Summary
Background
Cancer immunotherapy shows unique efficacy kinetics that differs from conventional treatment. These characteristics may lead to the prolongation of trial duration, hence reliable surrogate endpoints are urgently needed. We aimed to systematically evaluate the study-level performance of commonly reported intermediate clinical endpoints for surrogacy in cancer immunotherapy.
Methods
We searched the Embase, PubMed, and Cochrane databases, between database inception and October 18, 2022, for phase 3 randomised trials investigating the efficacy of immunotherapy in patients with advanced solid tumours. An updated search was done on July, 15, 2023. No language restrictions were used. Eligible trials had to set overall survival (OS) as the primary or co-primary endpoint and report at least one intermediate clinical endpoint including objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and 1-year overall survival. Other key inclusion and exclusion criteria included: (1) adult patients (>18 years old) with advanced solid tumour; (2) no immunotherapy conducted in the control arms; (3) follow-up is long enough to achieve OS; (4) data should be public available. A two-stage meta-analytic approach was conducted to evaluate the magnitude of the association between these intermediate endpoints and OS. A surrogate was identified if the coefficient of determination (R2) was 0.7 or greater. Leave-one-out cross-validation and pre-defined subgroup analysis were conducted to examine the heterogeneity. Potential publication bias was evaluated using the Egger's and Begg's tests. This trial was registered with PROSPERO, number CRD42022381648.
Findings
52,342 patients with 15 types of tumours from 77 phase 3 studies were included. ORR (R2 = 0.11; 95% CI, 0.00–0.24), DCR (R2 = 0.01; 95% CI, 0.00–0.01), and PFS (R2 = 0.40; 95% CI, 0.23–0.56) showed weak associations with OS. However, a strong correlation was observed between 1-year survival and clinical outcome (R2 = 0.74; 95% CI, 0.64–0.83). These associations remained relatively consistent across pre-defined subgroups stratified based on tumour types, masking methods, line of treatments, drug targets, treatment strategies, and follow-up durations. No significant heterogeneities or publication bias were identified.
Interpretation
1-year milestone survival was the only identified surrogacy endpoint for outcomes in cancer immunotherapy. Ongoing investigations and development of new endpoints and incorporation of biomarkers are needed to identify potential surrogate markers that can be more robust than 1-year survival. This work may provide important references in assisting the design and interpretation of future clinical trials, and constitute complementary information in drafting clinical practice guidelines.
Funding
None.
Keywords: Cancer, Immunotherapy, Surrogate endpoint, Biomarker, Overall survival
Research in context.
Evidence before this study
Immunotherapy shows unique efficacy kinetics such as delayed clinical effect and long-term favorable outcome. Accordingly, conventional endpoints based on Response Evaluation Criteria in Solid Tumours (RECIST) criteria fail to represent the long-term benefit of immunotherapy. In the last two years, many of the US Food and Drug Administration (FDA) accelerated drug approvals based on objective response rate (ORR) or progression-free survival (PFS) study data have been withdrawn by FDA. Here we conducted a systematic search in Embase, PubMed and Cochrane databases for phase 3 randomised trials investigating the efficacy of immunotherapy in patients with advanced solid tumours from database inception to July 2023. The keywords included “cancer”, “immunotherapy”, “randomised trial” et al. No language restrictions were used. Totally, 37,244 relevant records were identified from the initial search.
Added value of this study
Our study revealed that, in cancer immunotherapy, there is a strong correlation between clinical outcome and 1-year milestone survival rate, but weak associations with other intermediate endpoints including ORR, disease control rate, and PFS. Moreover, these associations remained relatively consistent across pre-defined subgroups stratified based on tumour types, masking methods, line of treatments, drug targets, treatment strategies, and follow-up durations.
Implications of all the available evidence
Our findings have potential implications for the design and interpretation of clinical trials, which could subsequently accelerate the drug development process and assist in drafting the clinical practice guidelines. 1-year survival was the only identified surrogate endpoint to date for cancer immunotherapy, ongoing investigations and development of new endpoints and incorporation of biomarkers are needed to identify potential surrogacy that can be more robust than 1-year survival.
Introduction
The application of immune checkpoint inhibitors (ICIs) has revolutionised cancer treatment in the last decade.1 Agents targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1) can rehabilitate or activate self-immunity against tumour cells,2 which result in delayed clinical effect and long-term favorable outcome.3 Currently, ICIs are widely used in clinical practice and become the standard treatments in multiple tumour types.1,4 Moreover, the broad efficacy of ICIs has led to unprecedented levels of research of immunotherapy, such as the combination with other treatment,5 and the development of new immune-based targets.6
Overall survival (OS) is a universally recognised endpoint to determine the clinical benefit in oncology trial. However, the long natural histories of some tumours make it difficult to achieve enough follow-up. Accordingly, intermediate endpoints that could serve as surrogates for OS are needed to prioritise combinations, detect signals of early activity, and interpret exploratory results. Intermediate endpoints based on tumour measurement, such as objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), have been routinely applied to assess new therapies in clinical trials and served as the endpoints to predict OS for the consideration of accelerated approval of drugs. In immunotherapy, it is well-known that conventional approaches such as Response Evaluation Criteria in Solid Tumours (RECIST) cannot fully characterise the clinical benefit.7 Additionally, numerous trials revealed that early death could occur in the first several months of immunotherapy.8 The novel mechanisms and unique patterns of anti-tumour activities in immunotherapy have renewed great interest in exploring surrogate endpoints to assist in on/off decision-making. Intermediate endpoints, including ORR, DCR, PFS, modified PFS, and milestone survival, have been proposed as surrogate endpoints for immunotherapy in clinical trials.9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 However, these studies were specific to one tumour type, one country/region, or with limited high-qualities trials, and the results were often ambiguous or conflicted due to the biological complexity of cancer. Here, though a comprehensive analysis with phase 3 randomised control trials (RCTs), our primary objective was to assess the study-level of ORR, DCR, PFS, and 1-year OS as surrogate endpoint for outcomes in cancer immunotherapy.
Methods
Search strategy and selection criteria
Our meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.21 This study, and its associated protocol, is registered with PROSPERO, number CRD42022381648 (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022381648). A systematic search of PubMed, Embase, and Cochrane databases for trails investigating cancer immunotherapy and describing overall survival and at least one intermediate clinical endpoint from inception to October 18, 2022 was conducted. An updated search was done on July, 15, 2023. The keywords used were: cancer, immunotherapy, randomised trial, adebrelimab, atezolizumab, avelumab, camrelizumab, cemiplimab, dostarlimab, durvalumab, envafolimab, ipilimumab, nivolumab, pembrolizumab, relatlimab, sintilimab, sugemalimab, tremelimumab, toripalimab, and tislelizumab. The detailed search terms were shown in Table S1. All investigators carried out the initial search independently, carefully reviewed the title and abstract for relevance, and classified the potential articles as included, uncertain and excluded. For uncertain studies, the full-texts were reviewed for the confirmation of eligibility.
Both inclusion and exclusion criteria were pre-specified. To be eligible, studies had to meet the following criteria: (1) study design: phase 3 randomised trials irrespective of blindness, tumour type, and line of treatment, OS as the primary or co-primary endpoint. (2) population: adult patients (>18 years old) with advanced solid tumour. (3) intervention: random assignment of patients to immunotherapy (monotherapy or combination treatments) or control treatment irrespective of dosage and duration. The immunotherapy combination treatments included immunotherapy + chemotherapy, immunotherapy + targeted therapy, immunotherapy + radiotherapy, and different ICIs combination, while the control arms included all the conventional treatment but immunotherapy. (4) outcomes: OS and information regarding intermediate clinical endpoints, and follow-up is long enough to achieve its primary endpoint. Of note, we conducted a subgroup analysis based on median follow-up duration (<24 months vs. ≥ 24 months). Trials published online ahead of print were eligible, but meeting abstracts were excluded. When multiple publications of the same databases appeared or if there was a case mix between different publications, we removed the overlapping data and only the most recent and/or complete report was included. Studies were excluded if they were: (1) other studies on this topic, including review articles, conference abstract, editorials, pre-clinical papers, phase 1 or phase 2 trials, quality of life studies, and cost effectiveness analyses; (2) studies in the pediatric population, or patients with hematological disease; (3) data from unpublished studies; (4) subgroup or post hoc analyses of clinical trials.
Rik of bias of eligible trials were evaluated by the Cochrane risk of bias tools,22 which covered the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
When disagreements occurred in terms of study selection, data extraction, and risk of bias assessment, all investigators double checked the original data independently, and discuss the potential problems together. The discrepancies were resolved when all authors came to an agreement.
Data extraction and outcome measures
For surrogacy assessment, the control and experimental arms were predefined for each RCT. All authors independently extracted study-level information regarding study characteristics (study name, tumour type, masking method, line of treatment, and sample size), treatment strategy, median follow-up, clinical endpoints information regarding ORR, DCR, PFS, 1-year survival rate, and OS. Treatment effects on OS and PFS were presented as OS hazard ratio (HROS) and HRPFS, respectively. Treatment effects on 1-year OS was expressed as Ratio1y-OS as previously reported,12 Ratio1y-OS = 1-year OS rate in the control arm/1-year OS rate in the experimental arm. Treatment effects on objective response and disease control were presented as OR relative risk (RROR) and RRDC. All analysis were conducted on intention-to-treat population.
