Abstract
After decades studying the microbial “deep biosphere” in subseafloor oceanic crust, the growth and life strategies in this anoxic, low energy habitat remain poorly described. Using both single cell genomics and metagenomics, we reveal the life strategies of two distinct lineages of uncultivated Aminicenantia bacteria from the basaltic subseafloor oceanic crust of the eastern flank of the Juan de Fuca Ridge. Both lineages appear adapted to scavenge organic carbon, as each have genetic potential to catabolize amino acids and fatty acids, aligning with previous Aminicenantia reports. Given the organic carbon limitation in this habitat, seawater recharge and necromass may be important carbon sources for heterotrophic microorganisms inhabiting the ocean crust. Both lineages generate ATP via several mechanisms including substrate-level phosphorylation, anaerobic respiration, and electron bifurcation driving an Rnf ion translocation membrane complex. Genomic comparisons suggest these Aminicenantia transfer electrons extracellularly, perhaps to iron or sulfur oxides consistent with mineralogy of this site. One lineage, called JdFR-78, has small genomes that are basal to the Aminicenantia class and potentially use “primordial” siroheme biosynthetic intermediates for heme synthesis, suggesting this lineage retain characteristics of early evolved life. Lineage JdFR-78 contains CRISPR-Cas defenses to evade viruses, while other lineages contain prophage that may help prevent super-infection or no detectable viral defenses. Overall, genomic evidence points to Aminicenantia being well adapted to oceanic crust environments by taking advantage of simple organic molecules and extracellular electron transport.

Subject terms: Microbial ecology, Environmental microbiology
Introduction
Recent estimates find ~80% of all microbial life exists in subsurface environments [1]. With 70% of Earth covered by ocean, the oceanic crust represents the largest subsurface environment, indicating its potential as a large reservoir of microbial life [2, 3]. Due to the relative inaccessibility of oceanic crust, few opportunities exist to learn about crustal microbial life and their metabolic processes. Three decades of exploration have revealed images of microorganisms attached to crustal rocks and within the surrounding fluid, while environmental genomics has demonstrated microorganisms from all domains of life inhabit oceanic crust [2, 4–9]. Several recent studies revealed crustal microorganisms have the potential to use amino acid supplemented acetogenesis and dissimilatory carboxydotrophy to live in the subsurface [8, 10]. In the absence of cultured isolates, metabolic reconstructions from environmental genomes can increase our understanding of the microbial influence on the ocean crust, and may lead to successful cultivation attempts [2, 10].
A well-studied subseafloor oceanic crust environment is found on the eastern flank of the Juan de Fuca Ridge (JdFR) and composed of 3.5 Ma basalt [5, 11–13]. Three decades of research with subseafloor borehole observatories has revealed the dynamic nature of subseafloor fluid flow and fluid-rock-microbe interactions [5, 11–13]. These observatories allow for in situ experimentation and collection of pristine fluid to examine microbial processes in the crustal deep biosphere [14]. Several studies of the microbial community diversity at this site [6, 15, 16] reveal the utilization of ancient carbon metabolic pathways [8, 11], highlight synergies between rock-attached and planktonic communities [17], and support novel virus-host interactions in the crust [7]. However, uncertainties remain about how specific microbial groups survive in this reductant depleted habitat [18–20].
One regularly observed yet enigmatic group in this environment is the Aminicenantia class of Acidobacteriota [21–26], formerly referred to as Obsidian Pool 8 (OP8; [27]). This class has been found in diverse anoxic environments including hot springs, terrestrial aquifers, marine sediment, and marine cold seeps [23, 27–30], but no cultured representatives exist [22]. Investigations of Aminicenantia in anoxic aquatic environments demonstrate the genomic potential for degradation and fermentation of complex carbohydrates and proteins, Wood-Ljungdahl driven acetate oxidation, or CO2 production through the oxidation of formate [23, 28, 31]. These studies concluded an ecological role of Aminicenantia could be to scavenge and deconstruct organic matter [28, 31]. The anoxic crustal fluid of the JdFR flank contains low concentrations of organic carbon (12 μM DOC) compared to overlying deep seawater (39 μM DOC) [32], raising questions about which metabolic strategies Aminicenantia use to live in this environment.
Previous work identified two distinct clades of Aminicenantia in subsurface oceanic crust of the Juan de Fuca Ridge flank, based on metagenomic interpretation [16]. Here, this previous work is used as a foundation to investigate the potential metabolic characteristics of the enigmatic uncultivated Aminicenantia persisting in oceanic crust by using a combination of publicly available and new metagenome and single cell genome datasets. Our data reveal life strategies for nineteen JdFR Aminicenantia that form two lineages, JdFR-78 and JdFR-80, in this low energy habitat, provide evidence that the subseafloor is a reservoir for early evolved lineages, and hint at possible approaches for cultivation.
Materials and methods
Sample collection
Borehole observatories accessing subseafloor crustal fluids were installed on the eastern flank of the JdFR in 2004 and 2010 during IODP Expeditions 301 and 327, respectively [33, 34]. These observatories penetrate ~240 m of sediment and ~110–290 m of underlying basement to enable crustal fluid collection from 8–190 m below the sediment-basement interface [6, 33, 34]. Crustal fluids in this region are consistently 62–64 °C, anoxic, and sulfate-replete (~18 mmol sulfate per kg water) [35].
New crustal fluid sampling occurred in May 2019 during expedition AT42-11 on R/V Atlantis with ROV Jason II, both operated by Woods Hole Oceanographic Institute. Single cell genomic data was generated from cells collected from borehole observatory U1362B using established syringe sampling approaches [8, 36]. Briefly, the top plug on the wellhead of U1362B [33] was removed to freely vent crustal fluid from the top of the observatory. After the borehole dead volume had been flushed, crustal fluid was collected using a sterilized syringe [36]. Once recovered, the fluid was distributed in a nitrogen-flushed glove bag into cryovials, amended with 5% glycerol and 1X-Tris-EDTA buffer (final concentrations), and immediately flash-frozen with liquid nitrogen before long term storage at −70 °C. In addition to the new sample, we examined existing single cell genomic and metagenomic data generated from samples collected from borehole observatories in International Ocean Discovery Program (IODP) Holes U1301A, U1362A, and U1362B in 2010 (AT15-66) and 2011 (AT18-07). Methods for previous pristine fluid sampling from these borehole observatories were described elsewhere for the 2010 [21] and 2011 [6, 8, 14, 16] sampling.
Genomic sequencing, assembly, and phylogenomic analysis
Nineteen JdFR Aminicenantia MAGs and SAGs were identified across three independent sampling expeditions that occurred over a 9-year period. Two new metagenome assembled genomes (MAGs 3300037598_45, 3300037558_26) were sequenced, assembled, and binned from 2010 and 2011 metagenomes by the Joint Genome Institute [21, 37]. Four MAGs from 2011 sampling (labeled as JdFR-##; Supplementary Data 1) were produced previously [6, 16]. Single cell amplified genomes (SAGs) from 2011 sampling were created as previously described from Holes U1362A and U1362B [6, 8]. This study examines ten of these 2011 SAGs from U1362B (IDs with AC-334- and AC-335-; Supplementary Data 1) and two SAGs from U1362A (AC-708-). One new SAG was generated from the 2019 Hole U1362B fluid sampling (AC-873) following the same cell sorting, lysis, amplification, sequencing, and assembly approach as described elsewhere [8], and based on the standard workflow of the Single Cell Genomics Center at Bigelow Laboratory for Ocean Sciences (East Boothbay, Maine, USA) [38]. Nine SAGs within the Aminicenantia order JdFR-78 are nearly identical (average pairwise nucleotide identity, ANI, >99%), prompting the generation of a nearly complete composite genome assembly from these SAGs named JDF1 (see Supplementary Methods). Assessment of phylogenetic and phylogenomic taxonomic placement and read mapping of the JdFR Aminicenantia in comparison to other environmental sequences is described in the Supplementary Materials.
Genome annotation and assessment of viral interactions
JdFR Aminicenantia SAGs and MAGs were annotated with KOFAMSCAN using KEGG Orthology Hidden Markov Model (KO HMM) version 4.3, -mapper option using default settings [39]. Single KEGG Orthology IDs were reported for each open reading frame (ORF) that met default thresholds [40]. Annotations were input into KEGGDecoder and KEGG Mapper—Reconstruct Pathway visualization tools [39, 41]. Glycosyl hydrolases were identified using the dbCRAN meta server with HMMER via CAZy standard settings [42].
