Abstract
Background and Aims
Antifibrotic therapies reduce lung function decline in patients with idiopathic pulmonary fibrosis (IPF). This single‐arm, open‐label, nonrandomized study aimed to determine the influence of antifibrotic treatment on patients' reported symptoms and expectations of the therapy.
Methods
Fifty‐two patients with confirmed IPF at a mean age of 65 ± 8.63 years (73% male) completed the following surveys at baseline and after 12 months of Pirfenidone treatment: Short Form Healthy Survey (SF‐36), St. George's Respiratory Questionnaire (SGRQ), Baseline Dyspnea Index (BDI), Fatigue Assessment Scale (FAS), Leicester Cough Questionnaire (LCQ), and Patient's Needs and Expectations Authors' Survey.
Results
The most important patients' needs were access to novel therapy, fast and easy access to health centers specializing in IPF treatment, and the improvement of the general condition or the maintenance of its level. These needs did not change with time, except for the significantly more important right of deciding on disease management after 12 months of treatment (p = 0.014). The quality of life per SF‐36, after 1 year of Pirfenidone treatment, significantly improved in the physical cumulative score (p = 0.004) and mental cumulative score (p = 0.003). Significant deteriorations were observed in bodily pain and vitality. For the remaining questionnaires (SGRQ, BDI, FAS, and LCQ), no significant changes in the course of the study were noticed. Around one in 10 patients subjected to Pirfenidone therapy had achieved general symptom improvement in all areas; that is, quality of life improvement as well as cough and dyspnea reduction.
Conclusions
One year of antifibrotic treatment resulted in a general improvement in the quality of life per the SF‐36 questionnaire. Patients' expectations of disease management did not change; also, access to novel therapies and easy access to health centers specializing in IPF management remained their top needs.
Keywords: antifibrotic therapies, cough, dyspnea, fatigue, idiopathic pulmonary fibrosis, patients' expectations, Pirfenidone, quality of life
1. INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive interstitial lung disease (ILD) that results in severe disability and death in the majority of cases. 1 The recent approval of antifibrotic drugs (Pirfenidone and Nintedanib) that slow disease progression has given some hope to patients. Pirfenidone was approved for the treatment of IPF in 2011; however, a lack of reimbursement in Poland resulted in significant limitations in access to this therapy. In January 2017, a therapeutic program applying Pirfenidone for patients with mild‐to‐moderate IPF refunded by the National Health Foundation (NHF) was introduced in Poland. Hitherto, several randomized clinical trials on the safety and efficacy of Pirfenidone in the treatment of patients with IPF have been published. 2 , 3 , 4 , 5 While it is established that available antifibrotic therapies slow down the progression of the disease, little is known about the impact of antifibrotic therapy on health‐related quality of life, 6 , 7 reported symptoms (i.e., dyspnea, fatigue, and cough), and other patient‐reported outcomes. Regarding Nintedanib, it had been already demonstrated that the therapy slowed down the deterioration in symptoms and health‐related quality of life. 6 On the other hand, secondary outcomes measured in the Pirfenidone trial did not demonstrate any impact of antifibrotic therapy on quality of life scores. 2 , 3 Moreover, experiences and expectations of antifibrotic therapy in IPF have not been explored. It must be recognized that patient needs and experiences are as important as objective measures in health outcomes. Therefore, in the present study, we aimed to determine whether the implementation of Pirfenidone therapy in IPF patients has any impact on their quality of life and expectations. Secondarily, we focused on estimating how many patients receiving Pirfenidone would experience improvements in the quality of life and/or reductions in symptoms and also assessing whether improvements in one area must be accompanied by reductions in other disease symptoms.
2. MATERIALS AND METHODS
This single‐arm, open‐label, nonrandomized study was approved by the Bioethics Committee of the Medical University of Silesia in Katowice (Act No. KNW/0022/KB1/85/I/17 from 19.12.2017) and written informed consent was obtained from all participants. Eighty‐seven patients with IPF were referred to the Lung Diseases Department of the Medical University of Silesia and to the Department of Pneumology of the Medical University of Lodz between 2017 and 2019, receiving Pirfenidone therapy for at least 12 months in the setting of the NHF therapeutic program was enrolled in the study. The diagnosis of IPF was confirmed based on the multidisciplinary approach proposed by the international ATS/ERS/JRS/ALAT. 8 Patients with a confirmed diagnosis of IPF who were eligible for the therapeutic program funded by the NHF were included in this study. Therefore, according to the NHF requirements, the study's inclusion criteria were as follows: a confirmed diagnosis of IPF by a multidisciplinary team according to the actual guidelines, 8 forced vital capacity (FVC) above 50% of the predicted value and the transfer factor of the lung for carbon monoxide (TLCO) above 30% of the predicted value, and patient consent to administer antifibrotic therapy. Our exclusion criteria were as follows: patient refusal to receive antifibrotic treatment, failure to meet the criteria for antifibrotic therapy funded by the NHF, and the discontinuation of Pirfenidone prematurely for any reason (before 12 months of the therapy).
On account of the progressive character of the disease, all patients with a diagnosis of IPF, that met the inclusion criteria for antifibrotic therapy received the abovementioned therapy. Hence, it was not possible to create a comparable control group as the patients would differ significantly regarding lung function abnormalities and, as such, would present different disease‐related clinical manifestations, expectations, and quality of life.
Clinical data, pulmonary function tests (including spirometry and TLCO), the 6‐min walking test (6MWT), and survey research consisting of assessments of patients' needs and expectations, quality of life, dyspnea, fatigue, and cough questionnaires were filled out collected at baseline and after 12 months of treatment.
Authors' survey on patients' needs and expectations (ASPNE).
All study participants were asked to fill in the ASPNE of Pirfenidone treatment at baseline and after 12 months of treatment. The ASPNE consisted of 15 phrases describing the patient's needs and expectations toward the antifibrotic treatment.
-
1.
Life extension regardless of its quality
-
2.
Complete and reliable information about the disease, its cause, and prognosis
-
3.
Fast and easy access to health centers specializing in IPF treatment
-
4.
Improvement of comfort and quality of life or the maintenance of its level
-
5.
Decrease in cough intensity
-
6.
Decrease in dyspnea‐induced discomfort
-
7.
Improvement of the general condition or the maintenance of its level
-
8.
Access to novel therapy
-
9.
Access to oxygen therapy at home
-
10.
Easy access to reference centers
-
11.
Ability to stay self‐dependent
-
12.
Availability of psychological support
-
13.
Everyday social assistance
-
14.
Well‐tolerate pharmacological treatment
-
15.
The right to make decisions on disease management.
