Abstract
Background
Existing reporting guidelines pay insufficient attention to the detail and comprehensiveness reporting of surgical technique. The Surgical techniqUe rePorting chEcklist and standaRds (SUPER) aims to address this gap by defining reporting standards for surgical technique. The SUPER guideline intends to apply to articles that encompass surgical technique in any study design, surgical discipline, and stage of surgical innovation.
Methods
Following the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network approach, 16 surgeons, journal editors, and methodologists reviewed existing reporting guidelines relating to surgical technique, reviewed papers from 15 top journals, and brainstormed to draft initial items for the SUPER. The initial items were revised through a three-round Delphi survey from 21 multidisciplinary Delphi panel experts from 13 countries and regions. The final SUPER items were formed after an online consensus meeting to resolve disagreements and a three-round wording refinement by all 16 SUPER working group members and five SUPER consultants.
Results
The SUPER reporting guideline includes 22 items that are considered essential for good and informative surgical technique reporting. The items are divided into six sections: background, rationale, and objectives (items 1 to 5); preoperative preparations and requirements (items 6 to 9); surgical technique details (items 10 to 15); postoperative considerations and tasks (items 16 to 19); summary and prospect (items 20 and 21); and other information (item 22).
Conclusions
The SUPER reporting guideline has the potential to guide detailed, comprehensive, and transparent surgical technique reporting for surgeons. It may also assist journal editors, peer reviewers, systematic reviewers, and guideline developers in the evaluation of surgical technique papers and help practitioners to better understand and reproduce surgical technique.
Trial Registration
Keywords: Surgical technique, surgical innovation, reporting guideline, reporting checklist, Surgical techniqUe rePorting chEcklist and standaRds (SUPER)
Highlight box.
Key findings
• The SUPER reporting guideline uses 22 items to clearly define the requirements for detailed reporting of surgical technique.
What is known and what is new?
• Existing guidelines that might guide surgical technique reporting recommend a detailed and complete description of surgical technique without setting clear criteria on the requirements for a detailed report.
• The SUPER sets the criteria.
What is the implication, and what should change now?
• The SUPER reporting guideline can assist surgeons to improve their reporting of surgical technique thus facilitating the refinement and reproducibility; help readers better understand surgical technique; and assist journal editors, peer reviewers, systematic reviewers, and guideline developers to better evaluate surgical technique.
• The literature related to surgical technique, regardless of the surgical discipline, study design, novelty level of surgical technique or stage of surgical innovation, are recommended to follow SUPER reporting guideline for better reporting.
Introduction
Background
Surgery plays a vital role in clinical treatment and is increasingly needed worldwide (1,2). Surgical technique, the craft and art of surgery, has previously been defined as “the specific way and skills of performing a particular medical operation” (3). Surgical technique is critical to achieving good outcomes and making them more reliable and reproducible (4). Patients treated by surgeons with a higher level of surgical technique have lower postoperative complication and reoperation rates (5,6). However, surgical technique is usually complex, involving many steps and factors that affect the outcomes of patients, which makes its reporting extremely challenging (7-9). The effectiveness of reporting guidelines in improving the reporting quality of publications has been proven (10). However, despite the availability of guidelines that might guide surgical technique reporting, most of the studies related to interventional surgical technique lacked complete and detailed descriptions of the surgical interventions, and some even provided only the names of the interventions (11-15).
Rationale and knowledge gap
One reason behind the non-significant improvement of surgical technique reporting is that we currently lack a guideline detailing how to report surgical technique. As of 2021, the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network (https://www.equator-network.org/) had published nearly 500 reporting guidelines and had over 100 under development. Through a systematic search, we identified over 50 reporting guidelines related to surgical technique. These guidelines include the CONSORT-NPT guideline for randomized controlled trials in non-pharmacological treatment (16), the STROCSS guideline for observational study in surgery (17), the PROCESS guideline for surgical case series (18), the SCARE guideline for surgical case reports (19), the TIDieR guideline for detailed reporting of interventional studies (20), and the IDEAL guideline for surgical innovation (21). While most of these guidelines recommend a detailed description of surgical technique, they do not provide sufficiently precise information on the requirements for a detailed report. The findings in our scoping review further indicate that a standardized reporting guideline that focuses on details of surgical technique is warranted (22).
