Summary
The African shrimp (Atya gabonensis) uses elongated setae to filter feed, adapting to high flow velocities. The setae’s stability stems from carefully designed geometric and structural parameters, notably a specialized wall and distribution principle. This study highlights the robust filtration mechanism in the shrimp and potential for developing stable structures in underwater environments.
Subject areas: Biological sciences, Biophysics, Zoology
Graphical abstract

Highlights
-
•
African shrimp setae stability test
-
•
Geometric and distributional characteristics of African shrimp setae
-
•
The stability mechanism of African shrimp setae
-
•
Filtration efficiency-device stability positive correlation confirmation
Biological sciences; Biophysics; Zoology
Introduction
Filter feeders, widely distributed in lakes, rivers, and oceans, mainly feed on plankton by filtering water.1 They have made outstanding contributions to water treatment2 and ecological management,3 and are indispensable for aquatic ecosystem.4 Filter feeders cover sponges,5 sea squirts,6 comb jellies,7 bivalves,8 crustaceans,9 cetaceans,10 and other species.11,12,13,14 They are equipped with a range of filtration apparatus to adapt to different living environments.15 Some filtration apparatus, consisting of cilia, cirrus, tentacles, or attachments with hairs, are mainly used to generate a feed stream for directional capture of suspended objects. However, most filtration apparatus consisting of mucus nets, gill filaments, cilia, or filter setae are primarily used for direct screening of suspended particles.16 For this type of filtration apparatus, the structural stability is of great significance which may affect the filter area of the filtration apparatus, consequently influencing the filtration efficiency.17,18 For instance, crustacean filter feeders use this type of filtration apparatus for feeding. In the past 500 million years, although crustacean filter feeders have evolved different forms of filtration apparatus, most of their filtration apparatus feature in converged morphology, namely they are made of filter setae and setules, and the main function of these filtration apparatus is to directly screen suspended solids in water.19
The African shrimp, Atya gabonensis (Decapoda: Atyidae) belongs to filter-feeding crustaceans, with a body length ranging from 51 to 138 mm, and in an average weight of 30.3 ± 18.7 g, mainly live in fast-flowing rivers or streams in South Africa and West Africa.20,21,22 The first and second cheliped of an African shrimp have chelae coated in soft filter setae, which function as filtration apparatus,20 used to directly capture suspended solids, and also to create feeding water flow. The African shrimp have extremely slender filter setae, with an aspect ratio ranging from 186 to 384, higher than that of the setae or gill filaments of most filter feeders. The extremely slender and soft filter setae pose a great challenge for maintaining stability.23 However, studies on the African shrimp have been limited to the anatomical24,25 and ecological26,27 aspects, and the stability of its filtration apparatus remains unknown.
Here, in this combined experimental and theoretical investigation, we reveal the mechanism by which the filter setae of African shrimp remain stable in the feeding stream. We first study the cross section at geometry and material properties of setae. Next by taking use of the micro-computed tomography (Micro-CT) scanning, we quantify spatial distribution characteristics of the setae on the chela. We use experimental methods and fluid-structure interaction simulation to investigate the effects of the geometric and structural characteristics of setae on their stiffness. In addition, we developed conceptual ideas for potential applications based on the filtration apparatus of the African shrimp. These applications would incorporate structures that possess both high softness and high aspect ratios, while also maintaining stability in unpredictable fluid environments.
Results
Stability of the setae in water flow
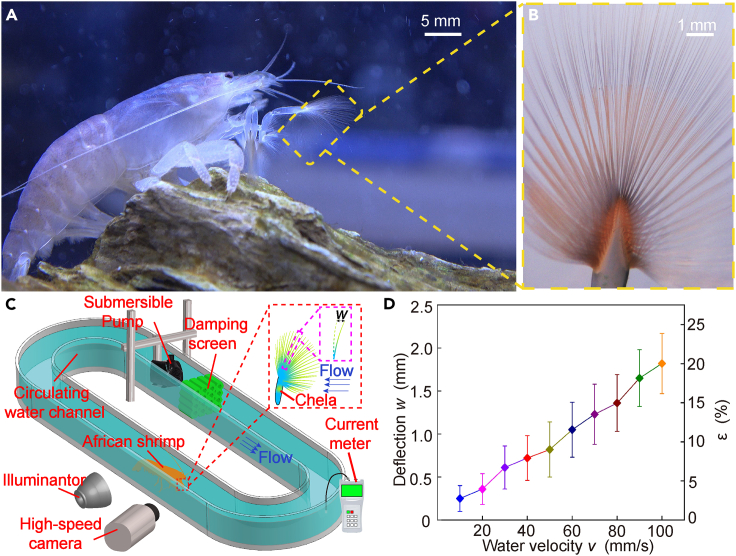
We raised ten living samples of African shrimp (A. gabonensis) in a tank (Figure 1A). The chela of an African shrimp is a meshed filtration apparatus composed of multiple slender filter setae (Figure 1B), which exhibit outstanding structural stability under impact of the maximum flow velocity of 40 mm/s in the tank (Video S1). To assess the stability of the filtration apparatus in African shrimp, we place them in a circulating water channel and vary the flow rate using a submersible pump to examine the impact on the apparatus. Using a high-speed camera equipped with a micro-lens, we record the average deflection of three randomly selected setae located in the middle part of the filtration apparatus (average length = 8.5 mm) under the flow conditions (Figure 1C). Our tests indicate that the seta is not largely deformed induced by water flow. In detail, when the water velocity reaches v = 40 mm/s (about 1600 times the length of the long axis of the setae cross section/s), the maximum deflection w of the seta only accounts for about 8.5% of the total length of the seta (Figure 1D). The filtration apparatus of the African shrimp may have specified morphological or structural characteristics to enhance specific stiffness, which is not clearly understood.
