Abstract
A human papillomavirus type 16 E7 DNA vaccine with the open reading frame encoding mutations in two zinc-binding motifs expressed a rapidly degraded E7 protein. This vaccine induced a significantly stronger E7-specific cytotoxic T-lymphocyte response and better tumor protection in mice than did a wild-type E7 DNA vaccine expressing a stable E7 protein.
Human papillomavirus (HPV) genes and their products have been identified in most anogenital cancers, and HPV type 16 (HPV 16) is the most common one associated with severe cervical dysplasia and cancers (25, 45). Certain early viral genes, such as HPV 16 E6 and E7, are expressed constantly in cervical cancer cells (13, 35). Immunogenicity of HPV 16 E7 has been demonstrated by using overlapping peptides spanning the full length of E7, by recombinant E7 proteins, by recombinant vaccinia virus containing the E7 open reading frame (ORF), or by CD80+ and HPV 16 E7+ tumor cells (1, 6, 8–10, 15–18, 20, 21, 23, 27, 34, 37, 38). Certain mouse and human T-cell epitopes of the E7 protein have been identified (1, 10, 15, 21, 31, 37, 38).
DNA-based immunization can induce host immune responses (2, 4, 19, 32, 41). DNA vaccination is accomplished by the expression of inoculated DNA encoding the gene of interest, with a mammalian promoter or enhancer and other DNA sequences that enable the gene to be expressed within mammalian cells (11, 12, 26, 43). To prevent viral infections and to treat viral diseases, cytotoxic T lymphocytes (CTL) are crucial. DNA immunization can potentially be very effective at inducing a major histocompatibility complex (MHC) class I-restricted CTL response because DNA encoding the antigen of interest is delivered directly into the cell, where its gene product accesses the MHC class I antigen presentation pathway. DNA vaccines have the following advantages over other vaccines. (i) The vaccines can be prepared easily. Genes inserted into the vector (plasmid) can be modified easily, allowing convenient removal or insertion of certain sequences. (ii) DNA vaccines are temperature stable, which allows economical transportation, especially in underdeveloped countries. (iii) The immune responses induced by DNA vaccines are long lasting. (iv) DNA immunization will not induce an immune response against the DNA vector itself, so the DNA vaccine can be used repeatedly. This feature is important because treatment of cancer may require repeated immunization, and an immune response against the vector might reduce the efficacy of the vaccine. Although DNA vaccines have been shown to induce CTL responses, not all immunized individuals develop CTL (7, 30, 33, 44). Therefore the potency of DNA vaccines in inducing CTL must be improved.
Because cervical cancers express the E7 antigen, we explored whether a DNA vaccine can be used to induce an E7-specific CTL response and protect against tumor challenge. However, experimental evidence demonstrates that HPV 16 E7 has transforming potential. HPV 16 E7 has been shown to transform established cells (22, 42). The HPV 16 E7 ORF is predicted to encode a 98-amino-acid protein that is phosphorylated, e.g., in the casein kinase II phosphorylation motifs just downstream of the retinoblastoma protein interaction motif in conserved region 2 (28, 36). A region within the E7 protein contains two Cys-X-X-Cys motifs. Mutations affecting only one of the Cys-X-X-Cys repeats, which are conserved between different HPV E7 proteins, severely reduced the transforming activity but did not totally destroy it. Double mutants in which both motifs were disrupted had little transforming activity (14). Further, the motifs are believed to form a zinc finger and thus may be important in maintaining the stability of the E7 protein (3, 5). An unstable protein has greater potential to generate CTL responses than a stable one (39, 40). To develop a safe DNA vaccine with low or no transforming activity and to increase the instability of the E7 protein, we produced a plasmid that contains an HPV16 E7 double mutation in the two Cys-X-X-Cys repeats (58 Cys → Gly, 91 Cys → Gly).
Construction of HPV 16 E7 DNA vaccines.