Statistical analysis
Candidacy for surrogacy was assessed with a widely-accepted two-stage meta-analytical approach,23,24 which required two conditions to evaluate the magnitude of the association or treatment effect estimates on the intermediate endpoint and OS. Condition 1 required a strong correlation between surrogacy and endpoint (ORR vs. Median OS, DCR vs. Median OS, Median PFS vs. Median OS, and 1-year survival rate vs. Median OS). Condition 2 required a strong correlation of treatment effects between the surrogacy and the clinical endpoint (RROR vs. HROS, RRDC vs. HROS, HRPFS vs. HROS, and Ratio1y-OS vs. HROS). The strength of correlation was quantified with coefficient of determination (R2), weighting each trial by the sample size.25 We used the TrialLevelMA function of R package Surrogate to calculate R2 and its associated 95% CI (Surrogate: Evaluation of Surrogate Endpoints in Clinical Trials. https://CRAN.R-project.org/package=Surrogate). Additionally, as sensitivity analysis we also evaluated another weighting system based on the numbers of events reported or derived from each trial. According to the Systematic Review and Recommendation for Reporting of Surrogate Endpoint Evaluation using Meta-analysis (ReSEEM) guidelines,26 R2 ≥ 0.7 suggest strong correlations (and thus surrogacy), R2 between 0.50 and 0.69 mean moderate correlations, and R2 < 0.5 represent weak correlations. Leave-one-out cross-validation was conducted as a sensitivity analysis.27 Funnel plots were generated to show any potential source of reporting bias. The 95% prediction interval for the regression line was developed by accessing the limits of the regression function over a sequence of possible values for the intermediate clinical endpoint, using the same trial level weights as for calculating R2.
Pre-planned subgroups were assessed for the candidacy of each intermediate clinical endpoint, including tumour types, masking methods, line of treatment, treatment strategy, drug target, and the duration of median follow-up. No post hoc analyses were conducted in this study.
Potential publication bias was assessed by visual inspection of a funnel plot, and also evaluated using the Egger's and Begg's tests.28,29 Two-sided p values < 0.05 were considered statistically significant. Data were acquired and analysed with MedCalc 18.2.1 and R 4.0.1 software.
Ethics
Ethics approval was not required because all data included in this study were publicly available de-sensitised data.
Role of the funding source
No funding was received.
Results
The initial search from Embase, PubMed and Cochrane databases identified 29,857 relevant records in October 18, 2022. We conducted a second search in July 2023 and found 7387 related manuscripts. After carefully screening and reviewing based on our inclusion and exclusion criteria, 77 phase 3 RCTs were eligible for the final analysis (Fig. 1). All data used for analysis were obtained from published manuscripts, and the baseline characteristics of eligible trials were illustrated in Table 1. Totally, 52,342 patients with cancer were enrolled, 29,294 were treated with ICIs, the rest 23,048 patients were in the controlled arms. The median age at the trial-level was 63 years old. 15 types of tumours were identified, including lung cancer (n = 29), gastric cancer/gastroesophageal junction cancer/esophageal cancer (GC/GEJC/EC, n = 10), renal cancer (n = 6), urothelial cancer (n = 5), hepatocellular cancer (n = 4), melanoma (n = 4), ovarian cancer (n = 3), breast cancer (n = 3), prostate cancer (n = 3), head and neck cancer (n = 3), glioblastoma (n = 2), cervical cancer (n = 2), endometrial cancer (n = 1), biliary tract cancer (n = 1), and mesothelioma (n = 1). A total of 89 comparisons were included because 10 studies had three arms; 2 trials had four arms. Immunotherapy were administrated as first-line of treatment in 50 trials, as second-line or later treatment in 26 RCTs. ICIs were applied as monotherapy in 37 trials, as immune-combination treatments in 48 studies. Treatment targeting CTLA-4 were found in 4 trials, PD-1 in 42 RCTs, PD-L1 in 28 studies, and the combination of PD-1/PD-L1 and CTLA-4 in 11 trials. The treatment effects of the eligible trials were shown in Table S2. Most trials (43/77, 55.84%) had over 24 months' median follow-up. Generally, the eligible RCTs had moderate or low risk of bias. The main issue affecting the method quality was lack of blinding giving 54 trials (70.13%) were open-labeled. Both Egger's and Begg's tests were conducted to evaluate the potential publication bias, and no significant bias was discovered (Figure S1).
Fig. 1.
Flowchart diagram of selected trials included in this study.
Table 1.
Baseline characteristics of eligible phase 3 trials.
| Study | Underlying malignancy | Masking | Line of treatment | Treatment agents | No. of patients | Median age (range), years | Sex, m/f | Median follow-up, months |
|---|---|---|---|---|---|---|---|---|
| A367100930 | Melanoma | Open label | 1 | Tremelimumab | 328 | 57 (22–90) | 190/138 | >40.0 |
| Chemotherapy | 327 | 56 (22–90) | 182/145 | |||||
| ARCTIC31 | Lung cancer | Open label | 3+ | Durvalumab | 62 | 64 (35–79) | 42/20 | >18.0 |
| Chemotherapy | 64 | 62 (41–81) | 48/16 | |||||
| Durvalumab + tremelimumab | 174 | 63 (26–81) | 115/59 | |||||
| Chemotherapy | 118 | 65 (42–83) | 81/37 | |||||
| ATTRACTION-432,33 | GC/GEJC | Double blind | 1 | Nivolumab + chemotherapy | 362 | 64 (25–86) | 253/109 | 26.6 |
| Placebo + chemotherapy | 362 | 65 (27–89) | 270/92 | |||||
| CA184-02434,35 | Melanoma | Double blind | 1 | Ipilimumab + dacarbazine | 252 | 58 (33–87) | 152/98 | 36.6 |
| Placebo + dacarbazine | 250 | 61 (31–76) | 149/103 | |||||
| CA184-09536 | Prostate Cancer | Double blind | 1 | Ipilimumab | 400 | 70 (44–91) | 400/0 | <54.0 |
| Placebo | 202 | 69 (42–92) | 202/0 | |||||
| CA184-10437 | Lung cancer | Double blind | 1 | Ipilimumab + chemotherapy | 388 | 64 (28–84) | 326/62 | 12.5 |
| Placebo + chemotherapy | 361 | 64 (28–85) | 309/52 | 11.8 | ||||
| CAPSTONE-138 | Lung cancer | Double blind | 1 | Adebrelimab + chemotherapy | 230 | 62 (55–66) | 184/46 | 14.4 |
| Placebo + chemotherapy | 232 | 62 (56–67) | 188/44 | 12.8 | ||||
| CASPIAN39, 40, 41 | Lung cancer | Open label | 1 | Durvalumab + tremelimumab + chemotherapy | 268 | 63 (58–68) | 202/66 | 25.1 |
| Durvalumab + chemotherapy | 268 | 62 (58–68) | 190/78 | |||||
| Placebo + chemotherapy | 269 | 63 (57–68) | 184/85 | |||||
| CheckMate 9LA42,43 | Lung cancer | Open label | 1 | Nivolumab + ipilimumab + chemotherapy | 361 | 65 (59–70) | 252/109 | 30.7 |
| Chemotherapy | 358 | 65 (58–70) | 252/106 | |||||
| CheckMate 01744, 45, 46, 47 | Lung cancer | Open label | 2 | Nivolumab | 135 | 62 (39–85) | 111/24 | 69.5 |
