Abstract
Purpose:
Radiation therapy remains part of the standard of care for breast, lung, and esophageal cancers. While radiotherapy improves local control and survival, radiation-induced heart dysfunction is a common side effect of thoracic radiotherapy. Cardiovascular dysfunction can also result from non-therapeutic total body radiation exposures. Numerous studies have evaluated the relationship between radiation dose to the heart and cardiotoxicity, but relatively little is known about whether there are differences based on biological sex in radiation-induced heart dysfunction (RIHD).
Materials and Methods:
We evaluated whether male and female inbred Dahl SS rats display differences in RIHD following delivery of 24 Gy in a single fraction to the whole heart using a 1.5 cm beam-size. We also compared 2.0 cm vs. 1.5 cm collimator in males. Pleural and pericardial effusions and normalized heart weights were measured, and echocardiograms were performed.
Results:
Female SS rats displayed more severe RIHD relative to age-matched SS male rats. Normalized heart weight was significantly increased in females, but not in males. A total of 94% (15/16) of males and 55% (6/11) of females survived 5 months after completion of radiotherapy (P<0.01). Among surviving rats, 100% of females and 14% of males developed moderate-to-severe pericardial effusions at 5 months. Females demonstrated increased pleural effusions, with the mean normalized pleural fluid volume for females and males being 56.6 mL/kg±12.1 and 10.96 mL/kg±6.4 in males (P=0.001), respectively. Echocardiogram findings showed evidence of heart failure, which was more pronounced in females. Because age-matched female rats have smaller lungs, a higher percentage of total lung was treated with radiation in females than males using the same beam size. After using a larger 2 cm beam in males which results in higher lung exposure, there was not a significant difference between males and females in terms of development of moderate-to-severe pericardial effusions or pleural effusions. Treatment of males with a 2 cm beam resulted in comparable increases in LV mass and reductions in stroke volume.
Conclusion:
Together, these results illustrate that there are differences in radiation-induced cardiotoxicity between male and female SS rats and add to data that lung radiation doses may play an important role in cardiac dysfunction following heart radiation exposure. These factors may be important to factor into future mitigation studies of radiation-induced cardiotoxicity.
Keywords: radiation, cardio-oncology, radiation-induced cardiotoxicity, sex differences, pericardial effusion, lung radiation, heart radiation
Introduction
Heart disease and cancer are the two leading causes of death in the United States, accounting for over 40% of all deaths (Heron 2017). The population of patients with coexisting cancer and heart disease is predicted to increase exponentially in the future, due to improved treatments and increased life expectancy (Driver et al. 2008). More than 50% of cancer patients receive radiation therapy (RT), which has significantly improved outcomes in breast, lung, and esophageal cancers (Antonia et al. 2018; Early Breast Cancer Trialists’ Collaborative et al. 2011; Shapiro et al. 2015). However, RT can cause a wide range of side effects including radiation-induced heart dysfunction (RIHD) (Filopei et al. 2012). The risk of cardiac toxicity is increased in cancer patients who receive incidental radiation to the heart, such as patients with left-sided breast cancer tumors or patients with locally-advanced lung cancer (Bradley et al. 2015; Sardaro et al. 2012). Given that cancer-specific survival has improved in recent decades (Antonia et al. 2018; Miller et al. 2016), there is an ever-increasing number of survivors who have received thoracic radiation to the heart and are at risk for RIHD. The use of improved RT techniques over time has allowed decreases in incidental heart radiation doses (Bergom et al. 2018; Chang et al. 2006; Desai et al. 2019), but even low doses of radiation exposure can still lead to chronic cardiac dysfunction (Darby et al. 2013; Saiki et al. 2017). Moreover, the risk of RIHD is further exacerbated by cardiotoxic chemotherapies, including anthracyclines and trastuzumab, which are often prescribed to patients with breast cancer and lymphoma (Aleman et al. 2007; Bates et al. 2019; Clements et al. 2002; Hooning et al. 2007; Marinko et al. 2018; Moulin et al. 2015). In addition, increased cardiovascular dysfunction was seen in studies of atomic bomb survivors and some occupationally exposed radiation workers (Moseeva et al. 2014; Shimizu et al. 2010).
Radiation-induced cardiotoxicity can manifest as coronary artery disease, pericarditis, myocardial fibrosis, cardiomyopathy, pericardial effusion, and/or arrhythmias (Schlaak et al. 2020). Pericardial disease is the most common manifestation of RIHD, with an incidence of 20–45% (Ning et al. 2017; Wang et al. 2017; Xue et al. 2019). It also is one of the earliest manifestations of RIHD. Pericardial disease can present as pericarditis, pericardial effusions, and/or delayed thickening and constrictive pericarditis, which is associated with a particularly poor prognosis (George et al. 2012). Pericardial effusions can be asymptomatic or progress to shortness of breath and even cardiac tamponade (Boerma et al. 2008; Boerma et al. 2015). Retrospective studies have used the whole heart dose to develop several RT dose parameters predictive of RIHD (Darby et al. 2013; Speirs et al. 2017), but studies only recently have begun to evaluate doses to critical heart substructures and their associations with pericardial toxicities (Hayashi et al. 2015; McWilliam et al. 2017; Wang et al. 2017).
Beyond the potential association of cardiac radiation doses and RIHD, other factors can also influence toxicities from incidental cardiac irradiation, including genetic background (Schlaak et al. 2019), concurrent systemic therapy (Aleman et al. 2007; Bates et al. 2019; Clements et al. 2002; Hooning et al. 2007; Marinko et al. 2018), lung radiation doses (Cella et al. 2015; Cella et al. 2015), and potentially sex (Bates et al. 2019; Mulrooney et al. 2009). However, few studies have explored reasons for differences in normal tissue radiosensitivity between males and females. Prior studies have demonstrated sex-specific differences in side effects from chemotherapeutic treatments (Clements et al. 2002; Lipshultz et al. 1995; Moulin et al. 2015), but reporting of sex-specific differences in acute and late toxicities after radiotherapy has rarely been investigated in a systematic manner, and thus data remains scarce. If differences do exist, then radiation dose constraints could be specifically tailored to men and women to better optimize treatment safety. Therefore, we compared male versus female severity of RIHD in a pre-clinical rat model we previously developed (Schlaak et al. 2019; Schlaak et al. 2020; Schlaak et al. 2020). Our findings demonstrate that age-matched female rats exhibit significantly worse pleural and pericardial effusions than males, as well as other measures of RIHD. These differences may in part be due to increased relative volumes of lung receiving higher doses of radiation, as treatment of male rats with a larger beam size increased pleural and pericardial effusions to levels similar to females treated with a smaller beam size that still covered the heart volume. These results highlight potential sex differences in cardiac dysfunction after cardiac radiation exposure.
Materials and Methods
Rats and irradiation procedure.
All procedures were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Procedures were used as described previously (Schlaak et al. 2019). Briefly, Dahl SS (Dahl salt-sensitive/Mcwi) rats, aged 10 to 12 wk, were randomly allocated to different treatment groups. Local cardiac radiation was performed with the high-precision image-guided X-RAD SmART irradiator (Precision X-Ray, North Branford, CT). A calibrated ionization chamber was used to regularly check the output of the irradiator. Rats were anesthetized using 3% isoflurane/room temperature air inhalation for the duration of each radiation treatment. Pilot V1.8 Imaging Software (University Health Network, Toronto, Canada) was used for planning. Two-dimensional projections over 360° were created to provide CT scans in sagittal, transverse, and frontal views. The heart projection was centered to fit in the collimator. Rats were in the prone position. A circular 1.5 cm or 2.0 cm diameter collimator was used. The isocenter was set in the center of the heart, with isocenter dose of 24 Gy administered in 1 fraction with equally weighted (1:1:1) anterior-posterior and 2 lateral beams (0.32 mm Cu, 225 kVp, 13 mA, 2.69 Gy/min). Control rats received sham irradiation. Some data from 1.5 cm collimator-treated rats has been reported previously, but male and female results have never been directly compared (Schlaak et al. 2019). In separate additional studies performed over the same time period, male rats were administered localized cardiac radiation in the same fashion as the 1.5 cm collimator-treated rats but were treated with a circular 2.0 cm collimator. Monte Carlo-based treatment planning (MAASTRO Radiotherapy Clinic, Maastricht, the Netherlands) was used to precisely calculate radiation doses. Rats were maintained in single ventilated cages with pathogen-free conditions. The environment was maintained at a temperature of 23°C on a 12-h:12-h light-dark cycle. Rats had access to a standard diet (Teklad, low-salt (0.4%) diet) and water.
