Abstract
The matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) has been widely applied in routine clinical microbiology laboratories as an efficient and reliable technique for diagnostic purpose. In this work, we evaluated the performance of the newly developed Zybio EXS3000 (Zybio Inc., China) in microbial identification and compared it with VITEK MS (bioMérieux, France). For this study, a total of 1340 isolates from various clinical specimens were collected. These isolates were analyzed simultaneously on both EXS3000 and VITEK MS. The inconsistent or unidentifiable data were further identified using the help of either 16S rRNA gene or ITS region sequencing. During the study, we observed that EXS3000 and VITEK MS provided positive confirmatory diagnostics for 95.0% and 96.5% of the isolates, respectively, which were consistent with the sequencing results. However, it is worth noting that the EXS3000 system needs to improve the identification performance of Candida albicans in the follow-up. There are no significant differences between the two devices in terms of microbial identification performance. The advantage of EXS3000 over VITEK MS is in its ability to perform in significantly lesser time period. In conclusion, the results of this investigation showed that EXS3000 can be used to identify microorganisms in clinical microbiology laboratories.
Highlights
-
•
The newly developed EXS3000 was evaluated for the identification of microorganisms.
-
•
EXS3000 showed reliable performance for microbial identification.
-
•
EXS3000 spent significantly less time in the procedure of microbial identification.
-
•
Database upgrade will aid in improving the identification accuracy of EXS3000.
1. Introduction
Clinically, the identification of pathogenic microorganisms plays a crucial role in the diagnosis and treatment of infectious diseases, and the accuracy and speed of microbial diagnostic greatly affect the treatment procedure. The identification of pathogens in clinical laboratories has long relied on phenotypic and biochemical analysis. Although these traditional methods have high sensitivity, they are cumbersome, time-consuming and only applicable to the identification of common pathogens [[1], [2], [3], [4]]. The application of 16S rRNA gene sequencing is of great significance for the identification of rare and atypical species, but it still cannot meet the clinical needs due to its tedious process and high cost [5,6].
Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) as an emerging high-throughput technique for the identification of microorganisms has been widely used in medical laboratories because of its speed, accuracy and low per-sample cost [7,8]. This technique analyses the total protein extract of microorganisms and matches against the protein fingerprint databases. This allows MALDI-TOF MS to rapidly and accurately identify microorganisms [9,10]. At present, the most widely used MS-based microbial identification systems worldwide are the BioTyper (Bruker Daltonics GmbII, Germany) and VITEK MS (bioMérieux, France) systems [11]. Other more recently introduced MALDI-TOF MS devices based on similar principles, such as MicroIDSys system (ASTA corp., South Korea), Clin-TOF (Bioyong Technologies, China), and Autof ms1000 (Autobio Diagnostics, China) are also gradually put into use in clinical laboratories [[12], [13], [14]].
Another new MALDI-TOF MS instrument with the trade name Zybio EXS3000 (Zybio Inc., China) was approved by the China Food and Drug Administration (CFDA) and was CE-IVD (European CE Marking for In Vitro Diagnostic devices) marked in 2020. The instrument EXS3000 is purposed to give a higher signal-to-noise ratio (SNR) and stability owing to factors such dustless air duct and temperature compartmentalization. Besides, the instrument provides a dual-ion mode handling to enable extended diagnostic applications. The Zybio EXS3000 was reported to show good resolution and accuracy in the identification of Shewanella species in China [15], but so far no evaluation has been done for routine identification in clinical microbiology laboratories.
The purpose of the present study is to assess the molecular diagnostic performance of Zybio EXS3000 in a real clinical microbiology set-up, by comparing its outcomes with that of bioMérieux VITEK MS. The results will serve as a reference for further assessment of this instrument in the medical market.
2. Materials and methods
2.1. Strains, cultivation methods and instruments
A total of 1340 non-repetitive isolates were collected from various clinical specimens (e.g. blood, urine, stool, cerebrospinal fluid, secretion and respiratory tract samples) between October 2021 to May 2022 at Guangdong Provincial Hospital of Traditional Chinese Medicine (Guangzhou, China). The 1340 clinical isolates consist of 201 staphylococci and related Gram-positive cocci, 229 Streptococcus spp., Enterococcus spp., and streptococcal-like organisms, 140 coryneform Gram-positive bacilli, 293 Enterobacterales and other fermenting bacteria, 168 nonfermenting Gram-negative bacilli, 52 anaerobic bacteria, 12 mycobacteria-aerobic actinomycetes, 82 fastidious Gram-negative bacilli and 163 fungi. The samples recovered from the clinic were inoculated on 5% sheep blood agar, chocolate agar or sabouraud dextrose agar. Most specimens required 18–24 h incubation at 37 °C under 5%CO2, while some required up to 72–96 h incubation for reliable species-level identification [16,17]. The cultivation of the anaerobic bacteria was performed in an anaerobic chamber with a longer incubation time. Escherichia coli ATCC 8739 and Candida albicans ATCC 10231 were used as quality control strains. Alpha-cyano-4-hydroxycinnamic acid matrix (CHCA) was used as the negative control. The VITEK MS (bioMérieux, France) was used as a reference system to evaluate the performance of Zybio EXS3000 (Zybio Inc., China). This study was approved by the Scientific ethical committee of the Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangdong, China (ZM2019–280).
