Abstract
Background
The predisposing factors and clinical presentations of fungal foot infections caused by non-dermatophytes and dermatophytes are challenging to differentiate. Definite diagnoses of non-dermatophyte infections at first visits facilitate their treatment.
Objectives
This study aimed to develop diagnostic criteria to differentiate fungal foot infections caused by Neoscytalidium dimidiatum and dermatophytes.
Methods
Diagnostic prediction research based on a retrospective, observational, cross-sectional study. The reviewed patients were aged ≥18 and underwent a mycological examination for fungal foot infections. A fungal culture at the initial visit was the gold standard for determining causative organisms.
Results
Analyses were carried out on the data from 371 patients. N. dimidiatum accounted for 184 (49.6%) infections, and dermatophytes caused the remaining 187 (50.4%) cases. Five significant predefined predictors were used to develop the diagnostic criteria and score. They were immunocompetence status, no family history of fungal infections, the absence of pruritus, the absence of other concurrent fungal skin infections, and agricultural work. The lower score cutoff was <8 (sensitivity 97.8% and specificity 25.7%). The higher cutoff was >11 (sensitivity 83.7% and specificity 57.8%). The score showed an area under the receiver operating characteristic curve of 0.755 and was well calibrated.
Conclusions
The criteria and score show promise for clinical use, with acceptable discriminative performance and good calibration. They will help physicians differentiate the causative organisms in patients with fungal foot infections at the first visit, enabling the determination of appropriate antifungal treatment.
Keywords: Neoscytalidium dimidiatum, Dermatophytes, Superficial fungal infection, Diagnosis, Non-dermatophytes, tinea pedis, fungal foot infection
1. Introduction
Fungal foot infections are common worldwide, with reported prevalences 0f 3.5%–40.6% among patients visiting health centers [[1], [2], [3], [4], [5]]. Diabetes mellitus, peripheral vascular disease, wearing slippers, and repetitive foot injuries have been associated with a higher risk of fungal foot infections [1]. The infections are caused by dermatophytes and non-dermatophyte molds and yeasts [4,[6], [7], [8], [9]]. The prevalence of non-dermatophyte foot infections is reportedly increasing, with studies finding that non-dermatophytes cause between 17% and 58% of fungal foot infections [2,10]. The variation in causative agents may reflect differences in the diagnostic methods employed and in the agents’ geographic distribution [1,10,11].
Neoscytalidium dimidiatum is a dematiaceous ascomycete and is usually transmitted to humans from the soil and the environment. The mold is commonly found in tropical countries, including Thailand, Singapore, and Brazil, and it causes nail and superficial skin lesions [1,[11], [12], [13], [14], [15]]. The presence of dematiaceous hyphae in a potassium hydroxide examination of skin lesion scrapings from skin lesions cannot identify causative species. Rather, fungal culture is the gold standard for diagnosis. Unfortunately, only some hospitals have the resources to perform and expertly interpret fungal cultures.
Notably, several studies have reported indistinguishable risk factors and clinical findings of dermatophyte and non-dermatophyte fungal foot infections [[1], [2], [3],10,16,17]. Regarding treatment and outcomes, topical antifungal medications, such as azoles and allylamines, are the primary treatment for dermatophyte foot infections. Systemic antifungal agents are reserved for patients who do not respond to topical therapy. In contrast to non-dermatophyte foot infections, particularly Neoscytalidium infection, which typically resist traditional topical treatment, a combination of topical and systemic antifungal medications or that of topical antifungal and keratolytic medication is recommended as the first treatment for non-dermatophyte foot infections [14,18]. Early diagnosis of non-dermatophyte infections would facilitate their treatment.
This study aimed to elucidate the risk factors, clinical features, and appropriate treatment of patients with Neoscytalidium dimidiatum foot infections. Moreover, diagnostic criteria and scores were developed from relevant predictive factors for differentiating between diagnoses of Neoscytalidium dimidiatum and dermatophyte foot infections.
2. Materials and methods
2.1. Design and setting
Diagnostic prediction research was conducted. It was based on a retrospective observational cross-sectional study. The reviewed patients attended the dermatology outpatient clinic of an academic university hospital between January 1, 2010 and December 31, 2021. All were ≥18 years of age and underwent a mycological examination for a fungal foot infection. Before this research began, informed consent was obtained, and its protocol was approved by the Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand Ethics Committee (Si-328/2022).
