Abstract
Immobilization of enzymes is one of the protein engineering methods used to improve their thermal and long-term stabilities. Immobilized pectinase has become an essential biocatalyst for optimization in the food processing industry. Herein, nanostructured magnetic nanoparticles were prepared in situ for use as supports to immobilize pectinase. The structural, morphological, optical and magnetic features and the chemical compositions of the nanoparticles were characterized. Nanoparticle agglomeration and low porosity were observed due to the synthetic conditions. These nanoparticles exhibited superparamagnetic behavior, which is desirable for biotechnological applications. The maximum retention rate for the enzyme was observed at pH 4.5 with a value of 1179.3 U/mgNP (units per milligram of nanoparticle), which was equivalent to a 65.6% efficiency. The free and immobilized pectinase were affected by the pH and temperature. The long-term instability caused 40% and 32% decreases in the specific activities of the free and immobilized pectinase, respectively. The effects of immobilization were analyzed with kinetic and thermodynamic studies. These results indicated a significant affinity for the substrate, a decreased reaction rate, and improved thermal stability of the immobilized pectinase. The reusability of the immobilized pectinase was preserved effectively during cycling, with only a 21.2% decrease in activity observed from the first to the last use. Therefore, alternative magnetic nanoparticles are presented for immobilizing and maintaining the thermostability of pectinase.
Keywords: Pectinase, Enzyme immobilization, Magnetic nanoparticles, Cross-linking
1. Introduction
Enzymes are macromolecular proteins that accelerate biochemical reactions [1]. They are classified into various categories according to their molecular activities, such as oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases [2]. Pectinase is an enzyme that breaks down pectin, a complex carbohydrate found in the cell walls of plants [3]. In the food industry, pectinase is used to improve the textures and clarities of fruit juices, wine, and beer via removal of haze-causing proteins and polysaccharides [4,5] as well as in the production of jams and jellies to disintegrate pectin and create a smoother consistency [6].
For industrial application, the lack of reusability and enzyme instability must be overcome, since they result in higher production costs [7]. Different protein engineering technologies, such as stereoselectivity, chemoselectivity, and regioselectivity, have been developed to optimize long-term stability [8]. Immobilization can address these challenges by linking the enzyme to a support matrix to ensure improved performance, rapid recovery, and reutilization [9]. Enzyme immobilization has been used successfully for several decades [10]. However, in the twenty-first century, new matrixes and methods are still required to enhance the catalytic capabilities of enzymes. These qualities, which provide better yields of the intended products, include selectivity, specificity, and decreased inhibition [11]. According to the literature, nanoparticles are used as improved immobilization matrixes due to their high surface areas and high mass transfer capacities, and they are easily recovered by applying a magnetic field [12].
A decline in enzymatic activity can be avoided by using the immobilization procedure, which consists of adsorption, covalent bonding, cross-linking, and nanoparticle immobilization. Literature reports indicate that when an enzyme is attached to a nanoparticle surface in an aqueous medium, the enzymatic activity is enhanced due to the Brownian motions of the nanoparticles. The collisions created by these movements could increase the reaction rates [13,14]. Moreover, magnetic nanoparticles have been researched to reuse enzymes due to their biocompatibility [15]. However, the residual activity of the immobilized enzyme can be affected by enzyme inactivation after the recycling process [16]. Numerous enzymes have been immobilized on Fe3O4, including cholesterol oxidase, laccase, amylase, lipase, and pectinase [17,18]. However, nanoparticle enzyme-based systems have been applied in a variety of processes, including the production of biofuels, bioremediation, and starch degradation, among others [[19], [20], [21]]. The magnetic-chitosan system has attracted interest for decades due to its versatility and broad application range. Because of its high magnetic susceptibility and saturation magnetization, Fe3O4 (magnetite) is the most frequently utilized magnetic material in biotechnology and related fields [22,23]. However, as an alternative to magnetite, Fe2O3 (ferric oxide) is an ideal material for the biomedical and biotechnology sectors. γ-Fe2O3 (maghemite) is one of the four polymorphic forms of ferric oxide. Maghemite has mainly been employed in medical treatments, with applications including magnetic hyperthermia, in vitro diagnostics, and drug delivery [24,25]. Additionally, the Food and Drug Administration (FDA) of the United States and Europe has approved the use of superparamagnetic nanoparticles for these purposes [26]. Immobilized enzymes are used as sweeteners (d-glucose), antioxidants (lipase B), and cocoa butter equivalents (lipase from RM), among other applications [27].
This study employed an innovative approach to create superparamagnetic nanobiocatalysts directly within a chitosan matrix. Extensive characterization was conducted to evaluate the structural, morphological, magnetic, and optical properties of these nanobiocatalysts. Subsequently, these nanoparticles were utilized to immobilize pectinase, and the impacts of various factors, such as the pH, temperature, time, and reusability, were investigated. Through kinetic and thermodynamic analyses, valuable insights were obtained into the nanocatalytic activity resulting from the synthetic process. The comprehensive findings obtained from this research underscore the remarkable potential for use of synthesized magnetic nanoparticles as an exceptionally effective support matrix for pectinase in the fruit processing industry.
