Abstract
Background
Cholecystectomy is a common surgical procedure to treat symptomatic gallstones; however, the long-term outcomes after cholecystectomy are unknown. Therefore, we aimed to investigate whether incident metabolic syndrome (MetS) is associated with cholecystectomy through a large, population-based, longitudinal study.
Methods
Subjects aged ≥20 years who underwent cholecystectomy from 2010 to 2014 (n=76,485) and controls (n=76,485), matched for age and sex, were identified from the Korean National Health Insurance Corporation. Cox proportional hazards analyses were performed to evaluate the association between cases and incident MetS, and hazard ratios and 95% confidence intervals (CIs) were calculated.
Results
A total of 152,970 patients were included. Mean age was 52.47±12.76 years, and 50.65% of participants were male. During the follow-up period, there were 38,979 (25.48%) newly diagnosed MetS cases in the study participants. The risk of MetS in the cholecystectomy group was approximately 20% higher than that in the control group [adjusted odds ratio (OR), 1.20; 95% CI: 1.17–1.23]. In the fully adjusted models, the corresponding ORs for new-onset high waist circumference (WC), low high-density lipoprotein cholesterol (HDL-C) levels, high triglycerides (TG) levels, high blood pressure (BP), and high blood glucose levels were 1.16 (1.13–1.19), 1.19 (1.16–1.22), 1.25 (1.22–1.28), 1.27 (1.23–1.31), and 1.21 (1.18–1.24), respectively. Cholecystectomy was an independent risk factor of incident MetS, after adjusting for potential confounding factors. In the subgroup analyses, the cholecystectomy group had a higher risk of MetS than the control group in subjects without hypertension or dyslipidemia, respectively.
Conclusions
In this large, population-based study, cholecystectomy was associated with an increased risk of developing MetS, independent of other confounding factors. Therefore, careful monitoring of metabolic variables and long-term follow-up are required to evaluate MetS risk after cholecystectomy.
Keywords: Cholecystectomy, metabolic syndrome (MetS), obesity, diabetes, cardiovascular risk
Introduction
Cholecystectomy is the standard treatment for symptomatic gallstones and is one of the most commonly performed operations worldwide (1). Gallstones and cholecystitis admissions rose by 135 percent between 2005 and 2019, from 40,592 to 95,337, according to the Korean National Health Insurance Service (NHIS) (2). The clinical outcomes of cholecystectomy are known to be overall good, and the surgical procedure has been considered to be safe, with minimal rates of mortality and morbidity. However, there is a growing body of evidence suggesting that cholecystectomy may lead to an increased risk for the development of metabolic abnormalities, including dyslipidemia (3), and nonalcoholic fatty liver disease (NAFLD) (4), and hyperglycemia (5). Moreover, one recent cross-sectional study found that the prevalence of metabolic syndrome (MetS) was 53.2% among subjects with gallstone disease and 63.5% among cholecystectomized subjects (6). These findings suggest that cholecystectomy itself may be a risk factor for metabolic disease, such as insulin resistance, independent of the pathogenesis of gallstones. However, there have been few studies that have investigated the impact of cholecystectomy on incident MetS.
MetS refers to a cluster of specific cardiovascular disease risk factors whose underlying pathophysiology is thought to be related to insulin resistance; these factors include abdominal obesity, dyslipidemia, impaired glucose tolerance, and hypertension. MetS is common, and its increasing prevalence worldwide relates largely to the increasing number of individuals with obesity and sedentary lifestyles. In Korea, 20.3% of adults aged ≥19 years have MetS in 2013–2015 according to the Korean National Health and Nutrition Examination Surveys (7). Because MetS directly promotes the development of cardiovascular diseases (8), diabetes (9), and chronic kidney disease (10), and even increases mortality (11), it has become an important public health problem that must be managed globally. Therefore, it is important to investigate possible risk factors for MetS, and identifying subjects at higher risk of developing MetS is important in clinical practice.
Recently, the roles of the gallbladder and bile acids in systemic metabolic homeostasis have become highlighted (12). Thus, it is necessary to examine the potential negative clinical outcomes of gallbladder removal, especially those associated with the risk of the subsequent development of MetS. Despite the emerging recognition of the association between gallbladder loss and insulin resistance, few large-scale, longitudinal, cohort studies have been conducted to determine whether there is an association between cholecystectomy and MetS. Thus, the present large-scale, population-based, longitudinal cohort study aimed to determine whether such an association exists, using data from the Korean NHIS database, which is a nationwide, representative cohort of the Korean population. We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-201/rc)
Methods
NHIS database and NHIS health check-up program
In our cohort study, we used data from the NHIS, which is a government policy program that was first implemented in the year 2000 and covers approximately 98% of the Korean population. The NHIS collects patients’ demographic data, such as that related to the region, age, sex, medical utilization/transaction information, claims and deduction data, and insurers’ payment coverage. The NHIS database has been described in detail in previous studies (13-15). The NHIS manages a biennial health check-up program for all insured Koreans aged >40 years, and an annual NHIS health check-up is recommended for employee subscribers who are aged >20 years. The NHIS health check-up program has four possible components that include a general health check-up, a neonatal/infant health check-up, cancer screening, and lifetime transition period health check-ups. The NHIS health check-up programs include anthropometric measurements, hearing, and visual acuity checks, laboratory tests, and assessments of past family, medical, and surgical histories, as well as social history. Hospitals perform these health check-ups after being certified by the NHIS, which also regularly qualifies trained examiners.
