Abstract
Excellent biocompatibility, mechanical properties, chemical stability, and elastic modulus close to bone tissue make polyetheretherketone (PEEK) a promising orthopedic implant material. However, biological inertness has hindered the clinical applications of PEEK. The immune responses and inflammatory reactions after implantation would interfere with the osteogenic process. Eventually, the proliferation of fibrous tissue and the formation of fibrous capsules would result in a loose connection between PEEK and bone, leading to implantation failure. Previous studies focused on improving the osteogenic properties and antibacterial ability of PEEK with various modification techniques. However, few studies have been conducted on the immunomodulatory capacity of PEEK. New clinical applications and advances in processing technology, research, and reports on the immunomodulatory capacity of PEEK have received increasing attention in recent years. Researchers have designed numerous modification techniques, including drug delivery systems, surface chemical modifications, and surface porous treatments, to modulate the post-implantation immune response to address the regulatory factors of the mechanism. These studies provide essential ideas and technical preconditions for the development and research of the next generation of PEEK biological implant materials. This paper summarizes the mechanism by which the immune response after PEEK implantation leads to fibrous capsule formation; it also focuses on modification techniques to improve the anti-inflammatory and immunomodulatory abilities of PEEK. We also discuss the limitations of the existing modification techniques and present the corresponding future perspectives.
Keywords: Polyetheretherketone, Biological inertness, Anti-inflammatory, Immunoregulation, Modification
Graphical abstract
Highlights
-
•
The FBR produced by PEEK implantation in the body leads to the formation of fibrous capsule and inhibits the binding of PEEK to bone.
-
•
The drug molecule delivery system constructed on PEEK surface can steadily release anti-inflammatory drugs for a long time, and inhibit the inflammatory response and the formation of fibrous capsule after implantation.
-
•
The surface chemical modification technique improves the surface properties of PEEK while retaining the structure and properties of the PEEK body.
-
•
Nanopore structure can change the surface roughness of PEEK and convert mechanical signals into biological signals to further modulate the local immune microenvironment.
1. Introduction
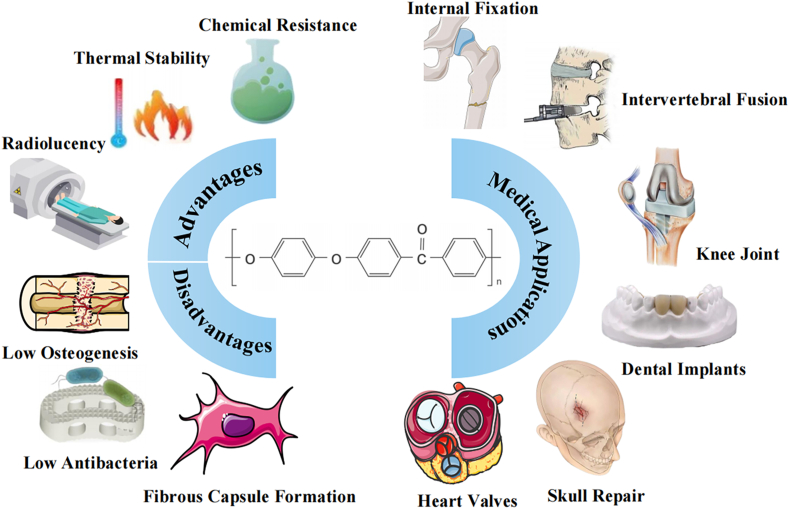
Biomaterials are widely used in implant materials in the surgical treatment of orthopedic patients. PEEK is an organic synthetic polymer with promising applications in bioengineering and clinical medicine. The material was approved by the United States Food and Drug Administration (FDA) as an implant material in the 1980s and is widely used in intervertebral fusions, joint replacements, bone defect repairs, and dental implants [1]. PEEK is a two-phase semicrystalline polymer consisting of amorphous and crystalline phases, which gives it good mechanical properties, chemical resistance, thermal stability, mechanical stability, and processing properties as a bioimplant material [[2], [3], [4]]. The advantage of PEEK over other bio-implantation materials is that its modulus of elasticity approximates that of human bone tissue. The elastic modulus of human cortical bone is about 15 GPa, and that of cancellous bone is about 1 GPa. PEEK has an elastic modulus of about 3.7 GPa, which is closer to that of human bone than titanium, another classic biological implantation material (with an elastic modulus of about 110 GPa). Thus, PEEK reduces stress-masking effects and the functional degradation of bone tissue [2,5]. PEEK is also a radiolucent material, allowing it to produce high-resolution images without artifacts on X-ray or magnetic resonance imaging (MRI) scans, facilitating the observation of bone formation and the detection of problems in the bone healing process around PEEK implants [6]. These advantages have caused PEEK to emerge as an alternative material to titanium and titanium alloy and play a broader role in the medical field (Fig. 1) .
Fig. 1.
Advantages, disadvantages, and applications of PEEK and its composite biomaterials.
Despite its excellent properties, the clinical applications of PEEK face many challenges due to biological property deficiencies that prevent it from functioning well after implantation, resulting in implant failure or poor medical outcomes. The molecular structure of PEEK dictates its surface hydrophobic properties. PEEK's hydrophobic surface reduces the ability of cells to adhere and affects the function of growth factors. Thus, PEEK has been identified as a biologically inert material [7,8]. Some studies showed that implant infection [9] and poor osseointegration [10] were the main factors leading to implant failure. The application scenarios of implanted materials are mainly trauma, infection, and lower immune function of the patient. These factors place high demands on the infection-resisting ability of PEEK, and improper handling can lead to localized pain, compromised wound healing, and implant failure [11]. The biological inertness of PEEK impedes its integration with natural bone tissue in vivo, and enhancing the osteogenic activity of PEEK was shown to improve peri-implant bone regeneration and subsequent bone remodeling after implantation [2].
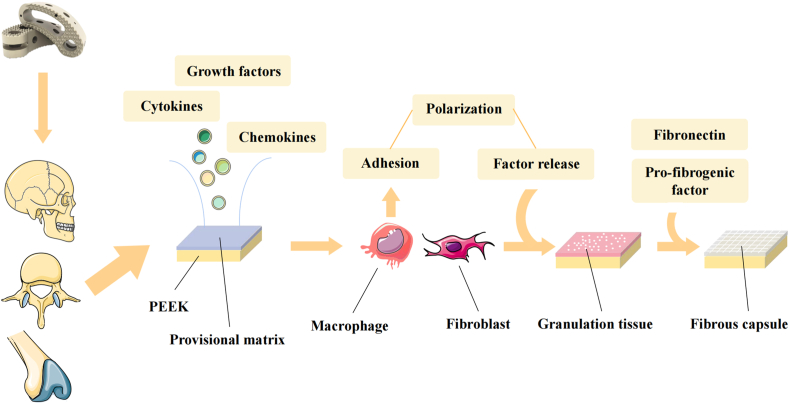
Foreign body reaction (FBR) significantly affects the osseointegration of biomaterials. The presence of many immune cells in the implant-tissue region affects the immune microenvironment there, causing aggregation, adhesion, and the secretion of cytokines, resulting in PEEK encapsulation by collagenous avascular fibrous tissue [9]. Fibrous encapsulation can adversely affect osseointegration and reduce the therapeutic expectations of PEEK by suppressing integration, decreasing the blood supply, causing discomfort, and preventing long-term medication release [12]. As research on PEEK implantation has progressed, the focus has shifted from biomechanical properties to improved biological inertness and enhanced osseointegration, which are critical for long-term stable function after PEEK implantation (Fig. 2).
Fig. 2.
The process of forming fibrous capsule after PEEK implantation in human body.
This article reviews the principles that lead to the inflammatory response and fibrous wrapping after PEEK implantation. Based on this rationale, we identified multiple factors involved in the inflammatory response and modified PEEK in different ways to modulate the response and reduce the generation of fibrous wrapping. Ultimately, our goal was to describe ways to improve the biological inertness of PEEK, avoid implantation failure, and improve the functional outcomes of PEEK as a bioimplantation material.
2. Inflammation and immune mechanisms
Implantation into the body activates the body's immune system to produce an FBR, a multifactorial immunomodulatory-related process involving many cells and cytokines. In the early stages of implantation, blood interacts with the biomaterial and forms a provisional matrix, in which a primary thrombus or clot is formed on the biomaterial's surface at the contact surface of the tissue and material [13]. The provisional matrix is rich in cytokines, growth factors, chemotactic agents, and other bioactive agents. It provides a rich biochemical basis for an FBR, as well as the proliferation and activation of other cell populations involved in inflammation [14,15]. First, a layer of adhesion proteins is formed on the surface of the implant, with components such as albumin, globulin, fibrinogen, fibronectin, and complement [16], followed by the involvement of complement and leukocytes in the development of the primary thrombus or clot associated with the biomaterial, forming the provisional matrix. This process is a manifestation of injury. It activates innate immunity, leads to the development of an inflammatory response, and results in the activation of coagulation, fibrinolysis, replenishment, and kinin-producing systems. The main purpose of the early provisional matrix is to create a polymer that may be assembled into a mechanically stable network, allowing the network to trap platelet plugs to prevent blood loss and provide a temporary scaffold for subsequent cell migration [17]. After provisional matrix formation, acute inflammation occurs at the tissue and implant contact surfaces, with neutrophil-mediated predominance, aided by mast cell degranulation, histamine release, and fibrinogen adsorption. The release of these cytokines and substances has a significant effect on the subsequent inflammatory response [18,19].
In the late provisional matrix, fibronectin is replaced mainly by fibronectin and proteoglycans. Fibronectin acts as a scaffold signaling protein, with downstream signals initiated by fibronectin assembly, capable of regulating cell adhesion dynamics, maintaining collagen fibril stability, and integrin binding. Fibronectin is a crucial link associated with permanent matrix deposition [20]. The extravasation and migration of monocytes and macrophages to the implantation site are critical for the progression of inflammation and the FBR. Macrophages enter the tissue and colonize the implant site in response to various chemotactic agents, such as chemokines, transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), CXCL4, leukotrienes (LTB4), and interleukin (IL-1) [21]. C3b is a component of the provisional matrix and is expressed by many inflammatory and immune cells. C3b causes the implant surface to be recognized by the immune system in the inflammatory response, thus assisting macrophage recruitment and adhesion to the blood and material contact surfaces [22,23]. After adhesion to the implant surface, macrophages express chemotactic cytokines and growth factors such as PDGF, tumor necrosis factor-α (TNF-α), IL-6, granulocyte colony-stimulating factor (G-CSF), and granulocyte monocyte colony-stimulating factor (GM-CSF), in response to IL-1 stimulation, thereby recruiting more macrophages to complete aggregation [21]. PDGF and vascular endothelial growth factor (VEGF) act synergistically to stimulate fibroblast migration, maturation, and angiogenesis [24]. Platelets are the most important source of CXCL4 in the body. CXCL4 can also be released by macrophages during the inflammatory response, and CXCL4 is one of the factors commonly upregulated in the pro-fibrotic macrophage process [25]. Integrins are a family of proteins widely found on the cell surface that mediate cell-extracellular matrix and intercellular interactions [26]. Integrins bind to the adhesion protein layer on the implant surface, providing signals within macrophages. Downstream signaling pathways influence cytoskeletal rearrangement and the formation of adhesion structures, facilitating macrophage adhesion and distribution on the implant surface [27,28].
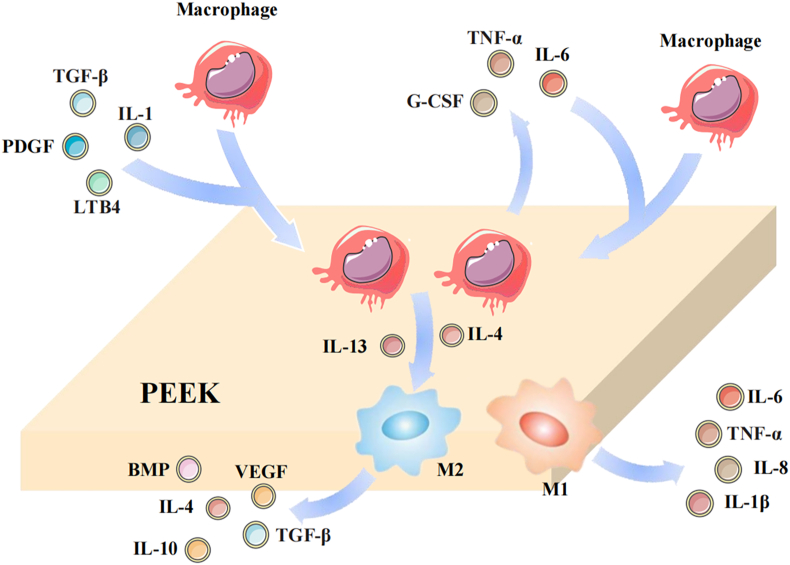
Macrophages are activated when they are involved in the local tissue inflammatory response and are classified as M1 macrophages or M2 macrophages according to their polarization-forming phenotype [29]. Macrophage polarization is an interaction between M1 and M2 macrophage phenotypes and has been suggested to be a determinant of the FBR. M1 macrophages exhibit a proinflammatory phenotype and are capable of secreting toxic reactive oxygen intermediates, such as TNF-α, IL-6, and cytokines, which can lead to the up-regulation of genes involved in the intracellular killing of pathogens, thereby enhancing the inflammatory response and promoting bone resorption [30]. M2 macrophages exhibit an anti-inflammatory phenotype and secrete bone morphogenic protein (BMP), VEGF, and TGF-β to attenuate the inflammatory response and inhibit osteoclast activity [31,32]. Although M1 and M2 macrophages are usually considered distinct phenotypes, local microenvironments may lead to the simultaneous expression of M1 and M2 markers [33]. Multiple cytokines are capable of inducing macrophage M2 polarization, typically IL-4 and IL-13 [34]. However, the polarization of macrophages may change after implantation due to the poor biocompatibility of PEEK. Thus, altering the biocompatibility of PEEK to induce the polarization of the anti-inflammatory macrophage phenotype is a viable strategy. When macrophages encounter a substance that is too large to be phagocytosed, they fuse into foreign body giant cells (FBGCs) to facilitate substance clearance [35]. IL-4 and IL-13 are expressed at high levels in regions where FBGCs develop and macrophages fuse, suggesting that the combined action of cytokines may promote macrophage fusion (Fig. 3) [36].
