TO THE EDITOR:
Allogeneic hematopoietic cell transplantation (HCT) is paramount to the treatment of relapsed refractory hematologic malignancies. In the absence of a matched related or unrelated donor, a mismatched unrelated donor (MMUD) increases the donor options, particularly for patients from ethnic minorities. However, MMUD HCTs have historically conveyed an increased risk of severe (grade 3-4) acute graft-versus-host disease (aGVHD) and treatment-related mortality (TRM).1, 2, 3, 4
Abatacept, cytotoxic T-cell lymphocyte-4-immunoglobulin, is a T-cell costimulation blockade agent5 that was granted US Food and Drug Administration (FDA) approval for the prevention of aGVHD in patients receiving unrelated donor HCT, in part based on the results of the Abatacept 2 trial (ABA2, NCT01743131). ABA2 was a multicenter phase 2 trial that evaluated abatacept in addition to standard GVHD prophylaxis with a calcineurin inhibitor and methotrexate (CNI/MTX) for GVHD prevention in 8/8 HLA-matched unrelated donor (MUD) HCT and 7/8 MMUD HCT. Patients in the 8/8 MUD cohort were assigned to a randomized, double-blinded, placebo-controlled stratum, whereas patients in the 7/8 MMUD cohort were assigned to a single-arm open-label stratum compared with a prespecified Center for International Blood and Marrow Transplant Research (CIBMTR) cohort. The 7/8 MMUD cohort demonstrated a significant reduction in grade 3 to 4 aGVHD compared with the CIBMTR cohort receiving CNI/MTX alone. The lower rates of grade 3 to 4 aGVHD translated into significant improvements in TRM and overall survival (OS) at 2 years for patients receiving abatacept.6
The ABA2 trial and subsequent FDA approval paved the way for real-world data that could validate these results and overcome the limitations of this study. One such limitation was that the ABA2 trial included pediatric and adult patients in the analysis, but outcomes were not reported separately for each group, which could raise translation queries for either group. Furthermore, patients treated on clinical trials may benefit from selection bias associated with study eligibility and participation, which could lead to improved outcomes compared with patients treated off study. The objective of this analysis was to determine the real-world impact of abatacept combined with CNI/MTX in patients not enrolled on the ABA2 trial, focusing on the development of aGVHD and chronic GVHD (cGVHD), survival, and relapse in pediatric and adult patients with hematologic malignancies receiving 7/8 MMUD HCT.
We conducted a multicenter retrospective analysis of outcomes for pediatric and adult patients receiving a 7/8 MMUD HCT with bone marrow (BM) or peripheral blood stem cells for hematologic malignancies who received GVHD prophylaxis with abatacept (4 doses, 10 mg/kg per dose, on days −1, +5, +14, and +28) combined with CNI/MTX. The analysis included adult patients who underwent transplantation at Emory University and pediatric patients who underwent transplantation at the Children’s Health care of Atlanta and Boston Children’s Hospital between January 2015 and December 2021. Data were analyzed for all patients and for pediatric and adult patients as separate cohorts. Patients were excluded if they were on the ABA2 trial, not in remission at the time of HCT, received a prior HCT, or received GVHD prophylaxis different from CNI/MTX + abatacept. The institutional review boards of Children’s Health care of Atlanta, Emory University, and the Dana-Farber Cancer Institute approved this study, which was conducted in accordance with the Declaration of Helsinki.
The patient characteristics are described in Table 1. Continuous variables were calculated as medians and interquartile ranges, whereas categorical variables were presented as counts and percentages. Group comparisons were performed using the Wilcoxon rank-sum tests and χ2 tests. Grade 2 to 4 aGVHD, grade 3 to 4 aGVHD, moderate and severe cGVHD, TRM, and relapse were described as cumulative incidence estimates. These variables were estimated using competing risk analysis for all patients, in which death or relapse acted as a competing event. Gray's tests were used to compare the outcomes between adult and pediatric patients. OS, disease-free survival (DFS), and the composite end point of grade 3 to 4 aGVHD-free, severe cGVHD-free, relapse-free survival (GRFS) are described as Kaplan-Meier survival probability estimates. Log-rank tests were used to compare the curves between adult and pediatric patients. Patients contributed person-time until the date of the event, the competing event if applicable, or until their last follow-up, whichever occurred first. All estimates were accompanied by 95% confidence intervals (CI). Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Table 1.