Objective
This study aimed to develop a guideline for guiding the reporting of surgical technique, the Surgical techniqUe rePorting chEcklist and standaRds (SUPER), and to clearly define the requirements for good and informative reporting of surgical technique, regardless of the surgical discipline, type of article, novelty, or stage of surgical innovation.
Methods
Surgical technique definition and scope of the SUPER reporting guideline
The surgical technique was defined as “the specific way and skills of performing a particular medical operation” in the published protocol (3). The SUPER reporting guideline emphasizes on the intraoperative process rather than the perioperative care and focuses on the intervention and treatment of abnormalities rather than diagnosis. The guideline aims to improve the reporting of novel, adapted and conventional surgical technique in all surgical disciplines. Surgical technique that reported in case reports, case series, observational studies or randomized controlled trials are all within the scope of the SUPER reporting guideline.
Development flow
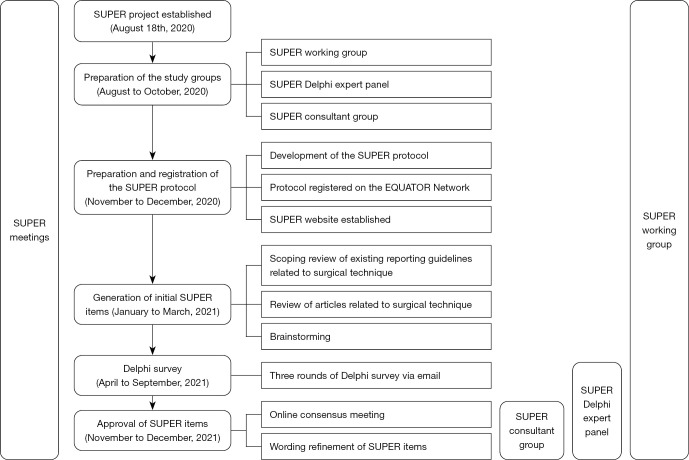
The SUPER guideline was developed following the EQUATOR Network approach (23) and a published protocol (3). The entire development process is shown in Figure 1. In each phase, independent ideas were generated first, then integrated, and finally discussed at a meeting to reach a consensus. Each SUPER group member had the opportunity to express their opinions fully and objectively.
Figure 1.
Flow chart showing the process of developing SUPER items. SUPER, Surgical techniqUe rePorting chEcklist and standaRds; EQUATOR Network, enhancing the quality and transparency of health research network.
Preparation of the study groups
The study includes three groups: the SUPER working group, the SUPER Delphi expert panel, and the consulting group. The responsibilities of each group are summarized in Figure 1. In brief, the SUPER working group is a multidisciplinary team comprising nine surgeons, four journal editors, and three methodologists. The working group takes responsibility for the entire project of SUPER, including developing the research protocol, drafting initial items, and then writing and disseminating the final SUPER checklist. The working group recruited the Delphi expert panel by sending emails to authors of surgical technique-related articles published in journals under the AME publishing company (https://www.amegroups.com/medicine-service/journal#journals). A total of 21 surgeons from 13 countries/regions ultimately joined the Delphi expert panel, responsible for the Delphi survey (Table S1). Another five surgeons were invited as consultants to contribute to the consensus meeting and wording refinement. Although the number of Delphi expert panel and consultants is not large, the specialties and geographical locations of the Delphi expert panel and consultants fit the global frequency of procedures and the geographical distribution of difficult-to-access surgeries (1).
Preparing the protocol, registration, and establishing the SUPER website
To ensure methodological rigor and transparency in developing the SUPER guideline, a protocol (3) was developed at the outset. Upon completion of the protocol, we registered the project on the EQUATOR Network (https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-other-study-designs/#SUPER) to avoid research duplication. Subsequently, we set up the SUPER website (https://www.thesuper.org/), which enabled us to post the project progress and receive advice in real time.
