Abstract
Membrane vesicles are produced by Gram-negative and Gram-positive bacteria. While membrane vesicles are potent elicitors of eukaryotic cells and involved in cell-cell communication, information is scarce about their general biology in the context of community members and the environment. Streptococcus sanguinis, a Gram-positive oral commensal, is prevalent in the oral cavity and well-characterized for its ability to antagonize oral pathobionts. We have found that production and dissemination of membrane vesicles by S. sanguinis is dependent on environmental and community factors. Co-culture with interacting commensal Corynebacterium durum, as well as with the periodontal pathobiont Filifactor alocis had no effect on S. sanguinis vesicle number and size, whereas the periodontal pathobiont Porphyromonas gingivalis abolished S. sanguinis vesicle production. Using both correlation and differential expression analyses to examine the transcriptomic changes underlying vesicle production, we found that differential expression of genes encoding proteins related to the cytoplasmic membrane and peptidoglycan correlate with the abundance of membrane vesicles. Proteomic characterizations of the vesicle cargo identified a variety of proteins, including those predicted to influence host interactions or host immune responses. Cell culture studies of gingival epithelial cells demonstrated that both crude and highly purified membrane vesicles could induce the expression of IL-8, TNF-α, IL-1β, and Gro-α within 6 hours of inoculation at levels comparable to whole cells. Our findings suggest that production of membrane vesicles by S. sanguinis is heavily influenced by community and environmental factors and plays an important role in communication with host cells.
Subject terms: Bacterial secretion, Microbial ecology
Introduction
Membrane vesicles (MVs) are small particles packed with biomolecules. Secreted by both Gram-negative and Gram-positive bacteria, MVs play diverse roles in cell-to-cell communication, biofilm formation, host-cell interactions, and long distance signaling (reviewed in [1]). Bacterial MVs were first described in Escherichia coli [2]. Since then, studies of MV derived from both Gram-negative and Gram-positive bacteria have demonstrated their importance in establishing infection of host cells through delivery of various effector molecules [3]. Several oral microorganisms produce membrane vesicles that affect immune responses, often to the same extent as the producer bacterial cells [4–6]. Moreover, MVs can enter eukaryotic cells of various types to induce inflammatory responses, including macrophages and epithelial cells [7]. A significant effect was demonstrated with P. gingivalis MVs, which can increase the permeability of vascular tissue, leading to death in zebrafish larvae [8]. In contrast, MVs produced by the oral commensal C. durum play an important morphological role during interspecies interactions with S. sanguinis [9], which may ultimately affect biofilm community development and potentially oral health maintenance.
The mechanisms supporting vesicle production are less clear and may involve multiple pathways. The general model for vesicle production from Gram-negative bacteria involves disruption of the peptidoglycan layer followed by separation and budding of the outer membrane layer, resulting in pinching off of MVs. Other hypotheses suggest that the loss of symmetry between inner- and outer-layer phospholipids results in bulging and eventual vesicle release [10]. Far less is currently known about vesicle formation in Gram-positive bacteria. Gram-positive MV were not discovered until 2009 [11], largely due to the assumption their production was not possible due to the thick peptidoglycan layer of Gram-positive bacteria. As such, current models suggest that it is necessary to weaken or degrade the peptidoglycan layer, which could result in either cell lysis or vesicles being forced through pores in the cell wall via turgor pressure (reviewed in [3, 12]). B. subtilis cell wall pores are formed by the action of prophage-encoded endolysins, resulting in MV release [3]. Similarly, studies in Staphylococcus aureus have shown that MV production can be induced by modulins and autolysins, which alter membrane fluidity [13]. Like B. subtilis, the S. aureus cell wall can be permeabilized via phage induction, resulting in MV release [14]. The use of antibiotics has been shown to facilitate or enhance MV release (reviewed in [15]). For example, the addition of mitomycin C resulted in peptidoglycan degradation in Bacillus subtilis, forming pores that allowed for vesicle release [16]. The phage-dependent release of S. aureus MVs is enhanced by β-lactam antibiotics flucloxacillin and ceftaroline [14]. Currently, genetic control mechanisms for vesiculation remain unclear, raising the question whether MV production is an accidental by-product of cell lysis or a specific genetically controlled process. Two studies suggest that MV genetic regulators do exist in Gram-positive bacteria, as disruption of Streptococcus pyogenes two-component system CovRS and transcription factor σB in Listeria monocytogenes both altered vesicle production [17, 18]. These studies all suggest that there may be several mechanisms resulting in release of MVs from both Gram-positive and Gram-negative bacteria.
The Gram-positive facultative anaerobe S. sanguinis can occupy several niches within the human body. In the oral cavity, S. sanguinis is a pioneer colonizing commensal that forms close associations with the commensal C. durum in addition to other oral microbes [9, 19]. S. sanguinis also antagonizes colonization by cariogenic species such as S. mutans through the production of hydrogen peroxide [20, 21] in addition to triggering homeostatic levels of cytokines in eukaryotic cells [22]. However, if S. sanguinis gains access to the bloodstream, it can colonize the cardiac endothelium causing infective endocarditis [23–26].
In this study, we have found that S. sanguinis differentially produces MV in response to distinct environmental and community stimuli. By combining RNA sequencing data with phenotypic measurements of vesicle production, we have investigated the underlying transcriptional responses regulating vesicle production. Furthermore, we demonstrated that S. sanguinis MV contain several putative virulence factors and can elicit inflammatory responses from gingival epithelial cells.
Results
S. sanguinis produces extracellular membrane vesicles both in single culture and when co-cultured with associating commensal C. durum
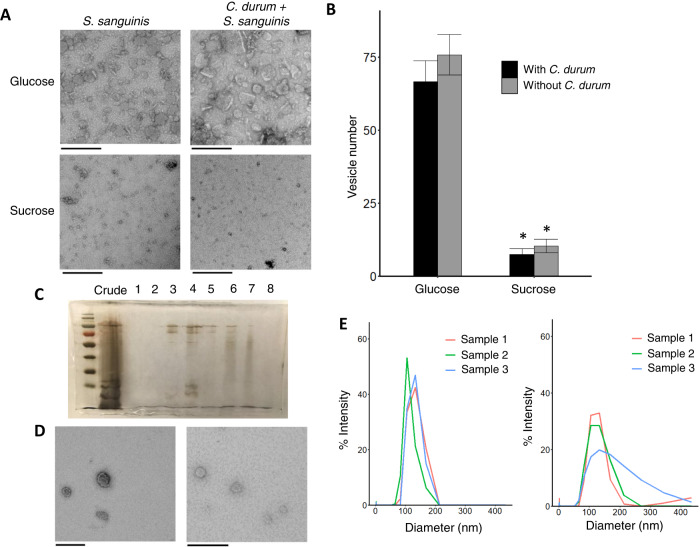
A variety of environmental and community effects were examined for influence upon S. sanguinis MV production. S. sanguinis SK36 was grown in chemically defined medium (CDM; [27]; [28]) with either 50 mM (1.8%) sucrose or glucose ± the interacting commensal C. durum [9], and resulting MV were visualized by TEM (Fig. 1a). TEM images from both monospecies S. sanguinis cultures and dual C. durum – S. sanguinis cultures show similar degrees of vesicle production in either sucrose or glucose regardless of the MVs morphological change. Published data showed that C. durum produces MVs in BHI + glucose, but not in CDM + 3.4% glucose when grown in static culture (Supplemental Fig. 1) suggesting that the vesicles originate from S. sanguinis. This is supported by quantitative PCR showing that C. durum only comprises a small percentage (1–8% of total biomass) relative to S. sanguinis (Supplemental Table 1). To more closely examine the effects of glucose on MV production, a wide variety of glucose concentrations (0.5%, 1.0%, 1.7%, 3.4%) was tested. S. sanguinis and C. durum – S. sanguinis cultures yielded moderate amounts of MV under all tested concentrations of glucose (Supplemental Fig. 2). CDM + 3.4% glucose was subsequently chosen for further characterizations of MV production. MV quantification was performed by counting MV from S. sanguinis and C. durum + S. sanguinis cultures grown with either 1.7% sucrose or 3.4% glucose, and substantially more MV were found in both glucose-supplemented cultures (75.7 vs 10.3 vesicles per 1.7µm2 frame with S. sanguinis alone; 66.5 vs 7.5 vesicles per 1.7µm2 frame from C. durum-S. sanguinis co-cultures) (Fig. 1b). Quantification of MVs via nanoparticle tracking analysis and protein concentration showed similar results (Supplemental Fig. 3a, c). MVs purified by density gradient centrifugation produced a prominent protein band at about ~15 kDa that correlated with MV presence detected via TEM (Fig. 1c, d). Subsequent MV DLS measurements produced similar unimodal histograms between the layers, with the majority of vesicles measuring 80-140 nm in diameter (Fig. 1e). These layers were pooled and used in further analyses.
Fig. 1. S. sanguinis produces membrane vesicles in a dual culture with C. durum in media supplemented with glucose.