Figure 1.
Structural stability of filtration apparatus of African shrimp
(A) A living African shrimp (Atya gabonensis).
(B) Microscopic image of a chela as the filtration apparatus.
(C) Setae deflection of an African shrimp tested by a circulating water channel.
(D) Deflection of the seta w with respect to the water velocity v. Here w is defined as the deflection occurring on the seta tip (C), and is the ratio of the seta deflection w to the seta length L = 8.5 mm.
Morphological characteristics of setae
We observe the overall morphological characteristics of filtration apparatus of African shrimp by SEM (Figure 2A), and find that the setae are densely distributed on the filtration apparatus and of varying lengths. We put the fresh-cut filtration apparatus in a Petri dish with MgCl2 solution (s = 10% wt./wt.) that results in complete unfolding of the setae. Upon examination under the microscope, the setae on the chela are found to have varying lengths and exhibit a consistent transition in length along the circumference. The setae are evenly distributed into five regions in the circumferential direction (Figure 2B). The aspect ratios of 15 setae are computed from each region through the extraction process (see STAR methods). We unexpectedly find that the aspect ratios of setae in African shrimp are extremely high, in which 226 ± 40 for the region I, 245 ± 33 for the region II, 287 ± 37 for the region III, 324 ± 25 for the region IV, and 362 ± 22 for the region V (Figure 2C), which exceed those of filter setae or gill filaments in other filter feeders (Figure 2D).19,28,29,30,31,32,33,34 In addition, we carefully examine the morphology of setae through the SEM. We find that the seta is conically structured,35 becoming tapered from the base to the tip. The base of the seta exhibits a hollow and elliptical cross section, with the wall unilaterally thickening along the long axis of the ellipse (Figure 2E). The thickening gradually diminishes from the base to the tip (Figures 2E and S1).
Figure 2.
Morphology of the setae on the filtration apparatus of an African shrimp
(A) SEM image of the chela covered by dense setae.
(B) Distribution of setae with different aspect ratios indicated by a spectrum of colors.
(C) Aspect ratio of setae with colors corresponding to (B).
(D) Aspect ratio of setae or gill filaments on filtration apparatus of various filter feeders. The filtration elements in Figure 1F are setae of the Barnacle (Balanus), gill cirrus of the Oyster (Crassostrea virginica), limb trunk setae of the Fairy shrimp (Artemia), setules of the Daphnia (Ditrupa arietina), gill filaments of the Fan worm (Sabellidae), gill filaments of the silver carp (Hypophthalmichthys molitrix), forefoot setae of the Krill (Meganyctiphanes norvegica), Baleen bristles (Eubalaena australis), and setae on chela of the African shrimp (Atya gabonensis).
(E) SEM image of seta. The cross section of the setae is elliptic with a unilateral thickened wall along the long axis.
Material characteristics of setae
To explore the stiffening mechanism of setae, we apply the energy dispersive spectroscopy and confocal laser scanning microscope to measure the element composition and distribution of the setae. Examinations show that the setae are mainly composed of C, O, N, S, Cl, Ca, and Na (Figures 3A and S2), and the material is evenly distributed with no observable material gradient (Figure 3B). In addition, we measure Young’s modulus of the setae by atomic force microscopy (Figure 3C). Notably, the average Young’s modulus of the setae is only about 11.6 MPa, which is lower than the filter setae and gill filaments of most filter feeders including Scyphozoa,36 Sabellidae,37 Actiniaria,38 Eubalaena australis,39 Mytilus edulis,40 and phylum Porifera41 (Figure 3D). The setae of the African shrimp characterized in both extremely high aspect ratio and with low Young’s modulus, yet they can remain stable under the impact of water flow. Due to the significant influence of cross-sectional features on the bending properties of biological structures,42,43 we hypothesize that the distinctive geometric cross-sectional properties of setae might potentially increase their stiffness.
Figure 3.
Material characteristics of the setae
(A) EDS maps of elements C, O, N, S, Cl, Ca, and Na on setae surface.
(B) CLSM image of seta. The image suggests that the elements are evenly distributed.
(C) Young’s modulus of setae measured by AFM test. The average Young’s modulus of the setae is 11.6 MPa.
(D) Young’s modulus of filter setae or gill filaments for filter feeders. The filtration elements in Figure 1F belong to the microfibrils of jellyfish (Scyphozoa), the gill filaments of fan worm (Sabellidae), the setae on the chela of African shrimp (Atya gabonensis), the cirri of a crinoid (Actiniaria), Baleen bristles (Eubalaena australis), the gill filaments of Mytilidae (Mytilus edulis), and spicules of sponges (Phylum Porifera) (from left to right).