2.2. Study population
Patients with suspected fungal foot infections were enrolled. The scales from their foot lesions were examined using a potassium hydroxide preparation and a fungal culture. Direct microscopic examination using 20% potassium hydroxide was employed to detect the presence of fungal hyphae and arthrospores, supporting the diagnosis of fungal foot infection. In addition, fungal cultures were utilized to identify the molds by observing their microscopic and macroscopic characteristics. Sabouraud dextrose agar (SDA) with cycloheximide was employed to grow dermatophytes, while SDA without cycloheximide was used for non-dermatophytes. Chloramphenicol was added to inhibit bacterial growth. The cultures were incubated at a temperature of 27 °C and assessed for fungal growth every 2 weeks over 6 weeks. The identification of fungi was based on the consensus agreement of three experienced fungal microscopists/technicians with over 5 years of expertise in mycological laboratory work. In case of any controversial results, the cultures are further analyzed using molecular methods. The participants were classified into two groups based on the initial culture results: Neoscytalidium and dermatophyte foot infection groups. Diagnoses of a Neoscytalidium foot infection followed the non-dermatophyte onychomycosis criteria [6]. Two consecutive positive cultures for Neoscytalidium dimidiatum, within 1–2 weeks, were used to confirm Neoscytalidium dimidiatum as the true pathogen. Diagnoses of a dermatophyte foot infection were based on dermatophyte growth in one culture. Patients were excluded from the analyses if they had a negative mycological culture, a mixed non-dermatophyte and dermatophyte infection, or an infection caused by a non-dermatophyte other than Neoscytalidium dimidiatum.
2.3. Data collection and predictors
Demographic data at the baseline visit, including age and gender, were collected. The following predisposing factors for fungal feet infection were reviewed.
-
•
history of previous fungal foot infections
-
•
agricultural work
-
•
using occlusive shoes for >6 h daily
-
•
foot hyperhidrosis for >6 months
-
•
history of using a topical steroid on the feet for >1 week during the previous 3 months
-
•
contact with animals
-
•
walking barefoot
Data on the symptoms at initial visits were collected. The symptoms included pruritus, pain, and clinical findings (for example, the type of fungal foot infection, bilateral or unilateral involvement, and the infection site). Concurrent fungal nail and other skin infections were recorded. The presence of peripheral vascular disease, lymphedema, malalignment of the feet, diabetes mellitus, obesity, or an immunocompromised status were considered comorbidities. Laboratory results were reviewed at the initial visit, the treatment administered, and the clinical outcomes.
2.4. Study size estimation
The sample calculation was from a study comparing the proportions of patients with Neoscytalidium and dermatophyte foot infections who worked in agriculture, a factor identified as a potentially significant predictor [12]. The proportions of patients involved in agricultural work were 0.29 and 0.16 in the Neoscytalidium and dermatophyte infection groups, respectively. To achieve 80% statistical power and a two-sided alpha error of 0.05, a sample size of 322 patients was required.
2.5. Statistical analysis
Analyses were performed using Stata Statistical Software, release 17 (StataCorp LLC, College Station, TX, USA). Descriptive statistical analysis was used for patients' demographic and clinical characteristics. We described continuous variables using the mean and standard deviation and categorical variables using their frequency and percentage. For comparisons between Neoscytalidium and dermatophyte infections, Fisher's exact probability test was used for categorical variables, while an independent t-test was used for continuous variables. The area under the receiver operating characteristic (AuROC) curve was used to examine the discriminative ability of each potential predictor. Statistical significance was set at p < 0.05.
Potential predictors for the diagnostic score were selected based on a literature review and variables reaching statistical significance in univariate analysis. A multivariate logistic model included all predictors with a univariate p-value <0.05. A full multivariate model without elimination was used to develop the diagnostic score. Each diagnostic factor was allocated a weighted score calculated from the logit coefficient of each predictor. Predictive performances, including model discrimination and calibration, were evaluated using the sum of the scores for the individual factors. The AuROC curve was analyzed to determine discriminative ability. A calibration plot and a Hosmer–Lemeshow goodness-of-fit test were then used to prove the score calibration. A score calibration plot was created by contrasting the sum of the total scores with the observed proportion of Neoscytalidium infections within each score stratum.