Expasy ScanProsite was used to search representative genomes for amino acid motifs that indicate siroheme (PS00365 for Nitrite/Sulfite reductases iron-sulfur/siroheme-binding site or heme utilization) or heme (PS51008 for multiheme cytochromes) utilization [43]. Transmembrane HMM (TMHMM) was used to identify transmembrane motifs in siroheme or heme utilizing proteins [44]. Several flagged genes with heme-binding motifs appeared to be misannotated as nitrite/nitrate reduction genes. To identify nearest-neighbors of these genes, NCBI RefSeq proteins that matched the original KOFAMSCAN annotation and top NCBI BlastP results were aligned with MUSCLE and bootstrapped phylogenies were constructed using RaXML -f a -p 1989 -m PROTCATLG -x 12345 -# 100 [45, 46].
Evidence of viral interactions with the 19 JdFR Aminicenantia MAGs and SAGs were examined using VirSorter v1.0.6 [47] and flagged viral sequences were annotated using Viral Orthologous Group HMM profiles. The CRISPR recognition tool [48] and CRISPRCasfinder [49] were used to identify Clustered Regular Interspaced Palindromic Repeats (CRISPR) bacterial defense systems. A detailed description of these methods can be found in the Supplementary Methods.
Results and discussion
Two Aminicenantia lineages persist in anoxic subseafloor oceanic crust
Phylogenetic (16S rRNA gene) and phylogenomic (concatenated marker protein) analyses place JdFR Aminicenantia into two separate lineages within the class Aminicenantia (Fig. 1 and Supplementary Fig. 1). This finding is supported by the Genome Taxonomy Database (GTDB) [25] which assigned the lineages to genus JdFR-78 (within the order JdFR-78) and genus JdFR-80 (within the order Aminicenantales). Fourteen JDFR MAGs and SAGs from this study grouped within the order JdFR-78, while five grouped within the genus JdFR-80 (Supplementary Data 1), previously named Class OP8_1 [23]. The JdFR-80 lineage contains Aminicenantia derived from marine environments [23], while the JdFR-78 lineage is a unique single-species order that branches near the root of the Aminicenantia tree (Fig. 1 and Supplementary Fig. 1, Supplementary Data 2 and 3). These lineage names come from publicly available MAGs JdFR-78 and JdFR-80 [16], which were used in the construction of the current GTDB tree. However, MAGs 3300037598_45, 3300037558_26 and no SAGs from this study were previously published. For downstream analyses and metabolic interpretations, representative JdFR Aminicenantia genomes were selected from both lineages. The order JdFR-78 was represented by JDF1 and the genus JdFR-80 was represented by MAG 3300037558-26, henceforth called JDF2. Read mapping publicly available subsurface metagenomes to the JDF1 and JDF2 genomes revealed that only JdFR metagenomic reads map to the representative genomes at a >95% identity match (Supplementary Fig. 2). These results indicate that the JdFR likely harbors endemic Aminicenantia lineages.
Fig. 1. Aminicenantia 16S rRNA gene phylogenetic tree reveals two lineages living in the Juan de Fuca Ridge flank oceanic crust.
Building on Farag et al. [23] (colored dots in inner ring), Juan de Fuca Aminicenantia 16S rRNA genes were compared to all other Aminicenantia available in public databases and over 800 bp (Supplementary Data 2). Aminicenantia 16S rRNA gene sequences were collected from a range of aquatic environments (colored squares in outer ring). The Juan de Fuca Aminicenantia form two separate clades, named JdFR-78 (orange) and JdFR-80 (blue). Bootstrap values of 70% or higher are represented by red dots. This pattern is supported by phylogenomic analysis of concatenated conserved marker proteins (Supplementary Fig. 1).
Heterotrophic pathways using simple carbon compounds
Representative genomes JDF1 and JDF2 encode nearly identical mechanisms for carbon import and incorporation into central carbon metabolism (Fig. 2 and Supplementary Fig. 3). Both representative genomes contain multiple gene copies of at least 32 unique peptidases that have the capability to hydrolyze secreted and periplasmic oligopeptides (Supplementary Data 4). Subsequently, cleaved amino acids may enter JdFR Aminicenantia via seven different symporters or antiporters (Supplementary Data 5). Both representative genomes contain the potential to catabolize 10 of 20 canonical amino acids (Supplemental Data 6).
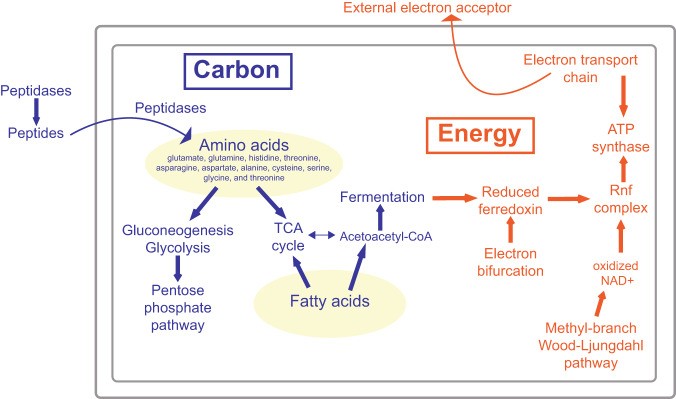
Fig. 2. Carbon utilization and energy generation metabolisms within the Juan de Fuca Aminicenantia.
All crustal Aminicenantia contain the metabolic potential to use amino acids and fatty acids as carbon sources (highlighted yellow) that feed into gluconeogenesis or the TCA Cycle. ATP synthesis can happen via two mechanisms: substrate-level phosphorylation through butyrate or propanoate fermentation, or an electrochemical gradient created with an electron transport chain or an Rnf Complex. The Rnf Complex relies on reduced ferredoxin produced from two different electron bifurcating complexes and oxidized NAD+ maintained by the methyl-branch of the Wood-Ljungdahl Pathway. Genomic evidence indicates an iron or sulfur based external terminal electron acceptor. For more detail see Supplementary Fig. 3 and Supplementary Data 4–11.
Fatty acids may be another carbon source for JdFR Aminicenantia. Both representative genomes contain nine enzymes necessary to cleave odd and even fatty acid chains [50] (Supplementary Data 7). Evidence of amino and fatty acid catabolism suggests JdFR Aminicenantia are heterotrophs, consistent with previous studies [24, 28], and similar strategies are observed in uncultivated JdFR acetogenic Firmicutes [10]. Additionally, both genomes contain 7–14 copies of glycosyl hydrolases (GH), and a full glycolysis/gluconeogenesis pathway exists in both representative genomes (Fig. 2 and Supplementary Fig. 3). From genomic data, it is unclear which direction this pathway runs. Amino and fatty acid catabolism can contribute to pyruvate pools that drive gluconeogenesis, and GH may create carbon pools that drive glycolysis (Supplementary Fig. 3). With fewer GH compared to other annotated and published Aminicenantia genomes [28, 31, 51], clear genomic evidence of amino acid incorporation and degradation, and the history of this class, we hypothesize gluconeogenesis is the predominate use of this metabolism. Fifteen of 20 standard amino acids were detected at 0.2–17 nM in JdFR crustal fluid [52], making up ~2% of measurable dissolved organic carbon and comparable to concentrations of these amino acids in deep seawater. Seawater recharge may be one source of amino acids for heterotrophic activity in the crust [52]. Other amino acid sources may be carbon diffusion across the sediment-basement interface, in situ production from chemolithoautotrophs, or microbial necromass [32, 52–55]. JdFR is a net sink for bottom seawater dissolved organic carbon, and heterotrophic microbial activity and abiotic reactions are thought to contribute to its removal [32, 56]; it appears JdFR Aminicenantia can contribute to this removal.
Use of external terminal electron acceptors
For energy generation, JDF1 and JDF2 may anaerobically respire iron. Both have 14 Nuo NADH dehydrogenase subunits, fumarate reductase, menaquinol biosynthesis, and F-type ATPase (Fig. 2 and Supplementary Fig. 3 and Supplementary Data 8). Additionally, a heme biosynthesis operon is followed by 14 ORFs containing 74 individual heme-binding motifs (CXXCH) and 10 ORFs containing transmembrane helices (Supplementary Fig. 4 and Supplementary Data 9). These indicate this operon is embedded in the membrane and involved in electron transfer [44]. KOFAMSCAN annotations for heme-binding motif ORFs indicated involvement in cytochrome C activities (Supplementary Fig. 4). This operon’s gene content and orientation resembles one in Pelobacter carbinolicus, family Geobacteraceae, known for extracellular iron reduction [57]. Phylogenetically, JdFR Aminicenantia heme-binding ORFs resemble Geobacteraceae sequences, known for iron oxide reduction, but the closest RefSeq quality sequence is cultured Thermotomaculum hydrothermale (Acidobacteriota) that reduces elemental sulfur [58] (Supplementary Figs. 5 and 6). The second most abundant mineral phase in JdFR basaltic crust is iron oxide and iron oxyhydroxide, while only some sulfur compounds (e.g., pyrite) were detected [59]. Due to genomic evidence of a membrane complex capable of moving electrons externally, and limited sulfur, we speculate that JdFR crustal Aminicenatia use iron oxyhydroxides as external electron acceptors.