Fig. 3.
Macrophage involvement in the FBR.
Macrophages play an important role in bone regeneration and new bone formation. They drive inflammation, promote angiogenesis, mitosis, and stromal mineralization, and guide osteoblast differentiation and maturation. Their contributions include the removal of excess stroma [37], the modification of the stromal microenvironment through paracrine cytokines [38], and the release of cytokines that direct the accumulation and maturation of osteoblasts. The coordination of these functions determines the quality and structure of bone regeneration. When lipopolysaccharide (LPS) or other endogenous ligands activate Toll-like receptors (TLRs), M1-type macrophages produce oncostatin M (OSM) by regulating cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) [39]. OSM is a class of secreted protein factors from the IL-6 family that promote osteoblast differentiation through the transcription factor signaling and transcriptional activator 3 (STAT3) differentiation of osteoblasts [40].
After the inflammatory response subsides, reparative macrophages and fibroblasts continue to absorb debris and form a new matrix, which, together with the released factors that promote angiogenesis, forms granulation tissue [41]. Granulation tissue is a precursor to fibrous capsule formation. The cell layer formed by monocytes, macrophages, and FBGCs separates granulation tissue from biological material [14,42]. Macrophages actively participate in the process of tissue remodeling by expressing high levels of extracellular matrix proteins, such as fibronectin, during the healing process [43]. Activated macrophages also produce pro-fibrogenic factors that enhance fibrogenesis in fibroblasts, while the release of IL-4 and IL-13 promotes M2 polarization and can significantly reduce fibrous tissue production [44]. As a result, macrophages adhering to the biomaterial can secrete proteins that regulate fibrosis, and thus, a fibrous capsule forms around the material after implantation. Fibrous capsules can impair the performance of medical devices by obstructing integration, decreasing blood flow, producing constriction and pain, and preventing the long-term release of drugs [12].
3. Drug delivery system
Anti-inflammatory drug molecules suppress the inflammatory response and modulate the immune microenvironment after PEEK implantation. Due to the hydrophobicity and biological inertness of the PEEK surface, anti-inflammatory drug molecules cannot be stably stored and released directly from the PEEK surface. Thus, suitable surface structures have been constructed to carry anti-inflammatory substances and enable their stable release. This section describes the current research on drug delivery systems. The constructed surface carrier structures can be divided into those with poly(lactic acid glycolic acid) (PLGA)/polyvinyl alcohol (PVA) hydrogel coating, liposome coating, polypropylene glycol carbonate coating, and PDA coating (Table 1).
Table 1.
PEEK-modified drug delivery system.
| Drug-controlled release vector | Drugs carried | Experimental category | Evidence of improved anti-inflammatory effects | Reference |
|---|---|---|---|---|
| PLGA/PVA hydrogel coating | DEX | in vitro | Reduced fibrous tissue formation | [45] |
| Liposomes | DEX/minocycline | in vitro | Down-regulated the expression of proinflammatory cytokines TNF-α, IL-6, IL-8, and COX-2 | [46] |
| Supporting lipid bilayers | Phosphatidylserine | in vitro | Down-regulated the expression of TNF-α and up-regulated the expression of TNF-β | [47] |
| PTMC | DEX/IL-10 | in vitro | Down-regulated the expression of CCR7 and IL-1β, up-regulated the expression of CD206, VEGF, and BMP2, and promoted M2 polarization of macrophages | [48] |
| in vivo | Promoted M2 polarization of macrophages and reduced the inflammatory response | [48] | ||
| PDA | GS | in vitro | Promoted M2 polarization of macrophages | [49] |
| in vivo | Down-regulated the expression of proinflammatory cytokines TNF-α, IL-1β, and IL-6 and up-regulated the expression of anti-inflammatory cytokines IL-4 and IL-10 | [49] | ||
| EUP-Sr | in vitro | Down-regulated the expression of proinflammatory cytokines IL-1β and IL-18 | [50] | |
| ICA | in vitro | Down-regulated the expression of proinflammatory cytokines TNF-α and IL-6 and up-regulated the expression of anti-inflammatory cytokines IL-4 and IL-10 | [51] | |
| in vivo | Promoted M2 polarization of macrophages and improved the bone immune microenvironment | [51] |
3.1. Drug release from microgel coating
Semi-permeable hydrogels have unique characteristics of high water content, easy solute transport, and different active groups that can be further chemically modified, which has led to extensive research on semi-permeable hydrogels as contamination-free implant coatings [52,53]. Bridges et al. [52] proposed a film coating strategy based on poly(n-isopropyl acrylamide) hydrogel particles cross-linked with polyethylene glycol diacrylate. The microgel coating effectively reduced the adhesion of leukocytes and fibrinogen and down-regulated the expression of proinflammatory cytokines. The topography of the hydrogel coating improved the biological inertness of the PEEK surface, enabling the hydrogel to exert anti-inflammatory effects and enhance the hydrophilicity of the surface [54,55].
PLGA particles, which can integrate multiple drugs and control the effective release of drugs, have been embedded into a PVA hydrogel coating to form a hydrogel coating that releases anti-inflammatory drugs autonomously and continuously [56]. The drug release pattern of PLGA particles is a classical three-phase release pattern, including a rapid drug release phase, a delayed drug release phase, and another rapid drug release phase [[57], [58], [59]]. The initial burst release phase may be due to the release of the drug adsorbed on the surface and the pore-closing effect due to PLGA swelling [60,61]. Gu et al. [62] reported that many drugs were diffused from microspheres and interfered with the intrinsic gel structure during the burst release phase. Thus, the expansion of microspheres might be a promoting factor for the release of drugs. The onset of the expansion of microspheres and the change in the pore structure of microspheres also play an essential role in the expansion of microspheres [62]. In the hysteresis phase, PLGA microspheres swell after forming internal pores. As the degradation products gradually accumulate, increasingly high osmotic pressure is generated, which attracts more water molecules into the particles. This leads to the deformation of the internal structure of the particles and triggers the next stage of the drug-release process [57]. The degree to which the particles expand determines the mobility of the drug molecules in the system and, thus, the rate of drug release. As the particles begin to expand significantly, their water content rises greatly, increasing the mobility of the drug molecules. Substantial changes in the morphology and size of the microparticles begin during the third drug-release phase. Gasmi et al. [63] indicated that the beginning of particle expansion coincided with the beginning of the third drug-release phase and that PLGA particle expansion could control the resulting drug release rate; when particle swelling is limited, the drug release rate slows.
Dexamethasone (DEX) is a potent corticosteroid with safe systemic immunosuppressive effects [64]. DEX has a positive effect on the formation of fibrotic scars caused by implants, inhibiting the formation of collagen layers and the expression of fibrotic proteins and cellular markers on the implant surface [65]. However, systemic injections of DEX have well-documented side effects and are unsuitable for long-term systemic therapy [66]. Topical anti-inflammatory drug release solves the safety problem, but controlling the release of DEX over a period of time remains a problem. DEX can be incorporated into PLGA microspheres and embedded in PVA hydrogel coatings, resulting in a hydrogel coating that can autonomously and continuously release DEX. Siddesh et al. [45] tested the in vitro and in vivo release properties of a PLGA microsphere/PVA hydrogel compound delivery system in a rat model, as well as the pharmacodynamic response at the implant and tissue interface in a one-month study. DEX release from the PLGA/PVA hydrogel coating was linear in both in vivo and in vitro experiments and significantly reduced the infiltration of inflammatory mediator cells and fibrous inclusion formation around the implant. Many other studies reached the same conclusion and found delayed FBR around the implant after complete DEX release [67]. This demonstrated that the sustained release and action of DEX were necessary during the implantation cycle. PLGA microspheres prepared by mixing PLGA with different molecular weights achieved continuous effective drug delivery for more than six months [58]. When the low-molecular-weight components were degraded, the more hydrophilic PLGA fragments increased the acidity and absorption of water by the polymer matrix, promoting the autocatalytic degradation of the high-molecular-weight PLGA components in these microspheres and successfully achieving the continuous release of DEX [58].
3.2. Drug release from liposomes
Liposomes are a popular drug delivery vehicle with the advantages of biodegradability, low toxicity, and low immunogenicity. Liposomes effectively deliver drug molecules as vectors and have continuous release properties [68]. A classical surface modification method is to use liposomes with DEX and minocycline in combination with polydopamine (PDA) coating to effectively ameliorate the FBR induced by PEEK implantation [69]. DEX and minocycline released from the surface of DEX/minocycline-modified liposomes effectively inhibited the expression of LPS-induced proinflammatory mediators at the mRNA and protein levels and decreased the levels of TNF-α, IL-6, IL-8, and COX-2. DEX/minocycline liposomes effectively reduced inflammatory cell infiltration around the implant [46]. Another study modulated macrophage M2 polarization by constructing phosphatidylserine (PS)-containing supporting lipid bilayers (SLBs) bound to the implant surface [70]. Macrophages express a PS-specific receptor that binds to PS on apoptotic cells and promotes phagocytosis. Macrophages not only prevented the leakage of proinflammatory cytokines through the binding of PS and PS receptors but also stimulated the release of anti-inflammatory cytokines [47]. The interaction of PS in liposomes with macrophages up-regulated the expression of TGF-β and down-regulated the expression of TNF-α, thus reducing the inflammatory response [47].
3.3. Release of polytrimethylene carbonate
Aliphatic polytrimethylene carbonate (PTMC) is gaining attention as a class of biodegradable and absorbable materials. PTMC is commonly used to improve the biocompatibility of biomaterials and drug delivery. Xie et al. [48] constructed a PTMC carrier coating containing DEX and IL-10 and combined it with a PEEK surface. The degradation of the PTMC coating enabled the sequential release of DEX and IL-10. The PTMC coating rapidly released IL-10 the first week and slowly released DEX over four weeks, which reduced the level of inflammatory factors and down-regulated the expression of various proinflammatory genes, such as CCR7 and IL-1β [48]. PTMC vector modification up-regulated the expression of various anti-inflammatory genes, such as CD206, VEGF, and BMP2, promoted macrophage M2 polarization, and effectively suppressed inflammatory responses near the implant [48].
3.4. Molecular release of PDA
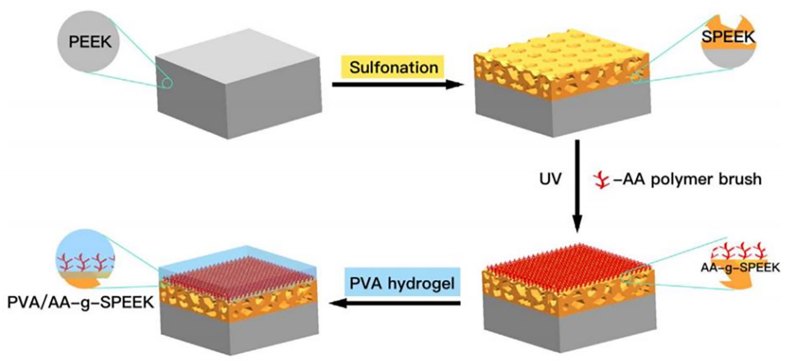
The strong adhesive properties of PDA make it a viable vehicle for drug delivery, enabling the sustained release and maintenance of the activity of various active substances. PDA is biocompatible and can facilitate other therapeutic and diagnostic modalities, and these advantages have led to a wide interest in PDA [71]. PDA used as a delivery coating carrying gentamicin (GS), enabled the sustained release of GS and induced the M2 polarization of macrophages on the PEEK surface [49]. Immunohistochemical analysis of rat femurs after six weeks demonstrated that the modified PEEK implantation sites expressed less proinflammatory TNF-α and IL-6 and more anti-inflammatory IL-4 and IL-10, effectively controlling bacterial infections while reducing inflammatory responses [49]. The biological inertness and smoothness of the PEEK surface weaken the combination of various ions on the PEEK surface, limiting the anti-inflammatory capacity. PDA, as an ion-carrying matrix, enhances the anti-inflammatory capacity by directly contacting the PEEK surface (Fig. 4) [72].
Fig. 4.
Schematic of a PVA hydrogel layer on sulfonated PEEK with an acrylic acid matrix. Reproduced with permission [54]. Copyright 2018 Journal of the Mechanical Behavior of Biomedical Materials.
EUP-Sr is a newly developed bioactive polysaccharide complex that was shown to promote osteogenesis and modulate bone immunity [73]. Zhang et al. [50] introduced Eucommia ulmoides-strontium polysaccharide (EUP-Sr) onto a PEEK surface using a PDA coating as a carrier. In vitro experiments showed that PDA-modified PEEK containing EUP-Sr could down-regulate the expression of inflammatory genes IL-1β and IL-18 and positively inhibit the inflammatory response [50]. However, it exhibited cytotoxicity in a specific concentration range, presumably due to the high concentration of Sr2+ [74]. PDA coatings can also carry a variety of other drugs. Icariin (ICA) is the main active ingredient of Herba epimedium and an effective therapeutic agent for osteoporosis [75]. ICA-PDA coating was shown to cause macrophages to produce fewer inflammatory factors (TNF-α and IL-6) and more anti-inflammatory factors (IL-4, IL-10), which attenuated the inflammatory response through immunomodulation [51]. In vivo experiments in mice showed that the ICA-PDA coating promoted macrophage M2 polarization and facilitated osseointegration [51].
4. Surface chemical modification or physical modification
Surface chemical modification improves the surface properties of PEEK while retaining the structure and properties of the PEEK body. This technique widely exists in the application of PEEK internal implants. This section is divided into three parts according to the different substances involved in surface modification: inorganic ion modification, organic and functional group modification, and biological material coating. Each section describes the relevant technology in the area (Table 2).
Table 2.
