Abstract
Light intensity, wavelength, and photoperiod have a combined effect on chicken incubation. This study was conducted to evaluate the effect of 12-h light, 12-h dark (12L:12D) photoperiod of white light (380–780 nm, WL), blue light (455/447.5–462.5 nm, BL), and green light (525/515–535 nm, GL) in chicken perceived light intensity during layer incubation on hatching performance, embryo development, eye structure, and melatonin concentration. Three batches of eggs from Jinghong No. 1 layer breeder were used in this experiment. Light stimulation had no effect on hatchability, and no consistent effect on embryo weight and newly hatched chick weight. However, the average hatching time of white light group and green light group was 7.3 h and 5.5 h later than that of the control group. Therefore, the holding period of chicks was significantly shortened (P = 0.001) in these 2 light groups. Light stimulation had a significant effect on the thickness of retinal layers (P < 0.05), retinal layers of white light group was thicker than that of the other 3 groups. Melatonin levels of chicks hatched in the green light and blue light were significantly higher than that of chicks hatched in the white light and darkness (P < 0.05). It indicated that the monochrome green and blue light promoted the expression of melatonin in chicken embryos. No significant diurnal rhythms were found at the level of plasma melatonin in 4 groups on d 21 using cosine analysis. It was concluded that green light has a positive effect on embryo development and melatonin secretion, while white light probably has positive effect on eye development. Furthermore, both green and white light stimulation resulted in late hatch for layer egg incubation. The obtained results are important in determining the light protocol for chicken incubation.
Key words: incubation light, chicken perceived light, eye structure, hatching time, diurnal rhythms
INTRODUCTION
Commercial hatcheries normally recognize the importance of adjustments to temperature, relative humidity, ventilation, and carbon dioxide rather than light manipulation for better hatch performance of chicken eggs. However, in nature, hens need to forage and turn eggs regularly. Therefore, eggs are certainly exposed to light during incubation (Duncan et al., 1978; Rogers, 1995). It has been known that eggshell blocks some harmful wavelengths of light from entering the developing embryo, such as ultraviolet B radiation which causes DNA damage and near-Infrared which overheats the embryos. Therefore, only some wavelengths of light have a positive effect on chick embryonic development (Maurer et al., 2011). In addition, research has shown that external light stimuli could be recognized early by chicken embryo and have a positive effect on hatch performance (Zhang et al., 2014). Manipulation of light in broiler egg incubation has also attracted more and more attention (Li et al., 2021). Study has shown that white, blue, and red light (250 lux) stimulation during incubation improved hatchability in broiler (Archer, 2018). Other studies have reported that white light stimulation (200–500 lux) increased embryo weight in broiler (Cooper, 1972; Ozkan et al., 2012b). Previous research has proved that green light (100–130 lux/1,200–1,400 lux) provided on the first 18 d of incubation for broiler eggs promoted the development of the embryo and initiated the hatching time by 3.4 h earlier (Tong et al., 2018).
The biological clock system regulates the secretion of melatonin and ultimately controlled the circadian rhythm (Kumar et al., 2004). The biological clock system of birds have 3 oscillators: the pineal gland, the hypothalamus, and the retina of eye (Gwinner and Brandst Tter, 2001). These oscillators synchronize with the external light-dark cycle through photoreceptors in the eyes and pineal gland, as well as multiple sites deep in the brain (Cassone and Menaker, 1984). Rods and cones are 2 types of photoreceptor cell in the retina of the eye. Different from human, poultry have 4 types of cone and are more sensitive to light at the spectrum of 415 nm, 450 nm, 555 nm, and 700 nm (Bowmaker et al., 1997). A specific unit “gallumiance” was derived by Nuboer et al. (1992) for poultry perceived light intensity instead of photometric units (lux), which considered the spectral sensitivity of the domestic fowl rather than that of human. However, Prescott and Wathes (1999) suggested a more accurate term “clux” for poultry.
Circadian rhythm of melatonin secretion is timed by signals transmitted via changes in the photoperiod (Li and Cassone, 2015). The rhythm has an effect on the physiological and behavioral activities of birds (Saito et al., 2005). Melatonin promotes the proliferation of lymphocytes, enhances the immunity and reduces the stress in chicks. Provision of constant light (24L:0D, 200 lux) for the first week has been reported to reduce melatonin concentration, and leaded to fearful behavior in chicken (Yang et al., 2022).
The existent of light in prenatal may have potential effect on chicken embryonic development and cause change in melatonin synthesis, and thereafter affect the hatch progress. The impact is normally comprehensive effect of light intensity, wavelength, and photoperiod. This study evaluated the effects of different wavelengths of light in chicken perceived light intensity during the incubation period on hatching performance, embryo development, and eye structure of layer eggs. The daily profile of plasma melatonin level was also analyzed to determine the effect of prenatal photoperiod on circadian rhythm of chicken embryo.
MATERIALS AND METHODS
Ethics Statement
The study proposal was approved by The Laboratory Animal Ethical Committee of China Agricultural University (AW712022-5-1). Experiments were conducted in accordance with the approved guidelines.