Alternative energy generation with fermentation, reduced ferredoxin, electron bifurcation, and an Rnf complex
ATP synthesis through substrate-level phosphorylation is a second method for energy generation in both lineages; however, their fermentative capabilities vary. JDF1 contains all enzymes required to convert pyruvate to butyrate, including butyryl-CoA dehydrogenase (Supplementary Fig. 3). Without a terminal electron acceptor, JDF1 may catabolize five amino acids or even chain fatty acids to pyruvate or acetoacetyl-CoA, and these subsequently enter butyrate fermentation (Supplementary Data 8) [60]. Butyryl-CoA dehydrogenase are homotetrameric and associate with electron transfer flavoproteins to form an electron bifurcating complex [61, 62]. JDF1 contains three subunits involved in the butyryl-CoA dehydrogenase/electron bifurcating complex (Supplementary Data 8). This complex couples simultaneous reduction of ferredoxin and crotonyl-CoA to oxidation of NADH [62], providing reduced ferredoxin, butyrate formation, and oxidized electron carrier NAD+ (Supplementary Fig. 3).
JDF2 does not contain evidence for butyryl-CoA dehydrogenase, but shows potential to ferment propanoate (Supplementary Fig. 3). Threonine or methionine catabolism can synthesize α-ketobutyrate used by pyruvate ferredoxin oxidoreductase, CoA ligase, and phosphate acetyltransferase to generate propanoate and ATP (Supplementary Data 8). Pyruvate ferredoxin oxidoreductase produces reduced ferredoxin [63]. Using different fermentative pathways, both lineages generate reduced ferredoxin, capitalizing on its low redox potential that makes it a valuable electron donor in anoxic environments (Fig. 2) [64]. Electron bifurcation increases the efficiency of substrate level and electron transport chain phosphorylation in anaerobic organisms [62]. These processes are in the genomes of metal reducing and thermophilic heterotrophic bacteria [65], characteristics that resemble JdFR Aminicenantia.
A second electron bifurcating complex present in each representative genome is NiFe hydrogenase/heterodisulfide reductase (Mvh; Fig. 2, Supplementary Fig. 3 and Supplementary Data 10). Hydrogenase classifier HydDB (version 1) listed this NiFe hydrogenase in group 3 subgroup c, a classification generally associated with electron bifurcating reactions [62, 66]. Bifurcating NiFe hydrogenases and heterodisulfide reductases couple simultaneous reduction of ferredoxin and CoM-CoB heterodisulfide to H2 oxidation [62]. Heterodisulfide reductases are ancient enzymes associated with the anaerobic core of the prokaryotic common ancestor [67]. Heterodisulfide reductases are present in metabolically diverse prokaryotes and are hypothesized to perform diverse physiological functions due to homolog detection in sulfate-reducing and halophilic bacteria [62, 67]. JdFR crustal fluids have H2 concentrations of 0.05–1.8 µmol kg−1 [68], making hydrogen available to drive the electron bifurcation activity of Mvh.
JdFR Aminicenantia may use an incomplete Wood-Ljungdhal Pathway (WLP) to maintain oxidized pools of electron carriers (e.g., NAD+). Both representative genomes contain a modified WLP containing only methyl branch steps (Fig. 2, Supplementary Fig. 3 and Supplementary Data 11). Fermenting Clostridium difficile studies found an increased activity in the WLP methyl branch as reductants accumulated because three of its enzymatic steps utilize electrons [69, 70]. During fermentation, using the methyl branch as an oxidant source helps maximize ATP generation and increase available energy [69]. JdFR Aminicenantia could use the WLP methyl branch to maintain a pool of oxidized electron carriers in the highly reduced crustal environment [71].
Rnf complexes may use reduced ferredoxin to create an electrochemical gradient. Both representative genomes contain six Na+ translocating ferredoxin-NAD reductase (Rnf) ORFs (Fig. 2, Supplementary Fig. 3 and Supplementary Data 8). The Rnf complex is an inner membrane electrochemical Na+ pump that oxidizes reduced ferredoxin to transport Na+ ions into the periplasm, generating an electrochemical gradient [64, 72]. This complex acts independent of anaerobic respiration or fermentation, and is a third metabolic route for electrochemical gradient generation that drives F-type ATP synthase [73]. Multiple mechanisms for low potential electron transport and electrochemical gradient production drive JdFR Aminicenantia energy generation.
These JdRF Aminicenantia metabolisms have also been found in three separate JdFR Firmicutes lineages that are predicted to utilize hydrogenotrophic acetogenesis supplemented by anaerobic respiration or fermentation [10, 74, 75]. JdFR Chloroflexi use electron bifurcation to drive an ion-translocating Rnf complex to achieve net ATP synthesis [75], similar to how JdFR Aminicenantia may use this metabolism. Similarly, JdFR Hydrothermarchaeota utilize a hydrogen driven WLP that is supplemented by anaerobic sulfate reduction [8]. Lastly, thermodynamic studies found organic material increased the favorability of JdFR crustal iron reduction [55, 76]. Thus, JdFR Aminicenantia metabolic energy strategies align with the collective narrative that JdFR microbes use a combination of autotrophic and heterotrophic processes to derive energy in the crust.
Viruses influence Juan de Fuca Aminicenantia
The abundance of putative viruses is higher than prokaryotic cell counts in JdFR, indicating bacteria experience phage predation [7]. To search for viral infection and defense mechanisms within JdFR Aminicenantia, the presence of viral-like scaffolds and CRISPR-Cas viral defense systems were investigated. VirSorter identified 22 viral-like contigs at VirSorter confidence levels 2–3 within thirteen JdFR Aminicenantia genomes (Supplementary Data 1). Nine similar viral-like scaffolds had 100% pairwise identity and identical sites, 61–100% query coverage, and e-values of 0 (Supplementary Data 12). Thirteen remaining viral-like scaffolds were considered unique (query coverage <50%), indicating diverse viruses interact with JdFR Aminicenantia. All viral-like scaffolds were identified within JdFR Aminicenantia genomes, providing evidence of prophage. Annotations of viral scaffolds identified integrase and transposase within JDF1 and JDF2 genomes in addition to known prophage genes (i.e., baseplate, capsid, and tail proteins) (Supplementary Data 13) [77]. The observation of prophage suggest that Aminicenantia viruses use a lysogenic strategy. In similar low biomass environments (~104 cells ml−1) [6], it has been shown that viruses prefer integrating into their host in place of lysing them [4, 78]. Other studies found lysogeny is prevalent in adverse conditions where host cell number and activity are low and viral particle decay rates are high due to high temperature [79].
Prophage can also increase the fitness of the host cell by encoding genes that confer resistance to additional virulence [80]. Two potential prophage defense strategies exist within the JdFR Aminicenantia (Supplementary Data 13). Lineage JdFR-78 contain viral-associated deoxynucleoside triphosphate triphosphohydrolase, a known dNTPase [81]. In mammals, dNTPases diminish viral activity by degrading cellular dNTP, making nucleotides less available for replicating viruses [81]. The dNTPase within lineage JdFR-78 prophage could be used to keep dNTP levels low, making it harder for competing viruses to replicate within an already claimed host. Lineage JdFR-80 contain viral-associated glucose-1-phosphate thymidylyltransferase, dTDP-4-dehydrorhamnose 3,5-epimerase, and bifunctional protein HldE (Supplemental Data 13). These three proteins are involved in lipopolysaccharide O-antigen sugar biosynthesis, aiding the cell in outer membrane signaling [82]. Viruses use the O-antigen component of lipopolysaccharide to identify, target, and bind to bacterial hosts [80]. Experiments with Pseudomonas aeruginosa have found prophages alter the O-antigen of their host to decrease the number of additional free viruses successfully infecting the cell [80]. The prophage O-antigen modifying genes in lineage JdFR-80 could “disguise” the host to prevent subsequent viral infection.
