Abstract
Rationale & Objective
The development of new therapies for autosomal dominant polycystic kidney disease requires clinical trials to be conducted efficiently. In this study, the factors affecting the recruitment and retention of participants enrolled in a 3-year randomized controlled trial in autosomal dominant polycystic kidney disease were investigated.
Study Design
Qualitative study.
Setting & Participants
All participants (N=187) were invited to complete a 16-item questionnaire at the final study visit of the primary trial. Participants were recruited to complete a semistructured interview using purposeful sampling according to age, self-reported gender, and randomization group.
Analytical Approach
Descriptive statistics were used for demographic data and questionnaires. The interview transcripts underwent inductive thematic coding.
Results
One hundred and forty-six of the 187 randomized participants (79%) completed the post-trial questionnaire, and 31 of the 187 participants (21%) completed the interview. Most participants (94%) rated their global satisfaction with the trial as high (a score of 8 or more out of 10). Altruism, knowledge gain, and access to new treatments were the main motivators for recruitment. The main reasons for considering leaving the study were concerns about the risk of intervention and family or work issues. Strategies that favored retention included flexibility in attending different study sites, schedule flexibility, staff interactions, and practical support with parking and reminders. The main burden was time away from work with lost wages, and burden associated with magnetic resonance imaging scans and 24-hour urine output collections.
Limitations
The study population was restricted to participants in a single nondrug clinical trial, and the results could be influenced by selection and possible social desirability bias.
Conclusions
Participants reported high levels of satisfaction that occurred as a function of the trial meeting participants’ expectations. Furthermore, retention was a balance between the perceived benefits and burden of participation. Consideration of these perspectives in the design of future clinical trials will improve their efficiency and conduct.
Plain-Language Summary
Advances in the clinical practice of autosomal dominant polycystic kidney disease (ADPKD) require affected individuals to voluntarily participate in long-term multicenter randomized controlled trials (RCTs). In this qualitative post hoc study of a 3-year RCT of increased water intake in ADPKD, altruism, knowledge gain, and access to a nondrug treatment positively influenced the decision to volunteer. Ongoing participation was enabled by building flexibility into the study protocol and staff prioritizing a participant’s needs during study visits. Although participants completed the required tests, most were considered burdensome. This study highlights the importance of incorporating protocol flexibility into trial design; the preference for interventions with a low risk of adverse effects; and the urgent requirement for robust surrogate noninvasive biomarkers to enable shorter RCTs in ADPKD.
Index Words: Autosomal dominant polycystic kidney disease, clinical trials, patient-centered care, participant experience, participant perspective, qualitative research, trial design
Current treatments for autosomal dominant polycystic kidney disease (ADPKD) are only partially effective, and thus randomized controlled trials are vital for developing new therapeutic advances.1 The timely recruitment of participants and maintaining their retention are 2 of the main key performance indicators that define the efficient conduct of a clinical trial.2 Although multiple factors are involved, the intrinsic and extrinsic motivators of participants, together with their overall experience during the study, are central to achieving this goal.3 The motivation for the general patient population to participate in research includes trust in their treating doctor, improving future medical care, and contributing to research.4 In general, this altruism and motivation apply to people affected by ADPKD who additionally have a desire to improve outcomes for both themselves and their families.5,6 Conversely, in clinical trials of chronic kidney disease, barriers to recruitment and retention include time commitments and the complexity of study procedures.7 Furthermore, participants fear the risks associated with an experimental intervention and being randomized to the placebo arm.7 Limited research on participant perspectives in ADPKD clinical trials has been undertaken, and therefore the aim of the current study was as follows: (i) to determine the experiences of participants in a long-term trial8; (ii) identify factors influencing reasons to enroll, remain, and adhere to trial procedures; and (iii) develop preliminary recommendations for improving future clinical trials.
Methods
Participant Selection and Recruitment
This was a prespecified sub-study of a 3-year randomized control trial that investigated the efficacy of increased water intake on the progression of ADPKD (PREVENT-ADPKD, ACTRN12614001216606) (2015-21), and published elsewhere.8,9 At the final study visit of the trial, all participants were invited to complete a post-trial questionnaire regarding their experiences. To complement these data, between February 2019, and June 2020, participants were also recruited for post-trial semistructured interviews. A standard purposeful sampling approach for qualitative studies10 was used for interview recruitment, and the stratification factors were age (greater or less than 45 years old), gender, and randomization group (intervention or control group). These demographic characteristics and trial-specific factors were selected by the investigators to ensure that maximal variation in responses could be obtained.10 Recruitment ceased when the number of participants in each stratification group reached at least 6. The study was approved by the Western Sydney Local Health District (WSLHD) Human Research Ethics Committee (HREC, AU RED HREC/14/WMEAD/414). The Consolidated Criteria for Reporting Qualitative (COREQ) studies was used to report this study.11
Data Collection
Questionnaire
The 16-item questionnaire was developed and modified from previous studies.12,13 The initial questionnaire was revised after the first 10 participants were recruited to incorporate themes that emerged from the first 5 semistructured interviews (Item S1). The dimensions of the questionnaire included factors hypothesized to influence participant recruitment (questions 1, 3, 4, 8, and 9) and retention (questions 2, 5, 6-7, and 10-15), and ranked the utility and difficulty of individual components.12 The questionnaire also included an overall global satisfaction score (1-10) (question 16) and a provision for free text comments. The questionnaire was provided in a paper format at the final study visit of the trial,8,9 and not all fields were mandatory.