Orientation of the seta cross section against water flow
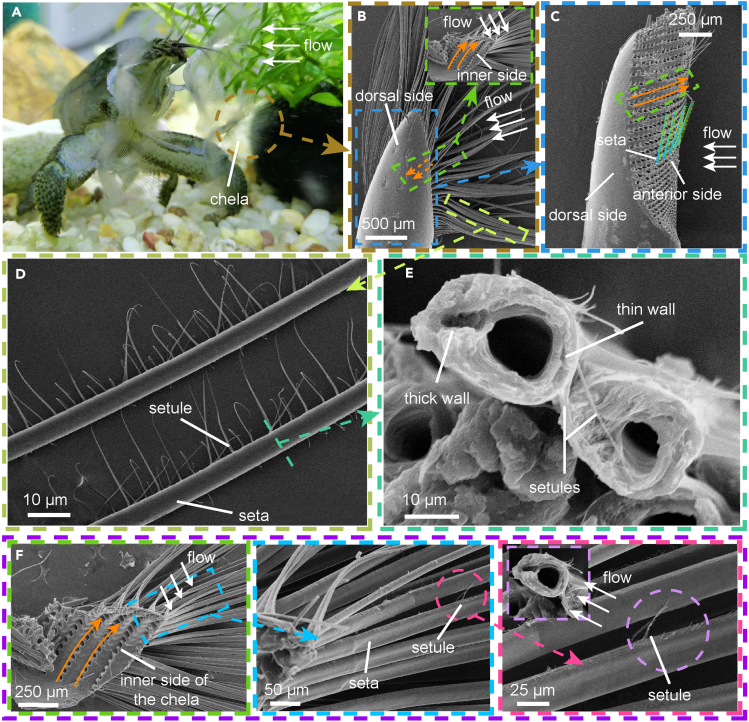
To investigate the effects of cross-sectional geometry on setae stiffness, it is essential to first establish the orientation of the setae cross section with respect to the direction of water flow. We find that anterior side of chela of the African shrimp is oriented toward the direction of the water flow during filtration from Video S1 and Figures 4A–4C. The SEM images show a row of dense setules bearing merely on the outer side of a seta’s thinner wall, which are distributed evenly along the long axis of the seta (Figures 4D and 4E). We cut out a piece of the basal part with setae from the chela of the African shrimp (indicated by the green dashed box in Figures 4C and 4F) and observe it using SEM. By combining the arrangement direction of the setae pores (indicated by orange arrows in Figures 4B, 4C, and 4F), we can clearly observe that the setules are distributed on the anterior side of the chela. Taking into account the direction of water flow, we can deduce that in the initial state when the African shrimp opens its chelae for filter feeding, the major axis of the setae’s elliptical cross section aligns parallel to the direction of water flow. Figure S3A illustrates the methodology used to determine the direction of flow toward the cross section of the seta. In our study, we consider the direction of the major axis of the setae’s elliptical cross section to be parallel to the direction of water flow. For specific details, please refer to Figure S3B.
Figure 4.
Identification of the water flow direction to the setae on the chela
(A–C) The African shrimp capturing particles with the anterior side of their chela facing the direction of the water flow. The orange arrow shows seta-pore distribution on the chela. The basal part with setae inside the green dashed box in (B) corresponds to (F). The perspective of the basal part with setae in (F) is obtained by cutting and flipping the basal part with setae within the dashed box in (B) from the chela, rotating it 180° in an upward direction.
(D and E) The setules are found only on the thin-walled side of the long axis of the setae cross section.
(F) The setules are distributed on the anterior side of the chela. The basal part with setae is taken from the position of the green dashed box in (C), where the orange arrows in (F) correspond to the orange arrows in (C).
Effects of specified cross-sectional geometry
To verify effects of the cross-sectional geometry on enhancing the setae stiffness, a bundle of setae (n = 30) in the region V are analyzed (Figure 2B; Table S1). The SEM images indicate that the cross section of the setae is elliptical and the wall thickens unilaterally along the long axis of the elliptic cross section (Figures 2E and 4E). This is comparable to how a uniform elliptical cross section can increase the thickness of the wall (ranging from 2 to 10 μm) along its long axis (as depicted in Figure 5A). The polar moment of inertia of elliptic cross section with uniform wall thickness is , and the increase of wall thickness may affect the polar moment of inertia of cross-section. Comparatively, we figure out four wall thickening fashions and analyze the influence on the polar moment of inertia of the cross-section, respectively. We use (i = 1,2,3,4) to represent the polar moment of inertia when the wall thickness increases unilaterally along the long axis, bilaterally along the long axis, unilaterally along the short axis, and bilaterally along the short axis of the elliptic cross-section, respectively (Figure 5B, Note S1). Then, (i = 1,2,3,4) is used to represent the increased ratio of polar moment of inertia when the wall thickness increases in the four fashions listed previously, respectively (Figure 5B, Note S1). Our numerical simulation shows that the natural thickening pattern of the setae on the African shrimp, which thickens unilaterally along the long axis of the elliptic cross-section, is the most beneficial in enhancing the polar moment of inertia of the cross section (Figure 5B), when the water flow direction remains parallel to the long axis of the cross section (Figure 4F), The four comparison models in Figure 5B have equal areas. In addition, we establish two types of seta simulation models with cross-sectional shape (Table S2), namely the ellipse with thickened wall thickness noted as A1, and circle with the same area as A1 noted as A2, respectively (Figure 5A; Table S2). The two models are put into the flow field with an attack angle of 90° for fluid-structure interaction simulation (Figure 5C). We find that when the water speed gets v = 40 mm/s, the deflection of the seta with A1 cross section is about 66% smaller than that of the seta with A2 cross section (Figure 5D), suggesting that the unilaterally thickened wall design of the setae is more effective than the other designs.
Figure 5.
Stiffness enhancement by the specialized geometry of the setae cross section
(A) Geometrical models of the elliptical setae cross section. Cross section of the setae of African shrimp thickens along one side (case A1). The elliptic cross section with universal wall thickness is marked as case A0 and is the circular cross section with an equal area as that of the case A2.
(B) Impact of different ways of increasing the wall on the polar moment of inertia of the cross section. Thickening along the long axis of the ellipse plays the most predominate role in enhancing the polar moment of inertia. In the comparative model, we apply the principle of equal area to ensure that the four comparison models in (B) have the same area, which is given by .
(C) Schematics of the fluid-seta interaction model with a single seta at an angle of attack of 90°.
(D) The fluid-structure interaction simulation of setae with different cross-sections shows that the deflection of the seta with elliptic cross section with thickened walls (A1) is about 66% smaller than that of the seta with circular cross section of the same area as A1 (A2).