Chemical surface modification or physical modification of PEEK.
| Substances involved in modification | Modification strategy | Experimental category | Evidence of improved anti-inflammatory effects | Reference |
|---|---|---|---|---|
| Ca2+ | Poly(norepinephrine) wrapping and Ca(OH)2 solution immersion | in vitro | Decreased TNF-α and IL-1α levels and increased IL-10 levels | [76] |
| IL-4 and Ca2+ grafted onto a hybrid coating consisting of polylactic acid, ALN and nano-HA | in vitro | Promoted M2 polarization of macrophages, decreased the level of TNF-α, and increased the level of IL-10 | [77] | |
| in vivo | Promoted M2 polarization of macrophages | [77] | ||
| Phosphate group | Plasma treatment and subsequent phosphorylation reaction | in vitro | Decreased TNF-α and increased IL-10 production by macrophages | [78] |
| Phosphonate group | Diazonium chemistry grafting | in vivo | Decreased fibrous capsule production | [79] |
| Sulfonate | One-step ultraviolet-initiated graft polymerization | in vitro | Reduced secretion and gene expression levels of the proinflammatory cytokine TNF-α | [80] |
| β-TCP | Construction of β-TCP coating | in vitro | Promoted M2 polarization of macrophages | [81] |
| Zn2+ | Customized magnetron sputtering technique | in vitro | Decreased expression levels of the inflammatory factors TNF-α and IL-6 and increased expression levels of the anti-inflammatory cytokines IL-4 and IL-10 | [82] |
| Mg2+ | Complex PA/Mg ion coating | in vivo | Promoted M2 polarization of macrophages and inhibited fibrous tissue formation | [83] |
| Sr2+ | Formation of 3D porous nanomesh structures by concentrated sulfuric acid and plasma treatment | in vitro | Down-regulated the expression of proinflammatory genes, such as COX-2 and TNFα, up-regulated the expression of the anti-inflammatory gene IL-10, and promoted M2 polarization of macrophages | [84] |
| CS-Sr | Integration into PEEK surfaces via PDA | in vitro | Promoted cell adhesion and diffusion, improved angiogenic activity, reduced the mRNA expression of proinflammatory factors TNF-α and IL-1β | [85] |
| in vivo | Reduced the inflammatory response | [85] | ||
| Cu2+ | Deposited on a sulfonated PEEK surface by magnetron sputtering technique | in vitro | Promoted M2 polarization of macrophages | [86] |
| Ag+ | Integration into PEEK surfaces via PDA | in vitro | Down-regulated the mRNA expression of proinflammatory cytokines IL-1β, IL-6, iNOS, and TNF-α and promoted M2 polarization of macrophages | [87] |
| SB | Sulfonated PEEK immersed in SB solution | in vitro | Promoted M2 polarization of macrophages | [88] |
| in vivo | Reduced fibrous tissue formation and neutrophil infiltration | [88] | ||
| COOH | COOH attached to the PEEK surface using diazonium-based chemistry reactions | in vitro | Down-regulated proinflammatory TNF-α, IL-1β, and IL-6 expression and up-regulated BMP-2 expression | [89] [90] |
| in vivo | Down-regulated proinflammatory gene expression | [91] | ||
| BMP2 | Construction of a DOPA4 adherent layer and integration by a DBCO-azide bio-click reaction | in vitro | Promoted M2 polarization of macrophages | [92] |
| TiO2 | TiO2 deposited on the PDA surface to form a PDA/TiO2 hybrid coating | in vitro | Reduced macrophage adhesion and decreased expression of the inflammatory factor TNF-α | [93] |
| AIP | in vivo | Reduced fibrous tissue formation and promoted the mechanical association of PEEK with bone tissue | [94] | |
| Ti | Construction of Ti coating on PEEK surfaces using plasma spraying technology | in vitro | Promoted M2 polarization of macrophages, down-regulated the expression of inflammatory factors, and regulated the osteoimmune environment | [95] |
| in vivo | Reduced fibrous tissue formation | [95] | ||
| HA | Synthesis of HA powder and PEEK powder mixed materials and sulfonation | in vitro | Reduced the proportion of M1 macrophages, inhibited the expression of proinflammatory genes, and promoted the expression of anti-inflammatory genes | [96] |
| in vivo | Reduced fibrous tissue formation | [97] | ||
| Nano HA/Silica | A silicone sol was sprayed onto the PEEK surface using the principle of sol-gel technology. After heat treatment, the PEEK melted and penetrated the interconnected pore structure of the coating material | in vivo | Reduced fibrous tissue formation | [98] |
4.1. Inorganic ion modification
4.1.1. Calcium ion (Ca2+) modification
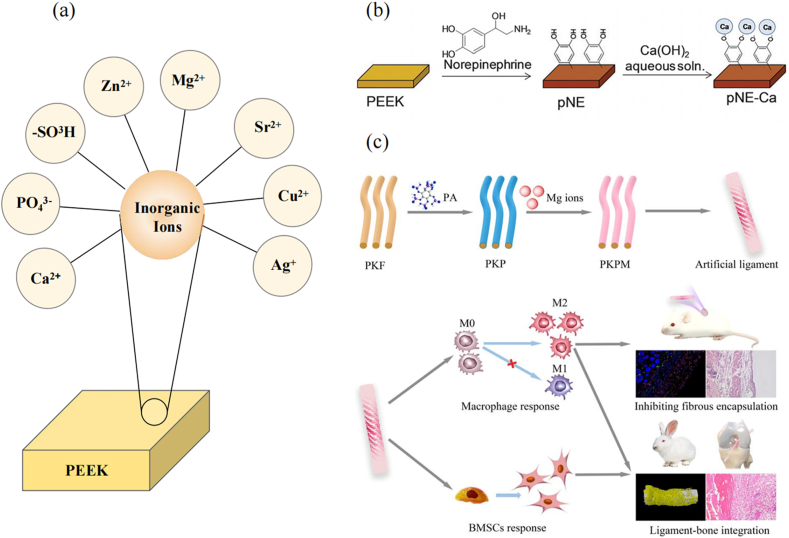
Calcium has an essential impact on the inflammatory response, especially during the M1 and M2 polarization of macrophages. Ca2+ can affect many inflammatory signaling pathways, such as the Wnt/Ca2+ signaling pathway and the Ca2+/CaSR signaling pathway [99,100]. Recent studies by Lv et al. showed that transient receptor potential vanilloid 1 (TRPV1), a cation channel, plays an important role in the polarization of M1 macrophages [101]. TRPV1 activation leads to calcium influx, which promotes calcium/calmodulin-dependent protein kinase II (CaMKII) phosphorylation. This process further promotes the nuclear localization of nuclear factor-erythroid 2-related factor 2 (Nrf2), thereby inhibiting M1 macrophage polarization [101]. By affecting these signaling pathways, macrophages are shifted toward M2 polarization, resulting in the down-regulation of corresponding inflammatory cytokines and the up-regulation of anti-inflammatory cytokines [102]. Therefore, Ca2+ may be used for its anti-inflammatory effects after PEEK implantation.
Toita et al. [76] modified Ca2+ on the surface of PEEK by coating PEEK with poly(norepinephrine) and soaking in Ca(OH)2 solution. Ca2+ activates pre-osteoblasts and human mesenchymal stem cell (MSC) responses and promotes switching from the M1 macrophage phenotype to the M2 phenotype. In vitro experiments demonstrated that Ca2+-modified PEEK-induced macrophages produced fewer TNF-α and IL-1α inflammatory factors, but the level of the anti-inflammatory factor IL-10 increased, thus creating a more favorable environment for anti-inflammatory wound healing (Fig. 5) [76]. Ca2+, in combination with other anti-inflammatory substances, still had an anti-inflammatory effect after PEEK implantation. Zheng et al. [77] constructed a biodegradable hybrid coating consisting of polylactic acid, alendronate (ALN), and nanohydroxyapatite (HA) on the surface of PEEK and grafted IL-4 and Ca2+ onto the surface of the coating. The cellular experiment showed an increase in M2 macrophages on the modified PEEK surface from 27% to 69%, a decrease in TNF-α levels, and an increase in IL-10 levels, consistent with the results of previous experiments. In vivo experiments in distal rat femurs confirmed that IL-4, ALN, and Ca2+ could act synergistically to alleviate the early acute inflammatory response and create a bone immunomodulatory micro-environment that promoted osteogenesis within the first few days of release [77]. Similarly, a reduction in the acute inflammatory response was derived from the promotion of M2 macrophage polarization .
Fig. 5.
(a) The inorganic ion modification on the peek surface. (b) Schematic of poly(norepinephrine) coating and Ca modification. Reproduced with permission [76]. Copyright 2015 Journal of Materials Chemistry B. (c) Schematic of PEEK fiber (PKF) surface coated with PA/Mg2+ complex (PKPM) and PKPM-modulating macrophage and bone mesenchymal stem cell (BMSC) responses. Reproduced with permission [83]. Copyright 2022 Biomaterials Advances.
4.1.2. Inorganic ion groups
Better bioactivity and anti-inflammatory capacity from the phosphate ion modification of biomaterials have been widely reported [103,78]. After modification with phosphate groups, the PEEK surface changes from hydrophobic to hydrophilic. In vitro experimental results showed that macrophages on phosphorylated PEEK surfaces produced lower levels of TNF-α and higher levels of IL-10 compared to bare-treated PEEK [78]. Another study reported that an increase in phosphate concentration enhanced the polarization of M2 macrophages [104]. These results indicated that phosphate-based modified PEEK significantly reduced the inflammatory response of macrophages.
Phosphonic acids are derivatives in which one or two hydroxyl groups in the phosphate molecule are substituted with hydrocarbon groups and are often grafted onto the PEEK surface by diazonium chemistry [105]. Mahjoubi et al. [79] grafted phosphonate groups onto the PEEK surface by diazonium chemistry to repair defective cranial bone in rats. In vivo experiments showed that implanted phosphonic acid-modified PEEK did not form a fibrous capsule beneath, while the control group exhibited fibrous capsule formation.
Sulfonate-modified PEEK surfaces can effectively reduce the inflammatory reaction after implantation. One-step ultraviolet-initiated graft polymerization has been used to introduce sulfonic acid groups onto the PEEK surface [106]. In vitro experiments showed that the secretion and gene expression of the proinflammatory cytokine TNF-α by macrophages cultured on a sulfonate-modified PEEK surface for three days were lower than those in the control group [80].
In addition to the direct modification of PEEK by calcium and phosphate groups to improve the anti-inflammatory capacity, another common treatment involves binding PEEK to inorganic calcium and phosphorus to modulate immunity using ion release from the inorganics. A previous study reported the positive modulatory effects of calcium/phosphorus inorganics on macrophage M2 polarization [102]. β-tricalcium phosphate (β-TCP) coating induced M2 macrophage phenotype polarization and significantly elevated the gene expression of the anti-inflammatory factor IL-1ra, thereby inhibiting inflammation and osteoclastogenesis and enhancing the osteogenic differentiation of bone marrow MSCs [81]. Feng et al. prepared three-phase scaffolds by mixing β-TCP, PEEK, and poly(l-lactide) (PLLA) powders [107]. In vivo experiments showed that the PEEK/β-TCP/PLLA scaffold improved osteoblast adhesion and proliferation with good bioactivity, cytocompatibility, and new bone formation [107]. β-TCP-infused PEEK has good prospects and technical feasibility as a fusion material for anterior cervical discectomy fusion [108]. However, the calcium phosphate fragments isolated in the experiment could cause osteoclast inflammation and bone resorption, leading to osteolysis and implant failure [109]. Therefore, more studies on the effects of other Ca2+ inorganic-modified PEEK on the post-implantation FBR and inflammatory responses are needed.
4.1.3. Zinc ion (Zn2+) modification
Zn2+ plays an essential role in immune cell homeostasis and the regulation of immune function and can enhance the secretion of anti-inflammatory cytokines in the body [110]. Zinc‐regulated transporter/iron‐regulated transporter-like protein transporters (ZIPs) are responsible for the cellular uptake of zinc ions [111]. Zip9 can enhance the phosphorylation level of STAT6, thus promoting the polarization of M2-type macrophages. It can also inhibit the phosphorylation of IκBα/β, thus inhibiting the polarization of M1-type macrophages [111]. Liu et al. [82] coated Zn2+ on sulfonated PEEK and cultured macrophages on the PEEK surface. ELISA assays revealed that modifying the PEEK surface with Zn2+ decreased the expression levels of the inflammatory factors TNF-α and IL-6 and increased the expression levels of the anti-inflammatory factors IL-4 and IL-10 by macrophages [82]. Flow cytometry also suggested that the percentage of the M2 macrophage fraction on the surface of Zn2+-modified PEEK was significantly higher than that of the bare PEEK group, while the expression of the typical macrophage M2 fractionation marker CD206 was up-regulated on Zn2+-modified PEEK, demonstrating that Zn2+-modified PEEK promoted M2 macrophage polarization [82].
4.1.4. Magnesium ion (Mg2+) modification
Mg is a widely used bioactive material. Mg2+ is present in bone tissue and has essential effects on bone development and the immune system. Mg2+ mediates bone immune regulation, forming a tissue microenvironment followed by the down-regulation of proinflammatory and the up-regulation of anti-inflammatory responses, thereby coordinating bone regeneration and effectively inhibiting the formation of fibrous inclusions [112]. Wang et al. [83] prepared PEEK fibers coated with a phytic acid (PA)/Mg2+ complex to regulate the inflammatory response at the PEEK implant site by the stable release of Mg2+. PA/Mg2+ complex-modified PEEK significantly stimulated macrophage M2 polarization and reduced the production of proinflammatory cytokines. In vivo experiments demonstrated fewer fibrous inclusions [83]. PA has anti-inflammatory and antibacterial abilities and a strong ability to chelate metal ions, which can be used to construct PA/metal ion complexes to regulate bone immunity in synergy with Mg2+ (Fig. 5) [113].
4.1.5. Strontium ion (Sr2+) modification
Studies confirmed that Sr2+ boosted the proliferation of human umbilical vein endothelial cells (HUVECs) by up-regulating VEGF expression, thereby regulating angiogenesis [114]. Sr2+ can inhibit the NF-κB signaling pathway to decrease the expression of proinflammatory factors. Sr2+ was also shown to regulate immunity and effectively inhibit inflammation [115,116]. Hu et al. [84] formed three-dimensional (3D) porous nanomesh structures on a PEEK surface by concentrated sulfuric acid and plasma treatment, allowing Sr2+ to bind tightly to the porous PEEK surface and stably release Sr2+ into the solution over a long period. In vitro experiments confirmed that Sr2+-modified PEEK significantly down-regulated the expression of proinflammatory genes, such as COX-2 and TNFα, and up-regulated the expression of the anti-inflammatory gene IL-10. Additionally, Sr2+-modified PEEK had a high capacity to promote macrophage differentiation toward the M2 phenotype [84]. Another study described a technique to enable the stable release of Sr2+ from materials using PDA as an intermediary to embed SrCO3 nanoparticles in micropores on the surface of sulfonated PEEK [117]. Sr2+ and other agents, such as EUP-Sr and strontium chondroitin sulfate (CS–Sr), act synergistically on the PEEK surface, displaying excellent anti-inflammatory properties and angiogenic activity [73,85].
