multiple times'.
Secondary outcomes
Duration of delirium was reported by 12 studies (de Jonghe 2014; Fukata 2014; Jeffs 2013; Kalisvaart 2005; Larsen 2010; Liptzin 2005; Lundstrom 2007; Marcantonio 2001; Martinez 2012; Mouzopoulos 2009; Sieber 2010; Watne 2014). Severity of delirium was reported by 11 studies (Al‐Aama 2011; de Jonghe 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Kalisvaart 2005; Larsen 2010; Marcantonio 2011; Mouzopoulos 2009; Watne 2014).
Fourteen studies reported data on cognitive outcomes (Ashraf 2015; Beaussier 2006; Bonaventura 2007; Chan 2013; de Jonghe 2014; Diaz 2001; Larsen 2010; Li 2013; Munger 2008; Papaioannou 2005; Pesonen 2011; Radtke 2013; Sieber 2010; Watne 2014). Mode of cognitive assessment varied: Ashraf 2015; Bonaventura 2007, Diaz 2001, Larsen 2010; Munger 2008; Papaioannou 2005 and Sieber 2010 used the Mini Mental State Examination (MMSE) (Folstein 1975); Beaussier 2006 assessed the number of days for MMSE to return to preoperative level; Chan 2013; Li 2013; Radtke 2013 and Watne 2014 assessed for postoperative cognitive dysfunction; Pesonen 2011 used the CAM‐ICU score on day five; and de Jonghe 2014 used IQCODE (Jorm 1989) and MMSE (Folstein 1975) assessment at three months follow‐up.
Length of hospital admission was a commonly used outcome measure, with only 11 of the included studies not reporting on this outcome (Bonaventura 2007; Diaz 2001; Fukata 2014; Gauge 2014; Hatta 2014; Larsen 2010; Leung 2006; Lurati 2012; Marcantonio 2011; Mouzopoulos 2009; Urban 2008).
Other secondary outcomes which were reported less frequently included: activities of daily living (Abizanda 2011; Watne 2014); behavioural disturbance (Aizawa 2002); activities of daily living performance (Abizanda 2011; de Jonghe 2014; Watne 2014); psychotropic medication use (Al‐Aama 2011; de Jonghe 2014; Gruber‐Baldini 2013; Pesonen 2011); return to previous residence or independent living (Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001); and institutionalisation (Watne 2014).
Adverse Outcomes
Only 15 of the included studies reported data on mortality, either in hospital or at follow‐up at three or 12 months (Abizanda 2011; Al‐Aama 2011; Beaussier 2006; Boustani 2012; Chan 2013; de Jonghe 2014; Hatta 2014; Lundstrom 2007; Lurati 2012; Mouzopoulos 2009; Radtke 2013; Sieber 2010; Stoppe 2013; Watne 2014; Whitlock 2015).
Other adverse outcomes reported include: adverse events (Abizanda 2011; Hatta 2014; Kalisvaart 2005; Marcantonio 2011; Sampson 2007); physical morbidity (Berggren 1987; Boustani 2012; Gruber‐Baldini 2013; Larsen 2010; Watne 2014); psychological morbidity (Berggren 1987; Chan 2013; Hempenius 2013; Lundstrom 2007); postoperative complications (Chan 2013; Hempenius 2013; Jia 2014; Papaioannou 2005; Sieber 2010; Whitlock 2015); falls (Boustani 2012; Hempenius 2013; Lundstrom 2007; Martinez 2012; Watne 2014); and pressure ulcers (Berggren 1987; Boustani 2012; Lundstrom 2007; Watne 2014).
Exclusion of prevalent delirium at baseline
Failure to exclude delirium at enrolment to the study was a common problem among included studies. Only 10 studies clearly excluded or accounted for prevalent cases of delirium at baseline (Abizanda 2011; Ashraf 2015; Boustani 2012; de Jonghe 2014; Hatta 2014; Gruber‐Baldini 2013; Jeffs 2013; Kalisvaart 2005; Martinez 2012; Sieber 2010).
Funding sources and declarations of interest
Most of the studies (24 out of 39) were funded via academic or governmental research institutions or grant funding schemes. Four studies were solely industry funded (Boustani 2012; Liptzin 2005; Munger 2008; Sampson 2007) and two received joint academic and industry funding (Lurati 2012; Radtke 2013). In nine studies the funding source was not reported (Aizawa 2002; Ashraf 2015; Bonaventura 2007; Diaz 2001; Gauge 2014; Jia 2014; Martinez 2012; Mouzopoulos 2009; Sieber 2010).
Eight studies reported there were potential interests to declare related to their publication (Boustani 2012; Gruber‐Baldini 2013; Hatta 2014; Larsen 2010; Leung 2006; Liptzin 2005; Lurati 2012; Stoppe 2013), which are listed in the Characteristics of included studies tables. Fourteen studies did not report on a declaration of interest (Aizawa 2002; Ashraf 2015; Beaussier 2006; Berggren 1987; Bonaventura 2007; Gauge 2014; Li 2013; Lundstrom 2007; Marcantonio 2001; Munger 2008; Papaioannou 2005; Sampson 2007; Sieber 2010;Urban 2008).
Excluded studies
We excluded 69 studies. Reasons for exclusion are given in Characteristics of excluded studies. Details of 27 studies identified as ongoing are given in Characteristics of ongoing studies.
Risk of bias in included studies
'Risk of bias' assessments are presented for each study in the 'Characteristics of included studies' table and are summarised in the text below and graphically in Figure 2. Only one study (Whitlock 2015) was assessed as at low risk of bias across all domains.
2.
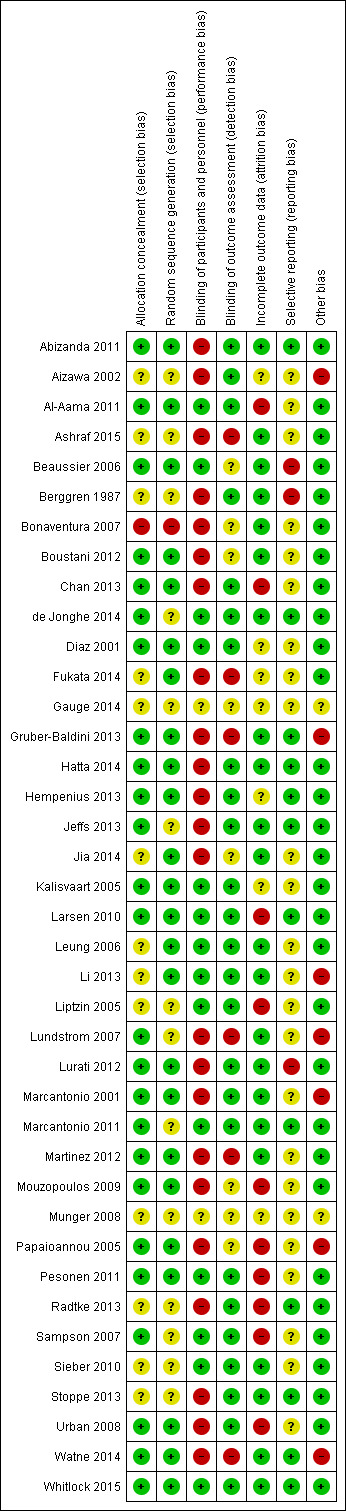
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one study (Bonaventura 2007) was assessed as high risk for selection bias considering both allocation concealment and random sequence generation. This was as a consequence of using the day of admission as the basis for their randomisation, which cannot be concealed. Nine studies (Aizawa 2002; Ashraf 2015; Berggren 1987; Gauge 2014; Liptzin 2005; Munger 2008; Radtke 2013; Sieber 2010; Stoppe 2013) were considered as unclear risk for selection bias on both criteria. This assessment was primarily made on the grounds of a lack of detail in the published report around the methods of generating the sequence and allocating participants to groups.
Blinding
Twenty‐three of the included studies (Abizanda 2011; Aizawa 2002; Ashraf 2015; Berggren 1987; Bonaventura 2007; Boustani 2012; Chan 2013; Fukata 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Jia 2014; Lundstrom 2007; Lurati 2012; Marcantonio 2001; Martinez 2012; Mouzopoulos 2009; Papaioannou 2005; Radtke 2013; Stoppe 2013; Urban 2008; Watne 2014) were assessed as high risk for performance bias as participants and personnel were not blinded to their allocation, often due to the nature of the intervention precluding such concealment. However, only six studies (Ashraf 2015; Fukata 2014; Gruber‐Baldini 2013; Lundstrom 2007; Martinez 2012; Watne 2014) were assessed at high risk of both performance and detection bias due to the assessors being unblinded in addition to participants and personnel. A further eight studies (Beaussier 2006; Bonaventura 2007; Boustani 2012; Gauge 2014; Jia 2014; Mouzopoulos 2009; Munger 2008; Papaioannou 2005) were assessed as unclear risk for detection bias due to a lack of reporting.
Incomplete outcome data
Ten studies were assessed as high risk for attrition bias (Al‐Aama 2011; Chan 2013; Larsen 2010; Liptzin 2005; Mouzopoulos 2009; Papaioannou 2005; Pesonen 2011; Radtke 2013; Sampson 2007; Urban 2008). This was due to incomplete reporting of losses or concerns about reasons for exclusion of participants. In particular, there were concerns about exclusions which may influence ascertainment of the primary outcome (delirium incidence) e.g. participants being too unwell to be assessed or developing postoperative complications. A further seven studies were considered at unclear risk for attrition bias (Aizawa 2002; Diaz 2001; Fukata 2014; Gauge 2014; Hempenius 2013; Kalisvaart 2005; Munger 2008. In these cases it was not possible to assess the potential bias associated with loss of participants due to a lack of detail in study reports.
Selective reporting
Three studies were assessed as high risk of reporting bias (Beaussier 2006; Berggren 1987; Lurati 2012). In all cases this was due to the reporting of outcomes not stated in the protocol or the methods for the study. Twelve studies were considered at low risk of reporting bias (Abizanda 2011; de Jonghe 2014; Gruber‐Baldini 2013; Hatta 2014; Hempenius 2013; Jeffs 2013; Larsen 2010; Marcantonio 2011; Radtke 2013; Stoppe 2013; Watne 2014; Whitlock 2015), with evidence of published protocols, formal trial registration or clear statement in relation to reporting contained in the published text. The remainder were assessed as unclear risk.
Other potential sources of bias
Seven studies were assessed as high risk of bias in this category (Aizawa 2002; Gruber‐Baldini 2013; Li 2013; Lundstrom 2007; Marcantonio 2001; Papaioannou 2005; Watne 2014).
In Aizawa 2002 no account was taken of how delirium assessment may have been affected by the sedating effects of the delirium‐free protocol. Similarly in Papaioannou 2005, there were concerns about unbalanced use of neuraxial analgesia between groups, affecting delirium assessment. Li 2013 administered supplementary morphine to both groups depending on pain scores, but use of this is significantly unbalanced and this is not accounted‐for in the interpretation of delirium findings. In Watne 2014, there are concerns about the integrity of the intervention delivered as the trial was conducted pragmatically and when beds were not available in the specialist unit, patients were cared‐for in the corridor, but are counted in the intervention group.
The proportion of included participants with dementia was imbalanced in three studies (Gruber‐Baldini 2013; Lundstrom 2007; Marcantonio 2001). In all cases there was a lower proportion of individuals with dementia in the intervention arm than the control arm. This has the potential to affect rates of incident delirium as delirium is known to be more common in individuals with dementia (Fong 2015).
Publication of two studies as abstracts (Gauge 2014; Munger 2008) gave insufficient information to allow for other sources of bias to be assessed, resulting in an assessment of unclear risk.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
1. Multi‐component interventions versus usual care
Seven studies investigated the effectiveness of multi‐component interventions for the prevention of delirium (Abizanda 2011; Bonaventura 2007; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012). A summary of findings for key outcomes is presented in Table 1.
a. Primary outcome
Available case analysis was performed on 1950 of 2018 randomised participants, using data from all seven studies. Pooled analysis showed evidence of a reduction in the incidence of delirium for multi‐component interventions compared to usual care (risk ratio (RR) 0.69, 95% confidence interval (CI) 0.59 to 0.81, I2 = 0%; 1950 participants. We assessed this as moderate‐quality evidence (downgraded due to risk of bias) (Analysis 1.1; Figure 3).
1.1. Analysis.
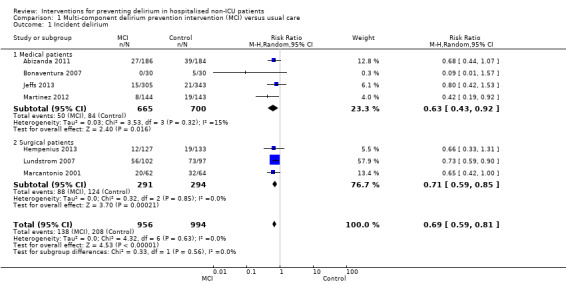
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 1 Incident delirium.
3.
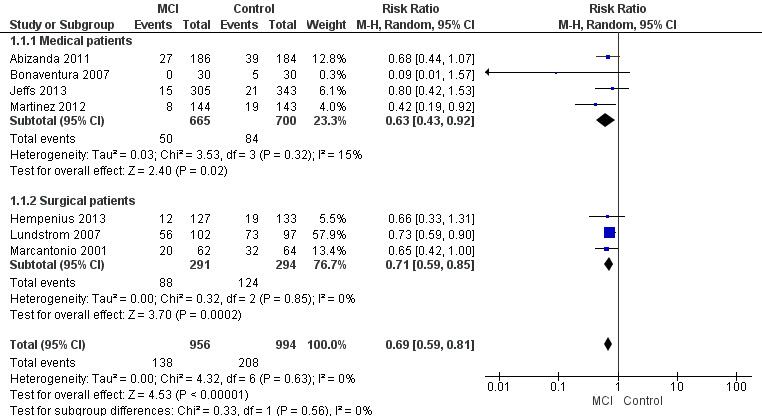
Forest plot of comparison: 1 Multi‐component delirium prevention intervention (MCI) versus usual care, outcome: 1.1 Incident delirium.
b. Secondary outcomes
We pooled data on the duration of delirium from four trials (Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012). The mean difference between groups was ‐1.16 days (shorter in the intervention group) but there was uncertainty about the size and direction of the effect (mean difference (MD) ‐1.16, 95% CI ‐2.96 to 0.64, I2 = 58%; 244 participants; assessed as very low‐quality evidence due to imprecision, risk of bias and inconsistency) (Analysis 1.3).
1.3. Analysis.
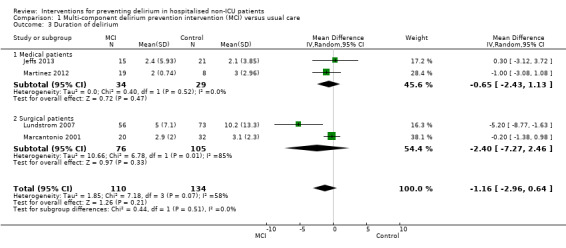
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 3 Duration of delirium.
Delirium severity was reported as an outcome in only two multi‐component intervention trials, each of which used different measures of severity (Hempenius 2013; Jeffs 2013). Compared with usual care the standardised mean difference (SMD) in delirium severity was ‐1.04 (lower with multi‐component interventions) (SMD ‐1.04, 95% CI ‐1.65 to ‐0.43, I2 = 25%; 67 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 1.4).
1.4. Analysis.
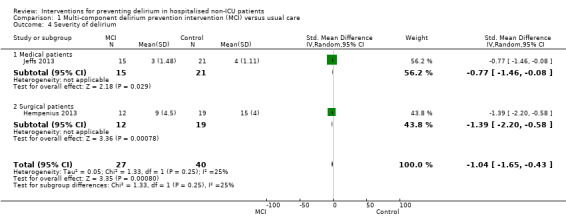
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 4 Severity of delirium.
We pooled data from six studies, which reported length of hospital admission (Abizanda 2011; Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001; Martinez 2012). The mean length of hospital admission was 0.01 days longer in the intervention compared to the usual care group (MD 0.01, 95% CI ‐0.48 to 0.51, I2 = 13%; 1920 participants; moderate‐quality evidence due to risk of bias) (Analysis 1.5).
1.5. Analysis.
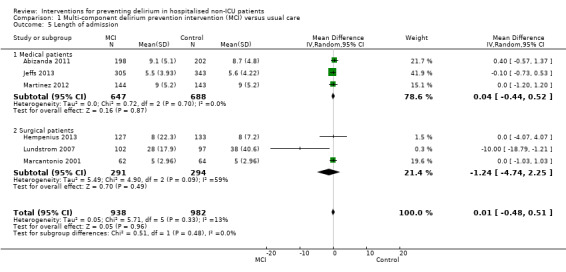
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 5 Length of admission.
One study assessed cognition (Bonaventura 2007); there was a clinically important difference in the mean MMSE score favouring those receiving multi‐component interventions compared to usual care (MD 9.10, 95% CI 7.20 to 11.00; 60 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 1.6).
1.6. Analysis.
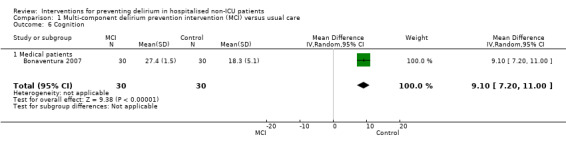
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 6 Cognition.
Abizanda 2011 reported on the number of participants whose Barthel Index score (Mahoney 1965) improved by 10 points during admission, comparing this between the groups. There was no evidence of effect of multi‐component interventions on improvements in activities of daily living compared to usual care (RR 1.15, 95% CI 0.91 to 1.47; 341 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 1.7).
1.7. Analysis.
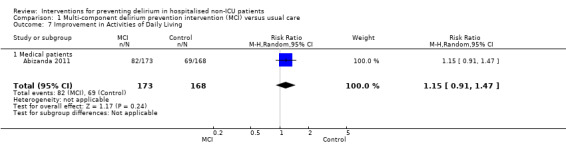
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 7 Improvement in Activities of Daily Living.
Four studies (Hempenius 2013; Jeffs 2013; Lundstrom 2007; Marcantonio 2001) reported on return to independent living. Again, there was no evidence of effect of multi‐component interventions compared to usual care (RR 0.95, 95% CI 0.85 to 1.06, I2 = 30%; 1116 participants; moderate‐quality evidence, downgraded due to risk of bias) (Analysis 1.8).
1.8. Analysis.
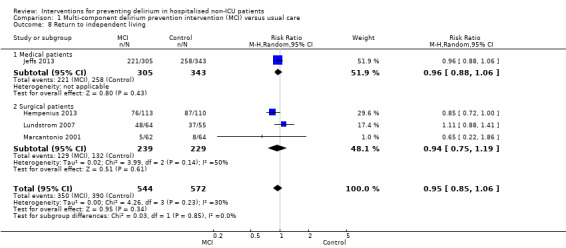
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 8 Return to independent living.
Lundstrom 2007 assessed depression with the Geriatric Depression Scale‐15 (GDS‐15) (Sheikh 1986), but found no evidence of any important effect of the intervention on this outcome (MD 0.70, 95% CI ‐0.44 to 1.84; 149 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 1.9).
1.9. Analysis.
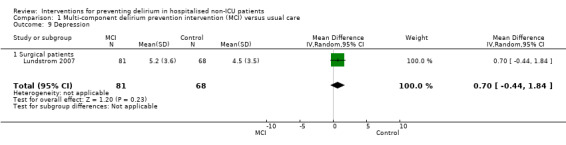
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 9 Depression.
One study reported no withdrawals from 126 participants (Marcantonio 2001) (Analysis 1.10).
1.10. Analysis.
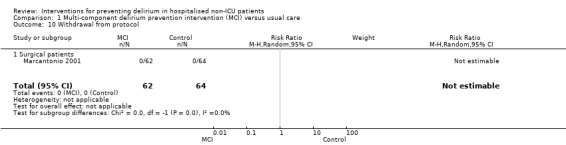
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 10 Withdrawal from protocol.
c. Adverse outcomes
Data on falls were only available from three studies (Hempenius 2013; Lundstrom 2007; Martinez 2012), there was no evidence of effect from multi‐component interventions compared to usual care (RR 0.57, 95% CI 0.16 to 2.01, I2 = 50%; 746 participants; very low‐quality evidence, downgraded due to risk of bias, serious imprecision and inconsistency) (Analysis 1.11).
1.11. Analysis.
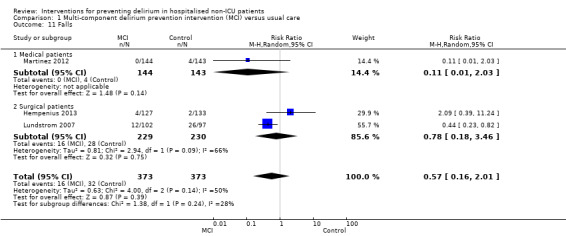
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 11 Falls.
Rates of pressure ulcers were only reported in two studies (Hempenius 2013; Lundstrom 2007) where there was evidence of a reduced risk of pressure ulcer formation in those receiving multi‐component interventions compared to usual care (RR 0.48, 95% CI 0.26 to 0.89, I2 = 0%; 457 participants; low‐quality evidence downgraded, due to risk of bias and imprecision) (Analysis 1.12).
1.12. Analysis.
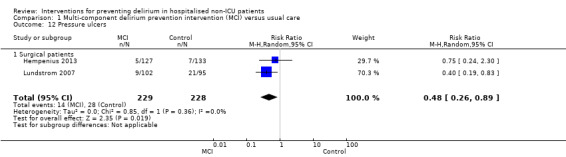
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 12 Pressure ulcers.
Inpatient mortality was reported in three studies (Abizanda 2011; Hempenius 2013; Lundstrom 2007), with no evidence of effect of multi‐component interventions on inpatient mortality (RR 0.90, 95% CI 0.56 to 1.43, I2 = 57%; 859 participants; very low‐quality evidence, downgraded due to risk of bias, imprecision and inconsistency) (Analysis 1.13).
1.13. Analysis.
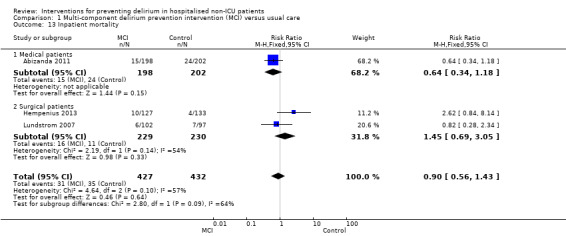
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 13 Inpatient mortality.
Lundstrom 2007 also reported on 12‐month mortality and found no evidence of effect of multi‐component interventions (RR 0.85, 95% CI 0.46 to 1.56; 199 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 1.14).
1.14. Analysis.
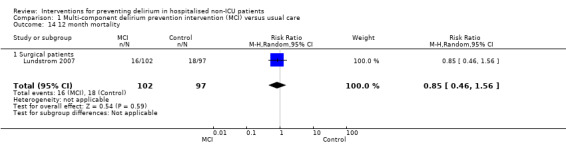
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 14 12 month mortality.
Hempenius 2013 reported on postoperative complications and there was no evidence of effect of multi‐component interventions on cardiovascular adverse events (RR 1.13, 95% CI 0.78 to 1.65; 260 participants; moderate‐quality evidence due to imprecision) or urinary tract infections (RR 1.20, 95% CI 0.45 to 3.20; 260 participants; low‐quality evidence due to serious imprecision) (Analysis 1.15; Analysis 1.16). Hempenius 2013 also reported on psychological morbidity, reporting SF‐36 scores for mental health (Ware 1992), dichotomized to having worsened versus improvement/stayed the same and there was no evidence of effect found (RR 0.88, 95% CI 0.64 to 1.20; 246 participants; moderate‐quality evidence due to imprecision) (Analysis 1.17).
1.15. Analysis.
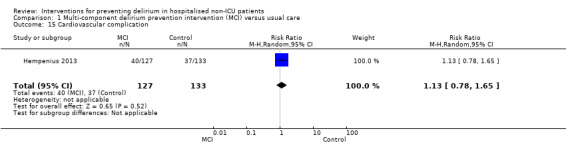
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 15 Cardiovascular complication.
1.16. Analysis.
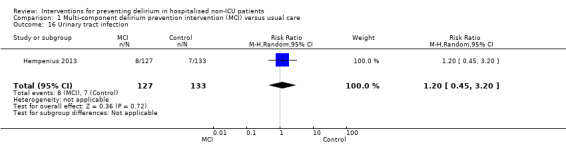
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 16 Urinary tract infection.
1.17. Analysis.
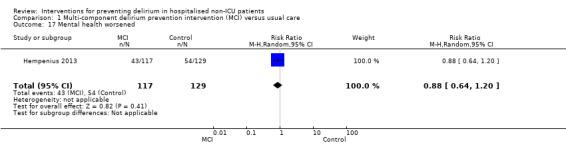
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 17 Mental health worsened.
Subgroup analysis by setting
The pre‐planned subgroup analysis assessed multi‐component delirium prevention trials in four medical (Abizanda 2011; Bonaventura 2007; Jeffs 2013; Martinez 2012) and three surgical (Hempenius 2013; Lundstrom 2007; Marcantonio 2001) settings. There were similar effect sizes in medical (RR 0.63, 95% CI 0.43 to 0.92; 1365 participants) and surgical (RR 0.71, 95% CI 0.59 to 0.85; 585 participants) settings in favour of the intervention reducing incident delirium (moderate‐quality evidence due to risk of bias for both) (Analysis 1.1; Figure 3).
Subgroup analysis by cognitive impairment
Only one trial (Marcantonio 2001) reported incident delirium in patients with pre‐existing dementia. In the intervention group 37% of participants were known to have dementia, compared to 51% of those in the control group. Delirium incidence was lower in patients receiving a multi‐component intervention in this subgroup also. However, the results are too imprecise to allow a conclusion to be drawn (RR 0.90, 95% CI 0.59 to 1.36; 50 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 1.2).
1.2. Analysis.
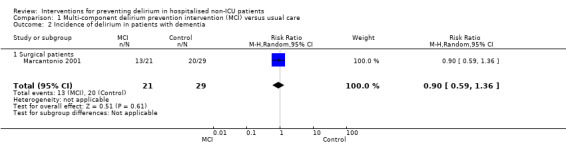
Comparison 1 Multi‐component delirium prevention intervention (MCI) versus usual care, Outcome 2 Incidence of delirium in patients with dementia.
2. Cholinesterase inhibitors versus placebo
Four studies investigated the effect of the cholinesterase inhibitor donepezil in the prevention of delirium (Liptzin 2005; Marcantonio 2011; Munger 2008; Sampson 2007). A 'Summary of findings' table for key outcomes is presented in Table 2.
a. Primary outcome
Data from only two of these four studies (Liptzin 2005; Sampson 2007) could be used to estimate the primary outcome, delirium incidence, as Marcantonio 2011 reported repeated CAM measures within the same individuals, and Munger 2008 reported mean CAM scores only. There was no evidence of effect of cholinesterase inhibitors on incident delirium (RR 0.68, 95% CI 0.17 to 2.62, I2 = 60%; 113 participants; very low‐quality evidence due to risk of bias, serious imprecision and inconsistency) (Analysis 2.1; Figure 4).
2.1. Analysis.
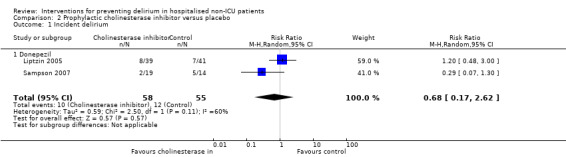
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 1 Incident delirium.
4.
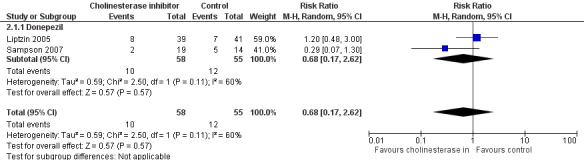
Forest plot of comparison: 2 Prophylactic cholinesterase inhibitor versus placebo, outcome: 2.1 Incident delirium.
b. Secondary outcomes
The effect of cholinesterase inhibitors on the duration of delirium episodes was assessed by Liptzin 2005, but no summary estimate was calculable due to the limited data available (Analysis 2.2).
2.2. Analysis.
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 2 Duration of delirium.
The effect of cholinesterase inhibitors on the severity of delirium episodes was assessed by Marcantonio 2011 who reported no evidence of effect (MD ‐0.30, 95% CI ‐4.17 to 3.57; 16 participants; low‐quality evidence, downgraded two levels due to serious imprecision) (Analysis 2.3).
2.3. Analysis.
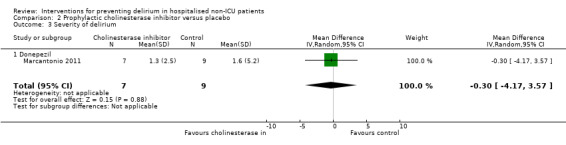
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 3 Severity of delirium.
Pooled data from three studies reporting length of hospital admission (Liptzin 2005; Munger 2008; Sampson 2007) showed a mean difference of ‐0.34 days with cholinesterase inhibitor treatment compared to placebo (MD ‐0.34, 95% CI ‐1.54 to 0.86, I2 = 45%; 128 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 2.4).
2.4. Analysis.
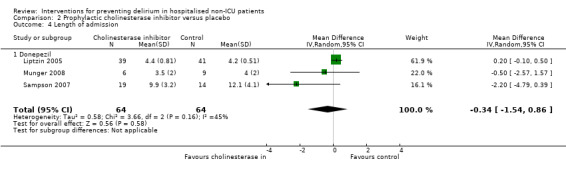
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 4 Length of admission.
One study examining the effect of cholinesterase inhibitor on cognition (Munger 2008) found no evidence of effect on MMSE (Folstein 1975) scores (MD ‐1.40, 95% CI ‐4.45 to 1.65; 15 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 2.5).
2.5. Analysis.
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 5 Cognition.
Two studies reported withdrawals from protocol (Liptzin 2005; Marcantonio 2011), finding no evidence of effect with cholinesterase inhibitor use compared to placebo (RR 0.95, 95% CI 0.49 to 1.87, I2 = 0%; 96 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 2.6).
2.6. Analysis.
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 6 Withdrawal from protocol.
c. Adverse outcomes
Adverse events were reported in two studies in different formats. Sampson 2007 reported the mean adverse events in each group and found no evidence of difference in occurrence between groups (MD 0.13, 95% CI ‐0.26 to 0.52; 33 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 2.7). Marcantonio 2011 reported adverse events as a binary outcome and found a higher rate of adverse events in the cholinesterase inhibitor group compared to placebo (RR 6.25, 95% CI 0.35 to 112.52; 16 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 2.8).
2.7. Analysis.
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 7 Adverse events (continuous).
2.8. Analysis.
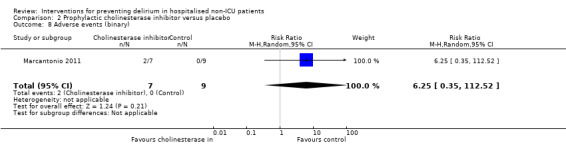
Comparison 2 Prophylactic cholinesterase inhibitor versus placebo, Outcome 8 Adverse events (binary).
3. Antipsychotics versus placebo
Three studies investigated the effect of antipsychotic medication in the prevention of delirium (Fukata 2014; Kalisvaart 2005; Larsen 2010). A 'Summary of findings' table for key outcomes is presented in Table 3.
a. Primary outcome
Two large studies evaluated antipsychotic medication versus placebo in elderly orthopaedic patients and one smaller study assessed those undergoing abdominal or orthopaedic surgery. Kalisvaart 2005 assessed oral haloperidol, a first generation (typical) antipsychotic preparation in 430 participants; data were available for 395 participants for available case analysis. Fukata 2014 administered prophylactic intravenous haloperidol to 121 patients from postoperative days one to three. Larsen 2010 tested oral olanzapine, a second generation (atypical) antipsychotic in 495 participants, with data for available case analysis for 400.
Pooled analysis of all three studies was inconclusive regarding an effect of antipsychotic treatment on incident delirium, but there was moderate heterogeneity between the studies (RR 0.73, 95% CI 0.33 to 1.59, I2= 90%; 916 participants; very low‐quality evidence due to risk of bias, imprecision and inconsistency) (Analysis 3.1; Figure 5).
3.1. Analysis.
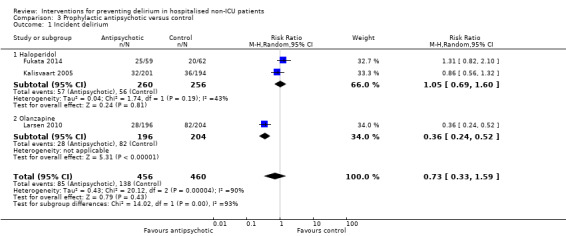
Comparison 3 Prophylactic antipsychotic versus control, Outcome 1 Incident delirium.
5.
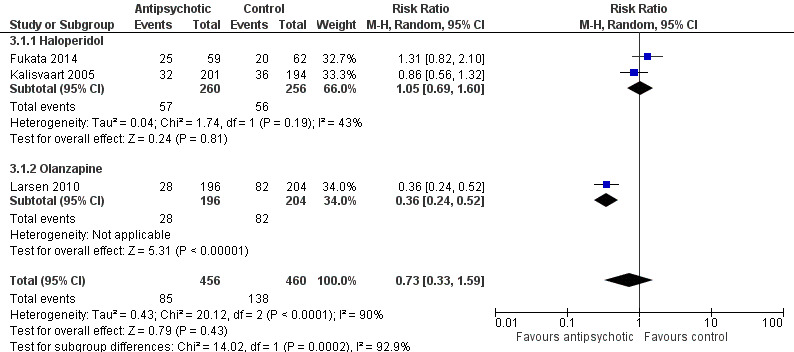
Figure 5Forest plot of comparison: 3 Prophylactic antipsychotic versus control, outcome: 3.1 Incidence of delirium.
Subgroup analysis
The pre‐planned subgroup analysis assessed the effect of typical and atypical antipsychotics separately on delirium incidence. There was no evidence of effect of haloperidol on delirium incidence (RR 1.05, 95% CI 0.69 to 1.60, I2= 43%; two studies; 516 participants; low‐quality evidence downgraded due to risk of bias and inconsistency). However, the risk of incident delirium was lower with olanzapine than with placebo (RR 0.36, 95% CI 0.24 to 0.52; one study; 400 participants; moderate‐quality evidence due to risk of bias) (Figure 5).
b. Secondary outcomes
All three studies reported duration of delirium episodes. However, Fukata 2014 present mean duration data without a standard deviation so they could not be included in the quantitative analysis. Between the other two studies there was serious heterogeneity in duration findings. Haloperidol showed a large effect size, with a shorter duration of delirium in the intervention group compared to control (MD ‐6.40 days, 95% CI ‐9.38 to ‐3.42; one study; 68 participants). Olanzapine showed a longer duration for the intervention group (MD 0.60 days, 95% CI 0.10 to 1.10; one study; 110 participants). The pooled analysis of both showed a mean difference in delirium duration between intervention and control groups of ‐2.74 days (95% CI ‐9.59 to 4.11, I2 = 95%; 178 participants; very low‐quality evidence due to serious imprecision and inconsistency) (Analysis 3.2).
3.2. Analysis.
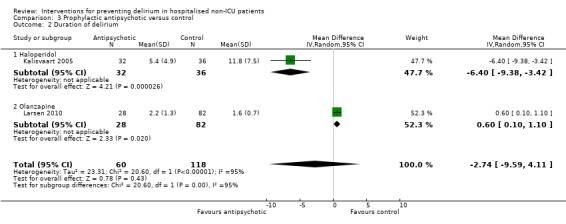
Comparison 3 Prophylactic antipsychotic versus control, Outcome 2 Duration of delirium.
Both Kalisvaart 2005 and Larsen 2010 reported severity of delirium episodes, although there was serious heterogeneity between studies as before. Haloperidol showed a large effect size, with a reduction in severity of delirium in the intervention group compared to control (MD ‐4.00, 95% CI ‐5.86 to ‐2.14; 68 participants). Olanzapine showed an increased severity for the intervention group (MD 1.90, 95% CI 0.41 to 3.39; 110 participants). Pooled analysis showed no evidence of effect in delirium severity with antipsychotic treatment (MD ‐1.02, 95% CI ‐6.80 to 4.76, I2 = 96%; 178 participants; very low‐quality evidence due to serious imprecision and inconsistency) (Analysis 3.3).
3.3. Analysis.
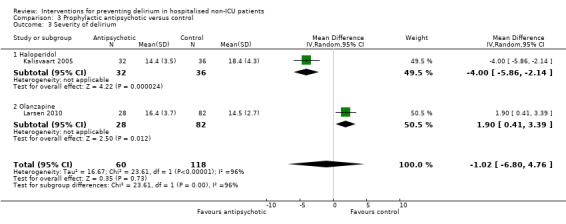
Comparison 3 Prophylactic antipsychotic versus control, Outcome 3 Severity of delirium.
Length of admission was only reported in one study (Kalisvaart 2005), which showed a mean difference of ‐5.50 days for haloperidol compared to placebo (95% CI ‐12.17 to 1.17; 68 participants; low‐quality evidence, downgraded two levels due to serious imprecision in results (Analysis 3.4).
3.4. Analysis.
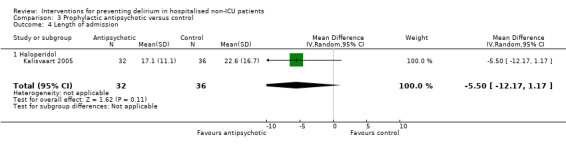
Comparison 3 Prophylactic antipsychotic versus control, Outcome 4 Length of admission.
Cognitive testing, using MMSE (Folstein 1975) was performed on the first day of the delirium episode by Larsen 2010. Those who received olanzapine had lower MMSE scores (poorer cognitive function) than those treated with placebo (MD ‐4.90, 95% CI ‐7.42 to ‐2.38; 110 participants; low‐quality evidence due to serious imprecision) (Analysis 3.5).
3.5. Analysis.
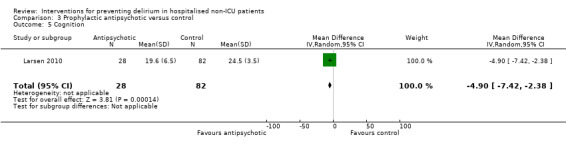
Comparison 3 Prophylactic antipsychotic versus control, Outcome 5 Cognition.
There was no evidence of effect of treatment allocation on withdrawal from protocol in pooled analysis including Kalisvaart 2005 & Larsen 2010 (RR 0.92, 95% CI 0.68 to 1.24, I2 = 0%; 925 participants; moderate‐quality evidence due to risk of bias) (Analysis 3.6).
3.6. Analysis.
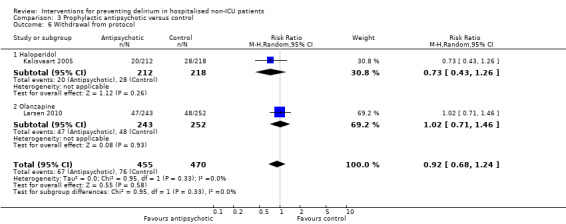
Comparison 3 Prophylactic antipsychotic versus control, Outcome 6 Withdrawal from protocol.
c. Adverse outcomes
Adverse events were reported by Kalisvaart 2005; there was no evidence of effect of haloperidol on adverse events (RR 0.39, 95% CI 0.10 to 1.43; 430 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 3.7). Larsen 2010 report data on the occurrence of pneumonia (RR 7.28, 95% CI 0.38 to 140.11; 400 participants), urinary tract infection (RR 0.26, 95% CI 0.03 to 2.31; 400 participants) and congestive heart failure (RR 1.04, 95% CI 0.07 to 16.52; 400 participants) and there was no evidence of effect of olanzapine on the risk of developing these adverse events (Very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 3.8; Analysis 3.9; Analysis 3.10).
3.7. Analysis.
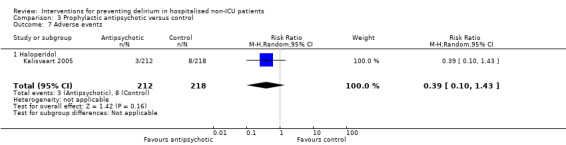
Comparison 3 Prophylactic antipsychotic versus control, Outcome 7 Adverse events.