2. Experimental section
2.1. Materials
Chitosan (Mw = 190–310 kDa, deacetylation degree of 75–85%), iron (III) chloride hexahydrate (FeCl3·6H2O), iron (II) chloride hexahydrate (FeCl2·6H2O), glutaraldehyde (GA, OHC(CH2)3CHO, 25% v/v), ammonium hydroxide solution (NH4OH, 25 wt%), methanol (CH4O), acetic acid (CH3CO2H), hydrogen peroxide solution (H2O2, 30% w/w), sodium hydroxide (NaOH), 3-APTES (H2N(CH2)3Si(OC2H5)3), pectinase, monobasic potassium phosphate (KH2PO4), dibasic potassium phosphate (K2HPO4), 3,5-dinitrosalicylic acid ((O2N)2C6H2-2-(OH)CO2H) and Bradford's reagent were obtained from Sigma‒Aldrich.
2.2. Synthesis of the magnetic nanoparticles
The magnetic nanoparticles (labeled MNPs) were prepared in two steps. First, 3 g of chitosan was dissolved in 100 mL of a 2% (v/v) acetic acid solution with continuous stirring. Subsequently, 0.4 mL of a 25% glutaraldehyde (GA) solution was added. After 24 h, a chitosan hydrogel was formed. In the second step, 8.34 g of FeCl3 and 3.08 g of FeCl2 were dissolved in 100 mL of deionized water, and the pH was adjusted to 1 with HCl. This solution was added to the hydrogel. Next, 100 mL of a NaOH solution (1.25 mol/L) was added to provide the magnetic nanoparticles. Finally, 100 mL of 8% (v/v) H2O2 in acetic acid was added to the degraded chitosan. The MNPs were separated by magnetic decantation after several washes with deionized water until the pH became neutral (pH 7). However, Fe3O4 nanoparticles are unstable and prone to oxidation. Therefore, the MNPs were calcined in air to form maghemite (γ-Fe2O3), which was stable [28]. The following Eq. (1) and Eq. (2) describe the process:
| (1) |
| (2) |
2.3. Nanoparticle characterization
The crystal structure of the MNP powder was characterized by X-ray diffraction (XRD) using an Empyrean diffractometer (PANalytical) with a Cu anode (λ = 1.5406 Å). The XRD patterns were obtained from 20° to 60° (2θ) with a 0.01° step size. Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy was employed to assess the presence of organic matter in the MNPs. ATR-FTIR spectra were recorded in the 4000–400 cm−1 range using an IRAffinity-1S (Shimadzu) spectrometer. The morphology of the MNPs was investigated with field emission scanning electron microscopy (FESEM, Hitachi, SU3500) operated at 15 kV and equipped with an Aztec X-Max (Oxford) energy dispersive X-ray spectrometry (EDS) system. The specific surface areas (SBET) were determined with the Brunauer–Emmett–Teller (BET) method and a Nova 4200e system (Quantachrome). The magnetic properties of the nanoparticles were determined with a Physical Properties Measurement System MS2024 (Mettler) in a mode-vibrating sample magnetometer (VSM) with applied fields of −20.0 to 20.0 kOe at room temperature. The optical properties were investigated with absorption spectra obtained with a Cary-5000 UV–vis (Agilent Technologies) spectrometer equipped with a polytetrafluoroethylene (PTFE) integration sphere. X-ray photoelectron spectroscopy (XPS) (Thermo Scientific, K-Alpha) was performed with a monochromatic Al Kα source (hv = 1486 eV) to determine the bonding states of the MNPs. All spectra were calibrated with the C 1s signal at 284.8 eV, which was produced by adventitious carbon. The Fe 2p and O 1s core levels were determined at 90° with a pass energy of 15 eV.
2.4. Functionalization of the magnetic nanoparticles
One gram of the MNPs was ultrasonicated in 50 mL of methanol for 1 h. After that, 3 mL of ammonium hydroxide (25 wt%) was added and ultrasonicated for 30 min. Then, 2 mL of 3-APTES was incorporated, and the solution was kept under continuous stirring at 50 °C for 9 h under reflux to prevent methanol evaporation. The MNPs were recovered with magnetic decantation and washed with deionized water until the pH became neutral.
2.5. Pectinase immobilization and activity assay
Enzymatic coupling and glutaraldehyde crosslinking were used to immobilize pectinase. The MNPs were incubated with 3% glutaraldehyde (GA) for 4 h, washed with distilled water three times, and separated by magnetic decantation (Scheme 1). The resulting MNPs were incubated at different pHs (4.5, 5, 6, 7, and 8) at 30 °C for 24 h with 1800 units of pectinase enzyme and stirring at 150 rpm. The MNPs-pectinase nanocatalyst was separated with a magnet and used for total protein quantification with the Bradford assay. The retention percentage was calculated based on the free protein before and after immobilization, and MNPs-pectinase was subjected to enzyme analysis. The pectinase activity was determined by spectrophotometrically measuring the d-galacturonic acid liberated from pectin at 595 nm with dinitrosalicylic acid. The effect of pH (4–8) at 30 °C, time (0–120 h) at 30 °C, temperature (25–55 °C), and reusability of the free and immobilized enzyme were analyzed separately by incubation at 30 °C.
Scheme 1.
Schematic representation of pectinase immobilization on magnetic nanoparticles.
