Abstract
Ginger contains bioactive compounds that possess anti-inflammatory and antimicrobial properties. In this study, 432-day-old Ross 708 broiler male chicks were randomly allocated to 6 dietary treatments to investigate the effect of ginger root extract (GRE) on immunocompetence and growth performance to 6 wk of age. Treatment 1 (CON) consisted of chicks fed a corn–soybean meal (SBM), a base diet without GRE. Treatment 2 (MX) chicks were given basal diets containing bacitracin methylene disalicylate (BMD) at 0.055 g/kg. Treatments 3 (GRE-0.375%), 4 (GRE-0.75%), 5 (GRE-1.5%), and 6 (GRE-3%) were fed similar diet to control with GRE supplemented at 0.375%, 0.75%, 1.5%, and 3%, respectively. Moreover, HPLC analysis of GRE was carried out to determine the concentration of bioactive compounds found in GRE. Each treatment consisted of 6 replicate pens with 12 chicks/pen. Bodyweight (BW) and feed conversion ratio (FCR) were recorded. Results show that the concentration of bioactive compounds increased with increasing GRE supplementation. Likewise, dietary GRE supplementation did not have any detrimental effect on growth performance parameters up to 1.5%, as values for BWG was not different from CON and MX; however, 3% GRE had the poorest FCR and a lower BWG as compared to other treatments. On d 27 and d 41, fecal and cecal concentrations of total bacteria count (TBC), Escherichia coli, Lactobacillus spp., and Bifidobacterium spp enumerated using selective plating media showed that GRE supplementation significantly reduced (P < 0.05) the amount of TBC and E. coli but increased the number of beneficial microorganisms such as Lactobacillus spp. and Bifidobacterium spp. On d 20, no significant differences were observed (P > 0.05) among all treatments for antibody titer against Newcastle disease virus and total IgY antibodies; however, on d 27, GRE-0.75% had the highest value for both immune indicators and was not different from MX. Dietary supplementation of GRE up to 1.5% enhanced the immune system and suppressed E. coli while promoting the growth of healthy bacteria, without any detrimental effect on growth performance.
Key words: GRE, immunocompetence, gingerol, lactobacillus, broiler chicken
INTRODUCTION
The poultry industry is growing at a fast pace, but such growth is limited by challenges caused by diseases of economic importance such as salmonellosis and infectious bronchitis (Agunos et al., 2016; Fasina et al., 2021; Adetunji et al., 2022; Biagini et al., 2022). Most especially, they affect humans through the consumption of undercooked eggs or poultry meat and other products (Kimura et al., 2004; Feye et al., 2020). The gastrointestinal tract of poultry contains an extensive microbial population. While some of these bacteria are nonpathogenic, some microorganisms within the population may become opportunistic pathogens during certain conditions (Aruwa et al., 2021). Therefore, to ensure the sustainability of the poultry industry, it is essential to mitigate the effects of pathogenic diseases.
Over the years, dietary supplementation of growth-promoting antibiotics has become a common commercial practice resulting in financial gain and low cost of poultry products. Bacitracin has been used to improve feed conversion and growth rate, and combat many infectious diseases of poultry (Proctor and Phillips, 2019). However, public concern about antibiotic resistance in humans has resulted in the decline in the use of antibiotics in animal feed (Lhermie et al., 2017; Kim and Lillehoj, 2019). It is therefore imperative that alternatives to dietary supplementation of antibiotics be found that maintain gut health, promote growth, and limit foodborne pathogens so as to minimize the risk of the emergence of antibiotic resistant disease (Yadav and Jha, 2019). Alternatives to antibiotics include the use of phytonutrients and herbs that serve as natural antimicrobial agents when incorporated into animal diets (Patra et al., 2019). These phytogenic additives contain bioactive constituents that are capable of improving growth performance, energy availability, nutrient digestibility, immune function, and stabilizing gut microbiome (Patra et al., 2019).
Ginger (Zingiber officinale Roscoe, Zingiberaceae) rhizome is a spice and has also been used in traditional medicine (Mohamad et al., 2019). In addition, it contains bioactive compounds such as shogaols, gingerols, gingerdione, and phenolic ketone derivatives (Prasad and Tyagi, 2015). Studies on the use of ginger and its bioactive compounds have reported its antiapoptotic, antioxidant, immunomodulatory, anti-inflammatory, antilipidemic, and antitumorigenic properties (Mashhadi et al., 2013; Bischoff-Kont and Fürst, 2021). Furthermore, ginger has been reported to stimulate gastric juice secretion and the overall activities of the digestive system and consequently enhance nutrient digestion and absorption, as well as cell viability (Anh et al., 2020). However, little information in the scientific literature is available on the immune-enhancing effects of dietary GRE in poultry production. This study evaluated the comparative effects of dietary ginger root extract (GRE; 0.375%–3%) and bacitracin methylene disalicylate (BMD) antibiotic (0.055 g/kg diet) on growth performance, innate immunity (differential leukocyte counts), humoral adaptive immunity (i.e., circulating antibody concentrations), cell-mediated adaptive immunity (delayed-type hypersensitivity [DTH] reaction), and indicator pathogenic (i.e., Escherichia coli) and healthy (i.e., Lactobacillus spp. and Bifidobacterium) fecal microorganisms in broiler chickens.
MATERIALS AND METHODS
The animal care and use procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of North Carolina Agricultural and Technical State University.
Experimental Design, Diet, and Bird Management
In a 6-wk experiment, day-old Ross 708 broiler male chicks (432) were randomly allocated to 6 treatments, CON, MX, 3.75 g/kg, 7.5 g/kg, 15 g/kg, and 30 g/kg in a completely randomized design (Table 1). Treatment 1 (CON) consisted of chicks fed a corn–soybean meal (SBM)-based diet. Treatment 2 (MX) consisted of chicks fed a similar diet that contained BMD (Zoetis Services LLC, Parsipanny, NJ) added at 0.055 g/kg diet. Treatments 3 (GRE-1), 4 (GRE-2), 5 (GRE-3), and 6 (GRE-4) consisted of chicks fed a diet similar to the unmedicated control, but with GRE added at 0.375 g/kg, 0.75 g/kg, 1.5 g/kg, and 3 g/kg level of the diet, respectively. The GRE used in this study was a gift from Sabinsa Corporation (East Windsor, NJ) and its bioactive compounds were analyzed to determine content in each experimental diet (Table 3). Subsequent to mixing, all feed were steam conditioned to 85°C (Model C15LL4/F6, California Pellet Mill, Crawfordsville, IN) and then pelleted using a 30 HP pellet mill (Model PM1112-2, California Pellet Mill, Crawfordsville, IN) equipped with a 4.4 × 35.2 mm pellet mill die. Starter feeds were fed as crumbles from placement to 21 d. Grower feeds were fed as whole pellets from d 22 to 42.
Table 1.