The patients in the dataset were grouped into three risk categories for Neoscytalidium foot infection: low, moderate, and high, to determine the clinical applicability of the proposed score. The score cutoffs were based on sensitivities and specificities suitable for clinical practice. A higher sensitivity with acceptable specificity was required for a lower cutoff, whereas a higher specificity with acceptable sensitivity was needed for a higher cutoff. The positive likelihood ratio (LHR+) with its corresponding 95% confidence interval (CI) was analyzed to determine the ability of each score category to predict Neoscytalidium foot infections. Internal validation of the proposed score with bootstrap resampling procedures was used to quantify the optimism of the derived score in diagnosing Neoscytalidium foot infections.
3. Results
3.1. Baseline characteristics
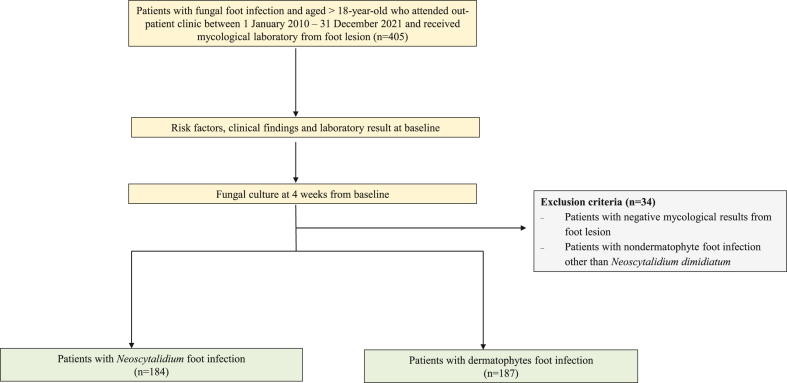
A total of 405 patients with fungal foot infections were enrolled. Thirty-four patients were subsequently excluded due to having yeast, Fusarium, and Aspergillus infections (Fig. 1). The remaining 371 patients were included in the analysis. Their mean age was 63.3 (SD ± 12.4) years, and 214 (57.5%) were male. Neoscytalidium dimidiatum was the causative agent in 184 cases (49.6%) and dermatophytes in 187 cases (50.4%). Among the 187 patients with dermatophyte foot infections, Trichophyton interdigitale predominated (101 cases; 54%), followed by T. rubrum (81 cases; 43.3%), T. tonsurans (three cases; 1.6%) and Microsporum canis (two cases; 1.1%). The demographic data, risk factors, and clinical characteristics of the patients with Neoscytalidium dimidiatum and dermatophyte infections are summarized in Table 1. The patients with Neoscytalidium infections were older than those with dermatophyte infections (mean ages, 66.4 vs 64.2 years; p = 0.078). Neoscytalidium foot infections were significantly associated with patients engaged in agricultural work (p = 0.003). Dermatophyte infections were statistically associated with a family history of fungal infections, an immunocompromised status, pruritus symptoms, and other concurrent skin infections. Regarding laboratory results, no dematiaceous hyphae were detected in any foot lesion specimens.
Fig. 1.
Study flow diagram of patient details.
Table 1.
Baseline demographic data, risk factors, and clinical characteristics of patients with Neoscytalidium dimidiatum and dermatophyte foot infections.
| Clinical characteristics | Neoscytalidium dimidiatum (n = 184) | Dermatophytes (n = 187) | Odds ratio (95% CI) | p-value | AuROC (95% CI) |
|---|---|---|---|---|---|
| Age (years) | 66.4 (10.9) | 64.2 (13.6) | 1.02 (0.99–1.03) | 0.078 | 0.54 (0.48–0.59) |
| Male | 105 (57.1) | 109 (58.3) | 0.95 (0.63–1.44) | 0.812 | 0.49 (0.44–0.54) |
| Risk factors | |||||
| - Agricultural work | 78 (42.4) | 52 (27.8) | 1.91 (1.24–2.94) | 0.003* | 0.57 (0.52–0.62) |
| - Use of occlusive shoes >6 h/days | 16 (8.7) | 26 (13.9) | 0.59 (0.31–1.14) | 0.116 | 0.47 (0.44–0.51) |
| - Hyperhidrosis of feet | 49 (26.6) | 47 (25.3) | 1.07 (0.67–1.71) | 0.765 | 0.51 (046–0.55) |
| - Use of topical corticosteroids on the feet within the previous 3 months | 7 (3.8) | 9 (4.8) | 0.78 (0.29–2.15) | 0.633 | 0.49 (0.46–0.52) |