4.1.6. Copper ion (Cu2+) modification
Cu2+ is an essential component of human enzymes and plays an essential role in bone metabolism and innate immunity. PEEK sulfonation imparts porous properties, and Cu2+ can be deposited on the sulfonated PEEK surface by magnetron sputtering technology [86]. Cu2+ coating was shown to up-regulate the M2 polarization of macrophages and improve the anti-inflammatory function of PEEK. However, a very rapid release of these ions can create a high Cu2+ concentration environment, resulting in cytotoxicity [91]. Therefore, researchers need to improve Cu2+-coating methods to gain better control over Cu2+ release.
4.1.7. Silver ion (Ag+) modification
Ag+ has a wide range of applications in the antibacterial field, but its immunomodulatory function also needs attention [118]. PDA-based Ag+ nanoparticle coating can bind to a PEEK surface and release Ag+ steadily. In vitro experiments demonstrated that Ag + significantly reduced the mRNA expression levels of proinflammatory cytokines IL-1β, IL-6, inducible nitric oxide synthase (iNOS), and TNF-α while contributing to the polarization of macrophages toward the M2 phenotype [87].
4.2. Organic and functional group modification
4.2.1. Sodium butyrate (SB) modification
Organics regulate inflammation and balance the immune system response. The technical approach to modifying PEEK with organic substances differs from that of inorganic substances due to the diverse molecular structures and the variety of groups carried by organic and inorganic substances. Butyrate has anti-inflammatory and immunomodulatory functions and is involved in many biological processes [119,120]. Loading SB onto sulfonated PEEK was found to promote M2 macrophage polarization and reduce TNF-α and IL-6 concentrations in the culture medium [88]. In further in vivo experiments, SB-modified sulfonated PEEK reduced fibrous tissue and neutrophil infiltration at the implant site [88]. Sulfonated PEEK has a porous structure and is an excellent delivery platform, which was shown to promote a sustained and effective release of SB [121]. However, high SB concentrations can produce cytotoxicity and poor osteogenic effects.
4.2.2. Carboxyl (COOH) modification
Various functional groups with different functions can modulate the inflammatory response after implantation by affecting the properties of the PEEK surface. Studies showed that COOH could inhibit the inflammatory response of macrophages and down-regulate the expression of genes that release proinflammatory factors (IL-1β, IL-6, and TNF-α) from macrophages [122]. Buck et al. [89] demonstrated that COOH-modified PEEK could down-regulate TNF-α, IL-1β, and IL-6 and up-regulate BMP-2 in vitro. Subsequent in vivo experiments demonstrated that COOH-modified PEEK down-regulated the expression of almost all proinflammatory genes three days after implantation [89]. COOH modulates the integrin signaling pathway by altering PEEK surface-adsorbed proteins, thereby regulating the inflammatory response of macrophages. Some of these proteins, such as 2-macroglobulin (A2M) and copper cyanide (CP), were shown to regulate cytokine release and promote wound healing, which contributed to promoting the anti-inflammatory function of macrophages [89,123]. Another study by Buck et al. [90] stimulated macrophages on a PEEK surface functionally modified with LPS, which resulted in enhanced M1 macrophage polarization. Other researchers calculated the ratio of TNF-α to IL-10 after 24 h of LPS stimulation and found that the COOH-modified PEEK surface promoted higher IL-10 secretion, reducing the inflammatory response [90].
4.2.3. BMP2 modification
BMP2 can induce the osteogenic process in vivo and has been widely used in bone tissue engineering [124]. BMP2 also has bone immunomodulatory functions, inducing macrophage recruitment and M2 polarization [125]. Zhao et al. [92] constructed a DOPA4 adhesion layer on a PEEK surface. The azide group at the end of DOPA4 underwent a diarylcyclooctyne (DBCO)-zide bio-click reaction with the DBCO moiety, thus integrating DBCO carrying BMP2 of DBCO onto the PEEK surface [92]. This technique offers a new way to bond many organic substances to PEEK surfaces.
4.3. Biomaterial coating
4.3.1. Titanium dioxide (TiO2) coating
TiO2 is an excellent biocompatible material with certain antibacterial and anti-inflammatory abilities and good osseointegration [126,127]. TiO2 enhances the bioactivity of other biomaterials when used to construct surface coatings or synthesize nanocomposites [128,93]. PDA-modified PEEK was dipped into a saturated deposition solution of TiO2 to deposit it onto the PDA surface, forming a hybrid PDA/TiO2 coating with the biological properties of both materials and synergistic effects. In vitro experiments showed that the PDA/TiO2 coating reduced macrophage adhesion after PEEK implantation, decreased the expression of the inflammatory factor TNF-α, and further improved the anti-inflammatory capacity compared to PDA coating without TiO2 [93]. Some studies proposed that arc ion plating (AIP) technology could be used to deposit TiO2 coatings on PEEK surfaces [129]. AIP uses pulsed arc discharge to ionize conductive materials, generating high-energy ions and depositing them on the substrate to prepare nanoscale thin film coatings or nanoparticles with a high ionization rate, high ion energy, and strong film adhesion [130]. The construction of TiO2 deposition coating can reduce the production of fiber tissues after PEEK implantation and promote the mechanical association of PEEK and bone tissues [94].
4.3.2. Titanium (Ti) coating
Ti is a classical biomaterial that adsorbs proteins and cells better than PEEK [131]. Plasma spraying is a widely used technique for biomaterial modification. The surface coating formed on the surface of biomaterials by plasma spraying can eliminate surface defects and impart excellent properties to the material [132]. Titanium coating by plasma spraying was reported to reduce the amount of fibrous tissue produced after PEEK implantation and enhance bone growth [131,133]. The adhesion and proliferation of macrophages to the titanium coating became stronger, exhibiting enhanced M2 macrophage polarization [95]. The expression levels of various inflammatory factors were also down-regulated, effectively modulating the bone immune environment and suppressing the acute inflammatory response after implantation. The surface roughness of Ti coatings is related to bioactivity. Some studies reported that Ti-coated surfaces must be very rough to maximize cell adhesion [134]. Torstrick et al. constructed Ti-coated modified PEEK [135], which did not show signs of enhanced osteogenic differentiation like other rough titanium surfaces, suggesting that finer Ti-coated surface features could better enhance osteogenic differentiation [135].
4.3.3. HA coating
HA, a naturally occurring inorganic substance, is the most abundant inorganic component in human bones. Various techniques are used to combine HA with PEEK, thereby altering the bioactivity and biocompatibility of PEEK. Conventional techniques use a mixture of PEEK and HA powder for compression molding [136], and other techniques, such as ion beam-assisted deposition (IBAD) [137] and selective laser sintering (SLS) [138], are used to construct blends of HA and PEEK. The sulfonation of PEEK/HA hybrid material synthesized from HA powder and PEEK powder significantly reduced the proportion of M1 macrophages, inhibited the expression of proinflammatory genes, and promoted the expression of anti-inflammatory genes [96]. Sulfonation increased the exposed area of HA and enhanced the cell adhesion ability of the HA-PEEK composite [96]. In vivo experiments showed that the HA-PEEK composite reduced fibrous tissue on the implant and bone growth surfaces [97]. Modifying the PEEK surface by HA coating reduced the expression of proinflammatory factors after PEEK implantation and suppressed the peri-implant inflammatory response. However, the lack of chemical bonding between HA and PEEK resulted in a loose bond between the HA coating and the PEEK surface [139]. Another study reported a hybrid coating constructed of nano HA/silica (SiO2) to address this problem. Nano HA/silica (SiO2) formed interconnected nanopores throughout the composite material [98]. SiO2 creates a transition zone between the coating and the implant, firmly connecting the coating material to the implant surface. The nanopores also produce a larger surface area and reduce the production of fibrous tissue around the implant [98]. Further studies are needed on the function and mechanism of HA/SiO2 coating and its stability in vivo.
HA coating has excellent anti-inflammatory activity, and it has been combined with various materials for clinical applications. However, PEEK also has a variety of orthopedic applications, and HA coating needs to be created in different geometric shapes. At the same time, the mechanical wear of the HA coating may decrease the anti-inflammatory function of the material. PEEK materials with HA blends provide a more stable anti-inflammatory effect. Unlike HA coating, which has little effect on PEEK mechanical properties, the proportion of HA powder changes the elastic modulus and tensile strength of PEEK-mixed materials. Thus, the clinical application of HA coating and PEEK materials with HA blends needs further research [140].
5. Techniques for changing surface roughness
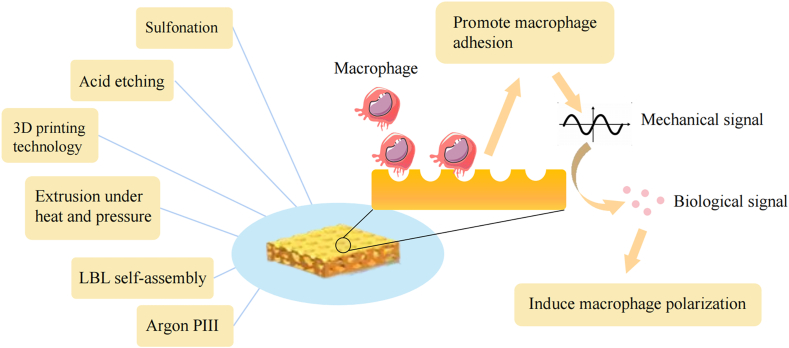
The smooth surface of conventional PEEK implants usually forms during mechanical processing, but this smooth implant surface is not conducive to osseointegration and tends to cause local inflammation at the implant site and form a fibrous capsule, which ultimately leads to implant failure [141]. Surface nanoporous biomaterial structures can modify the surface roughness of implants and improve surface wettability [142]. A porous structure increases the adhesion and diffusion of macrophages by increasing the attachment area and translates physical and mechanical signals into biological signals to trigger macrophage polarization, further modulating the local immune microenvironment [143,144]. Similarly, studies on the surface of other implants, such as Ti, support the conclusion that porous surfaces can provide better osseointegration [145]. Six different technologies for forming porous surfaces are presented in this section: sulfonation, acid etching, 3D printing prepared porous surface, extrusion under heat and pressure, layer-by-layer self-assembly, and argon plasma immersion ion implantation (Table 3)(Fig. 6).
Table 3.
Techniques for changing surface roughness.
| Techniques for porous surface preparation | Modification strategy | Experimental category | Evidence of improved anti-inflammation | Reference |
|---|---|---|---|---|
| Sulfonation | Concentrated sulfuric acid treatment | in vivo | Decreased TNF-α concentration, increased IL-10 concentration, and promoted M2 macrophage polarization | [144] |
| Concentrated sulfuric acid treatment followed by immersion in ASP solution and grafting of BFP onto sulfonated PEEK surface | in vitro | Down-regulated COX-2 and IL-6 expression | [146] | |
| Acid etching | HNO3 treatment | in vitro | Enhanced cell adhesion | [147] |
| HF and HNO3 treatment | in vitro | Down-regulated the expression of proinflammatory cytokines, promoted M2 macrophage polarization | [148] | |
| 3D printing technology prepared porous PEEK | 3D printed PEEK etched with sulfuric acid | in vivo | Reduced fibrous tissue formation, decreased the expression of IL-1β and TNF-α | [149] |
| in vitro | Promoted M2 macrophage polarization and enhanced secretion of anti-inflammatory cytokines IL-4 and IL-10 | [150] | ||
| Extrusion under heat and pressure | Sodium chloride powder and PEEK powder were extruded under high temperature and high pressure and dissolved in sodium chloride in water after cooling | in vivo | Reduced fibrous tissue formation | [141] |
| LBL self-assembly | Alternate dipping in PAA/PAH solution, then rinsing in acid solution | in vitro | Reduced the level of proinflammatory factors, down-regulated the TNF-α and JAK-STAT signaling pathways, and promoted M2 macrophage polarization | [151] |
| Argon PIII | Argon PIII and subsequent hydrogen peroxide treatment | in vitro | Reduced fibrous tissue formation around the implant | [152] |
Fig. 6.
The techniques of changing the roughness of the PEEK surface and the process of inducing cell polarization in macrophages.
Nanopores can mediate immune responses by regulating macrophage autophagy and changing the shape of adherent cells [143]. They also enhance the expression of autophagy signaling pathway components. The autophagy of macrophages decreased the activation of NF-κB and inhibited the expression of the pro-inflammatory cytokines IL-1β and IL-18 [143]. Immune cells can change their shape in response to different mechanical and chemical signals from different nanoforms to adapt to a particular physicochemical environment. Changes in cell shape can lead to changes in the microenvironment within the cell. Porous polycaprolactone/β-TCP composites were reported to regulate the morphology of macrophages. Large, round macrophages expressed M1 macrophage markers at high levels, whereas elongated fusiform macrophages tended to express M2 macrophages [153]. However, other studies arrived at the opposite conclusion, i.e., the surface nanopores of the material induced circular macrophage M2 morphology [143,154]. This may have been due to differences in materials and modification methods. Thus, the relationship between cell shape and the inflammatory state requires further study.
5.1. Sulfonation
Sulfonation is a common surface modification technique in which sulfonation forms a 3D porous network structure on the smooth surface of PEEK and introduces a sulfonyl group (SO3H) on the surface. This modification improves the bioactivity of PEEK [147]. Concentrated sulfuric acid treatment is commonly used for sulfonation, but concentrated sulfuric acid-treated PEEK surfaces have residual sulfur, which can harm human tissues [155]. Wei et al. [144] constructed a mouse air pouch model subcutaneously on the back of mice and implanted sulfonated PEEK into the pouch to observe the effect of sulfonated PEEK on macrophage polarization. In vivo experiments demonstrated that sulfonated porous surfaces on PEEK significantly reduced TNF-α concentrations, increased IL-10 concentrations, and promoted M2 macrophage polarization. In contrast, untreated PEEK elicited a significant M1 macrophage-mediated inflammatory response [144].