Enrolled patients assessed how the needs and expectations presented in the ASPNE survey were important for them on a scale of 1–5 (1, not important; 5, very important). Additionally, patients were asked to mark the three most important needs and expectations from all presented in the ASPNE survey.
2.1. Quality of life
The generic Short Form Healthy Survey (SF‐36) questionnaire was used to assess patients' health‐related quality of life. It is made up of eight domains (PF—physical function, RP—role‐physical, BP—bodily pain, GH—general health, VT—vitality, SF—social functioning, RE—role‐emotional, and MH—mental health) and two psychometric‐based summary components, each derived from four domain scores, which are the physical cumulative score (PCS) and the mental cumulative score (MCS). Domain and summary component scores range from 0 to 100, with higher scores corresponding to better health status or well‐being. A licensed computer software program was used to convert numbers to validated points. The minimal important difference (MID), according to Swigris et al., 9 , 10 was accepted as a 3‐point difference between each measurement for the PCS and MCS.
The second instrument used in this study, which was developed for patients with chronic lung diseases and dedicated to measuring patients' health‐related quality of life, was the St. George's Respiratory Questionnaire (SGRQ). It consists of the symptoms, activity, and impact of the disease on life domains. According to Swigris et al., 11 the MID was adopted as 7‐points for the total score, 8‐points for symptoms, 5‐points for activity, and 7‐points for the impact on life. This questionnaire has been validated for patients with ILDs. 11
2.2. Dyspnea
Dyspnea was assessed using the Baseline Dyspnea Index (BDI). 12 , 13 Using the BDI, the Transitional Dyspnea Index (TDI), which displays changes in dyspnea symptoms in different activities of everyday life (i.e., changes in functional impairment, the magnitude of the task, and the magnitude of the effort) was determined. During the transition period, changes in dyspnea were rated using 7 grades, ranging from −3 (major deterioration) to +3 (major improvement). 14
2.3. Cough
Cough was assessed using the Leicester Cough Questionnaire (LCQ). 15 This questionnaire was validated in patients with ILD in 2003. 15 A 7‐point Likert scale was used throughout the development of the LCQ, ranging from 1 = all the time to 7 = none of the time. A higher score indicates better health status. The MID for the LCQ was accepted as 2.56 according to previous data. 15
2.4. Fatigue
Fatigue was assessed using the Fatigue Assessment Scale (FAS). The questionnaire has 10 questions, with a maximum of 50‐points to score and MID equal to 4‐points. Results in the range of 22–34 points indicate average‐to‐moderate fatigue, with higher values correspond to significant fatigue. 16
Primary outcomes measured were IPF patients' expectations and quality of life at baseline and after 1 year of Pirfenidone treatment and assessments of their changes over time.
2.5. Statistical analysis
Statistical analyses were performed using STATISTICA 13.1 (Statsoft Inc., License SUM JPZ710A903825AR‐F). The Shapiro–Wilk test was used for testing the normality of data distribution. The differences between lung function test results and questionnaires at baseline and after 1 year of treatment were analyzed using the Wilcoxon signed‐rank test. A p value of <0.05 is considered statistically. Additionally, the effect size and power of tests were calculated using GPower software.
3. RESULTS
Out of the 87 eligible patients enrolled in the study who filled out all required questionnaires at the beginning of the analysis, four were disqualified from Pirfenidone therapy due to the occurrence of severe side effects (hepatotoxicity, a significant decrease in weight or radiological progression), two died from exacerbations of the underlying respiratory failure, 22 either did not fill out all required questionnaires or provided incomplete information and seven refused to continue the study. The baseline demographic, clinical, GAP index, comorbidities of the patients, and side effects of Pirfenidone therapy are reviewed in Tables 1 and 2. As a consequence of the side effects of this therapy, Pirfenidone dose reduction to 801 mg twice per day was required in 7 (13%) patients, while others received 801 mg three times per day.
Table 1.
The baseline demographic and clinical characteristics of the study participants.
| Parameter | n (%) |
|---|---|
| Age, years, mean (SD) | 65 (8.63) |
| Sex (Male:Female) | 38 (73):14 (27) |
| Supplemental oxygen use, n (%) | 4 (7.69) |
| Smoking status | |
| Never, n (%) | 18 (34.62) |
| Current, n (%) | 4 (7.69) |
| Former, n (%) | 30 (57.69) |
| FVC % predicted, mean (SD) | 82.02 (17.65) |
| TLco, % predicted, mean (SD) | 60.25 (18.47) |
| 6MWT, m, mean (SD) | 487.81 (93.69) |
| GAP index (%) | |
| I | 34 (65.39) |
| II | 17 (32.69) |
| III | 1 (1.92) |
Abbreviations: FVC, forced vital capacity; GAP, GAP index for idiopathic pulmonary fibrosis; n, number; TLCO, lung transfer factor for carbon monoxide; 6MWT, 6‐min walk test.
Table 2.
Comorbidities and side effects of Pirfenidone therapy.
| Comorbid condition | n (%) |
|---|---|
| Respiratory disorders other than IPF | 9 (17.31) |
| Chronic obstructive pulmonary disease | 1 (1.92) |
| Respiratory failure | 8 (15.38) |
| Gastroesophageal reflux disease | 10 (19.23) |
| Metabolism disorders | 19 (36.54) |
| Diabetes mellitus | 10 (19.23) |
| Hypercholesterolemia | 9 (17.31) |
| Vascular disorders | 36 (69.23) |
| Hypertension | 24 (46.15) |
| Cardiac disorders | 19 (36.54) |
| Osteoarthritis | 18 (34.62) |
| Infections developed during the course of the study | 2 (3.85) |
| Chronic sinusitis | 2 (3.85) |
| Nervous system disorders | 3 (5.77) |
| Psychiatric disorders | 2 (3.85) |
| Depression | 10 (19.23) |
| Side effects of Pirfenidone therapy | |
| Nausea | 14 (26.92) |
| Decrease in appetite | 21 (40.38) |
| Diarrhea | 6 (11.54) |
| Vomiting | 3 (5.77) |
| Dyspepsia | 13 (25) |
| Weight loss | 40 (76.92) |
| Rash | 11 (21.15) |
| Photosensitivity | 14 (26.92) |
| Hepatotoxicity | 2 (3.85) |
Abbreviation: n, number.
After 1 year of Pirfenidone treatment in the study group, nonsignificant changes in the mean FVC % pred. (82.02 ± 17.65 vs. 82.09 ± 17.63), TLCO % pred. (60.25 ± 17.65 vs. 53.88 ± 17.63), and distance in the 6MWT (487.81 ± 93.69 m vs. 483.29 ± 97.3 m) were observed. The GAP index after 1 year of the therapy increased in three patients (stage I‐32 pts, stage II‐18 pts, stage III‐2 pts, respectively).
