Abstract
Natural killer (NK) cells display a unique inherent ability to identify and eliminate virus-infected cells and tumor cells. They are particularly powerful for elimination of hematological cancers, and have attracted considerable interests for therapy of solid tumors. However, the treatment of solid tumors with NK cells are less effective, which can be attributed to the very complicated immunosuppressive microenvironment that may lead to the inactivation, insufficient expansion, short life, and the poor tumor infiltration of NK cells. Fortunately, the development of advanced nanotechnology has provided potential solutions to these issues, and could improve the immunotherapy efficacy of NK cells. In this review, we summarize the activation and inhibition mechanisms of NK cells in solid tumors, and the recent advances in NK cell-based tumor immunotherapy boosted by diverse nanomaterials. We also propose the challenges and opportunities for the clinical application of NK cell-based tumor immunotherapy.
Keywords: NK cells, Nanomaterials, Immunotherapy, Exogenous NK cells, Endogenous NK cells
Graphical abstract

Highlights
-
•
First comprehensive summary of the recent advances in the use of nanomaterials for enhancing NK cell-based tumor immunotherapy.
-
•
The mechanisms of NK cell activation and inhibition for immunotherapy.
-
•
Rational design and fabrication of nanomaterials to elaborately enhance NK cell-based tumor immunotherapy.
-
•
Challenges and opportunities for NK cell-based tumor immunotherapy in clinical applications.
Abbreviations
- ADO
Adenosine
- ADCC
Antibody dependent cell-mediated cytotoxicity
- ACT
Adoptive cell therapy
- CRS
Cytokine release syndrome
- CAR
Chimeric antigen receptor
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- CaP
CpG loaded-phosphate calcium
- COX2
Cyclooxygenase-2
- DCs
Dendritic cells
- DCNP
Down-conversion nanoparticles
- DDEs
DC-derived exosomes
- DNase I
Deoxyribonuclease I
- ECM
Extracellular matrix
- FasL
Fas ligand
- FLI
Fluorescence imaging
- GVHD
Graft-versus-host disease
- GOX
Glucose oxidase
- GNSs
Gold nanoclusters
- HCC
Hepatocellular carcinoma
- HLA
Human leukocyte antigen
- HSP
Heat shock protein
- IDO1
Indoleamine 2,3-dioxygenase 1
- IFN-γ
Interferon gamma
- IL
Interleukin
- iPSC
Induced pluripotent stem cells
- ITAMs
Immunoreceptor tyrosine-based activation motifs
- KIR
Killer cell immunoglobulin-like receptor
- LDHA
Lactate dehydrogenase A
- MDSC
Myeloid-derived suppressor cells
- MHC I
Major histocompatibility complex class I molecules
- MICA
MHC I polypeptide-related sequence A
- MICB
MHC I polypeptide-related sequence B
- MRI
Magnetic resonance imaging
- NAD+
Nicotinamide adenine dinucleotide
- NDEs
NK cell-derived exosomes
- NETs
Neutrophil extracellular traps
- NK
Natural killer
- NKCEs
Natural killer cell engagers
- NMN
Nicotinamide mononucleotide
- PAI
Photoacoustic imaging
- PDT
Photodynamic therapy
- PD-1
Programmed death-1
- PET
Positron emission tomography
- PFC
Perfluorohexane
- PGE2
Prostaglandin E2
- PTT
Photothermal therapy
- RBCs
Red blood cells
- ROS
Reactive oxygen species
- SHP-1
Src homology 2 domain-containing protein tyrosine phosphatase-1
- TCRs
T cell receptors
- TDEs
Tumor cell-derived exosomes
- TIGIT
T-cell Ig and ITIM domain
- TIM3
T cell immunoglobulin domain and mucin domain-3
- TLR4
Toll-like receptor 4
- TME
Tumor microenvironment
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- Treg
Regulatory T cells
- ULBPs
UL16-binding proteins
1. Introduction
Natural killer (NK) cells are innate lymphoid cells with strong cytolytic function to identify and kill tumor cells directly without antigen presentation, which is different to the other adaptive immune cells such as T cells and B cells [1]. In addition, NK cells lack surface T cell receptors (TCRs) and cannot cause cytokine release syndrome (CRS), and graft-versus-host-disease (GVHD) [2], which provides an opportunity to use the autologous (from the patients) or allogeneic (from the healthy donors) sources of NK cells for adoptive cellular therapy [3]. Chimeric antigen receptor (CAR) NK cells have been successfully investigated in clinical studies. The effects of NK cell infusion combined with IL-15 on solid and hematologic tumors, as well as the effects of anti-CD19 CAR-engineered NK cells on leukemia patients were investigated [4,5]. For example, 7/11 patients with relapsed or refractory CD19-positive cancers had a complete remission [5]. CAR-NK cells may have advantages over CAR-T cells, such as less induced side effects. In this context, recent studies have shown that CAR-NK cells did not cause any associated cytokine storm in patients with tumors, which makes the NK cell-based tumor immunotherapy very attractive and promising [6].
NK cell effector function is controlled by the surface activating and inhibiting receptors that can distinguish tumor cells from healthy cells. The most important inhibitory receptors on the surface of NK cells are the members of the killer cell immunoglobulin-like receptor (KIR) family and CD94/NKG2A, which recognize the major histocompatibility complex class I molecules (MHC I; MHC is also known as human leukocyte antigen, HLA). MHC I molecules expressed on the normal healthy cells act as ligands of inhibitory receptors and contribute to the self-tolerance of NK cells [7]. For tumor cells, their expression of MHC I molecules are often downregulated, allowing them escaping from the immune surveillance of CD8+ T cells. In contrast, with the absence of the main ligands of NK cells inhibitory receptors (CD94/NKG2A and KIRs), NK cells are not inhibited and become activated. They can directly kill tumor cells through NK cell-mediated cytotoxicity, or indirectly kill them through secretion of cytotoxic mediators (Granzyme B), pore-forming proteins (perforin), cytokines, and chemokines [8]. Granzyme B and perforin are the core proteins required for NK cell mediated tumor killing, although the death-receptor pathways (involving FasL and TRAIL) are sometimes involved [9]. Furthermore, NK cells secrete a large number of cytokines (such as IFN-γ, TNF-α, TNF-β, IL-6, GM-CSF) and chemokines (such as CCL3, CCL4, CCL5), which can shape B cell and T cell activation, and impact the function of macrophages, dendritic cells (DCs), and neutrophils [10]. The wealth properties of NK cells indicate their related complicated biological mechanism network and support the great potential of NK cell-based tumor immunotherapy.
Based on the powerful selective anti-tumor efficacy and immune-surveillance function, NK cell-based immunotherapy has been widely investigated both in hematologic and solid malignant cancers, such as myeloid leukemia [11,12], cervical cancer [13,14], melanoma [15,16], glioblastomas [17], and hepatocellular carcinoma [18,19]. However, NK cell-based immunotherapy is less effective in treatment of solid tumors because of the following challenges [20,21]. The tumor microenvironment (TME) and suppressive immunity of solid tumors lead to the dysfunction and poor infiltration of NK cells into the tumors [[22], [23], [24]]. Furthermore, the expression of ligands on tumor cell surface for activation of NK cells is reduced, which is contrast to the expression of inhibitory ligands [[25], [26], [27]]. Therefore, development of innovative methods to boost NK cell-based tumor immunotherapy is highly desired.
A possible solution for improving the efficacy of NK cell-based tumor immunotherapy is the nanotechnology. Over the past few decades, the application of nanotechnology in immunotherapy has attracted extensive attention [[28], [29], [30], [31], [32]]. The unique physical and chemical properties of nanoscale materials lay a solid foundation for tumor therapy [[33], [34], [35]]. Nanomaterials can be modified to selectively penetrate into tumor tissue to overcome several limitations of tumor immunotherapy [36,37]. They can selectively target immune or tumor cells by modification with targeting molecules, peptides or antibodies, and thus enhance the efficacy of immunotherapy [[38], [39], [40]]. In this article, we will review the recent advances in NK cell-based tumor immunotherapy, including the mechanism of NK cell activation and inhibition in tumor, rational design, fabrication and application of bionanomaterials for enhancing efficacy of NK cell-based tumor immunotherapy, and the new trends and clinical challenges (Fig. 1).
Fig. 1.
Illustrative representation of NK cell dysfunction in tumor and nanomaterials mediated NK cell-based tumor immunotherapy. The left half of Fig. 1 shows several major causes of NK cell dysfunction: (1) Downregulation of activating receptors on NK cells; (2) Upregulation of inhibitory ligands on tumor cells; (3) Inhibitory cytokines secreted by tumor cells, myeloid-derived suppressor cells (MDSC), and regulatory T cells (Treg); (4) Immunosuppressive tumor microenvironment. The right half of Fig. 1 shows several nanomaterial-mediated strategies for enhancing NK cell immunotherapy: (1) Encapsulation of core materials with the cell membrane from tumor cells, macrophages, red blood cells, or NK cells; (2) Use of exosomes and exosome-related nanomaterials from different parental cells (NK cells, DCs, or tumor cells); (3) Engineering of exogenous NK cells by nanomaterials to enhance their tumor targeting capability; (4) Regulation of tumor microenvironment, tumor or/and NK cells by nanomaterials to boost endogenous NK cells.
2. Sources, expansion, activation and inhibition of NK cells
2.1. Sources, expansion, and clinical trials of clinical-grade NK cells
NK cells used for infusion can be obtained from either the patients themselves or the donors. The purification/expansion of clinical-grade NK cells can be produced by using the peripheral blood, cord blood, and embryonic stem cells [[41], [42], [43], [44]]. In autologous transplantation, NK cells from the patients are activated and expanded in vitro in the presence of cytokines. The research results suggest that the combination of IL-12, IL-15, and IL-18 may produce NK cells with stronger functions and memory properties. Irradiated human lymphoblastocytes K562 are often used as feeder cells in the medium and can be modified to express cytokines (such as IL-15 and IL-21) and/or co-stimulatory molecules. Then, the activated and amplified NK cells are transferred back into the patients, who are typically administrated with cytokine administration (IL-2) to maintain the expansion and function of the injected NK cells [45]. In allogeneic transfer, NK cells can be obtained from HLA-matched or haploidentical donors.
NK cells are expanded through the processes similar to those used for autologous transfer, but T cells should be removed to avoid GVHD [46]. In recent years, various NK cell-mediated tumor therapies were tested in clinical trials and yielded promising results in patients (Table 1). A growing number of clinical studies have shown the safety and efficacy of NK cells derived from induced pluripotent stem cells (iPSC), peripheral blood mononuclear cells, cord blood, and cells lines in the treatment of malignant tumors [[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67]]. For example, in a phase I trial, iPSC-NK cells expressing CAR showed encouraging results in treatment of patients with relapsed or refractory B-cell lymphoma [48,68].
Table 1.
Clinical trials of tumor immunotherapy by using NK cells in recent years.
| Cell source | Cancer type | Study phase | Trail Name | Ref |
| Not disclosed | Relapsed or refractory B cell non-hodgkin lymphoma | Early Phase I | NCT04639739 | [47] |
| iPSC | Relapsed/refractory B-cell lymphoma; Chronic lymphocytic leukemia | Phase I | NCT04245722 | [48] |
| iPSC | Relapsed/refractory acute myelogenous leukemia; Relapsed/refractory multiple myeloma | Phase I | NCT04614636 | [49] |
| Peripheral blood | Acute myeloid leukemia; Myelodysplastic syndrome | Phase I | NCT04623944 | [50] |
| iPSC | Ovarian cancer | Phase I | NCT04630769 | [51] |
| iPSC | Advanced solid tumors | Phase I | NCT04551885 | [52] |
| Peripheral blood | Relapsed/refractory non-hodgkin lymphoma; chronic lymphocytic leukemia; B cell acute lymphoblastic leukemia | Phase I | NCT05020678 | [53] |
| Peripheral blood from HLA-haplo-identical donor | Refractory/relapsed B-cell NHL | Phase I | NCT04887012 | [54] |
| Cord blood | Relapsed or refractory hematological malignancies | Phase I | NCT04796675 | [55] |
| Not disclosed | Relapsed or refractory hematological malignancies | Phase I | NCT04796688 | [56] |
| Not disclosed | Relapsed/refractory acute myeloid leukemia | Phase I | NCT05008575 | [57] |
| Cord blood | Relapse/refractory hematological malignances | Phase I | NCT05092451 | [58] |
| Not disclosed | Relapsed or refractory multiple myeloma | Early phase I | NCT05008536 | [59] |
| Modified NK-92 | Recurrent/metastatic gastric or head and neck cancer | Phase II | NCT04847466 | [60] |
| Not disclosed | Relapsed/refractory B-cell acute lymphoblastic leukemia | Phase I | NCT05379647 | [61] |
| iPSC | Relapsed/refractory CD19-positive B-cell malignancies | Phase I | NCT05336409 | [62] |
| Not disclosed | Acute myeloid leukemia | Early phase I | NCT05215015 | [63] |
| iPSC | Relapsed/refractory multiple myeloma | Phase I | NCT05182073 | [64] |
| Not disclosed | Refractory metastatic colorectal cancer | Phase I | NCT05213195 | [65] |
| Cord blood | Relapsed/refractory acute myeloid leukemia | Not Applicable | NCT05247957 | [66] |
| Not disclosed | Advanced solid tumors | Early phase 1 | NCT05194709 | [67] |
2.2. Receptors, cytokines and small molecule drugs used for NK cell activation
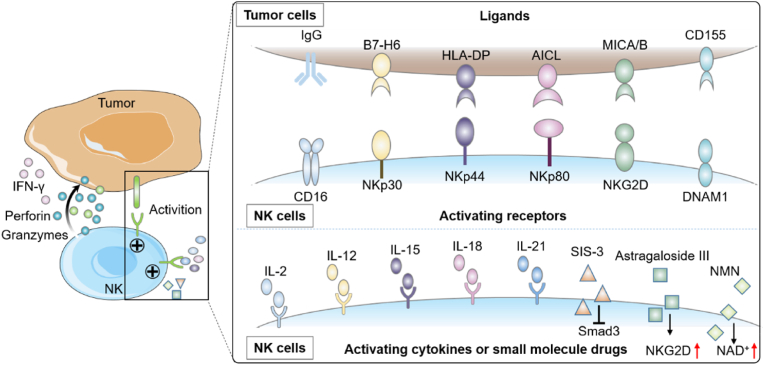
NK cell activation and inhibition is mainly controlled by a series of activating, co-stimulatory and inhibitory receptors. The net balance of activating and inhibiting signals of these receptors determines the state of NK cells. The activating receptors include the Fcγ receptor CD16, the natural cytotoxicity receptors family (NKp30, NKp44 and NKp80, etc.), C-type lectin receptors (NKG2D, CD94/NKG2C, etc.), killer cell immunoglobulin-like receptors (KIRs) (KIR-2DS, KIR-3DS, etc.) (Fig. 2).
Fig. 2.
Schematic illustration of receptors, cytokines and small molecule drugs used for activating NK cells. (1) Activating receptor transduce signals through various pathways. The interactions between activating receptors and their ligands can activate NK cells and trigger the apoptosis of tumor cells. Activated NK cells can release chemokines for attracting multiple subsets of immune cells, and the release of cytolytic granules containing granzyme B and perforin. (2) The anti-tumor activity of NK cells can also be enhanced by cytokines (IL-2, IL-12, IL-15, IL-18, IL-21) or specific small molecules (SIS-3, Astragaloside III, NMN, etc.).
CD16 is a potent activating receptor of NK cells, which can recognize the Fc portion of IgG antibodies specifically for activating NK cells through a process termed antibody dependent cell-mediated cytotoxicity (ADCC) [69]. It is the only receptor that can activate NK cells independently, without any additional activation through other receptors [70]. Preventing the down-regulation of CD16 in the NK cells might enhance their activity [71]. In addition to CD16, members of natural cytotoxicity receptors family (NKp30, NKp44 and NKp80, etc.) can also directly bind to tumor associated ligands to trigger the apoptosis of tumor cells [72]. NKG2D is a widely studied homodimeric C-type lectin receptor, and its ligands on tumors include MHC I polypeptide-related sequence A (MICA), MHC I polypeptide-related sequence B (MICB), and UL16-binding proteins (ULBPs) [73]. Inhibition of MICA and MICB shedding on the tumor surface by antibodies may promote NK cell-based tumor immunotherapy [74]. CD94/NKG2C is a heterodimer C-type lectin receptor and binds to HLA-E ligand, which is typically associated with inhibition signals via the NK cell inhibitory receptor NKG2A [75]. KIRs family of NK receptors can be divided into activating receptors and inhibitory receptors, depending on their transmembrane and intracellular domain. The activating receptors (KIR-2DS, KIR-3DS etc.) can produce activating signals through their association with adaptor proteins that contain ITAMs [76]. NK cell activation also depends on co-stimulation molecules such as 4-1BB (also known as CD137) [77], 2B4 (also known as CD244) [78], DNAM1 (also known as CD226) [79], and OX40 (also known as CD134) [80].
Ligation of individual activating receptors (except CD16) is usually not enough to trigger the cytotoxicity or cytokine secretion of naive NK cells. The secretion of cytokines is the key to pre-activation of NK cells [81]. Various cytokines, including IL-2, IL-12, IL-15, IL-18, and IL-21, can be used for enhancing the activation of NK cells (Fig. 2) [[82], [83], [84]]. In autologous NK cell transfer, IL-2 has been usually used for activation and expansion of NK cells [85]. Patients received infusion of NK cells are usually given IL-2 to promote in vivo expansion [86]. However, the anti-tumor effect of individual IL-2, IL-12 or IL-18 is rather limited. In contrast, the combination of IL-12, IL-15 and IL-18 may induce considerable biological changes and a population of memory NK cells, including enhanced responses to IL-2, more secretion of IFN-γ and greater cytotoxicity [45,81]. In addition, the combination of IL-21 and tumor targeted monoclonal antibodies can enhance the activity of NK cells and improve their anti-tumor effects [[87], [88], [89]]. However, among these cytokines, IL-15 is the most promising one for activating NK cells. Infusion of IL-15 into the patients with metastatic malignancies in clinical trials showed proliferation and substantial increase of NK cells [90].
In addition to cytokines, small molecule drugs can also effectively enhance the anti-tumor activity of NK cells. SIS-3, a specific Smad3 inhibitor, is used as an immunotherapeutic stimulant for promoting the activity and proliferation of NK cells [[91], [92], [93]]. It has been found that the dysregulated NAD+ metabolism contributes to the dysfunction of tumor-infiltrating NK cells. Supplementation of the NAD+ precursor nicotinamide mononucleotide (NMN) significantly enhanced NK cell-based tumor immunotherapy [94]. Natural compounds extracted from plants also possess the capability of activating NK cells [95], mainly including the vitamins and phytochemicals. Vitamins play crucial functions in cell metabolism. Retinoids (Vitamin A) can effectively increase the activity of splenic NK cells and suppress tumor growth [96]. Another study showed that the NK cell activity was increased after oral administration of vitamin C and returned to normal level after 48 h [97]. Apart from several vitamins, various phytochemicals have been identified as activators of NK cells [98]. Astragaloside III, a natural compound from astragalus, can significantly elevate the expressions of NKG2D, Fas, and IFN-γ in NK cells, leading to the increased infiltration of NK cells into tumor to upregulate the anti-tumor response [99]. Although various natural compounds can effectively activate NK cells for immunotherapy, their activation molecular mechanisms are still elusive and need to be comprehensively investigated.