| - Contact with animals | 74 (40.2) | 80 (42.8) | 0.90 (0.60–1.36) | 0.616 | 0.49 (0.44–0.54) |
| - Barefoot walking in public places | 47 (25.5) | 35 (18.7) | 1.49 (0.91–2.44) | 0.114 | 0.53 (0.49–0.58) |
| - Obesity (BMI >30) | 21 (11.4) | 21 (11.2) | 1.02 (0.54–1.94) | 0.956 | 0.5 (0.46–0.53) |
| - Family history of fungal infections | 7 (3.8) | 30 (16) | 0.21 (2.06–11.31) | <0.001* | 0.43 (0.41–0.47) |
| Underlying diseases | |||||
| - Immunocompetent status | 182 (98.9) | 172 (92.0) | 7.9 (1.8–35.2) | 0.006* | 0.53 (0.51–0.56) |
| - Peripheral vascular disease | 40 (21.7) | 41 (21.9) | 0.99 (0.6–1.62) | 0.965 | 0.5 (0.46–0.54) |
| - Atherosclerosis | 15 (8.1) | 15 (8.0) | 1.02 (0.48–2.15) | 0.963 | 0.5 (0.47–0.53) |
| - Malalignment of the feet | 51 (27.7) | 57 (30.5) | 0.87 (0.56–1.37) | 0.558 | 0.49 (0.44–0.53) |
| - Dyslipidaemia | 98 (53.3) | 96 (51.3) | 1.08 (0.72–1.62) | 0.711 | 0.51 (0.46–0.56) |
| - Diabetes mellitus | 54 (29.4) | 50 (26.7) | 1.14 (0.72–1.79) | 0.576 | 0.51 (0.47–0.56) |
| Symptoms at baseline | |||||
| - Pruritus | 22 (12.0) | 58 (31.0) | 0.3 (0.18–0.52) | <0.001* | 0.4 (0.36–0.45) |
| - Pain | 1 (0.5) | 3 (1.6) | 0.34 (0.04–3.25) | 0.346 | 0.49 (0.48–0.51) |
| Feet examination | |||||
| - Type of infection | |||||
| - Moccasin | 176 (96.7) | 183 (97.9) | 0.48 (0.14–1.62) | 0.239 | 0.49 (0.47–0.51) |
| - Interdigital | 20 (10.9) | 25 (13.4) | 0.79 (0.42–1.48) | 0.462 | 0.49 (0.45–0.52) |
| - Vesiculobullous | 12 (6.5) | 23 (12.3) | 0.5 (0.24–1.03) | 0.061 | 0.47 (0.44–0.5) |
| Both feet infection | 109 (59.2) | 109 (58.3) | 1.04 (0.69–1.57) | 0.468 | 0.5 (0.45–0.55) |
| Concurrent toenail infection | 135 (73.4) | 148 (79.1) | 0.73 (0.45–1.17) | 0.192 | 0.47 (0.43–0.51) |
| Other concurrent skin infection | 2 (1.1) | 42 (22.5) | 0.04 (0.01–0.16) | <0.001* | 0.39 (0.36–0.42) |
Numbers are n (%) or mean ± SD. Abbreviations: AuROC, area under the receiver operating characteristic curve; BMI, body mass index.
3.2. Multivariate analysis and modeling
The significant diagnostic predictors identified in the univariate analysis were subjected to multivariate analysis (Table 2). Each predictor in the final model was assigned a weighted score based on each factor's logit coefficient. Scores were approximated to the nearest whole number. The sum of the transformed scores ranged from 2 to 12, and the overall mean score of all patients was 10.0 ± 2.2. There was a significant difference in the mean scores of patients with Neoscytalidium dimidiatum infections and those with dermatophyte infections (11.0 ± 1.1 vs 9.1 ± 2.6; p < 0.001).
Table 2.
Multivariable regression analysis of predictive factors for differentiating Neoscytalidium dimidiatum foot infections from dermatophyte foot infections.
| Potential predictors | Odds ratio | 95% CI | p-value | β | Item score |
|---|---|---|---|---|---|
| Doing agricultural work | 1.87 | 1.15–3.07 | 0.012 | 0.63 | 1 |
| No family history of fungal infections | 4.29 | 1.71–10.75 | 0.002 | 1.46 | 2 |
| Immunocompetent status | 4.10 | 0.82–20.5 | 0.086 | 1.41 | 2 |
| No pruritus | 3.37 | 1.89–6.00 | <0.001 | 1.21 | 2 |
| No other concurrent fungal skin infections | 21.95 | 5.11–94.29 | <0.001 | 3.09 | 5 |
Abbreviations: β, beta-coefficient; CI, confidence interval.
