Abstract
Sodium/glucose cotransporter 2 (SGLT2) inhibitors have demonstrated a class effect in improving serum magnesium levels in patients with diabetes. Additionally, recent reports have shown their promising beneficial effects in the treatment of refractory hypomagnesemia in patients with diabetes. However, their role in treating hypomagnesemia in patients without diabetes remains unexplored. Here, we report 4 cases of severe and refractory hypomagnesemia that showed dramatic improvement after initiating SGLT2 inhibitors in patients without diabetes. Case 1 had calcineurin inhibitor-associated severe hypomagnesemia. Cases 2, 3, and 4 had refractory hypomagnesemia associated with platinum-based chemotherapy with or without gastrointestinal losses. Case 1 was able to withdraw from high-dose oral magnesium supplementation. Cases 2 and 3 achieved independence from intravenous magnesium supplementation, whereas case 4 had decreased intravenous magnesium requirements. All the cases demonstrated sustainably improved serum magnesium levels. Withdrawal of SGLT2 inhibitors in case 4 resulted in worsening serum magnesium levels and intravenous magnesium requirements. The extraglycemic benefit of this group of medications not only suggests the need for further studies to better understand the effect of SGLT2 inhibitors on magnesium homeostasis but also supports expanded use in a larger patient population.
Index Words: SGLT2 inhibitors, empagliflozin, dapagliflozin, hypomagnesemia, magnesium, type 2 diabetes
Introduction
Sodium/glucose cotransporter 2 (SGLT2) inhibitors, which initially emerged as a treatment option for patients with type 2 diabetes, have also been shown to improve clinical outcomes in patients with a variety of heart and kidney diseases with or without diabetes.1 The beneficial effect of SGLT2 inhibitors on magnesium balance in patients with diabetes with or without hypomagnesemia has been noted as a class effect in recent meta-analysis data from randomized clinical trials.2, 3, 4 Moreover, some reports have demonstrated their role in the treatment of refractory hypomagnesemia in patients with diabetes with or without overt urinary magnesium wasting.5,6 However, their role in the treatment of hypomagnesemia in patients without diabetes remains unexplored. Here, to the best of our knowledge, we report the first series of 4 patients without diabetes with severe hypomagnesemia successfully treated with SGLT2 inhibitors.
Case Report
Case 1
A woman in her 50s with a history of orthotropic liver transplantation developed severe hypomagnesemia accompanied by calcineurin inhibitor use (tacrolimus trough levels of 8-10 ng/mL), which required aggressive oral and intravenous magnesium supplementation. After reducing her tacrolimus trough levels to 4-6 ng/mL 4 months after transplantation, she achieved independence from intravenous magnesium sulfate (IV MgSO4) supplementation but remained dependent on a high-dose regimen of oral magnesium (Table 1). Further work up showed a 24-hour magnesium urine level of 90 mg with a fractional excretion of magnesium (FEMg) of 9.73%. Later, empagliflozin 10 mg daily was initiated and increased to 25 mg daily after 2 months. Her oral magnesium supplements were completely withdrawn 2 months after empagliflozin was initiated, and serum magnesium levels remain stable without any further episodes of hypomagnesemia at 5 months of follow-up. Repeat FEMg was reduced to 4.88% after the administration of empagliflozin (Table 1).
Table 1.
