Abstract
Quinoa bran is a by-product during quinoa processing, which is not well used due to its high content of antinutritional factors. The nutritional, antinutritional, antioxidative and mineral content were analyzed in quinoa bran from five producing areas (Hebei, Shanxi, Qinghai, Inner Mongolia and Gansu Province) in China. The results showed that the mean values of protein, starch, fat, fiber, reducing sugar, ash, moisture and energy in quinoa bran were 9.35%, 47.37%, 8.26%, 10.74%, 3.68%, 6.25%, 9.29% and 360.2 kcal/100 g, respectively. Although the protein content in quinoa bran is lower than that in quinoa grain, it is comparable to that in other grains (rice, corn, millet and sorghum) and brans (wheat, oat and rice), so it has the commercial potential to be processed into animal feed or other edible food. The contents of antioxidant flavonoids (460.9 mg/100g) and polyphenols (477.8 mg/100 g) in quinoa bran were higher than those in quinoa grain, suggesting that quinoa bran had better antioxidant capacity. The contents of saponins, tannins and phytic acid in quinoa bran were 18.65, 0.30 and 0.73%, respectively. The content of saponins was nearly one times higher than that in quinoa grain, the contents of tannins and phytic acid, however, were lower than those in quinoa grain. Therefore, the removal of saponins is the key to eliminate the antinutritional properties of quinoa bran. The contents of macroelements (sodium, potassium, calcium, magnesium, phosphorus) and microelements (iron, manganese, copper, zinc, cobalt, molybdenum, selenium, barium) in quinoa bran were generally higher than those in quinoa grain, which was consistent with the results of ash determination. In summary, quinoa bran was found to be a rich source of nutritional and bioactive components and minerals. If the antinutritional problem can be overcome, quinoa bran has great potential for application in the food industry.
Keywords: Quinoa bran, Nutrition, Antinutrition, Antioxidant, Macroelement, Microelement
Graphical abstract
Highlights
-
•
Quinoa bran was a rich source of nutritional and bioactive components and minerals.
-
•
Quinoa bran contains more antinutritional factors than quinoa grains.
-
•
The removal of saponins from quinoa bran is the key to reduce its adverse effect.
1. Introduction
Quinoa is a new food crop introduced in China from South America in recent years. After more than ten years of development, quinoa has been expanded to more than 10 provinces, and the planting area has reached more than 20,000 hm2 (Liu et al., 2021; Chen, 2021). According to the yield of quinoa per hectare at 2.25 t/hm2, the total annual yield of quinoa in China has reached 45,000 tonnes (Cui et al., 2019). Globally, the annual yield of quinoa stands at 175,188 tonnes (Chaudhary et al., 2023). Quinoa is a kind of pseudocereal of Chenopodiaceae family (Thakur et al., 2021), in which the achenes (seeds) are the traditionally edible parts. However, the seed coat contains saponins with bitter taste (Galwey et al., 1990; Han et al., 2019; Wang et al., 2020a), which not only affects its consumption, but also has certain antinutritional effects on animal and human health (Reichert et al., 1986; Ma et al., 1989; Vo et al., 2017; Hou et al., 2018). Therefore, the saponins should be removed before consumption. Currently, the main methods for the removal of saponin from quinoa were mechanical pearling, water washing and combinations of both (Ridout et al., 1991; Nickel et al., 2016; Ma, 2020; Cao et al., 2023), among which the mechanical peeling is the most widely used method. During the quinoa seeds hulling process, approximately 10% (weight percentage) of quinoa bran is produced (Carlson et al., 2012). According to the annual yield of quinoa in China (45,000 tonnes) (Cui et al., 2019) and the world (175,188 tonnes) (Chaudhary et al., 2023), the annual output of quinoa bran is about 4500 and 17518 tonnes, respectively.
Quinoa bran has high nutritional value (Zhang et al., 2020; Song et al., 2022). Xue et al. (2019) reported that the crude protein content of quinoa bran prepared by milling method was up to 30%, which has the potential to be used in animal feed. However, the antinutritional factors such as saponins, phytic acids and tannins (Thakur et al., 2021; Ruales and Nair, 1993a) in quinoa bran may affect the animal appetite and inhibit the bioavailability and absorption of nutrients and some of them are toxic (Improta and Kellems, 2001; Zhang et al., 2020). In the case of saponins, although the husk of quinoa accounted for only 10–12% of the seed weight (Hemalatha et al., 2016), it contained 86% of the total saponins (Ando et al., 2002; Ruiza et al., 2017; Sun et al., 2019; Rafik et al., 2021). Therefore, the saponin content of quinoa bran is higher than that of quinoa grain. Carlson et al. (2012) showed that the saponin content in hull meal from quinoa grown in South America was as high as 28.7%, and the bitter taste of quinoa bran affected the appetite of domestic piglets. When the piglets had been fed a diet containing 500 mg/kg quinoa hull meal, the feed intake and weight gain were numerically lower, indicating that the concentration of quinoa hull meal should not exceed this level to avoid negative effects on the production. Therefore, it is of practical significance to systematically evaluate the nutritional, antinutritional and biological activities of quinoa bran for its comprehensive utilization.
However, the potential use of quinoa bran in the food industry has so far escaped the attention of researchers. In order to evaluate the nutritional value and application potential of quinoa bran and to provide ideas for solving this problem, five representative samples of quinoa bran were collected from the major quinoa planting provinces (Qinghai, Gansu, Inner Mongolia, Shanxi and Hebei) in China. The contents of nutrients (protein, starch, fat, crude fiber, reducing sugar, ash), antioxidant components (flavonoids, polyphenols), antinutritional components (saponin, tannin, phytic acid) and minerals were comprehensively analyzed to explore the application potential of quinoa bran in food and feed, and to provide important reference for the development and utilization of quinoa bran.
2. Materials and methods
2.1. Materials
The quinoa bran samples and their sources are shown in Fig. 1. Quinoa bran of HB was provided by North Wheat Ecological Agriculture Limited Company, Guyuan County, Hebei Province. Quinoa bran of SX was provided by Shanxi Yilong Quinoa Development Limited Company, Jingle County, Shanxi Province. Quinoa bran of QH was provided by Qinghai Rongqia Ecological Agriculture Science and Technology Limited Company, Huzhu County, Qinghai Province. Quinoa bran of NM was provided by Inner Mongolia Yiji Biotechnology Limited Company, Hohhot City, Inner Mongolia Autonomous Region. Quinoa bran of GS was provided by Gansu Purity Plateau Agricultural Technology Limited Company, Wuwei City, Gansu Province. The chemical reagents used in the present study were of analytical grade and purchased from Sigma Chemical Co., Merck China and Xilong Scientific Co., China.
Fig. 1.
Quinoa bran from five provinces and autonomous regions in China.
SX: Shanxi Province; GS: Gansu Province; QH: Qinghai Province.
HB: Hebei Province; NM: Inner Mongolia.
2.2. Proximate composition of quinoa bran
The protein content of quinoa bran was determined by Kjeldahl method and the amount of protein was calculated as 6.25 × N (Nitrogen content) (Pedrali et al., 2023; Ruales and Nair, 1992). Fat was measured in a Soxtec system by extraction with petroleum ether (boiling range 30–60 °C). The reducing sugar content was determined on the basis of cupric reduction (Bhinder et al., 2021). The starch content of quinoa bran was determined according to GB/T 5009.9-2016 (State Food and Drug Administration of China, 2016). The starch was hydrolyzed into monosaccharides by amylase and hydrochloric acid successively, then the content of reducing sugar was determined. The content of starch was calculated based on the reducing sugar. The total ash content was determined by Muffle furnace burning method at 550 ± 25 °C for 4 h. The moisture content was determined by drying method at 105 ± 2 °C for 2 h. The crude fiber content of quinoa bran was determined by acid hydrolysis method (Zerlasht et al., 2023).