2.3. Receptors, cytokines and immunosuppressive environment for NK cell inhibition
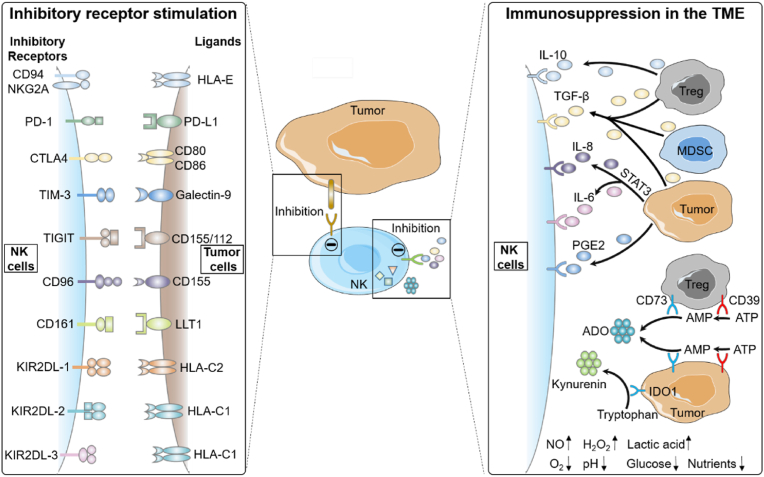
NK cells also express various inhibitory receptors, which provide immune self-tolerance and negative feedback mechanisms, and counteract the activation signals from activating receptors (Fig. 3). There are multiple families of NK cells inhibitory receptors, and the autoreactivity in human NK cells is controlled by KIRs and CD94/NKG2A inhibitory receptors specifically for recognizing human leukocyte antigen class I (HLA I) molecules. Under normal conditions, the interactions between these inhibitory receptors and their specific HLA I ligands inactivate NK cells, thus preventing cytolysis of healthy cells [7]. On the contrary, the overexpression of HLA-E in some tumors is associated with the worse outcome. Reducing the function of inhibitory KIRs by specifically blocking ligand recognition in the treatment of patients with HLA-E overexpression tumors would be particularly effective [100,101]. Other inhibitory immune checkpoints have also been found in NK cells, which are responsible for maintaining the homeostasis of immune cells, include PD-1, T-cell Ig and ITIM domain (TIGIT), CTLA4, TIM-3, CD96, etc. Several tumor mouse models showed that PD-1/PD-L1 interactions potently suppress NK cell-mediated immunotherapy [102]. TIGIT and CD96 are members of a group of Ig superfamily receptors. Their corresponding ligands CD112 and CD155, are usually upregulated in tumor cells. The upregulation of TIGIT and PD-1 has been reported in different malignancies, and they are usually related to the immunosuppression of T cells and NK cells in tumor [103]. It has been shown that TIGIT blocked with specific mAbs can induce excellent anti-tumor immune activity when it is combined with other checkpoints [104]. T cell immunoglobulin domain and mucin domain-3 (TIM-3) is a type 1 glycoprotein co-expressed with PD-1 on matured NK cells, and its ligand is Galecin-9 [105]. Previous studies have demonstrated that the upregulation of TIM-3 on NK cells in tumor impaired their effector function. The blockade of TIM-3 could restore T-cell effector function in preclinical models and result in an increased NK cytotoxicity [106,107]. In summary, blocking the interactions of inhibitory receptors or their ligands is still an attractive strategy in NK cell-based tumor immunotherapy.
Fig. 3.
Schematic illustration of receptors, cytokines and immunosuppressive environment for inhibiting NK cells. The left half of Fig. 3 shows the interactions of NK cell inhibitory receptors with their ligands. The overexpression or overstimulation of inhibitory receptors leads to inhibition of infiltrating NK cell activity in many malignant solid tumors. The right half of Fig. 3 shows that NK cell inhibition is associated with the immunosuppression of cytokines, immunomodulatory enzymes, and TME.
In addition to inhibitory receptor, intracellular checkpoint molecules (e.g. SHP-1 and Cbl-b/c-Cbl) are also engaged and functioned in the downstream of inhibitory and activating receptors of NK cells. Src homology 2 domain-containing protein tyrosine phosphatase-1(SHP-1) has been shown to inhibit NK cell activity by dephosphorylating the guanine nucleotide exchange factor (Vav1) [108], and the acute loss of SHP-1 abundance or activity is needed for enhancing anti-tumor function of NK cells [109,110]. In addition to SHP-1, the E3 ubiquitin ligases Cbl-b and c-Cbl, as the key negative regulators of NK cell activity, can block NK cells recognizing and killing tumor cells [111,112]. For example, a series of small-molecule Cbl-b inhibitors activate NK cell activity by inhibiting ubiquitination of TAM receptors [113,114].
During tumor progression, tumor cells in the TME have several ways to either evade from NK-cell recognition, or induce dysfunction of NK cells. The TME consists of immunosuppressive cytokines (e.g. TGF-β, IL-6, IL-8, IL-10, prostaglandin E2 (PGE2), adenosine (ADO)), immunomodulatory enzymes (e.g. CD39, CD73, indoleamine 2,3-dioxygenase 1 (IDO1), low nutrients (e.g. glucose and amino acid), hypoxia and acidity, which synergistically suppress the maturation, proliferation and activation of NK cells (Fig. 3) [[115], [116], [117], [118]]. In addition, the accumulation of extracellular matrix and increased interstitial fluid pressure can prevent the tissue penetration of immune cells [119].
TGF-β is a major immunosuppressive cytokine that reduces the anti-tumor activity of NK cells. In glioblastoma, TGF-β can reduce the expression of the major activating receptors on NK cells [117,120]. Patients with colorectal and lung cancers have higher level of plasma TGF-β than healthy volunteers, which leads to the decreased NKG2D expression on NK cells and the damage to NK cell function [121]. STAT3 signaling plays a key role in the secretion of IL-6 and IL-8, downregulates the activation receptors (NKG2D and NKp30) on NK cells in various primary tumors, decreases NK cell activity, and contributes to tumor progression and poor survival [122]. Apart from immunosuppressive cytokines, various cell types in the TME can downregulate immune response including regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSC), and fibroblasts [[123], [124], [125]]. Treg cells play an immunosuppressive role by inhibiting NK cells and CD8+ T cells via secretion of the IL-10 and TGF-β [126,127]. MDSC of tumor patients significantly inhibits NK cell-mediated ADCC by producing nitric oxides [128]. PGE2 is a small molecule upregulated in various cancers, produced by tumor cells and tumor-associated mesenchymal stem cells, which can reduce NK cytotoxicity [129,130].
In the TME, the immunoregulatory enzymes can produce immunosuppression through their catalytic products, which act as negative regulators of T cells and NK cells to inhibit their metabolism and effector functions. CD39 and CD73 are two immunoregulatory enzymes expressed in the TME, which contribute to the conversion of extracellular ATP into the immunosuppressive metabolite ADO [131]. IDO1 as a metabolic enzyme overexpressed in various tumors can convert the tryptophan into kynurenine to suppress the activation of NK cells [132,133]. The strategies for overcoming immunosuppressive factors mediated NK cell dysfunction include inhibiting immunosuppressive cytokines and immunoregulatory enzymes via small molecule inhibitors, or blocking metabolic enzymes with antagonistic antibodies [[134], [135], [136], [137]].
Apart from immunosuppressive cytokines and enzymes, most solid tumors are featured with hypoxia, low nutrients, and acidity due to their heterogeneity. Hypoxia is a common driver of NK cell dysfunction in solid tumors [138,139]. It promotes tumor cells to exhibit an abnormal metabolic behavior and result in a high level of lactic acid, over consumption of vital nutrients, high concentrations of toxic catabolites and the formation of tumor acid microenvironment, which further affects the recognition and response of the immune system to tumor cells [140]. To overcome the detrimental effects of hypoxia and metabolic immunosuppression, the current strategies mainly focus on two aspects: improving hypoxia or changing the metabolic constitutions of tumors to boost NK cells in the immunosuppressive TME. The cytotoxic activity of NK cells can be promoted by reducing the immunosuppressive metabolites [141,142]. For example, the lactate in the TME can be decreased by targeting lactate transporters, leading to improved anti-tumor cytotoxicity of T cells and NK cells in vivo [143,144].
It is important to keep physiological balance during the regulation of hypoxia and immune metabolism in TME, because some metabolites are important components of normal metabolism. Although many strategies are still in the stage of basic research or clinical trials, the combined methods involving TME regulation and NK cell immunotherapy could reduce immunosuppression to achieve better anti-tumor therapy.
Among the NK cell-based tumor immunotherapy strategies, the recent trend is to develop effective targeted nanomaterials to promote anti-tumor immune response of NK cells. Because of the unique physical and chemical properties, nanomaterials have been widely explored in tumor immunotherapy. They can target specific immune cells to transport immunomodulators, kill tumor cells to enhance antigen release, regulate immunosuppressive microenvironment and enhance the efficacy of immunotherapy. In this review, we will focus on the recent advances in nanomaterials mediated NK cell immunotherapy, along with the perspectives and challenges.
3. Nanomaterials for enhancing NK cell immunotherapy
Benefited from the excellent biocompatibility and biodegradability, various bioactive nanomaterials have shown great potential in enhancing efficacy of NK cell immunotherapy. This section introduces different strategies for enhancing engineering NK cell-based tumor immunotherapy by different types of nanomaterials, including cell membrane coated nanomaterials, exosome related nanomaterials, nanomaterials engineer exogenous NK cells, and nanomaterials boost endogenous NK cells (Fig. 4).
Fig. 4.
Schematic illustration of the nanomaterials boosting NK cell-based tumor immunotherapy. Four types of nanomaterials were used for enhancing NK cell immunotherapy: (1) Nanoparticles coated with cell membranes from different sources show the advantages of immune evasion, long blood circulation, tumor targeting, and good biocompatibility; (2) Exosomes and exosome-related nanomaterials from different parental cells (NK cells, DCs, or tumor cells) show the advantages of excellent biocompatibility, low toxicity, and biostability; (3) Nanomaterials promote the infiltration and activation of engineered exogenous NK cells in tumor site; (4) Nanomaterials boost the performance of endogenous NK cells in the following methods: (a) Regulating the tumor microenvironment; (b) Reducing the negative effects by regulating tumor cells or NK cells; (c) Acting as nanoengagers to co-regulate tumor and endogenous NK cells; (d) Promoting the immunogenic cell death of tumor cells for enhancing NK cell-based tumor immunotherapy.
3.1. Cell membrane coated nanomaterials
Coating nanomaterials by the natural cell membrane has attracted great attention to improve tumor therapy [36,145,146]. Tumor cells, macrophages, red blood cells (RBCs), NK cells are different sources for extraction of cell membrane. Tumor cell membrane modified nanomaterials can provide tumor associated antigen to activate immune response. Chen et al. synthesized CpG loaded-phosphate calcium nanoparticles coated with artificial membrane-mimicking phospholipid bilayer, which can be incorporated with B16OVA cell membrane and HSP70p in a self-assembled manner (Fig. 5A). The biomimetic nanomaterials can be delivered to the lymph nodes efficiently to activate the immune response of both innate NK cells and adaptive cytotoxic T cells [147]. Macrophages play an important role in the TME and affect the tumor progression and metastasis directly. Macrophage membrane modification can promote the infiltration of nanomaterials into tumor and synergize with anti-PD-L1 to restore their tumoricidal function for cancer immunotherapy (Fig. 5B) [148]. RBC membrane modified nanomaterials show long circulation life, they can also evade the immune system due to the presence of self-markers in the membrane [149,150]. Zhang et al. constructed the artificial NK cells (aNK) by emulsifying the mixture of RBC membrane, perfluorohexane (PFC), and glucose oxidase (GOX). On the one hand, aNK could directly kill tumor cells by the exhaustion of glucose and the generation of toxic H2O2. On the other hand, the produced H2O2 could re-educate macrophages and generate inflammation to activate the immune system, similar to the secretion of cytokines and chemokines by NK cells (Fig. 5C) [151]. NK cells can induce the polarization of pro-inflammatory M1 macrophages and target tumors through activating receptors on the NK cell membrane. Cai et al. synthesized the NK cell membrane cloaked TCPP-loaded mPEG-PLGA polymeric nanoparticles (NK-NPs) for targeting tumors and enhancing the M1 macrophage polarization to generate anti-tumor immune response (Fig. 5D). Furthermore, the photosensitizer TCPP loaded in the NK-NPs not only induced tumor cell death through photodynamic therapy (PDT), but also promoted the release of tumor-associated antigens for enhancing the NK cell-mediated immunotherapy (Fig. 5D) [152]. Table 2 provides the information of different cell membranes and the encapsulated core materials.
Fig. 5.
Cell membrane coating nanomaterials for NK cell-based tumor immunotherapy. (A) B16OVA cell membrane proteins coated adjuvant CpG and an a-helix peptide modified HSP70p for activating the innate NK cells and adaptive cytotoxic T cells [147]. (B) Schematic illustration of the macrophage membrane coated nano-gemcitabine system (MNGs) for promoting intratumor permeation and lymphocyte infiltration to synergistically treat tumor by combining with anti-PD-L1 therapy [148]. (C) The red blood cell membrane cloaked perfluorohexane (PFC) and glucose oxidase (GOX) to construct the aNK for killing tumor cells and educating M2 macrophages into M1 macrophages [151]. (D) Schematic illustration of NK cell membrane coated nanoparticles for PDT-enhanced immunotherapy [152]. Adapted with permission [147], Copyright 2018, Elsevier; adapted with permission [148], Copyright 2023, American Chemical Society; adapted with permission [151], Copyright 2019, Wiley-VCH; adapted with permission [152], Copyright 2018, American Chemical Society.
Table 2.
Summary of encapsulation of core materials with the cell membrane from different cells.
| Source cells | Core materials | Application | Tumor cell type | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Tumor cells | CpG, αHSP70p, phosphate calcium | Kill tumor cells and activate the innate NK cells and adaptive cytotoxic T cells immune response for tumor regression | B16OVA | 200 | [147] |
| Macrophages | Nano-gemcitabine System | Promote the CD8+ T cells and NK cells infiltration in tumors and synergize anti-PD-L1 to restore their tumoricidal function for cancer immunotherapy | 4T1, CT-26, PANC02 | 94 | [148] |
| Red blood cells | Perfluorohexane, glucose oxidase | Exhaust glucose to produce the toxicity of H2O2 for killing tumor cells and re-educating M2-type TAMs into M1 type | 4T1 | 190 | [151] |
| NK cells | TCPP, mPEG-PLGA | Cause tumor cell death through photodynamic therapy and enhance the efficacy of anti-tumor immunity | 4T1 | 80 ± 1.5 | [152] |
| NK cells | DOX | Extend the circulation half-life and enhance tumor homing efficiency of nanomaterials for tumor chemotherapy and immunotherapy | MCF-7 | 70 | [153] |
| NK cells | AIEdots | Cross the BBB for high contrast tumor imaging and photothermal therapy of glioma | U-87 MG | 78 | [154] |
| NK cells | Hollow mesoporous silica loaded with AIPH and (TA)/Fe3+ | Consume GSH and enhance free radical therapy | HepG2 | 153.2 ± 16.2 | [155] |
To sum up, the cell membrane coated NPs exhibit the characteristics of immune evasion, long blood circulation, tumor targeting capability, and good biocompatibility, all of which play great roles in NK cell-based tumor immunotherapy. Cell membrane coating can combine the merits of cell membrane and nanomaterials. The sources of cell membrane effects the function of cell membrane coated NPs, and specific cell membrane can be selected according to the desired application. The cell membrane can also be modified using various methods, thus adding additional opportunity and flexibility for the treatment. However, since membrane coated NPs are relatively new for NK cell-based tumor immunotherapy, the investigation of their pharmacokinetics or pharmacodynamics is limited. In addition, it is great of interests to develop the functional modification and hybridization of membranes for enhancing NK cell-based tumor therapy.
3.2. Exosome related nanomaterials
Exosomes are a group of nano-sized membrane vesicles (30–200 nm), which can transfer proteins, cytokines, and nucleic acids to nearby cells, and play an important role in the intercellular communication within the extracellular environment [[156], [157], [158]]. The exosome functions are determined by the specific carried cargos. Therefore, incorporation of therapeutic molecules into exosomes or exosome-mimetics have been widely explored. In this section, we will review recent advances in exosomes from different parental cells and exosome-related nanomaterials for enhancing NK cell-based tumor immunotherapy (Table 3).
Table 3.
Summary of exosomes from different parental cells and exosome-related nanomaterials.
| Cell types | Exosome-containing materials | Therapeutic effect | Target | Tumor cell type | Ref. |
|---|---|---|---|---|---|
| NK | Perforin, Granzyme A/B, granulysin, FasL | Mediate cytotoxicity against cancer cells | Tumor cells | SupB15, CHLA255 | [160] |
| CD16, CD69, NKp44, NKG2D, IFN-γ, LFA-1, DNAM1, PD-1 | Promote tumor cell apoptosis through DNAM1 pathway | Tumor cells | NALM-18 | [161] | |
| CD56, Perforin, FasL | Enhance cytotoxic activity against tumor cells | Tumor cells | Jurkat, K562, DAUDI, SKBR3 | [162] | |
| Perforin, Granulysin, Granzymes A/B | Activate multiple caspase pathways in tumor cells | Tumor cells | NALM-6, SupB15, CHLA-255, CHLA-136, MCF-7 |
[163] | |
| FasL, Perforin | Promote anti-tumor effect | Tumor cells | B16F10 | [164] | |
| Perforin, Granzyme B, FasL, TRAIL | Enhance the anti-tumor effects in HCC | Tumor cells | Hep3B, HepG2; Huh7 | [165] | |
| CD56, NKG2D, FasL, NKp30, NKp44, NKp46 | Enhance cytotoxicity against neuroblastoma tumor in vivo | Tumor cells | Neuroblastoma | [166] | |
| miR-186 | Inhibit neuroblastoma growth | Tumor cells | CHLA-136 | [167] | |
| Dendrimer, miRNA | Promote the synergistic apoptosis of tumor cells | Tumor cells | MDA-MB-231 | [168] | |
| BCL-2 siRNAs | Promote tumor apoptosis | Tumor cells | HEK293T, K562, MCF-7, MEC-1, SKBR3, MCF-10 A, MDA-MB-231 | [169] | |
| siRNA, Ce6 | Enhance tumor photodynamic and immunotherapy | Tumor cells | HepG2 | [170] | |
| DCs | |||||
| HLA-B-Associated Transcript-3 | Promote NK cells activation | NK cells | Not mentioned | [173] | |
| TNF, FasL, TRAIL | Promote NK cells activation and tumor apoptosis | NK cells | B16 melanoma cells | [174] | |
| IL-15 Rα, ULBP | Promote the IL-15 Rα and NKG2D-dependent NK cells proliferation and activation | NK cells | B16F10 | [175] | |
| TLR4 and TLR1/2 ligand | Promote NK and Dendritic cells activation | NK cells | Not mentioned | [176] | |
| Lysates of RAE-1γ-expressing CML cells | Simultaneously activate NK cells and T lymphocytes | NK and T cells | BP210, BP210-T315I | [177] | |
| IFN-γ, MHC I/II restricted cancer antigens | Nkp30-related NK cells response | NK cells | NSCLC | [178] | |
| Tumor | |||||
| J588HSP cells line | HSP70 | Simultaneously activate NK cells and CD8+ T cells | NK and T cells | J558 | [186] |
| Human pancreas (Colo357), colon (CX2) carcinoma cells | HSP70, Bag-4 | Stimulate the migratory capacity and cytolytic activity of NK cells | NK cells | Colo357; CX2 | [187] |
| The human melanoma cell line Ge (Ge-HSP70, Ge-con sublines) | HSP70 | Promote mouse NK cells activation | NK cells | YAC-1, Ge-con | [188] |
| Human hepatocellular carcinoma (HepG2) | HSP60, HSP70, HSP90 | Promote NK cells activation | NK cells | HepG2 | [189] |
| Human multiple myeloma cell lines (SKO-007 (J3), ARK) | HSP70 | Promote the secretion of IFN-γ by CD56high NK cell | NK cells | SKO-007 (J3), ARK, | [190] |
| Human multiple myeloma cell lines (SKO-007 (J3), ARK, and RPMI8226) | IL-15 R A, IL-15 | Promote NK cells activation and proliferation | NK cells | Not mentioned | [191] |
| K562 cells | IL15, IL-18, 4-1BBL | Promote NK cells proliferation in a short time (4 h) | NK cells | HepG2, HeLa, MCF-7, K562, Jurkat | [192] |
3.2.1. Immune cell-derived exosomes
Immune cell derived-exosomes as a potential therapeutic agents have been extensively investigated to verify their anti-tumor effect [159]. For NK cell-based tumor immunotherapy, they are mainly from NK cells and DCs.
