Highlights
-
•
6-APB was a strong locomotor stimulant, but 5-APDB and 5-MAPB were weak stimulants.
-
•
6-APB, 5-APDB and 5-MAPB produced MDMA-like discriminative stimulus effects.
-
•
6-APB, 5-APDB and 5-MAPB produced weak cocaine or methamphetamine-like effects.
-
•
Abuse liability of benzofurans may be similar to MDMA.
-
•
3 to 5 bullet points (maximum 85 characters, including spaces, per bullet point).
Keywords: MDMA, Methamphetamine, Cocaine, Rats, Drug discrimination
Abstract
Aims
Benzofurans are used recreationally, due their ability to cause psychostimulant and/or entactogenic effects, but unfortunately produce substantial adverse effects, including death. Three benzofurans 5-(2-aminopropyl)-2,3-dihydrobenzofuran (5-APDB), 5-(2-aminopropyl)-2,3-dihydrobenzofuran (5-MAPB) and 6-(2-aminopropyl) benzofuran (6-APB) were tested to determine their behavioral effects in comparison with 2,3-methylenedioxymethamphetamine (MDMA), cocaine, and methamphetamine.
Methods
Locomotor activity was tested in groups of 8 male Swiss-Webster mice in an open-field task to screen for locomotor stimulant or depressant effects and to identify behaviorally active doses and times of peak effect. Discriminative stimulus effects were tested in groups of 6 male Sprague-Dawley rats trained to discriminate MDMA (1.5 mg/kg), cocaine (10 mg/kg), or methamphetamine (1 mg/kg) from saline using a FR 10 for food in a two-lever operant task.
Results
In the locomotor activity test, MDMA (ED50 = 8.34 mg/kg) produced peak stimulant effects 60 to 80 min following injection. 5-MAPB (ED50 = 0.92 mg/kg) produced modest stimulant effects 50 to 80 min after injection, whereas 6-APB (ED50 = 1.96 mg/kg) produced a robust stimulant effect 20 to 50 min after injection. 5-APDB produced an early depressant phase (ED50 = 3.38 mg/kg) followed by a modest stimulant phase (ED50 = 2.57 mg/kg) 20 to 50 min after injection. In the drug discrimination tests, 5-APDB (ED50 = 1.02 mg/kg), 5-MAPB (ED50 = 1.00 mg/kg) and 6-APB (ED50 = 0.32 mg/kg) fully substituted in MDMA-trained rats, whereas only 5-MAPB fully substituted for cocaine, and no compounds fully substituted for methamphetamine.
Conclusions
The synthetic benzofuran compound 5-APDB and 5-MAPB produced weak locomotor effects, whereas 6-APB produced robust locomotor stimulant effects. All compounds were more potent than MDMA. All three compounds fully substituted in MDMA-trained rats suggesting similar subjective effects. Taken together, these results suggest that these benzofuran compounds may have abuse liability as substitutes for MDMA.
1. Introduction
Benzofurans are comprised of a benzene ring and a heterocyclic furan ring. A large number of substituted benzofurans have been created, many of which are psychoactive due to their structural similarity to amphetamines. A subset is similar in structure to 3,4-methylenedioxymethamphetamine (MDMA), having a furan ring with one oxygen atom rather than the methylenedioxy ring found in MDMA with two oxygens (Fig. 1). Placement of the oxygen and presence or absence of a double bond in the furan ring allows for several variants. In addition, these compounds can also be modified like amphetamines and cathinones by substitutions on the ethylamine chain (Monte et al., 1993).
Fig. 1.
Structures of MDMA and the benzofurans tested in the current study.
There has been increasing concern over the hazards of benzofurans when used recreationally, and three compounds have been flagged as of concern by the United States Drug Enforcement Administration (DEA). 5-APDB (also known as 3-desoxy-MDA, EMA-4), 6-APB, and 5-MAPB. 6-APB and 5-MAPB have been available for nearly a decade, appearing in national surveys, online and in the dance scene (Odoardi et al., 2016; Palamar et al., 2016; 2017; van der Gouwe et al., 2017). 5-MAPB has been found in drivers under the influence in Belgium Wille et al., 2018).
Toxicities have been reported, with a case of acute psychotomimetic effects in an individual taking 6-APB and marijuana (Chan et al., 2013), and three fatalities were associated with 6-APB combined with other substances (Seetohul et al., 2013). Signs of intoxication following oral administration of 5-MAPB included being pale, cold or hyperthermic, sweating, mydriasis, agitation, hallucinations, and convulsions (Hofer et al., 2017). Further, all three compounds produce hepatoxicity and oxidative stress in cell models (Roque Bravo et al., 2020; Nakagawa et al., 2017; 2018). The pharmacodynamics, kinetics, subjective effects, and toxicity in humans has been succinctly described by Roque Bravo et al. (2019).
