Abstract
Hyperpigmentation disorders, such as melasma and freckles, are highly prevalent and draw increasing attention. Patients thus tend to seek effective and safe cosmetic whitening agents. Fraxin, a bioactive substance extracted from Cortex Fraxini, possesses anti-inflammation and antioxidant properties. In this study, we further explored the anti-melanogenic activities of fraxin were explored in vitro and in vivo. We found that pretreatment with fraxin decreased the melanin content of MNT1 cells and zebrafishes. In MNT1 cells, melanogenesis-related proteins, such as MITF, TYR, TYRP1, and DCT were down-regulated and tyrosinase activity was reduced under fraxin treatment. Further exploration of the mechanism revealed that fraxin could inhibit the phosphorylation of ERK, which is closely related to melanogenesis. Besides, fraxin also protected MNT1 cells from H2O2-induced apoptosis via scavenging reactive oxygen species (ROS) in cells. Further experimentation revealed that fraxin could activate NRF2 and upregulate antioxidase CAT and HO-1. In conclusion, fraxin could be an effective agent with anti-melanogenesis and antioxidant properties for hyperpigmentation disorders.
Keywords: Fraxin, Melanogenesis, ROS, ERK, NRF2
1. Introduction
Melanin protects the skin from the environmental ultraviolet irradiation, but its abnormal production and accumulation contribute to hyperpigmentation diseases, such as melasma and freckles [1,2]. Those diseases are difficult to treat and are prone to recur in clinical practice, seriously affecting the physical and mental health of patients. Patients are eager to find effective drugs to treat these diseases. Traditional skin anti-browning agents, such as kojic acid [3,4], hydroquinone [5], and arbutin [6], can inhibit the activity of tyrosinase (TYR) from achieving a lightning effect. However, their irritation to the skin cannot be ignored. In the wake of much more attention to appearance nowadays, safe and effective skin whitening products need to be developed.
Melanin is mainly synthesized in human epidermal melanocytes; it is a complex biochemical process regulated by several enzymes. TYR is a key rate-limiting enzyme of this process, catalyzing l-tyrosine to L-3,4-dihydroxyphenylalanine (l-DOPA) and further to DOPA-quinone, which then changes into melanin by the catalysis of tyrosinase-related protein 1 (TYRP1) and tyrosinase-related protein 2 (TYRP2, also named DCT). TYR, TYRP1, and DCT are all regulated by microphthalmia-transcription factor (MITF), a central transcription factor for melanogenesis [7]. Those genes are regulated by several singling pathways. Including the mitogen-activated protein kinase (MAPK) pathway [8].
Fraxin, a traditional Chinese medicine, is mainly extracted from Cortex Fraxin. Fraxin prevented ischemia/reperfusion-induced acute kidney and acute respiratory distress syndrome through its antioxidant and anti-inflammatory effect [9,10]. It also exerted strong protective activity against hepatotoxicity by inhibiting inflammation response, oxidative stress, and lipid peroxidation [11,12]. Until now, there has been no study on the effects of fraxin on melanogenesis and antioxidants in pigment cells. In this study, we evaluated the role and mechanisms of action of fraxin in pigmentation and antioxidation.
2. Materials and methods
2.1. Cell lines and their culture
MNT1 melanoma cell line enriched in melanin were cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone, UT) with fetal bovine serum (20%) and penicillin-streptomycin (1%, Gibco). The cells were cultured in an incubator, at 37 °C, in the presence of 5% CO2.
2.2. Antibodies and chemicals
The primary antibodies of MITF, GAPDH, TYR, TYRP1, DCT, ras-related protein Rab-27A (RAB27A), nuclear factor E2-related factor 2 (NRF2), catalase (CAT), and heme oxygenase 1 (HO-1) were purchased from Cell Signaling Technology. The primary antibodies for BCL2 and BAX were from ZEN-BIOSCIENCE. Fraxin was purchased from Chemface (Hubei, China) and was stored at −20 °C after dissolving in dimethyl sulphoxide (DMSO) at 320 mM concentration. ML385 was purchased from Adooq Bioscience. Hydrogen peroxide (H2O2), DMSO, and Cell Counting Kit-8(CCK8) were procured from Biosharp (Hefei, China). The Fontana-Masson Stain Kit was purchased from G-CLONE. Hoechst staining solution (100 × ) was purchased from Beyotime Company.
