Abstract
The prevalence of fibrotic diseases and the lack of pharmacologic modalities to effectively treat them impart particular importance to the discovery of novel anti-fibrotic therapies. The repurposing of drugs with existing mechanisms of action and/or clinical data is a promising approach for treatment of fibrotic diseases. One paradigm that pervades all fibrotic disease is the pathological myofibroblast, a collagen-secreting, contractile mesenchymal cell that is responsible for deposition of fibrotic tissue. Here we use a gene expression paradigm characteristic of activated myofibroblasts in combination with the Connectivity Map in order to select compounds that are predicted to reverse the pathological gene expression signature associated with the myofibroblast and, thus, contain potential for use as anti-fibrotic compounds. We tested a small list of these compounds in a first-pass screen, applying them to human fibroblasts, and identified the retinoic acid receptor agonist Ch55 as potential hit. Further investigation exhibited and elucidated anti-fibrotic effects of Ch55 in vitro, as well as demonstrated anti-scarring activity upon intradermal application in a pre-clinical rabbit ear hypertrophic scar model. We hope that similar predictions to uncover anti-scarring compounds may yield further pre-clinical and, ultimately, clinical success.
Keywords: Ch55, scar, fibrosis, connectivity map, drug repurposing
INTRODUCTION
Fibrosis denotes the process by which damaged tissue seeks to heal via deposition of a scar. In the skin, fibrotic dermal manifestations such as hypertrophic scars and keloids are frequent results of injury resulting from burns, avulsions, and surgical incisions. Skin fibrosis leads to cosmetic disfigurement and potential further complications such as pain, pruritis, and contracture (Lee and Jang, 2018). A variety of injuries and insults can lead to the formation of fibrosis not only in skin but in numerous other organs as well, resulting in nearly half of reported deaths in the industrialized world (Wynn, 2008). Therefore, therapeutic modalities that seek to prevent or treat fibrosis are critically needed. Unfortunately, the complexity and redundancy inherent in the fibrotic response has complicated the successful development of anti-fibrotic therapeutics (Walraven and Hinz, 2018). Therefore, new targets and molecules with the potential to modulate processes critical to the development of fibrosis are of key interest for translational research. Recently, a single-cell RNA-seq dataset was published analyzing fibroblasts from multiple homeostatic and diseased tissues in human and mouse (Buechler et al., 2021). The authors identified gene expression signatures conserved across, and unique to, different populations of these fibroblasts. Here we utilized a subset of this transcriptomic data and subjected it to analysis by the Connectivity Map (CMap), a tool that screens existing high-throughput transcriptomic data to predict compounds that may mimic or reverse a given gene expression profile (Subramanian et al., 2017), in order to identify compounds to screen for anti-fibrotic potential in fibroblasts in vitro. We then follow the screen with further in vitro validation and mechanistic data exploring the most promising target, followed by further demonstration of its anti-fibrotic potential in a preclinical model of hypertrophic scar in rabbit ear.
RESULTS
Myofibroblast gene expression profile predicts potential anti-fibrotic compounds
The activity of myofibroblasts is a critical causative factor underlying all fibrotic pathological states. Activation of myofibroblasts is characterized by dysregulation of common and unique sets of genes in fibroblasts or in other cells that can differentiate into myofibroblasts, depending on the tissue in question (Hinz et al., 2007). We hypothesized that a set of genes commonly upregulated across myofibroblasts irrespective of origin might be a useful signature to predict compounds that antagonize myofibroblast activation and, therefore, suppress fibrosis. Recently, Buechler et al. (Buechler et al., 2021) used single-cell RNA-seq to characterize a conserved myofibroblast population found across several pathological states in varied tissues and organs in humans (LRRC15+) and in mice (Lrrc15+). In order to maximize generalizability of predictions made using this signature, we extracted the intersection of the overexpressed gene sets in both human and mouse, yielding a combined signature of 117 genes (Fig. 1A, genes and their encoded proteins listed in Table S1). Subjecting this gene signature to CMap analysis (accessed at https://clue.io, (Subramanian et al., 2017)) yielded a list of compounds predicted to reverse this gene expression signature. Several high-ranking compounds in this list included known TGF-βR inhibitors (SB-431542, LY-2157299, LY-364947, and D-4476), the DPP4 inhibitor sitagliptin, and the clinically approved anti-fibrotic tyrosine kinase inhibitor nintedanib, among many other compounds with myriad known targets and/or mechanisms of action. The well-characterized roles in fibrosis of the targets of several of these drugs, such as TGF-βRs, lent greater confidence to the ability of this screen to reveal other compounds with anti-fibrotic activity as well. We chose five high-ranking compounds from the list without immediately obvious connections to tissue fibrosis and decided to further investigate their potential as antifibrotic agents (Fig. 1A). Chemical structures of these compounds and their corresponding CAS identifiers are presented in Fig. S1.
Figure 1. Pilot screen for anti-fibrotic effects in primary fibroblasts.
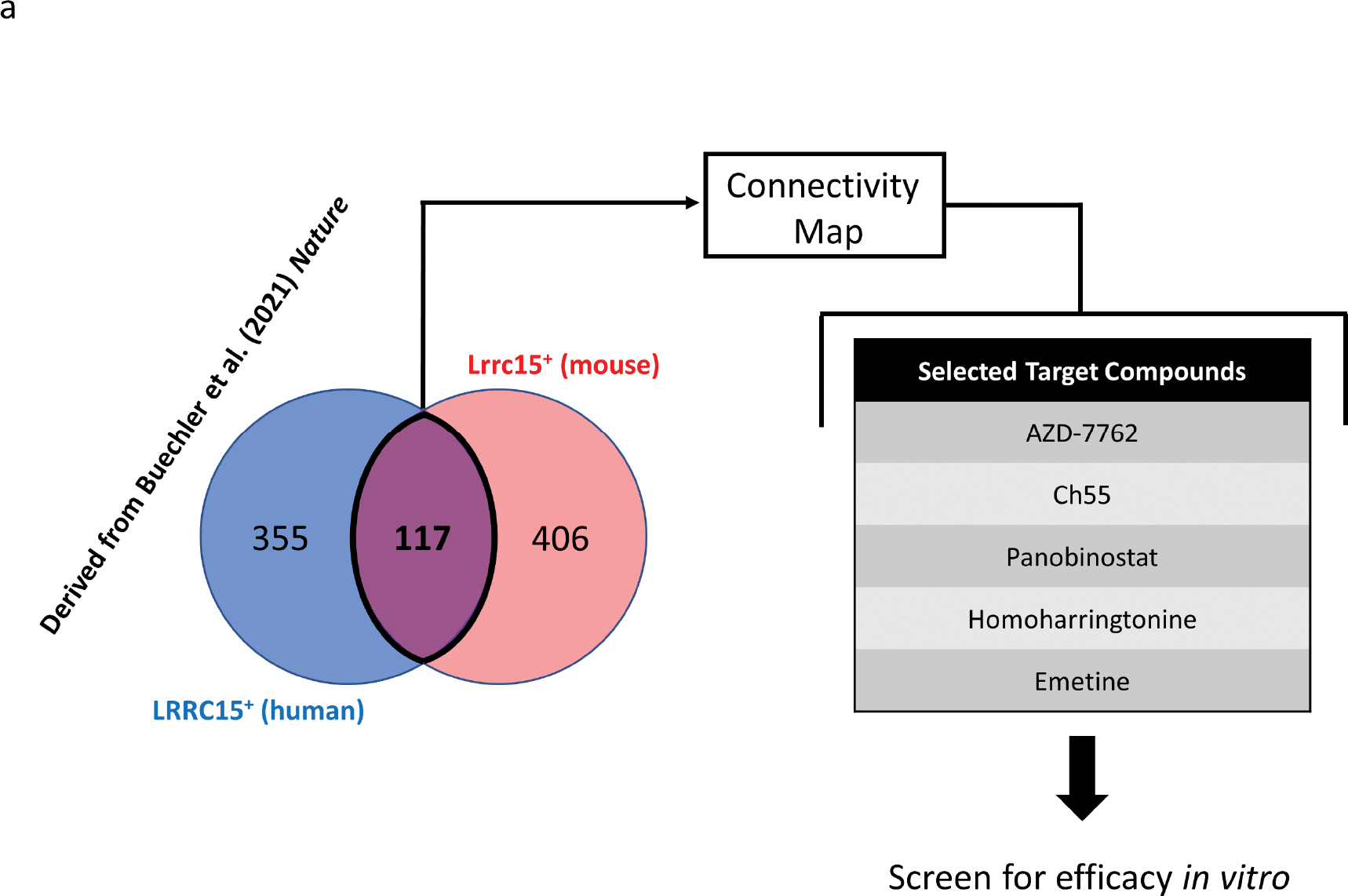
(A) The gene expression signature of interest was extracted by taking the intersection of human LRRC15+ fibroblast-enriched marker genes (472 genes) and mouse Lrrc15+ fibroblast-enriched marker genes (523 genes), which yielded a common signature of 117 genes. This signature was used to query the CMap in order to predict small molecules to reverse the transcriptional paradigm. Five target compounds were then manually selected for screening in fibroblasts in vitro.