2.2. Identification of Discrepant results
In case of discrepancies between the results of VITEK MS and Zybio EXS3000 at the species level identification of specimen or if one of the results was implausible, the specimen was subjected to sequencing analyses of the 16S rRNA gene for bacteria or internal transcription spacer (ITS) region for fungi. Sequencing reactions were carried out on an ABI 3730XL sequencer (ABI, Carlsbad, CA, USA). The primers used for 16S rRNA gene amplification were 5′-AGAGTTTGATCMTGGCTCAG-3′(27F) and 5′-TACGGYTACCTTGTTACGACTT-3′(1492R), while the primers used for ITS region amplification were 5′-TCCGTAGGTGAACCTGCGG-3′(ITS1) and 5′-TCCTCCGCTTATTGATATGC-3′(ITS4). The sequences obtained are submitted to the BLAST software (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for comparison with the sequences in the GenBank database and interpreted following CLSI standards [16]. For bacteria that cannot be accurately identified by 16S rRNA sequencing, such as Salmonella spp. and Streptococcus pneumoniae, further phenotypic methods, biochemical tests and serological tests according to CLSI M58 were performed [17].
2.3. MALDI-TOF MS analysis
Identification of the isolates in Zybio EXS3000 and VITEK MS systems were performed as indicated in the manufacturer's instructions, with quality control following the CLSI M58 standard [17]. Most bacteria were detected using the direct transfer method. For others like filamentous fungi, mycobacteria, Nocardia, and other aerobic actinomycetes, screening was done using the ethanol/formic acid (EtOH/FA) extraction method [18]. Comparative analyses were carried out at both the species and genus levels. It may be mentioned that our clinical microbiology laboratory is a certified biosafety level II laboratory. All procedures were performed in the biosafety cabinet.
Zybio EXS3000 The process of the direct transfer method is described as below: a single colony was evenly smeared onto the target plate, following which 1 μL of matrix solution (α-cyano-4-hydroxycinnamic acid, CHCA) was added dropwise. After drying at room temperature, the target plate was put in the instrument for analysis. However, for some Gram-positive bacilli, mucoid bacteria, and yeast-like fungi, it is necessary to pretreat the microbial membrane on the target plate with 1 μl of 70% formic acid (FA) solution before adding the matrix solution. In the EtOH/FA extraction method, a few colonies were transferred to an Eppendorf tube containing 300 μl of ultrapure water with a sterile pipette tip. Then, 900 μl of absolute ethanol was added and mixed thoroughly. The mixture was then centrifuged at 14000×g for 5 min, and the supernatant was drained from the Eppendorf tube. After drying the precipitate at room temperature, an equal amount of 70% FA and acetonitrile was constantly added and thoroughly mixed. The mixture was centrifuged at 12000×g for 3min and the supernatant transferred to a new tube. 1 μl of the extracted supernatant was added to a 96-well target plate (Zybio Inc., China), and covered with 1 μl of matrix solution after air drying. Finally, the target plate was put into the instrument for detection. All MS spectra were obtained in linear mode ranging from 2000 to 20000 Da. MS data were analyzed by EX-Accuspec V1.0.21.6 and EXS 3000 database v1.2. The manufacturer's instructions state that log scores ≥2.0 were reported as creditable species-level identification, while log scores <2.0 but ≥1.7 were considered identification at genus or presumptive species level. Additionally, log scores <1.7 were indicated as unreliable.
VITEK MS For both the direct transfer method and the EtOH/FA extraction method, VITEK MS followed similar steps to Zybio EXS3000. E. coli ATCC 8739 and Candida albicans ATCC 10231 were utilized as quality control strains on each target slide. Mass spectra were recorded with the mass range of 2000–20000 Da. The spectra captured by the VITEK MS system were compared with the IVD database v3.2. The analysis was carried out using VITEK®2 software v5.0.1. The results were presented in one of three ways: (i) the green frame (confidence value of 60.0%–99.9%) denotes a reliable species-level identification, (ii) the yellow triangle indicates a low resolution, and (iii) the red circle means no identification. It is important to note that our clinical microbiology laboratory is a biosafety level II laboratory. And all procedures were performed in the biosafety cabinet.
2.4. Comparison of the time to test
We also compared the time spent on the VITEK MS and EXS3000 in actual applications. The time taken by the steps including analysis preparation (slide loading and vacuum preparation) and target analysis (16-spot, 48-spot and 96-spot) independently measured.