Echocardiography
Echocardiography was used to determine cardiac function, as described previously (Schlaak et al. 2019). Briefly, two-dimensional strain and M-mode analysis performed on control and radiated rats at baseline, 3 mo, and 5 or 6 mo after treatment. A Vivid 7 echocardiograph (General Electric, Wauwatosa, WI) with an M12L (11-MHz) linear-array transducer was used with EchoPac software (General Electric, Wauwatosa WI). Percent ejection fraction (EF) was measured using left ventricle end-diastolic volume (LVEDV) and left ventricle end systolic volume (LVESV) with the following formula EF=LVEDV - LVESV/LVEDV x 100%. Percent fractional shortening (FS) was calculated using the formula: LVEDD - LVESD/LVEDD x 100, where LVESD is left ventricle end systolic diameter and LVEDD is left ventricle end-diastolic diameter. Three consecutive heartbeats were assessed for each measurement, and the average was reported. For strain, EchoPac Q analysis software (General Electric, Wauwatosa, WI) was used for image processing. The cardiac cycle was demarcated from the peak of two consecutive R waves. For circumferential and radial strain, the endocardial border was defined in an end-systolic frame at mid-ventricle, identified by prominent papillary muscles, in the short-axis view. Outer border adjustments were made to approximate the epicardial border. Subsequently a profile of circumferential (myocardial deformation along the curvature) and radial (myocardial deformation toward the center) strain (%) with time was provided. The average of three consecutive heartbeats were analyzed and reported (Migrino et al. 2007; Schattke et al. 2014). Pericardial effusions were quantified based on the American Society for Echocardiography consensus statement for quantification of human pericardial disease, with categorization of zero, mild, moderate, or large based on circumferential location, anatomic position, and the size of the effusion in the echolucent space seen on echocardiogram (Klein et al. 2013). At end diastole, mild effusions were <3 mm large in any one-dimension, moderate effusions were 3–6 mm, and large effusions were >6 mm (Klein et al. 2013).
Histologic analysis of cardiac tissue
Ten weeks after sham or radiation treatment, rats were euthanized using isoflurane overdose. The hearts were subsequently excised and rinsed with PBS. A short-axis mid-ventricular section was then excised for histology. Heart sections were fixed in zinc formalin for 48 hours and subsequently transferred to 70% ethanol. These sections were then embedded in paraffin. Four micrometer sections were used, and hematoxylin and eosin (H&E) staining was performed using standard methods. The H&E slides of the heart were examined for cellular necrosis (N=4 to 5 per condition). These changes were scored blindly by a board-certified pathologist (C.L.).
Statistical analysis
The means of two independent groups were compared using Student’s t-tests for continuous variables and Fisher exact test for categorical variables. One-way ANOVA was used to compare the means of three or more independent groups. Fisher’s Least Significant Difference (LSD) test was used to compare the means of one group with another. Data are reported as mean ± SE. Criteria for significance was P˂0.05.
Results
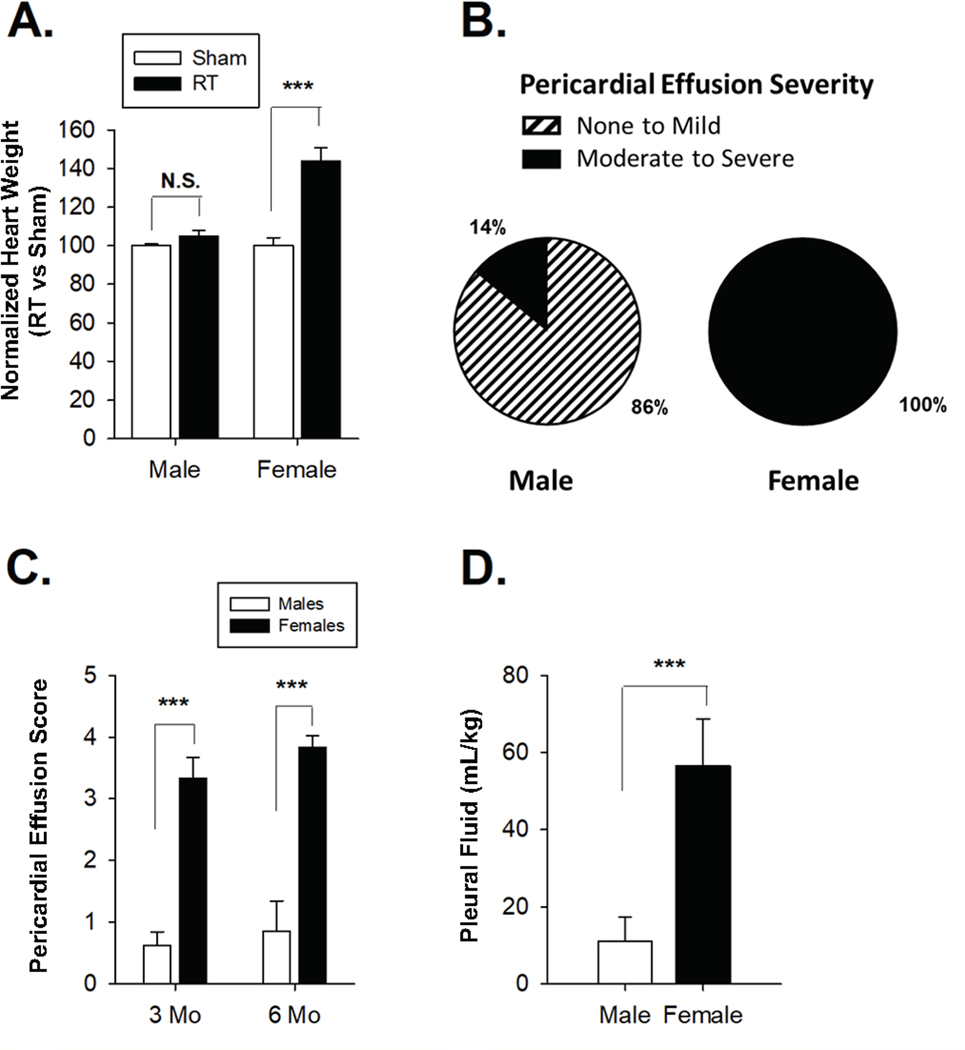
We previously reported that Dahl SS male and female rats treated with 24 Gy of localized cardiac RT using a 1.5 cm collimator develop signs of RIHD, including pericardial effusions, pleural effusions, and echocardiographic changes (Schlaak et al. 2019; Schlaak et al. 2020). Here we directly compared these age-matched adult male and female SS rats after treatment with 24 Gy of RT using the 1.5 cm collimator versus sham treatment. Heart weights were determined at 5–6 months following radiation and normalized to total body weight. The average normalized heart weight in RT vs. sham treated rats was 143.7% ± 7.2% and 105.0 ± 3.0% in females and males, respectively (Figure 1A). Female rats had significantly heavier heart weights relative to males (P=1.5 × 10−5). Pericardial effusion severity was assessed by scoring effusions as mild (1), moderate (2–3), or severe (4–5) during echocardiograms by skilled ultrasound operator blinded to treatment group, using scores derived previously that were adapted from clinical recommendations (Klein et al. 2013; Schlaak et al. 2019; Schlaak et al. 2020). At 5–6 months post-radiation, more moderate-to-severe pericardial effusions were present in females relative to males (100% vs 14.2%, P<0.001) (Figure 1B). The frequency and severity of pericardial effusions was significantly greater in females compared to males at 3 months and 5–6 months post-radiation (Figure 1C). We next compared pleural effusions (Schlaak et al. 2020), normalized to body weight (mg/kg). Normalized pleural fluid was 56.6 mL/kg ± 12.1 and 11.0 mL/kg ± 6.4 in females and males, respectively, demonstrating significantly larger pleural effusions in females (P=0.001; Figure 1D). Survival data was also suggestive of increased toxicity in males vs. females, with female survival 55% (6/11) and male survival 94% (15/16) at 5 months (P<0.01) (Schlaak et al. 2019). While the cause of death could not be definitively proven, animals that met euthanasia criteria had very large pleural effusions and evidence of worse cardiac function via echocardiogram, suggesting cardiopulmonary failure may have caused the deaths. Of note, no SS sham-treated rats died during the same time periods (not shown).
Figure 1: Female SS (Dahl salt-sensitive/Mcwi) rats exhibit worse mortality and increased effusions after localized cardiac irradiation compared to males.
(A) Increased normalized heart weight in females compared to males after 24 Gy localized cardiac radiation therapy vs. sham treatment. (B) Increased frequency of moderate to severe pericardial effusions in female SS rats compared to males. (C) Average pericardial effusions severity was higher in females relative to males at 12 weeks and 5–6 months post-radiation. (D) Normalized pleural fluid volume was higher in females relative to males 5–6 months post-radiation. Values are the mean ± SE. *P<0.05, **P<0.01, ***P< 0.001; ns, nonsignificant.