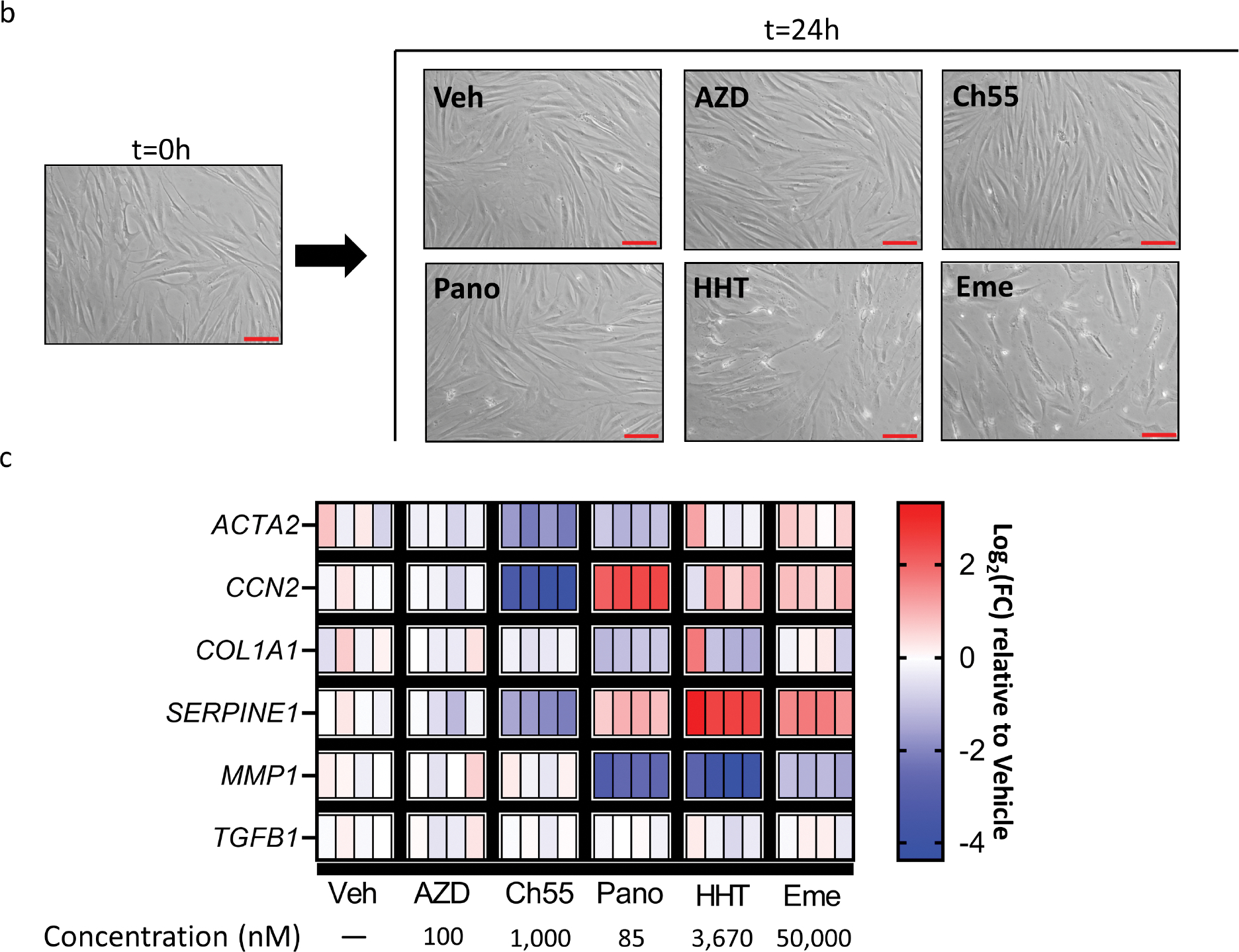
(B,C) Primary human foreskin fibroblasts were grown in culture and exposed to vehicle control or indicated drugs at a single concentration for 24 hours. (B) Representative brightfield microscopy before (left) and after (right) treatment. Scale bar=100μm. (C) Heatmap representing log2fold change values of each replicate relative to mean expression of vehicle control replicates for indicated genes. GAPDH was used as an internal control. Veh=vehicle. AZD=AZD-7762. Ch55=Ch55. Pano=panobinostat. HHT=homoharringtonine. Eme=emetine. n=4 replicates per condition.
In vitro screen of predicted compounds indicates Ch55 as a potential molecule of interest
In order to initially test the identified drugs, we applied each drug or DMSO vehicle control at a single concentration to primary human foreskin fibroblasts in vitro for 24 hours, before harvesting and measuring relative expression of selected myofibroblast marker genes by qRT-PCR. The checkpoint kinase inhibitor AZD-7762 (Mitchell et al., 2010), the retinoic acid receptor agonist Ch55 (Li Xiang et al., 2017, Ye et al., 2016), the histone deacetylase inhibitor panobinostat (Korfei et al., 2018), the protein synthesis inhibitor homoharringtonine (Li Xiaolei et al., 2017, Sun et al., 2021), and the protein synthesis inhibitor emetine (Yang et al., 2018) were applied based on concentrations previously demonstrated to have effects on cultured cells in vitro. Culture of human foreskin fibroblasts in the presence of emetine or homoharringtonine resulted in notable detachment of cells from the tissue culture plate by the time of harvest, while none of the other drugs yielded obvious cellular detachment at the tested concentrations (Fig. 1B). Quantification of relative expression of some common myofibroblast and fibrosis-associated genes (Pakshir et al., 2020, Samarakoon et al., 2013) (Fig. 1C) revealed that emetine and homoharringtonine, in addition to their detachment effects, also induced myofibroblast activation, as assessed by increased expression of CCN2 (the gene encoding connective tissue growth factor), increased expression of SERPINE1 (the gene encoding plasminogen activator inhibitor 1), and decreased expression of MMP1 (the gene encoding matrix metalloproteinase 1), a signature also shared by panobinostat. While AZD-7762 did not show a similar effect of fibroblast activation, neither did it appear to downregulate myofibroblast-associated genes. In contrast, application of Ch55 resulted in clear and consistent downregulation of ACTA2 (the gene encoding smooth muscle α-actin), CCN2, and SERPINE1 across replicates, without downregulating MMP1, compared to expression in vehicle-treated fibroblasts. None of the drugs tested appeared to affect expression of TGFB1 (the gene encoding transforming growth factor-beta 1) at the examined concentrations. Taken together, these data suggested that Ch55 was a putative anti-fibrotic molecule worthy of further investigation.
Ch55-mediated antagonism of fibroblast activation indicates a concentration-dependent response and is not donor-specific
We next sought to understand the concentration range over which Ch55 was active in fibroblasts. We treated primary human foreskin fibroblasts for 24 hours with DMSO vehicle control or log10 diluted concentrations of Ch55 from 10,000nM to 0.1nM. While no obvious cellular detachment was observed in fibroblasts treated with 0–1,000nM Ch55, consistent with our initial experiment presented in Fig. 1B, treatment with 10,000nM Ch55 resulted in near complete detachment of fibroblasts from the tissue culture plate (Fig. S2A). Analysis of expression of several myofibroblast markers and pro-fibrotic genes by qRT-PCR demonstrated concentration dependence of the antagonistic effects of Ch55. This effect was maximal at 1,000nM among tested concentrations, but significant decreases in expression of ACTA2 and TAGLN (the gene encoding transgelin) were also demonstrated at 100nM, CCN2 and CNN1 (the gene encoding calponin 1) as low as 10nM, and SERPINE1 as low as 1nM, while the decrease in expression of IL6 was only statistically significant at 1,000nM (Fig. S2B). RNA was not harvested from cells treated with 10,000nM Ch55 due to near-complete cell detachment after 24 hours of treatment. Treatment of primary human foreskin fibroblasts isolated from another tissue donor yielded comparable effects (Fig. S2C,D), as did treatment of primary rabbit dermal fibroblasts (Fig. S3A–D), suggesting that these effects were not due to a donor-specific idiosyncrasy.