| Docetaxel | 137 | 64 (42–84) | 97/40 | |||||
| CheckMate 02548, 49, 50 | Renal cancer | Open label | 2+ | Nivolumab | 410 | 62 (23–88) | 315/95 | 72.0 |
| Everolimus | 411 | 62 (18–86) | 304/107 | |||||
| CheckMate 03751,52 | Melanoma | Open label | 2+ | Nivolumab | 272 | 59 (23–88) | 176/96 | 24.0 |
| Chemotherapy | 133 | 62 (29–85) | 85/48 | |||||
| CheckMate 05744,53 | Lung cancer | Open label | 2 | Nivolumab | 292 | 61 (37–84) | 151/141 | 69.4 |
| Docetaxel | 290 | 64 (21–85) | 168/122 | |||||
| CheckMate 06654, 55, 56, 57 | Melanoma | Open label | 1 | Nivolumab | 210 | 64 (18–86) | 121/89 | 32 |
| Dacarbazine | 208 | 66 (26–87) | 125/83 | 10.9 | ||||
| CheckMate 07858, 59, 60 | Lung cancer | Open label | 2+ | Nivolumab | 338 | 60 (27–78) | 263/75 | >37.3 |
| Docetaxel | 166 | 60 (38–78) | 134/32 | |||||
| CheckMate 14161, 62, 63 | Head and neck cancer | Open label | 2 | Nivolumab | 240 | 59 (29–83) | 197/43 | >24.2 |
| Chemotherapy | 121 | 61 (28–78) | 103/18 | |||||
| CheckMate 21464, 65, 66, 67 | Renal cancer | Open label | 1 | Nivolumab + ipilimumab | 550 | 62 (26–85) | 413/137 | 43.6 |
| Sunitinib | 546 | 62 (21–85) | 395/151 | 32.3 | ||||
| CheckMate 22768, 69, 70 | Lung cancer | Open label | 1 | Nivolumab + ipilimumab | 396 | 64 (26–84) | 255/141 | 54.8 |
| Nivolumab | 396 | 64 (27–85) | 272/124 | |||||
| Chemotherapy | 397 | 64 (29–87) | 260/137 | |||||
| CheckMate 45971 | HCC | Open label | 1 | Nivolumab | 371 | 65 (57–71) | 314/57 | 15.2 |
| Sorafenib | 372 | 65 (58–72) | 317/55 | 13.4 | ||||
| CheckMate 49872 | Glioblastoma | Open label | 1 | Nivolumab + radiotherapy | 280 | 60 (18–83) | 190/90 | 13.0 |
| Temozolomide + radiotherapy | 280 | 56 (23–81) | 175/105 | 14.2 | ||||
| CheckMate 54873 | Glioblastoma | Double blind | 1 | Nivolumab + radiotherapy + temozolomide | 358 | 60 (24–79) | 205/153 | >12.5 |
| Placebo + radiotherapy + temozolomide | 358 | 60 (18–81) | 197/161 | >19.5 | ||||
| CheckMate 64874 | EC | Open label | 1 | Nivolumab + chemotherapy | 321 | 64 (40–90) | 253/68 | >13.0 |
| Nivolumab + ipilimumab | 325 | 63 (28–81) | 269/56 | |||||
| Chemotherapy | 324 | 64 (26–81) | 275/49 | |||||
| CheckMate 64975,76 | GC/GEJC/EC | Open label | 1 | Nivolumab + chemotherapy | 473 | 63 (54–69) | 331/142 | >24.0 |
| Chemotherapy | 482 | 62 (54–68) | 349/133 | |||||
| CheckMate 64975,76 | GC/GEJC/EC | Open label | 1 | Nivolumab + ipilimumab | 234 | 62 (22–84) | NR | >35.7 |
| Chemotherapy | 239 | 61 (23–90) | NR | |||||
| CheckMate 74377,78 | Mesothelioma | Open label | 1 | Nivolumab + ipilimumab | 303 | 69 (65–75) | 234/69 | 29.7 |
| Chemotherapy | 302 | 69 (62–75) | 233/69 | |||||
| CONTACT-0379 | Renal cancer | Open label | 2+ | Atezolizumab + Cabozantinib | 263 | 62 (20–85) | 204/59 | 15.2 |
| Cabozantinib | 259 | 63 (18–89) | 197/62 | |||||
| COSMIC-31280 | HCC | Open label | 1 | Cabozantinib + atezolizumab | 432 | 64 (57–71) | 360/72 | 13.3 |
| Sorafenib | 217 | 67 (60–73) | 186/31 | |||||
| DANUBE81 | Urothelial cancer | Open label | 1 | Durvalumab | 346 | 67 (60–73) | 249/97 | 41.2 |
| Durvalumab + tremelimumab | 342 | 68 (60–73) | 256/86 | |||||
| Chemotherapy | 344 | 68 (60–73) | 274/70 | |||||
| EMPOWER-Cervical 182 | Cervical cancer | Open label | 2 | Cemiplimab | 304 | 51 (22–81) | 0/304 | 18.2 |
| Chemotherapy | 304 | 50 (24–87) | 0/304 | |||||
| EMPOWER-Lung 383 | Lung cancer | Double blind | 1 | Cemiplimab + chemotherapy | 312 | 63 (57–68) | 268/44 | 16.3 |
| Chemotherapy | 154 | 63 (57–68) | 123/31 | 16.7 | ||||
| ESCORT-1st84 | EC | Double blind | 1 | Camrelizumab + chemotherapy | 298 | 62 (56–66) | 260/38 | 10.8 |
| Placebo + chemotherapy | 298 | 62 (56–67) | 362/35 | |||||
| IMagyn05085 | Ovarian cancer | Double blind | 1 | Atezolizumab + bevacizumab + chemotherapy | 651 | 60 (29–84) | 0/651 | 19.9 |
| Placebo + bevacizumab + chemotherapy | 650 | 59 (18–83) | 0/650 | 19.8 | ||||
| IMbassador25086 | Prostate cancer | Open label | 2 | Atezolizumab + enzalutamide | 379 | 70 (51–91) | 379/0 | 15.2 |
| Enzalutamide | 380 | 70 (40–92) | 380/0 | 16.6 | ||||
| IMbrave15087,88 | HCC | Open label | 1 | Atezolizumab + Bevacizumab | 336 | 64 (56–71) | 277/59 | 15.6 |
| Sorafenib | 165 | 66 (59–71) | 137/28 | |||||
| IMmotion15189,90 | Renal cancer | Open label | 1 | Atezolizumab + Bevacizumab | 454 | 62 (56–69) | 317/137 | >40.0 |
| Sunitinib | 461 | 60 (54–66) | 352/109 | |||||
| IMpassion13091, 92, 93 | Breast cancer | Double blind | 1 | Atezolizumab + Nab-Paclitaxel | 451 | 55 (20–82) | 3/448 | 18.8 |
| Placebo + Nab-Paclitaxel | 451 | 56 (26–86) | 1/450 | |||||
| IMpower11094,95 | Lung cancer | Open label | 1 | Atezolizumab | 277 | 64 (30–81) | 196/81 | 30.0 |
| Chemotherapy | 277 | 65 (30–87) | 193/84 | |||||
| IMpower13096 | Lung cancer | Open label | 1 | Atezolizumab + chemotherapy | 451 | 64 (18–86) | 266/185 | 18.5 |
| chemotherapy | 228 | 65 (38–85) | 134/94 | 19.2 | ||||
| IMpower13197 | Lung cancer | Open label | 1 | Atezolizumab + carboplatin + nab-paclitaxel | 343 | 65 (23–83) | 280/63 | 26.8 |
| Carboplatin + nab-paclitaxel | 340 | 65 (38–86) | 277/63 | 24.8 | ||||
| IMpower13298 | Lung cancer | Open label | 1 | Atezolizumab + chemotherapy | 292 | 64 (31–85) | 192/100 | 28.4 |
| Chemotherapy | 286 | 63 (33–83) | 192/94 | |||||
| IMpower13399,100 | Lung cancer | Double blind | 1 | Atezolizumab + chemotherapy | 201 | 64 (28–90) | 129/72 | 23.1 |
| Chemotherapy | 202 | 64 (26–87) | 132/70 | 22.6 | ||||
| IMpower150101, 102, 103, 104 | Lung cancer | Open label | 1 | Atezolizumab + chemotherapy | 402 | 63 (32–85) | 241/161 | 38.8 |
| Atezolizumab + bevacizumab + chemotherapy | 400 | 63 (31–89) | 240/160 | 39.8 | ||||
| Bevacizumab + chemotherapy | 400 | 63 (31–90) | 239/161 | 40.0 | ||||
| IMvigor211105,106 | Urothelial cancer | Open label | 2+ | Atezolizumab | 467 | 67 (33–88) | 110/357 | 33.0 |
| Chemotherapy | 464 | 67 (31–84) | 103/361 | |||||
| IPSOS107 | Lung cancer | Open label | 1 | Atezolizumab | 302 | 75 (69–81) | 108/43 | 41.0 |
| Chemotherapy | 151 | 75 (68–80) | 108/43 | |||||
| JAVELIN Bladder 100108 | Urothelial cancer | Open label | 1 | Avelumab | 350 | 68 (37–90) | 266/84 | >19.0 |
| Placebo | 350 | 69 (32–89) | 275/75 | |||||
| JAVELIN Gastric 100109 | GC/GEJC | Open label | 1 | Avelumab | 249 | 62 | 164/85 | 24.1 |
| Chemotherapy | 250 | 61 | 167/83 | 24.0 | ||||
| JAVELIN Lung 200110,111 | Lung cancer | Open label | 2 | Avelumab | 396 | 64 (58–69) | 269/127 | 18.9 |
| Docetaxel | 396 | 63 (57–69) | 273/123 | 17.8 | ||||
| JAVELIN Ovarian 200112 | Ovarian cancer | Open label | 2 | Avelumab + chemotherapy | 188 | 60 (53–67) | 0/188 | 18.4 |
| Avelumab | 188 | 61 (53–70) | 0/188 | 18.2 | ||||
| Chemotherapy | 190 | 60 (53–69) | 0/190 | 17.4 | ||||