There has been some limited study of the behavioral effects of these and related compounds. In an early study, several benzofurans were synthesized and their discriminative stimulus effects were tested (Monte et al., 1993). In that study, rats were trained to discriminate the entactogens N-methyl-1,3-benzodioxolylbutanamine (MBDB), 5‑methoxy-6-methyl-2-aminoindane (MMAI), the psychostimulant amphetamine or the hallucinogen lysergic acid diethylamide (LSD). 5-APDB and 6-APDB produced full substitution for both entactogens, but did not substitute for either amphetamine or LSD. Our laboratory previously tested two other benzofurans, 5-APB and 6-APDB (Dolan et al., 2017), along with 4-fluoroamphetamine which was often found in combination with these two compounds (Palamar et al., 2016; 2017; van der Gouwe et al., 2017; Salomone et al., 2016; Hondebrink et al., 2015). Similar to the earlier study, 6-APDB and 5-APB fully substituted for MDMA, but not cocaine, methamphetamine or the hallucinogen 2,5-dimethoxy-4-methylamphetamine (DOM) (Dolan et al., 2017). Finally, 5-APB was noted to produce conditioned place preference and limited self-administration. (Cha et al., 2016).
5-APDB and 6-APB act at monoamine transporters, inhibiting norepinephrine transporters (NET) and serotonin transporters (SERT) more than dopamine transporters (DAT), similar to MDMA (Rickli et al., 2015). In this study, both compounds released serotonin (5-HT), but 5-APDB also released norepinephrine (NE) and not dopamine (DA), whereas 6-APB released DA but not NE. Both compounds were found to bind to alpha1A and 2A adrenergic receptors, H1 histamine receptors, 5-HT1A, 5-HT2A, 2B and 2C serotonin receptors as well as the trace amine receptor (Rickli et al., 2015). Other studies reported that 6-APB had equal affinity at all three transporters and was a full agonist at 5-HT2B receptors (Iversen et al., 2013) or that 6-APB released NE and DA with equal potencies, but more than 5-HT (Brandt et al., 2020).
5-MAPB is a monoamine releaser, with potencies NET>DAT>SERT (Brandt et al., 2020). It also binds to DAT, slowing DA reuptake in the nucleus accumbens and reversing transport (Sahai et al., 2017). 5-MAPB caused increased 5-HT release in the corpus striatum, with resultant rise in body temperature and lethality (Fuwa et al., 2016). The authors suggested its toxic effects may be related to its metabolite, 5-APB. Similar to 5-APDB and 6-APB, 5-MAPB in highly non-selective, binding to monoamine transporters and acting with high efficacy, binding to 5-HT2A,B and C receptors with low efficacy activation, and binding to neuronal nicotinic ACh receptor α4β2 and alpha adrenergic receptors (Shimshoni et al., 2017).
The purpose of this study was to evaluate three synthetic benzofurans (5-APDB, 6-APB and 5-MAPB) to determine their preclinical potency and efficacy relative to the commonly abused psychostimulants cocaine and methamphetamine and to the club drug, MDMA. The locomotor activity test was used to evaluate whether compounds produce locomotor hyperactivity similar to that of the abused psychostimulants like cocaine and methamphetamine or to more serotonergic compounds like MDMA, and to rapidly identify the effective dose range and time course. The drug discrimination assay was used to evaluate whether compounds have interoceptive effects similar to that of MDMA, cocaine and methamphetamine, and this assay has been found to accurately predict human use (Horton et al., 2013). The DEA requested evaluation of these compounds for potential abuse liability due to their structural similarity to other abused and controlled psychostimulants and their toxic effects in recreational users.
2. Materials and methods
2.1. Subjects
Male Swiss-Webster mice (n = 200) and male Sprague-Dawley rats (n = 35) were purchased from Envigo (Indianapolis, IN) at approximately 2 months of age. All animals were allowed to acclimatize in the vivarium for about 2 weeks prior to behavioral testing. Mice were group housed (n = 4/cage) and allowed free access to food. Rats were housed individually, and weight was restricted to 320–350 g by limiting their access to food. All rats received a total of approximately 15 g of food per day including the food pellets they received during operant sessions.
Animals were maintained on a 12/12 light/dark cycle (lights on at 7:00 AM) and had free access to water. All housing and procedures were approved by the University of North Texas Health Science Center Institutional Animal Care and Use Committee and were in agreement with the guidelines set for the care and use of laboratory animals.
2.2. Locomotor activity
The study was conducted using Digiscan (Omnitech Electronics, Columbus, OH) locomotor activity testing chambers as previously described (Gatch et al., 2021). Separate groups of 8 mice were injected via the intraperitoneal route with either vehicle (0.9% saline) or a dose of MDMA (1, 2.5, 5, 12, 25, or 50 mg/kg), 6-APB (1, 2.5, 5, or 10 mg/kg), 5-APDB (1, 2.5, 5, 10, or 25 mg/kg), or 5-MAPB (0.5, 1, 2.5, 5, 10, or 25 mg/kg). Each compound was tested with a separate group of vehicle-injected controls.