Ch55 maintains potential to antagonize fibroblast activation in the presence of TGF-β1
Since myofibroblast activation occurs in the context of active TGF-β signaling, we next determined whether Ch55 maintained its effectiveness to antagonize fibroblast activation and collagen deposition in fibroblasts treated with TGF-β1. Primary human foreskin fibroblasts were cultured in the presence of vehicle control, TGF-β1, Ch55, or TGF-β1 and Ch55. At 24 hour harvest, analysis by qRT-PCR demonstrated that treatment with Ch55 significantly downregulated both basal and TGF-β1-induced expression of ACTA2, CCN2, and SERPINE1 (Fig. 2A). Culture for 48 hours demonstrated that Ch55 appeared to antagonize fibroblast activation, with decreased spread area per cell, as assessed by brightfield microscopy (Fig. 2B). Western blot analysis of lysates prepared from these samples at harvest confirmed that Ch55 dramatically antagonized fibroblast activation and collagen deposition as assessed by expression of α-SMA and type I collagen (Fig. 2C). Immunofluorescent staining and analysis revealed that Ch55 treatment resulted in loss of α-SMA protein from filamentous actin stress fibers (Fig. 2D), as well as greatly diminished deposition of collagen I (Fig. 2E), in both the presence and absence of exogenous TGF-β1.
Figure 2. Effects of Ch55 antagonistic to TGF-β1 stimulation in human fibroblasts.
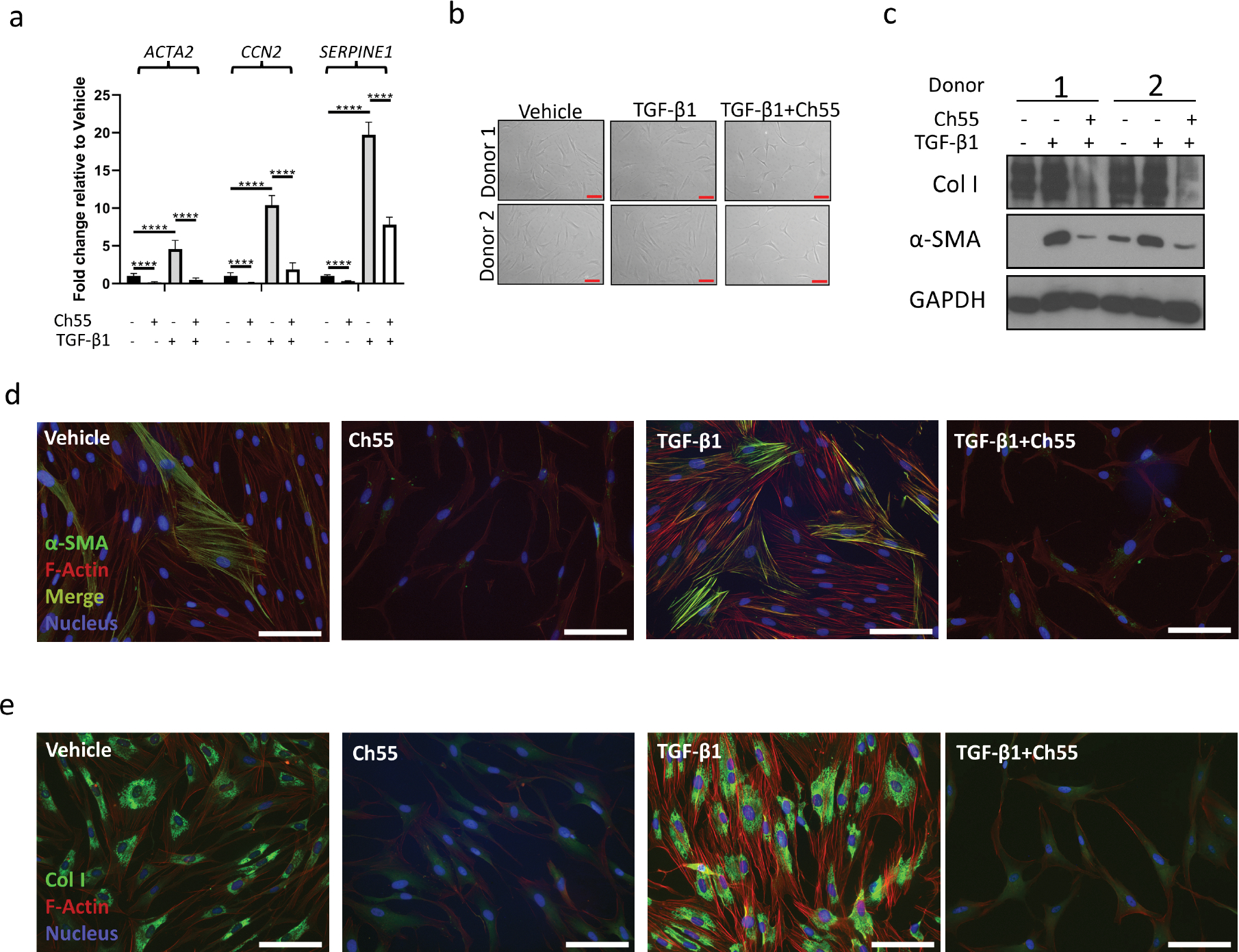
Primary human foreskin fibroblasts were grown in culture and exposed to vehicle control (Vehicle), vehicle+10ng/mL rhTGF-β1 (TGF-β1), 1,000nM Ch55 (Ch55), or 10ng/mL rhTGF-β1+1,000nM Ch55 (TGF-β1+Ch55) in vitro. (A) Transcript quantification of ACTA2, CCN2, and SERPINE1 by qRT-PCR in cells harvested after 24 hours of treatment, expressed relative to vehicle-treated cells. GAPDH was used as an internal control. n=6 replicates per group. Statistical analysis was performed by one-way ANOVA followed by Tukey’s post-hoc test, with selected statistical comparisons visualized in the figure. (B) Representative brightfield microscopy of fibroblasts from two donors immediately before harvest at 48 hours. Scale bar=100μm. (C) Western blot analysis of samples in (B), analyzing expression of α-SMA and type I collagen. GAPDH was used as an internal control. (D,E) Representative immunostaining of (D) α-SMA or (E) Collagen I in cells treated and fixed at 48 hour harvest. F-actin was counterstained with Alexafluor-568-conjugated phalloidin, and nuclei were counterstained with DAPI. Scale bar=100μm.
Ch55 reduces collagen deposition in fibroblasts sourced from hypertrophic scar and keloid
Since overproduction of collagen is characteristic of fibroblasts in tissue fibrosis, including hypertrophic scar and keloid, we wished to determine whether Ch55 also affects deposition of collagen I by these fibroblasts. Analysis of collagen I expression by Western blot (Fig. S4A) and immunofluorescence (Fig. S4B) demonstrated dramatic reduction of collagen I deposition by Ch55 in HTS and keloid fibroblasts, both in the presence and absence of exogenous TGF-β1. This suggests that Ch55 is also effective at reducing collagen deposition in pathological scar fibroblasts.
RAR agonist all-trans retinoic acid antagonizes myofibroblast activation
We then wished to see whether another RAR agonist could antagonize myofibroblast activation. Treatment of primary human foreskin fibroblasts with 10,000 nM all-trans retinoic acid (ATRA) substantially decreased α-SMA and collagen I protein, as well as F-actin stress fiber formation, as determined by Western blot (Fig. S5A) and immunofluorescence (Fig. S5B,C). Treatment with ATRA also dramatically decreased expression of collagen I in primary human fibroblasts isolated from HTS and keloid (data not shown). This suggests that the effects of Ch55 antagonistic to fibroblast activation and collagen deposition are not unique to Ch55 among RAR agonists.