3.1. ASPNE
The results of the ASPNE survey at baseline and after 12 months of antifibrotic therapy are presented in Table 3.
Table 3.
The ASPNE survey results before the initiation of treatment and after 12 months of antifibrotic therapy with Pirfenidone.
| Patients' needs and expectations | Before Pirfenidone treatment (baseline) | After 12 months of Pirfenidone treatment | p Value | Effect size/power |
|---|---|---|---|---|
| 1. Life extension regardless of its quality | 3.96 ± 1.50 | 3.96 ± 1.37 | 0.13 | 0.00001/0.050 |
| 2. Complete and reliable information about the disease, its cause, and its prognosis | 4.39 ± 1.10 | 4.35 ± 1.10 | 0.89 | 0.036/0.083 |
| 3. Fast and easy access to health centers specializing in IPF treatment | 4.70 ± 0.58 | 4.77 ± 0.60 | 0.21 | 0.118/0.212 |
| 4. Improvement of comfort and quality of life or the maintenance of its level | 4.65 ± 0.75 | 4.71 ± 0.92 | 0.29 | 0.071/0.127 |
| 5. Decrease in cough intensity | 4.00 ± 1.39 | 4.02 ± 1.41 | 0.99 | 0.014/0.061 |
| 6. Decrease of dyspnea‐related discomfort | 3.92 ± 1.35 | 4.30 ± 1.13 | 0.31 | 0.303/0.695 |
| 7. Improvement in the general condition or the maintenance of its level | 4.7 ± 0.74 | 4.73 ± 0.70 | 0.83 | 0.042/0.089 |
| 8. Access to novel therapy | 4.84 ± 0.51 | 4.86 ± 0.46 | 0.56 | 0.041/0.088 |
| 9. Access to oxygen therapy at home | 3.37 ± 1.70 | 3.58 ± 1.65 | 0.95 | 0.125/0.226 |
| 10. Easy access to reference centers | 3.41 ± 1.58 | 3.69 ± 1.55 | 0.73 | 0.178/0.355 |
| 11. Ability to stay self‐dependent | 4.47 ± 1.12 | 4.58 ± 1.05 | 0.22 | 0.101/0.177 |
| 12. Psychological support | 2.92 ± 1.55 | 3.15 ± 1.56 | 0.66 | 0.148/0.276 |
| 13. Everyday social assistance | 2.27 ± 1.45 | 2.74 ± 1.65 | 0.15 | 0.301/0.691 |
| 14. Ability to adequately tolerate pharmacological treatment | 4.49 ± 0.82 | 4.67 ± 0.75 | 0.12 | 0.229/0.493 |
| 15. The right to decide on disease management | 3.96 ± 1.37 | 4.19 ± 1.12 | 0.014 | 0.182/0.363 |
| Total | 58.14 ± 13.81 | 61.71 ± 11.28 | 0.08 | 0.280/0.637 |
Note: Data presented as mean ± SD. Bold values are statistically significant at p < 0.05.
Abbreviations: p, probability value; SD, standard deviation.
Before the initiation of treatment, the most important needs for patients were access to novel therapy (4.84 ± 0.51), fast and easy access to health centers specializing in IPF treatment (4.70 ± 0.58), and improvements in the general condition or the maintenance of its level (4.7 ± 0.74). The least essential needs were everyday social assistance (2.27 ± 1.45), psychological support (2.92 ± 1.55), and access to oxygen therapy at home (3.37 ± 1.70).
After 12 months of Pirfenidone treatment, patients' needs and expectations were exactly the same as those before the initiation of treatment. The most important needs of patients were access to novel therapy, fast and easy access to health centers specializing in IPF treatment, and improvements in their general condition or the maintenance of its level. The least essential needs were everyday social assistance, psychological support, and access to oxygen therapy at home.
No significant differences between average levels of patients' needs and expectations scored in the ASPNE survey before and after 12 months of treatment were observed, except for the right of deciding on disease management, which was significantly more important after 12 months of therapy compared to the baseline (3.96 ± 1.37 vs. 4.19 ± 1.12; p = 0.014).
3.2. SF‐36
After 12 months of Pirfenidone treatment, significant improvements in the mean values of PCS (42.33 ± 8.73 vs. 48.45 ± 13.84; p = 0.004) and MCS (44.6 ± 11.85 vs. 53.82 ± 17.32; p = 0.003) were observed in RP, GH, RE, and MH. Significant deteriorations in BP and VT were observed (Table 4).
Table 4.
Quality of life, dyspnea, cough, and fatigue in the study group before and after 12 months of Pirfenidone treatment.
| Before treatment (n = 52) | After treatment (n = 52) | p | Effect size/power | |
|---|---|---|---|---|
| SF‐36 | ||||
| SF‐36 PCS | 42.33 ± 8.73 | 48.45 ± 13.84 | 0.004 | 0.505/0.974 |
| SF‐36 MCS | 44.60 ± 11.85 | 53.82 ± 17.32 | 0.003 | 0.601/0.996 |
| PF | 39.85 ± 9.08 | 42.03 ± 13.75 | 0.12 | 0.180/0.358 |
| RP | 44.73 ± 23.66 | 74.07 ± 50.54 | 0.001 | 0.670/0.999 |
| BP | 43.84 ± 12.77 | 29.94 ± 18.53 | 0.001 | 0.846/1.000 |
| GH | 39.91 ± 10.08 | 46.24 ± 11.86 | 0.001 | 0.571/0.992 |
| VT | 45.20 ± 10.77 | 40.19 ± 9.95 | 0.01 | 0.482/0.963 |
| SF | 40.74 ± 9.6 | 40.24 ± 7.64 | 0.60 | 0.057/0.108 |
| RE | 47.76 ± 31.23 | 96.87 ± 66.95 | <0.001 | 0.846/1.000 |
| MH | 42.96 ± 10.09 | 39.98 ± 8.52 | 0.015 | 0.317/0.729 |
| SGRQ—total | 46.69 ± 20.62 | 47.09 ± 20.27 | 0.68 | 0.020/0.066 |
| Symptoms | 52.04 ± 21.24 | 54.24 ± 21.37 | 0.48 | 0.103/0.181 |
| Activity | 55.63 ± 23.31 | 57.58 ± 23.81 | 0.65 | 0.083/0.145 |
| Impact | 39.54 ± 23.95 | 37.56 ± 21.87 | 0.65 | 0.086/0.151 |
| BDI Total | 7.22 ± 2.45 | 7.34 ± 2.50 | 0.49 | 0.048/0.097 |
| Functional impairment | 2.56 ± 1.07 | 2.58 ± 1.08 | 0.50 | 0.019/0.065 |
| Magnitude of task | 2.58 ± 0.91 | 2.67 ± 0.84 | 0.56 | 0.103/0.180 |
| Magnitude of effort | 2.32 ± 0.91 | 2.26 ± 0.94 | 0.72 | 0.065/0.118 |
| LCQ | 14.47 ± 3.74 | 15.24 ± 3.61 | 0.59 | 0.209/0.439 |
| Fatigue assessment scale | 24.02 ± 7.06 | 23.98 ± 7.84 | 0.59 | 0.005/0.054 |
Note: Data presented as mean ± SD. Bold values are statistically significant at p < 0.05.