2.3. Cell viability assay
CCK8 regents were used to evaluate cell viability. MNT1 cells were plated at 5 × 103 cells/well (96-well plates) and treated with gradient concentrations of fraxin at 50% confluency for 24 h, 48 h, and 72 h. Then the culture medium was removed, and CCK8 solution were added to 96-well plates. Cells were placed in a 37 °C incubator for 30 min, and the absorbance was detected at 450 nm using a multimode plate reader (PerkinElmer).
In antioxidation assessment, the cells were treated with different concentrations of H2O2 to simulate cellular oxidative stress. To determine the suitable concentration of fraxin, cells were pretreated with various concentrations of fraxin for 24 h, then H2O2 was added to the culture medium and incubated for another 24 h before detecting the cell viability.
2.4. Zebrafish experiment
Zebrafish embryos and culture medium were purchased from EzeRinka Biotech. The protocol was approved by the Ethics Committee of Central South University (NO.2021-S137). The zebrafish eggs were incubated and then treated with fraxin at different concentrations for 5 days. The melanin pigments of zebrafish were observed and photographed with an inverted microscope, the culture medium was replaced each day. The melanin content was evaluated by the software of ImageJ.
2.5. Fontana-Masson melanin staining
MNT1 cells were treated with fraxin at varying concentrations in a 6-well plate for 48 h and fixed for 15 min with paraformaldehyde. The cells were washed with distilled water for 5 min (repeat five times) and then incubated with Fontana ammonia-silver solution at 55 °C away from light for 30 min. Next, cells were given a hyposulphite (500 μL) wash for 5 min. After discarding hyposulphite, distilled water was added. Finally, the melanin was detected with an inverted microscope.
2.6. Measurement of tyrosinase activity
MNT1 cells were cultured in a 100-mm Petri dish and given fraxin treatment for 48 h. Cells were washed with phosphate-buffered saline (PBS) after being digested and centrifuged, and then 106 cells were counted for the sample in every group. After incubation for 10 min, the samples were placed at 37 °C for 15 min. Per sample, l-DOPA (1 mL of 1% solution) was added, and then 200 μL was removed and placed in a 96-well plate. Absorbance was detected at 475 nm with a multimode plate reader.
2.7. Extraction of protein and western blotting
The cells were grown in Petri dish (100-mm) and treated with fraxin for 48 h. The supernatant was discarded and washed twice with PBS. Then, 200 μL of the RIPA lysis buffer (Biosharp) containing with 1 mM phenylmethylsulphonyl fluoride (Thermo Fisher) and 1 mM diluted phosphatase inhibitor cocktail (Roche) was added to each group. To each well, proteins were added to be separated by electrophoresis using a 10% sodium dodecyl sulfate-polyacrylamide gel and then transferred to a polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were blocked with BSA (5%) and incubated with (primary) antibodies. Membranes were washed with Tris-buffered solution (with Tween 20) (TBS-T). After being washed thrice for 10 min each, PVDF membranes were incubated for 60 min with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G at room temperature. After washing thrice with TBS-T, the proteins bands on the PVDF membranes were detected by chemiluminescence.
2.8. Cell apoptosis measurement
2.8.1. Flow cytometry
MNT1 cells were pretreated with various concentrations of fraxin (20, 40, 80 μM) for 24 h. After being treated with H2O2 (1 mM) for 24 h, cells were trypsinized (ethylenediamine tetraacetate-free trypsin). Then the cells were washed twice with PBS, and 100 μL PBS was added to resuspend them. The cells were incubated with propidium iodide and Annexin IV for 15 min and then detected by a flow cytometer. The apoptosis rate was analyzed by Flowjo software.
2.8.2. Hoechst 33825 nuclear assay
The cells were given fraxin treatment as above in a 6-well plate and fixed with paraformaldehyde for 30 min. Cells were washed three times with PBS and stained with Hoechst 33825 stain for 10 min. After washed with PBS for three times, the fluorescence was observed under an inverted microscope.