NK cell-derived exosomes (NDEs): As mentioned above, NK cells can kill tumor cells and stimulate adaptive immune response by secreting pro-inflammatory cytokines and chemokines. Similar to parental cells, NDEs express NK cell markers (e.g. CD56) and receptors (e.g. NKG2D), which can specifically bind to ligands expressed by malignant tumor cells. In addition, NDEs contain cytolytic molecules such as FasL, perforin, granzymes, and TRAIL (Fig. 6) [160,161]. In the early research of NDEs, Lugini et al. reported that NDEs contained not only NK cell marker CD56, but also FasL and perforin, and showed anti-tumor and immune homeostatic activities in hematological malignancies [162]. The exosomes isolated from the activated NK cells showed their cytotoxic activity against several cancer types via activating multiple caspase pathways [163]. In addition, NDEs can also induce apoptosis of various tumor cells and inhibit the growth of their xenograft tumors, such as melanoma [164], HCC [165], and neuroblastoma [166,167]. Ahn et al. found that NDEs effectively inhibited the growth of xenografted melanoma [164]. Choi et al. observed that NDEs regulated the active targeting and cell death of HCC through two different mechanisms, i.e. membrane fusion (perforin and granzyme B) and ligand receptor interaction (FasL and TRAIL) [165]. In addition, NDEs carried the tumor suppressor microRNA (miR)-186 exhibited cytotoxicity against neuroblastoma in vitro and in vivo [167]. These results support the therapeutic potential of NDEs against various tumors.
Fig. 6.
Schematic illustration of exosomes and exosome-related nanomaterials from different parental cells for enhancing NK cell-based tumor immunotherapy. NK cell-derived exosomes, which express NKp44, FasL, CD56, TRAIL or NKG2D and contain cytotoxic molecules such as perforin, granztmes A/B, IFN-γ, and miRNA, induce tumor cell death and inhibit tumor growth. DC-derived exosomes induce the activation of T cells and NK cells through the expression of some ligands such as TLR4 ligands, FasL, MHC/Peptide, TRAIL, TNF and co-stimulatory molecules. Tumor cell-derived exosomes induce the activation of NK cells through presenting HSP60, HSP70, HSP90, BAG-4, or 4-1BBL and cytokines such as IL-15, IL-18, etc.
In addition to exosomes themselves, their loaded nanomaterials, nucleic acid (mi RNA, siRNA), and other therapeutic drugs can further enhance their efficacy in tumor therapy. Xu et al. developed a “cocktail therapy” for neuroblastoma, based on the self-assembly of NDEs and dendrimers loaded with therapeutic miRNA [168]. NDEs loaded with BCL-2 siRNAs enhanced the intrinsic apoptosis of breast cancer cells, without affecting normal cells [169]. Combined with external light stimulation, NDEs loaded with siRNA and hydrophobic photosensitizer Ce6 can effectively induce tumor cell death via NK cell-like cytotoxicity [170]. Meanwhile, ROS generated upon laser irradiation not only achieved an effective PDT, but also facilitated polarization of tumor-associated macrophages from M2 type into M1 type, and maturation of DCs in the TME. In addition, the released siRNA triggered robust gene silencing of PD-L1 for activating CD4+ T cells and CD8+ T cells in the TME [170].
DC-derived exosomes (DDEs): Dendritic cells are the main sentinel, antigen presenting and regulating cells of the immune system, and play the role in communication between innate and adaptive immunity [171]. Therefore, DDEs can serve as tumor vaccines. DDEs haven been proved to eradicate tumors in a T cell-dependent and MHC-restrictive manner (Fig. 6) [172]. DDEs contain a variety of ligands on their surface, such as BAT3, TNF, FasL, TRAIL, NKG2D ligands etc., leading to the direct binding and activation of NK cells [[173], [174], [175]]. In a phase I clinical trial, DDEs based vaccines displayed the capability of restoring the number and NKG2D-dependent function of NK cells in 7/14 patients [175]. Furthermore, the capability of DDEs boosting NK cells can be enhanced by modification [[176], [177], [178]]. Chaput et al. synthesized the IFN-γ-DDEs loaded with class I- and class II restricted cancer antigens to boost the NK cells for anti-tumor immunotherapy in a phase II clinical trial. The results confirmed that IFN-γ-DDEs enhanced the anti-tumor immunity of NK cells in patients with advanced NSCLC [178].
In conclusion, immune cell-derived exosomes have been extensively used for cell-free immunotherapy due to their higher stability under the same storage conditions, lower risks of CRS, higher resistance to the immunosuppressive TME, and potential therapeutic effects on various solid tumors [[179], [180], [181]].
3.2.2. Tumor cell-derived exosomes
Tumor cell-derived exosomes (TDEs): Compared with immune cell-derived exosomes, tumor cell-derived exosomes (TDEs) are mostly described by their immunosuppressive roles in tumor [182,183]. Nevertheless, some studies show that TDEs may also take part in the activation of cytotoxic immune cells, mainly by presenting antigens to DCs, and then triggering the activation of CD8+ T cells, or directly activating NK cells [[184], [185], [186], [187]]. In this section, we will focus on their roles in activating NK cells (Fig. 6). TDEs are capable of stimulating the NK cells in the TLR4 (or TLR2)/HSP70-dependent manner. Multhoff et al. showed that exosomes presenting HSP70/Bag-4 on their surface stimulated migration and lytic activity in NK cells against HSP70 membrane-positive tumors [187]. Similar results were observed, in which the cytotoxic of NK cells induced by HSP70-positive TDEs was dependent on the expression of NKG2D ligands on the surface of target tumor cells. Compared with HSP70-negative TDEs, HSP70-positive TDEs can activate mouse NK cells in vitro, thus killing YAC-1 cells with expression of NKG2D ligands [188]. Interestingly, TDEs isolated from HepG2 cells pretreated with anti-tumor drugs can efficiently stimulate the cytotoxicity and granzyme B of NK cells, and confer superior immunogenicity of HSP-specific NK cell response [189]. Nevertheless, melphalan-treated multiple myeloma cells released more exosomes, which can stimulate the production of IFN-g by NK cells through the activation of the NF-kB pathway in a TLR-2/HSP70-dependent manner, but not affect the cytotoxic activity of NK cells [190].
Apart from HSP proteins, IL-15, IL-15RA, IL-18, and 4-1BBL are also expressed on the TDE isolated from multiple myeloma cells, promoting the proliferation of human NK cells [191,192]. However, with the extension of treatment time (48 h), the TDEs could inhibit the cytotoxicity of NK cells by inhibiting the expression of activated receptors on NK cells after a long-term treatment (48 h) [192]. This suggests that TDEs-mediated immune activation may trigger immune cell exhaustion due to the long exposure to stimuli.
To sum up, exosomes as therapeutic agents for enhancing NK cell-based tumor immunotherapy have distinguished advantages, including excellent biocompatibility, low toxicity, and biostability. Several clinical studies have been carried out to verify the anti-tumor effect of exosomes [175,178,193]. However, one of the bottlenecks in the exosome-based clinical practice is how to produce large-scale clinical grade exosomes [194]. In addition, in the absence of specific modification and natural targeting ability, most exosomes are captured by liver after intravenous administration, resulting in a significant decrease in the delivery efficiency. Therefore, studies on the generation of clinical-grade exosomes in a large scale, decoration of targeting motifs and the mechanism of exosomes from different parental cells will be an important topic of exosomes for enhancing NK cell-based tumor immunotherapy.
3.3. Nanomaterials engineer exogenous NK cells
During the process of NK cell-based tumor immunotherapy, the tumor vascular structure, chemokine expressions, and stromal tissue barriers in the TME prevent NK cells from homing, infiltration, proliferation, and activation. Highly localized abundant NK cells in tumor are essential for NK cell-based tumor immunotherapy. Therefore, engineering NK cells via nanotechnology for improving the enrichment of activated NK cells in tumor has become an important strategy for tumor therapy. Recently, several nanomaterials (such as hydrogel, Fe3O4, DCNP, etc.) have been successfully constructed to engineer exogenous NK cells for promoting the infiltration and activation of NK cells in tumor (Table 4). In this section, we will review recent advances in nanomaterials used for engineering exogenous NK cells for tumor therapy.
Table 4.
Summary of nanomaterials in combination with engineered exogenous NK cells.
| Source of NK cells | Nanomaterials and modifications of NK cells | Application methods of NK cells | Functions of nanomaterials | Tumor cell type | Ref. |
|---|---|---|---|---|---|
| NK-92 cell lines | Hyaluronic acid based niche loaded unmodified NK cells | Postsurgical implantation | Promote the NK cells proliferation, release cytokines, and enhance tumor-lytic ability | MDA-MB-231 | [195] |
| Primary human NK cells | DNase I@MBGNs and unmodified NK cells | Intravenous injection | DNase I@MBGNs can neutralize tumor acidity and destruct NETs, assist NK cell infusion | Hepa1-6 | [196] |
| Primary human NK cells | AuNSP@ αCD16 and unmodified NK cells | Intravenous injection | Mark CD16 antibody on tumor cells for intravenously injected NK cell recognition | Hela | [197] |
| NK-92 cell lines | SSB NMs and unmodified NK cells | Intravenous injection | Upregulate the expression of activation receptor-NKG2D on NK92 cells and its ligand-NKG2DL on tumor cells | MDA-MB-231 | [198] |
| NK-92 cell lines | Ru complex and unmodified NK cells | Intravenous injection | Activate multiple receptors (TNF-R1,DR5, Fas) on tumor cells and upregulating NKG2D and its ligand | MDA-MB-231 | [199] |
| NK-92 cell lines | Selenium-bearing ruthenium complex and unmodified NK cells | Intravenous injection | Upregulate DR5 and Fas expression, activate NK cells via TRAIL/TRAIL-R and Fas/FasL pathway | PC3 | [200] |
| NK-92 cell lines | NK cells reinforced acid-responsive micelles loaded with DOX | Intraperitoneal injection | NK cells against solid tumors is reinforced with the site-specific diffusion of DOX | MDA-MB-231 | [201] |
| Primary human NK cells | NK cells loaded with GNS@CaCO3/Ce6 | Intravenous injection | Bimodal imaging directed PTT, PDT, IT | A549 | [202] |
| Primary human NK cells | NK cells loaded with DCNP@786s | Intravenous injection | NIR-II FLI for assessing intracellular ROS generation and track NK cell viability | MHCC97H | [203] |
| NK-92 cell lines | NK cells loaded with liposomal ICG NPs | Experiment in vitro | NK cell-based tumor immunotherapy and PTT | A549, Hela, MCF-7 | [204] |
| NK-92 cell lines/Murine NK cells | NK cells incubated with magnetic NPs | Experiment in vitro | Not mentioned | Not mentioned | [205] |
| Primary human NK cells | NK cells coated with iron oxide NPs | Experiment in vitro | Increase the homing of NK cells at tumor site | SHSY5Y | [206] |
| Primary human NK cells | NK cells loaded with Fe3O4@PDA NPs | Intravenous injection | Reduce the expression of Ki-67 and increase the apoptosis of A549 cancer cells by magnetic targeting therapy | A549 | [207] |
| NK-92 cell lines | NK cells loaded with Magnetic Cationic NPs | Intratumors injection | Upregulate the expression of CCR4 and CXCR4 of NK cells | MDA-MB-231 | [208] |
| NK-92 cell lines | NK cells loaded with Fe3O4/SiO2 NPs | Intravenous injection | Promote NK cells infiltrating into tumor site by magnetic targeting therapy | RPMI8226 | [209] |
| NK-92 cell lines | NK cells loaded with Zn/Fe magnetic NPs | Intravenous injection | MRI, FLI, and increase the expression of EGFR targeting chimeric antigen receptor | MDA-MB-231 | [210] |
| Rat primary NK cells | NK cells loaded with HAPF complex | Intra-arterial injection | MRI guided magneto-activated NK cells therapy to suppress solid tumor growth | N1S1 | [211] |
| Primary human NK cells | PTAs nanosheets combined with TLS11a aptamer modified NK cells | Intravenous injection | Combine the artificial NK cell adaptive therapy with PTT treatment | HepG2 | [212] |
| NK-92 cell lines | Nanobody 7D12 modified NK cells | Intravenous injection | Specifically recognize and kill the EGFR-positive tumor cells | LoVo | [213] |
| NK-92 cell lines | NK cells modified with Azide (N3) and IL-21 NPs | Intravenous injection | Promote proliferation, activation, and persistence of NK cells | Raji | [214] |
| Mouse NK cells | NK cells modified with adenoviruses | Intravenous injection | Activate NK cells by upregulating the type I interferon signaling in a STAT4-granzyme B-dependent manner | 4T1 | [215] |
| Primary human NK cells | NK cells modified with Protein-DNA | Experiment in vitro | Eliminate the immunodepression from inhibitor sialic acid ligands at the NK-tumor cell synapse | Not mentioned | [216] |
3.3.1. Nanohybrids enhance the tumor homing ability of exogenous NK cells
NK cells are highly cytotoxic immune effectors, which can generate and release cytotoxic cytokines to directly kill tumor cells without antigen-restriction. As the innate immune cells, NK cells have a well-established homing ability to accumulate in tumor. Thus, a promising strategy combining the homing capability of NK cells with nanotechnology for tumor therapy has been developed. In a recent work, a biocompatible 3D-engineered hyaluronic acid based niche was constructed to culture NK cells after post-resection implantation (Fig. 7A) [195]. In addition to carrying NK cells, the hydrogel can be loaded with nanomaterials for tumor therapy. A dual pH-responsive hydrogel containing tumor acidity neutralizer and neutrophil extracellular traps (NETs) lyase (Deoxyribonuclease I, DNase I) was developed, and used in combination with NK cell infusion for preventing the recurrence of post-resected HCC. The hydrogel injected at tumor resection margin can neutralize tumor acidity to reduce tumor infiltration. The DNase I released by a pH-responsive manner can degrade NETs for enhancing NK cell infusion to combat the recurrence of post-resected HCC [196]. In line with the regulation of TME, a nano-immunomodulator AuNSP@αCD16 was explored to remodel the TME by modifying tumor cell surface with CD16 antibody for enhancing the recruitment and infiltration of intravenously injected NK cells in tumor (Fig. 7B) [197]. Combined with the regulation of TME, upregulating the expression of activated receptors and ligands also can induce significant anti-tumor effects [[198], [199], [200]]. Chen et al. developed a nanoemulsion system (SSB NMs) to co-deliver TGF-β inhibitor and selenocysteine to improve anti-tumor efficacy. SSB NMs can effectively inhibit TGF-β/TGF-β RI/Smad2/3 signaling, which thus enhanced NKG2DL expression on tumor cells and stimulated NKG2D surface expression on NK92 cells. The synergistic use of SSB NMs and intravenously injected NK cells can induce significant anti-tumor effect in vivo [198].
Fig. 7.
Tumor homing capability of NK cells combined with nanomaterials for NK cell-based tumor immunotherapy. (A) Schematic illustration of NK cell expansion in 3D-ENHANCE, followed by postsurgical implantation [195]. (B) Schematic illustration of AuNSP@αCD16 mediated TME modulation for enhancing the recruitment and infiltration of intravenously injected NK cells in tumor [197]. (C) Schematic illustration of NK cells reinforced with DOX-loaded acid-responsive micelles (ReNK) and their anti-tumor effect [201]. (D) Schematic illustration of DCNP@786s with ratiometric NIR-II fluorescence for tracking NK cell viability in vivo [203]. Adapted with permission [195], Copyright 2020, Elsevier; adapted with permission [197], Copyright 2022, Elsevier; adapted with permission [201], Copyright 2020, Wiley-VCH; adapted with permission [203], Copyright 2021, Wiley-VCH.
In addition to combination with exogenous NK cells without any modification, nanomaterials can also be directly modified or loaded onto NK cells to enhance the therapeutic effect. In this strategy, NK cells act as carriers of nanomaterials [[201], [202], [203], [204]]. For example, the DOX-loaded acid-responsive micelles were loaded onto the NK cells to prepare reinforced NK cells (ReNK) (Fig. 7C). The ReNK showed highly tumor-specific release of DOX to enhance therapeutic effect in solid tumors, inhibit tumor growth and reduce systemic toxicity [201]. Cui et al. designed a nanoplatform by using the characteristics of human NK cells in conjunction with CaCO3-coated gold nanoclusters (GNSs) carried with hydrophobic photosensitizer Ce6 (GNS@CaCO3/Ce6-NK) for enhancing the photothermal therapy (PTT)/photodynamic therapy (PDT) and immunotherapy of tumor [202]. In addition to enhancing the therapeutic efficacy of NK cells, it is also important to monitor NK cell viability during treatment. Song et al. developed a NIR-II fluorescence imaging strategy to quantitively track and visualize the adoptive NK cell viability in vivo in real-time [203]. The lanthanide-based down-conversion nanoparticles (DCNP) modified with IR786s (a broad-spectrum ROS-sensitive probe) were loaded onto the NK cells. Upon cell death, excessive ROS was produced in NK cells, along with IR786s degradation, resulting in the increase of NIR-II fluorescence at 1550 nm under 808 nm excitation; while the NIR-II fluorescence at 1550 nm under 980 nm excitation was stable (Fig. 7D). Therefore, the intracellular ROS-induced ratiometric NIR-II fluorescence was explored to track NK cells viability in vivo for NK cell-based tumor immunotherapy.
3.3.2. Magnetic nanomaterials enhance the tumor homing ability of exogenous NK cells
To further enhance the accumulation of exogenous NK cells in tumor, the NK cells can also be engineered with magnetic nanomaterials and guided to tumor site for targeted therapy under an external magnetic field. The magnetic NPs loaded onto NK cells did not seriously affect the function of NK cells [[205], [206], [207], [208]]. Cheng et al. synthesized the Cy5.5-conjugated and silica coated magnetic iron oxide (Fe3O4/SiO2) NPs to engineer NK cells, and then controlled the NK cells by the magnetic field [209]. Under the guidance of external magnetic field, the relative ratio of NK cells infiltrated into tumor was increased by 17 folds compared with the unmodified NK cells (Fig. 8A). In another work, magnetic Zn/Fe NPs modified with EGFR-CAR plasmids (MF-NPs) were prepared to engineer NK cells [210]. The MF-NPs could deliver plasmid DNA to NK cells and induce the expression of EGFR targeting chimeric antigen receptors on the NK cell surface. Furthermore, they were used to track NK cells in vivo through MRI and fluorescence imaging (FLI). Finally, the engineered NK cells enhanced cytotoxicity against xenografted tumor of MDA-MB-231 cells in vivo (Fig. 8B).
Fig. 8.
Magnetic nanomaterials enhance the tumor homing ability of NK cells. (A) Schematic illustration of NK cells loaded with the Cy5.5-conjugated and silica coated magnetic iron oxide (Fe3O4/SiO2) NPs for magnetic targeting and in vivo fluorescence imaging [209]. (B) Schematic illustration of the NK cells loaded with EGFR-CAR plasmids modified with magnetic Zn/Fe NPs for inducing the expression of EGFR and tracking NK cells in vivo through MRI and fluorescence imaging [210]. (C) Magneto-activated HAPF-labeled NK cells for MRI guided local NK cell therapy to treat hepatocellular carcinoma [211]. Adapted with permission [209], Copyright 2012, Elsevier; adapted with permission [210], Copyright 2019, Elsevier; adapted with permission [211], Copyright 2021, American Chemical Society.
In addition to magnetic targeting and MRI, the magnetic NPs loaded onto NK cells can also be used to activate NK cells. Magnetic nanocomplexes (HAPF) combining hyaluronic acid (HA), protamine (P), and ferumoxytol (F) were prepared for labeling NK cells [211]. Application of an external magnetic field could effectively activate NK cells, promote the secretion of perforin and granzyme B, as well as upregulate the expression of actin polymerization and mechanosensitive NKG2D for kill tumor cells effectively (Fig. 8C). In general, the NK cells engineered by magnetic nanomaterials can achieve the targeted delivery to enhance the NK cell-based tumor immunotherapy through the guidance of noninvasive imaging.