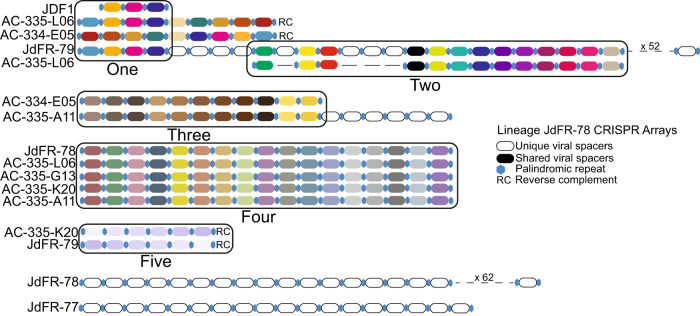
To defend against viral infection, CRISPR-Cas viral immunity systems are found within 10–45% of sequenced bacterial genomes, many originating from thermophilic environments [83, 84]. In this study, evidence for CRISPR arrays and associated-Cas proteins were found only within JdFR-78 lineage members (8/14 genomes; Fig. 3). Fifteen separate CRISPR arrays were dispersed across 8 JdFR-78 lineage members and contained a total of 332 viral spacers. Of the 332 viral spacers, 167 were shared across CRISPR arrays and 165 were unique (Fig. 3).The 167 shared viral spacers were organized into 5 similarity groups supporting the hypothesis that similar viruses infect multiple lineage JdFR-78 Aminicenantia members.
Fig. 3. Comparison of CRISPR arrays in Juan de Fuca crustal fluid Aminicenantia.
Shown are all 15 CRISPR arrays found in nine genomes within lineage JdFR-78. The CRISPR arrays contained 332 total viral spacers with 167 viral spacers shared across genomes. The shared viral spacers form five similarity groups (labeled one to five). Each colored oval represents a shared viral spacer within a cluster. The viral diversity within the individual genomes was not captured in composite genome JDF1 (listed in cluster one). Viral spacers were only shared amongst lineage JdFR-78 members that were nearly identical (99% ANI: AC-335-L06, AC-334-E05, JdFR-79, AC-335-A11, JdFR-78, AC-335-G13, AC-335-K20, JDF1). Lineage JdFR-80 contained no CRISPR arrays.
Of the eight JdFR-78 lineage members with CRISPR arrays, five were used to create JDF1 composite genome, and comparison of these CRISPR arrays indicates viral spacer diversity was not captured (Fig. 3). This unexpected finding supports increased evidence that complete clonal populations of cells do not exist in the ocean and algorithmically combining their sequence data can result in misrepresentations (like viral infection) [85]. The assemblies used to generate composite genome JDF1 were nearly identical (99% ANI) and infected by the same viruses, due to the presence of identical CRISPR array viral spacers. However, other JdFR-78 assemblies with only a 79% ANI similarity contained no shared CRISPR viral spacers (Fig. 3). This highlights the viral diversity and specificity interacting with JdFR Aminicenantia and supports previous metagenomic analyses that found JdFR Aminicenantia make up both viral hosts and CRISPR sources within this environment [7].
CRISPR-Cas defense against viral infection was only observed in the JdFR-78 lineage (Supplementary Data 14). Lineage JdFR-78 has CRISPR-Cas1-7 and CRISPR- Cmr1-6, Cas10 systems. The Cas proteins associated with CRISPR arrays are involved in the degradation of foreign DNA, while the Cmr proteins associated with CRISPR arrays are involved in foreign RNA degradation [86, 87]. CRISPR-Cas activity is well described in other microorganisms (e.g., Archaeoglobus and E. coli), resulting in this cluster of genes being recognized as a Class 1 Type 1 CRISPR-Cas system [86]. Alternatively, the type III B CRISPR-Cmr system has been best studied in archaea (e.g., Sulfolobus islandicus and Pyrococcus furiosus) but has also been found in the thermophilic bacterium Thermus thermophilus [88, 89]. The benefits of the CRISPR-Cmr complex include its targeting mechanism that degrades both foreign RNA as it is transcribed, and any single-stranded DNA associated with the transcription [90]. Through this mechanism, the CRISPR-Cmr complex displays a higher tolerance for mutations or mismatches between foreign DNA/RNA and the protospacer sequence within the CRISPR array [90]. The presence of CRISPR-Cas and CRISPR-Cmr defense systems indicate that DNA and RNA viruses patrol the JdFR crust. Previous modeling efforts found CRISPR microbial immune systems work optimally in high temperature and pressure environments that limit viable viral genome mutations necessary to evade CRISPR recognition [83]. Documented bacterial fitness costs associated with CRISPR immunity include autoimmunity, as host-DNA may be incorporated as a spacer, and prevention of beneficial gene acquisition through horizontal gene transfer [91]. These costs outweigh the benefits when viral genome mutations are not restricted [83]. The presence and similarity between CRISPR systems in lineage JdFR-78 indicate moderate viral diversity exists in the JdFR potentially due to the environmental constraints preventing viral mutations from outpacing CRISPR spacer acquisition [83]. These observations align with the models, indicating the JdFR thermophilic ocean crust environment supports the effectiveness of CRISPR based immunity systems.
Signatures of early evolution of Aminicenantia
Despite their similarities, representative genomes JDF1 and JDF2 have notable differences in their pathways used for heme biosynthesis (Fig. 4C and Supplementary Data 9). JDF2 is predicted to synthesize heme through a traditional mechanism with coproporphyrinogen-III as a biosynthesis intermediate, most common in bacteria (Fig. 4C). JDF1 uses less common precorrin-2 and siroheme as heme biosynthesis intermediates, common in Archaea and a small proportion (<10%) of Bacteria [92]. Upon further investigation, members within lineage JdFR-78 were the only group in the Aminicenantia class to generate siroheme as an intermediate in heme biosynthesis.
Fig. 4. Genomic and phylogenomic properties of Aminicenantia order JdFR-78 indicate evolutionary differences.

A Graph of GC percentage and genome length of all Aminicenantia families. Lineage JdFR-78 has the smallest genomes and lowest GC percentage. Family Aminicenantaceae_A contains lineage JDFR-80. B Phylogenomic root to tip distance of Aminicenantia families. Lineage JdFR-78 has the shortest root to tip distance. C Heme biosynthetic intermediates differ between lineage JdFR-78 and all other Aminicenantia families. Lineage JdFR-78 utilizes the oxygen intolerant siroheme intermediate. All other Aminicenantia families utilize oxygen tolerant intermediates. For more detail see Supplementary Data 9.
Siroheme is a co-factor in sulfite and nitrite reductases, but JDF1 did not contain these enzymes. JDF1 does not contain siroheme-binding motif PS00365, indicating siroheme acts as a heme biosynthetic intermediate and likely serves no other purpose [93]. The siroheme-based heme synthesis pathway may be a “vestigial structure” from an ancestor that used siroheme for a purpose no longer present in JDF1, such as sulfate or nitrate reduction [93]. Because siroheme is oxygen sensitive, its presence supports the idea that the entire evolutionary history of lineage JdFR-78 occurred in anoxic ocean crust. Due to separation from oxygen, there was no need to use oxygen tolerant heme biosynthetic mechanisms like the coproporphyrinogen-III route.
Additional evidence indicating lineage JdFR-78 represents an ancient lineage of Aminicenantia include lineage JdFR-78 branching near the root of Aminicenantia phylogenetic trees (Fig. 1 and Supplementary Fig. 1), and JdFR-78 lineages consistently have the shortest measured root-to-tip distances from the root of the tree (see Supplementary Materials; Fig. 4B). This indicates lineage JdFR-78 are the least divergent of any Aminicenantia group from the root of the tree [94]. Lineage JdFR-78 has the smallest genomes with the lowest GC content (Fig. 4A), potentially indicative of genome streamlining or originating from smaller ancestral genomes [95].
The presumably earlier-evolved JdFR-78 lineage of Aminicenantia may be limited in dispersal by habitable environmental constraints (Fig. 5). Microbial dispersibility throughout the ocean has been tracked using thermophilic spores [96, 97]. The oceanic crust releases microorganisms into the water column based on regional hydrology, and subsequent microbial survivability depends on ocean currents transporting cells to a habitable environment [96, 97]. Genomic studies found similar microbial community members in seawater, ocean sediment, and ocean crust at different relative abundances [98, 99]. This indicates the ocean crust can inoculate seawater and overlying sediment with microbes, and survivability depends on their metabolic needs [98, 99]. Aminicenantia 16S rRNA genes have been detected deep in the overlying sediment of JdFR [100], supporting our hypothesis that lineage JdFR-78 members surviving in anoxic hydrothermal oceanic crust may retain signatures of early evolutionary reducing conditions. This lineage could have either experienced genome streamlining to increase efficiency in nutrient limited ocean crust or adapted from a small ancestral genome. The lack of observation of lineage JdFR-78 in other habitats (Supplementary Fig. 2) suggests barriers to dispersal include absence of oxygen tolerant metabolisms, making it difficult to survive transport outside the subsurface. Lastly, as described above, lineage JdFR-78 contains CRISPR immunity systems, which have been shown to prevent acquisition of new genes through horizontal gene transfer [83]. It is possible this system has also contributed to lineage JdFR-78 constraint within the subsurface by limiting new gene acquisition. By contrast, lineage JdFR-80 has no evidence of CRISPR immunity preventing horizontal gene transfer. Additional genomic signatures indicative of evolutionary development and tolerance for oxygenated conditions include the presence of oxygen tolerant coproporphyrinogen-III in heme biosynthesis, and closer taxonomic similarity to marine Aminicenantia. All enabling survival in oxidized environments and indicating lineage JdFR-80 may have a wider dispersal capacity.