Incubation and Light Protocol
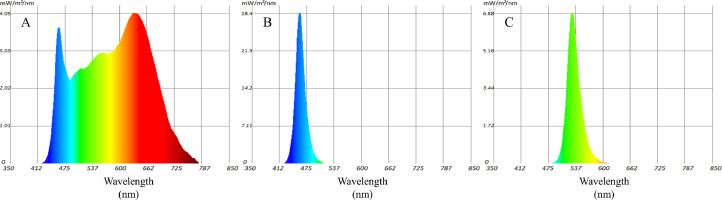
In total, 3 batches of fertile brown shell eggs (Jinghong No. 1, Beijing Huadu Yukou Poultry Industry Co. Ltd., Beijing, China) were used to evaluate the hatch performance and embryo development under different LEDs in the commercial incubator (EIFXDZ-75600 and EICXDH-15120, Xingyi Electronic Equipment Co., Ltd., Qingdao, China). A total of 504 eggs (60.3 ± 0.2 g) in each batch were distributed randomly into 4 groups and incubated under 4 different light treatments, respectively. In this study, monochromatic green light (525 nm/515–535 nm, GL), monochromatic blue light (455 nm/447.5–462.5 nm, BL) and 4,000 K white light (380–780 nm, WL) were selected as the light groups (Figure 1), and no light (darkness, NL) was used as the control group. The light was provided by low power LEDs (GaN LED S-30MBMUD-C and GaN LED S-20 CGMUD; San'an Optoelectronics Co., Ltd., Beijing, China) mounted on a board, which was fixed at the racks and placed above the eggs. The illumination scheme was 12 h of continuous illumination and 12 h of continuous darkness (12L:12D) for entire 21-days incubation. The light period consisted of 4 h of low intensity (200 ± 50 clux), followed by 1 h continuous illumination at a high intensity (2,000 ± 500 clux) and the remaining 7 h at the low intensity (Tong et al., 2018). The light intensity at egg level was realized using an iLMS software (Innoev New Energy Technology Nanjing Co., Ltd., Nanjing, China). The light intensity (lux) and chicken perceived light intensity (clux) were measured at 9 points of the egg surface in each group of first batch using a light meter (SRI-2000, Optimum Optoelectronics Crop., Taiwan, ROC). The incubation period was 21 d, of which 1 to 19 d were carried out in the setter and 19 to 21 d (457–504 h) were in the hatcher. Each group with 126 sample eggs were placed at the top tray of the setter trolley and top basket of the hatcher trolley which were separated by shading paper to avoid cross-influence of different light (Figure 2). The eggs in control treatment were place at the same position of the trolley next to the light treatments trolley in the same room. All the incubation conditions were maintained equally in different batches. During incubation, the temperature of the incubator was about 37°C to 38°C and the relative humidity was about 55 to 65%.
Figure 1.
Light spectral distributions of 3 light-emitting diode (LED) lights (white (A), blue (B), and green (C)).
Figure 2.
A photo of the experimental trays of setter (A) and baskets of hatcher (B) under the light treatments.
Hatch Performance and Embryo Development
All eggs were handled on 19 d when transferred from the turning trays to hatching baskets and incubation were stopped at 504 h of incubation. Chicks were weighted at the end of incubation. Hatchability and mortality at different stage were determined based on breakout results (Tong et al., 2018). On 12th, 16th, and 20th day of incubation (E12, E16, E20), 3 eggs in each group per batch were selected randomly and weighed. Then embryos or chicks were euthanized, and organs (heart, liver, and whole stomach with proventriculus and gizz) were dissected and weighed. The length of the beak and third toe as well as the crown and hip length was measured using caliper. Eggshell temperatures of 12 focal eggs in each group per batch were monitored and the hatching time of individual focal eggs was determined using eggshell temperature as previously described by Romanini et al. (2013). The incubator was stopped around 502 ± 2 h. Therefore, the time from hatching to pull (holding period) was calculated.
Eye and Melatonin
In order to explore the influence of different light on the eye structure, left eye of chick in each group from the last batch on d 21 was dissected and fixed in 4% paraformaldehyde solution for hematoxylin-eosin (H&E) staining after euthanized. Chicken eye samples were dehydrated in ethanol and embedded in paraffin wax, which was then cut into serial sections of 4-μm thicknesses and stained with H&E (Beijing Abace-biology Co., Ltd., Beijing, China). Observation and descriptions were made based on the image scanning using 3D HISTECH Pannoramic MIDI (made in Hungary). The eye structure and retina were labeled and measured using Image J. The thickness of retina, retina pigment epithelium, photoreceptor layer, outer limiting membrane, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, nerve fiber layer, and inner limiting membrane in 3 locations of eye was compared.
The blood of 3 chicks of each group per batch was sampled at every 2 h over a 24-h period (from 492 to 516 h). Circadian time (CT) is a standard time maker based on oscillations or rhythms in continuous darkness. CT0 and CT12 corresponded to the start time of subjective night and day, respectively. The blood sample was collected into a heparin tube and centrifuged at 3,000 rpm for 10 min. The plasma was stored at −20°C for melatonin measurement, melatonin was measured using ELISA (NY-D0042, Beijing Sino-uk Institute of Biological Technology, Beijing, China).