A TEM images of crude MV isolations from single S. sanguinis and dual C. durum + S. sanguinis cultures, grown in CDM with either 1.7% sucrose or 3.4% glucose. Bars represent 200 nm. B Vesicle counts from single S. sanguinis (gray bars) and dual C. durum + S. sanguinis (black bars) cultures, supplemented with either 1.7% sucrose or 3.4% glucose. Bars represent the average from 3 frames of 1.7 µm2, 3 replicates. Error bars show the standard error of each treatment. C Silver-stained SDS Page gel of Optiprep purification of S. sanguinis vesicles. Each number shows the 500 μl layer pipetted sequentially off the top of the ultracentrifuge column, with the second to leftmost lane showing an aliquot of the crude MV isolation. D TEM images showing the diluted, pre-washed Optiprep layers 3 (left) and 4 (right). Bars represent 200 nm. E Dynamic light scattering histograms showing the percentage of vesicles (Intensity) with each diameter (nm). Layer 3 shown on left, layer 4 on right from Optiprep purification. Each sample from a biologically independent preparation, labeled by color in legend.
P. gingivalis, but not F. alocis interferes with S. sanguinis MV production
Oral streptococci closely associate with Corynebacterium in dental plaque [19], but other species have been shown to closely associate as well, especially Porphyromonas. Therefore, S. sanguinis and S. sanguinis + C. durum were co-cultured with the periodontal pathobionts P. gingivalis and F. alocis in CDM + 3.4% glucose to examine MV production with TEM (Fig. 2a). Incubation of single strains of F. alocis ATCC 35896 and P. gingivalis W83 showed slow proliferation in CDM media, with OD600 peaking at 0.3 24 hours post inoculation (data not shown). Accordingly, qPCR showed that S. sanguinis made up more than 95% of total biomass in all culture combinations similar to what we reported before (Supplemental Table 1) [22]. Cultures of S. sanguinis and S. sanguinis + C. durum produced significantly higher numbers of MV compared to other species combinations (63.9 and 75.1 per 1.7 µm2 frame, respectively), while S. sanguinis + F. alocis and S. sanguinis + C. durum + F. alocis produced a slightly lower number (52.5 and 53.4 per frame, significant only from S. sanguinis + C. durum culture). The addition of P. gingivalis to S. sanguinis and S. sanguinis + C. durum cultures resulted in a substantial reduction of vesicles (20.1 and 10.7 per frame), which revealed that certain oral bacteria can affect S. sanguinis vesicle production regardless of environmental conditions (Fig. 2b). Quantification of MVs via nanoparticle tracking analysis and protein concentration showed similar results (Supplemental Fig. 3b, d).
Fig. 2. S. sanguinis vesicle production is varied in the presence of pathobionts F. alocis or P. gingivalis.
A Representative TEM images of single S. sanguinis and dual C. durum + S. sanguinis cultures with pathogens F. alocis or P. gingivalis W83. Bars represent 200 nm. B Vesicle counts from cultures of S. sanguinis with and without C. durum, with either F. alocis or P. gingivalis. Bars represent counts from 3 frames of 1.7 μm2 per sample, 3 biological replicates each. C Representative TEM images of dual C. durum + S. sanguinis cultures with variations of P. gingivalis, including the supernatant from W83, addition of ATCC 33277, and gingipain null mutant KDP128. Bars represent 200 nm. D Vesicle counts from cultures of C. durum + S. sanguinis with variations of P. gingivalis (Control: C. durum + S. sanguinis; Pg W83: C. durum + S.sanguinis + P. gingivalis W83; Pg W83 SupN: C. durum + S. sanguinis + P. gingivalis W83 supernatant; Pg 33277: C. durum + S.sanguinis + P. gingivalis 33277; Pg KDP128: C. durum + S sanguinis + P. gingivalis KDP128).Bars represent the average counts from 3 frames of 1.7 μm2 per sample, 2 biological replicates each. Letters represent different significance groups at p < 0.05.
We sought to reveal the cause of MV reduction in the presence of P. gingivalis, first by testing if the reduction is due to P. gingivalis whole cells or secreted external factors. S. sanguinis + C. durum (control) cultures were incubated with either P. gingivalis W83 cultured in FAB medium (OD600 > 1.0) or the corresponding conditioned supernatants. Resulting vesicle counts showed no difference between control cultures inoculated with W83 whole cells or W83 supernatant (SupN) (12.8 vs 8.4 vesicles per frame), suggesting that MV reduction is likely due to a secreted external factor from P. gingivalis. Consequently, we focused upon gingipains, which are secreted cysteine proteases of P. gingivalis that serve as major virulence factors. Incubation of S. sanguinis cultures with either a gingipain null mutant KDP128 or the parent wild type strain ATCC 33277 elicited substantially different levels of vesicle production, with KDP128 yielding 87.5 vesicles per 1.7 µm2 frame vs. 10.5 vesicles per 1.7 µm2 frame from the wild-type ATCC 33277 (Fig. 2c, d). These results show that the presence of extracellular virulence factors secreted by small volumes of P. gingivalis can drastically affect S. sanguinis MV production within a microbial community.
RNA sequencing of high/low vesicle production conditions
We screened for gene responses important for the control of vesicle production in S. sanguinis by sequencing mRNA isolated across all environmental and community groups compared in our prior MV quantification studies. The experimental design is shown in Table 1, and the total alignment scores range from 80.5% to 92.9% (average 88.3%) in S. sanguinis single-culture libraries, and 53.9% to 91.8% (average 79.2%) in multi-culture libraries (Supplemental Table 2 and Supplemental Fig. 4). Patterns from the resulting principal component analysis (PCA) plot (Supplemental Fig. 5) showed no distinction between S. sanguinis and S. sanguinis + C. durum samples. Of the S. sanguinis and C. durum + S. sanguinis treatments, oxic and anoxic - incubated samples are segregated across the PC1 direction (28% variance), with little to no separation between glucose and sucrose-treated samples. Cultures that include F. alocis, P. gingivalis, and P. gingivalis supernatant are segregated and clustered in the PC2 direction (17% variance). From here, we used the visual patterns in the PCA plot to keep the S. sanguinis and C. durum + S. sanguinis treatments combined into a single “S. sanguinis” treatment. We next performed separate differential expression analyses to examine each treatment within our full experiment. Addition of pathobionts yielded a higher number of significant genes, with 219 genes (9.6% of the CDS genome) differentially expressed in the S. sanguinis vs. P. gingivalis + S. sanguinis cultures (Supplemental Table 5) and 135 genes (5.9% of the CDS genome) differentially expressed in the S. sanguinis vs. F. alocis + S. sanguinis cultures (Supplemental Table 6). In contrast, the S. sanguinis cultures comparing sucrose vs. glucose had fewer significant genes, with 83 genes differentially expressed in anoxic conditions (Supplemental Table 3) and 54 differentially expressed in oxic conditions (Supplemental Table 4) (3.6% and 2.3% of the CDS genome).
Table 1.
Experimental design of RNA sequencing experiment.
| Culture | Carbon | Atmosphere | Vesicle count | Environment | Community |
|---|---|---|---|---|---|
| S.sanguinis | Sucrose | Aerobic | 6.1 ± 5.9 | Suboptimal | Optimal |
| C.durum + S.sanguinis | Sucrose | Aerobic | 6.31 ± 1.7 | Suboptimal | Optimal |
| S.sanguinis | Sucrose | Anaerobic | 13.25 ± 15.2 | Suboptimal | Optimal |
| C.durum + S.sanguinis | Sucrose | Anaerobic | 13.1 ± 15.4 | Suboptimal | Optimal |
| S.sanguinis | Glucose | Aerobic | 61.33 ± 20.4 | Optimal | Optimal |
| C.durum + S.sanguinis | Glucose | Aerobic | 54.4 ± 17.1 | Optimal | Optimal |
| S.sanguinis + P.gingivalis | Glucose | Anaerobic | 21.7 ± 2.2 | Optimal | Suboptimal |
| C.durum + S.sanguinis + P.gingivalis | Glucose | Anaerobic | 6.5 ± 5.8 | Optimal | Suboptimal |
| S.sanguinis | Glucose | Anaerobic | 48.6 ± 21.7 | Optimal | Optimal |
| C.durum + S.sanguinis | Glucose | Anaerobic | 68.1 ± 3.6 | Optimal | Optimal |
| S.sanguinis + F.alocis | Glucose | Anaerobic | 52.1 ± 0.6 | Optimal | Optimal |
| C.durum + S.sanguinis + F.alocis | Glucose | Anaerobic | 55.9 ± 3.5 | Optimal | Optimal |
| S.sanguinis + P.gingivalis supernatant | Glucose | Anaerobic | 27.3 ± 1.6 | Optimal | Suboptimal |
| C.durum + S.sanguinis + P.gingivalis supernatant | Glucose | Anaerobic | 12.9 ± 0.7 | Optimal | Suboptimal |
Culture refers to the strains included in each culture. Carbon refers to either 1.7% sucrose or 3.4% glucose. Oxic conditions are 37 °C, 5% CO2, anoxic conditions are 90% N2, 5%CO2, 5% H2, 37 °C. Vesicle count refers to the average number of vesicles in three 1.7 µm2 frames, two biological replicates. The Environment and Community columns refer to the conditions optimal (>20 vesicles per frame) or suboptimal (< 20 vesicles per frame) for vesicle number. Environment specifies the carbon conditions and Community specifies the combination of strains in each culture.
Quantitative reverse transcriptase PCR was used to check the validity and reproducibility of the transcriptomic dataset. The coefficient of variation for each gene across all libraries with an average count >10 was calculated and used to select an internal control [29]. SSA_1403 was selected, with the coefficient at 14.8%. A handful of biologically relevant genes were selected to test, including SSA_0329, SSA_0334, SSA_0326, SSA_0611, SSA_0613, SSA_1362, and SSA_2154. Supplemental Fig. 6 compares the differential expression data with the qRT-PCR data and shows close agreement.
