Summary
Acute-on-chronic liver failure (ACLF) is the most severe form of acutely decompensated cirrhosis and is characterised by the presence of one or more organ failures, intense systemic inflammation, peripheral blood lymphopenia, and a high risk of death without liver transplantation within 28 days. Herein, we propose the hypothesis that intense systemic inflammation may lead to organ failures through five different non-mutually exclusive mechanisms. First, pathogen-associated molecular patterns and inflammatory mediators (i.e. cytokines and lipid mediators) stimulate the production of the vasorelaxant nitric oxide in the walls of splanchnic arterioles, leading to enhanced splanchnic and systemic vasodilation which, in turn, induces enhanced activity of endogenous vasoconstrictor systems causing renal vasoconstriction and acute kidney injury. Second, neutrophils that reach the systemic circulation are prone to adhere to the vascular endothelium. Cytokines and lipid mediators act on the endothelium in microvessels of vital organs, an effect that favours the migration of neutrophils (and probably other leukocytes) to surrounding tissues where neutrophils can cause tissue damage and thereby contribute to organ failure. Third, cytokines and lipid mediators promote the formation of microthrombi that impair microcirculation and tissue oxygenation. Fourth, acute inflammation stimulates intense peripheral catabolism of amino acids whose products may be metabotoxins that contribute to hepatic encephalopathy. Fifth, acute inflammatory responses, which include the production of a broad variety of biomolecules (proteins and lipids), and an increase in biomass (i.e., granulopoiesis requiring de novo nucleotide synthesis), among others, are energetically expensive processes that require large amounts of nutrients. Therefore, immunity competes with other maintenance programmes for energy. The brain stem integrates the energy demand of each organ system, with immunity considered a top priority. The brain stem may “decide” to make a trade-off which involves the induction of a dormancy programme that permits the shutdown of mitochondrial respiration and oxidative phosphorylation in peripheral organs. In the context of acutely decompensated cirrhosis, the consequence of a shutdown of mitochondrial respiration and ATP production would be a dramatic decrease in organ function.
Keywords: Decompensation of cirrhosis, ACLF, inflammation, inflammatory mediators, immune cells, metabolism
Key points.
-
•
Acute-on-chronic liver failure (ACLF) is characterised by the presence of systemic inflammation and high risk of death within 28 days. In this review, we propose a hypothesis linking systemic inflammation with organ failure through the following mechanisms.
-
•
Pathogen-derived molecules and inflammatory mediators (i.e. cytokines and lipid mediators) stimulate the production of the vasorelaxant nitric oxide which induces splanchnic and systemic vasodilation that in turn activate endogenous vasoconstrictors causing renal vasoconstriction and acute kidney injury.
-
•
Inflammatory mediators activate neutrophils to adhere to vascular endothelium and migrate to tissues, causing tissue damage and contributing to organ failure.
-
•
Inflammatory mediators promote formation of microthrombi that impair microcirculation and tissue oxygenation.
-
•
Acute inflammation stimulates intense peripheral catabolism of amino acids whose products may be metabotoxins that contribute to hepatic encephalopathy.
-
•
Acute inflammatory responses, which are energetically demanding compete with other maintenance energy programmes. The brain stem prioritises the immune system over the peripheral organs which enter a dormancy state that leads to organ dysfunction.
Introduction
Acutely decompensated cirrhosis, which is the most frequent cause of non-elective hospital admission of patients with cirrhosis,1 refers to the recent development of ascites, encephalopathy, gastrointestinal haemorrhage, or any combination of these disorders.2,3 Acute-on-chronic liver failure (ACLF) is the most severe form of acutely decompensated cirrhosis. Compared to patients without ACLF, those with ACLF are characterised by a higher frequency of ongoing clinically apparent pro-inflammatory precipitants (bacterial sepsis, alcohol-related hepatitis, or both); more intense acute systemic inflammation (indicated by leukocytosis with neutrophilia, higher blood levels of C-reactive protein [CRP], as well as a variety of cytokines and chemokines); the presence of hepatic failure, extrahepatic organ failures, or both; and a much higher risk of short-term death, i.e. death without liver transplantation within 28 days after hospital admission.[2], [3], [4], [5]
Several studies that have been performed since the description of ACLF in 2013 suggest that systemic inflammation plays a causal role in its development.[5], [6], [7], [8], [9], [10], [11] In parallel, important progress has been made by immunologists in their understanding of the general principles of inflammatory responses to different stimuli, primarily infections.[12], [13], [14], [15], [16], [17], [18], [19], [20] In addition, immunologists have uncovered the major role played by changes in the metabolism of nutrients (glucose, amino acids and fatty acids) in support of energy-demanding inflammatory responses.17,[21], [22], [23] Here, we first provide general information about principles of inflammatory responses and associated changes in metabolism. Then, we review the available data from the ACLF medical literature and finally propose a theory explaining how systemic inflammation drives the development of ACLF.
General principles of inflammatory responses
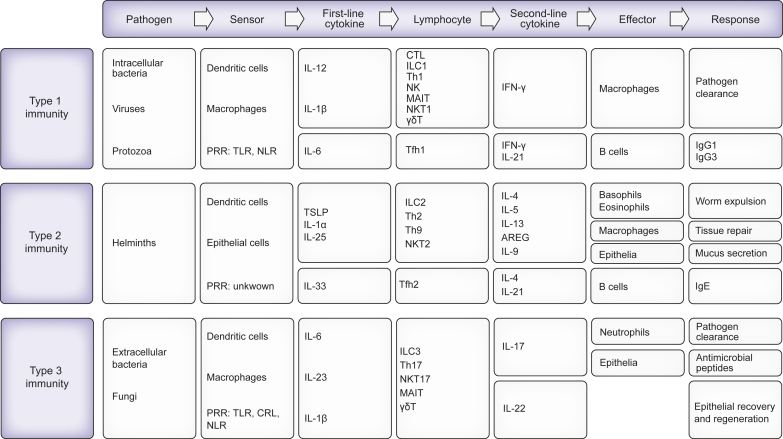
We will first comment on paradigmatic inflammatory responses to acute infections which are the most common triggers of acute inflammation and have been extensively investigated. As with any acute inflammatory response, the response elicited by infections generally involves four components that are successively and coordinately involved.14,15 The four components include the pathogen byproducts (including pathogen-associated molecular patterns [PAMPs; which are unique molecular structures associated with the pathogens] and virulence factors) that trigger the response, receptors located in immune cells (sensor cells) that detect them, inflammatory signals secreted by sensor cells, and effector cells that are targets of inflammatory signals. Inflammatory signals elicit activation, recruitment, and differentiation of effector immune cells capable of eliminating the causal pathogen. Therefore, the inflammatory response is an immune response whose objective is to eliminate the causal pathogen. Immunologists distinguish three types of inflammatory responses (type 1, type 2, and type 3) depending on the nature of the triggers of these responses15 (Fig. 1). Each type of immune response is generally a two-tiered process in which tissue-resident innate immune cells (dendritic cells [DCs], macrophages) are activated by pathogens to secrete cytokines that act on tissue-resident differentiated lymphocytes of both innate classes (e.g., natural killer [NK] cells) and adaptive classes (tissue-resident memory T cells). These lymphocytes, in turn, secrete cytokines to activate effector cells.14 There is a cytokine signature for each of the three types of immune responses and accordingly, cytokines are classified into type 1, type 2, and type 3 cytokines15 (Fig. 1).
Fig. 1.
Three types of immune responses to infections have been well documented.