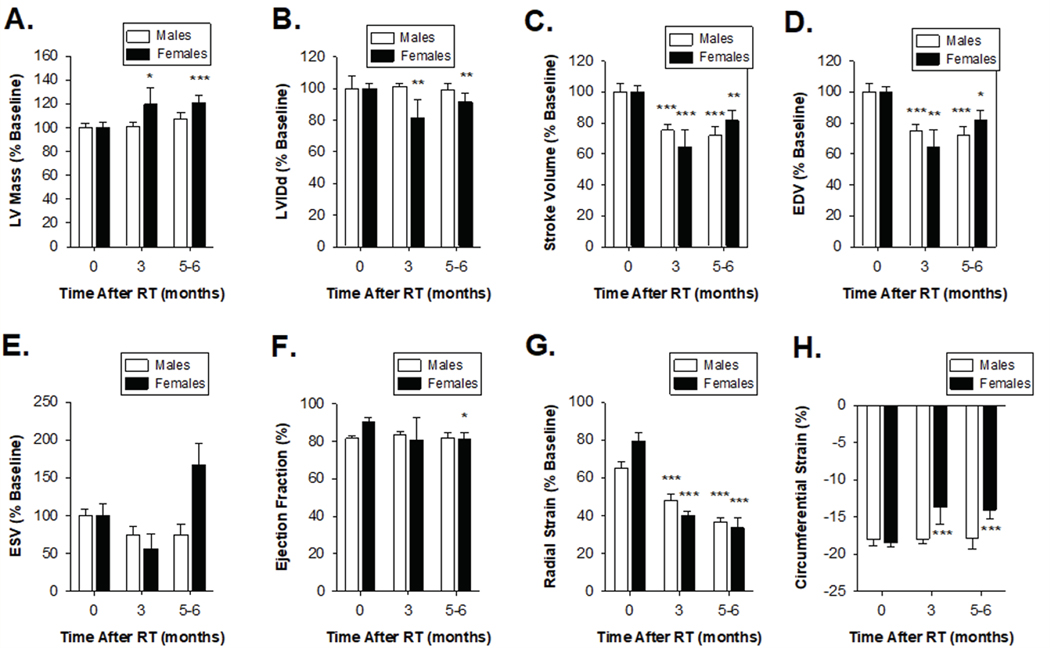
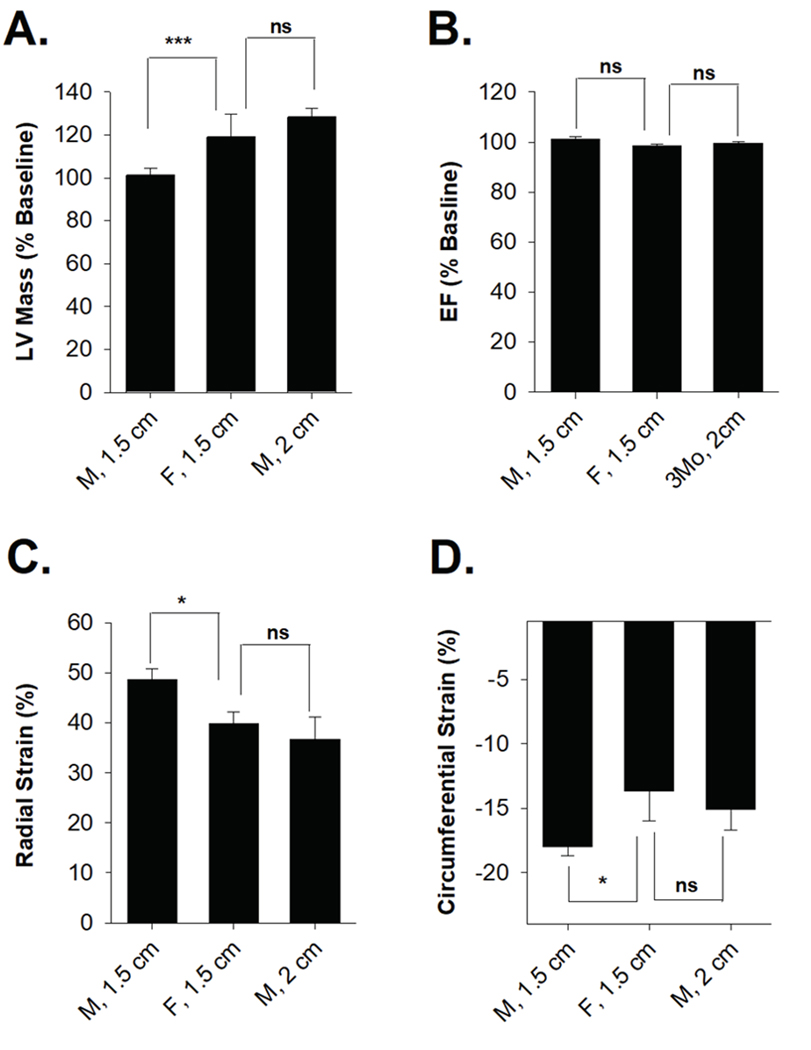
Cardiac function following RT was further evaluated using serial echocardiograms at 3 and 5–6 months (Schlaak et al. 2019; Schlaak et al. 2020), and results from the female and male rats were directly compared. Multiple echocardiographic parameters showed evidence of systolic heart dysfunction 5–6 months after completion of radiotherapy (Figure 2). Female rats had significantly increased LV mass and reduced left ventricular internal diameter at end systole (LVIDs), while males showed no significant change (Figure 2A–B). Both males and females exhibited reduced stroke volume and end-diastolic volume (EDV), but only females showed a trend toward higher end-systolic volumes (ESV, P=0.052, Figure 2C–E). However, females had significantly higher end-systolic volume relative to males at 5 months after RT (P=0.008). Female rats (but not male) showed a small but significant reduction in ejection fraction at 5 months after RT (EF, Figure 2F). Analysis of both radial and circumferential strain indicated that both male and female rats have significantly reduced myocardium deformation, consistent with decreased systolic function (Figure 2G). Female rats had more rapid onset and severity of cardiac dysfunction following radiation. Three months after radiation, female rats had significantly higher estimated LV mass (1.2% ± 3.4% vs 19.5% ± 13.7%; P=4.8 × 10−5, Figure 2A) and significantly reduced LV internal dimensions (+1.1% vs −29.6%; P=0.03, Figure 2B).
Figure 2: Echocardiogram measurements indicate similar reductions in cardiac performance in male and female SS rats (Dahl salt-sensitive/Mcwi) at 24 Gy localized heart radiation.
M-mode echocardiogram measurements of SS male and female rats that received 24 Gy of localized heart radiation therapy at baseline, 3 mo, and 5–6 months post-RT. (A) Left ventricular mass was significantly increased at 3 months and 6 months post-RT female rats, but not in male rats. (B) There was no significant change in left ventricular internal diameter end diastole (LVIDd) following radiation in male and female rats. (C) Stroke volume and (D) end-diastolic volume (EDV) were decreased in male and female rats. (E) End-systolic volume (ESV) increased in females, but not in males, 5–6 following RT. (F) Ejection fraction (%EF) significantly decreased at 6 months in females, but not in males. (G) Radial strain was significantly lower in male and female rats 3- and 6-months following RT. (H) Circumferential strain showed male and female rats had a decreased ability to contract, indicated by a smaller negative percentage. Values are the mean ± SE. *P<0.05, **P< 0.01, ***P<0.001 for values compared to baseline.