Stability facilitated by spatial distribution of setae
The analysis and simulations show that the stiffness of each strand of seta can be enhanced by the cross-sectional geometry. However, when it comes to a large group of setae forming a specialized 3D configuration, there may be another mechanism that augments the stiffness. To test this hypothesis, we capture microscopic images of setae and find that the setae distributed on the chela intersecting and twisting (Figures 6A and 6B). We devise an approach to uncover the spatial distribution information of setae. We first reconstruct the three-dimensional model of a chela with setae gently removed by Micro-CT, which exhibits an appearance of basal pores on the chela surface (Figure 6C, Video S2, STAR methods). We can reconfigure orientation of the setae by identifying spatial orientation of normal vector of the pores. After selecting the four rows of pores in the middle of the chela (Figures 6D–6F; Table S3), we can determine the orientations of the setae on these chela pores. We use this information to create a 3D model of the setae positions (Figures 6D–6F) and orientation vectors (Figure 6E), which are then plotted on an azimuth map (Figure 6G). This analysis allows us to observe a complex distribution of intersecting and twisting setae. The four rows of pores in the middle of the chela (Figures 6D–6F; Table S3) are selected to characterize orientations of the setae bearing on these chela pores. We then sketch the 3D position (Figures 6D–6F) and orientation vector of the setae (Figure 6E) in an azimuth map (Figure 6G), through which we can identify a combined intersecting and twisting distribution of setae. We proceed to create a simulation model of the setae bundle, taking into account the variations in both the number of setae and the twisted angle between adjacent setae (see Table S4 for details of model dimensions), and perform a fluid-structure interaction simulation (Figure 6H). Our simulation results show that the quantity of the intersecting setae has a negative correlation to the setae deflection on average. On the other hand, the twisted angle between the two adjacent setae is positively correlated to the average deflection of the setae (Figure 6I). The chela of the African shrimp has 7–15 setae on each row, and the twisted angle between the setae is 3°–5°. When the number of setae intersecting is 15 and the twisted angle is 3°, the average deflection is about 30% smaller than that of a single seta. The combination of the specific distribution pattern of setae and their cross section plays a crucial role in increasing stiffness. As a result, the average deflection of setae in the African shrimp’s filtration apparatus is reduced by about half, enabling it to maintain stability even under the pressure of rushing water flow.
Figure 6.
Effects of setae distribution characteristics on setae stiffness enhancement
(A) The stereoscopic image of the setae-coated chela. The setae show an appearance of intersecting and twisting pattern.
(B) Schematics showing geometry of the intersecting and twisting setae.
(C) Micro-CT image of the bald chela with setae amputated by which orientation of the setae can be quantified.
(D) Coordinates of setae base projected in the XOZ plane.
(E) Normal vectors indicating orientations of setae radiating out.
(F) Coordinates of setae base projected in the XOY plane.
(G) Schematic diagram of the normal vector showing position distribution of the setae.
(H) Fluid-structure interaction model of intersecting and twisting setae that emulates the spatial setae distribution law of the shrimp.
(I) Relationship among the number of intersecting setae, twisted angle of the setae, and the setae deflection acquired by fluid-structure interaction simulation.
Effect of structural stability on filtration efficiency
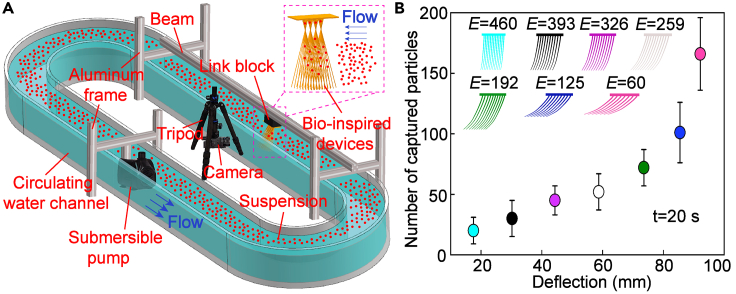
To further verify the benefits of structural stability for filtration efficiency, we build an experimental setup for particle capture. We print a series of bio-inspired, 50-times-scaled-up, rubber filtration devices (Figure S6) based on the geometric and distribution characteristics of the setae of the African shrimp with different Young’s modulus (E = 60, 125, 192, 259, 326, 393, 460 MPa) (Figures 2E and 6C–6G). These devices are then placed in a circulating channel filled with suspension (concentration: 8.45 × 10−4 g/mL) at a flow velocity v = 40 mm/s for particle capture (Figure 7A). Statistical analysis suggests that the devices with a high Young’s modulus can both perform a higher structural stability and reach a bigger number of captured particles in a duration of 20 s. For instance, the number of particles captured by the device with Young’s modulus E = 460 MPa is about 8 times that of the device with Young’s modulus E = 60 MPa (Figure 7B, S6, and S7). The findings indicate that an increase in the stiffness of the setae leads to a higher particle filtration rate, highlighting the crucial role of stiffness in animal appendages for filter feeding.
Figure 7.
Effects of structural stability on filtration efficiency
(A) Experimental setup. The particle capture number by the man-made filtration devices with varying stiffnesses is tested.
(B) The experimental findings demonstrate a positive correlation between the stiffness of the filtration devices and the number of captured particles, highlighting the critical role of stiffness in animal appendages for efficient filter feeding where E is Young’s modulus (MPa).
Discussion
Inhabiting in rushing streams or rivers, African shrimps encounter a stability challenge because of the extreme slenderness and softness of the setae on their filtration apparatus. This study demonstrates for the first time that the African shrimp increases the stiffness of the setae by combining geometric and structural features, enabling the filtration apparatus to maintain its stability in dynamic flows. The asymmetrically thickened cross section of the setae can reduce the deflection of the setae by approximately 66% compared to a hollow cylinder with a universal wall thickness. Moreover, intersecting and twisting distribution of the setae decreases the deflection of the setae by approximately 30% compared to a single seta.