Type 1 immune responses are triggered by intracellular microbes (bacteria, protozoa, and some viruses) whose PAMPs are sensed by dendritic cells (DCs) and macrophages through specific pattern recognition receptors (PRRs; which are germ-line encoded receptors), such as TLRs and nucleotide-binding oligomerisation domain [Nod]-, leucine-rich repeat-containing receptors (NLRs). These cells secrete first-line cytokines (here IL-12, IL-1β or IL-18) which act on different lymphocytes including innate lymphocytes (here group 1 innate lymphoid cells [ILC1 cells] and NK cells), innate-like lymphocytes (also known as unconventional T cells including type 1 natural killer (NKT1) cells, mucosal-associated invariant T [MAIT cells] cells, γδ T cells) and adaptive lymphocytes such as T helper type 1 (Th1) memory cells and CD8 memory T cells in the tissue, to produce the second-line cytokine INF-γ. INF-γ activates effector cells (here macrophages) to kill pathogens. Cytotoxic T cells and NK cells can also kill virus-infected cells. IL-12 can also stimulate follicular helper T cells (Tfh1 cells) to secrete INFγ which drives IgG responses in B cells. Type 2 immune responses are triggered by helminths. Although the mechanisms by which helminths are detected are still unclear, some components of the immune response to these multicellular parasites have been identified. First, the damage of epithelial cells and endothelial cells caused by helminths results in the release of first-line cytokines (here IL-33, IL-1α, IL-25 [also known as IL-17E], thymic stromal lymphopoietin [TSLP]). Second, a specific subset of DCs seems to be indispensable to mount a type 2 inflammatory response. Third, first-line cytokines stimulate ILC2 cells, NKT2 cells, γδ T cells, and Th2 memory cells to secrete second-line cytokines IL-5, IL-13, and amphiregulin (AREG). IL-4, another second-line cytokine, is secreted by memory Th2 cells in response to engagement of T-cell antigen receptors (TCRs). First-line cytokines also stimulate tissue-resident Th9 cells to produce IL-9. Second-line cytokines activate effector cells including mast cells, basophils, eosinophils, and macrophages, as well as IgE antibody production (which is driven by IL-4-producing Tfh2 cells), and therefore promote tissue repair and immunity against helminths. Type 3 immune responses are triggered by extracellular bacterial and fungi whose PAMPs are sensed by DCs and macrophages through PRRs including TLRs, NLRs and C-type lectin receptors (CLRs). These cells secrete first-line cytokines (here IL-1β, IL-23, IL-21) which activate ILC3 cells, NKT3 cells, MAIT cells, γδ T cells, and memory Th17 cells to secrete second-line cytokines IL-17 and IL-22. These cytokines, in turn, stimulate effector cells (here neutrophils) to kill extracellular pathogens. In addition, IL-17 and IL-22 activate endothelial cells, epithelial cells, fibroblasts and tissue-resident macrophages to produce matrix metallopeptidases, nitric oxide, cytokines, antimicrobial peptides and CXCL8 (a neutrophil-attractant chemokine also known as IL-8). ILC3-produced IL-22 also stimulates epithelial proliferation.
Triggers of the inflammatory response in ACLF
A recent prospective study (PREDICT study) revealed that among 420 patients with ACLF, 273 (65%) had clinically apparent triggers of systemic inflammation, including proven bacterial infection, severe alcohol-related hepatitis, and gastrointestinal haemorrhage with shock.10 Importantly, compared to patients with no clinically apparent trigger or those with a single apparent trigger, patients with two triggers or more had greater white cell, neutrophil and monocyte blood counts as well as more elevated CRP levels, suggesting a cumulative effect of triggers on systemic inflammation. More details are available in the original publication by Trebicka et al..10 While bacterial infections or active alcoholism are the most common precipitants of ACLF in Western countries, hepatotropic viral infections, e.g. hepatitis B virus reactivation and super-infection with hepatitis A or hepatitis E viruses, are more commonly responsible in Asian countries.24 Extrahepatic viral infections, such as influenza virus infection, have also been associated with the development of organ failure, secondary infections and death in patients with cirrhosis.25
Bacterial infections that trigger inflammatory responses in patients with ACLF are primarily caused by extracellular bacteria, including Gram-negative and Gram-positive bacteria.26,27 The most common Gram-negative bacteria are Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumanii. Gram-positive bacteria are Staphyloccocus aureus, Enterococcus faecium, and Enterococcus faecalis. Infections caused by fungi such as Candida albicans can also trigger inflammation. The mechanisms by which these bacteria and fungi induce inflammation have been reviewed elsewhere.14,28 Of note, the prevalence of Gram-positive or Gram-negative bacteria that are resistant to antibiotics is increasing in patients with ACLF.26 Resistance to antibiotics contributes to the intensity, prolongation and severity of inflammatory responses to bacteria.10 Finally, it is important to mention the potential role of changes in the gut microbiome as a source of bacterial products that might trigger systemic inflammation in patients with ACLF. It is widely known that intestinal bacterial overgrowth and dysbiosis in conjunction with impaired gut barrier function and increased bacterial translocation are central mechanisms by which bacterial products entering the systemic circulation trigger systemic inflammation (reviewed in ref.29). In fact, in the PREDICT study, 35% of the patients with ACLF did not have clinically apparent triggers of inflammatory responses.10 However, these patients had features of systemic inflammation, although less intense than those in patients with clinically apparent inflammatory triggers.6,10 Intestinal translocation of bacterial PAMPs has been documented in patients with cirrhosis,3,4 and therefore it has been suggested that translocated PAMPs may induce inflammatory responses in some patients with ACLF, in the absence of clinically apparent infection.4
A broad variety of mechanisms have been identified as potential triggers of acute inflammation in patients with severe alcohol-related hepatitis. Features of liver injury that characterise this disease include hepatocyte death (which is indicated, for example, by the release of keratin 18, a “passive” marker of cell death),30 resulting in the release of high-mobility group protein 1, a DAMP that triggers inflammatory responses through inflammasome activation.31 In addition, bulk liver transcriptome analysis in patients with severe alcohol-related hepatitis revealed RNA features related to senescent cells,32 which are known to secrete inflammatory mediators, including various cytokines, chemokines, extracellular matrix proteins and growth factors, collectively referred to as the senescence-associated secretory phenotype.33 Of note, excessive alcohol consumption causes major alterations in the intestinal barrier and changes in the microbiome, including increased pathogenic bacteria (in particular E. faecalis). A virulence factor (i.e., the exotoxin cytolysin) from E. faecalis can promote alcohol-induced liver injury.34 In addition, the case has been made that alterations in intestinal viruses35 and fungi36 may be associated with poor outcomes in patients with alcohol-related liver disease, suggesting that viral and fungal byproducts may play a role in the induction of inflammation in these patients. As mentioned earlier, patients with severe alcohol-related hepatitis often present with bacterial infection, which thereby contributes to inflammatory responses in these patients. In addition, a high incidence of infections caused by the saprophytic fungus Aspergillus fumigatus has been reported among patients with severe alcohol-related hepatitis.37
Patients with gastrointestinal haemorrhage and shock may have tissue damage, for example in the liver and kidneys, resulting in the release of pro-inflammatory DAMPs. These patients may also have intestinal ischaemia-reperfusion injury resulting in the translocation of bacterial PAMPs from the intestinal lumen to the systemic circulation, which in turn may induce inflammatory responses.38
Soluble mediators of inflammation in ACLF
Protein mediators
In 2016, Clària, Stauber et al. were the first to report the existence of a cytokine storm in a large series of patients (n = 237) who presented with ACLF.6 The term “cytokine storm” refers to the presence of elevated circulating cytokine levels that lead to secondary organ dysfunction (e.g., renal, hepatic, pulmonary),39 which is a collateral damage caused by an excessive inflammatory response (Box 1). In the study by Clària, Stauber et al., patients with ACLF simultaneously had organ failures and elevated levels of 14 out of the 17 cytokines detected in plasma.6 These included pro-inflammatory cytokines or chemokines, such as interleukin (IL)-6, tumour necrosis factor-α (TNF-α), C–C motif chemokine ligand 2 (CCL2, also known as MCP-1, chemotactic for monocytes), C-X-C motif chemokine ligand 8 (CXCL8, also known as IL-8, chemotactic for neutrophils); anti-inflammatory cytokines (IL-10, IL-1RA); the type-1 immune markers interferon-γ and CXCL10 (also known as IP-10, chemotactic for activated Th1 cells); the type 2 immune marker eotaxin; and the type 3 immune marker granulocyte-macrophage colony-stimulating factor, among others. In addition, this study showed that the circulating levels of molecules that comprised the cytokine storm were roughly similar whichever precipitants were present, with the exception of three cytokines (IL-6, IL-8 and TNFα), which had different levels according to the precipitants.6 In particular, the levels of IL-6 and TNFα were higher among patients with bacterial infection alone or combined with alcohol-related hepatitis than among those with severe alcohol-related hepatitis alone or those without clinically apparent precipitants. In contrast, IL-8 levels were higher among patients with severe alcohol-related hepatitis alone or combined with bacterial infection than among those with bacterial infection alone or those without clinically apparent precipitants.