Composition of experimental starter diets (% “as is”).1
| Ingredients | Control diet | BMD diet | GRE 0.375% | GRE-0.75% | GRE-1.5% | GRE-3% |
|---|---|---|---|---|---|---|
| Corn | 51.46 | 51.45 | 55.72 | 56.34 | 55.90 | 59.40 |
| Soybean meal (48%) | 40.39 | 40.39 | 40.23 | 40.08 | 39.78 | 39.17 |
| Ginger root extract (GRE) | 0.38 | 0.75 | 1.50 | 3.00 | ||
| Poultry fat | 3.64 | 3.64 | 3.63 | 3.61 | 3.59 | 3.53 |
| Limestone | 1.07 | 1.07 | 1.07 | 1.06 | 1.05 | 1.04 |
| Mono-dicalcium phosphate | 2.03 | 2.03 | 2.02 | 2.01 | 1.99 | 1.97 |
| Sodium chloride | 0.40 | 0.40 | 0.40 | 0.40 | 0.39 | 0.39 |
| Sodium bicarbonate | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| L-Lysine HCl 98% | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| DL-Methionine 99.0% | 0.34 | 0.34 | 0.34 | 0.33 | 0.33 | 0.33 |
| L-Threonine 98.5% | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| NCSU Poultry Vitamin Premix2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| NCSU Poultry Mineral Premix3 | 0.20 | 0.20 | 0.19 | 0.20 | 0.20 | 0.19 |
| Bacitracin (antibiotic, g/kg) | 0.055 | |||||
| Choline chloride 60% | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Selenium Premix* | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Analyzed nutrient composition4 | ||||||
| Metabolizable energy (Kcal/kg) | 3,150 | 3,155 | 3131 | 3197 | 3142 | 3201 |
| Crude protein, % | 23.13 | 24.88 | 23.69 | 24.69 | 23.69 | 23.63 |
| Crude fat, % | 5.15 | 5.47 | 10.71 | 5.30 | 6.00 | 6.87 |
| Crude fiber, % | 2.5 | 2.7 | 2.3 | 2.4 | 2.6 | 2.4 |
| Ash, % | 5.59 | 5.46 | 5.21 | 5.47 | 5.67 | 5.47 |
| Calculated nutrient composition | ||||||
| Total sulfur amino acids, % | 1.03 | 1.03 | 1.03 | 0.94 | 0.95 | 0.92 |
| Lysine, % | 1.42 | 1.42 | 1.41 | 1.26 | 1.25 | 1.23 |
| Calcium, % | 0.96 | 0.96 | 0.96 | 0.74 | 0.74 | 0.73 |
| Available phosphorus, % | 0.48 | 0.48 | 0.48 | 0.44 | 0.43 | 0.43 |
Diets used in this study included the following: 1) unmedicated corn–soybean meal (SBM) basal without GRE (Control (CON) diet); 2) unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055 g/kg diet (MX diet); and 3) GRE-1, GRE-2, GRE-3, and GRE-4 diets into which GRE was incorporated into unmedicated corn–SBM basal at 0.375%, 0.75%, 1.5%, and 3.0% level, respectively. Each of these 6 diets was separately formulated for the Starter (D 1–21) phase of broiler production cycle.
Vitamin Premix, supplied per kilogram of diet: vitamin A (6,600 IU), vitamin D (1,980 IU), vitamin E (33 IU), vitamin B12 (0.02 mg), biotin (0.13 mg), menadione (1.98 mg), thiamine (1.98 mg), riboflavin (6.60 mg), d-Pantothenic acid (11.0 mg), vitamin B6 (3.96 mg), niacin (55.0 mg), folic acid (1.1 mg).
Mineral Premix, supplied per kilogram of diet: manganese (Mn), 60 mg; zinc (Zn), 60 mg; iron (Fe), 40 mg; copper (Cu), 5 mg; iodine (I), 1.2 mg; cobalt (Co), 0.5 mg.
Experimental diets were analyzed for proximate nutrient composition by Eurofins Scientific Inc. Nutrient Analysis Center, 2200 Rittenhouse Street, Suite 150, Des Moines, IA.
Selenium Premix provides 0.3 mg Selenium/kg of feed as sodium selenite.
Table 3.
The concentration of 6 major ginger compounds in different diets supplemented with GRE.
| Starter | 6-gingerols (%) | 8-gingerols (%) | 10-gingerols (%) | 6-shogaols (%) | 8-shogaols (%) | 10-shogaols (%) | |
|---|---|---|---|---|---|---|---|
| 0.375% diet | 0.048 | 0.0052 | 0.013 | 0.0087 | 0.003 | 0.0084 | |
| 0.75% diet | 0.066 | 0.011 | 0.022 | 0.017 | 0.0062 | 0.017 | |
| 1.5% diet | 0.13 | 0.039 | 0.063 | 0.069 | 0.0374 | 0.081 | |
| 3.0% diet | 0.15 | 0.052 | 0.084 | 0.078 | 0.047 | 0.098 | |
| Grower | |||||||
| 0.375% diet | 0.04 | 0.0082 | 0.014 | 0.0099 | 0.0028 | 0.0062 | |
| 0.75% diet | 0.089 | 0.017 | 0.022 | 0.028 | 0.0051 | 0.013 | |
| 1.5% diet | 0.12 | 0.035 | 0.056 | 0.053 | 0.023 | 0.054 | |
| 3.0% diet | 0.23 | 0.059 | 0.10 | 0.105 | 0.037 | 0.092 | |
Each treatment consisted of 6 replicate pens, with each housing 12 chicks. The temperature was set at 92°F from d 1 to d 7, 87°F from d 8 to d 21, and 77°F from d 22 to d 42. Photoperiod consisted of continuous (23L: 1D) lighting at 30 lux from placement to 21 d and then reduced to 12L: 12D lighting from d 22 d to 42 d. As the chicks grew, light intensity was gradually reduced until it reached 5 lux during the last week of the experiment. All experimental diets (Tables 1 and 2) were formulated to meet or slightly exceed nutrient requirements recommended in the Ross broiler nutrition specification handbook (Aviagen, 2022). Birds were allowed ad libitum access to feed and water throughout the 42-d experiment.
Table 2.
Composition of experimental grower diets (% “as is”).1
| Ingredients | Control diet | BMD diet | GRE-0.375% | GRE-0.75% | GRE-1.5% | GRE-3% |
|---|---|---|---|---|---|---|
| Corn | 56.77 | 56.76 | 56.55 | 56.34 | 55.91 | 55.06 |
| Soybean meal (48%) | 35.43 | 35.43 | 35.29 | 35.16 | 34.89 | 34.37 |
| Ginger root extract (GRE) | 0.38 | 0.75 | 1.50 | 3.00 | ||
| Poultry fat | 4.00 | 4.00 | 3.98 | 3.97 | 3.94 | 3.88 |
| Limestone | 0.64 | 0.64 | 0.64 | 0.64 | 0.63 | 0.62 |
| Mono-dicalcium phosphate | 1.84 | 1.84 | 1.83 | 1.83 | 1.81 | 1.80 |
| Sodium chloride | 0.40 | 0.40 | 0.40 | 0.40 | 0.39 | 0.39 |
| Sodium bicarbonate | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.19 |
| L-Lysine HCl 98% | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| DL-Methionine 99.0% | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.29 |
| L-Threonine 98.5% | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 |
| NCSU Poultry Vitamin Premix2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| NCSU Poultry Mineral Premix3 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.19 |
| Bacitracin (antibiotic, g/kg) | 0.055 | |||||
| Choline chloride 60% | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Selenium Premix⁎ | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Analyzed nutrient composition4 | ||||||
| Metabolizable energy (Kcal/kg) | 3109 | 3232 | 3131 | 3197 | 3142 | 3201 |
| Crude protein, % | 22.13 | 22.94 | 23.69 | 22.00 | 21.94 | 20.88 |
| Crude fat, % | 5.36 | 8.22 | 5.80 | 6.57 | 6.61 | 7.31 |
| Crude fiber, % | 2.6 | 2.6 | 2.6 | 2.6 | 2.7 | 2.7 |
| Ash, % | 5.18 | 5.19 | 5.46 | 5.35 | 5.03 | 4.82 |
| Calculated nutrient composition | ||||||
| Total sulfur amino acids, % | 1.03 | 1.03 | 1.03 | 0.94 | 0.94 | 0.91 |
| Lysine, % | 1.42 | 1.42 | 1.40 | 1.26 | 1.25 | 1.23 |
| Calcium, % | 0.96 | 0.96 | 0.96 | 0.74 | 0.74 | 0.73 |
| Available phosphorus, % | 0.48 | 0.48 | 0.48 | 0.44 | 0.43 | 0.43 |
Diets used in this study included the following: 1) unmedicated corn–soybean meal (SBM) basal without GRE (Control (CON) diet); 2) unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055 g/kg diet (MX diet); and 3) GRE-1, GRE-2, GRE-3, and GRE-4 diets into which GRE was incorporated into unmedicated corn–SBM basal at 0.375%, 0.75%, 1.5%, and 3.0% level, respectively. Each of these 6 diets was separately formulated for the Grower (D 22–42) phase of broiler production cycle.