Abbreviations: BDI, baseline dyspnea index; BP, bodily pain; GH, general health; LCQ, Leicester Cough Questionnaire; MCS, mental cumulative score; MH, mental health; n, number; p, probability value; PCS, physical cumulative score; PF, physical function; RP, role‐physical; RE, role‐emotional; SF, social functioning; SGRQ, St. George's Respiratory Questionnaire; VT, vitality.
Taking into account individual responses to the questions in the SF‐36 questionnaire with respect to the MID, we observed that more patients reported improvement than deterioration in the MCS and PCS (Figure 1A).
Figure 1.
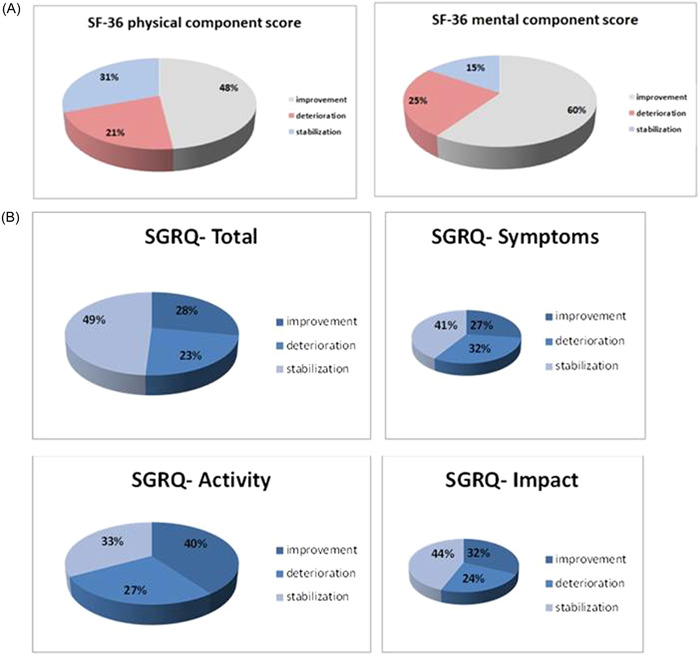
(A) Short Form Healthy Survey (SF‐36) and (B) St. George's Respiratory Questionnaire (SGRQ) score changes compared between the baseline and after 12 months of Pirfenidone treatment.
3.3. SGRQ
After 12 months of Pirfenidone treatment, no significant changes in the mean values of the SGRQ scores were observed (Table 4). Taking into account individual responses to the SGRQ concerning the MID, we observed that more patients reported improvement than deterioration in the activity score (40% vs. 27%; MID 5), impact score (32% vs. 24%; MID 7), and total score (28% vs. 23%; MID 7, Figure 1B).
3.4. Dyspnea
After 12 months of Pirfenidone treatment, no significant changes in the mean BDI scores (total score, functional impairment, magnitude of task, and magnitude of effort) were observed. Taking into account individual responses to the BDI concerning the TDI, we observed that more patients reported improvement than deterioration in the total BDI (41% vs. 36%) and BDI‐magnitude of task (36% vs. 33%). Stabilization was mainly observed in BDI‐magnitude of effort (63%) and BDI‐functional impairment (50%, Figure 2).
Figure 2.
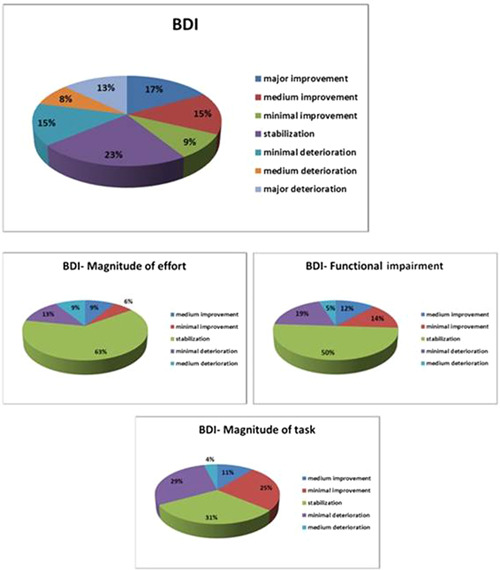
Baseline Dyspnea Index (BDI) changes compared between the baseline and after 12 months of Pirfenidone treatment.
3.5. Cough
After 12 months of Pirfenidone treatment, better mean health status with respect to cough in the LCQ was observed; however, the difference was not statistically significant (14.47 ± 3.74 vs. 15.24 ± 3.61; p = 0.26). In summary, 28% of patients presented with deterioration of cough, 25% presented with improvement of cough sensation, and 47% declared stabilization of cough sensation. Overall, more than two‐thirds of patients with IPF reported stabilization or improvement of cough sensation after 12 months of treatment (Figure 3).
Figure 3.
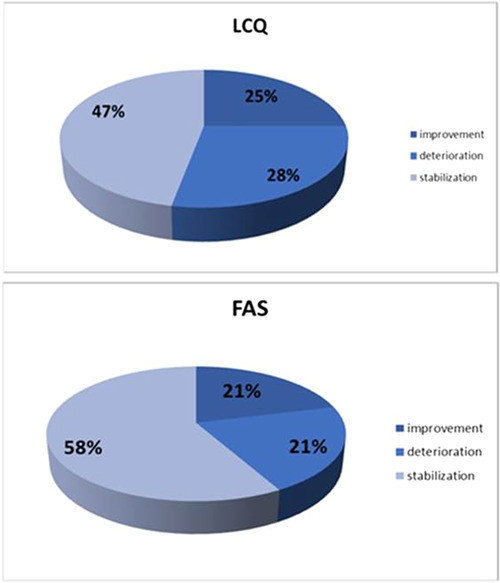
Changes in the Leicester Cough Questionnaire (LCQ) and the Fatigue Assessment Scale (FAS) scores after 12 months of Pirfenidone treatment.