2.5. Statistical analysis
SPSS19 statistical analysis software (IBM Corporation, Armonk, NY, USA) was used for the statistical analysis. Categorical variables were compared using Chi-squared or Fisher's exact tests. Continuous variables were compared using Student's t-test or Mann–Whitney test. When the two-tailed p-value <0.05, it is considered to be statistically significant. Figures were generated using GraphPad Prism version 6.0 (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. Isolate identification
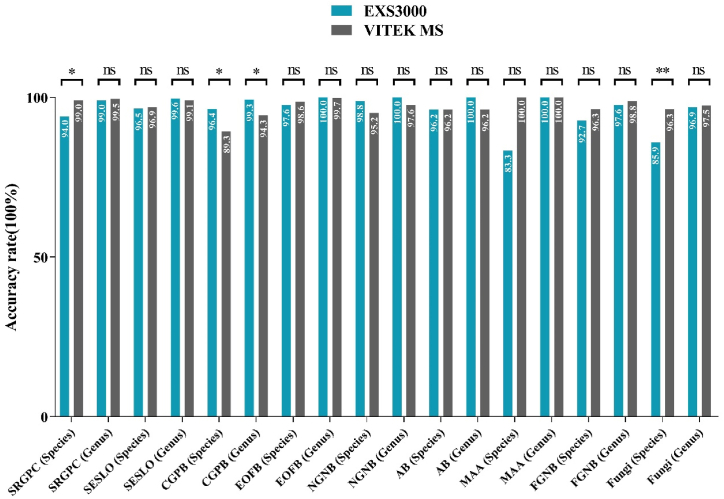
These two MS systems performed comparably well in the identification of all the 1340 isolates. EXS3000 identified 95.0% and 99.2% of the isolates to species (complex) and genus levels, respectively, while VITEK MS identified 96.5% and 98.3% of isolates to species and genus levels, respectively. The total coincidence rate of EXS3000 and VITEK MS was 95.0%, which indicated that they could identify clinical isolates equally well. Detailed results were shown in Fig. 1 and Table S1.
Fig. 1.
Comparison of microbial diagnostic results determined by the Zybio EXS3000 and bioMérieux VITEK MS systems. SRGPC: Staphylococci and Related Gram-Positive Cocci; SESLO: Streptococcus spp., Enterococcus spp., and Streptococcal-Like Organisms; CGPB: Coryneform Gram-Positive Bacilli; EOFB: Enterobacterales and Other Fermenting Bacteria; NGNB: Nonfermenting Gram-Negative Bacilli; AB: Anaerobic Bacteria; MAA: Mycobacteria-Aerobic Actinomycetes; FGNB: Fastidious Gram-Negative Bacilli; ns: not significant; *: P < 0.05; **: P < 0.01.
3.2. Comparison of identification performance in common bacteria and yeast
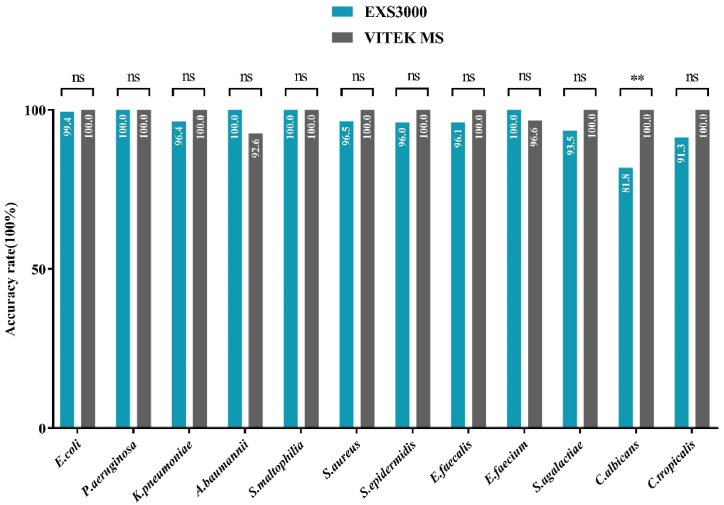
679 of the 1340 isolates, including Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Stenotrophomonas maltophilia, Staphylococcus aureus, S. epidermidis, Enterococcus faecalis, E. faecium, Streptococcus agalactiae, Candida albicans and C. tropicalis, were common clinical microbial isolates. The EXS3000 and VITEK MS identified these isolates with 96.5% and 99.6% accuracy respectively, at species level. The evaluation of the MS is greatly impacted by the capacity to identify these strains. Detailed results were shown in Fig. 2 and Table S2.
Fig. 2.
Comparative analysis of identification of common bacteria and yeast by the Zybio EXS3000 and bioMérieux VITEK MS. E. coli: Escherichia coli; P. aeruginosa: Pseudomonas aeruginosa; K. pneumoniae: Klebsiella pneumoniae; A. baumannii: Acinetobacter baumannii; S. maltophilia: Stenotrophomonas maltophilia; S. aureus: Staphylococcus aureus; S. epidermidis: Staphylococcus epidermidis; E. faecalis: Enterococcus faecalis; E. faecium: Enterococcus faecium; S. agalactiae: Streptococcus agalactiae; C. albicans: Candida albicans; C. tropicalis: Candida tropicalis; ns: not significant; **: P < 0.01.
3.3. Inconsistent results between the Zybio EXS3000 and the bioMérieux VITEK MS. The Zybio EXS3000 incorrectly identified or failed to identify 5.0% (67/1340) of the isolates at the species level
However, 55 of these isolates were identified at the generic level. In comparison, the VITEK MS misidentified or failed to identify 3.5% (47/1340) of the isolates, of which 25 were accurately identified to the generic level. Table 1 summarized the discrepancies in species identification by the two instruments.
Table 1.