Generating the initial SUPER items
Each member of the SUPER working group disclosed any conflicts of interest before participating in the generation of initial items. The initial SUPER items came from three sources: (I) a scoping review of all reporting guidelines related to surgical technique. Information was independently extracted from these reporting guidelines by seven surgeons, journal editors with medical backgrounds, and methodologists. (II) A review of surgical technique-related articles published between December 1, 2019, and November 30, 2020, in 15 top journals. The articles included all surgical technique-related articles between this timeframe, not only those when the study purpose was to describe a novel technique. The 15 journals included general journals, general oncology journals, general surgery journals, and journals focusing on particular disciplines, including orthopedics, gastroenterology, cardiology, and neurology (3)—a disciplinary selection that takes fully account of the global specialty distribution and frequency of procedures (1). Information related to surgical technique was extracted from articles in the journals by eight surgeons, journal editors with medical backgrounds, and methodologists. Due to the substantial number of articles, the team was divided into two groups to extract the information separately. (III) All SUPER working group members brainstormed initial SUPER items independently. Intra- and inter-group aggregation and de-duplication of the extracted data were conducted for each source. Finally, the information from all three sources was synthesized and subsequent removal of duplicates was conducted, and the initial SUPER items were formed.
Delphi survey
The SUPER Delphi panel used a 5-point Likert scale (1= not important, 5= very important) (24) to score the initial SUPER items, propose new items, and offer comments and suggestions for each item. Survey questions in all three rounds of surveys can be found in the website: https://cdn.amegroups.cn/static/public/hbsn-22-509-01.zip. Each expert disclosed any conflicts of interest before participating in the Delphi survey. Data obtained from each round of the Delphi survey were analyzed and discussed by the SUPER working group. Blinding was achieved by K.P.Z. replacing the experts’ names with random numbers and then by two other group members (Y.F.M. and J.L.W. in round 1, Y.F.M. and X.Z.Z. in round 2, Q.L.S. and X.Z.Z. in round 3) entering the Delphi survey data independently. The data were subsequently verified by K.P.Z. After that, a consensus on each item was determined according to the following rule “Consensus on any item is conditional to at least 66% of the Delphi survey responses having agreed on the rating”, as set in the protocol (3). Items for which a consensus was not reached were moved to the next round.
Approval of SUPER items
A virtual consensus meeting was held to discuss items failed to reach consensus. After a consensus on the content of all items had been reached, three rounds of wording refinement were arranged via email. Finally, after revision, the final SUPER items were finalized.
Results
Three rounds of Delphi survey
The response rates for the first, second, and third Delphi surveys were 91% (21/23), 74% (17/23), and 61% (14/23), respectively. The original data for the three Delphi surveys are available in the website: https://cdn.amegroups.cn/static/public/hbsn-22-509-02.zip, and the survey scores for the items in each round can be found in Table S2.
SUPER checklist description
The SUPER checklist comprises 22 items that we consider essential for the good reporting of surgical technique (Table 1). These items are distributed across six sections: background, rationale, and objectives (items 1 to 5); preoperative preparations and requirements (items 6 to 9); surgical technique details (items 10 to 15); postoperative considerations and tasks (items 16 to 19); summary and prospect (items 20 and 21); and other information (item 22). The draft of the SUPER explanation and elaboration statement is completed and provides the reader with a rationale and explanation for each item as well as a rich set of reporting examples from specialties. The SUPER guideline and the SUPER explanation and elaboration statement will both be available on the SUPER website (https://www.thesuper.org/).
Table 1. Surgical technique reporting checklist and standards.