Statistical Analysis
Data were analyzed with IBM SPSS Statistics 25.0 and expressed as mean ± SEM. All variables were tested for normality using the Shapiro-Wilk analysis. One-way ANOVA was used to test the difference in light intensity (lux), chicken perceived light intensity (clux), hatchability, different stage mortality and thickness of retinal layers among 4 light treatment groups.
A linear mixed model was used to analyze the effect of light treatments on chick weight and hatching time:
where Yjk is the egg weight, chick weight, and hatching time, μ is the overall mean, Lj is the fix effect of light treatment (j = the white light group, blue light group, green light group or no light group), Bk is the random effect of batch (k = 1–3), LBjk is the interaction effect, and ε is error effect.
A second linear mixed model was used to analyze the effect of light treatments and sampling time on embryonic parameters and melatonin levels.
where Yijk is the embryo weight, the organ weight, the length of the beak and third toe, the crown and hip length, and the melatonin level. μ is the mean, Ti is the sampling time factor (i = E12, E16, E20 for egg weight and embryo parameters; or i = 1–12 for melatonin), Lj is the fix effect of light treatment (j = the white light group, blue light group, green light group, or no light group), Bk is the random effect of batch (k = 1–3), TLij, LBjk, and TLBijk are the interaction effects, and ε is error effect.
The interaction was removed from the original model when it was not significant. When the effect was statistically different (P < 0.05), the means were further compared using LSD test or nonparametric statistics. Cosine analyzes are based on unimodal cosine regression was used to determine whether melatonin had rhythmicity in the 24-h cycle, where A, B, and C stand for median, amplitude, and peak phase, respectively.
RESULTS
Actual Illumination Level and Hatch Performance
The low illumination and high illumination in light intensities (lux) and chicken perceived light intensity (clux) of 3 light treatment groups were shown in Table 1 and realized the target light protocol. There were significant differences between light intensity and chicken perceived intensity in white, blue and green light, with the ratios of the latter to the former being about 1.5, 9.0, and 1.02, respectively. However, the low or high illumination in 3 groups was within the required range (low illumination: 200 ± 50 clux; high illumination: 2000 ± 500 clux).
Table 1.
The low and high illumination in light intensity and chicken perceived light intensity of 3 groups.
| Type of illumination | WL | BL | GL | SEM | Pvalue |
| Low illumination | |||||
| Light intensity (lux) | 109 | 24 | 206 | 8.67 | <0.001 |
| Chicken perceived light intensity (clux) | 163 | 216 | 210 | 14.97 | <0.001 |
| High illumination | |||||
| Light intensity (lux) | 1237 | 229 | 2028 | 66.20 | <0.001 |
| Chicken perceived light intensity (clux) | 1855 | 2061 | 2068 | 144.18 | 0.011 |
| Ratio | 1.5 | 9.0 | 1.02 |
Abbreviations: BL, monochromatic blue light (455 nm/447.5–462.5 nm); GL, monochromatic green light (525 nm/515–535 nm); WL, 4,000 K white light (380–780 nm).
Ratio: Chicken perceived light intensity (clux)/Light intensity (lux) in low illumination or high illumination.
There was no effect of light treatments on hatchability and different stages of mortality for 3 batches (Table 2). Hatchability from fertile eggs of the 4 groups was about 92%, while in fact, commercial incubation generally ranged from 90 to 96%. Therefore, the provision of light during incubation did not affect the overall hatchability. Furthermore, egg weight at setting (P = 0.121) and chick weight at hatch (P = 0.308) had no significant difference among the 4 groups.
Table 2.
The effects of different light treatments during incubation on the hatch performance.
| Light stimulation |
SEM | P value | ||||
| Hatch performance | NL | WL | BL | GL | ||
| Hatchability (%) | 93.0 | 92.2 | 92.0 | 92.5 | 0.47 | 0.931 |
| ED (%) | 1.1 | 1.3 | 1.2 | 1.0 | 0.18 | 0.959 |
| MD (%) | 0.3 | 0.5 | 0.5 | 0.4 | 0.13 | 0.934 |
| LD (%) | 1.2 | 1.3 | 1.2 | 1.6 | 0.14 | 0.787 |
| Embryo weight (g) | ||||||
| E12 | 5.8 | 5.5 | 5.5 | 5.8 | 0.10 | 0.126 |
| E16 | 18.0 | 17.3 | 17.4 | 18.2 | 0.17 | 0.477 |
| E20 | 39.8ab | 38.2ab | 35.0b | 40.7a | 0.91 | 0.035 |
| Egg weight at setting (g) | 59.7 | 60.2 | 61.3 | 60.2 | 0.24 | 0.121 |
| Chick weight at hatch (g) | 40.0 | 40.5 | 41.6 | 40.6 | 0.23 | 0.308 |
Abbreviations: BL, monochromatic blue light (455 nm/447.5–462.5 nm); E12, E16, and E20 on the 12th, 16th, 20th day of incubation; ED, early death; GL, monochromatic green light (525 nm/515–535 nm); LD, late death; MD, middle death; NL, no light; WL, 4,000 K white light (380–780 nm).