DNA vaccines used in our study were the plasmids that contain the HPV 16 wild-type E7 ORF or double mutant E7 (58 Cys → Gly, 91 Cys → Gly) ORF under the control of the cytomegalovirus immediate-early promoter and enhancer. The ORF encoding the wild-type E7 protein or the E7 double mutant was amplified by PCR from HPV 16 DNA. For wild-type E7, 5′ primer AGTCGCATGCATCATGCATGGAGAT and 3′ primer CCCGCATGCTTATGGTTTCTGAGAAC were used. For the E7 double mutant, overlapping PCR was used. We generated the first mutant fragment (58 Cys → Gly) by using 5′ primer AGTCGCATGCATCATGCATGGAGAT and 3′ primer ACTTGCAACCAAAGGTTAC. The second mutant fragment (58 Cys → Gly, 91 Cys → Gly) was generated by using 5′ primer GTAACCTTTGGTTGCAAGT and 3′ primer CCCGCATGCTTATGGTTTCTGAGAACAGATGGGGCCCAC. We then performed overlapping PCR to combine two fragments by using 5′ primer AGTCGCATGCATCATGCATGGAGAT and 3′ primer CCCGCATGCTTATGGTTTCTGAGAACAGATGGGGCCCAC. The ORF of E7 or E7 mutant was inserted into the plasmid BC219 SphI site to generate BC219-E7 or BC219-E7 mutant (29). For large-scale preparations of plasmid DNA, transformed Escherichia coli DH5α, bacteria were grown in LB medium in the presence of ampicillin, and plasmids were extracted by the alkaline lysis method followed by two rounds of purification on cesium chloride density gradients. DNA concentrations were determined by measuring the optical density at 260 nm, and the integrity of the plasmids as well as the absence of contaminating E. coli DNA or RNA were checked by agarose gel electrophoresis. DNA was stored at −20°C in Tris-EDTA buffer. For injection, DNA was diluted in phosphate-buffered saline to a final concentration of 2 μg/μl.
Mutant E7 DNA vaccine expresses an unstable E7 protein.
To determine whether both wild-type E7 and mutant E7 DNA vaccines express E7 protein, we transfected a cell line (RMA) with the plasmids and determined expression of the E7 protein by immunoprecipitation.
RMA cells were transfected with each plasmid (BC219-E7 mutant and BC219-E7, BC219), using a SuperFect transfection kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. At 48 h after transfection, hygromycin B (final concentration, 600 μg/ml) was added to the cell culture medium. After cloning by limiting dilution, the transfectants were maintained in a medium with hygromycin B for 2 weeks.
Stable transfectants (RMA-BC219-E7 mutant, RMA-BC219-E7, and RMA-BC219) were harvested and washed once with cold phosphate-buffered saline. Then 106 cells from each cell line were starved for 30 min in methionine-free medium, after which 35S-labeled methionine was added (0.1 mCi/ml, final activity; Amersham, Arlington Heights, Ill.). Cells were incubated for 4 h and then lysed on ice with lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris-HCl [pH 8.0]), and the same amount of cellular proteins from each cell line were immunoprecipitated on ice for 2 h with 6 μl of mouse anti-HPV 16 E7 monoclonal antibody (Zymed Laboratories Inc., South San Francisco, Calif.). Fifty microliters of protein A-agarose beads (Sigma, St. Louis, Mo.) was added to the antibody-antigen products, and then the mixture was incubated for 1 h at 4°C with rocking. Beads were washed three times with the lysis buffer, and samples were electrophoresed under reducing conditions on sodium dodecyl sulfate–15% polyacrylamide gels. Gels were dried and exposed to photographic film. As shown in Fig. 1, the E7 protein was detected in the cell line transfected with BC219-E7, whereas the mutant E7 protein was barely detectable in the cell line transfected with BC219-E7 mutant.
FIG. 1.
Immunoprecipitation of wild-type E7 and mutant E7 proteins. Transfectants (RMA-BC219, RMA-BC219-E7, and RMA-BC219-E7 mutant) were metabolically labeled with [35S]methionine. For the right-hand set of transfectants, the proteasome inhibitor ALLN was added at 20 μg/ml 30 min prior to addition of the label and was maintained at this concentration in all further manipulations. An immunoprecipitation was carried out with a monoclonal anti-HPV 16 E7 antibody, and the products were separated by electrophoresis. The absence (−) or presence (+) of ALLN is indicated.
To further distinguish failure of synthesis from degradation of E7 protein, we used proteasome inhibitor N-acetyl-leucyl-norleucinal (ALLN) in the immunoprecipitation. Thus, calpain inhibitor I (ALLN), which is a cell-permeable synthetic tripeptide with an aldehyde at its C terminus and specifically inhibits the activity of cysteine proteases, was added to cells at a final concentration of 20 μg/ml 30 min before addition of radiolabel and was maintained at this concentration throughout labeling, harvesting, and immunoprecipitation. As shown in Fig. 1, the quantity of E7 protein in cells transfected with BC219-E7 was not significantly changed in the presence of the inhibitor, suggesting that the E7 protein is stable in the cell. In contrast, in the presence of ALLN, mutant E7 protein was detected at levels similar to those of wild-type E7. Our data demonstrate that plasmid BC219-E7 mutant can express E7 protein, but the mutant E7 protein is rapidly degraded in the proteasome.