Semistructured Interview
The interviews were conducted face-to-face (or by telephone within 2 weeks of their last study visit) by personnel with no previous contact with the participant during the trial. An interview guide was designed to complement the questionnaire and provide richer details on the trial experience (Item S2). The participants were asked about expectations and motivations before enrolling, adequacy of informed consent, overall experience, perceived adherence, individual study components, interactions with study staff, and recommendations for future trial designs. The interview guide was revised after the first 5 interviews to expand on follow-up questions and prompts for each theme. The interviews were audio-recorded, transcribed verbatim, and entered into QSR Nvivo 12 (QSR International Pty Ltd).
Data Analysis
The descriptive data from the questionnaire were entered and analyzed in Microsoft Excel. Univariate and multivariable analyses were performed using JMP Pro (version 16.2, SAS Institute). For analysis of the interview, a coding framework was created in QSR Nvivo 12 based on the main themes covered (Item S3). Inductive coding was used, whereby broad, higher-order codes were established to create a preliminary coding framework. Transcripts were read and coded line-by-line by 2 researchers (SA and IS) independently for first-round coding, and disagreements in coding decisions were reviewed. New distinct sub-themes were generated inductively, and miscellaneous themes were created to minimize early dismissal. Collapsing and redefining continued until no new themes emerged. Verbatim quotes that represented the essence of each major theme were selected from the interviews.
Results
Participant Characteristics
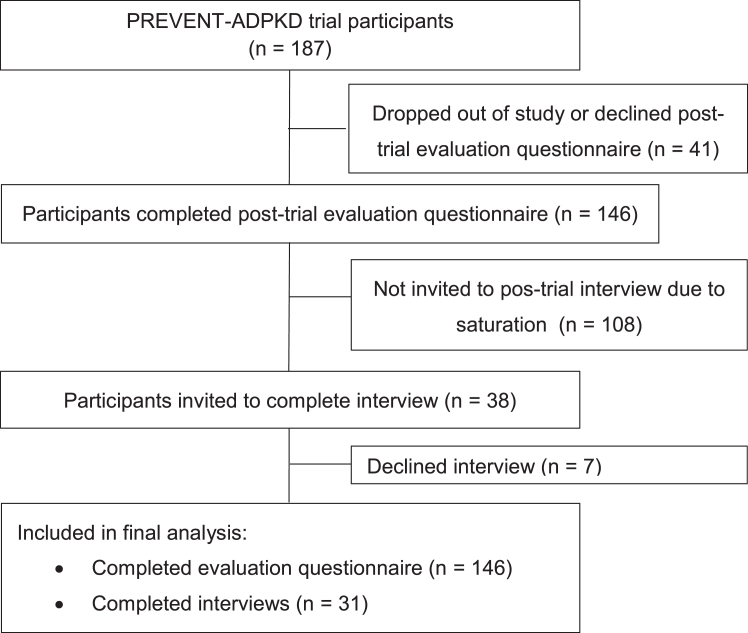
One hundred and forty-six of the total 187 participants (78.1%) randomized in the primary trial8 completed the questionnaire with equal numbers from each treatment arm (n=73 each) (Fig 1, Table 1). Thirty-eight of the 146 participants were invited to undertake the post-trial interview and 7 declined, leaving 31 who completed the interview (Fig 1). The demographic characteristics of the cohort are shown in Table 1 and were similar to those of the participants who did not complete the sub-study.
Figure 1.
CONSORT flow diagram showing recruitment of participants with ADPKD for the post-trial questionnaire and semistructured interview. As described in our previous publication8, 158 participants (n=81 in the water intake ad libitum group; n=77 in the prescribed water intake group; reasons for discontinuation of the trial are described in the previous publication). In the current study, an additional 12 participants declined to participate in the post-trial evaluation questionnaire. In other words: 92% (146/158) of participants who completed the initial randomized controlled trial (ie, the PREVENT-ADPKD clinical) agreed to undertake the post-trial questionnaire and 78% (146/187) of participants who were randomized in the initial randomized controlled trial were available for the post-trial questionnaire study.
Table 1.