Baseline Characteristics, Metabolic Profile and Changes Observed in Patients Treated with SGLT2 Inhibitors
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Sex, age (ye) | Female, 50s | Female, 70s | Male, 50s | Female, 50s |
| Comorbid conditions | Alcohol-associated cirrhosis of liver post orthotropic liver transplantation, hypertension, hypothyroidism, gastroesophageal reflux disease, and chronic kidney disease | Stage IIIC high-grade serous ovarian cancer, hypertension, and hypothyroidism | Left supraglottic cancer, depression, hypothyroidism, and osteoarthritis | Breast cancer, vulvo-vaginal atrophy, migraine, gastroesophageal reflux disease, irritable bowel syndrome, adrenal insufficiency, and lumbar spinal stenosis |
| Cause of hypomagnesemia | CNI | Platinum-based chemotherapy and/or GI events | Platinum-based chemotherapy and/or GI events | Platinum-based chemotherapy and/or GI events |
| Therapy for hypomagnesemiaa | Maximum tolerated oral magnesium [magnesium L-lactate dihydrate sustained-release caplets (Mag-Tab SR) 84 mg 4 caplets twice a d (a total of 672 mg of elemental magnesium per d)] | Maximum tolerated oral magnesium (gluconate 500 mg 3 tablets twice a d [a total of 162 mg of elemental magnesium per d] and magnesium sulfate 2 g IV 3 times per wk), amiloride 10 mg twice a d | Maximum tolerated oral magnesium (magnesium oxide 400 mg 2 tablets twice a d [a total of 964 mg of elemental magnesium per d]), intermittent magnesium sulfate 2-4 g IV, amiloride 10 mg twice a d | Maximum tolerated oral magnesium (magnesium 84 mg extended-release tablets twice a d), eplerenone 25 mg daily, magnesium sulfate 2 g IV 3 times a wk |
| SGLT2 inhibitor | Empagliflozin 25 mg/d | Dapagliflozin 10 mg/d | Dapagliflozin 10 mg/d | Dapagliflozin 10 mg/d |
| Pre-SGLT2 inhibitor | Post-SGLT2 inhibitor | Pre-SGLT2 inhibitor | Post-SGLT2 inhibitor | Pre-SGLT2 inhibitor | Post-SGLT2 inhibitor | Pre-SGLT2 inhibitor | Post-SGLT2 inhibitor | |
|---|---|---|---|---|---|---|---|---|
| FBS (mg/dL) | 101 | 98 | 90 | 83 | 99 | 104 | 80 | NA |
| Potassium (mEq/L) | 4.4 | 4.4 | 4.2 | 4.6 | 3.3 | 4.1 | 4.1 | 4.0 |
| Calcium (mg/dL) | 10.3 | 10.4 | 8.8 | 8.9 | 9.6 | 8.9 | 9.2 | 9.7 |
| Albumin (gm/dL) | 4.2 | 4.4 | 4.3 | 3.7 | 4.1 | 4.1 | 3.9 | 4.0 |
| Creatinine (mg/dL) | 1.36 | 1.40 | 0.7 | 0.63 | 0.73 | 0.70 | 0.85 | 0.84 |
| 24-h urine output (mg) | 90 | 56 | NA | NA | NA | NA | NA | NA |
| FEMg | 9.73% | 4.88% | NA | NA | NA | NA | NA | NA |
Note: Factional excretion of magnesium (FEMg) was calculated as 100 (uMg sCr)/(0.7 sMg uCr), where uMg and uCr represent urinary magnesium and creatinine concentrations measured in 24-hour urine collections, respectively, and sMg and sCr represent serum magnesium and creatinine levels, respectively.
Abbreviations: CNI, calcineurin inhibitor; FBS, fasting blood sugar; GI events, gastrointestinal events; NA, not available; SGLT2, sodium/glucose cotransporter 2.
Details of medications pre- and post-SGLT2 inhibitor use can be found in Table S1.
