Abstract
Adenosquamous proliferation (ASP) is known to occur in the central nidus of radial sclerosing lesions (RSL) of the breast. However, their significance is debated and remains largely unknown. In addition, there is a histologic overlap between ASP and low-grade adenosquamous carcinomas (LGASC).
We conducted a large retrospective review of 247 RSLs to evaluate the prevalence of ASP and quantitatively analyze associated histologic features of RSLs including size, stromal cellularity, and presence of chronic inflammation.
The central nidus of RSLs were classified as hyalinized in 121 cases (49%), cellular in 37 cases (15%), and equally mixed hyalinized and cellular in 89 (36%). ASP occurred in 92 of 247 RSLs (37.2%). Cases with ASP were significantly associated with a cellular stroma; 78.4% of RSLS with cellular stroma had ASP versus just 11.6% of hyalinized RSLs. In our large cohort, inflammation is commonly found in RSLs with ASP (p= <0.001).
In conclusion, we confirm that ASP is statistically more likely to be found in RSLs with a cellular stroma. In addition, ASP is commonly associated with chronic inflammation. The finding challenges the notion that prominent lymphocytes are a diagnostic clue to LGASC on limited biopsy material.
Keywords: Sclerosing lesions, Adenosquamous proliferation, Breast
Highlights
-
•
Adenosquamous proliferation is more likely to be found in radial sclerosing lesions with a cellular central nidus.
-
•
Adenosquamous proliferation is commonly associated with chronic inflammation.
1. Introduction
Radial sclerosing lesions (RSL) of the breast include radial scars, composed of a fibroelastotic core with radiating ducts in a stellate configuration, and complex sclerosing lesions (CSL), which are larger and more disorganized. Both lesions comprise entrapped benign glands showing various degrees of epithelial proliferation [1,2]. Commonly found in the central nidus of these lesions is the adenosquamous proliferation (ASP), defined as a benign proliferative lesion with squamous and glandular features [3]. ASP is not unique to RSL and has been observed in association with a variety of sclerosing breast lesions, including sclerosing papillomas [4].
While a recognized phenomenon, the significance of ASP remains elusive. From a practical standpoint, the ASP is significant because it histologically mimics the rare low-grade adenosquamous carcinoma (LGASC). LGASC of the breast was first described in 1987 in a case series by Rosen and Ernsberger [5]. LGASC is classified as a triple-negative breast carcinoma that paradoxically exhibits a favorable prognosis [6]. LGASCs comprise infiltrating small glands and squamous nests with low-grade nuclear features. The proportion of glands to squamous nests varies across cases. The histologic appearance of LGASC easily resembles a radial scar with ASP, especially in core needle biopsy (CNB) specimens where the key infiltrative nature of LGASC may be difficult to appreciate [6]. Further complicating assessment is the notable association of LGASC with RSLs, as first confirmed by Denley et al. [7]. Furthermore, immunohistochemistry is unreliable in distinguishing between these two entities, as both benign entrapped glands in RSLs and malignant glands in LGASC show ‘consistently inconsistent’ expression of myoepithelial markers [6,8]. Additionally, like LGASC, ASP is negative for estrogen and progesterone receptors and HER2 [4].
As a result of this morphologic overlap between ASP and LGASC, it has been proposed that ASP in RSLs actually represent a form of nascent LGASC, and that consequently the two are clonally related [9]. Indeed, a close relationship between ASP and LGASC occurs at the molecular level, as both ASP and LGASC contain PIK3CA mutations [[10], [11], [12]]. Therefore, based on concordance in histomorphology, shared location in RSLs, similar myoepithelial marker immunohistochemistry and identical PIK3CA mutations, a strong argument can be made that ASP is a direct precursor of LGASC. Some breast pathologists view ASP as preneoplastic lesions clonally related to LGASC, while others have described the distinction between ASP and LGASC as “artificial” [13].
In light of the current recommendations that radial scars and CSLs with radiology-pathology concordance and without atypia do not require excision [1,14,15], further evaluation of RSLs on CNBs and on resections could yield diagnostically helpful features that would enable accurate identification of ASP (requires no additional surgery) versus LGASC (complete excision required).
Additionally, the defining features of ASP in RSLs remain untested in a large series, because only relatively small case series have been reported [9]. We therefore conducted a large retrospective case series review of RSLs to determine ASP prevalence in RSLs, quantitatively analyze ASP histologic features and compare concordance of ASP features in CNBs and resections.