Ch55-dysregulated transcriptional paradigms antagonistic to TGF-β1 stimulation are enriched for pathways relevant to tissue fibrosis
In order to more completely understand the effects of Ch55 stimulation, we performed bulk sequencing on RNA isolated from primary human foreskin fibroblasts treated for 24 hours with vehicle, 10ng/mL TGF-β1, 1,000nM Ch55, or both 10ng/mL TGF-β1 and 1,000nM Ch55 (Analysis output tables in Supplementary Data 1). Myofibroblast marker genes examined initially by qRT-PCR in Figure S2 were confirmed to be significantly downregulated by Ch55 (Fig. S6), and many of the LRRC15+/Lrrc15+ signature-enriched genes (Table S1) initially used to query the CMap database were also found to be significantly downregulated by Ch55 (67/117, ~57% of genes, Table S2). Additionally, many genes known to be targets or regulators of retinoic acid signaling exhibited broad upregulation by Ch55 (Fig. S7). Visualization of transcriptional profiles by two-dimensional principal component analysis (PCA, Fig. 3A) revealed clear separation among all treatment groups. Interestingly, relative placement of treatment groups on the PCA suggested that substantial groups of genes were differentially regulated both discordantly and concordantly by Ch55 and TGF-β1. We extracted the set of genes differentially expressed by Ch55 and regulated in the same direction by TGF-β1 (TGF-β-mimicking signature, Fig. 3B). We also extracted the set of genes that was differentially expressed by Ch55, and the expression of which was regulated in the opposite direction by TGF-β1 (TGF-β-antagonizing signature, Fig. 3C). Signatures and their associated relative expression values are presented in Supplementary Data 2. We then used goseq (Young et al., 2010) to query the KEGG database with the TGF-β-mimicking signature and the TGF-β-antagonizing signature. Extraction of KEGG annotations and associated pathways enriched in the TGF-β-mimicking signature (Fig. 3D) tended to refer to either general categories (e.g. “proteasome,” “cell cycle,” “phagosome”) or categories relevant to other specific cell types (e.g. “Epithelial cell signaling in H. pylori infection,” “Collecting duct acid secretion,” “Axon guidance”). In contrast, KEGG annotations for the TGF-β-antagonizing signature revealed several specific categories with clear relevance to fibroblast activation and fibrosis (Fig. 3E). We next computed normalized gene expression values for the DEGs extracted from the TGF-β-antagonizing signature and overlaid Ch55-induced relative expression effects on potentially fibrosis-relevant pathways of interest using Pathview Web (Luo et al., 2017). This analysis demonstrated broad reversal of TGF-β-mediated effects and pathway dysregulation for the KEGG terms “Focal adhesion” (Fig. S8), “TGF-β signaling pathway” (Fig. S9), “ECM-Receptor interaction” (Fig. S10), and “Regulation of actin cytoskeleton” (Fig. S11). Taken together, these data suggest that Ch55 may be able to antagonize fibrosis by dysregulating cellular processes associated with fibroblast activation and cytoskeletal-ECM interactions.
Figure 3. RNA-seq analysis of Ch55 effects in primary human foreskin fibroblasts.
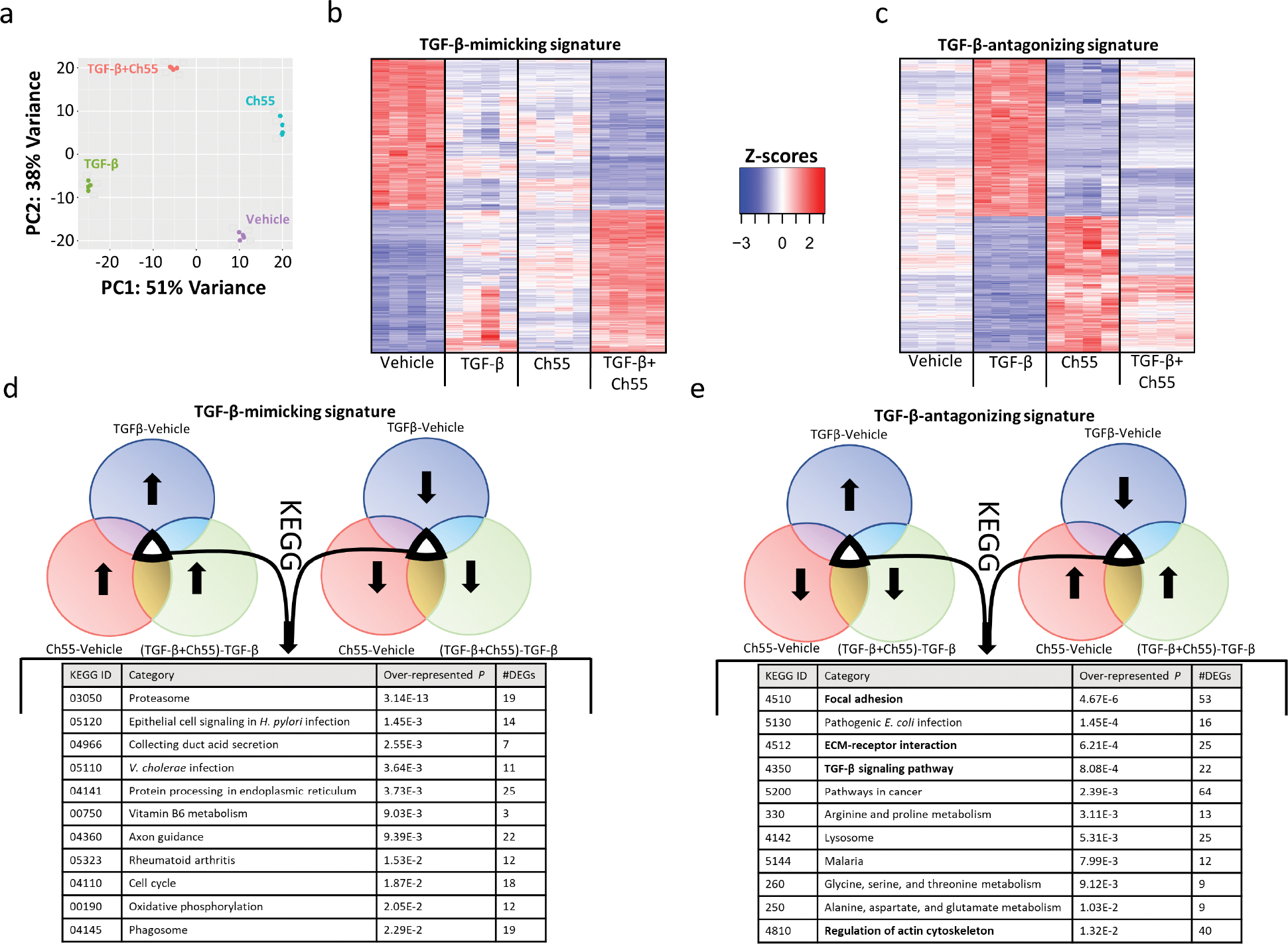
Primary human foreskin fibroblasts were grown in culture and exposed to vehicle control (Vehicle), vehicle+10ng/mL rhTGF-β1 (TGF-β1), 1,000nM Ch55 (Ch55), or 10ng/mL rhTGF-β1+1,000nM Ch55 (TGF-β1+Ch55) in vitro. RNA was harvested and expression profiling performed by RNA-seq. (A) PCA representation of variations in transcriptional profiles between and among treatment groups. (B,C) Heatmaps depicting normalized gene expression, represented as Z-scores, of Ch55-induced signatures (B) mimicking and (C) antagonizing TGF-β-induced effects. (D,E) Results of KEGG database query of signatures depicted in (B) and (C), respectively.
