Abstract
Introduction
Improvement of treatments for patients suffering from colorectal carcinoma and extended liver metastases has increased the overall survival and enables more patients to undergo surgical therapy. If the future liver remnant (FLR) is expected to be low, Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) is a potential treatment with high feasibility and an increase in overall survival. The evolving mixed reality technology could support hepatobiliary surgery. This case report demonstrates for the first time the combination of mixed reality technology and ALPPS procedure for a patient with low expected FLR.
Presentation of case
A 49-year-old patient is presented with adenocarcinoma of the caecum with bilateral liver metastasis. After colon resection, a palliative chemotherapy was administered with good response and partial remission, so curative liver resection was intended. Based on the low expected FLR, calculated from the 3D-model of the liver, we decided to perform an in-situ split resection supported by mixed reality intraoperatively. The total operation time was 6 + 2 h. During both steps no blood transfusion was required and no major complication occurred. The patient was discharged 15 days after the second step. Final pathology revealed multiple predominantly necrotic metastases of the pre-existing colon carcinoma (ypM1, R0).
Discussion
After the first step of ALPPS, an increase of the FLR up to 57 % was achieved, so the second step was performed on postoperative day (POD)11. The 3D-model and the intraoperative use of mixed reality supported our decision making and intraoperative navigation. This technique could be implemented on a larger scale to support complex liver resections.
Conclusion
The combination of mixed reality with ALPPS resulted in a good surgical outcome and should be considered as a potential alternative for liver resections.
Keywords: Case report, Liver surgery, Mixed reality, ALLPS, Colorectal cancer
Highlights
-
•
A sufficient future liver remnant volume is a main criteria for extendend hepatobiliary surgery.
-
•
The ALPPS-Procedure can induce rapidly high rates of hypertrophy of the future liver remnant.
-
•
Mixed reality can be used intraoperatively to better understand the anatomy and reduce errors during resection.
-
•
Considering strict selection criteria, ALPPS can be an appropriate treatment for advanced colorectal liver metastasis.
1. Introduction
Advances in chemotherapy, targeted oncologic therapy, perioperative management and development of new secure surgical techniques have increased the survival of patients suffering from malignancies of the liver, especially for colorectal metastases (CRLM). Main criteria for the resectability of CRLM are adequate vascular in- and outflow, biliary drainage and sufficient future liver remnant volume (FLRV) [1].
An accurate estimation of the FLR is required to minimize the risk of the feared post hepatectomy liver failure (PHLF), which is defined as the impaired ability of the liver for its synthetic, excretory and detoxifying functions, characterized by hyperbilirubinemia on day 5 after surgery and is graded based on its impact on clinical management [2].
Treatment of patients with bilobal liver metastasis and small FLR is still challenging. In 2012, Schnitzbauer et al. presented the two-staged hepatectomy, consisting of a portal vein ligation combined with a parenchymal transection as the first step and resection of the diseased hemiliver as second step after a few days. This procedure, later named ALPPS, can induce rapidly high hypertrophy rates of the remaining liver (volumetric increase of 74 % over a median of 9 days) after the first step [3].
In general, a FLR ≥ 20 % of volume in case of the normal liver and ≥ 30–40 % for impaired liver function as cholestasis or steatosis is recommended to reduce the risk for PHLF [4]. Most common for the calculation of the FLR is by using images of a CT-Scan or MRT. For preoperative estimation of liver function, we use LiMAx (which estimates liver function by measuring metabolized 13C-methacetin in breath) [5] and generate a patient-specific 3-dimensional model of the liver by uploading the anonymized preoperative CT or MRI-data to Visible Patient, a software developed in France from IRCAD. Total liver volume (TLV) is estimated with and without tumor mass and the FLR is calculated by choosing different branches of Vena portae which can be virtually ligated. Additionally, we upload the 3D models to our HoloLens 2 glasses, a Head-Mounted-Display, through the software platform VSI HoloMedicine® (apoQlar, Hamburg, Germany). Major liver resection can be assessed intraoperatively as mixed reality to lower the risk for a small remaining liver and therefore avoid complications by increasing navigational speed and reducing navigational error [6].
This is the first report worldwide combining ALPPS with the innovative technology of holomedicine and intraoperative navigation using mixed reality after 3D-reconstruction of the liver by using Visible Patient. This work has been reported in line with the SCARE criteria and is compliant to the PROCESS Guidelines [7,8].