2.9. Reactive oxygen species detection
MNT1 cells were pretreated with fraxin in a plate (6-wells) for 24 h and treated with H2O2 (1 mM) for another 24 h. Then 2′-7′dichlorofluorescin diacetate (DCFH-DA) probe was diluted to 1:1000 with DMEM and added to the 6-well plate and incubated for 1 h. The cells were given PBS wash five times and observed by an fluorescent microscope.
2.10. Immunofluorescence
The cells in 6-well plate were fixed with neutral paraformaldehyde (4%) for 30 min. After those cells were permeabilized with 0.3% Triton-100 for 30 min, they were washed three times with PBS, each time for 5 min. Then the antigens were blocked using BSA (5%) for 1 h, and incubated overnight with NRF2 primary antibody at 4 °C. On the second day, cells were washed thrice with PBS, each time for 5 min, and then incubated with secondary antibody (fluorochrome-conjugated) for 1 h. Finally, nuclei of cells were DAPI (4′,6 - diamidino 2 - phenylindole) - stained, and observed with an fluorescennt microscope.
2.11. Statistical analysis
In this study, the data were presented as mean ± standard deviation. For statistical analysis, GraphPad Prism was used. Statistical comparisons between multiple groups were conducted using ordinary one-way ANOVA, and student-t test was used in comparison between two samples. P < 0.05 was considered statistically significant. *p < 0.05, **p < 0.01.
3. Results
3.1. Fraxin inhibited melanogenesis in MNT1 cells
In this study, we first used the CCK8 kit to evaluate the effects of the fraxin on cell viability. MNT1 was cultured with varying concentrations of fraxin (from 2.5 to 640 μM) for 24, 48, or 72 h each. No significant change was observed when treated with a low concentration of fraxin (from 2.5 to 320 μM); however, the MNT1 viability decreased significantly when treated with 640 μM fraxin for 48 h and 72 h (Fig. 1a). Therefore, we used less than 640 μM of fraxin in the subsequent experiments.
Fig. 1.
Melanogenesis in MNT1 cells under fraxin treatments. (a) MNT1 cells were cultured with fraxin at varying concentrations for 24, 48, and 72 h, respectively, and the cytotoxicity was detected with CCK8 assay. MNT1 cells were cultured with fraxin for 48 h, then (b) the melanin content was observed by Fontana-Masson staining, (c) the tyrosinase (TYR) activity was detected, (d) the levels of melanogenesis-related protein (TYR, MITF, TYRP1, RAB27A, DCT), and (e) p-ERK, ERK, p-JNK, JNK, p-p38, and p38 were detected by western blotting. *p < 0.05, **p < 0.01.
Then, the effect of fraxin on melanogenesis was explored. MNT1 cells were given fraxin treatment at gradient concentrations (0, 40, 80, 160, 320 μM) for 48 h, and melanin staining was performed. The staining became weaker as the concentration of fraxin increased, suggesting that fraxin attenuated melanin production (Fig. 1b).
TYR is the key rate-limited enzyme for melanogenesis. As we measured the TYR activity. TYR activity decreased when cells were treated with fraxin (Fig. 1c). Meanwhile, western blotting assay was performed to determine protein levels of genes related to melanogenesis (TYR, TYRP1, DCT, and RAB27A), results showed down-regulated expression of these protein (Fig. 1d). Statistical results were displayed in supplementary materials (Fig. S1). Thus, fraxin decreased melanin production by inhibiting TYR activity and downregulated melanogenesis-related proteins.
To further explore mechanisms of inhibition of melanin content due to fraxin, protein from MNT1 cells was harvested after treatment with fraxin for 48 h, and then the changes in the MAPK signaling pathway were explored. We found down-regulated levels of phosphorylated protein of ERK (p-ERK), although phosphorylated c-Jun N-terminal kinase (JNK) and p38 did not change significantly (Fig. 1e). The corresponding quantitative statistical results were showed in Fig. S2.