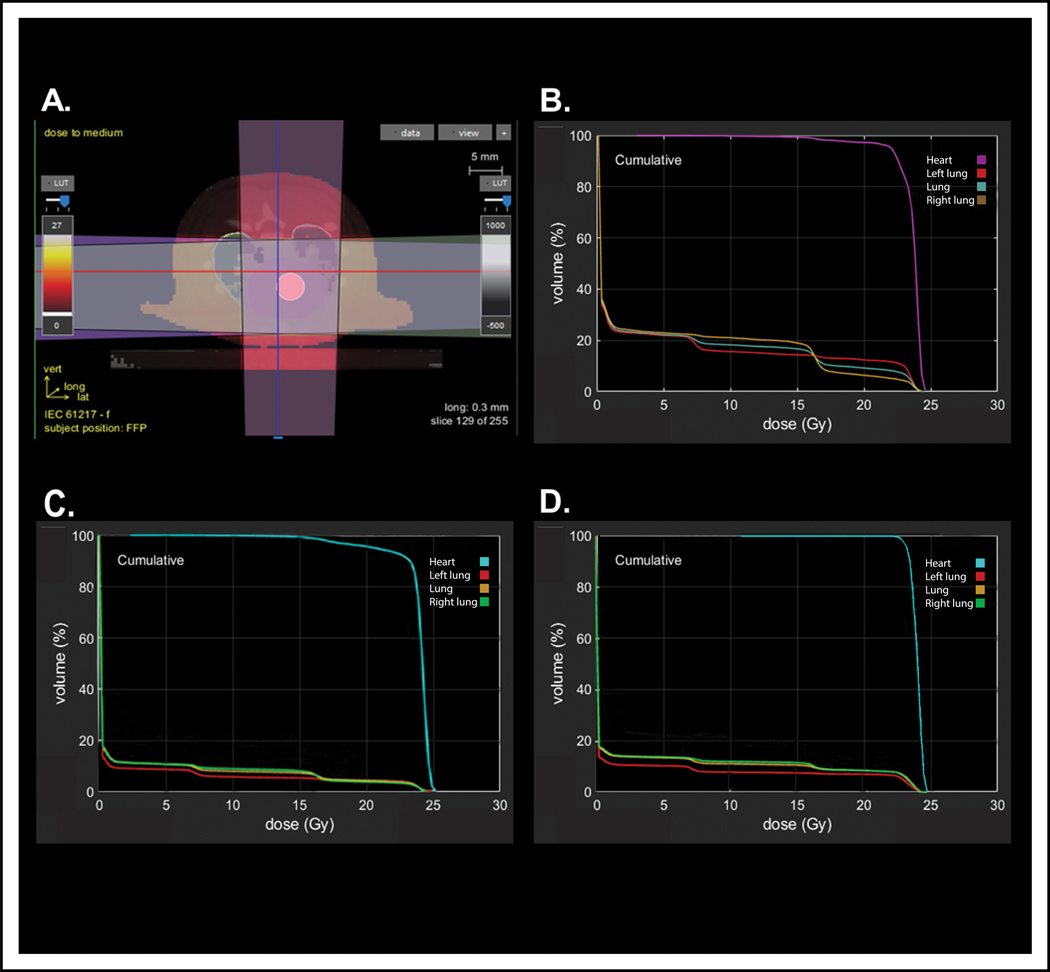
Dose-volume histograms of representative male and female rats receiving 24 Gy of radiation using a 1.5 cm collimator were compared (Table 1 and Figure 3). Dosimetric comparisons of male and female rats showed similar heart doses, but females received significantly higher lung doses. The higher lung doses in female rats are likely because in representative animals, females had approximately 40% less lung volume compared to age-matched male rats. Thus, the proportion of total lung in a representative male rat receiving 5 Gy of radiation was 8% (V5 = 8%) and total lung volume receiving 20 Gy was 5% (V20 = 5%), while the total lung volume in a representative female receiving 5 Gy of radiation was 16% (V5 = 22%) and total lung volume receiving 20 Gy was 12% (V20 = 10%, Table 1). The hearts of the males and females received similar doses (Figure 3B and 3C, Table 1). We hypothesized that the dramatic differences in pleural and pericardial effusions in male and female SS rats might be due to the large differences in total lung doses. To test this, male rats were administered localized cardiac radiation using the same beam arrangement and methods but using a 2 cm radiation beam to increase the radiation field size, thereby increasing the total radiation dose delivered to the lungs. DVH analysis showed that females treated with a 1.5 cm collimator had similar lung doses to male rats treated with a 2 cm collimator (Figure 3, Table 1), with comparable, although slightly less, heart coverage by doses greater than 16 Gy (98% coverage versus 100%, D95 21.9 Gy versus 22.9 Gy for males and females, respectively, Figure 3B vs. 3D and Table 1).
Table 1:
Comparison of dosimetric parameters between male and female rats treated with 24 Gy of whole-heart RT using a 1.5 cm collimator and a 2.0 cm collimator.
| Females (1.5 cm) | Males (1.5 cm) | Males (2.0 cm) | |
|---|---|---|---|
| Lung, Mean (Gy) | 4 | 1.8 | 2.6 |
| Lung, V5 (%) | 22 | 8 | 16 |
| Lung, V20 (%) | 10 | 5 | 10 |
| Heart, Mean (Gy) | 23.4 | 23.2 | 23.6 |
| Heart, D5 (Gy) | 24.2 | 24.2 | 24.1 |
| Heart, D95 (Gy) | 21.9 | 20.1 | 22.9 |
Abbreviations: Gy, Gray.
Figure 3: Dosimetric comparisons of heart radiation plans.
(A) Representative axial slice demonstrating radiotherapy setup using a 3-beam arrangement to deliver 24 Gy in one fraction to the whole heart. Representative dose-volume histograms (DVH) for (B) a female rat treated with a 1.5 cm collimator, (C) a male rat treated with a 1.5 cm collimator, and (D) a male rat treated with a 2.0 cm collimator.
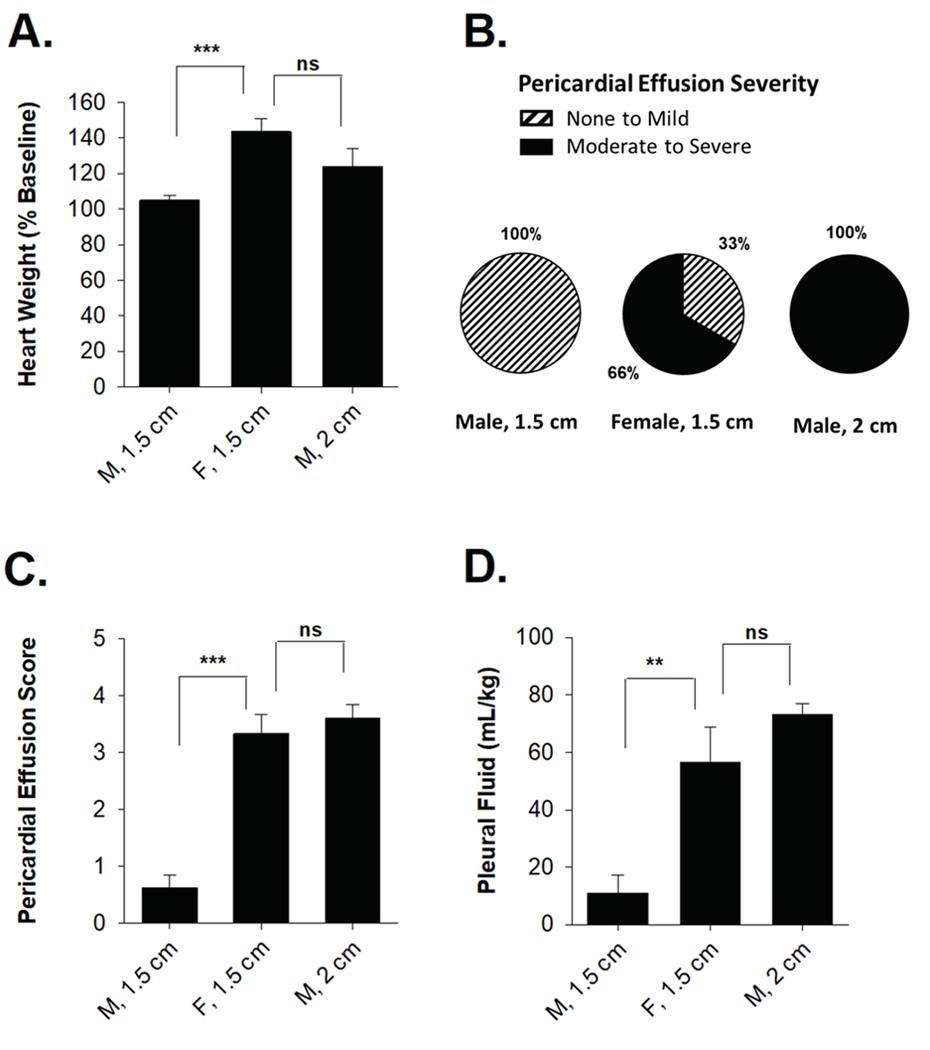
We next assessed whether increasing the total lung dose in male rats would lead to increased pericardial and pleural effusions. Male rats treated with 2 cm beam relative to a 1.5 cm beam had significantly increased heart weight 6 months after radiation (P=0.02; Figure 4A). Normalized heart weights did not differ significantly between males treated with a 1.5 cm beam and female treatment with a 2 cm beam (P=0.12). Whereas no male rats treated with a 1.5 cm beam developed moderate to severe pericardial effusions at 3 months, 100% of male rats treated with a 2 cm beam developed moderate to severe pericardial effusions (Figure 4B). Relative to males treated with a 1.5 cm beam, males treated with a 2 cm beam had significantly higher pericardial effusion index scores (3.6 vs 0.9; P=1 × 10−6, Figure 4C), but these scores were not significantly different in males treated with 2 cm beam and females treated with a 1.5 cm beam (P=0.60). Normalized pleural fluid volume was significantly higher in males treated with a 2 cm beam relative to a 1.5 cm beam (73.3 vs 11.0; P=9.8 × 10−6), but volumes were not significantly different in males treated with a 2 cm beam and females treated with a 1.5 cm beam (73.3 vs. 56.6, P=0.31) (Figure 4D). These results indicate that treating males with a 2.0 cm radiation beam resulted in similar pericardial and pleural effusions to females treated with a 1.5 cm beam.