Isolates misidentified at the species level or not identified by the EXS3000 and VITEK MS.
| Reference | N | EXS3000 |
VITEK MS |
||||
|---|---|---|---|---|---|---|---|
| Result | Score | Agreement | Result | Range | Agreement | ||
| Acinetobacter baumannii | 2 | Acinetobacter baumannii | 2.10-2.40 | ID species | Acinetobacter nosocomialis | 99.9% | MisID species |
| Acinetobacter nosocomialis | 1 | A. baumannii | 2.17 | MisID species | A. nosocomialis or A. baumannii | 50/50 | MisID species |
| Rothia amarae | 4 | Rothia amarae | 2.16-2.24 | ID species | No reliable ID | No ID | |
| Brachybacterium muris | 1 | Brachybacterium muris | 2.30 | ID species | No reliable ID | No ID | |
| Candida rugosa | 1 | No reliable ID | No ID | Candida rugosa | 99.9% | ID species | |
| Salmonella enterica | 1 | Salmonella spp. | 2.31 | ID genus | Salmonella enterica ssp enterica | 99.9% | ID genus |
| Durban Salmonella | 1 | Salmonella spp. | 2.14 | ID genus | Salmonella enterica ssp enterica | 99.9% | MisID species |
| Microbacterium proteolyticum | 1 | Microbacterium proteolyticum | 2.40 | ID species | Microbacterium testaceum | 99.9% | MisID species |
| Corynebacterium striatum | 2 | Corynebacterium striatum | 2.15-2.22 | ID species | Corynebacterium striatum or Corynebacterium tuberculostearicum | 50/50 | MisID species |
| Corynebacterium striatum | 1 | Corynebacterium striatum | 2.30 | ID species | No reliable ID | No ID | |
| Corynebacterium striatum | 1 | No reliable ID | 1.60 | No ID | Corynebacterium striatum | 99.9% | ID species |
| Neisseria gonorrhoeae | 1 | No reliable ID | 1.46 | No ID | No reliable ID | No ID | |
| Elizabethkingia anophelis | 1 | Elizabethkingia anophelis | 2.23 | ID species | Elizabethkingia anophelis or Brucella spp. | 50/50 | MisID species |
| Nocardia beijinggensis | 1 | Nocardia brasiliensis | 1.81 | MisID species | Nocardia beijinggensis | 99.9% | ID species |
| Acinetobacter bereziniae | 1 | Acinetobacter bereziniae | 2.38 | ID species | No reliable ID | No ID | |
| Corynebacterium propinquum | 1 | Corynebacterium propinquum | 2.44 | ID species | Corynebacterium propinquum or Corynebacterium pseudodiphtheriticum | 50/50 | MisID species |
| Corynebacterium pyruviciproducens | 1 | Corynebacterium pyruviciproducens | 2.46 | ID species | No reliable ID | No ID | |
| Corynebacterium resistens | 1 | Corynebacterium resistens | 2.40 | ID species | Corynebacterium auriscanis | 99.9% | MisID species |
| Corynebacterium minutissimum | 1 | Corynebacterium minutissimum | 2.31 | ID species | No reliable ID | No ID | |
| Corynebacterium propinquum | 1 | Corynebacterium propinquum | 2.15 | ID species | Corynebacterium pseudodiphtheriticum | 99.9% | MisID species |
| Streptococcus mitis | 1 | Streptococcus pneumoniae | 2.14 | MisID species | Streptococcus mitis/Streptococcus orails | 99.9% | ID species |
| Streptococcus peroris | 1 | Streptococcus peroris | 1.95 | ID genus | Streptococcus mitis/Streptococcus orails | 99.9% | MisID species |
| Streptococcus parasanguinis | 1 | Streptococcus parasanguinis | 2.27 | ID species | Streptococcus mitis/Streptococcus orails | 99.9% | MisID species |
| Streptococcus mitis | 1 | Streptococcus mitis | 2.07 | ID species | No reliable ID | No ID | |
| Streptococcus gallolyticus | 1 | Streptococcus gallolyticus | 2.33 | ID species | No reliable ID | No ID | |
| Streptococcus urinalis | 1 | Streptococcus urinalis | 2.67 | ID species | Streptococcus anginosus | 99.9% | MisID species |
| Streptococcus intermedius | 1 | Streptococcus intermedius | 2.39 | ID species | Streptococcus constellatus | 99.9% | MisID species |
| Candida tropicalis | 1 | Pichia farinosa | 1.73 | MisID species | Candida tropicalis | 99.9% | ID species |
| Candida colliculosa | 1 | No reliable ID | 1.62 | No ID | Candida colliculosa | 99.9% | ID species |
| Trichophyton tonsurans | 1 | Trichophyton tonsurans | 2.41 | ID species | trichophyton interdigitale | 99.9% | MisID species |
| Aspergillus pseudoglaucus | 1 | Aspergillus pseudoglaucus | 2.40 | ID species | Aspergillus glaucus (Eurotium herbariorum) | 99.9% | MisID species |
| Penicillium vermiculatum | 1 | No reliable ID | 1.56 | No ID | Penicillium vermiculatum | 99.9% | ID species |
| Aspergillus unguis | 1 | No reliable ID | 1.15 | No ID | Aspergillus unguis | 99.9% | ID species |
| Aspergillus restrictus | 1 | Aspergillus restrictus | 2.10 | ID species | No reliable ID | No ID | |
| Mucor indicus | 1 | Mucor indicus | 2.05 | ID species | No reliable ID | No ID | |
| Exophiala jeanselmei | 1 | Exophiala jeanselmei | 2.04 | ID species | No reliable ID | No ID | |
| Schizophyllum commune | 1 | Schizophyllum commune | 1.88 | ID genus | No reliable ID | No ID | |
| Enterococcus lactis | 1 | Enterococcus faecium | 2.20 | MisID species | Enterococcus lactis | 99.9% | ID species |