3.8. Analysis.
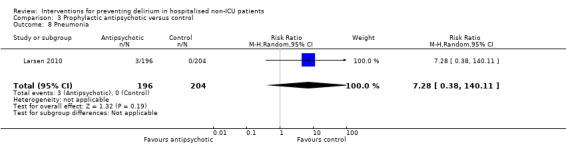
Comparison 3 Prophylactic antipsychotic versus control, Outcome 8 Pneumonia.
3.9. Analysis.
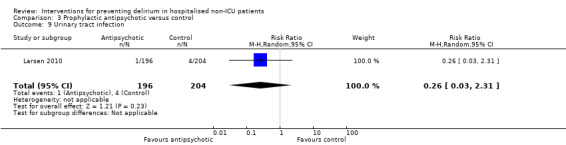
Comparison 3 Prophylactic antipsychotic versus control, Outcome 9 Urinary tract infection.
3.10. Analysis.
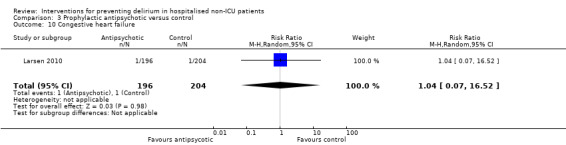
Comparison 3 Prophylactic antipsychotic versus control, Outcome 10 Congestive heart failure.
4. Melatonin or melatonin agonists versus placebo
Three studies investigated the effect of melatonin or melatonin agonists in the prevention of delirium (Al‐Aama 2011; de Jonghe 2014; Hatta 2014). Outcome data relevant to this review were obtained from the authors of Hatta 2014 for 43 participants who were cared for in acute medical wards rather than ICU. A 'Summary of findings' table for key outcomes is presented in Table 4.
a. Primary outcome
All three studies reported the primary outcome, delirium incidence. The pooled analysis showed no evidence of effect of melatonin on incident delirium (RR 0.41, 95% CI 0.09 to 1.89 I2 = 78%; 529 participants; very low‐quality evidence due to risk of bias, imprecision and inconsistency) (Analysis 4.1; Figure 6).
4.1. Analysis.
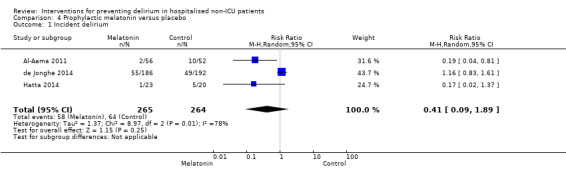
Comparison 4 Prophylactic melatonin versus placebo, Outcome 1 Incident delirium.
6.
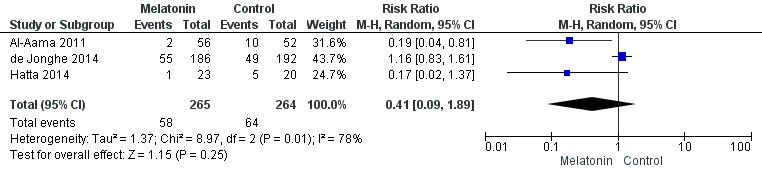
Forest plot of comparison: 4 Prophylactic melatonin versus placebo, outcome: 4.1 Incident delirium.
b. Secondary outcomes
Duration of delirium was only reported in one study (de Jonghe 2014). There was no evidence of a difference between melatonin and placebo groups in delirium duration (MD 0.00, 95% CI ‐0.57 to 0.57; 104 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 4.2) .
4.2. Analysis.
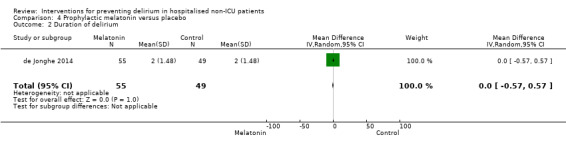
Comparison 4 Prophylactic melatonin versus placebo, Outcome 2 Duration of delirium.
Severity of delirium was reported in all three studies but each in a different way. de Jonghe 2014 reported delirium severity as a binary outcome, severe or not severe (defined as >= 3 mg haloperidol administered during delirium episode). There was no evidence of a difference between melatonin and placebo groups in the occurrence of severe delirium (RR 0.86, 95% CI 0.58 to 1.27; 104 participants; moderate‐quality evidence due to imprecision) (Analysis 4.3) Al‐Aama 2011 reported delirium severity using MDAS (Breitbart 1997), however their results include those with prevalent as well as incident delirium and have not been included in the quantitative summary. Hatta 2014 reported delirium severity using the DRS‐R‐98 (Trzepacz 2001). There appeared to be a reduction in delirium severity in those receiving the melatonin agonist (RR ‐4.10, 95% CI ‐19.47 to 11.27; six participants), but the evidence was of low quality, downgraded two levels due to serious imprecision (Analysis 4.4).
4.3. Analysis.
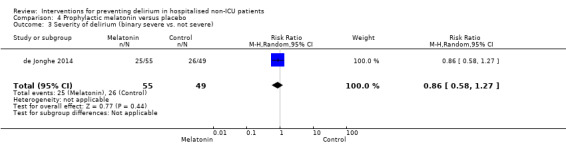
Comparison 4 Prophylactic melatonin versus placebo, Outcome 3 Severity of delirium (binary severe vs. not severe).
4.4. Analysis.
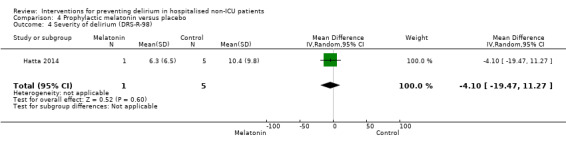
Comparison 4 Prophylactic melatonin versus placebo, Outcome 4 Severity of delirium (DRS‐R‐98).
Length of admission was reported in two studies, and there was no evidence of difference in admission duration between intervention and control groups (MD 0.09 days, 95% CI ‐1.20 to 1.39 days, I2 = 0%; 500 participants; moderate‐quality evidence due to imprecision) (Analysis 4.5).
4.5. Analysis.
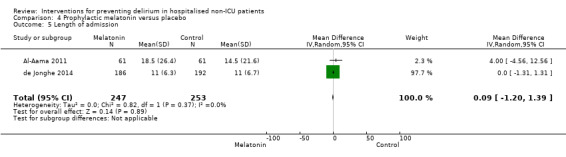
Comparison 4 Prophylactic melatonin versus placebo, Outcome 5 Length of admission.
de Jonghe 2014 assessed cognitive impairment using the Charlson index (Charlson 1994), IQCODE (Jorm 1989) and MMSE (Folstein 1975) at three‐month follow‐up. It appeared that those in the melatonin group had a lower risk of cognitive impairment, compared to those receiving placebo (RR 0.86, 95% CI 0.70 to 1.04; 378 participants). However, this evidence was of moderate quality due to imprecision of the result from a single study (Analysis 4.6).
4.6. Analysis.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 6 Cognitive impairment.
There was no evidence of difference in performance of activities of daily living, using the Katz index (Katz 1970), in those receiving melatonin found by de Jonghe 2014 (MD 0.00, 95%CI ‐1.20 to 1.20; 369 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 4.7).
4.7. Analysis.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 7 Activities of daily living.
Al‐Aama 2011 examined rates of psychotropic medication use, and reported a high proportion of participants in both melatonin and control groups were prescribed these drugs (33/61 in melatonin group and 38/61 in the placebo group). There was no evidence of a difference in use, however, between groups (RR 0.87, 95% CI 0.64 to 1.18; 122 participants; moderate‐quality evidence due to imprecision) (Analysis 4.8). de Jonghe 2014 reported use of anti‐psychotic medications and benzodiazepines on a cumulative basis, looking at mean consumption of each drug class. There was evidence of reduced use of both anti‐psychotic medications (MD ‐1.00 mg, 95% CI ‐1.79 to ‐0.21 mg; 378 participants; moderate‐quality evidence downgraded as from a single study) and benzodiazepines (MD ‐11.60 mg, 95% CI ‐24.34 to 1.14 mg; 378 participants). However, in the case of benzodiazepine use the evidence was of low quality, downgraded due to serious imprecision (Analysis 4.9; Analysis 4.10).
4.8. Analysis.
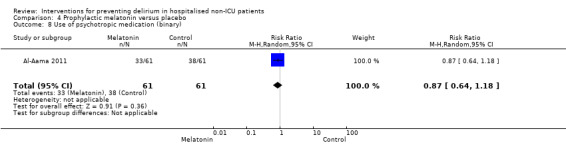
Comparison 4 Prophylactic melatonin versus placebo, Outcome 8 Use of psychotropic medication (binary).
4.9. Analysis.
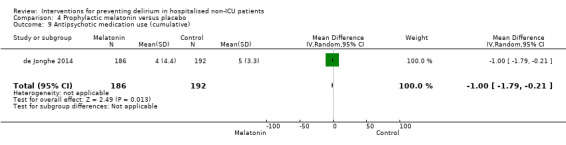
Comparison 4 Prophylactic melatonin versus placebo, Outcome 9 Antipsychotic medication use (cumulative).
4.10. Analysis.
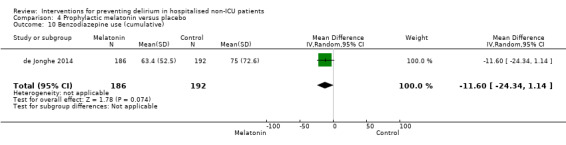
Comparison 4 Prophylactic melatonin versus placebo, Outcome 10 Benzodiazepine use (cumulative).
Al‐Aama 2011 and Hatta 2014 also compared withdrawals from the study and found no evidence of a difference between melatonin and placebo groups (RR 1.00, 95% CI 0.15 to 6.87; 165 participants; low‐quality evidence, due to serious imprecision) (Analysis 4.11).
4.11. Analysis.
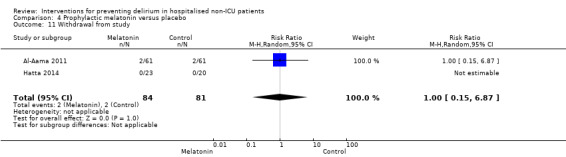
Comparison 4 Prophylactic melatonin versus placebo, Outcome 11 Withdrawal from study.
c. Adverse events
In‐hospital mortality was reported in all three studies and mortality at three months only by de Jonghe 2014. There was no evidence of effect on mortality rates with melatonin compared to placebo at either time‐period: In‐hospital mortality (RR 0.84, 95% CI 0.37 to 1.88, I2 = 0%; 543 participants; low‐quality evidence due to imprecision and low event rate) (Analysis 4.12) and three‐month mortality (RR 0.98, 95% CI 0.67 to 1.45; 378 participants; moderate‐quality evidence, downgraded due to imprecision) (Analysis 4.13).
4.12. Analysis.
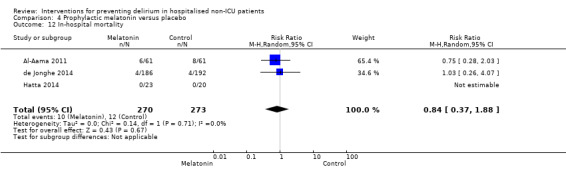
Comparison 4 Prophylactic melatonin versus placebo, Outcome 12 In‐hospital mortality.
4.13. Analysis.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 13 Mortality by 3 months.
Hatta 2014 reported adverse events and there were none reported in either group.
5. Citicoline versus placebo
One study tested the use of citicoline (Diaz 2001).
a. Primary outcome
The incidence of delirium was lower in the group treated with citicoline, but the results were too imprecise to allow a conclusion to be drawn (RR 0.68, 95% CI 0.22 to 2.06; 80 participants; moderate‐quality evidence) (Analysis 5.1).
5.1. Analysis.
Comparison 5 Prophylactic citicoline versus placebo, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no clear evidence of effect on cognitive status with citicoline treatment using MMSE score (MD ‐1.47, CI ‐3.85 to 0.91; 81 participants; moderate‐quality evidence, downgraded due to imprecision) (Analysis 5.2).
5.2. Analysis.
Comparison 5 Prophylactic citicoline versus placebo, Outcome 2 Cognitive status.
c. Adverse outcomes
No data were reported for adverse outcomes.
6. Oral premedication with diazepam and diphenhydramine versus no premedication
One study of 49 participants undergoing inpatient elective cardiac catheterisation compared the effect of premedication with diazepam and diphenhydramine with no premedication (Ashraf 2015).
a. Primary outcome
There were no cases of incident delirium in either group (49 participants; low‐quality evidence, downgraded due to risk of bias and evidence from single small study).
b. Secondary outcomes
No data are reported on secondary outcomes.
c. Adverse outcomes
No data are reported on adverse outcomes.
7. Intravenous (IV) methylprednisolone versus placebo
One large multicentre study of 7507 participants undergoing cardiopulmonary bypass procedures who were at high risk of morbidity and mortality compared the effect of intravenous (IV) methylprednisolone versus placebo and incorporated incidence of delirium as a safety outcome (Whitlock 2015).
a. Primary outcome
IV Methylprednisolone has no effect on the incidence of delirium for patients undergoing high‐risk cardiopulmonary bypass procedures (RR 1.02, 95% CI 0.87 to 1.19; 7507 participants; high‐quality evidence) (Analysis 7.1).
7.1. Analysis.
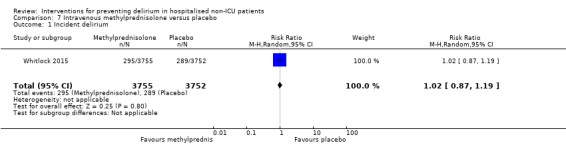
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 1 Incident delirium.
b. Secondary outcomes
IV methylprednisolone has no effect on the length of stay for patients undergoing high‐risk cardiopulmonary bypass procedures (RR 0.00, 95% CI ‐0.20 to 0.20; 7507 participants; high‐quality evidence) (Analysis 7.2).
7.2. Analysis.
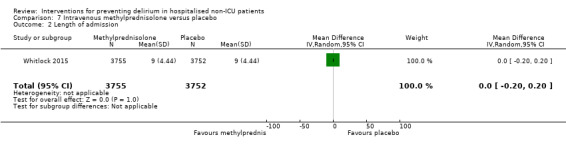
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 2 Length of admission.
c. Adverse outcomes
IV methylprednisolone has no effect on 30‐day mortality for patients undergoing high‐risk cardiopulmonary bypass procedures (RR 0.87, 95% CI 0.70 to 1.07; 7507 participants; high‐quality evidence) (Analysis 7.3).
7.3. Analysis.
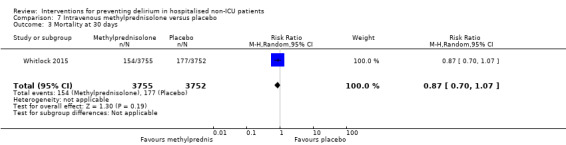
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 3 Mortality at 30 days.
Evaluating postoperative complications, IV methylprednisolone appears to increase the risk of myocardial injury compared to placebo (RR 1.22, 95% CI 1.07 to 1.38; 7507 participants; high‐quality evidence) and has no effect on the risk of respiratory failure (RR 0.91, 95% CI 0.80 to 1.05; 7507 participants; high‐quality evidence) and infection (RR 0.94, 95% CI 0.84 to 1.06; 7507 participants; high‐quality evidence).
8. Gabapentinoids versus placebo
Two studies tested gabapentinoids agents. One assessed gabapentin in 21 patients (Leung 2006), and the other tested the more potent preparation, pregabalin, in 70 patients (Pesonen 2011). However, results for these studies could not be pooled as each measured different outcomes.
a. Primary outcome
In Leung 2006, the incidence of delirium was lower in the group treated with gabapentin, but the results were too imprecise to allow a conclusion to be drawn (RR 0.12, 95% CI 0.01 to 1.90; 21 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 8.1).
8.1. Analysis.
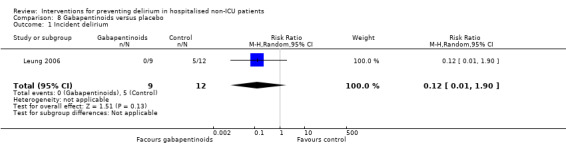
Comparison 8 Gabapentinoids versus placebo, Outcome 1 Incident delirium.
Pesonen 2011 tested for postoperative delirium using a Finnish modified CAM‐ICU but reported only median scores, precluding use of these data in the analysis .
b. Secondary outcomes
Pesonen 2011 reported effect of pregabalin compared to placebo on length of hospital admission (MD ‐0.60 days 95% CI ‐2.12 to 0.92; 60 participants) (Analysis 8.2); cognition (measured with the CAM‐ICU on day five), (MD 1.00 95% CI ‐2.76 to 4.76; 60 participants) (Analysis 8.3); and use of psychotropic medication, (RR 0.53 95% CI 0.21 to 1.38; 60 participants) (Analysis 8.4). For all three outcomes, results were inconclusive and we judged the evidence to be low‐quality, downgraded due to imprecision and risk of bias.
8.2. Analysis.
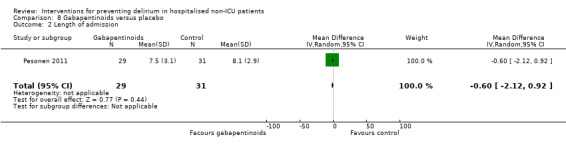
Comparison 8 Gabapentinoids versus placebo, Outcome 2 Length of admission.
8.3. Analysis.
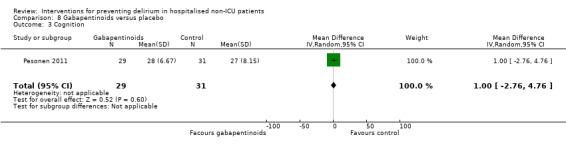
Comparison 8 Gabapentinoids versus placebo, Outcome 3 Cognition.
8.4. Analysis.
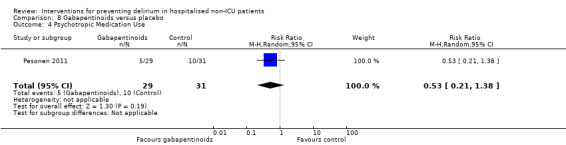
Comparison 8 Gabapentinoids versus placebo, Outcome 4 Psychotropic Medication Use.
Withdrawal from protocol appeared higher in the intervention group; however the results were too imprecise to allow a conclusion to be drawn (RR 9.0 95% CI 0.50 to 161.13; 70 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 8.5).
8.5. Analysis.
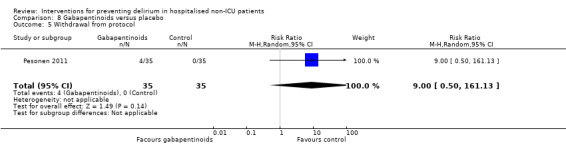
Comparison 8 Gabapentinoids versus placebo, Outcome 5 Withdrawal from protocol.
c. Adverse outcomes
No data were reported for adverse outcomes.
9. Ketamine versus placebo
One study (Urban 2008) tested the use of ketamine in 26 patients undergoing lumbar spinal fusion.
a. Primary outcome
Rates of incident delirium appeared higher among those treated with ketamine compared to control. However, the results are too imprecise to allow a conclusion to be drawn (RR 2.00, 95% CI 0.21 to 19.23; 24 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 9.1).
9.1. Analysis.
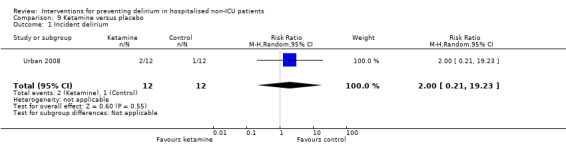
Comparison 9 Ketamine versus placebo, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no evidence of effect of ketamine treatment on withdrawals from protocol (RR 1.00, 95% CI 0.07 to 14.34; 26 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 9.2).
9.2. Analysis.
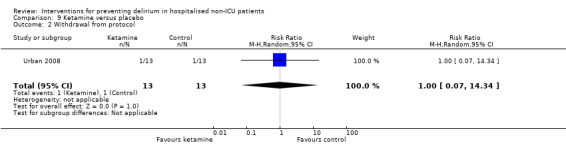
Comparison 9 Ketamine versus placebo, Outcome 2 Withdrawal from protocol.
c. Adverse outcomes
No data were reported for adverse outcomes.
10. Intravenous (IV) parecoxib sodium analgesia versus morphine and saline
One study of 80 participants admitted as an emergency for femoral head replacement surgery compared administration of IV parecoxib 12‐hourly versus IV morphine (single dose) followed by IV saline (Li 2013).
a. Primary outcome
The incidence of delirium was lower in those receiving parecoxib compared to those receiving morphine and saline (RR 0.50, 95% CI 0.26 to 0.98; 80 participants; low‐quality evidence due to indirectness [as the comparison tests regular analgesia to one dose of analgesia then placebo], risk of bias and this being a single small study) (Analysis 10.1).
10.1. Analysis.
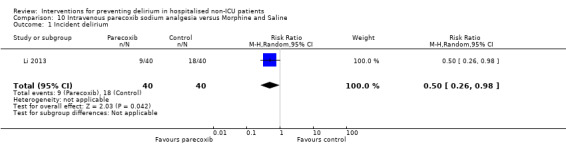
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 1 Incident delirium.
b. Secondary outcomes
Individuals receiving parecoxib had a shorter length of admission than those receiving morphine and saline (MD ‐0.90 days, 95% CI ‐1.58 to ‐0.22 days; 80 participants; low‐quality evidence due to indirectness and results from a single small study) (Analysis 10.2). Data are presented for rates of postoperative cognitive dysfunction (POCD) at three days, one week, three months, and six months, with evidence of a reduction in the risk of POCD at one week (RR 0.38, 95% CI 0.15 to 0.98; 80 participants; low‐quality evidence downgraded due to indirectness, imprecision and results being from a single small study) (Analysis 10.4).
10.2. Analysis.
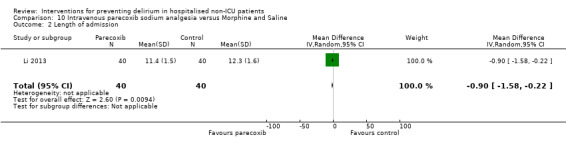
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 2 Length of admission.
10.4. Analysis.
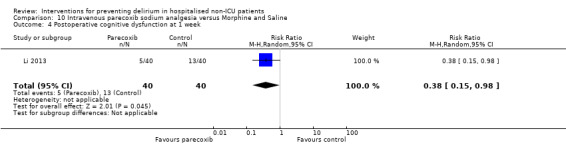
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 4 Postoperative cognitive dysfunction at 1 week.
c. Adverse outcomes
No data were reported for adverse outcomes.
11. Intrathecal morphine and patient controlled analgesia (PCA) versus saline and PCA
One study (Beaussier 2006) tested the administration of intrathecal morphine preoperatively in addition to postoperative patient‐controlled intravenous morphine for pain control in 59 patients. Both groups received postoperative PCA, but the intervention group were given intrathecal morphine, and the control group, a similar volume of saline preoperatively.
a. Primary outcome
There was no evidence of effect on intrathecal and PCA morphine on rates of incident delirium (RR 0.90, 95% CI 0.44 to 1.85; 52 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 11.1).
11.1. Analysis.
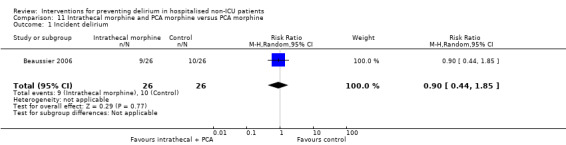
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 1 Incident delirium.
b. Secondary outcomes
Data were presented on length of admission (MD ‐0.50 days, 95% CI ‐1.51 to 0.51; 52 participants) (Analysis 11.2); days for cognition to return to preoperative level (MD 0.20, 95% CI ‐1.03 to 1.43; 52 participants) (Analysis 11.3); and withdrawals from protocol (RR 0.78, 95% CI 0.19 to 3.17; 59 participants) (Analysis 11.4) for intrathecal PCA morphine compared to saline and PCA. For all these outcomes, there was no clear evidence of effect from the intervention. We judged the evidence to be of low quality, downgraded due to risk of bias and imprecision.
11.2. Analysis.
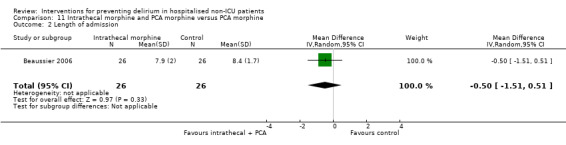
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 2 Length of admission.
11.3. Analysis.
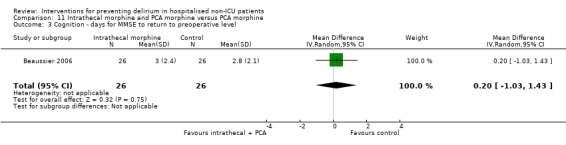
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 3 Cognition ‐ days for MMSE to return to preoperative level.
11.4. Analysis.
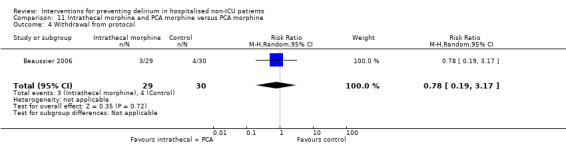
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 4 Withdrawal from protocol.
c. Adverse outcomes
Mortality appeared lower in those in the intrathecal and PCA morphine group, but the results were too imprecise for any conclusions to be drawn (RR 0.34, 95% CI 0.01 to 8.13; 59 participants; low‐quality evidence, downgraded two levels due to serious imprecision) (Analysis 11.5).
11.5. Analysis.
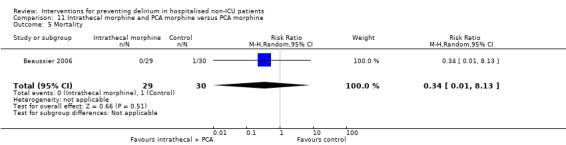
Comparison 11 Intrathecal morphine and PCA morphine versus PCA morphine, Outcome 5 Mortality.
12. Fascia iliaca compartment block (FICB) versus placebo
One study (Mouzopoulos 2009) with 219 participants tested administration of fascia iliaca compartment block (FICB) to manage pain in hip fracture patients assessed as being at intermediate or high risk of delirium.
a. Primary outcome
Use of a FICB reduced the risk of incident delirium compared to placebo (RR 0.45, 95% CI 0.24 to 0.87; 207 participants; moderate‐quality evidence due to risk of bias) (Analysis 12.1).
12.1. Analysis.
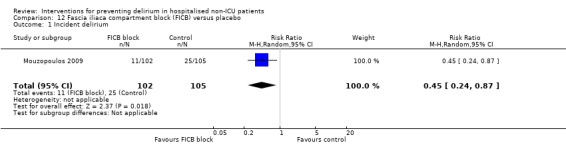
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 1 Incident delirium.
b. Secondary outcomes
Use of a FICB reduced the severity of delirium episodes (MD ‐4.30, 95% CI ‐6.81 to ‐1.79; 36 participants) (Analysis 12.2) and duration of delirium episodes (MD ‐5.70 days, 95% CI ‐9.50 to ‐1.90; 36 participants) (Analysis 12.3). However, we judged the evidence to be of very low‐quality, downgraded due to risk of bias and serious imprecision.
12.2. Analysis.
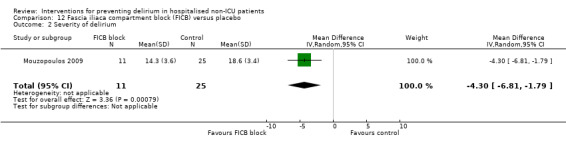
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 2 Severity of delirium.
12.3. Analysis.
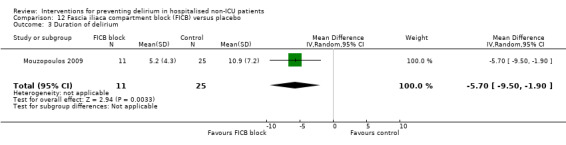
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 3 Duration of delirium.
c. Adverse outcomes
There was no evidence of effect of the intervention on risk of mortality (RR 0.51, 95% CI 0.05 to 5.58; 219 participants; low‐quality evidence downgraded two levels due to serious imprecision (Analysis 12.4).
12.4. Analysis.
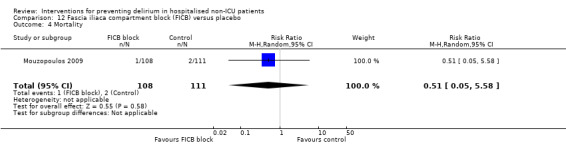
Comparison 12 Fascia iliaca compartment block (FICB) versus placebo, Outcome 4 Mortality.
13. Light versus deep propofol sedation
One study compared the effect of light and deep propofol sedation on the prevalence of postoperative delirium in 114 older adult patients who underwent hip fracture repair under spinal anaesthesia (Sieber 2010).
a. Primary outcome
The incidence of delirium was lower in those receiving light propofol sedation compared to deep propofol sedation (RR 0.48, 95% CI 0.26 to 0.89; 114 participants; moderate‐quality evidence due to risk of bias) (Analysis 13.1).
13.1. Analysis.
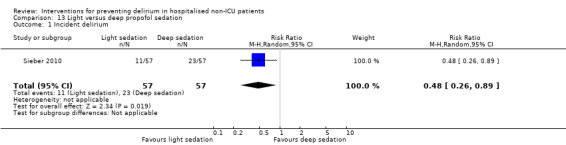
Comparison 13 Light versus deep propofol sedation, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no clear evidence of effect of level of sedation on delirium duration (MD ‐0.60 days, 95% CI ‐3.30 to 2.10; 34 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 13.2).
13.2. Analysis.
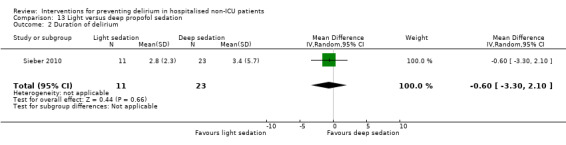
Comparison 13 Light versus deep propofol sedation, Outcome 2 Duration of delirium.
There was no evidence of effect on level of sedation on length of admission (MD 0.20 days, 95% CI ‐0.80 to 1.20 days; 114 participants; moderate‐quality evidence, downgraded due to risk of bias) (Analysis 13.3).
13.3. Analysis.
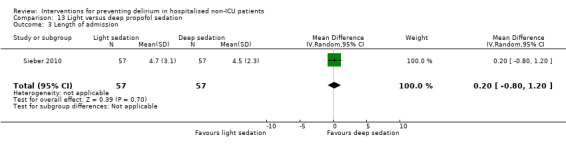
Comparison 13 Light versus deep propofol sedation, Outcome 3 Length of admission.
Light propofol sedation improved cognitive performance (on day two postoperatively, assessed using MMSE score (Folstein 1975)) (MD 3.10, 95% CI 0.30 to 5.90; 114 participants; moderate‐quality evidence due to risk of bias) (Analysis 13.4).
13.4. Analysis.
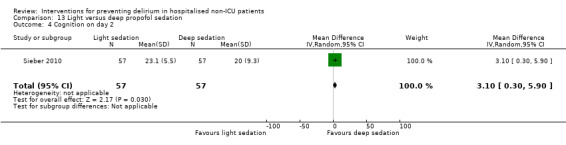
Comparison 13 Light versus deep propofol sedation, Outcome 4 Cognition on day 2.
c. Adverse outcomes
There was no evidence of effect of level of sedation on inpatient mortality (RR 0.50, 95% CI 0.05, to 5.36; 114 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 13.5). There was no evidence of effect of the intervention on the risk of experiencing >=1 postoperative complication (RR 0.87, 95% CI 0.60 to 1.26; 114 participants; low‐quality evidence due to risk of bias and imprecision) (Analysis 13.6).
13.5. Analysis.
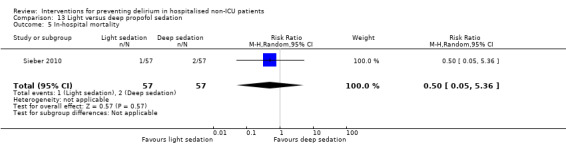
Comparison 13 Light versus deep propofol sedation, Outcome 5 In‐hospital mortality.
13.6. Analysis.
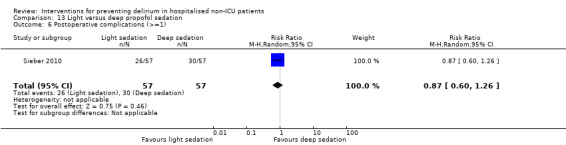
Comparison 13 Light versus deep propofol sedation, Outcome 6 Postoperative complications (>=1).
14. Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia or clinical judgement
Three studies Chan 2013 (925 participants), Radtke 2013 (1277 participants) and Gauge 2014 (81 participants) investigated the use of BIS in anaesthesia. Only two of these presented useable data for inclusion in the review (Chan 2013; Radtke 2013) as insufficient data were reported in Gauge 2014 (conference abstract). A summary of findings for key outcomes is presented in Table 5.
a. Primary outcome
BIS‐guided anaesthesia was effective in reducing incident delirium (RR 0.71, 95% CI 0.60 to 0.85, I2 = 0%; 2057 participants; moderate‐quality evidence due to risk of bias) (Analysis 14.1; Figure 7).
14.1. Analysis.
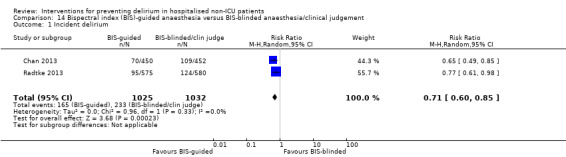
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 1 Incident delirium.
7.
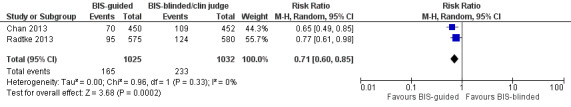
Forest plot of comparison: 11 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia, outcome: 11.1 Incident delirium.
b. Secondary outcomes
BIS‐guided anaesthesia resulted in a shorter length of admission than those receiving BIS‐blinded anaesthesia/clinical judgement (MD ‐0.94 days, 95% CI ‐1.45 to ‐0.43 days, I2 = 0%; 2057 participants; moderate‐quality evidence, downgraded due to risk of bias) (Analysis 14.2).
14.2. Analysis.
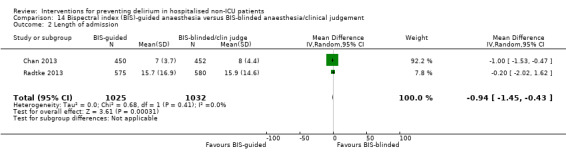
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 2 Length of admission.
Use of BIS‐guided anaesthesia showed evidence of reducing rates of cognitive impairment at seven days (RR 0.87, 95% CI 0.71 to 1.05, I2 = 0%; 1938 participants) (Analysis 14.3) and at three months (RR 0.71, 95% CI 0.53 to 0.97; 1990 participants) (Analysis 14.4). However, we considered the evidence to be of low quality, downgraded due to risk of bias and imprecision.
14.3. Analysis.
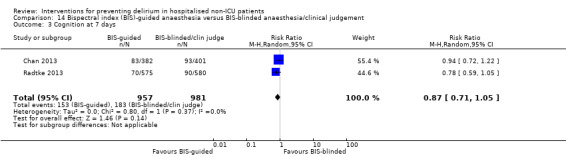
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 3 Cognition at 7 days.
14.4. Analysis.
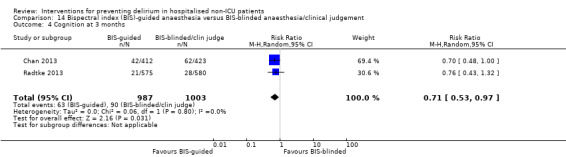
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 4 Cognition at 3 months.
c. Adverse outcomes
Chan 2013 reported SF‐36 mental summary scores (Ware 1992) at follow‐up and the BIS‐guided group had lower scores, indicating a poorer assessment of their own mental health (MD ‐1.90, 95% CI ‐3.40 to ‐0.40; 902 participants; moderate‐quality evidence downgraded as from a single study) (Analysis 14.5).
14.5. Analysis.
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 5 SF‐36 mental summary score.
One study reported mortality at seven days (Chan 2013); there was no clear evidence of any effect on mortality (RR 1.49, 95% CI 0.42 to 5.25; 921 participants; low‐quality evidence, downgraded two levels due to serious imprecision) (Analysis 14.6).
14.6. Analysis.
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 6 Mortality at 7 days.
Two studies reported mortality at three months (Chan 2013; Radtke 2013); there was no evidence of reduction in mortality (RR 1.10, 95% CI 0.77 to 1.59, I2 = 0%; 1938 participants; moderate‐quality evidence due to imprecision) (Analysis 14.7).
14.7. Analysis.
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 7 Mortality at 3 months.
Chan 2013 reported rates of cardiac, respiratory and infectious adverse events. There was no evidence of a reduction in cardiac (RR 0.85, 95% CI 0.52 to 1.39; 902 participants) or respiratory adverse events (RR 0.79, 95% CI 0.59 to 1.07; 902 participants), but infectious adverse events were lower in the group receiving BIS‐guided anaesthesia (RR 0.72, 95% CI 0.55 to 0.95; 902 participants). However, the evidence was deemed of low quality due to risk of bias and being from a single study.
15. Sevoflurane versus propofol anaesthesia
Lurati 2012 compared sevoflurane, an inhalational anaesthetic versus propofol, an intravenous anaesthetic to reduce perioperative myocardial ischaemia in 385 patients undergoing noncardiac surgery.
a. Primary outcome
There was no evidence of effect on rates of incident delirium with sevoflurane anaesthesia compared to propofol anaesthesia (RR 0.79, 95% CI 0.47 to 1.34; 385 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 15.1).
15.1. Analysis.
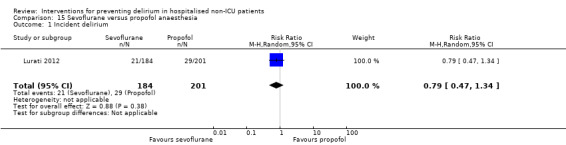
Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 1 Incident delirium.
b. Secondary outcomes
No data were reported for secondary outcomes.
c. Adverse outcomes
There was no evidence of a difference in mortality at 12 months between intervention and control groups (RR 1.19, 95% CI 0.70 to 2.02; 385 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 15.2).
15.2. Analysis.
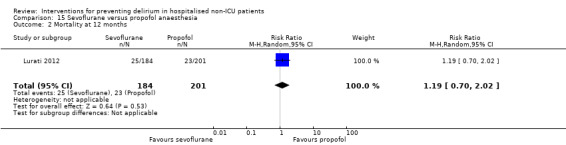
Comparison 15 Sevoflurane versus propofol anaesthesia, Outcome 2 Mortality at 12 months.
16. Xenon versus sevoflurane anaesthesia
Stoppe 2013 conducted a pilot trial to determine the feasibility and safety of xenon, a novel anaesthetic gas with neuroprotective and cardioprotective properties compared with sevoflurane a conventional inhalational anaesthetic in 30 patients undergoing elective coronary artery bypass grafting.
a. Primary outcome
There was no evidence of a difference in incidence of postoperative delirium between the xenon and sevoflurane groups. The highest incidence of delirium occurred on the second postoperative day (RR 0.75, 95% 0.20 to 2.79; 30 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 16.1).
16.1. Analysis.
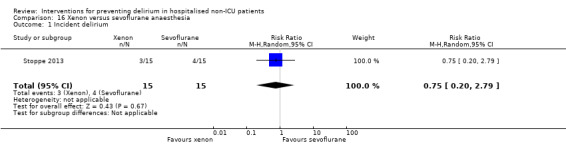
Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 1 Incident delirium.
b. Secondary outcomes
Hospital admission appeared to be longer in those treated with xenon, but the results were too imprecise to allow conclusions to be drawn (MD 4.00 days, 95% CI ‐1.72 to 9.72 days; 30 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 16.2).
16.2. Analysis.
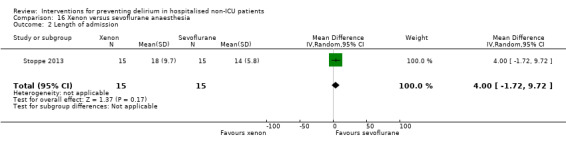
Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 2 Length of admission.
c. Adverse outcomes
There were no in‐hospital deaths amongst study participants (Analysis 16.3). There was no evidence of effect on adverse events (RR 0.75, 95% CI 0.34 to 1.64; 30 participants; low‐quality evidence downgraded due to risk of bias and imprecision) or the incidence of sepsis (RR 1.50, 95% CI 0.29 to 7.73; 30 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 16.4; Analysis 16.5).