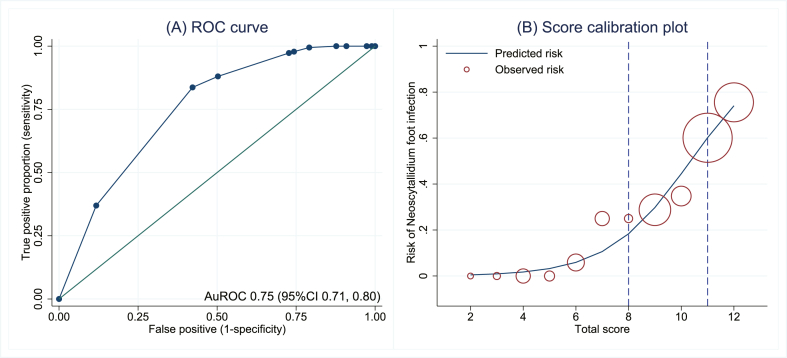
Two cutoffs were used to group the patients with fungal foot infections into three risk categories for Neoscytalidium foot infections: low, moderate, and high (Table 3). The lower cutoff score was <8, with a sensitivity of 97.8% (95% CI, 94.5–99.4) and a specificity of 25.7% (95% CI, 19.6–32.6). The higher cutoff was >11, with a sensitivity of 83.7% (95% CI, 77.5–88.7) and a specificity of 57.8% (95% CI, 50.3–64.9). The prevalence of Neoscytalidium dimidiatum foot infections and the LHR + for each category are detailed in Table 3. The confidence intervals of LHR + for the risk categories did not overlap. The score showed acceptable criteria to distinguish patients with Neoscytalidium dimidiatum and those with dermatophyte infections, based on an AuROC at 0.755 (95% CI, 0.71–0.80; Fig. 2A). After internal validation with the bootstrap procedure, the test AuROC was 0.753 (95% CI, 0.70–0.80). Regarding score calibration, the Hosmer–Lemeshow goodness-of-fit statistic revealed a non-significant result (no statistical evidence of lack-of-fit; p = 0.772), representing an almost perfect fit of the model to the observed data. The score calibration plot also showed good agreement between the calculated score and the observed risk for a Neoscytalidium dimidiatum foot infection. The predicted risk scores and the observed risks for a Neoscytalidium dimidiatum foot infection are illustrated in Fig. 2B to represent the relative number of patients for each score.
Table 3.
Distribution of Neoscytalidium dimidiatum foot infections, dermatophyte foot infections, and likelihood ratios of Neoscytalidium dimidiatum foot infections (LHR+) in low-, moderate-, and high-risk categories.
| Risk categories | Score | Prevalence (%) | Neoscytalidium infections (n = 184) | Dermatophyte infections (n = 187) | LHR+ | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Low | <8 | 14.0 | 4 (2.3) | 48 (25.6) | 0.08 | 0.02–0.24 | <0.001 |
| Moderate | 8–11 | 61.7 | 112 (60.7) | 117 (62.6) | 0.97 | 0.69–1.37 | 0.870 |
| High | >11 | 24.3 | 68 (37.0) | 22 (11.8) | 3.14 | 1.82–5.56 | <0.001 |
| Mean ± SD | 11.0 (1.1) | 9.1 (2.6) | <0.001 |
Numbers are n (%) or mean ± SD. Abbreviations: CI, confidence interval; LHR+, positive likelihood ratio; SD, standard deviation.
Fig. 2.
ROC curve (parametric) and 95% confidence band for predicting Neoscytalidium foot infections by the derived score (A). Predicted risk of Neoscytalidium foot infections by score (solid line) and observed risk of Neoscytalidium foot infections (hollow circles). The circle size depicts the relative number of patients for each score. (B) Abbreviations: AuROC, area under the receiver operating characteristic curve; CI, confidence interval.
4. Discussion
This study developed diagnostic criteria and scores that facilitate the differential diagnosis of Neoscytalidium dimidiatum from dermatophyte foot infections, thereby guiding appropriate management. The significant diagnostic predictors for Neoscytalidium dimidiatum foot infections were patient involvement in agricultural work, an immunocompetent status, the absence of pruritus, other concurrent fungal skin infections, or a family history of fungal infections. The score shows promise for clinical use, with acceptable discriminative performance and good calibration.