Ch55 ameliorates hypertrophic scar formation in vivo
In order to determine whether the in vitro activity of Ch55 to antagonize fibroblast activation and fibrosis-associated pathways was accompanied by anti-fibrotic potential in vivo, we utilized a well-characterized model of excisional wound-induced hypertrophic scarring in the ears of New Zealand White rabbits. Once excisional wounds closed, we performed three sequential intradermal injections of either a high dose (10μg) or low dose (2μg) of Ch55, or their corresponding vehicles, into the developing scars and harvested scar tissues on post-operative day 28 (POD28, Fig. 4A). Spectroscopic measurements performed immediately prior to harvest and normalized to measurements of unwounded skin on the same ear revealed that administration of high dose Ch55 significantly decreased erythema in developed scars, while the decrease resulting from low dose Ch55 was not statistically significant (Fig. S12). Harvested scars were processed and analyzed to determine scar elevation index (SEI), a common quantitative histological measurement used to assess scar hypertrophy (Fig. S13). Administration of either high or low dose Ch55 was sufficient to significantly reduce resultant hypertrophy as assessed by SEI, relative to vehicle controls (Fig. 4B,C). Visualization of representative harvested tissues stained with modified Masson’s Trichrome (Fig. S14A) and picrosirius red/fast green (Fig. S14B) suggested a possible decrease in collagen density of the scars of Ch55-treated wounds. Subsequently, detection by Western blot and quantification by densitometry confirmed that Ch55 administration at both high and low doses was sufficient to reduce the amount of type I collagen in the dermis at time of harvest (Fig. 4D,E). Taken together, these data demonstrated that dermal administration of Ch55 to developing scars was sufficient to limit hypertrophy and type I collagen deposition.
Figure 4. Anti-fibrotic effects of Ch55 in a rabbit ear hypertrophic scar model in vivo.
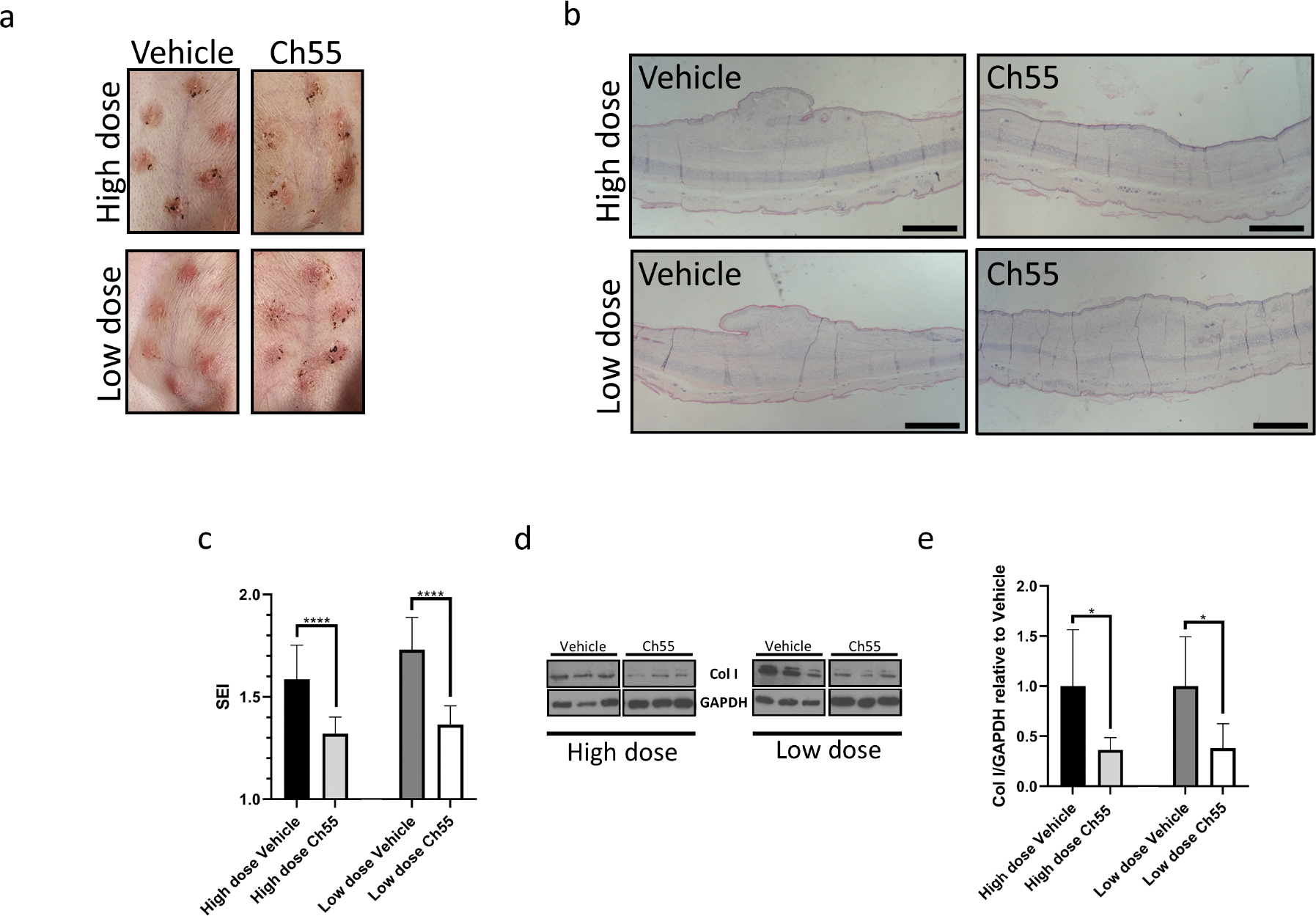
Rabbit ear excisional wounds were performed and treated with either Ch55 (high or low dose, 10μg/wound or 2μg/wound, respectively) or corresponding vehicle control via intradermal injections to closed wounds over the course of scar development, before terminating experiments on POD28. (A) Representative photographs of rabbit ear scars at time of harvest. (B) Visualization of representative hypertrophic scar cross-sections by H&E staining. Scale bar=1mm. (C) Quantification of scar elevation index (SEI) for scar tissues at harvest. n=11–12 samples/condition. (D) Representative Western blot detecting expression of type I collagen in protein isolated from scar dermis at harvest for high dose and low dose Ch55 treatment groups and their respective controls. GAPDH was used as a loading control. (E) Densitometric quantification of type I collagen relative to GAPDH, as detected by Western blot. Density values for each sample are normalized to the mean of the respective vehicle controls for that dose. n=5–6 samples per group.
DISCUSSION
As transcriptomic approaches become utilized more frequently in experimental research, particularly at the single-cell level, our understanding of gene expression signatures and cell types contributing to tissue pathology becomes more nuanced and sophisticated. Additionally, accessible repositories of raw and curated large-scale data and user friendly tools for analysis now exist. This enables a wide variety of researchers of varying backgrounds and skillsets to process, analyze, and build predictions from these data and construct testable experimental hypotheses (Brown et al., 2018). Here we extracted a conserved, cross-tissue gene expression profile characteristic of pathological myofibroblasts described in human and mouse (Buechler et al., 2021) and used CMap in order to predict small molecules that might reverse this gene expression profile, in order to identify potential anti-fibrotic compounds. Advantages to this method include repurposing of compounds with established mechanisms of action (MOA), therapeutic indices, pharmacokinetic and safety profiles, and, in some cases, clinical data. This may potentially bypass otherwise key concerns regarding drug safety, due to potential toxicity, or triviality, due to overlap with other compounds that share an MOA.
It is crucial to note that the absence of an anti-fibrotic effect of particular compounds in our initial screen does not necessitate that these compounds lack anti-fibrotic activity, since the initial screen was performed manually under a single, highly simplified condition (24 hour in vitro stimulation of one concentration of each molecule performed in primary fibroblasts from one donor). Therefore, this data should not be interpreted broadly as a lack of potential anti-fibrotic efficacy of these compounds. Future work will also seek to perform similar predictive analyses on other pathological transcriptional paradigms from other data sources and disease models in order to expand the list of potentially efficacious compounds. In addition, we will aim to select compounds predicted to ameliorate multiple pathological gene expression paradigms, reducing the rate of false positive compounds to enter the initial screening stage.