16.3. Analysis.
Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 3 In‐hospital mortality.
16.4. Analysis.
Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 4 Adverse events.
16.5. Analysis.
Comparison 16 Xenon versus sevoflurane anaesthesia, Outcome 5 Sepsis.
17. Epidural anaesthesia versus general anaesthesia
Two studies compared epidural versus general anaesthesia (Berggren 1987; Papaioannou 2005).
a. Primary outcome
We pooled data from both studies for the primary outcome of incident delirium, but the result was too imprecise to determine an effect (RR 1.19, 95% CI 0.69 to 2.03, I2 = 0%; 104 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 17.1).
17.1. Analysis.
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no evidence of reduction in admission length, evaluated as those with a length of stay >10 days versus not (RR 0.59, 95% CI 0.28 to 1.24; 47 participants) (Analysis 17.2) and cognitive decline (MD 0.15, 95% CI 0.02 to 1.06; 47 participants) (Analysis 17.3) from one study (Papaioannou 2005). For both outcomes the result was inconclusive and we judged the evidence to be low quality, downgraded due to risk of bias and imprecision.
17.2. Analysis.
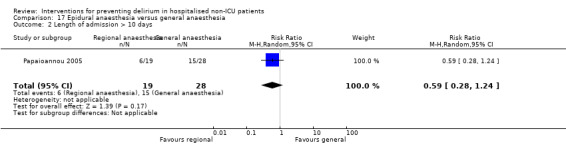
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 2 Length of admission > 10 days.
17.3. Analysis.
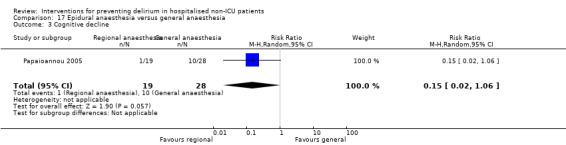
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 3 Cognitive decline.
c. Adverse outcomes
Berggren 1987 examined physical morbidity and found no evidence of reduction in urinary tract infection (MD 1.33, 95% CI 0.57 to 3.09; 57 participants) and psychological morbidity (depression) (RR 1.04; 95% CI 0.23 to 4.71; 57 participants). The evidence for both outcomes was of low quality downgraded two levels due to serious imprecision of results) (Analysis 17.4; Analysis 17.5).
17.4. Analysis.
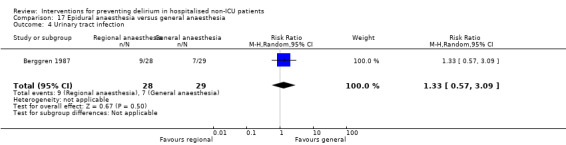
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 4 Urinary tract infection.
17.5. Analysis.
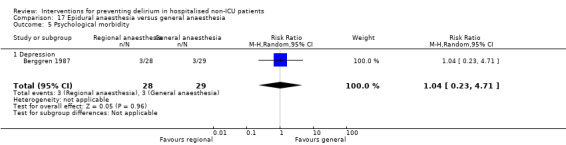
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 5 Psychological morbidity.
There was no evidence for reduction in postoperative complications using epidural versus general anaesthesia reported by Papaioannou 2005 (RR 0.92, 95% CI 0.35 to 2.39; 47 participants; very low‐quality evidence due to risk of bias and serious imprecision) (Analysis 17.6).
17.6. Analysis.
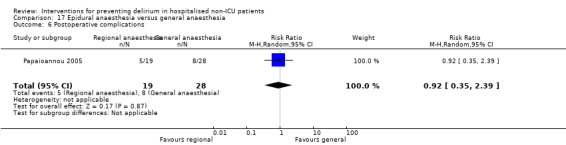
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 6 Postoperative complications.
Berggren 1987 investigated the impact on pressure ulcers and reported no evidence of effect of reduction in pressure ulcer formation between epidural and general anaesthesia groups (RR 0.62, 95% CI 0.16 to 2.36; 57 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 17.7).
17.7. Analysis.
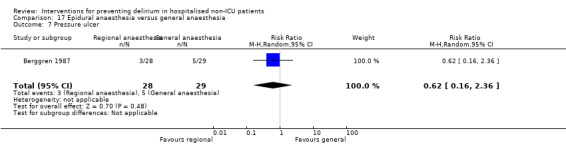
Comparison 17 Epidural anaesthesia versus general anaesthesia, Outcome 7 Pressure ulcer.
18. Liberal versus restrictive blood transfusion thresholds
One study Gruber‐Baldini 2013 with 139 participants compared the use of liberal versus restrictive blood transfusion thresholds for individuals undergoing surgical repair of hip fracture. There was significant overlap in the volume of blood received by participants in the liberal and restrictive groups.
a. Primary outcome
There was no evidence to support liberal transfusion thresholds on rates of incident delirium (RR 0.75, 95% CI 0.45 to 1.27; 108 participants; moderate‐quality evidence due to risk of bias) (Analysis 18.1).
18.1. Analysis.
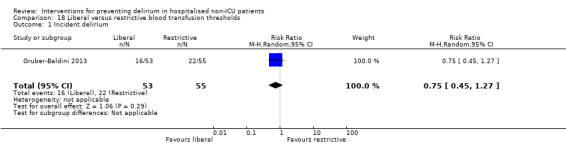
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no evidence that liberal transfusion thresholds affected the severity of delirium (MD ‐0.10 points, 95% CI ‐2.99 to 2.79; 38 participants; low‐quality evidence due to risk of bias and imprecision) or length of admission (MD ‐0.10 days, 95% CI ‐1.36 to 1.16 days; 138 participants; low‐quality evidence downgraded due to imprecision and risk of bias) (Analysis 18.2; Analysis 18.3). Use of psychoactive medication appeared balanced between the liberal and restrictive transfusion groups (RR 0.99, 95% CI 0.87 to 1.12; 138 participants; low‐quality evidence downgraded due to risk of bias and as results from a single small study) (Analysis 18.4).
18.2. Analysis.
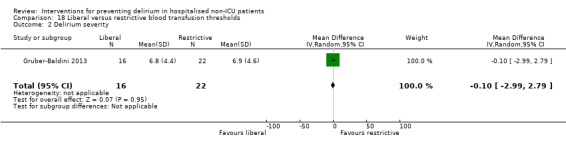
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 2 Delirium severity.
18.3. Analysis.
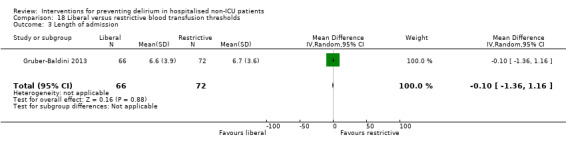
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 3 Length of admission.
18.4. Analysis.
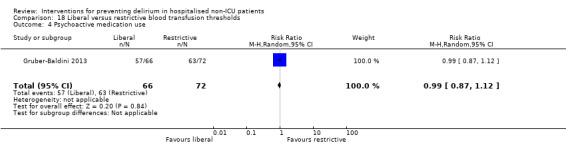
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 4 Psychoactive medication use.
c. Adverse outcomes
Data were reported on the occurrence of post‐randomisation adverse events, specifically infections and congestive heart failure. There was no evidence that liberal transfusions reduced the risk of infections (RR 1.09, 95% CI 0.23 to 5.22; 138 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) or congestive heart failure (RR 0.55, 95% CI 0.05 to 5.88; 138 participants; very low‐quality evidence downgraded due to risk of bias and serious imprecision) (Analysis 18.5; Analysis 18.6).
18.5. Analysis.
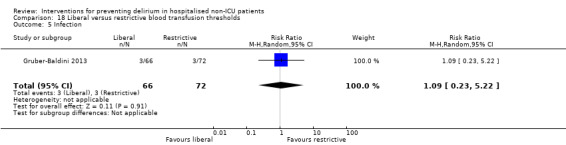
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 5 Infection.
18.6. Analysis.
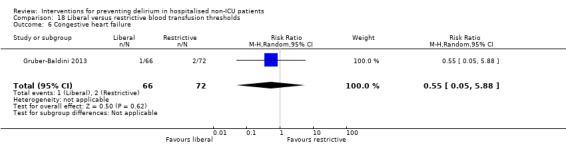
Comparison 18 Liberal versus restrictive blood transfusion thresholds, Outcome 6 Congestive heart failure.
19. Fast‐track surgery versus usual care
One study Jia 2014 with 240 participants evaluated the effects of fast‐track surgery for older adults with colorectal cancer compared to usual care.
a. Primary outcome
Evidence from this study supports fast‐track surgery as an intervention to reduce incident delirium (RR 0.26, 95% CI 0.09 to 0.77; 233 participants; low‐quality evidence, downgraded due to imprecision of results and risk of bias) (Analysis 19.1).
19.1. Analysis.
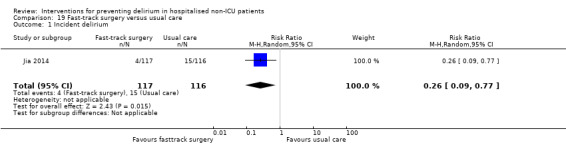
Comparison 19 Fast‐track surgery versus usual care, Outcome 1 Incident delirium.
b. Secondary outcomes
There is evidence to support fast‐track surgery in reducing length of admission (MD ‐4.20 days, 95% CI ‐4.60 to ‐3.80 days; 233 participants; high‐quality evidence) (Analysis 19.2).
19.2. Analysis.
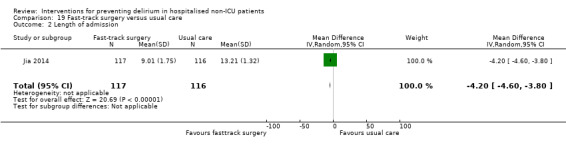
Comparison 19 Fast‐track surgery versus usual care, Outcome 2 Length of admission.
c. Adverse outcomes
The study reports on the occurrence of urinary tract infection and heart failure. It appeared that fast‐track surgery reduced the rate of urinary tract infection (RR 0.38, 95% CI 0.14 to 1.04), but this was low‐quality evidence as the result was too imprecise to draw a conclusion and there was risk of bias in outcome assessment (Analysis 19.3). There is evidence to support fast‐track surgery reducing the occurrence of heart failure compared to usual care (RR 0.31, 95% CI 0.10 to 0.91; 233 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 19.4)
19.3. Analysis.
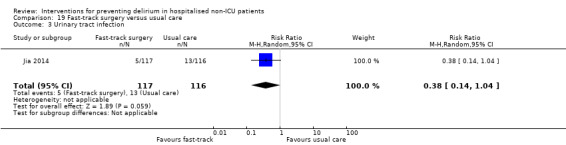
Comparison 19 Fast‐track surgery versus usual care, Outcome 3 Urinary tract infection.
19.4. Analysis.
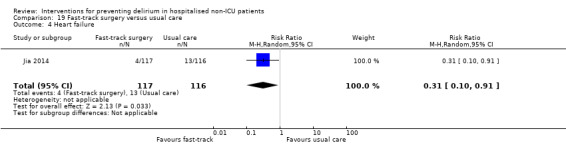
Comparison 19 Fast‐track surgery versus usual care, Outcome 4 Heart failure.
20. Postoperative delirium‐free protocol (DFP) versus usual care
One small study Aizawa 2002 with 42 participants evaluated a 'delirium‐free protocol' which was comprised of overnight infusions of diazepam, flunitrazepam and pethidine to older postoperative surgical patients.
a. Primary outcome
DFP use was associated with a lower rate of incident delirium, but the result was imprecise (RR 0.14, 95% CI 0.02 to 1.06; 40 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 20.1).
20.1. Analysis.
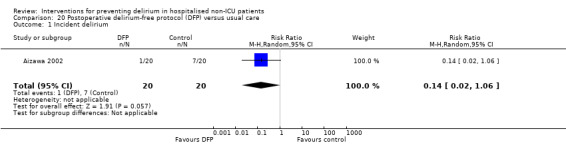
Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no evidence of effect of the DFP on length of admission (MD ‐4.30 days, 95% CI ‐12.51 to 3.91 days; 40 participants; very low‐quality evidence, downgraded due to risk of bias and serious imprecision) (Analysis 20.2).
20.2. Analysis.
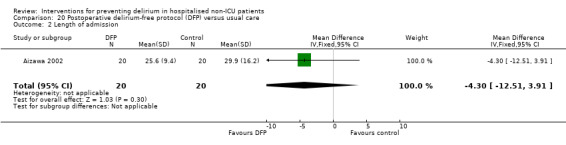
Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 2 Length of admission.
There was no evidence of effect of the DFP on the risk of behavioural disturbance (RR 0.20, 95% CI 0.03 to 1.56; 40 participants; low‐quality evidence, downgraded due to risk of bias and imprecision) (Analysis 20.3).
20.3. Analysis.
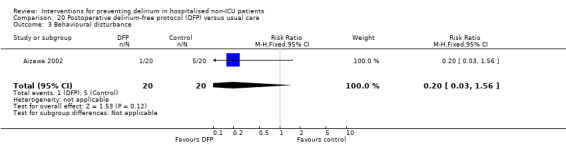
Comparison 20 Postoperative delirium‐free protocol (DFP) versus usual care, Outcome 3 Behavioural disturbance.
c. Adverse outcomes
No data were reported for adverse outcomes.
21. Computerised clinical decision support system (CCDSS) versus usual care
One study Boustani 2012 assessed the use of a computerised clinical decision support system (CCDSS) on the management of 427 older adults with cognitive impairment compared to usual care.
a. Primary outcome
There was no evidence of the effect of CCDSS in reducing incident delirium (RR 1.08, 95% CI 0.82 to 1.43; 424 participants; moderate‐quality evidence due to risk of bias) (Analysis 21.1).
21.1. Analysis.
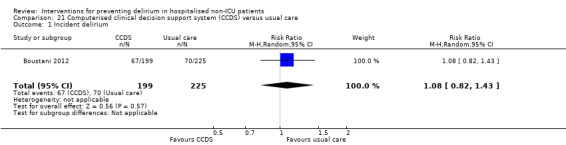
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no evidence of reduction in the length of admission (MD 0.90 days, 95% CI ‐0.35 to 2.15 days; 424 participants; low‐quality evidence, downgraded due to serious imprecision) (Analysis 21.2).
21.2. Analysis.
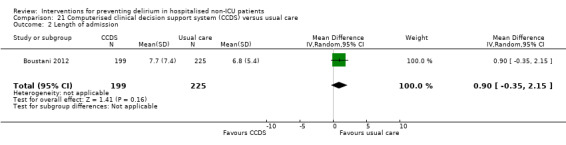
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 2 Length of admission.
c. Adverse outcomes
There was no evidence of a change in rates of mortality within 30 days of discharge (RR 1.04, 95% CI 0.49 to 2.23; 424 participants; low‐quality evidence downgraded due to serious imprecision) (Analysis 21.3).
21.3. Analysis.
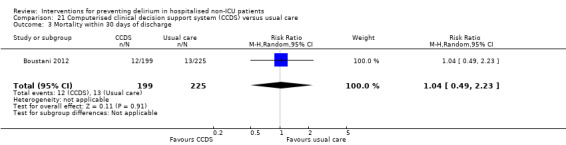
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 3 Mortality within 30 days of discharge.
There was no evidence of effect on rates of falls (RR 0.93, 95% CI 0.39 to 2.19; 424 participants) or pressure ulcers (RR 1.09, 95% CI 0.64 to 1.84; 424 participants) with use of the CCDSS with moderate‐quality evidence downgraded due to imprecision. (Analysis 21.4; Analysis 21.5)
21.4. Analysis.
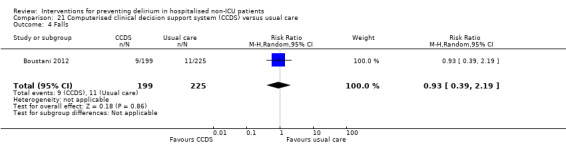
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 4 Falls.
21.5. Analysis.
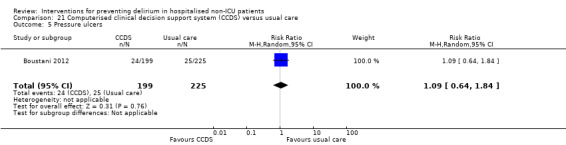
Comparison 21 Computerised clinical decision support system (CCDS) versus usual care, Outcome 5 Pressure ulcers.
22. Geriatric unit care versus orthopaedic unit care
One trial of 329 older adults following hip fracture compared care in a specialist geriatric unit and comprehensive geriatric assessment to care in their orthopaedic unit (Watne 2014).
a. Primary outcome
There was no evidence that care in the geriatric unit reduced the incidence of delirium compared to care in the orthopaedic unit (RR 0.98, 95% CI 0.79 to 1.22; 329 participants; low‐quality evidence downgraded due to risk of bias and imprecision) (Analysis 22.1).
22.1. Analysis.
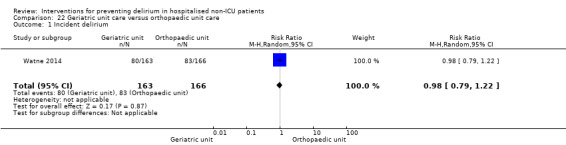
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 1 Incident delirium.
b. Secondary outcomes
There was no evidence that care in the geriatric unit reduced the duration (MD ‐1.00 days, 95% CI ‐2.04 to 0.04 days; 163 participants) (Analysis 22.2) or severity of delirium episodes (MD 1.50 points, 95% CI ‐1.00 to 4.00 points; 163 participants) (Analysis 22.3) compared to the orthopaedic unit, low‐quality evidence for both outcomes, downgraded due to risk of bias and imprecision.
22.2. Analysis.
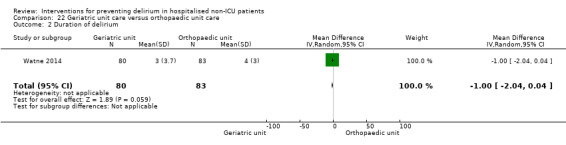
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 2 Duration of delirium.
22.3. Analysis.
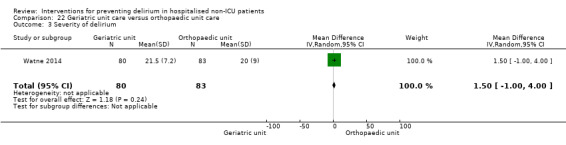
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 3 Severity of delirium.
Care in the geriatric unit increased length of hospital admission by a mean of three days (RR 3.00, 95% CI 1.94 to 4.06 days; moderate‐quality evidence downgraded due to risk of bias) compared to the orthopaedic unit (Analysis 22.4).
22.4. Analysis.
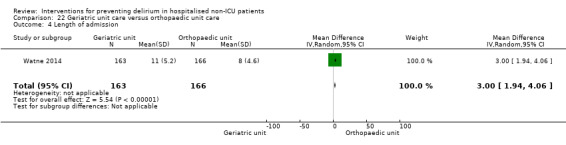
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 4 Length of admission.
Outcome assessments at four and 12 months were conducted blinded to original allocation, unlike those conducted while in hospital.
There was no evidence that care in the geriatric unit affected cognitive function (using a composite score) at four months follow‐up (MD 1.80 points, 95% CI ‐5.92 to 9.52 points; 228 participants; low‐quality evidence downgraded two levels due to serious imprecision) (Analysis 22.5). Care in the geriatric unit appeared to increase the rate of incident dementia at 12 months (RR 2.26, 95% CI 0.60 to 8.49; 193 participants) (Analysis 22.6), however, the evidence was deemed to be of low quality and was downgraded two levels due to serious imprecision.
22.5. Analysis.
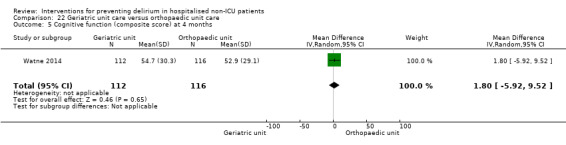
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 5 Cognitive function (composite score) at 4 months.
22.6. Analysis.
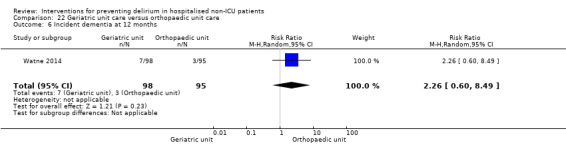
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 6 Incident dementia at 12 months.
There was no evidence that activities of daily living (measured by Barthel Index (Mahoney 1965)) were affected by allocation to the geriatric unit or the orthopaedic unit (MD 1.00, 95% CI ‐0.70 to 2.70; moderate‐quality evidence downgraded due to imprecision) (Analysis 22.7).
22.7. Analysis.
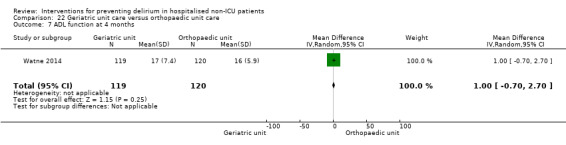
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 7 ADL function at 4 months.
There was no evidence that care in the geriatric unit affected risk of Institutionalisation at four (RR 1.06, 95% CI 0.58 to 1.91; 242 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 22.8) and 12 months (RR 0.86, 95% CI 0.47 to 1.59; 193 participants; moderate‐quality evidence downgraded due to imprecision) (Analysis 22.9).
22.8. Analysis.
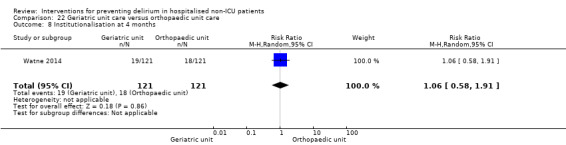
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 8 Institutionalisation at 4 months.
22.9. Analysis.
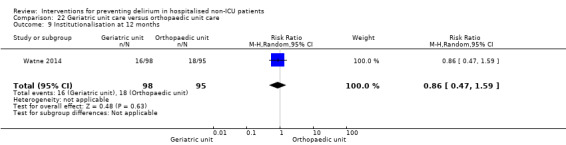
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 9 Institutionalisation at 12 months.
c. Adverse outcomes
There was no evidence that care in the geriatric unit improved the rate of in‐hospital mortality (RR 0.56, 95% CI 0.21 to 1.47; 329 participants; moderate‐quality evidence downgraded due to imprecision) compared to the orthopaedic unit (Analysis 22.10).
22.10. Analysis.
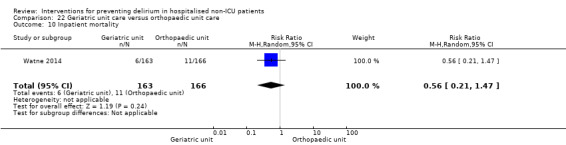
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 10 Inpatient mortality.
Evaluating other adverse outcomes there was no evidence that care in the geriatric unit reduced the rate of falls (RR 1.30, 95% CI 0.61 to 2.77; 329 participants) (Analysis 22.11); pressure ulcer formation (RR 0.38, 95% CI 0.10 to 1.41; 329 participants) (Analysis 22.12); other medical adverse events (RR 0.96, 95% CI 0.76 to 1.23; 329 participants) (Analysis 22.13); or postoperative complications (RR 0.68, 95% CI 0.20 to 2.36; 329 participants) (Analysis 22.14) with low‐quality evidence for each comparison, downgraded due to risk of bias and imprecision.
22.11. Analysis.
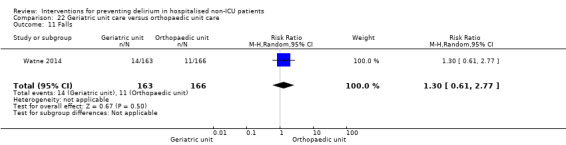
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 11 Falls.
22.12. Analysis.
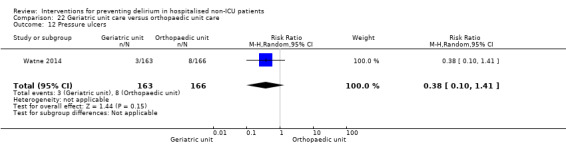
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 12 Pressure ulcers.
22.13. Analysis.
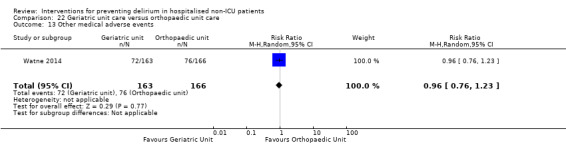
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 13 Other medical adverse events.
22.14. Analysis.
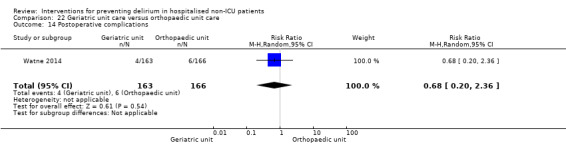
Comparison 22 Geriatric unit care versus orthopaedic unit care, Outcome 14 Postoperative complications.
Discussion
Summary of main results
Evidence for the effectiveness of most interventions for preventing delirium remains uncertain, with the exception of multi‐component interventions.
Multi‐component interventions
There is moderate‐quality evidence from seven randomised controlled trials that multi‐component interventions reduce delirium incidence, with an overall reduction in the risk of delirium by about 30% compared with usual care. Moreover, they appear to have similar effect sizes in medical and surgical study populations.
Despite the higher risk of delirium in patients with dementia, only one trial reported data on the incidence of delirium in this subgroup (for 50 participants); and in this study, dementia prevalence was unbalanced between intervention and control groups (Marcantonio 2001). The effectiveness of these interventions in patients with dementia remains uncertain.
Effects on delirium duration, length of hospital admission, institutionalisation and severity of delirium are also uncertain. There is no clear evidence of effect on mortality (either inpatient, or at 12 months); 12‐month mortality was only reported in one trial (Lundstrom 2007). Clinically important differences are reported for cognition (in one study; 60 participants, Bonaventura 2007) and pressure ulcers (two studies; 457 participants, Hempenius 2013; Lundstrom 2007), all in a direction favouring multi‐component interventions, although there is uncertainty in these results due to imprecision.
Pharmacological interventions
Cholinesterase inhibitors
We found no clear evidence of benefit for a cholinesterase inhibitor, donepezil, in preventing delirium in an elective orthopaedic population without cognitive impairment. The available evidence was judged to be very low‐quality due to imprecision and considerable inconsistency.
Antipsychotic medication
Overall, there is no clear evidence for effectiveness of antipsychotic medications as a group in delirium prevention, although there is uncertainty in this result because of imprecision and inconsistency.
The pre‐planned subgroup analysis indicates that an atypical antipsychotic drug (olanzapine) may reduce incidence of delirium, with a potentially large effect size, but there is no clear evidence supporting effectiveness of the typical antipsychotic, haloperidol. However, it is possible that in one study of haloperidol, optimisation of non‐pharmacological delirium prevention in both the intervention and control arms precluded detection of any additional benefit from medication. In the other study, haloperidol was administered on the first postoperative day for three days and this may have been too late for any preventive benefits, although this study was also at high risk of bias due its unblinded nature.
The impact on severity and duration of delirium also differed between two studies of haloperidol and olanzapine, but paradoxically, favoured the intervention group for haloperidol, and the control group for olanzapine. There is no clear evidence for effect of antipsychotic medication on length of hospital admission.
Melatonin
There is no clear evidence to support effectiveness of melatonin or melatonin agonists in delirium prevention. However, there is considerable heterogeneity in results, which may have been a result of differing study populations and different dosages. Al‐Aama 2011 reported a clinically important effect size in reducing delirium incidence in medical inpatients using 0.5 mg melatonin daily, (low‐quality evidence because of incomplete follow‐up); whilst de Jonghe 2014 reported no effect using melatonin 3 mg daily in hip fracture patients undergoing acute surgery. Ramelteon, a melatonin agonist, has previously been proposed as a safer treatment for insomnia (Miyamoto 2009), but we found no evidence of benefit in delirium prevention in one trial.
Other pharmacological interventions
We found no evidence to support effectiveness of citicoline in reducing delirium incidence.
Methylprednisolone had no effect on delirium incidence.
In one small trial of premedication using diazepam and diphenhydramine for elective inpatient cardiac catheterisation there were no cases of delirium in either group; thus the evidence that choice of premedication affects delirium incidence remains inconclusive.
Perioperative interventions
Opioid‐sparing measures
The evidence about the effect of gabapentin, ketamine or intrathecal and patient controlled analgesia (PCA) morphine for delirium prevention is inconclusive.
There was evidence that intravenous (IV) parecoxib reduced the incidence of delirium compared to morphine and saline. However, the evidence was of low quality, from a single study and affected by potential confounding related to the administration of supplementary morphine.
There is evidence that fascia iliaca compartment block (FICB) to manage pain in hip fracture patients is effective in reducing incidence of delirium. Lower‐quality evidence also suggested that it could reduce the severity and length of delirium episodes.
Reducing/controlling the depth of anaesthesia
Reduction in depth of general anaesthesia or controlling the depth is effective in preventing delirium. Both use of light propofol sedation compared to deep, and Bispectral index (BIS)‐guided anaesthesia compared to BIS‐blinded anaesthesia/clinical judgement were effective approaches.
Changing the mode of anaesthesia
There is no evidence of difference in effect on delirium incidence of using propofol or xenon compared to sevoflurane anaesthesia.
Avoiding general anaesthesia
The evidence for effectiveness of epidural anaesthesia compared to general anaesthesia in delirium prevention is uncertain.
Miscellaneous perioperative interventions
There was no evidence from one study that liberal versus restrictive blood transfusion was effective in preventing delirium.
One study of fast‐track surgery in elderly cancer patients suggested that it reduces the incidence of delirium and length of hospital admission.
One study which used a 'delirium‐free protocol' for older adults undergoing open laparotomy is likely to have resulted in sedation of participants and failed to demonstrate any evidence of benefit on delirium incidence.
Computerised clinical decision support system (CCDSS)
One study using a computerised clinical decision support system conducted in general and geriatric medical patients did not result in improvement in delirium incidence.
Geriatric unit care versus orthopaedic unit
There was no evidence that care in the geriatric medicine unit reduced the incidence duration or severity of delirium or other cognitive and functional outcomes. However, geriatric unit care increased length of hospital stay compared to care in the orthopaedic unit.
Overall completeness and applicability of evidence
Although 39 trials were identified for inclusion in this review, the body of evidence for delirium prevention in hospitalised non‐ICU patients remains limited, except for multi‐component interventions (seven trials). Most other interventions were only investigated in one or two small trials, with considerable heterogeneity in the interventions, outcomes, populations and settings studied, precluding meta‐analyses. Only one study (of a multi‐component intervention in surgical patients) presented results for people with dementia, an important subgroup to study in delirium prevention. The effectiveness of delirium interventions might be expected to differ given the higher prevalence of delirium and poorer outcomes in dementia.
For multi‐component interventions, it is likely that the included trials and meta‐analyses were underpowered to detect mortality and institutionalisation (both relatively rare outcomes), and this may explain the lack of observed impact on these endpoints, despite the reduction in incident delirium.
Although there was evidence suggesting FICB, controlling depth of anaesthesia and fast‐track surgery could reduce postoperative delirium incidence, it is important to note that in clinical practice, there will be a range of considerations apart from effectiveness in delirium prevention (including co‐morbidities, falls risk, and rehabilitation requirements) guiding choice of approaches to surgery and anaesthesia. Recommendations regarding surgery and anaesthetic practice cannot, therefore, be made based on the evidence from this review alone.
Most studies included delirium incidence as an outcome, and both cognition and length of hospital admission were also frequently reported. However, other important outcomes including delirium duration and severity, mortality, institutionalisation, activities of daily living (ADL) performance, and adverse outcomes were not commonly reported. No studies investigated the impact on quality of life, carers' psychological morbidity, staff psychological morbidity, or costs. Future studies need to address these gaps in the interventions, settings and outcomes studied.
Failure to exclude prevalent delirium at enrolment was a common limitation of the majority of included studies (29/39). This has the potential to reduce precision in the results as interventions cannot prevent cases of delirium already present in recruited participants.
Quality of the evidence
We used GRADEpro software (GRADEpro 2014) to inform the generation of evidence quality statements for five comparisons: i) multi‐component interventions versus usual care; ii) cholinesterase inhibitors versus placebo; iii) antipsychotic medication versus placebo; iv) melatonin versus placebo and v) BIS‐guided versus BIS‐blinded anaesthesia/clinical judgement. Full tabulations for each outcome are available in: Table 1, Table 2, Table 3, Table 4 and Table 5.
On the basis of seven randomised controlled trials (RCTs) (four in medical patients and three in surgical patients) n = 1950 participants, there is moderate‐quality evidence that multi‐component delirium prevention interventions can reduce rates of incident delirium; this is consistent across the included trials. Evidence has been downgraded due to the possibility of performance bias (the nature of the intervention precludes blinding of participants and those delivering intervention). Outcome assessors were unblinded to the intervention in two studies, including the study with the largest weighting and highest event rate. Furthermore, there is a risk of other bias in two of the included studies due to an imbalance between the intervention and control groups in respect to the prevalence of pre‐existing dementia.
Heterogeneity in the multi‐component interventions studied makes it difficult to ascertain whether specific components of the interventions are particularly effective in the prevention of delirium.
There is moderate‐quality evidence that multi‐component interventions have no effect on length of hospital stay (six studies, n = 1920 participants) and moderate‐quality evidence of no effect on the likelihood of return to independent living (four studies, n = 1116). There is considerable uncertainty regarding the effect of multi‐component interventions on the duration of delirium due to unblinded outcome assessment in two studies, imbalance in the prevalence of dementia in two studies and imprecise results.
On the basis of two RCTs (n = 113 participants), there is considerable uncertainty regarding the effect of prophylactic cholinesterase inhibitors on reducing delirium incidence due to very low‐quality evidence. Both of these studies have missing outcome data; evidence was downgraded due to imprecision and inconsistency in the results. There is low‐quality evidence for the effect of prophylactic cholinesterase inhibitors on the outcome of delirium severity (one study; n = 16 participants) and length of admission (two studies; n = 128 participants). Evidence was downgraded due to serious imprecision of the delirium severity results and for imprecision and risk of bias in length of admission.
On the basis of three RCTs (n = 916 participants), there is considerable uncertainty regarding the effect of antipsychotic medications on the incidence of delirium due to low‐quality evidence that has been downgraded because of risk of bias, inconsistency and imprecise results. There is very low‐quality evidence on the effect of antipsychotic medications on the severity (two studies, n = 178 participants) and duration of delirium (two studies, n = 178 participants), and low‐quality evidence on length of stay because of inconsistent and very imprecise results (one study, n = 68 participants).
On the basis of three RCTs (n = 529 participants), there is considerable uncertainty regarding the effect of prophylactic melatonin/melatonin agonists on the incidence of delirium due to very low‐quality evidence that has been downgraded because of risk of bias, imprecise and inconsistent results. There is moderate‐quality evidence that melatonin does not affect the duration of delirium, downgraded as the results are from a single study (n = 104). There is uncertainty regarding the effect of melatonin on severity of delirium due to moderate‐quality evidence from one study using a binary outcome (n = 104) and low‐quality evidence from a second study downgraded due to serious imprecision (n = 6). There is moderate‐quality evidence that melatonin does not reduce the length of stay (two studies; 500 participants); results were downgraded for inconsistency. There is uncertainty regarding the effect of melatonin on in‐hospital mortality due to low‐quality evidence from three studies that was downgraded because of imprecise results and a very small number of events (n = 543 participants).
On the basis of two RCTs (n = 2057 participants), there is moderate‐quality evidence that BIS‐guided anaesthesia reduces the incidence of delirium compared to BIS‐blinded anaesthesia/clinical judgement. The evidence was downgraded due to the risk of bias associated with participants and personnel being unblinded and incomplete outcome assessment. There was also an unclear risk of selection bias in Radtke 2013. There is also moderate‐quality evidence that BIS‐guided anaesthesia resulted in a shorter length of hospital admission compared to BIS‐blinded anaesthesia/clinical judgement (two studies, n = 2057 participants), also downgraded due to risk of bias.
Potential biases in the review process
This review has followed Cochrane procedures and there were only a small number of amendments to the review process, which are outlined in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
The previous version of this review (Siddiqi 2007) only included six studies, none of which assessed the same intervention. The review highlighted the potential role for multi‐component intervention (a Geriatric Consultation Service) and the use of atypical antipsychotic medication, but identified the need for a larger body of evidence before drawing conclusions or practice recommendations. The evidence base for multi‐component interventions for the prevention of incident delirium in hospitalised non‐ICU patients has expanded considerably since the previous version, and the evidence summarised in this update supports the use of multi‐component interventions. However, we found a continuing lack of evidence to support the use of antipsychotic medication as a group in the prevention of delirium.
Our principal review finding of the positive role of multi‐component interventions to prevent delirium is consistent with the wider published literature (Abraha 2015). The multi‐component intervention programme known as the Hospital Elder Life Program (HELP) for Prevention of Delirium has demonstrated effective reductions in the incidence of delirium in non‐randomised trials (Inouye 1999a; Inouye 2000). Hshieh 2015 published a meta‐analysis of intervention studies using multi‐component non‐pharmacological interventions and, although identifying similar issues with heterogeneity limiting reporting, found evidence to support reductions in delirium incidence and falls. Two recent systematic reviews have reached similar conclusions to those of this review. Martinez 2015 identified that multi‐component interventions were effective in reducing incident delirium and accidental falls for hospitalised adults. Zhang 2013 specifically reviewed the role of interventions to prevent postoperative delirium and identified that multi‐component interventions were beneficial, although the review also identified positive benefits from sedation and antipsychotic medications not replicated by our findings.
Multi‐component interventions for delirium prevention are now also recognised and recommended in practice guidelines. The UK National Institute for Health and Care Excellence (NICE) guidelines for delirium were published in 2010 (NICE 2010). These identified multi‐component interventions as having a critical role in identifying and addressing modifiable, clinical risk factors for delirium prevention. Multi‐component assessment and intervention is recommended within 24 hours of admission for those at risk; the intervention should be personalised to the needs of the individual and delivered by a multidisciplinary team (NICE 2010). Cost savings are identified to be anticipated, although we found no data on this in our review.
The lack of impact of multi‐component interventions on mortality and institutionalisation, despite a reduction in delirium is a surprising finding. Falls and institutionalisation are thought to be associated with frailty and may represent complications of the frailty syndrome (Clegg 2013; Eeles 2012; Fried 2001). Death and institutionalisation as endpoints may, therefore, represent non‐modifiable manifestations of frailty, and be relatively insensitive to a reduction in incident delirium, although a recent study has questioned the association of delirium with frailty (Joosten 2014). Reporting baseline frailty in future trials (measured with a validated frailty assessment instrument) would help to clarify this relationship.
Our findings for cholinesterase inhibitors are consistent with previous related studies. A large trial of another cholinesterase inhibitor, rivastigmine, for treatment of delirium in intensive care patients was halted in 2010 following safety concerns and no evidence of effectiveness (Sheldon 2010; van Eijk 2010).
Findings for antipsychotics are also consistent with a recent published review (Fok 2015).
The heterogeneity of our results for melatonin has also been reported by Chen and colleagues (Chen 2015). They conducted a subgroup analysis, and concluded that melatonin was effective in preventing delirium in medical, but not surgical patients.
Authors' conclusions
Implications for practice.
The evidence base for multi‐component interventions to prevent delirium in patients admitted to medical and surgical wards is strong and supports the adoption of systems of care that incorporate multi‐component interventions to prevent delirium in hospitals as part of routine care.
Implications for research.
Further “proof of concept” randomised controlled trials investigating the effectiveness of multi‐component interventions to prevent delirium in hospitalised non‐ICU patients are unwarranted (and unethical, as an effective treatment is denied to the control group). The focus of future research should be trials of implementation and to identify the key 'active' components to improve our understanding of the determinants for successful and efficient deployment of multi‐component interventions. Such trials should consider cluster randomisation (to minimise performance bias); incorporate more discriminatory baseline descriptors (to better account for delirium, frailty, and dementia interactions); and have at least a medium‐term follow‐up period (to assess the personal and system‐level impact of delirium prevention). Preliminary evidence for the content of multi‐component interventions suggests that they should include as a minimum: staff education; individualised care (sometimes referred to as person‐centred care); re‐orientation at frequent intervals; and early mobilisation, but this needs further investigation. These areas are familiar aspects of care but are currently poorly and unreliably delivered.