3.2. Fraxin reduced melanin production in zebrafishes
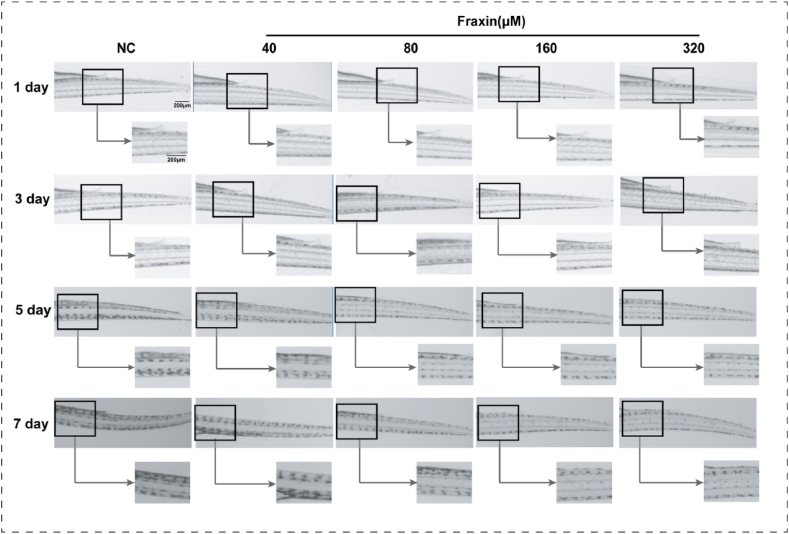
To verify the inhibitory effect of fraxin on melanogenesis, zebrafishes were treated with fraxin for 7 days. The melanin granules in zebrafish were observed every day. The melanin granules of all groups were almost the same at first 3 days. Then, as the zebrafishes grew, melanin density in the tails increased gradually, but was relatively lower in fraxin-treated groups, the changes showed a concentration-dependent manner (Fig. 2 and Fig. S3).
Fig. 2.
Inhibition of melanogenesis in zebrafishes after fraxin treatments. The zebrafish embryos were hatched and treated with fraxin at varying concentrations (40, 80, 160, and 320 μM) or zebrafish medium respectively (NC), and the melanin of zebrafish was observed by an inverted microscope for 7 days.
3.3. Fraxin protected MNT1 cells from H2O2-induced cytotoxicity
The antioxidative effect of fraxin on cells was also explored. The suitable concentration of H2O2 in stimulating apoptosis was assessed by CCK8 assay, and 1 mM H2O2 was used in subsequent experiments because cell damage was obvious at this concentration (Fig. S4a). Then the optimum concentration of fraxin was also explored with CCK8 assay. For this, MNT1 cells were given fraxin pretreatment at varying concentrations (2.5–640 μM) for 24 h before adding 1 mM H2O2. Fraxin, at 20, 40, and 80 μM concentrations, provided the best protection against the damage caused by H2O2 (Fig. S4b). The cell morphology of MNT1 cells contracted and floated after adding H2O2, while pretreatment with fraxin alleviated this situation (Fig. 3a). To investigate whether fraxin alleviated H2O2-induced damage by reducing apoptosis, flow cytometry was performed to detect the apoptotic rate. There was a significant increase in the rate of apoptosis in the H2O2 group, but decreased when pretreated with fraxin for 24 h prior to H2O2 treatment (Fig. 3b). Hoechst 33258 staining was used to evaluate the nuclei morphology during apoptosis. After treatment with H2O2, the characteristic of apoptosis - nuclear pyknosis and nuclear fragmentation - increased, and fraxin pretreatment reduced this change (Fig. 3c). The apoptosis related proteins of BCL2 and BAX were determinmined by western blotting. Result showed a decrease in BAX and increased in BCL2 in fraxin treated cell compared to H2O2(Fig. 3d-e). These findings indicated that pre-treatment with fraxin could alleviated the H2O2-induced cytotoxicity and apoptosis.
Fig. 3.