Immediately following injection, each mouse was placed in a testing chamber for measurement of its horizontal locomotor activity (photocell beam interruption). Locomotor activity was recorded every 10-minutes to resolve time-related shifts in activity related to drug time course, and the test lasted 8 h to ensure capture of the full potential time course of the benzofurans for comparison to that of the abused drug standard, MDMA. The locomotor stimulant effects of MDMA and other abused psychostimulants yield inverted-U-shaped dose response curves. Accordingly, for accurate estimation of comparative time-course, potency and efficacy of each benzofuran, it was necessary to identify a relatively short time window after injection that could be inferred to contain the maximal locomotor response at the peak of the concentration time curve. To accomplish this, we began testing at 1 mg/kg and continued to test additional doses in separate groups of mice until the earliest time window could be identified during which: (i) an ascending dose response to a maximum was identified (ii) at least one dose had been tested on the descending limb of the dose-effect curve and (iii) the lowest dose tested was not significantly different from the vehicle control. For the highest dose of each compound tested, mice were observed for unusual behaviors within the chambers after approximately 30, 120 and 480 min of testing.
2.3. Discrimination procedures
Adult male Sprague-Dawley rats were trained to discriminate either MDMA (1.5 mg/kg, i.p.), methamphetamine (1 mg/kg, i.p.) or cocaine (10 mg/kg, i.p.) from 0.9% saline using a FR 10 schedule of food reinforcement (45 mg food pellets; Bio-Serve, Frenchtown, NJ) with a two-lever choice procedure. A reinforcer was available for every 10 responses on a designated injection-appropriate lever. The rats received approximately 60 of these 20-min sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 9 of 10 sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug-drug-saline-saline-drug, etc.). After the completion of training phase, substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test (drug-saline-test-saline-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions. Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA, Model E10–10) connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT) were used for testing.
The compounds 6-APB (0.1, 0.25, 0.5, 1 mg/kg), 5-APDB (0.25, 0.5, 1, 2.5 mg/kg), and 5-MAPB (0.05, 0.1, 0.25, 0.5 mg/kg) were tested for substitution in groups of six MDMA-trained rats. 6-APB (0.1, 0.25, 0.5, 1 mg/kg), 5-APDB (0.5, 1, 2.5, 5 mg/kg), and 5-MAPB (0.25, 0.5, 1, 2.5 mg/kg) were tested for substitution in groups of six methamphetamine-trained rats. 6-APB (0.25, 0.5, 1, 2.5 mg/kg), 5-APDB (0.5, 1, 2.5, 5, 10 mg/kg), and 5-MAPB (0.25, 0.5, 1, 2.5 mg/kg) were tested for substitution in groups of six cocaine-trained rats.
In contrast with training sessions, both levers were active, such that 10 responses on either lever led to reinforcement. Data were collected until all 20 reinforcers were obtained, or for a maximum of 20 min. A repeated-measures design was used, such that each rat was tested at all doses of a given drug, including vehicle and training-drug controls. The dose effect of each compound was tested from no effect to full effect or rate suppression ( < 20% of vehicle control) or adverse effects. Starting doses and pretreatment times were inferred from the locomotor activity testing. MDMA was administered 15 min prior to testing, 6-APB was administered 30 min before testing, 5-APDB was administered 50 min before testing, and 5-MAPB was administered 60 min before testing. The pretreatment times were determined based on locomotor activity data.
2.4. Drugs
(+)-Methamphetamine hydrochloride, (-)-cocaine hydrochloride, (±)-methylenedioxy-methamphetamine hydrochloride, 5-APDB hydrochloride (1-(benzofuran-5-yl)-N-methylpropan-2-amine, 6-APB hydrochloride (6-(2-aminopropyl)benzofuran), 5-MAPB hydrochloride (5-(2-aminopropyl)−2,3-dihydrobenzofuran) were all supplied by the National Institute on Drug Abuse Drug Supply Program. All compounds were dissolved in saline.
2.5. Data analysis
The time course of locomotor stimulant or depressant effects at each dose was evaluated by plotting the mean horizontal activity counts (labeled on graphs as Ambulation Counts) as a function of 10-min periods. Time-related effects on ambulation counts after different doses were confirmed by significance of the two-way interaction of Dose and Time within ANOVAs (with Dose as a between-groups factor and 10-min Period as a within-subjects factor). Potency and efficacy of each compound were estimated independently of time course using the average ambulation counts/10-min within the earliest 30-minute time window containing the ascending dose-response and the maximal effect, excluding doses on the descending limb. For depressant effects, dose-response data were analyzed within the earliest 30-min time period during which maximal depression was evident. Dose-response data were considered in a one-way ANOVA for each compound, including all doses and the vehicle control, followed by planned individual comparisons of each dose against the vehicle control group. Efficacy of each compound for locomotor stimulation was estimated by ambulation counts at the dose producing the largest effect minus the mean of the vehicle control group, for the 30-min window of the dose response analysis. The stimulant effect magnitude over time for each compound was determined by calculating the area under the curve (AUC) for locomotor activity counts (minus vehicle control counts) across the full 8-h session for the dose with maximal efficacy. The benzofurans and MDMA were compared within separate one-way ANOVAs on the measures of efficacy and stimulant effect magnitude over time (AUC). Statistical significance was set at p < .05.
For the drug discrimination data, the mean percentage of drug-lever responses and response rate were calculated, and these measures were plotted against dose using a log scale. The percentage of drug-lever responding data were not considered if less than three rats completed the first fixed ratio. Full substitution was defined as mean percent drug-lever responding ≥ 80%. Rates of responding were expressed as the number of responses divided by the total session time (in seconds). Response rate data were analyzed by one-way repeated measures ANOVA. The mean response rate after each dose was compared to that of the vehicle control value using planned comparisons.