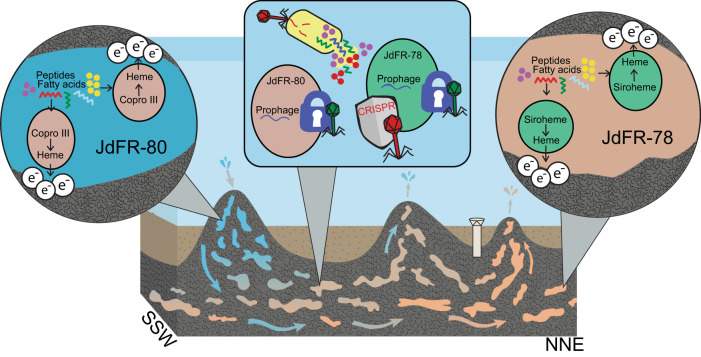
Fig. 5. Life strategies for two lineages of Aminicenantia living in the warm and anoxic crustal fluids of the Juan de Fuca Ridge flank.
Both lineages of Aminicenantia have the genomic potential to utilize peptides and fatty acids for carbon, which may derive from bottom seawater or necromass. Multiple lines of evidence indicate the use of an external terminal electron acceptor, which may be iron oxyhydroxides present in the surrounding rock. Both lineages synthesize heme to transport electrons, but the heme synthesis pathways utilize different intermediates. The JdFR-78 lineage uses an oxygen intolerant siroheme intermediate that, in addition to other evidence, drives our hypothesis that this lineage is older than the JdFR-80 lineage, and retains signatures of early evolution in a similar reducing environment. Other studies have found evidence of viruses in the ocean crust, both Aminicenantia lineages contain evidence of prophage insertion and prophage prevention of additional viral infection (as indicated by blue padlocks). Only the JdFR-78 lineage contain genomic evidence of CRISPR-Cas viral defense systems. Overall, these Aminicenantia populations appear to have evolved to take advantage of the limited resources available in the ocean crust.
Unique genomic signatures of JdFR Aminicenantia
Aminicenantia have no cultured representatives, but have been found in aquatic and soil ecosystems across the world (Fig. 1), usually making up less than 1% of the microbial community [23]. Despite their wide distribution, few studies have detailed the genomic potential of Aminicenantia. Exceptions to this come from two studies, hydraulically fractured well fluids in Australia (Aminicenantes-PK28) [31] and oil exploration borehole fluid in Russia (BY38 Aminicenantes) [28] where Aminicenantia made up to 10% of the microbial community and their metabolic potential was investigated in detail. These studies provide a framework for comparison of genome traits and metabolisms with JdFR Aminicenantia.
GTDB taxonomically classifies Aminicenantia PK28, BY38 and JDF2 within the order Aminicenantales, and family Saccharicenantaceae (PK28 & BY38) or Aminicenantaceae_A (JDF2). Separately, JDF1 falls within its own unique order that only includes Aminicenantia derived from the JdFR ocean crust (Supplementary Data 15). The genome size of these four Aminicenantia range from 2.46 to 2.90 Mb and encode 2064 to 2509 proteins (Supplementary Data 15) [28]. Core metabolic tools shared across all four genomes include peptidases capable of cleaving amino acids from oligopeptides, GH involved with carbon degradation, complete glycolysis/gluconeogenesis, fermentation capabilities, a partial TCA cycle, and hydrogenase utilization to generate a proton gradient (Supplementary Data 15) [28, 31]. These conserved metabolisms emphasize the scavenging nature of Aminicenantia and their conserved utilization of hydrogenases.
Key differences between these four genomes include the quantity of GH encoded in each genome, the types of hydrogenases utilized, the presence of CRISPR-Cas viral defense systems, the ability to anaerobically respire, and the utilization of the Wood-Ljungdahl pathway or Rnf complex (Supplementary Data 15). Aminicenantia PK28 and BY38 have more copies of GH compared to JdFR Aminicenantia, suggesting GH are utilized less in the JdFR compared to other subsurface environments. Regarding hydrogenases, only JdFR Aminicenantia use an electron bifurcating hydrogenase to generate reduced ferredoxin, emphasizing the need for low potential reductants within JdFR crustal fluid. Aminicenantia viral defense systems may be more variable with only PK28 and JDF1 containing CRISPR-Cas systems. Lastly, only JdFR Aminicenantia have the genomic potential for anaerobic respiration, Wood-Ljungdahl pathway utilization, or a Rnf complex, suggesting metabolic diversity may be advantageous in the isolated JdFR.
Conclusion
The two Aminicenantia lineages (JdFR-78 and JdFR-80; Fig. 5) characterized represent microbial lineages that contribute to heterotrophic strategies predicted to dominate warm and anoxic ocean crust (Fig. 5) [32, 52]. Previous work in this habitat has focused on the utilization of organic carbon to drive sulfate reduction, yet here we see evidence for extracellular iron oxyhydroxide or sulfur oxide reduction enabling microbial metabolism, which has only been thermodynamically predicted [54, 55]. As supported by previous work, amino acids appear to be the most available form of organic carbon, which JdFR Aminicenantia are predicted to use for carbon and to drive anaerobic energy generation [10, 52]. Use of the Wood-Ljungdahl Pathway consistently appears useful for life in the JdFR crust [8, 10], but only genes for the methyl-branch were found in JdFR Aminicenantia genomes, potentially due to its use outside of acetogenesis. Here, the methyl-branch may be working with two separate electron bifurcating complexes to generate oxidized electron carriers and reduced ferredoxin. These products drive the activity of an ion-translocating Rnf complex that can contribute to the electrochemical gradient driving ATP synthase. This work expands the metabolic toolkit known to be used by microorganisms in this environment, and may act as a framework for future laboratory-based culturing and experimental verification efforts.
Recent work described the presence of both lytic and lysogenic viruses in the JdFR crust [7]. Our observations provide supporting evidence of crustal viruses through the identification of prophage and CRISPR-Cas defense systems within JdFR Aminicenantia genomes. Some JdFR Aminicenantia combat viral infection with CRISPR-Cas systems, others through adaptive immunity provided via a prophage, and a few genomes contained neither. It seems viruses do influence the ecology of the deep subsurface through direct host manipulation as a prophage or as an additional stressor to combat as a phage.
Read mapping comparisons indicate Aminicenantia are endemic to the Juan de Fuca Ridge flank. Yet, genome size, GC content, phylogenetic placement, and metabolic differences hint that the lineages entered the subsurface at different time points, one potentially with little exposure to oxygen and the other after oxygen exposure. The remnants of oxygen intolerant, primordial biosynthetic intermediates supports the theory that the marine crustal biosphere may be used as a proxy to understand the evolution of early life. Overall, this study supports previous findings and aims to stitch them together to clarify how a group of recurring heterotrophic bacteria can live in the crust.
Supplementary information
Supplemental methods and figures associated with investigating Juan de Fuca Ridge Flank Aminicenantia
Acknowledgements
The authors thank the science parties and crews of expeditions AT18-07, AT26-18 and AT42-11 for their support to collect the samples used in this study. We also thank Jennifer Gladstone, Anna Chen, Melody Lindsay and Julia McGonigle for discussions that helped shape the direction of this study, the staff of the Single Cell Genomics Center at the Bigelow Laboratory for Ocean Science and the Joint Genome Institute (JGI) for all sequencing, and Ruth Booker and Orion Thomas for extensive proof-reading. Samples from 2019 were collected with the consent of the Government of Canada, as reviewed by Global Affairs Canada.
Author contributions
AEB, BNO, JMB, and TD conceived the project and designed the analyses. BNO, MSR, and RS secured funding. AEB, TD, AAB, JMB performed the analyses. All authors interpreted the results and wrote the manuscript.
Funding
Funding for the expeditions was provided by the U.S. National Science Foundation (awards OCE-1260723 to MSR, OCE-1260548 to C. Geoff Wheat, and OCE-1737017 to BNO). Funding for analyses was provided in part from the NSF (award OCE-173017 to BNO, OCE-1851582 to MSR, and OIA-1826734 to RS and BNO), from the NASA Exobiology program (80NSSC19K0466 to BNO), from the Center for Dark Energy Biosphere Investigation (C-DEBI; subaward from OCE-0939654 to BNO), and from the Rodney White Fellowship provided by Bigelow Laboratory for Ocean Sciences (to AEB and AAB). The work conducted by the US Department of Energy JGI, a US Department of Energy Office of Science User Facility, is supported under contract no. DE-AC02-05CH11231. This is HIMB contribution number 1933 and SOEST contribution number 11682.