The resulting data was organized following two different strategies to search for mechanisms of MV production. First, we correlate the number of vesicles produced by each culture in Table 1 with the normalized gene counts for each library, and second, we examine the different comparisons that gave us MV+ and MV- phenotypes. This organization allowed us to perform a separate differential expression analysis between these groups. We compared these separate analyses in order to determine if there are any overlapping transcriptomic mechanisms that control MV production and number across different environmental conditions. With this, we acknowledge there may be universal controls that affect MV production, as well as distinct mechanisms that determine MV number.
Quantitative assessment of transcriptomic changes associated with MV production
To examine the transcriptomic changes associated with the quantitative number of MV produced by each sample, we used the “Pheno-RNA” method [30], which correlates the strength of a measured phenotype to the transcriptional profile of a sample. A total of 93 genes were significantly correlated with vesicle quantity at a cutoff of BH-adjusted p < 0.05; 57 genes exhibited a positive correlation ranging from r = 0.39 to r = 0.56, while 36 genes exhibited a negative correlation ranging from r = −0.38 to r = −0.67. Supplemental table 7 shows the detailed list of genes and correlation values, and Supplemental Fig. 7 displays the correlation plots of 8 representative genes with a range of r values (−0.62 to +0.57). An exploration of COG categories (Fig. 3a) showed that more than 10% of positively correlated genes were predicted to be involved in defense mechanisms (category V), and 5-10% were involved in transport and metabolism of amino acids, nucleotides, and carbohydrates (categories E, F, G), and translation (category J). Of the negatively correlated genes, 5–10% were predicted to play roles in lipid transport and metabolism (category I), and the mobilome (category X). pSORTbv3.0 output of the predicted amino acid sequences of positively correlated genes showed that the majority were predicted to be localized in the cytoplasmic membrane (50.8%), followed by the cytoplasm (33.1%) (Fig. 3b, left). In contrast, the majority of negatively correlated genes were predicted to be localized to the cytoplasm (43.9%), followed by the cytoplasmic membrane (36.1%) (Fig. 3b, right).
Fig. 3. Transcriptomic changes associated with MV number.
A Distribution of COG categories across all genes significant and/correlated for MV number. Positive values represent genes that are positively correlated with MV number, negative values represent genes negatively correlated with MV number. Categories R (Predicted general function) and S (Function unknown) are omitted from graph. B Predicted cellular localization of genes positively correlated (top) for MV number, downregulated (bottom) for MV number.
Distinguishing among genes in treatment groups that are correlated with +/− MV phenotypes
In the second part of our transcriptomic analysis, we wanted to examine the individual differential expression comparisons against the correlation coefficients of the 1028 genes with a mean count value < 10. We focused on three datasets that had a significant high/low MV phenotype; P. gingivalis + S. sanguinis vs S. sanguinis, S. sanguinis sucrose vs. glucose anoxic conditions, and S. sanguinis sucrose vs. glucose oxic conditions. The scatterplots in Fig. 4 illustrate the relationship between each log2 fold change and correlation coefficient for all genes for each dataset listed above, highlighting genes that are significant for correlation with MV number (“Corr”), differential expression (“DE”), or both (“Both”) (Genes that are not significant in either analysis are indicated “NotSig”). The P. gingivalis + S. sanguinis vs. S. sanguinis dataset (Fig. 4a, Supplemental Fig. 8) showed a weak negative correlation with MV production (r = -0.20). Only 12 genes (6.4% of the 187 differentially regulated genes included in correlation dataset) were significant in both the MV correlation dataset and the P. gingivalis + S. sanguinis vs S. sanguinis dataset. The S. sanguinis sucrose vs. glucose anoxic conditions dataset (Fig. 4b, Supplemental Fig. 8) showed a moderate negative correlation with MV production (R = -0.43). Nineteen genes (22.8% of the 83 differentially regulated genes) were significant in both datasets. The S. sanguinis sucrose vs. glucose oxic conditions dataset showed the strongest correlation with MV production (R = −0.66) (Fig. 4c, Supplemental Fig. 8). Thirty-one genes (57.4% of the 54 differentially regulated genes) were significant in both datasets. We next examined all of the above comparisons (Fig. 4d) and selected genes significant in at least 2 of the above datasets, including the correlation to MV number. This yielded 46 genes of interest (Table 2). Among the genes upregulated in MV+ and correlation phenotype groups is a putative deoxyribose aldolase (deoC), which was previously shown in Streptococcus mutans to be essential for resistance to neutrophil killing [31]. In addition, there are two carbohydrate ABC transporters, likely in response to the glucose concentration in the media. There are also two FmtA-like genes. In S. aureus, FmtA was found to interact with teichoic acids in the bacterial cell wall [32], likely playing a role in altering the dynamics of the bacterial envelope. Downregulated genes include several genes potentially involved in the mevalonate pathway, including mvaD, mvaK2, and SSA_0336 (isopentenyl-diphosphate delta-isomerase, fni), which are likely involved in peptidoglycan synthesis. Also downregulated are rgg (SSA_0612), glucosyltransferase gtfP (SSA_0613), and an arginine/histidine ABC transporter (SSA_1360). For upregulated/negatively correlated gene responses, we identified three putative transposons, ORFA ISSsa1, ORFA ISSsa2, and ORFA ISSsa2 (SSA_0265, SSA_1361, SSA_1362).
Fig. 4. Comparison of differential expression and correlation analysis.
A Scatterplots showing the significant correlation between the differential expression values (log2 fold change) and correlation values with MV number for three comparison groups: i) P. gingivalis + S. sanguinis vs. S. sanguinis (r- -0.20); ii) S. sanguinis sucrose vs. glucose, anoxic conditions (r = −0.43); iii) S. sanguinis sucrose vs. glucose, oxic conditions (r = −0.67). For all scatterplots, black dots show genes not significant for correlation or differential expression (“NotSig”), green dots show genes significant for differential expression (“DE”), blue dots depict genes significant for correlation (“Corr”), and red dots show genes significant for both differential expression and correlation (“Both”). B 4-way Venn diagram showing the intersection of significant genes common for the correlation analysis, P. gingivalis + S. sanguinis vs. S. sanguinis, S. sanguinis sucrose vs glucose anoxic conditions, and S. sanguinis sucrose vs glucose oxic conditions.
Table 2.
List of genes significant in the correlation analysis and at least one of the differential expression analyses.
| Gene | COG | Product | Localization | Correlation with MV number | log2FC SK36 Suc/Glu O2 | log2FC SK36 Suc/Glu N2 | log2FC Pg + SK36/SK36 |
|---|---|---|---|---|---|---|---|
| SSA_0140 | P | Copper-translocating P-type ATPase, putative ctpA | CytoplasmicMembrane | 0.42 | −0.75 | NS | NS |
| SSA_0204 | TK | Nisin biosynthesis two-component response transcriptional regulator nisR, putative | Cytoplasmic | 0.4 | −1.16 | NS | NS |
| SSA_0205 | T | NisK (sensor-receptor histidine kinase domain), putative | CytoplasmicMembrane | 0.52 | NS | NS | −1.25 |
| SSA_0917 | E | Cobalamin-independent methionine synthase II, putative | Cytoplasmic | 0.39 | −0.9 | NS | −0.67 |
| SSA_1035 | S | Pyrimidine nucleoside phosphorylase, putative pdp | Cytoplasmic | 0.49 | −1.35 | NS | NS |
| SSA_1036 | F | Deoxyribose-phosphate aldolase, putative deoC | Cytoplasmic | 0.4 | −1.42 | NS | NS |
| SSA_1038 | F | Lipoprotein, putative | Unknown | 0.51 | −1.29 | −0.77 | NS |
| SSA_1039 | R | Sugar ABC transporter, ATP-binding protein, putative | CytoplasmicMembrane | 0.54 | −1.15 | NS | −0.77 |
| SSA_1040 | R | Sugar ABC transporter, permease protein, putative | CytoplasmicMembrane | 0.49 | NS | −0.78 | −1.16 |
| SSA_1369 | V | FmtA-like protein, putative | CytoplasmicMembrane | 0.51 | NS | −1.37 | NS |
| SSA_1371 | V | FmtA-like protein, putative | CytoplasmicMembrane | 0.54 | NS | −1.04 | NS |
| SSA_1571 | J | Threonyl-tRNA synthetase, thrS | Cytoplasmic | 0.43 | −1.31 | NS | NS |
| SSA_1572 | S | Conserved hypothetical protein | Cytoplasmic | 0.43 | −1.81 | −1.3 | NS |
| SSA_1679 | V | ABC-type multidrug transport system, ATPase component, putative cylA | CytoplasmicMembrane | 0.54 | −0.68 | NS | NS |