The total carbohydrate content of quinoa bran was calculated by subtracting the percent content of protein, fat, crude fiber, ash content and moisture from 100. Energy value was estimated based on the contents of fat, protein, total carbohydrate and crude fiber using the Atwater factors of 9.0, 4.0, 4.0 and 2.0 kCal/g of each component, respectively (Thakur et al., 2021; Gómez et al., 2021, 2023). The results are expressed as kCal 100 g−1 fresh weight.
2.3. Antioxidant potential evaluation
2.3.1. Determination of flavonoid content
The flavonoid content of quinoa bran was determined according to the method of Sharma et al. (2022) with some modifications. Instead of refluxing extraction twice with 20 mL acidified methanol, the quinoa bran (1.0 g) was extracted with 30 mL 80% ethanol (acidified with 0.1% HCl) at 50 °C for 120 min. After centrifugation and filtration through a 0.22 μm membrane, the supernatant was diluted 30 times. The diluted flavonoid extract solution (0.5 mL) was transferred into a test tube, followed by 0.4 mL NaNO2 solution (5%), 0.4 mL Al(NO3)3 solution (5%), and 4 mL NaOH solution (4%). The volume of the solution was adjusted to 10 mL with 75% ethanol and left to stand for 15 min after evenly mixed. The absorbance of the sample solution was measured at 510 nm by using ultraviolet–visible spectrophotometer (TU-1810, Beijing Puxi General Instrument Co., Ltd, China). Rutin was used as the standard and the concentration of rutin in the standard curve ranged from 0 to 0.05 mg/mL. The content of flavonoids in quinoa bran was expressed as mg rutin equivalents per 100 g of dry sample (Abeysinghe et al., 2007; Lim et al., 2020).
2.3.2. Determination of total polyphenol content
The total polyphenol content of quinoa bran was measured according to the method of Hirose et al. (2010). The quinoa bran was extracted with ethanol: water (2:1 v/v) at 50 °C for 60 min. The extract was centrifuged and filtered through a 0.22 μm membrane. Ten millilitre of water and 0.5 mL polyphenol solution were mixed with 1.0 mL Folin-Ciocalteu reagent. After 5 min, 1.0 mL 10% sodium carbonate solution was added. The volume of the mixture was adjusted to 20 mL by using distilled water. Absorbance was measured at the wavelength of 765 nm against a blank by using ultraviolet–visible spectrophotometer (TU-1810, Beijing Puxi General Instrument Co., Ltd, China), and the polyphenol content in the sample was calculated by gallic acid standard curve. The concentration of gallic acid in the standard curve ranged from 0 to 0.1 mg/mL. The total polyphenol content in the quinoa bran was expressed as mg gallic acid equivalents of per 100 g of dry sample.
2.3.3. Antioxidant activity
Antioxidant activity of quinoa bran was measured by DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging percentage (Sharma et al., 2022). Three milliliters of DPPH standard solution (0.2 mmol/mL) and 0.5 mL sample extract solution was mixed with 2.0 mL anhydrous ethanol. The mixtures were allowed to react in the dark for 30 min. The absorbance (A1) was measured at the wavelength of 517 nm by using ultraviolet–visible spectrophotometer (TU-1810, Beijing Puxi General Instrument Co., Ltd, China). In the second test tube, the absorbance (A2) was measured by replacing the DPPH standard solution with anhydrous ethanol. In the third test tube, the absorbance (A0) was measured by replacing the sample extract solution with anhydrous ethanol. The DPPH free radical scavenging percentage was calculated according to following formula.
2.4. Antinutritional factors evaluation
2.4.1. Determination of saponin content in quinoa bran
The saponin content of quinoa bran was performed according to the method of Medina-Meza et al. (2016) with some modifications. Instead of reflux extraction with 80% methanol for 3 h, the quinoa bran (1.0 g) was transferred into a conical flask (150 mL) and extracted with 30 mL ethanol (75%) in a water bath shaker at 50 °C for 6 h. After centrifugation, the extract solution was diluted 30 times. Under the condition of ice bath, a saponin extract solution (0.5 mL) was transferred into a test tube and mixed with 0.5 mL vanillin-anhydrous ethanol solution (8%) and 4 mL sulfuric acid solution (77%). After mixing evenly, the mixtures were heated at 60 °C in a water bath for 30 min. The test tube was taken out and put into an ice bath to terminate the color reaction. The absorbance of the sample solution was determined at 535 nm by using ultraviolet–visible spectrophotometer (TU-1810, Beijing Puxi General Instrument Co., Ltd, China). The saponin content in quinoa bran was calculated using oleanolic acid standard curve, and the concentration of oleanolic acid in the standard curve ranged from 0 to 0.02 mg/mL.
2.4.2. Determination of tannin content in quinoa bran
Quinoa bran (1.0 g) was placed in a conical flask (150 mL) and extracted with 20 mL dimethylformamide solution (75%) in a water bath shaker at 30 °C for 1 h. After extraction, the conical flask was allowed to stand for 10 min. Six milliliters of solution were taken out from the upper layer of the extract and transferred into a 50 mL centrifuge tube. After centrifugation (5000 g for 5 min), the supernatant was filtered through a 0.22 μm membrane. Then 1.0 mL of tannin extract solution was mixed with 4.0 mL of distilled water, 1.0 mL of ferric ammonium citrate solution (3.5 g/L) and 1.0 mL of ammonia solution (8 g/L). The mixtures reacted at room temperature for 10 min away from light. The absorbance of the reaction solution was measured at wavelength 525 nm by using ultraviolet–visible spectrophotometer (TU-1810, Beijing Puxi General Instrument Co., Ltd, China), and the tannin content was calculated by the tannic acid standard curve (Wang et al., 2020b; Martinez et al., 2020; Bhinder et al., 2021). The concentration of tannic acid in the standard curve ranged from 0 to 0.07 mg/mL.
2.4.3. Determination of phytic acid content in quinoa bran
Quinoa bran (1.0 g) was placed in a conical flask (150 mL) and mixed with 30 mL hydrochloric acid solution (2.4%, w/w). After being treated with ultrasonic water bath for 5 min, they were transferred to a shaker and extracted at 30 °C for 3 h. After centrifugation and filtration (0.22 μm membrane), 1.0 mL of extract solution was mixed with 5.0 mL of hydrochloric acid solution (2.4%), 0.6 mL of ammonium ferric sulfate solution (0.76 mmol/L) in a test tube. They were put into a boiling water bath to react for 20 min, and then quickly cooled to room temperature. 0.2 mL 10% ammonium thiocyanate solution was added to the test tube for color reaction. The absorbance of the samples was determined at 500 nm against distilled water by using ultraviolet–visible spectrophotometer (TU-1810, Beijing Puxi General Instrument Co., Ltd, China). The phytic acid content of the samples was calculated by using the standard curve of sodium phytic acid (Haug and Lantzsch, 1983; Wang et al., 2020b; Bhinder et al., 2021). The concentration of sodium phytic acid in the standard curve ranged from 0 to 0.04 mg/mL.
2.5. Determination of mineral contents
Quinoa bran samples were analyzed for macroelements (sodium, potassium, calcium, magnesium, phosphorus) and microelements (iron, manganese, copper, zinc, cobalt, selenium, molybdenum and barium) by inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7900, Agilent Technologies Co. Ltd., USA). Briefly, quinoa bran (0.6 g) was placed in a microwave digestion tube, and then added 8.0 mL of nitric acid (guaranteed reagent). After overnight soaking, 2.0 mL of hydrogen peroxide (30%) was added and left to stand for 0.5 h before the digestion tube was put into the microwave oven. The microwave digestion procedure was shown in Table 1.
Table 1.
Microwave digestion program of quinoa bran.
| Procedures | Powder/w | Heating up time/min | Temperature/°C | Heating time/min |
|---|---|---|---|---|
| 1 | 1200 | 6 | 120 | 5 |
| 2 | 1200 | 4 | 150 | 3 |
| 3 | 1200 | 3 | 180 | 20 |
After the digestion of samples was completed, the tube was taken out from the microwave oven and left to cool at room temperature. The tubes were transferred into the heating furnace to evaporate the remaining acid to about 1.0 mL. After cooling to room temperature, the solution in tube was transferred to a volumetric bottle (25 mL) with deionized water. The content of mineral elements was determined by ICP-MS.