Vitamin Premix, supplied per kilogram of diet: vitamin A (6,600 IU), vitamin D (1,980 IU), vitamin E (33 IU), vitamin B12 (0.02 mg), biotin (0.13 mg), menadione (1.98 mg), thiamine (1.98 mg), riboflavin (6.60 mg), d-Pantothenic acid (11.0 mg), vitamin B6 (3.96 mg), niacin (55.0 mg), folic acid (1.1 mg).
Mineral Premix, supplied per kilogram of diet: manganese (Mn), 60 mg; zinc (Zn), 60 mg; iron (Fe), 40 mg; copper (Cu), 5 mg; iodine (I), 1.2 mg; cobalt (Co), 0.5 mg.
Experimental diets were analyzed for proximate nutrient composition by Eurofins Scientific Inc. Nutrient Analysis Center, 2200 Rittenhouse Street, Suite 150, Des Moines, IA.
Selenium Premix provides 0.3 mg Selenium/kg of feed as sodium selenite.
Determination of the Concentrations of Major Bioactive Ginger Compounds in Experimental Diets
An HPLC ESA electrochemical detector (ECD) (ESA, Chelmsford, MA) consisting of an ESA model 584 HPLC pump, an ESA model 542 autosampler, an ESA organizer, and an ESA ECD coupled with 2 ESA model 6210 four sensor cells was used for analyzing the extract of ginger in different diets. A Gemini C18 column (150 × 4.6 mm, 5 µm; Phenomenex, Torrance, CA) was used for chromatographic analysis at a flow rate of 1.0 mL/min. The mobile phases consisted of solvent A (30 mM sodium phosphate buffer containing 1.75% acetonitrile and 0.125% tetrahydrofuran, pH 3.35) and solvent B (15 mM sodium phosphate buffer containing 58.5% acetonitrile and 12.5% tetrahydrofuran, pH 3.45). The gradient elution had the following profile: 5% solvent B from 0 to 3 min; 5%–40% solvent B from 3 to 10 min; 40%–80% solvent B from 10 to 40 min; 80%–100% solvent B from 40 to 50 min; 100% solvent B from 50 to 52 min; and then balance to 5% solvent B from 52 to 54 min and keep for 6 min. The cells were then cleaned at a potential of 1,000 mV for 1 min. The injection volume of the sample was 10 µL. The eluent was monitored by the Coulochem electrode array system (ESA) with potential settings at 100, 200, 300, 400, 450, 500, 550, and 600 mV. Quantification was performed with an external standard. The peak areas of ginger compounds at 300 mV channel were picked and their concentrations were calculated according to their linear curves.
Growth Performance
Bodyweight (BW), body weight gain (BWG), and feed intake (FI) of chicks were recorded on d 7, d 21, and d 42 for the evaluation of broiler growth performance. From these data, the feed conversion ratio (FCR) was calculated. Mortality was also recorded daily throughout the 42-d experiment.
Blood Collection and Plasma Harvest
On d 20 and d 27 of experiment, 2 birds were randomly taken from each pen (totaling 12 birds/treatment), and blood was collected from the brachial (wing) vein using a sterile 23 gauge 1″ needle attached to prelabeled sterile Ethylenediaminetetraacetic acid vacutainer tubes. Thereafter, the blood samples were centrifuged at 1,500 × g for 10 min to recover plasma. The plasma (supernatant) was collected and stored in 1.5 mL Eppendorf tubes at −80°C until time to determine total IgY concentration and antibody titers to Newcastle disease virus (NDV).
Differential Leukocyte Count Analysis
On d 25, blood was collected from 2 chicks per pen (totaling 12 birds/treatment) for differential leukocyte counts (DLC) analysis. Specifically, sterile 23 gauge 1″ needle were used to collect 2 mL of blood from the brachial vein into sterile prelabeled Ethylenediaminetetraacetic acid (anticlotting agent)-coated blood collection tubes. Thereafter, a thin smear of each blood sample was created on glass slides that were subsequently stained using the HEMA 3 Wright-Giemsa staining kit (Fisher Scientific, Pittsburgh, PA) according to manufacturer's instruction. The smear on each slide was air-dried and a drop of immersion oil was added onto each slide for viewing under the microscope (100× magnification) (Leica DME Side by Side Pathology 2×, Leica microsystems). Leukocytes (100 per slide) were counted, and the percentage of heterophil and lymphocyte was calculated in addition to the heterophil:lymphocyte ratio (Thiam et al., 2022).
Determination of Total IgY Concentration and Antibody Titers to NDV
Plasma samples were thawed on ice and assayed for total IgY concentration and antibody titers to NDV. Plasma IgY concentrations and NDV-specific antibody titers were determined using commercial sandwich ELISA kit (450 nm; Bethyl Laboratory, Montgomery, TX) and NDV ELISA kit (405 nm; BioChek, Scarborough, ME), respectively, according to manufacturer's instructions. The IgY concentrations and NDV titers were determined relative to standard curve and expressed as nanograms per milliliter plasma and sample to positive ratio (S/P ratio), respectively.
Evaluation of Phaseolus Vulgaris Lectin-Induced Cutaneous DTH
On d 25, the DTH analysis was performed on 2 birds/replicate pen, totaling 8 birds per treatment. Prior to injecting PHAP on d 24, a constant tension micrometer caliper was used to measure the thickness of the toe web between the second and third digits of both feet to get the initial preinjection reading. Thereafter, 100 µL of Phaseolus vulgaris (red kidney bean) lectin (PHAP; Sigma-Aldrich Inc., St. Louis, MO) was injected between the second and third digits of the right foot. The left foot (or the control foot) was injected with 100 µL of sterile PBS. Thickness of the toe web was measured at 24 h postinjection using a micrometer.
Microbiological Analysis of Indicator Microorganisms in the Ceca and Feces
On d 27 and d 41, 1 bird/replicate pen was randomly selected, euthanized by CO2 asphyxiation, and aseptically necropsied for the removal of ceca lobes. The ceca lobes from each bird was placed in a Whirl-Pak filter bag, weighed, and placed on ice. At the time of analysis, 25 mL 0.1% peptone solution was added into each Whirl-Pak filter bag, and the content of each bag were homogenized in a Stomacher 80 Microbiomaster at medium speed (approx. 230 rpm) for 60 s. Thereafter, 1 mL of filtrate from each Whirl-Pak bag (or sample) was serially diluted (i.e., serial 10-fold dilutions up to 106) in 10 mL peptone solution. Next, 0.1 mL of each dilution was surface-plated onto Brain Heart Infusion (BHI; Beckton Dickinson, Pittsburgh, PA), MacConkey (Remel Inc., Lenexa, KS), deMan–Rogosa–Sharpe (MRS; Oxoid Ltd., Lenexa, KS), and modified Bifidobacterium Iodoacetate Medium-25 (mBIM-25; HiMedia Labs, Kennett Square, PA) agars to enumerate total bacteria, E. coli, Lactobacillus spp., and Bifidobacterium, respectively. Plates were incubated at 37°C for 24 h for the total bacteria count and E. coli, whereas MRS and BIM-25 plates were incubated anaerobically for 48 to 72 h before bacteria counts from all samples were determined by the plate counting method.