Demographic and Participant Characteristics
| Characteristic | Parent Study Characteristics at Baseline | Questionnaire (n=146) | Interview (n=31) | |
|---|---|---|---|---|
| Age, n (%) | <45 y | – | 48 (33) | 12 (39) |
| ≥45 y | – | 98 (67) | 19 (61) | |
| Gender, n (%) | Male | 93 (50) | 75 (51) | 15 (48) |
| Female | 94 (51) | 71 (49) | 16 (52) | |
| Treatment Arm, n (%) | Control (A)a | 92 (50) | 73 (50) | 14 (45) |
| Intervention (B)b | 92 (50) | 73 (50) | 17 (55) | |
Usual water intake
Prescribed (increased) water intake
Quantitative Analysis of Factors Influencing Recruitment and Retention
General Characteristics
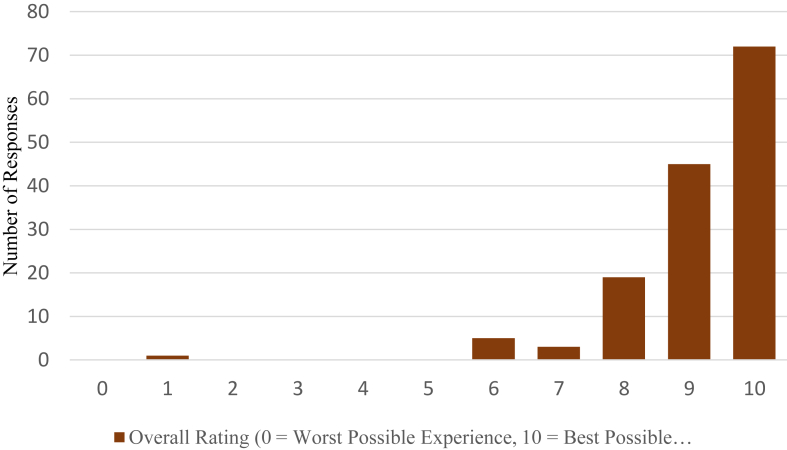
Only 14% (21/147) of participants had previously been involved in clinical research. Most participants (94%) rated their global satisfaction with the trial as high (8 or more out of 10) (Fig 2), and this was not correlated with age, gender, or treatment allocation. The majority felt valued as research partners and would recommend being involved in research to others (99.2% and 97.8%, respectively) (Fig S1).
Figure 2.
Global satisfaction score of participants’ self-reported assessment of their overall experience during 3-year clinical trial.
Factors Influencing Recruitment
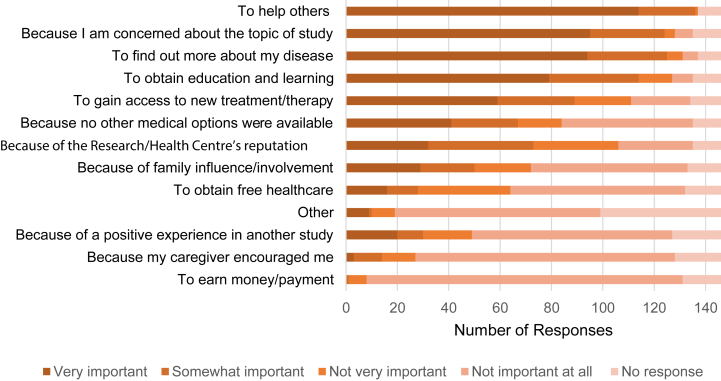
The main reasons to enroll in the primary trial related to altruism (to help others), knowledge gain (to find out more about my disease), and treatment benefit (to gain access to new treatment/therapy) (Fig 3). Conversely, participants rated financial incentive and encouragement from caregivers as not being important (Fig 3). The informed consent form and verbal discussions prepared most of the participants (91.3% and 92%, respectively) about what to expect in the study (Fig S2). During these initial discussions, 5.8% perceived pressure from the research staff to enroll (Fig S2). However, none of these factors were associated with the global satisfaction score.
Figure 3.
Reasons for participants to enroll in clinical trials of ADPKD.