| JAVELIN Renal 101113,114 | Renal cancer | Open label | 1 | Avelumab + axitinib | 270 | 62 (29–83) | 203/67 | 19.3 |
| Sunitinib | 290 | 61 (27–88) | 224/66 | 19.2 | ||||
| KESTR EL115 | Head and Neck cancer | Open label | 1 | Durvalumab | 204 | 62 (26–89) | 175/29 | NR |
| Durvalumab + tremelimumab | 413 | 61 (25–87) | 340/73 | |||||
| Chemotherapy | 206 | 61 (22–84) | 174/32 | |||||
| KEYLYNK-010116 | Prostate cancer | Open label | 2+ | Pembrolizumab + Olaparib | 529 | 71 (40–89) | 529/0 | 15.7 |
| Chemotherapy | 264 | 69 (49–84) | 264/0 | |||||
| KEYNOTE-010117, 118, 119 | Lung cancer | Open label | 3+ | Pembrolizumab | 690 | 63 (56–69) | 425/265 | 67.4 |
| Docetaxel | 343 | 62 (56–69) | 209/134 | |||||
| KEYNOTE-033120 | Lung cancer | Open label | 2+ | Pembrolizumab | 213 | 61 (28–83) | 157/56 | 22.3 |
| Chemotherapy | 212 | 61 (34–81) | 164/48 | |||||
| KEYNOTE-042121 | Lung cancer | Open label | 1 | Pembrolizumab | 637 | 63 (57–69) | 450/187 | 12.8 |
| Chemotherapy | 637 | 63 (57–69) | 452/185 | |||||
| KEYNOTE-045122,123 | Urothelial cancer | Open label | 2 | Pembrolizumab | 270 | 67 (29–88) | 200/70 | 27.7 |
| Chemotherapy | 272 | 65 (26–84) | 202/70 | |||||
| KEYNOTE-048124,125 | Head and neck cancer | Open label | 1 | Pembrolizumab + chemotherapy | 281 | 61 (55–68) | 224/57 | 13.0 |
| Cetuximab + chemotherapy | 278 | 61 (55–68) | 242/93 | 10.7 | ||||
| KEYNOTE-062126 | GC/GEJC | Partial-Blind | 1 | Pembrolizumab | 256 | 61 (20–83) | 180/76 | 29.4 |
| Pembrolizumab + chemotherapy | 257 | 62 (22–83) | 195/62 | |||||
| Chemotherapy | 250 | 63 (23–87) | 179/71 | |||||
| KEYNOTE-063127 | GC/GEJC | Open label | 2 | Pembrolizumab | 47 | 61 (32–75) | 32/15 | 24.0 |
| Paclitaxel | 47 | 61 (37–91) | 37/10 | |||||
| KEYNOTE-119128 | Breast cancer | Open label | 2+ | Pembrolizumab | 312 | 50 (43–59) | 0/312 | 31.4 |
| Chemotherapy | 310 | 53 (44–61) | 2/308 | 31.5 | ||||
| KEYNOTE-189129, 130, 131 | Lung cancer | Double blind | 1 | Pembrolizumab + chemotherapy | 410 | 65 (34–84) | 256/156 | 31.0 |
| Chemotherapy | 206 | 64 (34–84) | 109/97 | |||||
| KEYNOTE-240132 | HCC | Double blind | 2 | Pembrolizumab | 278 | 67 (18–91) | 226/52 | 13.8 |
| Placebo | 135 | 65 (23–89) | 112/23 | 10.6 | ||||
| KEYNOTE-355133,134 | Breast cancer | Double blind | 1 | Pembrolizumab + chemotherapy | 220 | 52 (44–62) | 0/220 | 44.1 |
| Chemotherapy | 103 | 55 (43–63) | 0/103 | |||||
| KEYNOTE-361135 | Urothelial cancer | Open label | 1 | Pembrolizumab + chemotherapy | 351 | 69 (62–75) | 272/79 | 31.7 |
| Pembrolizumab | 307 | 68 (61–74) | 228/79 | |||||
| Chemotherapy | 352 | 69 (61–75) | 262/90 | |||||
| KEYNOTE-407136,137 | Lung cancer | Double blind | 1 | Pembrolizumab + chemotherapy | 278 | 65 (29–87) | 220/58 | 14.3 |
| Chemotherapy | 281 | 65 (36–88) | 235/46 | |||||
| KEYNOTE-426138,139 | Renal cancer | Open label | 1 | Pembrolizumab + axitinib | 432 | 62 (30–89) | 308/124 | 30.6 |
| Sunitinib | 429 | 61 (26–90) | 320/109 | |||||
| KEYNOTE-590140 | EC | Double blind | 1 | Pembrolizumab + chemotherapy | 373 | 64 (28–94) | 306/67 | 22.6 |
| Chemotherapy | 376 | 62 (27–89) | 319/57 | |||||
| KEYNOTE-604141 | Lung cancer | Double blind | 1 | Pembrolizumab + chemotherapy | 228 | 64 (24–81) | 152/76 | 21.6 |
| Chemotherapy | 225 | 65 (37–83) | 142/83 | |||||
| KEYNOTE-775142 | Endometrial cancer | Open label | 2+ | Pembrolizumab + lenvatinib | 411 | 64 (30–82) | 0/411 | 12.2 |
| Chemotherapy | 416 | 65 (35–86) | 0/416 | 10.7 | ||||
| KEYNOTE-826143 | Cervical cancer | Double blind | 1 | Pembrolizumab + chemotherapy | 308 | 51 (25–82) | 0/308 | 22.0 |
| Chemotherapy | 309 | 50 (22–79) | 0/309 | |||||
| KEYNOTE-966144 | Biliary tract cancer | Double blind | 1 | Pembrolizumab + chemotherapy | 533 | 64 (57–71) | 280/253 | 25.6 |
| Chemotherapy | 536 | 63 (55–70) | 272/264 | |||||
| MYSTIC145 | Lung cancer | Open label | 1 | Durvalumab | 163 | 64 (32–84) | 113/50 | 30.2 |
| Durvalumab + tremelimumab | 163 | 65 (34–87) | 118/45 | |||||
| Chemotherapy | 162 | 65 (35–85) | 106/56 | |||||
| NINJA146 | Ovarian cancer | Open label | 2+ | Nivolumab | 157 | 58 (29–84) | 0/157 | <48.0 |
| Chemotherapy | 159 | 60 (34–80) | 0/159 | |||||
| OAK147, 148, 149, 150, 151 | Lung cancer | Open label | 2+ | Atezolizumab | 425 | 63 (33–82) | 261/164 | 47.7 |
| Docetaxel | 425 | 64 (34–85) | 259/166 | |||||
| ORIENT-15152 | EC | Double blind | 1 | Sintilimab + chemotherapy | 327 | 63 (57–67) | 279/48 | 16.0 |
| Placebo + chemotherapy | 332 | 63 (56–67) | 288/44 | 16.9 | ||||
| PACIFIC153, 154, 155, 156 | Lung cancer | Double blind | 3+ | Durvalumab | 476 | 64 (31–84) | 334/142 | 34.2 |
| Placebo | 237 | 64 (23–90) | 166/71 | |||||
| ORIENT-3157 | Lung cancer | Open label | 2 | Sintilimab | 145 | 61 (38–74) | 136/9 | 23.6 |
| Chemotherapy | 135 | 60 (34–75) | 122/13 | |||||
| RATIONALE 303158 | Lung cancer | Open label | 2+ | Tislelizumab | 535 | 61 (28–88) | 416/119 | 16.0 |
| Chemotherapy | 270 | 61 (32–81) | 206/64 | 10.7 | ||||
| RATIONALE 306159 | EC | Double blind | 1 | Tislelizumab + chemotherapy | 326 | 64 (59–68) | 282/44 | 16.3 |
| Chemotherapy | 323 | 65 (58–70) | 281/42 | 9.8 |
CRC, colorectal cancer; EC, Oesophageal cancer; GC, gastric cancer; GEJC, gastroesophageal junction cancer; HCC, Hepatocellular cancer.
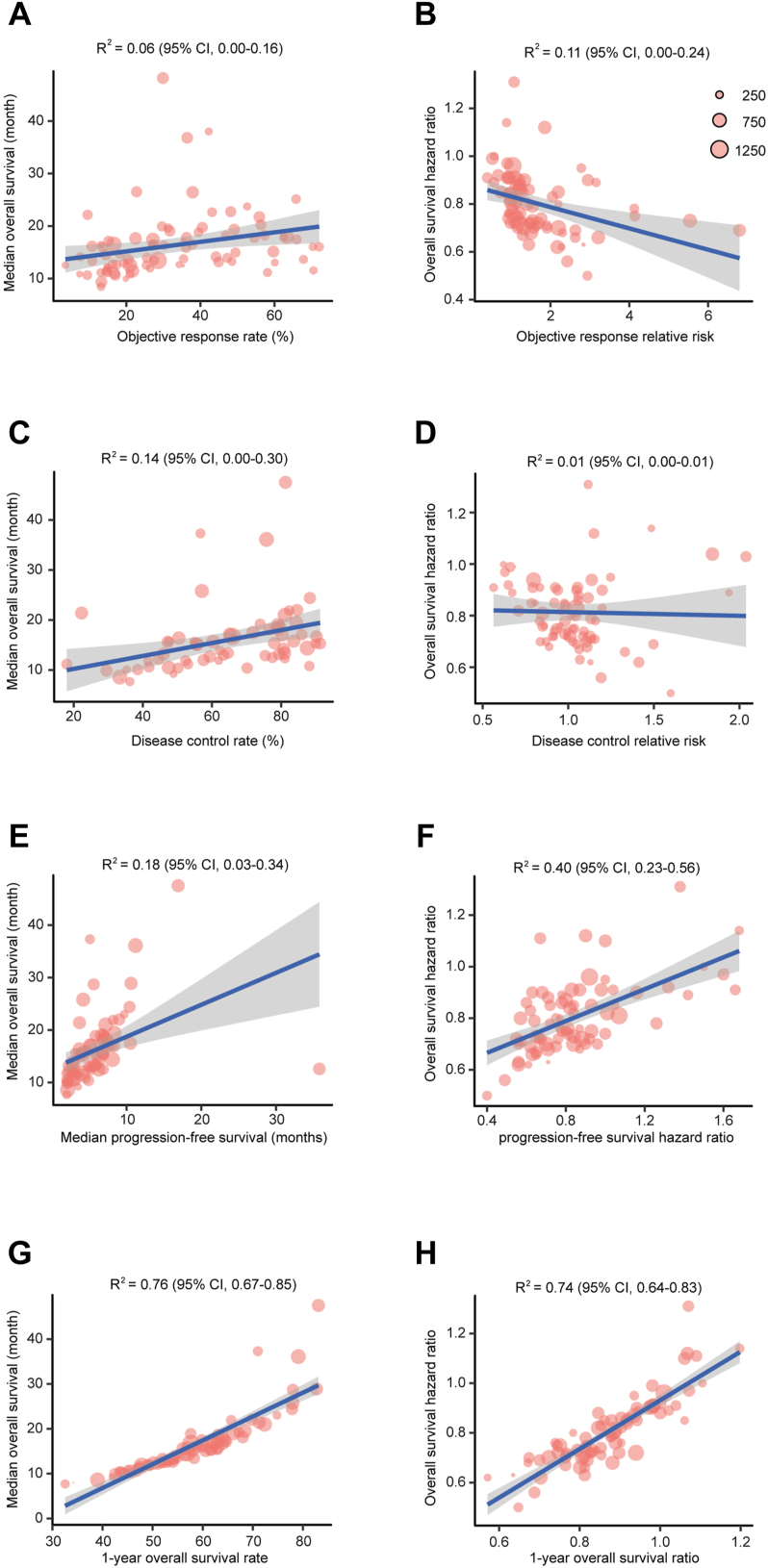
Objective responses were reported in 75 studies with 51,024 patients. There were weak associations between ORR and Median OS (Fig. 2A, R2 = 0.06; 95% CI, 0.00–0.16) and between RROR and HROS (Fig. 2B, R2 = 0.11; 95% CI, 0.00–0.24). Information regarding disease controls were presented in 64 trials with 43,109 individuals. Weak correlations were identified between DCR and Median OS (Fig. 2C, R2 = 0.14; 95% CI, 0.00–0.30) and between RRDC and HROS (Fig. 2D, R2 = 0.01; 95% CI, 0.00–0.01). With data from 73 RCTs with 49,379 patients, we found weak associations between median PFS and median OS (Fig. 2E, R2 = 0.18; 95% CI, 0.03–0.34) and between HRPFS and HROS (Fig. 2F, R2 = 0.40; 95% CI, 0.23–0.56). In contrast, strong correlations were observed between 1-year survival rate and Median OS (Fig. 2G, R2 = 0.76; 95% CI, 0.67–0.85) and between Ratio1y-OS and HROS (Fig. 2H, R2 = 0.74; 95% CI, 0.64–0.83). To evaluate whether any single RCT was more influential in the trial-level correlation between Ratio1y-OS and HROS, leave-one-out cross-validation by excluding 1 study at a time was conducted. The median R2 from the cross-validation is 0.75 (range, 0.72–0.78). As sensitivity analysis we also evaluated the surrogate endpoints with another weighting systems, which was based on the numbers of events reported or derived from each trial (Table S3). As expected, the strongest correlations were observed between 1-year survival rate and Median OS (R2 = 0.76) and between Ratio1y-OS and HROS (R2 = 0.73).