2. Materials and methods
2.1. Cohort selection
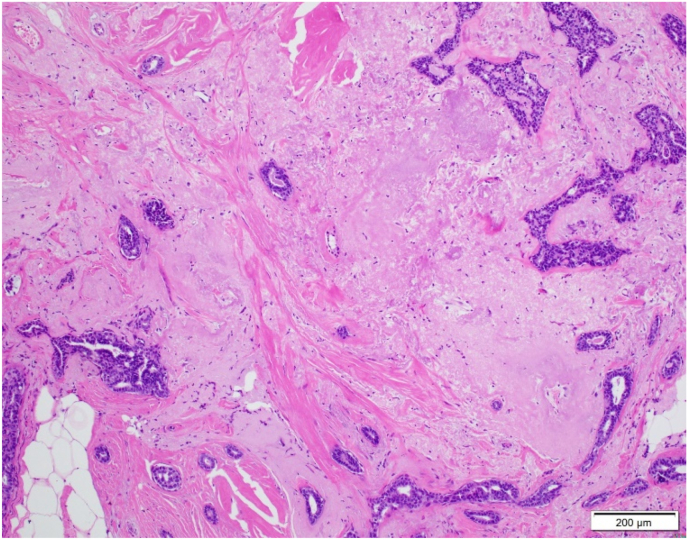
This study was reviewed and approved by the University of Kentucky Institutional Review Board. A natural language search of the pathology database identified 312 breast specimens with a diagnosis of “radial scar” or “complex sclerosing lesion” from the years 2000–2019. Every slide from each specimen was reviewed simultaneously by three surgical pathologists. From the 312 original cases identified, 247 unique radial scars/CSLs met diagnostic criteria (Fig. 1, Fig. 2). These 247 lesions derived from 167 accessioned cases, including up to eight radial scars/CSLs identified in a single specimen (as incidental lesions in a mastectomy specimen). These 167 cases occurred in 163 patients, as four patients had paired CNB and subsequent resection specimens included for review. Data recorded included patient age, other associated diagnoses in the specimen, and the type of surgical specimen (CNB versus resection).
Fig. 1.
Low power magnification of radial scar involved by usual ductal hyperplasia.
Fig. 2.
Flow chart of specimen selection and demographics.
2.2. Histologic analysis
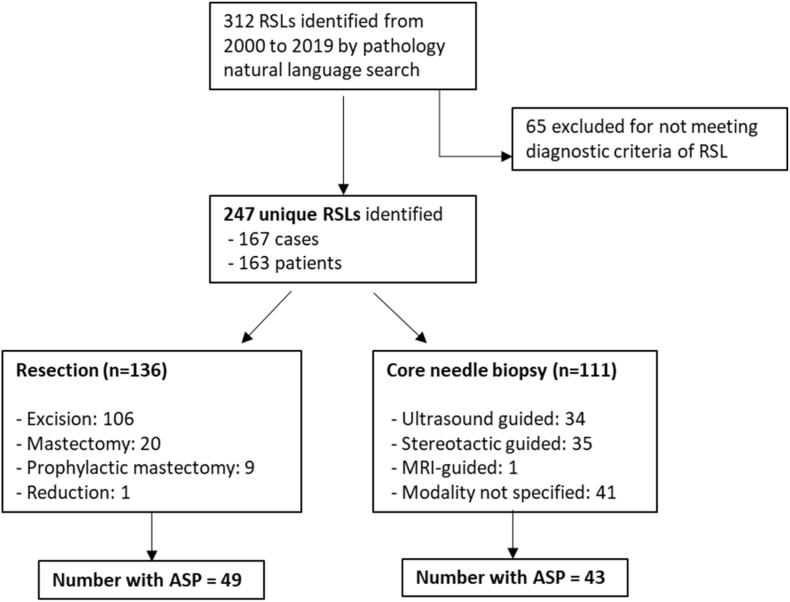
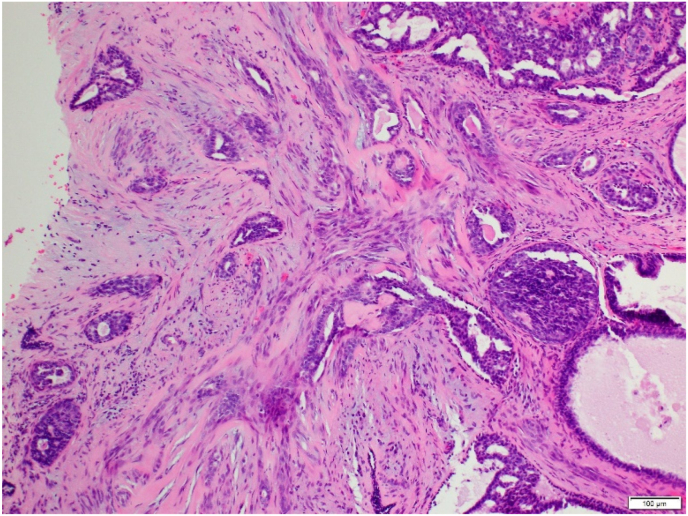
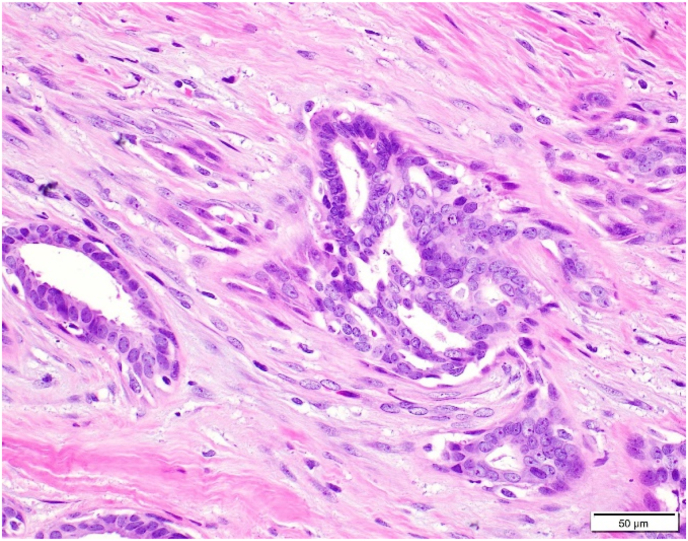
Three surgical pathologists (SB, VC, and TB) evaluated each of these 247 RSLs for: 1) overall RSL size, 2) size of the central nidus, 3) presence of lymphocyte clusters (chronic inflammation), 4) presence of ASP, and 5) stromal composition of the central nidus. The size of the overall lesion and central nidus was measured in millimeters (mm) of greatest linear dimension on the largest representative section of the lesion. Chronic inflammation was defined as an aggregate of greater than 10 lymphocytes and/or plasma cells located within or immediately adjacent to the central nidus (Fig. 3A–B). ASP was characterized by the presence of benign-appearing