Case 2
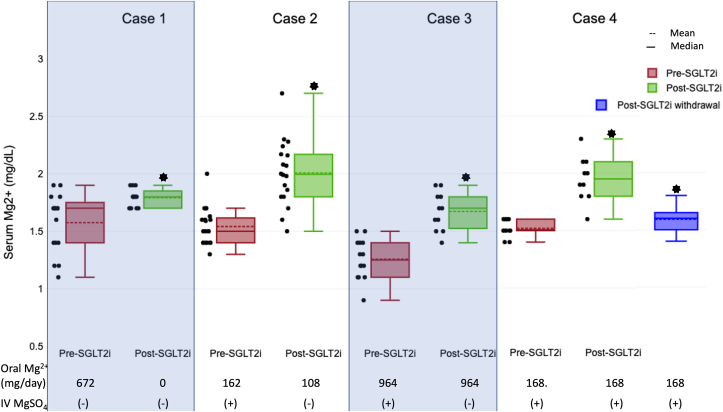
A woman in her 70s presented with a history of locally advanced serous ovarian cancer status post debulking procedure, including radical abdominal hysterectomy with bilateral salpingo-oophorectomy and diverting loop ileostomy. She received chemotherapy with docetaxel and carboplatin. Following chemotherapy, the patient developed multiple electrolyte abnormalities, including hypokalemia and hypomagnesemia, that partially improved after ostomy closure. She continued to have refractory hypomagnesemia requiring 2 g of IV MgSO4 3 times a week for 9 months in addition to maximum tolerated doses of oral magnesium supplements and amiloride (Table 1). Later, she was started on dapagliflozin with normalization of serum magnesium levels within 3 weeks and remained off intravenous supplementation at 10 months of follow-up (Fig 1).
Figure 1.
Sodium/glucose cotransporter 2 (SGLT2) inhibition was associated with increased serum magnesium levels in case 1 (1.60 ± 0.24, n=15 vs 1.79 ± 0.079, n=12; P = 0.02), case 2 (1.54 ± 0.16, n=20 vs 2.0 ± 0.28, n=18; P < 0.001), case 3 (1.25 ± 0.17, n=14 vs 1.67 ± 0.16, n=11; P < 0.001) and case 4 (1.52 ± 0.07, n=10 vs 1.95 ± 0.19, n=10; P <0.001). SGLT2 inhibition was associated with a decrease in oral magnesium supplementation in case 1 (672 mg vs 0 mg) and case 2 (162 mg vs 108 mg), allowed withdrawal from intravenous magnesium sulfate (IV MgSO4) dependence in case 2 and case 3, and decreased the need for IV MgSO4 supplementation in case 4. Withdrawal of SGLT2 inhibition in case 4 was associated with a decrease in serum magnesium levels (1.95 ± 0.19, n=10 vs 1.59 ± 0.10, n=12; P < 0.001) and an increase in requirements for IV MgSO4 (12 gm vs 20 gm IV MgSO4 at 1 month pre- vs post-SGLT2i withdrawal, respectively). Abbreviations: IV MgSO4, intravenous magnesium sulfate; SGLT2i, sodium-glucose cotransporter 2 inhibitor; oral Mg, oral elemental magnesium.
Case 3
A man in his 50s with left supraglottic cancer was treated with cisplatin, 5-fluorouracil, and pembrolizumab. Since chemotherapy, he was noted to have hypokalemia and hypomagnesemia, which failed to improve with amiloride. He remained dependent on oral and intravenous magnesium supplementation for refractory hypomagnesemia requiring over 60 g of IV MgSO4 over 2 months (Table 1). The patient was started on dapagliflozin, which normalized serum magnesium levels off intravenous magnesium supplementation with at least 2 months of follow-up (Fig 1).
Case 4
A woman in her 50s with a history of breast cancer developed severe hypomagnesemia after treatment with carboplatin, which was refractory to amiloride, eplerenone and maximally tolerated oral magnesium supplements requiring IV MgSO4 3 times per week over 4 years. Later, she was started on dapagliflozin with sustainably improved serum magnesium levels with decreased IV MgSO4 requirement (approximately 1-2 times per week) over 1 month of follow-up. Dapagliflozin had to be stopped after 1 month because of severe vaginal fungal infection resulting in a decline in serum magnesium levels and increased requirements for IV MgSO4 (Fig 1).