Altering the smooth surface of PEEK can increase the hydrophilicity and biocompatibility of the PEEK surface. Further modification of PEEK surfaces by sulfonation has been used to translate theory into practical applications. The previously mentioned PDA coatings and metal ions on sulfonated PEEK were shown to exert anti-inflammatory effects [51,82,84]. Yu et al. [146] constructed sulfonated PEEK-ASP-BFP by immersing sulfonated PEEK in an aspirin (ASP) solution and grafting a bone-forming peptide (BFP) onto porous PEEK. In vitro experiments revealed that the expression of inflammatory factors, such as COX-2 and IL-6, was down-regulated and that sulfonated PEEK-ASP-BFP had lower levels of inflammatory factors compared to sulfonated PEEK-ASP without BFP. Thus, the synergistic effect of ASP and BFP on sulfonated PEEK may further suppress the expression of inflammatory factors [146].
5.2. Acid etching
The process of using concentrated sulfuric acid treatment to sulfonate PEEK suggests that it can improve PEEK by both forming porous surface structures and introducing groups onto the surface, which raises expectations for modifications generated by co-treating PEEK with two acids. Nitric acid (HNO3) is a strong inorganic acid with strong oxidizing and corrosive properties, and nitration by HNO3 can also be used to construct porous PEEK. Smooth PEEK can be successively sulfonated and nitrated to form irregular nanoporous monolayer structures. Similar to sulfonation, nitrocellulose groups are accessed on the surface of PEEK by nitration. Nitrocellulose-treated PEEK had good biocompatibility and stronger cell adhesion ability due to the nanoporous structure and nitrocellulose groups [147]. Hydrofluoric acid (HF) is a weak acid that is strongly corrosive [156]. PEEK treated with HF and HNO3 formed corrosive structures on the surface and had F-functional groups and nitroxides. In vitro experiments showed enhanced M2 polarization and the down-regulation of proinflammatory cytokine expression in macrophages grown on the surface of PEEK treated with HF and HNO3 [148]. These studies provide additional schemes for acid-treating PEEK surfaces to enhance their immunomodulatory capacity.
5.3. 3D printing technology prepared porous surface
3D printing technology has a wide range of applications in bioengineering. It offers unique advantages in designing internal secondary structures and accurate materials, representing a promising technology for creating biomaterials for clinical applications. The production of PEEK with an internal network produced by 3D printing technology after acid-etching to form micropores promoted early integration with soft tissues without creating fibrous inclusions [149]. Fused deposition modeling (FDM) is a technique to fabricate 3D-printed porous PEEK with advantages such as a simple process and low cost [157]. Feng et al. [149] concluded that the internal 3D cross-linked structure of PEEK implants would provide a suitable channel for promoting soft tissue growth. Experiments showed that PEEK that had only undergone acid-etching without the internal cross-linked structure conferred by 3D printing technology had a low level of mechanical binding to soft tissue. Yuan et al. [150] found that 3D sulfonated PEEK facilitated M2 macrophage polarization and enhanced the secretion of anti-inflammatory cytokines IL-4 and IL-10. Changing the surface roughness did not significantly affect the anti-inflammatory effects or macrophage polarization-modulating ability of 3D sulfonated PEEK, but different surface roughness played an influential role in chondrocyte adhesion, proliferation, and exosomal secretion [150]. 3D printing technology has unique advantages over other technologies. 3D printing can specifically adjust to different loads for specific anatomical sites and more accurately fit the implant site.
5.4. Extrusion under heat and pressure
Introducing porosity into the entire PEEK implant through powder sintering or compression molding can promote the migration of various cells [158,159]. However, the high porosity and relatively weak connections produced during powder sintering can reduce the strength of PEEK [159]. Evans et al. [141] introduced a new method to produce surface porosity. They mixed sodium chloride powder and PEEK powder in a specific ratio in a mold, extruded it under high temperature and pressure conditions, and after cooling, the embedded sodium chloride crystals were immersed in water, dissolving them and leaving a porous surface layer. In vivo experiments showed that porous PEEK prepared in this manner was specifically resistant to fibrous tissue formation in the rat femur injury model [141]. This porous PEEK had advantages in terms of strength and fatigue resistance. Although its ability to enhance osteogenesis and anti-inflammation needs further investigation, it provides a new technique for producing surface porosity.
5.5. Layer-by-layer self-assembly
Layer-by-layer (LBL) self-assembly is a coating preparation technique used to fabricate functional multilayer nanocomposites for biomedical applications. LBL technology allows the building of sequentially coated surfaces or structures on a nanoscale, with the advantage of building multilayer films with specific chemical compositions [160]. LBL coating retains the physical properties of the internal PEEK components and transforms the biologically inert surface of PEEK into a biocompatible surface. Dip-coating is the best method currently available for preparing LBL coatings. Gao et al. [151] used electrostatic interactions to prepare multilayer adherent films on a PEEK surface by dipping PEEK alternately into PAA/PAH solution and then rinsing the surface with an acidic solution to make the surface appear nanoporous. LBL coating effectively reduced the levels of proinflammatory factors and down-regulated the inflammation-associated TNF-α and JAK-STAT signaling pathways, which further induced M2 macrophage polarization [151]. The retention of PEEK coating is an essential factor in choosing the coating technique and material. LBL coating was largely retained during in vitro simulated surgical implantation, and the surface roughness caused by nanopores during the preparation of LBL coating also affected the adhesion strength of the LBL coating [151,161].
5.6. Argon plasma immersion ion implantation (PIII)
Plasma treatment can effectively change the surface structure of biomaterials, including increasing the surface roughness and changing the surface charge distribution [162]. Ouyang et al. [152] used argon PIII and subsequent H2O2 treatment to construct nanostructures on a PEEK surface. In vitro experiments showed that argon PIII and H2O2-treated PEEK surfaces generated less fibrous tissue than smooth PEEK or PEEK treated with argon PIII alone [152].
6. Conclusion
PEEK has good biocompatibility, chemical stability, and elastic modulus close to that of bone tissue, making it a promising implantable biomaterial for a wide range of applications. However, when PEEK and its composites are used as clinical implants, fibrous tissue can proliferate at the implant site, resulting in fibrous inclusions that lead to implant failure. The occurrence of this phenomenon is closely related to the biological inertness of PEEK. Developing technical means to improve the anti-inflammatory and immunomodulatory capabilities of PEEK implants is essential in preventing the formation of fibrous wraps after implantation. We conducted significant research on exploring and investigating methods to enhance the anti-inflammatory properties of PEEK materials and improve the biological inertness of PEEK using techniques such as drug delivery systems, surface chemical modification, and surface porous treatment. Many experimental results showed that the anti-inflammatory and immunomodulatory capabilities of modified PEEK were improved compared to pure PEEK.
7. Future outlook
Despite these advancements, the exploration of the anti-inflammatory ability of modified PEEK is still in its infancy due to the complexity of the processing techniques, experimental limitations, and lack of long-term experimental observations of PEEK materials.
PEEK is mainly produced by the UK Victrex, Belgium Solvay, and Germany Evonik companies. Examples include PEEK-Optima (UK Victrex), HA-reinforced PEEK-Optima polymer (UK Victrex), and Zeniva PEEK (Belgium Solvay). At present, the clinical applications of PEEK mainly include the use of unmodified PEEK, carbon fiber-reinforced PEEK, and HA-mixed PEEK in fusion apparatuses, rods, filaments, film, and products. No clinical applications of modifications to enhance the immune function and anti-inflammatory function of PEEK have been reported, and clinicians have paid more attention to the osteogenic ability of PEEK. However, basic research on the immune and anti-inflammatory effects of PEEK has provided a theoretical basis for its clinical application (Table 4).
Table 4.
PEEK products for clinical applications.
| Company | Nation | Commodity | Reinforcers Addition |
|---|---|---|---|
| Invibio | British | PEEK-OPTIMA | – |
| PEEK-OPTIMA HA Enhanced | HA | ||
| Solvay | Belgium | Zeniva PEEK | – |
| Evonik | German | VESTAKEEP PEEK | – |
| Junsun | China | NATUREGEN | – |
| JunHua chinaPEEK | China | AKSOPEEK Natural | – |
| AKSOPEEK HA | HA-enhanced PEEK | ||
| AKSOPEEK CF | Chopped carbon fiber | ||
| AKSOPEEK LCF | Continuous carbon fiber |
Many of the existing experiments were limited to in vitro cellular assays, and the anti-inflammatory capacity of the implant in the post-implantation organism needs to be validated. The complete FBR process culminates in the formation of granulation tissue followed by fibrous encapsulation, which is difficult to demonstrate in in vitro experiments. The construction of surface coatings presents several challenges due to the weak binding of PEEK itself. Enhancing the stability of the binding of PEEK and its modified coatings is a crucial issue in the coating construction process. PEEK can also function as a therapeutic implant material in several parts of the body, and different parts of the implant undergo different post-implantation wear on the coating. For example, PEEK intervertebral fusion devices can undergo varying degrees of coating wear when placed. Future research should analyze the degree of coating wear in in vivo trials and reduce the degree of coating wear by improving the coating bonding technique and PEEK implantation method.
Drug delivery systems make it possible to release drug molecules with potent anti-inflammatory effects locally at the implantation site and modulate the FBR. In addition to the coating-like issues mentioned above, the method of releasing drug molecules in a controlled manner requires further investigation. The concentration and duration of drug molecule release are essential factors influencing the inflammatory response to the implant. More precise release of drug molecules allows concentrations to reach optimal anti-inflammatory benefits. At the same time, the release of high concentrations of drugs by different drug delivery and surface coating systems has the potential to produce bio-toxicity. More intensive experiments are needed to investigate the drug concentration with optimal efficacy and improve the stability of the surface modifications at the technical level. Other excellent biomaterials, such as Ti and silicon nitride, face post-implantation fiber encapsulation problems similar to those of PEEK. Many studies have demonstrated that the surface modifications of these biomaterials were effective in controlling the FBR and reducing fibrous tissue proliferation. In recent years, carbon fiber PEEK has gradually become an attractive material for clinical applications. Carbon fiber PEEK has the advantages of abrasion resistance, corrosion resistance, strong osteogenic ability, and good biocompatibility at a greatly reduced weight while maintaining excellent mechanical properties. Unfortunately, few studies have reported on the immune and anti-inflammatory functions of carbon fiber PEEK. Thus, improving the immune and anti-inflammatory functions of carbon fiber PEEK through modification is a future research direction.
Further demonstration of the effectiveness of these PEEK surface modification techniques is necessary. Methods to achieve reproducible processing and control cost-effectiveness while maintaining anti-inflammatory efficacy are also needed. Further innovations and research efforts are needed to develop implant materials with enhanced bioactivity to address the limitations of the current techniques.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China (82272468 and 52103163). This study was supported by the program of the First Hospital of Jilin University (grant number: JDYYJCHX2020020 and 2022YYGFZJC012).
Contributor Information
Zilin Zhang, Email: 18844116426@163.com.
Xingmin Zhang, Email: zhangxingmin1998@163.com.
Zhi Zheng, Email: zhengzhi20@mails.jlu.edu.cn.
Jingguo Xin, Email: xinjg1998@163.com.
Song Han, Email: hansong1843752634@163.com.
Jinwei Qi, Email: qjw0878@163.com.
Tianhui Zhang, Email: zhangtianhui@jlu.edu.cn.
Yongjie Wang, Email: wangyongjie0218@163.com.
