Abstract
The guanosine analogue ribavirin was tested for antiviral activity in two neural cell lines, human oligodendrocytes and rat glia, against Borna disease virus (BDV) strains V and He/80. Ribavirin treatment resulted in lower levels of virus and viral transcripts within 12 h. Addition of guanosine but not adenosine resulted in a profound reduction of the ribavirin effect. Ribavirin appears to be an effective antiviral agent for treatment of BDV infection in vitro. A likely mechanism for its activity is reduction of the intracellular GTP pool, resulting in inhibition of transcription and capping of BDV mRNAs.
Borna disease virus (BDV) is a nonsegmented negative-strand RNA virus that transcribes and replicates in the nucleus of infected cells (4, 7) and employs a number of strategies to direct expression of its genome including RNA splicing, overlap of transcription units and transcriptional signals, readthrough of transcription initiation sites, and differential use of translational initiation codons (reviewed in reference 39). In concert, these result in noncytolytic replication and persistence and may contribute to BDVs neurotropism. Although BDV is classically associated with meningoencephalitis of horses and sheep in central Europe, more recent data (3, 5, 26, 27, 29, 46) indicate that the host range of BDV probably extends to most warmblooded animals and suggest that the geographic distribution includes Asia and North America as well as greater Europe (14, 21, 26, 31, 32, 46).
The spectrum of clinical disease due to BDV infection ranges from subtle impairment of learning and memory to progressive, immune-mediated, frequently fatal meningoencephalitis (1, 9, 33, 38, 41). Asymptomatic infection has also been described (14, 21, 32, 37). Recognition of BDV’s broad host range and predilection to target higher integrative circuits of the brain and the observation that disturbances in infected animals are reminiscent of some aspects of human neuropsychiatric diseases led to the proposal that BDV might be implicated in their pathogenesis. Although there is consensus that humans are likely to be susceptible to BDV infection, the epidemiology and clinical consequences of human infection remain controversial (reviewed in reference 18).
Amantadine has been reported to be effective in vivo and in vitro against one strain of BDV. Bode and coworkers (2) described clearance of strain Hu-HI from cultured oligodendrocytes and from peripheral blood mononuclear cells of a patient with bipolar disorder. However, an antiviral effect of amantadine was not confirmed with other BDV isolates in studies of infected cultured cells, rodents, or horses (6, 11, 13, 16, 42).
In an effort to identify antiviral agents for BDV, we tested an encoded panel of 10 nucleoside analogues (Table 1) for activity against the two best-characterized laboratory strains of BDV, strains V and He/80, in two neural cell lines, OL (human oligodendrocytes) and C6 (rat glia). Strain V was originally isolated from naturally infected horse brain in 1929 (48), and strain He/80 was originally isolated from naturally infected horse brain in 1980 (19). Both viruses were later passaged in rabbits, rats, and a variety of cultured cells. Each of the compounds was initially tested with uninfected cells to establish a toxicity threshold defined as growth arrest (Table 1). Thereafter, efficacy against BDV was tested by using 10-fold dilutions of drug beginning at the toxicity threshold. One compound of the 10 examined, ribavirin, significantly reduced viral load at concentrations of 2 to 200 μM. No effect was seen with the other compounds, including 2′,3′-dideoxyinosine (ddI) (Table 1).
TABLE 1.
Toxic threshold and efficacy of nucleoside analogues tested for properties against BDV
| Compound | Toxic thresholda (μM) for:
|
Antiviral activity (fold)b | |
|---|---|---|---|
| OL | C6 | ||
| 3-Deazaguanine | 20 | 200 | |
| ICN 10169 | 2 | 2 | |
| ICN 10776 | 20 | 2 | |
| ICN 15100 | 200 | 200 | |
| 2′,3′-Dideoxyinosine (ddI) | 200 | 200 | |
| Ribavirin | 200 | 200 | >10 |
| ICN 17261c | 20 | 200 | |
| FTCd | 200 | 200 | |
| Pyrazofurin | 2 | 2 | |
| ICN 17377c | 200 | 20 | 5–10 |
Maximum concentration at which replication of treated cells was within 10% of that of the vehicle-treated cells after 1 week.
Reduction of virus load determined after 1 week of treatment and compared to vehicle-treated control at the highest nontoxic concentration.
Ribavirin analogue.