glands/tubules as well as squamoid cells. Squamoid cells were defined as both single cells and small groups of cells with vesicular nuclei and eosinophilic or orange cytoplasm, rounded contour and/or small tight clusters identifiable at 100x magnification. This magnification was chosen because the ASP would be readily identifiable to pathologists and therefore may avoid a diagnostic dilemma. The stroma of the central nidus was classified into three groups: 1) hyalinized-predominant (sclerotic), 2) cellular-predominant (desmoplastic), or 3) mixed-equal hyalinized and cellular components (Fig. 4A–C).
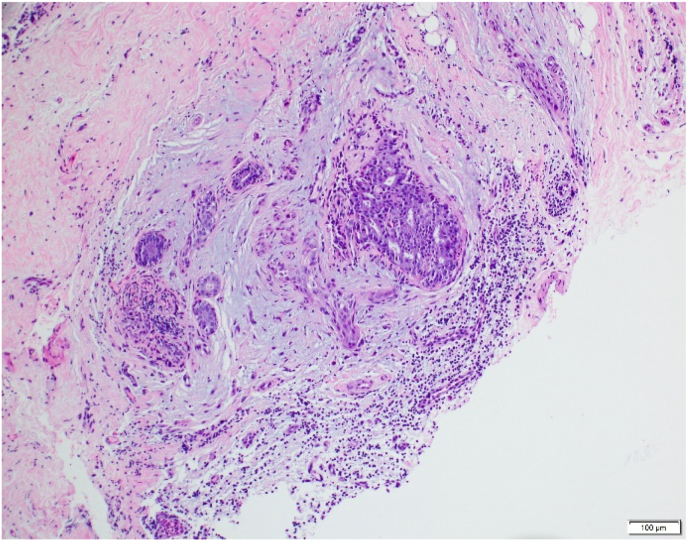
Fig. 3.
A: Low power magnification of lymphoid aggregates surrounding central nidus in radial sclerosing lesion with stroma classified as mixed-hyalinized predominant.
B: Additional representative lymphoid aggregate abutting central nidus.
Fig. 4.
A: Cellular stroma (100x magnification) B: Hyalinized stroma (40× magnification). C: Mixed stroma. Cellular component of stroma through center of picture with hyalinized stroma in bottom left and top right corners. (100x magnification).
Chi-square and t-tests tests were performed to compare the RSL characteristics. Results with p-values <0.05 were considered statistically significant.
3. Results
A total of 247 unique RSLs were identified from 167 specimens. Cases excluded for not meeting diagnostic criteria comprised alternate diagnoses preferred by the reviewers at re-review, which most commonly included sclerosing adenosis and biopsy site changes. Average patient age at time of diagnosis was 53 years (range: 20–81 years). 111 of the 247 RSLs (45%) were identified on CNB (10-, 12- and 14-gauge needles used, when recorded). The remaining 136 RSLs included excisions, mastectomies, and breast reduction procedures (Fig. 2). The RSL was the primary reason for excision in 59% of these cases, and was noted as an incidental finding in the remaining.