2.6. Statistical analysis
All experiments were performed in quintuplicate. Data were analyzed using one-way analysis of variance (ANOVA) with SPSS software (IBM Corporation, NY, USA). Results were reported as means ± Standard Deviation (SD). The significance level is p < 0.05. The correlation coefficients between the content of flavonoids or polyphenols and the antioxidant activity (DPPH) were calculated by Microsoft Office Excel (2019), and the significance was tested by unitary linear regression method.
3. Results and discussion
3.1. Proximate composition of quinoa bran
The results of proximate composition of five kinds of quinoa bran in China are shown in Table 2.
Table 2.
Proximate composition of quinoa bran in China.
| Origin zone | Protein (%) | Starch (%) | Fat (%) | Fiber (%) | RS* (%) | Ash (%) | Moisture (%) | CH* (%) | Energy (kCal/100g) |
|---|---|---|---|---|---|---|---|---|---|
| HB | 6.61 ± 0.22d | 57.61 ± 1.73a | 4.33 ± 0.27c | 8.87 ± 1.47c | 3.77 ± 0.64b | 5.72 ± 0.39b | 11.64 ± 0.27a | 62.83 ± 1.27a | 334.5 ± 4.2c |
| SX | 8.66 ± 0.32c | 45.25 ± 1.68b | 8.06 ± 0.72ab | 11.9 ± 0.94ab | 2.99 ± 0.43b | 5.53 ± 0.80b | 10.68 ± 0.34a | 51.17 ± 2.03b | 357.7 ± 6.0b |
| QH | 12.25 ± 1.06a | 44.26 ± 1.52b | 10.89 ± 0.90a | 8.33 ± 0.87c | 3.46 ± 0.57b | 8.14 ± 0.46a | 8.16 ± 0.11b | 51.86 ± 1.85b | 380.1 ± 6.4a |
| NM | 10.84 ± 1.02b | 43.48 ± 1.86b | 9.59 ± 1.07a | 11.1 ± 1.05b | 2.68 ± 0.61b | 5.83 ± 0.46b | 9.73 ± 0.16b | 51.85 ± 3.04b | 373.7 ± 10.5a |
| GS |
8.39 ± 0.67c |
46.26 ± 1.51b |
8.45 ± 0.66b |
13.33 ± 0.72a |
5.49 ± 0.62a |
6.04 ± 0.31b |
9.35 ± 0.15b |
53.68 ± 0.76b |
355.0 ± 5.6b |
| Average | 9.35 ± 2.09 | 47.37 ± 5.41 | 8.26 ± 2.31 | 10.74 ± 2.10 | 3.68 ± 1.11 | 6.25 ± 1.06 | 9.29 ± 1.68 | 54.28 ± 4.69 | 360.2 ± 17.1 |
RS*: Reducing sugar; CH*: Carbohydrates. All data were represented as the means and standard deviations of quintuplicate determinations. Superscript (a-d) indicate significant differences (P < 0.05) in the same column.
The protein content of quinoa bran ranged from 6.61 to 12.25%, showing significant differences in origin zones (p < 0.05) (except SX and GS). Of the five origin zones, the quinoa bran from QH and HB had the highest and lowest protein content (p < 0.05), respectively. The average protein content was 9.35%, which was lower than that of quinoa grain (11.51–18.8%) (Navruz-Varli and Sanlier, 2016; Bhinder et al., 2021; Pedrali et al., 2023), but was comparable to the protein content of rice, corn, millet and sorghum (6.8–9.8%) in dairy feeding (The Ministry of Agriculture of the People's Republic of China, 2004). The protein content of quinoa bran was slightly lower than other brans such as rich, wheat and oat brans (10.05, 10.1 and 11.6% respectively) (Zerlasht et al., 2023). The average starch content of quinoa bran was 47.37% (43.48–57.61%), which was lower than that of quinoa grain (52–69%) (Navruz-Varli and Sanlier, 2016; Lu et al., 2023). The quinoa bran from HB had a higher starch content (p < 0.05) than those from SX, QH, NM and GS. No statistical differences were found between SX, QH, NM and GS (p > 0.05). The fat content of quinoa bran ranged from 4.33 to 10.89% with significant difference among Hebei, Inner Mongolia and Gansu. The average fat content of quinoa bran was 8.26%, which was higher than that of quinoa grain (4.9–7.7%) (Pedrali et al., 2023; Pereira et al., 2019). The fat content of quinoa bran was lower than that of rice bran (14.72%) but was higher than those of wheat and oat brans (5.20 and 7.23% respectively) (Zerlasht et al., 2023). The crude fiber content of quinoa bran was between 8.33% and 13.33% with significant difference among Hebei, Gansu and Inner Mongolia. The average crude fiber content of quinoa bran was 10.74%, which was higher than that of quinoa grain (2.8–10.32%) (Stikic et al., 2012; Valcárcel-Yamani and Caetano da Silva Lannes, 2012; Navruz-Varli and Sanlier, 2016; Li et al., 2022; Jiang et al., 2022) and other brans such as rich, wheat and oat brans (7.22, 1.50 and 3.54% respectively) (Zerlasht et al., 2023). The animal experiment indicated that the dietary fiber could protect the gastric mucosa (Stikic et al., 2012), therefore, quinoa bran is suitable for animal feed. The average content of reducing sugar in quinoa bran was 3.68%, which was slightly higher than that in quinoa grain (about 2%) (Pereira et al., 2019; Fu et al., 2020), and was similar to the soluble sugar content (2.30–3.44 g 100 g−1 fresh weight) in quinoa grain (Gómez et al., 2021).
The average ash content of quinoa bran was 6.25%, which was twice as high as that of quinoa grain (2.7–3.8%) (Valcárcel-Yamani and Caetano da Silva Lannes, 2012; Navruz-Varli and Sanlier, 2016), meaning that the quinoa bran contains more mineral elements. The ash content of quinoa bran was lower than that of rice bran (7.50%) but was higher than those of wheat and oat brans (4.99 and 3.85% respectively) (Zerlasht et al., 2023). The average moisture content of quinoa bran was 9.29%, which was higher than that of quinoa grain (6.1–8.3%) (Pedrali et al., 2023). This may be due to the stronger water absorption of quinoa bran. Therefore, the storage of quinoa bran needs to be moisture-proof treatment. The average energy of quinoa bran was 360.2 kcal/100 g, which was slightly higher than that of quinoa grain (315.2–355 kcal/100 g) (Gómez et al., 2021, 2023). This may be due to the high content of protein, fat and fiber in quinoa bran.
3.2. Antioxidant properties of quinoa bran
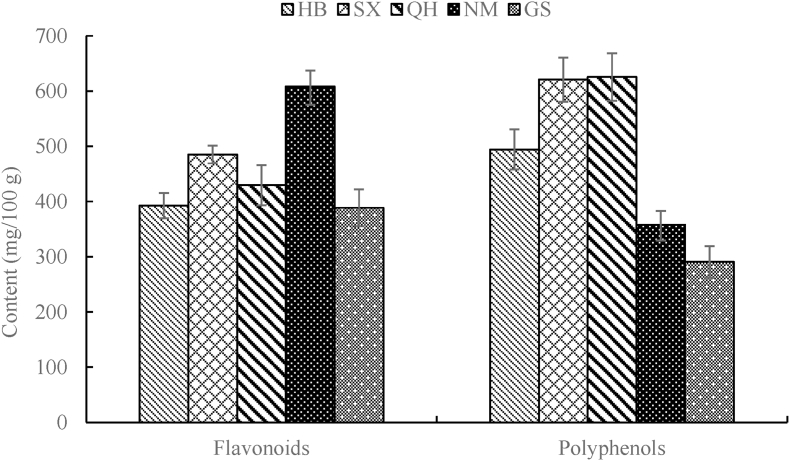
The content of flavonoids and polyphenols in quinoa bran are shown in Fig. 2.