3.6. FAS
Assessment of fatigue using the FAS indicated moderate fatigue at baseline, which did not change significantly after 12 months of treatment (24.02 ± 7.06 vs. 23.98 ± 7.84; p = 0.93). In 58% of patients, the mean FAS score was stable. Deterioration and improvement were observed in an equal proportion (both 21%) of patients (Figure 3).
Significant improvement/deterioration or stabilization based on MID of individual questionnaires during 12 months of Pirfenidone treatment are presented in Table 5. The relationships between improvement and deterioration in the scores of the main questionnaires (SGRQ, LCQ, and BDI) are presented in Figure 4. Patients not included in these figures reported stabilization in the SGRQ, LCQ, and/or BDI (described as not reaching the MID for deterioration or improvement). In all three questionnaires, about 12% of patients achieved improvement and 6% of patients reported deterioration simultaneously.
Table 5.
Significant improvement/deterioration or stabilization based on MID of individual questionnaires during 12 months of Pirfenidone treatment.
| MID | Number of patients | Percentage of patients | Cl 95% | |
|---|---|---|---|---|
| SF‐36 PCS | Important improvement | 25 | 48 | 0.004 ÷ 0.149 |
| Stabilization | 16 | 31 | 0.773 ÷ 0.958 | |
| Important deterioration | 11 | 21 | −0.006 ÷ 0.121 | |
| SF‐36 MCS | Important improvement | 31 | 60 | 0.029 ÷ 0.202 |
| Stabilization | 8 | 15 | 0.724 ÷ 0.93 | |
| Important deterioration | 13 | 25 | −0.006 ÷ 0.121 | |
| SF‐36 PF | Important improvement | 5 | 10 | 0.016 ÷ 0.176 |
| Stabilization | 45 | 86 | 0.773 ÷ 0.958 | |
| Important deterioration | 2 | 4 | −0.014 ÷ 0.091 | |
| SF‐36 RP | Important improvement | 5 | 10 | 0.016 ÷ 0.176 |
| Stabilization | 44 | 84 | 0.748 ÷ 0.944 | |
| Important deterioration | 3 | 6 | −0.006 ÷ 0.121 | |
| SF‐36 BP | Important improvement | 9 | 17 | 0.07 ÷ 0.276 |
| Stabilization | 40 | 77 | 0.655 ÷ 0.884 | |
| Important deterioration | 3 | 6 | −0.006 ÷ 0.121 | |
| SF‐36 GH | Important improvement | 4 | 8 | 0.004 ÷ 0.149 |
| Stabilization | 43 | 82 | 0.724 ÷ 0.93 | |
| Important deterioration | 5 | 10 | 0.016 ÷ 0.176 | |
| SF‐36 VT | Important improvement | 6 | 12 | 0.029 ÷ 0.202 |
| Stabilization | 44 | 84 | 0.748 ÷ 0.944 | |
| Important deterioration | 2 | 4 | −0.014 ÷ 0.091 | |
| SF‐36 SF | Important improvement | 8 | 15 | 0.056 ÷ 0.252 |
| Stabilization | 41 | 79 | 0.677 ÷ 0.899 | |
| Important deterioration | 3 | 6 | −0.006 ÷ 0.121 | |
| SF‐36 RE | Important improvement | 5 | 10 | 0.016 ÷ 0.176 |
| Stabilization | 42 | 81 | 0.701 ÷ 0.915 | |
| Important deterioration | 5 | 9 | 0.016 ÷ 0.176 | |
| SF‐36 MH | Important improvement | 8 | 15 | 0.056 ÷ 0.252 |
| Stabilization | 41 | 79 | 0.677 ÷ 0.899 | |
| Important deterioration | 3 | 6 | −0.006 ÷ 0.121 | |
| SGRQ total | Important improvement | 15 | 28 | 0.235 ÷ 0.496 |
| Stabilization | 25 | 49 | 0.326 ÷ 0.597 | |
| Important deterioration | 12 | 23 | 0.07 ÷ 0.276 | |
| SGRQ symptoms | Important improvement | 14 | 27 | 0.1 ÷ 0.323 |
| Stabilization | 21 | 41 | 0.403 ÷ 0.674 | |
| Important deterioration | 17 | 32 | 0.1320.368 | |
| SGRQ activity | Important improvement | 21 | 40 | 0.182 ÷ 0.433 |
| Stabilization | 17 | 33 | 0.345 ÷ 0.617 | |
| Important deterioration | 14 | 27 | 0.101 ÷ 0.323 | |
| SGRQ impact | Important improvement | 17 | 32 | 0.132 ÷ 0.368 |
| Stabilization | 23 | 44 | 0.423 ÷ 0.693 | |
| Important deterioration | 12 | 24 | 0.085 ÷ 0.299 | |
| LCQ | Important improvement | 13 | 25 | 0.07 ÷ 0.276 |
| Stabilization | 15 | 28 | 0.504 ÷ 0.765 | |
| Important deterioration | 24 | 47 | 0.085 ÷ 0.299 | |
| FAS | Important improvement | 11 | 21 | 0.085 ÷ 0.299 |
| Stabilization | 30 | 58 | 0.701 ÷ 0.915 | |
| Important deterioration | 11 | 21 | 0.341 ÷ 0.126 | |
| Borg | Important improvement | 11 | 21 | 0.101 ÷ 0.323 |
| Stabilization | 32 | 62 | 0.483 ÷ 0.748 | |
| Important deterioration | 9 | 17 | 0.07 ÷ 0.276 | |
| MRC | Important improvement | 14 | 27 | 0.149 ÷ 0.39 |
| Stabilization | 26 | 50 | 0.364 ÷ 0.636 | |
| Important deterioration | 12 | 23 | 0.116 ÷ 0.345 |
Note: Data presented as mean.
Abbreviations: BP, bodily pain; FAS, Fatigue Assessment Scale; GH, general health; LCQ, Leicester Cough Questionnaire; MCS, mental cumulative score; MH, mental health; MID, minimally important difference; MRC, Medical Research Council Dyspnoea Scale; PCS, physical cumulative score, PF, physical function, RE, role‐emotional; RP, role‐physical, SF, social functioning; SGRQ, St. George's Respiratory Questionnaire, VT, vitality.
Figure 4.
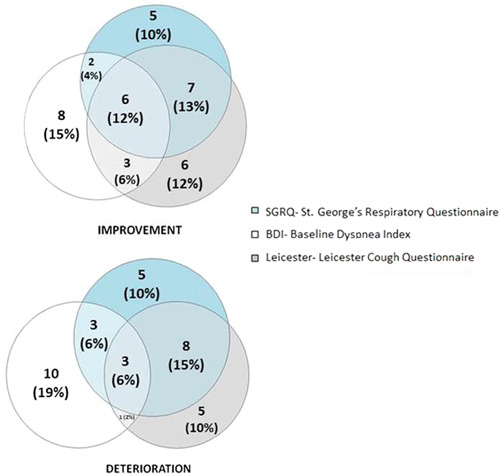
Significant improvements and deteriorations in reported outcomes of patients after treatment.