3.3.3. Nanomaterials engineer NK cell surface
Besides the above strategies for engineered NK cells, modification of NK cell surface with aptamers, nanobodies, bio-orthogonal groups, adenoviruses, DNA etc. Has also been used to enhance NK cell-based tumor immunotherapy [[212], [213], [214], [215], [216]]. Engineering of NK cells with the hepatocellular carcinoma specific targeting TLS11a-aptamer was carried out to eliminate residual tumor cells after nano-enhanced PTT, systematically improve anti-tumor efficacy and prevent tumor relapse (Fig. 9A) [212]. Nanobodies are novel antibody-like biomolecules with high antigen affinity, low immunogenicity, deep tumor tissue permeability. In a recent study, the nanobody (7D12) was conjugated with NK cells (7D12-NK92MI) via bio-orthogonal click chemistry [213]. The 7D12 was used to specifically recognize the human EGFR overexpressed by many solid tumors. The 7D12-NK92MI cells showed high specificity and affinity to EGFR-overexpressing tumor cells and efficiently lysed them (Fig. 9B). Additionally, the bio-orthogonal groups can also be used to enhance the infiltration and migration of NK cells in solid tumors. Cai et al. constructed a bio-orthogonal targeted living cell nanocarrier (N3-NK-NPs) by coupling azide (N3) on the surface of NK cells with hitchhiked IL-21 delivery system [214]. The N3-NK-NPs could efficiently recognize and infiltrate into the bicyclo [6.1.0] nonyne (BCN) modified Raji cells through bio-orthogonal reaction. The bio-orthogonal chemical groups (N3 and BCN) could act as artificial targeting receptors/ligands to achieve efficient recognition between the NK cells and tumors, and further promote the infiltration of NK cells into tumor. Meanwhile, the IL-21 loaded into nanoparticles was released into the surrounding of NK cells, promoting the sustainable and effective activation of NK cells in tumor site (Fig. 9C).
Fig. 9.
Modification strategies for engineering NK cell surface. (A) Schematic illustration of antiheat endurance strategy combined with artificial engineered NK cells for improving the therapeutic efficacy of nano-enhanced PTT [212]. (B) Schematic illustration of NK cells modified with the anti-EGFR nanobody 7D12 [213]. (C) Schematic illustration of live-cell nano-carrier N3-NK-NPs based on bio-orthogonal metabolic glycol-engineering and hitchhiking delivery system for improving NK cell-based tumor immunotherapy [214]. (D) Schematic illustration of adenoviruses infected NK cells for conducting a potent tumor targeted virotherapy in combination with immunotherapy [215]. (E) Schematic illustration of DNA-mediated protein anchoring (DMPA) on NK cell surface for enhancing immune response [216]. Adapted with permission [212], Copyright 2019, Wiley-VCH; adapted with permission [213], Copyright 2021, Wiley-VCH; adapted with permission [214], Copyright 2022, Wiley-VCH; adapted with permission [215], Copyright 2022, Wiley-VCH; adapted with permission [216], Copyright 2023, American Chemical Society.
Oncolytic adenoviruses have received extensively attention in tumor therapy because they can directly cause oncolytic infection and indirectly induce anti-tumor immunity [217]. Adenoviruses infected NK cells (Ad@NK) were used for loading, protection, replication, amplification, and release of adenoviruses at tumor site. As a return, adenoviruses infection provided an enhanced anti-tumor immunity to NK cells by activating type I interferon signal in a STAT4-granzyme B-dependent manner (Fig. 9D) [215]. The complementary interaction between adenoviruses and NK cells in combined immunotherapy and viral therapy is a promising therapeutic strategy in oncology.
Membrane protein engineering shows great potential in functionalization of NK cells. Zhang et al. designed a strategy of anchoring exogenous proteins on NK cell membrane through DNA-template [216]. The engineered NK cells displayed the capability of eliminating the immune checkpoints, thus significantly enhanced the activation efficiency of NK cells in the process of immunotherapy (Fig. 9E).
In sum up, the engineered exogenous NK cells have been widely used in tumor treatment, but their efficacy is poor because of the short survival in vivo. In the future, combined application of exogenous NK cells with nanomaterials will attract considerable attention for tumor therapy to enhance NK cell priming and expansion, improve their persistence and homing efficiency, and enhance their activation.
3.4. Nanomaterials boost endogenous NK cells
Since the exogenous NK cells could be depleted, apoptotic or unable to survive after infusion, diverse nanomaterials have been developed to promote the targeted delivery of functional small molecules to activate endogenous NK cells or enhance the proliferation of NK cells in vivo. In this section, we will summarize the methods of stimulating endogenous NK cell activity by nanomaterials for NK cell-based tumor immunotherapy (Table 5).
Table 5.
Summary of nanomaterials activating endogenous NK cells.
| Applications | Nanomaterials | Size | Functions of nanomaterials | Tumor cell type | Ref. |
|---|---|---|---|---|---|
| Regulate the TME | Vesicular CLAN with siLdha | 90 – 100 nm | Knockdown LDHA, reprogram pyruvate metabolism, reduce the production of lactate, neutralize the tumor pH | 4T1 | [229] |
| Nano-carriers encapsulate siRNAs | 130 ± 9 nm | Genetically silence the key intrinsic inhibitory NK cell molecules, SHP-1, Cbl-b, and c-Cbl | 221-Cw4 | [230] | |
| PCM@APAP@RNGs | 128.4 ± 0.8 nm | Release acetaminophen to inhibit PGE2 secretion for promoting the activity of NK cells | Panc02 | [231] | |
| Fraxinellone loaded nanoemulsion | 145 nm | Remold the TME by anti-fibrotic, combine with BRAF peptide vaccine to enhance anti-tumor efficacy | BPD6 | [232] | |
| Neobavaisoflavone nanoemulsion | 100 nm | Reduce ROS production, decrease ECM deposition via suppressing TGF-β/SMADs pathway | A549 | [233] | |
| Nanowires coupled with IL-2 antibody | 18 ± 4.3 μm (long) and 6 ± 2.1 μm (short) | Capture and potentiate endogenous IL-2 for stimulating NK and T cells | Not mentioned | [234] | |
| Na–IVAl-DMSN | 240 nm | Activate DCs to release cytokines IL-18 and IL-1β for recruit NK cells and T cells. | CT26 | [235] | |
| DAL4-LNP-IL-12 + IL-27 mRNA | 130 nm | Intratumorally deliver IL-12 and IL-27 mRNAs induced robust infiltration of immune effector cells | B16F10 | [236] | |
| CLPP/mIL-15 | 221.3 ± 2.5 nm | Promote the express of IL-15 | C26 | [237] | |
| HA/pIL-12/DOX-PMet | 122.1 ± 1.1 nm | Promote the express of IL-12 and enhance the M1 macrophage polarization | 4T1 | [238] | |
| Regulate the tumor cells | Pem/Se | 47 ± 2 nm | Suppress the HLA-E expression and release of pemetrexed | MDA-MB-231 | [243] |
| Pem/Se | 150 – 200 nm | Suppress the HLA-E expression, release of pemetrexed, and increase of ROS generation | A549 | [244] | |
| SeP/DOX | 240 ± 1.3 nm | Suppress the HLA-E expression and release of DOX at the tumor site | MDA-MB-231 | [245] | |
| PSeR/DOX | 87 nm | Suppress the HLA-E expression and release of DOX at the tumor site | MDA-MB-231 | [246] | |
| PSeLYAb NPs | 199.7 nm | Suppress the HLA-E expression and release of Cetuximab at the tumor site | HCT-116 | [247] | |
| Regulate the NK cells | CrvpPS-nano-Se | 1 μm | Increase the thymus index, T cell subpopulations and NK cells | Not mentioned | [248] |
| SeNPs@LNT | 160 nm | Restore the immunocompetence of dysfunctional immune cells (CD4+ T cells, NK cells, T cells, and B cells) | Lung adenocarcinoma | [249] | |
| NGO-α-hCD16 | 150 nm | Stimulate NK cells via the CD16 receptor | Not mentioned | [250] | |
| Cowpea mosaic virus, anti-4-1 B B | 36.8 nm | Stimulate tumor-resident and CPMV-recruited NK cells within TME | CT26, B16F10 | [251] | |
| Chiral nanoparticles | 140 nm | L-type NPs enhance the activation of CD8+ T and NK cells by stimulating DCs | EG7.OVA | [252] | |
| Co-regulate tumor and NK cells | Trifunctional NK-cell engagers | Not mentioned | Bring tumor cells and NK cells together | Raji | [253] |
| IMNs | 46 ± 10 nm | Modify the tumor cell surface with NK cell-activating signals to activate tumor-infiltrating NK cells | B16F10 | [254] | |
| α-EGFR/α-CD16/α-4-1 B B EPI NPs | 112 ± 7 nm | Target EGFR overexpressing tumors and promote the recruitment and activation of NK cells to eradicate tumor | B16F10 | [255] | |
| Pro-αCD25-NDs | 190.1 – 220.2 nm | Increase CD25 expression in target cell to enhance αCD25 binding and NK cell-mediated killing | J-Lat 10.6 | [256] | |
| SeNPs | 102 ± 9.6 nm | Upregulate the expression of activation receptor-NKG2D and its ligand-NKG2DL | HepG2 | [257] | |
| (As + Ce6)@MSNs-PEG | 121 nm | Activate adaptive immunity and release As to enhance the function of innate immunity in the tumor | CT26 | [258] | |
| Lip-CTN-DNase I–SIS3 | 37.2 ± 13 nm | Photothermal agents, killing cancer cells to induce ICD, promote activation of NK cells | 4T1 | [259] | |
| Other methods to activate NK cells | AuNC@MnO2 | 91 nm | FLI/PAI/MIR guided oxygen-boosted PDT & anti-tumor immunity | 4T1 | [260] |
| AuNPs on fluidic liposomes | Not mentioned | NIR(II) PTT to trigger ICD to potentiate tumor immunotherapy | 4T1 | [261] | |
| CD@MSNs | 50 – 60 nm | Photothermal imaging guided PTT cooperated with anti-tumor immunity | 4T1 | [262] | |
| Nanoscale immunoconjugates | 28.0 – 28.5 nm | Increase CD8+ T cells, NK cells and macrophages with a decrease of Tregs in the brain tumor area | GL261 | [263] | |
| ChA CQDs | 2 – 5 nm | Enhance ferroptosis to activate anti-tumor immunity | H22 | [264] |
3.4.1. Nanomaterials regulate the tumor microenvironment
The TME is heterogeneously composed of vasculature, various cell subtypes (such as immune cells, fibroblasts and lymphocytes, endothelial and inflammatory cells), extracellular matrix (ECM), interstitial fluid and other tumor-associated components (such as chemokines, secreted proteins, metabolites, etc.) [218,219]. The TME plays a key role in the progression of tumor and the development of drug resistance [220]. Recently, reprogramming TME has been used to enhance the efficacy of existing cancer treatment (such as chemotherapy, radiotherapy, immunotherapy [[221], [222], [223], [224], [225]]). Nanomaterials have notable advantages over the traditional platforms, and can target and reprogram the TME by changing its acidity, hypoxia, ROS, and immune modulators [[226], [227], [228]]. For example, vesicular cationic lipid-assisted nanoparticles (CLAN) mediated gene silence efficiently downregulated LDHA expression, decreased lactate secretion, normalized the tumor acidity, increased infiltration of immune cells, and restored the anti-tumor responses of T cells and NK cells (Fig. 10A) [229]. The strategies of regulating immune modulators also have the potential to restore NK cell activity [230,231]. The TME responsive nanogels can release acetaminophen to suppress the secretion of COX2-derived PGE2 in tumor cells and induce the activation of endogenous NK cells (Fig. 10B) [231]. The natural medicines delivered by nanomaterials can inhibit tumor-related fibroblasts activation, reduce extracellular matrix deposition, improve TME, and enhance endogenous NK cells activation [232,233].
Fig. 10.
Nanomaterials regulate the tumor microenvironment for enhancing the endogenous NK cell-based tumor immunotherapy. (A) Schematic illustration of micellar CLAN with siLdha for reversing the tumor immunosuppressive microenvironment and restoring the anti-tumor responses of T cells and NK cells [229]. (B) Schematic illustration of NK activation and DCs recruitment by restraining the secretion of COX2-derived PGE2 in tumor cells [231]. (C) Schematic illustration of nanowires immobilized anti-IL-2 antibody as an effective tissue-specific immune regulation platform through passive accumulation of endogenous IL-2 for activating targeted specific natural killer and T cell subsets [234]. Adapted with permission [229], Copyright 2019, American Chemical Society; adapted with permission [231], Copyright 2022, Wiley-VCH; adapted with permission [234], Copyright 2017, American Chemical Society.
In addition to improving the immunosuppressive TME, increasing the cytokines can also effectively activate endogenous NK cells [[234], [235], [236], [237], [238]]. For example, nanowires coupled with anti-IL-2 antibody was used for enhancing endogenous IL-2 and activating targeted specific endogenous NK and T cell subsets (Fig. 10C) [234]. In addition to capturing endogenous activating cytokines, increasing their secretion in tumor through immunogenetic therapy is also very effective. Nanomaterials encapsulated with mRNAs encoding cytokines were developed to provide efficient concentration and delivery capability for transportation of mRNA into tumor [[236], [237], [238]]. Dong et al. generated lipid NPs loaded with IL-12 and IL-27 mRNA (DAL-LNP). The intratumor administration of DAL-LNP showed a synergistic effect on production of INF-γ and TNF-α, promoting the infiltration of T cells and NK cells in tumor [236].
Different from killing tumor cells directly, regulating TME is a less toxic alternative way for tumor treatment, which can effectively promote the treatment efficacy [[239], [240], [241], [242]]. However, the TME characteristics of different types and different size of tumors may be different. Therefore, there is a huge opportunity to explore innovative nanomaterials to effectively and accurately regulate TME for optimizing NK cell-based tumor immunotherapy.
3.4.2. Nanomaterials regulate the tumor and endogenous NK cells
The activation and proliferation of NK cells is usually achieved through cytokine-based stimulation, inhibition of inhibitory ligands in tumor cells, and increase of activating receptors in NK cells. However, the direct in vivo administration of cytokines, antibodies, and genes can cause serious side effects and degradation during blood circulation. Therefore, nanomaterials are designed to reduce the negative effects by regulating tumor cells and NK cells for enhancing NK cell-based tumor immunotherapy.
Regulate the tumor cells: NK cells can kill tumor cells without pre-sensitization or affinity maturation, which offers great potential for clinical trials of NK cell-based tumor immunotherapy. However, the overexpression of ligands in tumor cells for inhibitory NK cell receptors, such as human leukocyte antigen E (HLA-E), a ligand for the inhibitory receptor NKG2A, can lead to NK cell dysfunction and exhaustion. Therefore, reducing the expression of inhibitory ligands on tumor cells is expected to improve the effectiveness of NK cell-based tumor immunotherapy. Xu et al. prepared diselenide-pemetrexed (Pem/Se) self-assemblies combining cancer immunotherapy with radiotherapy and chemotherapy. Under the irradiation of γ-rays, diselenides in Pem/Se were converted into seleninic acid for suppressing the expression of HLA-E, pemetrexed was released from the assemblies to achieve the combined radio-chemotherapy (Fig. 11A) [243]. In line with this strategy, some selenium-containing organic nanomaterials were used to enhance NK cell-based tumor immunotherapy [[244], [245], [246], [247]].
Fig. 11.
Nanomaterials regulate the tumor and endogenous NK cells. (A) Schematic illustration of diselenide-pemetrexed assemblies for combined NK cell-based tumor immunotherapy with radiotherapy and chemotherapy [243]. (B) Schematic illustration of SeNPs@LNT regulating various MPE immune cells to enhance immune response [249]. (C) Schematic illustration of recruitment and activation of NK cells in the TME through combined treatment of CPMV and anti-4-1 B B antibody [251]. (D) Schematic illustration of L-type NPs exerted mechanical forces on dendritic cells and stimulated cytokine expression to induce cytotoxic activity of NK cells [252]. Adapted with permission [243], Copyright 2020, Wiley-VCH; adapted with permission [249], Copyright 2021, Wiley-VCH; adapted with permission [251], Copyright 2022, American Chemical Society; adapted with permission [252], Copyright 2022, Wiley-VCH.
Regulate the endogenous NK cells: In addition to the downregulation of the recognition ligands on the tumor surface, the low activity of NK cells in the TME will also limit the efficacy of NK cell-based tumor immunotherapy. Recently, nanoparticles have been used to directly activate NK cells by regulating NK-cell functions [[248], [249], [250], [251], [252]]. The earliest research showed that intragastric administration of selenium-containing NPs could increase the thymus index, T cell subpopulations and NK cells [248]. In line with this strategy, Chen et al. synthesized selenium containing NPs for potentiating the proliferation and activation of NK cells by increasing expression of NKG2D and NKp30 (Fig. 11B) [249]. Nanomaterials can be also used to deliver antibodies for in situ activation of NK cells. For example, Dunlop et al. prepared nanoscale graphene oxide templated nanoclustering of α-hCD16 (NGO-α-hCD16), which could stimulate NK cells via CD16 receptor [250]. In another work, plant virus Cowpea mosaic virus (CPMV) and anti-4-1BB monoclonal antibody were combined to improve NK cell function and in situ cancer vaccination efficacy (Fig. 11C) [251]. Furthermore, the chirality of nanomaterials can influence their interactions with immune cells. Kuang et al. synthesized the chiral L-type Au NPs with a g-factor of 0.44 to enhance the activation of CD8+ T and NK cells by stimulating DCs. The interactions between L-type NPs and immune cells were higher than that of D-type NPs, which further promoted the activation of NK cells and CD8+ T cells and their infiltration into tumor (Fig. 11D) [252].
Co-regulate tumor and endogenous NK cells: The modifiable surface property, adjustable size, and biodegradable property of nanomaterials show great potential in enhancing the binding affinity between tumor cells and endogenous NK cells. Nanoengagers have been designed for binding tumor cells with NK cells [[253], [254], [255], [256]]. The trifunctional natural killer cell engagers (NKCEs) were developed to target an antigen on cancer cells and two activating receptors of NK cells (NKp46 and CD16). They can trigger tumor-cell destruction in solid and transformed tumor models (Fig. 12A) [253]. In line with this strategy, Zhang et al. prepared the immunomodulating NPs (IMN) as an engager, which could modify the tumor cell surface with IgG (activating ligand of CD16) to spatially regulate the interaction between NK cells and tumor cells for more effective activation of NK immunity against the tumor growth (Fig. 12B) [254]. Similar to this strategy, selenium nanoparticles (Se NPs) were explored to upregulate the expressions of activation receptor-NKG2D on NK cells and its ligand-NKG2DL on tumor cells (Fig. 12C) [257]. Nanomaterials can be also used as therapeutic agents to kill tumors and release stimulants to activate NK cells for combined immunotherapy of tumor [258,259].
Fig. 12.
Nanomaterials regulate the tumor and endogenous NK cells. (A) Schematic illustration of multifunctional engagers (NKCEs) for bringing tumor cells and NK cells together and triggering tumor-cell destruction [253]. (B) Schematic illustration of in situ modification of tumor cell surface with immune activation to trigger NK cells for lysis via the nano-engager [254]. (C) Schematic illustration of SeNPs enhancing the therapeutic effect of cytokine-induced killer (CIK) cells against liver cancer and the possible mechanism [257]. Adapted with permission [253], Copyright 2019, Elsevier; adapted with permission [254], Copyright 2019, Wiley-VCH; adapted with permission [257], Copyright 2020, Elsevier.
3.4.3. Other methods to activate endogenous NK cells
Besides the above strategies of activating endogenous NK cells with nanomaterials, other joint strategies based on nanomaterials can also activate NK cells [[260], [261], [262], [263], [264], [265], [266]]. For example, nanomaterials under laser irradiation can effectively elicit immunogenic death, promote DCs maturation, and induce activation of CD8+ T cells and NK cells [[260], [261], [262]]. Additionally, nanomaterials combined with checkpoint inhibitors can increase the infiltration of CD8+ T cells and NK cells into tumor [263].