Linear regressions of the linear portion of the dose-response data were performed on the pooled data for each compound, and potencies were calculated (ED50 ± SE) using OriginGraph (OriginLab Corporation, Northampton, MA). Locomotor activity potencies were based on 50% of the maximum effect of the given drug whereas drug discrimination potencies were based on 50% drug-appropriate responding. Potency was compared among the benzofurans and MDMA using one way ANOVAs and individual comparisons.
3. Results
3.1. Locomotor activity
Time course data for 4–5 doses per compound are depicted in Fig. 2. Some doses are omitted for clarity. Dose-response data for the time window of maximal effect are depicted in Fig. 3. An ANOVA comparing maximal effects, corrected for differences in baseline (vehicle control), indicated a significant overall effect F(3,28) = 16.196, p < 001. The maximal effects of 6-APB and 5-MAPB were not statistically different from MDMA. However, the maximal effect of 5-APDB was lower than those of the other compounds and the maximal effect of 6-APB was higher than that of both 5-APDB and 5-MAPB. The rank order of stimulant potency was 5-MAPB > 6-APB ≥ 5-APDB > MDMA. There was a significant effect when considering the total magnitude of effect over time (AUC), F(3,28) = 20.44, p < .001. The rank order of the compounds for AUC of the maximal dose was: 6-APB >> MDMA ≥ 5-MAPB > 5-APDB.
Fig. 2.
Time course of locomotor stimulant effects. Average horizontal activity counts/10 min (ambulation counts) as a function of time and dose for 6-APB, 5-APDB, and 5-MAPB. Each panel shows the effects of one dose of compound versus the vehicle; n = 8 for each dose. The shaded bars indicate the earliest 30-minute time window during which: (i) an ascending dose response to a maximum was identified and (ii) at least one dose had been tested on the descending limb of the dose-effect curve. * Indicates stimulant effects (p < .05) against vehicle control within the time period indicated by the shaded bars. show times of peak stimulant and/or depressant effects, as defined as the ambulation counts at the dose yielding the largest stimulant effect (or largest depressant effect) during the 30-min when effects were first seen at the lowest effective dose.
Fig. 3.
Locomotor activity dose effect. Average horizontal activity counts/10 min (ambulation counts) at the time of maximal effect (as defined as the ambulation counts at the dose yielding the largest stimulant effect during the 30-min when effects were first seen at the lowest effective dose) as a function of dose for 6-APB, 5-APDB, 5-MAPB, and MDMA. n = 8 for each dose.
MDMA (ED50 = 8.34 ± 0.08 mg/kg) produced locomotor stimulation lasting 150 min beginning approximately 60–80 min following injection of 5 or 10 mg/kg. Stimulant effects of 25 and 50 mg/kg began earlier, within the first 10 min after injection. Locomotor stimulation after 25 mg/kg MDMA occurred continuously for approximately 4 h, whereas the stimulant effect of 50 mg/kg MDMA was attenuated during the period of 70–100 min, but thereafter stimulated activity for most of the remaining session. The time- and dose-related effects of MDMA contributed to a significant interaction of [Treatment F(6,46) = 11.30, p < .001, 10-Min Periods F(47,2162) = 31.83, p < .001, and the interaction of Periods and Treatment F(282,2162) = 2.39, p < .001] when considered in a two-way ANOVA. The period 70–100 min was selected for analysis of dose-response for potency and efficacy based on the appearance of an inverted U-shaped dose response and maximal stimulant effect during this window. Planned individual comparisons within one-way ANOVA on these data [Treatment F(6,46) = 10.0, p < .001] indicated a significant stimulant effect for 5, 10 and 25 mg/kg. Maximum stimulant effects of 4145 ± 728 counts were observed following 25 mg/kg. Lethality occurred in 3 of 8 mice following 50 mg/kg MDMA. Data from these mice were not included in the analysis.
5-MAPB (ED50 = 0.92 ± 0.10 mg/kg, Fig. 2) produced time- and dose-dependent stimulation of locomotor activity beginning approximately 50 min after injection for the dose range of 1 to 10 mg/kg. These effects lasted 80–220 min, whereas the effect of 25 mg/kg began later (after 80 min) and continued for the remainder of the testing period. This pattern resulted in a significant interaction of Periods and Treatment F(282,2303) = 4.25, p < .001] when considered in the two-way ANOVA. The period 50–80 min was selected for analysis of dose-response for potency and efficacy based on the appearance of an inverted U-shaped dose response and maximal stimulant effect during this window. A locomotor stimulant effect was observed following 1 to 10 mg/kg [F(6,49) = 4.03, p = .002], with a maximal effect of 2912 ± 273 counts following 5 mg/kg that was 70% of the maximal effect of MDMA. It is noteworthy, however, that the stimulant effect of 25 mg (which occurred after the dose response analysis window) was greater than the maximal effect at 5 mg/kg.