Apoptosis of MNT1 cells under H2O2and fraxin treatments. MNT1 cells were pretreated with fraxin at varying concentrations (0, 20, 40, and 80 μM) for 24 h and then treated with H2O2 (1 mM) for 24 h, (a) the cell morphology was observed by an inverted microscope, (b–c) the apoptosis rate was detected by flow cytometry and Hoechst staining. (d) The levels of BCL2 and BAX proteins were detected by western blotting (e) and quantitative analyzed. *p < 0.05, **p < 0.01.
3.4. Fraxin decreased H2O2-induced oxidatvie stress in MNT1 cells
To determine how fraxin antagonizes H2O2-induced cytotoxicity and apoptosis, the levels of cellular ROS in MNT1 were detected. As detected, ROS levels in H2O2-treated (1 mM) cells were increased, but pretreatment with fraxin alleviated ROS generation (Fig. 4a). Furthermore, we detected the nucleus translocation of NRF2 by immunofluorescence and found that the levels of nuclear NRF2 in the fraxin pretreatment group was higher than that in H2O2-treated group, suggesting that fraxin enhanced nuclear translocation of NRF2 (Fig. 4b). Furthermore, western blotting revealed that fraxin pretreatment promoted NRF2 expression and downstream antioxidase HO-1 and CAT (Fig. 4c and d).
Fig. 4.
The changes of ROS and antioxidant proteins in H2O2-treated MNT1 cells under fraxin treatments. MNT1s were given fraxin pretreatment at varying concentrations (0, 20, 40, and 80 μM) for 24 h, then treated with H2O2 (1 mM) for 24 h. (a) The intracellular level of ROS was detected by DCFH-DA probes, (b) the expression and location of NRF2 was measured by immunofluorescence. (c) The expression of NRF2, CAT, and HO-1 protein was measured by western blotting, and (d) quantitative analyzed. *p < 0.05, **p < 0.01.
3.5. Fraxin inhibited UVB-induced melanogenesis by scavenging intracellular ROS
In this study, we found that fraxin had both antioxidant and anti-melanogenesis abilities, so we hypothesized that fraxin may reduce melanin synthesis through scavenging ROS. UVB exposure can lead to ROS production and melanogenesis in skin melanocytes. According to our previous study, treated with UVB at 30 mJ/cm2 for 2 days could induce melanin production without affecting cell viability. Therefore, we used UVB at 30 mJ/cm2 for 2 days to induce melanogenesis. As we found, the levels of intracellular ROS in MNT1 cells were increased after UVB irradiation (Fig. S5). After UV irradiation, melanin content and ROS in MNT1 cells were indeed increased, but that could be decreased by fraxin pretreatment (Fig. 5a–b). However, NRF2 inhibitor (ML385) could reverse the effects of fraxin by reducing the expression of CAT and HO-1 and ROS scavenging (Fig. 5a–c). These results suggest that fraxin may reduce melanogenesis through its antioxidant ability.
Fig. 5.
The role of fraxin on melanogenesis and intracellular ROS in UVB-treated MNT1 cells. (a) MNT1 cells was pretreatment with fraxin for 1 day, then treated with UVB (30 mJ/cm2) and ML385 (20 μM) for 2 days, (a) the melanin content was observed by Fontana-Masson staining, (b) the level of intracellular ROS was observed by DCFH-DA fluorescencent probes, (c) the expression of NRF2, CAT, and HO-1 proteins was measured by western blotting. (d) The schematic diagram of the molecular mechanism.
4. Discussion
The pursuit of agents and safe agents, with dual effects of antioxidant and anti-melanogenesis, has become a trending research topic. In this study, we comfirmed the inhibitory effects of fraxin on melanogenesis and oxidative stress in MNT1 cells for the first time, also revealed that fraxin can suppress the ERK/MAPK signaling pathway and activating NRF2 signaling pathway.
The anti-melanogenesis ability of fraxin was observed in both cells and zebrafishes. As we found in MNT1 cells, fraxin could increase the expression of melanogenesis-related proteins, including MITF, TYR, TRP1, and DCT. RAB27A is an another important protein for melanosomes transport that regulated by MITF [13]. In our study, fraxin suppressed the expression of MITF and its target genes and further affected the melanin production in MNT1 cells. More important, TYR, the rate-limiting enzyme of melanogenesis, has become a main target for developing of skin whitening agents. In this study, its activity was significantly inhibited by fraxin.