Fig. 2.
The content of flavonoids, polyphenols of quinoa bran in China
All experiments were performed as quintuplicate and the values are given as their mean values.
Flavonoids and polyphenols were associated to antioxidant activities (Repo-Carrasco-Valencia, et al., 2010). The content of flavonoids in quinoa bran ranged from 388.4 to 608.6 mg/100 g, showing significant differences in origin zones (p < 0.05) (except HB, 392.5 mg/100 g). Of the five origin zones, the quinoa bran from NM and GS had the highest and lowest flavonoids content (p < 0.05), respectively. The average flavonoids content in quinoa bran was 460.9 mg/100 g, which was higher than that in quinoa grain (224.56 mg/100 g) (Han et al., 2019). The content of polyphenols in quinoa bran ranged from 290.5 to 625.4 mg/100 g, showing significant differences in origin zones (p < 0.05). Nevertheless, no statistical differences were found between SX and QH. Of the five origin zones, the quinoa bran from QH and GS had the highest and lowest polyphenols content (p < 0.05), respectively. The average polyphenols content quinoa bran was 477.8 mg/100 g, which was higher than that in quinoa grain (97.6–200.4 mg/100 g) (Nickel et al., 2016; Han et al., 2019). The above results meant that the antioxidant activities of quinoa bran may be higher than that of quinoa grain. The antioxidant activity (DPPH radical scavenging percentage) of five kinds of quinoa bran (HB, SX, QH, NM and GS) were 84.81, 88.68, 90.67, 72.74 and 79.97% respectively, showing certain significant differences in the origin zone (p < 0.05).
The correlation coefficients between the content of flavonoids or polyphenols and the antioxidant activity (DPPH) were 0.5527 and 0.8767 (Figs. are not shown), respectively, which did not reach the significant level (P > 0.05). Since the critical value of correlation coefficient was 0.878 (α = 0.05), the correlation coefficient between the contents of polyphenols and DPPH was close to the significance level. In addition, its correlation coefficient was higher than that between the contents of flavonoids and DPPH, suggesting that the antioxidant activity of polyphenols was higher than that of flavonoids.
3.3. Antinutritional characteristics of quinoa bran
The saponins, tannins and phytic acids are important antinutrients of quinoa (Thakur et al., 2021; Bhinder et al., 2021; Ruales and Nair, 1993a), therefore, the anti-nutritional characteristics of quinoa bran can be predicted by analyzing the contents of these substances.
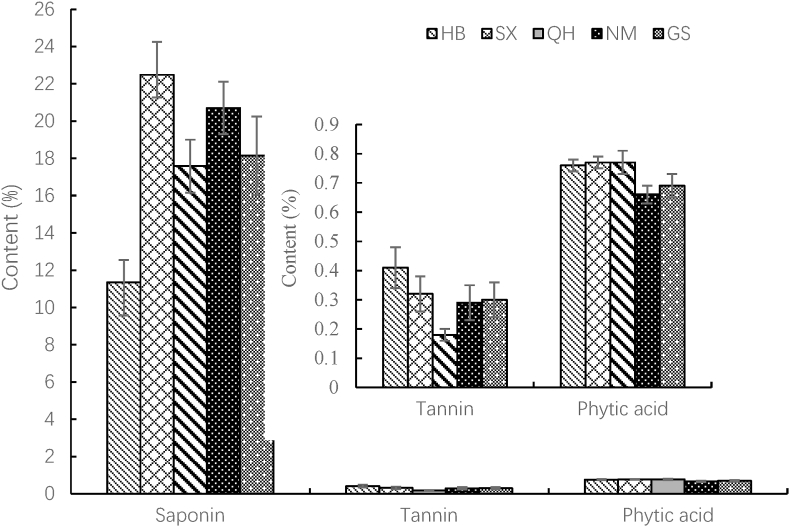
The content of saponins in quinoa bran ranged from 11.34 to 22.48% (Fig. 3), showing certain significant differences in origin zones (p < 0.05). Of the five origin zones, the quinoa bran from SX and HB had the highest and lowest saponins content (p < 0.05), respectively. The average saponins content in quinoa bran was 18.65%, which was much higher than that in quinoa seeds (5.6–7.5%) (Gómez-Caravaca, et al., 2011). Interestingly, there was no significant difference in saponin content between Inner Mongolia and Shanxi, or Qinghai and Gansu, but there was a significant difference between other provinces. The possible reasons for the above phenomena are that Inner Mongolia and Shanxi, or Qinghai and Gansu are geographically adjacent. The tannin content in quinoa bran ranged from 0.18 to 0.41%, and no statistical differences were found between SX, NM and GS (p > 0.05). Of the five origin zones, the quinoa bran from HB and QH had the highest and lowest saponins content (p < 0.05), respectively. The average tannin content in quinoa bran was 0.30%, which was lower than that in quinoa seed (0.53–1.7%) (González et al., 1989) and in quinoa hull (0.92%) (Chauhan, et al., 1992). The phytic acid content in quinoa bran was between 0.66% and 0.77%, the highest value being for HB, SX and QH with respect to NM and GS (p < 0.05). The average saponins content quinoa bran was 0.73%, which was lower than that in quinoa grains (1.03–1.04%) (Ruales and Nair, 1993a; Thakur et al., 2021). Sharma et al. (2022) reported that the phytic acid will be harmful to health when its content was greater than 1%. Since the phytic acid content in quinoa bran is below the harmful limit, it does not affect the nutrient absorption.
Fig. 3.
Content of saponins, tannins and phytic acids in quinoa bran in China
All experiments were performed as quintuplicate and the values are given as their mean values.
Through the above analysis, saponins are the main antinutrients in quinoa bran. Besides the bitter taste, saponins can form insoluble complexes with iron, zinc, calcium and other mineral ions, or form insoluble substances with fat soluble vitamins (VE, VA, VD3) to affect intestinal absorption (Milgate and Roberts, 1995; West and Greger, 1978; West et al., 1978). Therefore, the removal of saponins from quinoa bran is the key to reduce its antinutritional effect. The extraction of saponins by water (Gil-Ramirez et al., 2018), alcohol (Xue et al., 2020) and deep eutectic solvent (Taco et al., 2022) is worthy of further study.
3.4. The content of macroelements in quinoa bran
Mineral elements are divided into macroelements and microelements according to their content in animal body (Zhang and Zhang, 2021). Macroelements are also called major elements, whose content accounts for more than one in ten thousand of the total body weight. The macroelements mainly include sodium, potassium, calcium, magnesium and phosphorus (Tkacz et al., 2021). The content of macroelements in quinoa bran in China are shown in Table 3.
Table 3.
The content of macroelements in quinoa bran in China.
| Origin zones | Content of macroelements (mg/100 g) |
||||
|---|---|---|---|---|---|
| Sodium | Potassium | Calcium | Magnesium | Phosphorus | |
| HB | 19.5 ± 2.4d | 5136 ± 111d | 327 ± 12d | 494 ± 12b | 273 ± 14d |
| SX | 49.8 ± 4.4a | 6155 ± 124b | 490 ± 15b | 392 ± 15c | 432 ± 14a |
| QH | 31.8 ± 3.5c | 819 ± 22e | 770 ± 16a | 473 ± 17b | 364 ± 23b |
| NM | 31.6 ± 3.7c | 7477 ± 136a | 400 ± 22c | 406 ± 30c | 355 ± 24b |
| GS | 41.1 ± 3.6b | 5303 ± 125c | 411 ± 15c | 529 ± 21a | 304 ± 16c |
All data were represented as the means and standard deviations of quintuplicate determinations. Superscript (a-d) indicate significant differences (P < 0.05) in the same column.
As can be seen from Table 3, the macroelement with highest and lowest content in quinoa bran was potassium (819–7477 mg/100 g) and sodium (19.5–49.8 mg/100 g), respectively. The contents of calcium (327–770 mg/100 g), magnesium (392–529 mg/100 g) and phosphorus (273–432 mg/100 g) were between potassium and sodium.