Different lowercase letters represent significant difference within a row at P < 0.05.
The light treatment had an effect on the average hatching time (P < 0.05). Further analysis showed that the majority of chicks in the white (489.1 ± 1.24 h) and green (487.3 ± 1.12 h) light groups were hatched 7.3 h and 5.5 h later than that of the control group (481.8 ± 1.35 h), respectively (Table 3).
Table 3.
The average hatching time and holding period of white, blue, green, and no light treatment incubation.
| Light stimulation |
||||||
| Hatch performance | NL | WL | BL | GL | SEM | P vale |
| Hatching time (h) | 481.8c | 489.1a | 484.9bc | 487.3ab | 0.69 | 0.001 |
| Holding period (h) | 20.2a | 13.0c | 17.1ab | 14.7bc | 0.69 | 0.001 |
Abbreviations: BL, monochromatic blue light (455 nm/447.5–462.5 nm); GL, monochromatic green light (525 nm/515–535 nm); NL, no light; WL, 4,000 K white light (380–780 nm).
Different lowercase letters represent significant difference within a row at P < 0.05.
Embryo Development
The batch had no significant impact on embryo parameters. However, chicken embryo weight, heart weight, liver weight, stomach weight, beak length, third toe length, and the crown and hip length increased significantly with embryo age (P < 0.01). Furthermore, the light treatment had an effect on embryo weight (P = 0.008) and a significant difference among 4 groups were observed on E20 (P = 0.035). The GL had the highest embryo weight, which was significantly higher than that of WL, BL, and NL that were statistically equal among them (Table 2).
Eye Structure
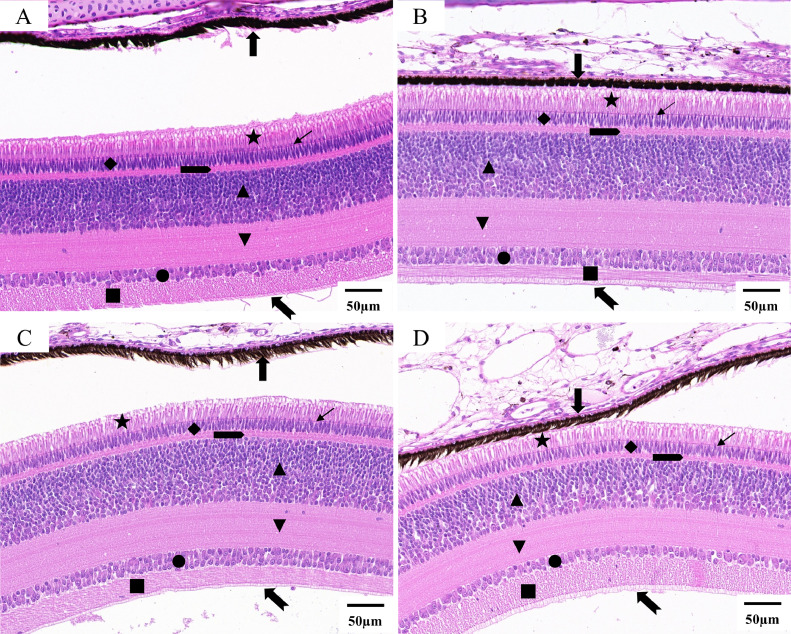
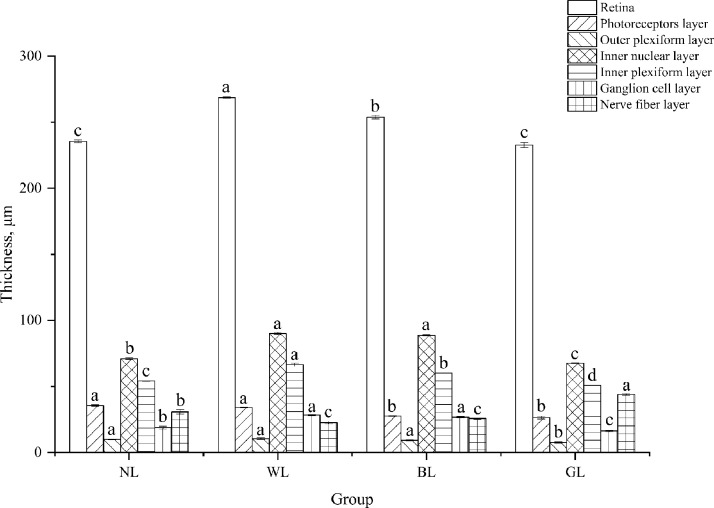
The images of H&E-stained whole eyeball from 4 groups were shown in Figure 3. The eyes of WL, BL, and GL were relatively intact, with cornea, iris, lens, vitreous, retina, choroid, and sclera. The retinal layers of 4 groups were well defined (Figure 4), from the sclera toward the vitreous direction, these were: the retina pigment epithelium, photoreceptor layer, outer limiting membrane, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, nerve fiber layer, and inner limiting membrane. The retina pigment epithelium was separated from the photoreceptor layers except for the WL group. There was a significant effect of light treatments on the thickness of the retina (except for the retina pigment epithelium), photoreceptor layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, and nerve fiber layer (P < 0.05) (Figure 5). The average thickness of the retina in NL, WL, BL, and GL groups were 235.45 ± 0.96 μm, 268.64 ± 0.55 μm, 253.80 ± 1.47 μm, and 232.65 ± 1.99 μm, respectively. Furthermore, thickness of photoreceptor in WL (34.11 ± 0.34 μm) and NL (35.49 ± 0.57 μm) were significantly higher than that in BL (27.49 ± 0.29 μm) and GL (26.35 ± 1.04 μm).