ERK is a key member of the MAPK family. Phosphorylated ERK can activate MITF [14] to promote melanogenesis. Down-regulation of MAPK signaling is a promising way to develop agents for hyperpigmentation disorders [15]. In previous studies, some herbal extracts, such as selaginellin [16], isoliquiritigenin [17], can inhibited melanogenesis by deactivating ERK. Fraxin has also been reported to down-regulate ERK protein phosphorylation to protect hepatocytes from carbon tetrachloride (CCL4)-induced damage and to antagonize cerebral ischemia-reperfusion injury [11,18]. Our findings of fraxin's effects on ERK/MAPK in MNT1 cells are consistent with these reports, thus we speculate that fraxin may reduce ERK phosphorylation and further downregulate MITF.
ROS are important substances in cell functions. However, factors such as UV radiation can lead to excessive ROS production and destroy cell structures [19], while the antioxidant system is an important strategy to maintain the equivalence. To tackle oxidative stress, NRF2 will enter the nucleus and attach to the antioxidant response elements, activate the expression of antioxidant enzymes, including HO-1 and CAT, thus neutralize ROS and protect cells [20,21]. Previous studies have reported that fraxin could eliminate oxidative stress in diabetic renal fibrosis and CCL4-induced hepatoma cell injury by activating the NRF2/ARE pathway [9,12]. In this study, we found that fraxin could ameliorate cell apoptosis, it is possible by activating NRF2 pathways and scavenging intracellular ROS.
As mentioned above, ROS overproduction could also been triggered by UV radiation in skin melanocytes, which participate in activating MAPK signaling pathway and MITF-induced melanogenesis [22]. In addition, some natural substances, such as maclurin, and hesperidin have inhibitory effects on melanogenesis by reducing ROS [23,24]. Antioxidant genes such as forkhead Box O6 can inhibit oxidative stress by upregulating intracellular antioxidant genes (including the catalase gene), thus achieving the effect of melanogenesis inhibition [25]. In our study, fraxin could upregulate NRF2, CAT, and HO-1 expression and reduce ROS production, along with suppressing melenogenesis, but its anti-melanogenesis effect could be reversed by NRF2 inhibitor. Therefore, scavenging ROS may be another important way of fraxin to inhibit melanogenesis.
This study also has some shortcomings. Firstly, we did not verify the findings in primary human melanocytes. Secondly, in vivo experiments were not conducted in mice or human. In addition, our mechanism exploration is superficial and needs further reasoning in the future.
In summary, this study demonstrated that fraxin may reduce melanin production by suppressing ERK phosphorylation to further inhibit melanogenesis-related proteins and TYR activity. Fraxin could also eliminate excessive ROS-induced oxidative stress by activating NRF2 signaling pathway, and that is another possible way to reduce melanogenesis (Fig. 5d). Therefore, fraxin may serve as a safe and effective antioxidant and a skin-whitening agents.
Author contribution statement
Liping Luo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Xing Yu: Performed the experiments.
Hongliang Zeng, Yibo Hu, Jinhua Huang: Contributed reagents, materials, analysis tools or data.
Ling Jiang, Chuhan Fu: Analyzed and interpreted the data.
Jing Chen, Qinghai Zeng: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
Qinghai Zeng was supported by The Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University {YX202007}.
Qinghai Zeng was supported by The Science and Technology Innovation Program of Hunan Province {2021RC3035}.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18929.
Contributor Information
Jing Chen, Email: 43700351@qq.com.