Study population
Using the NHIS database, we created a cohort of patients who underwent cholecystectomy between January 1, 2010, and December 31, 2014. We selected subjects who were aged >20 years who had undergone cholecystectomy from 2010 to 2014, as identified by the national health insurance claim codes for surgical procedure (Q7380) (n=284,829) (16). Subsequently, we selected subjects who received NHIS health check-ups within two years before the index date (n=157,741) and also selected individuals who received health check-ups again after the index date (n=134,685). Among these eligible subjects, we excluded subjects who had already received a MetS diagnosis within the 2 years before the index date (n=52,969), as well as subjects who had any missing data related to MetS variables (n=5,228). As a result, a total of 76,485 subjects who underwent cholecystectomy from 2010 to 2014, according to the medical records, were recruited in this study. Moreover, we subsequently recruited control individuals from among the subjects in the same cohort database who did not undergo a cholecystectomy, using a 1:1 matching ratio based on age and sex. Subsequently, we excluded those controls that fulfilled any of the exclusion criteria described for the cholecystectomy cases, including those with a MetS diagnosis within two years before the index date. For the participants who had undergone cholecystectomy, the index date was defined as the date of cholecystectomy. The index date of each control was assigned as the index date of the matched case. Finally, a total of 152,970 eligible individuals (76,485 cholecystectomy cases and 76,485 controls) were included in our study analysis. The flow chart of the selection process is shown in Figure S1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Hallym University Sacred Heart Hospital Institutional Review Board (IRB No. HALLYM 2020-05-001), and permission was granted to use the NHIS health check-up data (NHIS-REQ000047373-008). The requirement for written informed consent was waived by the review board because anonymous and de-identified information was used for the analysis.
Data collection
Detailed information pertaining to individuals’ demographic and lifestyle data were obtained from standardized self-report questionnaires. Income level was dichotomized at the lower 25%. Smoking status was classified as a non-smoker, ex-smoker, or current smoker. Alcohol consumption status was evaluated using a self-reported questionnaire at the first health examinations. Data on the frequency of alcohol intake per week (0–7) and amount of alcohol intake per session (0 to <32 g, 32 to <56 g, 56 to <112 g, and ≥112 g) were obtained from the self-reported questionnaire. Based on the frequency per week and amount per session, we classified the amount of alcohol consumption per day as none (0 g/day), mild (>0 g/day, <30 g/day), and heavy (≥30 g/day), respectively. Regular exercise was defined as >30 minutes of moderate-intensity physical activity ≥5 times a week (e.g. brisk walking, slow cycling, or tennis doubles) or >20 minutes of strenuous physical activity ≥5 times per week (e.g., jogging or running, bicycling >15 km/h, climbing briskly up a hill, or participating in an aerobics class). The health examinations provided by the NHIS include anthropometric and laboratory measurements. Height, weight, and waist circumference (WC) were measured, and body mass index (BMI) was calculated by dividing the weight (kg) by the square of the height (m). Systolic and diastolic blood pressure (BP) were measured in a seated position after at least five minutes at rest. Blood sampling was conducted after overnight fasting, and serum levels of glucose, total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and creatinine were measured.
Ascertainment of concomitant comorbidities
Disease diagnosis was based on the International Classification of Diseases, 10th Revision (ICD-10), Clinical Modification; medical treatment; and health examination results. Hypertension was defined as a BP of ≥140/90 mmHg or at least one claim per year for an antihypertensive medication prescription under ICD-10 codes I10–I15. Dyslipidemia was defined as a total cholesterol level of ≥240 mg/dL or at least one claim per year for the prescription of lipid-lowering agents under ICD-10 code E78 (17). Diabetes was defined as a fasting blood glucose level of ≥126 mg/dL or a prescription for anti-diabetic medication under ICD-10 codes E11–14 (18). Obesity was defined as a BMI of ≥25 kg/m2 in accordance with the Asia-Pacific criteria of the World Health Organization (WHO) guidelines.