The setae of the African shrimp are made of extremely soft materials and their structural stability is enhanced by geometric and structural properties. The way in which the setae are made of soft materials instead of hard materials may enable the setae to absorb impulse through deformation and prevent the setae from breaking when subjected to a large impact. In addition, from a physiological perspective, flexible setae in animals tend to grow more easily than rigid setae, which can compensate for the loss of setae during feeding. Furthermore, the twisting and intersecting distribution principle of the setae enhance the setae stiffness while also creating varied spacing between the setae, which may extend the filtration adaptability to a wider range of suspended particle sizes compared to setae arranged with fixed spacing. Additionally, the African shrimp have multiple rows of setae on the filtration apparatus, separated by curved channels that are more effective at capturing particles than the straight channel.44
In addition to the stiffness enhancement mechanisms mentioned previously, there may be other internal stiffness enhancement mechanisms in African shrimp that have yet to be explored, such as muscle regulation or internal pressurization, enabling their filtering apparatus to remain stable under the impact of fast flow.
Conclusion
The geometric and structural features of the setae on the filtration apparatus of African shrimp enhance the structural stability in random flow, thus possibly ensuring high filtration efficiency. The stability of the filtration apparatus allows the African shrimp to switch between active and passive filtration fashions, and may facilitate a trade-off between energy expenditure and filter-feeding efficiency. The ability of the filtration apparatus to maintain stability makes the African shrimp more adaptive to the rushing water environments, and may inspire next-generation marine robots equipped with slender structures.
Limitations of the study
In this study, only the stiffening mechanism of the setae of African shrimp under constant flow field is considered, and the resistance of the setae to deformation under random flow is not considered, which will be investigated in the following work.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Atya gabonensis (Decapoda: Atyidae) samples | Collected from an aquarium market in Guangzhou, Guangdong, China | N/A |
| Software and algorithms | ||
| Ansys 18.2 | Ansys Inc. | https://www.ansys.com/ |
| MATLAB r2019b | Mathworks Inc. | https://www.mathworks.com/ |
| Tracker 6.0.9 | Tracker Inc. | https://physlets.org/tracker/ |
| Other | ||
| SEM | HITACHI | SU8010 |
| CLSM | Carl Zeiss Microscopy GmbH | LSM880 |
| AFM | Bruker Daltonics Inc. | RTESP-300 |
| EDS | AZtecLive | Ulive Maxa 100 |
| Micro-CT | ZEISS Xradia | 510VERSA |
| Microscope | Olympus | CX33 |
| Speed-regulating pump | Smart Xiaoli | XDP-7500 |
| Current meter | Kuju | LS300-A |
| High-speed camera | Vision Research Inc. | Phantom VEO-E 310L |
Resource availability
Lead contact
Further information requests should be directed to the lead contact, Associate Professor Jianing Wu (wujn27@mail.sysu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
We collected Atya gabonensis (Decapoda: Atyidae) samples in body lengths of about 6–10 cm from an aquarium market in Guangzhou, Guangdong, China, and fed them in a shrimp tank (360 mm × 200 mm×220 mm) in Sun Yat-sen University, Guangzhou, China. The gravels were placed at the bottom of the shrimp tank for the attachment of the African shrimps to stabilize their bodies during filtration. A heater (25 W) was used to keep the water temperature at 25°C and A filter pump (with power p = 3 W and flow rate Q = 400 L/h) was used to generate water flow in the tank. We confirm that no special permission is required for these activities and that the research does not involve any endangered or protected species.
Method details
Calculation of aspect ratio of setae
The freshly-cut chela was immersed in a culture dish with MgCl2 solution (s = 10% wt./wt.) for 2 h to relax the muscle fibers and allow the setae to fully unfold.45 Then we put the chela under the microscope (Olympus, CX33, Japan) and divided the setae evenly into five regions in the circumferential direction. We used surgical scissors to extract 15 setae from each region with caution, and subsequently placed them under the SEM to gauge the length of the setae and the dimensions of their cross-section at the midpoint. We use to define the aspect ratio of the sample setae, where (i = I,II,III,IV,V, j = 1,2, …,15) is the length of the setae in the corresponding region (Figure 2B) and (i = I,II,III,IV,V) is the average diameter of the equivalent circular cross-section of the middle section of the setae in the corresponding region (Figure S8).
Stability test of the setae on living sample
Living African shrimps were placed in a circular tank, starved for 24 h, and induced to filter feed by adding wheat stalk fungus. To create a steady flow of different velocities, we used a speed-regulating pump (Smart Xiaoli XDP-7500, China) and a damping screen. The velocity in the circular tank was detected using a current meter (Kuju LS300-A, China). A high-speed camera (Phantom VEO-E 310L, USA) was then used to record deformation of the setae in region V at different flow rates. Then the Tracker 6.0.9 was used to extract the deflections of the three setae in the middle of the region V, and the average value of these three setae represented the amount of setae deformation.
AFM test
In order to measure Young’s modulus of setae on chela of the African shrimp, a strand of fresh seta sample was cut from the chela and examined under an atomic force microscopy (AFM, Bruker, Dimension Fast Scan Bio, Germany) equipped with two types of probes (RTESP-300 and PFQNM-SMPKIT-12M, Bruker).46 We tested 40 points uniformly distributed on one seta. All statistics were performed in the software NanoScope Analysis 2.0.