Qinghai Zeng, Email: zengqinghai@csu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rachmin I., et al. Topical treatment strategies to manipulate human skin pigmentation. Adv. Drug Deliv. Rev. 2020;153:65–71. doi: 10.1016/j.addr.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slominski A., et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 3.Saeedi M., Eslamifar M., Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. = Biomedecine & pharmacotherapie. 2019;vol. 110:582–593. doi: 10.1016/j.biopha.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., et al. Anti-melanogenesis of novel kojic acid derivatives in B16F10 cells and zebrafish. Int. J. Biol. Macromol. 2019;123:723–731. doi: 10.1016/j.ijbiomac.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Bolognia J., et al. Enhancement of the depigmenting effect of hydroquinone by cystamine and buthionine sulfoximine. Br. J. Dermatol. 1995;133(3):349–357. doi: 10.1111/j.1365-2133.1995.tb02660.x. [DOI] [PubMed] [Google Scholar]
- 6.Boo Y. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants (Basel, Switzerland) 2021;10(7) doi: 10.3390/antiox10071129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slominski A., et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 8.D'Mello S.A., et al. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016;17(7) doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topdağı Ö., et al. Preventive effects of fraxin on ischemia/reperfusion-induced acute kidney injury in rats. Life Sci. 2020;242 doi: 10.1016/j.lfs.2019.117217. [DOI] [PubMed] [Google Scholar]
- 10.Ma X., et al. Fraxin alleviates LPS-induced ARDS by downregulating inflammatory responses and oxidative damages and reducing pulmonary vascular permeability. Inflammation. 2019;42(5):1901–1912. doi: 10.1007/s10753-019-01052-8. [DOI] [PubMed] [Google Scholar]
- 11.Niu X., et al. Hepatoprotective effect of fraxin against carbon tetrachloride-induced hepatotoxicity in vitro and in vivo through regulating hepatic antioxidant, inflammation response and the MAPK-NF-Κb signaling pathway. Biomed. Pharmacother. = Biomedecine & pharmacotherapie. 2017;vol. 95:1091–1102. doi: 10.1016/j.biopha.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Chang B., et al. Fraxin prevents chemically induced hepatotoxicity by reducing oxidative stress. Molecules. 2017;22(4) doi: 10.3390/molecules22040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiaverini C., et al. Microphthalmia-associated transcription factor regulates RAB27A gene expression and controls melanosome transport. J. Biol. Chem. 2008;283(18):12635–12642. doi: 10.1074/jbc.M800130200. [DOI] [PubMed] [Google Scholar]
- 14.Singh S., et al. Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigm. Cell Res. 2005;18(2):113–121. doi: 10.1111/j.1600-0749.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim D., et al. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J. Cell Sci. 2003;116:1699–1706. doi: 10.1242/jcs.00366. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y., et al. Selaginellin inhibits melanogenesis via the MAPK signaling pathway. J. Nat. Prod. 2022;85(4):838–845. doi: 10.1021/acs.jnatprod.1c00971. [DOI] [PubMed] [Google Scholar]
- 17.Lv J., et al. Isoliquiritigenin inhibits melanogenesis, melanocyte dendricity and melanosome transport by regulating ERK-mediated MITF degradation. Exp. Dermatol. 2020;29(2):149–157. doi: 10.1111/exd.14066. [DOI] [PubMed] [Google Scholar]
- 18.Yao H., Zhao J., Song X. Protective effects of fraxin on cerebral ischemia-reperfusion injury by mediating neuroinflammation and oxidative stress through PPAR-γ/NF-κB pathway. Brain Res. Bull. 2022;187:49–62. doi: 10.1016/j.brainresbull.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Ichihashi M., et al. UV-induced skin damage. Toxicology. 2003;189(1–2):21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., et al. Nrf2, a multi-organ protector? Faseb. J. 2005;19(9):1061–1066. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M., Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 22.Hu S., et al. Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell. Physiol. 2019;234(5):7330–7340. doi: 10.1002/jcp.27492. [DOI] [PubMed] [Google Scholar]
- 23.Lee H.J., et al. Hesperidin, A popular antioxidant inhibits melanogenesis via Erk1/2 mediated MITF degradation. Int. J. Mol. Sci. 2015;16(8):18384–18395. doi: 10.3390/ijms160818384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon K.M., et al. Maclurin Exhibits antioxidant and anti-tyrosinase activities, suppressing melanogenesis. Antioxidants. 2022;11(6) doi: 10.3390/antiox11061164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon K.M., et al. FoxO6 inhibits melanogenesis partly by elevating intracellular antioxidant capacity. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.