Abstract
Key Clinical Message
This is the first case of a promyelocytic sarcoma diagnosed on pleural effusion and exposed the difficulty of demonstrating a leukemic phase in patients with bone diseases, such as Gorham's disease. It also showed that promyelocytic sarcoma can be treated by ATRA/ATO‐based therapy with an efficient and tolerated response.
Abstract
Myeloid sarcoma (MS) is a rare extramedullary tumoral infiltration of immature myeloid cells and can occur in different sites of the body, without leukemic infiltration. A 38‐year‐old woman patient presented at emergency with a pleural effusion, bicytopenias, and Gorham's disease, a very rare bone disorder. In the following days, she worsened with a chylothorax and pancytopenias. Pleural puncture cytologically revealed promyelocytes with Auer rods. Cytogenetic and molecular analyses subsequently confirmed the presence of the t(15:17) translocation. However, no circulating phase of these atypical promyelocytes was found. Similarly, no other origin was identified. We conclude that the patient had a MS of unknown etiology in the form of a pleural effusion with pathological promyelocytes. The patient was treated with a combination of oral all‐trans retinoic acid (ATRA) and arsenic trioxide (ATO) with a cytological and molecular remission persisting 3 months after diagnosis. We report here the first case of a promyelocytic MS of pleural origin without concomitant evidence of acute promyelocytic leukemia. We also show the efficacy of ATRA/ATO treatment in this etiology.
Keywords: all‐trans retinoic acid, myeloid sarcoma, promyelocytes
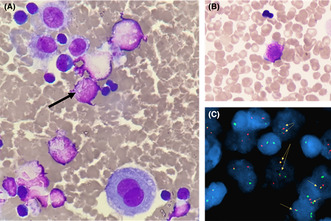
Pleural effusion showed atypical promyelocytes with Auer bodies. This finding was confirmed by FISH with the identification of the t(15:17) translocation.
INTRODUCTION
Myeloid sarcoma (MS), also known as granulocytic sarcoma or chloroma, is a rare extramedullary tumoral infiltration of immature myeloid cells that can occur in different sites of the body, without leukemic infiltration of the bone marrow (BM) (which, however, can be observed subsequently). The most common affected sites are skin, lymph nodes, gastrointestinal tract, bone, soft tissues, and testes. MS is characterized by a slight male predominance (sex ratio 1.2:1) and affects patients at any age. MS may develop de novo or in association with acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPN), myelodysplastic/myeloproliferative neoplasms (MDS/MPNs). It could be detected months or years before the myeloid malignancies. MS can also be the initial manifestation of relapse in patients treated for AML while in remission. 1 Without intensive chemotherapy, most patients with MS have a higher risk to develop AML in association with shorter survival. 2 Some cases were reported in the literature of MS with t(15;17) promyelocytes. The most common infiltrated sites in APL with MS were the spine, skin, and tongue. 3 All cases evolved in acute promyelocytic leukemia (APL), and a combination ATRA–ATO was made. This combination is the reference to treat APL. 4
We report here the first case of de novo MS arising from abnormal promyelocytes with t(15;17) (q24;q21) diagnosed on pleural fluid.
A 38‐year‐old woman was admitted in emergency for dyspnea and neutropenia. She had a history of Gorham's disease with lytic pelvis involvement. Three years ago, she was diagnosed with an invasive ductal carcinoma HER2+ of the left breast. She was initially treated with a combination of Epirubicin and Cyclophosphamide (4 cycles of EC) followed by Taxol plus Trastuzumab®. Subsequently, she had a mastectomy, parietal radiotherapy, and hormonal maintenance therapy. A rapidly—in two weeks—progressive dyspnea revealed a right pleural effusion without pulmonary embolism. A chylous pleural effusion was diagnosed with no sign of bacterial infection or the presence of breast malignant cells. CA 15–3 dosage was normal.
The patient also presented bicytopenia with neutrophils = 1.7 × 109/L and hemoglobin = 11.3 g/dL without abnormal circulating cells. On the following days, a thrombocytopenia appeared (platelets = 136 × 109/L). Hemostasis tests showed (Table 1) normal value of prothrombin time (PT), normal fibrinogen, increased D‐dimers (Ddi), and prolonged activated partial thromboplastin time (aPTT) related to prophylactic heparin treatment. A sternal BM aspiration was realized on D10 but it was non‐contributive due to hemodilution. No qualitative cytological abnormality on blood smear was noticed. We performed next‐generation sequencing (NGS) on BM sample which did not detect any mutation. Taken together, these results did not allow us to identify the origin of cytopenias.
TABLE 1.