3. Results and discussion
3.1. Structural and morphological characterization of the MNPs
The XRD patterns of the MNPs prepared in situ are shown in Fig. 1a. The peaks at 30°, 35°, 39°, 43°, 53°, and 57° corresponded to the (220), (311), (321), (400), (422), and (511) planes, which were consistent with JCPDS card No. 39–1346 for γ-Fe2O3. The XRD pattern showed broad diffraction peaks, indicating the formation of nanoparticles. The crystal system of γ-Fe2O3 is cubic with lattice parameters a = b = c = 8.29 Å, α = β = γ = 90°, and space group P4132. The average crystallite size was calculated using Scherrer's formula [29], resulting in 10 ± 2 nm. These results were evaluated with the position of the diffraction peak of the (311) plane.
Fig. 1.
Structural and morphological characterization of the MNPs and chitosan; a) XRD pattern, b) FTIR spectra, c) FE-SEM image, d) EDS layered image, e) EDS spectrum and elemental maps of the MNPs.
FTIR spectroscopy was used to determine the functional groups present in pure chitosan and the MNPs (Fig. 1b). The absorption bands for chitosan appeared at ∼3274 cm−1 (O–H, N–H stretching vibrations), ∼2923 cm−1 (C–H, stretching vibrations), ∼1651 to 1541 cm−1 (N–H, bending vibrations), ∼1430 cm−1 (C–O stretching vibration), ∼1362 cm−1 (C–N, stretching) and ∼1060 cm−1 (C–O–C, stretching vibrations) [30,31]. For the MNPs, the peak at 585 cm−1 was related to the Fe–O bond [32]. Strong absorption bands were located at ∼1574 cm−1, ∼1398 cm−1, and ∼1024 cm−1, which corresponded to the amide II peak and –CH, and –CH3 peaks for the epoxy groups of GA. Additionally, FE-SEM and EDS were used to determine the morphology and composition of the MNPs (Fig. 1c). An acetic acid/H2O2 solution was used to break down the chitosan, as described in the synthetic process. Specific chitosan residues persisted on the nanoparticle surfaces (Fig. 1b). The chitosan matrix was primarily responsible for the aggregated MNPs, as seen from the high-magnification FESEM image (Fig. 1c). The average grain size was calculated in 29 ± 5 nm (inset Fig. 1C, grain size distribution). Fe, O, and C were found in the sample, as seen in the EDS layered picture and individual maps (Fig. 1d), which depicted uniform distributions of Fe and O and some traces of C at this level. Finally, the EDS spectrum (Fig. 1e) showed an elemental composition of Fe (52.1%), O (26.3%), and C (21.6%).
Fig. 2 shows the N2 adsorption and desorption isotherms and (insets) the pore-size distributions of the MNPs and pectinase-MNPs. Fig. 2a demonstrates that the base material, i.e., without pectinase, exhibited type IV N2 adsorption and desorption isotherms, as per the International Union of Pure and Applied Chemistry (IUPAC) classification [33]. These isotherms suggested the absence of a discernible monolayer, indicating that the adsorbed molecules formed clusters around the most favorable surface sites. The specific surface area of the base material was determined to be 31.15 m2/g based on the BET method. The samples exhibited type H3 hysteresis loops predominantly, indicating that wedge-shaped pores were the primary pores present in the sample. Fig. 2a (inset) shows the pore size distribution based on the Barrett‒Joyner‒Halenda (BJH) model. A narrow pore size distribution with a maximum peak at approximately 3 nm indicated that the sample was a microporous and mesoporous material. Fig. 2b shows the adsorption-desorption isotherm and pore-size distribution (inset) of the material containing pectinase. There was no noticeable difference with respect to the base material without pectinase (SBET = 31.2 m2/g).
Fig. 2.
Nitrogen adsorption and desorption isotherms and (inset) pore-size distributions of the a) MNPs and b) pectinase-MNPs.
3.2. Magnetic properties of the MNPs
The magnetic properties of the MNPs and bare nanoparticles (BP) were determined by VSM at room temperature (Fig. 3). The mass saturation magnetization (Ms) of the MNPs was 22.7 emu/g, which was than the 67.02 emu/g observed for the bare nanoparticles. The decreased Ms value was due to the presence of nonmagnetic materials, such as carbon, which weakened the magnetic properties. The magnetization curves for the bare and in situ prepared nanoparticles did not exhibit hysteresis, which was consistent with formation of the superparamagnetic material required for biotechnological and medical applications. The MNP suspension is shown in the inset (Fig. 3) both before and after exposure to an external magnet, which indicated the potential for reuse of the MNPs and demonstrated their sustainability.
Fig. 3.
Magnetic hysteresis curve for the as-prepared MNPs and bare nanoparticles.
3.3. Optical properties of the MNPs
UV–visible absorption spectroscopy was utilized to analyze the optical properties of chitosan and the MNPs (Fig. 4). The band gap (Eg) of the MNPs was computed from the absorbance information with the Kubelka-Munk function according to Tauc's relation. The values measured for the MNPs and chitosan were Eg = 2.15 eV and Eg = 3.1 eV, respectively, which were consistent with previously published values. Due to the lack of conjugated double bonds in the chitosan structure, the sample did not exhibit absorbance bands in the 420–1200 nm region [34]. In addition, the MNPs displayed substantial visible-wavelength absorption (Fig. 4a). The maximum absorbance wavelength was related to the π→π* transition of C]O groups [35]. Based on the Eg value for the MNPs, the relative energies of the valence band (VB) and conduction band (CB) were calculated [36], and the values were 0.3 and 2.44 eV, respectively (Fig. 4b).