Mutant E7 vaccine induces a stronger CTL response against HPV 16 E7 than wild-type E7 vaccine.
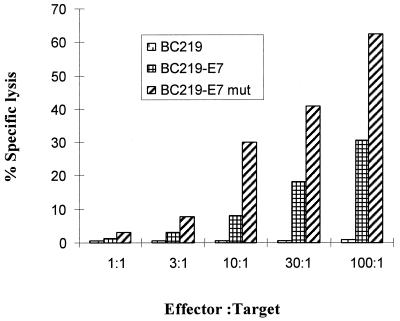
We immunized C57BL/6 mice (H-2b) intramuscularly with 100 μg of either BC219-E7, BC219-E7 mutant, or control BC219 plus incomplete Freund’s adjuvant (five mice per group) to determine whether these vaccines induce an HPV 16 E7-specific CTL response. Six- to 8-week-old female C57BL/6 mice (purchased from The Jackson Laboratory or Harlan) were used. All mice were kept under pathogen-free conditions. Two weeks after the DNA immunization, mice (five per group) were boosted subcutaneously with 2.5 × 104 HPV 16 E7-expressing tumor cells (RMA-E7 cells). Four days later, spleen cells were isolated from each mouse. We boosted mice in vivo with the E7-positive cells to shorten in vitro stimulation time and generate repeatable results. After incubation in a nylon wool column for 1 h at 37°C in 5% CO2, T cells were washed through the column with complete cell culture medium. The cells were cultured at 37°C in 5% CO2 for 7 days in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), interleukin-2 (10 U/ml), and E7 peptide49-57 (RAHYNIVTF, H-2Db-restricted epitope; 5 μg/ml). The CTL specificity was determined in a standard cytotoxicity assay (24). If the mice had not been primed by the DNA vaccines, no E7-specific CTL activity was detected at day 4 after the boost. If the mice had been effectively immunized, E7-specific CTL could be measured easily at day 4 after the boost. As shown in Fig. 2, lymphocytes isolated from both BC219-E7 and BC219-E7 mutant groups showed lytic activity against RMA-E7 cells, whereas lymphocytes isolated from BC219-immunized mice did not kill RMA-E7 cells. In comparison, immunization with BC219-E7 mutant induced a significantly stronger specific CTL response against RMA-E7 than BC219-E7. The lymphocytes from all three groups were unable to kill the control cell line RMA-neo (data not shown).
FIG. 2.
Spleen lymphocytes from mice immunized with control vector (BC219) or HPV 16 E7 DNA vaccines (BC219-E7 and BC219-E7 mutant [mut]) were isolated and stimulated in vitro for 1 week with HPV 16 E7 peptide (amino acids 49 to 57). The specific lysis of the spleen cells against RMA-E7 cells (target cells) was determined in a standard 6-h 51Cr release assay at different effector-to-target ratios. Results are the means of specific lysis from five different mice, and standard deviations were ≤10%. The CTL response induced by the mutant E7 vaccine is significantly stronger than the one induced by the wild-type E7 vaccine (P < 0.01).
HPV 16 E7 mutant vaccine protected 100% of mice against tumor challenge.
In the next experiment, we determined whether E7 DNA immunization could induce protective immunity against challenge with HPV 16 E7-positive tumors. First we developed an E7-positive tumor model by transfecting RMA cells (B6 syngeneic tumor cell line) with plasmid pCI-neo-E7. A control cell line, RMA-neo, was developed by transfecting RMA cells with plasmid pCI-neo. The cells were maintained in RPMI 1640 (GIBCO-BRL, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml), and G418 (800 μg/ml; Bio-Rad, Hercules, Calif.).
Mice (10 per group) were immunized intramuscularly with 100 μg of BC219-E7 mutant, BC219-E7, or BC219 plus incomplete Freund’s adjuvant. On day 14 after immunization, the mice were challenged subcutaneously with 2.5 × 104 RMA-E7 tumor cells and monitored every 3 days. This dose of tumor cells for challenge is the minimal dose to induce tumors in 100% of inoculated mice, based on our previous experiments. Tumor diameter was measured by two investigators blinded to the groups.