β-l-2′,3′-Dideoxy-5-fluoro-3′-thiacytidine.
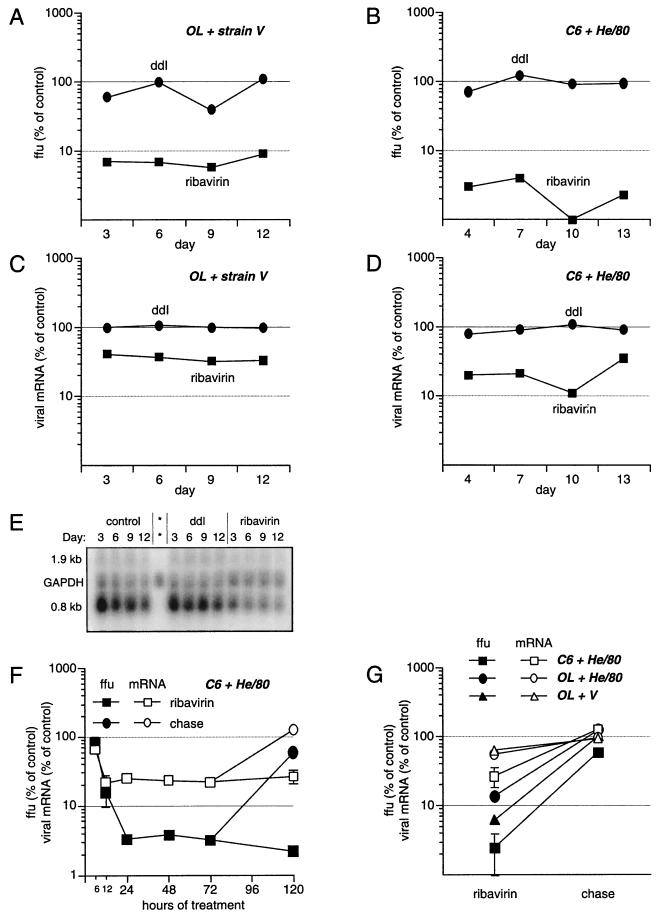
To further characterize the antiviral effect of ribavirin, OL cells persistently infected with strain V (Fig. 1A and C) and C6 cells persistently infected with strain He/80 (Fig. 1B and D) were treated for up to 13 days with 20 μM ribavirin or ddI (negative control). At 3, 6, 9, and 12 days (OL cells) or 4, 7, 10, and 13 days (C6 cells) the numbers of host cells were determined and infectious virus and total cellular RNA were isolated from cells treated with ribavirin, ddI, or vehicle (dimethyl sulfoxide without antiviral drug). Virus was titered by focus immunoassay using fetal rat glial cells and antiserum against viral nucleo- and phosphoproteins (P) (35). mRNA originating from the second transcription unit encoding the BDV P was quantitated by Northern hybridization (24) and PhosphorImager (Molecular Dynamics) analysis. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as host cell mRNA control.
FIG. 1.
Effect of ribavirin on viral titers and RNA levels. BDV strain V- (A and C) or He/80- (B and D) infected cells were treated with 20 μM ribavirin (squares) or 20 μM ddI (circles). Virus and RNA were isolated in parallel. All experiments were done in duplicate. (A and B) Viral titers in focus-forming units (ffu) normalized to the number of cells were determined in a focus immunoassay and are given as the percentages of the titers for vehicle-treated controls (with 100% corresponding to approximately 5 × 106 ffu/ml from 4 × 106 cells). (C and D) Amounts of viral 0.8-kb mRNA were determined by quantification of Northern blots using a PhosphorImager (Molecular Dynamics) and are given as the percentages of the amounts for vehicle-treated controls, normalized to the mRNA encoding GAPDH. (E) Representative Northern blot used for quantification of mRNA levels in strain V-infected OL cells. The asterisks indicate a negative control with RNA isolated from uninfected cells. The 1.9-kb RNA is a viral, non-mRNA also containing the P open reading frame. (F) Viral titers (filled squares) and amount of 0.8-kb mRNA (open squares) in the first 6, 12, 24, 48, and 72 h of ribavirin treatment, followed by a release from ribavirin for 2 days (filled circle for viral titer and open circle for viral mRNA). (G) Effect of the host cell on ribavirin susceptibility. Cells in the acute phase of infection with strain V or He/80 were treated for 2 weeks with ribavirin.