Fig. 4.
a) UV–Vis absorption spectra for chitosan and the MNPs and b) band gaps estimated with the Kubelka-Munk function.
3.4. Surface analyses with X-ray photoelectron spectroscopy
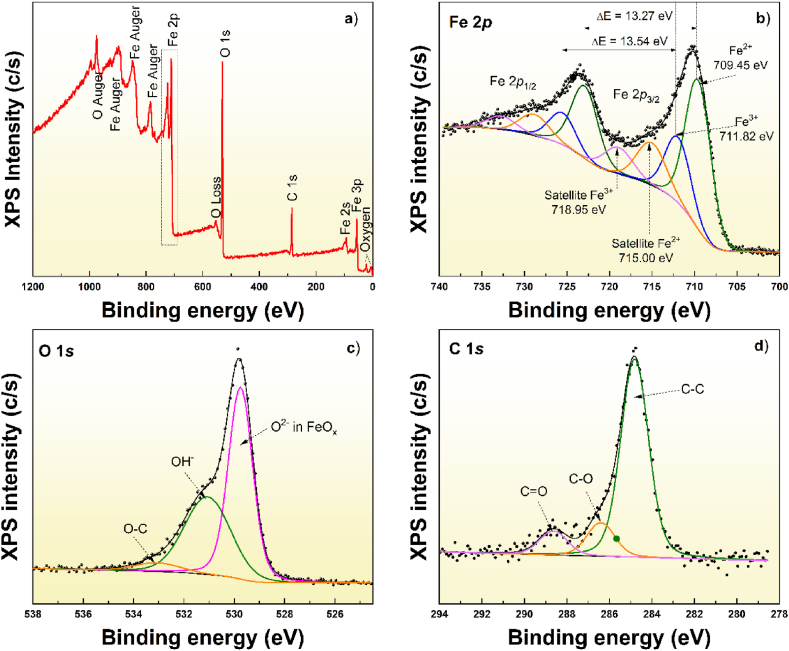
Fig. 5 shows the low- and high-resolution XPS spectra for the MNPs. Fig. 5a displays the spectrum from which it was possible to observe peaks related to the different core levels of the elements present in the sample. The presence of iron (Fe) and oxygen (O) was consistent with the elemental composition of the MNPs. The presence of carbon could be associated with organic residues from the synthetic process or adventitious carbon due to exposure of the sample to the environment. The high-resolution XPS spectra were deconvolved using Analyzer software to determine the chemical states on the nanoparticle surface [37]. In particular, the Fe 2p core level showed a stepped background and complex characteristics that were difficult to deconvolute (Fig. 5b). However, through proper background analysis, it was possible to identify the chemical components and the oxidation states of iron. A Shyrley-type background enabled reproduction of both sides of the Fe 2p core level. It was necessary to use four doublet peaks in fitting this region. The first two peaks, centered at 709.45 eV and 711.82 eV, were related to the Fe2+ and Fe3+ oxidation states [38]. The satellites centered at 715.0 eV and 718.95 eV were fingerprints of the Fe2+ and Fe3+ oxidation states [39]. Fig. 5c shows the O 1s core level, which was fitted with three single peaks. The first peak centered at 529.74 eV was associated with O2− ions in an FeOx structure, while the peak at 531.0 eV was associated with OH radicals, and the third was associated with O–C bonds. The C 1s core level (Fig. 5d) was fitted with three single peaks, which were related to C–C bonds (284.8 eV), C–O bonds (286.38 eV), and C]O bonds (288.61 eV). The presence of the Fe2+ and Fe3+ ions confirmed the formation of Fe2O3 and Fe3O4, as revealed by the XRD analysis. However, it is worth noting that the Fe2+:Fe3+ ratio was 2:1.
Fig. 5.
a) XPS spectra of the MNPs, b) Fe 2p core level spectrum, c) O 1s core level spectrum and d) C 1s core level spectrum.