Fig. 2.
Associations of the treatment effects between intermediate clinical endpoints and overall survival in cancer immunotherapy. The association between (A) objective response rate and median overall survival; (B) objective response relative risk and overall survival hazard ratio; (C) disease control rate and median overall survival; (D) disease control relative risk and overall survival hazard ratio; (E) median progression-free survival and median overall survival; (F) progression-free survival hazard ratio and overall survival hazard ratio; (G) 1-year overall survival rate and median overall survival; and (H) 1-year overall survival ratio and overall survival hazard ratio. Each dot represents one eligible phase 3 trials, size of the dot is proportional to the sample size in the trial. Blue lines indicate the correlation (R2), gray shaded area indicates 95% prediction intervals.
To assess the robustness of our findings, we further conducted 6 pre-defined subgroup analyses including tumour types, masking method, line of treatment, drug target, treatment strategy, and follow-up duration (Table 2). Generally, the correlations of the treatment effects between intermediate clinical endpoints and OS remained relatively consistent across these subgroup analyses. Of note, PFS showed moderate association with OS in some specific conditions including GC/GEJC/EC tumour type, ICIs were applied as monotherapy or as second or later line of treatment, immunotherapy targeting PD-1, and median follow-up duration over 2 years. Additionally, for some unknown reason, 1-year survival showed weak association with outcomes when patients were treated with multiple ICIs.
Table 2.
Subgroup surrogacy analysis of intermediate clinical endpoints.
| Trials, n | Comparisons, n | Sample size, n | Correlation of treatment effects (R2, 95% CI) |
||||
|---|---|---|---|---|---|---|---|
| RROR vs. HROS | RRDC vs. HROS | HRPFS vs. HROS | Ratio1y-OS vs. HROS | ||||
| Tumour types | |||||||
| Lung cancer | 29 | 34 | 19,006 | 0.11 (0.00–0.36) | 0.01 (0.00–0.14) | 0.25 (0.03–0.53) | 0.81 (0.64–0.90) |
| GC/GEJC/EC | 9 | 12 | 6482 | 0.52 (0.06–0.84) | 0.52 (0.05–0.85) | 0.56 (0.09–0.86) | 0.79 (0.41–0.94) |
| Urothelial cancer | 5 | 7 | 4215 | 0.51 (0.01–0.91) | 0.47 (0.02–0.90) | 0.44 (0.01–0.68) | 0.78 (0.15–0.97) |
| Masking | |||||||
| Open-label | 54 | 65 | 37,336 | 0.14 (0.02–0.32) | 0.00 (0.00–0.08) | 0.45 (0.25–0.63) | 0.71 (0.57–0.81) |
| Double-blind | 22 | 23 | 14,750 | 0.05 (0.00–0.36) | 0.14 (0.00–0.54) | 0.53 (0.20–0.77) | 0.81 (0.60–0.92) |
| Line of treatment | |||||||
| 1 | 50 | 60 | 36,339 | 0.09 (0.02–0.25) | 0.00 (0.00–0.09) | 0.32 (0.12–0.52) | 0.70 (0.54–0.81) |
| >1 | 26 | 28 | 15,254 | 0.17 (0.00–0.46) | 0.00 (0.00–0.18) | 0.57 (0.28–0.78) | 0.85 (0.69–0.92) |
| Treatment strategy | |||||||
| Monotherapy | 37 | 37 | 21,154 | 0.25 (0.00–0.52) | 0.02 (0.00–0.23) | 0.53 (0.24–0.74) | 0.84 (0.70–0.91) |
| Combination treatment | 48 | 52 | 32,850 | 0.02 (0.01–0.15) | 0.01 (0.00–0.14) | 0.39 (0.17–0.59) | 0.67 (0.49–0.81) |
| Drug target | |||||||
| PD-1 | 42 | 44 | 26,480 | 0.13 (0.01–0.36) | 0.25 (0.04–0.51) | 0.57 (0.35–0.74) | 0.74 (0.56–0.85) |
| PD-L1 | 28 | 30 | 18,262 | 0.11 (0.00–0.38) | 0.05 (0.00–0.30) | 0.43 (0.14–0.69) | 0.79 (0.61–0.89) |
| PD-1/PD-L1+CTLA-4 | 11 | 11 | 6794 | 0.00 (0.00–0.41) | 0.36 (0.00–0.81) | 0.40 (0.00–0.85) | 0.25 (0.00–0.75) |
| Median follow-up | |||||||
| >24 months | 43 | 52 | 30,221 | 0.22 (0.03–0.46) | 0.35 (0.10–0.60) | 0.40 (0.15–0.63) | 0.72 (0.52–0.84) |
| ≤24 months | 33 | 35 | 21,298 | 0.03 (0.00–0.24) | 0.02 (0.00–0.23) | 0.63 (0.41–0.81) | 0.78 (0.60–0.89) |
CI, confidential interval; CTLA-4, cytotoxic T lymphocyte antigen-4; DC, disease control; EC, oesophageal cancer; GC, gastric cancer; GEJC, gastroesophageal junction cancer; HR, hazard ratio; OR, objective response; OS, overall survival; PD-1, programmed death-1; PD-L1, programmed death ligand 1; PFS, progression-free survival; RR, relative risk.
Discussion
Based on 52,342 patients with 15 types of tumours enrolled in 77 phase 3 randomised trials, our study reveals that 1-year milestone survival is a strong surrogate for clinical outcomes in cancer immunotherapy. In contrast, other intermediate RECIST-based endpoints including ORR, DCR, and PFS, show weak associations with overall survival. These results may provide important references in accelerating the drug development process, assist in design and interpretation of clinical trials, and constitute complementary information in drafting the clinical practice guideline.
Endpoints based on RECIST criteria has been commonly applied as the surrogacy for OS to reduce the follow-up duration, the sample size, and the cost of clinical studies.160 However, it is well-established that, compared with other conventional treatments, immunotherapy shows unique efficacy kinetics such as delayed clinical effect and long-term favorable outcome.3 These characteristics may lead to the prolongation of trial duration and make it difficult to accelerate the drug development process.161 Consist with previous studies,18, 19, 20 here our data demonstrated that the conventional RECIST-based endpoints, including ORR, DCR and PFS, failed to represent the long-term benefit of immunotherapy. To further evaluate the robustness of these results, we conducted the sensitivity analysis using pre-defined subgroups, and could not identified any strong correlation between the RECIST-based endpoints and overall survival. Even in some cases PFS showed moderate associations with OS (Table 2), these associations usually had a wide range of confidential intervals, highlighted the inconsistency of RECIST-based endpoints. It was reported that ICIs could increase immune cell infiltration by activate the cytotoxic T-cell response, which might result in the initial upregulation of tumour volume or occurrence of new lesions followed by the shrinkage (pseudo-progression), or a severe and rapid pattern of progression (hyper-progression).162 The weak associations between OS and RECIST-based endpoints in our analysis might partly be due to the pseudo-progression and/or hyper-progression. It should be noted that many phase 3 randomised trials still set PFS as the primary solo-endpoint currently.163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174 Moreover, though Accelerated Approval Program, FDA had granted the application of ICIs in different types of tumours based on ORR or PFS.175 In the last two years, many of these accelerated approvals have been withdrawn and no longer FDA-approved (https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-cancer-accelerated-approvals).
Given that patients with modest or even absent PFS benefit may still gain long-term favorable outcomes from immunotherapy,176 several proposals aiming to optimise the existing RECIST criteria were emerging. A modified RECIST (mRECIST) was developed to improve response assessment that may predict outcomes.177 While RECIST defined progression by an increase in tumour diameters, mRECIST progression only required the disappearance or reduction of intra-tumoural arterial enhancement in tumour lesions. Immune-Related Response Criteria (irRC) combined the new lesions into the total tumour burden and included additional patterns of tumour response that appear after initial tumour expansion.178 In 2017, the RECIST Working Group recommend iRECIST guideline to assess the tumour response in immunotherapy studies.179 More recently, modified PFS, which omitted the events of progression within 3 months after randomisation, showed strong correlation with OS at both trial-level and patient-level.20 However, the robustness of these consensus guidelines needed further independent validation. In the present study, since only a small number of the eligible phase 3 trials reported both OS and these intermediate endpoints, it is not feasible to evaluate the powers of them as surrogacies for clinical outcomes in cancer immunotherapy.
We explored milestone survival analysis due to the following reasons. First, traditional metrics of response and progression by RECIST are insufficient in characterising the clinical benefit or prioritising immune-combination treatment. Additionally, patients treated with ICIs demonstrate unique pattern of response and prognosis. On the trial level, these patterns led to the delayed separation of survival curves beyond the median, non-proportional curves, and a subgroup of individuals can achieve long-term benefit. Furthermore, it is well-established that milestone analyses had several advantages including simplicity of analysis and predictability (time driven rather than event driven). The relative and absolute differences of the outcomes can be measured in the context of clinical studies. An example demonstrated that the long-term survival rate of approximately 15% between two treatments led to an additional 2-year follow-up.161 Moreover, milestone measured at a mature time point can avoid some conditions such as the survival curves are non-proportional or separate late. In some circumstances, the milestone survival rate may capture the sub-population who can derive favorable outcomes.176 Accordingly, the role of milestone survival at a given timepoint has been descriptive in nature in most studies.
One of the difficulties with milestone survival analysis is in milestone selection. The delayed clinical effect often occurred within 6 months after the application of ICIs, and the crossover of Kaplan–Meier curves may appear later. If the milestone placed at a timepoint before the treatment took effect, the potential benefit of immunotherapy would not have been discovered. Based on the clinical data derived from ipilimumab development program in which the OS were relatively stable beyond two years, a 2-year milestone was chosen in the retrospective real-time analysis.161 In 2017, a milestone survival study suggested that 1-year OS rate was a surrogate endpoint for non-small cell lung cancer (NSCLC). However, this investigation was conducted on trials with different treatment strategies including chemotherapy, targeted therapy, and immunotherapy.12 Our study is unique since we only included phase 3 immunotherapy trials, and we examined the correlation between 1-year survival and OS in several subgroups to validate our results. Since the biggest challenge with milestone survival analysis is the difficulty in maintaining the study integrity after the milestone timepoint, we specifically analysed the trials with follow-up over 2 years (Table 1). The correlation between 1-year milestone survival and OS remained robust in this subgroup.