EDS measurement
A chela was subjected to immersion in a 2.5% glutaraldehyde solution for 12 h at a temperature of 4°C.The chela was subsequently removed from the glutaraldehyde solution and the sample was rinsed three times with the phosphate buffer (0.1 mol/L, PH = 7) for 15 min each. The samples were fixed with 1% osmic acid solution for 1–2 h. After removed from the osmic acid solution, the samples were rinsed with phosphate buffer 3 times (15 min for each). The samples were dehydrated with ethanol solutions of gradient concentrations (including 30%, 50%, 70%, 80%, 90% and 95%) for 15 min of each concentration and then treated twice with 100% ethanol for 20 min each time. The samples were next treated with a mixture of ethanol and amyl acetate-iso (volume/volume = 1/1) for 30 min, and then treated with pure amyl acetate-iso for 1h or left overnight. The processed samples were detected by energy spectrometer (AZtecLive Ulive Maxa 100, UK). We turned to look into a length of 100 μm in the middle of a randomly-selected seta to test the elements of the seta (Figure S2). Scanned by the EDS, we obtained the element composition of the seta and the signal (cps) intensity of each element in the detection area (Figure S2).
CLSM observation
Bundles of setae were freshly excised from the chela of African shrimp and placed on slides containing glycerin and compacted with a coverslip, and then visualized with a confocal laser scanning microscopy (CLSM) (LSM 880, Carl Zeiss Microscopy, Germany) equipped with four stable solid-state lasers (laser lines: 405, 488, 561, and 633 nm) and four emission filters (410 BP−500, 490 BP−579, 570 BP−624, and 638 BP−747 nm) to collect the autofluorescence emitted by the samples.46 The material composition of the setae was characterized based on the autofluorescence of the samples. Colors were chosen according to the protocol suggested by Michels and Gorb,47 namely blue (excitation = 405 nm and emission = 420−480 nm), green (excitation = 488 nm and emission≥490 nm), and red (two settings: excitation = 555 nm and emission≥560 nm, and excitation = 639 nm and emission ≥560 nm).
Particle capture experiment of bio-inspired filtration device models
We poured water into the circulating water channel (2.3 m × 0.8 m x 0.16 m), and filling it up to 2/3 of its total volume, then added the nylon particles (d = 0.6 mm) into the water and used a submersible pump to create a flow rate v = 40 mm/s to make the nylon particles uniformly suspended in the water to form a suspension with moderate concentration (8.45 x 10−4 g/mL). The bio-inspired filtration device was then glued to the link block with adhesive tape and immersed in the suspension (Figure 7A). After filtering particles for 20 s, the bio-inspired filtration device was placed in a paper cup with water to detach the nylon particles from the device. We counted the number of nylon particles in the paper cup. According to the above methods, 7 bio-inspired filtration devices with different Young’s modulus were placed into suspension successively for particle capture experiments (Figures S6 and S7). Each bio-inspired filtration device captured particles 5 times, and then its particle capture capacity was expressed in the form of mean ± standard deviation of the number of captured particles.
Micro-CT scan of the chela
We soaked the chela in 8% solution for 1 h, and then removed the setae on the chela gently by tweezers under the microscope (Olympus, CX33, Japan). The 3D model of the chela was reconstructed by the Micro computed tomography (ZEISS Xradia 510VERSA, Germany), and then the software Mimics 17 (Materialise Inc.) was used to analyze and calculate the 3D model data.
Fluid-structure interaction (FSI) simulation
We constructed simulation models of setae and imported them into Ansys 18.2 for 3D fluid-structure interaction (FSI) simulations. In order to ensure the accuracy of simulation, we set a large enough computational domain (Figure S4), a long enough computing time, and carried out mesh verification (Figure S5). The fluid-structure interaction simulations were conducted corresponding to the setae with different cross-sections, numbers and twisted angles. Further information on simulation methods and grid verification is provided in Note S2 and Figures S4 and S5.
Quantification and statistical analysis
This study does not include statistical analysis or quantification.
Additional resources
This study does not have any additional resources.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 52275298, No. 51905556), and Shenzhen Science and Technology Program (Grant No. 20220817165030002 and No. GXWD2021B03).
Author contributions
Y.L., J. Wang, Z.W., and J. Wu conceived the project and designed the experiments. J. Wang and J.L. performed the flexing test of setae. Y.L. and J. Wang performed SEM and CLSM observation. Y.L. performed AFM test, EDS measurement, biological tissue section experiment, and Micro-CT scan. Y.L., J.L., and J. Wang conducted the data analysis and statistics. Y.L. performed the CFD analysis. J.L. performed the mathematical model. Y.L. and J. Wu wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: July 21, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107444.
Contributor Information
Yifeng Liao, Email: liaoyf26@mail2.sysu.edu.cn.
Zhigang Wu, Email: wuzhigang@mail.sysu.edu.cn.
Jianing Wu, Email: wujn27@mail.sysu.edu.cn.