3.5. Catalytic properties of free and immobilized pectinase
The purpose of immobilizing pectinase on the surfaces of the nanoparticles was to achieve optimal conditions for industrial application of the immobilized rather than free pectinase. Parameters such as the pH, temperature, time, and reusability were studied (Fig. 6). The Fourier transform infrared (FTIR) spectra of the pectinase and immobilized pectinase (IPE) are shown in Fig. 6a. The characteristic peaks for pectinase included those for the –NH2 (∼3500 cm−1) and –COOH vibrations. The stretching vibrational bands for the carboxyl groups were observed at ∼2930 (−OH) and ∼1650 cm−1 (−C═O) in the pectinase spectrum. According to the literature, GA and the enzyme form a Schiff base with a C]N group (∼1650 cm-1) [40]. Additionally, the shifted positions of the peaks for the immobilized pectinase indicated successful bonding between pectinase and the MNPs [41]. Maximum retention of the enzyme was observed at pH 4.5 with 1179.3 U/mgNP (units per milligram of nanoparticle) (Fig. 6b). The strongest catalytic performance occurred under strongly acidic conditions for the free and immobilized pectinase, specifically at a pH of 5 (Fig. 6c). Beyond this pH, the specific activity of the free pectinase gradually decreased to 0.184 UA/mg at pH 8. However, the immobilized pectinase retained its maximum specific activity at pH 5 and 6 (0.32 UA/mg). Beyond these values, the activity dropped to nearly 50% lower than that at pH 5. The free pectinase displayed higher specific activity across a wider range of pH values than the immobilized pectinase, indicating that the activities of both enzymes were affected by the pH. When comparing the activities of enzymes, thermal stability is a crucial consideration. A temperature range of 25 °C–55 °C was used in this analysis (Fig. 6d). Free and immobilized pectinase exhibited maximum activities at 45 °C (0.47 and 0.35 UA/mg, respectively). The activity of the free enzyme at 45 °C was almost double that seen at room temperature. However, at 55 °C, this activity fell by 5%. Similar behavior was seen with the immobilized enzyme. Again, the activity increased at all temperatures examined when free pectinase was present. The long-term stabilities of the free and immobilized enzymes were analyzed over 120 h (Fig. 6e). The specific activity of the free pectinase decreased by 40% from the initial value. In contrast, the immobilized pectinase showed a reduction of 32%. The reusability of an enzyme in an industrial process reduces the production costs. However, reutilization of the free pectinase was not possible. Instead, the specific activity of the immobilized pectinase was reduced by only 20% after 4 cycles (from 0.32 to 0.25 UA/mg) (Fig. 6f).
Fig. 6.
Immobilization and catalytic performance of the free and immobilized pectinase; a) FTIR spectra, b) enzyme retained expressed as units per milligram of nanoparticles (U/mgNP), c) effect of pH, d) effect of temperature, d) effect of time and e) reusability of the immobilized pectinase expressed as enzyme activity (U/mg).
3.6. Effects of immobilization on the kinetic and thermodynamic parameters of pectinase
The kinetic parameters for the free and immobilized pectinase were determined at pH 5 and 30 °C using the Lineweaver‒Burk model [42]:
| (3) |
where v0 is the initial velocity of an enzyme reaction, Km is the Michaelis constant, Vmax is the maximum velocity of the reaction, and S is the substrate concentration. From Eq. (3), the obtained values for the immobilized enzyme were Vmax = 0.41 U/mg and Km = 6.52 g/L, and for the free enzyme Vmax = 1.07 U/mg and Km = 17.65 g/L. The decrease in Km for the immobilized enzyme indicated a higher substrate affinity [43]. The observed increase in the substrate affinity could have resulted from better access to the active sites facilitated by immobilization [44]. On the other hand, the lower value of Vmax in the case of the immobilized enzyme was associated with stronger diffusional restrictions, i.e., the reaction could become a diffusion-limited process after immobilization. This is principally related to the structure (agglomerated nanoparticles), as shown with FE-SEM.
The thermal stabilities of the free and immobilized pectinase were analyzed at 36 °C for 120 h, and the kinetic and thermodynamic parameters were calculated. Based on the Arrhenius method, the deactivation energy (Eq. (4)) was obtained with the following equation [45]:
| (4) |
where kd is the deactivation rate constant, A is a constant, Ed is the deactivation energy, R (8.314 × 10−3 kJ mol−1 K−1) is the universal gas constant, and T is the absolute temperature. The time required for the enzyme to show a decrease to 50% of the initial activity (half-life) is t1/2 = ln 2/k. With the value of t1/2 for immobilized/free enzyme, it was possible to obtain the stabilization factor (SF). The thermodynamic parameters for both pectinases were calculated as follows [46]:
| (5) |
| (6) |
| (7) |
where h (6.6262 × 10−34 Js) is the Planck constant and kB (1.3806 × 10−23 JK−1) is the Boltzmann constant. The results are shown in Table 1.
Table 1.
Thermal inactivation and thermodynamic parameters for free and immobilized pectinase.
| Parameter | Free pectinase | Immobilized pectinase |
|---|---|---|
| Km (g/L) | 1.07 | 0.41 |
| Vmax (U/mg) | 17.65 | 6.52 |
| kd (h−1) | 0.0057 | 0.0044 |
| t1/2 (h) | 121.60 | 157.53 |
| SF | – | 1.30 |
| ΔG* (kJ/mol) | 102.54 | 103.22 |
| ΔH* (kJ/mol) | 21.84 | 22.52 |
| ΔS* (kJ/mol) | −0.253 | −0.254 |
The kinetic and thermodynamic data for the enzyme thermal deactivation process indicated an increase in the thermostability of the enzyme after immobilization. The half-life of the deactivation reaction increased from 121.6 to 157.53 h, thus increasing the SF by 30%, which established a slower thermal deactivation process for the immobilized enzyme. Regarding the thermodynamic parameters, Table 1 shows that ΔG* (Eq. (6)) increased slightly from 102.54 to 103.22 kJ/mol for the immobilized enzyme. This demonstrated that immobilization improved the thermal stability of the enzyme, making it more resistant to denaturation and conformational changes [47]. As seen in Eq. (7) and Table 1, ΔG* was positive because ΔH* (Eq. (5)) was also positive and ΔS* was negative, and both contributed to the stability of the enzyme after immobilization. While ΔS* remained virtually unchanged after immobilization, ΔH* showed a slight increase, demonstrating higher stability due to van der Waals-type interactions or hydrogen bonds [48].