Factors Influencing Retention
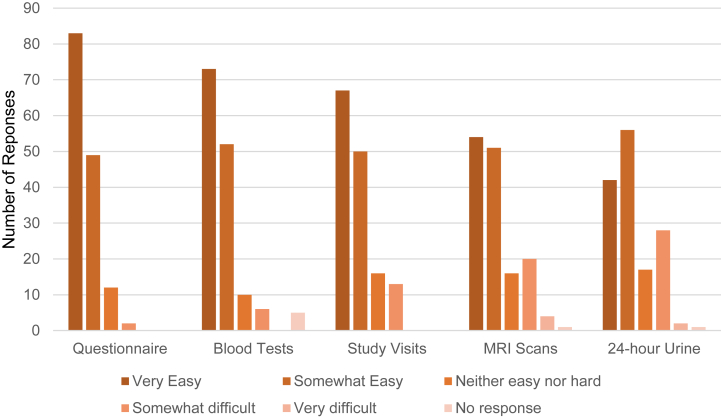
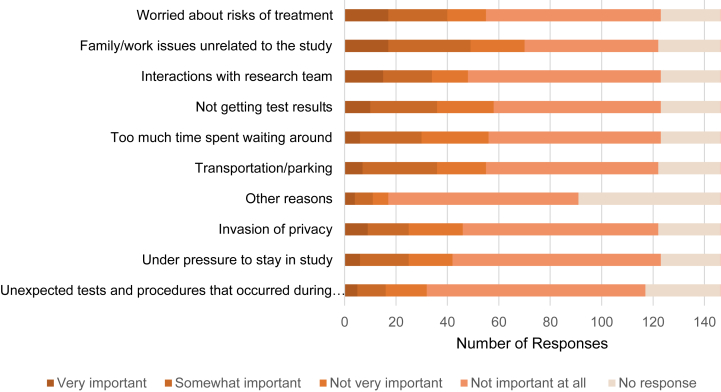
Participants ranked completing written questionnaires as the easiest study procedure, followed by undertaking blood tests, and study visits (Fig 4). The most difficult study procedures were completing the 24-hour urine output collections and magnetic resonance imaging (MRI) scans, which were rated as very difficult by only a couple of participants (2 and 4, respectively) (Fig 4). Of note, participants who ranked study procedures (blood tests, MRI scans, study visits, and questionnaires) as being easy to undertake were associated with a higher global satisfaction score (P < 0.05). In general, participants rated interactions with the research team, communication during the trial, and flexibility with study site location (as the primary trial was conducted at multiple geographic locations and participants could vary them to suit their preferences) as being positive. Fifty percent (73/146) of participants did not consider leaving the study. Of those that did consider withdrawal, most did not feel pressure from study staff to continue the trial. With reference to being involved in any clinical trial, the reasons participants would theoretically consider withdrawing were possible risks of intervention, personal or occupational issues, and if interactions with the research team were negative (Fig 5). Free text comments (Table S1) strengthened the points identified already (such as difficulty performing 24-hour urine output collections, importance of staff interactions, and flexibility of study visits).
Figure 4.
Participants’ rating of the ease of completing study procedures in clinical trials.
Figure 5.
Reasons for participants to consider withdrawing from clinical trials of ADPKD.
Qualitative Analysis of Factors Influencing Recruitment and Retention
Six themes emerged during the interview conducted with participants, as follows:
Previous Expectations
Some participants (7/31) had no previous expectations before commencing the clinical trial. For the others, 3 main themes emerged (Table S2) as follows:
Gain in Knowledge
First, it encompassed a personal desire to increase learning and general understanding of ADPKD, “my expectations was, well not much. Just hopefully find ways to improve myself, my health and see if it will help” (Male, 50s; participant #44). Second, participants wanted education on the clinical management of ADPKD, “when I'm done probably just a point in the right direction of what I should drink, how much I should drink, all that kind of stuff” (Male, 20s; participant #28).
Study Intervention
Participants had an expectation that the intervention showed a low risk of adverse effects compared with a drug: “it wasn’t a heavy drug and I already drink a lot of water… it didn’t seem that onerous or risky” (Female, 50s; participant #37).
Professionalism
Participants expected that the trial would be conducted according to high professional standards. An example quote is, “I guess just that it would be run professionally and that there would be certain things expected of me, but that they won’t be particular onerous” (Female, 30s; participant #2).
Motivations and Benefits of Participation
The motivation for recruitment aligned with the expected benefits of participation, which were divided into 3 main themes (Table S3).
Altruism, Organization, and Conscientiousness
Participants expressed altruism and a desire to find a cure. In addition, conscientiousness and organizational habits aided the decision to start and finish the trial: “I just wanted to help out. My mom passed away and I watched her die and I was approached if I'd help out, and after seeing that happen, I was just happy to do whatever needed to be done to help you guys with future patients.” (Female, 50s; participant #4).
Expected Personal and Family Health Benefits
Most participants also wanted to improve their own health: “I'm doing it for myself as much as I'm doing it for anyone else that has got this disease” (Male, 50s; participant #20). Moreover, the onus to participate arose from their family history and desire to reduce future family burden; “my ultimate goal was to help my children who have all got it” (Female, 50s; participant #19). Throughout the clinical trial, feedback on personal health and general education about ADPKD was also viewed as a benefit: “they explained it all well to me. I'm keen to learn more about what's going on in my body. So I'm pleased” (Male, 50s; participant #12).
Trust in Research
Finally, trust in research with a desire to create new knowledge was also viewed as an enabling factor for recruitment. The motivations comprised advancing medical research to improve treatment options: “knowing that my participation is going to hopefully help assist the medical field, you guys being able to work out how to better treat this disease” (Male, 50s; participant #12).