Treatment with 5-APDB resulted in both stimulation (ED50= 2.47 ± 0.1 mg/kg) and depression (ED50= 3.38 ± 0.05 mg/kg) of locomotor activity. Depressant effects of 5 to 25 mg/kg occurred within 10 min following injection and lasted 10–30 min. The depression of locomotor activity at these doses was followed by stimulation beginning 40 min after injection. The stimulant effect durations were dose-dependent, lasting from 80 min to 3 h. The stimulant/depressant pattern of time course for this compound yielded a significant interaction of Periods and Treatment F(235,1974) = 1.94, p < .001 when all data were considered in a two-way ANOVA.
The period 0–30 min was selected for analysis of dose-response for the depression effect of 5-APDB, whereas 50–80 min was used for consideration of stimulant potency and efficacy. ANOVA for each effect indicated a main effect of treatment (F ≥ 4.06, p < .005), and individual comparisons indicated a significant difference from vehicle for 5, 10, and 25 mg/kg. The maximal stimulant effect (2136 ± 273 counts) occurred following 5 mg/kg. This effect was 51% of the maximal stimulant effect of MDMA.
Treatment with 6-APB (ED50 = 1.96 ± 0.06 mg/kg) resulted in time- and dose-dependent stimulation of locomotor activity beginning within 10 min following 2.5 and 5 mg/kg, and after 2 h following 10 mg/kg. The duration of these effects was approximately 5 h. These effects yielded a significant interaction of Periods and Treatment [F(188,1645) = 9.53, p < .001] when considered in a two-way ANOVA.
Maximal locomotor stimulant effects and an inverted U-shaped dose response was evident during the 20–50 min time window following 6-APB, with a maximal effect of 6196 ± 342 counts following 5 mg/kg. A one-way ANOVA for data within this time period indicated a significant treatment effect [F(4,35) = 20.70, p < .001] driven by significant stimulant effects of 2.5 and 5 mg/kg 6-APB. The maximal effect of 6-APB was 149% of the maximal effect of MDMA.
3.2. Drug discrimination
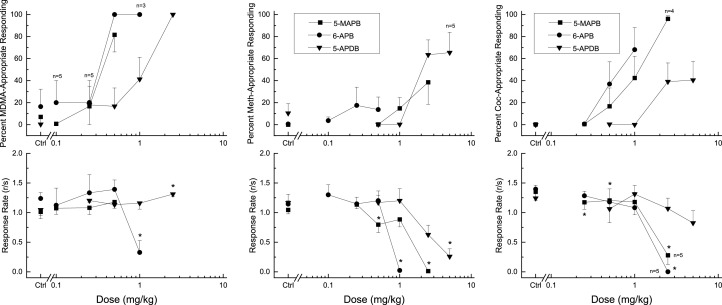
6-APB (ED50=0.32 ± 0.04 mg/kg), 5-APDB (ED50=1.02 ± 0.07 mg/kg) and 5-MAPB (ED50=0.33 ± 0.08 mg/kg) fully substituted for the discriminative stimulus effects of 1.5 mg/kg ±-MDMA (Fig 4). The dose of 6-APB that fully substituted (1 mg/kg) decreased response rate F(4,20) = 4.85, p = .007. 5-APDB and 5-MAPB did not alter rate of responding at the doses tested.
Fig. 4.
Substitution for the discriminative stimulus effects of 6-APB, 5-APDB, and 5-MAPB in rats trained to discriminate MDMA, methamphetamine, or cocaine from saline. The top graph in each panel shows the percentage of total responses made on the drug-appropriate lever. The bottom graph shows the rate of responding in responses per second (r/s). n = 6 for each compound, except where indicated. Ctrl indicates vehicle and training drug controls for each test compound. * indicates rate effects (p < .05) against vehicle control.
6-APB, 5-APDB and 5-MAPB failed to fully substitute for the discriminative stimulus effects produced by 1 mg/kg of (+)-methamphetamine (Fig 4). 6-APB produced maximum methamphetamine-appropriate responding of 18% following 0.25 mg/kg, and 1 mg/kg nearly completely suppressed responding F(4,20) = 36.30, p > .001. 5-APDB produced a peak of 66% following 5 mg/kg, which substantially decreased response rate F(4,28) = 10.58, p < .001. 5-MAPB produced peak methamphetamine-appropriate responding of 39% following 1 mg/kg. A higher dose, 2.5 mg/kg, suppressed responding F(4,20) = 28.95, p = < 0.001 and produced exophthalmos in all 6 rats.
5-MAPB (ED50 = 1.00 ± 0.08 mg/kg) fully substituted for the discriminative stimulus effects of 10 mg/kg cocaine (Fig 4). The dose of 5-MAPB that fully substituted (2.5 mg/kg) decreased response rate F(4,16) = 12.93, p < .001 and produced exophthalmos in 4 of 5 rats. One rat died before the end of the study and did not receive the 2.5 mg/kg dose. 6-APB, 5-APDB and failed to fully substitute for the discriminative stimulus effects produced by 10 mg/kg of cocaine. 6-APB produced maximum cocaine-appropriate responding of 68% following 1 mg/kg, and a higher dose (2.5 mg/kg) completely suppressed responding F(4,16) = 8.75, p < .001. The 2.5 mg/kg dose of 6-APB produced decreased muscle tone (4/5 rats), excessive salivation (2/5 rats) and convulsions (1/5 rats). Only 5 rats were tested at 2.5 mg/kg due to the adverse effects and response suppression. 5-APDB produced a peak of 41% following 5 mg/kg, and a higher dose (2.5 mg/kg) completely suppressed responding F(5,20) = 15.47, p < .001. The following were observed in 5/5 rats after 10 mg/kg 5-APDB: Decreased muscle tone, exophthalmos, salivation and piloerection.