Overall RSL size ranged from 1.5 to 20 mm with an average of 5.4 mm. The size of the central nidus ranged from 0.5 to 14 mm with an average of 3.5 mm. The average size of the RSLs on CNB specimens (4.2 mm) was smaller compared to those on resections (6.4 mm). Similarly, the central nidus of the RSL was smaller on CNB (2.8 mm) than resection (4.0 mm)).
For the stromal classification of the central nidus, 121 (49%) cases were hyalinized, 89 (36%) equally mixed, and 37 (15%) cellular.
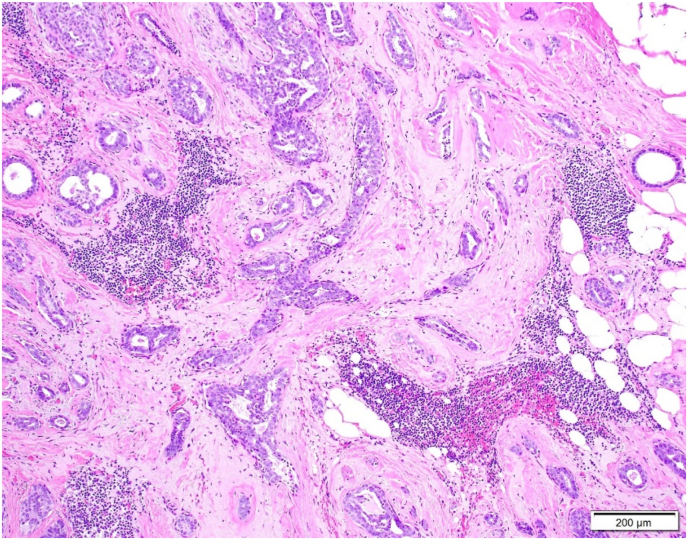
Ninety-two RSLs (37.2%, 92/247) contained ASP within or immediately adjacent to the nidus of the lesion (Fig. 5A–D). The average size of the ASP was 2.2 mm (range = 0.5–7 mm). ASP was identified in 29 (31.5%) cases with cellular stroma, 49 (53.3%) cases with mixed stroma, and 14 (15.2%) cases with hyalinized stroma (Table 1). The association of ASP being more commonly found in RSLs with a more cellular stroma was statistically significant (cellular vs mixed: p = 0.014, cellular vs hyalinized: p < 0.0001, and mixed vs hyalinized: p < 0.0001). Of note, in those RSLs classified as equally mixed stroma, the ASP was consistently found in the more cellular stromal component. The average size of the RSL and central nidus for lesions with and without squamoid cells was statistically similar, as shown in Table 2.
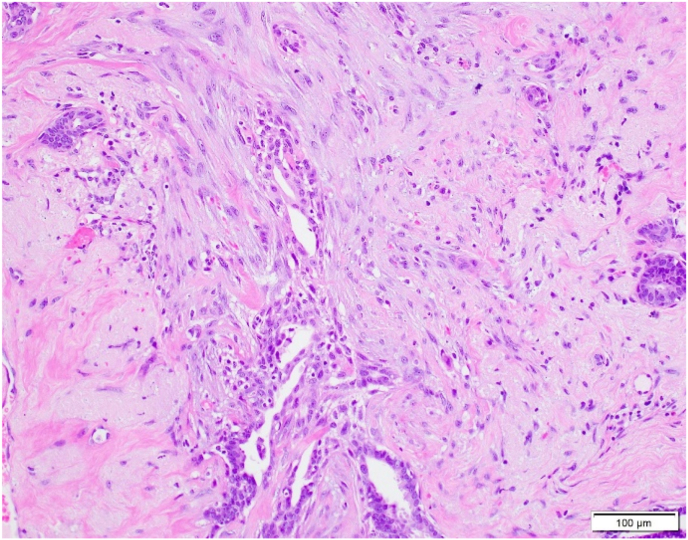
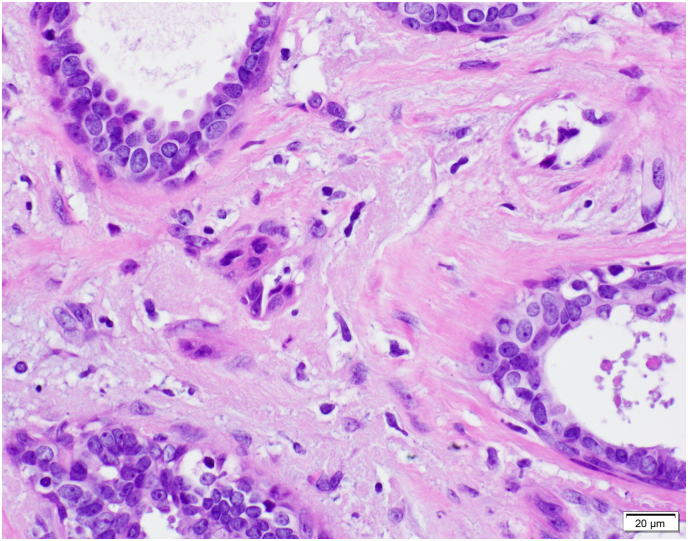
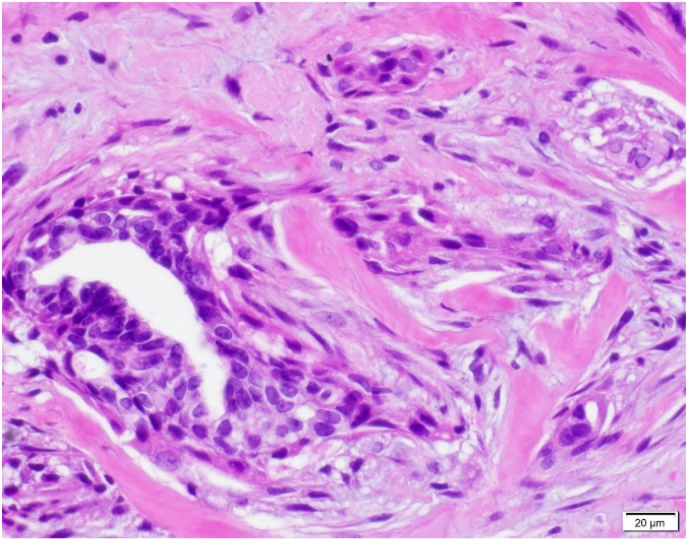
Fig. 5.
A: ASP with features visible at 100x magnification.B: ASP with focal squamoid features.C: ASP with squamoid features which appear to spin off adjacent benign gland.D: ASP with squamoid cells located variably distant from glandular component.
Table 1.
Features of radial sclerosing lesions by stromal category.
| Number of RSLs with histologic feature | Hyalinized (n = 121) | Mixed (n = 89) | Cellular (n = 37) |
|---|---|---|---|
| # with inflammation | 47 (38.8%) | 55 (61.8%) | 24 (64.9%) |