Laboratory values and biological follow‐up of the patient.
| Blood parameters | Reference values |
09/11/2022 On admission |
09/30/2022 D19 |
10/12/2022 D31 |
11/03/2022 D53 |
11/14/2022 D64 |
|---|---|---|---|---|---|---|
| White blood bell (WBC) count | 4.0–10.0 × 109/L | 1.7 × 109/L | 3.1 × 109/L | 2.0 × 109/L | 2.5 × 109/L | 5.3 × 109/L |
| Hemoglobin | 11.5–15.0 g/dL | 11.3 g/dL | 11.9 g/dL | 9.8 g/dL | 8.9 g/dL | 9.9 g/dL |
| Hematocrit | 34.0–45.0% | 32.3% | 35.1% | 29.6% | 25.2% | 29.9% |
| Mean corpuscular volume (MCV) | 75.0–96.0 fL | 92.0 fL | 96.4 fL | 99.0 fL | 97.3 fL | 105.3 fL |
| Mean corpuscular hemoglobin (MCH) | 24.0–33.0 pg | 32.2 pg | 32.7 pg | 32.8 pg | 34.4 pg | 34.9 pg |
| Mean corpuscular hemoglobin concentration (MCHC) | 32.0–36.0 g/dL | 35.0 g/dL | 33.9 g/dL | 33.1 g/dL | 35.3 g/dL | 33.1 g/dL |
| Reticulocytes | 25.0–100 × 109/L | / | 131.4 × 109/L | / | 368.1 × 109/L | 341.4 × 109/L |
| Platelets | 150–450 × 109/L | 152 × 109/L | 150 × 109/L | 189 × 109/L | 285 × 109/L | 250 × 109/L |
| Absolute neutrophil count (ANC) | 1.5–7.0 × 109/L | 0.8 × 109/L | 1.6 × 109/L | 0.8 × 109/L | 1.7 × 109/L | 4.0 × 109/L |
| Absolute lymphocyte count (ALC) | 1.2–4.0 × 109/L | / | 1.4 × 109/L | 0.5 × 109/L | 1.4 × 109/L | 0.6 × 109/L |
| Absolute monocyte count | 0.2–0.8 × 109/L | / | 0.1 × 109/L | 0.1 × 109/L | 0.2 × 109/L | 0.4 × 109/L |
| Lactate dehydrogenase (LDH) | <246 U/L | 237 U/L | 173 U/L | 209 U/L | 162 U/L | 221 U/L |
| Sodium | 133–145 mmol/L | 141 mmol/L | 139 mmol/L | 139 mmol/L | 128 mmol/L | 138 mmol/L |
| Potassium | 3.4–4.5 mmol/L | 2.8 mmol/L | 3.5 mmol/L | 4.4 mmol/L | 3.8 mmol/L | 4.2 mmol/L |
| Chloride | 99–111 mmol/L | 107 mmol/L | 107 mmol/L | 109 mmol/L | 101 mmol/L | 109 mmol/L |
| CO2 | 20–31 mmol/L | 21 mmol/L | 25 mmol/L | 28 mmol/L | 23 mmol/L | 22 mmol/L |
| Blood urea nitrogen (BUN) | 3.2–8.2 mmol/L | 3.4 mmol/L | 3.5 mmol/L | 6.0 mmol/L | 6.5 mmol/L | 7.0 mmol/L |
| Creatinine | 44–71 μmol/L | 43 μmol/L | 47 μmol/L | 55 μmol/L | 28 μmol/L | 30 μmol/L |
| Calcium | 2.18–2.60 mmol/L | 2.52 mmol/L | 2.11 mmol/L | 2.00 mmol/L | 1.68 mmol/L | 1.81 mmol/L |
| Alanine transaminase (ALT) | <40 U/L | 23 U/L | < 9 U/L | 33 U/L | 11 U/L | 23 U/L |
| Aspartate aminotransferase (AST) | <40 U/L | 26 U/L | 14 U/L | 36 U/L | 22 U/L | 31 U/L |
| Prothrombin time (PT) | 70%–100% | 86% | 96% | 90% | 87% | 100% |
| Activated partial thromboplastin time (aPTT) | <1.2 | 1.4 | 1.8 | 3.2 | 1.0 | 0.9 |
| Fibrinogen | 2.0–4.0 g/L | 2.3 g/L | 2.1 g/L | 1.8 g/L | 1.4 g/L | 2.6 g/L |
| FV | 70%–120% | / | 132% | 143% | 121% | / |
| D‐dimer | 0–0.50 μg/mL | / | 11.5 μg/mL | / | 3.98 μg/mL | / |
| C‐reactive protein (CRP) | <10 mg/L | 4 mg/L | / | 32.7 mg/L | < 4 mg/L | / |
| Protein | 57–82 g/L | / | 58 g/L | 46 g/L | / | 37 g/L |
| Albumin | 36.8–48.9 g/L (55.8–66.1%) | / | 31.7 g/L (54.9%) | 27.2 g/L (58.9%) | / | 22.0 g/L (59.9%) |
| Alpha‐1 globulin | 1.9–3.6 g/L (2.9–4.9%) | / | 3.3 g/L (5.8%) | 3.2 g/L (6.9%) | / | 2.8 g/L (7.5%) |