Monitoring the depth of anaesthesia through awareness of the Bispectral index (BIS) and the ability to control the level of anaesthesia reduced the incidence of postoperative delirium. However, the optimal level for depth of anaesthesia has not been established in the included studies and this remains an area for further research.
The role of drugs and other anaesthetic techniques (to reduce postoperative delirium) in the prevention of delirium remains uncertain with negative or conflicting findings. New research is justified, particularly regarding the role of typical and atypical antipsychotics and melatonin (including different settings, variations in physiological melatonin levels and different doses), but should account for developments in the understanding of the neuropathophysiology of delirium. In the case of atypical antipsychotics, the association between antipsychotics and increased mortality amongst older people with cognitive impairment may limit their usefulness as a prophylactic measure in this population (Huybrechts 2012). Furthermore, given the current evidence base supporting the use of multi‐component interventions, future trials of pharmacological agents for delirium prevention should optimise multi‐component non‐pharmacological delirium prevention in intervention and control arms to look for any additional benefit obtained from medication. The evidence does not support cholinesterase inhibitors for delirium prevention as a priority for further investigation.
What's new
| Date | Event | Description |
|---|---|---|
| 8 February 2016 | New search has been performed | Conclusions changed; authors changed |
| 31 January 2016 | New citation required and conclusions have changed | Review updated with results of searches in January 2013, February 2014, January 2015 and December 2015. Changes to authors as described in section 'Differences between protocol and review'. |
History
Protocol first published: Issue 4, 2005 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 23 January 2015 | New search has been performed | An update search was performed for this review on 23 January 2015. |
| 25 February 2014 | New search has been performed | An update search was performed for this review on 25 February 2014. |
| 20 January 2013 | New search has been performed | An update search was performed for this review on 20 January 2013. |
| 24 November 2010 | New search has been performed | An update search was performed for this review on 18 November 2010. The search retrieved new studies for consideration by the authors. |
| 18 March 2008 | New search has been performed | The update searches of March and October 2008 retrieved some studies for consideration by the authors. |
Acknowledgements
We are grateful to Anna Noel‐Storr who helped to develop the search strategy and performed the searches for this review.
We are also grateful to Dr de Jonghe who provided us with a copy of her group's publication (de Jonghe 2014) after correspondence about the abstract which had been identified by the update search in February 2014. We also thank Professor Kotaro Hatta for providing data specific to the subgroup of patients not taken to ITU to facilitate inclusion of quantitative data, not reported in the original paper (Hatta 2014).
We would also like to thank Robin W.M. Vernooij for his assistance with our 'Summary of findings' tables.
Appendices
Appendix 1. Search Strategy
| Source | Strategy |
| ALOIS www.medicine.ox.ac.uk/alois [last searched: 4 Dec 2015] |
delirium OR DEL |
| MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present (Ovid SP) [last search: 4 Dec 2015] |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. Delirium, Dementia, Amnestic, Cognitive Disorders/su [Surgery] 14. obnubilat*.ti,ab. 15. or/1‐14 16. Primary Prevention/ 17. prevent*.mp. 18. reduc*.ti,ab. 19. stop*.ti,ab. 20. taper*.ti,ab. 21. avoid*.ti,ab. 22. "cut* down".ti,ab. 23. or/16‐22 24. 15 and 23 25. randomized controlled trial.pt. 26. controlled clinical trial.pt. 27. randomi?ed.ab. 28. placebo.ab. 29. drug therapy.fs. 30. randomly.ab. 31. trial.ab. 32. groups.ab. 33. or/25‐32 34. (animals not (humans and animals)).sh. 35. 33 not 34 36. 35 and 34 |
| EMBASE 1974 to 2015 Week 01 (Ovid SP) [last search: 4 Dec 2015] |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. Delirium, Dementia, Amnestic, Cognitive Disorders/su [Surgery] 14. obnubilat*.ti,ab. 15. or/1‐14 16. primary prevention/ 17. prevent*.mp. 18. reduc*.ti,ab. 19. stop*.ti,ab. 20. taper*.ti,ab. 21. avoid*.ti,ab. 22. "cut* down".ti,ab. 23. or/16‐22 24. 15 and 23 25. randomized controlled trial/ 26. random*.ti,ab. 27. placebo.ti,ab. 28. trial.mp. 29. controlled clinical trial/ 30. or/25‐29 31. 24 and 30 |
| PsycINFO 1806 to December Week 1 2015 (Ovid SP) [last search: 4 Dec 2015] |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. obnubilat*.ti,ab. 14. or/1‐13 15. Prevention/ 16. prevent*.mp. 17. reduc*.ti,ab. 18. stop*.ti,ab. 19. taper*.ti,ab. 20. avoid*.ti,ab. 21. "cut* down".ti,ab. 22. or/15‐21 23. 14 and 22 24. random*.mp. 25. trial.mp. 26. placebo*.mp. 27. group.ab. 28. or/24‐27 29. 23 and 28 |
| CINAHL (EBSCOhost) [last search: 4 Dec 2015] |
1 deliri* 2 "acute psycho‐organic syndrome" or "clouded state" or "clouding of consciousness" or "exogenous psychosis" or "toxic psychosis" or "toxic confusion" 3 "acute brain confusion" or "acute brain failure" or "acute organic psychosyndrome" or "acute brain syndrome" or "metabolic encephalopathy" 4 "Delirium"/ without‐subheadings 5 #1 or #2 or #3 or #4 6 "Preventive‐Trials"/ without‐subheadings 7 prevent* or avoid* 8 #6 or #7 9 #5 and #8 10 random* or placebo* or control* or "normal care" or "standard care" or "normal treatment" or "standard treatment" 11 #9 and #10 12 "Alcohol‐Withdrawal‐Delirium"/ without‐subheadings 13 "delirium tremens" in TI 14 #12 or #13 15 #11 not #14 16 (animal in DE) not ((human in DE) and (animal in DE)) 17 #15 not #16 |
| LILACS (BIREME) [last search: 4 Dec 2015] |
deliri$ OR delirio OR loucura [Words] and randomly OR randomised OR randomized OR trial OR ensaio clínico [Words] |
| ISI Web of Science – all databases (ISI Web of Science) [last search: 4 Dec 2015] |
Topic=(deliri* OR "acute confusion*" OR "acute organic psychosyndrome" OR "acute brain syndrome" OR "metabolic encephalopathy" OR "acute psycho‐organic syndrome" OR "clouded state" OR "clouding of consciousness" OR "exogenous psychosis" OR "toxic psychosis" OR "toxic confusion" OR obnubilat*) AND Topic=(prevent* OR reduc* OR stop* OR taper* OR avoid* OR "cut* down") AND Topic=(randomised OR randomized OR randomly or placebo or "double‐blind" or trial OR groups OR "controlled study" OR RCT OR "single‐blind*") Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH. Lemmatization=On |
| CENTRAL (The Cochrane Library, Wiley) [last search: 4 Dec 2015] |
#1 MeSH descriptor Delirium, this term only #2 deliri* #3 "acute confusion*" #4 "acute organic psychosyndrome" #5 "acute brain syndrome" #6 "metabolic encephalopathy" #7 "acute psycho‐organic syndrome" #8 "clouded state" #9 "clouding of consciousness" #10 "exogenous psychosis" #11 "toxic psychosis" #12 "toxic confusion" #13 obnubilat* #14 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 MeSH descriptor Primary Prevention, this term only #16 prevent* #17 reduc* #18 stop* #19 taper* #20 avoid* #21 "cut* down" #22 (#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 (#14 AND #22) |
| ClinicalTrials.gov [last search: 4 Dec 2015] |
Search 1: randomized AND delirium AND hospital AND prevention | Interventional Studies | Adult, Senior |received Search 2: prevention AND (delirium OR toxic psychosis OR toxic confusion) | Interventional Studies | Adult, Senior | |
| ICTRP [last search: 4 Dec 2015] |
#1 Advanced search: Condition: delirium AND date rec: 01/10/2008‐23/01/2015 #2 Basic search: Prevention AND delirium #3 Basic search: prevent AND delirium |
Appendix 2. Summary of update searches and returned hits
| Source | December 2015 Hits |
January 2015 Hits |
February 2014 Hits |
January 2013 Hits |
November 2010 Hits |
| ALOIS | 2 | 0 | 29 | 99 | 31 |
| MEDLINE (Ovid SP) | 91 | 95 | 92 | 191 | 139 |
| EMBASE (Ovid SP) | 197 | 178 | 183 | 329 | 257 |
| PSYCINFO (Ovid SP) | 24 | 27 | 18 | 64 | 35 |
| CINAHL (EBSCO Host) | 25 | 13 | 21 | ‐ | 45 |
| LILACS (BIREME) | 0 | 0 | 15 | 1 | 54 |
| ISI Web of Knowledge (all databases) |
94 | n/a | 148 | 260 | 166 |
| CENTRAL (The Cochrane Library) |
39 | 27 | 22 | 41 | 33 |
| Clinicaltrials.gov | 2 | 0 | 6 | Search 1: 30 Search 2: 56 |
80 |
| ICTRP (WHO Portal) |
2 | 4 | 44 | ‐ | 74 |
Data and analyses
Comparison 1. Multi‐component delirium prevention intervention (MCI) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 7 | 1950 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.81] |
| 1.1 Medical patients | 4 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.92] |
| 1.2 Surgical patients | 3 | 585 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.59, 0.85] |
| 2 Incidence of delirium in patients with dementia | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 2.1 Surgical patients | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 3 Duration of delirium | 4 | 244 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.96, 0.64] |
| 3.1 Medical patients | 2 | 63 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐2.43, 1.13] |
| 3.2 Surgical patients | 2 | 181 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐7.27, 2.46] |
| 4 Severity of delirium | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.65, ‐0.43] |
| 4.1 Medical patients | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.46, ‐0.08] |
| 4.2 Surgical patients | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐2.20, ‐0.58] |
| 5 Length of admission | 6 | 1920 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.48, 0.51] |
| 5.1 Medical patients | 3 | 1335 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.44, 0.52] |
| 5.2 Surgical patients | 3 | 585 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐4.74, 2.25] |
| 6 Cognition | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| 6.1 Medical patients | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 9.10 [7.20, 11.00] |
| 7 Improvement in Activities of Daily Living | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| 7.1 Medical patients | 1 | 341 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.91, 1.47] |
| 8 Return to independent living | 4 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 8.1 Medical patients | 1 | 648 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.2 Surgical patients | 3 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.19] |
| 9 Depression | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| 9.1 Surgical patients | 1 | 149 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.44, 1.84] |
| 10 Withdrawal from protocol | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Surgical patients | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Falls | 3 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.16, 2.01] |
| 11.1 Medical patients | 1 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.03] |
| 11.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.18, 3.46] |
| 12 Pressure ulcers | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 12.1 Surgical patients | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 13 Inpatient mortality | 3 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.43] |
| 13.1 Medical patients | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.34, 1.18] |
| 13.2 Surgical patients | 2 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.69, 3.05] |
| 14 12 month mortality | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 14.1 Surgical patients | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.56] |
| 15 Cardiovascular complication | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.78, 1.65] |
| 16 Urinary tract infection | 1 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.45, 3.20] |
| 17 Mental health worsened | 1 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.64, 1.20] |
Comparison 2. Prophylactic cholinesterase inhibitor versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| 1.1 Donepezil | 2 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.17, 2.62] |
| 2 Duration of delirium | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severity of delirium | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| 3.1 Donepezil | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐4.17, 3.57] |
| 4 Length of admission | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| 4.1 Donepezil | 3 | 128 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.54, 0.86] |
| 5 Cognition | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| 5.1 Donepezil | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.45, 1.65] |
| 6 Withdrawal from protocol | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| 6.1 Donepezil | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.49, 1.87] |
| 7 Adverse events (continuous) | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| 7.1 Donepezil | 1 | 33 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.26, 0.52] |
| 8 Adverse events (binary) | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 6.25 [0.35, 112.52] |
Comparison 3. Prophylactic antipsychotic versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 3 | 916 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.59] |
| 1.1 Haloperidol | 2 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.60] |
| 1.2 Olanzapine | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.52] |
| 2 Duration of delirium | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐2.74 [‐9.59, 4.11] |
| 2.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐6.4 [‐9.38, ‐3.42] |
| 2.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 0.60 [0.10, 1.10] |
| 3 Severity of delirium | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐6.80, 4.76] |
| 3.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐5.86, ‐2.14] |
| 3.2 Olanzapine | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.41, 3.39] |
| 4 Length of admission | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| 4.1 Haloperidol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐5.5 [‐12.17, 1.17] |
| 5 Cognition | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐7.42, ‐2.38] |
| 6 Withdrawal from protocol | 2 | 925 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.24] |
| 6.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.26] |
| 6.2 Olanzapine | 1 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.71, 1.46] |
| 7 Adverse events | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| 7.1 Haloperidol | 1 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.43] |
| 8 Pneumonia | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 7.28 [0.38, 140.11] |
| 9 Urinary tract infection | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.03, 2.31] |
| 10 Congestive heart failure | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.07, 16.52] |
Comparison 4. Prophylactic melatonin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 3 | 529 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.09, 1.89] |
| 2 Duration of delirium | 1 | 104 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.57, 0.57] |
| 3 Severity of delirium (binary severe vs. not severe) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.58, 1.27] |
| 4 Severity of delirium (DRS‐R‐98) | 1 | 6 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐19.47, 11.27] |
| 5 Length of admission | 2 | 500 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐1.20, 1.39] |
| 6 Cognitive impairment | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.04] |
| 7 Activities of daily living | 1 | 369 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.20, 1.20] |
| 8 Use of psychotropic medication (binary) | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.18] |
| 9 Antipsychotic medication use (cumulative) | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.79, ‐0.21] |
| 10 Benzodiazepine use (cumulative) | 1 | 378 | Mean Difference (IV, Random, 95% CI) | ‐11.60 [‐24.34, 1.14] |
| 11 Withdrawal from study | 2 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.87] |
| 12 In‐hospital mortality | 3 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.88] |
| 13 Mortality by 3 months | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.67, 1.45] |
| 14 Adverse events | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.14. Analysis.
Comparison 4 Prophylactic melatonin versus placebo, Outcome 14 Adverse events.
Comparison 5. Prophylactic citicoline versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Incident delirium day 1 post surgery | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.22, 2.06] |
| 2 Cognitive status | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐3.85, 0.91] |
Comparison 6. Oral premedication with diazepam and diphenhydramine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
Comparison 6 Oral premedication with diazepam and diphenhydramine, Outcome 1 Incident delirium.
Comparison 7. Intravenous methylprednisolone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 2 Length of admission | 1 | 7507 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.20, 0.20] |
| 3 Mortality at 30 days | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.07] |
| 4 Myocardial injury | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.38] |
| 5 Respiratory failure | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.05] |
| 6 Infection | 1 | 7507 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.06] |
7.4. Analysis.
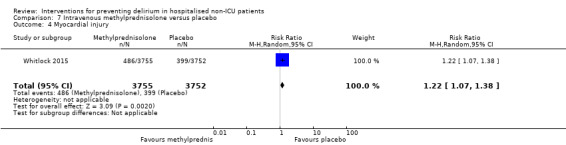
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 4 Myocardial injury.
7.5. Analysis.
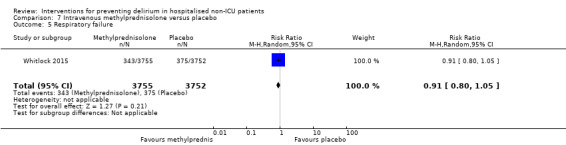
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 5 Respiratory failure.
7.6. Analysis.
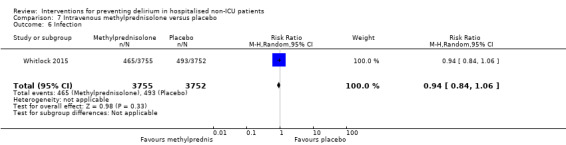
Comparison 7 Intravenous methylprednisolone versus placebo, Outcome 6 Infection.
Comparison 8. Gabapentinoids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 1.90] |
| 2 Length of admission | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.12, 0.92] |
| 3 Cognition | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐2.76, 4.76] |
| 4 Psychotropic Medication Use | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.38] |
| 5 Withdrawal from protocol | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.50, 161.13] |
Comparison 9. Ketamine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.21, 19.23] |
| 2 Withdrawal from protocol | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.34] |
Comparison 10. Intravenous parecoxib sodium analgesia versus Morphine and Saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.26, 0.98] |
| 2 Length of admission | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| 3 Postoperative cognitive dysfunction at 3 days | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.21, 1.02] |
| 4 Postoperative cognitive dysfunction at 1 week | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.98] |
| 5 Postoperative cognitive dysfunction at 3 months | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.01] |
| 6 Postoperative cognitive dysfunction at 6 months | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.11] |
10.3. Analysis.
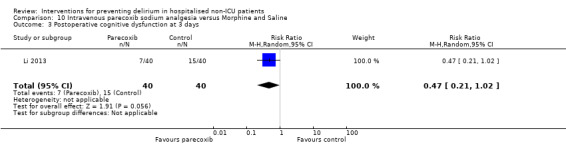
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 3 Postoperative cognitive dysfunction at 3 days.
10.5. Analysis.
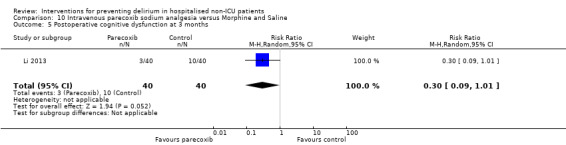
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 5 Postoperative cognitive dysfunction at 3 months.
10.6. Analysis.
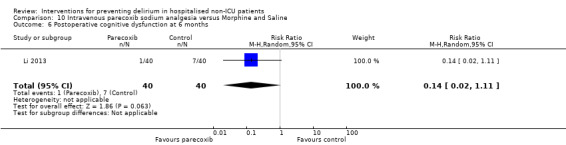
Comparison 10 Intravenous parecoxib sodium analgesia versus Morphine and Saline, Outcome 6 Postoperative cognitive dysfunction at 6 months.
Comparison 11. Intrathecal morphine and PCA morphine versus PCA morphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.9 [0.44, 1.85] |
| 2 Length of admission | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.51, 0.51] |
| 3 Cognition ‐ days for MMSE to return to preoperative level | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.03, 1.43] |
| 4 Withdrawal from protocol | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.17] |
| 5 Mortality | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.13] |
Comparison 12. Fascia iliaca compartment block (FICB) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.24, 0.87] |
| 2 Severity of delirium | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐6.81, ‐1.79] |
| 3 Duration of delirium | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐9.50, ‐1.90] |
| 4 Mortality | 1 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.05, 5.58] |
Comparison 13. Light versus deep propofol sedation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.89] |
| 2 Duration of delirium | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.30, 2.10] |
| 3 Length of admission | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.80, 1.20] |
| 4 Cognition on day 2 | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 3.10 [0.30, 5.90] |
| 5 In‐hospital mortality | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 5.36] |
| 6 Postoperative complications (>=1) | 1 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.60, 1.26] |
Comparison 14. Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 2 | 2057 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.85] |
| 2 Length of admission | 2 | 2057 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.45, ‐0.43] |
| 3 Cognition at 7 days | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.05] |
| 4 Cognition at 3 months | 2 | 1990 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.97] |
| 5 SF‐36 mental summary score | 1 | 902 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.40, ‐0.40] |
| 6 Mortality at 7 days | 1 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.42, 5.25] |
| 7 Mortality at 3 months | 2 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.59] |
| 8 Cardiac complications | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.39] |
| 9 Respiratory complications | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.07] |
| 10 Infective complications | 1 | 902 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.55, 0.95] |
14.8. Analysis.
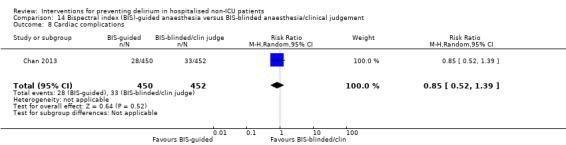
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 8 Cardiac complications.
14.9. Analysis.
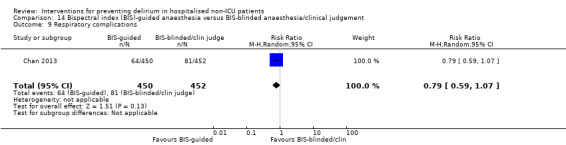
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 9 Respiratory complications.
14.10. Analysis.
Comparison 14 Bispectral index (BIS)‐guided anaesthesia versus BIS‐blinded anaesthesia/clinical judgement, Outcome 10 Infective complications.
Comparison 15. Sevoflurane versus propofol anaesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.34] |
| 2 Mortality at 12 months | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.70, 2.02] |
Comparison 16. Xenon versus sevoflurane anaesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.79] |
| 2 Length of admission | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐1.72, 9.72] |
| 3 In‐hospital mortality | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse events | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.34, 1.64] |
| 5 Sepsis | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.29, 7.73] |
Comparison 17. Epidural anaesthesia versus general anaesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 2 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.69, 2.03] |
| 2 Length of admission > 10 days | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.28, 1.24] |
| 3 Cognitive decline | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.06] |
| 4 Urinary tract infection | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.57, 3.09] |
| 5 Psychological morbidity | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| 5.1 Depression | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.23, 4.71] |
| 6 Postoperative complications | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.35, 2.39] |
| 7 Pressure ulcer | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.36] |
Comparison 18. Liberal versus restrictive blood transfusion thresholds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.45, 1.27] |
| 2 Delirium severity | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.99, 2.79] |
| 3 Length of admission | 1 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.36, 1.16] |
| 4 Psychoactive medication use | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.87, 1.12] |
| 5 Infection | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.23, 5.22] |
| 6 Congestive heart failure | 1 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.05, 5.88] |
Comparison 19. Fast‐track surgery versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.77] |
| 2 Length of admission | 1 | 233 | Mean Difference (IV, Random, 95% CI) | ‐4.20 [‐4.60, ‐3.80] |
| 3 Urinary tract infection | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.04] |
| 4 Heart failure | 1 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.10, 0.91] |
Comparison 20. Postoperative delirium‐free protocol (DFP) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.06] |
| 2 Length of admission | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐12.51, 3.91] |
| 3 Behavioural disturbance | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
Comparison 21. Computerised clinical decision support system (CCDS) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.82, 1.43] |
| 2 Length of admission | 1 | 424 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐0.35, 2.15] |
| 3 Mortality within 30 days of discharge | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.49, 2.23] |
| 4 Falls | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.39, 2.19] |
| 5 Pressure ulcers | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.84] |
Comparison 22. Geriatric unit care versus orthopaedic unit care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incident delirium | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.79, 1.22] |
| 2 Duration of delirium | 1 | 163 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.04, 0.04] |
| 3 Severity of delirium | 1 | 163 | Mean Difference (IV, Random, 95% CI) | 1.5 [1.00, 4.00] |
| 4 Length of admission | 1 | 329 | Mean Difference (IV, Random, 95% CI) | 3.0 [1.94, 4.06] |
| 5 Cognitive function (composite score) at 4 months | 1 | 228 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐5.92, 9.52] |
| 6 Incident dementia at 12 months | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.60, 8.49] |
| 7 ADL function at 4 months | 1 | 239 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐0.70, 2.70] |
| 8 Institutionalisation at 4 months | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.58, 1.91] |
| 9 Institutionalisation at 12 months | 1 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 10 Inpatient mortality | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.21, 1.47] |
| 11 Falls | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.61, 2.77] |
| 12 Pressure ulcers | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.10, 1.41] |
| 13 Other medical adverse events | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.23] |
| 14 Postoperative complications | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.20, 2.36] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abizanda 2011.
| Methods | Design: Randomised controlled trial of a short‐term occupational therapy intervention in an acute geriatric unit Date of study: November 2002 to June 2003 Power calculation: Yes Frequency of outcomes assessment: Daily during hospitalisation Inclusion criteria: All patients aged 65 and over consecutively admitted to the acute geriatric unit with an acute medical illness or exacerbation of existing chronic condition Exclusion criteria: None reported |
|
| Participants | Number in study: 400 Country: Spain Setting: One acute geriatric unit Age: Mean age 83.7 years (SD 6.1) in intervention group, 83.3 years (SD 6.5) in control group Sex: 43.4% male in intervention group, 43.1% male in control group Co‐morbidity: Number of previous chronic conditions 3.8 in intervention group, 3.5 in control group Dementia: 35.3% in intervention group, 31.4% in control group |
|
| Interventions | Intervention: Occupational therapy intervention (OTI) schedule consisted of a daily 45‐minute session with patient and relative/caregiver Monday‐Friday for the duration of admission. Activities were carried out according to needs and day of admission. Therapeutic plan included: cognitive stimulation; instruction on preventing complications including immobility, confusion, falls, urinary incontinence, pressure sores; retraining in ADL; assessment of technical aids for home. Control: All participants received medical treatment, nursing care, physical therapy and social assistance. |
|
| Outcomes | 1. Incident delirium, measured using CAM 2. Length of admission 3. Activities of daily living (ADL), measured using Barthel index 4. In‐hospital mortality 5. Adverse events |
|
| Notes | Funding source: Institute of Health Sciences, Junta de Comunidades de Castilla‐La Mancha. Declarations of interest: "All authors declare that there is not any personal, financial or potential conflict of interest, and therefore have nothing to declare." Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Assignment to randomised group by a geriatrician who did not participate in the clinical management of participants |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The geriatricians caring for the patients and providing their routine care were blinded to allocated group. Participants were not blinded due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor and the individual performing data analysis were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number with missing data are balanced between groups and there do not appear to be any systematic differences between the groups. |
| Selective reporting (reporting bias) | Low risk | No changes were made to trial outcomes after the trial was initiated |
| Other bias | Low risk | No evidence of other bias |
Aizawa 2002.
| Methods | Design: Randomised controlled trial of a delirium‐free protocol administered postoperatively in a general and colorectal surgery unit Date of study: November 1996 to March 1999 Power calculation: No Frequency of outcomes assessment: Twice daily screening interview after surgery for 7 consecutive days Inclusion criteria: Consecutive patients over 70 and under 86 years who underwent resection of gastric or colorectal cancer under general anaesthesia in one hospital department Exclusion criteria: Liver cirrhosis or dysfunction; renal dysfunction; respiratory disturbance; other poor risk factors; mental disorder; visual impairment; extended resection of other organs or emergency surgery |
|
| Participants | Number in study: n = 42 randomised, outcomes reported for n = 40 Country: Japan Setting: General surgery inpatients Age: Mean age 75.9 (SD 4.5) for intervention group; mean age 76.2 (SD 4.1) for control group Sex: 26 males and 14 females (15/20 males in intervention and 11/20 in control group) Co‐morbidity: Not reported Ilness severity: APACHE score 8.3 (SD 1.4) for intervention and 7.6 (SD 1.7) in control group Dementia: Not known |
|
| Interventions | Intervention: Delirium‐free protocol (DFP): Post surgery, Diazepam 0.1 mg/kg IM at 20.00, Flunitrazepam 0.04 mg/kg IV and Pethidine 1 mg/kg IV infusions 20.00‐04.00 for 3 nights Control: Treatment as usual. No placebo |
|
| Outcomes | 1. Incident delirium in 7 postoperative days by psychiatrist using DSM‐IV criteria 2. Behavioural disturbance in 7 postoperative days 3. Length of admission |
|
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium not excluded at enrolment Intervention used likely to sedate and therefore interfere with assessments for delirium Very specific patient group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation method unclear thus allocation is unclear |
| Random sequence generation (selection bias) | Unclear risk | Stated random assignment but method not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants and personnel unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment made by psychiatrist unaware of original allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two dropouts but not clear from which group and no data presented for these |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | High risk | The issue of how delirium was assessed in patients who might be sedated by the DFP is not addressed |
Al‐Aama 2011.
| Methods | Design: Randomised controlled trial of melatonin for 14 days or until discharge in a medical unit in a tertiary care hospital Date of study: October 2007 to February 2008 Power calculation: No Frequency of outcomes assessment: Every 24 to 48 hours during admission Inclusion criteria: admissions of 65 years and older to through the emergency department to Internal Medicine inpatient services Exclusion criteria: Expected stay or life expectancy <48 hours; unable to communicate in English; unable to take oral medications; had an intracranial bleed or seizures; INR <1 or >4 while on warfarin; known allergy to the study compounds |
|
| Participants | Number in study: 145 Country: Canada Setting: Internal Medicine inpatient services in a tertiary care hospital Age mean (SD): Intervention: 84.3 (5.9), Control 84.6 (6.2); P = 0.8 Sex: Male Intervention 46%, Control 39%; P= 0.58 Co‐morbidity: mean number(SD) Intervention 5.3 (2.3), 5.2 (1.9); P = 0.48 Dementia: Intervention 18%, Control 23%; P = 1.0 |
|
| Interventions | Intervention: Melatonin tablets half of 1 mg, rapid dissolving, daily for 14 days or until discharge Control: Lactose tablets 100 mg halved, similar in appearance |
|
| Outcomes | 1. Incident delirium measured using CAM 2. Delirium severity, measured using MDAS but included prevalent cases 3. Length of admission 4. Use of psychotropic medication 5. Withdrawal from protocol 6. Mortality |
|
| Notes | Funding source: Divison of Geriatric Medicine, University of Western Ontario Declarations of interest: "None of the authors or study team members has had any conflict of interest or any affiliation or relation with any melatonin producing organization" Delirium not excluded at enrolment, but data available for prevalent delirium Four participants not randomised‐ unclear why |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Pharmacy kept randomisation code |
| Random sequence generation (selection bias) | Low risk | Patients were assigned using computer‐generated blocked‐randomisation (block size: 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded. In case of emergency, an independent physician could request unmasking of the treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the assessments were carried out by research assistants and clinicians blinded to group assignment. The investigators did not become aware of treatment allocation until several months after study completion |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals and missing data for 11 in intervention group, 12 in control group. Reasons for missing data not separated by group, therefore difficult to tell whether reasons could be due to side effect of study medication, or more delirium episodes in one group. The results are presented as available case analysis rather than intention‐to‐treat. The authors present a sensitivity analysis to consider worst case figures for delirium incidence that all those missing from the intervention group have delirium and that none of those in the control group had delirium. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Ashraf 2015.
| Methods | Design: Randomised controlled trial of oral premedication with diazepam and diphenhydramine versus no premedication in older people undergoing cardiac catheterisation Date of study: Not reported Power calculation: Yes Frequency of outcomes assessment: 4 hours post‐procedure and 1‐day post‐procedure for inpatients Inclusion criteria: Aged > 70 years; elective cardiac catheterisation Exclusion criteria: MMSE <20; pre‐existing delirium on CAM; allergy to diphenhydramine, diazepam or midazolam |
|
| Participants | Number in study: 93 (53% inpatients; demographic data for entire sample) Country: USA Setting: Cardiac catheterisation facility within a single site medical centre Age: Mean age 78 years (SD 4.8) in intervention group; 77 years (SD 3.5) in control group Sex: Males 25 (53%) in intervention; 28 (61%) in control Co‐morbidity: Data reported on rates of hypertension, diabetes, hyperlipidaemia, coronary artery disease, anxiety, depression, delirium, COPD and atrial fibrillation. Imbalance on CAD 34% vs 52% and depression 13% vs 4% Dementia: Baseline MMSE comparable between groups. Excluded if MMSE < 20 |
|
| Interventions | Intervention: Oral premedication with diazepam 5 mg and diphenhydramine 25 mg Control: No premedication prior to procedure |
|
| Outcomes | 1. Incident delirium using CAM 2. Cognitive function using MMSE (data not fully reported in paper) 3. Length of stay (data not fully reported in paper) |
|
| Notes | Funding source: Not reported Declaration of interest: Not reported Delirium excluded at enrolment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo given to the control group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | States ‘the catheterization laboratory staff and nursing staff that took care of patients after the procedure and majority of the operators were unaware of the randomisation' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete reporting of all included participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Beaussier 2006.
| Methods | Design: Randomised controlled trial of intrathecal morphine versus patient‐controlled intravenous morphine for postoperative analgesia and recovery after major colorectal surgery Date of study: July 2001 to December 2003 Power calculation: Yes Frequency of outcomes assessment: Not reported Inclusion criteria: Cancer of left colon or rectum with surgical indication for resection in patients over 70 years with normal preoperative functional status Exclusion criteria: ASA III/IV, BMI > 30, IBD, contraindications to intrathecal morphine, preoperative mental dysfunction, chronic pain, preoperative opioid consumption, psychiatric disorders, inability to use PCA |
|
| Participants | Number in study: 59 Country: France Setting: One surgical department Age: Mean age 78 years (SD 5 years) in intervention group, 77 years (SD 5 years) in control group Sex: 58% male in intervention group, 46% male in control group Co‐morbidity: Not reported Dementia: Mean preoperative MMSE 27 (SD 2) in intervention group, 28 (SD 2) in control group |
|
| Interventions | Intervention: Preoperatively, a dose of 300 mcg of morphine was injected via the L4/L5 interspace. Postoperatively, patients had IV PCA. Control: Preoperatively, a 3 mL dose of saline was injected into the subcutaneous space between L4/L5. Postoperatively, patients had PCA. Postoperative management was identical for all patients. |
|
| Outcomes | 1. Incident delirium, measured using CAM 2. Cognitive status, defined as number of days for MMSE to return to preoperative value 3. Length of admission 4. Mortality 5. Withdrawal from protocol |
|
| Notes | Funding Source: Institutional grant from the Assistance Publique‐Hopitaux de Paris Declarations of interest: Not reported Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A physician independent from the study group opened a sealed letter that assigned the group of allocation according to the rank of inclusion |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded as already under general anaesthesia. Personnel providing care for the patient blinded to their assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind RCT but no statement of outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/59 patients not included in final analysis although reasons for exclusion reported |
| Selective reporting (reporting bias) | High risk | Reported outcomes which were not pre‐specified in the methods |
| Other bias | Low risk | No evidence of other bias |
Berggren 1987.
| Methods | Design: Randomised trial of epidural and general anaesthesia in patients operated on for fracture neck of femur Date of study: March 1983 to November 1984 Power calculation: No Frequency of outcomes assessment: First and seventh day postoperatively Inclusion criteria: All fully lucid, consenting patients admitted to an orthopaedic unit for fracture neck of femur Exclusion criteria: Score more than 6/36 on 12 item disorientation sub‐scale of Organic Brain Syndrome (OBS) assessed within 3 hours of admission |
|
| Participants | Number in study: 57 Country: Sweden Setting: Orthopaedic ward of one university hospital Age mean years (SD): Epidural 78(8), General 77(7) Sex M:F: Epidural 4/24, General 7/22 Co‐morbidity: No significant differences between groups (Chi2 test) for ischaemic heart disease, hypertension, diabetes mellitus, cerebrovascular disease, respiratory disease, depression, parkinsonism or sensory impairment Dementia: Not mentioned specifically but would in effect be excluded by exclusion criteria |
|
| Interventions | Intervention: Epidural anaesthesia Comparison: Halothane anaesthesia | |
| Outcomes | 1. Incident delirium measured using a modified version of the Organic Brain Syndrome Scale on postoperative days 1 and 7 2. Length of admission (data not fully reported) 3. Physical morbidity (stroke, urinary tract infection) 4. Psychological morbidity (depression) 5. Pressure ulcers |
|
| Notes | Funding source: Swedish Medical Council; King Gustav V Birthday Foundation; Umea University Research Foundation Declarations of interest: Not reported Delirium not excluded at enrolment No data presented for length of admission but reported as no difference between the two groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not described |
| Random sequence generation (selection bias) | Unclear risk | Method for random sequence generation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors did not know allocation of participants at time of testing for delirium |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in outcome reporting |
| Selective reporting (reporting bias) | High risk | Reported outcomes which were not pre‐specified in the methods |
| Other bias | Low risk | No evidence of other bias |
Bonaventura 2007.
| Methods | Design: Randomised controlled trial of a multi‐component intervention, the Intervention to Prevent Delirium (IPD) in older patients admitted to medical and geriatric wards Date of study: 2005 to 2006 Power calculation: No Frequency of outcomes assessment: Days 1, 2, 4 and 7 of admission Inclusion criteria: Age > or = to 65 years admitted to medical and geriatric wards in one hospital Exclusion criteria: MMSE score < or =25, at least 1 relative not present, transfer out of ward, pre‐existing dementia, blindness, deafness, aphasia or unable to understand Italian |
|
| Participants | Number in study: 60 Country: Italy Setting: Medical and geriatric wards Age: Not given Sex M:F: Intervention 12/18, Control 12/18 Co‐morbidity: comparable P = 0.77 Dementia: Excluded |
|
| Interventions | Intervention: Intervention to Prevent Delirium (IPD), a series of structured and standardised welfare actions based on existing guidelines, including support in the following areas: cognitive re‐orientation, sensory and environmental, mobilisation, hydration, and 'socio‐emotional' Control: Usual care, not described further |
|
| Outcomes | 1. Incident delirium measured using CAM & DRS‐R‐98 on days 1, 2, 4, 7 of hospital stay 2. Cogntive status using MMSE 3. Functional performance using Barthel Index |
|
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Odd and even days of admission used so concealment unlikely |
| Random sequence generation (selection bias) | High risk | Sequence generated using day of admission |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded, not possible given nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Boustani 2012.
| Methods | Design: Randomised controlled trial of a clinical decision support system to improve the care of hospitalised older adults with cognitive impairment Date of study: July 2006 to March 2008 Power calculation: No Frequency of outcomes assessment: Every weekday during hospital admission Inclusion criteria: At least 65 years of age, hospitalised on a medical ward, English‐speaking, and cognitive impairment at the time of hospital admission. Exclusion criteria: Patients were excluded if they had previously been enrolled in the study, were aphasic, or unresponsive at the time of screening |
|
| Participants | Number in study: 427 Country: USA Setting: Medical wards of Wishard Memorial University Hospital Age: Mean age 76.8 years (SD 7.9 years) in intervention group, 77.6 years (SD 8.3 years) in control group Sex: 39.7% male in intervention group, 28.9% male in control group Co‐morbidity: Mean Charlson comorbidity index 1.8 (SD 1.8) in intervention group, 2.4 (SD 2.1) in control group Dementia: Not reported |
|
| Interventions | Intervention: Electronically delivered clinical decision support system (CDSS) (1) Each time a physician enters an order for a patient randomised to the intervention arm, the physician received non‐interruptive alerts of the presence of CI, Foley catheter, physical restraints, anticholinergic drugs, or the need for ACE services; (2) If the physician orders a urinary catheter, s/he will receive interruptive alerts to recommending discontinuing the catheter; (3) If the physician orders physical restraints, s/he will receive interruptive alerts recommending substituting physical restraints with the use of a professional sitter or low dose trazodone; (4) If the physician orders any of the 18 inappropriate anticholinergics, s/he will receive interruptive alerts recommending stopping the drug, suggesting an alternative, or recommending dose modification. (5) The physician was required to make a decision to accept, reject, or modify any of the interruptive alerts. Control: Patients randomised into usual care did not receive CDSS |
|
| Outcomes | 1. Incident delirium, measured using CAM 2. Mortality 3. Length of hospital stay 4. Falls 5. Pressure ulcers |
|
| Notes | Funding source: NIA Paul B. Beeson K23 Career Development Award Declarations of interest: "Dr Boustani has work supported by grants from the NIA and AHRQ. He is also a member of the Pfizer speakers' bureau. Dr Buckley has provided expert testimony for local law firms. Mr Perkins owns stock in several pharmaceutical firms" Delirium assessed but not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Central process following computer generation |
| Random sequence generation (selection bias) | Low risk | A computer‐generated process was employed for sequence generation in a 1:1 ratio |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind personnel treating the patients in the CDSS group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of research assistants conducting outcome assessments not known |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 427 enrolled into trial, outcome data available for 424 with no account given for missing participants or which group they were assigned to. However, small as proportion of total sample. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Chan 2013.