Viral titers and viral transcripts rapidly declined in ribavirin- but not in ddI-treated cells. In OL cells, titers of strain V were reduced by more than 90% within 3 days and remained at this level through the end of the 12-day sampling period (Fig. 1A). The ribavirin effect was even more striking in C6 cells, where titers of He/80 were reduced by 97% within 4 days and 99% at day 10 (Fig. 1B). BDV transcripts were reduced by 60% in OL cells (Fig. 1C) and 80% in C6 cells (Fig. 1D).
To determine the kinetics of ribavirin’s antiviral activity, BDV P gene mRNA expression and titers were studied at shorter time intervals in C6 cells infected with strain He/80. Six hours into treatment with ribavirin, levels of P gene mRNA were reduced to 65% of untreated control values; steady-state levels of approximately 20% of untreated control values were observed within 12 h of initiating ribavirin treatment (Fig. 1F, open squares). Viral titers also fell rapidly but did not reach nadirs until 24 h after the addition of ribavirin. The reduction in viral titer was 20% at 6 h, 85% at 12 h, and 97% at 24 h (Fig. 1F, filled squares). The response of BDV to ribavirin was also brisk following withdrawal of treatment, suggesting rapid turnover of viral RNA. Viral titers and mRNA expression recovered fully within 2 days after removal of ribavirin from cultures (Fig. 1F, filled and open circles; chase from 72 [day 3] to 120 h [day 5] posttreatment). The time course for the effect of ribavirin on BDV P gene expression and titer was similar in strain V-infected OL cells (data not shown).
The antiviral effect of ribavirin was less pronounced on strain V in OL cells (Fig. 1A) than on strain He/80 in C6 cells (Fig. 1B). Thus, to assess the relative importance of viral strain and host cell phenotype on ribavirin effect, OL cells were infected with He/80 and treated with ribavirin. Although strain He/80 was highly susceptible to ribavirin in C6 cells (2.5% ± 1.5% of control titers after 2 weeks of treatment; Fig. 1G), it was less inhibited in OL cells (13.6% ± 1.7% of control values; Fig. 1G). Interestingly, in OL cells, strain V was more sensitive to ribavirin (6.2% ± 0.2% of control titers) than He/80. Together, these findings suggest that the potency of ribavirin is determined in part by virus-host interactions.
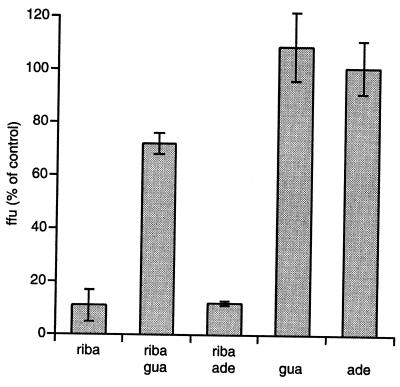
In X-ray diffraction studies, ribavirin closely resembles guanosine (36) and is postulated to interfere with the activity of the inosine monophosphate (IMP) dehydrogenase (43), the enzyme that converts IMP to xanthosine monophosphate, a precursor molecule in the biosynthesis of GTP and dGTP. The resulting depletion of the intracellular GTP pool suggests two mechanisms by which ribavirin might exert antiviral activity: (i) interference with viral transcription and (ii) inhibition of mRNA capping guanylylation after phosphorylation at the 5′ position (10, 12, 47). There is evidence that both mechanisms are operative in BDV-infected cells treated with ribavirin. Ribavirin-treated cells had lower levels of viral mRNAs (Fig. 1B and D), a result consistent with inhibition of transcription. An even more profound ribavirin effect was observed with respect to viral titers (Fig. 1A and C). The latter finding is consistent with an additional functional deficit in BDV transcripts due to inhibition of capping and reduced efficiency of translation. Support for the hypothesis that ribavirin acts through interference with GTP/dGTP biosynthesis has been found in other viral systems, for example, measles virus, where the antiviral effect of ribavirin in vitro is abrogated by addition of guanosine but not adenosine (43). To test whether a related mechanism applies to the antiviral effect of ribavirin in BDV, we measured viral titers in strain V-infected OL cells after 3 days of treatment with ribavirin, adenosine, or guanosine, individually or in combination (Fig. 2). Viral titers were reduced to 10% in the presence of ribavirin or a combination of ribavirin and adenosine. In contrast, an equimolar amount of guanosine significantly inhibited the antiviral activity of ribavirin, allowing viral titers to reach 70% of the level obtained in the negative controls (Fig. 2). Guanosine or adenosine had no effect on viral replication in the absence of ribavirin.