Similarly, on d 27 and d 41, fecal samples (10 g) were mixed with 90 mL of sterile 0.1% peptone solution and homogenized using a stomacher at 230 rpm for 1 min. Thereafter, samples were serially diluted 10-fold in peptone solution and plated on appropriate agar for the enumeration of total bacteria, E. coli, Lactobacillus spp., and Bifidobacterium as described for ceca samples. The concentration of each indicator bacteria was expressed as log10 CFU/g ceca content or log10 CFU/mL fecal filtrate.
Statistical Analysis
Growth performance, differential leukocyte analysis, total IgY concentration, antibody titers to NDV, and DTH data were subjected to 1-way ANOVA (Statistical Analysis Software, 2004, Version 9.2. SAS Institute Inc., Cary, NC). On the other hand, fecal and cecal microbiota data were subjected to log10 transformation before analysis by 1-way ANOVA. Means separation was done using Duncan's multiple range test. The results were expressed as mean ± SEM and the level of statistical significance was P < 0.05.
RESULTS
Concentration of Bioactive Compounds Found in Experimental Diets
The concentration of Shogaols and Gingerols present in different GRE diets are presented in Table 3. In the starter and grower diets the concentration of 6-Gingerol (6G), 8-Gingerol (8G), 10-Gingerol (10G) and, 6-Shogaol (6G), 8-Shogaol (8G), 10-Shogaol (10G) increased with increasing GRE supplementation (Table 3).
Growth Performance
From d 1 to d 7, BW and BWG were different (P < 0.05) among treatments (Table 4). Compared to the control and MX, GRE supplementation up to GRE-1.5% was not different, but GRE-3% had the lowest BW and BWG (Table 4). There was no difference (P > 0.05) in FI among all treatments. Furthermore, only GRE-0.75% treatment had superior FCR (1.080) compared to the CON treatment (Table 4). From d 8 to d 21, only GRE-3% had lower (P < 0.05) BW (0.735 kg) and BWG (0.695 kg) compared to CON treatment (BW, 0.819 kg; BWG, 0.782 kg), whereas the values for other treatments were in-between. However, although there were differences in FI among treatments (P < 0.05), FCR values were not different (P > 0.05; Table 4). From d 22 to d 42, only GRE-3% had lower (P < 0.05) BW (2.923 kg) and BWG (2.191 kg) compared to CON treatment (BW, 3.266 kg; BWG, 2.453 kg), whereas the values for other treatments were in-between (Table 5). Although there were no differences in FI, GRE-3% had the poorest FRC (1.952) compared to other treatments. Cumulative growth performance (d 1–42; Table 5) showed that GRE-3% had lower (P < 0.05) BWG (2.901 kg) and poorer FCR (1.821) compared to other treatments (BWG, 3.108–3.375 kg; FCR, 1.662–1.717).
Table 4.
Effect of dietary GRE supplementation on growth performance of broiler chicks (d 1–21).5
| Day 1–7 (parameters measured)2 |
Day 8–21 (parameters measured)2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatments1 | Body weight (BW, kg/bird)3 | Body weight gain (BWG, kg/bird) | Feed intake (FI, kg/bird) | FCR4 (kg:kg) | BW (kg/bird)3 | BWG (kg/bird) | FI (kg/bird) | FCR4 (kg:kg) |
| CON | 0.137ab | 0.097ab | 0.118 | 1.218a | 0.819a | 0.782a | 1.086ab | 1.396 |
| MX | 0.142ab | 0.108ab | 0.120 | 1.108ab | 0.816a | 0.766ab | 1.072ab | 1.400 |
| GRE-0.375% | 0.145a | 0.113a | 0.125 | 1.106ab | 0.829a | 0.785a | 1.116a | 1.423 |
| GRE-0.75% | 0.146a | 0.112a | 0.121 | 1.080b | 0.823a | 0.778a | 1.095a | 1.401 |
| GRE-1.5% | 0.138ab | 0.105ab | 0.117 | 1.120ab | 0.796ab | 0.750ab | 1.061ab | 1.423 |
| GRE-3% | 0.125b | 0.092b | 0.111 | 1.210a | 0.735b | 0.695b | 1.006b | 1.448 |
| SEM | 0.004 | 0.004 | 0.004 | 0.028 | 0.019 | 0.0207 | 0.155 | 0.014 |
| P-value | 0.0273 | 0.0042 | 0.3341 | 0.0043 | 0.0131 | 0.0105 | 0.0146 | 0.5069 |
Mean values bearing different superscript letters within a column are significantly different (P < 0.05). n = 12 birds per treatment.
Treatment CON consisted of chicks fed unmedicated corn–soybean meal (SBM) basal without GRE; Treatment MX consisted of chicks given unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055 g/kg diet; Treatment GRE-0.375% consisted of chicks given unmedicated corn–SBM basal into which GRE was added at 0.375% level; Treatments GRE-0.75%, GRE-1.5%, and GRE-3%, consisted of chicks that were given diets similar to GRE-0.375% at 0.75%, 1.5%, and 3%, respectively.
Values represent the mean of 6 replicate pens per treatment.
Values are based only on weight of live birds.
FCR = feed conversion ratio calculated as feed-to-gain ratio and adjusted for mortality by including the gains of dead birds in the calculations.
Cummulative = growth performance data from d 1 to 21
Table 5.
Effect of dietary GRE supplementation on growth performance of broiler chicks (d 22–42).
| Day 22–42 (parameters measured)2 |
Cumulative: Day 1–42 (parameters measured)2 |
||||||
|---|---|---|---|---|---|---|---|
| Treatments1 | Body weight (BW, kg/bird)3 | Body weight gain (BWG, kg/bird) | Feed intake (FI, kg/bird) | FCR4 (kg:kg) | BWG (kg/bird) | FI (kg/bird) | FCR4 (kg:kg) |
| CON | 3.266a | 2.453ab | 4.403 | 1.797b | 3.230ab | 5.490 | 1.702b |
| MX | 3.325a | 2.533ab | 4.428 | 1.747b | 3.310ab | 5.500 | 1.662b |
| GRE-0.375% | 3.376a | 2.580a | 4.522 | 1.750b | 3.375a | 5.637 | 1.669b |
| GRE-0.75% | 3.273a | 2.458ab | 4.503 | 1.832b | 3.260ab | 5.597 | 1.717b |
| GRE-1.5% | 3.143ab | 2.348bc | 4.260 | 1.814b | 3.108bc | 5.327 | 1.713b |
| GRE-3% | 2.923b | 2.191c | 4.270 | 1.952a | 2.901c | 5.278 | 1.821a |
| SEM | 0.057 | 0.059 | 0.093 | 0.024 | 0.072 | 0.101 | 0.020 |
| P-value | < 0.0001 | < 0.0001 | 0.2385 | <0.0001 | <0.0001 | 0.1068 | < 0.0001 |
Mean values bearing different superscript letters within a column are significantly different (P < 0.05). n = 12 birds per treatment.
Treatment CON consisted of chicks fed unmedicated corn–soybean meal (SBM) basal without GRE; Treatment MX consisted of chicks given unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055g/kg diet; Treatment GRE-0.375% consisted of chicks given unmedicated corn-SBM basal into which GRE was added at 0.375% level; Treatments GRE-0.75%, GRE-1.5%, and GRE-3% consisted of chicks that were given diets similar to GRE-0.375% consisted of chicks that were given diets similar to GRE1 at 0.75%, 1.5%, and 3%, respectively.
Values represent the mean of 6 replicate pens per treatment.
Values are based only on weight of live birds.
FCR = feed conversion ratio calculated as feed-to-gain ratio and adjusted for mortality by including the gains of dead birds in the calculations.