| Methods | Design: Prospective randomised double‐blinded parallel group study assessing BIS‐guided anaesthesia in elective surgical patients Date of study: January 2007‐December 2009 Power calculation: Not for delirium as delirium was a secondary outcome. Study underpowered given delirium rate of 20% Frequency of outcomes assessment: mornings after surgery, 1 week, 3 months Inclusion criteria: > 60yrs old; scheduled for elective major surgery anticipated to last > 2 hours or longer which has an anticipated hospital stay of at least 4 days Exclusion criteria: unavailable/unable to co‐operate with interviews; illiteracy; hearing/visual impairment; major psychosis; CNS diseases; suspected dementia/MMSE 23 or less |
|
| Participants | Number in study: 921 Country: Hong‐Kong Setting: General hospital Age: Mean age of 68.1 (SD 8.2) in intervention group 67.6 (SD 8.3) in control group Sex: 62.2% of intervention group and 60.4% of control group were male Co‐morbidity: no significant differences in pre‐existing medical conditions (cardiovascular, respiratory, endocrine or other) between intervention and control groups Dementia: Excluded is MMSE 23 or less |
|
| Interventions | Intervention: BIS‐guided anaesthesia ‐ anaesthetic dosage adjusted to maintain BIS value between 40‐60 from commencement of anaesthesia to the end of surgery; alarm sounded when out of range Control: Routine care, anaesthetic drug administration was titrated according to clinical judgment. BIS monitoring was continued in this group, but the BIS number, its trend, and the EEG waveform were omitted from the display, specifically designed for this trial |
|
| Outcomes | 1. Incident delirium, measured using CAM 2. Length of admission 3. Cognitive status (postoperative cognitive dysfunction) at 1 week and 3 months 4. Mortality at 1 week and 3 months 5. Postoperative complications 6. Psychological morbidity, measured using Short‐Form‐36 Mental Score |
|
| Notes | Funding source: Research Grants Council of Hong Kong and Health and Health Services Research Fund Declarations of interest: "The authors have no conflicts of interest to disclose" Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | No evidence that allocations know |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment accessed via intranet |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients, surgeons and all research staff were blinded but, concern re: anaesthetists and theatre team in view of alarm system for intervention group only |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data available for n = 783 at one week and n = 835 at 3 months but n = 921 were randomised. Reasons for exclusion reported: n = 80 were excluded in the intervention group and n = 58 in the control group at one week; n = 32 were excluded in the intervention group and n = 25 in the control group at three months. In n = 97 cases participants were not assessed at one week due to being 'unfit for testing', compared with n = 5 at three months |
| Selective reporting (reporting bias) | Unclear risk | Limited protocol available on Centre for Clinical Trials online registry |
| Other bias | Low risk | No evidence of other bias |
de Jonghe 2014.
| Methods | Design: Multi‐centre randomised controlled trial Date of study: November 2008‐May 2012 Power calculation: performed, study adequately powered Frequency of outcomes assessment: Daily following inclusion until discharge; 3‐month follow‐up Inclusion criteria: Patients 65 years and older admitted for surgical treatment of hip fractures; enrolment within 24 hours of admission; individual willing to participate; medically able to receive study medication according to the protocol for the duration of the study Exclusion criteria: Delirium at enrolment; patients transferred from another hospital; if postoperative admission to the ICU or coronary care unit was anticipated; inability to speak or understand Dutch; concomitant use of melatonin |
|
| Participants | Number in study: 452 Country: The Netherlands Setting: Teaching hospitals Age: Mean age 84.1 (SD 8.0) in intervention group, 83.4 (SD 7.5) in control group Sex: 53 (28.5%) male in intervention group, 62 (32.3%) of control group Co‐morbidity: Median Charlson Index 1.0 (IQR: 0.8‐2.0) in intervention group, 1.0 (IQR: 1.0‐2.0) in control group Dementia: Median MMSE 23 (IQR: 12‐28.8) in intervention group with 104 (55.9%) described as having cognitive impairment. Median MMSE 23 (IQR: 9.5‐28.0) in control group with 106 (55.2%) described as having cognitive impairment |
|
| Interventions | Intervention: 3 mg of melatonin Control: Placebo |
|
| Outcomes | 1. Incident delirium during the first eight days after initiation of the study medication using DSM‐IV and DOSS 2. Duration of delirium 3. 'Severe' delirium (defined as percentage of patients who received a total of ≥3mg haloperidol) 4. Length of admission 5. Use of psychotropic medications (reported as total dose rather than frequency of administration) 6. Cognitive outcomes at 3 months, using Charlson Index, IQCODE and MMSE 7. Functional outcomes at 3 months, using Katz ADL Index 8. In‐hospital mortality 9. Mortality at 3 months |
|
| Notes | Funding source: Dutch National Program of Innovative Care for vulnerable older persons (a program operated by ZonMw, a Dutch institute that funds health research) Declarations of interest: None declared Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation blinded, randomisation list maintained by the trial pharmacist |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified by study centre, with fixed blocks of 10 patients within each stratum. Before the start of the study, an independent statistician generated a randomisation schedule and the trial pharmacist maintained the randomisation list Not described method of sequence generation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, other staff members and patients remained blinded until after the last patient had completed the study and the follow‐up and data analyses had been completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above, blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 452 were randomised of which 70 did not complete the study, generally balanced between the groups although rates of prevalent delirium different between groups. Complete reporting of reasons for withdrawals and missing data. |
| Selective reporting (reporting bias) | Low risk | Outcome data presented as per pre‐published protocol |
| Other bias | Low risk | No evidence of other bias |
Diaz 2001.
| Methods | Design: Randomised controlled study of citicoline in hip fracture surgery patients Date of study: Study dates not reported Power calculation: Yes, indicates 88 patients needed, but results for 81 given Frequency of outcomes assessment: Immediately and on days 1, 2 and 3 postoperatively Inclusion criteria: 70 years or over, admitted with hip fracture Exclusion criteria: Organic brain disorder, major cerebrovascular disease, anaesthetic risk ASA IV |
|
| Participants | Number in study: 81 Country: Chile Setting: Multi‐centre orthopaedic or trauma departments Age mean years (SD): Citicoline 79.5 (6.6), Control 80.0 (5.9) P = 0.9 Sex M:F: Citicoline 4/31, Control 10/36; P = 0.2 Co‐morbidity: Specific conditions not described. Present in 28/35 in intervention group and 39/46 in control group Dementia: Excluded |
|
| Interventions | Intervention: Citicoline 400 mg orally 8 hourly, given between 24 hrs before and 4 days after surgery (n = 35). Control: Placebo matched for colour, consistency and flavour (n = 46) If anticholinergics and benzodiazepines were being used they were stopped, and anaemia and haemodynamic variables corrected in both groups | |
| Outcomes | 1. Incident delirium immediately, day 1, day 2 and day 3 postoperatively using MMSE, AMT, CAM 2. Cognitive status, using MMSE | |
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium excluded at enrolment using MMSE, AMT, CAM Study underpowered, as incidence of delirium much lower than the 20% used in power calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Carried out and codes kept by hospital pharmacy independently of researchers |
| Random sequence generation (selection bias) | Low risk | 'Lottery drawing' independently of researchers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample size reported but unclear how many randomised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Fukata 2014.
| Methods | Design: Randomised open‐label trial of postoperative low dose intravenous haloperidol in older patients undergoing abdominal, orthopaedic or other surgery Date of study: January 2007 ‐ December 2012 Power calculation: Yes Frequency of outcomes assessment: Daily from postoperative day 0 to day 7 Inclusion criteria: 75 years or older; elective abdominal surgery under general anaesthesia or elective orthopaedic surgery under general or spinal anaesthesia and who could consent to participate Exclusion criteria: Emergency surgery; preoperative NEECHAM score < 20; periodic dosing with newly added or switched antipsychotics, antidepressants, hypnotics or anti‐Parkinson agents within 2 weeks prior to surgery; previous treatment with haloperidol for delirium after surgery before the initiation of postoperative preventive haloperidol administration. |
|
| Participants | Number in study: 121 Country: Japan Setting: General and orthopaedic surgery units in five co‐operative hospitals Age: Mean age 80.5 years (SD 0.5) in intervention group versus 80.2 (SD 0.5) for controls Sex: Males: Intervention 32/59; Control: 32/62 Co‐morbidity: Abdominal surgery in 52 intervention and 55 controls; orthopaedic surgery in 5 intervention and 4 control; and other surgery in 2 intervention and 3 control patients; No differences in urinary incontinence, past history of excitement/hyperkinesia; or use of oral psychotropics Dementia: Not specifically assessed. MMSE score (mean (SD) in intervention = 23.3 (0.7) and 23.0 (0.7) in control patients |
|
| Interventions | Intervention: 2.5 mg/day of intravenous haloperidol dissolved in 100 mL of saline for first 3 days after surgery. Administered by infusion at 6 pm. Control: Usual care |
|
| Outcomes | 1. Delirium incidence using NEECHAM 2. Delirium incidence stratified by low MMSE score (data not fully reported in paper) 3. Delirium severity using NEECHAM (data not fully reported in paper) 4. Delirium duration (data not fully reported in paper) 5. Adverse events (data not fully reported in paper) |
|
| Notes | Funding source: Research Grant for Longevity Sciences (17C‐3, 21‐13) from the Ministry of Health, Labour and Welfare and The Research Funding for Longevity Sciences (23‐28) from the National Center for Geriatrics and Gerontology (NCGG), Japan Declaration of interest: The authors declare 'no conflicts of interest' Delirium not fully excluded at enrolment ‐ excluded if NEECHAM < 20 but this may not exclude all delirium Haloperidol given one day postoperatively rather than preoperatively or immediately postoperatively as in other studies, and prevalent delirium not excluded. Inclusion criteria only mention abdominal and orthopaedic surgery but results presented for 5 patients who underwent ‘other’ including vascular surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation, adjusted for age, gender and department |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unblinded to allocation; control group did not receive any IV medication/placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study; delirium assessment unblinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data reported on 119/121 patients. 2 patients in control group received haloperidol for delirium on day of surgery, therefore withdrawn |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
Gauge 2014.
| Methods | Design: Randomised controlled trial of optimisation of intraoperative depth of anaesthesia and cerebral oxygenation Date of study: Study dates not reported Power calculation: Yes ‐ powered as pilot study Frequency of outcomes assessment: Assessed at 3 +/‐ 1 days following surgery Inclusion criteria: Aged over 64 years, undergoing coronary artery bypass graft surgery Exclusion criteria: Not reported |
|
| Participants | Number in study: 81 Country: Not reported Setting: Not reported Age: Mean age 71.9 years (whole sample) Sex: 86% male (whole sample) Co‐morbidity: Not reported Dementia: Baseline MMSE ranged from 24 to 30 for whole sample |
|
| Interventions | Intervention: Intraoperative monitoring of depth of anaesthesia using bispectral index and cerebral oxygenation monitoring Control: Surgery performed blinded to bispectral index and cerebral oxygenation monitoring | |
| Outcomes | 1. Incidence of postoperative delirium using CAM | |
| Notes | Funding source: Not reported Declarations of interest: Not reported Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided ‐ abstract only |
| Random sequence generation (selection bias) | Unclear risk | No information provided ‐ abstract only |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided ‐ abstract only |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided ‐ abstract only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided ‐ abstract only |
| Selective reporting (reporting bias) | Unclear risk | No information provided ‐ abstract only |
| Other bias | Unclear risk | No information provided ‐ abstract only |
Gruber‐Baldini 2013.
| Methods | Design: Randomised controlled trial of liberal blood transfusion thresholds compared to restrictive transfusion practice for hip fracture patients Date of study: April 2008‐February 2009 Power calculation: Yes Frequency of outcomes assessment: multiple times within 5 days after randomisation or up to hospital discharge (if hospital stay was shorter) Inclusion criteria: aged 50 and older; undergoing surgical repair of hip fracture; Hb < 10 g/dL within 3 days after surgery; clinical evidence of cardiovascular disease or cardiovascular disease risk factors Exclusion criteria: non‐English speaking; unable to walk unaided before fracture; declined blood transfusions; multiple traumas; pathological hip fracture; clinical acute myocardial infarction within 30 days pre‐randomisation; previous participants in the trial; symptoms associated with anaemia; actively bleeding at time of potential randomisation |
|
| Participants | Number in study: 139 Country: USA and Canada Setting: 13 hospitals Age: Mean age 82.4 (SD 7.4) in intervention group compared to 80.6 (SD 10.4) in control group Sex: 81.8% of intervention group were female compared to 47% of control group Co‐morbidity: numbers and percentages of common co‐morbidities reported in paper (stroke/TIA, chronic lung disease, cancer, diabetes, atrial fibrillation, Parkinson's disease, hearing problems, visual problems and alcohol abuse or withdrawal) Dementia: 27.3% of intervention group had dementia compared to 36.1% of the control group |
|
| Interventions | Intervention (aka liberal treatment): One unit of packed red blood cells and as much blood as needed to maintain a haemoglobin concentration >10 g/dL Control (aka restrictive treatment): only transfused if symptoms of anaemia developed or at the study physicians discretion or if Hb < 8 g/dL |
|
| Outcomes | 1. Incident delirium, using CAM 2. Delirium severity, using MDAS 3. Length of admission 4. Psychoactive medication use 5. Physical morbidity (post‐randomisation adverse events) |
|
| Notes | Funding source: Research grant from National Heart Lung and Blood Institute Declarations of interest: "Dr Magaziner received support from Amgen, Eli Lilly, Glaxo SmithKline, Merck, Novartis and Sanofi Aventis to conduct research through his institution, provide academic consultation, or serve on an advisory board. Dr Roffey reports working as a consultant for Palladian Health. Dr Cardson reports receiving grant support to his institution from Amgen. Dr Marcantionio is a recipient of a Mid‐Career Investigator Award in Patient‐Oriented Research from the National Institute on Aging" Delirium assessed at baseline but not excluded >1/3 of the restrictive group received transfusion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | No evidence to suggest allocations revealed |
| Random sequence generation (selection bias) | Low risk | Automated central telephone randomisation system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research staff unblinded to treatment status except at one site |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 139 randomised, outcome assessment data available for 138 |
| Selective reporting (reporting bias) | Low risk | Data reported for all participants included in the study |
| Other bias | High risk | Imbalance in dementia prevalence between intervention and control groups (27.3% in intervention versus 36.1% in control) |
Hatta 2014.
| Methods | Design: Randomised controlled trial of ramelteon, a melatonin agonist Date of study: September 2011 to October 2012 Power calculation: Yes Frequency of outcomes assessment: Daily for up to seven days Inclusion criteria: aged 65‐89; newly admitted for serious medical problems; able to take oral medications Exclusion criteria: expected stay or life expectancy less than 48 hours; severe liver dysfunction; Lewy body disease; delirium at time of admission; patients taking fluvoxamine; those with mood disorders; drug or alcohol withdrawal |
|
| Participants | Number in study: 43 were admitted to acute medical wards (67 in total study cohort, 24 admitted to ICU) Country: Japan Setting: Acute medical wards in four university hospitals and one general hospital Age: Mean age 78.2 (SD 6.6) in the ramelteon group and 78.3 (SD 6.8) in the placebo group Sex: 48% of the intervention group were male compared with 32% of the placebo group Comorbidity: Charlson Index mean 3.2 (SD 2.4) in intervention group compared with 2.6 (SD 2.2) in placebo group Dementia: Clinical Dementia Rating mean score 0.5 (SD 0.7) in the intervention group compared with 0.6 (SD 0.9) in the placebo group |
|
| Interventions | Intervention: Ramelteon tablet 8 mg daily at 9 pm until development of delirium or up to seven days Control: Lactose powder 330 mg daily at 9 pm until development of delirium or up to seven days |
|
| Outcomes | 1. Incidence of delirium using DRS‐R‐98, cut‐off 14.5 2. Severity of delirium using DRS‐R‐98 3. Withdrawal from protocol 4. Adverse events 5. Inpatient mortality |
|
| Notes | Funding source: Japan Society for the Promotion of Science (Grant‐in‐Aid for Scientific Research) Declaration of interest: Authors declare receiving honoraria from & serving as consultants for Eli Lilly, Janssen, GlaxoSmithKline, Shionogi; Merck Sharp &Dohme; Otsuka; Pfizer; Mochida; Tsumura; Dainippon‐Sumitomo; Daiichi‐Sankyo; Eisai, and Ono Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed using envelope method |
| Random sequence generation (selection bias) | Low risk | Random number table, sealed opaque envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded, nurses administering medication not blinded; although other personnel blinded. Placebo not similar to active tablet |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | Reporting of outcomes as identified in the protocol published on the UMIN‐CTR registry 00005591 |
| Other bias | Low risk | No evidence of other bias |
Hempenius 2013.
| Methods | Design: multi‐centre, randomised controlled trial Date of study: June 2007‐June 2010 Power calculation: Yes but study underpowered Frequency of outcomes assessment: days 1‐10 postoperatively, 3 times per day Inclusion criteria: over 65 yrs; due to undergo elective surgery for a solid tumour, deemed to be frail (using Groningen Frailty Indicator >3) Exclusion criteria: unable to complete protocol; unable to complete follow‐up; unable to complete questionnaire |
|
| Participants | Number in study: 297 Country: The Netherlands Setting: 3 hospitals (1 university medical centre, 1 teaching hospital and 1 community hospital) Age: Mean age 77.45 (SD 6.72) in intervention group; 77.63 (SD 7.69) in usual care group Sex: 62.2% of intervention group were female compared with 65.8% of usual care group Co‐morbidity: stratified into < or equal to 2 co‐morbidities (39.6% of intervention group 40.4% of usual care group) or >2 co‐morbidities (60.4% in intervention group 59.6% of usual care group) Dementia: MMSE performed at baseline; mean score 26.6 in intervention group vs. 26.33 in usual care group (P = 0.49) |
|
| Interventions | Intervention: Multi‐component intervention focused on best supportive care and the prevention of delirium. Preoperative geriatric team assessment with daily monitoring during hospital stay, supported by the use of standardised checklists Usual care: only had access to geriatric care if treating physician requested referral |
|
| Outcomes | 1. Incident delirium, using DOSS ‐ if > 3 then had specialist assessment using DSM‐IV. Assessments performed up to 10 days postoperatively 2. Delirium severity, using DRS‐R‐98 3. Length of admission 4. Mortality 5. Return to independent living 6. Postoperative complications 7. Quality of life using Short‐Form‐36 8. Falls |
|
| Notes | Funding source: Netherlands Organisation for Health Research and Development Declarations of interest: "The authors declared that no competing interests exist" Delirium not excluded at enrolment No record of how many in usual care group received geriatrician input |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Central allocation system |
| Random sequence generation (selection bias) | Low risk | Interactive voice response telephone system for randomisation provided by university |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and research nurses unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Delirium assessment blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 297 participants randomised, outcome assessments available for 260 (n = 127 in intervention group and n = 133 in control group) ‐ no information provided, described as 'lost to follow‐up' |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per original protocol |
| Other bias | Low risk | No evidence of other bias |
Jeffs 2013.
| Methods | Design: Randomised controlled trial Date of study: May 2005‐December 2007 Power calculation: yes ‐ incorporating incident delirium and absolute risk reduction of 6% Frequency of outcomes assessment: every 48 hours Inclusion criteria: aged 65 years or older; admitted to a medical unit in the study area; in hospital < 48 hours Exclusion criteria: severe dysphasia rendering communication impossible; death expected within 24 hours; isolation for infection control; documented contraindication to mobilisation; admission to the Stroke Unit or to critical care; planned admission of < 48 hours; major psychiatric diagnosis; previous inclusion in the study; delirium documented in the admission notes; transfer from another hospital. |
|
| Participants | Number in study: 649 Country: Australia Setting: Acute medical wards, secondary referral centre Age: Mean age of 79.6 (SD 7.5) in intervention group, 79.1 (7.9) in control group Sex: 45% of intervention group were male, compared to 50% of control group Co‐morbidity: Charlson index of 2 (1‐3) in both groups at baseline Dementia: MMSE recorded at baseline in both groups: 25 (20‐28) in intervention group vs. 26 (19‐28) in control group |
|
| Interventions | Intervention: Participants randomised to the intervention arm received a graded physical activity and orientation programme twice daily, which was delivered in addition to usual care. A certified Allied Health Assistant, trained in administering exercise programmes, delivered the intervention after initial assessment of the participant by a physiotherapist. The programme started on the same day as the participant was randomised. Commensurate with ability, participants were prescribed one of four exercise programmes: bed, seated, standing or rails. All programmes were customised to the participant’s ability and were reviewed daily. Exercise programmes were modified to ensure suitable progression for those participants who made significant gains. The orientation programme comprised formal and informal elements. The formal element of the programme comprised a series of seven questions aimed at assessing and improving orientation (day, month, year, date, ward, bed number and name of primary nurse). The participant was asked the questions in sequence and prompted with the correct answer if they were not able to give a correct response. The informal element of the programme related to engaging in the exercise programme and in the social interaction with the Allied Health Assistant and/or Physiotherapist. Control: Usual care included 24‐hour nursing care, daily medical assessment and allied health referral by medical, nursing or other staff. Allied health input was provided on referral only, but daily ward meetings were held to review patient progress and facilitate referrals. Patients with significant functional, cognitive or social issues could be referred to the Aged Care medical consultation service that performed a daily round and could offer advice regarding the recognition, investigation and management of geriatric syndromes including delirium. |
|
| Outcomes | 1. Incidence of delirium, using CAM 2. Duration of delirium 3. Severity of delirium, using CAM 4. Length of stay 5. Return to previous residence |
|
| Notes | Funding source: HCF Health and Medical Research Foundation Declarations of interest: "No competing interests" Very low rates of delirium in both arms. Authors suggest may be due to 48 hourly assessments or not selecting those at high risk. Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes for allocation |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not clear, just states 'randomisation was achieved using sealed opaque envelopes' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not informed of allocation, but unable to fully blind due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n = 17 in intervention and n = 18 in control did not receive the intervention, but were assessed on an intention‐to‐treat analysis basis |
| Selective reporting (reporting bias) | Low risk | Trial protocol retrospectively registered with Australian New Zealand Clinical Trials Registry ACTRN 012605000044628; outcomes reported in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
Jia 2014.
| Methods | Design: Randomised controlled trial of fast‐track surgery for colorectal cancer compared to usual care Date of study: 2008‐2011 Power calculation: No Frequency of outcomes assessment: Day of admission and then daily from postoperative days 1 to 5 Inclusion criteria: patients aged 70 years and over with colorectal cancers admitted to the Fourth Hospital of Hebei Medical Univerity for open curative resection. Exclusion criteria: history of dementia; Parkinson's disease; alcohol intake of > or equal to 250 g/day; long‐term use of sleeping pills or anxiolytics; those who received anaesthesia within the past 30 days. Enrolled patients who were given intraoperative blood transfusions or were admitted to the ICU were excluded from analysis. |
|
| Participants | Number in study: 240 Country: China Setting: University hospital Age: Mean age of 75.6 (SD 4.2) in intervention group; 74.8 (SD 4) in control group Sex: 65% of intervention group were male, compared to 60% of the control group Co‐morbidity: Hypertension and diabetes were recorded at baseline, no significant differences between the groups (P = 0.275 and 0.511 respectively) Dementia: those with diagnosed dementia were excluded from the study |
|
| Interventions | Fast‐track surgery group: Bowel preparation with oral purgatives instead of a mechanical enema; thoracic epidural anaesthesia and postoperative analgesic maintenance via the epidural catheter maintained for 48h; no nasogastric tube insertion; no drainage tube placement with the exception of the low rectal anastomosis; water was allowed from 6 hours post operation, liquid diet in the morning and semi‐liquid diet at noon and evening of the first and second postoperative day (POD) with regular diet on POD 3; early urine catheter withdrawal; early out‐of‐bed mobilisation Traditional therapy group: usual preoperative and postoperative care |
|
| Outcomes | 1. Incidence of delirium, using DRS‐R‐98 2. Length of admission 3. Postoperative complications |
|
| Notes | Funding source: Not reported Declarations of interest: "No conflicts of interest" Delirium not clearly excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not clearly described |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel not blinded due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if psychiatrist performing outcome assessment was blinded to allocation or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n = 240 participants were randomised, outcome assessment available for n = 233. Three in intervention group and four in the control group did not receive their allocated intervention and were excluded from outcome assessment data ‐ these individuals did not meet study inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Kalisvaart 2005.
| Methods | Design: Randomised controlled study of haloperidol prophylaxis in patients undergoing hip surgery Date of study: August 2000 to August 2002 Power calculation: Yes Frequency of outcomes assessment: Daily Delirium Rating Scale Revised 98 (DRS‐R‐98), MMSE, Digit span by trained assessors Inclusion criteria: Patients aged 70 years or over admitted for acute or elective hip surgery, who were at intermediate or high risk of delirium postoperatively Exclusion criteria: Prevalent delirium, haloperidol allergy, prolonged QTc interval, use of cholinesterase inhibitors or levodopa, parkinsonism, epilepsy, inability to participate in interviews, delay in surgery more than 72 hrs from admission. |
|
| Participants | Number in study: 430 Country: The Netherlands Setting: 2 surgical and 3 orthopaedic wards in 1 teaching hospital Age mean (SD): Intervention 78.76.0), Control 79.66.3); P = 0.15 Sex M:F: Intervention 19.9%, Control 21.1% Co‐morbidity: Not reported Ilness severity: APACHE scores mean (SD) Intervention 13.4 (3.2), Control 13.3 (3.1) Dementia: Not reported |
|
| Interventions | Intervention: Haloperidol 0.5 mg orally three times daily on admission until 3 days postoperatively Control: Placebo tablets identical in appearance Proactive geriatric consultation offered to all patients in both groups If delirium occurred, patients treated with haloperidol or lorazepam (or both) 3 times daily in increasing doses depending on symptoms |
|
| Outcomes | 1. Incident delirium postoperatively using DSM‐IV and CAM
2. Delirium severity 3. Duration of delirium 4. Length of admission 5. Withdrawal from protocol 6. Adverse events |
|
| Notes | Funding source: Medical Center Alkmaar Declarations of interest: "Financial disclosure: none" Delirium at enrolment excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation by hospital pharmacy independent of researchers. Codes held in sealed envelopes. |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebos used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Members of the research team not involved in the clinical care of patients performed all baseline and outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete outcomes data available for n = 395, missing data for n = 35 (24 in control, 11 in intervention) 192/212 in intervention and 190/218 in control treated according to protocol. Outcome data available reported as intention‐to‐treat by study authors. More lost to follow‐up in placebo group than intervention group and lack of information about those who were lost. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Larsen 2010.
| Methods | Design: Randomised controlled trial of olanzapine to prevent postoperative delirium in elderly joint replacement patients Date of study: 2005 to 2007 Power calculation: Yes Frequency of outcomes assessment: Daily from postoperative day 1 to postoperative day 8 Inclusion criteria: All patients aged 65 years and over, patients aged less than 65 years with a history of delirium, impending joint‐replacement surgery, ability to speak English, and ability to provide informed consent Exclusion criteria: Diagnosis of dementia, active alcohol use (>10 drinks per week), a history of alcohol dependence or abuse, allergy to olanzapine, and current use of an antipsychotic medication |
|
| Participants | Number in study: 495 Country: USA Setting: Orthopaedic wards Age: Mean age 73.4 years (SD 6.1 years) in intervention group, 74.0 years (SD 6.2 years) in control group Sex: 48% female in intervention group, 60% female in control group Co‐morbidity: Not reported Dementia: Patients with dementia were excluded |
|
| Interventions | Intervention: First dose of olanzapine 5 mg (orally disintegrating tablet (ODT)) administered immediately before surgery in the pre‐anaesthesia care unit by nursing staff. Second dose of olanzapine 5 mg administered in the post‐anaesthesia care unit by nursing staff blind to the intervention arm. Control: Oral dispersible tablet placebo of similar appearance to the olanzapine tablet. |
|
| Outcomes | 1. Incident delirium, measured using CAM/DSM‐III‐R 2. Severity of delirium, measured using DRS‐R‐98 3. Duration of delirium 4. Withdrawal from protocol 5. Cognition using MMSE 6. Adverse events |
|
| Notes | Funding source: New England Baptist Hospital Research Department Declarations of interest: "Theodore A Stern, has been a consultant to and is on the speaker's bureau of Eli Lilly and Company, and has been a consultant to and shareholder of WiFiMed, the company that designed the Tablet PC data‐management software. No other authors reported conflicts of interest" Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence held in pharmacy department. Randomisation carried out by pharmacy department. |
| Random sequence generation (selection bias) | Low risk | Statistician provided pharmacy with a computer‐generated random‐number table. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Hospital pharmacy prepackaged the study drug and placebo in identical packages and blinded investigators and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments conducted by research assistants and nurses and verified by a clinical psychologist. All were blind to allocation group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 95 dropouts not included in final analysis (n = 47 in intervention, n = 48 in control). Reasons stated but imbalance between groups with loss due to anxiety, surgery cancelled and family pressure as significant factors. High rate of delirium (40% in placebo group vs 14.3% in intervention group), concern that some of the exclusions may influence outcome assessment |
| Selective reporting (reporting bias) | Low risk | Study protocol registered on ClinicalTrials.gov NCT000699946; outcomes reported in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
Leung 2006.
| Methods | Design: Pilot randomised controlled trial of gabapentin to decrease postoperative delirium in older patients Date of study: 2005 Power calculation: No Frequency of outcomes assessment: Daily from postoperative day 1 to postoperative day 3 Inclusion criteria: Consecutive patients who were > 45 years of age, undergoing surgery involving the spine, requiring general anaesthesia, and expected to remain in the hospital postoperatively for > 72 hours. Exclusion criteria: Patients who could not complete the delirium testing, already taking preoperative gabapentin, or with sensitivity to gabapentin. |
|
| Participants | Number in study: 21 Country: USA Setting: Elective spinal surgery Age: Mean age 59.6 years Sex: 48% female Co‐morbidity: Charlson co‐morbidity index 1.2 (SD 1.9) in intervention group, 0.5 (SD 1.0) in control group Dementia: Not reported |
|
| Interventions | Intervention: Gabapentin 900 mg administered by mouth 1 to 2 hours before surgery and anaesthesia. 900 mg dose continued daily for the first 3 postoperative days. Control: Placebo as control. Unclear whether matching placebo used. |
|
| Outcomes | 1. Incident delirium, measured using CAM | |
| Notes | Funding source: National Institute of Aging, National Institute of Health Declarations of interest: "Dr Rowbotham consults for, and owns stock in, a company developing an analogue of gabapentin, an investigational agent" Pilot trial Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Random number list given to the research pharmacist who prepared and delivered the designated drug to each study patient according to the randomised allocation. However, not clear how the random number list allocation was concealed from the pharmacist by the co‐investigator who created it. |
| Random sequence generation (selection bias) | Low risk | Computerised random number list generated by co‐investigator |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled so participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trained interviewer blinded to the study drug assignment measured the occurrence of delirium |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information presented to make judgment |
| Other bias | Low risk | No evidence of other bias |
Li 2013.
| Methods | Design: Randomised controlled trial of intravenous parecoxib sodium analgesia for those undergoing femoral head replacement Date of study: January 2011 ‐ May 2012 Power calculation: Unclear Frequency of outcomes assessment: 3 days, 1 month, 3 months & 6 months Inclusion criteria: age >70 years old; weight < 90 kg; diagnosed with femoral neck fracture caused by trauma and required for analgesia; anaesthetic risk ASA II or III; achieved satisfactory intraoperative anaesthesia outcome; sedation only by intravenous midazolam; maintain normal blood pressure and heart rate by ephedrine and atropine. Exclusion criteria: the score of MMSE < 23; have a history of psychosis or neurological disorder; severe peptic ulcer; long‐term use of antipsychotics or sedative medication; a history of alcohol abuse; a history of allergic to non‐steroid anti‐inflammatory drug; intraoperative blood transfusion; unable to accomplish preoperative cognitive function test due to communication disorders and poor educational background. |
|
| Participants | Number in study: 80 Country: China Setting: Recruited from the Emergency Department Age: Mean 76.6 (SD 2.6) Sex: Male sex 29 (36%) Co‐morbidity: Not described Dementia: Excluded those with low MMSE (< 23) and also those who could not perform pre‐op cognitive function tests (due to communication disorders and poor educational background) |
|
| Interventions | Intervention: Intravenous parecoxib sodium (non‐steroidal anti‐inflammatory medication). Dosage based by weight. Given 12 hourly over 3 days (total of 6 injections). Given up to 2 mg IV morphine if pain score elevated despite intervention. Control: Intravenous morphine 2 mg or 4 mg at first injection, thereafter given 5 injections of 2 mL of saline every 12 hours over 3 days (total of 6 injections). Could also be given up to 2 mg IV morphine if pain score elevated. |
|
| Outcomes | 1. Incident delirium using DSM‐IV 2. Length of admission 3. Postoperative cognitive dysfunction using APA criteria (3 days, 1 week, 3 months, 6 months) |
|
| Notes | Funding source: Science and Technology Development Project of Qingdao Science and Technology Bureau Declaration of interest: Not reported Unclear if delirium excluded at enrolment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Group assignment 'managed by one specific staff’ but not clear if allocation concealment maintained |
| Random sequence generation (selection bias) | Low risk | Random number tables used to generate randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, personnel administering medications and monitoring patient were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Paper states study was double‐blind, outcome assessment procedure not described in translation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Paper reports complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Potential confounding for unbalanced use of additional morphine doses between group; 7.9 mg in parecoxib group vs. 31.3 mg in morphine and saline group. |
Liptzin 2005.
| Methods | Design: Randomised controlled trial of donepezil in patients undergoing elective arthroplasty of the knee or hip Date of study: May 2000 to April 2003 Power calculation: Yes but used a higher estimate of delirium incidence than found in study Frequency of outcomes assessment: Daily pre‐ and postoperatively, and postoperative daily medical records review; delirium presence determined from this information at day 7 and 14 postoperatively Inclusion criteria: Patients over 50 years, able to give informed consent, admitted for elective knee or hip arthroplasty Exclusion criteria: Gastro‐oesophageal reflux disease, sick sinus syndrome, already using donepezil or intolerant to it, non‐English speaking |
|
| Participants | Number in study: 90 Country: USA Setting: Orthopaedic department in a medical academic centre Age mean(SD) years: Intervention 67.2 (8.7), Control 69.4 (8.9); P = 0.03 Sex M:F: Intervention 43%, Control 35%; P = 0.17 Co‐morbidity: Not reported Dementia: Not reported |
|
| Interventions | Intervention: Donepezil 5 mg once daily for 14 days before and after surgery, doubled to 10 mg if developed any symptoms of delirium Control: Placebo identical in appearance | |
| Outcomes | 1. Incident postoperative delirium, using DSM‐IV criteria from DSI and CAM 2. Duration of delirium (data not fully reported in paper) 3. Length of admission 4. Withdrawal from protocol | |
| Notes | Funding source: Pfizer Corporation Declarations of interest: "This study was supported by an unrestricted research grant from Pfizer Corporation. Dr Liptzin has also been a consultant or speaker for Pfizer, Novartis, Janssen, Forest Labs, and Bristol Myers Squibb" Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information on concealment not provided |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by research pharmacist, method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical capsules of active drug and placebo used so participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment by research assistant blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete follow‐up. Intention‐to‐treat analysis not conducted. Number of dropouts similar in both groups but sufficiently high to potentially affect results |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
Lundstrom 2007.
| Methods | Design: Randomised controlled trial of multi‐component delirium prevention intervention for older hip fracture patients Date of study: May 2000 to December 2002 Power calculation: Yes Frequency of outcomes assessment: All patients tested once between day 3 and day 5 postoperatively using organic brain scale, MMSE and geriatric depression scale. Delirium diagnosed retrospectively after the study had finished by specialist in geriatric medicine blind to allocation group on the basis of the nursing assessments by applying the DSM‐IV criteria. Inclusion criteria: Patients aged 70 years and older consecutively admitted to the orthopaedic department in Umea hospital, Sweden. Exclusion criteria: Age under 70, severe rheumatoid arthritis, severe hip osteoarthritis, severe renal failure, pathological fracture and patients who were bedridden before the fracture. |
|
| Participants | Number in study: 199 Country: Sweden Setting: Orthopaedic hip fracture patients Age: Mean age 82 years Sex: 74% female Co‐morbidity: No baseline between group differences in cardiovascular disease, respiratory disease, hypertension or diabetes. More patients in control group with depression (46% v 32%, P = 0.03) Dementia: 27.5 % in intervention group, 37.1% in control group |
|
| Interventions | Intervention: Multi‐disciplinary team providing comprehensive geriatric assessment, management and rehabilitation on a geriatric ward. Intervention comprising: staff education; teamwork; individual care planning; delirium prevention detection and treatment; prevention and treatment of complications; bowel/bladder function; sleep; decubitus ulcer prevention/treatment; pain management; oxygenation; body temperature measurement; nutrition; rehabilitation; secondary prevention of falls/fractures and osteoporosis prophylaxis. Control: Usual care on orthopaedic ward. |
|
| Outcomes | 1. Incident delirium, diagnosed retrospectively using DSM‐IV based on nursing notes (for the duration of the inpatient stay) and OBS (measured once between the 3rd and 5th postoperative day) 2. Duration of delirium, diagnosed retrospectively using DSM‐IV based on nursing notes and OBS 3. Length of admission 4. Cognitive status, measured using MMSE 5. Falls 6. New pressure ulcers 7. Psychological morbidity (Depression) 8. Mortality ‐ inpatient and at 12 months |
|
| Notes | Funding source: Swedish Research Council & Vardal Foundation Declarations of interest: Not reported Prevalent delirium not excluded at enrolment (21.8% intervention group, 30.9% control group) and patients with prevalent delirium appear to have been included in outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes to conceal allocation |
| Random sequence generation (selection bias) | Unclear risk | No information given on how randomisation sequence generated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All staff aware of allocation group, patients potentially aware due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff recording outcome measurements not blind to study arm. Blinded specialist made diagnosis of delirium retrospectively based on staff measurements and medical/ nursing records |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Imbalance in dementia prevalence between intervention and control groups (27.5% in intervention versus 37.1% in control) |
Lurati 2012.
| Methods | Design: Randomised controlled trial Date of study: February 2006‐October 2010 Power calculation: Yes Frequency of outcomes assessment: postoperative days 1, 2 and 7 or on the day of hospital discharge, whichever occurred first Inclusion criteria: patients scheduled for surgery under general anaesthesia were eligible if they either had proven coronary artery disease (CAD) and were scheduled for major surgery or had 2 or more risk factors for CAD and were scheduled for major vascular surgery Exclusion criteria: Current medication with sulphonylurea derivatives or theophylline unless stopped 2 or more days before surgery; current congestive heart failure; current unstable angina pectoris; preoperative haemodynamic instability, defined as the use of vasopressors; hepatic disease defined as alanine aminotransferase and/or aspartate aminotransferase values >100 U/L; renal insufficiency, defined as creatinine clearance < 30 mL/min; emergent surgery; severe chronic obstructive pulmonary disease, defined as forced expiratory volume in the first second of expiration < 1L; prior enrolment in the study; concurrent enrolment in another RCT; pregnancy; absence of written informed consent. |
|
| Participants | Number in study: 385 Country: Switzerland Setting: Tertiary referral hospital and two secondary care hospitals Age: Mean age 78 (SD 8) in sevoflurane group; 73 (SD 8) in propofol group Sex: 75% of sevoflurane group were male compared with 77.6% of propofol group Co‐morbidity: Numbers with history of CAD, TIA/Stroke, CHF and diabetes reported for both groups Dementia: not reported |
|
| Interventions | In both groups anaesthesia induction was with etomidate. The protocol did not regulate dosage for the induction or maintenance of anaesthesia or any other aspects of intraoperative management. Sevoflurane: Anaesthesia maintained using sevoflurane Propofol: Anaesthesia maintained using propofol |
|
| Outcomes | 1. Incidence of delirium using CAM 2. Mortality at 12 months |
|
| Notes | Funding source: University Hospital Basel; Roche Diagnostics; Abbot AG Declarations of interest: "Roche Diagnostics Switzerland provided in‐kind support (assay kits). Abbott AG Switzerland provided some financial support for the conduction of the study. No other potential conflicts of interest are to be disclosed for any of the authors." Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed opaque envelopes to conceal allocation |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants blinded to allocation, anaesthesiologists not blinded as able to work‐out allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Seventeen patients randomised in error, but reasons reported and excluded from analysis |
| Selective reporting (reporting bias) | High risk | Protocol for Trial of the Effect of Anesthetics on Morbidity and Mortality (TEAM‐Project) NCT00286585 but no information about reporting of delirium outcomes in original protocol |
| Other bias | Low risk | No evidence of other bias |
Marcantonio 2001.