Baseline characteristics of participants
| Characteristic | Level | Overall N = 50 |
Pediatric N = 26 |
Adult N = 24 |
|---|---|---|---|---|
| Age (y), median (range) | 20.8 (1.2-71.9) | 11 (1.2-21.6) | 61.7 (22.5-71.9) | |
| Sex, n (%) | Female | 14 (28) | 8 (31) | 6 (25) |
| Male | 36 (72) | 18 (69) | 18 (75) | |
| Race, n (%) | Non-White | 23 (46) | 14 (54) | 9 (38) |
| White | 27 (54) | 12 (46) | 15 (63) | |
| Disease, n (%) | ALL | 8 (16) | 7 (27) | 1 (4) |
| AML | 19 (38) | 12 (46) | 7 (29) | |
| CML | 3 (6) | 3 (12) | 0 (0) | |
| MDS | 16 (32) | 3 (12) | 13 (54) | |
| Other | 4 (8) | 1 (4) | 3 (13) | |
| CIBMTR disease stage, n (%) | Advanced | 13 (27) | 2 (8) | 11 (48) |
| Early | 29 (60) | 19 (73) | 10 (43) | |
| Intermediate | 7 (14) | 5 (19) | 2 (9) | |
| Conditioning regimen, n (%) | Busulfan/cyclophosphamide | 7 (14) | 7 (27) | 0 (0) |
| Busulfan/fludarabine | 11 (22) | 9 (35) | 2 (8) | |
| Fludarabine/melphalan | 22 (44) | 2 (8) | 20 (83) | |
| Other | 3 (6) | 1 (4) | 2 (8) | |
| Total body irradiation/cyclophosphamide | 7 (14) | 7 (27) | 0 (0) | |
| Conditioning intensity, n (%) | Myeloablative | 27 (54) | 24 (92) | 3 (13) |
| Reduced intensity | 23 (46) | 2 (8) | 21 (88) | |
| Graft type, n (%) | BM | 28 (56) | 24 (92) | 4 (17) |
| Peripheral blood | 22 (44) | 2 (8) | 20 (83) |
ALL, acute lymphatic leukemia; AML, acute myelogenous leukemia; CIBMTR, Center for International Blood and Marrow Transplantation Research; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome.
Fifty consecutive patients (26 pediatric patients and 24 adult patients) met the inclusion criteria. The “pediatric cohort” included 26 patients who were aged ≤21 years (1-21 years) and the “adult cohort” included 24 patients who were >21 years (22-72 years). The median follow-up was 759 days (22-2001 days). There were a few expected imbalances between the 2 cohorts in terms of patient, disease, and transplantation characteristics (Table 1). In the pediatric cohort, 73% had an early disease status compared with 43% in the adult group. The most common hematologic malignancies in the pediatric cohort were acute myelocytic leukemia, followed by acute lymphoblastic leukemia compared to myelodysplastic syndrome, and acute lymphoblastic leukemia in the adult cohort. In the pediatric cohort, 92% received myeloablative conditioning with busulfan/fludarabine as the most common, whereas 88% of the adult cohort received reduced-intensity conditioning with fludarabine/melphalan as the most common conditioning regimen. In the pediatric cohort, 92% received BM grafts, whereas 83% of the adult cohort received peripheral blood stem cell grafts.