| SSA_1918 | G | Phosphotransferase system, mannose-specific EIIAB, putative | Cytoplasmic | 0.44 | −1.24 | NS | NS |
| SSA_1919 | G | Phosphotransferase system, mannose-specific EIIC, putative | CytoplasmicMembrane | 0.43 | −1.05 | NS | NS |
| SSA_1920 | G | Phosphotransferase system, mannose-specific EIID, putative | CytoplasmicMembrane | 0.39 | −1.17 | NS | NS |
| SSA_1961 | K | Transcriptional regulator, XRE family, putative | Cytoplasmic | 0.45 | NS | NS | −0.69 |
| SSA_2153 | R | ABC-type transporter (uncharacterized), permease component, putative | CytoplasmicMembrane | 0.48 | NS | NS | −1.4 |
| SSA_2189 | O | Conserved hypothetical protein | Cytoplasmic | 0.44 | NS | NS | −0.98 |
| SSA_0265 | X | ORFA, transposon ISSsa1 | Cytoplasmic | −0.45 | 1.18 | 1.14 | NS |
| SSA_0287 | C | Glycerol dehydrogenase, NAD+ dependent, putative gldA | Cytoplasmic | −0.44 | 1.05 | NS | NS |
| SSA_0329 | G | Glycerol uptake facilitator/aquaporin protein, putative | CytoplasmicMembrane | −0.58 | 1.71 | NS | NS |
| SSA_0330 | S | Membrane protein, putative | CytoplasmicMembrane | −0.45 | 0.97 | NS | NS |
| SSA_0332 | R | Conserved hypothetical protein | Extracellular | −0.61 | 1.12 | NS | NS |
| SSA_0334 | I | Diphosphomevalonate decarboxylase, putative mvaD | Cytoplasmic | −0.49 | 0.85 | NS | NS |
| SSA_0335 | I | Phosphomevalonate kinase, putative mvaK2 | Cytoplasmic | −0.44 | 0.82 | NS | NS |
| SSA_0336 | C | Isopentenyl-diphosphate delta-isomerase, putative | Cytoplasmic | −0.48 | 0.96 | 0.69 | NS |
| SSA_0385 | R | ABC transporter, glycine-betaine/proline permease protein, putative opuAb | CytoplasmicMembrane | −0.62 | 0.91 | NS | NS |
| SSA_0386 | R | Glycine-betaine ABC transporter, ATPase component, putative opuAa | CytoplasmicMembrane | −0.67 | NS | NS | 0.97 |
| SSA_0387 | P | Transcriptional regulator, GntR family, putative | Cytoplasmic | −0.51 | 1.21 | NS | NS |
| SSA_0612 | L | Rgg protein, putative | Cytoplasmic | −0.39 | 1.93 | 1.16 | NS |
| SSA_0613 | G | Glucosyltransferase, putative gtfP | Extracellular | −0.42 | 1.44 | 0.87 | NS |
| SSA_1359 | R | Arginine/histidine ABC transporter, permease component, putative | CytoplasmicMembrane | −0.44 | 1.41 | 1.31 | NS |
| SSA_1360 | V | Arginine/histidine ABC transporter, ATPase component, putative | CytoplasmicMembrane | −0.44 | 1.33 | 1.52 | NS |
| SSA_1361 | X | ORFA, transposon ISSsa2 | Cytoplasmic | −0.41 | 1.47 | 1.26 | NS |
| SSA_1362 | X | ORFB, transposon ISSsa2 | Unknown | −0.47 | 1.87 | 1.64 | NS |
| SSA_1367 | P | Hypothetical protein | Cytoplasmic | −0.48 | 2.22 | 1.74 | NS |
| SSA_1819 | J | Valyl-tRNA synthetase, putative valS | Cytoplasmic | −0.47 | NS | NS | 1.23 |
| SSA_1928 | I | Acyl-CoA dehydrogenase, putative | Cytoplasmic | −0.48 | 1.08 | 1.23 | NS |
Gene refers to the Ensembl gene ID, COG refers to the cluster of orthologous groups. Localization is the predicted localization of each product, identified using pSORTbv3.0. Correlation with MV number refers to the Pearson’s correlation coefficient for each gene with MV number. Log2 FC SK36 suc/glu O2 lists the log2 fold change of each gene significantly different between sucrose and glucose media, oxic conditions. Log2 FC SK36 suc/glu N2 lists the log2 fold change of each gene significantly different between sucrose and glucose media, anoxic conditions. Log2 FC Pg+Ss/Ss lists the log2 fold change of each gene significantly different between S. sanguinis co-cultured with P. gingivalis, over S. sanguinis. NS = not significant.
Proteomic characterization of S. sanguinis membrane vesicles
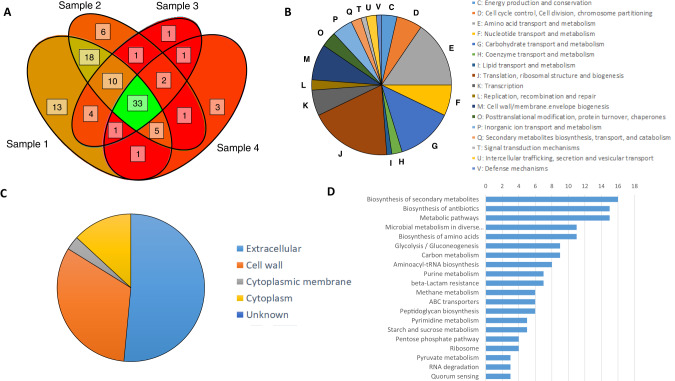
We used LC-MS/MS to examine the protein profile of SK36 membrane vesicles when grown in CDM + 3.4% glucose. We identified 99 unique proteins across the 4 independent preparations with quality posterior error probabilities of <0.05 (Supplemental Table 8). Of these 99 proteins, 33 were found in all 4 preparations, 47 in at least 3 preparations, 75 in at least 2 preparations, and 24 were found in only one preparation (Fig. 5a). Similarly, proteins were predicted to be from diverse cellular compartments, with 51 targeted to the cytoplasm, 27 to the cytoplasmic membrane, 5 in the extracellular space, 3 in the cell wall, and 12 with unknown locations (PSORTb, v3.0.2) (Fig. 5b). Analysis of the COG groups (Fig. 5c) showed that over 15% of proteins belong to category J (translation, ribosomal structure, and biogenesis) and category E (amino acid transport and metabolism). Ten to 15% were predicted to be involved in carbohydrate transport and metabolism (category G). Five to 10% of proteins were predicted to be involved in categories M (cell wall/envelope biogenesis), F (nucleotide transport and metabolism), K (transcription), and D (cell division). A Fisher’s exact test showed that proteins were significantly enriched for category D (cell division, p = 0.007) and trending for category J (p = 0.057). Unique proteins were categorized into a number of KEGG groups, with the top 3 categories as “Biosynthesis of secondary metabolites” (16 proteins), “Biosynthesis of antibiotics” (15 proteins), and “Metabolic pathways” (15 proteins) (Fig. 5d). STRINGdb v11.5 (https://string-db.org/) was used to look for functional associations between the proteins. Setting the significance threshold to “high” (0.7) revealed 3 observable clusters of proteins that separated into 4 enriched GO categories: “Glycolytic process” (GO:0006096, FDR = 0.0083), “tRNA aminoacylation for protein translation” and “translation” (GO:0006418, FDR = 0.0083; GO:0006412, FDR = 0.0140), and “Peptidoglycan metabolic process” (GO:0000270, FDR = 0.0140) (Fig. 6, full characterization shown in Supplemental Figure 9).
Fig. 5. Proteomic characterization of S. sanguinis MVs.
A Venn Diagram showing overlap of shared 99 unique predicted proteins found throughout the 4 preparations of S. sanguinis MVs. B The number of predicted proteins (N = 84) found within S. sanguinis MVs in each COG category. Categories R (General function prediction) and S (Function unknown) are omitted from graph. C Whole cell localization of the predicted proteins within S. sanguinis MVs (N = 99). D Organization of unique proteins into KEGG groups. X-axis denotes the number of proteins.
Fig. 6. Functional associations of proteins predicted in S. sanguinis MV cargo.
Functional associations between the 29 proteins found in S. sanguinis MVs classified as significantly enriched for GO categories: Red= Glycolytic process (GO:0006096), Blue = tRNA aminoacylation for protein translation (GO:0006418), yellow = translation (GO:0006412), Green = Peptidoglycan metabolic process (GO:0000270). Lines depict both functional and physical protein associates at high confidence (0.7 minimum score). Asterisks depict proteins of interest, black indicate proteins with a moonlighting function, blue indicate protein is in Virulence Factor Database (VFDB), red indicate proteins predicted to be virulent (VirulentPred). Full STRINGdbv3.0 ouput with all 99 unique proteins is shown in Supplemental Fig. 8).
Several predicted proteins showed similarities to virulence factors, so we used the prediction tool VirulentPred (http://bioinfo.icgeb.res.in/virulent/index.html) to assess the amino acid sequences of all proteins. Out of the 99 unique proteins, 24 were scored as “virulent” using the Cascade SVM prediction algorithm [33]. These included several putative proteins demonstrated in the literature as playing a role in pathogenicity: a putative C5a peptidase (SSA_1882), an N-acetylmuramoyl-L-alanine amidase (SSA_0860), a calcium-binding hemolysin (SSA_1099), and a zinc metalloprotease ZmpB (SSA_2004). Also included are putative sulfatase- and rhodanese domain-containing proteins, and several uncharacterized proteins. Next, we checked the list of predicted proteins against the Virulence Factor Database (VFDB, http://www.mgc.ac.cn/VFs/) to look for sequences that are annotated as virulence factors in other streptococci. Five matches were identified, including PrtS and ZmpB, in addition to an enolase (SSA_0886), a fructose-bisphosphate aldolase (SSA_1992), and an extracellular glucosyltransferase GtfP (SSA_0613). We were also interested to identify proteins that might moonlight as virulence factors. Moonlighting functions of proteins are alternative functions that occur without structural changes or gene fusions [34]. The database MoonProt (http://www.moonlightingproteins.org/; [35]) identified 18 proteins with at least 2 functions; several of these had secondary functions that facilitated attachments to host cells including adhesins (FusA, SSA_2109; AdhE, SSA_0068; Fba SSA_1992) and fibronectin- and plasminogen-binding proteins (enolase, SSA_0886; GpmA, SSA_0688, Pfk, SSA_0847, and Pgk, SSA_0302). The full list of these proteins is in Table 3, and labeled in Fig. 6and Supplemental Figure 9.