FIG. 2.
BDV strain V-infected OL cells were treated for 3 days with 10 μM ribavirin (riba), 10 μM adenosine (ade), and 10 μM guanosine (gua) individually or in the indicated combinations. Virus was quantified by focus-immunoassay titration and is given as the percentage of the vehicle-treated control.
While this article was in preparation, Mizutani and colleagues (30) showed through reverse transcription-PCR experiments that ribavirin treatment resulted in lower levels of BDV RNAs in a non-neural cell line (MDCK) infected with strain He/80 (30). Our experiments confirm and extend these findings by demonstrating that ribavirin has activity against two different strains of BDV (strain V and He/80) in two neural cell lines of different lineages (human oligodendrocytes and rat glia). The significance of our data is underscored by the controversy in the literature concerned with effects of amantadine on BDV replication where strains and cell lines are considered to be important variables. Furthermore, we have monitored the effect on virus titer as well as viral transcripts and provided data that suggest that ribavirin not only reduces the amount of viral RNA but also interferes with their function as messengers.
In vivo, ribavirin is effective in the treatment of respiratory syncytial virus infections (15, 44) and, in combination with interferon α, of chronic hepatitis C virus infections (8, 28). Although our in vitro studies indicate that ribavirin inhibits BDV at 2 μM (data not shown), a concentration well below that associated with toxicity in vitro (200 μM) and within the physiologic concentration in plasma of 0.5 to 10 μM (22), it is conceivable that use of ribavirin in BDV-infected animals may enhance rather than inhibit disease. Ribavirin was recently shown to promote Th1 responses (20, 25, 34, 45). It has been suggested that this Th1 effect may contribute to antiviral activity in hepatitis C virus infections (20). However, severity of disease in BDV infections is correlated with the Th1 immune response (17, 40). Indeed, although vaccination of rats with BDV nucleoprotein constructs results in enhanced Th1 responses and viral clearance, the clinical outcome is adverse due to increased mortality and morbidity (23). Thus, it is difficult to predict whether the antiviral or Th1 effect of ribavirin will prevail in vivo. Future work will be directed toward assessing the therapeutic utility of ribavirin by treatment of experimental BDV infection and exploring ribavirin analogues that have selective antiviral or immunomodulatory activity.
Acknowledgments
We thank Kanda Ramasamy and Guangyi Wang for providing the compounds listed in Table 1, and Georg Pauli for providing BDV strain V and human oligodendrocytes.
REFERENCES
- 1.Bautista J R, Schwartz G J, De La Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–40. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 2.Bode L, Dietrich D E, Stoyloff R, Emrich H M, Ludwig H. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet. 1997;349:178–179. doi: 10.1016/S0140-6736(05)60979-8. [DOI] [PubMed] [Google Scholar]
- 3.Bode L, Durrwald R, Ludwig H. Borna virus infections in cattle associated with fatal neurological disease. Vet Rec. 1994;135:283–284. doi: 10.1136/vr.135.12.283. [DOI] [PubMed] [Google Scholar]
- 4.Briese T, de la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplazi P, Waldvogel A, Stitz L, Braun U, Ehrensperger F. Borna disease in naturally infected cattle. J Comp Pathol. 1994;111:65–72. doi: 10.1016/s0021-9975(05)80112-4. [DOI] [PubMed] [Google Scholar]
- 6.Cubitt B, de la Torre J C. Amantadine does not have antiviral activity against Borna disease virus. Arch Virol. 1997;142:2035–2042. doi: 10.1007/s007050050220. [DOI] [PubMed] [Google Scholar]