Figure 4: Male and female SS rats demonstrated similar increases in heart weight, pericardial effusion frequency and severity, and pleural effusions when both receive comparable total lung doses.
(A) Change in total heart weight relative to baseline was significantly increased and comparable between male rats treated with a 2 cm collimator and females treated with a 1.5 cm collimator. (B) No male rats treated with a 1.5 cm collimator developed moderate to severe pericardial effusions, whereas all male rats treated with a 2 cm collimator developed moderate to serve pericardial effusions. (C) Pericardial effusion index scores were similar between male rats treated with a 2 cm collimator and female rats treated with a 1.5 cm collimator. (D) Normalized pleural fluid volume was similar in male rats treated with a 2 cm collimator and female rats treated with a 1.5 cm collimator. Values are the mean ± SE. *P<0.05, **P< 0.01, ***P<0.001; ns, nonsignificant.
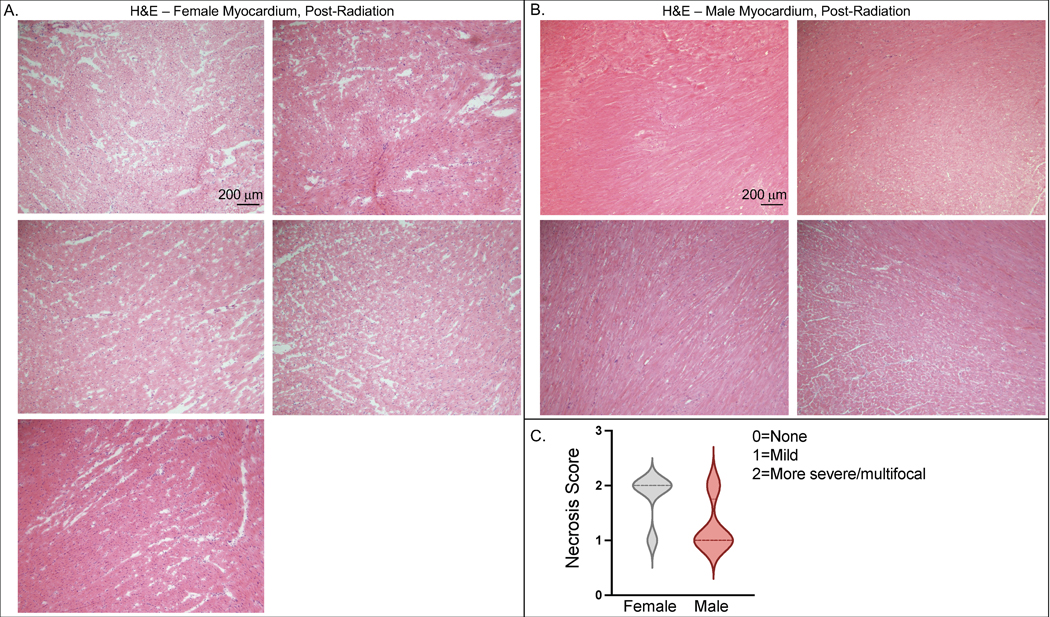
Serial echocardiograms were compared between the female and male 1.5 cm beam and the male 2.0 cm beam treatment groups. Three months after radiation, males treated with a 2 cm beam vs. 1.5 cm beam had significant increases in LV size (28.4% ± 4.2% vs 1.2% ± 3.4%, P=1.3 × 10−4), and males treated with a 2 cm beam were not significantly different from females treated with a 1.5 cm beam (28.4% ± 4.2% vs. 19.5% ± 13.7%, P=0.31) (Table 2 and Figure 5A). Similarly, males treated with a 2 cm beam vs. 1.5 cm beam had significant increases in interventricular septum thickness at end-diastole (IVSd) and left ventricular posterior wall (LVPWd) (Table 2). Left ventricular internal dimension at end-diastole was significantly increased in males treated with a 2 cm beam (LVIDd) (18% ± 6.0% vs 1.1 ± 2.3%, P=0.004), which has been previously reported as a strong risk factor for CHF and independent predictor of cardiac mortality in heart failure patients (Anselmino et al. 2009; Vasan et al. 1997). EF was unchanged in the males treated with a 2 cm beam (Figure 5B). Stroke volume, ESV, and EDV were also unchanged. Analysis of both radial and circumferential strain indicated that male and female rats have significantly reduced myocardium deformation, consistent with decreased systolic function (Figure 2G and 2H). Histologic assessment of male and female rat hearts after radiation also supported the findings that in animals treated with the same size 1.5 cm beam, females had more cardiac damage than males. In H&E-stained heart sections at 10 weeks after radiation, 5 of 6 female hearts had more severe/multifocal necrosis, while only 1 of 4 male hearts exhibited more severe/multifocal necrosis (Figure 6A–B), with the other animals exhibiting mild necrosis (Figure 6C).
Table 2:
Echocardiogram changes three months after RT in males and females treated with 1.5 cm beam-size and males treated with a 2 cm beam-size.
| Male | Female | ||||
|---|---|---|---|---|---|
|
|
|||||
| 1.5 cm Beam | 2.0 cm Beam | 1.5 cm Beam | |||
| Mean ± SE | Mean ± SE | P † | Mean ± SE | P ‡ | |
| LV Mass (%) Δ | 101.2 ± 3.4 | 128.4 ± 4.2 | 1.3 × 10 −4 | 119.5 ± 13.7 | 0.31 |
| IVSd (%) Δ | 100.1 ± 3.3 | 135.6± 6.0 | 2.4 × 10 −5 | 142.6 ± 18.0 | 0.03 |
| LVID (%) Δ | 101.1 ± 2.3 | 118.0 ± 6.0 | 0.004 | 81.4 ± 11.4 | 0.002 |
| LVPWd (%) Δ | 107.9 ± 5.2 | 127.6 ± 8.4 | 0.054 | 128.2 ± 12.0 | 0.15 |
| EF (%) | 83.1 ± 0.01 | 80.6 ± 0.03 | 0.44 | 83.5 ± 0.01 | 0.24 |
| Stroke Volume (%) Δ | 71.0 ± 2.6 | 75.4 ± 4.4 | 0.40 | 65.1 ± 8.9 | 0.11 |
| EDV (%) Δ | 75.2 ± 3.9 | 75.8 ± 3.9 | 0.93 | 64.3 ± 9.6 | 0.48 |
| ESV (%) Δ | 74.4 ± 11.2 | 53.0 ± 8.2 | 0.28 | 56.1 ± 19.0 | 0.37 |
| Radial Strain (%) | 48.0 ± 3.2 | 36.7 ± 4.4 | 0.02 | 39.8 ± 2.4 | 0.91 |
| Circumferential Strain (%) | −18.0 ± 0.7 | −15.10 ± 1.59 | 0.39 | −13.67 ± 2.30 | 0.64 |
Data presented as percent change from baseline;
P value is testing for significant difference between males treated with 1.5 cm vs 2.0 cm beam-size;
P value is testing for significant difference between males treated with 2.0 cm beam-size vs females treated with 1.5 cm beam-size. Bolded values are those with P<0.05. Abbreviations: IVSd, interventricular septal wall thickness at end-diastole; LVID, left ventricular internal dimensions at end-diastole; LVPwd, left ventricular posterior wall thickness at end-diastole; EF, ejection fraction; EDV, end-diastolic volume; ESV, end-systolic volume.
Figure 5: Male rats treated with a 2.0 cm beam size showed significant signs of heart failure on echocardiogram, which were comparable to female rats treated with a 1.5 cm beam size.
Female rats treated with a 1.5 cm collimator and male rats treated with a 2 cm collimator showed similar changes 3 months following radiation in terms of (A) left ventricular mass, (B) ejection fraction, (C) radial strain, and (D) circumferential strain. Values are mean ± SE. *P<0.05, **P< 0.01, ***P<0.001; ns, nonsignificant.
Figure 6. Female rats exhibit more histologic evidence of necrosis in the myocardium than male rats after 24Gy of localized cardiac RT.
Ten weeks after sham treatment or 24 Gy of localized cardiac RT, H&E staining was performed on fixed cardiac tissue. Female hearts (A, N=5) exhibited more pronounced necrosis than male rats (B, N=4), with a representative image from each rat shown (scale bar = 200 μm). There were 4 of 5 female hearts exhibiting more severe/multifocal necrosis and only 1 of 4 male rats exhibiting more severe/multifocal necrosis (C). Median value represented with hatched line.