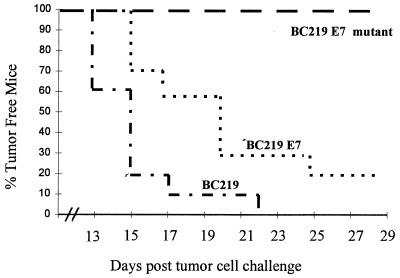
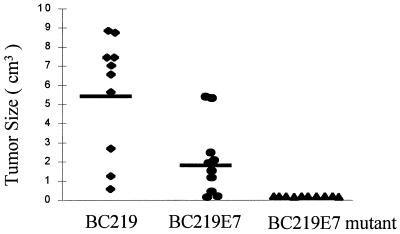
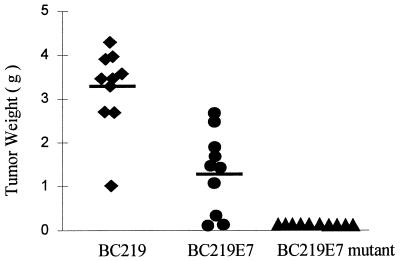
On day 13 of challenge, RMA-E7 tumors started growing in four mice immunized with BC219; by day 22, all of the mice in this group developed tumors. Three of the mice immunized with BC219-E7 developed RMA-E7 tumors on day 15 of tumor challenge, and only two mice in this group did not develop tumors by the date of sacrifice. In contrast, none of the mice immunized with BC219-E7 mutant developed an RMA-E7 tumor (Fig. 3). There was a statistically significant difference in tumor take between the groups of mice. The mice that grew tumors were sacrificed at day 28, and the tumor size and weight were assessed. Both tumor size (Fig. 4) and tumor weight (Fig. 5) of the mice immunized with BC219-E7 were significantly less than those of the mice immunized with BC219 (P < 0.01). The mice immunized with BC219-E7 mutant did not develop tumors for at least 6 months. As controls, mice (10 per group) immunized with the three different vaccines were challenged with RMA-neo cells in the same way. The RMA-neo tumors grew in all three groups of mice, and there was no significant difference in tumor take, tumor size, and tumor weight between the groups (data not shown).
FIG. 3.
Tumor incidence in mice (10 per group) immunized with BC219, BC219-E7, or BC219-E7 mutant DNA vaccine. The mice were challenged with 2.5 × 104 RMA-E7 cells 2 weeks after the immunization. There were significant differences between each group (P < 0.001).
FIG. 4.
Tumor size in mice immunized with BC219, BC219-E7, or BC219-E7 mutant DNA vaccine. Two weeks after the immunization, mice (10 mice per group) were challenged with 2.5 × 104 RMA-E7 cells. The data represent the size of individual tumors measured at the end of week 4 after tumor challenge. The median of the size is shown by bars. The tumor size from the mice immunized with BC219-E7 is significantly smaller than that from the mice immunized with BC219 (P < 0.01).
FIG. 5.
Tumor weight in mice immunized with BC219, BC219-E7, BC219-E7 mutant DNA vaccine. Two weeks after the immunization, mice (10 mice per group) were challenged with 2.5 × 104 RMA-E7 cells. The data represent the weight of individual tumors measured at the end of week 4 after tumor challenge. The median of the weight is shown by bars. The tumor weight from the mice immunized with BC219-E7 is significantly less than that from the mice immunized with BC219 (P < 0.01).
The differences in continuous measures among groups were compared by analysis of variance. Between-group comparisons were made with the Duncan test. Differences in time to tumor take were examined using Kaplan-Meier actuarial survival techniques. In all cases a two-sided alpha level of 0.05 was considered statistically significant.
Our data indicate that a DNA vaccine encoding the wild-type E7 protein can induce an E7-specific CTL response and some tumor protection. However, because the E7 protein is known to have transforming activity, this DNA vaccine might have oncogenic potential. Although any E7-expressing cells should be killed by the CTL induced by the DNA vaccine, some plasmids might be retained in MHC class I-low-expressing cells that might resist CTL lysis; thus, transformation of these cells could occur. Mutation in the E7 protein, which abrogates the transforming activity, helps to ensure the safety of the DNA vaccine.