Box 1. Cytokine storm.
CRP, C-reactive protein; IL-, interleukin-; TNFα, tumour necrosis factor-α.
More recently, Weiss, de la Grange et al. measured 37 inflammatory markers in 16 patients with ACLF at presentation (of whom seven had severe sepsis) and six healthy individuals and found that these patients had elevated circulating levels not only of all inflammatory mediators already identified by Clària, Stauber et al.6 but also of type 2 (IL-1α and IL-4) and type 3 (IL-17A) immune markers.5 In addition, several inflammatory markers that were undetectable in healthy individuals were elevated in patients with ACLF, including the B-cell activator CD40 ligand, the pro-inflammatory chemokines CCL22 (also known as MDC; chemotactic for activated T cells) and CXCL1 (chemotactic for neutrophils), the markers of macrophage activation CD163 and CD206, the marker of fibrinolysis inhibition t-PAI-1 (for tissue plasminogen activator inhibitor; with extremely high levels in patients), and markers of endothelial dysfunction such as intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 (which were both present at extremely high levels in patients). These authors also compared the blood levels of each inflammatory cytokine and chemokine with their expression at the mRNA level in whole blood and found a very weak correlation, a finding that suggests that elevated levels of circulating inflammatory mediators may be explained by their spillover from peripheral tissues (which are the sites of production) rather than increased production by circulating immune cells.
Together these findings show that patients with ACLF have deregulated tissue immunological responses indicated by a full-blown cytokine storm (in particular with simultaneous increases in circulating levels of immune markers for type 1, type 2 and type 3 immunity), which is associated with features of activation of macrophage function, inhibition of fibrinolysis (a prothrombotic effect) and endothelial dysfunction. Both alterations in fibrinolysis and endothelial function likely developed in the microvasculature of vital organs where they favoured the formation of microthrombi and leukocyte migration from blood to tissues, respectively.
Lipid mediators
Lipid mediators are bioactive lipids generated from structural lipid species (i.e. phospholipids containing polyunsaturated fatty acids), which compose the lipid bilayer of cell membranes.40 Most lipid mediators are eicosanoids, which means that they are derived from the essential omega-6 fatty acid arachidonic acid.41 The eicosanoid family consists of prostaglandins, thromboxane A2, leukotrienes, lipoxins and epoxyeicosatrienoic acids. Except for lipoxins, eicosanoids have potent pro-inflammatory properties and, in fact, prostaglandins and thromboxane A2 are the primary targets for non-steroidal anti-inflammatory drugs.42,43 Like cytokines, eicosanoids are released in large quantities by immune cells in response to infections or tissue injury, initiating the so-called “eicosanoid storm”.40 However, unlike cytokines, much less information is currently available on the role of lipid mediators in ACLF. The formation and actions of lipid mediators, mainly eicosanoids, in patients with acutely decompensated cirrhosis have been extensively studied in the past in the context of renal dysfunction,44 but their role in the context of systemic inflammation and immunosuppression in ACLF has only been tackled more recently. Recent evidence has shown that increased circulating levels of prostaglandin E2 (PGE2) may drive immunosuppression and magnify the risk of infection in patients with acutely decompensated cirrhosis.45 However, in a targeted lipidomic analysis of more than 100 lipid mediators in 200 patients with acutely decompensated cirrhosis with and without ACLF, López-Vicario et al. did not confirm increased plasma PGE2 levels nor identify any association of this lipid mediator with the presence or risk of developing infections during hospitalisation.46 Instead, these authors reported increased expression of the PGE2-degrading enzyme 15-hydroxy-PG dehydrogenase in patients with acutely decompensated cirrhosis.47
The plasma lipid mediator landscape of patients with ACLF is also characterised by a deficit in anti-inflammatory/pro-resolving lipid mediators.46,48 This is consistent with the presence in these patients of a higher omega-6 to omega-3 ratio, a surrogate marker of systemic inflammation and impaired resolution.49 Indeed, a deficit in lipoxin A4 formation and reduced levels of the pro-resolving lipid mediator lipoxin A5 have been described in these patients.46,48 Analysis of lipid mediators bound to serum albumin (lipid mediators travel in the circulation attached to this protein) from patients with acutely decompensated cirrhosis at risk of developing ACLF has confirmed a lower content of anti-inflammatory/pro-resolving lipid mediators in this condition.47 Likewise, a lower content of the pro-resolving lipid mediator resolvin E1 has recently been reported in extracellular vesicles from patients with ACLF.50 Finally, it has been shown that the survival of patients with ACLF is associated with a shifted profile in the levels of pro-resolving lipid mediators.51 Together, these findings suggest that systemic inflammation in ACLF can also be driven by a loss of anti-inflammatory and pro-resolving molecules involved in the control of acute inflammation.
Immune cells in ACLF
Characteristics of circulating immune cells
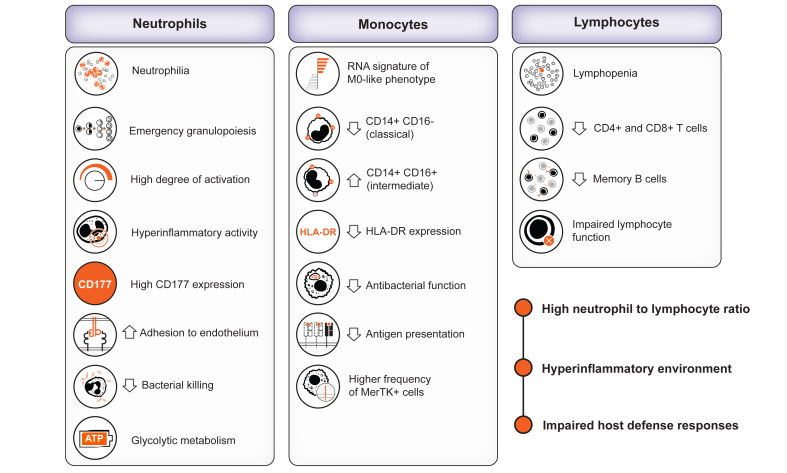
At presentation, patients with ACLF have marked changes in clinical blood counts characterised by leukocytosis and neutrophilia which contrast with lymphopenia5 (Fig. 2). Of note, these patients have no apparent changes in the clinical monocyte count. The dichotomy between leukocytosis (made up of neutrophils) and lymphopenia is a hallmark of ACLF. The blood white cell count is a component of the CLIF-C ACLF score which assesses the probability of death at 1 month and 3 months in patients with ACLF; the higher the white cell count the greater the probability of death. These findings underline the association between increased biomass (leukocytosis) and outcomes in patients with ACLF. Although the landscape of circulating immune cells associated with ACLF has not yet been investigated in-depth in large series of patients, the results obtained from microarray analysis of RNA expression in whole blood and neutrophils, RNA sequencing in specific monocyte subsets, and flow cytometry in small series of patients have provided the first clues of perturbations in the blood immune cell compartment in ACLF.
Fig. 2.
The immunopathological landscape in peripheral blood of patients with acutely decompensated cirrhosis and ACLF is characterised by neutrophilia and severe lymphopenia.