| Methods | Design: Randomised controlled trial of proactive geriatric consultation in patients with hip fracture Date of study: Study dates not reported Power calculation: Yes. Study adequately powered for bivariate analyses but not for the multivariate or stratified analyses. Frequency of outcomes assessment: Daily interviews from enrolment to discharge to complete MMSE, DSI, CAM, MDAS Inclusion criteria: All patients aged 65 years and older, admitted for primary surgical repair of hip fracture, who were at intermediate or high risk of delirium (presence of 1 or more delirium risk factors) Exclusion criteria: Metatstatic cancer or comorbid illness reducing life expectancy to less than 6 months; Unable to obtain consent (or proxy assent) within 24 hrs of surgery, or 48 hrs of admission |
|
| Participants | Number in study: 126 Country: USA Setting: One academic centre orthopaedic department Age mean (SD): Intervention 78 (8), Control 80 (8); P = 0.39 Sex M:F: Intervention 21%, Control 22%; P = 0.9 Co‐morbidity: Charlson Index > 4 Intervention 39%, Control 33%; P = 0.49 Dementia: Intervention 37%, Control 51%; P = 0.13. However, dementia assessment only reported for 90% of participants |
|
| Interventions | Intervention: Proactive consultation by Consultant Geriatrician, with daily visits starting preoperatively or within 24 hrs post operatively for duration of admission. Protocol based targeted recommendations over and above what was already being done by team, limited to 5 at initial visit and 3 at follow‐up visits. Controls: Usual care, consisting of management by orthopaedic team and consultation by internal medicine or geriatrics on reactive rather than proactive basis. | |
| Outcomes | 1. Delirium incidence‐ total cumulative during admission, using CAM (performed daily throughout inpatient stay) 2. Delirium incidence in dementia subgroup 3. Delirium duration 4. Length of admission 5. Return to independent living 6. Withdrawals from protocol |
|
| Notes | Funding source: Older Americans Independence Center; Charles Farnworth Trust;
Declarations of interest: Not reported Delirium examined but not reported at intake, making interpretation of results for primary outcome of cumulative delirium incidence difficult |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared with allocation |
| Random sequence generation (selection bias) | Low risk | Random number table used to generate sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nature of intervention precluded blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent researchers conducted delirium assessments and timed not to coincide with Geriatrician consultation. States blinding successfully maintained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Imbalance in dementia prevalence between intervention and control groups (37% in intervention and 51% in control) |
Marcantonio 2011.
| Methods | Design: Pilot randomised controlled trial of donepezil for delirium after hip fracture Date of study: January 2007 ‐ August 2008 Power calculation: No Frequency of outcomes assessment: Daily during hospital stay and at weeks 2, 4 and 6 Inclusion criteria: Admitted to the orthopaedic service for surgical repair of hip fracture and: age 70 and older, English speaking, residence within 40 mile radius of medical centre, life expectancy 6 months or greater, not currently taking cholinesterase inhibitor therapy Exclusion criteria: Pathological fracture due to metastatic cancer, advanced dementia, little potential for functional recovery |
|
| Participants | Number in study: 16 Country: USA Setting: Orthopaedic hip fracture patients Age: Mean age 88.0 years (SD 5.2) in intervention group; 87.0 (3.7) in control group Sex: 71% female in intervention group; 44% female in control group Co‐morbidity: Not reported Dementia: 43 % in intervention group, 44% in control group |
|
| Interventions | Intervention: 5 mg dose of donepezil initiated on the day before or within 24 hours of surgery and continued for a total of 30 days. Control: Matching placebo. All participants received perioperative co‐management from a geriatric team on orthogeriatric ward |
|
| Outcomes | 1. Incident delirium, measured using CAM but not included in meta‐analysis as reported as cumulative measures within individuals 2. Delirium severity, measured using MDAS 3. Withdrawal from trial 4. Adverse events |
|
| Notes | Funding Source: National Institute of Aging Declarations of interest: "The authors have no financial or any other kind of personal conflicts with this paper" Delirium not excluded at enrolment Only 16 participants in pilot trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate allocation concealment likely: on‐site pharmacy prepared and dispensed active medication and placebo; study team masked to treatment assignment. |
| Random sequence generation (selection bias) | Unclear risk | Permuted block randomisation used but method of sequence generation not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Delirium assessment conducted by trained research interviewer blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed, all randomised participants included in the analysis |
| Selective reporting (reporting bias) | Low risk | Protocol for Supporting the Health of Adults Undergoing Orthopedic Surgery During the Recovery Period (SHARP) NCT00586196; reporting in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
Martinez 2012.
| Methods | Design: Randomised controlled trial of a multi‐component delirium prevention intervention provided by family members Date of study: September 2009‐June 2010 Power calculation: Yes Frequency of outcomes assessment: Daily during hospital stay Inclusion criteria: All patients at risk for delirium (> 70 years, cognitive impairment (MMSE < 24 prior to admission) alcoholism or metabolic imbalance at admission) Exclusion criteria: Delirium at admission, no family support, admitted to ward other than general medicine, those in a room with more than two beds |
|
| Participants | Number in study: 287 Country: Chile Setting: Internal medicine ward of acute hospital Age: Mean age 78.1 years (SD 6.3) in intervention group; 78.3 years (6.1) in control group Sex: 42% female in intervention group; 33% female in control group Co‐morbidity: Median Charlson comorbidity index (CCI) 2 (interquartile range, IQR, 1‐4) in intervention group, median CCI 2 (IQR 1‐3) in control group Dementia: 9% in intervention group, 8% in control group |
|
| Interventions | Intervention: Multi‐component non‐pharmacological intervention provided by family members, including education regarding confusional syndromes; provision of a clock and calendar; avoidance of sensory deprivation (glasses, denture and hearing aids available as needed); presence of familiar objects in the room; re‐orientation of patient provided by family members; extended visiting times (5 hours daily). Control: Usual care from the attending physician |
|
| Outcomes | 1. Incident delirium, measured using CAM performed daily, throughout admission 2. Duration of delirium 3. Length of admission 4. Falls |
|
| Notes | Funding source: Not reported Declarations of interest: "No conflicts of interest declared" Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by a statistician who was not involved in data collection |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel unblinded due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐1 treat analysis performed, 5% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other forms of bias |
Mouzopoulos 2009.
| Methods | Design: Randomised placebo‐controlled trial of fascia iliaca compartment block (FICB) prophylaxis for hip fracture patients at risk for delirium. Date of study: July 2004‐March 2008 Power calculation: No Frequency of outcomes assessment: Daily during hospitalisation Inclusion criteria: Men and women aged 70 years and older admitted for hip fracture surgery Exclusion criteria: Delirium at admission, metastatic hip cancer, history of bupivacaine allergy, use of cholinesterase inhibitors, severe coagulopathy, Parkinsonism, epilepsy, levodopa treatment, delay of surgery of more than 72 hours after admission, and inability to participate in interviews (profound dementia, respiratory isolation, intubation, aphasia, coma or terminal illness). |
|
| Participants | Number in study: 219 Country: Greece Setting: Orthopaedic ward Age: Mean age 72.7 years Sex: 74% female Co‐morbidity: Not reported Dementia: Not reported |
|
| Interventions | Intervention: Fascia iliaca compartment block (FICB) using a 0.25 mg dose of 0.3 mL/kg bupivacaine at admission and repeated daily until either delirium developed or hip fracture surgery was performed. 24 hours after surgery, the same dose of FICB was administered and repeated every 24 hours until either delirium occurred or discharge. Control: Matching placebo using water for injection following same regimen. |
|
| Outcomes | 1. Incident delirium measured using DSM‐IV/CAM 2. Delirium severity, measured using DRS‐R‐98 3. Duration of delirium 4. Mortality |
|
| Notes | Funding source: Not reported Declarations of interest: "The authors declare that they have no conflict of interest related to the publication of this manuscript" Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by central allocation method |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single (participant) blinding. Orthopaedic surgeons performing the local anaesthetic injection do not appear to be blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who performed outcome assessments and if blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Nine patients not included in outcome assessment and lack of information about those lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other forms of bias |
Munger 2008.
| Methods | Design: Randomised controlled trial of donepezil in preventing delirium and postoperative cognitive decline following orthopaedic surgery. Date of study: Study dates not reported Power calculation: Not reported Frequency of outcomes assessment: Recorded on four occasions, but unclear when Inclusion criteria: Aged 65 years and over, no prior donepezil use and scheduled for hip fracture repair or elective hip or knee replacement surgery. Exclusion criteria: Not stated |
|
| Participants | Number in study: 15 Country: USA Setting: Orthopaedic surgery Age: Mean age 74.1 years Sex: 66% female Co‐morbidity: Not reported Dementia: Not reported |
|
| Interventions | Elective patients: donepezil 5 mg starting 7 days prior to surgery and tapering off during the third week following surgery Hip fracture patients: donepezil 5 mg starting on the day of surgery ending 5 days postoperatively Control: placebo |
|
| Outcomes | 1) Incident delirium, but reported using mean CAM rather than dichotomous data 2) Length of admission 3) Cognitive status using MMSE |
|
| Notes | Funding source: Clarian Values Fund, Pfizer Inc Declarations of interest: Not reported Pilot study, 15 participants. Mean CAM reported as opposed to numbers of people with delirium so limitations regarding interpretation of data. Although MMSE measured daily, frequency of CAM, MDAS not reported. Four time points were reported in the results table but not stated when these were. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided ‐ abstract data only |
| Random sequence generation (selection bias) | Unclear risk | No information provided ‐ abstract data only |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided ‐ abstract data only |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided ‐ abstract data only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided ‐ abstract data only |
| Selective reporting (reporting bias) | Unclear risk | No information provided ‐ abstract data only |
| Other bias | Unclear risk | No information provided ‐ abstract data only |
Papaioannou 2005.
| Methods | Design: Randomised trial of regional and general anaesthesia in elective surgery patients Date of study: Study dates not reported Power calculation: Yes Frequency of outcomes assessment: daily for first three postoperative days Inclusion criteria: Patients aged 60 years or over scheduled for elective surgery that could be performed under regional or general anaesthesia and who had agreed to be randomly allocated to receive either type of anaesthesia Exclusion criteria: Illiteracy, severe auditory or visual disturbances, central nervous system disorders, alcohol or drug dependence, treatment with tranquillisers or antidepressants, Parkinson's disease, and preoperative MMSE score less than 23 (indicative of dementia). |
|
| Participants | Number in study: 50 Country: Greece Setting: Unclear Age 60‐69/70 and over: Regional 14/5, General 15/13 Sex M/F: Regional 12/7, General 18/10 Co‐morbidity: Not reported ASA score: ASA I‐II/II‐IV: Regional 16/3, General 27/1 Dementia: Excluded |
|
| Interventions | Intervention: Regional anaesthesia (epidural or spinal) Control: General anaesthesia via propofol infusion or inhaled anaesthetic Both given to achieve a Ramsay sedation score of ≤2. Benzodiazepines not administered for premedication or intraoperative sedation. |
|
| Outcomes | 1. Incident delirium using DSM‐III criteria with informant history from attending relatives and nurses. Unclear whether patients interviewed 2. Length of admission 3. Cognitive status using MMSE 4. Postoperative complications |
|
| Notes | Funding source: European Commission BIOMED2 program BMH4‐98‐3335 and Greek Ministry of Health Declarations of interest: Not reported Delirium diagnosed using informant history from attending relatives and nurses. Unclear whether patients interviewed. Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by central |
| Random sequence generation (selection bias) | Low risk | Computer programme used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method of outcome assessment is unclear, "incidence of delirium was evaluated by asking the attending nurses and relatives for features fulfilling the DSM III criteria" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 50 patients randomised, 4 randomised to intervention crossed‐over to general anaesthesia. Delirium incidence results presented are per protocol, intention‐to‐treat not reported in original paper |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | High risk | Potential confounding from unbalanced neuraxial analgesia use 18 in regional anaesthesia, 3 in general anaesthesia group |
Pesonen 2011.
| Methods | Design: Randomised controlled trial of pregabalin as an opioid‐sparing agent in elderly patients after cardiac surgery. Date of study: April 2008‐September 2009 Power calculation: Yes Frequency of outcomes assessment: Preoperatively and on postoperative days 1‐5. Inclusion criteria: Aged 75 years and over and undergoing primary elective coronary artery bypass grafting with cardiopulmonary bypass (CPB) or single valve repair or replacement with CPB Exclusion criteria: Left ventricular ejection fraction < 30%, acute renal failure or chronic kidney disease (creatinine > 150 micromol/L), liver disease, congestive cardiac failure, type I diabetes mellitus, neurological disease other than transient ischaemic attack, preoperative infections, BMI > 35, psychiatric disease or alcohol abuse, chronic pain syndrome and recent use of gabapentinoids |
|
| Participants | Number in study: 70 Country: Finland Setting: Cardiac surgery patients at University teaching hospital Age: Median age 79.5 years (IQR 75‐89) in intervention group, 79.6 years (IQR 75‐91) in control group Sex: 40% female in intervention group, 54% female in control group Co‐morbidity: No baseline between‐group differences in TIA, hypertension, diabetes or COPD Dementia: Not reported |
|
| Interventions | Intervention: Patients were premedicated orally 1 hour before surgery with lorazepam (0.02‐0.03 mg/kg) and the study drug, pregabalin 150 mg (Lyrica 75 mg capsule, Pfizer GmbH, Freiburg, Germany) or placebo. Beginning on the first postoperative morning, patients received 75 mg pregabalin or placebo twice daily until the fifth postoperative day. Control: Patients received matching placebo |
|
| Outcomes | 1. Delirium, measured using CAM‐ICU (continuous score) ‐ not included in meta‐analysis 2. Length of admission 3. Cognition, mean CAM‐ICU score on day 5 4. Psychotropic medication use 5. Withdrawal from protocol |
|
| Notes | Funding source: Helsinki University Hospital Research Fund and Finska Lakaresallskapet (Finnish Medical Association). Declarations of interest: "No conflicts of interest declared" Continuous score of CAM‐ICU reported as opposed to delirium present/absent so unable to use data in outcome table. Continuous delirium score slightly higher on postoperative day 1 in intervention group (median 24 versus 21, P = 0.04), but no differences on days 2, 3, 4 or 5. Delirium not excluded at admission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Pharmacy conducted randomisation |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Identical placebo used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/70 patients randomised excluded from analysis; 6 from intervention, and 4 from control group. |
| Selective reporting (reporting bias) | Unclear risk | Insuffiecient information to assess |
| Other bias | Low risk | No evidence of other bias |
Radtke 2013.
| Methods | Design: parallel group randomised controlled trial Date of study: March 2009‐May 2010 Power calculation: Yes but stopped early so study underpowered Frequency of outcomes assessment: days 1‐7 postoperatively and at 3 months Inclusion criteria: aged 60 years or older; planned for elective surgery lasting at least 60 minutes Exclusion criteria: MMSE < 24; history of neurologic deficits; participation in pharmaceutical study; not planned for general anaesthesia; did not speak language of authors; unable to provide written consent |
|
| Participants | Number in study: 1277 Country: Berlin Setting: Two campuses of university hospital Age: Mean age 69.7 (SD 6.3) in intervention group, 70.1 (SD 6.5) in control group Sex: 44.7% of intervention group were female with 47.6% in the control group Co‐morbidity: Not reported Dementia: Excluded based on MMSE |
|
| Interventions | Intervention: BIS data were allowed to be included in the management of anaesthesia Control: Anaesthesia was provided with blinded BIS monitoring; unblinding of monitoring was allowed if it was deemed necessary for the patient's benefit |
|
| Outcomes | 1. Incident delirium, using DSM‐IV 2. Mortality, at 3 months 3. Length of admission 4. Cognitive status (Postoperative cognitive dysfunction) |
|
| Notes | Funding source: Charite‐Universitatsmedizin Berlin and Aspect Medical Systems (now Covidien) Declarations of interest: "None declared" Delirium not excluded at enrolment Stopped early due to lack of funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method for allocation concealment unclear |
| Random sequence generation (selection bias) | Unclear risk | Not clearly described ‐ "patients were consecutively recruited and after stratification electronically randomised into two study groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Allocation of anaesthetist dependent on whether for intervention or control so blinding not possible and unblinding of group in ˜10% of cases |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment performed by trained medical personnel under Psychiatrist supervision, blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | n = 1277 participants randomised, outcome assessment available for n = 1155. n = 45 in intervention group and n = 39 in control group did not receive their allocated intervention and were excluded from the analysis. Of n = 593 assigned to intervention n = 18 were lost to follow‐up (n = 575 analysed). Of n = 600 assigned to control n = 20 were lost to follow‐up (n = 580 analysed). 9.6% of randomised participants do not have outcome data |
| Selective reporting (reporting bias) | Low risk | ISRCTN registration with protocol, outcomes reported in accordance with protocol |
| Other bias | Low risk | No evidence of other bias |
Sampson 2007.
| Methods | Design: Randomised double‐blind controlled trial of donepezil in patients undergoing elective total hip replacement surgery Date of study: October 2003 to January 2004 Power calculation: No Frequency of outcomes assessment: Three times daily for duration of treatment + 1 day after Inclusion criteria: All consenting patients undergoing elective hip replacement and attending pre‐admission assessment clinic Exclusion criteria: MMSE less than 26, sensory impairment, hypersensitivity to donepezil or piperidine derivatives, or contraindications to donepezil |
|
| Participants | Number in study: 50 Country: UK Setting: One orthopaedic department in teaching hospital Age mean (SD) Intervention 69.7 (8.4), Placebo 65.1 (11.1) P = 0.1 Sex % male: Intervention 57.9, Placebo 42.9 P = 0.39 Co‐morbidity: Not reported Dementia: Not assessed, MMSE < 26 excluded |
|
| Interventions | Intervention: Donepezil 5 mg starting postoperatively on returning to orthopaedic ward, every 24 hours for 3 days Control: Identical placebo |
|
| Outcomes | 1. Incident delirium measured using Delirium Symptom Interview 2. Length of hospital admission 3. Adverse events |
|
| Notes | Funding source: Unrestricted educational grant from Pfizer Esai, UK Declarations of interest: Not reported Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation concealment managed centrally by pharmacy |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation method but sequence generation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebo used so participants and personnel blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors not aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 50 participants randomised, outcome assessment available for 33 (n = 19 in intervention group, n = 14 in control group). Surgery cancelled for 7 participants, 10 withdrew consent |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
Sieber 2010.
| Methods | Design: Randomised controlled trial of light sedation during spinal anaesthesia for reducing postoperative delirium in elderly hip fracture patients Date of study: April 2005‐October 2008 Power calculation: Yes Frequency of outcomes assessment: Daily from second postoperative day Inclusion criteria: Aged 65 years and over undergoing hip fracture repair with spinal anaesthesia and propofol sedation Exclusion criteria: Contraindications to spinal anaesthesia, prior hip surgery, mental or language barriers that would preclude data collection, severe heart failure, severe COPD |
|
| Participants | Number in study: 114 Country: USA Setting: Hip fracture patients Age: Mean age 81.2 years (SD 7.6) in intervention group, 81.8 years (SD 6.7) in control group Sex: 70% female in intervention group, 75% female in control group Co‐morbidity: Mean Charlson comorbidity index score 1.6 (1.2) in intervention group, 1.4 (1.4) in control group Dementia: 37% in intervention group, 33% in control group |
|
| Interventions | Intervention: Sedation was provided during surgery by a propofol infusion targeted to a bispectral index (BIS) of 80 or higher in the light sedation group Control: Sedation was provided during surgery by a propofol infusion targeted to a bispectral index (BIS) of approximately 50 in the deep sedation group. In general, these targets render the light sedation group responsive to voice and the heavy sedation group unresponsive to noxious stimuli. |
|
| Outcomes | 1. Incident delirium, measured using CAM 2. Duration of delirium 3. Length of admission 4. Mortality (in hospital, at 1‐year and overall) 5. Cognition using MMSE on postoperative day 2 6. Postoperative complications (Patients with >=1 complications) |
|
| Notes | Funding source: Not reported Declarations of interest: Not reported Light sedation group received significantly more midazolam (5.5 mg/kg vs 1.3 mg/kg, P = 0.02). Mean BIS in light sedation group 85.7 (11.3) vs 49.9 (13.5) control P < 0.001 Exclusion of patients with MMSE<15 limits generalisability of findings. Delirium excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealing allocation not clearly described |
| Random sequence generation (selection bias) | Unclear risk | Method of generating sequence not clearly described: "randomised block design with random length blocks.....incorporated a stratification scheme for age and cognitive impairment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All study team members, patient and physician blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Delirium assessments conducted by trained research nurse blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed. No withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Protocol for the study approved by John Hopkins Medicine Institutional Review Board but this is not publicly available |
| Other bias | Low risk | No evidence of other bias |
Stoppe 2013.
| Methods | Design: Randomised controlled trial Date of study: Study dates not reported Power calculation: Yes Frequency of outcomes assessment: daily postoperatively Inclusion criteria: undergoing elective isolated coronary artery bypass grafting (CABG) with the use of cardiopulmonary by‐pass (CPB); age > 50 years; ASA physical status II‐IV; preserved cardiac function (left ventricular ejection fraction > 50%) and EuroSCORE < or equal to 8 Exclusion criteria: cardiac, respiratory, liver or renal failure; acute coronary syndrome within 24 hours before surgery; haemodynamic instability; emergency operations; lack of informed consent; severe neurological dysfunction; depression; a geriatric depression score (GDS) > 5; MMSE <24; patients with a predisposition to malignant hyperthermia and/or hypersensitivity to the study drugs; women with childbearing potential or pregnancy. |
|
| Participants | Number in study: 30 Country: Germany Setting: Cardiac surgery inpatients Age: Mean age 66 (48‐81) in xenon group; 68 (51‐79) in sevoflurane group Sex: 80% of both groups were male Co‐morbidity: not reported at baseline Dementia: MMSE< 24 were excluded |
|
| Interventions | Both groups received induction of anaesthesia with propofol and sufentanil. Muscle relaxation was obtained with rocuronium. Anaesthetic depth was adjusted by titration of end‐expiratory xenon or sevoflurane concentrations according to changes in physiological parameters and BIS values. During CPB, patients received a propofol infusion instead of xenon or sevoflurane. Xenon: Maintenance of anaesthesia was achieved by continuous infusion of sufentanil and xenon (end‐expiratory concentrations of 45‐50 vol%) Sevoflurane: Maintenance of anaesthesia was achieved by continuous infusion of sufentanil and sevoflurane (end‐expiratory concentrations of 1‐1.4 vol%) |
|
| Outcomes | 1. Incidence of delirium, using CAM‐ICU 2. Mortality 3. Length of stay 4. Adverse events |
|
| Notes | Funding source: Deutsche Forschungsgemeinschaft (DFG) grants Declarations of interest: "MC and RR received lecture and consultant fees from Air Liquide Sante International, a company interested in developing clinical applications for medical gases, including xenon" Delirium not clearly excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Random sequence generation (selection bias) | Unclear risk | Method not described, states patients "randomly assigned to receive...." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and staff not clearly blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments conducted by trained study scientists blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Protocol registered on ClinicalTrials.gov and trial reported in accordance with published protocol |
| Other bias | Low risk | No evidence of other bias |
Urban 2008.
| Methods | Design: Randomised controlled trial of ketamine as an adjunct to postoperative pain management after spinal fusion Date of study: Study dates not reported Power calculation: Yes Frequency of outcomes assessment: Postoperative day 1 Inclusion criteria: Patients scheduled for elective lumbar spinal fusions who were taking opioids on a daily basis Exclusion criteria: Any patients who remained at a pain numerical rating scale of 10 after 2 hours |
|
| Participants | Number in study: 26 Country: USA Setting: Patients scheduled for elective lumbar spinal fusions Age: Mean age 53 years (SD 12) in intervention group, 48 years (SD 9) in control group Sex: Not reported Co‐morbidity: Not reported Dementia: Not reported |
|
| Interventions | Intervention: Patients in the ketamine group received 0.2 mg/kg on induction of general anaesthesia and then 2 mcg/kg/hr until discharge from the post‐anaesthesia care unit. Control: All patients received a general anaesthetic with midazolam 5 mg, 70% nitrous oxide, 0.4% isoflurane, fentanyl at 1‐2 mcg/kg/hr and propofol at 70‐100 mg/hr. Spinal morphine (10 mcg/kg) was administered at instrumentation. |
|
| Outcomes | 1) Incident delirium, measured using CAM on postoperative day 1 | |
| Notes | Funding source: Department of Anesthesia, Hospital for Special Surgery, New York Declarations of interest: Not reported Delirium not excluded at enrolment Study author conclusion: use of ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusion reduced postoperative pain. There was no effect on delirium. Small trial (n = 24). Only reported delirium on postoperative day 1. Concern about the integrity of the intervention 3 in control failed their initial pain management and were converted to IV ketamine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients blinded but the physicians and nurses were cognitive of the groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (physical therapists) blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis performed as there was cross‐over between intervention and control groups. However, two patients excluded after randomised so no outcome assessment data included Any patients who remained at a numerical rating scale of 10 after 2 hours were excluded |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to assess |
| Other bias | Low risk | No evidence of other bias |
Watne 2014.
| Methods | Design: Randomised controlled trial comparing care in an acute geriatric ward or standard orthopaedic ward following hip fracture Date of study: September 2009 ‐ January 2012 Power calculation: Yes but powered for primary outcome of cognitive function not delirium Frequency of outcomes assessment: Daily using CAM preoperatively and until the fifth postoperative day or for patients with delirium until discharge Inclusion criteria: All acute admissions to Oslo University Hospital with a hip fracture Exclusion criteria: Hip fracture due to high energy trauma (defined as a fall from higher than one metre) or if they were moribund on admission |
|
| Participants | Number in study: 332 randomised; 329 included in analyses Country: Norway Setting: University hospital Age: Mean age 84 years (range: 55 to 99) for intervention group and 85 years (range: 46 to 101) Sex: Male sex 42 (26%) for intervention group; 38 (23%) for controls Co‐morbidity: Not reported Dementia: 49% in both intervention and control groups diagnosis by expert evaluation |
|
| Interventions | Intervention: Acute geriatric ward – 20 bed ward mainly admitting patients suffering from acute medical disorder superimposed upon frailty, co‐morbidities and polypharmacy. Comprehensive Geriatric Assessment was the basis for treatment planning. Assessment by geriatrician, nurse, physiotherapist and occupational therapists was expected during their first day on the ward and this team had daily meetings to plan discharge. Checklists and clinical routines based on published literature and previous experience. These included medication reviews, optimal pain control, correction of physiological disturbances preoperatively and postoperatively (hypoxaemia, anaemia, electrolyte disturbances, acid–base disturbances, dehydration, hypotension, blood sugar etc), early and intensive mobilisation, optimising pre and postoperative nutrition and early discharge planning. Outpatient orthopaedic clinic at 4 months. Control: Usual care in orthopaedic ward setting. Staffing levels were similar but there was no multidisciplinary meetings and no geriatric assessments. Early mobilisation was emphasised and patients were seen by a physiotherapist soon after surgery. Outpatient orthopaedic clinic at 4 months. |
|
| Outcomes | 1. Incident delirium using CAM 2. Delirium duration (days) 3. Delirium severity using MDAS 4. Length of stay 5. In‐hospital mortality 6. New care home residence at four and 12 months 7. Cognitive function at four months using composite outcome 8. Incident dementia at 12 months 9. ADL function using Barthel Index at four months 10. Falls 11. Pressure ulcers 13. Postoperative complications |
|
| Notes | Funding source: Research Council of Norway through the program ‘Improving mental health of older people through multidisciplinary efforts’ (Grant No: 187980/H10) plus Oslo University Hospital, The Sophies Minde Foundation, The Norweigan Association for Public Health and Civitan’s Research Foundation Declaration of interest: The authors declare ‘they have no competing interests’ Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed opaque numbered envelopes |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (blocks of variable and unknown size) carried‐out by statistician not involved in clinical service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not possible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Delirium assessments; performed by study nurse/geriatrician aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 moribund patients erroneously randomised were excluded from the analysis (2 from intervention and 1 from control arm) |
| Selective reporting (reporting bias) | Low risk | Study reported in accordance with published protocol |
| Other bias | High risk | Where a bed was not available in the specialist geriatric unit, care was received in the corridor. As a result there are concerns about the fidelity of the intervention as a delirium prevention intervention as not all participants had the entire length of stay in either unit |
Whitlock 2015.
| Methods | Design: Randomised double‐blind controlled trial of methylprednisolone in patients at high risk of morbidity and mortality undergoing cardiac surgery with the use of cardiopulmonary bypass Date of study: June 2007 ‐ December 2013 Power calculation: Yes but based on primary outcome of 30‐day mortality Frequency of outcomes assessment: Once on postoperative day 3 Inclusion criteria: Patients aged 18 years or older with European System for Cardiac Operative Risk Evaluation (EuroSCORE) of at least 6 (or from 2011, at least 4 if from India or China) and providing written informed consent Exclusion criteria: Taking or expected to receive systemic steroids in immediate postoperative period; history of bacterial or fungal infection in preceding 30 days; allergy or intolerance to steroids; expected to receive aprotinin; previously participated in this study |
|
| Participants | Number in study: 7507 Country: Multinational, 18 countries Setting: Hospital‐based cardiac surgery practices Age: Mean age 67.5 years (SD 13.6) in intervention group; 67.3 years (SD 13.8) for controls Sex: Male sex 2257 (60%) in intervention group; 2280 (61%) in controls Co‐morbidity: Data reported on extensive list of coexisting medical conditions, no imbalances between groups Dementia: Not specifically assessed; participants had to provide written informed consent |
|
| Interventions | Intervention: Intravenous methylprednisolone (250 mg at anaesthetic induction and 250 mg at initiation of cardiopulmonary bypass) Control: Matched placebo |
|
| Outcomes | 1. Incident delirium on postoperative day 3 using CAM 2. Length of hospital stay 3. Mortality at 30 days 4. Physical morbidity (myocardial injury; stroke; respiratory failure; infection) |
|
| Notes | Funding source: Canadian Institutes of Health Research Declaration of interest: Authors report ‘no conflicts to declare’ Delirium not excluded at enrolment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Centralised computerised system with drug prepared by local pharmacy |
| Random sequence generation (selection bias) | Low risk | Block randomisation with random block sizes of 2, 4 or 6 stratified by centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants received intraoperative medication; healthcare providers blinded to medication administered |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collection and outcome assessment blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis presented |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per published protocol |
| Other bias | Low risk | No evidence of other bias |
ADL: activities of daily living; BIS: Bispectral index; BMI: body mass index; CAM: Confusion Assessment Method; CNS: central nervous system; COPD: chronic obstructive pulmonary disease; DRS‐R‐98: Delirium Rating Scale Revised 98; DSI: Delirium Symptom Interview; DSM: Diagnostic and Statistical Manual; FICB: fascia iliaca compartment block; Hb: haemoglobin; IM: intramuscular; INR: International Normalised Ratio; IQR: interquartile range; IV: intravascular; mcg: micrograms; MDAS: Memorial Delirium Assessment Scale; MMSE: Mini Mental State Examination; OBS: organic brain syndrome; PCA: patient controlled analgesia; SD: standard deviation; RCT: randomised controlled trial; TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al Tamimi 2015a | ICU study. |
| Astaneh 2007 | Not a randomised controlled trial. |
| Baldwin 2004 | The intervention was not designed to prevent delirium. Cognitive impairment rather than delirium was used as an outcome measure. |
| Benedict 2009 | Not a randomised controlled trial. |
| Bolotin 2014 | A validated method for diagnosis of delirium was not used. |
| Brueckmann 2015 | A validated method for diagnosis of delirium was not used. |
| Budd 1974 | A validated method for diagnosis of delirium was not used. |
| Caplan 2006 | Study not in hospitalised patients ‐ active intervention in community setting. |
| Cerchietti 2000 | Not a delirium prevention study. |
| Colak 2015 | A validated method for diagnosis of delirium was not used. |
| Cole 2002 | Not a delirium prevention study. |
| Culp 2003 | Randomisation not used and participants were long‐term care residents. |
| De Jonghe 2007 | Not a randomised controlled trial. |
| Del Rosario 2008 | Not a randomised controlled trial. |
| Ding 2015 | PACU study. |
| Ding 2015a | PACU study. |
| Ely 2004a | ICU study. |
| Ely 2004b | ICU study. |
| Finotto 2006 | ICU study. |
| Gamberini 2009 | ICU study. |
| Hsieh 2015 | ICU study. |
| Hu 2006 | Treatment study. |
| Hudetz 2009 | ICU study. |
| Hudetz 2015 | ICU study. |
| Hwang 2015 | ICU study. |
| Inouye 1993a | Not original research‐ review article. |
| Inouye 1999 | Randomisation not used. |
| Kaneko 1999 | A validated method for delirium diagnosis was not used. Although DSM‐IIIR diagnostic criteria used, data obtained from retrospective chart review. |
| Kat 2008 | Not a randomised controlled trial. |
| Lackner 2008 | Nursing home setting. |
| Landefeld 1995 | Outcomes examined did not include delirium. |
| Lili 2013 | Not delirium prevention. |
| Lundstrom 2005 | Randomisation not used. |
| Maneeton 2007 | Not a randomised controlled trial. |
| Marcantonio 2010 | Post‐acute care, not hospital setting. |
| Mardani 2013 | ICU study. |
| Marino 2009 | A validated method for diagnosis of delirium was not used. |
| Mentes 2003 | Randomisation not used. |
| Meybohm 2015 | ICU study. |
| Milisen 2001 | Not a randomised controlled trial. Before and after study. |
| Mudge 2008 | Not a randomised controlled trial. |
| Myint 2013 | Delirium not used as an outcome measure. |
| Naughton 2005 | Randomisation not used. |
| Neri 2010 | Not in hospitalised patients. |
| Oldenbeuving 2008 | Treatment study. |
| Overshott 2010 | Treatment study. |
| Pandharipande 2010 | ICU study. |
| Parker 2015 | A validated method for diagnosis of delirium was not used. |
| Parra Sanchez 2009 | ICU study. |
| Perkisas 2015 | Commentary. |
| Pitkala 2006 | Treatment study. |
| Prakanrattana 2007 | ICU study. |
| Pretto 2014 | A validated method for diagnosis of delirium was not used. |
| Ritchie 2008 | No recruitment, trial stopped. |
| Saager 2015 | ICU study. |
| Sauer 2014 | ICU study. |
| Short 2015 | Not a delirium prevention study. |
| Shu 2010 | ICU study and method of delirium diagnosis not validated. |
| Tabatabaie 2015 | Not a randomised controlled trial. Retrospective observational study. |
| Tabet 2005 | Randomisation not used. |
| Takeuchi 2007 | Treatment study and not randomised controlled trial. |
| Tokita 2001 | A validated method for diagnosis of delirium was not used. Delirium diagnosis relied on retrospective records review. |
| Torres 2015 | A validated method for diagnosis of delirium was not used. |
| van de Steeg 2014 | Primary outcome is screening for incidence of delirium; unable to report incidence of delirium as first date of delirium diagnosis is not recorded. |
| Wang 2012 | ICU study. |
| Wanich 1992 | Not a delirium prevention study. |
| Wong 2005 | Not a randomised controlled study. Before and after study. |
| Yamaguchi 2014 | ICU study. |
| Yang 2015 | ICU study. |
DSM‐IIR: Diagnostic and Statistical Manual ICU: Intensive Care Unit PACU: post‐anaesthesia care unit
Characteristics of ongoing studies [ordered by study ID]
Al Tmimi 2015.
| Trial name or title | Xenon for the prevention of post‐operative delirium in cardiac surgery: study protocol for a randomised controlled clinical trial |
| Methods | Randomised controlled trial |
| Participants | 190 patients, older than 65 years, and scheduled for elective cardiac surgery with use of cardiopulmonary bypass |
| Interventions | Group 1: General anaesthesia with xenon Group 2: General anaesthesia with sevoflurane |
| Outcomes | Primary outcome: Incidence of postoperative delirium during the first 5 postoperative days measured using 3D‐CAM or CAM‐ICU Secondary outcomes: Duration of postoperative delirium (total number of days and percentage of patients with duration of longer than 2 days; delirium severity; use of physical restraints; postoperative cognitive function; ADL; use of anti delirium medication; duration of sedation; duration of ICU and hospital stay; adverse events. |
| Starting date | May 2013 |
| Contact information | layth.altmimi@uzleuven.be 1 Department of Anesthesiology, KU Leuven – University of Leuven, University Hospitals of Leuven, Herestraat 49, B‐3000 Leuven, Belgium |
| Notes | EudraCT Identifier: 2014‐005370‐11. Will need to differentiate between ICU and non‐ICU delirium in results. |
Avidan 2009.
| Trial name or title | BAG‐RECALL Study: BIS or anesthesia gas to reduce explicit recall |
| Methods | Phase IV double‐blind multi‐centre randomised controlled trial |
| Participants | Patients aged over 18 undergoing surgery assessed as high risk for awareness requiring general anaesthesia |
| Interventions | Group 1: Bispectral index‐guided anaesthesia (target range 40‐60) Group 2: End‐tidal anaesthetic gas‐guided anaesthesia (target range 0.7‐1.3 age‐adjusted minimum alveolar concentration) |
| Outcomes | Primary outcome: Awareness with explicit recall during surgical and anaesthetic periods Secondary outcomes: postoperative delirium, postoperative mortality, psychological symptoms, postoperative pain |
| Starting date | March 2008 |
| Contact information | Michael Avidan avidanm@wustl.edu |
| Notes | ClinicalTrials.gov identifier: NCT00682825 Completed December 2010. Published N Engl J Med 2011 Aug 18;365(7):591‐601 but delirium outcome not reported yet. |
Avidan 2015.
| Trial name or title | The prevention of delirium and complications associated with surgical treatments multi‐centre clinical trial (PODCAST) |
| Methods | Phase 3 double‐blind randomised controlled trial |
| Participants | Patients 60 and over undergoing major surgery and able to provide informed consent |
| Interventions | Intervention: Drug: Low‐dose (sub‐anaesthetic) ketamine (0.5 mg/kg) following induction of anaesthesia or administration of sedative medications Placebo Comparator: Intravenous normal saline |
| Outcomes | Primary outcomes: Incidence of postoperative delirium within three days of surgery (assessed by the CAM or CAM‐ICU) Secondary outcomes: Postoperative acute pain within three postoperative days (assessed by visual analogue pain scale) |
| Starting date | November 2013 |
| Contact information | Michael Avidan avidanm@anest.wustl.edu |
| Notes | ClinicalTrials.gov identifier: NCT01690988 Estimated primary completion date June 2015 |
Beilin 2010.
| Trial name or title | The effect of physostigmine on cognitive functioning in the immediate period after sedation for colonoscopy |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients over18 years old, ASA I‐III, fluency in Hebrew, Russian, or Arabic, without serious hearing or visual impairment |
| Interventions | Intervention: Physostigmine Intravenous bolus of physostigmine 1 mg, 3‐5 minutes before completion of colonoscopy Comparator: no physostigmine |
| Outcomes | Primary outcome: Cognitive functioning at time of hospital discharge |
| Starting date | July 2010 |
| Contact information | beilinb@clalit.org.il Bezion Beilin, Hasharon Hospital, Rabin Medical Center |
| Notes | ClinicalTrials.gov identifier: NCT01121497 Estimated Primary Completion Date: July 2011 |
Bekker 2008.
| Trial name or title | Rivastigmine prophylaxis in elderly patients at risk for delirium: a randomised, double‐blind placebo‐controlled pilot study |
| Methods | Phase IV double‐blind randomised controlled trial |
| Participants | 65 years and older undergoing major elective surgery greater than 2 hours duration with any of preoperative cognitive impairment, age >70, use of psychotropic medications, previous history of delirium, severe illness/comorbidity. |
| Interventions | Intervention: Rivastigmine patch delivering 4.6 mg/24hrs applied to upper back preoperatively for 24 hrs. Control: A gauze and Tegaderm dressing applied to upper back within 3 hrs of surgery for 24 hrs |
| Outcomes | Primary outcome: postoperative delirium within 72 hours of surgery (CAM‐ICU) Secondary outcomes: delirium episodes, delirium severity (MDAS), length of hospital stay, cognitive function at 1 and 3 months postoperatively |
| Starting date | December 2008 |
| Contact information | Alex Bekker, NYU School of Medicine, New York |
| Notes | ClinicalTrials.gov identifier: NCT00835159 Data not available to us; manuscript in preparation. New York study, sponsored by Novartis. Study closed prematurely because of emerging safety concerns with this group of drugs, encouraged by Novartis |
Brzezinski 2012.