Table 3.
List of predicted proteins in S. sanguinis membrane vesicles that are either predicted as virulence factors in the Virulence Factor Database (VFDB), predicted moonlighting proteins, or predicted as virulence factors in VirPred.
| Ensembl Gene ID | Accession | COG | Number peptides | Number detected | Symbol | Description | VFDB | Moonlighting | VirulentPred |
|---|---|---|---|---|---|---|---|---|---|
| SSA_1882 | A3CQ08 | R | 9 | 4 | prtS | C5a peptidase (prtS) | Yes | Yes | Virulent |
| SSA_0688 | A3CLS0 | G | 2 | 2 | gpmA | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | No | Yes | Nonvirulent |
| SSA_0886 | A3CMA7 | G | 2 | 4 | eno | Enolase | Yes | Yes | Nonvirulent |
| SSA_0120 | A3CK77 | J | 2 | 4 | rpsH | 30 S ribosomal protein S8 | No | Yes | Nonvirulent |
| SSA_0176 | A3CKD3 | K | 2 | 4 | rpoB | DNA-directed RNA polymerase subunit beta | No | Yes | Nonvirulent |
| SSA_0068 | A3CK27 | C | 1 | 1 | adhE | Aldehyde-alcohol dehydrogenase | No | Yes | Nonvirulent |
| SSA_1949 | A3CQ69 | E | 1 | 1 | SSA_1949 | AliA protein | No | Yes | Nonvirulent |
| SSA_0302 | A3CKQ4 | G | 1 | 1 | pgk | Phosphoglycerate kinase | No | Yes | Nonvirulent |
| SSA_0908 | A3CMC7 | R | 1 | 1 | SSA_0908 | ABC-type uncharacterized transport system, periplasmic component, | No | Yes | Nonvirulent |
| SSA_0804 | A3CM30 | G | 1 | 2 | glmM | Phosphoglucosamine mutase | No | Yes | Nonvirulent |
| SSA_0848 | A3CM70 | G | 1 | 2 | pykF | Pyruvate kinase | No | Yes | Nonvirulent |
| SSA_0263 | A3CKL7 | O | 1 | 2 | pepO | Zinc metalloproteinase in scaA 5’region | No | Yes | Nonvirulent |
| SSA_0721 | A3CLV0 | P | 1 | 2 | sodA | Superoxide dismutase | No | Yes | Nonvirulent |
| SSA_0453 | A3CL46 | E | 1 | 3 | SSA_0453 | Alpha-dextrin endo-1,6-alpha-glucosidase | No | Yes | Nonvirulent |
| SSA_0847 | A3CM69 | G | 1 | 3 | pfk | ATP-dependent 6-phosphofructokinase | No | Yes | Nonvirulent |
| SSA_0860 | A3CM81 | M | 1 | 3 | SSA_0860 | Lysozyme, GN = SSA_0860 | No | Yes | Virulent |
| SSA_0015 | A3CJX9 | O | 1 | 3 | ftsH | ATP-dependent zinc metalloprotease | No | Yes | Nonvirulent |
| SSA_1992 | A3CQA8 | G | 1 | 4 | fba | Fructose-bisphosphate aldolase | Yes | Yes | Nonvirulent |
| SSA_1283 | A3CNC9 | C | 1 | 4 | nrd | Nitroreductase | No | Yes | Nonvirulent |
| SSA_1732 | A3CPK9 | F | 1 | 4 | upp | Uracil phosphoribosyltransferase | No | Yes | Nonvirulent |
| SSA_2109 | A3CQM2 | J | 1 | 4 | fusA | Elongation factor G | No | Yes | Nonvirulent |
| SSA_1817 | A3CPU4 | R | 1 | 4 | SSA_1817 | Conserved uncharacterized protein | No | Yes | Virulent |
| SSA_1099 | A3CMV6 | Q | 4 | 4 | SSA_1099 | Calcium binding hemolysin-like protein, GN = SSA_1099 | No | No | Virulent |
| SSA_0613 | A3CLJ9 | R | 3 | 4 | gtfP | Dextransucrase, glucosyltransferase gtfP | Yes | No | Nonvirulent |
| SSA_1797 | A3CPS4 | R | 2 | 4 | pabC | Endolytic murein transglycosylase | No | No | Virulent |
| SSA_1245 | A3CN93 | K | 1 | 1 | cpsY | Transcriptional regulator, LysR family | No | No | Virulent |
| SSA_1249 | A3CN97 | L | 1 | 1 | SSA_1249 | CRISPR system Cms endoribonuclease Csm3 | No | No | Virulent |
| SSA_2278 | A3CR35 | S | 1 | 1 | ukp | Ukp protein | No | No | Virulent |
| SSA_1505 | A3CNZ4 | M | 1 | 2 | SSA_1505 | Sulfatase domain-containing protein | No | No | Virulent |
| SSA_2004 | A3CQB9 | O | 1 | 3 | zmpB | Zinc metalloprotease zmpB | Yes | No | Virulent |
| SSA_1854 | A3CPY0 | D | 1 | 3 | SSA_1854 | Mid-cell-anchored protein Z | No | No | Virulent |
| SSA_1832 | A3CPV9 | J | 1 | 3 | SSA_1832 | UPF0374 protein | No | No | Virulent |
| SSA_0159 | A3CKB5 | S | 1 | 3 | SSA_0159 | Uncharacterized protein | No | No | Virulent |
| SSA_1909 | A3CQ29 | K | 1 | 4 | SSA_1909 | Transcriptional attenuator LytR | No | No | Virulent |
| SSA_0647 | A3CLN0 | P | 1 | 4 | SSA_0647 | Rhodanese domain-containing protein | No | No | Virulent |
| SSA_1291 | A3CND6 | V | 1 | 4 | SSA_1291 | Tox-GHH domain-containing protein | No | No | Virulent |
COG refers to the cluster of orthologous groups for each protein, Number peptides is the number of significant peptides of each predicted protein. Number detection is the number of independent vesicle preparations each predicted protein is found, in a total of 4 preparations.
Inoculation of gingival epithelial cells with MV elicits inflammatory responses
To test the host responses to S. sanguinis MVs, we inoculated gingival epithelial cells (hTERT TIGK line CRL3397) with 10 μg of purified MVs from S. sanguinis, F. alocis, and P. gingivalis, in parallel with whole cells from the same strains at an MOI of 100 (similar to [6]). Expression of immune-related genes IL-6, IL-8, IL-1β, TNF-α, Gro-α, and Pannexin-1, 6 hours post-inoculation are shown in Fig. 7. Genes encoding cytokines IL-6, IL-1β, and TNF-α showed significant differences between MVs of S. sanguinis and P. gingivalis, but not between S. sanguinis and F. alocis. The second pattern was observed for genes encoding IL-8, Gro-α, and Pannexin-1, in which S. sanguinis MVs induced expression of each gene to a significantly higher degree than both F. alocis and P. gingivalis MVs.
Fig. 7. Inoculation of gingival epithelial cells with S. sanguinis membrane vesicles induced expression of cytokines.
Expression of genes encoding cytokines and immune-related proteins 6 hours after inoculation by MVs and whole cells of S. sanguinis SK36, F. alocis 35896, and P. gingivalis W83, relative to a 1X PBS control. Bars represent the averages of 2 inoculated samples, 3 independent biological replicates. * = Significantly different than S. sanguinis MVs, p < 0.05.
Internalization of S. sanguinis MVs into gingival epithelial cells
Despite the comparatively robust inflammatory response to S. sanguinis MVs, the morphology of CRL3397 cells was not obviously different from the PBS-inoculated control cells after 24 hours incubation. In contrast to this, cells inoculated with P. gingivalis MVs showed widespread rounding and detachment from the flask surface (Fig. 8a). A trypan blue assay showed negligible/no cell death in SK36-inoculated cells, comparable to PBS-inoculated control cells (<3%). In contrast, 27% of cells showed a positive blue stain after inoculation with P. gingivalis MVs (Fig. 8b), significantly different than both PBS- and S. sanguinis MV-inoculated cells. This demonstrates that despite triggering an inflammatory response, S. sanguinis MVs did not trigger cell death in gingival epithelial cells. To determine whether internalization occurs with S. sanguinis MVs, we stained MVs with Vybrant DiO (Invitrogen) and incubated 20 μg with hTERT TIGK CRL3397 gingival epithelial cells. Microscopy images (Fig. 8c) showed that vesicles were internalized into gingival epithelial cells between 6 hours to 24 hours after addition. This suggests that MV internalization is not explicitly required to trigger an inflammatory response.
Fig. 8. Gingival epithelial cell visualization and MV internalization.