| # without inflammation | 74 (61.2%) | 34 (38.2%) | 13 (35.1%) |
| Hyalinized (n = 121) | Mixed (n = 89) | Cellular (n = 37) | |
|---|---|---|---|
| # with ASP | 14 (11.6%) | 49 (55.1%) | 29 (78.4%) |
| # without ASP | 107 (88.4%) | 40 (44.9%) | 8 (21.6%) |
P values: cellular vs hyalinized p = 0.005, hyalinized vs mixed stroma p = 0.001, mixed vs cellular p = 0.746. P values: cellular vs mixed: p = 0.014, cellular vs hyalinized: p < 0.0001, and mixed vs hyalinized: p < 0.0001.
Table 2.
Features of RSLs by presence or absence of adenosquamous proliferation (ASP).
| RSLs with ASP (n = 92) | RSLs without ASP (n = 155) | ||
|---|---|---|---|
| Average size of RSL | 5.5 mm | 5.4 mm | p = 0.70 |
| Average size of central nidus | 3.7 mm | 3.4 mm | p = 0.47 |
| # positive for lymphoid aggregates (n = 126) | 62 (67.4%) | 64 (41.2%) | p =<0.001 |
Lymphoid aggregates were identified in 126 RSLs (51.0%, 126/247). In parallel with the ASP, lymphoid aggregates also were more likely to be found in a cellular versus hyalinized stroma (Table 1). Lymphoid aggregates were identified in 24 (64.9%) cases with cellular stroma, 55 (61.8%) with mixed stroma, and 47 (38.8%) with hyalinized stroma. This difference was statistically significant in cellular vs hyalinized (p = 0.005) and hyalinized vs mixed stroma (p = 0.001). However, the difference in presence of lymphoid aggregates was not statistically different in RSLs with mixed vs cellular stroma (p = 0.746).
Furthermore, RSLs with ASP were more likely to have lymphoid aggregates, which reached statistical significance (p = <0.001). Of the 92 RSLs with ASP, 62 also had lymphoid aggregates (67.4%). In comparison, 64 of 155 RSLs without ASP had lymphoid aggregates (41.3%) (Table 2). However, the inverse relationship between inflammation and ASP was not statistically significant. Of RSLs with lymphoid aggregates (n = 126), 62 (49.2%) had ASP and 64 (51.0%) did not.