| Section/Item | Item | Recommendation |
|---|---|---|
| Background, rationale, and objectives | ||
| Background | 1 | Describe the background of the disease or condition (e.g., its definition, classification, clinical manifestations, epidemiological characteristics, and natural history) |
| Rationale | 2 | (I) Describe the pros and cons of existing treatments for the disease or condition, including currently used single or combined surgical techniques |
| (II) Explain whether the proposed surgical technique is a novel or modified procedure, including whether any modifications have been made to key devices or materials. If only a conventional surgical technique is used, a brief description should be accompanied by a citation of a source which describes the surgical technique in detail | ||
| Objectives | 3 | State what objectives and challenges the proposed surgical technique will address. Introduce what the surgical technique figure and video will cover |
| Classification | 4 | Classify the surgical technique, either by: (I) surgical approach: open, minimally invasive (e.g., thoracoscopic, robotic), or hybrid; or (II) treatment goal: curative or palliative |
| Name | 5 | Report the names of all involved surgical techniques in the title or abstract. If the surgical technique is the focus of the paper, also include “surgical technique” in the title |
| Preoperative preparations and requirements | ||
| Setting | 6 | (I) Report information or requirements of the surgical environment (e.g., the name of the hospital, the hospital grade such as tertiary hospital, the degree of cleanliness, and whether the procedure must be performed in an operating theatre) |
| (II) List and provide details of any special surgical equipment, supplies, drugs, or software used (e.g., the manufacturer, product model, quantity, dosage, route, duration, and parameters) | ||
| Operators | 7 | Provide information about the surgical team personnel, including their role (e.g., surgeon, anesthetist, nurse), learning curve (e.g., the number of cases), and training needed if applicable |
| Recipients | 8 | Report detailed indications and contraindications(I) Disease or condition: type, etiology, the location, shape and size of the lesion, etc. (II) Recipients: age, sex, clinical manifestations, disease stage and severity, comorbidities and related complications, surgical history and relevant family history, preoperative tests, pre-intervention, and other factors pertinent to successful practice |
| 9 | Provide detailed generic information and preparations(I) Generic information: de-identified demographic information, symptoms and signs, imaging findings, staging, comorbidities, and relevant therapy history, etc.(II) Preparations: cardiovascular, gastrointestinal and respiratory tract preparation, urinary catheterization, skin preparation, blood product preparation, anesthetic procedure and management, and patient positioning, etc. | |
| Surgical technique details | ||
| Surgical approach, key anatomic landmarks, and adjacent structures | 10 | (I) Describe in detail how to establish the surgical approach (e.g., devices and equipment used, the position of the surgeons, anatomic localization, and the incision type, length, size, depth, angle, and number) |
| (II) Describe the essential anatomic landmarks and adjacent structures, including areas, structures, blood vessels, and nerves, etc. (e.g., “use the Louis angle between the sternal manubrium and the sternal body to find the second costal notch”) | ||
| Intraoperative monitoring | 11 | Describe intraoperative monitoring specifically related to the surgical technique (e.g., near-infrared spectroscopy in aortic arch surgery) |
| Step-by-step description | 12 | Include all relevant details of each operative step in a step-by-step manner along with both quantitative and qualitative description |
| (I) Details may include the intraoperative findings, timeline, histomorphology, exposure of vital structures, extent of lymph node dissection, determination of surgical margins, suture pattern (running suture or single stitches; spacing of stitches), anastomosis, knot-tying, specimen handling, and devices/supplies/drugs/blood products used, etc. | ||
| (II) Note the operative time | ||
| (III) If a non-conventional maneuver was applied, specify the reason | ||
| Quality and consistency | 13 | Describe tips and skills for ensuring surgical quality and consistency, especially for the key steps and any conditions or variations that require uniform management (if applicable). For example, using standardized training, establishing quality control teams, and organizing multidisciplinary consultations |
| Safety | 14 | Describe tips and skills for ensuring safety. For example, how to prevent or deal with possible intraoperative complications and emergencies, or when and how to undertake a surgical conversion |
| Visualization | 15 | (I) Visualize the key steps in a step-by-step and self-explanatory manner. Consider using narrated video(s) and anatomic illustration(s) with designated symbols and illustrated text |
| (II) The key information in item 12 should be visualized; it can either be presented as a stand-alone figure or embedded in the video(s) | ||
| (III) Visualization of the key information in items 10, 13, and 14 is encouraged as appropriate | ||
| (IV) After peer review, add clips into the video(s) to present the video title, operator name, and operation date at the beginning, and the informed consent and the ethical approval statements at the end | ||
| Postoperative considerations and tasks | ||
| Evaluation | 16 | (I) Define the criteria for success and failure, and evaluate the efficacy or effectiveness of the surgical technique from both the technical aspect and the clinical outcome perspective (e.g., length of stay, improvements in short- and long-term mortality, recurrence, survival time, and patient impairment) |
| (II) When possible, include the perspective of the patient (e.g., symptoms and signs, postoperative pain, and aesthetic results) | ||
| Postoperative monitoring | 17 | Describe in detail postoperative monitoring specifically related to the surgical technique (e.g., monitoring indicators, devices, frequency or duration, examination, and nursing required) |