The monocyte population can be slightly increased or within the normal range. In addition to increased neutrophil counts, patients with acute-on-chronic liver failure (ACLF) exhibit an augmented number of immature neutrophil precursors, indicating enhanced emergency granulopoiesis. Blood neutrophils from patients with ACLF also show signs of hyperactivation, increased CD177 expression and adhesion to endothelium. Paradoxically, in this hyperinflammatory environment, the bactericidal activities of neutrophils are impaired. All these neutrophil activities are nurtured through a very intense glycolytic metabolism. The blood monocytes circulating in patients with ACLF also present reduced antigen presentation, HLA-DR expression and antibacterial function. Despite the number of monocytes not being decreased, patients with ACLF exhibit reduced numbers of CD14+CD16- (classical) and CD14+CD16+ (intermediate) monocytes. Also, a higher number of MerTK+ (immunosuppressed) monocytes is observed in this condition. On the other hand, in addition to decreased lymphocyte counts, mainly affecting CD4+ and CD8+ T lymphocytes, and memory B cells, patients with ACLF exhibit impaired lymphocyte function. The increased neutrophil to lymphocyte ratio and the above-described features set the ground for the presence of a concomitant hyperinflammatory and defective host defences in the peripheral blood of patients with acutely decompensated cirrhosis and ACLF.
Neutrophils
With the use of microarray analysis of whole blood RNA expression in patients with ACLF and healthy individuals, Weiss, de la Grange et al. identified a large number of genes that were upregulated in patients compared to healthy individuals.5 Enrichment analyses revealed that genes coding for components of neutrophil granules were overrepresented among genes that were upregulated in ACLF, features indicating that ACLF-associated neutrophilia was made up of activated neutrophils. The increased transcription of granule genes was confirmed by bulk transcriptome analysis in circulating neutrophils from patients with ACLF vs. healthy individuals.5 In addition, genes coding for key enzymes in glycolysis, another feature of neutrophil activation, were upregulated in neutrophils from the blood of patients with ACLF. Alterations in the neutrophil transcriptome observed in patients with ACLF were not found in patients with acutely decompensated cirrhosis without ACLF, a finding highlighting the specificity of transcriptional changes in ACLF. Together, these findings are consistent with dramatic increases in de novo production of neutrophils by bone marrow, a process called emergency granulopoiesis, in patients with ACLF; this results from enhanced myeloid precursor cell proliferation in response to PAMPs (e.g. lipopolysaccharide [LPS]) or cytokine storm (that includes elevated blood levels of granulocyte colony-stimulating factor [G-CSF]).52 Indeed, myeloid precursor cells express pattern recognition receptors that sense PAMPs (e.g. Toll-like receptor [TLR]4 that senses LPS) and cytokine receptors (e.g., receptors for G-CSF).52
Weiss, de la Grange et al. also showed that activated blood neutrophils from patients with ACLF overexpressed the protein CD177 and that CD177-overexpressing neutrophils firmly adhered to endothelial cells.5 This increased adhesion of neutrophils to the endothelium may be the first step in the migration of neutrophils to tissues, which is otherwise favoured by endothelial dysfunction combined with the overproduction of the master neutrophil-attracting chemokine IL-8 by inflamed tissues.5
Of note, additional functional studies indicated that, despite being activated at the transcriptional level, the production of reactive oxygen species (ROS) stimulated by N-formylmethionyl-leucyl-phenylalanine was markedly reduced in neutrophils from patients with ACLF.5 ROS release plays a crucial role in neutrophil-mediated bacterial killing and thus a defect in ROS production by neutrophils may contribute to the elevated prevalence of infections in patients with ACLF, at their presentation and during follow-up (secondary infections).
Monocytes
Weiss, de la Grange et al. showed that gene signatures related to monocytes were overrepresented among genes that were upregulated in blood from patients with ACLF, indicating that transcription was also increased in monocytes from these patients.5 Using the CIBERSORT software package to deconvolute the results of blood RNA expression, they found that increased RNA features were related to monocytes with a “macrophage M0-like phenotype”, whereas signatures related to other monocyte subsets (i.e., signatures for M1-like and M2-like phenotypes) were not changed. Thus, these findings suggest that alterations of the monocyte compartment may not be homogeneous in ACLF. The results of some studies are consistent with this hypothesis. A recent study which used flow cytometry in blood from patients with ACLF showed that these patients had decreases in the frequency of classical monocytes (CD14+CD16-), increases in intermediate monocytes (CD14+CD16+), and no changes in non-classical monocytes (CD14dimCD16+).53 Moreover, classical monocytes, but not the other monocyte subsets, exhibited reduced expression of the HLA-DR isotype. In addition, elevated frequencies of cells producing the anti-inflammatory cytokine IL-10 were found among the classical and intermediate monocyte populations. Finally, transcriptional profiling of isolated classical monocytes in ACLF revealed upregulation of an array of immunosuppressive parameters and compromised antibacterial and antigen presentation machinery.53 Other studies mainly based on flow cytometry have shown an increased frequency of circulating CD14+ monocytes expressing the receptor tyrosine kinase MerTK54 and CD14+CD15-HLADR- myeloid-derived suppressor cells55 in patients with ACLF. Both subsets of myeloid mononuclear cells had suppressed innate responses to bacterial PAMPs, suggesting that some mechanisms induced a tolerance to PAMP-induced activation of circulating mononuclear cells from patients with ACLF. Of note, MerTK is induced by tolerogenic stimuli such as glucocorticoids56 and is known to inhibit the innate immune response.57 The role for PGE2 in the suppression of innate immune responses in acutely decompensated cirrhosis is still controversial (see above).45,46 It has also been suggested that the tolerant phenotype of peripheral blood immune cells from patients with decompensated cirrhosis may be explained by prolonged exposure to LPS from gut bacteria.58 Therefore, tolerance of mononuclear cells, in addition to functional defects in neutrophils, may contribute to the elevated prevalence of infections reported in patients with ACLF.
Together, these findings suggest that the clinical blood monocyte count is apparently “normal” in patients with ACLF because a significant fraction of circulating monocytes might have left the circulation toward tissues in response to monocyte-attracting chemokines produced by these tissues. The case has been made that MerTK-overexpressing blood monocytes from patients with ACLF migrate to different tissues, including the liver, where they may contribute to tissue homeostasis through clearance of apoptotic cells.56
Dendritic cells
The ACLF-associated profile of circulating DCs is unclear. In their study of the whole-blood transcriptome, Weiss, de la Grange et al. found that genes assigned to DCs were enriched among genes that were upregulated in patients with ACLF compared to healthy individuals.5 There was no overrepresentation of DC genes among genes that were downregulated in patients with ACLF vs. healthy individuals. In contrast, another study found decreased frequencies of DCs and plasmacytoid DCs in patients with ACLF related to severe alcohol-related hepatitis.59
Lymphocytes
In their microarray analysis of whole-blood RNA expression in patients with ACLF and healthy individuals, Weiss, de la Grange et al. identified a large number of genes that were downregulated in patients.5 Genes assigned to T cells, NK cells and B cells were overrepresented among genes that were downregulated in ACLF, consistent with the presence of peripheral lymphopenia associated with ACLF. In addition, they performed deconvolution of whole blood transcriptome data using the CIBERSORT software and found that patients with ACLF had decreases in RNA signatures related to resting memory CD4 T cells, CD8 T cells, resting NK cells, and memory B cells. Together, these findings indicate that blood from patients with ACLF is characterised by a decrease in lymphocytes of both the innate (NK cells) and adaptive (T and B cells) immune systems. Of note, all the alterations of the lymphocyte compartment observed in ACLF were absent or much less marked in patients without ACLF. Importantly, these lymphocyte alterations were found at the presentation of ACLF, findings in sharp contrast with those observed in the general population of patients with sepsis in whom lymphopenia is delayed, i.e. observed only in patients with protracted sepsis. Future studies should determine whether peripheral blood lymphopenia associated with ACLF is the result of one or more of the following mechanisms: i) generalised lymphocyte death (a mechanism that has been proposed to explain lymphopenia associated with protracted sepsis60); (ii) the activation of the tryptophan-kynurenine pathway seen in ACLF61 (some kynurenine metabolites known to inhibit T-cell proliferation62 may cause defective renewal of a depleted lymphocyte compartment); iii) emergency granulopoiesis (the expansion of bone marrow granulopoiesis paralleled by a decrease in bone marrow lymphopoiesis52); iv) impaired lymphocyte egress from lymph organs63 and v) migration of lymphocytes from blood to lymphoid and non-lymphoid tissues in response to several lymphocyte-attractant chemokines identified in ACLF. Elucidating the mechanisms of peripheral lymphopenia associated with ACLF is of importance given the crucial role played by lymphocytes in the immune responses against pathogens (Fig. 1).