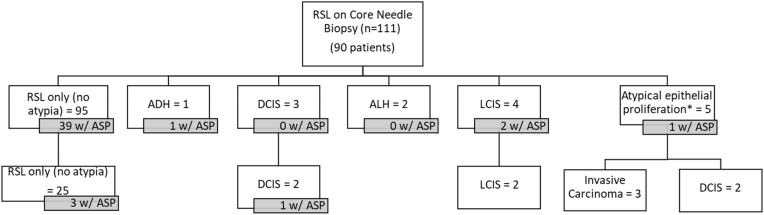
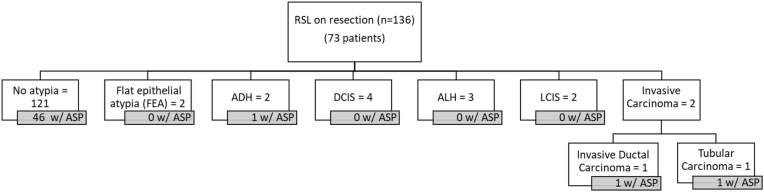
111 unique RSLs were diagnosed on CNB and included 90 patients. Only 34 of these RSLs (27 patients) underwent subsequent excision. In cases with no atypia on CNB (n = 95), 25 were excised and there were no upgrades to in situ- or invasive carcinoma at excision. Two RSLs with DCIS adjacent to the RSL underwent excision, and both showed residual DCIS. Furthermore, one contained an ASP on resection that was not present in the CNB sample. There were 5 RSLs with atypical epithelial proliferations on biopsy which were worrisome for carcinoma. All were excised, and included three well-differentiated invasive carcinomas, and two DCIS (Fig. 6). On CNB, ASP was present in 39 of the 95 RSLs without atypia (41.1%) and four of the 16 RSLs with atypia (25%). Similarly, in resections, ASP was present in 46 of the 121 RSLS without atypia (38%) and in three of the 15 RSLs with atypia (20%) (Fig. 7). Importantly, no cases were re-classified as LGASC on review for this study.
Fig. 6.
RSL on core needle biopsies with other associated diagnoses and follow-up excisions. There were 43 RSLs on biopsy with ASP. Four RSLs showed ASP present on excision that were not present on the CNB.
Fig. 7.
RSL in resections specimens and other associated diagnoses within or immediately adjacent to the RSL.
4. Discussion
Our study is the largest published case series which systematically evaluated and characterized the histologic features of ASP in RSLs. We found that ASP commonly occur in RSLs, occurring in 92 of 247 RSLs in our cohort. Wilsher reported a higher percentage of cases with ASP in RSLs (60% in their study) versus 37.2% in our cohort [9]. Possibly, the difference in number of RSLs evaluated (20 cases in their study versus 247 in the current study), population differences, and criteria for ASP identification (such as must be readily visible at 100x for our series) may account for this discrepancy observed between prevalence of ASP. RSL size was not a factor, as we found a similar distribution in the overall size of the RSL (1.5–20 mm in our cohort versus 1.7–21 mm in the Wilsher study) [9]. Furthermore, the ASP were always located within or immediately adjacent to the central nidus of the RSL.
Importantly, we showed that RSLs with ASP were significantly associated with a cellular stromal component (desmoplastic stroma). This observation may support the theory proposed by Wilsher et al. that ASP develop in early, more cellular RSLs and involute in late hyalinized or elastotic RSLs (i.e. display temporal lesional heterogeneity) [9,11]. Possibly, the cellular RSL stroma induces the development of squamoid epithelial cells. Supporting this conjecture is the location of squamoid cells predominantly in the cellular stromal areas in cases designated as “mixed” stroma. However, the association of cellular stroma and squamoid cells was not absolute, as a few cases of RSLs with hyalinized/sclerotic stroma did contain ASPs (14/121) (Table 1). Of the 92 RSLs demonstrating ASP, 43 were found in CNB specimens indicating the ASP and desmoplastic stroma were not attributable to biopsy site changes.
Possible explanations for the predilection for ASP squamoid cells to be associated with a cellular stroma include: 1) mesenchymal to epithelial transition (MET) occurs in the desmoplastic stroma from precursor fibroblast-like cells and/or 2) transformation of abluminal cells to squamoid cells is triggered by cytokine signaling from numerous adjacent fibroblasts in the desmoplastic stroma. Squamoid cells of RSLs (as opposed to fully developed squamous cells) may represent an intermediate state of MET [16]. Alternatively, if cells lining the abluminal layer of pre-existing tubules in the RSL are the source of squamoid cells, the sequence of stages of development of squamoid or true squamous cells in RSLs could comprise: 1) hypoxia of the RSL nidus induces an inflammatory cell response; 2) the inflammatory cells activate stromal fibroblasts which proliferate forming the hypercellular stroma; 3) activated fibroblasts release cytokines that cause the outer abluminal cell layer of ducts in the RSL to detach, traversing the basement membrane; 4) these detached cells undergo squamoid (and squamous) transformation in the conducive, hypercellular (desmoplastic) nidus stromal environment.