To sum up, precisely targeting tumor cells with nanomaterials can effectively activate endogenous NK cells for tumor therapy. However, there are abundant abnormal blood vessels, immunosuppressive cells and immunosuppressive cytokines in the solid tumors, which drastically inhibit the infiltration, proliferation and activation of immune cells in the solid tumors. Therefore, it is promising to design novel nanomaterials to promote the infiltration of endogenous NK cells in tumor and maintain the activation and function for a long time. When the novel inorganic nanomaterials were used for NK cell-based immunotherapy, the potential impacts of their chemical composition, shape, size, and surface property on the activation and function of NK cells should be comprehensively considered. In addition, most nanomaterials are enriched in the reticuloendothelial system (RES), which can lead to long-term side effects or toxicity. Therefore, the following aspects should be considered during the fabrication and application of inorganic nanomaterials for NK cell-based immunotherapy: (1) The inorganic nanomaterials should be made from the major or trace elements for maintaining good health. More importantly, these elements are conducive to the activation of NK cells and their functions; (2) They should have the ideal physicochemical properties. For example, in term of biodegradability, they should have a suitable degradation rate to not induce any side effects; (3) For the non-degradable nanoparticles, they should be excreted by renal clearance or hepatointestinal metabolism in a short time, after they deliver NK cells to the tumor.
4. Conclusion and perspectives
NK cells are a versatile cell population with “natural killing” character discovered more than 40 years ago. Their capability of killing tumor cells can be enhanced by in vitro expansion and gene modification [267]. Engineered NK cells with excellent activation or inhibition performance can enhance their anti-tumor activity. However, the engineered NK cells have been not successfully and fully applied in clinical practice, the challenges mainly include the following aspects. Firstly, it lacks appropriate models to investigate the complexity of human NK cell biology. Secondly, NK cells are poorly delivered to target tumor. Moreover, exogenous NK cells may be depleted, apoptotic, or survived for a short time after infusion. Finally, the expansion and cryopreservation of NK cells are expensive with a high risk of contamination.
To address the above challenges, various nanomaterials have been designed and fabricated to promote NK cell-based tumor immunotherapy. The excellent properties of nanomaterials include simplicity of synthesis, high specific surface area, surface modifiability, and versatility for drug delivery [31]. Different nanomaterials can activate exogenous and/or endogenous NK cells in different ways, and promote their applications for tumor immunotherapy. Unfortunately, the nanomaterials-mediated NK cell immunotherapy has rarely achieved the clinical translation. Extensive studies should be done in the following aspects (Fig. 13).
-
(I)
Design and fabrication of nanomaterials for the precise delivery of NK cells
Fig. 13.
The state-of-art and prospects of nanomaterials mediated NK cell-based tumor immunotherapy. Research on the application of nanomaterials for enhancing NK cell-based tumor immunotherapy has been extensively investigated, but few of them was used for clinical practice. Extensive studies should be done in the following aspects: (1) Design and fabrication of the precise delivery system for NK cells; (2) Sustainably retaining the activation and function of NK cells; (3) Imaging-guided NK cell adoptive therapy by nanomaterials.
The adoptive cell therapy (ACT) has been proved to be very effective in treatment of blood cancer, but the traditional ACT is poorly effective in treating solid tumors. The current research of adoptively transferred NK cells mainly focuses on their isolation, activation, propagation and infusion. However, the exogenous NK cells may be depleted and died after infusion, which prevents the homing and infiltration of NK cells into tumor. Hydrogel is an attractive carrier for releasing NK cells at tumor site after postsurgical implantation [195]. Additionally, bio-orthogonal targeted live-cell nanocarriers are also attractive for promoting the specific recognition and migration of NK cells in tumor [214]. Many synthetic methods have been developed to synthesize nanomaterials, but few of them focus on preparation of nanomaterials for promoting the efficient enrichment of exogenous NK cells in tumor. Therefore, the design and fabrication of nanomaterials for precise delivery of NK cells into tumor has shown promise in treatment of solid tumors when compared with the standard intravenous injection method.
-
(II)
Retention of the activation and function of NK cells
The challenges of NK cell-based tumor immunotherapy are not only their insufficient homing and infiltration capability, but also the rapid decline of survival and function in the presence of tumor immunosuppressive microenvironment. In section 3.4.1, we review the nanomaterials used for regulating the TME to enhance the NK cell-based tumor immunotherapy. However, nanomaterials cannot activate and maintain NK cell activity for a long time due to their short retention time at tumor site. Most strategies of maintaining the therapeutic efficacy are to increase the frequency or dosage of nanomaterials, which may increase the risks of side effects. Several methods have been developed to prolong the retention of nanomaterials in tumor. In addition to the traditional PEG modification, the use of size‐changeable and nano-biomembrane composites has become a mainstream and potential strategy [145,268]. Therefore, tuning the composition, size, morphology, and surface of nanomaterials for maintaining the activation and function of NK cells in the TME has been an important aspect.
-
(III)
Imaging-guided NK cell adoptive therapy by nanomaterials
Although ACT has been explored by promoting the efficient enrichment of exogenous NK cells, and activation of NK cells in tumor site, there are also some challenges for the real-time imaging of patient response during ACT to avoid the cytokine storms. To achieve the real-time monitoring the response of NK cells in vivo, immunohistochemistry biopsy is always used in the clinical and current research, which makes the ACT unmanageable. The NIR-IIb molecular imaging of CD8 and PD-L1 in tumor was used to reveal the cytotoxic T lymphocytes in immunotherapy response. The NK cell response and activity in the ACT can be predicted by contrast agents mediated molecular imaging such as immuno-positron emission tomography (PET), immuno-magnetic resonance imaging (MRI), immune-photoacoustic imaging (PAI), and so on [269]. Therefore, design of multimodal imaging nanoagents for in vivo real-time monitoring the response of NK cells is of great significance for the manageable ACT in future.
Ethics approval and consent to participate
The manuscript is a review article. The authors declare no experimentation on human or animals were designed.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (81971671, 32101110, 82101927), the National Key Research and Development Program of China (2018YFA0208800), the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (22KJA310004), and China Postdoctoral Science Foundation (2020M681720). The authors also are grateful for support from the Jiangsu Provincial Key Laboratory of Radiation Medicine and Protection, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Tingting Wang, Email: ttwang68@suda.edu.cn.
Zhen Li, Email: zhenli@suda.edu.cn.
References
- 1.Deng W., Gowen B.G., Zhang L., Wang L., Lau S., Iannello A., Xu J., Rovis T.L., Xiong N., Raulet D.H. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348(6230):136–139. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif K.L., Overman B., Thall P., Kaplan M., Nandivada V., Kaur I., Nunez C.A., Cao K., Daher M., Hosing C., Cohen E.N., Kebriaei P., Mehta R., Neelapu S., Nieto Y., Wang M., Wierda W., Keating M., Champlin R., Shpall E.J., Rezvani K. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker P.S.A., Suck G., Nowakowska P., Ullrich E., Seifried E., Bader P., Tonn T., Seidl C. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunol. Immunother. 2016;65(4):477–484. doi: 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu E., Tong Y., Dotti G., Shaim H., Savoldo B., Mukherjee M., Orange J., Wan X., Lu X., Reynolds A., Gagea M., Banerjee P., Cai R., Bdaiwi M.H., Basar R., Muftuoglu M., Li L., Marin D., Wierda W., Keating M., Champlin R., Shpall E., Rezvani K. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–531. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif K.L., Overman B., Thall P., Kaplan M., Nandivada V., Kaur I., Nunez C.A., Cao K., Daher M., Hosing C., Cohen E.N., Kebriaei P., Mehta R., Neelapu S., Nieto Y., Wang M., Wierda W., Keating M., Champlin R., Shpall E.J., Rezvani K. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie G., Dong H., Liang Y., Ham J.D., Rizwan R., Chen J., cells C.A.R.-N.K. A promising cellular immunotherapy for cancer. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raulet D.H., Vance R.E. Self-tolerance of natural killer cells. Nat. Rev. Immunol. 2006;6(7):520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 8.Myers J.A., Miller J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021;18(2):85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng M., Chen Y., Xiao W., Sun R., Tian Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013;10(3):230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalfa C., Paust S. Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.633205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H., Ham J.D., Hu G., Xie G., Vergara J., Liang Y., Ali A., Tarannum M., Donner H., Baginska J., Abdulhamid Y., Dinh K., Soiffer R.J., Ritz J., Glimcher L.H., Chen J., Romee R. Memory-like NK cells armed with a neoepitope-specific car exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl. Acad. Sci. USA. 2022;119(25) doi: 10.1073/pnas.2122379119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier L., Virone-Oddos A., Beninga J., Rossi B., Nicolazzi C., Amara C., Blanchard-Alvarez A., Gourdin N., Courta J., Basset A., Agnel M., Guillot F., Grondin G., Bonnevaux H., Bauchet A., Morel A., Morel Y., Chiron M., Vivier E. Control of acute myeloid leukemia by a trifunctional NKP46-CD16a-NK cell engager targeting CD123. Nat. Biotechnol. 2023 doi: 10.1038/s41587-022-01626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan R., Ryan J., Pan D., Wucherpfennig K.W., Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. 2022;185(9):1521–1538. doi: 10.1016/j.cell.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez-Hoya A., Soto-Cruz I. NK cell regulation in cervical cancer and strategies for immunotherapy. Cells. 2021;10(11):3104. doi: 10.3390/cells10113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mgrditchian T., Arakelian T., Paggetti J., Noman M.Z., Viry E., Moussay E., Van Moer K., Kreis S., Guerin C., Buart S., Robert C., Borg C., Vielh P., Chouaib S., Berchem G., Janji B. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl. Acad. Sci. USA. 2017;114(44):E9271. doi: 10.1073/pnas.1703921114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinavahi S.S., Chen Y., Punnath K., Berg A., Herlyn M., Foroutan M., Huntington N.D., Robertson G.P. Targeting WEEL/AKT restores p53-dependent natural killer-cell activation to induce immune checkpoint blockade responses in“cold”melanoma. Cancer Immunol. Res. 2022;10(6):757–769. doi: 10.1158/2326-6066.CIR-21-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimoto T., Nakazawa T., Matsuda R., Nishimura F., Nakamura M., Yamada S., Nakagawa I., Park Y., Tsujimura T., Nakase H. Evaluation of comprehensive gene expression and NK cell-mediated killing in glioblastoma cell line-derived spheroids. Cancers. 2021;13(19):4896. doi: 10.3390/cancers13194896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z., Yu X., Zhang J., Tian Z., Zhang C. TLR7/8 agonists promote NK-DC cross-talk to enhance NK cell anti-tumor effects in hepatocellular carcinoma. Cancer Lett. 2015;369(2):298–306. doi: 10.1016/j.canlet.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Wang S., Wu Q., Chen T., Su R., Pan C., Qian J., Huang H., Yin S., Xie H., Zhou L., Zheng S. Blocking CD47 promotes antitumour immunity through CD103+ dendritic cell-NK cell axis in murine hepatocellular carcinoma model. J. Hepatol. 2022;77(2):467–478. doi: 10.1016/j.jhep.2022.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Hu W., Wang G., Huang D., Sui M., Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh S., Lee J., Kwack K., Choi S. Natural killer cell therapy: a new treatment paradigm for solid tumors. Cancers. 2019;11(10):1534. doi: 10.3390/cancers11101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumino N., Nava Lauson C.B., Tiberti S., Besi F., Martini S., Fiore P.F., Scodamaglia F., Mingari M.C., Moretta L., Manzo T., Vacca P. The tumor microenvironment drives NK cell metabolic dysfunction leading to impaired antitumor activity. Int. J. Cancer. 2023;152(8):1698–1706. doi: 10.1002/ijc.34389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riggan L., Shah S., Sullivan T.E.O. Arrested development: suppression of NK cell function in the tumor microenvironment. Clin. Transl. Immunol. 2021;10(1):1003–1016. doi: 10.1002/cti2.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russick J., Joubert P., Gillard-Bocquet M., Torset C., Meylan M., Petitprez F., Dragon-Durey M., Marmier S., Varthaman A., Josseaume N., Germain C., Goc J., Dieu-Nosjean M., Validire P., Fournel L., Zitvogel L., Bindea G., Lupo A., Damotte D., Alifano M., Cremer I. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J. Immunother. Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raulet D.H., Gasser S., Gowen B.G., Deng W., Jung H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013;31(1):413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamiya T., Seow S.V., Wong D., Robinson M., Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Invest. 2019;129(5):2094–2106. doi: 10.1172/JCI123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun C., Xu J., Huang Q., Huang M., Wen H., Zhang C., Wang J., Song J., Zheng M., Sun H., Wei H., Xiao W., Sun R., Tian Z. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. OncoImmunology. 2017;6(1) doi: 10.1080/2162402X.2016.1264562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Wang L., Song Q., Ali M., Crowe W.N., Kucera G.L., Hawkins G.A., Soker S., Thomas K.W., Miller L.D., Lu Y., Bellinger C.R., Zhang W., Habib A.A., Petty W.J., Zhao D. Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti-PD-L1 therapy for malignant pleural effusion. Nat. Nanotechnol. 2022;17(2):206–216. doi: 10.1038/s41565-021-01032-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang C., Lin Y., Lee R., Lai Y., Cheng H., Hsieh C., Shyu W., Chen S. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018;13(8):746–754. doi: 10.1038/s41565-018-0146-7. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen N.T., Huang K., Zeng H., Jing J., Wang R., Fang S., Chen J., Liu X., Huang Z., You M.J., Rao A., Huang Y., Han G., Zhou Y. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat. Nanotechnol. 2021;16(12):1424–1434. doi: 10.1038/s41565-021-00982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhan M., Guo Y., Shen M., Shi X. Nanomaterial-boosted tumor immunotherapy through natural killer cells. Adv. Nanobiomed Res. 2022;2(12) [Google Scholar]
- 32.Raza A., Rossi G.R., Janjua T.I., Souza-Fonseca-Guimaraes F., Popat A. Nanobiomaterials to modulate natural killer cell responses for effective cancer immunotherapy. Trends Biotechnol. 2023;41:77–92. doi: 10.1016/j.tibtech.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Deng L., Xu Y., Sun C., Yun B., Sun Q., Zhao C., Li Z. Functionalization of small black phosphorus nanoparticles for targeted imaging and photothermal therapy of cancer. Sci. Bull. 2018;63(14):917–924. doi: 10.1016/j.scib.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Qu X., Zhou D., Lu J., Qin D., Zhou J., Liu H. Cancer nanomedicine in preoperative therapeutics: nanotechnology-enabled neoadjuvant chemotherapy, radiotherapy, immunotherapy, and phototherapy. Bioact. Mater. 2023;24:136–152. doi: 10.1016/j.bioactmat.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H., Liu H., Guo Y., Zai W., Li X., Xiong W., Zhao X., Yao Y., Hu Y., Zou Z., Wu J. Photosynthetic microorganisms coupled photodynamic therapy for enhanced antitumor immune effect. Bioact. Mater. 2022;12:97–106. doi: 10.1016/j.bioactmat.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang T., Zhang H., Qiu W., Han Y., Liu H., Li Z. Biomimetic nanoparticles directly remodel immunosuppressive microenvironment for boosting glioblastoma immunotherapy, Bioact. Materials. 2022;16:418–432. doi: 10.1016/j.bioactmat.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang T., Zhang H., Han Y., Liu H., Ren F., Zeng J., Sun Q., Li Z., Gao M. Light-enhanced O2-evolving nanoparticles boost photodynamic therapy to elicit antitumor immunity. ACS Appl. Mater. Interfaces. 2019;11(18):16367–16379. doi: 10.1021/acsami.9b03541. [DOI] [PubMed] [Google Scholar]
- 38.Chen J., Fang H.P., Hu Y.Y., Wu J.Y., Zhang S.J., Feng Y.J., Lin L., Tian H.Y., Chen X.S. Combining mannose receptor mediated nanovaccines and gene regulated PD-L1 blockade for boosting cancer immunotherapy, Bioact. Materials. 2022;7:167–180. doi: 10.1016/j.bioactmat.2021.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei J., Wu D., Zhao S., Shao Y., Xia Y., Ni D., Qiu X., Zhang J., Chen J., Meng F., Zhong Z. Immunotherapy of malignant glioma by noninvasive administration of TLR9 agonist CpG nano-immunoadjuvant. Adv. Sci. 2022;9(13) doi: 10.1002/advs.202103689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T., Zhang H., Han Y., Zheng Q., Liu H., Han M., Li Z. Reversing T cell dysfunction to boost glioblastoma immunotherapy by paroxetine-mediated GRK2 inhibition and blockade of multiple checkpoints through biomimetic nanoparticles. Adv. Sci. 2023 doi: 10.1002/advs.202204961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woll P.S., Grzywacz B., Tian X., Marcus R.K., Knorr D.A., Verneris M.R., Kaufman D.S. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood. 2009;113(24):6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker P.S.A., Suck G., Nowakowska P., Ullrich E., Seifried E., Bader P., Tonn T., Seidl C. Selection and expansion of natural killer cells for NK cell-based immunotherapy, Cancer Immunology. Immunotherapy. 2016;65(4):477–484. doi: 10.1007/s00262-016-1792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brownlie D., Scharenberg M., Mold J.E., Hård J., Kekäläinen E., Buggert M., Nguyen S., Wilson J.N., Al-Ameri M., Ljunggren H., Marquardt N., Michaëlsson J. Expansions of adaptive-like NK cells with a tissue-resident phenotype in human lung and blood. Proc. Natl. Acad. Sci. USA. 2021;118(11) doi: 10.1073/pnas.2016580118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shapiro R.M., Birch G.C., Hu G., Vergara Cadavid J., Nikiforow S., Baginska J., Ali A.K., Tarannum M., Sheffer M., Abdulhamid Y.Z., Rambaldi B., Arihara Y., Reynolds C., Halpern M.S., Rodig S.J., Cullen N., Wolff J.O., Pfaff K.L., Lane A.A., Lindsley R.C., Cutler C.S., Antin J.H., Ho V.T., Koreth J., Gooptu M., Kim H.T., Malmberg K., Wu C.J., Chen J., Soiffer R.J., Ritz J., Romee R. Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse. J. Clin. Invest. 2022;132(11) doi: 10.1172/JCI154334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romee R., Schneider S.E., Leong J.W., Chase J.M., Keppel C.R., Sullivan R.P., Cooper M.A., Fehniger T.A. Cytokine activation induces human memory-like NK cells. Blood. 2012;120(24):4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malmberg K., Carlsten M., Björklund A., Sohlberg E., Bryceson Y.T., Ljunggren H. Natural killer cell-mediated immunosurveillance of human cancer. Semin. Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 47.ClinicalTrials.gov, NCT04639739 2020. https://clinicaltrials.gov/study/NCT04639739