| Trial name or title | Effect of prophylactic, perioperative propranolol on peri‐ and postoperative complications in patients With Post Traumatic Stress Disorder |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | Patients over 40 with full or subthreshold PTSD of three months duration admitted for any surgical procedure (except open‐heart or intracranial surgery) requiring general or combined general‐regional anaesthesia and an overnight hospital stay. |
| Interventions | Experimental: Drug: Propranolol hydrochloride will be taken for a total of 14 days commencing on the morning of surgery Comparator: Placebo pill will be taken for a total of 14 days commencing on the morning of surgery |
| Outcomes | Primary outcomes: Postoperative delirium (assessed using CAM, CAM‐ICU), ICU length of stay, hospital length of stay, postoperative renal dysfunction Secondary outcomes: peri‐ and postoperative complications, pain intensity, PTSD symptoms, use of analgesics, length of mechanical ventilation, quality of life, functional status, sleep quality, depression symptoms, postoperative neurocognitive dysfunction score, mortality |
| Starting date | May 2012 |
| Contact information | brzezinm@anesthesia.ucsf.edu curt.johanson@va.gov |
| Notes | ClinicalTrials.gov identifier: NCT01555554 Estimated primary completion date December 2013 |
Chan 2010.
| Trial name or title | The effect of periarticular multi‐drug regimen on pain after partial hip replacement |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients admitted with femoral neck fracture, or for partial hip replacement |
| Interventions | Intervention: oral administration of oxycodone SR 10 mg and celecoxib 200 mg with 10 mL of water 1 hour before surgery and intraoperative periarticular injection of 50 mL solution containing ropivacaine 15 mg, epinephrine 0.3 mg, cefmetazole 1000 mg, ketorolac 30 mg and morphine HCL 10 mg before wound closure Control: no medication preoperatively or intraoperatively |
| Outcomes | Primary outcome: pain visual analogue scale (VAS) on postoperative days 1, 4 and 7 Secondary outcomes: opioid consumption on postoperative days 1, 4 and 7, frequency of use of patient controlled analgesia (PCA) on post operative days 1, 4 and 7, delirium (delirium rating scale) on postoperative days 1, 4 and 7 |
| Starting date | May 2010 |
| Contact information | Yong Chan Ha ksdeok@cau.ac.kr |
| Notes | ClinicalTrials.gov identifier: NCT01112436 Correspondence with author suggests patients are assessed on surgical wards. Estimated final data collection for primary outcome April 2012 |
Chaput 2009.
| Trial name or title | A randomised, double‐blind, placebo‐controlled trial to assess the safety and efficacy of the perioperative administration of pregabalin in reducing the incidence of postoperative delirium and improving acute postoperative pain management |
| Methods | Randomised double‐blind placebo‐controlled trial |
| Participants | Patients aged 60 years and older, admitted for major orthopaedic or vascular surgery with expected length of stay > 2 days |
| Interventions | Intervention: Pregabalin 75 mg given preoperatively, then either 50 mg or 25 mg every 8 hours for 3 days postoperatively (based on renal function) Control: Placebo |
| Outcomes | Primary outcome: Delirium (CAM‐ICU positive) Secondary outcomes: Interference with daily activities (BPI), pain at rest and on movement of the operative site (NRS), Narcotic analgesic requirements, Sedation (RSS), Narcotic‐related adverse effects (ORSDS), Recovery using the QoR, length of stay, Medical Outcome Study (MOS) sleep score |
| Starting date | May 2009 |
| Contact information | Dr. A. Chaput, Ottawa Hospital Research Institute |
| Notes | ClinicalTrials.gov identifier: NCT00819988 Correspondence with author suggests delirium assessed on wards. This study has been completed. |
Coburn 2012.
| Trial name or title | An international, multi‐centre randomised controlled trial evaluating the effect of xenon on post‐operative delirium in elderly patients undergoing hip fracture surgery |
| Methods | Multi‐centre double‐blind randomised controlled trial |
| Participants | Patients aged 75 and over with hip fracture and surgery planned within 48 hours and able to provide informed consent |
| Interventions | Intervention: Xenon 60% (1 MAC) in oxygen (FiO2 0.35‐0.45) Control: Sevoflurane 1.1‐1.4%(1 MAC) in oxygen (FiO2 = 0.35‐0.45) and medical air |
| Outcomes | Primary outcome: Postoperative delirium (CAM) within four days post‐surgery Secondary outcomes: Postoperative delirium (CAM) from day 5 postoperatively until discharge, sequential organ failure assessment from day 1 to day 4 post‐surgery, recovery parameters, safety and health economic parameters |
| Starting date | September 2010 |
| Contact information | Steffen Rex steffen.rex@uzleuven.be |
| Notes | ClinicalTrials.gov identifier NCT01199276 Estimated completion date December 2013 |
Diehl 2006.
| Trial name or title | Prevention of post‐operative delirium with donepezil |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients 70 Years and older, cognitively healthy, elective hip or knee replacement |
| Interventions | Intervention: Donepezil before (over 5‐7 days), during and after (over 7 days) surgery Control: Placebo |
| Outcomes | Primary outcome: Incidence of delirium Secondary outcome: Cognitive performance |
| Starting date | January 2006 |
| Contact information | Janine Diehl, M. D. Dept. of Psychiatry, Technische Universitaet Muenchen |
| Notes | ClinicalTrials.gov identifier NCT00220896 This study has now been completed |
Fernandez‐Robles 2012.
| Trial name or title | Usefulness of bright light therapy in the prevention of delirium in patients undergoing Hematopoietic Stem Cell Transplant (HSCT) |
| Methods | Pilot double‐blind randomised placebo‐controlled study |
| Participants | Patients aged 18 and over undergoing HSCT |
| Interventions | Intervention: Bright light therapy (2500 Lux gaze directed every morning from 8 am until 8:30 am) Control: Placebo sham light (<1000 Lux gaze directed every morning from 8 am until 8:30 am) |
| Outcomes | Primary outcome: Delirium incidence and time to development of delirium (Delirium Rating Scale‐Revised‐98 and/or Memorial Delirium Assessment Scale). Secondary outcomes: Length and severity of delirium episodes, dose of antipsychotic medications required to manage delirium, hospital length of stay, adverse events (falls, aspiration, infections, nutritional deficits). |
| Starting date | October 2012 |
| Contact information | Carlos Fernandez‐Robles
cfernandez‐robles@partners.org Justin Eusebio jeusebio@partners.org |
| Notes | ClinicalTrials.gov identifier: NCT01700816 Estimated primary completion date April 2014 |
Fischer 2009.
| Trial name or title | Tailored patient management guided with absolute cerebral oximetry to prevent neurocognitive injury in elderly patients undergoing cardiac surgery. |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients 65 and older admitted for elective cardiac or thoracic aortic surgery, able to provide informed consent |
| Interventions | Intervention: Optimisation of cerebral oxygenation within 5 minutes once cerebral desaturation (SctO2 < 60 %) has been established. Control: No intervention in this arm if the Sct02 falls below 60%. |
| Outcomes | Primary outcome: Postoperative delirium and postoperative cognitive dysfunction within 5 days of surgery. Secondary outcome: Postoperative morbidity and mortality |
| Starting date | September 2009 |
| Contact information | Gregory Fischer gregory.fischer@mountsinai.org |
| Notes | ClinicalTrials.gov identifier: NCT00991328 Estimated Primary Completion Date: June 2010 |
Foss 2006.
| Trial name or title | Incidence of delirium in hip fracture patients randomized to regular hypnotics vs placebo |
| Methods | Randomised controlled trial |
| Participants | 70 years and older admitted for hip fracture |
| Interventions | Intervention: Zolpidem 5 mg daily in perioperative period Control: Placebo tablet in perioperative period |
| Outcomes | Primary outcome: Incidence and severity of postoperative delirium. Secondary outcomes: Sleep quality. mobilisation, loss of functional ability, length of stay, sedation, nocturnal nursing events. |
| Starting date | February 2004 |
| Contact information | Nicolai B Foss, MD, Hvidovre University Hospital |
| Notes | Clinical trials identifier: NCT00286936 |
Hua 2010.
| Trial name or title | Influence of multi‐modal analgesia with parecoxib and morphine on post‐surgical delirium in elderly patients |
| Methods | Single‐blind randomised controlled trial |
| Participants | Patients aged 60 years and over admitted for elective non‐cardiac surgery |
| Interventions | Intervention: multi‐modal analgesia with parecoxib and morphine PCA Control: opioid PCA |
| Outcomes | Primary outcomes: Pain at rest and on movement, delirium diagnosis with CAM‐ICU from 1 to 7 days after operation Secondary outcomes: adverse postoperative events, 28 day survival, hepatic and renal function at 48 hours, delirium (CAM‐ICU) assessed twice daily with CAM‐ICU |
| Starting date | December 2010 |
| Contact information | Zhen Hua: hua1013@163.com |
| Notes | ChiCTR‐TRC‐10001063 http://www.chictr.org/en/proj/show.aspx?proj=342 |
Katznelson 2010.
| Trial name or title | Post‐operative melatonin administration and delirium prevention in patients undergoing vascular and cardiac surgery |
| Methods | Double‐blind randomised controlled trial |
| Participants | Patients over 60 admitted for non‐emergency vascular surgery with expected length of hospital stay > 48 hours, ASA category I to IV and able to provide informed consent |
| Interventions | Intervention: Melatonin 5 mg sublingually given at 9 pm for 5 days postoperatively or until discharge Control: placebo |
| Outcomes | Primary outcome: incidence of postoperative delirium (assessment up to day 7 postoperatively) Secondary outcome: pain visual analogue score |
| Starting date | August 2010 |
| Contact information | Rita Katznelson, Toronto General Hospital, UHN, Toronto, Ontario, Canada |
| Notes | ClinicalTrials.gov identifier: NCT01198938 Study completed February 2013 |
Mouchoux 2011.
| Trial name or title | CONFUCIUS Study : Impact of a multi‐faceted program to prevent postoperative delirium in the elderly |
| Methods | Stepped wedge cluster‐randomised controlled trial |
| Participants | Patients aged over 75 admitted for scheduled surgery |
| Interventions | Intervention: Preoperative geriatric consultation performed by a mobile geriatric team, training of surgical ward staff and implementation of HELP (Hospital Elder Life Program), morbidity and mortality conferences related to delirium cases. Control: Usual care |
| Outcomes | Primary outcome: Postoperative delirium rate within 7 days after surgery (assessed using the CAM) Secondary outcomes: Mean delirium intensity, length of hospital stay, postoperative complications 30 days after surgery incidence, mortality 6 months after surgery, feasibility of the multi‐disciplinary prevention program |
| Starting date | March 2011 |
| Contact information | christelle.mouchoux@chu‐lyon.fr |
| Notes | ClinicalTrials.gov identifier: NCT01316965 Estimated primary completion date March 2013 Sponsors: Hospices Civils de Lyon |
Nadler 2014.
| Trial name or title | Does positive airway pressure therapy reduce the incidence of post‐operative delirium in patients at risk for obstructive sleep apnoea? |
| Methods | Randomised controlled trial of continuous positive airways pressure |
| Participants | Patients at risk of obstructive sleep apnoea (OSA) (STOP‐BANG score>2, untreated for OSA undergoing elective joint replacement |
| Interventions | Continuous Positive Airway Pressure (CPAP) prior to surgery and on postoperative days 0, 1 and 2 vs. routine perioperative care |
| Outcomes | Incidence of delirium assessed using CAM and DRS‐R‐98 |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes |
Nanayakkara 2011.
| Trial name or title | Early pharmacological intervention to prevent delirium: Haloperidol prophylaxis in older emergency department patients |
| Methods | Multi‐centre double‐blind randomised placebo‐controlled trial |
| Participants | Patients aged 70 or over, admitted to a medical or surgical specialty and at risk of delirium according to one or more positive answers on the VMS delirium‐risk questions |
| Interventions | Intervention: Haloperidol 1 mg twice daily at 12 am and 8 pm, orally Control: Placebo 1 mg twice‐daily at 12 am and 8 pm, orally |
| Outcomes | Primary outcome: Incident delirium and delirium duration (measured with Delirium Observation Screening (DOS) score) Secondary outcome Measures: Time to develop delirium, length of stay, ; The (mean) number of days participants are admitted to the hospital; change from baseline function at 3 and 6 months (ADL scale), change from baseline instrumental activities at 3 and 6 months (Instrumental ADL scale); mortality. |
| Starting date | November 2012 |
| Contact information | p.nanayakkara@vumc.nl |
| Notes | ClinicalTrials.gov identifier: NCT01530308 Estimated primary completion date April 2014 |
Privitera 2006.
| Trial name or title | Namenda to prevent post‐operative delirium |
| Methods | Double‐blind randomised placebo‐controlled trial |
| Participants | Patients over 50, medically stable admitted for elective joint replacement under general anaesthetic |
| Interventions | Intervention: Memantine 10 mg once daily orally 8 days prior to procedure and 4 days postoperatively Control: Placebo orally once daily 8 days prior to procedure and 4 days postoperatively |
| Outcomes | Incidence and severity of delirium measured with the Delirium Rating Scale Revised‐98, MMSE, CAM, Clock Drawing Test, DSM‐IV‐TR criteria for delirium |
| Starting date | March 2006 |
| Contact information | M Privitera, University of Rochester, USA |
| Notes | ClinicalTrials.gov identifier: NCT00303433 Terminated early December 2009 (under‐recruitment) |
Schrijver 2014.
| Trial name or title | Efficacy and safety of haloperidol prophylaxis for delirium prevention in older medical and surgical at‐risk patients acutely admitted to hospital through the emergency department: study protocol of a multicenter, randomised, double‐blind, placebo‐controlled clinical trial (HARPOON study) |
| Methods | Randomised controlled trial |
| Participants | 390 patients aged 70 years and older admitted through the emergency department for general medicine and surgical specialties |
| Interventions | Prophylactic haloperidol 1 mg or placebo twice daily for seven days |
| Outcomes | Incidence of delirium, severity of delirium, duration of delirium, adverse events, length of stay, all cause mortality, institutionalisation, instrumental ADL, cognitive function |
| Starting date | TBC |
| Contact information | Edmee Schrijver. ej.schrijver@vumc.nl |
| Notes | ClinicalTrials.gov identifier NCT01530308 |
Silverstein 2008.
| Trial name or title | Perioperative cognitive function ‐ dexmedetomidine and cognitive reserve |
| Methods | Multi‐centre double‐blind randomised placebo‐controlled trial |
| Participants | 68 years and older, undergoing elective major surgery under general anaesthesia, ASA grade I‐III, MMSE >20 |
| Interventions | Intervention: Precedex (dexmedetomidine). 0.5/ug/kg/hr. Dexmedetomidine infusions will begin prior to the surgery (no loading dose), and will be maintained at 0.5 mcg/kg/hour throughout surgery and titrated postoperatively for 2 hrs postoperatively. Control: Placebo infusion. |
| Outcomes | Primary outcome: Delirium Battery post‐surgery and then daily for 5 days then at 3 and 6 months Secondary outcomes: Neuropsychological testing at 3 and 6 months |
| Starting date | February 2008 |
| Contact information | Jeff Silverstein, Mount Sinai School of Medicine jeff.silverstein@mountsinai.org |
| Notes | ClinicalTrials.gov identifier: NCT00561678 Estimated Primary Completion Date: June 2013 |
Spies 2009.
| Trial name or title | Perioperative physostigmine prophylaxis for liver resection patients at risk for delirium and postoperative cognitive dysfunction: a prospective, randomised, controlled, double‐blinded, two‐armed single‐centre trial |
| Methods | Phase IV double‐blind randomised placebo‐controlled trial |
| Participants | Patients over 18 undergoing elective liver resection with or without additional elective surgery in the same session, able to provide informed consent, negative pregnancy testing (beta‐human chorionic gonadotrophin [B‐HCG]). |
| Interventions | During liver resection: 1. 24‐hour perioperative intravenous administration of physostigmine (0.02 mg/kg BW as bolus and 0.01 mg/kg BW/hr (for 24 hours) from the beginning of the operation 2. 24‐hour perioperative intravenous administration of placebo over 24 hrs. |
| Outcomes | Primary outcomes: Incident delirium (DSM‐IV criteria), measured preoperatively and up to hospital discharge, Cambridge Neurophysiological Test Automated Battery (CANTAB), measured preoperatively, on the 7th, 90th and 365th postoperative day Secondary outcomes: Delirium; Evaluation of intensive care unit performance, Length of postoperative hospital stay, Length of postoperative ICU stay, pain, postoperative complications and organ dysfunction, rate of systemic inflammatory response syndrome (SIRS) and infection, quality of life questionnaires, mortality, postoperative survival at 90 days, 6 months and one year, immune parameters, perioperative assessment of sleep stage, parameters of haematology, parameters of renal function. |
| Starting date | August 2009 |
| Contact information | gerrit.fleige@charite.de |
| Notes | ISRCTN18978802 Anticipated end date: April 2016 |
Strijbos 2013.
| Trial name or title | Design and methods of the Hospital Elder Life Program (HELP), a multi component targeted intervention to prevent delirium in hospitalised older patients: efficacy and cost‐effectiveness in Dutch health care |
| Methods | Cluster‐randomised controlled trial (stepped wedge) |
| Participants | Patients aged 70 years and over at risk for delirium and admitted to cardiology, internal medicine, geriatrics, orthopedics and surgery |
| Interventions | Multi‐component targeted delirium prevention intervention (Hospital Elder Life Program) |
| Outcomes | Incidence of delirium, duration of delirium, severity of delirium, quality of life, length of stay, use of care services |
| Starting date | TBC |
| Contact information | m.strijbos@umcutrecht.nl |
| Notes | Netherlands trial register NTR3842 |
Thomas 2012.
| Trial name or title | Does femoral nerve catheterization reduce the incidence of post‐operative delirium in patients presenting for hip fracture repair? |
| Methods | Randomised controlled trial |
| Participants | Patients aged 50 and over presenting with a hip fracture |
| Interventions | Intervention: Preoperative femoral nerve catheterisation Control: Intravenous opioids given postoperatively |
| Outcomes | Primary outcome: Rate of postoperative delirium up to 3 days Secondary outcomes: length of stay, pain score (VAS) and consumption of analgesic medication |
| Starting date | March 2012 |
| Contact information | lesthomas@ochsner.org |
| Notes | ClinicalTrials.gov identifier: NCT01547468 Estimated date of primary completion March 2015 |
van der Burg 2005.
| Trial name or title | Randomised double‐blind placebo‐controlled study of post‐operative haloperidol versus placebo for prevention of post‐operative delirium after acute hip surgery |
| Methods | Double‐blind randomised placebo‐controlled study |
| Participants | Patients aged 75 and over undergoing surgery for hip fracture |
| Interventions | Intervention: Haloperidol 1 mg twice daily for 72 hours Control: Placebo 1 mg twice daily for 72 hours |
| Outcomes | Primary outcomes: Incidence of postoperative delirium in 72 hours postoperative period Secondary outcomes: Length of stay; mortality; ADL dependence at 3 months; adverse outcomes |
| Starting date | November 2005 |
| Contact information | Boke Linso Sjirk Borger van der Burg, Department of Surgery, Bronovo Hospital |
| Notes | ClinicalTrials.gov identifier: NCT00250237 Study completed October 2008. Results not published. |
Wang 2012a.
| Trial name or title | Effects of two different anaesthesia‐analgesia methods on the incidence of post‐operative delirium: a multi‐centre, randomized controlled trial |
| Methods | Multi‐centre randomised controlled trial |
| Participants | Patients aged 60‐90 years undergoing elective major (more than two hours) open abdominal or thoracic (non‐cardiovascular) surgery, able to provide informed consent. |
| Interventions | Intervention: Combined epidural and general anaesthesia (Epi‐GA) with postoperative patient controlled epidural analgesia (PGEA). Control: General anaesthesia and patient controlled intravenous analgesia (PCIA). |
| Outcomes | Primary outcome: Incidence of postoperative delirium. Secondary outcomes: Incidence of postoperative complications, 30‐day mortality, VAS pain score, duration of postoperative hospital stay, daily prevalence of postoperative delirium (7 days) |
| Starting date | November 2011 |
| Contact information | Yuan Zeng yuan_zeng@sina.com |
| Notes | ClinicalTrials.gov identifier: NCT01661907 Estimated primary completion date October 2014 |
Young 2015.
| Trial name or title | Prevention of Delirium (POD) for older people in hospital: protocol for a randomised controlled feasibility study |
| Methods | Cluster‐randomised controlled trial |
| Participants | Patients, aged 65 years and over, admitted to a participating orthopaedic trauma or geriatric medicine. |
| Interventions | Intervention: A manualised, multi‐component intervention and systematic implementation process Control: Usual care |
| Outcomes | Primary outcome: New onset delirium Secondary outcomes: Number, severity and length of delirium episodes (including persistent delirium); length of stay in hospital; in‐hospital mortality; destination at discharge; health‐related quality of life and health resource use; physical and social independence; anxiety and depression; patient experience. |
| Starting date | 13/03/2014 |
| Contact information | s.hartley@leeds.ac.uk Clinical Trials Research Unit, Leeds Institute of Clinical Trials Research, University of Leeds, Leeds LS2 9JT, UK |
| Notes | Trial registration: ISRCTN01187372 |
ADL: activities of daily living; CAM: Confusion Assessment Method; DSM‐IIR: Diagnostic and Statistical Manual; ICU: Intensive Care Unit; MDAS:Memorial Delirium Assessment Scale; MMSE: Mini Mental State Examination; PCA: patient controlled analgesia; PTSD: post‐traumatic stress disorder
Differences between protocol and review
The original protocol was published in 2005 and stated the analysis would be performed using an intention‐to‐treat approach and this was adopted in the original version of the review (Siddiqi 2007). However, for this update an available case analysis was performed consistently, including re‐analysing the six studies included in the original review.
We added adverse events (falls, pressure ulcers, mortality) as outcomes although this was not specified in the original published protocol. We also removed physical morbidity from secondary outcomes, and instead included infections (specifically wound infections, urinary tract infections, pneumonia) and cardiac adverse events (specifically myocardial infarction and cardiac failure) as adverse events.
'Summary of findings' tables were added in accordance with current Cochrane Collaboration Guidance utilising GRADE assessments.
We also specified studies conducted in ICU settings would be excluded in this update.
Authorship for this update has changed with the addition of AC, ET, JH, JY, SS, and JT. AB, JH and RS are no longer authors on this update.
Contributions of authors
NS, AC, ET, JH and SS reviewed search results and extracted data for included studies.
AC, ET and JH completed ’Summary of findings’ tables and generated GRADE Evidence Profiles.
JT reviewed and interpreted results for studies testing approaches to anaesthesia and pain management.
All authors contributed to write up of the review.
Sources of support
Internal sources
Health Sciences, University of York, Hull York Medical School, UK.
Bradford District Care NHS FoundationTrust, UK.
University of Leeds, UK.
-
The Alzheimer Scotland Dementia Research Centre and Centre for Cognitive Ageing and Cognitive Epidemiology, The University of Edinburgh, UK.
JKH is supported by a Clinical Research Fellowship funded by Alzheimer Scotland and The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/L501530/1). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) is gratefully acknowledged.
External sources
-
NIHR, UK.
This review update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abizanda 2011 {published data only}
- Abizanda P, León M, Domínguez‐Martín L, Lozano‐Berrio V, Romero L, Luengo C, et al. Effects of a short‐term occupational therapy intervention in an acute geriatric unit. Maturitas May 2011 Epub;69(3):273‐8. [DOI] [PubMed] [Google Scholar]
Aizawa 2002 {published data only}
- Aizawa K, Kanai T, Saikawa Y, Takabayashi T, Kawano Y, Miyazawa N, et al. A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surgery Today 2002;32(4):310‐4. [DOI] [PubMed] [Google Scholar]
Al‐Aama 2011 {published data only}
- Al‐Aama T, Brymer C, Gutmanis I, Woolmore‐Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo‐controlled trial. International Journal of Geriatric Psychiatry 2011;26(7):687‐94. [DOI] [PubMed] [Google Scholar]
Ashraf 2015 {published data only}
- Ashraf JM, Schweiger M, Vallurupalli N, Bellantonio S, Cook JR. Effects of oral premedication on cognitive status of elderly patients undergoing cardiac catheterization. Journal of Geriatric Cardiology 2015;12(3):257‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beaussier 2006 {published data only}
- Beaussier M, Weickmans H, Parc Y, Delpierre E, Camus Y, Funck‐Brentano C, et al. Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Regional Anesthesia and Pain Medicine 2006;31(6):531‐8. [DOI] [PubMed] [Google Scholar]
Berggren 1987 {published data only}
- Berggren D, Gustafson Y, Eriksson B, Bucht G, Hansson LI, Reiz S, et al. Postoperative confusion after anesthesia in elderly patients with femoral neck fractures. Anesthesia & Analgesia 1987;66(6):497‐504. [PubMed] [Google Scholar]
Bonaventura 2007 {published data only (unpublished sought but not used)}
- Bonaventura M, Zanotti, R. Effectiveness of "IPD" treatment for delirium prevention in hospitalized elderly. A controlled randomized clinical trial [Italian]. Professioni Infermieristiche 2007;60(4):230‐6. [PubMed] [Google Scholar]
Boustani 2012 {published data only}
- Boustani MA, Campbell NL, Khan BA, Abernathy G, Zawahiri M, Campbell T, et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. Journal of General Internal Medicine 2012;27(5):561‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013 {published data only}
- Chan MT, Cheng BC, Lee TM, Gin T and the CODA Trial Group. BIS‐guided anesthesia decreases postoperative delirium and cognitive decline. Journal of Neurosurgical Anesthesiology 2013;25(1):33‐42. [DOI] [PubMed] [Google Scholar]
de Jonghe 2014 {published data only (unpublished sought but not used)}
- Jonghe A, Munster BC, Goslings JC, Kloen P, Rees C, Wolvius R, et al. A randomized, double‐blind controlled trial of melatonin versus placebo in delirium. European Geriatric Medicine 2013 Conference: 9th Congress of the European Union Geriatric Medicine Society. Venice, Italy, 2013:S175‐S176.
- Jonghe A, Munster BC, Goslings JC, Kloen P, Rees C, Wolvius R, et al. on behalf of the Amsterdam Delirium Study Group. Effect of melatonin on incidence of delirium among patients with hip fracture: a multicentre, double‐blind randomized controlled trial. Canadian Medical Association Journal 2014;186(14):E547‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonghe A, Munster BC, Oosten HE, Goslings JC, Kloen P, Rees C, et al. The effects of melatonin versus placebo on delirium in hip fracture patients: study protocol of a randomised, placebo‐controlled, double blind trial. BMC Geriatrics 2011;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz 2001 {published data only}
- Diaz V, Rodriguez J, Barrientos P, Serra M, Salinas H, Toledo C, et al. [Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly A randomized controlled trial]. Revista de Neurologia 2001;33(8):716‐9. [PubMed] [Google Scholar]
Fukata 2014 {published data only}
- Fukata S. A study on the prevention of postoperative delirium for the elderly. WHO trial registry ID JPRN‐UMIN000002891 2009.
- Fukata S, Kawabata Y, Fujisiro K, Katagawa Y, Kuroiwa K, Akiyama H, et al. Haloperidol prophylaxis does not prevent postoperative delirium in elderly patients: a randomized, open‐label prospective trial. Surgery Today 2014;44(12):2305‐13. [DOI] [PubMed] [Google Scholar]
Gauge 2014 {published data only}
- Gauge N, Salaunkey K, Zhu J, Ferreira N, Aron J, Araujo H, et al. Optimization of intra‐operative depth of anaesthesia and cerebral oxygenation significantly reduces postoperative delirium after coronary artery bypass graft surgery. Applied cardiopulmonary pathophysiology 2014;Conference: 29th Annual Meeting of the European Association of Cardiothoracic Anaesthesiologists, EACTA 2014 and 14th International Congress on Cardiovascular Anesthesia, ICCVA 2014(29):68. [Google Scholar]
Gruber‐Baldini 2013 {published data only}
- Gruber‐Baldini AL, Marcantonio E, Orwig D, Magaziner J, Terrin M, Barr E, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. Journal of the American Geriatrics Society 2013;61:1286‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatta 2014 {published and unpublished data}
- Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, et al. Preventive effects of ramelteon on delirium: a randomized placebo‐controlled trial. JAMA Psychiatry 2014;71(4):397‐403. [DOI] [PubMed] [Google Scholar]
Hempenius 2013 {published data only}
- Hempenius L, Slaets JPJ, Asselt D, Bock GH, Wiggers T, Leeuwen BL. Outcomes of a geriatric liaison intervention to prevent the development of postoperative delirium in frail elderly cancer patients: report on a multicentre, randomized, controlled trial. PLOS One 2013;8(6):e64834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffs 2013 {published data only}
- Jeffs KJ, Berlowitz DJ, Grant S, Lawlor V, Graco M, Morton NA, et al. An enhanced exercise and cognitive programme does not appear to reduce incident delirium in hospitalised patients: a randomised controlled trial. BMJ Open 2013;3:e002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs KJ, Berlowitz DJ, Savige JA, Lim WK. Does an enhanced exercise and cognitive program reduce incident delirium in older hospital patients: results of a randomised controlled trial. Internal Medicine Journal 2008;38(Suppl 5):A121. [Google Scholar]
Jia 2014 {published data only}
- Jia Y, Jin G, Guo S, Gu B, Jin Z, Gao X, et al. Fast‐track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Archives of Surgery 2014;399:77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalisvaart 2005 {published data only}
- Kalisvaart KJ, Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TCG, Burger BJ, et al. Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study. Journal of the American Geriatrics Society 2005;53(10):1658‐66. [DOI] [PubMed] [Google Scholar]
Larsen 2010 {published data only}
- Larsen KA, Min D, Kelly SE, Stern TA, Bode RH Jr, Price LL, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint‐replacement patients: a randomized, controlled trial. Psychosomatics 2010;51(5):409‐18. [DOI] [PubMed] [Google Scholar]
Leung 2006 {published data only}
- Leung JM, Sands LP, Rico M, Petersen KL, Rowbotham MC, Dahl JB, et al. Pilot clinical trial of gabapentin to decrease postoperative delirium in older patients. Neurology 2006;67:1251‐3. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li J‐Z, Li X‐Z, Wang X‐M, Wang M‐S, Yu H‐F, Shi F, et al. Effects of parecoxib sodium analgesia on serum concentrations of neuron‐specific enolase and S‐100^b and postoperative cognitive function of elderly patients undergoing acute replacement of femoral head. [Chinese]. Zhonghua Yi Xue Za Zhi 2013;93(27):2152‐4. [PubMed] [Google Scholar]
Liptzin 2005 {published data only}
- Liptzin B, Laki A, Garb J, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post‐surgical delirium. American Journal of Geriatric Psychiatry 2005;13(12):1100‐6. [DOI] [PubMed] [Google Scholar]
Lundstrom 2007 {published data only}
- Lundstrom M, Olofsson B, Stenvall M, Karlsson S, Nyberg L, Englund U, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clinical and Experimental Research 2007;19(3):178‐86. [DOI] [PubMed] [Google Scholar]
Lurati 2012 {published data only}
- Lurati Buse GA, Schumacher P, Seeberger E, Studer W, Schuman RM, Fassl J, et al. Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation 2012;126:2696‐704. [DOI] [PubMed] [Google Scholar]
Marcantonio 2001 {published data only}
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. Journal of the American Geriatrics Society 2001;49(5):516‐22. [DOI] [PubMed] [Google Scholar]
Marcantonio 2011 {published data only}
- Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. Journal of the American Geriatric Society 2011;59(Suppl 2):S282‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2012 {published data only}
- Martinez F. Prophylactic Environmental Management of Delirium. https://clinicaltrials.gov/ct2/show/NCT01356810 2011.
- Martinez FT, Tobar C, Beddings CI, Vallejo G. Preventing delirium in an acute hospital using a non‐pharmacological intervention. Age Ageing 2012;41(5):629‐34. [DOI] [PubMed] [Google Scholar]
Mouzopoulos 2009 {published data only}
- Mouzopoulos G, Vasiliadis G, Lasanianos N, Nikolaras G, Morakis E, Kaminaris M. Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo‐controlled study. Journal of Orthopaedics and Traumatology 2009;10(3):127‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munger 2008 {published data only}
- Munger S, Boustani M, Parr J. The effectiveness of donepezil in preventing delirium and post‐ operative cognitive decline following orthopaedic surgery. American Geriatrics Society Scientific Conference. 2008.
Papaioannou 2005 {published data only}
- Papaioannou A, Fraidakis O, Michaloudis D, Balalis C, Askitopoulou H. The impact of the type of anaesthesia on cognitive status and delirium during the first postoperative days in elderly patients. European Journal of Anaesthesiology 2005;22(7):492‐9. [DOI] [PubMed] [Google Scholar]
Pesonen 2011 {published data only}
- Pesonen A, Suojaranta‐Ylinen R, Hammarén E, Kontinen VK, Raivio P, Tarkkila P, et al. Pregabalin has an opioid‐sparing effect in elderly patients after cardiac surgery: a randomized placebo‐controlled trial. British Journal of Anaesthesia 2011;106(6):873‐81. [DOI] [PubMed] [Google Scholar]
Radtke 2013 {published data only}
- Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. British Journal of Anaesthesia 2013;110(S1):i98‐i105. [DOI] [PubMed] [Google Scholar]
Sampson 2007 {published data only}
- Sampson EL, Raven PR, Ndhlovu PN, Vallance A, Garlick N, Watts J, et al. A randomized, double blind, placebo‐controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Internation Journal of Geriatric Psychiatry 2007;22(4):343‐9. [DOI] [PubMed] [Google Scholar]
Sieber 2010 {published data only}
- Brown CH 4th, Azman AS, Gottschalk A, Mears SC, Sieber FE. Sedation depth during spinal anesthesia and survival in elderly patients undergoing hip fracture repair. Anesthesia and Analgesia 2014;118(5):977‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proceedings 2010;85(1):18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoppe 2013 {published data only}
- Stoppe C, Fahlenkamp AV, Rex S, Veeck NC, Gozdowsky SC, Schalte G, et al. Feasibility and safety of xenon compared with sevoflurane anaesthesia in coronary surgical patients: a randomized controlled pilot study. British Journal of Anaesthesia 2013;111(3):406‐16. [DOI] [PubMed] [Google Scholar]
Urban 2008 {published data only}
- Urban MK, Ya Deau JT, Wukovits B, Lipnitsky JY. Ketamine as an adjunct to postoperative pain management in opioid tolerant patients after spinal fusions: a prospective randomized trial. HSS Journal: the musculoskeletal journal of Hospital for Special Surgery 2008;4(1):62‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watne 2014 {published data only}
- Watne LO, Torbergsen AC, Conroy S, Engedal K, Frihagen F, Hjorthaug GA, et al. The effect of a pre‐ and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial (Oslo Orthogeriatric Trial). BMC Medicine 2014;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyller TB, Watne LO, Torbergsen A, Engedal K, Frihagen F, Juliebø V, et al. The effect of a pre‐ and post‐operative orthogeriatric service on cognitive function in patients with hip fracture. The protocol of the Oslo Orthogeriatrics Trial. BMC Geriatrics 2012;12(36):doi:10.1186/1471‐2318‐12‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitlock 2015 {published data only}
- Whitlock RP, Devereaux PJ, Teoh KH, Lamy A, Vincent J, Pogue J, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double‐blind, placebo‐controlled trial. Lancet 2015;386(10000):1243‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al Tamimi 2015a {published data only}
- Al Tmimi L, Hemelrijck J, Velde M, Sergeant P, Meyns B, Missant C, et al. Xenon anaesthesia for patients undergoing off‐pump coronary artery bypass graft surgery: A prospective randomized controlled pilot trial. British Journal of Anaesthesia 2015;115(4):550‐9. [DOI] [PubMed] [Google Scholar]
Astaneh 2007 {published data only}
- Astaneh A, Khajehmougahi N, Pakseresht S. The multicomponent intervention to prevent postoperative delirium after open‐heart surgery. Pakistan Journal of Medical Sciences 2007;23(2):188‐92. [Google Scholar]
Baldwin 2004 {published data only}
- Baldwin R, Pratt H, Goring H, Marriott A, Roberts C. Does a nurse‐led mental health liaison service for older people reduce psychiatric morbidity in acute general medical wards? A randomised controlled trial. Age and Ageing 2004;33(5):472‐8. [DOI] [PubMed] [Google Scholar]
Benedict 2009 {published data only}
- Benedict L, Hazelett S, Fleming E, Ludwick R, Anthony M, Fosnight S, et al. Prevention, detection and intervention with delirium in an acute care hospital: a feasibility study. International Journal of Older Peoples Nursing 2009;4(3):194‐202. [DOI] [PubMed] [Google Scholar]
Bolotin 2014 {published data only}
- Bolotin G, Huber CH, Shani L, Mohr FW, Carrel TP, Borger MA, et al. Novel emboli protection system during cardiac surgery: A multi‐center, randomized, clinical trial. Annals of Thoracic Surgery 2014;98(5):1627‐33. [DOI] [PubMed] [Google Scholar]
Brueckmann 2015 {published data only}
- Brueckmann B, Sasaki N, Grobara P, Li MK, Woo T, Bie J, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: A randomized, controlled study. British Journal of Anaesthesia 2015;115(5):743‐51. [DOI] [PubMed] [Google Scholar]
Budd 1974 {published data only}
- Budd S, Brown W. Effect of a reorientation technique on postcardiotomy delirium. Nursing Research 1974;23(4):341‐8. [PubMed] [Google Scholar]
Caplan 2006 {published data only}
- Caplan GA, Coconis J, Board N, Sayers A, Woods J. Does home treatment affect delirium? A randomised controlled trial of rehabilitation of elderly and care at home or usual treatment (the REACH‐OUT trial). Age and Ageing 2006;35(1):53‐60. [DOI] [PubMed] [Google Scholar]
Cerchietti 2000 {published data only}
- Cerchietti L, Navigante A, Sauri A, Palazzo F. Hypodermoclysis for control of dehydration in terminal‐stage cancer.. International journal of palliative nursing 2000;6(8):370‐4. [DOI] [PubMed] [Google Scholar]
Colak 2015 {published data only}
- Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric‐Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: A randomized, prospective study. European Journal of Cardiothoracic Surgery 2015;47(3):447‐54. [DOI] [PubMed] [Google Scholar]
Cole 2002 {published data only}
- Cole MG, McCusker J, Bellavance F, Primeau FJ, Bailey RF, Bonnycastle MJ, Laplante J. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. Canadian Medical Association Journal 2002;167(7):753‐9. [PMC free article] [PubMed] [Google Scholar]
Culp 2003 {published data only}
- Culp K, Mentes J, Wakefield B. Hydration and acute confusion in long‐term care residents. Western Journal of Nursing Research 2003;25(3):251‐66; discussion 267‐73. [DOI] [PubMed] [Google Scholar]
De Jonghe 2007 {published data only}
- Jonghe JFM, Kalisvaart KJ, Dijkstra M, Dis H, Vreeswijk R, Kat MG, et al. Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. American Journal of Geriatric Psychiatry 2007;15(2):112‐21. [DOI] [PubMed] [Google Scholar]
Del Rosario 2008 {published data only}
- Rosario E, Esteve N, Sernandez MJ, Batest C, Aquilar JL. Does femoral nerve analgesia impact the development of postoperative delirium in the elderly? A retrospective investigation. Acute Pain 2008;10(2):59‐64. [Google Scholar]
Ding 2015 {published data only}
- Ding L, Zhang H, Mi W, Wang T, He Y, Zhang X, et al. Effects of dexmedetomidine on anesthesia recovery period and postoperative cognitive function of patients after robot‐assisted laparoscopic radical cystectomy. International Journal of Clinical and Experimental Medicine 2015;8(7):11388‐95. [PMC free article] [PubMed] [Google Scholar]
Ding 2015a {published data only}
- Ding L, Zhang H, Mi W, He Y, Zhang X, Ma X, et al. [Effects of dexmedetomidine on recovery period of anesthesia and postoperative cognitive function after robot‐assisted laparoscopicradical prostatectomy in the elderly people]. [Chinese]. Zhong Nan da Xue Xue Bao 2015;Yi Xue Ban = Journal of Central South University. Medical Sciences. 40(2):129‐35. [DOI] [PubMed] [Google Scholar]
Ely 2004a {published data only}
- Ely EW. A randomized, double‐blind trial in ventilated ICU patients comparing treatment with an Alpha2 Agonist versus a Gamma Aminobutyric Acid (GABA)‐Agonist to determine delirium rates, efficacy of sedation, analgesia and discharge cognitive status. ClinicalTrials.Gov 2004a.