To support this possible theory of the sequence of inciting events, we found that chronic inflammation and desmoplastic stroma are statistically significantly associated with RSLs that contain ASP. Studies in mouse models of mammary carcinoma have shown that squamous carcinomas, adenosquamous carcinomas and myoepitheliomas arise from basal or myoepithelial cells [[17], [19], [20], [21]]. In our series, we found squamoid cells that appeared to swirl off small ducts (Fig. 5C–D), suggesting a close relationship and possibly transition from abluminal cells of the ducts. Notably, this peeling away of squamoid cells from glands is a characteristic feature of ASP [4]. This phenomenon is also reported in salivary gland tumors wherein neoplastic myoepithelial cells merge abruptly into squamous nests suggesting an ability to transform to squamous cells [22]. In the breast, in low grade adenosquamous carcinoma, neoplastic glandular and squamoid cells appear to derive from pluripotent cells expressing p63, keratin 5 and keratin 14 [23]. Given the many similarities between LGASC and adenosquamous proliferations, an identical precursor cell may also serve as the source of ASP in RS/CSL.
It has been reported that large lymphoid aggregates are a diagnostic clue to LGASC rather than ASP [6,18]. However, one pathologist's “small cluster” may be another's “large cannonball.” As shown in Fig. 2, although uncommon, “large” lymphoid aggregates can be found in ASP. Therefore, the presence of sizable lymphoid aggregates should not serve as the discriminating feature between LGASC and ASP.
Likely because of the relatively small size of the RSLs in our series, in the CNBs as well as resections, it was possible to assess whether the squamoid cells and intermingled tubules appeared infiltrative, a key discriminating feature between LGASC and ASP (Table 3). In our cases, the ASP were located in the central nidus without drift beyond this area, confirming a non-infiltrative growth pattern. However, caution may be warranted in CNBs of RSLs that contain abundant squamoid cells and small tubules, as such florid ASP were not as commonly encountered in our case series and may reflect a more worrisome finding.
Table 3.
Common and differential parameters of LGASC versus RSL with ASP. Histologic features of infiltration into surrounding tissue for LGASC is the only distinguishing feature.
| Low-grade Adenosquamous Carcinoma | Radial sclerosing lesion with adenosquamous proliferation | |
|---|---|---|
| Morphology |
|
|
| Immunohistochemistry |
|
|
| Molecular | PIK3CA mutations in ∼50% | PIK3CA mutations in 77%a |
Ref 11. Wilsher et al., 2017.
Limitations of our study include low number of patients with follow-up resections that could be re-reviewed and small size of the RSLs. Additionally, CNBs comprised three levels to review, at a minimum, while resections comprised only one level in >95% of cases. However, we did not note a significant difference in rate of identification of ASP when three shallow levels versus one level were reviewed. Possibly, for some cases in which ASP was not identified, additional levels may have revealed them. We did not cut additional levels, as given the overall small nidus size, many lesions would potentially have been cut through with additional leveling. Finally, many patients did not undergo resection after CNB. Our institution was an early adopter of the surgical practice of not excising RSLs without atypia and with radiology/pathology correlation.
In summary, we evaluated the histologic features of ASP in a large case series and confirm the findings of Wilsher that ASP is statistically more likely to be found in RSLs that have a hypercellular (desmoplastic) central nidus [9]. In our series, ASP is commonly associated with chronic inflammation, including in some cases relatively sizeable aggregates, as is reported for LGASC. Therefore, the finding of multiple lymphocyte aggregates in an RSL is not necessarily indicative of LGASC, a finding which has been suggested to be a clue for malignancy in RSL [6]. Finally, CNBs, including larger, vacuum-assisted specimens, prove to be sufficient to confirm the benign nature of ASP in RSLs based on an average of at least three years of institutional follow-up of our patients. Determining whether ASP in reality are nascent LGASC or not may require expanded molecular genetic and epigenetic analyses. In either scenario, our series confirms that re-excisions are not necessary in RSLs found to have ASP that appear confined to the nidus on core needle biopsy.
References
- 1.Sahin A, Collins LC. Radial scar/complex sclerosing lesion. WHO Classification of Tumours: Breast Tumours. 5 ed. p. 30-31.