Participant Enablers and Barriers Affecting Completion of Study Procedures
Four factors were identified (representative quotations are shown in Table S4) as follows:
Variable External Support
Participants received variable support from family, friends, and workplaces: “I feel very positive about the level of support from my family, less positive about my workplace” (Female, 50s; participant #19).
Personal Availability to Complete Study Procedures
For most participants, the ability to complete study procedures was limited by external commitments from work, continuing education, and family. Some participants reported that their employer accommodated time required for the study, but others had to use “personal leave” (Female, 30s; participant #27).
General Time Requirements
Some participants described the trial as time-consuming, with the major burden being the loss of time from work and thus, lost wages: “It’s more about time and taking days off work, losing wages by coming here and doing all that stuff” (Male, 30s; participant #17). However, despite the burdens, in general, participants felt a personal responsibility, and along with the ease of completing individual study procedures, were stated as reasons to continue the trial, “I signed up for the inconvenience of coming here every six months and doing blood tests. I signed up for that. So in that sense, it was no inconvenience at all” (Male, 40s; participant #42).
External Situational Factors Leading to Consideration of Withdrawal
Participants reported situations when they considered withdrawal. In general, the situations were unrelated to the clinical trial, such as illness or work commitments. “I did [consider withdrawing] when my mother was very sick” (Male, 50s; participant #44).
Logistics of the Study Procedures
Participants provided several insights into factors that facilitated and hindered the completion of the study procedures. Representative quotations are shown in Table S5.
Access and Logistics of Study Procedures
The access and logistics of completing the study procedure, such as travel for study visits, pathology collection sites, and refrigerating the 24-hour urine output collection during work hours were described as being difficult and restrictive. For example, “the problem for me is that I live in…and so there’s three hours in travel or say five, five and half hours round trip travel there” (Male, 50s; participant #38) and “Christmas… it’s just such a busy, frantic time of year…so maybe it would be as awkward trying to do a 24 hour urine output collection and yeah, timing with menstruation” (Female, 50s; participant #43).
Multiple Locations for Study Visits
The availability of the clinical trial at multiple geographic locations within a city was identified as an enabler of completing the study visits. An illustrative quote: “there are a few hospitals available, so depending on where I was for work on a given day” (Female, 50s; participant #37).
Low Frequency of Procedures and Flexible Schedule
The routine nature and low frequency of the procedures with flexibility in appointment scheduling were deemed enablers, for example, “I think that there was just the pattern to it. So every couple of months you get a reminder, you’ve already got your envelope with the dates on it, it’s time to do the urine output collection and blood test. The MRIs came around less often, and I was always told when there was a change in the frequency” (Female, 50s; participant #37). The study schedule also allowed flexibility, which facilitated completion of trial procedures. “I had to do the collection a week late, everyone was fine with it. It was flexible so I didn’t have any issue” (Male, 30s; participant #28).
Adequate and Informative Consent
The participants reported positively about the dual nature of the consent process, with verbal and written information: “they explained it really well and it was all explained in the package and information that I got when I first started out” (Female, 50s; Participant #45).
Staff Support
The administrative support during the trial was considered an enabler of retention, particularly the provision of reminders and scheduling of appointments and the organization of parking. An illustrative quote is “very good in communicating. For example if there is any change, they use SMS or email and also near the visit day they will send reminder and all that. Yeah they take care of the, as I mentioned, the booking for parking spots and all that.” (Male, 60s; participant #3).
Interactions with Research Staff
Communication and interactions with the same research staff were considered important enablers of retention. Representative quotations are shown in Table S6.
Sensitive and Informative Communication
Most participants commented positively on the communication, approachability, and supportiveness of the research staff. The support was both for study procedures and emotional needs. It was commented that staff improved the participant’s experiences by overcoming hurdles and providing information in a sensitive manner and tailored to their personal circumstances. An illustrative quote: “Everyone was supportive and kind. I think when you’re dealing with a degenerative disease you have to be careful with the language that you use around decline of function and everyone was very sensitive to that” (Female, 30s; participant #27).
Staff Consistency
There was an equal split between a preference for the same or multiple research staff conducting the study interactions. The preference for the same person was based on the developing rapport and understanding: “Oh it was nice just to have the continuity… I think because you don’t have to establish rapport with a new person every single time, and there’s a history of understanding, and you kind of know how it works already and doesn’t change so you can pretty much pick up where you left off” (Female, 50s; participant #37). However, the participants who showed no preference reflected on the nature of research being team-based, “well, it’s research. Everyone’s learning and everyone’s sharing information, so I don’t have any preference. I would probably expect, one person to have all the information, easier. But with all the notes that one person left for the other, it’s not a problem” (Male, 60s; participant #12).