Definition of incident MetS
The endpoint of this study was newly developed MetS at the follow-up visit. MetS was defined based on the modified criteria of the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP III) (19), whereas the Asian-specific WC cutoff was adopted for abdominal obesity (20). Individuals meeting at least three of the following criteria were diagnosed with MetS: (I) WC ≥90 cm for men or ≥85 cm for women according to the cutoffs for abdominal obesity defined by the Korean Society of Obesity (21); (II) serum triglyceride levels ≥150 mg/dL or treatment with lipid-lowering medication; (III) serum HDL-C level <40 mg/dL for men or <50 mg/dL for women; (IV) systolic BP ≥130 mmHg, diastolic BP ≥85 mmHg, or treatment with antihypertensive medication; and (V) fasting plasma glucose level ≥100 mg/dL or use of anti-hyperglycemic agents.
Statistical analysis
The baseline characteristics of the subjects are expressed as mean ± standard deviation for continuous variables and as numbers (percentages) for categorical variables. The values for each variable were compared between two groups using independent t-tests for continuous variables and chi-square tests for categorical variables. We evaluated the association of cholecystectomy with the incidence of new cases of MetS and with the incidence of new cases of each MetS component at the follow-up visit. To evaluate the incidence of new cases of each MetS component, we excluded subjects who exhibited the presence of that specific component at baseline. Multivariable logistic regression analysis was used to assess the independent association of cholecystectomy with incident MetS. The multivariable-adjusted models were as follows: Model 1 was the crude model, Model 2 was adjusted for age, sex, low income, smoking status, alcohol consumption, and regular exercise. We also examined the associations between cholecystectomy status and incident MetS in several subgroups based on age, sex, obesity, smoking status, alcohol consumption, physical activity, and the presence of diabetes, hypertension, and dyslipidemia at baseline. The results are expressed as odds ratios (ORs) [95% confidence interval (CI)]. The OR (95% CI) for incident MetS of the cholecystectomy group was compared with that of the non-cholecystectomy group as a reference. P values <0.05 were considered statistically significant. Statistical analysis was performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics of the study population
The analysis included 152,970 study participants (76,485 patients with cholecystectomy and 76,485 matched patients without cholecystectomy). The baseline characteristics of the participants with/without cholecystectomy are described in Table 1; their mean age was 52.47±12.76 years, and 50.65% were male. The proportion of subjects categorized as having low income (the lowest quartile) was higher in the non-cholecystectomy group than in the cholecystectomy group. The proportions of non-smokers and regular exercisers were higher in the non-cholecystectomy group than in the cholecystectomy group. The proportion of heavy alcohol drinkers was higher in the non-cholecystectomy group than in the cholecystectomy group. The participants who underwent cholecystectomy exhibited higher values for the metabolic parameters (including BMI, WC, fasting glucose levels, LDL cholesterol levels, and TG levels) than those in the non-cholecystectomy group, whereas those who underwent cholecystectomy had lower HDL-C and total cholesterol levels than those who did not. The systolic and diastolic BP levels were similar between the two groups. The patients who received a cholecystectomy were more likely to exhibit a higher prevalence of hypertension, and diabetes at baseline than those without cholecystectomy (all P<0.001).
Table 1. Baseline characteristics of participants.
| Characteristics | Cholecystectomy (n=76,485) | Non-cholecystectomy (n=76,485) | P value |
|---|---|---|---|
| Age (years) | 52.47±12.76 | 52.47±12.76 | 1.000 |
| Sex, male | 38,736 (50.65) | 38,736 (50.65) | 1.000 |
| Body mass index (kg/m2) | 23.68±2.98 | 23.05±2.782 | <0.001 |
| Waist circumference (cm) | 80.07±8.15 | 78.42±7.95 | <0.001 |
| Fasting blood glucose (mg/dL) | 94.92±18.92 | 94.13±17.72 | <0.001 |
| Total cholesterol (mg/dL) | 195.57±35.69 | 196.35±34.9 | <0.001 |
| Triglyceride (mg/dL) | 100.43 (100.06–100.8) | 98.57 (98.19–98.95) | <0.0001 |
| HDL-C (mg/dL) | 56.06±20.05 | 57.77±19.86 | <0.001 |
| LDL-C (mg/dL) | 117.99±33.38 | 117.12±33.51 | <0.001 |
| Systolic blood pressure | 120.13±13.92 | 120.22±14.33 | 0.201 |
| Diastolic blood pressure | 74.92±9.43 | 74.94±9.61 | 0.743 |
| Comorbidities | |||
| Hypertension | 16,554 (21.64) | 15.426 (20.17) | <0.001 |
| Dyslipidemia | 9,459 (12.37) | 9,341 (12.21) | 0.358 |
| Diabetes mellitus | 4,238 (5.54) | 3,325 (4.35) | <0.001 |
| Smoking status | <0.001 | ||
| Non-smoker | 47,873 (62.59) | 48,872 (63.9) | |
| Ex-smoker | 12,609 (16.49) | 11,951 (15.63) | |
| Current | 16,003 (20.92) | 15,662 (20.48) | |
| Alcohol | <0.001 | ||
| None | 44,897 (58.7) | 42,346 (55.37) | |
| Mild | 27,033 (35.34) | 29,323 (38.34) | |
| Heavy | 4,555 (5.96) | 4,816 (6.3) | |
| Regular exercise | 14,760 (19.3) | 15,584 (20.38) | <0.001 |
| Low income (<25%) | 13,893 (18.16) | 14,829 (19.39) | <0.001 |
Data are expressed as the mean ± SD, n (%) or median (interquartile range). HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Risk of incident MetS according to the cholecystectomy status
During the mean follow-up period of 4.9±1.7 years [median follow-up duration: 4.87 (3.84–6.1) years], there were 38,979 (25.48%) individuals with newly diagnosed MetS among the study participants. The number of incident MetS were 18,212 (23.81%) in non-cholecystectomy group and 20,767 (27.15%) in cholecystectomy group. An increased risk of MetS was observed in the cholecystectomy group compared with that of the non-cholecystectomy group in the crude model (OR, 1.19; 95% CI: 1.17–1.22) (Table 2). After fully adjusting for cofounding factors including age, sex, income, smoking status, alcohol consumption, and regular exercise, the relationship between cholecystectomy and incident MetS remained significant (OR, 1.20; 95% CI: 1.17–1.23).