As shown in this study, working in agriculture or having contact with soil or an environment contaminated with Neoscytalidium dimidiatum predisposes to foot infection [1,7]. Contact with soil may also present an additional risk for geophilic dermatophyte infections; however, such dermatophytes rarely cause foot infections [1,2,19]. Close contacts or family members with histories of fungal infections have been reported in high proportions (72%–80%) of patients with dermatophyte infections [[20], [21], [22]]. Genetic susceptibility and close contact with family members or transmission through fomites or the environment may cause chronic and recurrent dermatophyte infections [20]. However, there have been limited reports on genetic predisposition or familial infections related to non-dermatophyte molds, especially Neoscytalidium dimidiatum.
Dysregulation of the host immune response was reported to be associated with dermatophyte infections [20,23,24]. The present study found that an immunocompromised status is a risk factor for dermatophyte infections, similar to previous studies. Conversely, non-dermatophytes, especially Neoscytalidium dimidiatum, are highly virulent in invading the skin and nails [1,6,8]. Hence, irrespective of an individual's immunocompetent status, aggressive non-dermatophytes can cause superficial, deep skin and nail infections [13,25].
Previous studies have reported that the clinical presentations of fungal foot infections caused by non-dermatophytes and dermatophytes were not distinguishable [12,26]. The vesicular form of fungal foot infections rarely occurred in cases of non-dermatophyte infections. However, one study reported that 9.7% of patients with Neoscytalidium dimidiatum foot infections presented with vesicles [1]. Consequently, the present study determined that 6.5% of the Neoscytalidium dimidiatum foot infections had vesiculobullous lesions. Nevertheless, this study found that vesiculobullous formation and pruritus were significantly less frequent among Neoscytalidium dimidiatum infections than dermatophyte infections.
Concerning widespread fungal infections, they are observed more frequently in dermatophyte nail infections than in non-dermatophyte nail infections [12]. Similarly, the current investigation revealed that other concurrent skin infections were observed in patients with dermatophyte foot infections but not in those with Neoscytalidium foot infections. Previous reports demonstrated that only 8.6%–9.4% of patients with Neoscytalidium infections presented with lesions on their palms, fingers, or groin [7,8]. Whole-body examinations should be performed in all patients with fungal foot infections to facilitate diagnosis and determine the appropriate treatment.
The newly developed diagnostic score shows promise for clinical use. The data for its predictors can be readily obtained in an outpatient setting. The diagnostic score can help physicians categorize patients into risk groups and manage the diseases accordingly. Patients with a low risk for a Neoscytalidium foot infection could be treated topically with allylamine, imidazole, ciclopirox, benzylamine, or tolnaftate. Systemic antifungal treatment may help the widespread or concurrent nail infections. Particular care should be taken in patients with a high risk of a Neoscytalidium foot infection. A consensus treatment for Neoscytalidium infections is yet to be established. In vitro studies showed the antifungal effects of amphotericin B, terbinafine, voriconazole, and luliconazole against Neoscytalidium dimidiatum [27,28]. Topical keratolytic and new topical antifungal agents, such as luliconazole, have shown promising outcomes [28]. A combination of terbinafine and topical treatments should be used in refractory cases. Mycological cure should also be monitored carefully.
This study has some limitations. First, the data were collected retrospectively, so bias and missing data could not be avoided. Patients with complete data were included in the analysis to develop the score. Second, fungal culture results at baseline were utilized to determine the causative agents. Molecular methods provide higher sensitivity and rapid detection; however, their high cost has hindered their routine use in general clinical practice. Consequently, we opted to employ the culture method for identifying the type of fungal infection in our study. To mitigate the potential for misclassification bias, we ensured that fungal diagnoses were made by well-trained and experienced microscopists/technicians. In cases where controversial identifications arose from the fungal culture results, molecular methods were used for further specimen identification. Regarding mixed infection, a previous study reported an eclipsed phenomenon, defined as a non-dermatophyte nail infection occurring after a dermatophyte nail infection [29]. Notably, some mixed dermatophyte and Neoscytalidium foot infections that subsequently occurred in the current investigation may have been misclassified. Finally, the present study was conducted at a single tertiary-care hospital that handles complex and recalcitrant cases. This means that the prevalence of non-dermatophyte infections may be higher than that at local hospitals. Therefore, the generalization of the diagnostic score to other clinical settings or populations should be validated before implementation.
5. Conclusions
This study proposes using diagnostic criteria that adopt five simple predictors to differentiate between Neoscytalidium dimidiatum and dermatophyte foot infections. The proposed diagnostic score demonstrated acceptable discriminative ability and calibration in determining causative agents, thereby supporting the delivery of appropriate management. A history of agricultural work, a family history of fungal infections, and an immunocompromised status should be evaluated in all patients. Moreover, itch symptoms should be investigated, and a holistic physical examination should be performed to identify any other concurrent skin infections.