Classical application of retinoic acid receptor (RAR) agonists for dermatologic indications such as acne vulgaris, psoriasis, and icthyosis generally revolve around their activity in the epidermis (Cosio et al., 2021, Kassir et al., 2020, Zouboulis, 2001). However, there is potential for pharmacologic use of retinoids for dermal pathologies as well. Previous reports have demonstrated anti-scarring effects of retinoid compounds applied topically to hypertrophic and keloid scars (Daly and Weston, 1986, Janssen de Limpens, 1980) and to scleroderma skin (Mizutani et al., 1999). Interestingly, it has been described that the epidermis in keloid scars demonstrates aberrant retinoic acid metabolism, dampening RAR transactivation and leading to a secretory profile that drives pro-fibrotic phenotypes in the dermis (Jumper et al., 2016). This finding suggests that application of retinoid compounds topically to fibrotic skin might have a dually beneficial effect not only through their direct effects on fibroblasts, provided that they are able to penetrate into the dermis, but also through regulating epidermal-dermal crosstalk via stimulation of epidermal RAR signaling. This is consistent with key roles of aberrant keratinocyte-fibroblast interactions recognized in the pathophysiology of hypertrophic scar, keloid, and scleroderma (Amiri et al., 2022, Dolivo et al., 2023, Russo et al., 2020, 2022). A recent report demonstrated that application of a pan-RAR agonist or pan-RAR antagonist to healing mouse excisional wounds potentiated or limited skin regeneration, respectively, as assessed by the degree of hair follicle neogenesis (Abbasi et al., 2020), demonstrating roles for both endogenous and pharmacologic stimulation of RAR signaling in regenerative wound healing. In addition to topical application, several studies have demonstrated beneficial effects of oral administration of retinoids for treatment of skin fibrosis as well. Case reports and small clinical studies have noted improvement in the skin of scleroderma patients provided oral isotretinoin (Bahmer and Zaun, 1985, Maurice et al., 1989) and oral etretinate (Ikeda et al., 2004, Shima et al., 2014). Consistent with this, oral retinoid administration also diminished skin fibrosis in tight-skin (Delany and Brinckerhoff, 1993) and bleomycin-induced (Ikeda et al., 2005) mouse models of scleroderma. These findings are also consistent with known roles of pharmacologic RAR activity in fibrotic disease states of some internal organs, as well (Zhou et al., 2013). In this report we demonstrated anti-fibrotic effects of Ch55 injected directly into the dermis in a rabbit ear hypertrophic scar model in vivo, as assessed by reductions in hypertrophy and collagen deposition (Figure 4). Of note, among RAR agonists, Ch55 is notable due to its comparably high binding affinity to all three RARs, as well as its lack of binding to cellular retinoic acid binding protein (CRABP) (Jetten et al., 1987, Sun et al., 1997). Nevertheless, our findings that Ch55 antagonizes fibroblast activation, though novel for this compound specifically as far as we are aware, are consistent with the above reports detailing pre-clinical and clinical effects of retinoid application to treat dermal fibrosis, as well as additional reports investigating effects of other retinoid compounds on dermal fibroblasts in vitro (Abergel et al., 1985, Kim and Stern, 1990, Ohta and Uitto, 1987, Xiao et al., 2008, Xiao et al., 2011).
Previous studies of retinoid effects on fibroblasts in vitro mostly have focused on their ability to decrease expression of collagen. While we have validated those findings (Fig. 2, S4, S5), our RNA-seq data also enables us to gain a fuller picture of the effects of Ch55 on fibroblasts. Here we demonstrated that Ch55 treatment regulates a large set of genes in the opposite direction of TGF-β1 (Fig. 3C). Enrichment analysis determined that these genes were overrepresented in KEGG category annotations for several cellular processes (Fig. 3E), including focal adhesions and ECM-receptor interactions. Closer inspection of these results revealed that, in addition to downregulating genes encoding ECM molecules and matricellular proteins themselves, Ch55 also downregulated expression of a large number of integrin genes (Fig. S8B, S10B). These pathways are critical to fibrosis due to the well-recognized roles of mechanotransduction in the maintenance and exacerbation of tissue fibrosis (Duscher et al., 2014, Humphrey et al., 2014); pharmacologic disruption of mechanical signaling has recently demonstrated efficacy in blunting fibrosis and driving regeneration in animal models (Chen et al., 2022, Mascharak et al., 2022). Several other reports have described anti-adhesion effects of retinoic acid treatment on various cell types including Schwann cell-like neuroblastoma cells (Voigt et al., 2000), melanoma cells (Edward et al., 1989), and breast cancer cells (Sanchez et al., 2016), among others. Interestingly, previous work has also implicated ligand-independent roles of RARs in maintaining functional adhesion and expression of related genes in mouse embryonic fibroblasts (Al Tanoury et al., 2014), demonstrating that the interplay between RAR pathways and regulation of cellular adhesion is more complicated than we currently understand. With the aid of our RNA-seq data and preliminary analysis, we will next seek to elucidate which pathways associated with focal adhesions and mechanotransduction, including integrin/FAK, YAP/TAZ, and myocardin/MRTF, are affected by Ch55 and other RAR agonists, in order to better understand the nature of their anti-fibrotic effects.
Another key limitation of our work is worth discussing in some detail and with appropriate context. Since both Ch55 and ATRA bind and activate all three RARs with high affinity (Sun et al., 1997), this does not enable us to discern which receptor(s) mediate the key anti-fibrotic effects of these compounds. Interestingly, a recent pre-print by Rinkevich and colleagues has shed some light on this possibility (Rinkevich et al., 2022). In this report, authors demonstrated that a decrease in endogenous retinoic acid signaling is associated with a transition from pro-inflammatory fibroblasts towards myofibroblasts, suggesting that pharmacologic RAR agonism may suppress fibrosis by preventing differentiation of myofibroblast precursors. Accordingly, authors demonstrated that treatment of fascia explants with agonists of either RARα, RARβ, or RARγ (or a pan-RAR agonist) was sufficient to antagonize contraction, indicative of impaired myofibroblast differentiation. Intriguingly, this suggests that shared RAR-mediated signaling pathways and/or non-canonical pathways may regulate the phenotypes of myofibroblasts. This is consistent with previous demonstration of partial redundancy of different RAR family members to regulate target genes in response to retinoic acid treatment in mouse embryonic fibroblasts (Al Tanoury et al., 2014). Analysis of our RNA-seq data in unstimulated fibroblasts demonstrates practically undetectable basal expression of RARB in comparison to RARA and RARG (Fig. S15A), consistent with previous analyses of RAR transcript expression in skin fibroblasts (Elder et al., 1991, Redfern and Todd, 1992, Rees and Redfern, 1989), though RARB expression is induced by Ch55 treatment. Interestingly, our data also demonstrate that RARA is upregulated, and RARG downregulated, by treatment with TGF-β1 (Fig. S15B). This suggests that fibrotic dermis containing pathological myofibroblasts may be enriched for RARA expression over RARG expression in comparison to healthy dermis. Thus, we plan to perform future work to determine which RARs are necessary and sufficient to observe anti-fibrotic effects of RAR agonists in myofibroblasts, and whether specific agonism of each RAR is sufficient to phenocopy these results in vitro and in vivo. Recognition that the vast majority of RARs in the epidermis are comprised of RARγ (Fisher et al., 1994) has led to a focus on the development of selective RARγ agonists for dermatological applications, culminating in demonstrated clinical efficacy and regulatory approval of the selective RARγ agonist trifarotene for topical application to treat acne vulgaris (Blume-Peytavi et al., 2020, Dreno et al., 2022, Scott, 2019, Tan et al., 2019). If specific activation of other RARs, namely RARα, is sufficient to exert antifibrotic effects, this may suggest a divergent strategy for therapeutic retinoid treatment for fibrotic dermal pathologies compared to epidermal pathologies. Indeed, drug development for oncologic indications has seen success in the synthesis and subsequent utilization of small molecules with highly specific agonism towards RARα (Ambinder et al., 2020, Beard et al., 2002, Hernandez et al., 2020), providing a repertoire of existing small molecules that might be evaluated for this purpose.
In this report, Ch55 was predicted from a myofibroblast gene expression profile that was found to be consistent across varied tissue-specific pathologies (Buechler et al., 2021). Since the emergence and activity of myofibroblasts is associated with numerous fibrotic pathologies affecting various organs, it may be worth investigating the potential for appropriate delivery of Ch55 (or other analogous compounds) to alleviate fibrosis in models of internal organ fibroses as well, alongside the potential to antagonize activation of myofibroblast progenitors in other tissues in vitro and in vivo. This will require both a better understanding of the chemical characteristics of Ch55, including drug stability and optimal conditions of storage, as well as development and utilization of appropriate delivery modalities to reach tissues of interest and limit off-target effects, as has been undertaken extensively for other retinoid-based therapies (Ferreira et al., 2020). Ultimately, our findings serve as a proof of concept demonstrating the use of a pathological gene expression signature to predict putative small molecule compounds to reverse subsets of that transcriptomic profile in vitro and, ultimately, pathological tissue phenotypes in vivo. Given the near-limitless promise of ever-increasing access to “healthy” and pathophysiological sequencing data, and the development of widely available tools to facilitate their analysis, we hope that approaches similar to ours can be harnessed for future identification and preclinical validation of compounds for a myriad of distinct pathologies.