A Representative photos of gingival epithelial cell line CRL3397 24 hours after inoculation, 20X magnification. B % of cells positively stained with trypan blue 24 hours after inoculation. *= Significantly different than PBS-inoculated gingival epithelial cells. C Visualization of CRL3397 after inoculation by 20 ng DiO-stained S. sanguinis MVs or 1X PBS. Actin is stained with Texas Red Phalloidin (2:500) and nucleic acids are stained with DAPI. Bars represent 20 µm. Experiments were repeated with similar results.
Discussion
In this study, we demonstrate that production MVs by S. sanguinis is strongly influenced by culture conditions. The highest number of vesicles were produced in a chemically defined medium with glucose as a carbon source, whereas sucrose suppressed vesicle formation. The addition of other commensal and/or pathogenic oral bacteria exerted no significant impact on S. sanguinis vesicle formation, with the exception of P. gingivalis, which significantly reduced MV number. We used both differential expression and correlation analyses to connect phenotypic data (vesicle number) to transcriptomic data (normalized counts), thereby identifying transcriptional patterns that are correlated with vesicle production. It is important to note that while our results do show transcriptomic changes correlated with MV production, we cannot conclude what happens at a systems level just by linking a genotype with a phenotype [36]. However, our work identifies potential mechanisms that result in vesicle production which require further verification, for example using specific deletions of potentially involved genes.
Our transcriptomic data showed few effects attributable to carbon sources, which potentially can be explained by the similar media backgrounds. Since our primary aim was to correlate gene responses with differential production of vesicles, we focused our downstream analyses on these comparisons. Transcriptomic patterns that correlated with MV number suggest key gene responses either directly or indirectly affect the peptidoglycan layer. This is seen with the downregulation of 3 genes involved in the mevalonate pathway (mvaK2, and mvaD, and fni), essential for isoprenoid and peptidoglycan biosynthesis ([37]; [38]). Another line of evidence comes from the positive correlation of 2 FmtA-like proteins, which are shown to affect cell envelope component teichoic acids in S. aureus, thus altering the charge of the bacterial surface [32]. The resulting pH change may trigger changes in the peptidoglycan layer that facilitate the release of MVs. Similarly, 2 osmoprotectants encoded by opuAa and opuAb are negatively correlated with MV release. The inhibition of osmoprotectants coupled with changes in the cell wall might all play a role in increased vesicle production. For Gram-positive bacteria, it is a requirement that vesicles must escape the thick peptidoglycan layer. Several studies have suggested that MV formation and release is a physiological process that involves more than one mechanism, this can be seen through studies investigating the effect of phage lysis and the use of antibiotics, all of which may affect the peptidoglycan layer ([39], reviewed in [15]). Our transcriptomic data are consistent with this notion, since there is only a moderate degree of overlap between each of our +/− MV differential expression analyses. Therefore, any transcriptomic changes affecting the peptidoglycan layer could potentially alter MV production.
Characterization of the protein cargo of S. sanguinis MVs revealed a wide variety of predicted proteins. STRINGdb functional analysis coupled with enrichment of COG groups revealed three distinct clusters of proteins involved in translational machinery, glycolysis, and peptidoglycan metabolism. Database and literature searches categorized 40% of the unique proteins as associated with virulence due to homology to well-characterized virulence factors. For example, prtS is encoding a C5a peptidase, which has been shown to cleave and inactivate the human phagocyte chemotaxin C5a, slowing neutrophil recruitment in several Group A and Group B streptococci [40]. Another potential virulence factor is the zinc metalloprotease ZmpB, which plays a role in increased TNFa production in Streptococcus pneumoniae [41]. A previously characterized virulence factor in S. sanguinis (SSA_1099) is also included as MV cargo. SSA_1099 encodes an RTX-like hemolytic protein. Knockout mutants lacking SSA_1099 were impaired in the ability to form vegetative plaques in endocardial tissue [26]. Other potential virulence factors were identified based upon their previous characterization as potential moonlighting proteins [34]. A number of proteins identified as MV cargo are primarily characterized as glycolytic enzymes. However, these were also found to have secondary roles in binding to host cell components such as plasminogen and fibronectin. Examples include enolase (S. suis, S. mutans, [42]), phosphoglycerate mutase (GpmA, Bifidobacterium, [43]), 6-phosphofructokinase (Pfk, S. oralis, [44]), phosphoglycerate kinase (Pgk, [45] (S. agalactiae), and pyruvate kinase (PykF, Lactococcus lactis, binds to invertase [46]). Our results are comparable to MVs cargo from Granulicatella adiacens, a Gram-positive coccus that also causes infective endocarditis. G. adiacens MVs were also enriched for glycolytic (enolase, pyruvate kinase, and phosphoglycerate mutase), and translational machinery (50 S ribosomal protein L10) [47]. We are currently investigating if the identified proteins are involved in MV biogenesis.
Given the content of the MVs, it is not surprising that they triggered a substantial immune response in gingival epithelial cells within hours of exposure. Our results parallel the reports of MVs from other commensal and pathogenic bacteria triggering immune responses in eukaryotic hosts (reviewed in [1]), with both IL-8 and Gro-α significantly upregulated compared to F. alocis and P. gingivalis MVs. This is noteworthy as both IL-8 and Gro-α serve as chemoattractants for neutrophils, which can further affect the subsequent immune response (reviewed in [48]). The comparatively weak response to F. alocis MVs and lack of response to P. gingivalis MVs gives a clue into the eventual fate of the cells; mainly that S. sanguinis triggers a defensive response while F. alocis and P. gingivalis are able to invade and eventually damage the cells. We do acknowledge that there may be biases in our measurement of host elicitation by P. gingivalis due to the levels of cell death in our assay, however our results agree with other studies that P. gingivalis has the ability to evade detection by host immune pathways [49–51]. The significant expression of IL-6 triggered by S. sanguinis MVs might suggest another outcome. Recent studies have examined the connection between dysbiosis and the development of malignancy. A number of oral pathobionts, including P. gingivalis, Fusobacterium spp., as well as cariogenic S. mutans have been associated with oral squamous cell carcinoma (OSCC) (reviewed in [52]). The mechanisms behind this are still unclear, but current evidence suggests that upregulation of IL-6 and TNF-α together with changes in NF-kB and Wnt pathways is associated with tumor development (reviewed in [52]). In contrast to this, the oral commensals Neisseria sicca and Corynebacterium matruchotii were shown to inhibit development of OSCC via several mechanisms, including downregulation of NF-kB and IL-6 in tumor cells [53]. Taking these findings into account, it would be interesting to examine the role of S. sanguinis whole cells as well as MVs in tumor development.
Given the apparent inflammatory potential of S. sanguinis MV, we wanted to see whether there are any downstream effects upon gingival epithelial cells. Trypan blue staining showed negligible morphological effects upon S. sanguinis MV-inoculated cells compared to the PBS-inoculated control, suggesting that they did not trigger cell death. Confocal microscopy confirmed that these MVs are internalized within 24 hours (Fig. 8c). The rate and effect of vesicle internalization may not be standard across cell lines. MV from S. pneumoniae were shown to be internalized into a variety of eukaryotic cells, including lung epithelial cells, dendritic cells, macrophages, and primary T cells, occurring as soon as 30 minutes in some cell lines, but 2-4 hours in others [39, 54]. The relatively slow uptake of S. sanguinis MVs into gingival epithelial cells is most similar to MVs of S. pneumoniae internalization into lung epithelial cells [54]. The dynamics of MV detection and internalization and their effect on host immune responses will be further explored in future studies.
Development and dissemination of extracellular membrane vesicles is an important part of microbial function. We sought to investigate the conditions in which the opportunistic Gram-positive bacterium S. sanguinis produces membrane vesicles, as well as the proteomic cargo and their immunostimulatory effect on gingival epithelial cells. In the oral microbiome, S. sanguinis can serve as an oral commensal, discouraging colonization of cariogenic pathogens through production of bioactive molecules. We have demonstrated that production of S. sanguinis MVs is dependent on carbon source and community members. In an environment as variable as the oral cavity, where temperature, micronutrient availability, oxygen availability, and pH is always in flux, the production and dissemination of MVs may vary. In addition, the clinical relevance of oral microbial MV is currently not known. Our research demonstrates that the context of the environment might play a significant role in any effects MV may have on the host and/or other members of the oral biofilm. Here we present a simplified model with three relevant oral microbial species, while the oral biofilm in each individual might harbor up to 300 different phylotypes [55]. This oral biofilm complexity and diversity inevitably will influence when and how MV are produced. Future research should aim to address the clinical relevance and determine the production in vivo, perhaps through detection of MV in saliva dependent on the oral health status of subjects. Judging from current research with eukaryotic extracellular vesicles it is to be expected that microbial MV play a similar role in health and disease [56].
Materials and methods
Full protocols are in supplemental file 1
Bacterial strains, cell cultures, and media conditions
Reference strains S. sanguinis SK36 and C. durum 33822 were cultured in BHI (brain-heart infusion) broth in oxic conditions (37 °C, 5% CO2). P. gingivalis W82 and F. alocis ATCC 35896 were maintained on BHI agar with 2% yeast extract, 5 µM hemin, and 0.5 µM menadione under anoxic (90% N2, 5% CO2, 5% H2) conditions. Immortalized normal human gingival keratinocyte cell line hTERT TIGKs (ATCC® CRL3397™) [57] were maintained in keratinocyte growth medium supplemented with the Lonza, KGM-Gold™ Bullet kit.