Table 2. Risk of metabolic syndrome according to the cholecystectomy status.
| Cholecystectomy status | Incident metabolic syndrome case, n (%) | Odds ratio (95% CI) | |
|---|---|---|---|
| Model 1 | Model 2 | ||
| Non-cholecystectomy | 18,212 (23.81) | 1 (reference) | 1 (reference) |
| Cholecystectomy | 20,767 (27.15) | 1.19 (1.17–1.22) | 1.20 (1.17–1.23) |
Model 1: age, sex (Crude model); Model 2: age, sex, low income, smoking status, alcohol intake, regular exercise. CI, confidence interval.
Risk of developing each individual MetS component according to the cholecystectomy status
Table 3 shows the developing risk for each MetS component during the follow-up period according to the cholecystectomy status. The numbers of subjects who developed new-onset high WC, low HDL-C levels, high TG levels, high BP, and high blood glucose levels were 10,495 (15.98%), 15,850 (25.86%), 16,813 (26.42%), 13,905 (28.06%), and 16,646 (28.48%), respectively. In the crude model, the risk for developing all of the components of MetS increased in the cholecystectomy group compared with the non-cholecystectomy group. Even after adjusting for age, sex, low income, smoking status, alcohol consumption, and regular exercise, the observed higher risk of developing a high WC, low HDL-C levels, high TG levels, high BP, and high blood glucose levels remained in the cholecystectomy group in the crude model. In the fully-adjusted models, the corresponding ORs for new-onset high WC, low HDL-C levels, high TG levels, high BP, and high blood glucose levels were 1.16 (1.13–1.19), 1.19 (1.16–1.22), 1.25 (1.22–1.28), 1.27 (1.23–1.31), and 1.21 (1.18–1.24), respectively.
Table 3. The incident risk of metabolic syndrome components according to the status of cholecystectomy.
| Components | Cholecystectomy | Incident cases, n (%) | Odds ratio (95% CI) | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| High waist circumference | No | 8,545 (12.34) | 1 (reference) | 1 (reference) |
| Yes | 10,495 (15.98) | 1.16 (1.13–1.19) | 1.16 (1.13–1.19) | |
| Low HDL-C | No | 14,685 (23.05) | 1 (reference) | 1 (reference) |
| Yes | 15,850 (25.86) | 1.19 (1.16–1.22) | 1.19 (1.16–1.22) | |
| High triglyceride | No | 16,010 (25.38) | 1 (reference) | 1 (reference) |
| Yes | 16,813 (26.42) | 1.25 (1.22–1.28) | 1.25 (1.21–1.28) | |
| High blood pressure | No | 13,354 (26.87) | 1 (reference) | 1 (reference) |
| Yes | 13,905 (28.06) | 1.27 (1.23–1.32) | 1.27 (1.23–1.31) | |
| High blood glucose | No | 15,823 (26.54) | 1 (reference) | 1 (reference) |
| Yes | 16,646 (28.48) | 1.21 (1.18–1.24) | 1.21 (1.17–1.24) | |
Model 1: age, sex (Crude model); Model 2: age, sex, low income, smoking status, alcohol intake, regular exercise. HDL-C, high density lipoprotein cholesterol; CI, confidence interval.