Data availability
All SAG data for this project can be found on NCBI under BioProject ID PRJNA842252 under accession numbers: JAMZRZ000000000 (JDF1 composite genome); JAMZSA000000000 (AH-873-B07); JAMZSB000000000 (AC-708-M15); JAMZSC000000000 (AC-708-I09); JAMZSD000000000 (AC-335-O07); JAMZSE000000000 (AC-335-L06); JAMZSF000000000 (AC-335-K20); JAMZSG000000000 (AC-335-G13); JAMZSH000000000 (AC-335-B20); JAMZSI000000000 (AC-335-A11); JAMZSJ000000000 (AC-334-K16); JAMZSK000000000 (AC-334-E05). All MAG data for this project can be found on IMG.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41396-023-01454-5.
References
- 1.Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci USA. 2018;115:6506–11. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orcutt BN, D’angelo T, Jungbluth SP, Huber JA, Sylvan JB. Microbial life in oceanic crust. 2020. 10.31219/osf.io/2wxe6.
- 3.Edwards KJ, Fisher AT, Wheat CG. The deep subsurface biosphere in igneous ocean crust: frontier habitats for microbiological exploration. Front Microbiol. 2012;3:8. doi: 10.3389/fmicb.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orcutt BN, Edwards KJ. Life in the ocean crust: lessons from subseafloor laboratories. Dev Mar Geol. 2014;7:175–96. 10.1016/B978-0-444-62617-2.00007-4.
- 5.Cowen JP, Giovannoni SJ, Kenig F, Johnson HP, Butterfield D, Rappé MS, et al. Fluids from aging ocean crust that support microbial life. Science. 2003;299:120–3. doi: 10.1126/science.1075653. [DOI] [PubMed] [Google Scholar]
- 6.Jungbluth SP, Bowers RM, Lin H-T, Cowen JP, Rappé MS. Novel microbial assemblages inhabiting crustal fluids within mid-ocean ridge flank subsurface basalt. ISME J. 2016;10:2033–47. doi: 10.1038/ismej.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigro OD, Jungbluth SP, Lin H, Hsieh C, Miranda JA, Schvarcz CR, et al. Viruses in the oceanic basement. mBio. 2017;8. 10.1128/mBio.02129-16. [DOI] [PMC free article] [PubMed]
- 8.Carr SA, Jungbluth SP, Eloe-Fadrosh EA, Stepanauskas R, Woyke T, Rappé MS, et al. Carboxydotrophy potential of uncultivated hydrothermarchaeota from the subseafloor crustal biosphere. ISME J. 2019;13:1457–68. doi: 10.1038/s41396-019-0352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivarsson M, Bengtson S, Neubeck A. The igneous oceanic crust—Earth’s largest fungal habitat? Fungal Ecol. 2016;20:249–55. doi: 10.1016/j.funeco.2016.01.009. [DOI] [Google Scholar]
- 10.Smith AR, Mueller R, Fisk MR, Colwell FS. Ancient metabolisms of a thermophilic subseafloor bacterium. Front Microbiol. 2021;12:764631. doi: 10.3389/fmicb.2021.764631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith AR, Kieft B, Mueller R, Fisk MR, Mason OU, Popa R, et al. Carbon fixation and energy metabolisms of a subseafloor olivine biofilm. ISME J. 2019;13:1737–49. doi: 10.1038/s41396-019-0385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher AT, Davis EE, Hutnak M, Spiess V, Zühlsdorff L, Cherkaoui A, et al. Hydrothermal recharge and discharge across 50 km guided by seamounts on a young ridge flank. Nature. 2003;421:618–21. doi: 10.1038/nature01352. [DOI] [PubMed] [Google Scholar]
- 13.Orcutt BN, Bach W, Becker K, Fisher AT, Hentscher M, Toner BM, et al. Colonization of subsurface microbial observatories deployed in young ocean crust. ISME J. 2011;5:692–703. doi: 10.1038/ismej.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher AT, Tsuji T, Petronotis K, Wheat CG, Becker K, Clark JF, et al. IODP expedition 327 and Atlantis expedition AT 18-07: observatories and experiments on the eastern flank of the Juan de Fuca Ridge. Sci Drill. 2012. 10.2204/iodp.sd.13.01.2011.
- 15.Smith AR, Fisk MR, Thurber AR, Flores GE, Mason OU, Popa R, et al. Deep crustal communities of the Juan de Fuca Ridge are governed by mineralogy. Geomicrobiol J. 2017;34:147–56. doi: 10.1080/01490451.2016.1155001. [DOI] [Google Scholar]
- 16.Jungbluth SP, Amend JP, Rappé MS. Metagenome sequencing and 98 microbial genomes from Juan de Fuca Ridge flank subsurface fluids. Sci Data. 2017;4:170037. doi: 10.1038/sdata.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramírez GA, Garber AI, Lecoeuvre A, D’Angelo T, Wheat CG, Orcutt BN. Ecology of subseafloor crustal biofilms. Front Microbiol. 2019;10:1983. doi: 10.3389/fmicb.2019.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robador A, LaRowe DE, Jungbluth SP, Lin H-T, Rappé MS, Nealson KH, et al. Nanocalorimetric characterization of microbial activity in deep subsurface oceanic crustal fluids. Front Microbiol. 2016;7:454. doi: 10.3389/fmicb.2016.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaRowe DE, Koch BP, Robador A, Witt M, Ksionzek K, Amend JP. Identification of organic compounds in ocean basement fluids. Org Geochem. 2017;113:124–7. doi: 10.1016/j.orggeochem.2017.07.017. [DOI] [Google Scholar]
- 20.Lu GS, LaRowe DE, Amend JP. Bioenergetic potentials in terrestrial, shallow-sea and deep-sea hydrothermal systems. Chem Geol. 2021;583:120449. doi: 10.1016/j.chemgeo.2021.120449. [DOI] [Google Scholar]
- 21.Jungbluth SP, Grote J, Lin HT, Cowen JP, Rappé MS. Microbial diversity within basement fluids of the sediment-buried Juan de Fuca Ridge flank. ISME J. 2013;7:161–72. doi: 10.1038/ismej.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castelle CJ, Banfield JF. Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell. 2018;172:1181–97. doi: 10.1016/j.cell.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Farag IF, Davis JP, Youssef NH, Elshahed MS. Global patterns of abundance, diversity and community structure of the Aminicenantes (Candidate Phylum OP8) PLoS ONE. 2014;9:e92139. doi: 10.1371/journal.pone.0092139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, Cheng J-F, et al. Insights into the phylogeny and coding potential of microbial dark matter. Nature. 2013;499:431–7. doi: 10.1038/nature12352. [DOI] [PubMed] [Google Scholar]
- 25.Parks DH, Chuvochina M, Rinke C, Mussig AJ, Chaumeil P-A, Hugenholtz P. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022;50:D785–94. doi: 10.1093/nar/gkab776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oren A, Garrity GM. Valid publication of the names of forty-two phyla of prokaryotes. Int J Syst Evol Microbiol. 2021;71. 10.1099/ijsem.0.005056. [DOI] [PubMed]
- 27.Hugenholtz P, Pitulle C, Hershberger KL, Pace NR. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–76. doi: 10.1128/JB.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadnikov VV, Mardanov AV, Beletsky AV, Karnachuk OV, Ravin NV. Genome of the candidate phylum Aminicenantes bacterium from a deep subsurface thermal aquifer revealed its fermentative saccharolytic lifestyle. Extremophiles. 2019;23:189–200. doi: 10.1007/s00792-018-01073-5. [DOI] [PubMed] [Google Scholar]
- 29.Begmatov S, Savvichev AS, Kadnikov VV, Beletsky AV, Rusanov II, Klyuvitkin AA, et al. Microbial communities involved in methane, sulfur, and nitrogen cycling in the sediments of the barents sea. Microorganisms. 2021;9:2362. doi: 10.3390/microorganisms9112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semler AC, Fortney JL, Fulweiler RW, Dekas AE. Cold seeps on the passive Northern U.S. Atlantic Margin host globally representative members of the seep microbiome with locally dominant strains of archaea. Appl Environ Microbiol. 2022;88:0046822. doi: 10.1128/aem.00468-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins SJ, Evans PN, Parks DH, Golding SD, Tyson GW. Genome-centric analysis of microbial populations enriched by hydraulic fracture fluid additives in a coal bed methane production well. Front Microbiol. 2016;7:731. doi: 10.3389/fmicb.2016.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin HT, Cowen JP, Olson EJ, Amend JP, Lilley MD. Inorganic chemistry, gas compositions and dissolved organic carbon in fluids from sedimented young basaltic crust on the Juan de Fuca Ridge flanks. Geochim Cosmochim Acta. 2012;85:213–27. doi: 10.1016/j.gca.2012.02.017. [DOI] [Google Scholar]
- 33.Fisher AT, Wheat CG, Becker K, Cowen J, Orcutt B, Hulme S, et al. Design, deployment, and status of borehole observatory systems used for single-hole and cross-hole experiments, IODP Expedition 327, eastern flank of Juan de Fuca Ridge. Proc Integr Ocean Drill Progr. 2011 doi: 10.2204/iodp.proc.327.107.2011. [DOI] [Google Scholar]