| Complication prevention and management | 18 | Report the possible or observed postoperative complications and their prevention and management, especially complications that differ from those related to conventional techniques |
| Follow-up | 19 | (I) Report the details of follow-up visits, including pathway, frequency, duration, and indicators (e.g., pathway-“telephone follow-up”; frequency-“radiological examinations every 3 months”; duration-“up to 3 years”; indicators-poor outcomes, complications, quality of life, and unexpected events) |
| (II) If applicable, compare the information in item 19a with those of conventional techniques | ||
| Summary and prospect | ||
| Strengths, limitations, and outlook | 20 | Discuss the main strengths and limitations of the surgical technique, and provide detailed suggestions for improvement and future outlooks |
| Impact and cost | 21 | (I) Summarize the key points and take-away lessons of the surgical technique and its impact in the clinical setting and on society (e.g., the economic cost) |
| (II) Consider in context the predominant cost and its potential impact on the implementation and adoption of the surgical technique | ||
| Other information | ||
| Conflicts of interest, ethical approval, and informed consent | 22 | (I) Specify any potential conflicts of interest; (II) include the ethics committee or institutional review board approval (and the number when applicable); and (III) provide the informed consent for publication |
Discussion
Key findings
The SUPER guideline provides a set of 22 generic items that can be applied to any surgical discipline, type of article, or stage of surgical innovation to guide detailed, comprehensive, and transparent reporting of surgical technique. It can assist surgeons to improve their reporting of surgical technique, thus facilitating refinement and reproducibility, and assisting journal editors, peer reviewers, systematic reviewers, and guideline developers to better evaluate surgical technique articles, ultimately benefiting patients.
Strengths and limitations
The SUPER reporting guideline has three main strengths. First, it takes 6 items to concentrate on defining in detail what specific components the surgical technique details should include. Surgical technique is complex and massive entity that may be presented as a black box, and one has difficulty telling what is happening inside. The SUPER guideline can be visualized as a computerized tomography scan that slices this massive entity into a series of thin, organized, and miniature compositions, breaking down its complexity and helping us to truly understand what is happening on the inside. Second, the SUPER reporting guideline was developed in strict accordance with the suggested three-round Delphi method (23). Third, the members developing the SUPER reporting guideline are representative at multiple facets, including geographic location, professional role (surgeon/journal editor/methodologist), specialty discipline, gender, and years of qualified, making the SUPER reporting guidelines highly representative.
Like other reporting guidelines, the SUPER guideline has certain limitations. First, while we tried to recruit a sufficient and representative number of experts to the Delphi survey, the number of the Delphi panel remains modest; the specialty coverage is still dominated by thoracic and gastrointestinal surgery; and female participation is also limited (Table S1). The undesirable number of participants in the Delphi survey may be attributed to the insufficient use of multiple channels for recruitment, which needs to be addressed in future updates and refinements of checklist. The suboptimal coverage of disciplines and gender in the Delphi survey may be due largely to the disciplinary distribution of the AME Publishing Company journals and the fact that more male surgeons enroll than female surgeons in surgical career. We have made efforts to address the suboptimal disciplinary and gender coverage of the Delphi survey: (I) we drew the initial items from 15 representative journals to make them applicable to various specialties; (II) we tried to ensure sufficient female participation in the SUPER working group—37.5% (6/16) of the SUPER working group were female. Third, the Delphi survey response rate dropped in each consecutive round, with only 61% of members responding in the third round. In the context of the coronavirus disease 2019 (COVID-19) pandemic, some members may have been far too occupied with patient care to participate in the Delphi survey, especially those surgeons in regions where healthcare personnel are in severe short supply.
Comparison with similar researches
The devil is in the details. Thus, the SUPER guideline clarifies concrete criteria for detailed surgical technique reporting, which differentiates it from other surgical reporting guidelines. Reporting guidelines related to surgical technique have facilitated better reporting, but clear and comprehensive criteria on the level of detail required for proper reporting is lacking. For instance, the SCARE, PROCESS, STROCSS, and TIDieR guidelines ask “what, where, when and how was it done” (17-20); the CONSORT-NPT guideline refers to “intervention details” (16); and the IDEAL guideline asks for “Clear and detailed description of new technique/device, including necessary pre- and post-procedure care” (21). In contrast, the SUPER guideline contains an entire section, “Surgical Technique Details”, containing items 10 to 15, to describe specific requirements for detailed reporting. Of note, TIDieR is the guideline used to improve the detailed reporting of all interventional studies. However, we concluded that it would be preferable to develop a new SUPER guideline specifically tailored to the surgical technique rather than to modify the TIDieR guideline, given the importance of the surgical technique and the substantial differences in the reporting dimensions and level of detail required for its reporting compared to other interventions. We encourage the use of the SUPER guideline in combination with these guidelines (Figure S1) to promote more detailed and reproducible reports for surgical technique-related literature.