- 2.Hoda S.A., Brogi E., Koerner F.C., Rosen P.P. fifth ed. Wolters Kluwer; 2021. Rosen's breast pathology. [Google Scholar]
- 3.Hicks D.G., Lester S.C. 2 ed. Elsevier; Salt Lake City, UT: 2016. Diagnostic pathology: breast. [Google Scholar]
- 4.Wilsher M.J. Significance of adenosquamous proliferation in breast lesions. J Clin Pathol. 2021;74(9):559–567. doi: 10.1136/jclinpath-2020-207097. [DOI] [PubMed] [Google Scholar]
- 5.Rosen P.P., Ernsberger D. Low-grade adenosquamous carcinoma. A variant of metaplastic mammary carcinoma. Am J Surg Pathol. 1987;11(5):351–358. doi: 10.1097/00000478-198705000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Shin S.J. Springer; Switzerland: 2016. A comprehensive guide to core needle biopses of the breast. [Google Scholar]
- 7.Denley H., Pinder S.E., Tan P.H., et al. Metaplastic carcinoma of the breast arising within complex sclerosing lesion: a report of five cases. Histopathology. 2000;36(3):203–209. doi: 10.1046/j.1365-2559.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 8.Hilson J.B., Schnitt S.J., Collins L.C. Phenotypic alterations in myoepithelial cells associated with benign sclerosing lesions of the breast. Am J Surg Pathol. 2010;34(6):896–900. doi: 10.1097/PAS.0b013e3181dd60d3. [DOI] [PubMed] [Google Scholar]
- 9.Wilsher M.J. Adenosquamous proliferation of the breast and low grade adenosquamous carcinoma: a common precursor of an uncommon cancer? Pathology. 2014;46(5):402–410. doi: 10.1097/PAT.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 10.Ang D.C., Warrick A.L., Shilling A., et al. Frequent phosphatidylinositol-3-kinase mutations in proliferative breast lesions. Mod Pathol. 2014;27(5):740–750. doi: 10.1038/modpathol.2013.197. [DOI] [PubMed] [Google Scholar]
- 11.Wilsher M.J., Owens T.W., Allcock R.J. Next generation sequencing of the nidus of early (adenosquamous proliferation rich) radial sclerosing lesions of the breast reveals evidence for a neoplastic precursor lesion. J. Pathol. Clin. Res. 2017;3(2):115–122. doi: 10.1002/cjp2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batallion G., Fuhrmann L., Girard E., et al. High rate of PIK3CA mutations but no TP53 mutations in low-grade adenosquamous carcinoma of the breast. Histopathology. 2018;73(2):273–283. doi: 10.1111/his.13514. [DOI] [PubMed] [Google Scholar]
- 13.Wilsher M.J., Snook K. Bilateral, multicentric low-grade adenosquamous carcinomas of the breast, each arising within a separate radial scar/complex sclerosing lesion. Pathology. 2014;46(1):85–88. doi: 10.1097/PAT.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 14.Sohn V.Y., Causey M.W., Steele S.R., et al. The treatment of radial scars in the modern era--surgical excision is not required. Am Surg. 2010;76(5):522–525. [PubMed] [Google Scholar]
- 15.Bacci J., MacGrogan G., Alran L., Labrot-Hurtevent G. Management of radial scars/complex sclerosing lesions of the breast diagnosed on vacuum-assisted large-core biopsy: is surgery always necessary? Histopathology. 2019;75(6):900–915. doi: 10.1111/his.13950. [DOI] [PubMed] [Google Scholar]
- 16.Pastushenko I., Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Soundararajan R., Fradette J.J., Konen J.M., et al. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers. 2019;11(5) doi: 10.3390/cancers11050714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Alfonso T.M., Shin S.J. Small glandular proliferations of the breast. Surgical pathol. clin. 2012;5(3):591–643. doi: 10.1016/j.path.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Drobysheva D., Smith B.A., McDowell M., et al. Transformation of enriched mammary cell populations with polyomas T antigen influences tumor subtype and metastatic potential. Breast Cancer Res. 2015;17:132–149. doi: 10.1186/s13058-015-0641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ince T.A., Richardson A.L., Bell G.W., et al. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer Cell. 2007;12:160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Molyneux G., Geher F.C., Magnay F.A., et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7:403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher C.D.M., editor. Chapter 7 tumors of the salivary glands by wah cheuk and john K.C. Chan. Elsevier Inc.; Philadelphia PA: 2021. Diagnostic histopathology of tumors; pp. 301–409. 5th Ed. [Google Scholar]
- 23.Boecker W., Stenman G., Loening T., et al. Differentiation and histogenesis of syringomatous tumour of the nipple and low-grade adenosquamous carcinoma: evidence for a common origin. Histopathology. 2014;65(1):9–23. doi: 10.1111/his.12358. [DOI] [PubMed] [Google Scholar]