- 48.ClinicalTrials.gov, NCT04245722 2020. https://clinicaltrials.gov/study/NCT04245722
- 49.ClinicalTrials.gov, NCT04614636 2020. https://clinicaltrials.gov/study/NCT04614636
- 50.ClinicalTrials.gov, NCT04623944 2020. https://clinicaltrials.gov/study/NCT04623944
- 51.ClinicalTrials.gov, NCT04630769 2020. https://clinicaltrials.gov/study/NCT04630769
- 52.ClinicalTrials.gov, NCT04551885 2020. https://clinicaltrials.gov/study/NCT04551885
- 53.ClinicalTrials.gov, NCT05020678 2021. https://clinicaltrials.gov/study/NCT05020678
- 54.ClinicalTrials.gov, NCT04887012 2021. https://clinicaltrials.gov/study/NCT04887012
- 55.ClinicalTrials.gov, NCT04796675 2021. https://clinicaltrials.gov/study/NCT04796675
- 56.ClinicalTrials.gov, NCT04796688 2021. https://clinicaltrials.gov/study/NCT04796688
- 57.ClinicalTrials.gov, NCT05008575 2021. https://clinicaltrials.gov/study/NCT05008575
- 58.ClinicalTrials.gov, NCT05092451 2021. https://clinicaltrials.gov/study/NCT05092451
- 59.ClinicalTrials.gov, NCT05008536 2021. https://clinicaltrials.gov/study/NCT05008536
- 60.ClinicalTrials.gov, NCT04847466 2021. https://clinicaltrials.gov/study/NCT04847466
- 61.ClinicalTrials.gov, NCT05379647 2021. https://clinicaltrials.gov/study/NCT05379647
- 62.ClinicalTrials.gov, NCT05336409 2022. https://clinicaltrials.gov/show/NCT05336409
- 63.ClinicalTrials.gov, NCT05215015 2022. https://clinicaltrials.gov/study/NCT05215015
- 64.ClinicalTrials.gov, NCT05182073 2022. https://clinicaltrials.gov/study/NCT05182073
- 65.ClinicalTrials.gov, NCT05213195 2022. https://clinicaltrials.gov/study/NCT05213195
- 66.ClinicalTrials.gov, NCT05247957 2022. https://clinicaltrials.gov/study/NCT05247957
- 67.ClinicalTrials.gov, NCT05194709 2022. https://clinicaltrials.gov/study/NCT05194709
- 68.Bachanova V., Ghobadi A., Patel K., Park J.H., Flinn I.W., Shah P., Wong C., Bickers C., Szabo P., Wong L., Valamehr B., Atwal S., Chu Y., Elstrom R., Strati P. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood. 2021;138(Supplement 1):823. [Google Scholar]
- 69.Ochoa M.C., Minute L., Rodriguez I., Garasa S., Perez Ruiz E., Inogés S., Melero I., Berraondo P. Antibody-dependent cell cytotoxicity: immunotherapy strategies enhancing effector NK cells, Immunol. Cell Biol. 2017;95(4):347–355. doi: 10.1038/icb.2017.6. [DOI] [PubMed] [Google Scholar]
- 70.Bryceson Y.T., March M.E., Ljunggren H., Long E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006;214(1):73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J., Mishra H.K., Walcheck B. Role of ADAM17 as a regulatory checkpoint of CD16A in NK cells and as a potential target for cancer immunotherapy. J. Leukoc. Biol. 2019;105(6):1297–1303. doi: 10.1002/JLB.2MR1218-501R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrow A.D., Martin C.J., Colonna M. The natural cytotoxicity receptors in health and disease. Front. Immunol. 2019;10:909. doi: 10.3389/fimmu.2019.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bauer S., Groh V., Wu J., Steinle A., Phillips J.H., Lanier L.L., Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 74.Ferrari D.A.L., Tay R.E., Pan D., Luoma A.M., Ito Y., Badrinath S., Tsoucas D., Franz B., May K.J., Harvey C.J., Kobold S., Pyrdol J.W., Yoon C., Yuan G.C., Hodi F.S., Dranoff G., Wucherpfennig K.W. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359(6383):1537–1542. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braud V.M., Allan D.S.J., O'Callaghan C.A., Söderström K., D'Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H., Lanier L.L., Mcmichael A.J. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391(6669):795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 76.Vivier E., Nunès J.A., Vély F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 77.Etxeberria I., Glez-Vaz J., Teijeira Á., Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2019;4:e733. doi: 10.1136/esmoopen-2020-000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song A., Kim H., Kim J.M., Hwang S., Ko D., Kim H.S. Bispecific antibody designed for targeted NK cell activation and functional assessment for biomedical applications. ACS Appl. Mater. Interfaces. 2021;13(36):42370–42381. doi: 10.1021/acsami.1c08986. [DOI] [PubMed] [Google Scholar]
- 79.Luu T.T., Wagner A.K., Schmied L., Meinke S., Freund J.E., Kambayashi T., Ravens I., Achour A., Bernhardt G., Chambers B.J., Höglund P., Kadri N. IL-15 and CD155 expression regulate lat expression in murine DNAM1+ NK cells, enhancing their effectors functions. Eur. J. Immunol. 2020;50(4):494–504. doi: 10.1002/eji.201948233. [DOI] [PubMed] [Google Scholar]
- 80.Pollmann J., Götz J., Rupp D., Strauss O., Granzin M., Grünvogel O., Mutz P., Kramer C., Lasitschka F., Lohmann V., Björkström N.K., Thimme R., Bartenschlager R., Cerwenka A. Hepatitis C virus-induced natural killer cell proliferation involves monocyte-derived cells and the OX40/OX40L axis. J. Hepatol. 2018;68(3):421–430. doi: 10.1016/j.jhep.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 81.Romee R., Rosario M., Berrien-Elliott M.M., Wagner J.A., Jewell B.A., Schappe T., Leong J.W., Abdel-Latif S., Schneider S.E., Willey S., Neal C.C., Yu L., Oh S.T., Lee Y., Mulder A., Claas F., Cooper M.A., Fehniger T.A. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016;8(357):123r–357r. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 2014;192(12):5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shemesh A., Pickering H., Roybal K.T., Lanier L.L. Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion. J. Exp. Med. 2022;219(8) doi: 10.1084/jem.20212434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou X., Yu J., Cheng X., Zhao B., Manyam G.C., Zhang L., Schluns K., Li P., Wang J., Sun S. The deubiquitinase otub1 controls the activation of CD8+ T cells and NK cells by regulating IL-15-mediated priming. Nat. Immunol. 2019;20(7):879–889. doi: 10.1038/s41590-019-0405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukherjee S., Jensen H., Stewart W., Stewart D., Ray W.C., Chen S., Nolan G.P., Lanier L.L., Das J. In silico modeling identifies CD45 as a regulator of IL-2 synergy in the NKG2D-mediated activation of immature human NK cells. Sci. Signal. 2017;10(485) doi: 10.1126/scisignal.aai9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burns L.J., Weisdorf D.J., Defor T.E., Vesole D.H., Repka T.L., Blazar B.R., Burger S.R., Panoskaltsis-Mortari A., Keever-Taylor C.A., Zhang M., Miller J.S. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32(2):177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 87.Steele N., Anthony A., Saunders M., Esmarck B., Ehrnrooth E., Kristjansen P.E., Nihlen A., Hansen L.T., Cassidy J. A phase 1 trial of recombinant human IL-21 in combination with cetuximab in patients with metastatic colorectal cancer. Br. J. Cancer. 2012;106(5):793–798. doi: 10.1038/bjc.2011.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seo H., Jeon I., Kim B., Park M., Bae E., Song B., Koh C., Shin K., Kim I., Choi K., Oh T., Min J., Min B.S., Han Y.D., Kang S., Shin S.J., Chung Y., Kang C. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat. Commun. 2017;8(1) doi: 10.1038/ncomms15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mcmichael E.L., Jaime-Ramirez A.C., Guenterberg K.D., Luedke E., Atwal L.S., Campbell A.R., Hu Z., Tatum A.S., Kondadasula S.V., Mo X., Tridandapani S., Bloomston M., Ellison E.C., Williams T.M., Bekaii-Saab T., Carson W.E. IL-21 enhances natural killer cell response to cetuximab-coated pancreatic tumor cells. Clin. Cancer Res. 2017;23(2):489–502. doi: 10.1158/1078-0432.CCR-16-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conlon K.C., Lugli E., Welles H.C., Rosenberg S.A., Fojo A.T., Morris J.C., Fleisher T.A., Dubois S.P., Perera L.P., Stewart D.M., Goldman C.K., Bryant B.R., Decker J.M., Chen J., Worthy T.A., Figg W.D., Peer C.J., Sneller M.C., Lane H.C., Yovandich J.L., Creekmore S.P., Roederer M., Waldmann T.A. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 2014;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang P.M., Zhou S., Meng X.M., Wang Q.M., Li C.J., Lian G.Y., Huang X.R., Tang Y.J., Guan X.Y., Yan B.P., To K.F., Lan H.Y. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat. Commun. 2017;8 doi: 10.1038/ncomms14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jinnin M., Ihn H., Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-β1-induced extracellular matrix expression. Mol. Pharmacol. 2006;69(2):597. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 93.Wu N., Lian G., Sheng J., Wu D., Yu X., Lan H., Hu W., Yang Z. Discovery of a novel selective water-soluble Smad3 inhibitor as an antitumor agent. Bioorg. Med. Chem. Lett. 2020;30(17) doi: 10.1016/j.bmcl.2020.127396. [DOI] [PubMed] [Google Scholar]
- 94.Guo X., Tan S., Wang T., Sun R., Li S., Tian P., Li M., Wang Y., Zhang Y., Yan Y., Dong Z., Yan L., Yue X., Wu Z., Li C., Yamagata K., Gao L., Ma C., Li T., Liang X. NAD+ salvage governs mitochondrial metabolism, invigorating natural killer cell antitumor immunity. Hepatology. 2022 doi: 10.1002/hep.32658. [DOI] [PubMed] [Google Scholar]
- 95.Lai H.C., Chang C.J., Yang C.H., Hsu Y.J., Chen C.C., Lin C.S., Tsai Y.H., Huang T.T., Ojcius D.M., Tsai Y.H., Lu C.C. Activation of NK cell cytotoxicity by the natural compound 2,3-butanediol. J. Leukoc. Biol. 2012;92(4):807–814. doi: 10.1189/jlb.0112024. [DOI] [PubMed] [Google Scholar]
- 96.Fraker L.D., Halter S.A., Forbes J.T. Effects of orally-administered retinol on natural-killer cell-activity in wild-type balb/c and congenitally athymic balb/c mice. Cancer Immunol. Immunother. 1986;21(2):114–118. doi: 10.1007/BF00199858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vojdani A., Ghoneum M. In vivo effect of ascorbic-acid on enhancement of human natural-killer-cell activity. Nutr. Res. 1993;13(7):753–764. [Google Scholar]
- 98.Grudzien M., Rapak A. Effect of natural compounds on NK cell activation. J. Immunol. Res. 2018 doi: 10.1155/2018/4868417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen X., Chen X., Gao J., Yang H., Duan Y., Feng Y., He X., Gong X., Wang H., Wu X., Chang J. Astragaloside III enhances anti-tumor response of NK cells by elevating NKG2D and IFN-γ. Front. Pharmacol. 2019;10:898. doi: 10.3389/fphar.2019.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ravindranath M.H., Selvan S.R., Terasaki P.I. Augmentation of anti-HLA-E antibodies with concomitant HLA-Iα reactivity in IFNγ-treated autologous melanoma cell vaccine recipients. J. Immunot. 2012;9(3):282–291. doi: 10.3109/1547691X.2011.645582. [DOI] [PubMed] [Google Scholar]
- 101.Li D., Brackenridge S., Walters L.C., Swanson O., Harlos K., Rozbesky D., Cain D.W., Wiehe K., Scearce R.M., Barr M., Mu Z., Parks R., Quastel M., Edwards R.J., Wang Y., Rountree W., Saunders K.O., Ferrari G., Borrow P., Jones E.Y., Alam S.M., Azoitei M.L., Gillespie G.M., Mcmichael A.J., Haynes B.F. Mouse and human antibodies bind HLA-E-leader peptide complexes and enhance NK cell cytotoxicity. Commun. Biol. 2022;5(1):271. doi: 10.1038/s42003-022-03183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsu J., Hodgins J.J., Marathe M., Nicolai C.J., Bourgeois-Daigneault M., Trevino T.N., Azimi C.S., Scheer A.K., Randolph H.E., Thompson T.W., Zhang L., Iannello A., Mathur N., Jardine K.E., Kirn G.A., Bell J.C., Mcburney M.W., Raulet D.H., Ardolino M. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 2018;128(10):4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou X., Li W., Wu Y., Han L., Cao X., Yang X., Wang H., Zhao W., Zhai W., Qi Y., Gao Y. Intrinsic expression of immune checkpoint molecule tigit could help tumor growth in vivo by suppressing the function of NK and CD8+ T cells. Front. Immunol. 2018;9:2821. doi: 10.3389/fimmu.2018.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hung A.L., Maxwell R., Theodros D., Belcaid Z., Mathios D., Luksik A.S., Kim E., Wu A., Xia Y., Garzon-Muvdi T., Jackson C., Ye X., Tyler B., Selby M., Korman A., Barnhart B., Park S.M., Youn J.I., Chowdhury T., Park C.K., Brem H., Pardoll D.M., Lim M. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. OncoImmunology. 2018;7(8) doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonçalves Silva I., Rüegg L., Gibbs B.F., Bardelli M., Fruehwirth A., Varani L., Berger S.M., Fasler-Kan E., Sumbayev V.V. The immune receptor Tim-3 acts as a trafficker in a Tim-3/galectin-9 autocrine loop in human myeloid leukemia cells. OncoImmunology. 2016;5(7) doi: 10.1080/2162402X.2016.1195535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Das M., Zhu C., Kuchroo V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017;276(1):97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang W., Li F., Jiang Y., Li S., Liu X., Xu Y., Li B., Feng X., Zheng C. Tim-3 blockade elicits potent anti-multiple myeloma immunity of natural killer cells. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.739976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stebbins C.C., Watzl C., Billadeau D.D., Leibson P.J., Burshtyn D.N., Long E.O. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol. Cell Biol. 2003;23(17):6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ben-Shmuel A., Biber G., Sabag B., Barda-Saad M. Modulation of the intracellular inhibitory checkpoint SHP-1 enhances the antitumor activity of engineered NK cells. Cell. Mol. Immunol. 2021;18(5):1314–1316. doi: 10.1038/s41423-020-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Viant C., Fenis A., Chicanne G., Payrastre B., Ugolini S., Vivier E. SHP-1-mediated inhibitory signals promote responsiveness and anti-tumour functions of natural killer cells. Nat. Commun. 2014;5(1):5108. doi: 10.1038/ncomms6108. [DOI] [PubMed] [Google Scholar]
- 111.Paolino M., Choidas A., Wallner S., Pranjic B., Uribesalgo I., Loeser S., Jamieson A.M., Langdon W.Y., Ikeda F., Fededa J.P., Cronin S.J., Nitsch R., Schultz-Fademrecht C., Eickhoff J., Menninger S., Unger A., Torka R., Gruber T., Hinterleitner R., Baier G., Wolf D., Ullrich A., Klebl B.M., Penninger J.M. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507(7493):508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim H.S., Das A., Gross C.C., Bryceson Y.T., Long E.O. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity. 2010;32(2):175–186. doi: 10.1016/j.immuni.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Offringa R., Kötzner L., Huck B., Urbahns K. The expanding role for small molecules in immuno-oncology. Nat. Rev. Drug Discov. 2022;21(11):821–840. doi: 10.1038/s41573-022-00538-9. [DOI] [PubMed] [Google Scholar]
- 114.Chirino L.M., Kumar S., Okumura M., Sterner D.E., Mattern M., Butt T.R., Kambayashi T. TAM receptors attenuate murine NK-cell responses via E3 ubiquitin ligase Cbl-b. Eur. J. Immunol. 2019;50(1):48–55. doi: 10.1002/eji.201948204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rautela J., Dagley L.F., de Oliveira C.C., Schuster I.S., Hediyeh-Zadeh S., Delconte R.B., Cursons J., Hennessy R., Hutchinson D.S., Harrison C., Kita B., Vivier E., Webb A.I., Degli-Esposti M.A., Davis M.J., Huntington N.D., Souza-Fonseca-Guimaraes F. Therapeutic blockade of activin-a improves NK cell function and antitumor immunity. Sci. Signal. 2019;12(596):eaat7527. doi: 10.1126/scisignal.aat7527. [DOI] [PubMed] [Google Scholar]
- 116.Young A., Ngiow S.F., Gao Y., Patch A., Barkauskas D.S., Messaoudene M., Lin G., Coudert J.D., Stannard K.A., Zitvogel L., Degli-Esposti M.A., Vivier E., Waddell N., Linden J., Huntington N.D., Souza-Fonseca-Guimaraes F., Smyth M.J. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78(4):1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 117.Close H.J., Stead L.F., Nsengimana J., Reilly K.A., Droop A., Wurdak H., Mathew R.K., Corns R., Newton-Bishop J., Melcher A.A., Short S.C., Cook G.P., Wilson E.B. Expression profiling of single cells and patient cohorts identifies multiple immunosuppressive pathways and an altered NK cell phenotype in glioblastoma. Clin. Exp. Immunol. 2020;200(1):33–44. doi: 10.1111/cei.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stiff A., Trikha P., Mundy-Bosse B., Mcmichael E., Mace T.A., Benner B., Kendra K., Campbell A., Gautam S., Abood D., Landi I., Hsu V., Duggan M., Wesolowski R., Old M., Howard J.H., Yu L., Stasik N., Olencki T., Muthusamy N., Tridandapani S., Byrd J.C., Caligiuri M., Carson W.E. Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing Fc receptor-mediated natural killer cell function. Clin. Cancer Res. 2018;24(8):1891–1904. doi: 10.1158/1078-0432.CCR-17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Clift R., Souratha J., Garrovillo S.A., Zimmerman S., Blouw B. Remodeling the tumor microenvironment sensitizes breast tumors to anti-programmed death-ligand 1 immunotherapy. Cancer Res. 2019;79(16):4149–4159. doi: 10.1158/0008-5472.CAN-18-3060. [DOI] [PubMed] [Google Scholar]
- 120.Song H., Hur D.Y., Kim K., Park H., Kim T., Kim C., Bang S., Cho D. IL-2/IL-18 prevent the down-modulation of NKG2D by TGF-β in NK cells via the c-Jun N-terminal kinase (JNK) pathway. Cell. Immunol. 2006;242(1):39–45. doi: 10.1016/j.cellimm.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Lee J., Lee K., Kim D., Heo D.S. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients1. J. Immunol. 2004;172(12):7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 122.Wu J., Gao F., Wang C., Qin M., Han F., Xu T., Hu Z., Long Y., He X., Deng X., Ren D., Dai T. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019;38(1):321. doi: 10.1186/s13046-019-1310-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tomar M.S., Singh R.K., Ulasov I.V., Kaushalendra, Acharya A. Refurbishment of NK cell effector functions through their receptors by depleting the activity of nTreg cells in dalton,s lymphoma-induced tumor microenvironment: an in vitro and in vivo study. Cancer Immunol. Immunother. 2022 doi: 10.1007/s00262-022-03339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Law A.M.K., Valdes-Mora F., Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells. 2020;9(3):561. doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iaia I., Brancato V., Caballero D., Reis R.L., Aglietta M., Sangiolo D., Kundu S.C. Fibroblasts impair migration and antitumor activity of NK-92 lymphocytes in a melanoma-on-chip model. Bioengineering. 2023;10(1):52. doi: 10.3390/bioengineering10010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smyth M.J., Teng M., Swann J., Kyparissoudis K., Godfrey D.I., Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J. Immunol. 2006;176(3):1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 127.Cózar B., Greppi M., Carpentier S., Narni-Mancinelli E., Chiossone L., Vivier E. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11(1):34–44. doi: 10.1158/2159-8290.CD-20-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stiff A., Trikha P., Mundy-Bosse B., Mcmichael E., Mace T.A., Benner B., Kendra K., Campbell A., Gautam S., Abood D., Landi I., Hsu V., Duggan M., Wesolowski R., Old M., Howard J.H., Yu L., Stasik N., Olencki T., Muthusamy N., Tridandapani S., Byrd J.C., Caligiuri M., Carson W.E. Nitric oxide production by myeloid-derived suppressor cells plays a role in impairing fc receptor-mediated natural killer cell function. Clin. Cancer Res. 2018;24(8):1891–1904. doi: 10.1158/1078-0432.CCR-17-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Park A., Lee Y., Kim M.S., Kang Y.J., Park Y.J., Jung H., Kim T.D., Lee H.G., Choi I., Yoon S.R. Prostaglandin E2 secreted by thyroid cancer cells contributes to immune escape through the suppression of natural killer (NK) cell cytotoxicity and NK cell differentiation. Front. Immunol. 2018;9:1859. doi: 10.3389/fimmu.2018.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galland S., Vuille J., Martin P., Letovanec I., Caignard A., Fregni G., Stamenkovic I. Tumor-derived mesenchymal stem cells use distinct mechanisms to block the activity of natural killer cell subsets. Cell Rep. 2017;20(12):2891–2905. doi: 10.1016/j.celrep.2017.08.089. [DOI] [PubMed] [Google Scholar]