- 34.Fisher AT, Urabe T, Klaus A, and the Expedition 301 Scientists. Expedition 301 summary. Proc Integr Ocean Drill Progr. 2005 doi: 10.2204/iodp.proc.301.101.2005. [DOI] [Google Scholar]
- 35.Wheat CG, Hulme SM, Fisher AT, Orcutt BN, Becker K. Seawater recharge into oceanic crust: IODP Exp 327 Site U1363 Grizzly Bare outcrop. Geochem Geophys Geosyst. 2013;14:1957–72. doi: 10.1002/ggge.20131. [DOI] [Google Scholar]
- 36.Wheat CG, Jannasch HW, Kastner M, Hulme S, Cowen J, Edwards KJ, et al. Fluid sampling from oceanic borehole observatories: design and methods for CORK activities (1990-2010) Proc Integr Ocean Drill Progr. 2011 doi: 10.2204/iodp.proc.327.109.2011. [DOI] [Google Scholar]
- 37.Clum A, Huntemann M, Bushnell B, Foster B, Foster B, Roux S, et al. DOE JGI metagenome workflow. mSystems. 2021;6. 10.1128/mSystems.00804-20. [DOI] [PMC free article] [PubMed]
- 38.Stepanauskas R, Fergusson EA, Brown J, Poulton NJ, Tupper B, Labonté JM, et al. Improved genome recovery and integrated cell-size analyses of individual uncultured microbial cells and viral particles. Nat Commun. 2017;8:84. doi: 10.1038/s41467-017-00128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanehisa M, Sato Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020;29:28–35. doi: 10.1002/pro.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aramaki T, Blanc-Mathieu R, Endo H, Ohkubo K, Kanehisa M, Goto S, et al. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics. 2020;36:2251–2. doi: 10.1093/bioinformatics/btz859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham ED, Heidelberg JF, Tully BJ. Potential for primary productivity in a globally-distributed bacterial phototroph. ISME J. 2018;12:1861–6. doi: 10.1038/s41396-018-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin Y, Mao X, Yang J, Chen X, Mao F, Xu Y. dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012;40:W445–51. doi: 10.1093/nar/gks479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, et al. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–5. doi: 10.1093/nar/gkl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krogh A, Larsson B, Von Heijne G, Sonnhammer E. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 45.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roux S, Enault F, Hurwitz BL, Sullivan MB. VirSorter: mining viral signal from microbial genomic data. PeerJ. 2015;3:e985. doi: 10.7717/peerj.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bland C, Ramsey TL, Sabree F, Lowe M, Brown K, Kyrpides NC, et al. CRISPR Recognition Tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinform. 2007;8:209. doi: 10.1186/1471-2105-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padilha VA, Alkhnbashi OS, Shah SA, de Carvalho ACPLF, Backofen R. CRISPRcasIdentifier: machine learning for accurate identification and classification of CRISPR-Cas systems. Gigascience. 2020;9. 10.1093/gigascience/giaa062. [DOI] [PMC free article] [PubMed]
- 50.Campbell JW, Morgan-Kiss RM, Cronan JE. A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic b-oxidation pathway. Mol Microbiol. 2003;47:793–805. doi: 10.1046/j.1365-2958.2003.03341.x. [DOI] [PubMed] [Google Scholar]
- 51.Sharon I, Kertesz M, Hug LA, Pushkarev D, Blauwkamp TA, Castelle CJ, et al. Accurate, multi-kb reads resolve complex populations and detect rare microorganisms. Genome Res. 2015;25:534–43. doi: 10.1101/gr.183012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin HT, Amend JP, LaRowe DE, Bingham JP, Cowen JP. Dissolved amino acids in oceanic basaltic basement fluids. Geochim Cosmochim Acta. 2015;164:175–90. doi: 10.1016/j.gca.2015.04.044. [DOI] [Google Scholar]
- 53.Lomstein BA, Langerhuus AT, D’Hondt S, Jørgensen BB, Spivack AJ. Endospore abundance, microbial growth and necromass turnover in deep sub-seafloor sediment. Nature. 2012;484:101–4. doi: 10.1038/nature10905. [DOI] [PubMed] [Google Scholar]
- 54.Robador A, Jungbluth SP, LaRowe DE, Bowers RM, Rappé MS, Amend JP, et al. Activity and phylogenetic diversity of sulfate-reducing microorganisms in low-temperature subsurface fluids within the upper oceanic crust. Front Microbiol. 2015;5:748. doi: 10.3389/fmicb.2014.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boettger J, Lin HT, Cowen JP, Hentscher M, Amend JP. Energy yields from chemolithotrophic metabolisms in igneous basement of the Juan de Fuca ridge flank system. Chem Geol. 2013;337-338:11–19. doi: 10.1016/j.chemgeo.2012.10.053. [DOI] [Google Scholar]
- 56.Lang SQ, Butterfield DA, Lilley MD, Paul Johnson H, Hedges JI. Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim Cosmochim Acta. 2006;70:3830–42. doi: 10.1016/j.gca.2006.04.031. [DOI] [Google Scholar]
- 57.Haveman SA, Holmes DE, Ding YHR, Ward JE, DiDonato RJ, Lovley DR. c-type cytochromes in Pelobacter carbinolicus. Appl Environ Microbiol. 2006;72:6980–5. doi: 10.1128/AEM.01128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izumi H, Nunoura T, Miyazaki M, Mino S, Toki T, Takai K, et al. Thermotomaculum hydrothermale gen. nov., sp. nov., a novel heterotrophic thermophile within the phylum Acidobacteria from a deep-sea hydrothermal vent chimney in the Southern Okinawa Trough. Extremophiles. 2012;16:245–53. doi: 10.1007/s00792-011-0425-9. [DOI] [PubMed] [Google Scholar]
- 59.Fisher AT, Tsujii T, Petronotis K, Expedition 327 Scientists. Site U1362. Proc Integr Ocean Drill Progr. 2011 doi: 10.2204/iodp.proc.327.103.2011. [DOI] [Google Scholar]
- 60.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5:00889. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chowdhury NP, Mowafy AM, Demmer JK, Upadhyay V, Koelzer S, Jayamani E, et al. Studies on the mechanism of electron bifurcation catalyzed by electron transferring flavoprotein (Etf) and butyryl-CoA dehydrogenase (Bcd) of Acidaminococcus fermentans. J Biol Chem. 2014;289:5145–57. doi: 10.1074/jbc.M113.521013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poudel S, Dunham EC, Lindsay MR, Amenabar MJ, Fones EM, Colman DR, et al. Origin and evolution of flavin-based electron bifurcating enzymes. Front Microbiol. 2018;9:1762. doi: 10.3389/fmicb.2018.01762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charon M-H, Volbeda A, Chabriere E, Pieulle L, Fontecilla-Camps JC. Structure and electron transfer mechanism of pyruvate:ferredoxin oxidoreductase. Curr Opin Struct Biol. 1999;9:663–9. doi: 10.1016/S0959-440X(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 64.Kuhns M, Trifunović D, Huber H, Müller V. The Rnf complex is a Na+ coupled respiratory enzyme in a fermenting bacterium, Thermotoga maritima. Commun Biol. 2020;3:431. doi: 10.1038/s42003-020-01158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stojanowic A, Mander GJ, Duin EC, Hedderich R. Physiological role of the F420-non-reducing hydrogenase (MvH) from Methanothermobacter marburgensis. Arch Microbiol. 2003;180:194–203. doi: 10.1007/s00203-003-0577-9. [DOI] [PubMed] [Google Scholar]
- 66.Søndergaard D, Pedersen CNS, Greening C. HydDB: a web tool for hydrogenase classification and analysis. Sci Rep. 2016;6:34212. doi: 10.1038/srep34212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan Z, Wang M, Ferry JG. A ferredoxin- and F420H2-dependent, electron-bifurcating, heterodisulfide reductase with homologs in the domains Bacteria and Archaea. mBio. 2017;8. 10.1128/mBio.02285-16. [DOI] [PMC free article] [PubMed]
- 68.Lin H-T, Cowen JP, Olson EJ, Lilley MD, Jungbluth SP, Wilson ST, et al. Dissolved hydrogen and methane in the oceanic basaltic biosphere. Earth Planet Sci Lett. 2014;405:62–73. doi: 10.1016/j.epsl.2014.07.037. [DOI] [Google Scholar]
- 69.Gencic S, Grahame DA. Diverse energy-conserving pathways in Clostridium difficile: growth in the absence of amino acid stickland acceptors and the role of the Wood-Ljungdahl pathway. J Bacteriol. 2020;202. 10.1128/JB.00233-20. [DOI] [PMC free article] [PubMed]
- 70.Ragsdale SW, Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta. 2008;1784:1873–98. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wheat CG, Jannasch HW, Fisher AT, Becker K, Sharkey J, Hulme S. Subseafloor seawater-basalt-microbe reactions: continuous sampling of borehole fluids in a ridge flank environment. Geochem Geophys Geosyst. 2010;11. 10.1029/2010GC003057.