MATERIALS AND METHODS
Detailed methods are described in the supplement.
Cell culture
Human fibroblasts were obtained with written, informed consent from donors (adult fibroblasts) or legal guardians (neonatal foreskin fibroblasts). Primary human foreskin fibroblasts, primary rabbit dermal fibroblasts, and primary human keloid-derived and hypertrophic scar-derived fibroblasts were cultured on tissue culture plastic in DMEM+10% FBS. Cultures were maintained in a humidified cell culture incubator at 37°C, 5% CO2, and ambient O2.
RNA isolation and qRT-PCR
RNA was extracted using Tri Reagent and subjected to phenol/chloroform extraction and isopropanol precipitation, according to manufacturer’s protocols. RNA was reverse-transcribed with Superscript III and subjected to qRT-PCR using a StepOnePlus Real Time PCR instrument with gene-specific primers (Table S3).
RNA-seq
Purified RNA harvested from primary cultured human foreskin fibroblasts pooled from three donors underwent TruSeq stranded mRNA-seq library preparation, followed by paired-end sequencing on an Illumina HiSeq 4000.
Rabbit ear hypertrophic scar model
All animal experiments were approved prior to initiation by the Northwestern University Institutional Animal Care and Use Committee (IACUC). Rabbit experiments were performed in female New Zealand White rabbits (Envigo, Indianapolis, IN) of mass 2.5–3kg. Briefly, on each ear, six full-thickness circular excisional wounds of 7-mm diameter were created. Intradermal injections (~50μL/injection using 30-gauge hollow needles) of Ch55 or vehicle control were performed to each wound on PODs 16, 19, and 22. Tissues were harvested on POD28.
Statistical analysis
All statistical analysis was performed with Graphpad Prism 9. All error bars represent population standard deviations. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Supplementary Material
ACKNOWLEDGMENTS
Chemical structures were constructed using Chemdraw 20.1.1 (PerkinElmer, Waltham, MA). We would like to acknowledge and thank the teams involved in the conceptualization, development, and maintenance of the Connectivity Map tools for enabling us to perform the predictive analyses described in this manuscript. We would also like to thank all of the researchers involved in the construction and maintenance of Galaxy, for promoting accessibility of RNA-seq data analysis for non-specialists. RNA-sequencing work was supported by the Northwestern University NUSeq Core Facility at the Center for Genetic Medicine.
Funding
Funding for this research was provided in part by NIAMS award number 1R21AR081475-01 to SJH.
Footnotes
CONFLICTS OF INTEREST
All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement
Raw RNA-seq data is available via the NCBI Sequence Read Archive (SRA) accession number PRJNA921850. Processed data are available as supplements to this manuscript, and any further data will be made available upon reasonable request to the corresponding author.
References
- Abbasi S, Sinha S, Labit E, Rosin NL, Yoon G, Rahmani W, et al. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell stem cell 2020;27(3):396–412. e6. [DOI] [PubMed] [Google Scholar]
- Abergel RP, Meeker CA, Oikarinen H, Oikarinen AI, Uitto J. Retinoid modulation of connective tissue metabolism in keloid fibroblast cultures. Arch Dermatol 1985;121(5):632–5. [PubMed] [Google Scholar]
- Al Tanoury Z, Piskunov A, Andriamoratsiresy D, Gaouar S, Lutzing R, Ye T, et al. Genes involved in cell adhesion and signaling: a new repertoire of retinoic acid receptor target genes in mouse embryonic fibroblasts. Journal of Cell Science 2014;127(3):521–33. [DOI] [PubMed] [Google Scholar]
- Ambinder AJ, Norsworthy K, Hernandez D, Palau L, Paun B, Duffield A, et al. A Phase 1 Study of IRX195183, a RARα-Selective CYP26 Resistant Retinoid, in Patients With Relapsed or Refractory AML. Frontiers in Oncology 2020;10:587062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri N, Golin AP, Jalili RB, Ghahary A. Roles of cutaneous cell-cell communication in wound healing outcome: An emphasis on keratinocyte-fibroblast crosstalk. Experimental Dermatology 2022;31(4):475–84. [DOI] [PubMed] [Google Scholar]
- Bahmer FA, Zaun H. Isotretinoin therapy for progressive systemic sclerosis. Archives of Dermatology 1985;121(3):308-. [PubMed] [Google Scholar]
- Beard RL, Duong TT, Teng M, Klein ES, Standevan AM, Chandraratna RA. Synthesis and biological activity of retinoic acid receptor-α specific amides. Bioorganic & medicinal chemistry letters 2002;12(21):3145–8. [DOI] [PubMed] [Google Scholar]
- Blume-Peytavi U, Fowler J, Kemény L, Draelos Z, Cook-Bolden F, Dirschka T, et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. Journal of the European Academy of Dermatology and Venereology 2020;34(1):166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Cambruzzi J, Cox PJ, Davies M, Dunbar J, Plumbley D, et al. Big data in drug discovery. Progress in medicinal chemistry 2018;57:277–356. [DOI] [PubMed] [Google Scholar]
- Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, et al. Cross-tissue organization of the fibroblast lineage. Nature 2021;593(7860):575–9. [DOI] [PubMed] [Google Scholar]
- Chen K, Henn D, Januszyk M, Barrera JA, Noishiki C, Bonham CA, et al. Disrupting mechanotransduction decreases fibrosis and contracture in split-thickness skin grafting. Science Translational Medicine 2022;14(645):eabj9152. [DOI] [PubMed] [Google Scholar]
- Cosio T, Di Prete M, Gaziano R, Lanna C, Orlandi A, Di Francesco P, et al. Trifarotene: a current review and perspectives in dermatology. Biomedicines 2021;9(3):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly TJ, Weston WL. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. Journal of the American Academy of Dermatology 1986;15(4):900–2. [DOI] [PubMed] [Google Scholar]
- Delany AM, Brinckerhoff CE. The synthetic retinoid (4-hydroxyphenyl) retinamide decreases collagen expression in vitro and in the tight-skin mouse. Arthritis and rheumatism 1993;36(7):983–93. [DOI] [PubMed] [Google Scholar]
- Dolivo DM, Sun LS, Rodrigues AE, Galiano RD, Mustoe TA, Hong SJ. Epidermal potentiation of dermal fibrosis: lessons from occlusion and mucosal healing. The American Journal of Pathology 2023. [DOI] [PubMed] [Google Scholar]
- Dreno B, Kang S, Leyden J, York J. Update: Mechanisms of Topical Retinoids in Acne. Journal of drugs in dermatology: JDD 2022;21(7):734–40. [DOI] [PubMed] [Google Scholar]
- Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, et al. Mechanotransduction and fibrosis. Journal of biomechanics 2014;47(9):1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward M, Gold JA, MacKIE RM. Modulation of melanoma cell adhesion to basement membrane components by retinoic acid. Journal of cell science 1989;93(1):155–61. [DOI] [PubMed] [Google Scholar]
- Elder JT, Fisher GJ, Zhang Q-Y, Eisen D, Krust A, Kastner P, et al. Retinoic acid receptor gene expression in human skin. Journal of investigative dermatology 1991;96(4):425–33. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Napoli J, Enver T, Bernardino L, Ferreira L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nature Communications 2020;11(1):4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Talwar HS, Xiao J-H, Datta SC, Reddy AP, Gaub M-P, et al. Immunological identification and functional quantitation of retinoic acid and retinoid X receptor proteins in human skin. Journal of Biological Chemistry 1994;269(32):20629–35. [PubMed] [Google Scholar]
- Hernandez D, Palau L, Norsworthy K, Anders NM, Alonso S, Su M, et al. Overcoming microenvironment-mediated protection from ATRA using CYP26-resistant retinoids. Leukemia 2020;34(11):3077–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170(6):1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nature reviews Molecular cell biology 2014;15(12):802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Ohtani T, Furukawa F. Vitamin A derivative etretinate improves bleomycin-induced scleroderma. Allergology International 2005;54(3):419–25. [Google Scholar]
- Ikeda T, Uede K, Hashizume H, Furukawa F. The vitamin A derivative etretinate improves skin sclerosis in patients with systemic sclerosis. Journal of dermatological science 2004;34(1):62–6. [DOI] [PubMed] [Google Scholar]