- 131.Goswami S., Walle T., Cornish A.E., Basu S., Anandhan S., Fernandez I., Vence L., Blando J., Zhao H., Yadav S.S., Ott M., Kong L.Y., Heimberger A.B., de Groot J., Sepesi B., Overman M., Kopetz S., Allison J.P., Pe Er D., Sharma P. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat. Med. 2020;26(1):39–46. doi: 10.1038/s41591-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li T., Yang Y., Hua X., Wang G., Liu W., Jia C., Tai Y., Zhang Q., Chen G. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and Ido. Cancer Lett. 2012;318(2):154–161. doi: 10.1016/j.canlet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 133.Wang D.D., Saga Y., Mizukami H., Sato N., Nonaka H., Fujiwara H., Takei Y., Machida S., Takikawa O., Ozawa K., Suzuki M. Indoleamine-2,3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int. J. Oncol. 2012;40(4):929–934. doi: 10.3892/ijo.2011.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shaim H., Shanley M., Basar R., Daher M., Gumin J., Zamler D.B., Uprety N., Wang F., Huang Y., Gabrusiewicz K., Miao Q., Dou J., Alsuliman A., Kerbauy L.N., Acharya S., Mohanty V., Mendt M., Li S., Lu J., Wei J., Fowlkes N.W., Gokdemir E., Ensley E.L., Kaplan M., Kassab C., Li L., Ozcan G., Banerjee P.P., Shen Y., Gilbert A.L., Jones C.M., Bdiwi M., Nunez-Cortes A.K., Liu E., Yu J., Imahashi N., Muniz-Feliciano L., Li Y., Hu J., Draetta G., Marin D., Yu D., Mielke S., Eyrich M., Champlin R.E., Chen K., Lang F.F., Shpall E.J., Heimberger A.B., Rezvani K. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Invest. 2021;131(14) doi: 10.1172/JCI142116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.ClinicalTrials.gov, NCT4148937 2020. https://ClinicalTrials.gov/show/NCT4148937
- 136.ClinicalTrials.gov, NCT3616886 2018. https://ClinicalTrials.gov/show/NCT3616886
- 137.Greene S., Robbins Y., Mydlarz W.K., Huynh A.P., Schmitt N.C., Friedman J., Horn L.A., Palena C., Schlom J., Maeda D.Y., Zebala J.A., Clavijo P.E., Allen C. Inhibition of mdsc trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin. Cancer Res. 2020;26(6):1420–1431. doi: 10.1158/1078-0432.CCR-19-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Terren I., Orrantia A., Vitalle J., Zenarruzabeitia O., Borrego F. NK cell metabolism and tumor microenvironment. Front. Immunol. 2019;10:2278. doi: 10.3389/fimmu.2019.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zalfa C., Paust S. Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.633205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lyssiotis C.A., Kimmelman A.C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27(11):863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murphy D.A., Cheng H., Yang T., Yan X., Adjei I.M. Reversing hypoxia with PLGA-encapsulated manganese dioxide nanoparticles improves natural killer cell response to tumor spheroids. Mol. Pharm. 2021;18(8):2935–2946. doi: 10.1021/acs.molpharmaceut.1c00085. [DOI] [PubMed] [Google Scholar]
- 142.Solocinski K., Padget M.R., Fabian K.P., Wolfson B., Cecchi F., Hembrough T., Benz S.C., Rabizadeh S., Soon-Shiong P., Schlom J., Hodge J.W. Overcoming hypoxia-induced functional suppression of NK cells. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tian L., Lin M., Zhong H., Cai Y., Li B., Xiao Z., Shuai X. Nanodrug regulates lactic acid metabolism to reprogram the immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Biomater. Sci. 2022;10(14):3892–3900. doi: 10.1039/d2bm00650b. [DOI] [PubMed] [Google Scholar]
- 144.ClinicalTrials.gov, NCT01791595 2013. https://ClinicalTrials.gov/show/NCT01791595
- 145.Wang T., Zhang H., Liu H., Yuan Q., Ren F., Han Y., Sun Q., Li Z., Gao M. Boosting H2O2-guided chemodynamic therapy of cancer by enhancing reaction kinetics through versatile biomimetic fenton nanocatalysts and the second near-infrared light irradiation. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 146.Liu S., Wu J., Feng Y., Guo X., Li T., Meng M., Chen J., Chen D., Tian H. CD47KO/CRT dual-bioengineered cell membrane-coated nanovaccine combined with anti-PD-L1 antibody for boosting tumor immunotherapy, Bioact. Materials. 2023;22:211–224. doi: 10.1016/j.bioactmat.2022.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kang T., Huang Y., Zhu Q., Cheng H., Pei Y., Feng J., Xu M., Jiang G., Song Q., Jiang T., Chen H., Gao X., Chen J. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials. 2018;164:80–97. doi: 10.1016/j.biomaterials.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 148.Li J., Wu Y., Wang J., Xu X., Zhang A., Li Y., Zhang Z. Macrophage membrane-coated nano-gemcitabine promotes lymphocyte infiltration and synergizes antiPD-L1 to restore the tumoricidal function. ACS Nano. 2023;17(1):322–336. doi: 10.1021/acsnano.2c07861. [DOI] [PubMed] [Google Scholar]
- 149.Oldenborg P., Zheleznyak A., Fang Y., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 150.Jiang Q., Liu Y., Guo R., Yao X., Sung S., Pang Z., Yang W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials. 2019;192:292–308. doi: 10.1016/j.biomaterials.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 151.Zou M.Z., Liu W.L., Gao F., Bai X.F., Chen H.S., Zeng X., Zhang X.Z. Artificial natural killer cells for specific tumor inhibition and renegade macrophage re-education. Adv. Mater. 2019;31(43) doi: 10.1002/adma.201904495. [DOI] [PubMed] [Google Scholar]
- 152.Deng G., Sun Z., Li S., Peng X., Li W., Zhou L., Ma Y., Gong P., Cai L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 153.Pitchaimani A., Nguyen T.D.T., Aryal S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials. 2018;160:124–137. doi: 10.1016/j.biomaterials.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 154.Deng G., Peng X., Sun Z., Zheng W., Yu J., Du L., Chen H., Gong P., Zhang P., Cai L., Tang B.Z. Natural-killer-cell-inspired nanorobots with aggregation-induced emission characteristics for near-infrared-II fluorescence-guided glioma theranostics. ACS Nano. 2020;14(9):11452–11462. doi: 10.1021/acsnano.0c03824. [DOI] [PubMed] [Google Scholar]
- 155.Lin J., Li Y., Wang P., Wu M., Zhu F., Zhang Y., Hou Z., Liu J., Liu X. Natural killer cell membrane-cloaked virus-mimicking nanogenerator with NIR-triggered shape reversal and •C/•OH storm for synergistic thermodynamic-chemodynamic therapy. Adv. Sci. 2022;9(5) doi: 10.1002/advs.202103498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J., Ma P., Kim D.H., Liu B., Demirci U. Towards microfluidic-based exosome isolation and detection for tumor therapy. Nano Today. 2021;37 doi: 10.1016/j.nantod.2020.101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li B., Chen X., Qiu W., Zhao R., Duan J., Zhang S., Pan Z., Zhao S., Guo Q., Qi Y., Wang W., Deng L., Ni S., Sang Y., Xue H., Liu H., Li G. Synchronous disintegration of ferroptosis defense axis via engineered exosome-conjugated magnetic nanoparticles for glioblastoma therapy. Adv. Sci. 2022;9(17) doi: 10.1002/advs.202105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Feng C., Xiong Z., Wang C., Xiao W., Xiao H., Xie K., Chen K., Liang H., Zhang X., Yang H. Folic acid-modified exosome-ph20 enhances the efficiency of therapy via modulation of the tumor microenvironment and directly inhibits tumor cell metastasis. Bioact. Mater. 2021;6(4):963–974. doi: 10.1016/j.bioactmat.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hong Y., Kim I. The therapeutic potential of immune cell-derived exosomes as alternative to adoptive cell transfer. Bmb Rep. 2022;55(1):39–47. [Google Scholar]
- 160.Wu C.H., Li J., Li L., Sun J., Fabbri M., Wayne A.S., Seeger R.C., Jong A.Y. Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J. Extracell. Vesicles. 2019;8(1) doi: 10.1080/20013078.2019.1588538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di Pace A., Tumino N., Besi F., Alicata C., Conti L., Munari E., Maggi E., Vacca P., Moretta L. Characterization of human NK cell-derived exosomes: role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers. 2020;12(3):661. doi: 10.3390/cancers12030661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lugini L., Cecchetti S., Huber V., Luciani F., Macchia G., Spadaro F., Paris L., Abalsamo L., Colone M., Molinari A., Podo F., Rivoltini L., Ramoni C., Fais S. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012;189(6):2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 163.Jong A.Y., Wu C.H., Li J.B., Sun J.P., Fabbri M., Wayne A.S., Seeger R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles. 2017;6:1–12. doi: 10.1080/20013078.2017.1294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhu L., Kalimuthu S., Gangadaran P., Oh J.M., Lee H.W., Baek S.H., Jeong S.Y., Lee S., Lee J., Ahn B. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732–2745. doi: 10.7150/thno.18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim H.Y., Min H., Song H., Yoo A., Lee S., Kim K., Park J., Choi Y.H., Choi E. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: an in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 2022;29(1):2897–2911. doi: 10.1080/10717544.2022.2118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shoae-Hassani A., Hamidieh A.A., Behfar M., Mohseni R., Mortazavi-Tabatabaei S.A., Asgharzadeh S. NK cell-derived exosomes from NK cells previously exposed to neuroblastoma cells augment the antitumor activity of cytokine-activated NK cells. J. Immunother. 2017;40(7):265–276. doi: 10.1097/CJI.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Neviani P., Wise P.M., Murtadha M., Liu C.W., Wu C., Jong A.Y., Seeger R.C., Fabbri M. Natural killer-derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019;79(6):1151–1164. doi: 10.1158/0008-5472.CAN-18-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen H., Shou X., Ye T. Cocktail strategy based on NK cell-derived exosomes and their biomimetic nanoparticles for dual tumor therapy. Cancers. 2019;11(10):1560. doi: 10.3390/cancers11101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kaban K., Hinterleitner C., Zhou Y., Salva E., Kantarci A.G., Salih H.R., Märklin M. Therapeutic silencing of BCL-2 using NK cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers. 2021;13(10):2397. doi: 10.3390/cancers13102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang M., Shao W., Yang T., Liu H., Guo S., Zhao D., Weng Y., Liang X.J., Huang Y. Conscription of immune cells by light-activatable silencing NK-derived exosome (lasneo) for synergetic tumor eradication. Adv. Sci. 2022;9(22) doi: 10.1002/advs.202201135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kapsenberg M.L. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003;3(12):984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 172.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat. Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 173.Simhadri V.R., Reiners K.S., Hansen H.P., Topolar D., Simhadri V.L., Nohroudi K., Kufer T.A., Engert A., von Strandmann E.P. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. Plos One. 2008;3(10):e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Munich S., Sobo-Vujanovic A., Buchser W.J., Beer-Stolz D., Vujanovic N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. OncoImmunology. 2012;1(7):1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Viaud S., Terme M., Flament C., Taieb J., Andre F., Novault S., Escudier B., Robert C., Caillat-Zucman S., Tursz T., Zitvogel L., Chaput N. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Rα. Plos One. 2009;4(3) doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sobo-Vujanovic A., Munich S., Vujanovic N.L. Dendritic-cell exosomes cross-present toll-like receptor-ligands and activate bystander dendritic cells, Cell. Immunol. 2014;289(1–2):119–127. doi: 10.1016/j.cellimm.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Du Z., Huang Z., Chen X., Jiang G., Peng Y., Feng W., Huang N. Modified dendritic cell-derived exosomes activate both NK cells and T cells through the NKG2D/NKG2D-L pathway to kill cml cells with or without T315I mutation. Exp. Hematol. Oncol. 2022;11(1):36. doi: 10.1186/s40164-022-00289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., Le Chevalier T., Livartoski A., Barlesi F., Laplanche A., Ploix S., Vimond N., Peguillet I., Thery C., Lacroix L., Zoernig I., Dhodapkar K., Dhodapkar M., Viaud S., Soria J.C., Reiners K.S., Pogge V.S.E., Vely F., Rusakiewicz S., Eggermont A., Pitt J.M., Zitvogel L., Chaput N. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology. 2016;5(4) doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., Valente N., Shreeniwas R., Sutton M.A., Delcayre A., Hsu D.H., Le Pecq J.B., Lyerly H.K. A phase i study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Karami Fath M., Azami J., Masoudi A., Mosaddeghi Heris R., Rahmani E., Alavi F., Alagheband Bahrami A., Payandeh Z., Khalesi B., Dadkhah M., Pourzardosht N., Tarhriz V. Exosome-based strategies for diagnosis and therapy of glioma cancer. Cancer Cell Int. 2022;22(1):262. doi: 10.1186/s12935-022-02642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim H., Park H., Chang H.W., Back J.H., Lee S.J., Park Y.E., Kim E.H., Hong Y., Kwak G., Kwon I.C., Lee J.E., Lee Y.S., Kim S.Y., Yang Y., Kim S.H. Exosome-guided direct reprogramming of tumor-associated macrophages from protumorigenic to antitumorigenic to fight cancer. Bioact. Mater. 2023;25:527–540. doi: 10.1016/j.bioactmat.2022.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alfonsi R., Grassi L., Signore M., Bonci D. The double face of exosome-carried micrornas in cancer immunomodulation. Int. J. Mol. Sci. 2018;19(4):1183. doi: 10.3390/ijms19041183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rong L., Li R., Li S., Luo R. Immunosuppression of breast cancer cells mediated by transforming growth factor-beta in exosomes from cancer cells. Oncol. Lett. 2016;11(1):500–504. doi: 10.3892/ol.2015.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Batista I.A., Quintas S.T., Melo S.A. The interplay of exosomes and NK cells in cancer biology. Cancers. 2021;13(3):473. doi: 10.3390/cancers13030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., Angevin E., Amigorena S., Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 186.Xie Y., Bai O., Zhang H., Yuan J., Zong S., Chibbar R., Slattery K., Qureshi M., Wei Y., Deng Y., Xiang J. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8+ CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J. Cell Mol. Med. 2010;14(11):2655–2666. doi: 10.1111/j.1582-4934.2009.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gastpar R., Gehrmann M., Bausero M.A., Asea A., Gross C., Schroeder J.A., Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65(12):5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Elsner L., Muppala V., Gehrmann M., Lozano J., Malzahn D., Bickeböller H., Brunner E., Zientkowska M., Herrmann T., Walter L., Alves F., Multhoff G., Dressel R. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J. Immunol. 2007;179(8):5523. doi: 10.4049/jimmunol.179.8.5523. [DOI] [PubMed] [Google Scholar]
- 189.Lv L., Wan Y., Lin Y., Zhang W., Yang M., Li G., Lin H., Shang C., Chen Y., Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012;287(19):15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vulpis E., Cecere F., Molfetta R., Soriani A., Fionda C., Peruzzi G., Caracciolo G., Palchetti S., Masuelli L., Simonelli L., D'Oro U., Abruzzese M.P., Petrucci M.T., Ricciardi M.R., Paolini R., Cippitelli M., Santoni A., Zingoni A. Genotoxic stress modulates the release of exosomes from multiple myeloma cells capable of activating NK cell cytokine production: role of HSP70/TLR2/NF-κB axis. OncoImmunology. 2017;6(3) doi: 10.1080/2162402X.2017.1279372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Borrelli C., Ricci B., Vulpis E., Fionda C., Ricciardi M.R., Petrucci M.T., Masuelli L., Peri A., Cippitelli M., Zingoni A., Santoni A., Soriani A. Drug-induced senescent multiple myeloma cells elicit NK cell proliferation by direct or exosome-mediated IL15trans-presentation. Cancer Immunol. Res. 2018;6(7):860–869. doi: 10.1158/2326-6066.CIR-17-0604. [DOI] [PubMed] [Google Scholar]
- 192.Li Q., Huang Q., Huyan T., Wang Y., Huang Q., Shi J. Bifacial effects of engineering tumour cell-derived exosomes on human natural killer cells. Exp. Cell Res. 2018;363(2):141–150. doi: 10.1016/j.yexcr.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 193.Escudier B., Dorval T., Chaput N., Andre F., Caby M.P., Novault S., Flament C., Leboulaire C., Borg C., Amigorena S., Boccaccio C., Bonnerot C., Dhellin O., Movassagh M., Piperno S., Robert C., Serra V., Valente N., Le Pecq J.B., Spatz A., Lantz O., Tursz T., Angevin E., Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase i clinical trial. J. Transl. Med. 2005;3 doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021;16(7):748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 195.Ahn Y.H., Ren L., Kim S.M., Seo S., Jung C., Kim D.S., Noh J., Lee S.Y., Lee H., Cho M.Y., Jung H., Yoon S.R., Kim J., Lee S.N., Kim S., Shin I.W., Shin H.S., Hong K.S., Lim Y.T., Choi I., Kim T. A three-dimensional hyaluronic acid-based niche enhances the therapeutic efficacy of human natural killer cell-based cancer immunotherapy. Biomaterials. 2020;247 doi: 10.1016/j.biomaterials.2020.119960. [DOI] [PubMed] [Google Scholar]
- 196.Cheng Y., Gong Y., Chen X., Zhang Q., Zhang X., He Y., Pan L., Ni B., Yang F., Xu Y., Zhou L., Yang Y., Chen W. Injectable adhesive hemostatic gel with tumor acidity neutralizer and neutrophil extracellular traps lyase for enhancing adoptive NK cell therapy prevents post-resection recurrence of hepatocellular carcinoma. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121506. [DOI] [PubMed] [Google Scholar]
- 197.Lin X., Li F., Gu Q., Wang X., Zheng Y., Li J., Guan J., Yao C., Liu X. Gold-seaurchin based immunomodulator enabling photothermal intervention and αCD16 transfection to boost NK cell adoptive immunotherapy. Acta Biomater. 2022;146:406–420. doi: 10.1016/j.actbio.2022.04.029. [DOI] [PubMed] [Google Scholar]
- 198.Liu C., Lai H., Chen T. Boosting natural killer cell-based cancer immunotherapy with selenocystine/transforming growth factor-beta inhibitor-encapsulated nanoemulsion. ACS Nano. 2020;14(9):11067–11082. doi: 10.1021/acsnano.9b10103. [DOI] [PubMed] [Google Scholar]
- 199.Chen Q., He L., Li X., Xu L., Chen T. Ruthenium complexes boost NK cell immunotherapy via sensitizing triple-negative breast cancer and shaping immuno-microenvironment. Biomaterials. 2022;281 doi: 10.1016/j.biomaterials.2022.121371. [DOI] [PubMed] [Google Scholar]