Metastasis or recurrence is an important step in tumour progression, and has long been known as the overwhelming majority of cancer-related deaths.180 According, metastasis-free survival (MFS) and recurrence-free survival (RFS) have been examined as surrogates for outcomes in numerous tumours, and show strong correlation with OS in nasopharyngeal carcinoma,181 prostate cancer,182 colorectal cancer,183 melanoma.184 Unfortunately, no or very few immunotherapy trials involved in these studies. In 2020, Goart et al. evaluated the performance of relapse-free survival as a surrogate in adjuvant therapy of melanoma treated with ipilimumab,185 and found a strong association at the patient-level but only moderate association at the trial-level. It should be noted that only 264 patients from EORTC trial were investigated in this study. Accordingly, more solid evidences were needed to confirm the strength of this association. Since the first ICI was approved only over 10 years ago,186 currently most available trials mainly focused on patients with late-stage tumours. Accordingly, it is very difficult to extract enough information regarding MFS and RFS in our analysis. However, ICIs were widely used in clinical practice nowadays and became the standard first-line or second-line treatment in multiple malignancies. We believe a systematic analysis on the correlation between MFS or RFS and OS can be conducted in the near future.
Our study has several strengths and clinical implication. We conducted a comprehensive meta-analysis and included the most up-to date published phase 3 RCTs of immunotherapy across multiple advanced solid tumours with over 50,000 patients. Hence our study had enhanced statistical power, which provide more reliable and precise estimates. Moreover, since agents targeting different checkpoints were examined in a heterogeneous population, we could investigate for variability in outcomes and improved the generalisability of our conclusion. Second, we tested all the commonly used surrogate endpoints in cancer trials including ORR, DCR, PFS, and 1-year survival, which meant our result were more extensive and valid than others. Third, the correlation between 1-year milestone survival and OS remained robust based on various classification criteria. With the application of 1-year milestone survival, further studies might need more patients, more resources, and longer follow-up to obtain these data. Even so, considering the relatively low success rate of phase 3 trials in cancer research,187 and the increasing number of trials using ICIs in tumours at early stage, the application of this surrogate is still cost-effective in guiding the selection of agents for a more time-consuming and costly study.
This study has several limitations. First, it was well-known that an optimal surrogacy should fulfill the condition of a strong correlation with the outcome at both the trial and patient level.188 The patient level association does not always consist with the results derived from the trial levels. For example, the pathological complete response is a surrogate endpoint in neoadjuvant studies of early-stage breast cancer at patient level,189 but not at trial level.190 Here, we were unable to access to the individual patient data and hence could not examine patient-level association. Second, comprehensive and reliable data on cross over between experimental arms and salvage immunotherapy for control arm patients are unclear in most trials, which could impact the robustness of surrogate endpoints. Currently, we cannot conduct sensitivity analysis according to the presence of cross over. However, considering patients are transferred from the control arms to the experimental arms in most cases, these crossovers are likely to weaken the power of the association between intermediate endpoints and OS. Third, the immune-related response evaluation criteria lack consensus and are not widely used in these eligible trials. Hence, we did not evaluate the power of immune-related ORR, DCR, and PFS as surrogate endpoints here. Fourth, the heterogeneity across studies may not be fully explained. Some confounders, such as the different properties of ICIs, study designs, patient population, and the distribution of tumour stage, can also be the source of the heterogeneity. Further analysis is needed to determine their influences on the robustness of surrogate endpoints. Fifth, the 1-year milestone survival analysis is a cross-sectional analysis and only considers a given timepoint as the primary endpoint. It cannot account for the totality of the OS times or the effects of censoring before this timepoint. Lastly, the included studies were conducted at different hospitals by various clinicians, who might be associated with some subjectivity and have potential bias in reporting these intermediate endpoints. Our study is subject to any errors or biases of the original researchers, and the results are generalisable only to the patient eligible for these trials.
In summary, our study revealed there is a strong correlation between clinical outcome and 1-year milestone survival rate in cancer immunotherapy.
Contributors
BZ conceived and designed the study. ZZ, QP, ML and BZ developed the protocol and performed the data analysis. ZZ, QP and BZ collected data. ZZ, QP, ML and BZ make the figures. BZ, ZZ and QP wrote the manuscript. BZ and ZZ accessed and verified the underlying data. BZ supervised this work. All authors read and approved the final manuscript. BZ has accessed and verified the data and responsible for the decision to submit the manuscript.
Data sharing statement
All original data discussed in the text of this paper is also included as figure and/or table either in the main text or in the appendix. No additional data are available.
Declaration of interests
We declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102156.
Appendix A. Supplementary data
Figure S1.
References
- 1.Zhao B., Zhao H., Zhao J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920937612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosellini M., Marchetti A., Mollica V., Rizzo A., Santoni M., Massari F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat Rev Urol. 2023;20(3):133–157. doi: 10.1038/s41585-022-00676-0. [DOI] [PubMed] [Google Scholar]
- 3.Shen X., Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. doi: 10.1136/bmj.k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao B., Zhao H., Zhao J. Impact of clinicopathological characteristics on survival in patients treated with immune checkpoint inhibitors for metastatic melanoma. Int J Cancer. 2019;144(1):169–177. doi: 10.1002/ijc.31813. [DOI] [PubMed] [Google Scholar]
- 5.Mollica V., Santoni M., Matrana M.R., et al. Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Targeted Oncol. 2022;17(1):61–68. doi: 10.1007/s11523-021-00861-y. [DOI] [PubMed] [Google Scholar]
- 6.Hegde P.S., Chen D.S. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Hodi F.S., Hwu W.J., Kefford R., et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viscardi G., Tralongo A.C., Massari F., et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. doi: 10.1016/j.ejca.2022.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Cabibbo G., Celsa C., Enea M., et al. Progression-free survival early assessment is a robust surrogate endpoint of overall survival in immunotherapy trials of hepatocellular carcinoma. Cancers. 2020;13(1):90. doi: 10.3390/cancers13010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hellmann M.D., Kris M.G., Rudin C.M. Medians and milestones in describing the path to cancer cures: telling “Tails”. JAMA Oncol. 2016;2(2):167–168. doi: 10.1001/jamaoncol.2015.4345. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie G., Gasper H., Man J., et al. Defining the most appropriate primary end point in phase 2 trials of immune checkpoint inhibitors for advanced solid cancers: a systematic review and meta-analysis. JAMA Oncol. 2018;4(4):522–528. doi: 10.1001/jamaoncol.2017.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal G.M., Zhang L., Zhang H., et al. Milestone analyses of immune checkpoint inhibitors, targeted therapy, and conventional therapy in metastatic non-small cell lung cancer trials: a meta-analysis. JAMA Oncol. 2017;3(8) doi: 10.1001/jamaoncol.2017.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu A.X., Lin Y., Ferry D., Widau R.C., Saha A. Surrogate end points for survival in patients with advanced hepatocellular carcinoma treated with immune checkpoint inhibitors. Immunotherapy. 2022;14(16):1341–1351. doi: 10.2217/imt-2022-0089. [DOI] [PubMed] [Google Scholar]
- 14.Hua T., Gao Y., Zhang R., Wei Y., Chen F. Validating ORR and PFS as surrogate endpoints in phase II and III clinical trials for NSCLC patients: difference exists in the strength of surrogacy in various trial settings. BMC Cancer. 2022;22(1):1022. doi: 10.1186/s12885-022-10046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talukder R., Makrakis D., Lin G.I., et al. Association of the time to immune checkpoint inhibitor (ICI) initiation and outcomes with second line ICI in patients with advanced urothelial carcinoma. Clin Genitourin Cancer. 2022;20(6):558–567. doi: 10.1016/j.clgc.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goring S., Varol N., Waser N., et al. Correlations between objective response rate and survival-based endpoints in first-line advanced non-small cell lung Cancer: a systematic review and meta-analysis. Lung Cancer. 2022;170:122–132. doi: 10.1016/j.lungcan.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S., Li S., Cui Y., Zhao P., Sun X., Cheng Y. Consideration of surrogate endpoints for overall survival associated with first-line immunotherapy in extensive-stage small cell lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.696010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mushti S.L., Mulkey F., Sridhara R. Evaluation of overall response rate and progression-free survival as potential surrogate endpoints for overall survival in immunotherapy trials. Clin Cancer Res. 2018;24(10):2268–2275. doi: 10.1158/1078-0432.CCR-17-1902. [DOI] [PubMed] [Google Scholar]
- 19.Nie R.C., Chen F.P., Yuan S.Q., et al. Evaluation of objective response, disease control and progression-free survival as surrogate end-points for overall survival in anti-programmed death-1 and anti-programmed death ligand 1 trials. Eur J Cancer. 2019;106:1–11. doi: 10.1016/j.ejca.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z.X., Wu H.X., Xie L., et al. Exploration of modified progression-free survival as a novel surrogate endpoint for overall survival in immuno-oncology trials. J Immunother Cancer. 2021;9(4) doi: 10.1136/jitc-2020-002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciani O., Davis S., Tappenden P., et al. Validation of surrogate endpoints in advanced solid tumors: systematic review of statistical methods, results, and implications for policy makers. Int J Technol Assess Health Care. 2014;30(3):312–324. doi: 10.1017/S0266462314000300. [DOI] [PubMed] [Google Scholar]
- 24.Buyse M., Molenberghs G., Paoletti X., et al. Statistical evaluation of surrogate endpoints with examples from cancer clinical trials. Biom J. 2016;58(1):104–132. doi: 10.1002/bimj.201400049. [DOI] [PubMed] [Google Scholar]
- 25.Xie W., Regan M.M., Buyse M., et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35(27):3097–3104. doi: 10.1200/JCO.2017.73.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie W., Halabi S., Tierney J.F., et al. A systematic review and recommendation for reporting of surrogate endpoint evaluation using meta-analyses. JNCI Cancer Spectr. 2019;3(1):pkz002. doi: 10.1093/jncics/pkz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker S.G., Kramer B.S. Surrogate endpoint analysis: an exercise in extrapolation. J Natl Cancer Inst. 2013;105(5):316–320. doi: 10.1093/jnci/djs527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 29.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribas A., Kefford R., Marshall M.A., et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Planchard D., Reinmuth N., Orlov S., et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31(5):609–618. doi: 10.1016/j.annonc.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Kang Y.K., Chen L.T., Ryu M.H., et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(2):234–247. doi: 10.1016/S1470-2045(21)00692-6. [DOI] [PubMed] [Google Scholar]