Discussion
Our prior studies have demonstrated that SS rats are sensitive to 24 Gy of localized cardiac RT, with male and female data presented separately to compare cardiac function between sham and irradiated animals, but the male and female cardiac function studies were never directly compared (Schlaak et al. 2019; Schlaak et al. 2020). In this study, direct comparisons of male and female cardiotoxicity data from age-matched rats clearly demonstrates that female rats have statistically significant differences in a number of cardiotoxicity metrics. Adult female SS rats administered localized cardiac radiation demonstrated reduced survival, significantly higher rates of pericardial and pleural effusion, and a more rapid decline in cardiac function following whole heart radiation than age-matched male SS rats administered the same radiation plan. Adult female SS rats were smaller than age-matched adult male SS rats, with corresponding smaller total lung volumes, and consequently the proportion of lung receiving lower dose (V5) or higher dose (V20) radiation was higher in females (Table 1). When the size of the radiation beam was increased in males from 1.5 to 2.0 cm to produce more comparable lung dose to females, with only very small changes in heart coverage (Figure 3, Table 1), male and female rats had a similar observed incidence and severity of pericardial and pleural effusions (Figure 4). To our knowledge, this is the first study to demonstrate that sex and/or increased lung dose leads to an increase in the frequency and severity of pericardial and pleural effusions. Our prior studies have demonstrated that the SS rats are sensitive to 24 gy of localized cardiac RT, and male and female data were presented separately but never directly compared. In this study, a comparison of male and female cardiotoxicity data from age-matched rats clearly demonstrated that female rats had statistically significant differences in a number of cardiotoxicity metrics.
Prior studies have described an association between heart dose and lung toxicity following radiotherapy (Cella et al. 2015; Ferreira-Machado et al. 2010; Yarnold et al. 2010), while other studies have reported improved cardiotoxicity models with the inclusion of lung variables (Cella et al. 2014; Cella et al. 2015). Together, these studies suggest a strong interrelationship between lung and cardiac toxicity. In addition, a retrospective analysis of 416 patients with locally advanced lung cancer showed the heart volume receiving 50 Gy (V50) and total lung volume receiving 5 Gy (V5) were both significant predictors of overall survival (Speirs et al. 2017). Consistent with these prior studies, we show that increasing the total lung dose leads to increased radiation-induced pericardial and pleural effusions.
Pericardial effusions are one of the most common toxicities of mediastinal radiotherapy associated with treatment of locally-advanced NSCLC (Wang et al. 2017; Xue et al. 2019), breast cancer (Marinko 2018; Marinko et al. 2018; McGale et al. 2011), lymphoma (Marks et al. 2018), and esophageal cancers (Fukada et al. 2013; Wei et al. 2008). Other pericardial pathologies, such as pericarditis and pericardial thickening, can also occur in patients after cardiac radiation (Mulrooney et al. 2009; Wang, Eblan, et al. 2017; Wang, Pearlstein et al. 2017). It remains unclear which anatomical structure is the best predictor for pericardial complications. A few studies have evaluated the pericardium dose itself, but wide variability in pericardial contours has confounded the ability to generate uniform dose-volume constraints (Fukada et al. 2013; Konski et al. 2012; Martel et al. 1998; Xue et al. 2019). Several studies have focused on whole heart dose and shown an increased risk of pericardial effusions associated with the whole heart volume receiving 35 Gy (V35) > 10%, volume receiving 10 Gy (V10) > 72.8%, and volume receiving 45 Gy (V45) > 15% (Hayashi et al. 2015; Ning et al. 2017; Ogino et al. 2016). In Wang et al, dose to left and right atrium substructures were shown to be significant predictors of pericardial events (Wang et al. 2017). The incidence of symptomatic pericardial effusions and pericarditis in NSCLC patients treated with RT was over 6% at 4 years, adjusting for competing risks. In addition, the incidence of asymptomatic pericardial effusions was 27% (Wang et al. 2017). Pericardial effusions also occurred in 30% of patients treated with cardiac SBRT for ventricular arrhythmias, which demonstrates that a radiating a small amount of heart with high doses can lead to pericardial effusions (Robinson et al. 2019). We believe this is the first study to suggest that total lung dose is a predictor of pericardial toxicity.
Our model demonstrates that female SS rats display more severe radiation-induced toxicity relative to age-matched SS male rats after receiving 24 Gy of localized cardiac radiation. This is evident when examining survival, with 94% (15 of 16) of male rats alive 6 months after 24 Gy of heart radiation, while only 55% (6 of 11) of females were alive at 5 months post-radiation (P<0.01). Given that the frequency and severity of pericardial effusions was also significantly higher in females compared to males, it raises the possibility that the reduced overall survival observed in females is a reflection of the significantly higher rate of pericardial effusions in females. In patients, one study found no association between the presence of pericardial effusion and survival (Xue et al. 2019), although this association has not been extensively evaluated.
Relatively little is known about how biologic sex influences normal tissue radiosensitivity. Roughly 60 years ago, studies of male and female mice receiving daily low-dose whole body radiation showed significantly reduced survival times in females, which was not observed in ovariectomized female mice, suggesting this effect was hormone-related (Hamilton et al. 1963; Sacher et al. 1964). Recent studies have identified sex-specific differences in radiation-induced gene and protein expression and global genome DNA methylation (Kovalchuk et al. 2004; Kovalchuk et al. 2004; Pogribny et al. 2004), but there are limited studies of tissue-specific male and female differences. One prior retrospective study of 144 patients treated with definitive chemoradiation for lung cancer reported a higher risk of severe pneumonitis in women (Robnett et al. 2000). C57BL/6J mice exposed to whole lung radiation, female mice had a 2.18 times higher risk of death relative to males (Jackson et al. 2016), but it remains unclear if reduced survival was secondary to lung or cardiac toxicity or both. Like lung studies, there are few studies on how biologic sex influences radiation-induced cardiotoxicity. A retrospective study of predictive modeling in esophageal cancer reported a threshold for cardiac toxicity (TD50) in female patients that was 19 Gy lower for females compared to males (Snyder 2012). In another study of more than 24,000 childhood cancer survivors showed that female survivors had greater risk of heart failure, possibly due to increased sensitivity to anthracycline-related heart failure, while male survivors were more likely to develop coronary artery disease (Bates et al. 2019). Our study therefore adds to the sparse literature on biologic sex-related differences in radiation toxicity.
We can draw several conclusions regarding biologic sex-specific differences in RIHD from the present study. First, for a given radiation field, women may be more susceptible to radiation-induced cardiotoxicity given their reduced total lung volume. Higher lung doses in our study could also be a surrogate for higher pericardial doses, although heart doses (including the pericardium) were relatively similar in our rats (Table 1). However, given that some studies have suggested women have higher rates of pneumonitis, it is also possible that normal lung tissue is more sensitive to radiation in females. Thus, there could be sex-specific modifiers that contribute to increased lung toxicity or radiation-induced pericarditis. Second, female rats had a more rapid decline in cardiac performance, with significantly increased LV size three months after completing radiotherapy. It remains unclear how increased pericardial effusions, pleural effusions, and cardiac dysfunction each contributed to the reduced survival observed in female rates. It is also possible that inflammatory or injury pathways have differing responses to radiation in males versus females. A prior study recently reported that men had better survival rates compared to women, despite similar LV systolic dysfunction, scar burden, and co-morbidities (Kwon et al. 2009). This raises the possibility that female rats may have worse survival with comparable cardiac dysfunction.
Our study has some limitations. First, we delivered a high cumulative dose of radiation to the whole heart, which yielded significant cardiotoxicity and a large difference between irradiated and unirradiated rats. While a higher dose provided a robust dynamic range among endpoints, the high radiation dose may have masked more subtle sex-specific differences that are apparent at lower doses. However, many lung and esophageal cancer patients may receive significant heart radiation exposure (Wang et al. 2017), and patients who were previously treated definitively with mantle field radiation therapy for Hodgkin lymphoma typically received high doses of radiation therapy to the heart (Aleman et al. 2007). In addition, patients who receive radiation therapy for refractory ventricular tachycardia receive a localized dose of radiation to the heart of 25 Gy (Robinson et al. 2019). The results in this study may be more relevant to what is seen with higher levels of radiation exposure in the heart, and additional studies with lower doses should be examined in the future to determine whether sex differences persist.
Second, the biological effects of radiation are dependent upon dose delivered per fraction. Modern radiation is delivered over the course of many days (i.e., fractionated radiotherapy) with ways to account for changes in patient setup and anatomy on a daily basis. While a multi-fraction approach would have been more generalizable, a single fraction approach eliminated intrafraction variation as a confounder. In addition, the use of anesthesia over many days is quite toxic to rats, which itself can confound results. While the dose used in this study is a higher single fraction dose, previously we have demonstrated that treating Dahl SS rats with 24 Gy x 1 of localized RT yields similar cardiac changes to treatment with 9 Gy x 5 of localized cardiac RT (Schlaak et al. 2019).