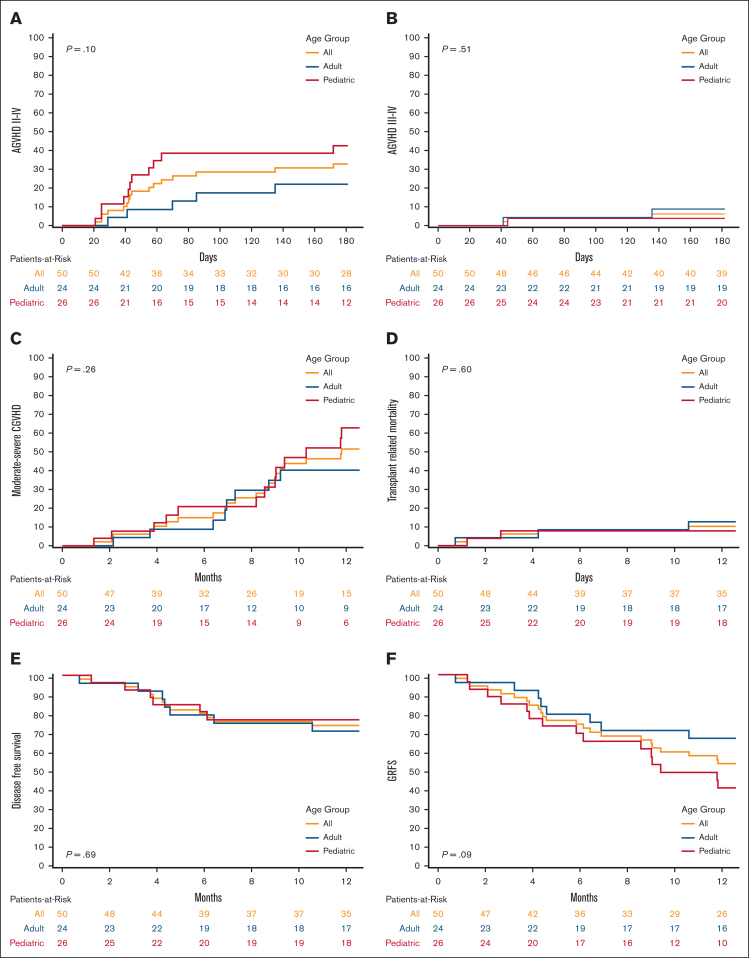
Grade 2 to 4 aGVHD at day 180 was 33% (95% CI, 20-46), 43% (95% CI, 23-61), and 22% (95% CI, 8-41) for all patients, pediatric patients, and adult patients, respectively (P = .10, Figure 1). Grade 3 to 4 aGVHD at day 180 was 6% (95% CI, 2-15), 4% (95% CI, 0.3-17), and 9% (95% CI, 1-25) for all, pediatric, and adult patients, respectively (P = .51). Moderate-to-severe cGVHD at 1 year was 51% (95% CI, 34-65), 61% (95% CI, 36-79), and 39% (95% CI, 18-60) for all, pediatric, and adult patients, respectively (P = .26). TRM at 1 year was 10% (95% CI, 4-20), 8% (95% CI, 1-22), and 13% (95% CI, 3-29) for all, pediatric, and adult patients, respectively (P = .6). The OS at 1 year was 82% (95% CI, 68-90), 84% (95% CI, 64-94), and 79% (95% CI, 57-91) for all, pediatric, and adult patients, respectively (P = .63). DFS at 1 year was 74% (95% CI, 59-84), 77% (95% CI, 55-89), and 71% (95% CI, 48-85) for all, pediatric, and adult patients, respectively (P = .69). GRFS at 1 year was 53% (95% CI, 39-66), 41% (95% CI, 22-59), and 67% (95% CI, 44-82) for all, pediatric, and adult patients, respectively (P = .09). Neither cohort had end-organ cytomegalovirus disease or posttransplantation lymphoproliferative disease.
Figure 1.
Cumulative incidence and Kaplan-Meier plots comparing all, adult and pediatric patients. (A) Cumulative incidence of grade 2 to 4 AGVHD; (B) Cumulative incidence of grade 3 to 4 AGVHD; (C) Cumulative incidence of moderate-to-severe CGVHD; (D) Cumulative incidence of TRM; (E) Kaplan-Meier plot of DFS; (F) Kaplan-Meier plot of GRFS.