2. Presentation of case
We present the case of a 49-year-old man with initial diagnosis of stenosing ileocecal carcinoma with primary bilobal multilocular liver metastasis. In December 2020, the laparoscopic right hemicolectomy and biopsy of liver metastasis were performed at the referring hospital. Histology report revealed stage IV adenocarcinoma (T3, N2b, M1, R0, G2) according to UICC 8th edition with positive Bethesda criteria with exclusion of MSI (mutation status: K-RAS-WT, BRAF-WT, Her2-neu negative, N-RAS mutated). Patient history showed no previous diseases or medication. Subsequently a palliative intended chemotherapy was administered with 12 cycles of Bevacizumab/FOLFOXIRI until August 2021 with partial remission in re-staging, so further therapy using Bevacizumab/Capecitabine followed. In May 2022 MRI of the liver demonstrated a total of seven metastases (S6, S8, in transition S8 to S4 and S3) which were size-regressed (Fig. 1). Our multidisciplinary tumor board recommended curative resection.
Fig. 1.
Preoperative imaging.
MRI showing metastases predominantly in the right liver lobe and also in Segment 3.
After 5 weeks of pausing systemic therapy and laparoscopic exclusion of peritoneal carcinomatosis, further preparation for planned metastasectomy was performed. Laboratory results showed normal liver values and tumor markers. The LiMAx test showed impaired but adequate liver function (308 μg/kg/h; reference value>315 μg/kg/h). To estimate the FLRV a conventional volumetry calculation was performed in the CT scan as well as a calculation based on the 3D model of the liver generated with Visible Patient (Fig. 2). The total expected FLRV was 30 % (TLV 1573 cm3; FLRV 473 cm3) in the CT scan and the expected healthy FLRV (volume of tumor mass excluded) was 21.2 % in the 3D model.
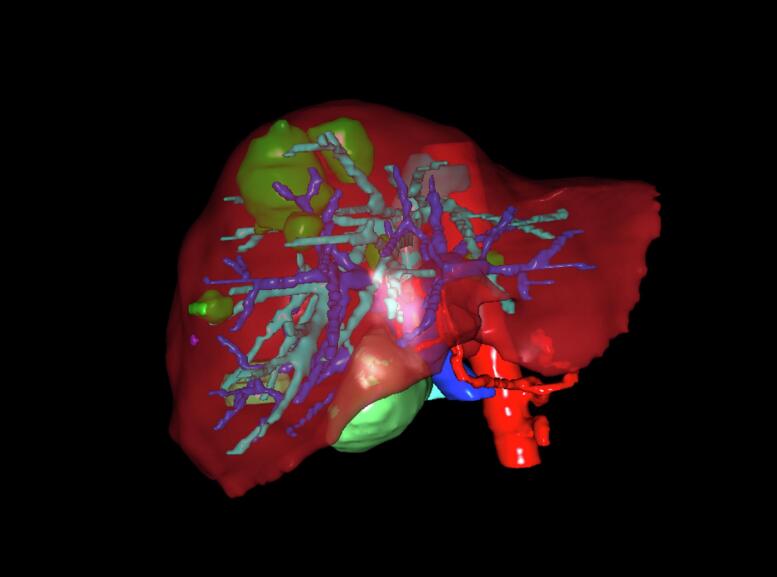
Fig. 2.
3D reconstruction.
Patient-specific 3D model of the liver, generated by the Visible Patient software prior surgery.
Due to the low values for the FLR in the volumetry, impaired LiMAx test and required metastatic resection in the left liver lobe, we decided to perform a two-stage hepatectomy according to ALPPS procedure.
The first step of ALPPS included resection of two metastases in the left lobe, cholecystectomy, and dissection of the two right main branches of the portal vein with parenchymal transection of the previously marked resection margin at S4. The liver was dissected with the crash-clamp-technique. Intraoperatively, the procedure was supported by imaging using color-coded duplex sonography (CCD) and the 3D model was scaled to the surgical site and projected with HoloLens (Fig. 3). This significantly facilitated the preparation of the central vessels in the liver hilum and provided the navigation during the transection. In the CDD of the left lobe an unknown lesion in S2 was found and resected. We placed two drains which were removed at POD2. The second step was planned for POD11. We performed an additional CT-scan at POD10 (Fig. 4) to recalculate the FLRV, which showed an increase up to 37 % (473 cm3 vs 730 cm3). By extrapolating the volume of the metastases and calculating only the healthy liver volumes using the 3D model, the increase in FLRV was 57 % (312 cm3 vs 730cm3).
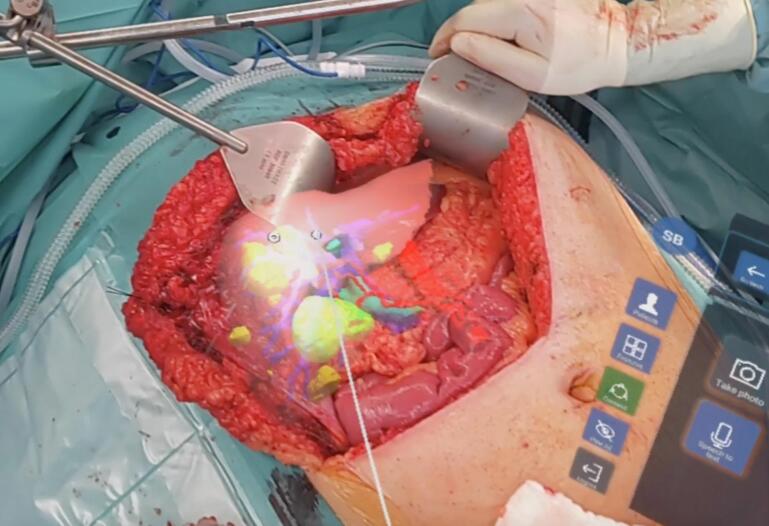
Fig. 3.