Metabolic changes in ACLF
Inflammatory responses, which are highly energy-demanding, represent a priority at the organismal level.19 In immune cells, inflammatory signals modulate cell-specific metabolic programmes, a process known as immunometabolism. In non-immune cells, inflammatory signals induce a reallocation of metabolic resources through different mechanisms, including a reduction in the utilisation of nutrients by peripheral organs and the induction of a dormancy programme (hypometabolism).22 Below, we describe the major metabolic alterations present in patients with ACLF.
Anabolic reprograming of innate myeloid cells
Metabolites have been identified with the use of untargeted metabolomics by liquid chromatography coupled to high-resolution mass spectrometry in the blood of a large series of patients prospectively enrolled in the CANONIC study (including patients with and without ACLF) and healthy individuals.64,65 Compared to the other study groups, patients with ACLF showed a generalised accumulation of metabolites in peripheral blood. The most distinctive features that characterise the metabolomic signature of patients with ACLF are as follows: First, in patients with ACLF, there is evidence of channelling of intracellular glucose into cytosolic aerobic glycolysis, as indicated by: i) increased blood levels of lactate (pyruvate, the end-product of glycolysis, being preferentially used to produce lactate rather than to fuel the mitochondrial Krebs cycle), and ii) increased blood levels of metabolites of the pentose phosphate pathway (a branch of glycolysis that produces NADPH and fuels de novo purine and pyrimidine synthesis through ribose-5-phosphate). All these features suggest a preferential cellular use of glucose for anabolism rather than for energy production (ATP) in mitochondria, a preference known as the Warburg effect. Second, a metabolite signature of activated serine-glycine one-carbon metabolism (folate cycle, which is also involved in nucleotide synthesis) has been observed in ACLF. Third, a metabolic network comprising the amino acids aspartate and glutamate and the methionine cycle is activated, contributing to increased nucleotide synthesis. In addition, activation of the methionine cycle is associated with engagement of the transsulfuration pathway, which is an antioxidant process. Together, these findings are consistent with the capture of the anabolic reprogramming of innate myeloid cells by the blood metabolome, as described in Box 2.
Box 2. High metabolic demand of activated innate myeloid cells and adaptation of non-immune organs to immune metabolic demand.
GDF15, growth and differentiation factor 15; IL-, interleukin-; PAMPs, pathogen-associated molecular patterns; TNFα, tumour necrosis factor-α.
A limitation of blood metabolomic studies in cirrhosis is that it is difficult to discern whether the metabolic changes are due to liver failure per se or to changes in the metabolic programmes within the circulating immune cells. However, we propose that the metabolic changes observed in the blood of patients with ACLF are likely related to granulopoiesis, metabolic alterations in circulating innate myeloid cells, or both. This view is supported by recent work by Zhang et al., which demonstrated similar changes in the blood metabolome and in immune cells freshly isolated from patients with ACLF.66 Indeed, using metabolic flux and gene expression analyses, these authors showed that leukocytes from patients with ACLF preferentially use intracellular glucose through aerobic glycolysis and the pentose phosphate pathway.66 They also confirmed that glutamine-derived glutamate fuelled reactions of the Krebs cycle, giving rise to aspartate which is involved in de novo nucleotide synthesis. They also found evidence of decreased mitochondrial fatty acid β-oxidation in these cells, suggesting that fatty acids were skewed to molecular synthesis instead of ATP production.66 Importantly, with the use of transmission electron microscopy, Zhang et al. demonstrated that the number of mitochondria per leukocyte was higher in patients with ACLF, accompanied by a reduction in their size and changes in mitochondrial ultrastructure distinguished by cristae rarefication and swelling (Fig. 3),66 findings that require further studies given the central role of mitochondria in immunometabolism. To better summarise all this information and integrate it with systemic inflammation and organ failure in peripheral organs, a simplified schematic representation of the major abnormalities in the metabolism of glucose, fatty acids and amino acids in patients with ACLF is provided (Fig. 4).
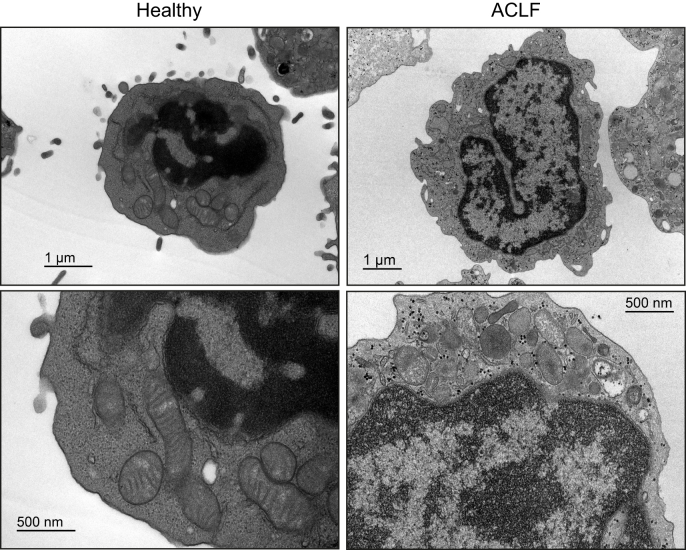
Fig. 3.
Representative electron microscopy images at different magnifications of leukocytes from healthy individuals and from patients with ACLF.
Leukocytes from patients with acute-on-chronic liver failure (ACLF) present higher numbers of mitochondria per cell, but of smaller size. These leukocytes also present altered mitochondrial morphology characterized by more apparent cristae rarefication, swelling and lack of connection. These alterations in mitochondrial ultrastructure indicate severely disorganized mitochondria and extensive degradation of these organelles in blood leukocytes from patients with ACLF. Images reproduced from Zhang et al. J Hepatol. 2022;76:93-106.66
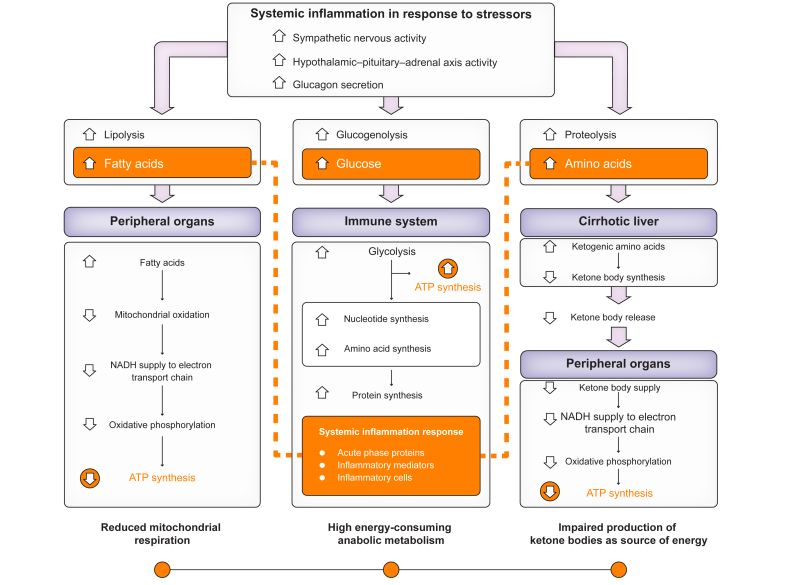
Fig. 4.
This figure shows the three major disorders of energetic metabolism in patients with ACLF.
i) Increased systemic catabolic metabolism in response to systemic inflammation (upper part of the panel), which leads to intense lipolysis, glycogenolysis and proteolysis and release of fatty acids, glucose and amino acids to the immune system and peripheral organs; ii) anabolic and increased energetic metabolism (ATP synthesis) by innate immune cells (central box); iii) reduced mitochondrial respiration and energy production by peripheral (non-immune) cells (lateral boxes). Figure adapted from Arroyo et al. J Hepatol. 2021;74:670-685.