4. Conclusion
MNPs were efficiently prepared in situ from a chitosan matrix, and their structural, morphological, and optical properties were characterized. These MNPs were then functionalized and utilized as a support to immobilize pectinase. The immobilized pectinase demonstrated enhanced affinity for the substrate and a lower reaction rate compared with free pectinase. However, the immobilized enzyme specific activity was lower than that of the free enzyme. This discrepancy was attributed to the distribution of nanoparticles and the insolubility of the MNPs, resulting in colloidal properties that caused the immobilized enzyme to settle at the bottom of the reaction tube. Consequently, there were reduced interactions between the immobilized enzyme and the substrate. Nonetheless, the pectinase immobilization process led to improved thermostability.
Author contribution statement
Diego Eloyr Navarro-López, Naveen Tiwari, Edgar R. López Mena: conceived and designed the experiments, performed the experiments, analyzed and interpreted the data, contributed reagents, materials, analysis tools or data, wrote the paper.
Alvaro R. Bautista-Ayala, Maria Fernanda Rosales-De la Cruz, Selina Martínez-Beltrán, Diego E. Rojas-Torres, M. Sepúlveda-Villegas: performed the experiments, analyzed and interpreted the data.
A. Sanchez-Martinez, O. Ceballos-Sanchez, Luis Marcelo Lozano, J. A. Jáuregui-Jáuregui: analyzed and interpreted the data, contributed reagents, materials, analysis tools or data, wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Alvaro R. Bautista-Ayala, M. Fernanda Rosales-De la Cruz, Selina Martínez-Beltrán, and Diego E. Rojas-Torres acknowledge Tecnologico de Monterrey for their undergraduate scholarship. This work was partially funded by Tecnológico de Monterrey, through the Challenge-Based Research Funding Program (Project ID: E090-EIC-GI04 -A-T3-E) and Nanodevices Research Groups. A. Sanchez-Martinez acknowledges CONACyT Mexico through the Catedras-CONACyT # 67 project. O. Ceballos-Sanchez thanks the Universidad de Guadalajara Mexico for the PRO–SNI–2022 project and CONACyT Mexico through CF2021-316883 Project.
Contributor Information
Naveen Tiwari, Email: naveen.tiwari@usc.es.
Edgar R. López-Mena, Email: edgarl@tec.mx.
References
- 1.Martínez Cuesta S., Rahman S.A., Furnham N., Thornton J.M. The classification and evolution of enzyme function. Biophys. J. 2015;109:1082–1086. doi: 10.1016/j.bpj.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald A.G., Tipton K.F. Fifty-five years of enzyme classification: advances and difficulties. FEBS J. 2014;281:583–592. doi: 10.1111/febs.12530. [DOI] [PubMed] [Google Scholar]
- 3.Satapathy S., Rout J.R., Kerry R.G., Thatoi H., Sahoo S.L. Biochemical prospects of various microbial pectinase and pectin: an approachable concept in pharmaceutical bioprocessing. Front. Nutr. 2020;7 doi: 10.3389/fnut.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dal Magro L., de Moura K.S., Backes B.E., de Menezes E.W., Benvenutti E.V., Nicolodi S., Klein M.P., Fernandez-Lafuente R., Rodrigues R.C. Immobilization of pectinase on chitosan-magnetic particles: influence of particle preparation protocol on enzyme properties for fruit juice clarification. Biotechnology Reports. 2019;24 doi: 10.1016/j.btre.2019.e00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladole M.R., Pokale P.B., Varude V.R., Belokar P.G., Pandit A.B. One pot clarification and debittering of grapefruit juice using co-immobilized enzymes@chitosanMNPs. Int. J. Biol. Macromol. 2021;167:1297–1307. doi: 10.1016/j.ijbiomac.2020.11.084. [DOI] [PubMed] [Google Scholar]
- 6.Lara-Espinoza C., Carvajal-Millán E., Balandrán-Quintana R., López-Franco Y., Rascón-Chu A. Pectin and pectin-based composite materials: beyond food texture. Molecules. 2018;23 doi: 10.3390/molecules23040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L.J., Xia W.J., Ma G.P., Chen Y.L., Ma Y.Y. A study on the enzymatic properties and reuse of cellulase immobilized with carbon nanotubes and sodium alginate. Amb. Express. 2019;9 doi: 10.1186/s13568-019-0835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao H., Li J., Sivakumar D., Kim T.S., Patel S.K.S., Kalia V.C., Kim I.W., Zhang Y.W., Lee J.K. NADH oxidase from Lactobacillus reuteri: a versatile enzyme for oxidized cofactor regeneration. Int. J. Biol. Macromol. 2019;123:629–636. doi: 10.1016/j.ijbiomac.2018.11.096. [DOI] [PubMed] [Google Scholar]