Although the wild-type E7 DNA vaccine protected mice against challenge with HPV 16 E7-positive tumor cells, the protection was not complete. There are two possible reasons: (i) RMA-E7 cells grow relatively fast and might override the CTL induced by the E7 DNA vaccine; and (ii) the number of CTL generated by the vaccine might be too low. Because the E7 plasmid is believed to be taken up by antigen-presenting cells, e.g., dendritic cells, the E7 protein is likely produced by these cells. Because E7 cannot be secreted, it enters the MHC class I pathway instead of the MHC class II pathway. Consequently, no E7-specific T helper cells are generated, which might limit the expansion of E7-specific CTL.
Our data indicate that mutation in the zinc-binding motifs near the C terminus of E7 leads to rapid degradation of the protein. This finding is based on the fact that the mutant E7 can be better detected in the presence of a proteasome inhibitor (ALLN). Because a zinc finger might be important in maintaining the stability of the protein, the mutation in the motifs might abrogate the zinc-binding activity and result in instability of the protein. Our data suggest that the mutant E7 may be degraded rapidly in the proteasome and a large amount of antigenic peptides may be transported to endoplasmic reticulum. Consequently, more MHC class I molecules of antigen-presenting cells present the E7 peptide (amino acids 49 to 57), resulting in an enhanced CTL response and tumor protection. Townsend et al. reported that defective presentation to class I-restricted CTL in vaccinia virus-infected cells is overcome by enhanced degradation of influenza virus hemagglutinin antigen (40). Tobery and Siliciano demonstrated that rapid intracellular degradation of human immunodeficiency virus type 1 Env or Nef protein induced an enhanced CTL response in vivo after recombinant vaccinia virus immunization (39). Recently it has been shown that ubiquitination of a viral protein enhances CTL induction and antiviral protection in DNA immunization (33). Thus, those data together with ours suggest that acceleration of protein degradation leads to enhanced antigen presentation in the context of MHC class I and subsequently CTL induction.
The potency of a DNA vaccine for the generation of CTL is relatively weak. It has been shown that only some individuals immunized with HIV Nef, Rev, or Tat DNA vaccines had CTL responses in their peripheral blood (7). It has also been shown that 50 to 75% of animals immunized with pCMV-NP DNA vaccines developed CTL (30, 33, 44). It is predictable that the individuals without a measurable CTL response will not have effective protection against viral infection. This was also true in a tumor system, as we showed that the wild-type E7 DNA vaccine did not protect all mice against tumor challenge, although it induced some CTL response specific for the E7. Therefore, CTL responses can be enhanced by mutation of encoded proteins or by ubiquitination in DNA vaccination, which will augment the protective effects of the vaccine, as shown by our data and others (33, 39, 40). Moreover, mutation of a viral protein will also result in abrogation of its function, which is often harmful to the host, further ensuring the safety of the vaccine.
Acknowledgments
This work was supported by the Breast and Cervical Cancer Research Fund (grant 83880356) from the Illinois Department of Public Health.
We thank David Stone for assistance in preparing the manuscript.