“Alarm response” and intense peripheral catabolism
Patients with ACLF exhibit metabolic features related to the “alarm response” mediated by the central nervous system. For example, metabolomic studies found that ACLF was associated with elevated blood levels of 4-hydroxy-3-methoxyphenylglycol sulphate, a marker of increased sympathetic nervous activity.64,65 Also, patients with ACLF have elevated levels of the mitokine GDF15 (growth and differentiation factor 15),66 which is known to be produced by immune and non-immune cells during acute inflammation and to act on the brain stem to stimulate sympathetic outflow to the liver and the hepatic release of triglycerides.67 In addition, the blood metabolome of patients with ACLF exhibits elevated levels of most proteinogenic amino acids released secondarily to skeletal muscle catabolism, which is a consequence of the “alarm response”.64,65 Furthermore, an untargeted lipidomics study showed that the circulating lipid landscape of patients with ACLF was characterised by marked increases in fatty acids, reflecting inflammation-enhanced lipolysis.68 Together, these findings are consistent with the view that signals related to acute inflammation stimulate the brain stem to elicit an “alarm response” which controls peripheral metabolism (see Box 2 for a summary of the most relevant features of the “alarm response”). Although more data need to be collected, we suggest that ACLF-associated acute inflammation is associated with an intense peripheral catabolism resulting in the release of nutrients (amino acids, fatty acids) from peripheral storage sites (skeletal muscles, adipose tissue) that likely fuel the vigorous anabolic demand of activated innate myeloid cells.
Another metabolic characteristic of patients with ACLF is blood accumulation of products of the catabolism of amino acids which are known to be neurotoxic, including quinolinic acid (a metabolite of the kynurenine pathway), pipecolate (a product of lysine degradation) and N-acetyl-L-aspartic acid (derived from aspartate).61,64,65 These metabolites may, therefore, be involved in the development of brain failure in patients with ACLF.
Reprograming of hepatic fatty acid β-oxidation
Ketone bodies become an important source of energy in ACLF because glucose is prioritised to fuel inflammatory responses and because of glucose scarcity (related to the sickness behaviour of anorexia). As expected in the context of acute systemic inflammation,22 patients with ACLF exhibit increased blood levels of acylcarnitines, reflecting the inhibition of hepatic β-oxidation and, therefore, suppression of this source of ketone bodies.64 These patients also exhibit features of increased catabolism of ketogenic amino acids, a logical mechanism to compensate for the inhibition of β-oxidation. However, blood metabolomics did not detect any increase in ketone bodies in blood from patients with ACLF,64 suggesting that the activation of ketogenic amino acid catabolism failed to give rise to its end-products, i.e. ketone bodies. These findings suggest that ACLF is associated with a dramatic decrease in the production of ketone bodies and, therefore, deprivation of an important source of energy for peripheral tissues.
Prognostic value of metabolic changes
Several blood metabolites have been shown to be associated with short-term death (by 28 and 90 days after presentation) in a large series of patients with acutely decompensated cirrhosis who had not received early liver transplantation.69 Moreover, with the use of multivariable analyses, a prognostic score was developed which included, age, the international normalised ratio, bilirubin, 4-hydroxy-3-methoxyphenylglycol sulphate and the acylcarnitine, hexanoylcarnitine; this score was more accurate for assessing short-term death than other prognostic scores, including the MELD-Na score, the CLIF-C ACLF score (for patients with ACLF) and the CLIF-C AD score (for patients with acutely decompensated cirrhosis without ACLF).69 Together these findings suggest that metabolites may be of prognostic value because they are markers of metabolic alterations that underlie poor outcomes in patients with acutely decompensated cirrhosis.
Unifying hypothesis for organ failure development in ACLF
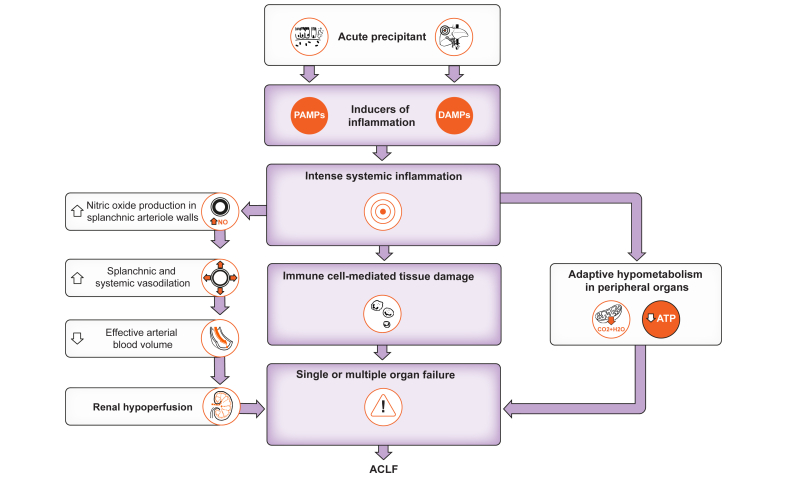
Portal hypertension and liver failure are determinants of hallmark complications of cirrhosis, including ascites, hyperammonaemia-related hepatic encephalopathy, and variceal haemorrhage. In addition, splanchnic and systemic vasodilation, both components of the hyperdynamic circulation in patients with advanced cirrhosis, stimulate endogenous anti-natriuretic systems that promote avid sodium retention by renal tubules and subsequent fluid accumulation (ascites).70 We suggest that, on top of these mechanisms, intense acute inflammation is a driving mechanism that explains the development of organ failure in patients with acutely decompensated cirrhosis. Our hypothesis is as follows (Fig. 5). Inflammation starts in a tissue because of infection, tissue damage (e.g. liver injury), or both (e.g. tissue damage caused by microbial pathogens). In the context of infection, the inducers of inflammation are PAMPs and virulence factors, whereas DAMPs are inducers of inflammation in the context of tissue damage. When local inflammatory responses (that involve various myeloid and lymphoid cells and a variety of cytokines and lipid mediators) fail to eliminate the source of perturbation (i.e. pathogens, tissue damage, or both), local inflammation becomes more intense (with a spillover of locally produced cytokines into the blood), and the immune system engages in intense systemic inflammation, which can cause organ failure through five different mechanisms that are not mutually exclusive. First, PAMPs, cytokines (e.g., TNFα) and lipid mediators (e.g. leukotriene B4) stimulate the production of the vasorelaxant nitric oxide in the walls of the splanchnic arterioles. This effect leads to enhanced splanchnic and systemic vasodilation, which induces enhanced activity of endogenous vasoconstrictor systems that cause renal vasoconstriction and acute kidney injury. Second, PAMPs, cytokines (e.g. G-CSF) and lipid mediators (e.g. PGE2) stimulate emergency granulopoiesis, which explains the neutrophilia seen in patients with ACLF. Neutrophils that reach the systemic circulation are rewired to adhere to the vascular endothelium (e.g. through the surface CD177 protein). Cytokines/chemokines (e.g. IL-8) and lipid mediators (e.g. leukotriene B4) activate the endothelium in microvessels of vital organs, an effect that favours the migration of neutrophils (and probably other leukocytes) in surrounding tissues where neutrophils can cause tissue damage (a process called immunopathology) and thereby contribute to organ failure. Third, cytokines and lipid mediators (e.g. thromboxane A2) promote the formation of microthrombi in microvessels, an effect that impairs tissue oxygenation and therefore function. Fourth, acute inflammation stimulates intense peripheral catabolism of amino acids, the products of which may be metabotoxins affecting central nervous system activity. These toxic metabolites may, therefore, contribute to brain failure in ACLF. Fifth, acute inflammatory responses, which include the production of a broad variety of biomolecules (protein and lipids), respiratory burst, acute-phase response, and increases in biomass (i.e., granulopoiesis requiring de novo nucleotide synthesis) are energetically expensive processes that require large amounts of nutrients. Therefore, immunity competes with other maintenance programmes for energy. In patients with ACLF, activated innate immune responses compete with energy-consuming processes such as an elevated resting cardiac output, active sodium reabsorption by renal tubules, and active transport systems within the liver, among others. In the liver, the induction of the hepatic acute-phase response (i.e., de novo synthesis of a broad variety of secretory proteins such as CRP, all involved in the host immune response)71 by IL-6 and TNFα competes for energy with several ATP-consuming transport systems. The brain stem integrates the energy demand of each organ system, with immunity considered a top priority. We hypothesise that the brain stem “makes the decision” of a trade-off, which is the induction of a dormancy programme (similar to torpor) allowing for the shutdown of mitochondrial oxygen consumption (i.e. respiration: the reduction of oxygen into water) and oxidative phosphorylation (i.e. ATP production) in peripheral organs. In the context of acutely decompensated cirrhosis, the consequence of a shutdown of mitochondrial respiration and ATP production is a dramatic reduction in organ function (e.g. decreases in kidney function or a decrease in cardiac output as described in ref.72) In our hypothesis, liver failure would indicate the engagement of an energy trade-off; in other words, liver failure would result in large part from the arrest of ATP-consuming transport systems as an adaptation to the increased energy demand related to de novo synthesis of a broad variety of acute-phase proteins which are prioritised because of their major role in host defence.71
Fig. 5.