Discussion
SGLT2 inhibitors have emerged as potential treatment options for hypomagnesemia in patients with type 2 diabetes. Data analysis performed on randomized controlled trials has demonstrated increased serum magnesium levels with SGLT2 inhibitors as a class effect in patients with type 2 diabetes compared with placebo.2, 3, 4 In 2020, Ray et al5 reported the beneficial effect of SGLT2 inhibitors in refractory hypomagnesemia in 3 patients with diabetes and overt urinary magnesium wasting. Later, we reported a similar effect of SGLT2 inhibitors in 2 patients with diabetes without overt urinary magnesium wasting.6 Of note, both the patients in this series demonstrated dramatic effects in the correction of refractory hypomagnesemia with SGLT2 inhibitors without significant changes in glycemic control. Here, to the best of our knowledge, we report the first series of 4 patients without diabetes with the substantial beneficial effect of SGLT2 inhibitors in the treatment of severe hypomagnesemia.
The etiology of hypomagnesemia in case 1 appears to be overt urinary magnesium wasting secondary to calcineurin inhibitors (FEMg 9.73%, Table 1). Calcineurin inhibitors are known to cause hypomagnesemia secondary to the downregulation of Mg2+ transport proteins, transient receptor potential cation channel subfamily M, member 6 (TRPM6), responsible for transcellular magnesium transport in the distal convoluted tubules of the kidney epithelium.7 For cases 2, 3, and 4, the etiology of hypomagnesemia could be urinary magnesium wasting due to the use of platinum-based chemotherapy. However, extrakidney losses because of gastrointestinal events are also possible and cannot be ruled out as the 24-hour urine magnesium levels were unavailable.
Several mechanisms can explain the beneficial effect of SGLT2 inhibitors on magnesium balance in patients with or without diabetes. Inhibition of the electrogenic sodium-glucose cotransporter with SGLT2 inhibitors would increase the intraluminal electrical potential in proximal tubules and drive passive paracellular magnesium reabsorption.8 However, only a relatively small amount of Mg2+ is reabsorbed in this segment.9 One may speculate that increased Na+ delivery to the thick ascending limb of the loop of Henle with resulting increased activity of the Na+/K+/2Cl− cotransporter (NKCC2) may increase transepithelial membrane potential in the thick ascending limb and drive passive paracellular Mg2+ reabsorption.9 However, 3 studies failed to show increased NKCC2 expression with SGLT2 inhibitor use but instead showed a trend toward decreased expression.10, 11, 12 Additionally, this mechanism fails to explain their beneficial effects on magnesium homeostasis in patients without a reduction in fractional excretion of Mg2+, as we reported previously.6 Hence, improved magnesium levels secondary to changes in luminal electrical potential provide an unsatisfactory or at least inadequate explanation. A study on an animal model of metabolic syndrome showed that dapagliflozin enhanced TRPM6-mediated transepithelial magnesium transport in kidney tubule cells.13 The mechanism of how SGLT2 inhibitors affect TRPM6 channels remains an area of further investigation. The binding of insulin to its receptors results in the increased insertion of TRPM6 in the plasma membrane.14 Thus, improved insulin resistance could be a potential mechanism. However, their beneficial effects in patients without significant change in glycemic control, as reported previously, and among the patients without diabetes, as reported here, make this an insufficient explanation.6
To explain the beneficial effects of SGLT2 inhibitors in nondiabetic patients, we speculate that potential mechanisms could include increased glucagon and arginine vasopressin secretion. SGLTs play a central role in pancreatic alpha cell function.15,16 They mediate sodium influx in the alpha cells, and their inhibition can result in improved intracellular pH and increased glucagon secretion. Increased glucagon levels, either due to a direct effect on alpha cells or indirectly in response to urinary glucose wasting, can lead to increased Mg2+ reabsorption in distal convoluted tubules.16,17 While the presence of SGLT1 is more consistently detected in pancreatic alpha cells, the same is not true for SGLT2.15 Because SGLT2 expression on alpha cells is highly variable among individuals and even in islets of the same individuals, some individuals/islets respond to SGLT2 inhibitors, whereas others are less responsive or unresponsive.16 Similar to in vitro experiments, studies have shown opposing effects of SGLT2 inhibitors: undereuglycemic hyperinsulinemic conditions, glucagon has been reported to either increase or remain unchanged.18, 19, 20 This finding may explain why the dramatic beneficial effect of SGLT2 inhibitors on hypomagnesemia may not be universally witnessed in all patients. Further studying the beneficial effect of selective SGLT2 inhibitors compared to nonselective SGLT1/2 inhibitors on magnesium homeostasis may help elucidate this better. Similarly, osmotic diuresis by SGLT2 inhibition can stimulate vasopressin, which in turn, can lead to increased Mg2+ reabsorption and improved serum magnesium levels.17,21 Studies performed under a controlled laboratory environment are necessary to understand these better.