The content of sodium in quinoa is related to the physical and chemical properties of soil. Because quinoa is a salt-tolerant crop, the absorption of sodium ions by quinoa is enhanced when the content of salt in soil is high (Roman et al., 2020; Derbali et al., 2021). It was reported that the sodium content in quinoa seeds ranged from 1.47 to 220 mg/100 g (Stikic et al., 2012; Marmouzi et al., 2015). It was even reported that sodium content in quinoa was lower than the detection limit (Nascimento et al., 2014). The above results were consistent with this study.
The content of potassium in quinoa bran (819–7477 mg/100 g) was higher than that in quinoa grains (452.72–1980 mg/100 g) (Kozioł, 1992; Palombini et al., 2013; Bhinder et al., 2021; Gómez et al., 2021), but the phosphorus content in quinoa bran (273–432 mg/100 g) is equivalent to that of the raw quinoa (330–357 mg/100 g) (Chauhan, et al., 1992; Nascimento et al., 2014; Mota et al., 2016). The contents of calcium (327–770 mg/100 g) and magnesium (392–529 mg/100 g) in quinoa bran were higher than those in most quinoa grains (the content of calcium was 20–390 mg/100 g, and the content of magnesium was 130–460 mg/100 g) (Kozioł, 1992; Palombini et al., 2013; Nascimento et al., 2014; Mota et al., 2016; Gómez et al., 2021; Bhinder et al., 2021), but lower than those in Ecuadorian quinoa grain (the content of calcium and magnesium was 874 and 2620 mg/100 g, respectively) (Ruales and Nair, 1993b).
3.5. The content of microelements in quinoa bran
Microelements refer to the content of less than 0.01% in the body, but they are essential for the animal life activities and play an important role in maintaining animal health (Wang et al., 2020c). Microelements mainly include iron, manganese, copper, zinc, cobalt, selenium, molybdenum, etc. (Zhang and Zhang, 2021). The content of microelements in quinoa bran in China are shown in Table 4.
Table 4.
The content of microelements in quinoa bran in China.
| Origin zones | Content of microelements (mg/100 g) |
Content of microelements (mg/kg) |
||||||
|---|---|---|---|---|---|---|---|---|
| Iron | Manganese | Copper | Zinc | Cobalt | Selenium | Molybdenum | Barium | |
| HB | 67.2 ± 2.6a | 23.8 ± 2.3a | 0.26 ± 0.04a | 1.68 ± 0.07b | 0.33 ± 0.08a | 0.13 ± 0.03a | 0.25 ± 0.03a | 1.9 ± 0.13c |
| SX | 51.7 ± 1.6b | 8.93 ± 1.6d | 0.39 ± 0.08a | 1.57 ± 0.13b | 0.26 ± 0.04ab | 0.08 ± 0.02b | 0.34 ± 0.06a | 1.9 ± 0.14c |
| QH | 11.9 ± 1.0c | 13.4 ± 1.4bc | 2.3 ± 0.2a | 13.8 ± 1.0a | 0.19 ± 0.04b | 0.06 ± 0.01b | 0.17 ± 0.07a | 3.4 ± 0.19a |
| NM | 7.21 ± 0.61d | 15.0 ± 1.4b | 0.48 ± 0.06a | 2.06 ± 0.32b | 0.21 ± 0.05b | 0.11 ± 0.02a | 0.26 ± 0.05a | 1.6 ± 0.14d |
| GS | 51.6 ± 1.0b | 12.2 ± 1.0c | 0.37 ± 0.06a | 1.42 ± 0.11b | 0.31 ± 0.06a | 0.13 ± 0.02a | 0.16 ± 0.04c | 2.3 ± 0.2b |
All data were represented as the means and standard deviations of quintuplicate determinations. Superscript (a-d) indicate significant differences (P < 0.05) in the same column.
The contents of manganese (8.93–23.8 mg/100 g) and iron (7.21–67.2 mg/100 g) in quinoa bran were higher than those in most quinoa grains (the content of manganese and iron was 1.89–3.35 mg/100 g and 2.6–13.96 mg/100 g, respectively) (Ogungbenle, 2003; Miranda et al., 2010; Stikic et al., 2012; Nascimento et al., 2014; Mota et al., 2016; Bhinder et al., 2021; Gómez et al., 2021), but lower than those in Ecuadorian quinoa grain (the content of manganese and iron was 33 ± 1.2 and 81 ± 0.9 mg/100 g, respectively) (Ruales and Nair, 1993b). The content of copper in quinoa bran ranged from 0.26 to 2.3 mg/100 g, which was similar to that in most quinoa grains (0.502–0.87mg/100 g) (Bruin 1964; Miranda et al., 2010; Nascimento et al., 2014; Mota et al., 2016; Bhinder et al., 2021), but lower than the results reported by Ogungbenle (2003) (7.5 mg/100 g) and Ruales and Nair (1993b) (10 ± 0.4 mg/100 g). The content of zinc in quinoa bran ranged from 1.42 to 13.8 mg/100 g, which was similar to that of most quinoa grains (Ranhotra et al., 1993; Ogungbenle, 2003; Nascimento et al., 2014; Mota et al., 2016; Bhinder et al., 2021), but which was lower than the results reported by Alamri et al. (2023) (the content of zinc was 45.0 ± 0.70 mg/100 g) and Ruales and Nair (1993b) (the content of zinc was 36 ± 2 mg/100 g).
The content of cobalt in quinoa bran was between 0.19 and 0.33 mg/kg, which was higher than that in most quinoa grains (0.035–0.051 mg/kg) (Bruin 1964; Kozioł, 1992; Nascimento et al., 2014), but lower than that reported by Ruales and Nair (1993b) (0.5 ± 0.02 mg/100 g). The content of selenium in quinoa bran was between 0.06 and 0.13 mg/kg, which was higher than the result reported by Nascimento et al. (2014) (lower than the limit of quantification). However, it was lower than the selenium content reported by Ruales and Nair (1993b) (3.6 mg/kg) and Zhao et al. (2019) (10–150 mg/kg). The content of molybdenum in quinoa bran was between 0.16 and 0.34 mg/kg, which was similar to the results reported by Nascimento et al. (2014) (0.228 ± 0.68 mg/kg), and it was slightly higher than that in quinoa seeds (0.1 mg/kg) (Bruin, 1964). However, the content of molybdenum was much lower than that in Ecuadorian quinoa grain (28 mg/kg) (Ruales and Nair, 1993b).
The content of barium in quinoa bran ranged from 1.6 to 3.4 mg/kg. Nevertheless, to our knowledge, the content of barium in quinoa grain has not been reported. Although the existing studies have not confirmed that barium does not have a recognized biological role in humans (Peana et al., 2021). Wang et al. (2009) reported that barium is a non-essential and non-toxic rare trace element. Unless the barium salt is over absorbed by the body, otherwise it will not produce acute and chronic toxic effects on the human and animal health.
4. Conclusions
Quinoa bran is a by-product of the processing of quinoa grain with rich nutritional value. Compared with quinoa grain, the quinoa bran had lower protein and starch content but higher fat, fiber content and energy value. Since the protein content of quinoa bran is comparable to that in other grains (rice, corn, millet and sorghum) and brans (wheat, oat and rice), it has commercial potential for processing into animal feed or other edible products. The contents of antioxidant flavonoids and polyphenols in quinoa bran were higher than those in quinoa grains, suggesting that quinoa bran had better antioxidant capacity. The correlation coefficient between polyphenols and DPPH radical scavenging was higher than that of flavonoids, meaning that the polyphenols had stronger antioxidant capacity. The content of antinutritional saponins in quinoa bran was much higher than that in quinoa grains, the content of tannin and phytic acid, however, was lower than that in quinoa grains. Therefore, the removal of saponins was the key to eliminate the antinutritional properties of quinoa bran. The contents of macroelements (sodium, potassium, calcium, magnesium, phosphorus) and microelements (iron, manganese, copper, zinc, cobalt, molybdenum, selenium, barium) in quinoa bran were generally higher than those in quinoa grains, which was consistent with the results of ash content. The study also showed that the nutritional, antinutritional, antioxidative, and mineral content characteristics of quinoa bran have a certain relationship with the planting regions. In summary, quinoa bran was found to be a rich source of nutritional and bioactive components and minerals. Nevertheless, since it contains more antinutritional factors than quinoa grains, the removal of antinutrients is key to the commercial development of quinoa bran. If saponins can be developed into pesticides, medicines and cosmetics, the further processing of quinoa bran will achieve a win-win situation.