- 7.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis G L, Esteban-Mur R, Rustgi V, Hoefs J, Gordon S C, Trepo C, Shiffman M L, Zeuzem S, Craxi A, Ling M H, Albrecht J. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 9.Dittrich W, Bode L, Ludwig H, Kao M, Schneider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989;26:818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson B, Helgstrand E, Johansson N G, Larsson A, Misiorny A, Noren J O, Philipson L, Stenberg K, Stening G, Stridh S, Oberg B. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother. 1977;11:946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Dunia D, Cubitt B, de la Torre J C. Mechanism of Borna disease virus entry into cells. J Virol. 1998;72:783–788. doi: 10.1128/jvi.72.1.783-788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami B B, Borek E, Sharma O K, Fujitaki J, Smith R A. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun. 1979;89:830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- 13.Grabner A, Herzog S, Hafner A, Schmidt P. Presented at the Second International Bornavirus Meeting, Freiburg, Germany. 1998. BDV infections of horses in Germany: clinical and epidemiological aspects. [Google Scholar]
- 14.Hagiwara K, Kawamoto S, Takahashi H, Nakamura Y, Nakaya T, Hiramune T, Ishihara C, Ikuta K. High prevalence of Borna disease virus infection in healthy sheep in Japan. Clin Diagn Lab Immunol. 1997;4:339–344. doi: 10.1128/cdli.4.3.339-344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall C B, McBride J T, Walsh E E, Bell D M, Gala C L, Hildreth S, Ten Eyck L G, Hall W J. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind study. N Engl J Med. 1983;308:1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 16.Hallensleben W, Zocher M, Staeheli P. Borna disease virus is not sensitive to amantadine. Arch Virol. 1997;142:2043–2048. doi: 10.1007/s007050050221. [DOI] [PubMed] [Google Scholar]
- 17.Hatalski C G, Hickey W F, Lipkin W I. Evolution of the immune response in the central nervous system following infection with Borna disease virus. J Neuroimmunol. 1998;90:137–142. doi: 10.1016/s0165-5728(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 18.Hatalski C G, Lewis A J, Lipkin W I. Borna disease. Emerg Infect Dis. 1997;3:129–135. doi: 10.3201/eid0302.970205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 20.Hultgren C, Milich D R, Weiland O, Sallberg M. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol. 1998;79:2381–2391. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 21.Kao M, Hamir A N, Rupprecht C E, Fu Z F, Shankar V, Koprowski H, Dietzschold B. Detection of antibodies against Borna disease virus in sera and cerebrospinal fluid of horses in the USA. Vet Rec. 1993;132:241–244. doi: 10.1136/vr.132.10.241. [DOI] [PubMed] [Google Scholar]
- 22.Khakoo S, Glue P, Grellier L, Wells B, Bell A, Dash C, Murray-Lyon I, Lypnyj D, Flannery B, Walters K, Dusheiko G M. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol. 1998;46:563–570. doi: 10.1046/j.1365-2125.1998.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis A J, Whitton J L, Hatalski C G, Weissenböck H, Lipkin W I. Effect of immune priming on Borna disease. J Virol. 1999;73:2541–2546. doi: 10.1128/jvi.73.3.2541-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipkin W I, Travis G H, Carbone K M, Wilson M C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci USA. 1990;87:4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M F, Ning Q, Pope M, Mosmann T, Leibowitz J, Ding J W, Fung L S, Rotstein O, Gorczynski R, Levy G A. Resistance of naive mice to murine hepatitis virus strain 3 requires development of a Th1, but not a Th2, response, whereas pre-existing antibody partially protects against primary infection. Adv Exp Med Biol. 1998;440:415–423. doi: 10.1007/978-1-4615-5331-1_52. [DOI] [PubMed] [Google Scholar]
- 26.Lundgren A L, Czech G, Bode L, Ludwig H. Natural Borna disease in domestic animals other than horses and sheep. Zentbl Vetmed. 1993;40:298–303. doi: 10.1111/j.1439-0450.1993.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 27.Malkinson M, Weisman Y, Perl S, Ashash E. A Borna-like disease of ostriches in Israel. Curr Top Microbiol Immunol. 1995;190:31–38. doi: 10.1007/978-3-642-78618-1_3. [DOI] [PubMed] [Google Scholar]
- 28.McHutchison J G, Gordon S C, Schiff E R, Shiffman M L, Lee W M, Rustgi V K, Goodman Z D, Ling M H, Cort S, Albrecht J K. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 29.Metzler A, Ehrensperger F, Wyler R. Natural Borna virus infection in rabbits. Zentbl Vetmed. 1978;25:161–164. [PubMed] [Google Scholar]