- 9.Ansari S.A., Husain Q. Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol. Adv. 2012;30:512–523. doi: 10.1016/j.biotechadv.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Homaei A.A., Sariri R., Vianello F., Stevanato R. Enzyme immobilization: an update. J Chem Biol. 2013;6:185–205. doi: 10.1007/s12154-013-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheldon R.A. Enzyme immobilization: the quest for optimum performance. Adv. Synth. Catal. 2007;349:1289–1307. doi: 10.1002/adsc.200700082. [DOI] [Google Scholar]
- 12.Bilal M., Zhao Y., Rasheed T., Iqbal H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: a review. Int. J. Biol. Macromol. 2018;120:2530–2544. doi: 10.1016/j.ijbiomac.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Block S. Brownian motion at lipid membranes: a comparison of hydrodynamic models describing and experiments quantifying diffusion within lipid bilayers. Biomolecules. 2018;8 doi: 10.3390/biom8020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jee A.Y., Cho Y.K., Granick S., Tlusty T. Catalytic enzymes are active matter. Proc. Natl. Acad. Sci. U.S.A. 2018;115 doi: 10.1073/pnas.1814180115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S.K.S., Anwar M.Z., Kumar A., Otari S.V., Pagolu R.T., Kim S.Y., Kim I.W., Lee J.K. Fe2O3 yolk-shell particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem. Eng. J. 2018;132:1–8. doi: 10.1016/j.bej.2017.12.013. [DOI] [Google Scholar]
- 16.Patel S.K.S., Kalia V.C., Lee J.K. Laccase immobilization on copper-magnetic nanoparticles for efficient bisphenol degradation. J. Microbiol. Biotechnol. 2023;33:127–134. doi: 10.4014/jmb.2210.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otari S.V., Patel S.K.S., Kalia V.C., Lee J.K. One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour. Technol. 2020;302 doi: 10.1016/j.biortech.2020.122887. [DOI] [PubMed] [Google Scholar]
- 18.Patel S.K.S., Gupta R.K., Kim S.Y., Kim I.W., Kalia V.C., Lee J.K. Rhus vernicifera laccase immobilization on magnetic nanoparticles to improve stability and its potential application in bisphenol A degradation. Indian J. Microbiol. 2021;61:45–54. doi: 10.1007/s12088-020-00912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basso A., Serban S. Industrial applications of immobilized enzymes—a review. Mol. Catal. 2019;479 doi: 10.1016/j.mcat.2019.110607. [DOI] [Google Scholar]
- 20.Shokrollahi H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn Mater. 2017;426:74–81. doi: 10.1016/j.jmmm.2016.11.033. [DOI] [Google Scholar]
- 21.Ansari S.A., Husain Q. Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol. Adv. 2012;30:512–523. doi: 10.1016/j.biotechadv.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Kikani B.A., Singh S.P. Enzyme stability, thermodynamics and secondary structures of α-amylase as probed by the CD spectroscopy. Int. J. Biol. Macromol. 2015;81:450–460. doi: 10.1016/j.ijbiomac.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Salem S.S., Fouda A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Elem. Res. 2021;199:344–370. doi: 10.1007/s12011-020-02138-3. [DOI] [PubMed] [Google Scholar]
- 24.Shokrollahi H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn Mater. 2017;426:74–81. doi: 10.1016/j.jmmm.2016.11.033. [DOI] [Google Scholar]
- 25.Kour S., kumar Sharma R., Jasrotia R., Singh V.P. AIP Conf Proc. American Institute of Physics Inc.; 2019. A brief review on the synthesis of maghemite (?-Fe2O3) for medical diagnostic and solar energy applications. [DOI] [Google Scholar]
- 26.Di Corato R., Aloisi A., Rella S., Greneche J.M., Pugliese G., Pellegrino T., Malitesta C., Rinaldi R. Maghemite nanoparticles with enhanced magnetic properties: one-pot preparation and ultrastable dextran shell. ACS Appl. Mater. Interfaces. 2018;10:20271–20280. doi: 10.1021/acsami.7b18411. [DOI] [PubMed] [Google Scholar]
- 27.Basso A., Serban S. Industrial applications of immobilized enzymes—a review. Mol. Catal. 2019;479 doi: 10.1016/j.mcat.2019.110607. [DOI] [Google Scholar]
- 28.Behram T., Pervez S., Nawaz M.A., Ahmad S., Jan A.U., Rehman H.U., Ahmad S., Khan N.M., Khan F.A. Development of pectinase based nanocatalyst by immobilization of pectinase on magnetic iron oxide nanoparticles using glutaraldehyde as crosslinking agent. Molecules. 2023;28 doi: 10.3390/molecules28010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanosains J. Derivation of scherrer relation using an approach in. Basic Physics Course. 2008;1:28–32. [Google Scholar]
- 30.Kumar S., Koh J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012;13:6103–6116. doi: 10.3390/ijms13056102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalaycıoğlu Z., Torlak E., Akın-Evingür G., Özen İ., Erim F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017;101:882–888. doi: 10.1016/j.ijbiomac.2017.03.174. [DOI] [PubMed] [Google Scholar]