Subgroup analysis of the risk of incident MetS
Because there were some differences in the baseline characteristics between the case (cholecystectomy) and control (non-cholecystectomy) groups, we investigated the risk of incident MetS using subgroup analyses stratified by age, sex, smoking status, alcohol consumption, regular exercise, and the presence or absence of obesity, diabetes, hypertension, and dyslipidemia (Figure 1). The cholecystectomy group exhibited higher risks of incident MetS in most of the subgroups (except in the subjects who were ex-smokers or heavy drinkers) compared with those in the control group. The increased risk of incident MetS in the cholecystectomy group was significantly higher in females than in males (adjusted OR: 1.27, 95% CI: 1.22–1.31 in females vs. adjusted OR: 1.14, 95% CI: 1.11–1.18 in males, P for interaction <0.0001) and in the younger age group than in the older age group (adjusted OR: 1.24, 95% CI: 1.20–1.27 in <65 years vs. adjusted OR: 1.08, 95% CI: 1.03–1.13 in ≥65 years, P for interaction <0.0001). Furthermore, the risk was also higher in non-heavy drinkers than in heavy drinkers (adjusted OR: 1.24, 95% CI: 1.20–1.28 in non-heavy drinkers vs. adjusted OR: 1.14, 95% CI: 1.10–1.18 in heavy drinkers, P for interaction =0.0005). The risk of MetS was also higher in non-current smokers (adjusted OR: 1.21, 95% CI: 1.18–1.25, P for interaction =0.0447) than the current smokers (adjusted OR: 1.15, 95% CI: 1.09–1.20). The increased risk of MetS in the cholecystectomy group was constantly observed regardless of presence or absence of obesity. In the cholecystectomy group, the higher adjusted OR of incident MetS were more remarkable among the subgroups without hypertension (adjusted OR: 1.23, 95% CI: 1.20–1.27 in patients without hypertension vs. adjusted OR: 1.07, 95% CI: 1.03–1.12 in patients with hypertension, P for interaction: <0.001), diabetes (adjusted OR: 1.20, 95% CI: 1.7–1.23 in patients without diabetes vs. adjusted OR: 0.993, 95% CI: 0.906–1.089 in patients with diabetes, P for interaction: 0.0001), or dyslipidemia (adjusted OR: 1.22, 95% CI: 1.19–1.25 in patients without dyslipidemia vs. adjusted OR: 1.10, 95% CI: 1.04–1.17 in patients with dyslipidemia, P for interaction: 0.0014) than in those with hypertension, diabetes, or dyslipidemia.
Figure 1.
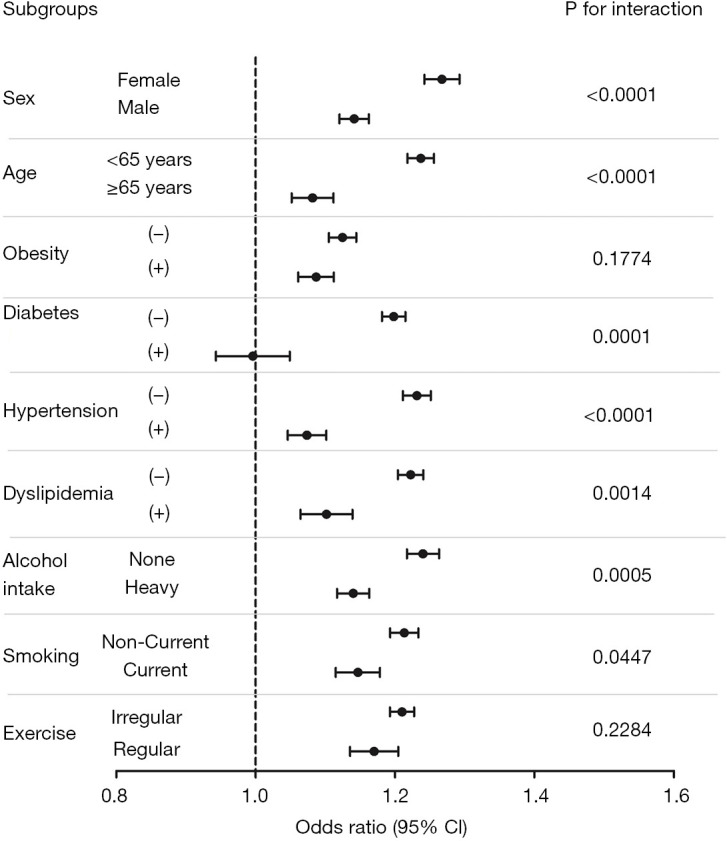
Subgroup analysis of metabolic syndrome risk in patients with cholecystectomy compared to those in patients without cholecystectomy. Model was adjusted for age, sex, low income, smoking status, alcohol intake, regular exercise. CI, confidence interval.
Discussion
In this large-scale, longitudinal cohort study comprising 152,970 Korean patients from the general population, we investigated the relationship between cholecystectomy and the development of MetS. We observed that individuals who underwent cholecystectomy exhibited an approximately 21% higher risk of incident MetS than those who did not undergo cholecystectomy. Cholecystectomy was an independent predictor of incident MetS in the future, even after adjusting for potential confounding factors. In the subgroup analyses, we observed that the increased risk of MetS in cholecystectomy patients was more prominent in those with lower cardiovascular risk factors (e.g., female sex, younger age, none-heavy alcohol drinkers, non-smokers, and the absence of hypertension, diabetes, and dyslipidemia) than in those with higher cardiovascular risk factors. Our findings suggest that the absence of a gallbladder by itself may be a risk factor for MetS. To the best of our knowledge, this is the first and largest nationwide, longitudinal, cohort study to examine the relationship between cholecystectomy and the risk of MetS in the general population.