We have previously demonstrated the Dahl SS strain used here is more sensitive to cardiac radiation than the Brown Norway strain (Schlaak et al. 2019). Strain-specific differences in baseline cardiovascular indices may contribute to the differences seen after radiation and between male and female rats. For example, the Dahl SS rats used in this study have a higher blood pressure than some other rat strains (Lenarczyk et al. 2020). However, when kept on a low salt diet (0.4%), such as was used in this study, prior studies have shown that the mean arterial pressure does not significantly increase, while a high salt diet (4%) causes hypertension over a three-week period (Cowley et al. 2013). If the Dahl SS rats are more sensitive to cardiac radiation in part due to baseline differences in cardiovascular health, the present study is still important and relevant, as many individuals who receive incidental cardiac radiation exposure have cardiovascular risk factors. Related to these issues, our study describes cardiotoxicity differences between males and females using animal models, but it remains unclear whether there are biologic sex-related differences at the molecular and cellular level for lung and/or cardiac tissue. The role of hormonal changes in female versus male rats was not explicitly studied. These changes will be the focus of future studies. Finally, it must be noted that with respect to the International Commission on Radiological Protection (ICRP) policy, gender-specific data are not recommended at this time for the purpose of radiological protection (ICRP Recommendations, 2007).
In conclusion, this study demonstrates that sex differences can play an important role in cardiac normal tissue radiation sensitivity. Differences in the percent of lung receiving high dose radiation in age-matched male and female animals administered the same radiation treatments may also be important in determining the severity of different types of RIHD, especially pericardial effusions, which are one of the most common manifestations of incidental radiation to the heart. Additional studies will help to determine the effect of lung doses and sex differences on pericardial effusions and other manifestations of RIHD. This study highlights that factors such as sex may be important to factor into future mitigation studies of radiation-induced cardiotoxicity.
Acknowledgements:
This work was supported by NIH R01HL147884 (CB). Additional support was provided by the Susan G. Komen® CCR17483233 (CB), the Mary Kay Foundation Award Grant No. 017-29 (CB), the Nancy Laning Sobczak, PhD, Breast Cancer Research Award (CB), the Michael H. Keelan, Jr., MD, Research Foundation Grant (CB), and the Cardiovascular Center at the Medical College of Wisconsin (CB).
Footnotes
Supplementary Materials: None
Manuscript Contribution to the Field
Radiation therapy remains part of the standard of care for many types of thoracic cancers. While radiotherapy can improve local control and survival, radiation-induced heart dysfunction is a common side effect of thoracic radiotherapy. Numerous studies have evaluated the relationship between radiation dose to the heart and cardiotoxicity, but relatively little is known about whether there are biologic sex-related differences in radiation-induced heart dysfunction. Our results suggest that animal sex influences at least some of the changes associated with RIHD, but that the proportion of the lung receiving radiation in preclinical models, which can be different in male and female rats for a given collimator size centered on the heart, can also influence cardiac toxicity.
Conflicts of Interest: The authors declare no relevant conflicts of interest. M.J.F. currently is a Principal Research Scientist at Abbvie, but at the time of his contributions to the manuscript, he was employed at the Medical College of Wisconsin.
References
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication Annal ICRP. 37:1–332. [DOI] [PubMed] [Google Scholar]
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, van ‘t Veer MB, Baaijens MH, de Boer JP, Hart AA, Klokman WJ, Kuenen MA, Ouwens GM, et al. 2007. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 109(5):1878–1886. [DOI] [PubMed] [Google Scholar]
- Anselmino M, De Ferrari GM, Massa R, Manca L, Tritto M, Molon G, Curnis A, Devecchi P, Sarzi Braga S, Bartesaghi G, et al. 2009. Predictors of mortality and hospitalization for cardiac causes in patients with heart failure and nonischemic heart disease: a subanalysis of the ALPHA study. Pacing Clin Electrophysiol. 32 Suppl 1:S214–218. [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. 2018. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 379(24):2342–2350. [DOI] [PubMed] [Google Scholar]
- Bates JE, Howell RM, Liu Q, Yasui Y, Mulrooney DA, Dhakal S, Smith SA, Leisenring WM, Indelicato DJ, Gibson TM, et al. 2019. Therapy-Related Cardiac Risk in Childhood Cancer Survivors: An Analysis of the Childhood Cancer Survivor Study. J Clin Oncol. 37(13):1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergom C, Currey A, Desai N, Tai A, Strauss JB. 2018. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front Oncol. 8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma M, Roberto KA, Hauer-Jensen M. 2008. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys. 72(1):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma M, Singh P, Sridharan V, Tripathi P, Sharma S, Singh SP. 2015. Effects of Local Heart Irradiation in a Glutathione S-Transferase Alpha 4-Null Mouse Model. Radiat Res. 183(6):610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A, et al. 2015. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella L, Palma G, Deasy JO, Oh JH, Liuzzi R, D’Avino V, Conson M, Pugliese N, Picardi M, Salvatore M, et al. 2014. Complication probability models for radiation-induced heart valvular dysfunction: do heart-lung interactions play a role? PLoS One. 9(10):e111753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella L, D’Avino V, Palma G, Conson M, Liuzzi R, Picardi M, Pressello MC, Boboc GI, Battistini R, Donato V, et al. 2015. Modeling the risk of radiation-induced lung fibrosis: Irradiated heart tissue is as important as irradiated lung. Radiother Oncol. 117(1):36–43. [DOI] [PubMed] [Google Scholar]
- Cella L, Oh JH, Deasy JO, Palma G, Liuzzi R, D’Avino V, Conson M, Picardi M, Salvatore M, Pacelli R. 2015. Predicting radiation-induced valvular heart damage. Acta Oncol. 54(10):1796–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, Mohan R, Komaki R, Cox JD. 2006. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 65(4):1087–1096. [DOI] [PubMed] [Google Scholar]
- Clements IP, Davis BJ, Wiseman GA. 2002. Systolic and diastolic cardiac dysfunction early after the initiation of doxorubicin therapy: significance of gender and concurrent mediastinal radiation. Nucl Med Commun. 23(6):521–527. [DOI] [PubMed] [Google Scholar]
- Cowley AW Jr., Ryan RP, Kurth T, Skelton MM, Schock-Kusch D, Gretz N.2013. Progression of glomerular filtration rate reduction determined in conscious Dahl salt-sensitive hypertensive rats. Hypertension. 62(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al. 2013. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 368(11):987–998. [DOI] [PubMed] [Google Scholar]
- Desai N, Currey A, Kelly T, Bergom C. 2019. Nationwide Trends in Heart-Sparing Techniques Utilized in Radiation Therapy for Breast Cancer. Adv Radiat Oncol. 4(2):246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver JA, Djousse L, Logroscino G, Gaziano JM, Kurth T. 2008. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ. 337:a2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative G, Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al. 2011. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 378(9804):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Machado SC, Rocha Nde N, Mencalha AL, De Melo LD, Salata C, Ribeiro AF, Torres Tda S, Mandarim-De-Lacerda CA, Canary PC, Peregrino AA, et al. 2010. Up-regulation of angiotensin-converting enzyme and angiotensin II type 1 receptor in irradiated rats. Int J Radiat Biol. 86(10):880–887. [DOI] [PubMed] [Google Scholar]
- Filopei J, Frishman W. 2012. Radiation-induced heart disease. Cardiol Rev. 20(4):184–188. [DOI] [PubMed] [Google Scholar]
- Fukada J, Shigematsu N, Takeuchi H, Ohashi T, Saikawa Y, Takaishi H, Hanada T, Shiraishi Y, Kitagawa Y, Fukuda K. 2013. Symptomatic pericardial effusion after chemoradiation therapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys. 87(3):487–493. [DOI] [PubMed] [Google Scholar]
- George TJ, Arnaoutakis GJ, Beaty CA, Kilic A, Baumgartner WA, Conte JV. 2012. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 94(2):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KF, Sacher GA, Grahn D. 1963. A sex difference in mouse survival under daily gamma irradiation and its modification by gonadectomy. Radiat Res. 18:12–16. [PubMed] [Google Scholar]
- Hayashi K, Fujiwara Y, Nomura M, Kamata M, Kojima H, Kohzai M, Sumita K, Tanigawa N. 2015. Predictive factors for pericardial effusion identified by heart dose-volume histogram analysis in oesophageal cancer patients treated with chemoradiotherapy. Br J Radiol. 88(1046):20140168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M.2017. Deaths: Leading Causes for 2015. Natl Vital Stat Rep. 66(5):1–76. [PubMed] [Google Scholar]
- Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW, van Leeuwen FE. 2007. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 99(5):365–375. [DOI] [PubMed] [Google Scholar]
- Jackson IL, Zhang Y, Bentzen SM, Hu J, Zhang A, Vujaskovic Z. 2016. Pathophysiological mechanisms underlying phenotypic differences in pulmonary radioresponse. Sci Rep. 6:36579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, Hung J, Garcia MJ, Kronzon I, Oh JK, et al. 2013. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 26(9):965–1012 e1015. [DOI] [PubMed] [Google Scholar]
- Konski A, Li T, Christensen M, Cheng JD, Yu JQ, Crawford K, Haluszka O, Tokar J, Scott W, Meropol NJ, et al. 2012. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother Oncol. 104(1):72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk O, Burke P, Besplug J, Slovack M, Filkowski J, Pogribny I. 2004. Methylation changes in muscle and liver tissues of male and female mice exposed to acute and chronic low-dose X-ray-irradiation. Mutat Res. 548(1–2):75–84. [DOI] [PubMed] [Google Scholar]
- Kovalchuk O, Ponton A, Filkowski J, Kovalchuk I. 2004. Dissimilar genome response to acute and chronic low-dose radiation in male and female mice. Mutat Res. 550(1–2):59–72. [DOI] [PubMed] [Google Scholar]
- Kwon DH, Halley CM, Popovic ZB, Carrigan TP, Zysek V, Setser R, Schoenhagen P, Flamm SD, Starling RC, Desai MY. 2009. Gender differences in survival in patients with severe left ventricular dysfunction despite similar extent of myocardial scar measured on cardiac magnetic resonance. Eur J Heart Fail. 11(10):937–944. [DOI] [PubMed] [Google Scholar]
- Lenarczyk M, Laiakis EC, Mattson DL, Johnson BD, Kronenberg A, North PE, Komorowski R, Mader M, Baker JE. 2020. Irradiation of the kidneys causes pathologic remodeling in the nontargeted heart: A role for the immune system. FASEB Bioadv. 2(12):705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. 1995. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 332(26):1738–1743. [DOI] [PubMed] [Google Scholar]
- Marinko T.2018. Pericardial disease after breast cancer radiotherapy. Radiol Oncol. 53(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinko T, Borstnar S, Blagus R, Dolenc J, Bilban-Jakopin C. 2018. Early Cardiotoxicity after Adjuvant Concomitant Treatment with Radiotherapy and Trastuzumab in Patients with Breast Cancer. Radiol Oncol. 52(2):204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LJ, McCarten KM, Pei Q, Friedman DL, Schwartz CL, Kelly KM. 2018. Pericardial effusion in Hodgkin lymphoma: a report from the Children’s Oncology Group AHOD0031 protocol. Blood. 132(11):1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MK, Sahijdak WM, Ten Haken RK, Kessler ML, Turrisi AT. 1998. Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys. 40(1):155–161. [DOI] [PubMed] [Google Scholar]
- McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, Fornander T, Gigante B, Jensen MB, Peto R, et al. 2011. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 100(2):167–175. [DOI] [PubMed] [Google Scholar]
- McWilliam A, Kennedy J, Hodgson C, Vasquez Osorio E, Faivre-Finn C, van Herk M. 2017. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer. 85:106–113. [DOI] [PubMed] [Google Scholar]
- Migrino RQ, Zhu X, Pajewski N, Brahmbhatt T, Hoffmann R, Zhao M. 2007. Assessment of segmental myocardial viability using regional 2-dimensional strain echocardiography. J Am Soc Echocardiogr. 20(4):342–351. [DOI] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. 2016. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 66(4):271–289. [DOI] [PubMed] [Google Scholar]
- Moseeva MB, Azizova TV, Grigoryeva ES, Haylock R. 2014. Risks of circulatory diseases among Mayak PA workers with radiation doses estimated using the improved Mayak Worker Dosimetry System 2008. Radiat Environ Biophys. 53(2):469–477. [DOI] [PubMed] [Google Scholar]
- Moulin M, Piquereau J, Mateo P, Fortin D, Rucker-Martin C, Gressette M, Lefebvre F, Gresikova M, Solgadi A, Veksler V, et al. 2015. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodeling. Circ Heart Fail. 8(1):98–108. [DOI] [PubMed] [Google Scholar]
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, et al. 2009. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning MS, Tang L, Gomez DR, Xu T, Luo Y, Huo J, Mouhayar E, Liao Z. 2017. Incidence and Predictors of Pericardial Effusion After Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 99(1):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino I, Watanabe S, Iwahashi N, Kosuge M, Sakamaki K, Kunisaki C, Kimura K. 2016. Symptomatic radiation-induced cardiac disease in long-term survivors of esophageal cancer. Strahlenther Onkol. 192(6):359–367. [DOI] [PubMed] [Google Scholar]
- Pogribny I, Raiche J, Slovack M, Kovalchuk O. 2004. Dose-dependence, sex- and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem Biophys Res Commun. 320(4):1253–1261. [DOI] [PubMed] [Google Scholar]
- Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, Goddu SM, Lang A, Cooper DH, Faddis M, et al. 2019. Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation. 139(3):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. 2000. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 48(1):89–94. [DOI] [PubMed] [Google Scholar]
- Sacher GA, Grahn D. 1964. Survival of Mice under Duration-of-Life Exposure to Gamma Rays. I. The Dosage-Survival Relation and the Lethality Function. J Natl Cancer Inst. 32:277–321. [PubMed] [Google Scholar]
- Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. 2017. Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation. 135(15):1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardaro A, Petruzzelli MF, D’Errico MP, Grimaldi L, Pili G, Portaluri M. 2012. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol. 103(2):133–142. [DOI] [PubMed] [Google Scholar]
- Schattke S, Xing Y, Lock J, Brechtel L, Schroeckh S, Spethmann S, Baumann G, Borges AC, Knebel F. 2014. Increased longitudinal contractility and diastolic function at rest in well-trained amateur Marathon runners: a speckle tracking echocardiography study. Cardiovasc Ultrasound. 12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak RA, Frei A, Schottstaedt AM, Tsaih SW, Fish BL, Harmann L, Liu Q, Gasperetti T, Medhora M, North PE, et al. 2019. Mapping genetic modifiers of radiation-induced cardiotoxicity to rat chromosome 3. Am J Physiol Heart Circ Physiol. 316(6):H1267–H1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak RA, Frei A, Fish BL, Harmann L, Gasperetti T, Pipke JL, Sun Y, Rui H, Flister MJ, Gantner BN, et al. 2020. Acquired Immunity Is Not Essential for Radiation-Induced Heart Dysfunction but Exerts a Complex Impact on Injury. Cancers (Basel). 12(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak RA, Frei A, SenthilKumar G, Tsaih SW, Wells C, Mishra J, Flister MJ, Camara AKS, Bergom C. 2020. Differences in Expression of Mitochondrial Complexes Due to Genetic Variants May Alter Sensitivity to Radiation-Induced Cardiac Dysfunction. Front Cardiovasc Med. 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaak RA, SenthilKumar G, Boerma M, Bergom C. 2020. Advances in Preclinical Research Models of Radiation-Induced Cardiac Toxicity. Cancers (Basel). 12(2):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, et al. 2015. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 16(9):1090–1098. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, Grant EJ, Sugiyama H, Sakata R, Moriwaki H, et al. 2010. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 340:b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M, Burmeister J, Joiner M, Meyer J, Tait L, Cohen S, McSpadden E, Konski A.2012. Potential gender differences in a normal tissue complication probability model for heart toxicity during radiation therapy for esophageal Cancer. Int J Radiat Oncol Biol Phys. 84(3):S757–S758. [Google Scholar]
- Speirs CK, DeWees TA, Rehman S, Molotievschi A, Velez MA, Mullen D, Fergus S, Trovo M, Bradley JD, Robinson CG. 2017. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 12(2):293–301. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. 1997. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 336(19):1350–1355. [DOI] [PubMed] [Google Scholar]
- Wang K, Eblan MJ, Deal AM, Lipner M, Zagar TM, Wang Y, Mavroidis P, Lee CB, Jensen BC, Rosenman JG, et al. 2017. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol. 35(13):1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Pearlstein KA, Patchett ND, Deal AM, Mavroidis P, Jensen BC, Lipner MB, Zagar TM, Wang Y, Lee CB, et al. 2017. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiother Oncol. 125(2):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Liu HH, Tucker SL, Wang S, Mohan R, Cox JD, Komaki R, Liao Z. 2008. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 70(3):707–714. [DOI] [PubMed] [Google Scholar]
- Xue J, Han C, Jackson A, Hu C, Yao H, Wang W, Hayman J, Chen W, Jin J, Kalemkerian GP, et al. 2019. Doses of radiation to the pericardium, instead of heart, are significant for survival in patients with non-small cell lung cancer. Radiother Oncol. 133:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnold J, Brotons MC. 2010. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 97(1):149–161. [DOI] [PubMed] [Google Scholar]