Figure 3.
Slices of chick's eye from no light (A), white light (B), blue light (C), and green light (D) incubation.
Figure 4.
Retina of chick's eye from no light (A), white light (B), blue light (C), and green light (D) incubation (scale bar = 50 μm, 24.4×). Retina pigment epithelium ( ), Photoreceptor layer (★), Outer limiting membrane (
), Photoreceptor layer (★), Outer limiting membrane ( ), Outer nuclear layer (◆), Outer plexiform layer (
), Outer nuclear layer (◆), Outer plexiform layer ( ), Inner nuclear layer (▲), Inner plexiform layer (▼), Ganglion cell layer (●), Nerve fiber layer (▪), and Inner limiting membrane (
), Inner nuclear layer (▲), Inner plexiform layer (▼), Ganglion cell layer (●), Nerve fiber layer (▪), and Inner limiting membrane ( ).
).
Figure 5.
The thickness of retinal layers from no light (NL), white light (WL), blue light (BL), and green light (GL) incubation. Data are presented as the mean ± SEM. a–dValues tissue thickness within all groups differ significantly at P < 0.05.
Melatonin
The level of melatonin was proved to have no significant diurnal rhythms in 4 treatments from 492 to 516 h using cosine analysis (Figure 6). Furthermore, the mixed effects models, taking into account batch effect, showed that there was no effect of sampling time on melatonin. However, light treatment had a significant impact on melatonin (P = 0.002). The average plasma melatonin levels of chicks from NL, WL, BL, and GL groups were 57.06 ± 1.55 pg/mL, 52.10 ± 1.24 pg/mL, 68.01 ± 1.72 pg/mL, and 75.87 ± 1.79 pg/mL, respectively. As shown in Figure 6, during the whole 12L:12D period, the melatonin levels of chicks hatched in the GL and BL was significantly higher than that of chicks hatched in the NL (P < 0.05), while the melatonin levels of chicks hatched in WL was significantly lower than that of chicks hatched in the NL (P < 0.05).
Figure 6.
Melatonin levels of chicks hatched in white light (WL), blue light (BL), green light (GL), and no light (NL) treatment. CT: circadian time. Data are presented as the mean ± SEM.
DISCUSSION
Although the maximum spectral sensitivity of poultry is close to that of human at 545 to 575 nm (green), poultry also has higher spectral sensitivity at 400 to 480 nm (blue) and 580 to 700 nm (yellow-red) than human (Bowmaker et al., 1997). Poultry and human have different spectral sensitivities, and poultry might perceive parts of the spectrum better than human. It means that poultry perceived higher brightness than human did under certain light sources (Duncan et al., 1978). Therefore, the illuminance (lux) could not reflect the real perceived illuminance level of the poultry effectively in the light environment in poultry house (Rogers, 1995). In this study chicken perceived light intensity (clux) was applied in order to evaluate the effects of light during incubation more effectively. However, for achieving the similar illumination in chicken perceived light intensity (clux) on the egg surface, the actual light intensity in human perceived light (lux) among the 3 light groups was significant different. And the ratios of the former to the later in white, blue and green light were about 1.5, 9.0 and 1.02, respectively. Lewis et al. (2000) found that the ratio of chicken perceived light intensity to human perceived light intensity in green light (545–575 nm) was about 1.0, while that of blue light (440–550 nm) fluctuated between 3.24 and 13.3. It is depended on the light source. Furthermore, the embryo perceived light would be further affected by the characteristic of eggshell especially in wavelength and intensity for different light treatment groups, but the influence is not clear and needs to be further investigated.