Explanations of findings
Although the SUPER guideline focuses primarily on the detailed description of surgical technique, it goes well beyond this. Surgeries are complex and the chosen surgical technique is not the only factor affecting the patient’s outcome—careful patient selection, appropriate settings, safe anesthesia, appropriate postoperative settings, and protocols, for instance, all impact the surgical results. The entire scheme has to be defined and documented to enable its reproducibility by others and achieve similar positive outcomes. Therefore, although technical skills and details are the cornerstone of SUPER, the guideline also includes an important description of the environment, surgeon, patient selection, anesthesia procedures, postoperative monitoring, complications management, follow-up, and other key factors that influence outcomes (items 6–9 and items 16–19). It is also worth mentioning that SUPER includes a specific expectation for the visualization of the surgical technique (item 15). Historically, the development of surgical technique was initially largely dependent on textbooks and hands-on training. These methods have since evolved into richer modalities, such as watching surgical videos remotely or online. With the COVID-19 pandemic, a growing number of surgeons have developed a positive attitude towards learning surgical technique on streaming media such as YouTube (25,26). Further, surgical videos and images can be disseminated separately from an article. Therefore, we believe that standardization of surgical videos and images is warranted now more than ever.
Implications and actions needed
There are several key clarifications on the use of the SUPER guideline:
❖ SUPER is not a tool for assessing the quality of surgical technique; it only provides suggestions for better reporting of surgical technique.
❖ SUPER does not prescribe a specific reporting sequence and only specifies which items should be clearly and sufficiently presented within the article.
❖ The requirements for creating surgical images and videos may seem difficult to achieve for users. However, the item does not demand sophisticated technology—manual drawings, medical illustrations, videos recorded by cell phone or professional equipment are all appropriate as long as they comprehensively represent essential information. Step-by-step instructions have already been available detailing how to record and make a publishable surgical video (27).
❖ There may be some concerns that the SUPER guideline mandates the standardization of procedures by advocating detailed reporting, which potentially conflicts with the idea of individualized treatment. While variations in surgical technique are common, not unfavorable or discouraged, and are completely acceptable, variation and heterogeneity in patient outcomes are unacceptable (28). Standardized and detailed reporting of a surgical technique is therefore not a deterrent to progress or personalized care, but rather an attempt to make the technique more stable and progressive by reducing variation that may induce unsatisfactory patient outcomes. It can then be followed by further integration of patient-centered therapy, evidence-based evaluation, and surgeons’ ideas. Detailed reporting of the surgical technique is therefore crucial to the goal of optimal integration.
Extensive endorsement and thorough implementation make the SUPER guideline work. Regarding endorsement of the SUPER guideline, we recommend that journals advocate and, further, require that reporting of surgical technique follows the SUPER guidelines in their author instructions. Evidence suggests that the majority (62%) of surgical journals do not mention reporting guidelines in their author instructions, and of those that do (38%), only 14% require explicit and relevant reporting guidelines (29). This situation warrants urgent improvement. Current literature has found insufficient evidence of an association between journal endorsement of reporting guidelines and the reporting completeness of published health research reports (30). Rather than dismissing the importance of journal endorsement, this may indirectly reflect that endorsement by journals at present may be more of a slogan than a real practice. To promote the implementation of the SUPER guideline, we advise journals to take the following steps: arrange for editors to check the reporting quality item by item; send the reporting checklist to peer reviewers; publish the completed reporting checklist online together with the article; encourage authors to detail and fully describe the surgical technique in the supplement. This guideline may also face practical challenges in its application, such as users feeling overwhelmed in using a reporting guideline, not knowing standards of good compliance, and using the reporting guideline too late (31). The SUPER collaborative group also plans to facilitate implementation by developing online writing tools and strengthening education (32).