- 30.Mizutani T, Inagaki H, Araki K, Kariwa H, Arikawa J, Takashima I. Inhibition of Borna disease virus replication by ribavirin in persistently infected cells. Arch Virol. 1998;143:2039–2044. doi: 10.1007/s007050050440. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Asahi S, Nakaya T, Bahmani M K, Saitoh S, Yasui K, Mayama H, Hagiwara K, Ishihara C, Ikuta K. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells derived from domestic cats in Japan. J Clin Microbiol. 1996;34:188–191. doi: 10.1128/jcm.34.1.188-191.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura Y, Kishi M, Nakaya T, Asahi S, Tanaka H, Sentsui H, Ikeda K, Ikuta K. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells from healthy horses in Japan. Vaccine. 1995;13:1076–1079. doi: 10.1016/0264-410x(95)00050-b. [DOI] [PubMed] [Google Scholar]
- 33.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent Borna virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 34.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding J W, Liu M F, Rotstein O, Phillips M J, Levy G. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 35.Pauli G, Grunmach J, Ludwig H. Focus-immunoassay for Borna disease virus-specific antigens. Zentbl Vetmed. 1984;31:552–557. doi: 10.1111/j.1439-0450.1984.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 36.Prusiner P, Sundaralingam M. A new class of synthetic nucleoside analogues with broad-spectrum antiviral properties. Nat New Biol. 1973;244:116–118. doi: 10.1038/newbio244116a0. [DOI] [PubMed] [Google Scholar]
- 37.Richt J A, Herzog S, Haberzettl K, Rott R. Demonstration of Borna disease virus-specific RNA in secretions of naturally infected horses by the polymerase chain reaction. Med Microbiol Immunol. 1993;182:293–304. doi: 10.1007/BF00191945. [DOI] [PubMed] [Google Scholar]
- 38.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 39.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 40.Sobbe M, Bilzer T, Gommel S, Nöske K, Planz O, Stitz L. Induction of degenerative brain lesions after adoptive transfer of brain lymphocytes from Borna disease virus-infected rats: presence of CD8+ T cells and perforin mRNA. J Virol. 1997;71:2400–2407. doi: 10.1128/jvi.71.3.2400-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sprankel H, Richarz K, Ludwig H, Rott R. Behavior alterations in tree shrews (Tupaia glis, Diard 1820) induced by Borna disease virus. Med Microbiol Immunol. 1978;165:1–18. doi: 10.1007/BF02121228. [DOI] [PubMed] [Google Scholar]
- 42.Stitz L, Planz O, Bilzer T. Lack of antiviral effect of amantadine in Borna disease virus infection. Med Microbiol Immunol. 1998;186:195–200. doi: 10.1007/s004300050064. [DOI] [PubMed] [Google Scholar]
- 43.Streeter D G, Witkowski J T, Khare G P, Sidwell R W, Bauer R J, Robins R K, Simon L N. Mechanism of action of 1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci USA. 1973;70:1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taber L H, Knight V, Gilbert B E, McClung H W, Wilson S Z, Norton H J, Thurson J M, Gordon W H, Atmar R L, Schlaudt W R. Ribavirin aerosol treatment of bronchiolitis associated with respiratory syncytial virus infection in infants. Pediatrics. 1983;72:613–618. [PubMed] [Google Scholar]
- 45.Tam, R. C., B. Pai, J. Bard, C. Lim, D. R. Averett, U. Phan, and T. Milocanovic. Ribavirin polarizes human T cell responses towards a type 1 cytokine profile. J. Hepatol., in press. [DOI] [PubMed]
- 46.Weissenböck H, Nowotny N, Caplazi P, Kolodziejek J, Ehrensperger F. Borna disease in a dog with lethal meningoencephalitis. J Clin Microbiol. 1998;36:2127–2130. doi: 10.1128/jcm.36.7.2127-2130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wray S K, Gilbert B E, Knight V. Effect of ribavirin triphosphate on primer generation and elongation during influenza virus transcription in vitro. Antiviral Res. 1985;5:39–48. doi: 10.1016/0166-3542(85)90013-0. [DOI] [PubMed] [Google Scholar]
- 48.Zwick W, Seifried O, Witte J. Weitere Beiträge zur Erforschung der Bornaschen Krankheit des Pferdes. Arch Wissensch Prakt Tierheilk. 1929;59:511–545. [Google Scholar]