Intraoperative imaging.
3D rendering of patient liver in situ superimposed on the patient by mixed reality using an HMD during step one. Highlighted are the metastases (yellow) and the vascular structures (green: bile ducts, red: arteries, blue: portal veins).
Fig. 4.
Imaging after step one.
CT-Scan 10 days after the first step, showing the parenchymal transection zone (yellow) and resection area of the metastasis in Segment 3 of the remnant liver (white).
During the second step we performed the right hemihepatectomy, again supported by mixed reality. Intraoperative CCD showed undisturbed perfusion to the residual liver. The total operation time was 360 min for the first step and 130 min for the second step. No blood transfusion was required. Postoperatively the patient received intensive medical care for two days after the second step. Blood values initially showed increasing liver transaminases and INR on POD5 but normal bilirubin and lactate. No biliary leak was detected. The postoperative LiMAx test on POD7 after step two indicated impaired liver function (121 μg/kg/h). However, no other major postoperative complications (Clavien-Dindo ≥3a) [9] occurred. Computertomography on POD10 after step two showed an increasing hypertrophy of the remaining liver up to 1000 cm3. After further normalization of liver function we discharged the patient two weeks after step two. Final pathology revealed tumor necrosis in S2 and S3 without vital tumor cells and multiple predominantly necrotic metastases measuring up to 7.5 cm including vital residuals of the pre-existing colon carcinoma (ypM1, R0). The short-term follow-up after three months showed no recurrence of the tumor disease.
3. Discussion
Despite advances in chemotherapy and antibody therapy, radical resection of CRLM remains the only curative therapy [10]. After ALPPS emerged, there was great enthusiasm for the surgical treatment of extended tumor diseases of the liver and liver metastases [11]. The previously reported higher rates for postoperative complications, including higher mortality and reports of early and aggressive tumor recurrence are still subject of discussion [11,12]. The recent results of the LIGRO trial demonstrate that ALPPS has comparable complications and mortality in the treatment of CRLM to the more common two staged hepatectomy (TSH), consisting of a portal vein embolization and resection after a couple of weeks, and is superior in resection rate [13]. Contrary to previous studies, which found no difference in survival comparing ALPPS to TSH [12], the first follow-up of the LIGRO trial showed an improved disease-free and overall survival for patients treated with ALPPS (estimated median survival 46 vs 26 months), probably due to the higher resection rate and growing experience with this procedure [14]. In addition strong patient selection seems to improve the outcome. Patients suffering from CRLM at a younger age, having a good response to systemic treatment, absence of severe disease or liver dysfunction and an FLR < 30 % seem to be most suitable for ALPPS [15].
In the presented case, after systemic treatment with partial remission, a multidisciplinary decision was made for curative intended resection for this initially as unresectable defined case. In order to perform a parenchyma-sparing resection, a 3D model of the liver was generated, processing the preoperative imagery. This model increased our understanding of the location of the metastases, allowed us to calculate the FLR more accurately and supported our decision making to find the appropriate procedure for our patient. In our clinical practice for hepatic surgery, we upload the generated 3D models to HMD-Glasses to project these models as mixed reality for a better intraoperative navigation. Drawing from our experience, this innovative technology proves to be beneficial in facilitating the localization of liver metastases, particularly for those situated in more central areas. This advantage is attributed to the color-highlighted vessels and metastases, which enhance the accuracy of identification. Unlike the traditional use of CDD, this technology allows the surgeon to maintain focus on the surgical field without interruptions to handle a transducer or divert attention to a separate monitor.
Through preoperative volumetry, functional tests and achieving a good intraoperative 3D visualization, these technologies have become a key factor in improving our preoperative and intraoperative decision making, even if no differences in operation time or blood loss were observed [16]. The impact of these evolving technologies on postoperative morbidity, mortality and oncological outcome after extended hepatobiliary surgery needs still to be further evaluated. The present case demonstrates the feasibility and potential benefit of combining mixed reality with ALPPS.
4. Conclusion
Considering strict selection criteria, ALPPS can be an appropriate treatment for patients suffering from CRLM with a low expected FLR. The presented 3D-visualization by mixed reality can support preoperative decision making and intraoperative navigation. This case shows that new techniques of liver surgery such as ALPPS can be usefully combined with the evolving mixed reality technology for better surgical outcomes.