REFERENCES
- 1.Altmann A, Jochmus-Kudielka I, Frank R, Gausepohl H, Moebius U, Gissmann L, Meuer S C. Definition of immunogenic determinants of the human papillomavirus type 16 nucleoprotein E7. Eur J Cancer. 1992;28:326–333. doi: 10.1016/s0959-8049(05)80047-4. [DOI] [PubMed] [Google Scholar]
- 2.Bagarazzi M L, Boyer J D, Ayyavoo V, Weiner D B. Nucleic acid-based vaccines as an approach to immunization against human immunodeficiency virus type-1. Curr Top Microbiol Immunol. 1998;226:107–143. doi: 10.1007/978-3-642-80475-5_8. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa M S, Lowy D R, Schiller J T. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989;63:1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton P A, Kennedy R C. DNA vaccines strategies for the treatment of cancer. Curr Top Microbiol Immunol. 1998;226:1–20. doi: 10.1007/978-3-642-80475-5_1. [DOI] [PubMed] [Google Scholar]
- 5.Berezutskaya E, Bagchi S. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26 S proteasome. J Biol Chem. 1997;272:30135–30140. doi: 10.1074/jbc.272.48.30135. [DOI] [PubMed] [Google Scholar]
- 6.Borysiewicz L K, Fiander A, Nimako M, Man S, Wilkinson G W, Westmoreland D, Evans A S, Adams M, Stacey S N, Boursnell M E, Rutherford E, Hickling J K, Inglis S C. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18 E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 7.Calarota S, Bratt G, Nordlund S, Hinkula J, Leandersson A-C, Sandstrom E, Wahren B. Cellular cytotoxic response induced by DNA vaccination in HIV-1 infected patients. Lancet. 1998;351:1320–1325. doi: 10.1016/S0140-6736(97)09440-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen L P, Thomas E K, Hu S-L, Hellstrom I, Hellstrom K E. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc Natl Acad Sci USA. 1991;88:110–114. doi: 10.1073/pnas.88.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L P, Mizuno M T, Singhal M C, Hu S-L, Galloway D A, Hellstrom I, Hellstrom K E. Induction of cytotoxic T lymphocytes specific for a syngeneic tumor expressing the E6 oncoprotein of human papillomavirus type 16. J Immunol. 1992;148:2617–2621. [PubMed] [Google Scholar]
- 10.Comerford S A, McCance D J, Dougan G, Tite J P. Identification of T- and B-cell epitopes of the E7 protein of human papillomavirus type 16. J Virol. 1991;65:4681–4690. doi: 10.1128/jvi.65.9.4681-4690.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danko I, Wolff J A. Direct gene transfer into muscle. Vaccine. 1994;12:1499–1502. doi: 10.1016/0264-410x(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 12.Davis H L, Whalen R G, Demeneix B A. Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther. 1993;4:151–159. doi: 10.1089/hum.1993.4.2-151. [DOI] [PubMed] [Google Scholar]
- 13.Dyson N, Howley P M, Münger K M, Harlow E. The human papillomavirus-16 E7 protein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 14.Edmonds C, Vousden K H. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989;63:2650–2656. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feltkamp M C W, Smits H L, Vierboom M P M, Minnaar R P, de Jongh B M, Drijfhout J W, ter Schegget J, Melief C J, Kast W M. Vaccination with cytotoxic T lymphocyte epitope containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 16.Feltkamp M C W, Vreugdenjil G R, Vierboom M P M, Ras E, van der Burg S H, ter Schegget J, Melief C J M, Kast W M. Cytotoxic T lymphocytes raised against a subdominant epitope offered as a synthetic peptide eradicate human papillomavirus type 16-induced tumors. Eur J Immunol. 1995;25:2638–2642. doi: 10.1002/eji.1830250935. [DOI] [PubMed] [Google Scholar]
- 17.Frazer I H. Immunology of papillomavirus infection. Curr Opin Immunol. 1996;8:484–491. doi: 10.1016/s0952-7915(96)80035-5. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Chain B, Sinclair C, Crawford L, Zhou J, Morris J, Zhu X, Stauss H. Immune response to human papillomavirus type 16 E6 gene in a live vaccinia vector. J Gen Virol. 1994;75:157–164. doi: 10.1099/0022-1317-75-1-157. [DOI] [PubMed] [Google Scholar]
- 19.Hassett D E, Whitton J L. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 20.Herd K, Fernando G J, Dunn L A, Frazer I H, Lambert P, Tindle R W. E7 oncoprotein of human papillomavirus type 16 expressed constitutively in the epidermis has no effect on E7-specific B- or Th-repertoires or on the immune response induced or sustained after immunization with E7 protein. Virology. 1997;231:155–165. doi: 10.1006/viro.1997.8491. [DOI] [PubMed] [Google Scholar]
- 21.Kadish A S, Romney S L, Ledwidge R, Tindle R, Fernando G J, Zee S Y, Van Ranst M A, Burk R D. Cell-mediated immune responses to E7 peptides of human papillomavirus type 16 are dependent on the HPV type infecting the cervix whereas serological reactivity is not type-specific. J Gen Virol. 1994;75:2277–2284. doi: 10.1099/0022-1317-75-9-2277. [DOI] [PubMed] [Google Scholar]