Isolation and purification of extracellular membrane vesicles
Differential centrifugation was used to isolate MVs from 500-1000 ml cultures at late log-phase (OD600 > 1.5), similarly to protocol used in [9]. Purification of membrane vesicles was done using an Optiprep:1X PBS gradient. Layers consisting of 1 ml each of 15%, 25%, and 35% Optiprep were carefully pipetted on top of 45% Optiprep + crude MV isolation and ultracentrifuged for 18 hours at 100,000xg at 4 °C. Layers with a volume of 500 μl containing MVs were pooled and washed at least twice via ultracentrifugation in 1X PBS, then stored as specified above.
Visualization and quantification of extracellular membrane vesicles
Both crude and purified MV isolations were visualized using transmission electron microscopy. MVs were quantified and measured using image analysis (ImageJ, [58]) with three frames per preparation. All statistical analyses were done using R software packages ggplot2 [59] and PMCMRplus [60]. To visualize protein banding patterns, MV isolations were separated on a 15% SDS-PAGE gel and stained with the ProteoSilver Plus Silver Staining kit (Sigma). Protein quantification was done using a BCA, with protein concentration calculated against a standard curve generated from known concentrations of bovine serum albumin. The size distribution of vesicles was assessed using Dynamic Light Scattering (DLS) and measured using DynaPro NanoStar (Wyatt Technologies).
Proteomic analysis of S. sanguinis MVs
Proteomic analysis of S. sanguinis MVs was done at the Mass Spectrometry facility at Oregon State University, Corvalis, OR, using an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific). The SequestHT search engine was used to search against the Swissprot S. sanguinis reference proteome (UP000275495). Protein abundances were calculated as a sum of abundances of unique peptides detected.
RNA isolation and quantitative reverse transcriptase PCR
Total RNA was isolated using TRIzol reagent as described in [22]. Genomic DNA was degraded using DNase I, followed by a column clean-up using the RNeasy Mini Kit (Qiagen). cDNA synthesis was carried out with the qScript cDNA synthesis kit (Quanta Biosciences) according to the manufacturer’s specifications. Quantitative RT-PCR was carried out using a protocol as described in [22]. A list of all primer sequences is in Supplemental table 6.
RNA-Seq generation and data analysis
Total RNA from samples described in Table 1 was isolated, treated with DNaseI and column-cleaned as specified above. Sequencing was carried out using a 100 bp paired-end protocol on a NovaSeq 6000 platform (Illumina) at the Massively Parallel Sequencing Shared Resource at Oregon Health and Science University. Details about replicates can be found in Supplemental Fig. 1. Reads were processed using Cutadapt v4.0 software [61], and sequences were aligned to the S. sanguinis SK36 genome (accession GCA_000014205.1) using BWA-MEM software [62]. Counts were generated using samtools htseq-count [63], and normalization and downstream differential expression analysis was done in DESeq2 [64]. A Pearson correlation analysis was used to correlate MV number with transcriptional profile and significance values were calculated using the standard R platform (v4.0.4).
Inoculation of gingival epithelial cells and transcript analysis
Inoculation set-up was similar to that described in [22]. In brief, 5×105 cells were seeded into each well of a 6-well tray and were inoculated with either 10 μg of membrane vesicles or whole cells at 100 MOI. For transcriptomic analysis, cells were removed via scraping and suspended in 1 ml Trizol for RNA extraction, followed by genomic DNA degradation, cDNA synthesis, and quantitative real-time PCR as specified above. For cell death analysis, the supernatant containing de-attached cells was harvested 24 hpi and pelleted via centrifugation. Cells were resuspended in 50 µl 0.2% trypan blue + 1X PBS and stained cells were quantified by hemocytometer. A live cell count was taken by removal of the supernatant followed by trypsinization and count of viable adhered cells from a 1X PBS control.
Staining and internalization of S. sanguinis membrane vesicles
Internalization assay was performed similarly to [54]. Briefly, SK36 MVs were isolated from 1 L of CDM + 3.4% glucose, and labeled as per the manufacture’s protocol with Vybrant-DiO (Invitrogen). To visualize F-actin, TIGK 3397 cells were stained with 10:200 Texas Red-phalloidin in 1X PBS (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions. Coverslips with adhered, fixed cells were placed onto a slide containing ProLong Gold Antifade reagent with DAPI (Invitrogen) and visualized on a Zeiss Laser-Scanning Confocal Microscope with Airyscan.2, and images were processed using Fiji [65].
Supplementary information
Acknowledgements
We would like to thank the following: OHSU Massive Parallel Sequencing Core (Robert Searles, Amy Carlos), OHSU Microscropy Facility (Claudia Lopez, Steven Adamou), OHSU Imaging Facility (Stefanie Kaech Petrie) and Oregon State University Proteomics Core Facility (Stanislau Stanisheuski). We are grateful to Özlem Yilmaz (Medical University of South Carolina) for sharing F. alocis, Richard J. Lamont (University of Louisville) for sharing P. gingivalis gingipain mutants, and Delaney Shea for assistance with nanoparticle tracking analysis. This work was supported by an NIH-NIDCR grant DE021726, DE029492 and DE029612 to JK and NIH-NIDCR grant DE028252 to J.M.
Author contributions
E.H., J.M., and J.K .developed and designed the research. EH performed the experiments. E.H., D.C., and JK analyzed data. E.H. drafted the manuscript. E.H., D.C., J.M. and J.K. edited and revised the manuscript. All authors reviewed and approved the results and the revisions made to the manuscript.
Data availability
All raw and normalized RNA sequence data and associated metadata are available on NCBI Gene Expression Omnibus (GEO), under accession GSE225861. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [1] partner repository with the dataset identifier PXD041791.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emily Helliwell, Email: helliwel@ohsu.edu.
Jens Kreth, Email: kreth@ohsu.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41396-023-01456-3.
References
- 1.Caruana JC, Walper SA. Bacterial membrane vesicles as mediators of microbe – microbe and microbe – host community interactions. Front Microbiol. 2020;11:432. doi: 10.3389/fmicb.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Work E, Knox KW, Vesk M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann N. Y Acad Sci. 1966;133:438–49. doi: 10.1111/j.1749-6632.1966.tb52382.x. [DOI] [PubMed] [Google Scholar]
- 3.Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 2019;17:13–24. doi: 10.1038/s41579-018-0112-2. [DOI] [PubMed] [Google Scholar]
- 4.Cecil JD, O’Brien-Simpson NM, Lenzo JC, Holden JA, Chen YY, Singleton W, et al. Differential responses of pattern recognition receptors to outer membrane vesicles of three periodontal pathogens. PLoS ONE. 2016;11:e0151967. doi: 10.1371/journal.pone.0151967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, et al. Selective sorting of cargo proteins into bacterial membrane vesicles. J Biol Chem. 2011;286:1269–76. doi: 10.1074/jbc.M110.185744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HY, Lim Y, An SJ, Choi BK. Characterization and immunostimulatory activity of extracellular vesicles from Filifactor alocis. Molecular Oral. Microbiology. 2020;35:1–9. doi: 10.1111/omi.12272. [DOI] [PubMed] [Google Scholar]
- 7.Cecil JD, Sirisaengtaksin N, O’Brien-Simpson NM, Krachler AM. Outer membrane vesicle-host cell interactions. Microbiol Spectrum. 2019;7. 10.1128/microbiolspec.PSIB-0001-2018 [DOI] [PMC free article] [PubMed]
- 8.Farrugia C, Stafford GP, Murdoch C. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J Dent Res. 2020;99:1494–501. doi: 10.1177/0022034520943187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treerat P, Redanz U, Redanz S, Giacaman RA, Merritt J, Kreth J. Synergism between Corynebacterium and Streptococcus sanguinis reveals new interactions between oral commensals. ISME J. 2020;14:1154–69. doi: 10.1038/s41396-020-0598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E-Y, Choi D-Y, Kim D-K, Kim J-W, Park JO, Kim S, et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–36. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 12.Díaz-Garrido N, Badia J, Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracell Vesicles. 2021;10:e12161. doi: 10.1002/jev2.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlatterer K, Beck C, Hanzelmann D, Lebtig M, Fehrenbacher B, Schaller M, et al. The mechanism behind bacterial lipoprotein release: Phenol-soluble modulins mediate toll-like receptor 2 activation via extracellular vesicle release from Staphylococcus aureus. mBio. 2018;9:1–13. doi: 10.1128/mBio.01851-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreoni F, Toyofuku M, Menzi C, Kalawong R, Shambat SM, François P, et al. Antibiotics Stimulate Formation of Vesicles in Staphylococcus aureus in both Phage-Dependent and-Independent Fashions and via Different Routes. 2019. 10.1128/AAC [DOI] [PMC free article] [PubMed]