Supplemental information
Data and code availability
-
•
Original data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
References
- 1.Giovannoni S.J., Stingl U. Molecular diversity and ecology of microbial plankton. Nature. 2005;437:343–348. doi: 10.1038/nature04158. [DOI] [PubMed] [Google Scholar]
- 2.Ren L., Ma J., Chen M., Qiao Y., Dai R., Li X., Wang Z. Recent advances in electrocatalytic membrane for the removal of micropollutants from water and wastewater. iScience. 2022;25:104342. doi: 10.1016/j.isci.2022.104342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vidiella B., Solé R. Ecological firewalls for synthetic biology. iScience. 2022;25:104658. doi: 10.1016/j.isci.2022.104658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chislock M.F., Doster E., Zitomer R.A., Wilson A.E. Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nature Educ. Knowledge. 2013;4:10. [Google Scholar]
- 5.Godefroy N., Le Goff E., Martinand-Mari C., Belkhir K., Vacelet J., Baghdiguian S. Sponge digestive system diversity and evolution: filter feeding to carnivory. Cell Tissue Res. 2019;377:341–351. doi: 10.1007/s00441-019-03032-8. [DOI] [PubMed] [Google Scholar]
- 6.Satoh N. Nature Publishing Group; 2019. A Deep Dive into the Development of Sea Squirts. [DOI] [PubMed] [Google Scholar]
- 7.Jokura K., Sato Y., Shiba K., Inaba K. Two distinct compartments of a ctenophore comb plate provide structural and functional integrity for the motility of giant multicilia. Curr. Biol. 2022;32:5144–5152.e6. doi: 10.1016/j.cub.2022.09.061. [DOI] [PubMed] [Google Scholar]
- 8.Kelley N.P., Irmis R.B., DePolo P.E., Noble P.J., Montague-Judd D., Little H., Blundell J., Rasmussen C., Percival L.M.E., Mather T.A., Pyenson N.D. Grouping behavior in a Triassic marine apex predator. Curr. Biol. 2022;32:5398–5405.e3. doi: 10.1016/j.cub.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Yamamori L., Kato M. Shift of feeding mode in an epizoic stalked barnacle inducing gall formation of host sea urchin. iScience. 2020;23:100885. doi: 10.1016/j.isci.2020.100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fordyce R.E., Marx F.G. Gigantism precedes filter feeding in baleen whale evolution. Curr. Biol. 2018;28:1670–1676.e2. doi: 10.1016/j.cub.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Cade D.E., Kahane-Rapport S.R., Gough W.T., Bierlich K.C., Linsky J.M.J., Calambokidis J., Johnston D.W., Goldbogen J.A., Friedlaender A.S. Minke whale feeding rate limitations suggest constraints on the minimum body size for engulfment filtration feeding. Nat. Ecol. Evol. 2023;7:535–546. doi: 10.1038/s41559-023-01993-2. [DOI] [PubMed] [Google Scholar]
- 12.Letendre F., Cameron C.B. The capture of crude oil droplets by filter feeders at high and low Reynolds numbers. J. Exp. Biol. 2022;225:jeb243819. doi: 10.1242/jeb.243819. [DOI] [PubMed] [Google Scholar]
- 13.Matthews L.H. Filter Feeding in Flamingoes. Nature. 1957;180:128–129. [Google Scholar]
- 14.Ou Q., Shu D., Zhang Z., Han J., Van Iten H., Cheng M., Sun J., Yao X., Wang R., Mayer G. Dawn of complex animal food webs: A new predatory anthozoan (Cnidaria) from Cambrian. Innovation. 2022;3 doi: 10.1016/j.xinn.2021.100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerosey-Aubril R., Pates S. New suspension-feeding radiodont suggests evolution of microplanktivory in Cambrian macronekton. Nat. Commun. 2018;9:3774. doi: 10.1038/s41467-018-06229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riisgård H., Larsen P.S. Particle capture mechanisms in suspension-feeding invertebrates. Mar. Ecol. Prog. Ser. 2010;418:255–293. doi: 10.3354/meps08755. [DOI] [Google Scholar]
- 17.Repka S., Veen A., Vijverberg J. Morphological adaptations in filtering screens of Daphnia galeata to food quantity and food quality. J. Plankton Res. 1999;21:971–989. doi: 10.1093/plankt/21.5.971. [DOI] [Google Scholar]
- 18.Repka S., Veselá S., Weber A., Schwenk K. Plasticity in filtering screens of Daphnia cucullata× galeata hybrids and parental species at two food concentrations. Oecologia. 1999;120:485–491. doi: 10.1007/s004420050881. [DOI] [PubMed] [Google Scholar]
- 19.Riisgård H.U., Thiel M., Watling L. Vol. 2. 2015. Filter-feeding mechanisms in crustaceans. Life styles and feeding biology; pp. 418–463. (The natural history of the Crustacea). [Google Scholar]
- 20.Carvalho-Batista A., Oliveira C.M., Souza G., Carvalho F.L., Mantelatto F.L. Morphometric aspects of two coexisting amphidromous shrimps, Atya gabonensis Giebel, 1875 and Atya scabra (Leach, 1816), in the Paraíba do Sul River, Brazil. Nauplius. 2021;29 doi: 10.1590/2358-2936e2021018. [DOI] [Google Scholar]
- 21.Hobbs H.H., Jr. 1982. The Shrimp Genus Atya (Decapoda: Atyidae) [Google Scholar]
- 22.Obande R., Kusemiju K. Food and feeding habits of Atya gabonensis from Lower River Benue in Northern Nigeria. West African Journal of Applied Ecology. 2008;13:77–82. doi: 10.4314/wajae.v13i1.40582. [DOI] [Google Scholar]
- 23.Lazarus B.S., Chadha C., Velasco-Hogan A., Barbosa J.D.V., Jasiuk I., Meyers M.A. Engineering with keratin: A functional material and a source of bioinspiration. iScience. 2021;24:102798. doi: 10.1016/j.isci.2021.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fryer G. X.—The Feeding Mechanism of Some Atyid Prawns of the Genus Caridina. Trans. R. Soc. Edinb. Earth Sci. 1960;64:217–244. doi: 10.1017/S0080456800100225. [DOI] [Google Scholar]