CRediT authorship contribution statement
Xueyong Zhou: Conceptualization, Resources, Investigation, Writing – review & editing, Funding acquisition, Supervision. Ting Yue: Resources, Data curation, Investigation, Writing – original draft, Formal analysis. Zuofu Wei: Writing – original draft, Software, Formal analysis, Resources. Liyan Yang: Resources, Data curation, Investigation, Validation. Lihong Zhang: Investigation, Writing – review & editing, Data curation. Baomei Wu: Investigation, Formal analysis, Software, Photos, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research is funded by the projects of the central government guides the development of local science and technology in Shanxi Province, China (YDZJSX2022A055), the Fundamental Research Program of Shanxi Province, China (202203021221122 and 20210302123328), the Scientific and Technological Innovation Programs of Higher Education Institution in Shanxi Province, China (2019L0465 and 2020L0236).
Handling Editor: Dr. Yeonhwa Park
Data availability
Data will be made available on request.
References
- Abeysinghe D.C., Li X., Sun C.D., Zhang S.W., Zhou C.H., Chen K.S. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007;104:1338–1344. doi: 10.1016/j.foodchem.2007.01.047. [DOI] [Google Scholar]
- Alamri E., Amany B., Bayomy H. Quinoa seeds (Chenopodium quinoa): nutritional value and potential biological effects on hyperglycemic rats. J. King Saud Univ. Sci. 2023;35 doi: 10.1016/j.jksus.2022.102427. [DOI] [Google Scholar]
- Ando H., Chen Y.C., Tang H., Shimizu M., Watanabe K., Mitsunaga T. Food components in fractions of quinoa seed. Food Sci. Technol. Res. 2002;8:80–84. doi: 10.3136/fstr.8.80. [DOI] [Google Scholar]
- Bhinder S., Kumari S., Singh B., Kaur A., Singh N. Impact of germination on phenolic composition, antioxidant properties, antinutritional factors, mineral content and Maillard reaction products of malted quinoa flour. Food Chem. 2021;346 doi: 10.1016/j.foodchem.2020.128915. [DOI] [PubMed] [Google Scholar]
- Bruin A.D. Investigation of the food value of quinua and cañihua seed. J. Food Sci. 1964;29:872–879. [Google Scholar]
- Cao H.W., Huang Q.L., Wang C., Guan X., Huang K., Zhang Y. Effect of compositional interaction on in vitro digestion of starch during the milling process of quinoa. Food Chem. 2023;403 doi: 10.1016/j.foodchem.2022.134372. [DOI] [PubMed] [Google Scholar]
- Carlson D., Fernandez J.A., Poulsen H.D., Nielsen B., Jacobsen S.E. Effects of quinoa hull meal on piglet performance and intestinal epithelial physiology. J. Anim. Physiol. Anim. Nutr. 2012;96:198–205. doi: 10.1111/j.1439-0396.2011.01138.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary N., Walia S., Kumar R. Functional composition, physiological effect and agronomy of future food quinoa (Chenopodium quinoa Willd.): a review. J. Food Compos. Anal. 2023;118 doi: 10.1016/j.jfca.2023.105192. [DOI] [Google Scholar]
- Chauhan G.S., Eskin N.A.M., Tkachuk R. Nutrients and antinutrients in quinoa seed. Cereal Chem. 1992;69:85–88. [Google Scholar]
- Chen X.Q. Cultivation techniques of quinoa. Agric. Sci-Technol. Info. 2021;62–63 doi: 10.15979/j.cnki.cn62-1057/s.2021.13.023. [DOI] [Google Scholar]
- Cui H.L., Xing B., Yao Q., Zhang Q.P., Yang X.S., Yao Y., Ren G.X., Qin P.Y. SWTO analysis of quinoa industry development in Xinjiang Yili Valley. Crop J. 2019;32–37 doi: 10.16035/j.issn.1001-7283.2019.01.005. [DOI] [Google Scholar]
- Derbali W., Manaa A., Goussi R., Derbali I., Abdelly C., Koyro H.W. Post-stress restorative response of two quinoa genotypes differing in their salt resistance after salinity release. Plant Physiol. Biochem. 2021;164:222–236. doi: 10.1016/j.plaphy.2021.04.024. [DOI] [PubMed] [Google Scholar]
- Fu R.X., Zhou X.Y., Xiao J.Z., Cui Y., Wu H.Q., Rajasab A.H. Effects of germination temperature and germination time on nutritional components of quinoa. Food Ind. 2020;41:341–345. [Google Scholar]
- Galwey N.W., Leakey C.L.A., Price K.R., Fenwick G.R. Chemical composition and nutritional characteristics of quinoa (Chenopodium quinoa Willd) Food Sci. Nutr. 1990;42:245–261. doi: 10.1080/09543465.1989.11904148. [DOI] [Google Scholar]
- Gil-Ramirez A., Salas-Veizaga D.M., Grey C., Karlsson E.N., Rodriguez-Meizoso I., Linares-Pastén J.A. Integrated process for sequential extraction of saponins, xylan and cellulose from quinoa stalks (Chenopodium quinoa Willd.) Ind. Crop. Prod. 2018;121:54–65. doi: 10.1016/j.indcrop.2018.04.074. [DOI] [Google Scholar]
- Gómez M.J.R., Maestro-Gaitán I., Magro P.C., Sobrado V.C., Blázquez M.R., Prieto J.M. Unique nutritional features that distinguish Amaranthus cruentus L. and Chenopodium quinoa Willd seeds. Food Res. Int. 2023;164 doi: 10.1016/j.foodres.2022.112160. [DOI] [PubMed] [Google Scholar]
- Gómez M.J.R., Prieto J.M., Sobrado V.C., Magro P.C. Nutritional characterization of six quinoa (Chenopodium quinoa Willd) varieties cultivated in Southern Europe. J. Food Compos. Anal. 2021;99 doi: 10.1016/j.jfca.2021.103876. [DOI] [Google Scholar]
- Gómez-Caravaca A.M., Segura-Carretero A., Fernández-Gutiérrez A., Caboni M.F. Simultaneous determination of phenolic compounds and saponins in Quinoa (Chenopodium quinoa Willd.) by a liquid chromatography-diode array detection-electrospray ionization-time-of-flight mass spectrometry methodology. J. Agric. Food Chem. 2011;59:10815–10825. doi: 10.1021/jf202224j. [DOI] [PubMed] [Google Scholar]
- González J.A., Roldán A., Gallardo M., Escudero T., Prado F.E. Quantitative determinations of chemical compounds with nutritional value from Inca crops: Chenopodium quinoa (‘quinoa’) Plant Foods Hum. Nutr. 1989;39:331–337. doi: 10.1007/BF01092070. [DOI] [PubMed] [Google Scholar]
- Han Y.M., Chi J.W., Zhang M.W., Zhang R.F., Fan S.H., Dong L.H., Huang F., Liu L. Changes in saponins, phenolics and antioxidant activity of quinoa (Chenopodium quinoa Willd) during milling process. LWT--Food Sci. Technol. 2019;114 [Google Scholar]
- Haug W., Lantzsch H.J. Sensitive method for the rapid determination of phytate in cereals and cereal products. J. Sci. Food Agric. 1983;34:1423–1426. [Google Scholar]
- Hemalatha P., Bomzan D.P., Rao B.V.S., Sreerama Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2016;199:330–338. doi: 10.1016/j.foodchem.2015.12.025. [DOI] [PubMed] [Google Scholar]
- Hirose Y., Fujita T., Ishii T., Ueno N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010;119:1300–1306. doi: 10.1016/j.foodchem.2009.09.008. [DOI] [Google Scholar]