Conclusions
The SUPER guideline has been developed based on the consensus of a multidisciplinary team using a three-round Delphi approach. It offers direction and specific benchmarks for detailed and comprehensive reporting of surgical technique. Surgeons, journal editors, peer reviewers, systematic reviewers, and guideline developers are encouraged to adopt the SUPER guideline, provide feedback, and conduct relevant research. Such efforts will help to improve the transparency, availability, accessibility, safety, and reproducibility of surgical techniques, facilitating procedure evaluation and reducing resource waste in surgical practice.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the language polish by Jennifer Reynolds.
Funding: This project is supported by the AME Reporting Guidelines Research Fund (No. 2020-1016-885) and Lanzhou University Research Unit for Evidence-Based Evaluation and Guidelines, Chinese Academy of Medical Sciences Fund (2021RU017). The study sponsor participated in the design, with no involvement in collection, analysis, and interpretation of the data, in the writing of the article.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This manuscript does not need ethics approval.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-509/coif). KZ, GSL, XT and SDW are full-time employees of AME Publishing Company, the publisher of Hepatobiliary Surgery and Nutrition. RHP has received speaker fees from Medtronic, AMBU, Medela and AstraZeneca and is the Advisory Board member of AstraZeneca, MSD and Roche. CSHN has received consulting fees from Medtronic & Johnson and Johnson. AT reports consulting fee from Intuitive. MFJ has received consulting and lecture fees from Medtronic and Intuitive. AYO reports educational and research grants from Artivion and Terumo Aortic to his institution. The other authors have no conflicts of interest to declare.
References
- 1.Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015;386:569-624. 10.1016/S0140-6736(15)60160-X [DOI] [PubMed] [Google Scholar]
- 2.Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385 Suppl 2:S11. 10.1016/S0140-6736(15)60806-6 [DOI] [PubMed] [Google Scholar]
- 3.Zhang K, Ma Y, Shi Q, et al. Developing the surgical technique reporting checklist and standards: a study protocol. Gland Surg 2021;10:2591-9. 10.21037/gs-21-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosseinpour AR. The Importance of Surgical Technique. J Surg Tech Proced 2017;1:1001. [Google Scholar]
- 5.Birkmeyer JD, Finks JF, O'Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013;369:1434-42. 10.1056/NEJMsa1300625 [DOI] [PubMed] [Google Scholar]
- 6.Varban OA, Thumma JR, Finks JF, et al. Evaluating the Effect of Surgical Skill on Outcomes for Laparoscopic Sleeve Gastrectomy: A Video-based Study. Ann Surg 2021;273:766-71. 10.1097/SLA.0000000000003385 [DOI] [PubMed] [Google Scholar]
- 7.Agha RA, Fowler AJ, Lee SY, et al. Systematic review of the methodological and reporting quality of case series in surgery. Br J Surg 2016;103:1253-8. 10.1002/bjs.10235 [DOI] [PubMed] [Google Scholar]
- 8.Jacquier I, Boutron I, Moher D, et al. The reporting of randomized clinical trials using a surgical intervention is in need of immediate improvement: a systematic review. Ann Surg 2006;244:677-83. 10.1097/01.sla.0000242707.44007.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha S, Sinha S, Ashby E, et al. Quality of reporting in randomized trials published in high-quality surgical journals. J Am Coll Surg 2009;209:565-71.e1. 10.1016/j.jamcollsurg.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 10.Turner L, Shamseer L, Altman DG, et al. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane review. Syst Rev 2012;1:60. 10.1186/2046-4053-1-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans S, Rauh S, Jellison S, et al. Evaluation of the Completeness of Interventions Reported in Published Randomized Controlled Trials in Plastic Surgery: A Systematic Review. Aesthet Surg J 2021;41:707-19. 10.1093/asj/sjaa166 [DOI] [PubMed] [Google Scholar]
- 12.Blencowe NS, Boddy AP, Harris A, et al. Systematic review of intervention design and delivery in pragmatic and explanatory surgical randomized clinical trials. Br J Surg 2015;102:1037-47. 10.1002/bjs.9808 [DOI] [PubMed] [Google Scholar]