Concentration of Indicator Microorganisms in the Ceca and Fecal of Broiler Chicks
In Table 6, Table 7, Table 8 to 9, total bacteria and E. coli counts were highest for CON (7.38 and 7.94 Log10 CFU/g cecal content and 5.23 and 4.86 Log10 CFU/g cecal contents; 5.51 and 4.69 Log10 CFU/g fecal contents and 5.23 and 4.86 Log10 CFU/g fecal contents at d 27 and d 41, respectively). Specifically, E. coli counts gradually reduced (P < 0.05) with increasing concentration of dietary GRE. On the other hand, the concentrations of beneficial bacteria evaluated, namely Lactobacillus and Bifidobacteria, were higher in both ceca content and feces of MX and all GRE-supplemented diets (P < 0.05) compared to CON treatment.
Table 6.
Effect of GRE on the concentration of indicator microorganisms in the ceca of broiler chicks (Log10 CFU/g cecal content) at d 27.
| Treatments1 | Total bacteria | E. coli | Lactobacillus | Bifidobacteria |
|---|---|---|---|---|
| CON | 7.38a | 7.94a | 6.33c | 6.37c |
| MX | 6.73bc | 7.89a | 6.40c | 6.46c |
| GRE-0.375% | 6.97b | 7.31b | 6.48bc | 6.84b |
| GRE-0.75% | 6.92b | 7.04b | 6.59b | 7.29a |
| GRE-1.5% | 6.40c | 6.99bc | 7.14a | 7.26a |
| GRE-3% | 6.33c | 6.63c | 6.99a | 7.03ab |
| SEM | 0.071 | 0.072 | 0.031 | 0.055 |
| P-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Mean values bearing different superscript letters within a column are significantly different (P < 0.05). n = 12 birds per treatment.
Treatment CON consisted of chicks fed unmedicated corn–soybean meal (SBM) basal without GRE; Treatment MX consisted of chicks given unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055 g/kg diet; Treatment GRE-0.375% consisted of chicks given unmedicated corn–SBM basal into which GRE was added at 0.375% level; Treatments GRE-0.75%, GRE-1.5%, and GRE-3% consisted of chicks that were given diets similar to GRE-0.375% consisted of chicks that were given diets similar to GRE1 at 0.75%, 1.5%, and 3%, respectively.
Table 7.
Effect of GRE on the concentration of indicator microorganisms in the ceca of broiler chicks (Log10 CFU/g cecal content) at d 41.
| Treatments1 | Total bacteria | E. coli | Lactobacillus | Bifidobacteria |
|---|---|---|---|---|
| CON | 5.23a | 4.86a | 4.12c | 4.09c |
| MX | 4.52b | 4.23cd | 4.65b | 4.75ab |
| GRE-0.375% | 4.57b | 4.43b | 5.20a | 4.94a |
| GRE-0.75% | 4.58b | 4.29c | 4.70b | 4.82ab |
| GRE-1.5% | 4.55b | 4.14de | 4.63b | 4.70ab |
| GRE-3% | 4.35b | 4.09e | 4.57b | 4.58b |
| SEM | 0.073 | 0.022 | 0.033 | 0.059 |
| P-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Mean values bearing different superscript letters within a column are significantly different (P < 0.05). n = 12 birds per treatment.
Treatment CON consisted of chicks fed unmedicated corn–soybean meal (SBM) basal without GRE; Treatment MX consisted of chicks given unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055g/kg diet; Treatment GRE-0.375% consisted of chicks given unmedicated corn–SBM basal into which GRE was added at 0.375% level; Treatments GRE-0.75%, GRE-1.5%, and GRE-3% consisted of chicks that were given diets similar to GRE-0.375% consisted of chicks that were given diets similar to GRE1 at 0.75%, 1.5%, and 3%, respectively.
Table 8.
Effect of GRE on the concentration of indicator microorganisms in the ceca of broiler chicks (Log10 CFU/g feces) at d 27.
| Treatments1 | Total bacteria | E. coli | Lactobacillus | Bifidobacteria |
|---|---|---|---|---|
| CON | 5.51a | 4.69a | 4.14d | 4.10d |
| MX | 4.68bc | 4.29cd | 4.51c | 4.68bc |
| GRE-0.375% | 4.89bc | 4.55ab | 5.13a | 5.08a |
| GRE-0.75% | 4.66bc | 4.35abc | 4.91b | 4.84ab |
| GRE-1.5% | 4.57bc | 4.15cd | 4.62c | 4.86ab |
| GRE-3% | 4.34c | 4.09d | 4.46c | 4.45c |
| SEM | 0.098 | 0.052 | 0.050 | 0.062 |
| P-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Mean values bearing different superscript letters within a column are significantly different (P < 0.05). n = 12 birds per treatment.
Treatment CON consisted of chicks fed unmedicated corn–soybean meal (SBM) basal without GRE; Treatment MX consisted of chicks given unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055 g/kg diet; Treatment GRE-0.375% consisted of chicks given unmedicated corn–SBM basal into which GRE was added at 0.375% level; Treatments GRE-0.75%, GRE-1.5%, and GRE-3% consisted of chicks that were given diets similar to GRE-0.375% consisted of chicks that were given diets similar to GRE1 at 0.75%, 1.5%, and 3%, respectively.
Table 9.
Effect of GRE on the concentration of indicator microorganisms in the ceca of broiler chicks (Log10 CFU/g feces) at d 41.
| Treatments1 | Total bacteria | E. coli | Lactobacillus | Bifidobacteria |
|---|---|---|---|---|
| CON | 5.23a | 4.86a | 4.12c | 4.09c |
| MX | 4.52b | 4.23cd | 4.65b | 4.75ab |
| GRE-0.375% | 4.57b | 4.43b | 5.20b | 4.94a |
| GRE-0.75% | 4.58b | 4.29c | 4.70b | 4.82ab |
| GRE-1.5% | 4.55b | 4.14de | 4.63b | 4.70ab |
| GRE-3% | 4.35b | 4.09e | 4.57b | 4.58b |
| SEM | 0.073 | 0.022 | 0.033 | 0.059 |
| P-value | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Mean values bearing different superscript letters within a column are significantly different (P < 0.05). n = 12 birds per treatment.
Treatment CON consisted of chicks fed unmedicated corn–soybean meal (SBM) basal without GRE; Treatment MX consisted of chicks given unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055 g/kg diet; Treatment GRE-0.375% consisted of chicks given unmedicated corn–SBM basal into which GRE was added at 0.375% level; Treatments GRE-0.75%, GRE-1.5%, and GRE-3% consisted of chicks that were given diets similar to GRE-0.375% consisted of chicks that were given diets similar to GRE1 at 0.75%, 1.5%, and 3%, respectively.
Differential Leukocyte Counts
Data showing the influence of dietary GRE supplementation on differential leukocyte counts is presented in Table 10. On d 6, only GRE-0.75% treatment had lower percentage of heterophils (41%; P < 0.05) compared to MX (61.67%), but this difference was no longer apparent by d 13. On d 6 and d 13, percentage of lymphocytes was higher for GRE-0.375% (d 6, 23%; d 13, 28%; P < 0.05) compared to MX (d 6, 11.33%; d 13, 15%), whereas the percentages for other treatments were in-between. On d 6, only GRE-0.75% had higher H:L ratio (0.566; P < 0.05) compared to MX (0.189), and on d 13, only GRE-1.5% had higher H:L ratio (0.600; P < 0.05) compared to MX (0.286). This suggests that GRE influences the bird's hematological defense mechanism (Table 10).
Table 10.