The effects of LED in different spectrums, photoperiods, and intensities on hatch performance are not consistent and not clearly among different species. In this study, there was no significant difference among 4 treatments regarding hatchability and embryo mortality for layer incubation. It is same to those obtained by Li et al. (2021) when incubated under red, white and blue light (200 lux). Manet et al. (2023) found that green light had no negative effect on hatchability and hatching characteristics of layer eggs. Similarly, Tong et al. (2018) reported that green light stimulation (100–130 lux/1,200–1,400 lux, 12L:12D) had no negative effect on broiler egg hatchability. However, other study found that red and blue light (250 lux, 12L:12D) during incubation increased the hatchability by about 6% (82.9 ± 1.4%) and 4% (80.9 ± 1.0%), respectively, compared with dark incubation (Archer, 2018). Relevant studies have reported that applying light during incubation can also promote incubation and shorten hatch window. Tong et al. (2018) found that the individual hatching time in the green light group was 3.4 h earlier than that in the no light group. Wang et al. (2020) reported that the average hatching time with green light (200 lux, 12L:12D) stimulation was advanced by 5.34 h compared with no light. Shafey and AI-mohsen (2002) found that the hatching time in the green light was shortened by about 24 h, when applied monochromatic green light (1,340–1,730 lux) continuously to the Hybro broilers for 18 d. Contrary to previous studies which showed that hatch process of broiler eggs was accelerated when exposed to green light during incubation, current study showed that light manipulation during incubation altered the hatching process of layers and the hatching time of chicks was significantly delayed in GL and WL. It was reported by previous research that hatching time even varied among different layer strains under green light incubation (Wang et al., 2020). Genotype specific of chicken might be the possible reason of different response to light. Delayed hatching time indicated that chicks had shorter holding period and less unfavorable experience which including feed and water deprivation before pulling. If chicks hatched earlier, they would be dehydrated due to long-term water shortage and lost more weight during a longer holding time (Tong et al., 2018), which increased the early mortality of newly hatched chicks. And chicks failed to drink and eat in time after hatching, which damaged the immune system activation and digestive enzyme stimulation, and had a great impact on growth and production performance (Willemsen et al., 2010).
It was found in this study that green light had an effect on embryo development and significantly increased the embryonic weight on E20. The broiler embryo weight was also increased when incubated under green light (50 lux, 16L:8D) (Yu et al., 2018). Özkan et al. (2012a) also reported that white light (200–300 lux, 16L:8D) increased embryo weight compared with dark incubation. Moreover, other studies found similar results in turkey and layer (Cooper, 1972). Embryonic development was related to the amount and wavelength of light entering the eggshell. Therefore, the differences in embryonic development may be the combination effects of spectrum, photoperiod, light intensity, eggshell thickness and pigment. As part of the embryo development, the prenatal effect of light on mouse fetal eye was reported in late pregnancy, and found that light stimulation promoted the regression of embryonic hyaloid vasculature and formation of the retinal vasculature (Rao et al., 2013). Current study showed that light stimulation during chicken incubation had an effect on the thickness of retinal layers. White light significantly increased the thickness of retina layer and photoreceptor than did the blue and green light. It was suggested that white light enhanced the effect because of the wider spectrum. Previous study has shown that green light and blue light caused different changes in the number and distribution of retinal cone cells in guinea pigs in 2-wk old (Qian, 2010). In addition, Gorla and Nag (2021) reported that rod and cone outer segments were significantly damaged in chick under white light (700 lux, 12L:12D) for 1 to 7 d and 24 h continuous light (700 lux) for 7 to 18 d. All the above studies have shown that light has a certain prenatal and postnatal effect on the retina and photoreceptor. Further study may be needed for exploring more information about the prenatal effect of different light on the development of chicken eye.
Circadian rhythm is synchronized by ambient light and other signals through regulating the level of melatonin. A certain diurnal rhythm of pineal melatonin levels on E20 broiler embryos incubated under the 12L:12D photoperiod of white, red, green or blue light was found in the results of Drozdova et al. (2019). An expected diurnal rhythm of plasma melatonin was not identified in newly hatched layers in the present study. The effects of different spectrums are not consistent among different species, plus the difference in light source, breed, sampling time and tissue. However, the average plasma melatonin level of chicks on d 21 differed under different wavelengths of light during incubation. The highest plasma melatonin levels were determined under green and blue light, and lower levels under dark and white light. It indicated that the GL and BL promoted the expression of melatonin, while the WL inhibited its expression. Similar results were found in previous studies that green light promoted the concentration of melatonin either in culturing chick pineal gland (Ma et al., 2018) or in 2-wk-old broilers (Zhang et al., 2016). Although, it is agreed that blue light with the spectrum of 450 to 495 nm is the main signal for circadian rhythm (Rozenboim et al., 2013). It has been proved that green light improved the positive regulators expression of cellular clock (cCLOCK, cBMAL1, cBMAL2, and cAANAT) which is involved in novel mechanism of circadian rhythm, and finally promoted the secretion of melatonin in broiler (Cao et al., 2017). In addition, studies have found that green light promoted positive clock genes (CLOCK, BMAL1, and BMAL2) expression both in the pineal gland (Jiang et al., 2016) and hypothalamus (Jiang et al., 2017), which promoting the melatonin secretion.