4. DISCUSSION
The study revealed no significant changes in fatigue, dyspnea, or cough perception after 12 months of Pirfenidone treatment. With regard to patients' expectations, only the right of deciding on disease management was significantly more important after 1 year of therapy compared to the baseline, whereas other expectations remained unchanged. In relation to the quality of life, after 12 months of Pirfenidone treatment, the RP, RE, GH, and MH domains significantly improved.
However, taking into account individual answers, the majority of patients with IPF declared either improvement or stabilization rather than a deterioration in the quality of life, perception of dyspnea, incidence of cough, and fatigue. Our study demonstrated that after 12 months of antifibrotic treatment, more than two‐thirds of patients reported improvements in at least one of the domains (SGRQ, LCQ, or BDI), from which almost half in more than one questionnaire. The above indicates that all symptoms reported by patients influence their quality of life. Therefore, the quality of life should be considered as the composed outcome containing many components that could be disregarded by using a single questionnaire. To the best of our knowledge, this is the first study to demonstrate the impact of antifibrotic therapy on different symptom areas. In our study, 12% of patients experienced simultaneous improvements in their quality of life and also achieved cough and dyspnea reduction. This implies that when patients are put on Pirfenidone therapy, approximately 10% of them may achieve improvements in general clinical manifestations.
Clinicians and researchers are focused on the “objective outcomes” for the assessment of the efficacy of medical interventions, including the limitation of gradual lung function deterioration or reducing mortality in IPF. Surprisingly, multicenter prospective trials on the efficacy of antifibrotic treatment in IPF are lacking appropriate and dedicated tools, such as patient‐reported outcomes (PROs), PRO measures (PROMs), and patient‐reported experience measures (PREMs).
This is particularly important in the case of patients with IPF, where only a few relationships between lung function test results and symptoms influencing the quality of life were found. 17 Therefore, as those are the independent outcomes, improvements in patients' quality of life do not need to be accompanied by improvements in lung function test results. For these reasons, the studies should analyze the impact of a treatment in many areas.
Meanwhile, all major IPF trials are focused on objective measures such as lung function test results, whereas PROMs have only been used as the secondary endpoints. In fact, increasing identification of the needs in terms of including patients' perspectives among the outcomes evaluating the efficiency of the treatment and medical care are observed. Recently, Kalluri et al., in their review, 18 summarized the care pathway from the perspective of patients, identifying current gaps in care, education, support, and communication among patients with IPF. In conclusion, they summarized that there is a real need for the support of multidisciplinary work focused on identifying gaps across the treatment process of patients, and PREMs and PROMs can be instrumental to the achievement of this goal. In the last decade, increased use of PROMs in patients with IPF has been observed, generally including dyspnea, cough, quality of life, depression, and anxiety questionnaires. 19 In our study, we focused on generic PROMs using the SF‐36, disease‐specific PROMs using the SGRQ, and PREMs to gather data on patients' views of their healthcare experience and outcomes, which was acquired using the authors' questionnaire. Additionally, we collected data on the most important symptoms of IPF, such as cough, dyspnea, and fatigue, which have an influence on the quality of life and the quality of treatment.
4.1. SGRQ
The SGRQ was originally developed for use in patients with chronic obstructive pulmonary disease and asthma; however, nowadays, it is commonly used to assess the quality of life of patients with IPF. 9 , 10 , 11 The SGRQ was validated for use in patients with IPF, and the MID was determined. 9 , 10 It is interesting that, in different studies, the baseline SGRQ total score varies considerably (from 35.7 to 53.4). 11 In a large nationwide observational IPF registry (INSIGHT‐IPF), the baseline SGRQ total score was 45.9, 20 while the pivotal trials, INPULSIS‐1 and INPULSIS‐2, in which the efficacy and safety of Nintedanib were evaluated, reported 39.6 as a baseline total score for the SGRQ. 4 In our observational study, the baseline SGRQ total score was 46.7; consequently, our patients reported a worse quality of life at baseline than patients enrolled in the INPULSIS studies, even though both groups of patients met the same inclusion criteria and were recruited before the initiation of antifibrotic therapy. To the best of our knowledge, the best quality of life was presented by Japanese patients with IPF recruited to the INPULSIS study, where the total SGRQ baseline score was 35.1 and after 1 year of Nintedanib treatment increased by 5.81‐points. 21 In our study, the total SGRQ score increased by 0.4‐points after 1 year of antifibrotic treatment. Therefore, we observed no significant deterioration in quality of life. These results are consistent with the outcomes of the INSIGHT and INPULSIS studies, 4 , 20 where no significant deterioration in quality of life per the SGRQ was observed. It is interesting that, in our study, more than 70% of responding patients after 1 year of antifibrotic treatment reported no deterioration in the quality of life per the SGRQ, while 28% of patients reported an improvement in the total SGRQ score. The highest increase was noticed in the activity domain, where 40% of patients in the study reported an increase.
4.2. SF‐36
Unfortunately, there are only a few reports on the GH‐related quality of life (HRQL) in patients with IPF; therefore, knowledge of this matter remains insufficient. Martinez et al. 22 reported that the SF‐36 questionnaire is a valid instrument to evaluate HRQL in patients with IPF, and the MID in the SF‐36 for IPF was determined. 7 , 9 , 10 In a cross‐sectional, longitudinal Japanese study, Tomioka et al. 23 reported a significant decline in HRQL in two subscales of the SF‐36: physical function (PF) and bodily pain (BP). In our study, we also noted a significant deterioration in the BP domain, while in most other domains of the SF‐36, there were improvements in patients' quality of life. In the literature, we found only one report on changes in HRQL per the SF‐36 questionnaire in patients with IPF from the Saudi group. 24 The authors compared changes in the SF‐36 to the control group and postponed the analysis of the changes over time. However, when the results were compared with source data, insignificant improvements in all domains of the SF‐36, except MH, were observed. Similarly, based on the results of our study, we can conclude that 1 year of antifibrotic treatment results in a general improvement in the quality of life per the SF‐36 questionnaire.
4.3. Dyspnea
The first publications on antifibrotic treatment (the ASCEND and CAPACITY studies) reported that Pirfenidone therapy reduces disease progression in patients with IPF without causing differences in dyspnea scores. 3 However, the last study including patients with more advanced lung function impairment in the course of IPF, after 52 weeks of treatment with the same active substance, reported benefits in dyspnea as evaluated by the University of California‐San Diego Shortness of Breath (UCSD SOBQ) questionnaire. 25 In our study, we observed no significant changes in dyspnea as evaluated by the BDI. This notwithstanding, in our study, only approximately 30% of the responders reported the deterioration of dyspnea during follow‐up.