- 32.Liao H., Chen D., Yuan L., Zheng M., Zhu Y., Liu X. Immobilized cellulase by polyvinyl alcohol/Fe2O3 magnetic nanoparticle to degrade microcrystalline cellulose. Carbohydr. Polym. 2010;82:600–604. doi: 10.1016/j.carbpol.2010.05.021. [DOI] [Google Scholar]
- 33.Thommes M., Kaneko K., Neimark A.V., Olivier J.P., Rodriguez-Reinoso F., Rouquerol J., Sing K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) Pure Appl. Chem. 2015;87:1051–1069. doi: 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- 34.Thamilarasan V., Sethuraman V., Gopinath K., Balalakshmi C., Govindarajan M., Mothana R.A., Siddiqui N.A., Khaled J.M., Benelli G. Single step fabrication of chitosan nanocrystals using Penaeus semisulcatus: potential as new insecticides, antimicrobials and plant growth promoters. J. Cluster Sci. 2018;29:375–384. doi: 10.1007/s10876-018-1342-1. [DOI] [Google Scholar]
- 35.Fauzi N.I.M., Fen Y.W., Omar N.A.S., Saleviter S., Daniyal W.M.E.M.M., Hashim H.S., Nasrullah M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers. 2020;12:1–13. doi: 10.3390/polym12071497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khurram R., Wang Z., Ehsan M.F., Peng S., Shafiq M., Khan B. Synthesis and characterization of an α-Fe2O3/ZnTe heterostructure for photocatalytic degradation of Congo red, methyl orange and methylene blue. RSC Adv. 2020;10:44997–45007. doi: 10.1039/d0ra06866g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrera-Gomez A. 2014. AAnalyzer: an Analysis Software for Photoelectron and Infrared Spectra.http://qro.cinvestav.mx/∼aanalyzer/ [Google Scholar]
- 38.Fujii T., de Groot F.M.F., Sawatzky G.A., Voogt F.C., Hibma T., Okada K. In situ xps analysis of various iron oxide films grown by (formula presented)-assisted molecular-beam epitaxy. Phys. Rev. B Condens. Matter. 1999;59:3195–3202. doi: 10.1103/PhysRevB.59.3195. [DOI] [Google Scholar]
- 39.McIntyre N.S., Zetaruk D.G. X-Ray photoelectron spectroscopic studies of iron oxides. Anal. Chem. 1977;49:1521–1529. doi: 10.1021/ac50019a016. [DOI] [Google Scholar]
- 40.Miao Q., Zhang C., Zhou S., Meng L., Huang L., Ni Y., Chen L. Immobilization and characterization of pectinase onto the cationic polystyrene resin. ACS Omega. 2021;6:31683–31688. doi: 10.1021/acsomega.1c04374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soozanipour A., Taheri-Kafrani A., Barkhori M., Nasrollahzadeh M. Preparation of a stable and robust nanobiocatalyst by efficiently immobilizing of pectinase onto cyanuric chloride-functionalized chitosan grafted magnetic nanoparticles. J. Colloid Interface Sci. 2019;536:261–270. doi: 10.1016/j.jcis.2018.10.053. [DOI] [PubMed] [Google Scholar]
- 42.Lineweaver H., Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934;56:658–666. doi: 10.1021/ja01318a036. [DOI] [Google Scholar]
- 43.Molina-Fernández C., Péters A., Debecker D.P., Luis P. Immobilization of carbonic anhydrase in a hydrophobic poly(ionic liquid): a new functional solid for CO2 capture. Biochem. Eng. J. 2022;187 doi: 10.1016/j.bej.2022.108639. [DOI] [Google Scholar]
- 44.Gashtasbi F., Ahmadian G., Noghabi K.A. New insights into the effectiveness of alpha-amylase enzyme presentation on the Bacillus subtilis spore surface by adsorption and covalent immobilization. Enzym. Microb. Technol. 2014;64(65):17–23. doi: 10.1016/j.enzmictec.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 45.de Andrade B.C., Gennari A., Renard G., Nervis B.D.R., Benvenutti E.V., Costa T.M.H., Nicolodi S., da Silveira N.P., Chies J.M., Volpato G., Volken de Souza C.F. Synthesis of magnetic nanoparticles functionalized with histidine and nickel to immobilize His-tagged enzymes using β-galactosidase as a model. Int. J. Biol. Macromol. 2021;184:159–169. doi: 10.1016/j.ijbiomac.2021.06.060. [DOI] [PubMed] [Google Scholar]
- 46.Kikani B.A., Singh S.P. Enzyme stability, thermodynamics and secondary structures of α-amylase as probed by the CD spectroscopy. Int. J. Biol. Macromol. 2015;81:450–460. doi: 10.1016/j.ijbiomac.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 47.de Andrade B.C., Gennari A., Renard G., Nervis B.D.R., Benvenutti E.V., Costa T.M.H., Nicolodi S., da Silveira N.P., Chies J.M., Volpato G., Volken de Souza C.F. Synthesis of magnetic nanoparticles functionalized with histidine and nickel to immobilize His-tagged enzymes using β-galactosidase as a model. Int. J. Biol. Macromol. 2021;184:159–169. doi: 10.1016/j.ijbiomac.2021.06.060. [DOI] [PubMed] [Google Scholar]
- 48.Eijsink V.G.H., Bjørk A., Gåseidnes S., Sirevåg R., Synstad B., van den Burg B., Vriend G. Rational engineering of enzyme stability. J. Biotechnol. 2004;113:105–120. doi: 10.1016/j.jbiotec.2004.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.