Author contribution statement
Charussri Leeyaphan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Chatree Chai-Adisaksopha; Phichayut Phinyo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper. Napatra Tovanabutra; Sumanas Bunyaratavej: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding sources
None.
Prior presentation
None.
IRB approval status
Reviewed and approved by Siriraj IRB (Si 328/2022).
Reprint requests
Charussri Leeyaphan.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ungpakorn R., Lohaprathan S., Reangchainam S. Prevalence of foot diseases in outpatients attending the institute of dermatology, Bangkok, Thailand. Clin. Exp. Dermatol. 2004;29:87–90. doi: 10.1111/j.1365-2230.2004.01446.x. [DOI] [PubMed] [Google Scholar]
- 2.Gawdzik A., Nowogrodzka K., Hryncewicz-Gwozdz A., Maj J., Szepietowski J., Jankowska-Konsur A. Epidemiology of dermatomycoses in southwest Poland, years 2011-2016. Postepy Dermatol Alergol. 2019;36:604–608. doi: 10.5114/ada.2018.80615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster K.W., Ghannoum M.A., Elewski B.E. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J. Am. Acad. Dermatol. 2004;50:748–752. doi: 10.1016/s0190-9622(03)02117-0. [DOI] [PubMed] [Google Scholar]
- 4.Hazarika D., Jahan N., Sharma A. Changing trend of superficial mycoses with increasing nondermatophyte mold infection: a clinicomycological study at a tertiary referral center in Assam. Indian J. Dermatol. 2019;64:261–265. doi: 10.4103/ijd.IJD_579_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burzykowski T., Molenberghs G., Abeck D., Haneke E., Hay R., Katsambas A., et al. High prevalence of foot diseases in Europe: results of the achilles project. Mycoses. 2003;46:496–505. doi: 10.1046/j.0933-7407.2003.00933.x. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A.K., Drummond-Main C., Cooper E.A., Brintnell W., Piraccini B.M., Tosti A. Systematic review of nondermatophyte mold onychomycosis: diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Dermatol. 2012;66:494–502. doi: 10.1016/j.jaad.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Lacroix C., Kac G., Dubertret L., Morel P., Derouin F., de Chauvin M.F. Scytalidiosis in Paris, France. J. Am. Acad. Dermatol. 2003;48:852–856. doi: 10.1067/mjd.2003.454. [DOI] [PubMed] [Google Scholar]
- 8.Xavier A.P., Oliveira J.C., Ribeiro V.L., Souza M.A. Epidemiological aspects of patients with ungual and cutaneous lesions caused by Scytalidium spp. An. Bras. Dermatol. 2010;85:805–810. doi: 10.1590/s0365-05962010000600005. [DOI] [PubMed] [Google Scholar]
- 9.Kaur R., Panda P.S., Sardana K., Khan S. Mycological pattern of dermatomycoses in a tertiary care hospital. J. Trop. Med. 2015;2015 doi: 10.1155/2015/157828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grover S., Roy P. Clinico-mycological Profile of superficial mycosis in a hospital in north-east India. Med. J. Armed Forces India. 2003;59:114–116. doi: 10.1016/S0377-1237(03)80053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assadamongkol R., Lertwattanarak R., Wannachalee T., Bunyaratavej S., Leeyaphan C., Matthapan L. Prevalence, risk factors, and type of organism in fungal foot infection and toenail onychomycosis in Thai diabetic patients. J. Med. Assoc. Thai. 2016;99:659–664. [PubMed] [Google Scholar]
- 12.Bunyaratavej S., Prasertworonun N., Leeyaphan C., Chaiwanon O., Muanprasat C., Matthapan L. Distinct characteristics of Scytalidium dimidiatum and non-dermatophyte onychomycosis as compared with dermatophyte onychomycosis. J. Dermatol. 2015;42:258–262. doi: 10.1111/1346-8138.12768. [DOI] [PubMed] [Google Scholar]