- 13.Candy B, Vickerstaff V, Jones L, et al. Description of complex interventions: analysis of changes in reporting in randomised trials since 2002. Trials 2018;19:110. 10.1186/s13063-018-2503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray R, Sullivan M, Altman DG, et al. Adherence of trials of operative intervention to the CONSORT statement extension for non-pharmacological treatments: a comparative before and after study. Ann R Coll Surg Engl 2012;94:388-94. 10.1308/003588412X13171221592339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassar M, Page MJ, Glasbey J, et al. Evaluation of the completeness of intervention reporting in Cochrane surgical systematic reviews using the TIDieR-SR checklist: a cross-sectional study. BMJ Evid Based Med 2021;26:51-2. 10.1136/bmjebm-2020-111417 [DOI] [PubMed] [Google Scholar]
- 16.Boutron I, Altman DG, Moher D, et al. CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann Intern Med 2017;167:40-7. 10.7326/M17-0046 [DOI] [PubMed] [Google Scholar]
- 17.Mathew G, Agha R; STROCSS Group. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. 10.1016/j.ijsu.2021.106165 [DOI] [PubMed] [Google Scholar]
- 18.Agha RA, Sohrabi C, Mathew G, et al. The PROCESS 2020 Guideline: Updating Consensus Preferred Reporting Of CasESeries in Surgery (PROCESS) Guidelines. Int J Surg 2020;84:231-5. 10.1016/j.ijsu.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Agha RA, Franchi T, Sohrabi C, Mathew G, Kerwan A; SCARE Group. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg 2020;84:226-30. 10.1016/j.ijsu.2020.10.034 [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 21.Bilbro NA, Hirst A, Paez A, et al. The IDEAL Reporting Guidelines: A Delphi Consensus Statement Stage Specific Recommendations for Reporting the Evaluation of Surgical Innovation. Ann Surg 2021;273:82-5. 10.1097/SLA.0000000000004180 [DOI] [PubMed] [Google Scholar]
- 22.Shi Q, Ma Y, Zhang X, et al. Reporting guidelines for surgical technique could be improved: A scoping review and a call for action. J Clin Epidemiol 2022. [Epub ahead of print]. doi: . 10.1016/j.jclinepi.2022.11.012 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Schulz KF, Simera I, et al. Guidance for developers of health research reporting guidelines. PLoS Med 2010;7:e1000217. 10.1371/journal.pmed.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018;319:388-96. 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 25.Marcasciano M, Kaciulyte J, Mori FLR, et al. Breast surgeons updating on the thresholds of COVID-19 era: results of a multicenter collaborative study evaluating the role of online videos and multimedia sources on breast surgeons education and training. Eur Rev Med Pharmacol Sci 2020;24:7845- 54. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Fukunaga T, Oka S, et al. Concerns of quality, utility, and reliability of laparoscopic gastrectomy for gastric cancer in public video sharing platform. Ann Transl Med 2020;8:196. 10.21037/atm.2020.01.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pojskić M, Nguyen VN, Gienapp AJ, et al. Step-by-Step Guide on How to Make a 2-Dimensional Operative Neurosurgical Video: Microsurgical Resection of a Right Lateral Ventricle Subependymoma by an Anterior Interhemispheric Transcallosal Approach. Oper Neurosurg (Hagerstown) 2022;22:165-70. 10.1227/ONS.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 28.Skjold-Ødegaard B, Søreide K. Standardization in surgery: friend or foe? Br J Surg 2020;107:1094-6. 10.1002/bjs.11573 [DOI] [PubMed] [Google Scholar]
- 29.Agha RA, Barai I, Rajmohan S, et al. Support for reporting guidelines in surgical journals needs improvement: A systematic review. Int J Surg 2017;45:14-7. 10.1016/j.ijsu.2017.06.084 [DOI] [PubMed] [Google Scholar]
- 30.Stevens A, Shamseer L, Weinstein E, et al. Relation of completeness of reporting of health research to journals' endorsement of reporting guidelines: systematic review. BMJ 2014;348:g3804. 10.1136/bmj.g3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marušić A. A tool to make reporting checklists work. BMC Med 2015;13:243. 10.1186/s12916-015-0476-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struthers C, Harwood J, de Beyer JA, et al. GoodReports: developing a website to help health researchers find and use reporting guidelines. BMC Med Res Methodol 2021;21:217. 10.1186/s12874-021-01402-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as