The patient in case 4 developed a severe vaginal infection, likely predisposed by her history of vulvovaginal atrophy. Genital mycotic infections have been reported to be significantly increased (relative risk, 3.57; confidence interval, 3.14-4.06) with the use of SGLT2 inhibitors in a meta-analysis of large placebo-controlled trials.22 Patient education, including proper daily hygiene and monitoring for early signs of infection, are of utmost importance.
Although invented as a new class of glucose-lowering medications, the beneficial effects of SGLT2 inhibition extend beyond glycemic control. Extra glycemic beneficial effects of SGLT2 inhibitors have been reported to include improvements in blood pressure, body weight, uric acid concentrations, sleep apnea, and liver steatosis along with a significant reduction in the rate of cardiovascular events and improved kidney outcomes.23 Indeed, evidence supports that improved magnesium balance is also an important extraglycemic benefit of this group of medications. The improved magnesium balance as a class effect can, in part, also help explain the all-cause and cardiovascular mortality benefits observed with these medications.24 Our findings support grounds for further studies to better understand the effect of SGLT2 inhibitors on magnesium homeostasis and propose expanding their use in a larger patient population.
SGLT2 inhibitors can be considered for treatment of severe hypomagnesemia in patients without diabetes. Further studies to understand their effect on hypomagnesemia and magnesium homeostasis are needed.
Article Information
Authors’ Full Names and Academic Degrees
Chintan V. Shah, MD, Nour Hammad, MD, Bhavna Bhasin-Chhabra, MD, Arash Rashidi, MD.
Support
None.
Financial Disclosure
Dr Rashidi, MD is a member of the speaker bureau for AstraZeneca. The remaining authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from each patient reported in this article for publication of the information about him/her that appears within this Case Report and any associated supplementary material.
Peer Review
Received March 25, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form May 21, 2023.
Footnotes
Complete author and article information provided before references.
Table S1: Medications Pre- and Post-SGLT2 Inhibitor Use.
Supplementary Material
Table S1.
References
- 1.Brown E., Wilding J.P.H., Alam U., Barber T.M., Karalliedde J., Cuthbertson D.J. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53(1):2072–2089. doi: 10.1080/07853890.2020.1841281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang H., Zhang X., Zhang J., et al. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59(12):2546–2551. doi: 10.1007/s00125-016-4101-6. [DOI] [PubMed] [Google Scholar]
- 3.Toto R.D., Goldenberg R., Chertow G.M., et al. Correction of hypomagnesemia by dapagliflozin in patients with type 2 diabetes: a post hoc analysis of 10 randomized, placebo-controlled trials. J Diabetes Complications. 2019;33(10) doi: 10.1016/j.jdiacomp.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Huan Y., Leibensperger M., Seo B., Song Y. Comparative effects of sodium-glucose cotransporter 2 inhibitors on serum electrolyte levels in patients with type 2 diabetes: a pairwise and network meta-analysis of randomized controlled trials. Kidney360. 2022;3(3):477–487. doi: 10.34067/KID.0006672021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray E.C., Boyd-Shiwarski C.R., Liu P., Novacic D., Cassiman D. SGLT2 inhibitors for treatment of refractory hypomagnesemia: a case report of 3 patients. Kidney Med. 2020;2(3):359–364. doi: 10.1016/j.xkme.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah C.V., Robbins T.S., Sparks M.A. Sodium-glucose cotransporter 2 inhibitors and management of refractory hypomagnesemia without overt urinary magnesium wasting: a report of 2 cases. Kidney Med. 2022;4(10) doi: 10.1016/j.xkme.2022.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nijenhuis T., Hoenderop J.G., Bindels R.J. Downregulation of Ca(2+) and Mg(2+) transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol. 2004;15(3):549–557. doi: 10.1097/01.asn.0000113318.56023.b6. [DOI] [PubMed] [Google Scholar]