Shaokun Zhang, Email: shaokun@jlu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.He M., Huang Y., Xu H., Feng G., Liu L., Li Y., Sun D., Zhang L. Modification of polyetheretherketone implants: from enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021;1:18–32. doi: 10.1016/j.actbio.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S.M., Devine J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;2(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin L., Pei X.-Q., Bennewitz R., Schlarb A.K. Friction and wear of PEEK in continuous sliding and unidirectional scratch tests. Tribol. Int. 2018;122:108–113. doi: 10.1016/j.triboint.2018.02.035. [DOI] [Google Scholar]
- 4.He M., Chen X., Guo Z., Qiu X., Yang Y., Su C., Jiang N., Li Y., Sun D., Zhang L. Super tough graphene oxide reinforced polyetheretherketone for potential hard tissue repair applications. Compos. Sci. Technol. 2019;174:194–201. doi: 10.1016/j.compscitech.2019.02.028. [DOI] [Google Scholar]
- 5.Carpenter R.D., Klosterhoff B.S., Torstrick F.B., Foley K.T., Burkus J.K., Lee C.S.D., Gall K., Guldberg R.E., Safranski D.L. Effect of porous orthopaedic implant material and structure on load sharing with simulated bone ingrowth: a finite element analysis comparing titanium and PEEK. J. Mech. Behav. Biomed. Mater. 2018;80:68–76. doi: 10.1016/j.jmbbm.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korn P., Elschner C., Schulz M.C., Range U., Mai R., Scheler U. MRI and dental implantology: two which do not exclude each other. Biomaterials. 2015;53:634–645. doi: 10.1016/j.biomaterials.2015.02.114. [DOI] [PubMed] [Google Scholar]
- 7.Przykaza K., Wozniak K., Jurak M., Wiacek A.E., Mroczka R. Properties of the Langmuir and Langmuir-Blodgett monolayers of cholesterol-cyclosporine A on water and polymer support. Adsorption-Journal of the International Adsorption Society. 2019;25(4):923–936. doi: 10.1007/s10450-019-00117-2. [DOI] [Google Scholar]
- 8.Ji Y., Zhang H., Ru J., Wang F., Xu M., Zhou Q., Stanikzai H., Yerlan I., Xu Z., Niu Y., Wei J. Creating micro-submicro structure and grafting hydroxyl group on PEEK by femtosecond laser and hydroxylation to synergistically activate cellular response. Mater. Des. 2021;199 doi: 10.1016/j.matdes.2020.109413. [DOI] [Google Scholar]
- 9.Zhang S., Long J., Chen L., Zhang J., Fan Y., Shi J., Huang Y. Treatment methods toward improving the anti-infection ability of poly(etheretherketone) implants for medical applications. Colloids Surf. B Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112769. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Tian W., Chen J., Yu J., Zhang J., Chen J. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res. Bull. 2019;153:143–149. doi: 10.1016/j.brainresbull.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Kurz A., Sessler D.I., Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N. Engl. J. Med. 1996;334(19):1209–1215. doi: 10.1056/nejm199605093341901. [DOI] [PubMed] [Google Scholar]
- 12.Sussman E.M., Halpin M.C., Muster J., Moon R.T., Ratner B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 2014;42(7):1508–1516. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- 13.Ceccarelli J., Putnam A.J. Sculpting the blank slate: how fibrin's support of vascularization can inspire biomaterial design. Acta Biomater. 2014;10(4):1515–1523. doi: 10.1016/j.actbio.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wight T.N. Provisional matrix: a role for versican and hyaluronan, Matrix biology. journal of the International Society for Matrix Biology. 2017;60–61:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodbeck W.G., Colton E., Anderson J.M. Effects of adsorbed heat labile serum proteins and fibrinogen on adhesion and apoptosis of monocytes/macrophages on biomaterials. J. Mater. Sci. Mater. Med. 2003;14(8):671–675. doi: 10.1023/a:1024951330265. [DOI] [PubMed] [Google Scholar]
- 17.Barker T.H., Engler A.J. The provisional matrix: setting the stage for tissue repair outcomes, Matrix biology. journal of the International Society for Matrix Biology. 2017;60–61:1–4. doi: 10.1016/j.matbio.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zdolsek J., Eaton J.W., Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J. Transl. Med. 2007;5:31. doi: 10.1186/1479-5876-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang L., Jennings T.A., Eaton J.W. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc. Natl. Acad. Sci. U. S. A. 1998;95(15):8841–8846. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sottile J., Hocking D.C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell. 2002;13(10):3546–3559. doi: 10.1091/mbc.e02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broughton G., 2nd, Janis J.E., Attinger C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006;117(7 Suppl):12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson B., Ekdahl K.N., Mollnes T.E., Lambris J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 2007;44(1–3):82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Trindade R., Albrektsson T., Tengvall P., Wennerberg A. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin. Implant Dent. Relat. Res. 2016;18(1):192–203. doi: 10.1111/cid.12274. [DOI] [PubMed] [Google Scholar]
- 24.Mahon O.R., Browe D.C., Gonzalez-Fernandez T., Pitacco P., Whelan I.T., Von Euw S., Hobbs C., Nicolosi V., Cunningham K.T., Mills K.H.G., Kelly D.J., Dunne A. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials. 2020;239 doi: 10.1016/j.biomaterials.2020.119833. [DOI] [PubMed] [Google Scholar]
- 25.Hoeft K., Schaefer G.J.L., Kim H., Schumacher D., Bleckwehl T., Long Q., Klinkhammer B.M., Peisker F., Koch L., Nagai J., Halder M., Ziegler S., Liehn E., Kuppe C., Kranz J., Menzel S., Costa I., Wahida A., Boor P., Schneider R.K., Hayat S., Kramann R. Platelet-instructed SPP1(+) macrophages drive myofibroblast activation in fibrosis in a CXCL4-dependent manner. Cell Rep. 2023;42(2) doi: 10.1016/j.celrep.2023.112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delon I., Brown N.H. Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 2007;19(1):43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Cha B.H., Shin S.R., Leijten J., Li Y.C., Singh S., Liu J.C., Annabi N., Abdi R., Dokmeci M.R., Vrana N.E., Ghaemmaghami A.M., Khademhosseini A. Integrin-mediated interactions control macrophage polarization in 3D hydrogels. Adv Healthc Mater. 2017;6(21) doi: 10.1002/adhm.201700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaveri T.D., Lewis J.S., Dolgova N.V., Clare-Salzler M.J., Keselowsky B.G. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35(11):3504–3515. doi: 10.1016/j.biomaterials.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Ma J., Chen T., Mandelin J., Ceponis A., Miller N.E., Hukkanen M., Ma G.F., Konttinen Y.T. Regulation of macrophage activation. Cell. Mol. Life Sci. : CMLS. 2003;60(11):2334–2346. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trindade R., Albrektsson T., Galli S., Prgomet Z., Tengvall P., Wennerberg A. Bone immune response to materials, Part I: titanium, PEEK and copper in comparison to sham at 10 Days in rabbit Tibia. J. Clin. Med. 2018;7(12) doi: 10.3390/jcm7120526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trindade R., Albrektsson T., Galli S., Prgomet Z., Tengvall P., Wennerberg A. Bone immune response to materials, Part II: copper and polyetheretherketone (PEEK) compared to titanium at 10 and 28 Days in rabbit Tibia. J. Clin. Med. 2019;8(6) doi: 10.3390/jcm8060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., Hauch K.D., Laflamme M.A., Murry C.E., Ratner B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. U. S. A. 2010;107(34):15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokarram N., Merchant A., Mukhatyar V., Patel G., Bellamkonda R.V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33(34):8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henson P.M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. Journal of immunology (Baltimore, Md. 1950;107(6):1547–1557. [PubMed] [Google Scholar]
- 36.Higgins D.M., Basaraba R.J., Hohnbaum A.C., Lee E.J., Grainger D.W., Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am. J. Pathol. 2009;175(1):161–170. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pederson L., Ruan M., Westendorf J.J., Khosla S., Oursler M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20764–20769. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen C.K. Wound healing essentials: let there be oxygen, Wound repair and regeneration. official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(1):1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu L.Y., Loi F., Nathan K., Lin T.H., Pajarinen J., Gibon E., Nabeshima A., Cordova L., Jämsen E., Yao Z., Goodman S.B. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. : official publication of the Orthopaedic Research Society. 2017;35(11):2378–2385. doi: 10.1002/jor.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guihard P., Danger Y., Brounais B., David E., Brion R., Delecrin J., Richards C.D., Chevalier S., Rédini F., Heymann D., Gascan H., Blanchard F. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem cells (Dayton, Ohio) 2012;30(4):762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 41.Weber B., Saurer L., Schenk M., Dickgreber N., Mueller C. CX3CR1 defines functionally distinct intestinal mononuclear phagocyte subsets which maintain their respective functions during homeostatic and inflammatory conditions. Eur. J. Immunol. 2011;41(3):773–779. doi: 10.1002/eji.201040965. [DOI] [PubMed] [Google Scholar]
- 42.Sheikh Z., Brooks P.J., Barzilay O., Fine N., Glogauer M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials. 2015;8(9):5671–5701. doi: 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witherel C.E., Abebayehu D., Barker T.H., Spiller K.L. Macrophage and fibroblast interactions in biomaterial-mediated fibrosis. Adv Healthc Mater. 2019;8(4) doi: 10.1002/adhm.201801451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witherel C.E., Sao K., Brisson B.K., Han B., Volk S.W., Petrie R.J., Han L., Spiller K.L. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patil S.D., Papadimitrakopoulos F., Burgess D.J. Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol. Therapeut. 2004;6(6):887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 46.Xu X., Wang L., Luo Z., Ni Y., Sun H., Gao X., Li Y., Zhang S., Li Y., Wei S. Facile and versatile strategy for construction of anti-inflammatory and antibacterial surfaces with polydopamine-mediated liposomes releasing dexamethasone and minocycline for potential implant applications. ACS Appl. Mater. Interfaces. 2017;9(49):43300–43314. doi: 10.1021/acsami.7b06295. [DOI] [PubMed] [Google Scholar]
- 47.Borisenko G.G., Matsura T., Liu S.X., Tyurin V.A., Jianfei J., Serinkan F.B., Kagan V.E. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells--existence of a threshold. Arch. Biochem. Biophys. 2003;413(1):41–52. doi: 10.1016/s0003-9861(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 48.Xie L., Wang G., Wu Y., Liao Q., Mo S., Ren X., Tong L., Zhang W., Guan M., Pan H., Chu P.K., Wang H. Programmed surface on poly(aryl-ether-ether-ketone) initiating immune mediation and fulfilling bone regeneration sequentially. Innovation. 2021;2(3) doi: 10.1016/j.xinn.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun A., Lin X., Xue Z., Huang J., Bai X., Huang L., Lin X., Weng S., Chen M. Facile surface functional polyetheretherketone with antibacterial and immunoregulatory activities for enhanced regeneration toward bacterium-infected bone destruction. Drug Deliv. 2021;28(1):1649–1663. doi: 10.1080/10717544.2021.1960924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mengdi Z., Jiayi L., Canfeng L., Guofeng W., Yutong W., Pengzhou H., Yikun Z., Xintao Z., Bin T. Surface modification of polyetheretherketone (PEEK) to enhance osteointegration by grafting strontium Eucommia ulmoides polysaccharides. Int. J. Biol. Macromol. 2022;211:230–237. doi: 10.1016/j.ijbiomac.2022.05.048. [DOI] [PubMed] [Google Scholar]
- 51.Chai H., Sang S., Luo Y., He R., Yuan X., Zhang X. Icariin-loaded sulfonated polyetheretherketone with osteogenesis promotion and osteoclastogenesis inhibition properties via immunomodulation for advanced osseointegration. J. Mater. Chem. B. 2022;10(18):3531–3540. doi: 10.1039/d1tb02802b. [DOI] [PubMed] [Google Scholar]
- 52.Bridges A.W., Singh N., Burns K.L., Babensee J.E., Andrew Lyon L., García A.J. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials. 2008;29(35):4605–4615. doi: 10.1016/j.biomaterials.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collier T.O., Anderson J.M., Brodbeck W.G., Barber T., Healy K.E. Inhibition of macrophage development and foreign body giant cell formation by hydrophilic interpenetrating polymer network. J. Biomed. Mater. Res., Part A. 2004;69(4):644–650. doi: 10.1002/jbm.a.30030. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X., Xiong D., Liu Y. Improving surface wettability and lubrication of polyetheretherketone (PEEK) by combining with polyvinyl alcohol (PVA) hydrogel. J. Mech. Behav. Biomed. Mater. 2018;82:27–34. doi: 10.1016/j.jmbbm.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y., Xiong D. A tannic acid-reinforced PEEK-hydrogel composite material with good biotribological and self-healing properties for artificial joints. J. Mater. Chem. B. 2021;9(38):8021–8030. doi: 10.1039/d1tb01357b. [DOI] [PubMed] [Google Scholar]
- 56.Yin W.H., Zhou C.H., Ju X.J., Deng Y., Zhang L., Xie R., Wang W., Liu Z., Chu L.Y. Dual-functional polyetheretherketone surface with programmed sequential drug release coating. Colloids Surf. B Biointerfaces. 2022;219 doi: 10.1016/j.colsurfb.2022.112806. [DOI] [PubMed] [Google Scholar]
- 57.Gasmi H., Danede F., Siepmann J., Siepmann F. Does PLGA microparticle swelling control drug release? New insight based on single particle swelling studies. J. Contr. Release : official journal of the Controlled Release Society. 2015;213:120–127. doi: 10.1016/j.jconrel.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 58.Gu B., Papadimitrakopoulos F., Burgess D.J. PLGA microsphere/PVA hydrogel coatings suppress the foreign body reaction for 6 months. J. Contr. Release : official journal of the Controlled Release Society. 2018;289:35–43. doi: 10.1016/j.jconrel.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu B., Burgess D.J. Prediction of dexamethasone release from PLGA microspheres prepared with polymer blends using a design of experiment approach. Int. J. Pharm. 2015;495(1):393–403. doi: 10.1016/j.ijpharm.2015.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J., Wang B.M., Schwendeman S.P. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. J. Contr. Release : official journal of the Controlled Release Society. 2002;82(2–3):289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 61.Kang J., Schwendeman S.P. Pore closing and opening in biodegradable polymers and their effect on the controlled release of proteins. Mol. Pharm. 2007;4(1):104–118. doi: 10.1021/mp060041n. [DOI] [PubMed] [Google Scholar]