Numerous epidemiological studies have investigated whether gallbladder disease or cholecystectomy status is associated with the development of MetS or insulin resistance-related chronic metabolic disease. These previous studies have demonstrated that gallbladder disease or cholecystectomy is independently associated with NAFLD (22), MetS (23), and altered glucose homeostasis (24,25). However, most of these previous studies were small in scale and involved a cross-sectional design; consequently, a cause-effect relationship between cholecystectomy and MetS in the general population could not be clearly established. Furthermore, those previous studies could not investigate the isolated effect of cholecystectomy on future metabolic health, as they failed to differentiate between cholecystectomy and general gallstone disease. One recent review article did demonstrate that cholecystectomy per se may cause metabolic abnormalities, making cholecystectomized patients metabolically different from gallstone patients with a gallbladder in situ (26). Thus, it is important to investigate whether cholecystectomy itself, distinct from gallstone disease, contributes to the development of MetS or other chronic metabolic disorders. We, therefore, considered it crucial to conduct a longitudinal study to explore the actual relationship between the absence of a gallbladder caused by cholecystectomy and incident MetS using large-scale and longitudinal cohort data. Thus, our findings are capable of providing more concrete evidence of the association between cholecystectomy and MetS.
In our study, the subjects who underwent cholecystectomy had already higher cardio-metabolic risk factors at baseline than those who did not receive cholecystectomy. The main cause of cholecystectomy is usually gallstone disease, which has a shared pathogenesis with MetS; for example, insulin resistance and obesity. Therefore, the higher cardio-metabolic risk observed in the cholecystectomy group at baseline may have influenced the ultimate incidence of MetS. To overcome this limitation, we performed subgroup analyses, stratified by the presence or absence of various cardio-metabolic risk factors (e.g., old age, male sex, obesity, diabetes, hypertension and dyslipidemia) at baseline. As a result, we observed that cholecystectomy was consistently associated with incident MetS, regardless of the cardio-metabolic risk status at baseline. Moreover, we found that the association between cholecystectomy and incident MetS was more prominent in the participants without the traditional cardiovascular risk factors (such as female sex, younger age, absence of diabetes, hypertension, and dyslipidemia) than in those with the traditional cardiovascular risk factors. This finding suggests that even metabolically healthy individuals at baseline should be carefully monitored for incident MetS or metabolic abnormalities if they undergo cholecystectomy. Furthermore, when we conducted a propensity score (PS)-matched analysis including age, sex, income status, alcohol consumption, smoking and exercise status, and all metabolic parameters, we found that the risk of developing MetS increased by 10% among the cholecystectomy group than the non-cholecystectomy group (Table S1). This evidence makes it reasonable to speculate that cholecystectomy may directly stimulate or result in a deranged metabolic profile.
In terms of each metabolic component, we found that cholecystectomy was significantly associated with almost all of the components of MetS. The current study supports the data suggesting a link between cholecystectomy and MetS. This finding is in line with those of previous studies that reported a significant weight gain or elevation of glucose levels among cholecystectomized patients (3,27). Other studies showed that subjects with gall bladder disease or those who had undergone cholecystectomy had higher plasma TG levels, as well as higher levels of total cholesterol, but lower levels of HDL-C, than patients who had not undergone cholecystectomy (3,28). A previous study with a cross-sectional design also reported a significantly higher prevalence of hypertension among subjects with history of cholecystectomy than in patients without gallstone disease (29). Similarly, our study demonstrated that cholecystectomy increased the risk of high BP the most among five components of MetS. While, cholecystectomy increased the risk of abdominal obesity the least among five components of MetS. This suggests that other mechanisms besides weigh gain caused by the absence of the gallbladder may influence on the metabolism of glucose, lipid, and vascular resistance. To elucidate these relationships, further large-scale and long-term follow-up studies are warranted.
The underlying mechanisms by which cholecystectomy may cause MetS are not fully elucidated; however, there are several possible explanations for these relationships. The gallbladder is a physiological pacemaker that controls bile acid concentrations by contracting and relaxing rhythmically in relation with dietary intake (30). This function has an important role in regulating the whole-body homeostasis of TGs, bile acids, and cholesterol (31) through the nuclear farnesoid X receptor (FXR) and the gallbladder G-protein-coupled bile acid receptor 1 (GPBAR-1) (32). After cholecystectomy, the rhythm and intensity of bile acid flow are altered, and the rapid release of bile acids into the duodenum in response to food intake is impoverished (5). Ultimately, the gene expression of components of the bile acids/FXR and bile acids/GPBAR-1 axes that regulate glucose homeostasis and lipid metabolism becomes disturbed. Also, the level of circulating human protein fibroblast growth factor (FGF)-19, which regulates lipid, glucose, and energy metabolism, is decreased (33,34). Thus, removal of the gallbladder could influence the suppression of FXR- and GPBAR-1-dependent metabolic signaling and decrease of FGF-19 levels, resulting in unstable systemic metabolic homeostasis.