4.4. Cough
Cough, one of the most disabling symptoms in patients with IPF, affects up to 86% of subjects and is an independent predictor of disease progression. 26 Cough severity could be measured by two methods: objectively with 24 h cough recording using a cough monitor or subjectively with cough‐related quality of life questionnaires. In our study, we used the fully validated LCQ, which provides an estimation of the physical, psychological, and social impacts of cough. 27 The effect of Pirfenidone on cough in patients with IPF has already been evaluated in a study by van Manen et al. 28 Compared to the aforementioned study, in our analysis, patients reported a better perception of cough (14‐points vs. 12‐points). Surprisingly, in both studies conducted after Pirfenidone treatment, the results concerning the incidence of cough were similar (15‐points vs. 15‐points). However, in our study, the follow‐up period was 52 weeks; whereas, in the study conducted by van Manen et al., patients were re‐evaluated after 12 weeks of treatment. Considering particular patients, during antifibrotic treatment, only 25% of patients reported improvements in the cough sensation, while almost 50% of patients reported its stabilization.
4.5. Fatigue
Fatigue, just like dyspnea, is expected to be among the most prevalent symptoms in patients with IPF. Fatigue is also a registered side effect of Pirfenidone, one of two approved antifibrotic drugs. The prevalence of fatigue has been scarcely studied in patients with IPF. In a recent multicenter, retrospective observational study conducted in a large Polish cohort of patients with IPF treated with Pirfenidone, fatigue was the most frequently reported adverse effect among study participants. 5 In the clinical management of patients with IPF, it is recommended to assess fatigue because this symptom, alongside dyspnea, is associated with functional impairments in patients' physical activity. In our study, the mean fatigue perception did not change during the 12 months of Pirfenidone treatment. A small prospective study conducted in Denmark demonstrated that the majority of patients with IPF suffered from substantial fatigue at the time of diagnosis, which adequately corroborates our findings. However, contrary to our results, Augustson et al. 29 observed that fatigue progressed during antifibrotic treatment over six months of follow‐up. The possible explanation of the stable fatigue perception over the treatment period in our study could be a good response to antifibrotic therapy with no significant change in pulmonary function over 12 months of follow‐up.
The main strength of our study is a simultaneous assessment of different signs and symptoms affecting various areas of life. After 12 months of antifibrotic treatment, approximately 72% of patients reported improvement in at least one of the domains of the SGRQ, LCQ, or BDI, from which 35% improvements were noticed in more than one questionnaire. By contrast, about 68% of patients reported deteriorations in at least one of the domains, and 29% of patients in more than one questionnaire. It is noteworthy that some patients may have experienced improvements in one questionnaire and either deteriorations or stabilization in another; therefore, the sum of all of them is not equal to 100%. Interestingly, in all three questionnaires, approximately 12% of patients experienced improvements and 6% of patients reported deterioration. As mentioned, dyspnea is the symptom that most severely limits the physical activity of patients; therefore, it is no wonder that reducing dyspnea perception would influence the improvement of the SGRQ. Consequently, all symptoms influencing patients' quality of life (since they are indissoluble) should be assessed inherently, and selective analyses of only one of them may lead to the wrong conclusions. Future studies are needed to determine which aspects of antifibrotic treatment impact complete symptom improvement and if there are any predictors that may identify patients who will respond to treatment in terms of quality of life.
The identification of patient‐specific needs is critical in the patient‐physician relationship. Many basic conditions for IPF care are still frequently unmet. An initiative by the 11th European Patient Advocacy Group for pulmonary fibrosis identified five key areas of unmet needs: (1) better diagnosis, (2) better access to different treatment forms, (3) the availability of emotional support, (4) improved information resources, and (5) equal availability of palliative and end‐of‐life care. 30
In our study, before starting antifibrotic treatment, the most important thing for patients was to access novel therapy and easy access to health centers specializing in IPF management. Those preferences did not change over 1 year of treatment and dominated during the follow‐up period, after 12 months.
Nevertheless, our study had several limitations. The assessment of effectiveness was limited by the single‐arm study design. The number of patients enrolled in the study was relatively small; so, our study did not have sufficient power to demonstrate clinically significant differences regarding the treatment. For this reason, we could not analyze the impact of comorbidities and antifibrotic side effects on patients' quality of life. In addition, patients who discontinued treatment were excluded from the longitudinal follow‐up, which may have induced bias in data reporting. However, the study aimed to assess the impact of 1 year antifibrotic treatment on patients' quality of life; therefore, the results may be disturbed by the discontinuation of therapy. Future studies should investigate whether these outcomes are dependent on antifibrotic treatment.
In conclusion, our study provides real‐world data on the longitudinal expectations of treatment, clinical symptoms, and quality of life in patients with IPF subjected to antifibrotic therapy. Per the findings of this study, after 12 months of Pirfenidone treatment, the majority of patients with IPF declare either improvement or stabilization rather than a deterioration in the quality of life, perception of dyspnea, incidence of cough, and fatigue.
5. CONCLUSION
One year of antifibrotic treatment results in a general improvement in patients' quality of life per the SF‐36 questionnaire. Patients' expectations of disease management did not change, while access to novel therapies and easy access to health centers specializing in IPF management remained their greatest needs. Approximately 10% of patients subjected to Pirfenidone therapy may achieve improvements in general clinical manifestations in all areas (quality of life improvement as well as cough and dyspnea reduction).
AUTHOR CONTRIBUTIONS
Dariusz Jastrzębski: Conceptualization; formal analysis; investigation; methodology; supervision; validation; writing—original draft. Sabina Kostorz‐Nosal: Data curation; funding acquisition; investigation; software; writing—original draft. Dagmara Galle: Data curation; investigation. Alicja Gałeczka‐Turkiewicz: Data curation; investigation. Joanna Warzecha: Data curation; methodology; project administration; supervision. Sebastian Majewski: Methodology; resources; writing—original draft. Wojciech Piotrowski: Conceptualization; methodology; project administration; supervision; writing—review and editing. Dariusz Ziora: Conceptualization; supervision; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Bioethics Committee of the Medical University of Silesia in Katowice (Act No. KNW/0022/KB1/85/I/17 from 19.12.2017), and written informed consent was obtained from all participants. The trial was conducted per the principles of the Declaration of Helsinki.