Our real-world results using abatacept prophylaxis for 7/8 MMUD HCT compared favorably with the ABA2 trial results, including low incidences of grade 3 to 4 aGVHD and TRM and encouraging DFS. These results are promising given that the off-study patients had twice as much advanced disease as the patients in the ABA2 trial. This was especially true in the adult cohort, in which 48% had advanced disease stage. Notably, the cumulative incidence of moderate-to-severe cGVHD was numerically higher in the pediatric cohort, albeit not significantly (P = .26). This finding was surprising given that younger recipient age and the use of BM grafts have been associated with lower rates of cGVHD.7 A possible reason for this may be related to differences in grading cases of cGVHD due to center effect between pediatric and adult centers. Studies have reported inconsistencies in cGVHD evaluation, even among experienced transplant physicians, due to the complexities in cGVHD diagnosis and staging, which could have also contributed to these results.8,9 These reasons may explain the lower rates of TRM and higher rates of DFS in the pediatric cohort, despite having higher rates of moderate-to-severe cGVHD. Pediatric patients also had a lower relapse risk after transplantation, as evidenced by a lower advanced disease stage compared with the adult cohort (8% vs 48%, respectively), which could have also contributed to the higher DFS. Finally, the higher rates of moderate-to-severe cGVHD in the pediatric cohort contributed to a lower GRFS (41% vs 67%). Although this did not translate to increased TRM or decreased DFS in the pediatric cohort, it likely resulted in decreased quality of life (QOL).10 This analysis did not compare the QOL between pediatric and adult patients and further studies are needed to evaluate the impact of GVHD on QOL.
There are limitations to our analysis, including center effects and small sample sizes. The results of this analysis were based on 1 adult and 2 pediatric centers and therefore might not be generalizable to all patients. This analysis also included only 50 patients, and larger studies will be helpful in validating these results.
In summary, this study is the first to analyze and report outcomes separately for pediatric and adult patients receiving abatacept for GVHD prophylaxis and the results indicate that both pediatric and adult patients demonstrate favorable outcomes with abatacept. Similar to results from the ABA2 trial, cGVHD rates did not appear to be affected by the 4 dose abatacept regimen, and an ongoing study is evaluating extended dosing of abatacept to further improve cGVHD. The real-world results of this analysis suggest that the addition of abatacept to standard immunoprophylaxis for patients receiving 7/8 MMUD transplantation can substantially reduce severe aGVHD and improve transplant outcomes in both adult and pediatric patients.
Conflict-of-interest disclosure: M.L.S. reports being a consultant/receiving honorarium at Omeros and Alexion. M.Q. reports receiving honoraria at Vertex and Novartis. L.S.K. reports receiving research funding from Novartis, bluebird bio, EMD Serono, and Magenta; consultancy from Vertex and HiFiBio; patents and royalties: clinical trial, research funding from Bristol Myers Squibb; is a current equity holder in private company Mammoth Biosciences; and is a current holder of stock options in a privately held company. The remaining authors declare no competing financial interests.
Acknowledgments
Contribution: S.R., L.G., J.H., A.A.L., and B.W. designed the study, analyzed the data, and wrote the manuscript; and S.R., L.G., M.G., B.B., Y.S., S.G., A.L.W., K.M.W., M.L.S., L.S.K., J.H., A.A.L., M.Q., and B.W. reviewed the data and edited the manuscript.
Footnotes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 11 December 2022.
Data collected for this study will be made available with publication to anyone who wishes to access the data for any purpose from the corresponding author, Sharmila Raghunandan (sraghu2@emory.edu; sharmila.raghunandan@gmail.com).
References
- 1.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 2.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw PJ, Kan F, Woo Ahn K, et al. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood. 2010;116(19):4007–4015. doi: 10.1182/blood-2010-01-261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5(3):185–186. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 6.Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021;39(17):1865–1877. doi: 10.1200/JCO.20.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pusic I, Pavletic SZ. Challenges in conducting studies in chronic graft-versus-host disease. Clin Hematol Int. 2019;1(1):36–44. doi: 10.2991/chi.d.190314.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell SA, Jacobsohn D, Thormann Powers KE, et al. A multicenter pilot evaluation of the National Institutes of Health chronic graft-versus-host disease (cGVHD) therapeutic response measures: feasibility, interrater reliability, and minimum detectable change. Biol Blood Marrow Transplant. 2011;17(11):1619–1629. doi: 10.1016/j.bbmt.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Kim H, Ho V, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 2006;38(4):305–310. doi: 10.1038/sj.bmt.1705434. [DOI] [PubMed] [Google Scholar]