Nature of the Study Procedures
As identified in the quantitative analysis, the 24-hour urine output collection (difficult to complete, need for refrigeration, and return), MRI scan (claustrophobia and anxiety), blood tests, and completion of questionnaires (length and time required and frequency) were considered the most difficult by participants. Representative quotations are shown in Table S7. Participants were also asked to rate their perceived level of adherence. Of which, 71% stated they completed all the study procedures, 19% stated they completed the procedures most of the time, and 10% did not comment on their level of adherence.
Perspectives Regarding Future Clinical Trials
Additional suggestions to improve future clinical trial experiences included improving coordination of communication of study results to participants and their treating health care workers, providing of monetary incentives, increasing education opportunities about ADPKD, reducing the time commitment, improving the logistics of the 24-hour urine output collection, and reducing the length and complexity of questionnaires (Table S8). Factors that might affect future enrollment included the state of their personal health, time commitment required, the nature of the intervention, and potential risks. The desirable interventions for future trials are centered on lifestyle changes, medical and surgical treatments, and a miraculous cure (Table S9).
Discussion
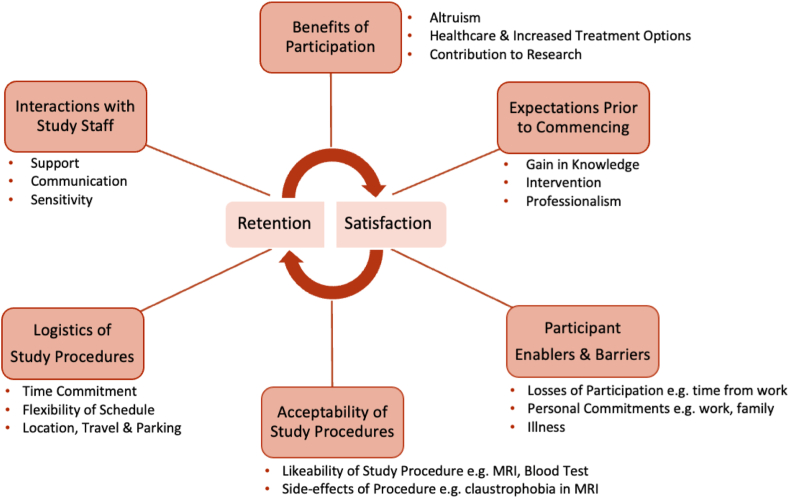
To our knowledge, the present study is one of the first detailed analyses of the perspectives of clinical trial participants in ADPKD. The main findings were as follows: (i) clinical trial participation was generally associated with high levels of satisfaction; (ii) altruism, knowledge gain, and enabling access to new treatments for the benefit of self and future generations were the factors that promoted recruitment; and (iii) retention was determined by perception of potential adverse effects of study intervention, nature of the study procedures, and time required. Taken together, fulfillment of participant expectations was linked with satisfaction, and retention was a balance between the perceived benefits and burden of undertaking study procedures. These findings are summarized in Fig 6.
Figure 6.
Thematic schema outlining the factors contributing to participant satisfaction and retention.
Clinical trials are a significant burden for participants, expensive (median cost US$3.4-21.4M for Phase 1-3 studies), and less productive when rare diseases are involved.14 Over the last 20 years, it is estimated that a total of 126 clinical trials in ADPKD involving 53,942 participants have been performed, and these have led to significant changes in clinical practice.1 In this study, many participants reported that the nature of the intervention and the potential side-effects would be primary considerations when deciding to enroll in future trials. Furthermore, factors driving a lack of satisfaction revolved around time commitments and the logistics of study procedures. The major burden was time commitment (0.5-2 hours per visit in the PREVENT-ADPKD trial) and lost hours from work, and participants stated this would influence their willingness to be involved in future trials. Strategies to aid retention included the ease of study procedures, a flexible schedule, and assistance with logistics (parking, booking appointments, and prepackaged information). These strategies have previously been shown to improve the study experience and increase retention, and they may be applicable depending on the time sensitivity of the measurements being collected and analyzed.7,15, 16, 17
Other factors influencing retention included research staff interactions and the type of study procedures required. As shown in this study, research staff are integral to the participant’s experience and are associated with high retention rates.16 This finding is consistent with trust in the research team and research institution being integral to participation in clinical trials, as showed in study populations with non-ADPKD (general), but the additional factors in participants with ADPKD include an interest in their disease and the desire to assist future generations.4, 5, 6 However, a cochrane review showed that nonblinded studies improve recruitment into randomized controlled trials.18 In our trial, it is likely that participants with ADPKD interested in nondrug therapies (such as water intake) would naturally be motivated to enroll in this trial, and this could influence their overall positive experience. In addition, we hypothesize that a key explanation could also be the involvement of a small research team that maintained continuity with all participants over the duration of the study. In this study, some participants found both 24-hour urine output collections and MRI scans to be a significant burden. Although financial incentives were not an important factor in facilitating enrollment, participants suggested the trial should be cost neutral and that there should be a way to compensate for time away from work.7,15, 16, 17
There are several strengths in this study. First, the questionnaire and interview guides have been used in previous studies.19 Second, representative sampling was used to recruit for the semistructured interview to capture diverse demographic characteristics and experiences. Third, the integration of both qualitative and quantitative methods provided a richer thematic understanding. Fourth, the interview and coding analyses were conducted by staff not directly involved in the primary trial, creating a space for open disclosure and reducing bias. The coding was also completed through an inductive method to allow new themes to emerge naturally.19 The key limitations were that the study population was confined to those that completed a single nondrug clinical trial in a high resource health care environment. The perceptions of participants who were not retained in the study are not reported in this study. Similarly, both the questionnaire and interviews required self-reporting, with potential for social desirability and recall bias.