| Alpha‐2 globulin | 4.7–8.7 g/L (7.1–11.8%) | / | 7.0 g/L (12.2%) | 6.5 g/L (14.1%) | / | 4.9 g/L (13.4%) |
| Beta‐1 globulin | 3.1–5.3 g/L (4.7–7.2%) | / | 3.6 g/L (6.2%) | 1.8 g/L (4.0%) | / | 2.7 g/L (7.3%) |
| Beta‐2 globulin | 2.1–4.8 g/L (3.2–6.5%) | / | 2.8 g/L (4.8%) | 2.2 g/L (4.7%) | / | 1.5 g/L (4.2%) |
| Gamma globulin | 7.3–13.9 g/L (11.1–18.8%) | / | 9.3 g/L (16.1%) | 5.3 g/L (11.4%) | / | 2.8 g/L (7.7%) |
On D13, another pleural puncture was carried out: a lactescent, hemorrhagic, and sterile liquid was collected with 1100 leucocytes/μL. Cytological analysis revealed a granulocytic contingent composed of promyelocytes containing intense azurophilic granulations and bundles of Auer rods (Figure 1A,B). These results suggested an extramedullary location of promyelocytic acute myeloid leukemia (AML). Flow cytometric analysis performed on this sample revealed an immature myeloid population expressing CD45dim, CD117, CD33, CD13, and partially CD7, whereas CD34 and HLA‐DR were negative. Fluorescence in situ hybridization (FISH) confirmed the presence of a t(15;17) (q24;q21) translocation in 20% of the analyzed nuclei (Figure 1C). Results of molecular biology subsequently confirmed the presence of the fusion transcript PML‐RARΑ (bcr2 breakpoint) at a high level in the pleural effusion sample, whereas blood and BM samples were negative.
FIGURE 1.
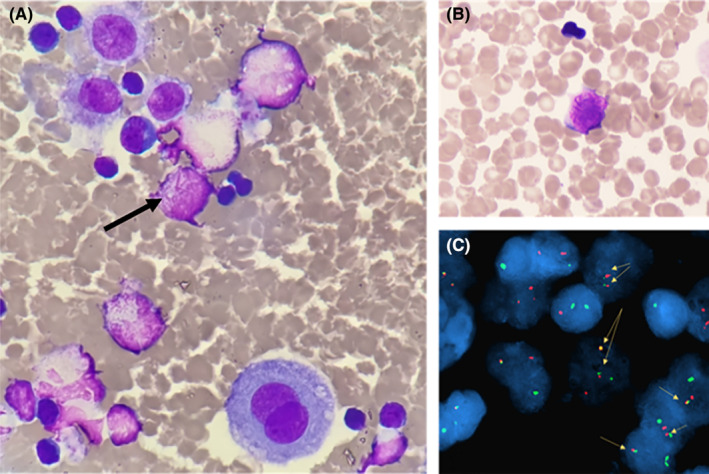
(A) May–Grünwald–Giemsa (MGG) stained cytospin (50×) from pleural puncture at D13 showing a pathological promyelocyte with Auer rods in a mixed inflammatory cells infiltrate including mesothelial cells and lymphocytes (B) MGG stained smear from the pleural puncture (50×) showing another pathognomonic APL promyelocyte with numerous Auer rods (C) FISH analysis with PML and RARA double fusion probes demonstrating the presence of PML‐RARA fusion rearrangement.
Retrospectively, we highlighted on CT‐scan a multinodular right breast and right axillary extension with retropectoral extension. Considering these results, the absence of circulating blasts, and the observation of typical pathological promyelocytes only in the pleural fluid, the diagnosis of MS (extramedullary APL) was retained. On D17, oral all‐trans retinoic acid (ATRA) treatment 45 mg/m2/day (60 mg) was started with addition 3 days later of arsenic trioxide (ATO) 0.15 mg/Kg intravenous over 2 h daily according to APL0406 protocol. Treatment was well tolerated.
Pleural fluid collected at D27 revealed differentiated granulocytic cells with persistent Auer rods, hypergranular cells, and a significant eosinophilic contingent of up to 35% of putative reactive origin. Thereafter, we confirmed the absence of pathological cells on two consecutive pleural punctures realized at D33 and D38. The treatment by ATRA/ATO combination was thus found to be well tolerated and efficient in our patient. Three months after the diagnosis, the patient was in cytological and molecular remission. She had oral ATRA (70 mg/day) 2 weeks per month as consolidation treatment.