4. Discussion
The benzofurans 5-APDB and 5-MAPB produced weak locomotor stimulant effects similar to those of MDMA, with rapid onset depressant effects at low doses, followed by slower-onset stimulant effects at higher doses. 5-APDB had a stronger depressant effect and a smaller and shorter-acting stimulant effect than MDMA or 5-MAPB. Adjusted for baseline, the peak effect of 5-MAPB was not different from MDMA. However, the peak effect of 5-APDB was lower than those of the other test compounds. The rank order of stimulant potency was 5-MAPB > 6-APB ≥ 5-APDB > MDMA. In contrast, 6-APB produced robust, long-lasting locomotor stimulant effects similar to those produced by methamphetamine in earlier studies (Gatch et al., 2019, Gatch et al., 2021; Shetty et al., 2023). Potency of 6-APB was greater than methamphetamine in those studies. The peak effect of 6-APB was higher than that of both 5-APDB and 5-MAPB. Although the present study demonstrated that 6-APB was more potent and had a longer duration of action compared to MDMA, it is worth noting that its efficacy was not significantly higher than MDMA.
The effects of 6-APB are comparable to those of 5-APB, which was also an efficacious and long-acting locomotor stimulant as observed in a previous study (Dolan et al., 2017). However, 5-MAPB, a structurally similar compound to 5-APB, with an additional methyl group at the N position, produced weaker locomotor stimulant effects compared to both 5-APB or 6-APB. In contrast, 6-APDB was an efficacious locomotor stimulant in an earlier study (Dolan et al., 2017), whereas 5-APDB in the current study produced a significant depressant effect during the initial phase of the time course.
All three compounds, namely 6-APB, 5-APDB and 5-MAPB exhibited MDMA-like discriminative stimulus effects, but not methamphetamine-like discriminative stimulus effects. Only one of the three (5-MAPB) produced cocaine-like discriminative stimulus effects. These observations are similar to those of a previous study (Dolan et al., 2017), in which 6-APDB and 5-APB fully substituted for MDMA, but not for cocaine, methamphetamine or DOM. However, in a study by Monte et al., (2003) it is should be noted that 5-APDB substituted for amphetamine (Monte et al., 1993). Taken together, these findings suggest that the benzofurans tested so far have discriminative effects that are more MDMA-like rather than psychostimulant-like.
As discussed earlier, benzofurans have structures similar to that of MDMA and hence their effects on monoaminergic systems are largely similar to MDMA. In a previous study, the investigators reported a relative potency of transporter inhibition in the following order NET>SERT>DAT (Rickli et al., 2015). Additionally, MDMA and benzofurans like 5-APB, 6-APB, 5-APDB and 6-APDB were reported to bind to serotonin receptors, alpha adrenergic receptors, H1 histamine receptors and trace amine receptor (TA1) (Iversen et al., 2013; Rickli et al., 2015). The strong selectivity of some benzofurans to SERT over DAT suggests that these compounds will produce MDMA-like entactogenic effects in humans. Strong psychostimulants like methamphetamine have a greater affinity for DAT over SERT resulting in higher dopamine-mediated effects (Gannon et al., 2018; Simmler et al., 2013; 2014). Therefore, non-selectivity of the benzofurans at DAT would explain the weak psychostimulant-like discriminative stimulus effects. Nevertheless, 5-APDB did produce full substitution for amphetamine (Monte et al., 1993) and 5-MAPB produced full substitution for cocaine in the present study.
Due to the strong locomotor stimulant effects of 5-APB, 6-APB and 6-APDB, it would be expected that these three compounds would have the most DAT activity. 5-APB and 6-APB did show the highest potency for DAT inhibition and 6-APDB, 6-APB and 5-APB produced significant amounts of DA release (Rickli et al., 2015). In another study, 6-APB had relative potencies of inhibition of DAT>NET>SERT (Brandt et al., 2020). However, 6-APDB showed low potency for DAT inhibition (Rickli et al., 2015) and 5-APB was selective for SERT and NET over DAT in another study (Brandt et al., 2020). The weak locomotor stimulant effects of 5-APDB were predicted by its relative potency of monoamine transporter inhibition, NET≥SERT>>DAT. The decreased muscle tone, exophthalmos, salivation and piloerection produced by 5-APDB, and 6-APB may be due to their activity at adrenergic or serotonergic receptors.
5-MAPB also acts at monoamine transporters, with relative inhibition potencies NET>DAT>SERT and acts as a releaser (Brandt et al., 2020; Sahai et al., 2017). Similar to the other benzofurans, 5-MAPB binds not only to the monoamine transporters, but to a wide variety of receptors, including 5-HT2A,B and C receptors, neuronal nicotinic acetylcholine (ACh) receptor α4β2, and alpha adrenergic receptors (Shimshoni et al., 2017). Interestingly, MDMA was also found to bind to the α4β2 nicotinic ACh receptor in that study. 5-MAPB has serotonergic effects, increasing 5-HT release in corpus striatum, with a resultant rise in body temperature and fatality (Fuwa et al., 2016). Taken together, it is not surprising that 5-MAPB was a weak locomotor stimulant and produced very little methamphetamine-like discriminative stimulus effects. The exophthalmos observed during testing may be the result of 5-MAPB's cholinergic or adrenergic activity.