- Hou S.H., Fu M.R., Zhang W.Y., Ren G.X. Research progress on saponins of quinoa (Chenopodium quinoa Willd.) J. Food Safty Qual. 2018;9:5146–5152. [Google Scholar]
- Improta F., Kellems R.O. Comparison of raw, washed and polished quinoa (Chenopodium quinoa Willd.) to wheat, sorghum or maize based diets on growth and survival of broiler chicks. Livest. Res. Rural Dev. 2001;13 Article 1. [Google Scholar]
- Jiang Q.Q., Dong X.J., Xu H.M., Jin Z.Y. Study on effects of frying heat treatment on the nutritional quality of quinoa. Cereals & oils. 2022;35:52–54. [Google Scholar]
- Kozioł M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.) J. Food Compos. Anal. 1992;5:35–68. [Google Scholar]
- Li X., Li X.F., Wang J.B., Zhu L.L., Zhang Y.M., Wang Q.C., Mao X.F., Chen Z.G. Effects of high altitude environment on the nutritional components of Chenopodium quinoa. J. Agric. For. 2022;12:56–63. [Google Scholar]
- Lim J.G., Park H.M., Yoon K.S. Analysis of saponin composition and comparison of the antioxidant activity of various parts of the quinoa plant (Chenopodium quinoa Willd.) Food Sci. Nutr. 2020;8:694–702. doi: 10.1002/fsn3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.J., Li Y.P., Zhang M., Zhang Y.M., Lin C. Study on introduction, cultivation and industrial development of quinoa in Yunnan province. Modern Agric. Sci. Technol. 2021;4–9 doi: 10.3969/j.issn.1007-5739.2021.10.002. [DOI] [Google Scholar]
- Lu S.Y., Li J., Ji J.Y., Wen Y.Y., Li H.Y., Wang J., Sun B.G. Endogenous protein and lipid facilitate the digestion process of starch in cooked quinoa flours. Food Hydrocolloids. 2023;134 doi: 10.1016/j.foodhyd.2022.108099. [DOI] [Google Scholar]
- Ma W.W., Heinstein P.F., Mclaughlin J.L. Additional toxic, bitter saponin from the seeds of Chenopodium quinoa. J. Nat. Prod. 1989;52:1132–1135. doi: 10.1021/np50065a035. [DOI] [PubMed] [Google Scholar]
- Ma Y.M. Master Dissertation of Inner Mongolia Agricultural University; Hohhot city, China: 2020. Study on Rubbing and Husking Process or Quinoa Seeds and Optimization of Husking Parameters. [Google Scholar]
- Marmouzi I., Madani N.E., Charrouf Z., Cherrah Y., Faouzi M.Y.E.A. Proximate analysis, fatty acids and mineral composition of processed Moroccan Chenopodium quinoa Willd. and antioxidant properties according to the polarity. Phytothérapie. 2015;13:110–117. doi: 10.1007/s10298-015-0931-5. [DOI] [Google Scholar]
- Martinez O.D.M., Toledo R.C.L., Queiroz V.A.V., Pirozi M.R., Martino H.S.D., Barros F.A.R.D. Mixed sorghum and quinoa flour improves protein quality and increases antioxidant capacity in vivo. LWT--Food Sci. Technol. 2020;129 [Google Scholar]
- Medina-Meza I.G., Aluwi N.A., Saunders S.R., Ganjyal G.M. GC-MS Profiling of triterpenoid saponins from 28 quinoa varieties (Chenopodium quinoa Willd.) grown in Washington state. J. Agric. Food Chem. 2016;64:8583–8591. doi: 10.1021/acs.jafc.6b02156. [DOI] [PubMed] [Google Scholar]
- Milgate J., Roberts D.C.K. The nutritional & biological significance of saponins. Nutr. Res. 1995;15:1223–1249. [Google Scholar]
- Miranda M., Vega-Gálvez A., López J., Parada G., Sanders M., Aranda M., Uribe E., Scala K.D. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.) Ind. Crop. Prod. 2010;32:258–263. doi: 10.1016/j.indcrop.2010.04.019. [DOI] [Google Scholar]
- Mota C., Nascimento A.C., Santos M., Delgado I., Coelho I., Rego A., Matos A.S., Torres D., Castanheira I. The effect of cooking methods on the mineral content of quinoa (Chenopodium quinoa), amaranth (Amaranthus sp.) and buckwheat (Fagopyrum esculentum) J. Food Compos. Anal. 2016;49:57–64. doi: 10.1016/j.jfca.2016.02.006. [DOI] [Google Scholar]
- Nascimento A.C., Mota C., Coelho I., Gueifão S., Santos M., Matos A.S., Gimenez A., Lobo M., Samman N., Castanheira I. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: proximates, minerals and trace elements. Food Chem. 2014;148:420–426. doi: 10.1016/j.foodchem.2013.09.155. [DOI] [PubMed] [Google Scholar]
- Navruz-Varli S., Sanlier N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.) J. Cereal. Sci. 2016;69:371–376. doi: 10.1016/j.jcs.2016.05.004. [DOI] [Google Scholar]
- Nickel J., Spanier L.P., Botelho F.T., Gularte M.A., Helbig E. Effect of different types of processing on the total phenolic compound content, antioxidant capacity, and saponin content of Chenopodium quinoa Willd grains. Food Chem. 2016;209:139–143. doi: 10.1016/j.foodchem.2016.04.031. [DOI] [PubMed] [Google Scholar]
- Ogungbenle H.N. Nutritional evaluation and functional properties of quinoa (Chenopodium quinoa) flour. Int. J. Food Sci. Nutr. 2003;54:153–158. doi: 10.1080/0963748031000084106. [DOI] [PubMed] [Google Scholar]
- Palombini S.V., Claus T., Maruyama S.A., Gohara A.K., Souza A.H.P., Souza N.E.D., Visentainer J.V., Gomes S.T.M., Matsushita M. Evaluation of nutritional compounds in new amaranth and quinoa cultivars. Food Sci. Technol. 2013;33:339–344. doi: 10.1590/S0101-20612013005000051. [DOI] [Google Scholar]
- Peana M., Medici S., Dadar M., Zoroddu M.A., Pelucelli A., Chasapis C.T., Bjørklund G. Environmental barium: potential exposure and health-hazards. Arch. Toxicol. 2021;95:2605–2612. doi: 10.1007/s00204-021-03049-5. [DOI] [PubMed] [Google Scholar]
- Pedrali D., Giupponi L., De la Peña-Armada R., Villanueva-Suárez M.J., Mateos-Aparicio I. The quinoa variety influences the nutritional and antioxidant profile rather than the geographic factors. Food Chem. 2023;402 doi: 10.1016/j.foodchem.2022.133531. [DOI] [PubMed] [Google Scholar]
- Pereira E., Encina-Zelada C., Barros L., Gonzales-Barron U., Cadavez V., Ferreira I.G.F.R. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: a good alternative to nutritious food. Food Chem. 2019;280:110–114. doi: 10.1016/j.foodchem.2018.12.068. [DOI] [PubMed] [Google Scholar]
- Rafik S., Rahmani M., Rodriguez J.P., Andam S., Ezzariai A., Gharous M.E., Karboune S., Choukr-Allah R., Hirich A. How does mechanical pearling affect quinoa nutrients and saponin contents? Plants. 2021;10:1133. doi: 10.3390/plants10061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhotra G.S., Gelroth J.A., Glaser B.K., Lorenz K.J., Johnson D.L. Composition and protein nutritional quality of quinoa. Cereal Chem. 1993;70:303–305. [Google Scholar]
- Reichert R.D., Tatarynovich J.T., Tyler R.T. Abrasive dehulling of quinoa (Chenopodium quinoa): effect on saponin content as determined by an adapted hemolytic assay. Cereal Chem. 1986;63:471–475. [Google Scholar]
- Repo-Carrasco-Valencia R., Hellström J.K., Pihlava J.M., Mattila P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus) Food Chem. 2010;120:128–133. doi: 10.1016/j.foodchem.2009.09.087. [DOI] [Google Scholar]