The major strengths of the current study include its large sample size, which comprised more than 125,000 subjects, and the use of longitudinal data. Furthermore, our study provides the first evidence that cholecystectomy may directly contribute to the development of MetS in the general population, as the NHIS cohort data are based on a nationally representative cohort database, comprising nearly the entire South Korean population. However, our study has several limitations that need to be addressed. First, we did not collect relevant information on dietary habits because these data were unavailable for the NHIS cohort. Second, we could not consider any changes in metabolic parameters and interventions or medication regarding each MetS component during the follow-up period. Third, because our study subjects were mostly Korean, the results might not be generalizable to other ethnic groups. Furthermore, we could not determine the cause of cholecystectomy among each of the study participants. Forth, the follow-up period was too short to determine the cause-effect relationship between cholecystectomy and MetS. Finally, we could not collect data on hormonal factors (such as FXR, GPBAR-1 and FGF-19) to elucidate the mechanism driving of the association between cholecystectomy and incident MetS.
Conclusions
We found that cholecystectomy is an independent risk factor for MetS in the general population. The subjects who underwent cholecystectomy exhibited a higher incidence of MetS compared with that of the matched controls, independent of other established risk factors for the development of MetS. Furthermore, cholecystectomy significantly increased the risk of abnormalities of each MetS component. Our finding supports the evidence that cholecystectomy is not a neutral event and could lead to various abnormal metabolic consequences. Careful monitoring of metabolic parameters and proper intervention for metabolic abnormalities may be necessary for patients who have undergone cholecystectomy in clinical practice. Moreover, cholecystectomy should be conducted with proper indications to avoid unnecessary long-term complications. As the prevalence of cholecystectomy is high worldwide, larger studies with longer follow-up periods are required to confirm whether cholecystectomy leads to the development of MetS and to identify subjects at higher risk of developing MetS after cholecystectomy.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and Information and Communications Technology) (No. 2017R1D1A1B03029575, to Jun Goo Kang) and supported by Hallym University Research Fund 2021 (No. HURF-2021-45, to Ji Hye Huh).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Hallym University Sacred Heart Hospital Institutional Review Board (IRB No. HALLYM 2020-05-001). The requirement for written informed consent was waived by the review board because anonymous and de-identified information was used for the analysis.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-201/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-201/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-201/coif). JHH reports that this study was supported by Hallym University Research Fund 2021 (HURF-2021-45). JGK reports that this study was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (2017R1D1A1B03029575). The other authors have no conflicts of interest to declare.
References
- 1.European Association for the Study of the Liver (EASL) . Electronic address: easloffice@easloffice. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016;65:146-81. 10.1016/j.jhep.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 2.Jeon CH, Hong J, Jung J, et al. Chronological trends in patients undergoing cholecystectomy in Korea: a nationwide health insurance claims study. Ann Surg Treat Res 2022;102:205-13. 10.4174/astr.2022.102.4.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chavez-Tapia NC, Kinney-Novelo IM, Sifuentes-Rentería SE, et al. Association between cholecystectomy for gallstone disease and risk factors for cardiovascular disease. Ann Hepatol 2012;11:85-9. 10.1016/S1665-2681(19)31490-5 [DOI] [PubMed] [Google Scholar]
- 4.Ruhl CE, Everhart JE. Relationship of non-alcoholic fatty liver disease with cholecystectomy in the US population. Am J Gastroenterol 2013;108:952-8. 10.1038/ajg.2013.70 [DOI] [PubMed] [Google Scholar]
- 5.Sonne DP, Hare KJ, Martens P, et al. Postprandial gut hormone responses and glucose metabolism in cholecystectomized patients. Am J Physiol Gastrointest Liver Physiol 2013;304:G413-9. 10.1152/ajpgi.00435.2012 [DOI] [PubMed] [Google Scholar]