In conclusion, white light and green light postpone hatching time, as a consequence, shortened the holding period of newly hatched layer chicks. Furthermore, green light promoted the secretion of plasma melatonin and improved embryo development, while white light probably has a positive effect on eye development during incubation. But these were not observed in blue light and darkness incubation. The difference in light intensity together with perceived light by embryo through the egg shell might be the key reason for different response of layer embryo to different light source. The obtained results were important to understanding the effect of different spectrums in chicken perceived light intensity on hatching process, eye structure, and melatonin secretion during incubation.
ACKNOWLEDGMENTS
Thanks are extended to our colleagues at the Key Laboratory of Agricultural Engineering in Structure and Environment of Agricultural Structure and Environmental Engineering, China Agricultural University, Beijing, China.
DISCLOSURES
All authors declare no conflicts of interest.
REFERENCES
- Archer G.S. Effect of two different commercially available white light LED fixtures on broiler hatchability and chick quality. Br. Poult. Sci. 2018;59:251–255. doi: 10.1080/00071668.2018.1436160. [DOI] [PubMed] [Google Scholar]
- Bowmaker J.K., Heath L.A., Wilkie S.E., Hunt D.M. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 1997;37:2183–2194. doi: 10.1016/s0042-6989(97)00026-6. [DOI] [PubMed] [Google Scholar]
- Cao J., Bian J., Wang Z.X., Dong Y.L., Chen Y.X. Effect of monochromatic light on circadian rhythmic expression of clock genes and arylalkylamine N-acetyltransferase in chick retina. Chronobiol. Int. 2017;34:1149–1157. doi: 10.1080/07420528.2017.1354013. [DOI] [PubMed] [Google Scholar]
- Cassone V.M., Menaker M. Is the avian circadian system a neuro-endocrine loop. J. Exp. Zool. 1984;232:539–549. doi: 10.1002/jez.1402320321. [DOI] [PubMed] [Google Scholar]
- Cooper J.B. Effect of light during incubation on hatchability of turkey eggs. Poult. Sci. 1972;51:1105. doi: 10.3382/ps.0511105. [DOI] [PubMed] [Google Scholar]
- Drozdova A., Okuliarova M., Zeman M. The effect of different wavelengths of light during incubation on the development of rhythmic pineal melatonin biosynthesis in chick embryos. Animal. 2019;13:1635–1640. doi: 10.1017/S1751731118003695. [DOI] [PubMed] [Google Scholar]
- Duncan I.J.H., Savory C.J., Wood-Gush D.G.M. Observations on the reproductive behaviour of domestic fowl in the wild. Appl. Anim. Ethol. 1978;4:29–42. [Google Scholar]
- Gorla S., Nag T.C. Effect of constant light on the morphology of the retina in domestic chick (Gallus g. domesticus): a scanning electron microscope study. Indian J. Biochem. Biophys. 2021;58:381–384. [Google Scholar]
- Gwinner E., Brandst Tter R. Complex bird clocks. Philos. Trans. R. Soc. B-Biol. Sci. 2001;356:1801–1810. doi: 10.1098/rstb.2001.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Wang Z.X., Cao J., Dong Y.L., Chen Y.X. Role of monochromatic light on daily variation of clock gene expression in the pineal gland of chick. J. Photochem. Photobiol. B-Biol. 2016;164:57–64. doi: 10.1016/j.jphotobiol.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Jiang N., Wang Z.X., Cao J., Dong Y.L., Chen Y.X. Effect of monochromatic light on circadian rhythmic expression of clock genes in the hypothalamus of chick. J. Photochem. Photobiol. B-Biol. 2017;173:476–484. doi: 10.1016/j.jphotobiol.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Kumar V., Singh B.P., Rani S. The bird clock: a complex, multi-oscillatory and highly diversified system. Biol. Rhythm. Res. 2004;35:121–144. [Google Scholar]
- Lewis P.D., Morris T.R. Poultry and coloured light. Worlds Poult. Sci. J. 2000;56:189–207. [Google Scholar]
- Li Y., Cassone V.M. Clock-controlled regulation of the acute effects of norepinephrine on chick pineal melatonin rhythms. J. Biol. Rhythm. 2015;30:519–532. doi: 10.1177/0748730415607060. [DOI] [PubMed] [Google Scholar]
- Li X., Rathgeber B., Mclean N., Macisaac J. Providing colored photoperiodic light stimulation during incubation: 1. Effects on embryo development and hatching performance in broiler hatching eggs. Poult. Sci. 2021;100:101336. doi: 10.1016/j.psj.2021.101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S.H., Wang Z.X., Cao J., Dong Y.l., Chen Y.X. Effect of monochromatic light on circadian rhythm of clock genes in chick pinealocytes. Photochem. Photobiol. 2018;94:1263–1272. doi: 10.1111/php.12963. [DOI] [PubMed] [Google Scholar]
- Manet M.W.E., Kliphuis S., van den Brand H., Nordquist R.E., Goerlich V.C. and Rodenburg T.B., Green light during incubation: effects on hatching characteristics in brown and white laying hens, Livest. Sci., 274, 2023,105270.