TRANSPARENCY STATEMENT
The lead author Sabina Kostorz‐Nosal affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by a grant for scientific research (PCN‐1‐194/N/0/K) from the Medical University of Silesia in Poland and a grant from the National Science Center in Poland (No 2016/23/N/NZ7/02002). The funders had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Jastrzębski D, Kostorz‐Nosal S, Galle D, et al. Expectations, symptoms, and quality of life before and after 1 year of Pirfenidone treatment in patients with idiopathic pulmonary fibrosis: A single‐arm, open‐label nonrandomized study. Health Sci Rep. 2023;6:e1449. 10.1002/hsr2.1449
DATA AVAILABILITY STATEMENT
The data analyzed in this study are available in Fig share https://doi.org/10.6084/m9.figshare.19119464.v1.
REFERENCES
- 1. Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431‐440. [DOI] [PubMed] [Google Scholar]
- 2. Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760‐1769. [DOI] [PubMed] [Google Scholar]
- 3. King TE, Bradford WZ, Castro‐Bernardini S, et al. A phase 3 trial of Pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083‐2092. [DOI] [PubMed] [Google Scholar]
- 4. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of Nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071‐2082. [DOI] [PubMed] [Google Scholar]
- 5. Majewski S, Białas AJ, Buchczyk M, et al. A multicentre retrospective observational study on polish experience of Pirfenidone therapy in patients with idiopathic pulmonary fibrosis: the PolExPIR study. BMC Pulm Med. 2020;20:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kreuter M, Wuyts WA, Wijsenbeek M, et al. Health‐related quality of life and symptoms in patients with IPF treated with Nintedanib: analyses of patient‐reported outcomes from the INPULSIS® trials. Respir Res. 2020;21:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Witt S, Krauss E, Barbero MAN, et al. Psychometric properties and minimal important differences of SF‐36 in idiopathic pulmonary fibrosis. Respir Res. 2019;20:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence‐based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piotrowski W, Bestry I, Białas A, et al. Guidelines of the polish respiratory society for diagnosis and treatment of idiopathic pulmonary fibrosis. Adv Respir Med. 2020;88:42‐94. 10.5603/ARM.2020.0081 [DOI] [PubMed] [Google Scholar]
- 10. Swigris JJ, Brown KK, Behr J, et al. The SF‐36 and SGRQ: validity and first look at minimum important differences in IPF. Respir Med. 2010;104:296‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swigris JJ, Esser D, Conoscenti CS, Brown KK. The psychometric properties of the st. george's respiratory questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: a literature review. Health Qual Life Outcomes. 2014;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahler DA, Harver A, Rosiello R, Daubenspeck JA. Measurement of respiratory sensation in interstitial lung disease. Chest. 1989;96:767‐771. [DOI] [PubMed] [Google Scholar]
- 13. Papiris SA, Daniil ZD, Malagari K, et al. Τhe medical research council dyspnea scale in the estimation of disease severity in idiopathic pulmonary fibrosis. Respir Med. 2005;99:755‐761. [DOI] [PubMed] [Google Scholar]
- 14. Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Chest. 1984;85:751‐758. [DOI] [PubMed] [Google Scholar]
- 15. Birring SS. Development of a symptom specific health status measure for patients with chronic cough: leicester cough questionnaire (LCQ). Thorax. 2003;58:339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vries J, Michielsen H, Heck GL, Drent M. Measuring fatigue in sarcoidosis: the fatigue assessment scale (FAS). Br J Health Psychol. 2004;9:279‐291. [DOI] [PubMed] [Google Scholar]
- 17. Tzanakis N, Samiou M, Lambiri I, Antoniou K, Siafakas N, Bouros D. Evaluation of health‐related quality‐of‐life and dyspnea scales in patients with idiopathic pulmonary fibrosis. correlation with pulmonary function tests. Eur J Intern Med. 2005;16:105‐112. [DOI] [PubMed] [Google Scholar]
- 18. Kalluri M, Luppi F, Ferrara G. What patients with idiopathic pulmonary fibrosis and caregivers want: filling the gaps with patient reported outcomes and experience measures. Am J Med. 2020;133:281‐289. [DOI] [PubMed] [Google Scholar]
- 19. Ferrara G, Luppi F, Birring SS, et al. Best supportive care for idiopathic pulmonary fibrosis: current gaps and future directions. Eur Respir Rev. 2018;27:170076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kreuter M, Swigris J, Pittrow D, et al. The clinical course of idiopathic pulmonary fibrosis and its association to quality of life over time: longitudinal data from the INSIGHTS‐IPF registry. Respir Res. 2019;20:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azuma A, Taniguchi H, Inoue Y, et al. Nintedanib in Japanese patients with idiopathic pulmonary fibrosis: a subgroup analysis of the INPULSIS® randomized trials. Respirology. 2017;22:750‐757. [DOI] [PubMed] [Google Scholar]
- 22. Martinez TY, Pereira CAC, dos Santos ML, Ciconelli RM, Guimarães SM, Martinez JAB. Evaluation of the short‐form 36‐item questionnaire to measure health‐related quality of life in patients with idiopathic pulmonary fibrosis. Chest. 2000;117:1627‐1632. [DOI] [PubMed] [Google Scholar]
- 23. Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health‐related quality of life in patients with idiopathic pulmonary fibrosis‐cross‐sectional and longitudinal study. Intern Med. 2007;46:1533‐1542. [DOI] [PubMed] [Google Scholar]
- 24. Alhamed EH. Pirfenidone treatment in idiopathic pulmonary fibrosis: a Saudi experience. Ann Thorac Med. 2015;10:38‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nathan SD, Costabel U, Albera C, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respir Med. 2019;153:44‐51. [DOI] [PubMed] [Google Scholar]
- 26. Turner‐Warwick M, Burrows B, Johnson A. Cryptogenic fibrosing alveolitis: clinical features and their influence on survival. Thorax. 1980;35:171‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Key AL, Holt K, Hamilton A, Smith JA, Earis JE. Objective cough frequency in idiopathic pulmonary fibrosis. Cough. 2010;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Manen MJG, Birring SS, Vancheri C, et al. Effect of Pirfenidone on cough in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2017;50:1701157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kølner‐Augustson L, Prior TS, Skivild V, Aalestrup A, Bendstrup E. Fatigue in idiopathic pulmonary fibrosis measured by the Fatigue Assessment Scale during antifibrotic treatment. Eur Clin Respir J. 2020;8:1853658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonella F, Wijsenbeek M, Molina‐Molina M, et al. European IPF patient charter: unmet needs and a call to action for healthcare policymakers. Eur Respir J. 2016;47:597‐606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data analyzed in this study are available in Fig share https://doi.org/10.6084/m9.figshare.19119464.v1.