- Janssen de Limpens A The local treatment of hypertrophic scars and keloids with topical retinoic acid. The British journal of dermatology 1980;103(3):319–23. [DOI] [PubMed] [Google Scholar]
- Jetten AM, Anderson K, Deas M, Kagechika H, Lotan R, Rearick J, et al. New benzoic acid derivatives with retinoid activity: lack of direct correlation between biological activity and binding to cellular retinoic acid binding protein. Cancer research 1987;47(13):3523–7. [PubMed] [Google Scholar]
- Jumper N, Hodgkinson T, Arscott G, Har-Shai Y, Paus R, Bayat A. The aldo-keto reductase AKR1B10 is upregulated in keloid epidermis, implicating retinoic acid pathway dysregulation in the pathogenesis of keloid disease. Journal of Investigative Dermatology 2016;136(7):1500–12. [DOI] [PubMed] [Google Scholar]
- Kassir M, Karagaiah P, Sonthalia S, Katsambas A, Galadari H, Gupta M, et al. Selective RAR agonists for acne vulgaris: A narrative review. Journal of Cosmetic Dermatology 2020;19(6):1278–83. [DOI] [PubMed] [Google Scholar]
- Kim R, Stern W. Retinoids and butyrate modulate fibroblast growth and contraction of collagen matrices. Investigative ophthalmology & visual science 1990;31(6):1183–6. [PubMed] [Google Scholar]
- Korfei M, Stelmaszek D, MacKenzie B, Skwarna S, Chillappagari S, Bach AC, et al. Comparison of the antifibrotic effects of the pan-histone deacetylase-inhibitor panobinostat versus the IPF-drug pirfenidone in fibroblasts from patients with idiopathic pulmonary fibrosis. PloS one 2018;13(11):e0207915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. International journal of molecular sciences 2018;19(3):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu D, Ma Y, Du X, Jing J, Wang L, et al. Direct reprogramming of fibroblasts via a chemically induced XEN-like state. Cell stem cell 2017;21(2):264–73. e7. [DOI] [PubMed] [Google Scholar]
- Li X, Wang S, Dai J, Yan L, Zhao S, Wang J, et al. Homoharringtonine prevents surgery-induced epidural fibrosis through endoplasmic reticulum stress signaling pathway. European Journal of Pharmacology 2017;815:437–45. [DOI] [PubMed] [Google Scholar]
- Luo W, Pant G, Bhavnasi YK, Blanchard SG Jr, Brouwer C. Pathview Web: user friendly pathway visualization and data integration. Nucleic acids research 2017;45(W1):W501–W8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascharak S, Talbott HE, Januszyk M, Griffin M, Chen K, Davitt MF, et al. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell stem cell 2022;29(2):315–27. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice P, Bunker C, Dowd PM. Isotretinoin in the treatment of systemic sclerosis. British Journal of Dermatology 1989;121(3):367–74. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Choudhuri R, Fabre K, Sowers AL, Citrin D, Zabludoff SD, et al. In vitro and In vivo Radiation Sensitization of Human Tumor Cells by a Novel Checkpoint Kinase Inhibitor, AZD7762Radiosensitization by Chk1/2 Inhibition. Clinical cancer research 2010;16(7):2076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani H, Yoshida T, Nouchi N, Hamanaka H, Shimizu M. Topical tocoretinate improved hypertrophic scar, skin sclerosis in systemic sclerosis and morphea. The Journal of Dermatology 1999;26(1):11–7. [DOI] [PubMed] [Google Scholar]
- Ohta A, Uitto J. Procollagen gene expression by scleroderma fibroblasts in culture. inhibition of collagen production and reduction of proα (i) and proα1 (III) collagen messenger RNA steady-state levels by retinoids. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 1987;30(4):404–11. [DOI] [PubMed] [Google Scholar]
- Pakshir P, Noskovicova N, Lodyga M, Son DO, Schuster R, Goodwin A, et al. The myofibroblast at a glance. Journal of Cell Science 2020;133(13):jcs227900. [DOI] [PubMed] [Google Scholar]
- Redfern C, Todd C. Retinoic acid receptor expression in human skin keratinocytes and dermal fibroblasts in vitro. Journal of Cell Science 1992;102(1):113–21. [DOI] [PubMed] [Google Scholar]
- Rees JL, Redfern CP. Expression of the α and β retinoic acid receptors in skin. Journal of investigative dermatology 1989;93(6):818–20. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Correa-Gallegos D, Ye H, Dasgupta B, Sardogan A, Ichijo R, et al. CD201+ fascia progenitors choreograph injury repair. 2022. [Google Scholar]
- Russo B, Brembilla NC, Chizzolini C. Interplay between keratinocytes and fibroblasts: a systematic review providing a new angle for understanding skin fibrotic disorders. Frontiers in immunology 2020;11:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo B, Brembilla NC, Chizzolini C. Contribution of keratinocytes to dermal fibrosis. Current Opinion in Rheumatology 2022;34(6):337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cellular signalling 2013;25(1):264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AM, Shortrede JE, Vargas-Roig LM, Flamini MI. Retinoic acid induces nuclear FAK translocation and reduces breast cancer cell adhesion through Moesin, FAK, and Paxillin. Molecular and Cellular Endocrinology 2016;430:1–11. [DOI] [PubMed] [Google Scholar]
- Scott LJ. Trifarotene: first approval. Drugs 2019;79(17):1905–9. [DOI] [PubMed] [Google Scholar]
- Shima T, Yamamoto Y, Ikeda T, Furukawa F. A patient with localized scleroderma successfully treated with etretinate. Case Reports in Dermatology 2014;6(3):200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell 2017;171(6):1437–52. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S-Y, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Research 1997;57(21):4931–9. [PubMed] [Google Scholar]
- Sun Y, Dai J, Jiao R, Jiang Q, Wang J. Homoharringtonine inhibits fibroblasts proliferation, extracellular matrix production and reduces surgery-induced knee arthrofibrosis via PI3K/AKT/mTOR pathway-mediated apoptosis. Journal of Orthopaedic Surgery and Research 2021;16(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Thiboutot D, Popp G, Gooderham M, Lynde C, Del Rosso J, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. Journal of the American Academy of Dermatology 2019;80(6):1691–9. [DOI] [PubMed] [Google Scholar]
- Voigt A, Hartmann P, Zintl F. Differentiation, proliferation and adhesion of human neuroblastoma cells after treatment with retinoic acid. Cell Adhesion and Communication 2000;7(5):423–40. [DOI] [PubMed] [Google Scholar]
- Walraven M, Hinz B. Therapeutic approaches to control tissue repair and fibrosis: Extracellular matrix as a game changer. Matrix Biol 2018;71–72:205–24. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008;214(2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Kanekura T, Yoshida N, Higashi Y, Yan KL, Fukushige T, et al. 9-Cis-retinoic acid exhibits antifibrotic activity via the induction of cyclooxygenase-2 expression and prostaglandin E2 production in scleroderma fibroblasts. Clinical and Experimental Dermatology: Experimental dermatology 2008;33(4):484–90. [DOI] [PubMed] [Google Scholar]
- Xiao R, Yoshida N, Higashi Y, LU QJ, Fukushige T, Kanzaki T, et al. Retinoic acids exhibit anti-fibrotic activity through the inhibition of 5-lipoxygenase expression in scleroderma fibroblasts. The Journal of dermatology 2011;38(4):345–53. [DOI] [PubMed] [Google Scholar]
- Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, He S, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell discovery 2018;4(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Ge J, Zhang X, Cheng L, Zhang Z, He S, et al. Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell research 2016;26(1):34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome biology 2010;11(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T-B, Drummen GP, Qin Y-H. The controversial role of retinoic acid in fibrotic diseases: analysis of involved signaling pathways. International journal of molecular sciences 2013;14(1):226–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis CC. Retinoids–which dermatological indications will benefit in the near future? Skin Pharmacology and Physiology 2001;14(5):303–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw RNA-seq data is available via the NCBI Sequence Read Archive (SRA) accession number PRJNA921850. Processed data are available as supplements to this manuscript, and any further data will be made available upon reasonable request to the corresponding author.