In summary, the benzofurans 5-APDB and 5-MAPB produced weak locomotor stimulant effects, whereas 6-APB produced robust locomotor stimulant effects. All three compounds produced MDMA-like discriminative stimulus effects, but not methamphetamine-like discriminative stimulus effects. Only one of the three (5-MAPB) produced cocaine-like discriminative stimulus effects. These findings suggest that these compounds may be used recreationally as entactogens and less as psychostimulants. Adverse effects at doses that produce maximal discriminative stimulus effects suggest may increase their risk of use. The unpleasant adrenergic- or serotonergic-like effects of 5-APDB and 6-APB may discourage user interest, and the possibility of convulsant effects of 6-APB may increase risk of harm.
Author Contribution
RDH and RAS conducted experiments, searched the literature, and wrote initial drafts of the manuscript.
MJF and MBG designed the experiments.
RDH, NS and MBG analyzed the data.
RDH and MBG wrote the final version of the manuscript. All authors approved the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Michael J. Forster reports financial support was provided by National Institute on Drug Abuse Division of Therapeutics and Medical Consequences.
Acknowledgements
Funding Sources: N01DA-18–8936.
References
- Brandt S.D., Walters H.M., Partilla J.S., Blough B.E., Kavanagh P.V., Baumann M.H. The psychoactive aminoalkylbenzofuran derivatives, 5-APB and 6-APB, mimic the effects of 3,4-methylenedioxyamphetamine (MDA) on monoamine transmission in male rats. Psychopharmacology (Berl) 2020;237(12):3703–3714. doi: 10.1007/s00213-020-05648-z. PMC7686291 PMID: 32875347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha H.J., Lee K.W., Eom J.H., Kim Y.H., Shin J., Yun J., Han K., Kim H.S. 5-(2-Aminopropyl) benzofuran and phenazepam demonstrate the possibility of dependence by increasing dopamine levels in the brain. Pharmacol. Biochem. Behav. 2016;149:17–22. doi: 10.1016/j.pbb.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Chan W.L., Wood D.M., Hudson S., Dargan P.I. Acute psychosis associated with recreational use of benzofuran 6-(2-aminopropyl)benzofuran (6-APB) and cannabis. J. Med. Toxicol. 2013;9(3):278–281. doi: 10.1007/s13181-013-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan S.B., Forster M.J., Gatch M.B. Discriminative stimulus and locomotor effects of para-substituted and benzofuran analogs of amphetamine. Drug Alcohol Depend. 2017;180:39–45. doi: 10.1016/j.drugalcdep.2017.07.041. PMID:28865391, PMC6463889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuwa T., Suzuki J., Tanaka T., Inomata A., Honda Y., Kodama T. Novel psychoactive benzofurans strongly increase extracellular serotonin level in mouse corpus striatum. J. Toxicol. Sci. 2016;41(3):329–337. doi: 10.2131/jts.41.329. [DOI] [PubMed] [Google Scholar]
- Gannon B.M., Baumann M.H., Walther D., Jiminez-Morigosa C., Sulima A., Rice K.C., Collins G.T. The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology. 2018;43:2399–2407. doi: 10.1038/s41386-018-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M.B., Dolan S.B., Forster M.J. Locomotor activity and discriminative stimulus effects of five novel synthetic cathinone analogs in mice and rats. Drug Alcohol Depend. 2019;199:50–58. doi: 10.1016/j.drugalcdep.2019.02.016. PMID: 30986635, PMCID: PMC6534427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M.B., Shetty R.A., Sumien N., Forster M.J. Behavioral effects of four novel synthetic cathinone analogs in rodents. Addict. Biol. 2021;26:e12987. doi: 10.1111/adb.12987. PMID: 33155384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer K.E., Faber K., Müller D.M., Hauffe T., Wenger U., Kupferschmidt H., Rauber-Lüthy C. Acute toxicity associated with the recreational use of the novel psychoactive benzofuran N-methyl-5-(2 aminopropyl)benzofuran. Ann. Emerg. Med. 2017;69(1):79–82. doi: 10.1016/j.annemergmed.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Hondebrink L., Nugteren-van Lonkhuyzen J.J., Van Der Gouwe D., Brunt T.M. Monitoring new psychoactive substances (NPS) in The Netherlands: data from the drug market and the poisons information centre. Drug Alcohol Depend. 2015;147:109–115. doi: 10.1016/j.drugalcdep.2014.11.033. [DOI] [PubMed] [Google Scholar]