- 33.Boku N., Ryu M.H., Kato K., et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4) Ann Oncol. 2019;30(2):250–258. doi: 10.1093/annonc/mdy540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robert C., Thomas L., Bondarenko I., et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 35.Maio M., Grob J.J., Aamdal S., et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 2015;33(10):1191–1196. doi: 10.1200/JCO.2014.56.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beer T.M., Kwon E.D., Drake C.G., et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 37.Govindan R., Szczesna A., Ahn M.J., et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Zhou C., Yao W., et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–747. doi: 10.1016/S1470-2045(22)00224-8. [DOI] [PubMed] [Google Scholar]
- 39.Goldman J.W., Dvorkin M., Chen Y., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 40.Paz-Ares L., Chen Y., Reinmuth N., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paz-Ares L., Dvorkin M., Chen Y., et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 42.Reck M., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 44.Borghaei H., Gettinger S., Vokes E.E., et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vokes E.E., Ready N., Felip E., et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;29(4):959–965. doi: 10.1093/annonc/mdy041. [DOI] [PubMed] [Google Scholar]
- 47.Horn L., Spigel D.R., Vokes E.E., et al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35(35):3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motzer R.J., Escudier B., George S., et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–4167. doi: 10.1002/cncr.33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motzer R.J., Escudier B., McDermott D.F., et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Escudier B., Sharma P., McDermott D.F., et al. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol. 2017;72(6):962–971. doi: 10.1016/j.eururo.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Weber J.S., D'Angelo S.P., Minor D., et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 52.Larkin J., Minor D., D'Angelo S., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383–390. doi: 10.1200/JCO.2016.71.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert C., Long G.V., Brady B., et al. Five-year outcomes with nivolumab in patients with wild-type BRAF advanced melanoma. J Clin Oncol. 2020;38(33):3937–3946. doi: 10.1200/JCO.20.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert C., Long G.V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 56.Long G.V., Weber J.S., Larkin J., et al. Nivolumab for patients with advanced melanoma treated beyond progression: analysis of 2 phase 3 clinical trials. JAMA Oncol. 2017;3(11):1511–1519. doi: 10.1001/jamaoncol.2017.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ascierto P.A., Long G.V., Robert C., et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2018;5(2):187–194. doi: 10.1001/jamaoncol.2018.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu S., Wang J., Cheng Y., et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer: 2-year follow-up from a randomized, open-label, phase 3 study (CheckMate 078) Lung Cancer. 2021;152:7–14. doi: 10.1016/j.lungcan.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Chang J., Wu Y.L., Lu S., et al. Three-year follow-up and patient-reported outcomes from CheckMate 078: nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer. Lung Cancer. 2021;165:71–81. doi: 10.1016/j.lungcan.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y.L., Lu S., Cheng Y., et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867–875. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gillison M.L., Blumenschein G., Jr., Fayette J., et al. CheckMate 141: 1-year update and subgroup analysis of nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist. 2018;23(9):1079–1082. doi: 10.1634/theoncologist.2017-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motzer R.J., Escudier B., McDermott D.F., et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Regan M.M., Jegede O.A., Mantia C.M., et al. Treatment-free survival after immune checkpoint inhibitor therapy versus targeted therapy for advanced renal cell carcinoma: 42-month results of the CheckMate 214 trial. Clin Cancer Res. 2021;27(24):6687–6695. doi: 10.1158/1078-0432.CCR-21-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Motzer R.J., Tannir N.M., McDermott D.F., et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albiges L., Tannir N.M., Burotto M., et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6) doi: 10.1136/esmoopen-2020-001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hellmann M.D., Ciuleanu T.E., Pluzanski A., et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paz-Ares L.G., Ramalingam S.S., Ciuleanu T.E., et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 Part 1 trial. J Thorac Oncol. 2022;17(2):289–308. doi: 10.1016/j.jtho.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Hellmann M.D., Paz-Ares L., Bernabe Caro R., et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 71.Yau T., Park J.W., Finn R.S., et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 72.Omuro A., Brandes A.A., Carpentier A.F., et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase 3 trial. Neuro Oncol. 2022;25(1):123–134. doi: 10.1093/neuonc/noac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim M., Weller M., Idbaih A., et al. Phase 3 trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 2022;24(11):1935–1949. doi: 10.1093/neuonc/noac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doki Y., Ajani J.A., Kato K., et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 75.Shitara K., Ajani J.A., Moehler M., et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942–948. doi: 10.1038/s41586-022-04508-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janjigian Y.Y., Shitara K., Moehler M., et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peters S., Scherpereel A., Cornelissen R., et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. 2022;33(5):488–499. doi: 10.1016/j.annonc.2022.01.074. [DOI] [PubMed] [Google Scholar]
- 78.Baas P., Scherpereel A., Nowak A.K., et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 79.Pal S.K., Albiges L., Tomczak P., et al. Atezolizumab plus cabozantinib versus cabozantinib monotherapy for patients with renal cell carcinoma after progression with previous immune checkpoint inhibitor treatment (CONTACT-03): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2023;402(10397):185–195. doi: 10.1016/S0140-6736(23)00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelley R.K., Rimassa L., Cheng A.L., et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6. [DOI] [PubMed] [Google Scholar]
- 81.Powles T., van der Heijden M.S., Castellano D., et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–1588. doi: 10.1016/S1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 82.Tewari K.S., Monk B.J., Vergote I., et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med. 2022;386(6):544–555. doi: 10.1056/NEJMoa2112187. [DOI] [PubMed] [Google Scholar]
- 83.Gogishvili M., Melkadze T., Makharadze T., et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med. 2022;28(11):2374–2380. doi: 10.1038/s41591-022-01977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo H., Lu J., Bai Y., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore K.N., Bookman M., Sehouli J., et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39) J Clin Oncol. 2021;39(17):1842–1855. doi: 10.1200/JCO.21.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Powles T., Yuen K.C., Gillessen S., et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med. 2022;28(1):144–153. doi: 10.1038/s41591-021-01600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng A.L., Qin S., Ikeda M., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 88.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 89.Rini B.I., Powles T., Atkins M.B., et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 90.Motzer R.J., Powles T., Atkins M.B., et al. Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol. 2022;8(2):275–280. doi: 10.1001/jamaoncol.2021.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Emens L.A., Adams S., Barrios C.H., et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32(8):983–993. doi: 10.1016/j.annonc.2021.05.355. [DOI] [PubMed] [Google Scholar]
- 92.Schmid P., Adams S., Rugo H.S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 93.Schmid P., Rugo H.S., Adams S., et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 94.Jassem J., de Marinis F., Giaccone G., et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol. 2021;16(11):1872–1882. doi: 10.1016/j.jtho.2021.06.019. [DOI] [PubMed] [Google Scholar]
- 95.Herbst R.S., Giaccone G., de Marinis F., et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 96.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 97.Jotte R., Cappuzzo F., Vynnychenko I., et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 98.Nishio M., Barlesi F., West H., et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 99.Liu S.V., Reck M., Mansfield A.S., et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133) J Clin Oncol. 2021;39(6):619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Horn L., Mansfield A.S., Szczęsna A., et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 101.Socinski M.A., Nishio M., Jotte R.M., et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16(11):1909–1924. doi: 10.1016/j.jtho.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 102.Reck M., Mok T.S.K., Nishio M., et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 103.Nogami N., Barlesi F., Socinski M.A., et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. 2022;17(2):309–323. doi: 10.1016/j.jtho.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 104.Socinski M.A., Jotte R.M., Cappuzzo F., et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 105.Powles T., Duran I., van der Heijden M.S., et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 106.van der Heijden M.S., Loriot Y., Durán I., et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: a long-term overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur Urol. 2021;80(1):7–11. doi: 10.1016/j.eururo.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 107.Lee S.M., Schulz C., Prabhash K., et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet. 2023;S0140-6736(23):00774. doi: 10.1016/S0140-6736(23)00774-2. [DOI] [PubMed] [Google Scholar]
- 108.Powles T., Park S.H., Voog E., et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 109.Moehler M., Dvorkin M., Boku N., et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966–977. doi: 10.1200/JCO.20.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park K., Özgüroğlu M., Vansteenkiste J., et al. Avelumab versus docetaxel in patients with platinum-treated advanced NSCLC: 2-year follow-up from the JAVELIN lung 200 phase 3 trial. J Thorac Oncol. 2021;16(8):1369–1378. doi: 10.1016/j.jtho.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 111.Barlesi F., Vansteenkiste J., Spigel D., et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 112.Pujade-Lauraine E., Fujiwara K., Ledermann J.A., et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22(7):1034–1046. doi: 10.1016/S1470-2045(21)00216-3. [DOI] [PubMed] [Google Scholar]
- 113.Choueiri T.K., Motzer R.J., Rini B.I., et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8):1030–1039. doi: 10.1016/j.annonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Motzer R.J., Penkov K., Haanen J., et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Psyrri A., Fayette J., Harrington K., et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann Oncol. 2023;34(3):262–274. doi: 10.1016/j.annonc.2022.12.008. [DOI] [PubMed] [Google Scholar]