| Enterococcus faecium | 1 | Enterococcus faecium | 2.19 | ID species | Enterococcus faecalis | 99.9% | MisID species |
| Staphylococcus lugdunensis | 1 | Staphylococcus haemolyticus | 1.85 | MisID species | Staphylococcus lugdunensis | 99.9% | ID species |
| Staphylococcus hominis | 1 | No reliable ID | 1.35 | No ID | Staphylococcus hominis | 99.9% | ID species |
| Pseudomonas otitidis | 1 | Pseudomonas otitidis | 2.40 | ID species | No reliable ID | No ID | |
| Pseudomonas monteilii | 1 | Pseudomonas monteilii | 2.21 | ID species | Pseudomonas putida | 99.9% | MisID species |
| Neisseria elongate | 1 | Neisseria elongate | 2.19 | ID species | Neisseria flava/perflava/subflava | 99.9% | MisID species |
| Citrobacter freundii | 1 | Citrobacter freundii | 2.43 | ID species | Citrobacter werkmanii | 99.9% | MisID species |
| Kocuria indica | 1 | Kocuria marina | 1.93 | MisID species | Kocuria rhizophila | 99.9% | MisID species |
| Dolosigranulum pigrum | 1 | No reliable ID | 1.34 | No ID | Dolosigranulum pigrum | 99.9% | ID species |
| Dermacoccus barathri | 1 | No reliable ID | 1.38 | No ID | Dermacoccus barathri | 99.9% | ID species |
| Providencia rettgeri | 1 | Providencia rettgeri | 2.30 | ID species | No reliable ID | No ID | |
| Haemophilus semen | 1 | Haemophilus influenzae | 2.43 | MisID species | Haemophilus haemolyticus/Haemophilus influenzae | 50/50 | MisID species |
| Brucella melitensis | 1 | Brucella melitensis | 2.41 | ID species | Brucella Spp. | 99.9% | ID genus |
| Lactobacillus gallinarum | 1 | Lactobacillus gallinarum | 1.92 | ID genus | No reliable ID | No ID | |
| Paenibacillus urinalis | 1 | Paenibacillus urinalis | 2.30 | ID species | Paenibacillus provencenis | 99.9% | MisID species |
| Cupriavidus pauculus | 1 | Cupriavidus pauculus | 1.85 | ID genus | No reliable ID | No ID | |
| Micrococcus luteus | 1 | Micrococcus luteus | 2.33 | ID species | No reliable ID | No ID | |
| Lactobacillus vaginalis | 1 | Lactobacillus vaginalis | 2.10 | ID species | No reliable ID | No ID | |
| Aeromonas hydrophila | 1 | Aeromonas caviae | 2.44 | MisID species | Aeromonas hydrophila | 99.9% | ID species |
| Campylobacter rectus | 1 | No reliable ID | 1.44 | No ID | Campylobacter rectus | 99.9% | ID species |
3.4. Comparison of the test time
The time required for test preparation, or the period from inserting the slide into the apparatus until the vacuum preparation range from 56s (range 52–58 s) for the EXS3000 to 1 min 35 s (range 1 min 8 s to 2 min 14 s) for the VITEK MS. Additionally, we compared the analysis time required by the two devices to analyze 16-spot, 48-spot and 96-spot. The 16-spot analysis was completed in an average time period of 1 min 50 s (1 min 36 s to 1 min 59 s) by the EXS3000 and 8 min 6 s (7 min 13 s to 8 min 28 s) by the VITEK MS, respectively. When running the 48-spot analysis, the EXS3000 and VITEK MS took on average 5 min 44 s (5 min 12 s to 6 min 9 s) and 22 min 49 s (21 min 13 s to 23 min 39 s), respectively. For the 96-spot analysis, EXS3000 required approximately 11 min 33 s (range 11 min 5 s to 11 min 58 s), while VITEK MS required 45 min 18 s (range 41 min 1 s to 47 min 33 s). The data is presented in Table 2.
Table 2.
Comparison of the time period required by Zybio EXS3000 and VITEK MS for each identification procedure.
| Description | Zybio EXS3000 | VITEK MS | P value |
|---|---|---|---|
| Test preparation time (n = 24) | 56 s (52 s–58 s) | 1 min 35 s (1 min 8 s-2 min 14 s) | <0.001 |
| 16-spot analysis time (n = 8) | 1 min 50 s (1 min 36 s-1 min 59 s) | 8 min 6 s (7 min 13 s-8 min 28 s) | <0.001 |
| 48-spot analysis time (n = 6) | 5 min 44 s (5 min 12 s-6 min 9 s) | 22 min 49 s (21 min 13 s-23 min 39 s) | <0.001 |
| 96-spot analysis time (n = 4) | 11 min 33 s (11 min 5 s-11 min 58 s) | 45 min 18 s (41 min 1 s-47 min 33 s) | <0.001 |
4. Discussion
Infectious diseases and related complications are the leading causes of mortality in critically ill patients. Timely and appropriate antibiotic administration is essential for the treatment of bacterial infections, which relies on the rapid and accurate identification of pathogenic microorganisms [19]. MALDI-TOF MS, as an emerging technology for microbial identification, has been increasingly recognized by clinical laboratories in recent years [[20], [21], [22], [23], [24], [25]]. Compared with conventional methods and molecular methods, this MS-based approach has the advantages of speed, accuracy, and cost-effectiveness [[26], [27], [28]]. Although the commonly utilized MALDI-TOF MS equipment has several limitations, it considerably increases the working efficiency of clinical microbiology laboratories and makes the identification of microorganisms more reliable.