Unifying hypothesis for organ failure development in ACLF.
The proposed hypothesis is a “snapshot” taken when ACLF has developed. Further studies should investigate patients longitudinally, before the development of ACLF, to better understand the dynamics of immune and metabolic changes that precede the development of organ failures.
Financial support
EF CLIF, a non-profit private organization, receives unrestricted donations from Cellex Foundation, Grifols and European Union’s Horizon 2020 research and innovation programme (825694 and 847949). JC laboratory is a Consolidated Research Group recognized by the Generalitat de Catalunya (2021 SGR 01323) and is supported by the Spanish Ministerio de Ciencia e Innovacion (PID2019-105240RB-I00 and PID2022-138970OB-I00).
Authors’ contributions
All authors participated in drafting and writing of the manuscript and were involved in its critical revision for intellectual content.
Conflict of interest
None of the authors have competing financial interests to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank Lídia García-Campmany and Marta Duran-Güell for assistance in the preparation of the manuscript.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100807.
Contributor Information
Joan Clària, Email: jclaria@clinic.cat.
Richard Moreau, Email: richard.moreau@inserm.fr.
Supplementary data
The following are the supplementary data to this article.
References
- 1.Gu W., Hortlik H., Erasmus H.P., Schaaf L., Zeleke Y., Uschner F.E., et al. Trends and the course of liver cirrhosis and its complications in Germany: nationwide population-based study (2005 to 2018) Lancet Reg Health Eur. 2021;12 doi: 10.1016/j.lanepe.2021.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreau R., Jalan R., Ginès P., Pavesi M., Angeli P., Cordoba J., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo V., Moreau R., Jalan R. Acute-on-Chronic liver failure. N Engl J Med. 2020;382:2137–2145. doi: 10.1056/NEJMra1914900. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo V., Moreau R., Kamath P.S., Jalan R., Ginès P., Nevens F., et al. Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.41. [DOI] [PubMed] [Google Scholar]
- 5.Weiss E., de la Grange P., Defaye M., Lozano J.J., Aguilar F., Hegde P., et al. Characterization of blood immune cells in patients with decompensated cirrhosis including ACLF. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.619039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clària J., Stauber R.E., Coenraad M.J., Moreau R., Jalan R., Pavesi M., et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–1264. doi: 10.1002/hep.28740. [DOI] [PubMed] [Google Scholar]
- 7.Trebicka J., Amoros A., Pitarch C., Titos E., Alcaraz-Quiles J., Schierwagen R., et al. Addressing profiles of systemic inflammation across the different clinical phenotypes of acutely decompensated cirrhosis. Front Immunol. 2019;10:476. doi: 10.3389/fimmu.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trebicka J., Fernández J., Papp M., Caraceni P., Laleman W., Gambino C., et al. The Predict study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro S., Grandt J., Uschner F.E., Kimer N., Madsen J.L., Schierwagen R., et al. Differential inflammasome activation predisposes to acute-on-chronic liver failure in human and experimental cirrhosis with and without previous decompensation. Gut. 2021;70:379–387. doi: 10.1136/gutjnl-2019-320170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C., et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74:1097–1108. doi: 10.1016/j.jhep.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Fernández J., Acevedo J., Wiest R., Gustot T., Amoros A., Deulofeu C., et al. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870–1880. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annunziato F., Romagnani C., Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626–635. doi: 10.1016/j.jaci.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Rankin L.C., Artis D. Beyond host defense: emerging functions of the immune system in regulating complex tissue physiology. Cell. 2018;173:554–567. doi: 10.1016/j.cell.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Wang A., Luan H.H., Medzhitov R. An evolutionary perspective on immunometabolism. Science. 2019;363:eaar3932. doi: 10.1126/science.aar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florsheim E.B., Sullivan Z.A., Khoury-Hanold W., Medzhitov R. Food allergy as a biological food quality control system. Cell. 2021;184:1440–1454. doi: 10.1016/j.cell.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374:1070–1075. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- 20.Mayassi T., Barreiro L.B., Rossjohn J., Jabri B. A multilayered immune system through the lens of unconventional T cells. Nature. 2021;595:501–510. doi: 10.1038/s41586-021-03578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganeshan K., Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganeshan K., Nikkanen J., Man K., Leong Y.A., Sogawa Y., Maschek J.A., et al. Energetic trade-offs and hypometabolic states promote disease tolerance. Cell. 2019;177:399–413. doi: 10.1016/j.cell.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanderson S.M., Gao X., Dai Z., Locasale J.W. Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nat Rev Cancer. 2019;19:625–637. doi: 10.1038/s41568-019-0187-8. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y., Yang Y., Hu Y., Wu W., Yang Q., Zheng M., et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232–242. doi: 10.1002/hep.27795. [DOI] [PubMed] [Google Scholar]
- 25.Schütte A., Ciesek S., Wedemeyer H., Lange C.M. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797–799. doi: 10.1016/j.jhep.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Fernández J., Piano S., Bartoletti M., Wey E.Q. Management of bacterial and fungal infections in cirrhosis: the MDRO challenge. J Hepatol. 2021;75(Suppl 1):101–117. doi: 10.1016/j.jhep.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Piano S., Singh V., Caraceni P., Maiwall R., Alessandria C., Fernandez J., et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019;156:1368–1380. doi: 10.1053/j.gastro.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Van der Merwe S., Chokshi S., Bernsmeier C., Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 2021;75(Suppl 1):S82–S100. doi: 10.1016/j.jhep.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Trebicka J., Bork P., Krag A., Arumugam M. Utilizing the gut microbiome in decompensated cirrhosis and acute-on-chronic liver failure. Nat Rev Gastroenterol Hepatol. 2021;18:167–180. doi: 10.1038/s41575-020-00376-3. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald S., Andreola F., Bachtiger P., Amoros A., Pavesi M., Mookerjee R., et al. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology. 2018;67:989–1002. doi: 10.1002/hep.29581. [DOI] [PubMed] [Google Scholar]
- 31.Bataller R., Arab J.P., Shah V.H. Alcohol-associated hepatitis. N Engl J Med. 2022;387:2436–2448. doi: 10.1056/NEJMra2207599. [DOI] [PubMed] [Google Scholar]