- 8.Kokko J.P. Proximal tubule potential difference. Dependence on glucose on glucose, HCO3, and amino acids. J Clin Invest. 1973;52(6):1362–1367. doi: 10.1172/JCI107308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Baaij J.H.F. Magnesium reabsorption in the kidney. Am J Physiol Renal Physiol. 2023;324(3):F227–F244. doi: 10.1152/ajprenal.00298.2022. [DOI] [PubMed] [Google Scholar]
- 10.Chen L., LaRocque L.M., Efe O., Wang J., Sands J.M., Klein J.D. Effect of dapagliflozin treatment on fluid and electrolyte balance in diabetic rats. Am J Med Sci. 2016;352(5):517–523. doi: 10.1016/j.amjms.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung S., Kim S., Son M., et al. Empagliflozin contributes to polyuria via regulation of sodium transporters and water channels in diabetic rat kidneys. Front Physiol. 2019;10:271. doi: 10.3389/fphys.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma C., de Baaij J.H.F., Millar P.J., et al. Effect of dapagliflozin treatment on the expression of renal sodium transporters/channels on high-fat diet diabetic mice. Nephron. 2019;142(1):51–60. doi: 10.1159/000496617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng H.Y., Kuo W.H., Tain Y.L., Leung F.F., Lee W.C., Lee C.T. Effect of dapagliflozin and magnesium supplementation on renal magnesium handling and magnesium homeostasis in metabolic syndrome. Nutrients. 2021;13(11):4088. doi: 10.3390/nu13114088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gommers L.M.M., Hoenderop J.G.J., Bindels R.J.M., de Baaij J.H.F. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. 2016;65(1):3–13. doi: 10.2337/db15-1028. [DOI] [PubMed] [Google Scholar]
- 15.Armour S.L., Frueh A., Knudsen J.G. Sodium, glucose and dysregulated glucagon secretion: the potential of sodium glucose transporters. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.837664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodson D.J., Rorsman P. A variation on the theme: SGLT2 inhibition and glucagon secretion in human islets. Diabetes. 2020;69(5):864–866. doi: 10.2337/dbi19-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai L.J., Bapty B., Ritchie G., Quamme G.A. Glucagon and arginine vasopressin stimulate Mg2+ uptake in mouse distal convoluted tubule cells. Am J Physiol. 1998;274(2):F328–F335. doi: 10.1152/ajprenal.1998.274.2.F328. [DOI] [PubMed] [Google Scholar]
- 18.Merovci A., Solis-Herrera C., Daniele G., et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrannini E., Muscelli E., Frascerra S., et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundkvist P., Pereira M.J., Kamble P.G., et al. Glucagon levels during short-term SGLT2 inhibition are largely regulated by glucose changes in patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(1):193–201. doi: 10.1210/jc.2018-00969. [DOI] [PubMed] [Google Scholar]
- 21.Masuda T., Muto S., Fukuda K., et al. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol Rep. 2020;8(2) doi: 10.14814/phy2.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuffield Department of Population Health Renal Studies Group SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. doi: 10.1016/S0140-6736(22)02074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonora B.M., Avogaro A., Fadini G.P. Extraglycemic effects of SGLT2 inhibitors: a review of the evidence. Diabetes Metab Syndr Obes. 2020;13:161–174. doi: 10.2147/DMSO.S233538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odutayo A., da Costa B.R., Pereira T.V., et al. Sodium-glucose cotransporter 2 inhibitors, all-cause mortality, and cardiovascular outcomes in adults with type 2 diabetes: a Bayesian meta-analysis and meta-regression. J Am Heart Assoc. 2021;10(18) doi: 10.1161/JAHA.120.019918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.