- 72.Buckel W, Thauer RK. Flavin-based electron bifurcation, a new mechanism of biological energy coupling. Chem Rev. 2018;118:3862–86. doi: 10.1021/acs.chemrev.7b00707. [DOI] [PubMed] [Google Scholar]
- 73.Mulkidjanian AY, Galperin MY, Makarova KS, Wolf YI, Koonin EV. Evolutionary primacy of sodium bioenergetics. Biol Direct. 2008;3:13. doi: 10.1186/1745-6150-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jungbluth SP, Glavina del Rio T, Tringe SG, Stepanauskas R, Rappé MS. Genomic comparisons of a bacterial lineage that inhabits both marine and terrestrial deep subsurface systems. PeerJ. 2017;5:e3134. doi: 10.7717/peerj.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fincker M, Huber JA, Orphan VJ, Rappé MS, Teske A, Spormann AM. Metabolic strategies of marine subseafloor Chloroflexi inferred from genome reconstructions. Environ Microbiol. 2020;22:3188–204. doi: 10.1111/1462-2920.15061. [DOI] [PubMed] [Google Scholar]
- 76.Bach W, Edwards KJ. Iron and sulfide oxidation within the basaltic ocean crust: implications for chemolithoautotrophic microbial biomass production. Geochim Cosmochim Acta. 2003;67:3871–87. doi: 10.1016/S0016-7037(03)00304-1. [DOI] [Google Scholar]
- 77.Kauffman KM, Chang WK, Brown JM, Hussain FA, Yang J, Polz MF, et al. Resolving the structure of phage–bacteria interactions in the context of natural diversity. Nat Commun. 2022;13:372. doi: 10.1038/s41467-021-27583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anderson RE, Brazelton WJ, Baross JA. Using CRISPRs as ametagenomic tool to identify microbial hosts of a diffuse flow hydrothermal vent viral assemblage. FEMS Microbiol Ecol. 2011;77:120–33. doi: 10.1111/j.1574-6941.2011.01090.x. [DOI] [PubMed] [Google Scholar]
- 79.Howard-Varona C, Hargreaves KR, Abedon ST, Sullivan MB. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J. 2017;11:1511–20. doi: 10.1038/ismej.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bondy-Denomy J, Qian J, Westra ER, Buckling A, Guttman DS, Davidson AR, et al. Prophages mediate defense against phage infection through diverse mechanisms. ISME J. 2016;10:2854–66. doi: 10.1038/ismej.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deutschmann J, Gramberg T. SAMHD1… And viral ways around it. Viruses. 2021;13:395. doi: 10.3390/v13030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gronow S, Brade H. Lipopolysaccharide biosynthesis: which steps do bacteria need to survive. J Endotoxin Res. 2001;7:3–23. doi: 10.1179/096805101101532468. [DOI] [PubMed] [Google Scholar]
- 83.Weinberger AD, Wolf YI, Lobkovsky AE, Gilmore MS, Koonin EV. Viral diversity threshold for adaptive immunity in prokaryotes. mBio. 2012;3:00456–12. doi: 10.1128/mBio.00456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGinn J, Marraffini LA. Molecular mechanisms of CRISPR–Cas spacer acquisition. Nat Rev Microbiol. 2019;17:7–12. doi: 10.1038/s41579-018-0071-7. [DOI] [PubMed] [Google Scholar]
- 85.Pachiadaki MG, Brown JM, Brown J, Bezuidt O, Berube PM, Biller SJ, et al. Charting the complexity of the marine microbiome through single-cell genomics. Cell. 2019;179:1623–35.e11. doi: 10.1016/j.cell.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makarova KS, Haft DH, Barrangou R, Brouns SJJ, Charpentier E, Horvath P, et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–77. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hale CR, Cocozaki A, Li H, Terns RM, Terns MP. Target RNA capture and cleavage by the Cmr type III-B CRISPR–Cas effector complex. Genes Dev. 2014;28:2432–43. doi: 10.1101/gad.250712.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han W, Stella S, Zhang Y, Guo T, Sulek K, Peng-Lundgren L, et al. A Type III-B Cmr effector complex catalyzes the synthesis of cyclic oligoadenylate second messengers by cooperative substrate binding. Nucleic Acids Res. 2018;46:10319–30. doi: 10.1093/nar/gky844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Staals RHJ, Agari Y, Maki-Yonekura S, Zhu Y, Taylor DW, van Duijn E, et al. Structure and activity of the RNA-targeting Type III-B CRISPR-Cas complex of Thermus thermophilus. Mol Cell. 2013;52:135–45. doi: 10.1016/j.molcel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Mo CY, Wasserman MR, Rostøl JT, Marraffini LA, Liu S. Dynamics of Cas10 govern discrimination between self and non-self in Type III CRISPR-Cas immunity. Mol Cell. 2019;73:278–90.e4. doi: 10.1016/j.molcel.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koonin EV, Wolf YI. Evolution of the CRISPR-Cas adaptive immunity systems in prokaryotes: models and observations on virus–host coevolution. Mol Biosyst. 2015;11:20–7. doi: 10.1039/C4MB00438H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O’Brian MR, et al. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev. 2017;81. 10.1128/mmbr.00048-16. [DOI] [PMC free article] [PubMed]
- 93.Murphy MJ, Siegel LM, Tove SR, Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci USA. 1974;71:612–6. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xavier JC, Gerhards RE, Wimmer JLE, Brueckner J, Tria FDK, Martin WF. The metabolic network of the last bacterial common ancestor. Commun Biol. 2021;4:413. doi: 10.1038/s42003-021-01918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giovannoni SJ, Thrash JC, Temperton B. Implications of streamlining theory for microbial ecology. ISME J. 2014;8:1553–65. doi: 10.1038/ismej.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hubert C, Loy A, Nickel M, Arnosti C, Baranyi C, Brüchert V, et al. A constant flux of diverse thermophilic bacteria into the cold Arctic seabed. Science. 2009;325:1541–4. doi: 10.1126/science.1174012. [DOI] [PubMed] [Google Scholar]
- 97.Müller AL, de Rezende JR, Hubert CRJ, Kjeldsen KU, Lagkouvardos I, Berry D, et al. Endospores of thermophilic bacteria as tracers of microbial dispersal by ocean currents. ISME J. 2014;8:1153–65. doi: 10.1038/ismej.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walsh EA, Kirkpatrick JB, Rutherford SD, Smith DC, Sogin M, D’Hondt S. Bacterial diversity and community composition from seasurface to subseafloor. ISME J. 2016;10:979–89. doi: 10.1038/ismej.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jørgensen SL, Zhao R. Microbial inventory of deeply buried oceanic crust from a young ridge flank. Front Microbiol. 2016;7:820. doi: 10.3389/fmicb.2016.00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Labonté JM, Lever MA, Edwards KJ, Orcutt BN. Influence of igneous basement on deep sediment microbial diversity on the Eastern Juan de Fuca Ridge flank. Front Microbiol. 2017;8:1434. doi: 10.3389/fmicb.2017.01434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods and figures associated with investigating Juan de Fuca Ridge Flank Aminicenantia
Data Availability Statement
All SAG data for this project can be found on NCBI under BioProject ID PRJNA842252 under accession numbers: JAMZRZ000000000 (JDF1 composite genome); JAMZSA000000000 (AH-873-B07); JAMZSB000000000 (AC-708-M15); JAMZSC000000000 (AC-708-I09); JAMZSD000000000 (AC-335-O07); JAMZSE000000000 (AC-335-L06); JAMZSF000000000 (AC-335-K20); JAMZSG000000000 (AC-335-G13); JAMZSH000000000 (AC-335-B20); JAMZSI000000000 (AC-335-A11); JAMZSJ000000000 (AC-334-K16); JAMZSK000000000 (AC-334-E05). All MAG data for this project can be found on IMG.