In conclusion, this study has identified several interrelated factors that influence recruitment and retention in ADPKD clinical trials. On the basis of these findings, the design of future clinical trials in ADPKD should consider being shorter in duration (<3 years); incorporating flexible study schedules; prioritizing disease progression biomarkers that are assessed by blood tests and spot urine tests rather than 24-hour urine output collections and imaging end points (total kidney volume); developing protocols for sharing results with the primary care providers; simplifying the questionnaires; and developing strategies for participant access to both treatment arms. The co-design of studies with consumers and consideration of platform trials for evaluating the most promising therapeutic interventions will help achieve these outcomes.13,20,21
Article Information
Authors’ Full Names and Academic Degrees
Sneha Amin, MBBS, Irene Sangadi, MND, Margaret Allman-Farinelli, PhD, Sunil V. Badve, MBBS, PhD, Neil Boudville, MBBS, PhD, Helen Coolican, MA, Susan Coulshed, MBBS, Sheryl Foster, MHSc, Mangalee Fernando, MBBS, PhD, Imad Haloob, MBBS, PhD, David C.H. Harris, MD, Carmel M. Hawley, MBBS, PhD, Jane Holt, MBBS, Martin Howell, PhD, Karthik Kumar, MBBS, David W. Johnson, MBBS, PhD, Vincent W. Lee, MBBS, PhD, Jun Mai, MBBS, Anna Rangan, PhD, Simon D. Roger, MBBS, MD, Kamal Sud, MBBS, Vicente Torres, MD, PhD, Eswari Vilayur, MBBS, Gopala K. Rangan, MBBS, PhD
Authors’ Contributions
Research idea and study design: GKR, SA, IS; participant recruitment: MA-F, SVB, NB, HC, SC, SF, MF, IH, DCHH, CMH, JH, MH, KK, DWJ, VWL, JM, AR, SDR, KS, VT, EV, data acquisition: IS, SA, GKR; data analysis: SA, IS, GKR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The PREVENT-ADPKD study was an investigator-initiated multicenter randomized controlled trial funded by the National Health and Medical Research Council of Australia (No. 1138533), Danone Research, PKD Australia, the University of Sydney and the Westmead Hospital Medical Research Foundation. Dr David W. Johnson is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant.
Financial Disclosure
Dr David W. Johnson has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Health care and Fresenius Medical care, consultancy fees from Astra Zeneca, Bayer, and AWAK, speaker’s honoraria from ONO and Boehringer Ingelheim and Lilly, and travel sponsorships from Ono and Amgen. Dr Gopala K. Rangan reports research grants and travel sponsorship from Danone Research, Otsuka, and Sanofi. The remaining authors declare that they have no relevant financial interests.
Acknowledgments
The authors thank all study participants of the PREVENT-ADPKD study. The authors also thank Ms Carly Mannix, Dr Annette Wong, Ms Alexandra Munt, and Ms Jennifer Zhang for assistance with study visits and the development of interview guide.
Peer Review
Received on January 23, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form May 9, 2023.
Footnotes
Complete author and article information provided before references.
Figure S1: Participant responses to whether they felt valued as research partners and if they would recommend joining the study to others.
Figure S2: Participant responses regarding factors influencing recruitment (information and discussions, informed consent process, and pressure from research staff).
Item S1: PREVENT-ADPKD clinical trial participation questionnaire.
Item S2: PREVENT-ADPKD clinical trial participation interview guide.
Item S3: Coding framework.
Table S1: Question 16 Additional Comments from Questionnaire.
Table S2: Selected Participant Quotations of Expectations Before Enrollment.
Table S3: Selected Participant Quotations of Motivations and Benefits.
Table S4: Selected Participant Quotations of Participant Enablers and Barriers for Completion of Study Procedures.
Table S5: Selected Participant Quotations Regarding Logistics of Study Procedures.
Table S6: Selected Participant Quotations Regarding Clinical Trial Staff.
Table S7: Selected Participant Quotations Regarding Clinical Trial Procedures.
Table S8: Selected Participant Quotations Regarding Opportunities for Improvement.
Table S9: Selected Participant Quotations Regarding Future Direction of ADPKD Research.