- Iversen L., Gibbons S., Treble R., Setola V., Huang X.P., Roth B.L. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 2013 Jan 30;700(1–3):147–151. doi: 10.1016/j.ejphar.2012.12.006. PMID: 23261499 PMCID: PMC3582025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte A.P., Marona-Lewicka D., Cozzi N.V., Nichols D.E. Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy) amphetamine. J. Med. Chem. 1993;36:3700–3706. doi: 10.1021/jm00075a027. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Suzuki T., Inomata A. Preventive effects of fructose and N-acetyl-l-cysteine against cytotoxicity induced by the psychoactive compounds N-methyl-5-(2-aminopropyl)benzofuran and 3,4-methylenedioxy-N-methamphetamine in isolated rat hepatocytes. J Appl Toxicol. 2018;38(2):284–291. doi: 10.1002/jat.3523. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Suzuki T., Tada Y., Inomata A. Cytotoxic effects of psychotropic benzofuran derivatives, N-methyl-5-(2-aminopropyl)benzofuran and its N-demethylated derivative, on isolated rat hepatocytes. J. Appl. Toxicol. 2017;37(3):243–252. doi: 10.1002/jat.3351. [DOI] [PubMed] [Google Scholar]
- Odoardi S., Romolo F.S., Strano-Rossi S. A snapshot on NPS in Italy: distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013-2015. Forensic Sci. Int. 2016;265:116–120. doi: 10.1016/j.forsciint.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Palamar J.J., Salomone A., Vincenti M., Cleland C.M. Detection of "bath salts" and other novel psychoactive substances in hair samples of ecstasy/MDMA/"Molly" users. Drug Alcohol Depend. 2016;161:200–205. doi: 10.1016/j.drugalcdep.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar J.J., Salomone A., Gerace E., Di Corcia D., Vincenti M., Cleland C.M. Hair testing to assess both known and unknown use of drugs amongst ecstasy users in the electronic dance music scene. Int. J. Drug Policy. 2017;48:91–98. doi: 10.1016/j.drugpo.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A., Kopf S., Hoener M.C., Liechti M.E. Pharmacological profile of novel psychoactive benzofurans. Br. J. Pharmacol. 2015;172(13):3412–3425. doi: 10.1111/bph.13128. PMID: 25765500 PMCID: PMC4500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque Bravo R., Carmo H., Carvalho F., Bastos M.L., Dias da Silva D. Benzo fury: a new trend in the drug misuse scene. J. Appl. Toxicol. 2019;39(8):1083–1095. doi: 10.1002/jat.3774. [DOI] [PubMed] [Google Scholar]
- Roque Bravo R., Carmo H., Silva J.P., Valente M.J., Carvalho F., Bastos M.L., Dias da Silva D. Emerging club drugs: 5-(2-aminopropyl)benzofuran (5-APB) is more toxic than its isomer 6-(2-aminopropyl)benzofuran (6-APB) in hepatocyte cellular models. Arch. Toxicol. 2020;94(2):609–629. doi: 10.1007/s00204-019-02638-9. [DOI] [PubMed] [Google Scholar]
- Sahai M.A., Davidson C., Khelashvili G., Barrese V., Dutta N., Weinstein H., Opacka-Juffry J. Combined in vitro and in silico approaches to the assessment of stimulant properties of novel psychoactive substances - The case of the benzofuran 5-MAPB. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;75:1–9. doi: 10.1016/j.pnpbp.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Salomone A., Gazzilli G., Di Corcia D., Gerace E., Vincenti M. Determination of cathinones and other stimulant, psychedelic, and dissociative designer drugs in real hair samples. Anal. Bioanal. Chem. 2016;408(8):2035–2042. doi: 10.1007/s00216-015-9247-4. [DOI] [PubMed] [Google Scholar]
- Seetohul L.N., Pounder D.J. Four fatalities involving 5-IT. J. Anal. Toxicol. 2013;37(7):447–451. doi: 10.1093/jat/bkt053. [DOI] [PubMed] [Google Scholar]
- Shetty R.A., Hoch A.C., Sumien N., Forster M.J., Gatch M.B. Comparison of locomotor stimulant and drug discrimination effects of four synthetic cathinones to commonly abused psychostimulants. J. Psychopharmacol. 2023;37:520–528. doi: 10.1177/02698811221142566. [DOI] [PubMed] [Google Scholar]
- Shimshoni J.A., Winkler I., Golan E., Nutt D. Neurochemical binding profiles of novel indole and benzofuran MDMA analogues. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390(1):15–24. doi: 10.1007/s00210-016-1297-4. [DOI] [PubMed] [Google Scholar]
- Simmler L.D., Buser T.A., Donzelli M., Schramm Y., Dieu L.H., Huwyler J., Chaboz S., Hoener M.C., Liechti M.E. Pharmacological characterization of designer cathinones in vitro. Brit. J. Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L.D., Rickli A., Hoener M.C., Liechti M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- van der Gouwe D., Brunt T.M., van Laar M., van der Pol P. Purity, adulteration and price of drugs bought on-line versus off-line in the Netherlands. Addiction. 2017;112(4):640–648. doi: 10.1111/add.13720. [DOI] [PubMed] [Google Scholar]
- Wille S.M.R., Richeval C., Nachon-Phanithavong M., Gaulier J.M., Di Fazio V., Humbert L., Samyn N., Allorge D. Prevalence of new psychoactive substances and prescription drugs in the Belgian driving under the influence of drugs population. Drug Test Anal. 2018;10(3):539–547. doi: 10.1002/dta.2232. [DOI] [PubMed] [Google Scholar]