CRediT authorship contribution statement
Darick Fidan: Conceptualization, Data curation, Writing – original draft. Genadi Mero: Software, Visualization. Laura Ioana Mazilescu: Resources, Formal analysis. Theodor Heuer: Writing – review & editing. Gernot Maximilian Kaiser: Supervision, Validation, Writing – review & editing.
Declaration of competing interest
None.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The name of the institution that waived ethical approval: St. Franziskus Stiftung, Münster, Germany.
Contributor Information
Darick Fidan, Email: darick.fidan@umm.de.
Laura Ioana Mazilescu, Email: laura.mazilescu@uk-essen.de.
Theodor Heuer, Email: theodor.heuer@st-bernhard-hospital.de.
Gernot Maximilian Kaiser, Email: gernot.kaiser@st-bernhard-hospital.de.
References
- 1.Regimbeau J.M., Cosse C., Kaiser G., Hubert C., Laurent C., Lapointe R., et al. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: a LiverMetSurvey analysis. HPB (Oxford). 2017;19(5):396–405. doi: 10.1016/j.hpb.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Sparrelid E., Olthof P.B., Dasari B.V.M., Erdmann J.I., Santol J., Starlinger P., et al. Current evidence on posthepatectomy liver failure: comprehensive review. BJS Open. 2022;6(6) doi: 10.1093/bjsopen/zrac142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnitzbauer A.A., Lang S.A., Goessmann H., Nadalin S., Baumgart J., Farkas S.A., et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 2012;255(3):405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 4.Memeo R., Conticchio M., Deshayes E., Nadalin S., Herrero A., Guiu B., et al. Optimization of the future remnant liver: review of the current strategies in Europe. Hepatobiliary Surg. Nutr. 2021;10(3):350–363. doi: 10.21037/hbsn-20-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockmann M., Lock J.F., Malinowski M., Niehues S.M., Seehofer D., Neuhaus P. The LiMAx test: a new liver function test for predicting postoperative outcome in liver surgery. HPB (Oxford). 2010;12(2):139–146. doi: 10.1111/j.1477-2574.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollen E, Awad L, Langridge B, Butler PEM. The intraoperative use of augmented and mixed reality technology to improve surgical outcomes: a systematic review. Int. J. Med. Robot. 202218(6):e2450. doi: 10.1002/rcs.2450. [DOI] [PubMed]
- 7.Agha R.A., Franchi T., Sohrab C., Mathew G., Kirwan A., Thomas A., et al. The SCARE 2020guideline: updating consensus surgical case report (SCARE) guidelines. Int. J. Surg. 2020;84(1):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., SCARE Group. The PROCESS Statement: updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int. J. Surg. 2018;2018(60):279–282. doi: 10.1016/j.ijsu.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliante F., Viganò L., De Rose A.M., Mirza D.F., Lapointe R., Kaiser G., et al. Liver-first approach for synchronous colorectal metastases: analysis of 7360 patients from the LiverMetSurvey registry. Ann. Surg. Oncol. 2021;28(13):8198–8208. doi: 10.1245/s10434-021-10220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang H., Baumgart J., Mittler J. Associated liver partition and portal vein ligation for staged hepatectomy (ALPPS) registry: what have we learned? Gut Liver. 2020;14(6):699–706. doi: 10.5009/gnl19233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldhafer K.J., Donati M., Jenner R.M., Stang A., Stavrou G.A. ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J. Surg. 2014;38(6):1504–1509. doi: 10.1007/s00268-013-2401-2. [DOI] [PubMed] [Google Scholar]
- 13.Sandström P., Røsok B.I., Sparrelid E., Larsen P.N., Larsson A.L., Lindell G., et al. ALPPS improves Resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian multicenter randomized controlled trial (LIGRO trial) Ann. Surg. 2018;267(5):833–840. doi: 10.1097/SLA.0000000000002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasselgren K., Røsok B.I., Larsen P.N., Sparrelid E., Lindell G., Schultz N.A., et al. ALPPS improves survival compared with TSH in patients affected of CRLM: survival analysis from the randomized controlled trial LIGRO. Ann. Surg. 2021;273(3):442–448. doi: 10.1097/sla.0000000000003701. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Alejandro R., Ruffolo L.I., Alikhanov R., Björnsson B., Torres O.J.M., Serrablo A. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure for colorectal liver metastasis. Int. J. Surg. 2020;82S:103–108. doi: 10.1016/j.ijsu.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Mero G., Donchev R., Banysch M., Hornstein M., Heuer T., Kaiser G.M. Mixed reality technology - initial expierence from liver surgery. Gastroenterologie. 2023;18:46–53. doi: 10.1007/s11377-022-00662-3. [DOI] [Google Scholar]