In this work, we evaluated the performance of the newly developed Zybio EXS3000 system and compared it to the widely used VITEK MS system. This study included a total of 1340 strains involving Enterobacter spp., Streptococcus spp., Staphylococcus spp., Corynebacterium spp., Enterococcus spp., Neisseria spp., and yeast, etc., which adequately reflect the capacity of the system to identify pathogenic microorganisms.
Of all 1340 isolates collected from a variety of clinical specimens, 1234 (92.0%) isolates obtained the same results with log score (≥2.0 of Zybio EXS3000) and confidence value (≥99.9% of VITEK MS). Compared to the EXS3000, VITEK MS identified more isolates to the species/complex level. However, this gap was not statistically significant. The performance of the EXS3000 in this study was comparable to that of the VITEK MS.
In the identification of staphylococci (Staphylococcus aureus, S. epidermidis, S. haemolyticus), the mean log score of the results was significantly lower compared to Gram-negative bacteria. The primary explanation is that laser ablation cannot destroy the thick peptidoglycan layer in the cell wall of staphylococci, resulting in the inability to ionize some of the bacterial proteins during MALDI [29]. As a result, the system is unable to obtain high quality spectral data. According to Wang et al. [30], we added a step of FA lysis to the direct transfer method. By this approach, we considerably improved the identification accuracy and identification scores of Staphylococcus spp. Our future work will focus on matrix solution optimization and check the effect of solvent composition for Gram-positive cocci. Furthermore, some species have relatively insufficient spectral data in the database, which may also affect the accuracy of identification, and we will build in-house library to further increase the identification rate.
Low-confidence identification of yeast-like fungi, particularly Candida albicans (18.2%, 10/55), also occurred with EXS3000. The primary reason was that the sample preparation method used to build the database was inconsistent with the method used in this study. In building the database, the FA/acetonitrile extraction method was used for sample preparation to obtain a sufficient number of characteristic peaks. The FA/acetonitrile extraction method is, however, not suitable for routine use in clinical laboratories due to its complex and time-consuming drawbacks. The spectra obtained using the direct transfer method have fewer characteristic peaks, which makes it impossible to match well with the spectra data in the database. We will address this issue by supplementing the spectral data of yeast-like fungi in the database with the direct transfer method.
In clinical practice, it is sometimes difficult to distinguish some closely related species accurately. Patients with Acinetobacter baumannii infection, for example, are frequently misdiagnosed with other Acinetobacter species, particularly Acinetobacter nosocomialis. In this research, EXS3000 incorrectly identified one A. nosocomialis as A. baumannii, and VITEK MS also misidentified two A. baumannii as A. nosocomialis. Aeromonas spp. are also susceptible to misidentification, with EXS3000 misidentifying a strain of Aeromonas hydrophila as Aeromonas caviae. Such closely related species have different pathogenic potentials, and a strict distinction between them is crucial for the treatment of the infection. Therefore, the database and algorithms must be optimized to focus on such problems. On the bright side, both EXS3000 and VITEK MS performed well in distinguishing S. pneumoniae from related Streptococcus spp., achieving a correct identification with more than 95.0% accuracy.
For Enterobacterales members such as E. coli, Salmonella spp. and Shigella spp., a single MALDI-TOF MS analysis may not always provide sufficiently accurate identification results. For Salmonella spp., both instruments can only provided genus-level identification, and species or serotypes identification can only be achieved with the help of serological tests. Several studies had also demonstrated that the present MALDI-TOF MS technology still does not adequately meet the needs of clinical microbiology laboratories for the identification of Salmonella species [3,4]. Although the Shigella species and E. coli are closely related, they must typically be differentiated because of their different clinical significance. Previous studies had pointed out that E. coli and Shigella spp. share almost all of their primary characteristic peaks, making it challenging for the MALDI-TOF MS to distinguish them accurately [31,32]. Therefore, it is still necessary to differentiate them with the help of laboratory methods based on physio-biochemical characteristics. For the time being, the identification of few Enterobacterales specimens required a combination of MALDI-TOF MS, biochemical methods, and serotype testing.
The EXS3000 and VITEK MS also performed well in indentifying rare and fastidious bacteria, with species-level identification rates of 94.2% and 92.4%, respectively, for all 172 strains (Table S1). Such bacteria were relatively difficult to isolate or cultivate, and it was challenging and time-consuming to identify them by conventional phenotypic and biochemical methods. The use of MALDI-TOF MS for identification will help to improve the identification rate of such bacteria and will reduce the time and economic costs. In addition, it is worth mentioning that EXS3000 achieved correct species-level identification of a Burkholderia pseudomallei and a Brucella melitensis, while VITEK MS did not. The two species were considered potential bioterrorism pathogens because they risked large-scale transmission [33]. Due to safety concerns, however, we do not recommend the identification of such microorganisms be performed in common laboratories.