- 200.Lai H., Zeng D., Liu C., Zhang Q., Wang X., Chen T. Selenium-containing ruthenium complex synergizes with natural killer cells to enhance immunotherapy against prostate cancer via activating TRAIL/FasL signaling. Biomaterials. 2019;219 doi: 10.1016/j.biomaterials.2019.119377. [DOI] [PubMed] [Google Scholar]
- 201.Im S., Jang D., Saravanakumar G., Lee J., Kang Y., Lee Y.M., Lee J., Doh J., Yang Z.Y., Jang M.H., Kim W.J. Harnessing the formation of natural killer-tumor cell immunological synapses for enhanced therapeutic effect in solid tumors. Adv. Mater. 2020;32(22) doi: 10.1002/adma.202000020. [DOI] [PubMed] [Google Scholar]
- 202.Liu B., Cao W., Cheng J., Fan S., Pan S., Wang L., Niu J., Pan Y., Liu Y., Sun X., Ma L., Song J., Ni J., Cui D. Human natural killer cells for targeting delivery of gold nanostars and bimodal imaging directed photothermal/photodynamic therapy and immunotherapy. Cancer Biol. Med. 2019;16(4):756–770. doi: 10.20892/j.issn.2095-3941.2019.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liao N., Su L., Zheng Y., Zhao B., Wu M., Zhang D., Yang H., Liu X., Song J. In vivo tracking of cell viability for adoptive natural killer cell-based immunotherapy by ratiometric NIR-II fluorescence imaging. Angew. Chem. Int. Ed. 2021;133(38):21056–21064. doi: 10.1002/anie.202106730. [DOI] [PubMed] [Google Scholar]
- 204.Huang S., Fong C.I., Xu M., Han B., Yuan Z., Zhao Q. Nano-loaded natural killer cells as carriers of indocyanine green for synergetic cancer immunotherapy and phototherapy. J. Innov. Opt. Health Sci. 2019;12(3) [Google Scholar]
- 205.Sanz-Ortega L., Rojas J.M., Portilla Y., Pérez-Yagüe S., Barber D.F. Magnetic nanoparticles attached to the NK cell surface for tumor targeting in adoptive transfer therapies does not affect cellular effector functions. Front. Immunol. 2019;10:2073. doi: 10.3389/fimmu.2019.02073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Burga R.A., Khan D.H., Agrawal N., Bollard C.M., Fernandes R. Designing magnetically responsive biohybrids composed of cord blood-derived natural killer cells and iron oxide nanoparticles. Bioconjugate Chem. 2019;30(3):552–560. doi: 10.1021/acs.bioconjchem.9b00048. [DOI] [PubMed] [Google Scholar]
- 207.Wu L., Zhang F., Wei Z., Li X., Zhao H., Lv H., Ge R., Ma H., Zhang H., Yang B., Li J., Jiang J. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater. Sci. 2018;6(10):2714–2725. doi: 10.1039/c8bm00588e. [DOI] [PubMed] [Google Scholar]
- 208.Kim K., Han J., Choi S.H., Jung H., Park J.D., An H., Kim S., Kim D., Doh J., Han D.K., Kim I., Park W., Park K. Cationic nanoparticle-mediated activation of natural killer cells for effective cancer immunotherapy. ACS Appl. Mater. Interfaces. 2020;12(51):56731–56740. doi: 10.1021/acsami.0c16357. [DOI] [PubMed] [Google Scholar]
- 209.Jang E., Shin J., Ren G., Park M., Cheng K., Chen X., Wu J.C., Sunwoo J.B., Cheng Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials. 2012;33:5584–5592. doi: 10.1016/j.biomaterials.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim K., Han J., Park J., Kim H., Choi S.H., Kim G.R., Song H., An H.J., Han D.K., Park W., Park K. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials. 2019;221 doi: 10.1016/j.biomaterials.2019.119418. [DOI] [PubMed] [Google Scholar]
- 211.Sim T., Choi B., Kwon S.W., Kim K., Choi H., Ross A., Kim D. Magneto-activation and magnetic resonance imaging of natural killer cells labeled with magnetic nanocomplexes for the treatment of solid tumors. ACS Nano. 2021;15(8):12780–12793. doi: 10.1021/acsnano.1c01889. [DOI] [PubMed] [Google Scholar]
- 212.Zhang D., Zheng Y., Lin Z., Lan S., Zhang X., Zheng A., Li J., Liu G., Yang H., Liu X., Liu J. Artificial engineered natural killer cells combined with antiheat endurance as a powerful strategy for enhancing photothermal-immunotherapy efficiency of solid tumors. Small. 2019;15(42) doi: 10.1002/smll.201902636. [DOI] [PubMed] [Google Scholar]
- 213.Gong L., Li Y., Cui K., Chen Y., Hong H., Li J., Li D., Yin Y., Wu Z., Huang Z. Nanobody-engineered natural killer cell conjugates for solid tumor adoptive immunotherapy. Small. 2021;17(45) doi: 10.1002/smll.202103463. [DOI] [PubMed] [Google Scholar]
- 214.Meng D., Pan H., He W., Jiang X., Liang Z., Zhang X., Xu X., Wang Z., Zheng J., Gong P., Li W., Cai L. In situ activated NK cell as bio-orthogonal targeted live-cell nanocarrier augmented solid tumor immunotherapy. Adv. Funct. Mater. 2022;32(29) [Google Scholar]
- 215.Ding L., Gao Q., Xu Z., Cai L., Chen S., Zhang X., Cao P., Chen G. An inter-supplementary biohybrid system based on natural killer cells for the combinational immunotherapy and virotherapy of cancer. Adv. Sci. 2022;9(2) doi: 10.1002/advs.202103470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xiong M., Kong G., Liu Q., Liu L., Yin Y., Liu Y., Yuan H., Zhang X., Tan W. Dna-templated anchoring of proteins for programmable cell functionalization and immunological response. Nano Lett. 2023;23(1):183–191. doi: 10.1021/acs.nanolett.2c03928. [DOI] [PubMed] [Google Scholar]
- 217.Sun M., Yang S., Huang H., Gao P., Pan S., Cheng Z., He Z., Wang Z., Sun J., Liu F. Boarding oncolytic viruses onto tumor-homing bacterium-vessels for augmented cancer immunotherapy. Nano Lett. 2022;22(12):5055–5064. doi: 10.1021/acs.nanolett.2c00699. [DOI] [PubMed] [Google Scholar]
- 218.Liu J., Chen Q., Feng L., Liu Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today. 2018;21:55–73. [Google Scholar]
- 219.Gulzar A., Xu J., Wang C., He F., Yang D., Gai S., Yang P., Lin J., Jin D., Xing B. Tumour microenvironment responsive nanoconstructs for cancer theranostic. Nano Today. 2019;26:16–56. [Google Scholar]
- 220.Yang Q., Xu J., Gu J., Shi H., Zhang J., Zhang J., Chen Z.S., Fang X., Zhu T., Zhang X. Extracellular vesicles in cancer drug resistance: roles, mechanisms, and implications. Adv. Sci. 2022;9(34) doi: 10.1002/advs.202201609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang H., Wang T., Liu H., Ren F., Qiu W., Sun Q., Yan F., Zheng H., Li Z., Gao M. Second near-infrared photodynamic therapy and chemotherapy of orthotopic malignant glioblastoma with ultra-small Cu2-xSe nanoparticles. Nanoscale. 2019;11(16):7600–7608. doi: 10.1039/c9nr01789e. [DOI] [PubMed] [Google Scholar]
- 222.Wang Y., Wu W., Mao D., Teh C., Wang B., Liu B. Metal-organic framework assisted and tumor microenvironment modulated synergistic image-guided photo-chemo therapy. Adv. Funct. Mater. 2020;30(28) [Google Scholar]
- 223.Xu Q., Zhang H., Liu H., Han Y., Qiu W., Li Z. Inhibiting autophagy flux and dna repair of tumor cells to boost radiotherapy of orthotopic glioblastoma. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121287. [DOI] [PubMed] [Google Scholar]
- 224.Wu J., Guo Z., Ni W., Feng Y., Guo X., Meng M., Yuan Y., Lin L., Chen J., Tian H., Chen X. Novel cocktail therapy based on a nanocarrier with an efficient transcytosis property reverses the dynamically deteriorating tumor microenvironment for enhanced immunotherapy. Nano Lett. 2022;22(17):7220–7229. doi: 10.1021/acs.nanolett.2c02724. [DOI] [PubMed] [Google Scholar]
- 225.Yang M., Li J., Gu P., Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 2021;6(7):1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zheng Y., Han Y., Wang T., Liu H., Sun Q., Hu S., Chen J., Li Z. Reprogramming tumor-associated macrophages via ros-mediated novel mechanism of ultra-small Cu2-xSe nanoparticles to enhance anti-tumor immunity. Adv. Funct. Mater. 2022;32(12) [Google Scholar]
- 227.Zhang H., Wang T., Liu H., Han Y., Zheng Q., Xu Q., Bao B., Xing W., Li Z. Boost therapy of hepatocellular carcinoma by amplifying vicious cycle between mitochondrial oxidative stress and endoplasmic reticulum stress via biodegradable ultrasmall nanoparticles and old drug. Nano Today. 2022;46 [Google Scholar]
- 228.Zhou X., Liu H., Zheng Y., Han Y., Wang T., Zhang H., Sun Q., Li Z. Overcoming radioresistance in tumor therapy by alleviating hypoxia and using the HIF-1 inhibitor. ACS Appl. Mater. Interfaces. 2020;12(4):4231–4240. doi: 10.1021/acsami.9b18633. [DOI] [PubMed] [Google Scholar]
- 229.Zhang Y., Zhao Y., Shen J., Sun X., Liu Y., Liu H., Wang Y., Wang J. Nanoenabled modulation of acidic tumor microenvironment reverses anergy of infiltrating T cells and potentiates anti-PD-1 therapy. Nano Lett. 2019;19(5):2774–2783. doi: 10.1021/acs.nanolett.8b04296. [DOI] [PubMed] [Google Scholar]
- 230.Biber G., Sabag B., Raiff A., Ben Shmuel A., Puthenveetil A., Benichou J.I.C., Jubany T., Levy M., Killner S., Barda Saad M. Modulation of intrinsic inhibitory checkpoints using nano-carriers to unleash NK cell activity. EMBO Mol. Med. 2022;14(1) doi: 10.15252/emmm.202114073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Deng J., Xu W., Lei S., Li W., Li Q., Li K., Lyu J., Wang J., Wang Z. Activated natural killer cells-dependent dendritic cells recruitment and maturation by responsive nanogels for targeting pancreatic cancer immunotherapy. Small. 2022;18(44) doi: 10.1002/smll.202203114. [DOI] [PubMed] [Google Scholar]
- 232.Hou L., Liu Q., Shen L., Liu Y., Zhang X., Chen F., Huang L. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics. 2018;8(14):3781–3796. doi: 10.7150/thno.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ye H., He X., Feng X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110369. [DOI] [PubMed] [Google Scholar]
- 234.Zamecnik C.R., Lowe M.M., Patterson D.M., Rosenblum M.D., Desai T.A. Injectable polymeric cytokine-binding nanowires are effective tissue-specific immunomodulators. ACS Nano. 2017;11(11):11433–11440. doi: 10.1021/acsnano.7b06094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tang J., Yang Y., Qu J., Ban W., Song H., Gu Z., Yang Y., Cai L., Theivendran S., Wang Y., Zhang M., Yu C. Mesoporous sodium four-coordinate aluminosilicate nanoparticles modulate dendritic cell pyroptosis and activate innate and adaptive immunity. Chem. Sci. 2022;13(29):8507–8517. doi: 10.1039/d1sc05319a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu J., Zhang C., Zhang X., Yan J., Zeng C., Talebian F., Lynch K., Zhao W., Hou X., Du S., Kang D.D., Deng B., Mccomb D.W., Bai X., Dong Y. Intratumoral delivery of IL-12 and IL-27 mrna using lipid nanoparticles for cancer immunotherapy. J. Contr. Release. 2022;345:306–313. doi: 10.1016/j.jconrel.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lei S., Zhang X., Men K., Gao Y., Yang X., Wu S., Duan X., Wei Y., Tong R. Efficient colorectal cancer gene therapy with IL-15 mRNA nanoformulation. Mol. Pharm. 2020;17(9):3378–3391. doi: 10.1021/acs.molpharmaceut.0c00451. [DOI] [PubMed] [Google Scholar]
- 238.Sun Y., Liu L., Zhou L., Yu S., Lan Y., Liang Q., Liu J., Cao A., Liu Y. Tumor microenvironment-triggered charge reversal polymetformin-based nanosystem co-delivered doxorubicin and IL-12 cytokine gene for chemo-gene combination therapy on metastatic breast cancer. ACS Appl. Mater. Interfaces. 2020;12(41):45873–45890. doi: 10.1021/acsami.0c14405. [DOI] [PubMed] [Google Scholar]
- 239.Yang Y., Zhu W., Cheng L., Cai R., Yi X., He J., Pan X., Yang L., Yang K., Liu Z., Tan W., Chen M. Tumor microenvironment (TME)-activatable circular aptamer-peg as an effective hierarchical-targeting molecular medicine for photodynamic therapy. Biomaterials. 2020;246 doi: 10.1016/j.biomaterials.2020.119971. [DOI] [PubMed] [Google Scholar]
- 240.Zhao Z., Yan J., Ling K., Shi R., Lv R., Nie B., Liu J. Triggering reactive oxygen species field effect transistor based on HIF‐1α signaling for enhanced chemodynamic therapy. Adv. Funct. Mater. 2021;31(49) [Google Scholar]
- 241.Zhu H., Liu Q., Miao L., Musetti S., Huo M., Huang L. Remodeling the fibrotic tumor microenvironment of desmoplastic melanoma to facilitate vaccine immunotherapy. Nanoscale. 2020;12(5):3400–3410. doi: 10.1039/c9nr09610h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chen Y., Wang L., Zheng M., Zhu C., Wang G., Xia Y., Blumenthal E.J., Mao W., Wan Y. Engineered extracellular vesicles for concurrent Anti-PDL1 immunotherapy and chemotherapy. Bioact. Mater. 2022;9:251–265. doi: 10.1016/j.bioactmat.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li T., Pan S., Gao S., Xiang W., Sun C., Cao W., Xu H. Diselenide-pemetrexed assemblies for combined cancer immuno-, radio-, and chemotherapies. Angew. Chem. Int. Ed. 2020;59(7):2700–2704. doi: 10.1002/anie.201914453. [DOI] [PubMed] [Google Scholar]
- 244.Pan S., Li T., Tan Y., Xu H. Selenium-containing nanoparticles synergistically enhance pemetrexed&NK cell-based chemoimmunotherapy. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121321. [DOI] [PubMed] [Google Scholar]
- 245.Wei Z., Yi Y., Luo Z., Gong X., Jiang Y., Hou D., Zhang L., Liu Z., Wang M., Wang J., Guo R., Yang J., Wang L., Wang H., Zhao Y. Selenopeptide nanomedicine activates natural killer cells for enhanced tumor chemoimmunotherapy. Adv. Mater. 2022;34(17) doi: 10.1002/adma.202108167. [DOI] [PubMed] [Google Scholar]
- 246.Gao S., Li T., Guo Y., Sun C., Xianyu B., Xu H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv. Mater. 2020;32(12) doi: 10.1002/adma.201907568. [DOI] [PubMed] [Google Scholar]
- 247.Pan S., Guan J., Xianyu B., Tan Y., Li T., Xu H. A nanotherapeutic strategy to reverse NK cell exhaustion. Adv. Mater. 2023 doi: 10.1002/adma.202211370. [DOI] [PubMed] [Google Scholar]
- 248.Shen W., Wang H., Guo G., Tuo J. Immunomodulatory effects of caulerpa racemosa var peltata polysaccharide and its selenizing product on T lymphocytes and NK cells in mice. Sci. China Life Sci. 2008;51(9):795–801. doi: 10.1007/s11427-008-0106-9. [DOI] [PubMed] [Google Scholar]
- 249.Song Z., Luo W., Zheng H., Zeng Y., Wang J., Chen T. Translational nanotherapeutics reprograms immune microenvironment in malignant pleural effusion of lung adenocarcinoma. Adv. Healthc. Mater. 2021;10(12) doi: 10.1002/adhm.202100149. [DOI] [PubMed] [Google Scholar]
- 250.Loftus C., Saeed M., Davis D.M., Dunlop I.E. Activation of human natural killer cells by graphene oxide-templated antibody nanoclusters. Nano Lett. 2018;18(5):3282–3289. doi: 10.1021/acs.nanolett.8b01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Koellhoffer E.C., Steinmetz N.F. Cowpea mosaic virus and natural killer cell agonism for in situ cancer vaccination. Nano Lett. 2022;22(13):5348–5356. doi: 10.1021/acs.nanolett.2c01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang W., Zhao J., Hao C., Hu S., Chen C., Cao Y., Xu Z., Guo J., Xu L., Sun M., Xu C., Kuang H. The development of chiral nanoparticles to target NK cells and CD8+ T cells for cancer immunotherapy. Adv. Mater. 2022;34(16) doi: 10.1002/adma.202109354. [DOI] [PubMed] [Google Scholar]
- 253.Gauthier L., Morel A., Anceriz N., Rossi B., Blanchard-Alvarez A., Grondin G., Trichard S., Cesari C., Sapet M., Bosco F., Rispaud-Blanc H., Guillot F., Cornen S., Roussel A., Amigues B., Habif G., Caraguel F., Arrufat S., Remark R., Romagné F., Morel Y., Narni-Mancinelli E., Vivier E. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177(7):1701–1713. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 254.Zheng C., Wang Q., Wang Y., Zhao X., Gao K., Liu Q., Zhao Y., Zhang Z., Zheng Y., Cao J., Chen H., Shi L., Kang C., Liu Y., Lu Y. In situ modification of the tumor cell surface with immunomodulating nanoparticles for effective suppression of tumor growth in mice. Adv. Mater. 2019 doi: 10.1002/adma.201902542. [DOI] [PubMed] [Google Scholar]
- 255.Au K.M., Park S.I., Wang A.Z. Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Sci. Adv. 2020;6(27) doi: 10.1126/sciadv.aba8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sweeney E.E., Balakrishnan P.B., Powell A.B., Bowen A., Sarabia I., Burga R.A., Jones R.B., Bosque A., Cruz C.R.Y., Fernandes R. PLGA nanodepots co-encapsulating prostratin and anti-CD25 enhance primary natural killer cell antiviral and antitumor function. Nano Res. 2020;13(3):736–744. doi: 10.1007/s12274-020-2684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu T., Xu L., He L., Zhao J., Zhang Z., Chen Q., Chen T. Selenium nanoparticles regulates selenoprotein to boost cytokine-induced killer cells-based cancer immunotherapy. Nano Today. 2020;35 [Google Scholar]
- 258.Wu X., Yang H., Chen X., Gao J., Duan Y., Wei D., Zhang J., Ge K., Liang X., Huang Y., Feng S., Zhang R., Chen X., Chang J. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120654. [DOI] [PubMed] [Google Scholar]
- 259.Chen S., Wang X., Lin M., Hou Y., Ding M., Kong D., Sun H., Zhang Q., Li J., Zhou Q. Liposome-based nanocomplexes with pH-sensitive second near-infrared photothermal property for combinational immunotherapy. Appl. Mater. Today. 2021;25 [Google Scholar]
- 260.Liang R., Liu L., He H., Chen Z., Han Z., Luo Z., Wu Z., Zheng M., Ma Y., Cai L. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149–160. doi: 10.1016/j.biomaterials.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 261.Ma Y., Zhang Y., Li X., Zhao Y., Li M., Jiang W., Tang X., Dou J., Lu L., Wang F., Wang Y. Near-infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano. 2019;13(10):11967–11980. doi: 10.1021/acsnano.9b06040. [DOI] [PubMed] [Google Scholar]
- 262.Qian M., Chen L., Du Y., Jiang H., Huo T., Yang Y., Guo W., Wang Y., Huang R. Biodegradable mesoporous silica achieved via carbon nanodots-incorporated framework swelling for debris-mediated photothermal synergistic immunotherapy. Nano Lett. 2019;19(12):8409–8417. doi: 10.1021/acs.nanolett.9b02448. [DOI] [PubMed] [Google Scholar]
- 263.Galstyan A., Markman J.L., Shatalova E.S., Chiechi A., Korman A.J., Patil R., Klymyshyn D., Tourtellotte W.G., Israel L.L., Braubach O., Ljubimov V.A., Mashouf L.A., Ramesh A., Grodzinski Z.B., Penichet M.L., Black K.L., Holler E., Sun T., Ding H., Ljubimov A.V., Ljubimova J.Y. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 2019;10(1):3850. doi: 10.1038/s41467-019-11719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yao L., Zhao M., Luo Q., Zhang Y., Liu T., Yang Z., Liao M., Tu P., Zeng K. Carbon quantum dots-based nanozyme from coffee induces cancer cell ferroptosis to activate antitumor immunity. ACS Nano. 2022;16(6):9228–9239. doi: 10.1021/acsnano.2c01619. [DOI] [PubMed] [Google Scholar]
- 265.Tang L., Zheng Y., Melo M.B., Mabardi L., Castaño A.P., Xie Y., Li N., Kudchodkar S.B., Wong H.C., Jeng E.K., Maus M.V., Irvine D.J. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 2018;36(8):707–716. doi: 10.1038/nbt.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Suarez-Kelly L.P., Sun S.H., Ren C., Rampersaud I.V., Albertson D., Duggan M.C., Noel T.C., Courtney N., Buteyn N.J., Moritz C., Yu L., Yildiz V.O., Butchar J.P., Tridandapani S., Rampersaud A.A., Carson W.E. Antibody conjugation of fluorescent nanodiamonds for targeted innate immune cell activation. ACS Appl. Nano Mater. 2021;4(3):3122–3139. doi: 10.1021/acsanm.1c00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016;17(9):1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 268.Jiang M., Liu Y., Dong Y., Wang K., Yuan Y. Bioorthogonal chemistry and illumination controlled programmed size-changeable nanomedicine for synergistic photodynamic and hypoxia-activated therapy. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121480. [DOI] [PubMed] [Google Scholar]
- 269.Zhong Y., Ma Z., Wang F., Wang X., Yang Y., Liu Y., Zhao X., Li J., Du H., Zhang M., Cui Q., Zhu S., Sun Q., Wan H., Tian Y., Liu Q., Wang W., Garcia K.C., Dai H. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 2019;37(11):1322–1331. doi: 10.1038/s41587-019-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]