- 6.Shen C, Wu X, Xu C, et al. Association of cholecystectomy with metabolic syndrome in a Chinese population. PLoS One 2014;9:e88189. 10.1371/journal.pone.0088189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huh JH, Kang DR, Jang JY, et al. Metabolic syndrome epidemic among Korean adults: Korean survey of Cardiometabolic Syndrome (2018). Atherosclerosis 2018;277:47-52. 10.1016/j.atherosclerosis.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 8.Jang YN, Lee JH, Moon JS, et al. Metabolic Syndrome Severity Score for Predicting Cardiovascular Events: A Nationwide Population-Based Study from Korea. Diabetes Metab J 2021;45:569-77. 10.4093/dmj.2020.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huh JH, Ahn SG, Kim YI, et al. Impact of Longitudinal Changes in Metabolic Syndrome Status over 2 Years on 10-Year Incident Diabetes Mellitus. Diabetes Metab J 2019;43:530-8. 10.4093/dmj.2018.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huh JH, Yadav D, Kim JS, et al. An association of metabolic syndrome and chronic kidney disease from a 10-year prospective cohort study. Metabolism 2017;67:54-61. 10.1016/j.metabol.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev 2008;29:777-822. 10.1210/er.2008-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chávez-Talavera O, Tailleux A, Lefebvre P, et al. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017;152:1679-94.e3. 10.1053/j.gastro.2017.01.055 [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Moon JS, Byun SJ, et al. Fatty liver index and development of cardiovascular disease in Koreans without pre-existing myocardial infarction and ischemic stroke: a large population-based study. Cardiovasc Diabetol 2020;19:51. 10.1186/s12933-020-01025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seong SC, Kim YY, Park SK, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017;7:e016640. 10.1136/bmjopen-2017-016640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MK, Han K, Lee SH. Current Trends of Big Data Research Using the Korean National Health Information Database. Diabetes Metab J 2022;46:552-63. 10.4093/dmj.2022.0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Choe S, Park JW, et al. The Risk of Colorectal Cancer After Cholecystectomy or Appendectomy: A Population-based Cohort Study in Korea. J Prev Med Public Health 2018;51:281-8. 10.3961/jpmph.18.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YH, Kang JG, Lee SJ, et al. Underweight Increases the Risk of End-Stage Renal Diseases for Type 2 Diabetes in Korean Population: Data From the National Health Insurance Service Health Checkups 2009-2017. Diabetes Care 2020;43:1118-25. 10.2337/dc19-2095 [DOI] [PubMed] [Google Scholar]
- 18.Ko SH, Han K, Lee YH, et al. Past and Current Status of Adult Type 2 Diabetes Mellitus Management in Korea: A National Health Insurance Service Database Analysis. Diabetes Metab J 2018;42:93-100. 10.4093/dmj.2018.42.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640-5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 20.Kim MK, Lee WY, Kang JH, et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul) 2014;29:405-9. 10.3803/EnM.2014.29.4.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SY, Park HS, Kim DJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75:72-80. 10.1016/j.diabres.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 22.Kwak MS, Kim D, Chung GE, et al. Cholecystectomy is independently associated with nonalcoholic fatty liver disease in an Asian population. World J Gastroenterol 2015;21:6287-95. 10.3748/wjg.v21.i20.6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latenstein CSS, Alferink LJM, Darwish Murad S, et al. The Association Between Cholecystectomy, Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease: A Population-Based Study. Clin Transl Gastroenterol 2020;11:e00170. 10.14309/ctg.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv J, Yu C, Guo Y, et al. Gallstone Disease and the Risk of Type 2 Diabetes. Sci Rep 2017;7:15853. 10.1038/s41598-017-14801-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, Wang J, Li Y, et al. Gallstone Disease and Type 2 Diabetes Risk: A Mendelian Randomization Study. Hepatology 2019;70:610-20. 10.1002/hep.30403 [DOI] [PubMed] [Google Scholar]
- 26.Garruti G, Wang DQ, Di Ciaula A, et al. Cholecystectomy: a way forward and back to metabolic syndrome? Lab Invest 2018;98:4-6. 10.1038/labinvest.2017.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houghton PW, Donaldson LA, Jenkinson LR, et al. Weight gain after cholecystectomy. Br Med J (Clin Res Ed) 1984;289:1350. 10.1136/bmj.289.6455.1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juvonen T, Kervinen K, Kairaluoma MI, et al. Effect of cholecystectomy on plasma lipid and lipoprotein levels. Hepatogastroenterology 1995;42:377-82. [PubMed] [Google Scholar]
- 29.Nervi F, Miquel JF, Alvarez M, et al. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol 2006;45:299-305. 10.1016/j.jhep.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 30.Portincasa P, Di Ciaula A, Wang HH, et al. Coordinate regulation of gallbladder motor function in the gut-liver axis. Hepatology 2008;47:2112-26. 10.1002/hep.22204 [DOI] [PubMed] [Google Scholar]
- 31.Housset C, Chrétien Y, Debray D, et al. Functions of the Gallbladder. Compr Physiol 2016;6:1549-77. 10.1002/cphy.c150050 [DOI] [PubMed] [Google Scholar]
- 32.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 2002;298:714-9. 10.1016/S0006-291X(02)02550-0 [DOI] [PubMed] [Google Scholar]
- 33.Barrera F, Azócar L, Molina H, et al. Effect of cholecystectomy on bile acid synthesis and circulating levels of fibroblast growth factor 19. Ann Hepatol 2015;14:710-21. 10.1016/S1665-2681(19)30766-5 [DOI] [PubMed] [Google Scholar]
- 34.Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004;145:2594-603. 10.1210/en.2003-1671 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