Effect of dietary GRE supplementation on differential leukocyte counts in broiler chicks (d 6 and 13).
| Day 6 (parameters measured)2 |
Day 13 (parameters measured)2 |
|||||
|---|---|---|---|---|---|---|
| Treatments1 | Heterophil (%) | Lymphocyte (%) | H:L ratio | Heterophil (%) | Lymphocyte (%) | H:L ratio |
| CON | 54.00ab | 13.50ab | 0.264b | 51.67 | 18.00bc | 0.357ab |
| MX | 61.67a | 11.33b | 0.189b | 56.33 | 15.00c | 0.286b |
| GRE-0.375% | 53.00ab | 23.00a | 0.443ab | 54.33 | 28.00a | 0.531ab |
| GRE-0.75% | 41.00b | 22.33ab | 0.566a | 43.33 | 25.17ab | 0.597ab |
| GRE-1.5% | 48.50ab | 18.83ab | 0.410ab | 45.00 | 25.17ab | 0.600a |
| GRE-3% | 54.67ab | 19.17ab | 0.371ab | 45.00 | 24.50abc | 0.565ab |
| SEM | 3.317 | 2.560 | 0.062 | 3.308 | 2.265 | 0.697 |
| P-value | 0.0043 | 0.0155 | 0.0031 | 0.0345 | 0.0022 | 0.0076 |
Mean values bearing different superscript letters within a column are significantly different (P < 0.05). n = 12 birds per treatment.
Treatment CON consisted of chicks fed unmedicated corn–soybean meal (SBM) basal without GRE; Treatment MX consisted of chicks given unmedicated corn–SBM basal into which bacitracin methylene disalicylate (BMD) was added at 0.055 g/kg diet; Treatment GRE-0.375% consisted of chicks given unmedicated corn–SBM basal into which GRE was added at 0.375% level; Treatments GRE-0.75%, GRE-1.5%, and GRE-3% consisted of chicks that were given diets similar to GRE-0.375% consisted of chicks that were given diets similar to GRE1 at 0.75%, 1.5%, and 3%, respectively.
Plasma Total IgY Concentration and NDV-Specific Antibody Titer
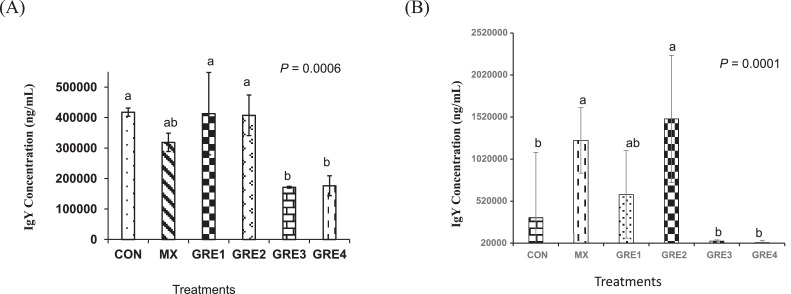
Total plasma IgY concentrations on d 20 and d 27 of experiment are presented in Figures 1A and 1B, respectively. On d 20, GRE-supplemented group had plasma IgY concentrations that were not different (P > 0.05) from that of MX. However, by d 27, GRE-1.5% (40,838 ng/mL) and GRE-3% (25,286 ng/mL) showed lower IgY concentrations (P < 0.05) compared to MX treatment (1,242,781 ng/mL Figure 1B). These results indicate that only dietary GRE up to 0.75% level had comparable efficacy to MX in enhancing plasma IgY concentrations.
Figure 1.
Effect of dietary ginger root extract on plasma IgY concentration (A) d 20 and (B) d 27. The data are expressed as means ± SEM, with n = 12 per treatment; a, b means without a common superscript are different. Statistically significant differences are indicated with P < 0.05. GRE-1, GRE-2, GRE-3, and GRE-4 consisted of chicks fed diets supplemented with ginger root extract at 0.375 g/kg, 0.75 g/kg, 1.5 g/kg, and 3 g/kg level of the diet, respectively.
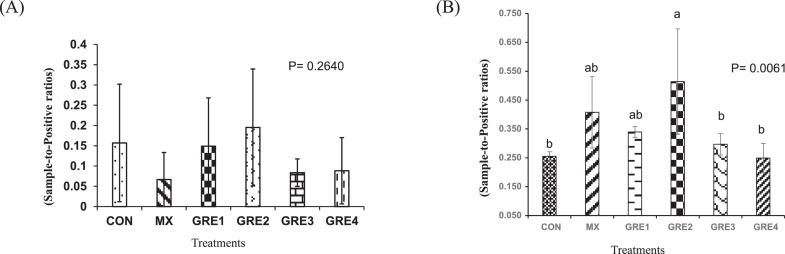
Antibody titer to NDV was measured to determine the influence of dietary GRE concentrations on humoral adaptive immune response to NDV vaccination. Results at d 20 showed no difference (P > 0.05) among the treatments (Figure 2A). However, by d 27, the GRE-0.75% treatment had a higher NDV-specific antibody titer (P < 0.05) than CON treatment, whereas the titers for other treatments were in-between (Figure 2B).
Figure 2.
Effect of dietary ginger root extract on NDV antibody titers (A) d 20 and (B) d 27. The data are expressed as means ± SEM, with n = 12 per treatment; a, b means without a common superscript are different. Statistically significant differences are indicated with P < 0.05. GRE-1, GRE-2, GRE-3, and GRE-4 consisted of chicks fed diets supplemented with ginger root extract at 0.375 g/kg, 0.75 g/kg, 1.5 g/kg, and 3 g/kg level of the diet, respectively.
DTH Reaction
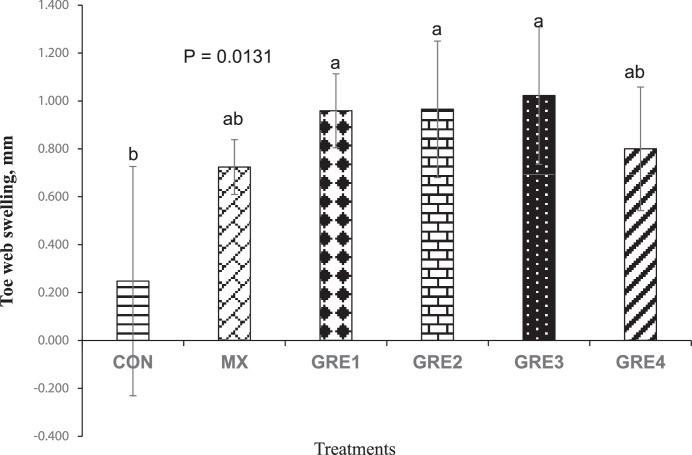
On d 24, DTH evaluation was carried out to assess the efficacy of the cell-mediated immune response of the birds. At 24 h post-PHAP injection, birds in the dietary treatments that had GRE supplementation up to 1.5% had higher toe web swelling (GRE-0.375%-0.96 mm; GRE-0.75%-0.97 mm; and GRE-1.5%-1.02 mm; P < 0.05) compared to CON (0.25 mm), but were not different from MX (0.72 mm). This suggests that dietary supplementation of GRE up to 1.5% level of the diet boosted cell-mediated immune response (Figure 3).
Figure 3.
Effect of dietary ginger root extract on delayed-type hypersensitivity response (24 h post-PHAP injection). The data are expressed as means ± SEM, with n = 12 per treatment; a, b means without a common superscript are different. Statistically significant differences are indicated with P < 0.05. GRE-1, GRE-2, GRE-3, and GRE-4 consisted of chicks fed diets supplemented with ginger root extract at 0.375 g/kg, 0.75 g/kg, 1.5 g/kg, and 3 g/kg level of the diet, respectively.
DISCUSSION
Despite the beneficial effect of antibiotics in improving chicken growth performance through modulation of the gut microbiota, their use has been limited and banned in some countries. In the present study, we showed that bioactive compounds, such as gingerols and shogaols, are present in GREs and their concentration is dependent on the level of supplementation in the diet. Because GRE contains compounds that perform biological functions, we supplemented GRE in broiler diet in a bid to investigate the effects of GRE on growth performance, its role in immune competence as well as its pharmacological properties at different levels of supplementation.