- 62.Gu B., Sun X., Papadimitrakopoulos F., Burgess D.J. Seeing is believing, PLGA microsphere degradation revealed in PLGA microsphere/PVA hydrogel composites. J. Contr. Release : official journal of the Controlled Release Society. 2016;228:170–178. doi: 10.1016/j.jconrel.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasmi H., Siepmann F., Hamoudi M.C., Danede F., Verin J., Willart J.F., Siepmann J. Towards a better understanding of the different release phases from PLGA microparticles: dexamethasone-loaded systems. Int. J. Pharm. 2016;514(1):189–199. doi: 10.1016/j.ijpharm.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 64.Colak O., Ozer K., Dikmen A., Ozakinci H., Ozkaya O. Evaluation of safe systemic immunosuppression created with dexamethasone in prevention of capsular contracture: a glance to distinct perspectives with Toll-like receptors. Aesthetic Plast. Surg. 2018;42(4):1133–1143. doi: 10.1007/s00266-018-1119-9. [DOI] [PubMed] [Google Scholar]
- 65.Pakshir P., Younesi F., Wootton K.A., Battiston K., Whitton G., Ilagan B., Louka D., Statham M., Mackey G., Daley A., Parrag I., Naimark W., Hinz B. Controlled release of low-molecular weight, polymer-free corticosteroid coatings suppresses fibrotic encapsulation of implanted medical devices. Biomaterials. 2022;286 doi: 10.1016/j.biomaterials.2022.121586. [DOI] [PubMed] [Google Scholar]
- 66.Polderman J.A., Farhang-Razi V., Van Dieren S., Kranke P., DeVries J.H., Hollmann M.W., Preckel B., Hermanides J. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 2018;8(8):Cd011940. doi: 10.1002/14651858.CD011940.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhardwaj U., Sura R., Papadimitrakopoulos F., Burgess D.J. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int. J. Pharm. 2010;384(1–2):78–86. doi: 10.1016/j.ijpharm.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 68.Richters C.D., Paauw N.J., Mayen I., van Bloois L., Metselaer J.M., Storm G., du Pont J.S., Hoekstra M.J., Kreis R.W., Kamperdijk E.W. Administration of prednisolone phosphate-liposomes reduces wound contraction in a rat partial-thickness wound model. Wound Repair Regen. : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006;14(5):602–607. doi: 10.1111/j.1743-6109.2006.00167.x. [DOI] [PubMed] [Google Scholar]
- 69.Xu X., Li Y., Wang L., Li Y., Pan J., Fu X., Luo Z., Sui Y., Zhang S., Wang L., Ni Y., Zhang L., Wei S. Triple-functional polyetheretherketone surface with enhanced bacteriostasis and anti-inflammatory and osseointegrative properties for implant application. Biomaterials. 2019;212:98–114. doi: 10.1016/j.biomaterials.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 70.Quan H., Kim Y., Park H.C., Yang H.-C. Effects of phosphatidylserine-containing supported lipid bilayers on the polarization of macrophages. J. Biomed. Mater. Res. 2018;106(10):2625–2633. doi: 10.1002/jbm.a.36454. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z., Duan Y., Duan Y. Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J. Contr. Release : official journal of the Controlled Release Society. 2018;290:56–74. doi: 10.1016/j.jconrel.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Gao C., Wang Y., Han F., Yuan Z., Li Q., Shi C., Cao W., Zhou P., Xing X., Li B. Antibacterial activity and osseointegration of silver-coated poly(ether ether ketone) prepared using the polydopamine-assisted deposition technique. J. Mater. Chem. B. 2017;5(47):9326–9336. doi: 10.1039/c7tb02436c. [DOI] [PubMed] [Google Scholar]
- 73.Deng Y., Ma F., Ruiz-Ortega L.I., Peng Y., Tian Y., He W., Tang B. Fabrication of strontium Eucommia ulmoides polysaccharides and in vitro evaluation of their osteoimmunomodulatory property. Int. J. Biol. Macromol. 2019;140:727–735. doi: 10.1016/j.ijbiomac.2019.08.145. [DOI] [PubMed] [Google Scholar]
- 74.Braux J., Velard F., Guillaume C., Bouthors S., Jallot E., Nedelec J.M., Laurent-Maquin D., Laquerrière P. A new insight into the dissociating effect of strontium on bone resorption and formation. Acta Biomater. 2011;7(6):2593–2603. doi: 10.1016/j.actbio.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Wang Q.S., Wang G.F., Lu Y.R., Cui Y.L., Li H., Li R.X., Zhang X.Z., Zhang C.Q., Liu T.J. The Combination of icariin and constrained dynamic loading stimulation attenuates bone loss in ovariectomy-induced osteoporotic mice. J. Orthop. Res. : official publication of the Orthopaedic Research Society. 2018;36(5):1415–1424. doi: 10.1002/jor.23777. [DOI] [PubMed] [Google Scholar]
- 76.Toita R., Rashid S.A.N., Tsuru K., Ishikawa K. Modulation of the osteoconductive property and immune response of poly(ether ether ketone) by modification with calcium ions. J. Mater. Chem. B. 2015;3(44):8738–8746. doi: 10.1039/c5tb01679g. [DOI] [PubMed] [Google Scholar]
- 77.Zheng Y., Gao A., Bai J., Liao Q., Wu Y., Zhang W., Guan M., Tong L., Geng D., Zhao X., Chu P.K., Wang H. A programmed surface on polyetheretherketone for sequentially dictating osteoimmunomodulation and bone regeneration to achieve ameliorative osseointegration under osteoporotic conditions. Bioact. Mater. 2022;14:364–376. doi: 10.1016/j.bioactmat.2022.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fukuda N., Tsuchiya A., Sunarso, Toita R., Tsuru K., Mori Y., Ishikawa K. Surface plasma treatment and phosphorylation enhance the biological performance of poly(ether ether ketone) Colloids Surf. B Biointerfaces. 2019;173:36–42. doi: 10.1016/j.colsurfb.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 79.Mahjoubi H., Buck E., Manimunda P., Farivar R., Chromik R., Murshed M., Cerruti M. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017;47:149–158. doi: 10.1016/j.actbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 80.Liu L., Zhang W., Yuan L., Liu Y., Zheng Y. Ameliorative antibacterial, anti-inflammatory, and osteogenic activity of sulfonate-bearing polyetheretherketone toward orthopedic and dental implants. Mater. Lett. 2021;305 doi: 10.1016/j.matlet.2021.130774. [DOI] [Google Scholar]
- 81.Chen Z., Mao X., Tan L., Friis T., Wu C., Crawford R., Xiao Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials. 2014;35(30):8553–8565. doi: 10.1016/j.biomaterials.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 82.Liu W., Li J., Cheng M., Wang Q., Yeung K.W.K., Chu P.K., Zhang X. Zinc-modified sulfonated polyetheretherketone surface with immunomodulatory function for guiding cell fate and bone regeneration. Adv. Sci. 2018;5(10) doi: 10.1002/advs.201800749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang F., Sun P., Xie E., Ji Y., Niu Y., Li F., Wei J. Phytic acid/magnesium ion complex coating on PEEK fiber woven fabric as an artificial ligament with anti-fibrogenesis and osteogenesis for ligament-bone healing. Biomaterials advances. 2022;140 doi: 10.1016/j.bioadv.2022.213079. [DOI] [PubMed] [Google Scholar]
- 84.Hu L., Ge Y., Cao Z., Tian Y., Sun Q., Li Z., Ma J., Wu Y., Wang N., Tang B. Strontium-modified porous polyetheretherketone with the triple function of osteogenesis, angiogenesis, and anti-inflammatory for bone grafting. Biomaterials advances. 2022;143 doi: 10.1016/j.bioadv.2022.213160. [DOI] [PubMed] [Google Scholar]
- 85.Zheng Z., Hu L., Ge Y., Qi J., Sun Q., Li Z., Lin L., Tang B. Surface modification of poly(ether ether ketone) by simple chemical grafting of strontium chondroitin sulfate to improve its anti-inflammation, angiogenesis, osteogenic properties. Adv Healthc Mater. 2022;11(13) doi: 10.1002/adhm.202200398. [DOI] [PubMed] [Google Scholar]
- 86.Liu W., Li J., Cheng M., Wang Q., Qian Y., Yeung K.W.K., Chu P.K., Zhang X. A surface-engineered polyetheretherketone biomaterial implant with direct and immunoregulatory antibacterial activity against methicillin-resistant Staphylococcus aureus. Biomaterials. 2019;208:8–20. doi: 10.1016/j.biomaterials.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Gao C., Wang Y., Han F., Yuan Z., Li Q., Shi C., Cao W., Zhou P., Xing X., Li B. Antibacterial activity and osseointegration of silver-coated poly(ether ether ketone) prepared using the polydopamine-assisted deposition technique. J. Mater. Chem. B. 2017;5(47):9326–9336. doi: 10.1039/c7tb02436c. [DOI] [PubMed] [Google Scholar]
- 88.Yang C., Ouyang L., Wang W., Chen B., Liu W., Yuan X., Luo Y., Cheng T., Yeung K.W.K., Liu X., Zhang X. Sodium butyrate-modified sulfonated polyetheretherketone modulates macrophage behavior and shows enhanced antibacterial and osteogenic functions during implant-associated infections. J. Mater. Chem. B. 2019;7(36):5541–5553. doi: 10.1039/c9tb01298b. [DOI] [PubMed] [Google Scholar]
- 89.Buck E., Lee S., Gao Q., Tran S.D., Tamimi F., Stone L.S., Cerruti M. The role of surface chemistry in the osseointegration of PEEK implants. ACS Biomater. Sci. Eng. 2022;8(4):1506–1521. doi: 10.1021/acsbiomaterials.1c01434. [DOI] [PubMed] [Google Scholar]
- 90.Buck E., Lee S., Stone L.S., Cerruti M. Protein adsorption on surfaces functionalized with COOH groups promotes anti-inflammatory macrophage responses. ACS Appl. Mater. Interfaces. 2021;13(6):7021–7036. doi: 10.1021/acsami.0c16509. [DOI] [PubMed] [Google Scholar]
- 91.Vimbela G.V., Ngo S.M., Fraze C., Yang L., Stout D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017;12:3941–3965. doi: 10.2147/ijn.S134526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao H., Wang X., Zhang W., Wang L., Zhu C., Huang Y., Chen R., Chen X., Wang M., Pan G., Shi Q., Zhou X. Bioclickable mussel-derived peptides with immunoregulation for osseointegration of PEEK. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.780609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xian P., Chen Y., Gao S., Qian J., Zhang W., Udduttula A., Huang N., Wan G. Polydopamine (PDA) mediated nanogranular-structured titanium dioxide (TiO2) coating on polyetheretherketone (PEEK) for oral and maxillofacial implants application. Surf. Coating. Technol. 2020;401 doi: 10.1016/j.surfcoat.2020.126282. [DOI] [Google Scholar]
- 94.Tsou H.K., Chi M.H., Hung Y.W., Chung C.J., He J.L. In vivo osseointegration performance of titanium dioxide coating modified polyetheretherketone using arc ion plating for spinal implant application. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/328943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Y., Zhang H., Komasa S., Kusumoto T., Kuwamoto S., Okunishi T., Kobayashi Y., Hashimoto Y., Sekino T., Okazaki J. Immunomodulatory properties and osteogenic activity of polyetheretherketone coated with titanate nanonetwork structures. Int. J. Mol. Sci. 2022;23(2) doi: 10.3390/ijms23020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X., Ouyang L., Chen L., Qiao Y., Ma X., Xu G., Liu X. Hydroxyapatite composited PEEK with 3D porous surface enhances osteoblast differentiation through mediating NO by macrophage. Regenerative biomaterials. 2022;9 doi: 10.1093/rb/rbab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walsh W.R., Pelletier M.H., Bertollo N., Christou C., Tan C. Does PEEK/HA enhance bone formation compared with PEEK in a sheep cervical fusion model? Clin. Orthop. Relat. Res. 2016;474(11):2364–2372. doi: 10.1007/s11999-016-4994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frankenberger T., Graw C.L., Engel N., Gerber T., Frerich B., Dau M. Sustainable surface modification of polyetheretherketone (PEEK) implants by hydroxyapatite/silica coating-an in vivo animal study. Materials. 2021;14(16) doi: 10.3390/ma14164589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.MacLeod R.J., Hayes M., Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293(1):G403–G411. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- 100.De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim. Biophys. Sin. 2011;43(10):745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 101.Lv Z., Xu X., Sun Z., Yang Y.X., Guo H., Li J., Sun K., Wu R., Xu J., Jiang Q., Ikegawa S., Shi D. TRPV1 alleviates osteoarthritis by inhibiting M1 macrophage polarization via Ca(2+)/CaMKII/Nrf2 signaling pathway. Cell Death Dis. 2021;12(6):504. doi: 10.1038/s41419-021-03792-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Z., Wu C., Gu W., Klein T., Crawford R., Xiao Y. Osteogenic differentiation of bone marrow MSCs by β-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014;35(5):1507–1518. doi: 10.1016/j.biomaterials.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 103.Toita Sunarso R., Tsuru K., Ishikawa K. Immobilization of calcium and phosphate ions improves the osteoconductivity of titanium implants, Materials science & engineering. C, Materials for biological applications. 2016;68:291–298. doi: 10.1016/j.msec.2016.05.090. [DOI] [PubMed] [Google Scholar]
- 104.Villa-Bellosta R., Hamczyk M.R., Andres V. Novel phosphate-activated macrophages prevent ectopic calcification by increasing extracellular ATP and pyrophosphate. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Griffete N., Lamouri A., Herbst F., Felidj N., Ammar S., Mangeney C. Synthesis of highly soluble polymer-coated magnetic nanoparticles using a combination of diazonium salt chemistry and the iniferter method. RSC Adv. 2012;2(3):826–830. doi: 10.1039/c1ra00577d. [DOI] [Google Scholar]
- 106.Zheng Y.Y., Liu L.H., Ma Y., Xiao L., Liu Y. Enhanced osteoblasts responses to surface-sulfonated polyetheretherketone via a single-step ultraviolet-initiated graft polymerization. Ind. Eng. Chem. Res. 2018;57(31):10403–10410. doi: 10.1021/acs.iecr.8b02158. [DOI] [Google Scholar]