Gorham's disease is a rare disorder of unknown etiology characterized by massive osteolysis, angiomatosis involving blood vessels and more rarely lymph vessels. 5 , 6 Bone involvement is variable and can lead to destruction of the osseous matrix. Pleural effusions or chylothorax may occur. Our patient was affected by a disabling osteolysis of pelvic bone treated by bisphosphonates which contraindicated iliac BM puncture or biopsy. The previous thoracic radiotherapy and the angiogenesis associated with Gorham's disease may explain the recurrent non‐contributive diluted sternal aspirations. Blood molecular tests did not allow us to confirm the diagnosis of APL, and NGS myeloid panel did not detect any somatic mutation in agreement with the absence of abnormal circulating cells. FISH analysis performed on D10 BM puncture was negative for PML‐RARA fusion.
When MS is diagnosed in association with BM leukemic infiltration in favor of APL, a circulating phase with the presence of blasts should be observed in association with t(15;17) (q24;q21) translocation and the presence of the PML‐RARA fusion transcript. In our case, no involvement of peripheral blood and diluted BM were observed. However, the demonstration of characteristic promyelocytes, without concomitant circulating blasts, nor cytogenetic anomaly or molecular criteria on blood and diluted BM, was in line with the diagnostic of de novo MS without concomitantly APL. This diagnosis of MS was supported by the nodular infiltration of the right breast, present a year earlier, which increased concomitantly with the presence of pathological promyelocytes in the pleural fluid. Due to the therapeutic emergency of this clinical presentation, ATRA–ATO‐based chemotherapy should be required and has been rapidly initiated with success and good tolerance.
AUTHOR CONTRIBUTIONS
Romain Loyaux: Conceptualization; data curation; formal analysis; methodology; validation; writing – original draft; writing – review and editing. Solene Lecolant: Conceptualization; data curation; formal analysis; methodology; validation; writing – original draft; writing – review and editing. Leila CYSIQUE FOINLAN: Data curation; investigation. Caroline Pradon: Investigation. Sophie Cotteret: Investigation. Jean‐Baptiste Micol: Investigation; supervision; writing – review and editing. Annabelle Stoclin: Investigation; supervision; writing – review and editing. Veronique SAADA: Formal analysis; methodology; supervision; writing – review and editing. CHRISTOPHE MARZAC: Formal analysis; methodology; project administration; supervision; validation; writing – review and editing. Ahmadreza Arbab: Conceptualization; formal analysis; methodology; supervision; validation; writing – review and editing.
FUNDING INFORMATION
The research leading to these results has not received funding.
CONFLICT OF INTEREST STATEMENT
Dr. Jean‐Baptiste Micol—Honoraria: Jazz Pharmaceuticals, AstraZeneca, Astellas Pharma; Consulting or Advisory Role: AbbVie, Gilead Sciences; Travel, Accommodations, Expenses: AbbVie; outside the submitted work. Dr. Christophe Marzac—Honoraria: Astellas Pharma, Celgene/Bristol Myers Squibb, Jazz Pharmaceuticals. Research Funding: FORMA Therapeutics (Inst); outside the submitted work.
All co‐authors have seen and agree with the contents of the manuscript, and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
ETHICS STATEMENT
There is no ethics approval statement for this case report. We have obtained informed consent from the patient. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
CONSENT
We have obtained informed consent from the patient. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Loyaux R, Lecolant S, Cysique Foilan L, et al. An atypical promyelocytic sarcoma and pleural effusion in a patient with Gorham's disease: Efficiency of ATRA/ATO‐based treatment. Clin Case Rep. 2023;11:e7785. doi: 10.1002/ccr3.7785
Romain Loyaux and Solène Lecolant have contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, AA, upon reasonable request.
REFERENCES
- 1. Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703‐1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico‐pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21(2):340‐350. [DOI] [PubMed] [Google Scholar]
- 3. Yamashita T, Nishijima A, Noguchi Y, Narukawa K, Oshikawa G, Takano H. Acute promyelocytic leukemia presenting as recurrent spinal myeloid sarcomas 3 years before developing leukemia: a case report with review of literature. Clin Case Rep. 2019;7(2):316‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lo‐Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111‐121. [DOI] [PubMed] [Google Scholar]
- 5. Patel DV. Gorham's disease or massive osteolysis. Clin Med Res. 2005;3(2):65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiang J, Zhong W. The molecular mechanism of Gorham syndrome: an update. Front Immunol. 2023;14:1165091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, AA, upon reasonable request.