Ely 2004b {published data only}
- Ely EW. Delirium in the ICU: a prospective, randomized, trial of placebo vs haloperidol vs ziprasidone. ClinicalTrials.gov 2004b.
Finotto 2006 {published data only}
- Finotto S, Artiolo G, Davoli L, Barbara B. Nursing interventions for the prevention of the delirium in intensive care unit (ICU): a randomized study. Professioni Infermieristiche 2006;59(4):228‐32. [PubMed] [Google Scholar]
Gamberini 2009 {published data only}
- Gamberini M, Bolliger S, Lurati Buse GA, Burkhart CS, Grapow M, Gagneux A, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery ‐ a randomized controlled trial. Critical Care Medicine 2009;37(5):1762‐8. [DOI] [PubMed] [Google Scholar]
Hsieh 2015 {published data only}
- Hsieh S. Intranasal Insulin for neuroprotection in elderly cardiac surgery patients. ClinicalTrials.gov: NCT01561378 2012.
- Hsieh SJ, Fuster D, D'Alessandro DA, Leff JD, Gong MN. Feasibility and efficacy of intranasal insulin for post‐operative delirium: The CNS‐elders randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2015;May 1:A4018‐A4018. [Google Scholar]
Hu 2006 {published data only}
- Hu H, Deng W, Yang H, Liu Y. Olanzepine and haloperidol for senile delirium: a randomised controlled observation. Chinese Journal of Clinical Rehabilitation 2006;10(42):188‐90. [Google Scholar]
Hudetz 2009 {published data only}
- Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Byrne AJ, Hudetz AG, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2009;23(5):651‐7. [DOI] [PubMed] [Google Scholar]
Hudetz 2015 {published data only}
- Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Pagel PS. Remote ischemic preconditioning prevents deterioration of short‐term postoperative cognitive function after cardiac surgery using cardiopulmonary bypass: results of a pilot investigation. Journal of Cardiothoracic and Vascular Anesthesia 2015;29(2):382‐8. [DOI] [PubMed] [Google Scholar]
Hwang 2015 {published data only}
- Hwang J‐Y, Bang J‐S, Oh C‐W, Joo J‐D, Park S‐J, Do S‐H, et al. Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: A randomized, double‐blind, and controlled study. World Neurosurgery 2015;83(1):108‐13. [DOI] [PubMed] [Google Scholar]
Inouye 1993a {published data only}
- Inouye SK. A controlled trial of a nursing‐centered intervention in hospitalized elderly medical patients: the Yale Geriatric Care Program. Journal of the American Geriatrics Society. 1993;41(12):1353. [DOI] [PubMed] [Google Scholar]
Inouye 1999 {published data only}
- Bogardus ST Jr, Desai MM, Williams CS, Leo Summers L, Acampora D, Inouye SK. The effects of a targeted multicomponent delirium. American Journal of Medicine 2003;114(5):383‐90. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Bogardus ST Jr, Charpentier PA, Leo‐Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients see comments. New England Journal of Medicine 1999;340(9):669. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Bogardus ST Jr, Williams CS, Leo‐Summers L, Agostini JV. The role of adherence on the effectiveness of nonpharmacologic interventions: Evidence from the delirium prevention trial. Archives of Internal Medicine 2003;163(8):958‐64. [DOI] [PubMed] [Google Scholar]
- Leslie DL, Zhang Y, Bogardus ST, Holford TR, Leo‐Summers LS, Inouye SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. Journal of the American Geriatrics Society 2005;53(3):405‐9. [DOI] [PubMed] [Google Scholar]
- Rizzo JA, Bogardus ST, Leo‐Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value?. Medical Care 2001;39(7):740‐52. [DOI] [PubMed] [Google Scholar]
Kaneko 1999 {published data only}
- Kaneko T, Cai J, Ishikura T, Kobayashi M, Naka T, Kaibara N. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica 1999;42(3):179‐84. [Google Scholar]
Kat 2008 {published data only}
- Kat MG, Vreeswijk R, Jonghe JF, Ploeg T, can Gool WA, Eikelenboom P, et al. Long term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dementia and Geriatric Cognitive Disorders 208;26(1):1‐8. [DOI] [PubMed] [Google Scholar]
Lackner 2008 {published data only}
- Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo‐controlled trial of the cognitive effect, safety, and tolerability of oral extended‐release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. Journal of the American Geriatrics Society 2008;56(5):862‐70. [DOI] [PubMed] [Google Scholar]
Landefeld 1995 {published data only}
- Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. New England Journal of Medicine 1995;332(20):1338‐44. [DOI] [PubMed] [Google Scholar]
Lili 2013 {published data only}
- Lili X, Zhiyong H, Jianjun S. A preliminary study of the effects of ulinastatin on early postoperative cognition function in patients undergoing abdominal surgery. Neuroscience Letters 2013;541:15‐9. [DOI] [PubMed] [Google Scholar]
Lundstrom 2005 {published data only}
- Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. Journal of the American Geriatrics Society 2005;53(4):622‐8. [DOI] [PubMed] [Google Scholar]
Maneeton 2007 {published data only}
- Maneeton B, Maneeton N, Srisurapanont M. An open‐label study of quetiapine for delirium. Journal of the Medical Association of Thailand 2007;90(10):2158‐208. [PubMed] [Google Scholar]
Marcantonio 2010 {published data only}
- Marcantonio ER, Bergmann MA, Kiely DK, Orav EJ, Jones RN. Randomized trial of a delirium abatement program for postacute skilled nursing facilities. Journal of the American Geriatrics Society 2010;58(6):1019‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mardani 2013 {published data only}
- Mardani D, Bigdelian H. Prophylaxis of dexamethasone protects patients from further post‐operative delirium after cardiac surgery: A randomized trial. Journal of Research in Medical Sciences 2013; Vol. 18, issue 2:137‐43. [PMC free article] [PubMed]
Marino 2009 {published data only}
- Marino J, Russo J, Kenny M, Herenstein R, Livote E, Chelly JE. Continuous lumbar plexus block for postoperative pain control after total hip arthoplasty. A randomised controlled trial. Journal of Bone and Joint Surgery 2009;91(1):29‐37. [DOI] [PubMed] [Google Scholar]
Mentes 2003 {published data only}
- Mentes JC, Culp K. Reducing hydration‐linked events in nursing home residents. Clinical Nursing Research 2003;12(3):210‐25; discussion 226‐8. [DOI] [PubMed] [Google Scholar]
Meybohm 2015 {published data only}
- Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. New England Journal of Medicine 2015;373(15):1397‐407. [DOI] [PubMed] [Google Scholar]
Milisen 2001 {published data only}
- Milisen K, Foreman MD, Abraham IL, Geest S, Godderis J, Vandermeulen E, et al. A nurse‐led interdisciplinary intervention program for delirium in elderly hip‐fracture patients. Journal of the American Geriatrics Society 2001;49(5):523‐32. [DOI] [PubMed] [Google Scholar]
Mudge 2008 {published data only}
- Mudge AM, Giebel AJ, Cutler AJ. Exercising body and mind: an integrated approach to functional independence in hospitalized older people. Journal of the American Geriatrics Society 2008;56(4):630‐5. [DOI] [PubMed] [Google Scholar]
Myint 2013 {published data only}
Naughton 2005 {published data only}
- Naughton BJ, Saltzman S, Ramadan F, Chadha N, Priore R, Mylotte JM. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. Journal of the American Geriatrics Society 2005;53(1):18‐23. [DOI] [PubMed] [Google Scholar]
Neri 2010 {published data only}
- Neri S, Bertino G, Petralia A, Giancarlo C, Rizzotto A, Calvagno GS, et al. A multidisciplinary therapeutic approach for reducing the risk of psychiatric side effects in patients with chronic hepatitis C treated with pegylated interferon α and ribavirin. Journal of Clinical Gastroenterology 2010;44(9):e210‐e217. [DOI] [PubMed] [Google Scholar]
Oldenbeuving 2008 {published data only}
- Oldenbeuving AW, Kort PL, Jansen BP, Kappelle LJ, Roks G. A pilot study of rivastigmine in the treatment of delirium after stroke: a safe alternative. BMC Neurology 2008;834:doi: 10.1186/1471‐2377‐8‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overshott 2010 {published data only}
- Overshott R, Vernon M, Morris J, Burns A. Rivastigmine in the treatment of delirium in older people: A pilot study. International Psychogeriatrics 2010;22(5):812‐8. [DOI] [PubMed] [Google Scholar]
Pandharipande 2010 {published data only}
- Pandharipande PP, Sanders PD, Girard TD, McGrane S, Thomptosn JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori‐designed analysis of the MENDS randomized controlled trial. Critical Care 2010;14(2):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2015 {published data only}
- Parker MJ, Griffiths R. General versus regional anaesthesia for hip fractures. A pilot randomised controlled trial of 322 patients. Injury 2015;46(8):1562‐6. [DOI] [PubMed] [Google Scholar]
Parra Sanchez 2009 {published and unpublished data}
- Parra Sanchez, Ivan. Intravenous lidocaine and postoperative outcomes after cardiac surgery. www.ClinicalTrials.gov (NCT00840918) 2009.
Perkisas 2015 {published data only}
- Perkisas SMT, Vandewoude MFJ. Ramelteon for prevention of delirium in hospitalized older patients. JAMA ‐ Journal of the American Medical Association 2015;313(17):1745‐6. [DOI] [PubMed] [Google Scholar]
Pitkala 2006 {published data only}
- Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized controlled trial. Journals of Gerontology Series A (Biological Sciences and Medical Sciences) 2006;61(2):176‐81. [DOI] [PubMed] [Google Scholar]
Prakanrattana 2007 {published data only}
- Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesthesia and Intensive Care 2007;35(5):714‐9. [DOI] [PubMed] [Google Scholar]
Pretto 2014 {published data only}
- Pretto G, Westphal GA, Silva E. Clonidine for reduction of hemodynamic and psychological effects of S+ ketamine anesthesia for dressing changes in patients with major burns: an RCT. Burns 2014;40(7):1300‐7. [DOI] [PubMed] [Google Scholar]
Ritchie 2008 {published data only}
- Ritchie C. A phase III, seven‐day randomised, double‐blind, placebo‐controlled, parallel group study to assess efficacy of Donsepezil for reducing the incidence and severity of post‐operative delirium after an elective total hip of knee replacement in patients over 65 years old. http://www.isrctn.com/ISRCTN55655483 2008.
Saager 2015 {published data only}
- Saager L, Duncan AE, Yared J‐P, Hesler BD, You J, Deogaonkar A, et al. Intraoperative tight glucose control using hyperinsulinemic normoglycemia increases delirium after cardiac surgery. Anesthesiology 2015;122(6, (NIH) *National Institutes of Health*):1214‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sauer 2014 {published data only}
- Sauer A‐MC, Slooter AJ, Veldhuijzen DS, Eijk MM, Devlin JW, Dijk D. Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Anesthesia and analgesia 2014;119(5):1046‐52. [DOI] [PubMed] [Google Scholar]
Short 2015 {published data only}
- Short TG, Leslie K, Chan MTV, Campbell D, Frampton C, Myles P. Rationale and design of the balanced anesthesia study: a prospective randomized clinical trial of two levels of anesthetic depth on patient outcome after major surgery. Anesthesia and Analgesia 2015;121(2):357‐65. [DOI] [PubMed] [Google Scholar]
Shu 2010 {published data only}
- Shu H. Effects of parecoxib on emergence of delirium and postoperative pain in elderly patients undergoing abdominal surgery after general anesthesia. NCT01221025 2010.
Tabatabaie 2015 {published data only}
- Tabatabaie O, Matin N, Heidari A, Tabatabaie A, Hadaegh A, Yazdanynejad S, et al. Spinal anesthesia reduces postoperative delirium in opium dependent. Acta Anaesthesiologica Belgica 2015;66(2):49‐54. [PubMed] [Google Scholar]
Tabet 2005 {published data only}
- Tabet N, Hudson S, Sweeney V, Sauer J, Bryant C, Macdonald A, et al. An educational intervention can prevent delirium on acute medical wards. Age and Ageing 2005;34(2):152‐6. [DOI] [PubMed] [Google Scholar]
Takeuchi 2007 {published data only}
- Takeuchi T, Furuta K, Hirasawa T, Masaki H, Yukizane T, Atsuta H, et al. Perospirone in the treatment of patients with delirium. Psychiatry and Clincial Neurosciences 2007;61(1):67‐70. [DOI] [PubMed] [Google Scholar]
Tokita 2001 {published data only}
- Tokita K, Tanaka H, Kawamoto M, Yuge O. [Patient‐controlled epidural analgesia with bupivacaine and fentanyl suppresses postoperative delirium following hepatectomy]. Masui [Japanese Journal of Anesthesiology] 2001;50(7):742‐6. [PubMed] [Google Scholar]
Torres 2015 {published data only}
- Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community‐acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015;313(7):677‐86. [DOI] [PubMed] [Google Scholar]
van de Steeg 2014 {published data only}
- Steeg L, IJkema R, Langelaan M, Wagner C. Can an e‐learning course improve nursing care for older people at risk of delirium: a stepped wedge cluster randomised trial. BMC Geriatrics 2014;14:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang W, Li HL, Wang DX, Zhu X, Li SL, Yao GQ, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Critical Care Medicine 2012;40(3):731‐9. [DOI] [PubMed] [Google Scholar]
Wanich 1992 {published data only}
- Wanich CK, Sullivan‐Marx EM, Gottlieb GL, Johnson JC. Functional status outcomes of a nursing intervention in hospitalized elderly. Image ‐ the Journal of Nursing Scholarship 1992;24(3):201‐7. [DOI] [PubMed] [Google Scholar]
Wong 2005 {published data only}
- Wong Tin Niam DM, Bruce JJ, Bruce DG. Quality project to prevent delirium after hip fracture. Australasian Journal on Ageing 2005;24(3):174‐7. [Google Scholar]
Yamaguchi 2014 {published data only}
- Yamaguchi Y, Mihara T, Taguri M, Yamaguchi O, Goto T. Melatonin receptor agonist for the prevention of postoperative delirium in elderly patients: A randomized, double‐blind, placebo‐controlled trial. Intensive care medicine 2014;Conference: 27th Annual Congress of the European Society of Intensive Care Medicine, ESICM 2014 Barcelona Spain. Conference Start: 20140927 Conference End: 20141001. Conference Publication:(var.pagings):S246. [Google Scholar]
Yang 2015 {published data only}
- Yang X, Li Z, Gao C, Liu R. Effect of dexmedetomidine on preventing agitation and delirium after microvascular free flap surgery: a randomized, double‐blind, control study. Journal of Oral and Maxillofacial Surgery 2015;73(6):1065‐72. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Al Tmimi 2015 {published data only}
- Al Tmimi L, Velde M, Herijgers P, Meyns B, Meyfroidt G, Milisen K, et al. Xenon for the prevention of postoperative delirium in cardiac surgery: study protocol for a randomized controlled clinical trial. Trials 2015;16:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avidan 2009 {published data only}
- Avidan M, Palanca B, Glick D, Jacobsohn E, Villafranca A, O'Connor M, et al. Protocol for the BAG‐RECALL clinical trial: a prospective, multi‐center, randomized, controlled trial to determine whether a bispectral index‐guided protocol is superior to an anesthesia gas‐guided protocol in reducing intraoperative awareness with explicit recall in high risk surgical patients. BMC Anesthesiology 2009;9(8):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avidan 2015 {published data only}
- Avidan M. The prevention of delirium and complications associated with surgical treatments multi center clinical trial. https://clinicaltrials.gov/ct2/show/NCT01690988 2013.
- Avidan MS, Fritz BA, Maybrier HR, Muench MR, Escallier KE, Chen Y, et al. The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicentre randomised controlled trial. BMJ Open 2015;4(9):e005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beilin 2010 {published data only}
- Beilin B. The effect of physostigmine on cognitive functioning in the immediate period after sedation for colonoscopy. ClinicalTrials.gov: NCT01121497 2010.
Bekker 2008 {published data only}
- Bekker A. Rivastigmine prophylaxis in elderly patients undergoing major surgery. ClinicalTrials.gov: NCT00835159 2008.
Brzezinski 2012 {published data only}
- Brzezinski M. Effect of prophylactic, perioperative propranolol on peri‐ and postoperative complications in patients with post traumatic stress disorder. ClinicalTrials.gov: NCT01555554 2012.
Chan 2010 {published data only}
- Chan Y. The effect of periarticular multi‐drug regimen on pain after partial hip replacement. https://clinicaltrials.gov/ct2/show/NCT01112436 2010.
Chaput 2009 {published data only}
- Chaput A, Yang H, Bryson GL, Evans H, Beaule P, Jetty P, et al. A randomized, double‐blind, placebo‐controlled trial to assess the safety and efficacy of the perioperative administration of pregabalin in reducing the incidence of postoperative delirium and improving acute postoperative pain management. https://clinicaltrials.gov/ct2/show/NCT00819988 2009.
Coburn 2012 {published data only}
- Coburn M, Sanders R, Maze M, Rossiant R. The Hip Fracture Surgery in Elderly Patients (HiPELD) study: protocol for a randomized, multicenter controlled trial evaluating the effect of xenon on postoperative delirium in older patients undergoing hip fracture surgery. Trials 2012;13(180):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diehl 2006 {published data only}
- Diehl J. Prevention of postoperative delirium with donepezil. https://www.clinicaltrials.gov/ct2/show/NCT00220896.
Fernandez‐Robles 2012 {published data only}
- Fernandez‐Robles C. Usefulness of bright light therapy in the prevention of delirium in patients undergoing Hematopoietic Stem Cell Transplant (HSCT). https://clinicaltrials.gov/ct2/show/NCT01700816 2012.
Fischer 2009 {published data only}
- Fischer G. Tailored patient management guided with absolute cerebral oximetry to prevent neurocognitive injury in elderly patients undergoing cardiac surgery. https://clinicaltrials.gov/ct2/show/NCT00991328 2009.
Foss 2006 {published and unpublished data}
- Foss NB. Incidence of delirium in hip fracture patients randomized to regular hypnotics vs placebo. ClinicalTrials.Gov NCT00286936 2006.
Hua 2010 {published data only}
- Hua Z. Influence of multimodal analgesia with parecoxib and morphine on post surgical delirium in elderly patients. ChiCTR‐TRC‐10001063 2010.
Katznelson 2010 {published data only}
- Katznelson R. Postoperative melatonin administration and delirium prevention in patients undergoing vascular and cardiac surgery. https://clinicaltrials.gov/ct2/show/NCT01198938 2013.
Mouchoux 2011 {published data only}
- Mouchoux C, Rippert P, Duclos A, Fassier T, Bonnefoy M, Comte B, et al. Impact of a multifaceted program to prevent postoperative delirium in the elderly: the CONFUCIUS stepped wedge protocol. BMC Geriatrics 2011;11(25):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nadler 2014 {published data only}
- Nadler J, Evans JL, Fang E, Preud'Homme X, Daughtry L, Chapman J, et al. Does positive airway pressure therapy reduce the incidence of postoperative delirium in patients at risk for obstructive sleep apnea? Interim analysis results from a randomized controlled clinical trial. Sleep 2014;Conference: 28th Annual Meeting of the Associated Professional Sleep Societies, LLC, SLEEP 2014 Minneapolis, MN United States. Conference Start: 20140531 Conference End: 20140604. Conference Publication:(var.pagings):A125. [Google Scholar]
Nanayakkara 2011 {published data only}
- Nanayakkara P. Early pharmacological intervention to prevent delirium: haloperidol prophylaxis in older emergency department patients. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3207 2011.
Privitera 2006 {published data only}
- Privitera. Namenda to prevent post‐operative delirium. https://clinicaltrials.gov/ct2/show/NCT00303433 2006.
Schrijver 2014 {published data only}
- Schrijver EJ, Vries OJ, Verburg A, Graaf K, Bet PM, Ven PM, et al. Efficacy and safety of haloperidol prophylaxis for delirium prevention in older medical and surgical at‐risk patients acutely admitted to hospital through the emergency department: study protocol of a multicenter, randomised, double‐blind, placebo‐controlled clinical trial. BMC Geriatrics 2014;14:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverstein 2008 {published data only}
- Silverstein S. Perioperative cognitive function ‐ dexmedetomidine and cognitive reserve. https://clinicaltrials.gov/ct2/show/NCT00561678 2008.
Spies 2009 {published data only}
- Spies C. Peri‐operative physostigmine prophylaxis for liver resection patients at risk for delirium and post‐operative cognitive dysfunction: a prospective, randomised, controlled, double‐blinded, two‐armed single centre trial. http://www.isrctn.com/ISRCTN18978802 2009.
Strijbos 2013 {published data only}
- Strijbos MJ, Steunenberg B, Mast RC, Inouye SK, Schuurmans MJ. Design and methods of the Hospital Elder Life Program (HELP), a multicomponent targeted intervention to prevent delirium in hospitalized older patients: efficacy and cost‐effectiveness in Dutch health care. BMC Geriatrics 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2012 {published data only}
- Thomas L. Does femoral nerve catheterization reduce the incidence of post‐operative delirium in patients presenting for hip fracture repair?. https://clinicaltrials.gov/ct2/show/NCT01547468 2012.
van der Burg 2005 {published data only}
- Burg BL. Post‐operative haloperidol versus placebo for prevention of post‐operative delirium after acute hip surgery. https://clinicaltrials.gov/ct2/show/NCT00250237 2005.
Wang 2012a {published data only}
- Wang D. Effects of two different anesthesia‐analgesia methods on the incidence of postoperative delirium: a multicenter, randomized controlled trial. ClinicalTrials.gov: NCT01661907 2012. [DOI] [PMC free article] [PubMed]
Young 2015 {published data only}
- Young J, Cheater F, Collinson M, Fletcher M, Forster A, Godfrey M. Prevention of delirium (POD) for older people in hospital: study. Trials 2015;16(1):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abraha 2015
- Abraha I, Trotta F, Rimland JM, Cruz‐Jentoft A, Lozano‐Montoya I, Soiza RL. Efficacy of non‐pharmacological interventions to prevent and treat delirium in older patients: a systematic overview.. PloS One 2015;10(6):e0123090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albert 1992
- Albert MS, Levkoff SE, Reilly C, Liptzin B, Pilgrim D, Cleary PD, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. Journal of Geriatric Psychiatry & Neurology 1992;5(1):14‐21. [DOI] [PubMed] [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition. Washington DC: American Psychiatric Association, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2013
- American Psychiatric Association. Statistical Manual of Mental Disorders. 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Aubrun 2007
- Aubrun F, Marmion F. The elderly patient and postoperative pain treatment. Best Practice & Research Clinical Anaesthesiology 2007;21(1):109‐27. [DOI] [PubMed] [Google Scholar]
Bell 2006
- Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004603.pub2] [DOI] [PubMed] [Google Scholar]
Bonnet 2005
- Bonnet F, Marret E. Influence of anaesthetic and analgesic techniques on outcome after surgery. British Journal of Anaesthesia 2005;95(1):52‐8. [DOI] [PubMed] [Google Scholar]
Bourne 2006
- Bourne RS, Mills GH. Melatonin: possible implications for the postoperative and critically ill patients. Intensive Care Medicine 2006;32(3):371‐9. [DOI] [PubMed] [Google Scholar]
Breitbart 1997
- Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. Journal of Pain & Symptom Management 1997;13(3):128‐37. [DOI] [PubMed] [Google Scholar]
Breitbart 2002
- Breitbart W, Gibson C, Tremblay A. The delirium experience: delirium recall and delirium‐related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002;43(3):183‐94. [DOI] [PubMed] [Google Scholar]
Carson 2011
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. FOCUS Investigators. Liberal or restrictive transfusion in high‐risk patients after hip surgery. New England Journal of Medicine 2011;365(26):2453‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Centeno 2004
- Centeno C, Sanz A, Bruera E. Delirium in advanced cancer patients. Palliative Medicine 2004;18(3):184‐94. [DOI] [PubMed] [Google Scholar]
Charlson 1994
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology 1994;47(11):1245‐51. [DOI] [PubMed] [Google Scholar]
Cheer 2001
- Cheer SM, Goa KL. Parecoxib (parecoxib sodium). Drugs 2001;61(8):1133‐41. [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen S, Shi Li, Lang F, Xu L, Desislava D, Wu Q, et al. Exogenous melatonin for delirium prevention: A meta‐analysis of randomized controlled trials. Molecular Neurobiology 2015;July ePub ahead of print:DOI 10.1007/s12035‐015‐9350‐8. [DOI] [PubMed] [Google Scholar]
Clegg 2013
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381(9868):752‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
Cole 1999
- Cole MG. Delirium: effectiveness of systematic interventions. Dementia and Geriatric Cognitive Disorders 1999;10(5):406‐11 1999. [DOI] [PubMed] [Google Scholar]
Dexter 2001
- Dexter PR, Perkins S, Overhage JM, Maharry K, Kohler RB, McDonald CJ. A computerized reminder system to increase the use of preventive care for hospitalized patients. New England Journal of Medicine 2001;345:965‐70. [DOI] [PubMed] [Google Scholar]
Dieleman 2012
- Dieleman JM, Nierich AP, Rosseel PM, Maaten JM, Hofland J, Diephuis JC, et al. Dexamethasone for Cardiac Surgery (DECS) Study Group. Intraoperative high‐dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA 2012;308(17):1761‐7. [DOI] [PubMed] [Google Scholar]
Douglas 2001
- Douglas WW, Kehlet H. Management of patients in fast track surgery. BMJ 2001;322(7284):473‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edelstein 2004
- Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval K. Effect of postoperative delirium on outcome after hip fracture. Clinical Orthopaedics & Related Research 2004;422:195‐200. [DOI] [PubMed] [Google Scholar]
Eeles 2012
- Eeles EMP, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older patients. Age Ageing 2012;41:412‐6. [DOI] [PubMed] [Google Scholar]
Elie 2000
- Elie M, Rousseau F, Cole M, Primeau F, McCusker J, Bellavance F. Prevalence and detection of delirium in elderly emergency department patients. Canadian Medical Association Journal 2000;163(8):977‐81. [PMC free article] [PubMed] [Google Scholar]
Ellis 2011
- Ellis G, Whitehead MA, Robinson D, O'Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ 2011;343:d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ely 2001
- Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment method for the Intensive Care Unit (CAM‐ICU). Critical Care Medicine 2001;27(7):1370‐9. [DOI] [PubMed] [Google Scholar]
Fick 2011
- Fick DM, Steis MR, Mion LC, Walls JL. Computerized decision support for delirium superimposed on dementia in older adults. Journal of Gerontological Nursing 2011;37(4):39‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figueroa‐Ramos 2009
- Figueroa‐Ramos, Milagros I. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Medicine 2009;35(5):781‐95. [DOI] [PubMed] [Google Scholar]
Fines 2006
- Fines DP, Severn AM. Anaesthesia and cognitive disturbance in the elderly. Continuing Education in Anaesthesia, Critical Care & Pain 2006;6(1):37‐40. [Google Scholar]
Fioravanti 2005
- Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP‐choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD000269.pub3] [DOI] [PubMed] [Google Scholar]
Fioravanti 2006a
- Fioravanti M, Buckley AE. Citicoline (Cognizin) in the treatment of cognitive impairment. Clinical Interventions in Aging 2006;1(3):247‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fok 2015
- Fok MC, Sepehry A, Frisch L, Sztramko R, Burg BBL, Vochteloo AJH, et al. Do antipsychotics prevent postoperative delirium? A systematic review. International Journal of Geriatric sychiatry 2015;30(4):333‐44. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [DOI] [PubMed] [Google Scholar]
Fong 2015
- Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. The Lancet Neurology 2015;14(8):823‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 1990
- Francis J, Kapoor WN. Delirium in hospitalized elderly. Journal of General Internal Medicine 1990;5(1):65‐79. [DOI] [PubMed] [Google Scholar]
Fried 2001
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 2001;56(3):M146‐56. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. McMaster University, 2014.
Gustafson 1985
- Gustafson L, Lindgren M, Westling B. A new rating scale of evaluation of confusional states and other organic brain syndromes. II International Congress of Psychogeriatric Medicine. Umea, August 28‐31, 1985; Vol. 57.
Hanania 2002
- Hanania M, Kitain E. Melatonin for treatment and prevention of postoperative delirium. Anesthesia and Analgesia 2002;94(2):338‐9. [DOI] [PubMed] [Google Scholar]
Holmes 2000
- Holmes JD, House AO. Psychiatric illness in hip fracture. Age and Ageing 2000;29(6):537‐46. [DOI] [PubMed] [Google Scholar]
Hshieh 2015
- Hshieh TT, Yue J, Oh E, Puelle M, Dowal S, Travison T, Inouye SK. Effectiveness of multicomponent nonpharmacological delirium interventions. JAMA Internal Medicine 2015;175(4):512‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hubbard 2004
- Hubbard RE, O’Mahony MS, Cross E, Morgan A, Hortop H, Morse R, et al. The ageing of the population: implications for multi‐disciplinary care in hospital. Age Ageing 2004;33:479‐82. [DOI] [PubMed] [Google Scholar]
Hustey 2003
- Hustey FM, Meldon SW, Smith MD, Lex CK. The effect of mental status screening on the care of elderly emergency department patients. Annals of Emergency Medicine 2003;41(5):678‐84. [DOI] [PubMed] [Google Scholar]
Huybrechts 2012
- Huybrechts KF, Gerhard T, Crystal S, Olfson M, Avorn J, Levin R, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ 2012;344:e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inouye 1990
- Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine 1990;113(12):941‐8. [DOI] [PubMed] [Google Scholar]
Inouye 1998a
- Inouye SK, Rushing JT, Foreman MD, Palmer RM, Pompei P. Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. Journal of General Internal Medicine 1998;13:234‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inouye 1998b
- Inouye SK. Delirium in hospitalized older patients: recognition and risk factors. Journal of Geriatric Psychiatry and Neurology 1998;11(3):118‐25; discussion 157‐8. [DOI] [PubMed] [Google Scholar]
Inouye 1999a
- Inouye SK, Bogardus ST Jr, Charpentier PA, Leo‐Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. New England Journal of Medicine 1999;340(9):669‐76. [DOI] [PubMed] [Google Scholar]
Inouye 1999b
- Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care Is failing older persons and a window to improve quality of hospital care. American Journal of Medicine 1999;106(5):565‐73. [DOI] [PubMed] [Google Scholar]
Inouye 1999c
- Inouye SK. Predisposing and precipitating factors for delirium in hospitalized older patients. Dementia & Geriatric Cognitive Disorders 1999;10(5):393‐400. [DOI] [PubMed] [Google Scholar]
Inouye 2000
- Inouye SK, Bogardus ST Jr, Baker DI, Leo‐Summers L, Cooney LM Jr. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Journal of the American Geriatrics Society 2000;48(12):1697‐706. [DOI] [PubMed] [Google Scholar]
Inouye 2014
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Institute for Innovation 2006
- Institute for Innovation and Improvement. Care pathways for frail older people. London: DH; 2006.. Care Pathways for Frail Older People. London: Department of Health, 2006. [Google Scholar]
Joosten 2014
- Joosten E, Demuynck M, Detroyer E, Milisen K. Prevalence of frailty and its ability to predict in hospital delirium, falls, and 6‐month mortality in hospitalized older patients. BMC Geriatrics 2014;14(1):doi: 10.1186/1471‐2318‐14‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorm 1989
- Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio‐demographic correlates, reliability, validity and some norms. Psychological Medicine 1989;19:1015‐22. [DOI] [PubMed] [Google Scholar]
Katz 1970
- Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10(1):20‐30. [DOI] [PubMed] [Google Scholar]
Koponen 1999
- Koponen HJ. Neurochemistry and delirium. Dementia and Geriatric Cognitive Disorders 1999;10(5):339–41. [DOI] [PubMed] [Google Scholar]
Krenk 2012
- Krenk L, Rasmussen LS, Kehlet H. Delirium in the fast‐track surgery setting. Best Practice and Research. Clinical Anaesthesiology 2012;26(3):345‐53. [DOI] [PubMed] [Google Scholar]
Levkoff 1991
- Levkoff S, Cleary P, Liptzin B, Evans DA. Epidemiology of delirium: an overview of research issues and findings. International Psychogeriatrics 1991;3(2):149‐67. [DOI] [PubMed] [Google Scholar]
Lewis 2004
- Lewis MC, Barnett SR. Postoperative delirium: the tryptophan dysregulation model. Medical Hypotheses 2004;63(3):402‐6. [DOI] [PubMed] [Google Scholar]
Ljubisavljevic 2003
- Ljubisavljevic V, Kelly B. Risk factors for development of delirium among oncology patients. General Hospital Psychiatry 2003;25(5):345‐52. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel D. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56‐61. [PubMed] [Google Scholar]
Martinez 2015
- Martinez F, Tobar C, Hill N. Preventing delirium: should non‐pharmacological, multicomponent interventions be used? A systematic review and meta‐analysis of the literature. Age Ageing 2015;44(2):196‐204. [DOI] [PubMed] [Google Scholar]
McCusker 2001
- McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. Canadian Medical Association Journal 2001;165(5):575‐83. [PMC free article] [PubMed] [Google Scholar]
McCusker 2002
- McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12‐month mortality. Archives of Internal Medicine 2002;162(4):457‐63. [DOI] [PubMed] [Google Scholar]
McCusker 2003a
- McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay?. Journal of the American Geriatrics Society 2003;51(11):1539‐46. [DOI] [PubMed] [Google Scholar]
McCusker 2003b
- McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. Journal of General Internal Medicine 2003;18(9):696‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milbrandt 2004
- Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Critical Care Medicine 2004;32(4):955‐62. [DOI] [PubMed] [Google Scholar]
Milisen 2005
- Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. Journal of Advanced Nursing 2005;52(1):79‐90. [DOI] [PubMed] [Google Scholar]
Miyamoto 2009
- Miyamoto M. Pharmacology of ramelteon, a selective MT1/MT2 receptor agonist: a novel therapeutic drug for sleep disorders. Clinical Neuroscience & Therapeutics 2009;15(1):32‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moller 1998
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long‐term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post‐Operative Cognitive Dysfunction. Lancet 1998;351(9106):857‐61. [DOI] [PubMed] [Google Scholar]
Neelon 1996
- Neelon VJ, Champagne MT, Carlson JR, Funk SG. The NEECHAM confusion scale: construction, validation and clinical testing. Nursing Research 1996;45(6):324‐30. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Clinical Excellence. Delirium: Diagnosis, prevention and management CG103. London: National Institute for Health and Clinical Excellence, 2010. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Reviw Manager (RevMan). Version 5.2. Copenhagen: The Cochrane Collaboration, 2012.
Rizzo 2001
- Rizzo JA, Bogardus ST Jr, Leo‐Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value?. Medical Care 2001;39(7):740‐52. [DOI] [PubMed] [Google Scholar]
Robinson 2009
- Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Annals of Surgery 2009;249(1):173‐8. [DOI] [PubMed] [Google Scholar]
Rockwood 1999
- Rockwood K. Educational interventions in delirium. Dementia and Geriatric Cognitive Disorders 1999;10(5):426‐9. [DOI] [PubMed] [Google Scholar]
Santos 2004
- Santos FS, Velasco IT, Fraguas R Jr. Risk factors for delirium in the elderly after coronary artery bypass graft surgery. International Psychogeriatrics 2004;16(2):175‐93. [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings; tables [In: Higgins JPT, Green S (editors),]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions [In: Higgins JPT, Green S (editors)]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org.
Schuurmans 2003
- Schuurmans MJ, Shortridge‐Baggett LM, Duursma SA. The Delirium Observation Screening Scale: A screening instrument for delirium. Research and Theory for Nursing Practice 2003;17(1):31‐50. [DOI] [PubMed] [Google Scholar]
Sheikh 1986
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. In: TL Brink editor(s). Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press, Inc, 1986:165‐73. [Google Scholar]
Sheldon 2010
Siddiqi 2006
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in‐patients: a systematic literature review. Age and Ageing 2006;35:350‐64. [DOI] [PubMed] [Google Scholar]
Stevens 1998
- Stevens LE, Moore GM, Simpson JM. Delirium in hospital: does it increase length of stay?. Australian and New Zealand Journal of Psychiatry 1998;32(6):805‐8. [DOI] [PubMed] [Google Scholar]
Tippana 2007
- Tippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety.. Anesthesia and Analgesia 2007;104:1545–56. [DOI] [PubMed] [Google Scholar]
Trzepacz 1988
- Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Research 1988;23(1):89‐97. [DOI] [PubMed] [Google Scholar]
Trzepacz 1996
- Trzepacz PT. Delirium. Advances in diagnosis, pathophysiology, and treatment. Psychiatric Clinics of North America 1996;19(3):429‐48. [DOI] [PubMed] [Google Scholar]
Trzepacz 2001
- Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale‐revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. Journal of Neuropsychiatry and Clinical Neurosciences 2001;13(2):229‐42. [DOI] [PubMed] [Google Scholar]
Tu 2006
- Tu RH, Grewall P, Leung JW, Suryaprasad AG, Sheykhzadeh PI, Doan C, et al. Diphenhydramine as an adjunct to sedation for colonoscopy: a double‐blind randomized, placebo‐controlled study. Gastrointestinal Endoscopy 2006;63(1):87‐94. [DOI] [PubMed] [Google Scholar]
Tune 1999
- Tune LE, Egeli S. Acetylcholine and delirium. Dementia and Geriatric Cognitive Disorders 1999;10(5):342‐4. [DOI] [PubMed] [Google Scholar]
Uldall 1997
- Uldall KK, Berghuis JP. Delirium in AIDS patients: recognition and medication factors. AIDS Patient Care and Standards 1997;11(6):435‐41. [DOI] [PubMed] [Google Scholar]
van Eijk 2010
- van Eijek, MMJ, Roes KC, Honing ML, Kuiper MA, Karakus A, Jagt M, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double‐blind, placebo‐controlled randomised trial. Lancet 2010;376(9755):1829–37. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30:473‐83. [PubMed] [Google Scholar]
Whitlock 2008
- Whitlock RP, Chan S, Devereaux PJ, Sun J, Rubens FD, Thorlund K, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: a meta‐analysis of randomized trials. European Heart Journal 2008;29(21):2592‐600. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. International Classification of Diseases, 10th revision. Geneva: World Health Organisation, 1992. [Google Scholar]
Williams‐Russo 1992
- Williams‐Russo P, Urquhart BL, Sharrock NE, Charlson ME. Post‐operative delirium: Predictors and prognosis in elderly orthopedic patients. Journal of the American Geriatrics Society 1992;40(8):759‐67. [DOI] [PubMed] [Google Scholar]
Witlox 2010
- Witlox J, Eurelings LS, Jonghe JF, Kalisvaart KJ, Eikelenboom P, Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA 2010;304(4):443‐51. [DOI] [PubMed] [Google Scholar]
Woodhead 2007
- Woodhead J, Harding SA, Simmonds M, Dee S, McBride‐Henry K. Premedication for cardiac catheterization and percutaneous coronary intervention: does it increase vascular access site complications?. Journal of Cardiovascular Nursing 2007;22(6):466‐71. [DOI] [PubMed] [Google Scholar]
Young 2007
- Young J, Inouye SK. Delirium in older people. BMJ 2007;334:842‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang H, Lu Y, Liu M, Zou Z, Wang L, Xu F‐Y, et al. Strategies for prevention of postoperative delirium: a systematic review and meta‐analysis of randomized trials. Critical Care 2013;17:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Siddiqi 2007
- Siddiqi N, Holt R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalised patients. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005563.pub2] [DOI] [PubMed] [Google Scholar]