- Ridout C.L., Price K.R., DuPont M.S., Parker M.L., Fenwick G.R. Quinoa saponins-analysis and preliminary investigations into the effects of reduction by processing. J. Sci. Food Agric. 1991;54:165–176. [Google Scholar]
- Roman V.J., Toom L.A.D., Gamiz C.C., Pijl N.V.D., Visser R.G.F., Loo E.N.V., Linden C.G.V.D. Differential responses to salt stress in ion dynamics, growth and seed yield of European quinoa varieties. Environ. Exp. Bot. 2020;177 [Google Scholar]
- Ruales J., Nair B.M. Nutritional quality of the protein in quinoa (Chenopodium quinoa, Willd) seeds. Plant Foods Hum. Nutr. (Dordr.) 1992;42:1–11. doi: 10.1007/BF02196067. [DOI] [PubMed] [Google Scholar]
- Ruales J., Nair B.M. Saponins, phytic acid, tannins and protease inhibitors in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem. 1993;48:137–143. [Google Scholar]
- Ruales J., Nair B.M. Content of fat, vitamins and minerals in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem. 1993;48:131–136. doi: 10.1016/0308-8146(93)90047-J. [DOI] [Google Scholar]
- Ruiza K.B., Khakimov B., Engelsen S.B., Bak S., Biondi S., Jacobsen S.E. Quinoa seed coats as an expanding and sustainable source of bioactive compounds: an investigation of genotypic diversity in saponin profiles. Ind. Crop. Prod. 2017;104:156–163. doi: 10.1016/j.indcrop.2017.04.007. [DOI] [Google Scholar]
- Sharma S., Kataria A., Singh B. Effect of thermal processing on the bioactive compounds, antioxidative, antinutritional and functional characteristics of quinoa (Chenopodium quinoa) LWT--Food Sci. Technol. 2022;160 doi: 10.1016/j.lwt.2022.113256. [DOI] [Google Scholar]
- Song Z.Q., Dai H.Y., Yang J.R., Zhu Y.B. Effect of quinoa bran insoluble dietary fiber on bread quality. Food Res. Dev. 2022;43:8–13. [Google Scholar]
- State Food and Drug Administration of China . 2016. Determination of Starch in Food. GB/T 5009.9-2016. Published on. [Google Scholar]
- Stikic R., Glamoclija D., Demin M., Vucelic-Radovic B., Jovanovic Z., Milojkovic-Opsenica D., Jacobsen S.E., Milovanovic M. Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulation. J. Cereal. Sci. 2012;55:132–138. [Google Scholar]
- Sun X.Y., Yang X.S., Xue P., Zhang Z.G., Ren G.X. Improved antibacterial effects of alkali-transformed saponin from quinoa husks against halitosis-related bacteria. BMC Compl. Alternative Med. 2019;19:46. doi: 10.1186/s12906-019-2455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taco V., Savarino P., Benali S., Villacrés E., Raquez J.-M., Gerbaux P., Duez P., Nachtergael A. Deep eutectic solvents for the extraction and stabilization of Ecuadorian quinoa (Chenopodium quinoa Willd.) saponins. J. Clean. Prod. 2022;363 [Google Scholar]
- Thakur P., Kumar K., Ahmed N., Chauhan D., Rizvi Q.U.E.H., Jan S., Singh T.P., Dhaliwal H.S. Effect of soaking and germination treatments on nutritional, anti-nutritional, and bioactive properties of amaranth (Amaranthus hypochondriacus L.), quinoa (Chenopodium quinoa L.), and buckwheat (Fagopyrum esculentum L.) Curr. Res. Food Sci. 2021;4:917–925. doi: 10.1016/j.crfs.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Ministry of Agriculture of the People's Republic of China . 2004. Dairy Cattle Feeding Standard; pp. 1–64. [Google Scholar]
- Tkacz K., Wojdyło A., Turkiewicz I.P., Nowicka P. Triterpenoids, phenolic compounds, macro- and microelements in anatomical parts of sea buckthorn (Hippophaërhamnoides L.) berries, branches and leaves. J. Food Compos. Anal. 2021;103 doi: 10.1016/j.jfca.2021.104107. [DOI] [Google Scholar]
- Valcárcel-Yamani B., Caetano da Silva Lannes S. Applications of quinoa (Chenopodium quinoa Willd.) and amaranth (amaranthus Spp.) and their influence in the nutritional value of cereal based foods. Food Publ. Health. 2012;2:265–275. [Google Scholar]
- Vo N.N.Q., Fukushima E.O., Muranaka T. Structural and hemolytic activity relationships of triterpenoid saponins and sapogenins. J. Nat. Med-Tokyo. 2017;71:50–58. doi: 10.1007/s11418-016-1026-9. [DOI] [PubMed] [Google Scholar]
- Wang H., Xiang Y.K., Zhang Y.W. Transport mechanism of trace elements in animals. Feed Res. 2020;43:109–112. doi: 10.13557/j.cnki.issn1002-2813.2020.11.027. [DOI] [Google Scholar]
- Wang Y., Gong X., Zhang Y., Geng D.H., Cao L.K., Ruan C.Q., Yu L.H., Zhang D.J., Tong L.T. Effect of peeling treatment on the physicochemical properties of quinoa flour. J. Food Process. Eng. 2020;43:1–9. doi: 10.1111/jfpe.13387. [DOI] [Google Scholar]
- Wang Y., Zuo Y.M., Zhou X.Y., Fu R.X., Xiao J.Z., Ren G.X., Zhou H.T. Quinoa germination promoted the release of its active ingredients. Modern Food Sci. Technol. 2020;36:126–133. doi: 10.13982/j.mfst.1673-9078.2020.8.1177. [DOI] [Google Scholar]
- Wang Y.B., Huang W.L., Li Y.C. Trace element barium and human health. Endemic diseases Bulletin. 2009;24:81–83. [Google Scholar]
- West L.G., Greger J.L. In vitro studies on saponin-vitamin complexation. J. Food Sci. 1978;43:1340–1341. [Google Scholar]
- West L.G., Greger J.L., White A., Nonnamaker B.J. In vitro studies on saponin-mineral complexation. J. Food Sci. 1978;43:1342–1343. [Google Scholar]
- Xue P., Zhao L., Jing J.J., Wang X., Zhang F.X. Amino acid analysis and nutritional evaluation of quinoa bran protein. Food Res. Dept. 2019;40:65–70. doi: 10.3969/j.issn.1005-6521.2019.05.012. [DOI] [Google Scholar]
- Xue P., Zhao L., Wang Y.J., Hou Z.H., Zhang F.X., Yang X.S. Reducing the damage of quinoa saponins on human gastric mucosal cells by a heating process. Food Sci. Nutr. 2020;8:500–510. doi: 10.1002/fsn3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlasht M., Javaria S., Murtaza M.A., Iqbal R.K., Quddoos M.Y., Azhar S., Syed A., Elgorban A.M. Antimicrobial potential and phyto-physio-chemical characterization of brans from wheat, oat, and rice. J. King Saud Univ. Sci. 2023;35 [Google Scholar]
- Zhang F.N., Zhang X.Z. Analysis on the effects of trace elements on animal immune function. Chinese Livest. Poultry Breeding. 2021;17:55–56. [Google Scholar]
- Zhang Q.P., Xing B., Zhou B.W., Sun M.H., Yao Y., Yang X.S., Ren G.X., Qin P.Y. Research progress and application prospect of quinoa. Chin. J. Grassl. 2020;42:162–168. doi: 10.16742/j.zgcdxb.20190214. [DOI] [Google Scholar]
- Zhao D.Q., Kai J.R., Lu J., Liu W. Analysis of main nutritional components and mineral element contents of different varieties of quinoa in different producing areas of Ningxia. Cereals & Ooils. 2019;32:62–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.