Some studies have reported the use of some natural herbs that contain bioactive compounds in the replacement of antibiotics in animal feeds (Abdel-Wareth et al. 2012; Dhama et al., 2015). In other animal studies, GRE influences growth and biological activities when consumed as it prevents the generation of free radicals, regulate inflammation, and enhances digestive enzyme activity and cell viability (Platel and Srinivasan, 2000; Penna et al., 2003). However, there have been discrepancies in reports on the effect of ginger on growth performance parameters. For instance, Bamidele and Omidiran (2012) reported a decrease in growth rate with the use of ginger during the starter growth phase when ginger was added to broiler feed at 20 and 60 g/kg, but Ademola et al. (2004) observed that the addition of 5, 10, or 15 g/kg of ginger to broiler diet improved growth performance slightly. Interestingly, our study showed that the effects of GRE supplementation up to 1.5% level on BW, BWG, and FCR was not different from that of the CON treatment. However, dietary GRE inclusion level beyond 1.5% resulted in poorer growth performance parameters. This suggests that the varied reports with the use of ginger was a result of the level of inclusion of ginger in the diets, as phytotoxicity set in at a higher supplementation rate. Moreover, the increase observed in the performance parameters with supplementation of GRE up to 1.5% was not different from that of the bacitracin-supplemented diet (MX).
Gut bacteria population includes a diverse and complex microbiota involved in digestion and absorption of nutrients, immune system development, and pathogen exclusion (Shang et al., 2018). Studies have shown that ginger suppresses the growth and proliferation of bacteria that colonize the digestive tract. According to Yadufashije et al. (2020), ginger extract can be used to treat infections as it inhibits the growth of bacteria in the digestive system in humans. Its bacteria growth inhibitory effect was also reported for Helicobacter pylori and oral pathogens such as Porphyromonas spp (Guerra et al., 2022; Park et al., 2008). We also observed similar findings that the use of GRE supplementation in broiler diet reduced the concentration of pathogenic E. coli and overall total bacteria count in both the cecal content and feces of the broiler chickens. According to Tintu et al. (2012) and Mahady et al. (2003), 10-gingerol and 6-gingerol are influential in mitigating and inhibiting the growth of these microbes. Intriguingly, higher levels of beneficial Lactobacillus spp. and Bifidobacterium were observed in our study. This aligns with Babu et al. (2018) that ascribed the growth-promoting potential of ginger extracts to the increase in beneficial bacteria such as Lactobacillus and Bifidobacterium on the bioactive compound constituents. Dietary addition of Z. officinale improved the population of beneficial bacteria, such as lactic acid bacteria in the small intestine mainly jejunum (Tekeli et al., 2011). The relationship between the increase in these beneficial bacteria in the gut and improvement in overall health and growth performance of livestock and humans has been widely reported. More so, the presence of oligosaccharides in bioactive compounds has been reported to be responsible for their contribution to the increase of beneficial bacteria in the gut (Markowiak and Śliżewska, 2017; Kondapalli et al., 2022).
White blood cells and other functionally related cells are saddled with the responsibility of stimulating an effective immune response to stimuli (Farag and Alagawany, 2018; Thiam et al., 2021). According to Tan and Vanitha (2004), essential oils extracted from ginger improved the phagocytic activity of heterophils. Likewise, Al-Khalaifah et al. (2022) reported an increase in WBC with an increased level of ginger in the diet at 5 wk. However, Ademola et al. (2007) reported a different finding, stating that ginger given to chickens at a concentration level of 1.0% resulted in a significant decrease in the total number of WBCs. In this study, the MX treatment had higher percentage of circulating heterophils compared to the GRE-treatments, but the reverse was the case for the percentage of circulating lymphocytes. It can be speculated that differences exist in the molecular mechanisms by which MX and GRE enhance immune surveillance in the body.
NDV antibody titers show the level of protection of the birds since the birds were vaccinated against Newcastle disease at the hatchery. Although at d 20, the NDV antibody titers was not different among treatments, by d 27, the GRE-0.75% group had higher antibody titers against NDV (P < 0.05) compared to control. An increase in the level of circulating plasma antibodies has been reported to be synonymous with an increase in the protection of the host against many enteric viral diseases (Zhu et al., 2019). Accordingly, the elevated IgY concentration and NDV antibody titers in the GRE-0.75% treatment suggests that birds in this treatment had a higher level of protection against NDV. Furthermore, GRE supplementation up to 1.5% supplementation level increased the delayed-type hypersensitivity response, which is a measure of immune responsiveness to sensitization (Vohr, 2005). Our finding is similar to that of Farhath et al. (2013) who reported increased delayed-type hypersensitivity response with the oral administration of 6-gingerol.
In conclusion, supplementation of GRE up to 1.5% level of the diet improved growth performance, immune response, and overall health status of broiler chickens. However, further study needs to be conducted to elucidate the mechanism by which the bioactive compounds in GRE confer immunity and selectively decimate microbes.
ACKNOWLEDGMENTS
The authors thank members of the Poultry Research Team at North Carolina Agricultural and Technical State University (Greensboro, NC) for their technical support.
DISCLOSURES
The authors declare no conflicts of interest.
References
- Abdel-Wareth A., Kehraus S., Hippenstiel F., Südekum K. Effects of thyme and oregano on growth performance of boilers from 4 to 42 days of age and on microbial counts in crop, small intestine and caecum of 42-day-old broilers. Anim. Feed Sci. Technol. 2012;178:198–202. [Google Scholar]
- Ademola S., Farinu G., Adewolo O., Lawal T., Babatunde G. Antimicrobial activity of garlic and ginger mixtures, serum lipid profile and growth performances of broilers fed the mixtures. Bowen J. Agric. 2007;4:103–113. [Google Scholar]
- Ademola S., Farinu G., Obe A.A., Babatunde G. Growth, haematological and biochemical studies on garlic and ginger-fed broiler chickens. Moor J. Agric. Res. 2004;5:122–128. [Google Scholar]
- Adetunji A., Casey T., Franco J., Shah D., Fasina Y. Proteomic analysis of the effect of Salmonella challenge on broiler chicken. Molecules. 2022;27:7277. doi: 10.3390/molecules27217277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agunos A., Pierson F.W., Lungu B., Dunn P.A., Tablante N. Review of nonfoodborne zoonotic and potentially zoonotic poultry diseases. Avian Dis. 2016;60:553–575. doi: 10.1637/11413-032416-Review.1. [DOI] [PubMed] [Google Scholar]
- Al-Khalaifah H., Al-Nasser A., Al-Surrayai T., Sultan H., Al-Attal D., Al-Kandari R., Al-Saleem H., Al-Holi A., Dashti F. Effect of ginger powder on production performance, antioxidant status, hematological parameters, digestibility, and plasma cholesterol content in broiler chickens. Animals. 2022;12:901. doi: 10.3390/ani12070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anh N.H., Kim S.J., Long N.P., Min J.E., Yoon Y.C., Lee E.G., Kim M., Kim T.J., Yang Y.Y., Son E.Y., Yoon S.J., Diem N.C., Kim H.M., Kwon S.W. Ginger on human health: a comprehensive systematic review of 109 randomized controlled trials. Nutrients. 2020;12:157. doi: 10.3390/nu12010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruwa C.E., Pillay C., Nyaga M.M., Sabiu S. Poultry gut health - microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. J. Anim. Sci. Biotechnol. 2021;12:119. doi: 10.1186/s40104-021-00640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen, W. 2022. Ross 708: Broiler management and nutrition specifications. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerNutritionSpecifications2022-EN.pdf. accessed August 5, 2023.