- 15.Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioactive Materials. 2022;14:169–81. doi: 10.1016/j.bioactmat.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, et al. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nat Commun. 2017;8:481. doi: 10.1038/s41467-017-00492-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Choi CW, Lee T, Kim SI, Lee JC, Shin JH. Transcription factor σB plays an important role in the production of extracellular membrane-derived vesicles in Listeria monocytogenes. PLoS ONE. 2013;8:e73196. doi: 10.1371/journal.pone.0073196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resch U, Tsatsaronis JA, Le Rhun A, Stübiger G, Rohde M, Kasvandik S, et al. A two-component regulatory system impacts extracellular membrane-derived vesicle production in group a streptococcus. mBio. 2016;7:e00207–16. doi: 10.1128/mBio.00207-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welch JLM, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci USA. 2016;113:E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreth J, Zhang Y, Herzberg MC. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 2008;190:4632–40. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redanz S, Treerat P, Mu R, Redanz U, Zou Z, Koley D, et al. Pyruvate secretion by oral streptococci modulates hydrogen peroxide dependent antagonism. ISME J. 2020;14:1074–88. doi: 10.1038/s41396-020-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redanz U, Redanz S, Treerat P, Prakasam S, Lin L-J, Merritt J, et al. Differential response of oral mucosal and gingival cells to Corynebacterium durum, Streptococcus sanguinis, and Porphyromonas gingivalis Multispecies Biofilms. Front Cell Infect Microbiol. 2021;11:686479. doi: 10.3389/fcimb.2021.686479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callahan JE, Munro CL, Kitten T. The Streptococcus sanguinis competence regulon is not required for infective endocarditis virulence in a rabbit model. PLoS ONE. 2011;6:e26403. doi: 10.1371/journal.pone.0026403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crump KE, Bainbridge B, Brusko S, Turner LS, Ge X, Stone V, et al. The relationship of the lipoprotein SsaB, manganese and superoxide dismutase in Streptococcus sanguinis virulence for endocarditis. Mol Microbiol. 2014;92:1243–59. doi: 10.1111/mmi.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge X, Kitten T, Chen Z, Lee SP, Munro CL, Xu P. Identification of Streptococcus sanguinis genes required for biofilm formation and examination of their role in endocarditis virulence. Infect Immun. 2008;76:2551–9. doi: 10.1128/IAI.00338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martini AM, Moricz BS, Ripperger AK, Tran PM, Sharp ME, Forsythe AN, et al. Association of novel Streptococcus sanguinis virulence factors with pathogenesis in a native valve infective endocarditis model. Front Microbiol. 2020;11:10. doi: 10.3389/fmicb.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van De Rijn I, Kessler RE. Growth characteristics of group a streptococci in a new chemically defined medium. Infection Immunity. 1980;27:444–8. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Standar K, Kreikemeyer B, Redanz S, Münter WL, Laue M, Podbielski A. Setup of an in vitro test system for basic studies on biofilm behavior of mixed-species cultures with dental and periodontal pathogens. PLoS ONE. 2010;5:e13135. doi: 10.1371/journal.pone.0013135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dos Santos KCG, Desgagné-Penix I, Germain H. Custom selected reference genes outperform pre-defined reference genes in transcriptomic analysis. BMC Genomics. 2020;21:35. doi: 10.1186/s12864-019-6426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darwiche R, Struhl K. Pheno-RNA, a method to associate genes with a specific phenotype, identifies genes linked to cellular transformation. Proc Natl Acad Sci USA. 2020;117:28925–9. doi: 10.1073/pnas.2014165117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Sun L, Liu W, Guo L, Liu Z, Wei X, et al. A nuclease from Streptococcus mutans facilitates biofilm dispersal and escape from killing by neutrophil extracellular traps. Front Cell Infect Microbiol. 2017;7:97. doi: 10.3389/fcimb.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman MM, Hunter HN, Prova S, Verma V, Qamar A, Golemi-Kotra D. The Staphylococcus aureus methicillin resistance factor FmtA is a D-amino esterase that acts on teichoic acids. mBio. 2016;7:02070–15. doi: 10.1128/mBio.02070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg A, Gupta D. VirulentPred: A SVM based prediction method for virulent proteins in bacterial pathogens. BMC Bioinforma. 2008;9:62. doi: 10.1186/1471-2105-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffery CJ. Protein moonlighting: What is it, and why is it important? Philos Trans R Soc B: Biol Sci. 2018;373:20160523. doi: 10.1098/rstb.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.C Chen, H Liu, S Zabad, N Rivera, E Rowin, M Hassan et al. MoonProt 3.0: an update of the moonlighting proteins database, Nucleic Acids Research, 49, 8 January 2021, D368-D372, 10.1093/nar/gkaa1101 [DOI] [PMC free article] [PubMed]
- 36.Papatheodorou I, Oellrich A, Smedley D. Linking gene expression to phenotypes via pathway information. J Biomed Semant. 2015;6:17. doi: 10.1186/s13326-015-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balibar CJ, Shen X, Tao J. The mevalonate pathway of Staphylococcus aureus. J Bacteriol. 2009;191:851–61. doi: 10.1128/JB.01357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto Y, Yasukawa J, Ishii M, Hayashi Y, Miyazaki S, Sekimizu K. A critical role of mevalonate for peptidoglycan synthesis in Staphylococcus aureus. Sci Rep. 2016;6:22894. doi: 10.1038/srep22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehanny M, Koch M, Lehr CM, Fuhrmann G. Streptococcal extracellular membrane vesicles are rapidly internalized by immune cells and alter their cytokine release. Front Immunol. 2020;11:80. doi: 10.3389/fimmu.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kent Brown C, Gu ZY, Matsuka YV, Purushothaman SS, Winter LA, Patrick Cleary P, et al. Structure of the streptococcal cell wall C5a peptidase. 2005. https://www.pnas.org [DOI] [PMC free article] [PubMed]
- 41.Blue CE, Paterson GK, Kerr AR, Bergé M, Claverys JP, Mitchell TJ. ZmpB, a novel virulence factor of Streptococcus pneumoniae that induces tumor necrosis factor alpha production in the respiratory tract. Infect Immun. 2003;71:4925–35. doi: 10.1128/IAI.71.9.4925-4935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones MN, Holt RG. Cloning and characterization of an alpha-enolase of the oral pathogen Streptococcus mutans that binds human plasminogen. Biochem Biophys Res Commun. 2007;364:924–9. doi: 10.1016/j.bbrc.2007.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Candela M, Bergmann S, Vici M, Vitali B, Turroni S, Eikmanns BJ, et al. Binding of human plasminogen to Bifidobacterium. J Bacteriol. 2007;189:5929–36. doi: 10.1128/JB.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinnby B, Booth NA, Svensäter G. Plasminogen binding by oral streptococci from dental plaque and inflammatory lesions. Microbiology. 2008;154:924–31. doi: 10.1099/mic.0.2007/013235-0. [DOI] [PubMed] [Google Scholar]
- 45.Boone TJ, Burnham CAD, Tyrrell GJ. Binding of group B streptococcal phosphoglycerate kinase to plasminogen and actin. Microb Pathogenesis. 2011;51:255–61. doi: 10.1016/j.micpath.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Katakura Y, Sano R, Hashimoto T, Ninomiya K, Shioya S. Lactic acid bacteria display on the cell surface cytosolic proteins that recognize yeast mannan. Appl Microbiol Biotechnol. 2010;86:319–26. doi: 10.1007/s00253-009-2295-y. [DOI] [PubMed] [Google Scholar]
- 47.Alkandari SA, Bhardwaj RG, Ellepola A, Karched M. Proteomics of extracellular vesicles produced by Granulicatella adiacens, which causes infective endocarditis. PLoSONE. 2020;15:e0227657. doi: 10.1371/journal.pone.0227657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64:456–60. [PubMed] [Google Scholar]
- 49.Lan C, Chen S, Jiang S, Lei H, Cai Z, Huang X, et al. Different expression patterns of inflammatory cytokines induced by lipopolysaccharides from Escherichia coli or Porphyromonas gingivalis in human dental pulp stem cells. BMC Oral Health. 2022;22:121. doi: 10.1186/s12903-022-02161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 51.Takashima K, Matsunaga N, Yoshimatsu M, Hazeki K, Kaisho T, Uekata M, et al. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Br J Pharm. 2009;157:1250–62. doi: 10.1111/j.1476-5381.2009.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.la Rosa GRM, Gattuso G, Pedullà E, Rapisarda E, Nicolosi D, Salmeri M. Association of oral dysbiosis with oral cancer development (Review) Oncol Lett. 2020;19:3045–58. doi: 10.3892/ol.2020.11441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen X, Zhang B, Hu X, Li J, Wu M, Yan C, et al. Neisseria sicca and Corynebacterium matruchotii inhibited oral squamous cell carcinomas by regulating genome stability. Bioengineered. 2022;13:14094–106. doi: 10.1080/21655979.2022.2078556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yerneni SS, Werner S, Azambuja JH, Ludwig N, Eutsey R, Lucas PC, et al. Pneumococcal extracellular vesicles modulate host immunity. mBio. 2021;12:0165721. doi: 10.1128/mBio.01657-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bik E, Long C, Armitage G, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010;4:962–74. doi: 10.1038/ismej.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 57.Moffatt-Jauregui CE, Robinson B, de Moya AV, Brockman RD, Roman AV, Cash MN, et al. Establishment and characterization of a telomerase immortalized human gingival epithelial cell line. J Periodontal Res. 2013;48:713–21. doi: 10.1111/jre.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org
- 60.Pohlert T (2022). _PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended_.
- 61.MARTIN, Marcel. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal, [S.l.], 17, 10-2, may 2011. ISSN 2226-6089. 10.14806/ej.17.1.200.
- 62.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anders S, Pyl PT, Huber W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw and normalized RNA sequence data and associated metadata are available on NCBI Gene Expression Omnibus (GEO), under accession GSE225861. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [1] partner repository with the dataset identifier PXD041791.