- Maurer G., Portugal S.J., Cassey P. Review: an embryo's eye view of avian eggshell pigmentation. J. Avian. Biol. 2011;42:494–504. [Google Scholar]
- Nuboer J.F.W., Coemans M.A.J.M., Vos J.J. Artificial lighting in poultry houses: are photometric units appropriate for describing illumination intensities? Br. Poult. Sci. 1992;33:135–140. doi: 10.1080/00071669208417449. [DOI] [PubMed] [Google Scholar]
- Ozkan S., Yalcin S., Babacanoglu E., Kozanoglu H., Karadas F., Uysal S. Photoperiodic lighting (16 hours of light:8 hours of dark) programs during incubation: 1. Effects on growth and circadian physiological traits of embryos and early stress response of broiler chickens. Poult. Sci. 2012;91:2912–2921. doi: 10.3382/ps.2012-02426. [DOI] [PubMed] [Google Scholar]
- Ozkan S., Yalcin S., Babacanoglu E., Uysal S., Karadas F., Kozanoglu H. Photoperiodic lighting (16 hours of light:8 hours of dark) programs during incubation: 2. Effects on early posthatching growth, blood physiology, and production performance in broiler chickens in relation to posthatching lighting programs. Poult. Sci. 2012;91:2912–2921. doi: 10.3382/ps.2012-02427. [DOI] [PubMed] [Google Scholar]
- Prescott N.B., Wathes C.M. Spectral sensitivity of the domestic fowl (Gallus g. domesticus) Br. Poult. Sci. 1999;40:332–339. doi: 10.1080/00071669987412. [DOI] [PubMed] [Google Scholar]
- Qian Y.F. Effects of different monochromatic lights on eye growth and retinal-cone distribution and quantity in guinea pigs. Uni. Fudan. 2010 [Google Scholar]
- Rao S., Chun C., Fan J.Q., Kofron J.M., Yang M.B., Hegde R.S., Ferrara N., Copenhagen D.R., Lang R.A. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.J. CAB International; Wallingford, UK: 1995. The Development of Brain and Behaviour in the Chicken. [Google Scholar]
- Romanini C.E.B., Exadaktylos V., Tong Q., Mcgonnel I., Demmers T.G.M., Bergoug H., Eterradossi N., Roulston N., Garain P., Bahr C. Monitoring the hatch time of individual chicken embryos. Poult. Sci. 2013;92:303–309. doi: 10.3382/ps.2012-02636. [DOI] [PubMed] [Google Scholar]
- Rozenboim E.l., Halawani M.E., Kashash Y., Piestum Y., Halevy O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen Comp Endocrinol. 2013;190:214–219. doi: 10.1016/j.ygcen.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Saito S., Tachibana T., Choi Y.H., Denbow D.M., Furuse M. ICV melatonin reduces acute stress responses in neonatal chicks. Behav. Brain Res. 2005;165:197–203. doi: 10.1016/j.bbr.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Shafey T.M., Al-Mohsen T.H. Embryonic growth, hatching time and hatchability performance of meat breeder eggs incubated under continuous green light. Asian Australas. J. Anim. Sci. 2002;15:1702–1707. [Google Scholar]
- Tong Q., Mcgonnell I.M., Demmers T.G.M., Roulston N., Bergoug H., Romanini C.E., Verhelst R., Guinebretière M., Eterradossi N., Berckmans D., Exadaktylos V. Effect of a photoperiodic green light programme during incubation on embryo development and hatch process. Animal. 2018;12:765–773. doi: 10.1017/S1751731117002117. [DOI] [PubMed] [Google Scholar]
- Wang P., Sun Y., Fan J., Zong Y., Chen J. Effects of monochromatic green light stimulation during embryogenesis on hatching and post-hatch performance of four strains of layer breeder. Poult. Sci. 2020;99:5501–5508. doi: 10.1016/j.psj.2020.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., Ecuypere E.D. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Yang Y., Cong W., Liu J., Zhao M., Xu P., Han W., Wang D., Zhao R. Constant light in early life induces fear-related behavior in chickens with suppressed melatonin secretion and disrupted hippocampal expression of clock- and BDNF-associated genes. J. Anim. Sci. Biotechnol. 2022;13:1–11. doi: 10.1186/s40104-022-00720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Li Z., Zhong Z., Jin S., Pan J., Rao X., Yu Y. Effect of monochromatic green LED light stimuli during incubation on embryo growth, hatching performance, and hormone levels. Trans. ASABE. 2018;61:661–669. [Google Scholar]
- Zhang L.W., Cao J., Wang Z.X., Dong Y.L., Chen Y.X. Melatonin modulates monochromatic light-induced GHRH expression in the hypothalamus and GH secretion in chicks. Acta Histochem. 2016;118:286–292. doi: 10.1016/j.acthis.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.J., Wang J., Wu S.G., Qiao X., Yue H.Y., Yao J.H., Qi G.H. Stimulation with monochromatic green light during incubation alters satellite cell mitotic activity and gene expression in relation to embryonic and posthatch muscle growth of broiler chickens. Animal. 2014;8:86–93. doi: 10.1017/S1751731113001882. [DOI] [PubMed] [Google Scholar]