Furthermore, EXS3000 could process the identification procedure faster in comparison to VITEK MS. More specifically, the EXS3000 required less time during the steps of test preparation and target analysis. The remarkable reduction in preparation time may be attributed to the following: First, the unique structural design of EXS3000 makes the volume of its vacuum chamber (the space formed by the target tray, sample loading hole and hatch) smaller than that of VITEK MS, so it takes less time to reach the vacuum state. Secondly, the high-precision EXS3000 platform module can support the rapid operation of the components, so the target plate can quickly reach the detection site after entering the cabin. In addition, the reduction of detection time is because EXS3000 adopts different process of spectrogram acquisition unlike VITEK MS. As for instance, VITEK MS requires calibration and quality control during each round of testing, while EXS3000 only requires daily calibration, considerably cutting down on testing time without affecting the results of identification. Besides, in each identification process of VITEK MS, it is necessary to accumulate 100 good profiles (5 laser shots in each spot position are counted as a profile) or acquire 109 spot positions with more than 30 good profiles before spectra acquisition can be judged as passing. However, EXS3000 only needs 50 laser shots in each spot position to synthesize a single spectrum and only 200 shots to generate qualified Cumulated spectra. The reduction of time will also help to save costs and improve work efficiency. Besides, quicker identification aids in confirming the diagnosis and implementing proper medication as soon as possible, which is crucial for critically ill patients in the ICU. The application of cloud databases is also one of the advantages of the EXS3000 platform. The cloud database is continually updated by the manufacturer, and the most recent version is always accessible online without cost to the clinical laboratory.
In this study, we also admit that 16S rRNA sequencing or ITS sequencing were not performed for all the 1340 isolates, but only for those isolates with inconsistent identification results or low confidence scores from both instruments. In addition, the number of Salmonella spp. and filamentous fungal strains included in this study was limited. To improve the limitation present in this research, we need to incorporate more samples in our future work.
5. Conclusion
In conclusion, the current investigation reveals that the novel microbial identification system Zybio EXS3000 exhibits good identification performance which is comparable to VITEK MS while drastically lowering detection time. It demonstrates that the EXS3000 is capable of performing microbial identification in clinical laboratories. With the increasing quantity and quality of spectral data in the database, it is expected to become a valuable platform for microbial identification.
Declaration
Author contribution statement
Song Li: Conceived and designed the experiments; Analyzed and interpreted the data. Pinghua Qu: Conceived and designed the experiments; Analyzed and interpreted the data. Dexing Han: Wrote the paper; Perform.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
This study was partially funded by Zybio and one of the spectrometers studied is a Zybio product. The funder had no role in study design, data collection and analysis, publication decisions, or manuscript preparation.
Additional information
Supplementary content related to this article has been published online at [URL].
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:This study was partially funded by Zybio and one of the spectrometers studied is a Zybio product. The funder had no role in study design, data collection and analysis, publication decisions, or manuscript preparation.
Acknowledgments
We highly appreciate the financial support and technical help provided by Zybio, inc. And we also thank bioMérieux for their contribution in this study. This study was also supported by the Guangzhou Science and Technology Plan Project (grant number 202002020038, and 202103000025) and the Guangdong Basic and Applied Basic Research Foundation (grant number 2021A1515110857).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e18990.
Contributor Information
Pinghua Qu, Email: ququtdr@163.com.
Cha Chen, Email: chencha906@163.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Hu Shoukui, et al. Identification of Acinetobacter baumannii and its carbapenem-resistant gene blaOXA-23-like by multiple cross displacement amplification combined with lateral flow biosensor. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-54465-8. article 17888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasanov Uta, et al. Methods for the isolation and identification of Listeria spp. and Listeria monocytogenes: a review. FEMS Microbiol. Rev. 2005;29(5):851–875. doi: 10.1016/j.femsre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Yeh Hwey-Chin, et al. Identification of microbiota in peri-implantitis pockets by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Sci. Rep. 2019;9(1):774. doi: 10.1038/s41598-018-37450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngeow Yun Fong, et al. Short chain N-acylhomoserine lactone production by clinical multidrug resistant Klebsiella pneumoniae strain CSG20. Sensors. 2013;13(11):15242–15251. doi: 10.3390/s131115242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Weiping, et al. Performance of mass spectrometric identification of bacteria and yeasts routinely isolated in a clinical microbiology laboratory using MALDI-TOF MS. J. Thorac. Dis. 2014;6(5):524–533. doi: 10.3978/j.issn.2072-1439.2014.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bizzini A., et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry as an alternative to 16S rRNA gene sequencing for identification of difficult-to-identify bacterial strains. J. Clin. Microbiol. 2011;49(2):693–696. doi: 10.1128/JCM.01463-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhal Neelja, et al. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang Kyoung-Soon, Young Hwan Kim. Rapid and robust MALDI-TOF MS techniques for microbial identification: a brief overview of their diverse applications. J. Microbiol. 2018;56(4):209–216. doi: 10.1007/s12275-018-7457-0. [DOI] [PubMed] [Google Scholar]