Supplementary Material (PDF)
Figure S1-S2; Item S1-S3; Table S1-S9.
References
- 1.Rangan G.K., Raghubanshi A., Chaitarvornkit A., et al. Current and emerging treatment options to prevent renal failure due to autosomal dominant polycystic kidney disease. Exp Opinion Orph Drugs. 2020;8(8):285–302. [Google Scholar]
- 2.Jacques R.M., Ahmed R., Harper J., et al. Recruitment, consent and retention of participants in randomised controlled trials: a review of trials published in the National Institute for Health Research (NIHR) Journals Library (1997-2020) BMJ Open. 2022;12(2) doi: 10.1136/bmjopen-2021-059230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daykin A., Clement C., Gamble C., et al. ‘Recruitment, recruitment, recruitment’—the need for more focus on retention: a qualitative study of five trials. Trials. 2018;19(1):76. doi: 10.1186/s13063-018-2467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann F., Matzneller P., Weber M., et al. Perception of clinical research among patients and healthy volunteers of clinical trials. Eur J Clin Pharmacol. 2022;78(10):1647–1655. doi: 10.1007/s00228-022-03366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho Y., Sautenet B., Gutman T., et al. Identifying patient-important outcomes in polycystic kidney disease: an international nominal group technique study. Nephrology (Carlton) 2019;24(12):1214–1224. doi: 10.1111/nep.13566. [DOI] [PubMed] [Google Scholar]
- 6.Tran W.-C., Huynh D., Chan T., Chesla C.A., Park M. Understanding barriers to medication, dietary, and lifestyle treatments prescribed in polycystic kidney disease. BMC Nephrol. 2017;18(1):214. doi: 10.1186/s12882-017-0641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natale P., Gutman T., Howell M., et al. Recruitment and retention in clinical trials in chronic kidney disease: report from national workshops with patients, caregivers and health professionals. Nephrol Dial Transplant. 2020;35(5):755–764. doi: 10.1093/ndt/gfaa044. [DOI] [PubMed] [Google Scholar]
- 8.Rangan G.K., Wong A.T., Munt A., et al. Prescribed water intake in autosomal dominant polycystic kidney disease. NEJM Evid. 2022;1(1) doi: 10.1056/EVIDoa2100021. [DOI] [PubMed] [Google Scholar]
- 9.Wong A.T.Y., Mannix C., Grantham J.J., et al. Randomised controlled trial to determine the efficacy and safety of prescribed water intake to prevent kidney failure due to autosomal dominant polycystic kidney disease (PREVENT-ADPKD) BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-018794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palinkas L.A., Horwitz S.M., Green C.A., Wisdom J.P., Duan N., Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533–544. doi: 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong A., Sainsbury P., Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 12.Partridge S.R., Allman-Farinelli M., McGeechan K., et al. Process evaluation of TXT2BFiT: a multi-component mhealth randomised controlled trial to prevent weight gain in young adults. Int J Behav Nutr Phys Act. 2016;13(1):7. doi: 10.1186/s12966-016-0329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yessis J.L., Kost R.G., Lee L.M., Coller B.S., Henderson D.K. Development of a research participants’ perception survey to improve clinical research. Clin Transl Sci. 2012;5(6):452–460. doi: 10.1111/j.1752-8062.2012.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin L., Hutchens M., Hawkins C., Radnov A. How much do clinical trials cost? Nat Rev Drug Discov. 2017;16(6):381–382. doi: 10.1038/nrd.2017.70. [DOI] [PubMed] [Google Scholar]
- 15.Ross S., Grant A., Counsell C., Gillespie W., Russell I., Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 16.Abshire M., Dinglas V.D., Cajita M.I.A., Eakin M.N., Needham D.M., Himmelfarb C.D. Participant retention practices in longitudinal clinical research studies with high retention rates. BMC MDS Res Methodol. 2017;17(1):30. doi: 10.1186/s12874-017-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard N.R., Lester P., Rotheram-Borus M.J., Mattes K., Gwadz M., Ferns B. Successful recruitment and retention of participants in longitudinal behavioral research. AIDS Education and Prevention. 2003;15(3):269–281. doi: 10.1521/aeap.15.4.269.23827. [DOI] [PubMed] [Google Scholar]
- 18.Treweek S., Pitkethly M., Cook J., et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2(2) doi: 10.1002/14651858.MR000013.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elo S., Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 20.Vanderloo L.M., Vanderhout S.M., Tavares E., Maguire J., Straus S., Birken C.S. Parent engagement in co-design of clinical trials: the PARENT trial. Trials. 2021;22(1):347. doi: 10.1186/s13063-021-05305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotwal S., Perkovic V., Heerspink H.J.L. Platform clinical trials within nephrology-interpreting the evidence. Am J Kidney Dis. 2022;80(1):143–146. doi: 10.1053/j.ajkd.2022.01.430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S2; Item S1-S3; Table S1-S9.