- 25.Meyer R. lmu; 2014. Integrative Taxonomy of Decapod Crustaceans with Traditional and Modern Methods. [Google Scholar]
- 26.Madeleine K.A.M., Gervais N., Gouli G.B. Weight-length relationships and condition factor of Atya gabonensis Giebel, 1875 in Bandama River-Côte d’Ivoire. Int. J. Fish. Aquat. Stud. 2015;3:283–286. [Google Scholar]
- 27.Nwosu F.M. Aspects of biology of the Gabon shrimp Atya gabonensis, Giebel, 1875 in the Cross River, Nigeria. J. Fisheries International. 2009;4:58–61. doi: 10.3923/jfish.2009.58.61. [DOI] [Google Scholar]
- 28.Cohen K.E., George A.E., Chapman D.C., Chick J.H., Hernandez L.P. Developmental ecomorphology of the epibranchial organ of the silver carp, Hypophthalmichthys molitrix. J. Fish. Biol. 2020;97:527–536. doi: 10.1111/jfb.14409. [DOI] [PubMed] [Google Scholar]
- 29.Dawdry N.E. 2004. Diel Vertical Migration and Feeding by Krill Meganyctiphanes Norvegica. [Google Scholar]
- 30.Loch C., Vaz Viegas S., Waddell J.N., Kemper C., Cook R.B., Werth A.J. Structure and properties of baleen in the Southern right (Eubalaena australis) and Pygmy right whales (Caperea marginata) J. Mech. Behav. Biomed. Mater. 2020;110 doi: 10.1016/j.jmbbm.2020.103939. [DOI] [PubMed] [Google Scholar]
- 31.Ribelin B.W., Collier A. Studies on the gill ciliation of the American oyster Crassostrea virginica (Gmelin) J. Morphol. 1977;151:439–449. doi: 10.1002/jmor.1051510308. [DOI] [PubMed] [Google Scholar]
- 32.Riisgård H., Grémare A., Amouroux J., Charles F., Vétion G., Rosenberg R., Nielsen C. Comparative study of water-processing in two ciliary filter-feeding polychaetes (Ditrupa arietina and Euchone papillosa) from two different habitats. Mar. Ecol. Prog. Ser. 2002;229:113–126. doi: 10.3354/meps229113. [DOI] [Google Scholar]
- 33.Rogers D.C., Olesen J., Weaver J., Quinney D.L. A new giant species of predatory fairy shrimp from Idaho, USA (Branchiopoda: Anostraca) J. Crustac Biol. 2006;26:1–12. doi: 10.1651/C-2509.1. [DOI] [Google Scholar]
- 34.Vo M., Mehrabian S., Villalpando F., Etienne S., Pelletier D., Cameron C.B. The fluid dynamics of Balanus glandula barnacles: Adaptations to sheltered and exposed habitats. J. Biomech. 2018;71:225–235. doi: 10.1016/j.jbiomech.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y., Zhao P., Cai X., Rong J., Dong Z., Chen H., Wu P., Hu H., Jin X., Zhang D., Liu H. Bristled-wing design of materials, microstructures, and aerodynamics enables flapping flight in tiny wasps. iScience. 2022;25:103692. doi: 10.1016/j.isci.2021.103692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Megill W.M. University of British Columbia; 2002. The Biomechanics of Jellyfish Swimming. [Google Scholar]
- 37.Knight-Jones P., Mackie A.S.Y. A revision of Sabellastarte (Polychaeta: Sabellidae) J. Nat. Hist. 2003;37:2269–2301. doi: 10.1080/00222930110120629. [DOI] [Google Scholar]
- 38.Szewciw L.J., de Kerckhove D.G., Grime G.W., Fudge D.S. Calcification provides mechanical reinforcement to whale baleen α-keratin. Proc. Biol. Sci. 2010;277:2597–2605. doi: 10.1098/rspb.2010.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumiller T.K., Labarbera M. Mechanical properties of the stalk and cirri of the sea lily Cenocrinus asterius. Comp. Biochem. Physiol. Physiol. 1993;106:91–95. doi: 10.1016/0300-9629(93)90045-6. [DOI] [Google Scholar]
- 40.Aldred N., Wills T., Williams D.N., Clare A.S. Tensile and dynamic mechanical analysis of the distal portion of mussel (Mytilus edulis) byssal threads. J. R. Soc. Interface. 2007;4:1159–1167. doi: 10.1098/rsif.2007.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aluma Y., Ilan M., Sherman D. Comments on a skeleton design paradigm for a demosponge. J. Struct. Biol. 2011;175:415–424. doi: 10.1016/j.jsb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan T.N., Pissarenko A., Herrera S.A., Kisailus D., Lubarda V.A., Meyers M.A. A lightweight, biological structure with tailored stiffness: The feather vane. Acta Biomater. 2016;41:27–39. doi: 10.1016/j.actbio.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W., Xu J., Yu T. Dynamic behaviors of bio-inspired structures: Design, mechanisms, and models. Eng. Struct. 2022;265 doi: 10.1016/j.engstruct.2022.114490. [DOI] [Google Scholar]
- 44.Yuk J., Chakraborty A., Cheng S., Chung C.-I., Jorgensen A., Basu S., Chamorro L.P., Jung S. On the design of particle filters inspired by animal noses. J. R. Soc. Interface. 2022;19 doi: 10.1098/rsif.2021.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Namba K., Kabayashi M., Aida S., Uematsu K., Yoshida M., Kondo Y., Miyata Y. Persistent relaxation of the adductor muscle of oyster Crassostrea gigas induced by magnesium ion. Fish. Sci. 1995;61:241–244. doi: 10.2331/fishsci.61.241. [DOI] [Google Scholar]
- 46.Wei J., Liang Y., Chen X., Gorb S.N., Wu Z., Li H., Wu J. Enhanced flexibility of the segmented honey bee tongue with hydrophobic tongue hairs. ACS Appl. Mater. Interfaces. 2022;14:12911–12919. doi: 10.1021/acsami.2c00431. [DOI] [PubMed] [Google Scholar]
- 47.Michels J., Gorb S.N. Detailed three-dimensional visualization of resilin in the exoskeleton of arthropods using confocal laser scanning microscopy. J. Microsc. 2012;245:1–16. doi: 10.1111/j.1365-2818.2011.03523.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Original data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.