- 9.Uhlik Ondrej, et al. Matrix-assisted laser desorption ionization (MALDI)-time of flight mass spectrometry- and MALDI biotyper-based identification of cultured biphenyl-metabolizing bacteria from contaminated horseradish rhizosphere soil. Appl. Environ. Microbiol. 2011;77(19):6858–6866. doi: 10.1128/AEM.05465-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Qiutao, et al. Screening for potential serum-based proteomic biomarkers for human type 2 diabetes mellitus using MALDI-TOF MS. Proteonomics Clin. Appl. 2017;11:3–4. doi: 10.1002/prca.201600079. [DOI] [PubMed] [Google Scholar]
- 11.Jang Kyoung-Soon, Young Hwan Kim. Rapid and robust MALDI-TOF MS techniques for microbial identification: a brief overview of their diverse applications. J. Microbiol. 2018;56(4):209–216. doi: 10.1007/s12275-018-7457-0. [DOI] [PubMed] [Google Scholar]
- 12.Chung Yousun, et al. Comparative evaluation of bruker biotyper and ASTA MicroIDSys for species identification in a clinical microbiology laboratory. Diagnostics. 2021;11(9):1683. doi: 10.3390/diagnostics11091683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Yong-Lu, et al. Evaluation of an in-house MALDI-TOF MS rapid diagnostic method for direct identification of microorganisms from blood cultures. J. Med. Microbiol. 2019;68(1):41–47. doi: 10.1099/jmm.0.000866. [DOI] [PubMed] [Google Scholar]
- 14.Ma Qiong, et al. Evaluation of the Autof MS1000 mass spectrometer in the identification of clinical isolates. BMC Microbiol. 2020;20(1):318. doi: 10.1186/s12866-020-02005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Keyi, et al. Establishment and application of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for detection of Shewanella genus. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.625821. article 625821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janda J Michael, Abbott Sharon L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J. Clin. Microbiol. 2007;45(9):2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute . first ed. CLSI guideline M58; Wayne, PA: 2017. Methods for the Identification of Cultured Microorganisms Using Matrix Assisted Laser Desorption/ionization Time of Flight Mass Spectrometry. [Google Scholar]
- 18.Zhou Longrong, et al. Performance of VITEK mass spectrometry V3.0 for rapid identification of clinical Aspergillus fumigatus in different culture conditions based on ribosomal proteins. Infect. Drug Resist. 2017;10:499–506. doi: 10.2147/IDR.S148121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buehler, Stephanie S., et al. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin. Microbiol. Rev. 2016;29(1):59–103. doi: 10.1128/CMR.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulthess Bettina, et al. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J. Clin. Microbiol. 2013;51(6):1834–1840. doi: 10.1128/JCM.02654-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tekippe McElvania, Erin, et al. Optimizing identification of clinically relevant Gram-positive organisms by use of the Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry system. J. Clin. Microbiol. 2013;51(5):1421–1427. doi: 10.1128/JCM.02680-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fothergill Amy, et al. Rapid identification of bacteria and yeasts from positive-blood-culture bottles by using a lysis-filtration method and matrix-assisted laser desorption ionization-time of flight mass spectrum analysis with the SARAMIS database. J. Clin. Microbiol. 2013;51(3):805–809. doi: 10.1128/JCM.02326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford Bradley A., D Burnham Carey-Ann. Optimization of routine identification of clinically relevant Gram-negative bacteria by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry and the Bruker Biotyper. J. Clin. Microbiol. 2013;51(5):1412–1420. doi: 10.1128/JCM.01803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan K.E., et al. Prospective evaluation of a matrix-assisted laser desorption ionization-time of flight mass spectrometry system in a hospital clinical microbiology laboratory for identification of bacteria and yeasts: a bench-by-bench study for assessing the impact on time to identification and cost-effectiveness. J. Clin. Microbiol. 2012;50(10):3301–3308. doi: 10.1128/JCM.01405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khot Prasanna D., et al. Optimization of matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis for bacterial identification. J. Clin. Microbiol. 2012;50(12):3845–3852. doi: 10.1128/JCM.00626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neville Stephen A., et al. Utility of matrix-assisted laser desorption ionization-time of flight mass spectrometry following introduction for routine laboratory bacterial identification. J. Clin. Microbiol. 2011;49(8):2980–2984. doi: 10.1128/JCM.00431-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Veen S.Q., et al. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J. Clin. Microbiol. 2010;48(3):900–907. doi: 10.1128/JCM.02071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherkaoui Abdessalam, et al. Comparison of two matrix-assisted laser desorption ionization-time of flight mass spectrometry methods with conventional phenotypic identification for routine identification of bacteria to the species level. J. Clin. Microbiol. 2010;48(4):1169–1175. doi: 10.1128/JCM.01881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smole Sandra C., et al. Sample preparation of Gram-positive bacteria for identification by matrix assisted laser desorption/ionization time-of-flight. J. Microbiol. Methods. 2002;48:2–3. doi: 10.1016/s0167-7012(01)00315-3. 107-115. [DOI] [PubMed] [Google Scholar]
- 30.Wang Jinghua, et al. Evaluation of three sample preparation methods for the identification of clinical strains by using two MALDI-TOF MS systems. J. Mass Spectrom.: JMS. 2021;56(2) doi: 10.1002/jms.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling Jin, et al. A novel short-term high-lactose culture approach combined with a matrix-assisted laser desorption ionization-time of flight mass spectrometry assay for differentiating Escherichia coli and Shigella species using artificial neural networks. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0222636. article e0222636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng Jiankai, et al. Comparison of MALDI-TOF MS, gene sequencing and the Vitek 2 for identification of seventy-three clinical isolates of enteropathogens. J. Thorac. Dis. 2014;6(5):539–544. doi: 10.3978/j.issn.2072-1439.2014.02.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotz Lisa D., et al. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 2002;8(2):225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.