- 13.Machouart M., Menir P., Helenon R., Quist D., Desbois N. Scytalidium and scytalidiosis: what's new in 2012? J. Mycol. Med. 2013;23:40–46. doi: 10.1016/j.mycmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Miqueleiz-Zapatero A., Santa Olalla C., Buendia B., Barba J. Dermatomycosis due to Neoscytalidium spp. Enferm. Infecc. Microbiol. Clín. 2017;35:130–131. doi: 10.1016/j.eimc.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Salakshna N., Bunyaratavej S., Matthapan L., Lertrujiwanit K., Leeyaphan C. A cohort study of risk factors, clinical presentations, and outcomes for dermatophyte, nondermatophyte, and mixed toenail infections. J. Am. Acad. Dermatol. 2018;79:1145–1146. doi: 10.1016/j.jaad.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 16.Bunyaratavej S., Srinonprasert V., Kiratiwongwan R., Wongdama S., Leeyaphan C. Onychomycosis in older adults: the age and associated factors affecting the complete cure rate. Australas. J. Dermatol. 2022;63:74–80. doi: 10.1111/ajd.13686. [DOI] [PubMed] [Google Scholar]
- 17.Silva L.B., de Oliveira D.B., da Silva B.V., de Souza R.A., da Silva P.R., Ferreira-Paim K., et al. Identification and antifungal susceptibility of fungi isolated from dermatomycoses. J. Eur. Acad. Dermatol. Venereol. 2014;28:633–640. doi: 10.1111/jdv.12151. [DOI] [PubMed] [Google Scholar]
- 18.Crawford F., Hollis S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD001434.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal U.S., Saran J., Agarwal P. Clinico-mycological study of dermatophytes in a tertiary care centre in Northwest India. Indian J. Dermatol. Venereol. Leprol. 2014;80:194. doi: 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]
- 20.Verma S.B., Panda S., Nenoff P., Singal A., Rudramurthy S.M., Uhrlass S., et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. epidemiology, risk factors and clinical features. Indian J. Dermatol. Venereol. Leprol. 2021;87:154–175. doi: 10.25259/IJDVL_301_20. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan S., Tilak R., Kaushal S.K., Mishra R.N., Pandey S.S. Clinico-mycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care center. Indian J. Dermatol. Venereol. Leprol. 2017;83:436–440. doi: 10.4103/ijdvl.IJDVL_519_16. [DOI] [PubMed] [Google Scholar]
- 22.Pathania S., Rudramurthy S.M., Narang T., Saikia U.N., Dogra S. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J. Dermatol. Venereol. Leprol. 2018;84:678–684. doi: 10.4103/ijdvl.IJDVL_645_17. [DOI] [PubMed] [Google Scholar]
- 23.Tainwala R., Sharma Y. Pathogenesis of dermatophytoses. Indian J. Dermatol. 2011;56:259–261. doi: 10.4103/0019-5154.82476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rai G., Das S., Ansari M.A., Singh P.K., Pandhi D., Tigga R.A., et al. The interplay among Th17 and T regulatory cells in the immune dysregulation of chronic dermatophytic infection. Microb. Pathog. 2020;139 doi: 10.1016/j.micpath.2019.103921. [DOI] [PubMed] [Google Scholar]
- 25.Moutran R., Maatouk I., Wehbe J., Abadjian G., Obeid G. [Subcutaneous infection spread by Scytalidium (Neoscytalidium) dimidiatum] Ann. Dermatol. Venereol. 2012;139:204–208. doi: 10.1016/j.annder.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Nowicka D., Nawrot U. Tinea pedis- an embarrassing problem for health and beauty-a narrative review. Mycoses. 2021;64:1140–1150. doi: 10.1111/myc.13340. [DOI] [PubMed] [Google Scholar]
- 27.Madrid H., Ruiz-Cendoya M., Cano J., Stchigel A., Orofino R., Guarro J. Genotyping and in vitro antifungal susceptibility of Neoscytalidium dimidiatum isolates from different origins. Int. J. Antimicrob. Agents. 2009;34:351–354. doi: 10.1016/j.ijantimicag.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Futatsuya T., Ogawa A., Anzawa K., Mochizuki T., Shimizu A. First isolation of Neoscytalidium dimidiatum from human dermatomycosis in Japan. Med. Mycol. J. 2022;63:71–75. doi: 10.3314/mmj.22-00005. [DOI] [PubMed] [Google Scholar]
- 29.Bunyaratavej S., Limphoka P., Kiratiwongwan R., Leeyaphan C. Eclipsed phenomenon: the relationship between nail and foot infections in patients presenting with nondermatophyte infections after dermatophyte infections in onychomycosis. Br. J. Dermatol. 2020;183:158–159. doi: 10.1111/bjd.18860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.