- 22.Kanda T, Furuno A, Yoshiike K. Human papillomavirus type 16 open reading frame E7 encodes a transforming gene for rat 3Y1 cells. J Virol. 1988;62:610–613. doi: 10.1128/jvi.62.2.610-613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kast W M, Brandt R M P, Sidney J, Drijfhout J W, Kubo R T, Grey H M, Melief C J, Sette A. Role of HLA-A motifs in identification of potential CTL epitopes in human papillomavirus type 16 E6 and E7 proteins. J Immunol. 1994;152:3904–3912. [PubMed] [Google Scholar]
- 24.Kaufmann A M, Gissmann L, Schreckenberger C, Qiao L. Cervical carcinoma cells transfected with the CD80 gene elicit a primary cytotoxic T lymphocyte response specific for HPV 16 E7 antigens. Cancer Gene Ther. 1997;4:377–382. [PubMed] [Google Scholar]
- 25.Koutsky L A, Galloway D A, Holmes K K. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–163. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- 26.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin K Y, Guarnieri F G, Staveley-O’Carroll K F, Levitsky H I, August J T, Pardoll D M, Wu T C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 28.Massimi P, Pim D, Storey A, Banks L. HPV-16 E7 and adenovirus E1a complex formation with TATA box binding protein is enhanced by casein kinase II phosphorylation. Oncogene. 1996;12:2325–2330. [PubMed] [Google Scholar]
- 29.Mucke S, Polack A, Pawlita M, Zehnpfennig D, Massoudi N, Bohlen H, Doerfler W, Bornkamm G, Diehl V, Wolf J. Suitability of Epstein-Barr virus-based episomal vectors for expression of cytokine genes in human lymphoma cells. Gene Ther. 1997;4:82–92. doi: 10.1038/sj.gt.3300363. [DOI] [PubMed] [Google Scholar]
- 30.Pedroza Martins L, Lau L L, Asano M S, Ahmed R. DNA vaccination against persistent viral infection. J Virol. 1995;69:2574–2582. doi: 10.1128/jvi.69.4.2574-2582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ressing M E, Sette A, Brandt R M P, Ruppert J, Wentworth P A, Hartman M, Oseroff C, Grey H M, Melief C J, Kast W M. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 32.Robinson H L, Tores C A. DNA vaccines. Semin Immunol. 1997;9:271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez F, Zhang J, Whitton J L. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J Virol. 1997;71:8497–8503. doi: 10.1128/jvi.71.11.8497-8503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadovnikova E, Zhu X, Collins S M, Zhou J, Vousden K, Crawford L, Beverley P, Stauss H J. Limitations of predictive motifs revealed by cytotoxic T lymphocyte epitope mapping of the human papillomavirus E7 protein. Int Immunol. 1995;6:289–296. doi: 10.1093/intimm/6.2.289. [DOI] [PubMed] [Google Scholar]
- 35.Seedorf K, Oltersdorf T, Krämmer G, Röwekamp W. Identification of early proteins of the human papillomaviruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987;6:139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smotkin D, Wettstein F O. The major human papillomavirus protein in cervical cancers is a cytoplasmic phosphoprotein. J Virol. 1987;61:1686–1689. doi: 10.1128/jvi.61.5.1686-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strang G, Hickling J K, McIndoe G A, Howland K, Wilkinson D, Ikeda H, Rothbard J B. Human T cell responses to human papillomavirus type 16 L1 and E6 synthetic peptides: identification of T cell determinants, HLA-DR restriction and virus type specificity. J Gen Virol. 1990;71:423–431. doi: 10.1099/0022-1317-71-2-423. [DOI] [PubMed] [Google Scholar]
- 38.Tindle R W, Fernando G J, Sterling J C, Frazer I H. A “public” T-helper epitope of the E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc Natl Acad Sci USA. 1991;88:5887–5891. doi: 10.1073/pnas.88.13.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobery T W, Siliciano R F. Targeting of HIV-1 antigens for rapid intracellular degradation enhances cytotoxic T lymphocyte (CTL) recognition and the induction of de novo CTL responses in vivo after immunization. J Exp Med. 1997;185:909–920. doi: 10.1084/jem.185.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Townsend A, Bastin J, Gould K, Brownlee G, Andrew M, Coupar B, Boyle D, Chan S, Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988;168:1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 42.Vousden K H, Doniger J, DiPaolo J A, Lowy D R. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988;3:167–175. [PubMed] [Google Scholar]
- 43.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 44.Zarozinski C C, Fynan E F, Selin L K, Robinson H L, Welsh R M. Protective CTL-dependent immunity and enhanced immunopathology in mice immunized by particle bombardment with DNA encoding an internal virion protein. J Immunol. 1995;154:4010–4017. [PubMed] [Google Scholar]
- 45.zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]