- Babu K.N., Hemalatha R., Satyanarayana U., Shujauddin M., Himaja N., Bhaskarachary K., Kumar B.D. Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J. Herb. Med. 2018;13:42–51. [Google Scholar]
- Bamidele O., Adejumo I.O. Effect of garlic (Allium sativum L.) and ginger (Zingiber officinale Roscoe) mixtures on performance characteristics and cholesterol profile of growing pullets. Int. J. Poult. Sci. 2012;11:217–220. [Google Scholar]
- Biagini L., Galosi L., Roncarati A., Attili A.R., Mangiaterra S., Rossi G. The role of nutraceuticals and phytonutrients in chickens' gastrointestinal diseases. Animals. 2022;12:892. doi: 10.3390/ani12070892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Kont I., Fürst R. Benefits of ginger and its constituent 6-shogaol in inhibiting inflammatory processes. Pharmaceuticals (Basel) 2021;14:571. doi: 10.3390/ph14060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Latheef S.K.S., Samad H.A., Karthik K., Tiwari T., Khan R.U., Alagawany M., Farag M.R., Gazi M.A., Laudadio V. Multiple beneficial applications and modes of action of herbs in poultry health and production-A review. Int. J. Pharmacol. 2015;11:152–176. [Google Scholar]
- Farag M.R., Alagawany M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018;76:101–106. doi: 10.1016/j.jtherbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Farhath S., Vijaya P., Vimal M. Immunomodulatory activity of geranial, geranial acetate, gingerol, and eugenol essential oils: evidence for humoral and cell-mediated responses. Avicenna J. Phytomed. 2013;3:224–230. [PMC free article] [PubMed] [Google Scholar]
- Fasina Y.O, Obanla T.O., Ferket P.R., Shah D.H. Comparative efficacy of spray-dried plasma and bacitracin methylene disalicylate in reducing cecal colonization by Salmonella Enteritidis in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feye K.M., Baxter M.F.A., Tellez-Isaias G., Kogut M.H., Ricke S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020;99:653–659. doi: 10.1016/j.psj.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra V., María O.P.Patricio, Guillermo P. Plant-based polyphenols: anti-Helicobacter pylori effect and improvement of gut microbiota. Antioxidants. 2022;1:109. doi: 10.3390/antiox11010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.H., Lillehoj H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2019;250:41–50. [Google Scholar]
- Kimura A.C., Reddy V., Marcus R., Cieslak P.R., Mohle-Boetani J.C., Kassenborg H.D., Segler S.D., Hardnett F.P., Barrett T., Swerdlow D.L., Emerging Infections Program FoodNet Working Group Chicken consumption is a newly identified risk factor for sporadic Salmonella enterica serotype Enteritidis infections in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 2004;38:S244–S252. doi: 10.1086/381576. [DOI] [PubMed] [Google Scholar]
- Kondapalli N.B., Hemalatha R., Uppala S., Yathapu S.R., Mohammed S., Venkata Surekha M., Rajendran A., Bharadwaj D.K. Ocimum sanctum, Zingiber officinale, and Piper nigrum extracts and their effects on gut microbiota modulations (prebiotic potential), basal inflammatory markers and lipid levels: oral supplementation study in healthy rats. Pharm. Biol. 2022;60:437–450. doi: 10.1080/13880209.2022.2033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermie G., Gröhn Y.T., Raboisson D. Addressing Antimicrobial Resistance: An Overview of Priority Actions to Prevent Suboptimal Antimicrobial Use in Food-Animal Production. Front. Microbiol. 2017;7:2114. doi: 10.3389/fmicb.2016.02114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahady G.B., Pendland S.L., Yun G.S., Lu Z.Z., Stoia A.G. (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699–3702. [PMC free article] [PubMed] [Google Scholar]
- Markowiak P., Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhadi N.S., Ghiasvand R., Askari G., Hariri M., Darvishi L., Mofid M.R. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int. J. Prev. Med. 2013;4:S36–S42. [PMC free article] [PubMed] [Google Scholar]
- Mohamad H.S., Wenli S., Qi C. Clinical aspects and health benefits of ginger (Zingiber officinale) in both traditional Chinese medicine and modern industry. Acta Agric. Scand. B Soil Plant Sci. 2019;69:546–556. [Google Scholar]
- Park M., Bae J., Lee D.-S. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008;22:1446–1449. doi: 10.1002/ptr.2473. [DOI] [PubMed] [Google Scholar]
- Patra A.K., Amasheh S., Aschenbach J.R. Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2019;59:3237–3266. doi: 10.1080/10408398.2018.1486284. [DOI] [PubMed] [Google Scholar]
- Penna S.C., Medeiros M.V., Aimbire F.S.C., Faria-Neto H.C.C., Sertié J.A.A., Lopes-Martins R.A.B. Anti-inflammatory effect of the hydralcoholic extract of Zingiber officinale rhizomes on rat paw and skin edema. Phytomedicine. 2003;10:381–385. doi: 10.1078/0944-7113-00271. [DOI] [PubMed] [Google Scholar]
- Platel K., Srinivasan K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung. 2000;44:42–46. doi: 10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Prasad S., Tyagi A.K. Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. 2015;2015 doi: 10.1155/2015/142979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor A., Phillips G.J. Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front. Vet. Sci. 2019;6:114. doi: 10.3389/fvets.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y., Kumar S., Oakley B., Kim W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistical Analysis Software (SAS). 2004. Version 9.2. SAS Institute Inc., Cary, NC.
- Tan B., Vanitha J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: a review. CMC. 2004;11:1423–1430. doi: 10.2174/0929867043365161. [DOI] [PubMed] [Google Scholar]
- Tekeli A., Kutlu H.R., Celik L. Effects of Z. officinale and propolis extracts on the performance, carcass and some blood parameters of broiler chicks. Curr. Res. Poult. Sci. 2011;1:12–23. [Google Scholar]
- Thiam M., Barreto Sánchez A.L., Zhang J., Zheng M., Wen J., Zhao G., Wang Q. Association of heterophil/lymphocyte ratio with intestinal barrier function and immune response to Salmonella Enteritidis infection in chicken. Animals. 2021;11:3498. doi: 10.3390/ani11123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam M., Wang Q., Barreto S.A.L., Zhang J., Ding J., Wang H., Zhang Q., Zhang N., Wang J., Li Q., Wen J., Zhao G. Heterophil/lymphocyte ratio level modulates Salmonella resistance, cecal microbiota composition and functional capacity in infected chicken. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.816689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintu I., Dileep K.V., Remya C., Augustine A., Sadasivan C. 6-gingerol inhibits fungal alpha amylase: enzyme kinetic and molecular modeling studies. Starch/Stärke. 2012;64:607–612. doi: 10.1111/j.1747-0285.2012.01426.x. [DOI] [PubMed] [Google Scholar]
- Vohr H.W. In: Encyclopedic Reference of Immunotoxicology. Vohr HW., editor. Springer; Berlin, Heidelberg: 2005. Delayed-type hypersensitivity. [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadufashije C., Niyonkuru A., Munyeshyaka E., Madjidi S., Mucumbitsi J. Antibacterial activity of ginger extracts on bacteria isolated from digestive tract infection patients attended Muhoza Health Center. Asian J. Med. Sci. 2020;11:35–41. [Google Scholar]
- Zhu Y., Ma Y., Lu M., Zhang Y., Li A., Liang X., Li J. Efficient production of human norovirus-specific IgY in egg yolks by vaccination of hens with a recombinant vesicular stomatitis virus expressing VP1 protein. Viruses. 2019;11:444. doi: 10.3390/v11050444. [DOI] [PMC free article] [PubMed] [Google Scholar]