- 116.Antonarakis E.S., Park S.H., Goh J.C., et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J Clin Oncol. 2023;41 doi: 10.1200/JCO.23.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herbst R.S., Garon E.B., Kim D.W., et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol. 2021;16(10):1718–1732. doi: 10.1016/j.jtho.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Herbst R.S., Garon E.B., Kim D.W., et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1‒positive, advanced non‒small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. 2020;38(14):1580–1590. doi: 10.1200/JCO.19.02446. [DOI] [PubMed] [Google Scholar]
- 119.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 120.Ren S., Feng J., Ma S., et al. KEYNOTE-033: randomized phase 3 study of pembrolizumab vs docetaxel in previously treated, PD-L1-positive, advanced NSCLC. Int J Cancer. 2023;153(3):623–634. doi: 10.1002/ijc.34532. [DOI] [PubMed] [Google Scholar]
- 121.Mok T.S.K., Wu Y.L., Kudaba I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 122.Bellmunt J., de Wit R., Vaughn D.J., et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fradet Y., Bellmunt J., Vaughn D.J., et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of > 2 years of follow-up. Ann Oncol. 2019;30(6):970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burtness B., Rischin D., Greil R., et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022;40(21):2321–2332. doi: 10.1200/JCO.21.02198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 126.Shitara K., Van Cutsem E., Bang Y.J., et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chung H.C., Kang Y.K., Chen Z., et al. Pembrolizumab versus paclitaxel for previously treated advanced gastric or gastroesophageal junction cancer (KEYNOTE-063): a randomized, open-label, phase 3 trial in Asian patients. Cancer. 2022;128(5):995–1003. doi: 10.1002/cncr.34019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Winer E.P., Lipatov O., Im S.A., et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(4):499–511. doi: 10.1016/S1470-2045(20)30754-3. [DOI] [PubMed] [Google Scholar]
- 129.Rodríguez-Abreu D., Powell S.F., Hochmair M.J., et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32(7):881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 130.Gadgeel S., Rodríguez-Abreu D., Speranza G., et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 131.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 132.Finn R.S., Ryoo B.Y., Merle P., et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 133.Cortes J., Cescon D.W., Rugo H.S., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 134.Cortes J., Rugo H.S., Cescon D.W., et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387(3):217–226. doi: 10.1056/NEJMoa2202809. [DOI] [PubMed] [Google Scholar]
- 135.Powles T., Csőszi T., Özgüroğlu M., et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931–945. doi: 10.1016/S1470-2045(21)00152-2. [DOI] [PubMed] [Google Scholar]
- 136.Paz-Ares L., Vicente D., Tafreshi A., et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 137.Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 138.Powles T., Plimack E.R., Soulières D., et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–1573. doi: 10.1016/S1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 139.Rini B.I., Plimack E.R., Stus V., et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 140.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 141.Rudin C.M., Awad M.M., Navarro A., et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–2379. doi: 10.1200/JCO.20.00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Makker V., Colombo N., Casado Herráez A., et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Colombo N., Dubot C., Lorusso D., et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385(20):1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 144.Kelley R.K., Ueno M., Yoo C., et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–1865. doi: 10.1016/S0140-6736(23)00727-4. [DOI] [PubMed] [Google Scholar]
- 145.Rizvi N.A., Cho B.C., Reinmuth N., et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamanishi J., Takeshima N., Katsumata N., et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA) J Clin Oncol. 2021;39(33):3671–3681. doi: 10.1200/JCO.21.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fehrenbacher L., von Pawel J., Park K., et al. Updated efficacy analysis including secondary population results for OAK: a randomized phase III study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(8):1156–1170. doi: 10.1016/j.jtho.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 148.Gadgeel S.M., Lukas R.V., Goldschmidt J., et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: exploratory analyses of the phase III OAK study. Lung Cancer. 2019;128:105–112. doi: 10.1016/j.lungcan.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 149.Gandara D.R., von Pawel J., Mazieres J., et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J Thorac Oncol. 2018;13(12):1906–1918. doi: 10.1016/j.jtho.2018.08.2027. [DOI] [PubMed] [Google Scholar]
- 150.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.von Pawel J., Bordoni R., Satouchi M., et al. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer. 2019;107:124–132. doi: 10.1016/j.ejca.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 152.Lu Z., Wang J., Shu Y., et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377 doi: 10.1136/bmj-2021-068714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Faivre-Finn C., Vicente D., Kurata T., et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC-an update from the PACIFIC trial. J Thorac Oncol. 2021;16(5):860–867. doi: 10.1016/j.jtho.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 154.Gray J.E., Villegas A., Daniel D., et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15(2):288–293. doi: 10.1016/j.jtho.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Antonia S.J., Villegas A., Daniel D., et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 157.Shi Y., Wu L., Yu X., et al. Sintilimab versus docetaxel as second-line treatment in advanced or metastatic squamous non-small-cell lung cancer: an open-label, randomized controlled phase 3 trial (ORIENT-3) Cancer Commun. 2022;42(12):1314–1330. doi: 10.1002/cac2.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou C., Huang D., Fan Y., et al. Tislelizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer (RATIONALE-303): a phase 3, open-label, randomized controlled trial. J Thorac Oncol. 2022;18(1):93–105. doi: 10.1016/j.jtho.2022.09.217. [DOI] [PubMed] [Google Scholar]
- 159.Xu J., Kato K., Raymond E., et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023;24(5):483–495. doi: 10.1016/S1470-2045(23)00108-0. [DOI] [PubMed] [Google Scholar]
- 160.Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13(Suppl 2):19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 161.Chen T.T. Milestone survival: a potential intermediate endpoint for immune checkpoint inhibitors. J Natl Cancer Inst. 2015;107(9) doi: 10.1093/jnci/djv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guaitoli G., Baldessari C., Bertolini F., Tomasello C., Cascinu S., Barbieri F. Are we ready to describe response or progression to immunotherapy in lung cancer? Crit Rev Oncol Hematol. 2019;138:112–119. doi: 10.1016/j.critrevonc.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 163.Choueiri T.K., Powles T., Burotto M., et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Peters S., Dziadziuszko R., Morabito A., et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort C randomized phase 3 trial. Nat Med. 2022;28(9):1831–1839. doi: 10.1038/s41591-022-01933-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou C., Chen G., Huang Y., et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305–314. doi: 10.1016/S2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 166.Ren S., Chen J., Xu X., et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncol. 2022;17(4):544–557. doi: 10.1016/j.jtho.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 167.Motzer R., Alekseev B., Rha S.Y., et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 168.Miles D., Gligorov J., André F., et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 169.Lee N.Y., Ferris R.L., Psyrri A., et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021;22(4):450–462. doi: 10.1016/S1470-2045(20)30737-3. [DOI] [PubMed] [Google Scholar]
- 170.Popat S., Curioni-Fontecedro A., Dafni U., et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol. 2020;31(12):1734–1745. doi: 10.1016/j.annonc.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 171.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ascierto P.A., Stroyakovskiy D., Gogas H., et al. Overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in BRAF(V600) mutation-positive advanced melanoma (IMspire150): second interim analysis of a multicentre, randomised, phase 3 study. Lancet Oncol. 2022;24(1):33–44. doi: 10.1016/S1470-2045(22)00687-8. [DOI] [PubMed] [Google Scholar]
- 173.Monk B.J., Colombo N., Oza A.M., et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(9):1275–1289. doi: 10.1016/S1470-2045(21)00342-9. [DOI] [PubMed] [Google Scholar]
- 174.Usmani S.Z., Schjesvold F., Oriol A., et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019;6(9):e448–e458. doi: 10.1016/S2352-3026(19)30109-7. [DOI] [PubMed] [Google Scholar]
- 175.Wu Q., Qian W., Sun X., Jiang S. Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J Hematol Oncol. 2022;15(1):143. doi: 10.1186/s13045-022-01362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anagnostou V., Yarchoan M., Hansen A.R., et al. Immuno-oncology trial endpoints: capturing clinically meaningful activity. Clin Cancer Res. 2017;23(17):4959–4969. doi: 10.1158/1078-0432.CCR-16-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Llovet J.M., Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. doi: 10.1016/j.jhep.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wolchok J.D., Hoos A., O'Day S., et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 179.Seymour L., Bogaerts J., Perrone A., et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hebert J.D., Neal J.W., Winslow M.M. Dissecting metastasis using preclinical models and methods. Nat Rev Cancer. 2023;23(6):391–407. doi: 10.1038/s41568-023-00568-4. [DOI] [PubMed] [Google Scholar]
- 181.Rotolo F., Pignon J.P., Bourhis J., et al. Surrogate end points for overall survival in loco-regionally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis. J Natl Cancer Inst. 2017;109(4) doi: 10.1093/jnci/djw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gharzai L.A., Jiang R., Wallington D., et al. Intermediate clinical endpoints for surrogacy in localised prostate cancer: an aggregate meta-analysis. Lancet Oncol. 2021;22(3):402–410. doi: 10.1016/S1470-2045(20)30730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kataoka K., Takahashi K., Takeuchi J., et al. Correlation between recurrence-free survival and overall survival after upfront surgery for resected colorectal liver metastases. Br J Surg. 2023;110(7):864–869. doi: 10.1093/bjs/znad127. [DOI] [PubMed] [Google Scholar]
- 184.Suciu S., Eggermont A.M.M., Lorigan P., et al. Relapse-free survival as a surrogate for overall survival in the evaluation of stage II-III melanoma adjuvant therapy. J Natl Cancer Inst. 2018;110(1) doi: 10.1093/jnci/djx133. [DOI] [PubMed] [Google Scholar]
- 185.Coart E., Suciu S., Squifflet P., et al. Evaluating the potential of relapse-free survival as a surrogate for overall survival in the adjuvant therapy of melanoma with checkpoint inhibitors. Eur J Cancer. 2020;137:171–174. doi: 10.1016/j.ejca.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 186.Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561. doi: 10.1038/471561a. [DOI] [PubMed] [Google Scholar]
- 187.Harrison R.K. Phase II and phase III failures: 2013-2015. Nat Rev Drug Discov. 2016;15(12):817–818. doi: 10.1038/nrd.2016.184. [DOI] [PubMed] [Google Scholar]
- 188.Buyse M., Molenberghs G., Burzykowski T., Renard D., Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1(1):49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- 189.Cortazar P., Zhang L., Untch M., et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 190.Conforti F., Pala L., Sala I., et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ. 2021;375 doi: 10.1136/bmj-2021-066381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.