- 107.Feng P., Wu P., Gao C., Yang Y., Guo W., Yang W., Shuai C. A multimaterial scaffold with Tunable properties: toward bone tissue repair. Adv. Sci. 2018;5(6) doi: 10.1002/advs.201700817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ofluoglu A.E., Erdogan U., Aydogan M., Cevik O.M., Ofluoglu O. Anterior cervical fusion with interbody cage containing beta-tricalcium phosphate: clinical and radiological results. Acta Orthop. Traumatol. Turcica. 2017;51(3):197–200. doi: 10.1016/j.aott.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Velard F., Braux J., Amedee J., Laquerriere P. Inflammatory cell response to calcium phosphate biomaterial particles: an overview. Acta Biomater. 2013;9(2):4956–4963. doi: 10.1016/j.actbio.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 110.Hojyo S., Fukada T. Roles of zinc signaling in the immune system. Journal of immunology research. 2016;2016 doi: 10.1155/2016/6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gou Y., Yang D., Tian T., Zhu X., Zhang R., Ren J., Tu D., Luo Y., Miao Y., Zhao H., Wang Y., Wei B. The transcription of ZIP9 is associated with the macrophage polarization and the pathogenesis of hepatocellular carcinoma. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.725595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin Z., Shen D., Zhou W., Zheng Y., Kong T., Liu X., Wu S., Chu P.K., Zhao Y., Wu J., Cheung K.M.C., Yeung K.W.K. Regulation of extracellular bioactive cations in bone tissue microenvironment induces favorable osteoimmune conditions to accelerate in situ bone regeneration. Bioact. Mater. 2021;6(8):2315–2330. doi: 10.1016/j.bioactmat.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cai K., Shen W., Ren B., He J., Wu S., Wang W. A phytic acid modified CoFe2O4 magnetic adsorbent with controllable morphology, excellent selective adsorption for dyes and ultra-strong adsorption ability for metal ions. Chem. Eng. J. 2017;330:936–946. doi: 10.1016/j.cej.2017.08.009. [DOI] [Google Scholar]
- 114.Gu Z., Xie H., Huang C., Peng H., Tan H., Li L., Yu X. Effects of strontium-doped calcium polyphosphate on angiogenic growth factors expression of co-culturing system in vitro and of host cell in vivo. RSC Adv. 2014;4(6):2783–2792. doi: 10.1039/c3ra44323j. [DOI] [Google Scholar]
- 115.Huang X., Guo X., Qu L., Wu Z., Yu T., Jiao Y., Zhou C. Gradient regulation of osteo-immune microenvironment by chitooligosaccharide-containing ion-doped mesoporous silica nanoparticles to accelerate osteogenesis. Appl. Mater. Today. 2021;23 doi: 10.1016/j.apmt.2021.101067. [DOI] [Google Scholar]
- 116.Zhu S., Hu X., Tao Y., Ping Z., Wang L., Shi J., Wu X., Zhang W., Yang H., Nie Z., Xu Y., Wang Z., Geng D. Strontium inhibits titanium particle-induced osteoclast activation and chronic inflammation via suppression of NF-κB pathway. Sci. Rep. 2016;6 doi: 10.1038/srep36251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sang S., Yang C., Chai H., Yuan X., Liu W., Zhang X. The sulfonated polyetheretherketone with 3D structure modified by two bio-inspired methods shows osteogenic and antibacterial functions. Chem. Eng. J. 2021;420 doi: 10.1016/j.cej.2021.130059. [DOI] [Google Scholar]
- 118.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 2010;23(2):366–384. doi: 10.1017/s0954422410000247. [DOI] [PubMed] [Google Scholar]
- 120.Chen G., Ran X., Li B., Li Y., He D., Huang B., Fu S., Liu J., Wang W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ouyang L., Sun Z., Wang D., Qiao Y., Zhu H., Ma X., Liu X. Smart release of doxorubicin loaded on polyetheretherketone (PEEK) surface with 3D porous structure. Colloids Surf. B Biointerfaces. 2018;163:175–183. doi: 10.1016/j.colsurfb.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 122.Huang Y.J., Hung K.C., Hsieh F.Y., Hsu S.H. Carboxyl-functionalized polyurethane nanoparticles with immunosuppressive properties as a new type of anti-inflammatory platform. Nanoscale. 2015;7(48):20352–20364. doi: 10.1039/c5nr06379e. [DOI] [PubMed] [Google Scholar]
- 123.Swartzlander M.D., Barnes C.A., Blakney A.K., Kaar J.L., Kyriakides T.R., Bryant S.J. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials. 2015;41:26–36. doi: 10.1016/j.biomaterials.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Y., Yang Y., Hua Z., Li S., Yang Z., Liu Q., Fu G., Ji P., Wu Q. BMP2 immune complexes promote new bone formation by facilitating the direct contact between osteoclasts and osteoblasts. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120890. [DOI] [PubMed] [Google Scholar]
- 125.Wei F., Zhou Y., Wang J., Liu C., Xiao Y. The immunomodulatory role of BMP-2 on macrophages to accelerate osteogenesis, tissue engineering. Part A. 2018;24(7–8):584–594. doi: 10.1089/ten.TEA.2017.0232. [DOI] [PubMed] [Google Scholar]
- 126.Jia Z., Xiu P., Li M., Xu X., Shi Y., Cheng Y., Wei S., Zheng Y., Xi T., Cai H., Liu Z. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials. 2016;75:203–222. doi: 10.1016/j.biomaterials.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 127.Li L.H., Kong Y.M., Kim H.W., Kim Y.W., Kim H.E., Heo S.J., Koak J.Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004;25(14):2867–2875. doi: 10.1016/j.biomaterials.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 128.Kong F., Nie Z., Liu Z., Hou S., Ji J. Developments of nano-TiO2 incorporated hydroxyapatite/PEEK composite strut for cervical reconstruction and interbody fusion after corpectomy with anterior plate fixation. J. Photochem. Photobiol. B Biol. 2018;187:120–125. doi: 10.1016/j.jphotobiol.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 129.Tsou H.K., Hsieh P.Y., Chung C.J., Tang C.H., Shyr T.W., He J.L. Low-temperature deposition of anatase TiO2 on medical grade polyetheretherketone to assist osseous integration. Surf. Coating. Technol. 2009;204(6–7):1121–1125. doi: 10.1016/j.surfcoat.2009.06.018. [DOI] [Google Scholar]
- 130.Chung C.J., Lin H.I., He J.L. Antimicrobial efficacy of photocatalytic TiO2 coatings prepared by arc ion plating. Surf. Coating. Technol. 2007;202(4–7):1302–1307. doi: 10.1016/j.surfcoat.2007.07.077. [DOI] [Google Scholar]
- 131.Cheng B.C., Jaffee S., Averick S., Swink I., Horvath S., Zhukauskas R. A comparative study of three biomaterials in an ovine bone defect model. Spine J. : official journal of the North American Spine Society. 2020;20(3):457–464. doi: 10.1016/j.spinee.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 132.Huang Y., He J., Gan L., Liu X., Wu Y., Wu F., Gu Z.W. Osteoconductivity and osteoinductivity of porous hydroxyapatite coatings deposited by liquid precursor plasma spraying: in vivo biological response study. Biomed. Mater. (Bristol, U. K.) 2014;9(6) doi: 10.1088/1748-6041/9/6/065007. [DOI] [PubMed] [Google Scholar]
- 133.Walsh W.R., Bertollo N., Christou C., Schaffner D., Mobbs R.J. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. : official journal of the North American Spine Society. 2015;15(5):1041–1049. doi: 10.1016/j.spinee.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 134.Yoon B.J., Xavier F., Walker B.R., Grinberg S., Cammisa F.P., Abjornson C. Optimizing surface characteristics for cell adhesion and proliferation on titanium plasma spray coatings on polyetheretherketone. Spine J. : official journal of the North American Spine Society. 2016;16(10):1238–1243. doi: 10.1016/j.spinee.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 135.Torstrick F.B., Lin A.S.P., Potter D., Safranski D.L., Sulchek T.A., Gall K., Guldberg R.E. Porous PEEK improves the bone-implant interface compared to plasma-sprayed titanium coating on PEEK. Biomaterials. 2018;185:106–116. doi: 10.1016/j.biomaterials.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 136.Wong K.L., Wong C.T., Liu W.C., Pan H.B., Fong M.K., Lam W.M., Cheung W.L., Tang W.M., Chiu K.Y., Luk K.D., Lu W.W. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials. 2009;30(23–24):3810–3817. doi: 10.1016/j.biomaterials.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 137.Durham J.W., III, Montelongo S.A., Ong J.L., Guda T., Allen M.J., Rabiei A. Hydroxyapatite coating on PEEK implants: biomechanical and histological study in a rabbit model. Materials Science & Engineering C-Materials for Biological Applications. 2016;68:723–731. doi: 10.1016/j.msec.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan K.H., Chua C.K., Leong K.F., Cheah C.M., Cheang P., Abu Bakar M.S., Cha S.W. Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials. 2003;24(18):3115–3123. doi: 10.1016/s0142-9612(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 139.Almasi D., Izman S., Assadian M., Ghanbari M., Kadir M.R.A. Crystalline ha coating on peek via chemical deposition. Appl. Surf. Sci. 2014;314:1034–1040. doi: 10.1016/j.apsusc.2014.06.074. [DOI] [Google Scholar]
- 140.Tang S.M., Cheang P., AbuBakar M.S., Khor K.A., Liao K. Tension-tension fatigue behavior of hydroxyapatite reinforced polyetheretherketone composites. Int. J. Fatig. 2004;26(1):49–57. doi: 10.1016/s0142-1123(03)00080-x. [DOI] [Google Scholar]
- 141.Evans N.T., Torstrick F.B., Lee C.S., Dupont K.M., Safranski D.L., Chang W.A., Macedo A.E., Lin A.S., Boothby J.M., Whittingslow D.C., Carson R.A., Guldberg R.E., Gall K. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015;13:159–167. doi: 10.1016/j.actbio.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khoury J., Selezneva I., Pestov S., Tarassov V., Ermakov A., Mikheev A., Lazov M., Kirkpatrick S.R., Shashkov D., Smolkov A. Surface bioactivation of PEEK by neutral atom beam technology. Bioact. Mater. 2019;4:132–141. doi: 10.1016/j.bioactmat.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Z., Ni S., Han S., Crawford R., Lu S., Wei F., Chang J., Wu C., Xiao Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale. 2017;9(2):706–718. doi: 10.1039/c6nr06421c. [DOI] [PubMed] [Google Scholar]
- 144.Wei R., Wu J., Li Y. Macrophage polarization following three-dimensional porous PEEK, Materials science & engineering. C, Materials for biological applications. 2019;104 doi: 10.1016/j.msec.2019.109948. [DOI] [PubMed] [Google Scholar]
- 145.Kelly C.N., Wang T., Crowley J., Wills D., Pelletier M.H., Westrick E.R., Adams S.B., Gall K., Walsh W.R. High-strength, porous additively manufactured implants with optimized mechanical osseointegration. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121206. [DOI] [PubMed] [Google Scholar]
- 146.Yu Y., Xie K., Xie L., Deng Y. Endowing polyetheretherketone with anti-inflammatory ability and improved osteogenic ability, Journal of biomaterials science. Polymer edition. 2021;32(1):42–59. doi: 10.1080/09205063.2020.1815634. [DOI] [PubMed] [Google Scholar]
- 147.Li Y., Wang J., He D., GuoxiongZhu, Wu G., Chen L. Surface sulfonation and nitrification enhance the biological activity and osteogenesis of polyetheretherketone by forming an irregular nano-porous monolayer. J. Mater. Sci. Mater. Med. 2019;31(1):11. doi: 10.1007/s10856-019-6349-0. [DOI] [PubMed] [Google Scholar]
- 148.Huo S., Meng X., Zhang S., Yue B., Zhao Y., Long T., Nie B., Wang Y. Hydrofluoric acid and nitric acid cotreatment for biofunctionalization of polyetheretherketone in M2 macrophage polarization and osteogenesis. J. Biomed. Mater. Res., Part A. 2021;109(6):879–892. doi: 10.1002/jbm.a.37079. [DOI] [PubMed] [Google Scholar]
- 149.Feng X., Yu H., Liu H., Yu X., Feng Z., Bai S., Zhao Y. Three-dimensionally-printed polyether-ether-ketone implant with a cross-linked structure and acid-etched microporous surface promotes integration with soft tissue. Int. J. Mol. Sci. 2019;20(15) doi: 10.3390/ijms20153811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan Z., Long T., Zhang J., Lyu Z., Zhang W., Meng X., Qi J., Wang Y. 3D printed porous sulfonated polyetheretherketone scaffold for cartilage repair: potential and limitation. Journal of orthopaedic translation. 2022;33:90–106. doi: 10.1016/j.jot.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao A., Liao Q., Xie L., Wang G., Zhang W., Wu Y., Li P., Guan M., Pan H., Tong L., Chu P.K., Wang H. Tuning the surface immunomodulatory functions of polyetheretherketone for enhanced osseointegration. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119642. [DOI] [PubMed] [Google Scholar]
- 152.Ouyang L.P., Chen M.L., Wang D.H., Lu T.Y., Wang H.Y., Meng F.H., Yang Y., Ma J.Z., Yeung K.W.K., Liu X.Y. Nano Textured PEEK surface for enhanced osseointegration. ACS Biomater. Sci. Eng. 2019;5(3):1279–1289. doi: 10.1021/acsbiomaterials.8b01425. [DOI] [PubMed] [Google Scholar]
- 153.Wu H., Wei X., Liu Y., Dong H., Tang Z., Wang N., Bao S., Wu Z., Shi L., Zheng X., Li X., Guo Z. Dynamic degradation patterns of porous polycaprolactone/β-tricalcium phosphate composites orchestrate macrophage responses and immunoregulatory bone regeneration. Bioact. Mater. 2023;21:595–611. doi: 10.1016/j.bioactmat.2022.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fu Z., Hou Y., Haugen H.J., Chen X., Tang K., Fang L., Liu Y., Zhang S., Ma Q., Chen L. TiO(2) nanostructured implant surface-mediated M2c polarization of inflammatory monocyte requiring intact cytoskeleton rearrangement. J. Nanobiotechnol. 2023;21(1):1. doi: 10.1186/s12951-022-01751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu D., Huang Y., Bu D., Liu A.D., Holmberg L., Jia Y., Tang C., Du J., Jin H. Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling. Cell Death Dis. 2014;5(5):e1251. doi: 10.1038/cddis.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen M., Ouyang L., Lu T., Wang H., Meng F., Yang Y., Ning C., Ma J., Liu X. Enhanced bioactivity and bacteriostasis of surface fluorinated polyetheretherketone. ACS Appl. Mater. Interfaces. 2017;9(20):16824–16833. doi: 10.1021/acsami.7b02521. [DOI] [PubMed] [Google Scholar]
- 157.Zhang C., Wang L., Kang J., Fuentes O.M., Li D. Bionic design and verification of 3D printed PEEK costal cartilage prosthesis. J. Mech. Behav. Biomed. Mater. 2020;103 doi: 10.1016/j.jmbbm.2019.103561. [DOI] [PubMed] [Google Scholar]
- 158.Karageorgiou V., Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 159.Converse G.L., Conrad T.L., Roeder R.K. Mechanical properties of hydroxyapatite whisker reinforced polyetherketoneketone composite scaffolds. J. Mech. Behav. Biomed. Mater. 2009;2(6):627–635. doi: 10.1016/j.jmbbm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 160.Ferreira A.M., Tonda-Turo C., Mancuso E., Gentile P. Multilayer nanoscale functionalization to treat disorders and enhance regeneration of bone tissue. Nanomed. Nanotechnol. Biol. Med. 2019;19:22–38. doi: 10.1016/j.nano.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 161.Liu Z., Tang Q., Liu R.T., Yu M.Z., Peng H., Zhang C.Q., Zhu Z.Z., Wei X.J. Laponite intercalated biomimetic multilayer coating prevents glucocorticoids induced orthopedic implant failure. Bioact. Mater. 2023;22:60–73. doi: 10.1016/j.bioactmat.2022.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Benčina M., Resnik M., Starič P., Junkar I. Use of plasma technologies for antibacterial surface properties of metals. Molecules. 2021;26(5) doi: 10.3390/molecules26051418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.