- 32.Sharma S., Maras J.S., Das S., Hussain S., Mishra A.K., Shasthry S.M., et al. Pre-therapy liver transcriptome landscape in Indian and French patients with severe alcoholic hepatitis and steroid responsiveness. Sci Rep. 2017;7:6816. doi: 10.1038/s41598-017-07161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan Y., Llorente C., Lang S., Brandl K., Chu H., Jiang L., et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang L., Lang S., Duan Y., Zhang X., Gao B., Chopyk J., al Set. Intestinal virome in patients with alcoholic hepatitis. Hepatology. 2020;72:2182–2196. doi: 10.1002/hep.31459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lang S., Duan Y., Liu J., Torralba M.G., Kuelbs C., Ventura-Cots M., et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–538. doi: 10.1002/hep.30832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karakike E., Moreno C., Gustot T. Infections in severe alcoholic hepatitis. Ann Gastroenterol. 2017;30:152–160. doi: 10.20524/aog.2016.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L.W., Egan L., Li Z.W., Greten F.R., Kagnoff M.F., Karin M. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 39.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 42.Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem. 2012;287:10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 44.Clària J., Arroyo V. Prostaglandins and other cyclooxygenase-dependent arachidonic acid metabolites and the kidney in liver disease. Prostaglandins Other Lipid Mediat. 2003;72:19–33. doi: 10.1016/s1098-8823(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien A.J., Fullerton J.N., Massey K.A., Auld G., Sewell G., James S., et al. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518–523. doi: 10.1038/nm.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Vicario C., Checa A., Urdangarin A., Aguilar F., Alcaraz-Quiles J., Caraceni P., et al. Targeted lipidomics reveals extensive changes in circulating lipid mediators in patients with acutely decompensated cirrhosis. J Hepatol. 2020;73:817–828. doi: 10.1016/j.jhep.2020.03.046. [DOI] [PubMed] [Google Scholar]
- 47.Casulleras M., Flores-Costa R., Duran-Güell M., Zhang I.W., López-Vicario C., Curto A., et al. Albumin lipidomics reveals meaningful compositional changes in advanced cirrhosis and its potential to promote inflammation resolution. Hepatol Commun. 2022;6:1443–1456. doi: 10.1002/hep4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clària J., Titos E., Jiménez W., Ros J., Ginès P., Arroyo V., Rivera F., Rodés J. Altered biosynthesis of leukotrienes and lipoxins and host defense disorders in patients with liver cirrhosis. Gastroenterology. 1998;115:147–156. doi: 10.1016/s0016-5085(98)70376-2. [DOI] [PubMed] [Google Scholar]
- 49.Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med. 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez-Rodríguez M.B., Téllez E., Casulleras M., Borràs F.E., Arroyo V., Clària J., Sarrias M.R. Reduced plasma extracellular vesicle CD5L content in patients with acute-on-chronic liver failure: interplay with specialized pro-resolving lipid mediators. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.842996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becares N., Härmälä S., China L., Colas R.A., Maini A.A., Bennet K., Skene S.S., Shabir Z., Dalli J., O'Brien A. Immune regulatory mediators in plasma from patients with acute decompensation are associated with 3-month mortality. Clin Gastroenterol Hepatol. 2020;18:1207–1215. doi: 10.1016/j.cgh.2019.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manz M.G., Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 53.Korf H., du Plessis J., van Pelt J., De Groote S., Cassiman D., Verbeke L., et al. Inhibition of glutamine synthetase in monocytes from patients with acute-on-chronic liver failure resuscitates their antibacterial and inflammatory capacity. Gut. 2019;68:1872–1883. doi: 10.1136/gutjnl-2018-316888. [DOI] [PubMed] [Google Scholar]
- 54.Bernsmeier C., Pop O.T., Singanayagam A., Triantafyllou E., Patel V.C., Weston C.J., et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology. 2015;148:603–615. doi: 10.1053/j.gastro.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 55.Bernsmeier C., Triantafyllou E., Brenig R., Lebosse F.J., Singanayagam A., Patel V.C., et al. CD14+ CD15- HLA-DR- myeloid-derived suppressor cells impair antimicrobial responses in patients with acute-on-chronic liver failure. Gut. 2018;67:1155–1167. doi: 10.1136/gutjnl-2017-314184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zagórska A., Través P.G., Lew E.D., Dransfield I., Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol. 2014;15:920–928. doi: 10.1038/ni.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothlin C.V., Ghosh S., Zuniga E.I., Oldstone M.B., Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 58.Weiss E., Rautou P.E., Fasseu M., Giabicani M., de Chambrun M., Wan J., et al. Type I interferon signaling in systemic immune cells from patients with alcoholic cirrhosis and its association with outcome. J Hepatol. 2017;66:930–941. doi: 10.1016/j.jhep.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Weichselbaum L., Azouz A., Smolen K.K., Das J., Splittgerber M., Lepida A., et al. Epigenetic basis for monocyte dysfunction in patients with severe alcoholic hepatitis. J Hepatol. 2020;73:303–314. doi: 10.1016/j.jhep.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 60.Hotchkiss R.S., Opal S.M. Activating immunity to fight a foe - a new path. N Engl J Med. 2020;382:1270–1272. doi: 10.1056/NEJMcibr1917242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clària J., Moreau R., Fenaille F., Amorós A., Junot C., Gronbaek H., et al. Orchestration of tryptophan-kynurenine pathway, acute decompensation, and acute-on-chronic liver failure in cirrhosis. Hepatology. 2019;69:1686–1701. doi: 10.1002/hep.30363. [DOI] [PubMed] [Google Scholar]
- 62.Belladonna M.L., Puccetti P., Orabona C., Fallarino F., Vacca C., Volpi C., et al. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation. 2007;84:S17–S20. doi: 10.1097/01.tp.0000269199.16209.22. [DOI] [PubMed] [Google Scholar]
- 63.Nakai A., Hayano Y., Furuta F., Noda M., Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreau R., Clària J., Aguilar F., Fenaille F., Lozano J.J., Junot C., et al. Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol. 2020;72:688–701. doi: 10.1016/j.jhep.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Zaccherini G., Aguilar F., Caraceni P., Clària J., Lozano J.J., Fenaille F., et al. Assessing the role of amino acids in systemic inflammation and organ failure in patients with ACLF. J Hepatol. 2021;74:1117–1131. doi: 10.1016/j.jhep.2020.11.035. [DOI] [PubMed] [Google Scholar]
- 66.Zhang I.W., Curto A., López-Vicario C., Casulleras M., Duran-Güell M., Flores-Costa R., et al. Mitochondrial dysfunction governs immunometabolism in leukocytes of patients with acute-on-chronic liver failure. J Hepatol. 2022;76:93–106. doi: 10.1016/j.jhep.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Luan H.H., Wang A., Hilliard B.K., Carvalho F., Rosen C.E., Ahasic A.M., Herzog E.L., Kang I., Pisani M.A., Yu S., Zhang C., Ring A.M., Young L.H., Medzhitov R. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. 2019;178:1231–1244. doi: 10.1016/j.cell.2019.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clària J., Curto A., Moreau R., Colsch B., López-Vicario C., Lozano J.J., et al. Untargeted lipidomics uncovers lipid signatures that distinguish severe from moderate forms of acutely decompensated cirrhosis. J Hepatol. 2021;75:1116–1127. doi: 10.1016/j.jhep.2021.06.043. [DOI] [PubMed] [Google Scholar]
- 69.Weiss E., de la Peña-Ramirez C., Aguilar F., Lozano J.J., Sánchez-Garrido C., Sierra P., et al. Sympathetic nervous activation, mitochondrial dysfunction and outcome in acutely decompensated cirrhosis: the metabolomic prognostic models (CLIF-C MET) Gut. 2023;72:1581–1591. doi: 10.1136/gutjnl-2022-328708. gutjnl-2022-328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gadano A., Moreau R., Heller J., Chagneau C., Vachiéry F., Trombino C., et al. Relation between severity of liver disease and renal oxygen consumption in patients with cirrhosis. Gut. 1999;45:117–121. doi: 10.1136/gut.45.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mantovani A., Garlanda C. Humoral innate immunity and acute-phase proteins. N Engl J Med. 2023;388:439–452. doi: 10.1056/NEJMra2206346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruiz-del-Arbol L., Monescillo A., Arocena C., Valer P., Ginès P., Moreira V., et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.