Highlights
-
•
Children previously infected with SARS-CoV-2 Omicron variant developed robust neutralizing antibody responses after only one dose of BNT162b2, comparable with that of two doses, one month apart.
-
•
Children with an interval of > 6 months from SARS-CoV-2 infection to vaccination had higher neutralizing antibody response than those with an interval of 3 – 6 months.
Keywords: SARS-CoV-2 vaccine, Child, Infant, Neutralizing antibody titer, Anti-SARS-CoV-2 IgG, BNT162b2 vaccine
Abstract
Background
Children 6 months to < 5 years old are recommended to receive 3-dose regimen of BNT162b2. Children previously infected with Omicron variant of SARS-CoV-2 develop immunity from natural infection, therefore may require fewer doses of vaccine.
Objective
To compare immunogenicity of 1- or 2-dose BNT162b2 in healthy children post COVID-19 with 3-dose BNT162b2 in COVID-naïve children.
Methods
Children aged 6 months to < 5 years who developed COVID-19 during the Omicron-predominant period were enrolled; Group A 3–6 months(N = 40) and Group B > 6 months(N = 40) prior to vaccination. Participants in Group A and B received 2-dose BNT162b2 intramuscularly 1 month apart. COVID-naïve children were enrolled as a control group (N = 40) and received 3-dose BNT162b2 at month 0,1,3. Neutralizing antibody against Omicron variant(BA.2.75 and BA.4/5) was determined by pseudovirus assays(pVNT) as reported by neutralization dilution for 50%inhibition (ID50) at 28 days after the 1st and 2nd dose.
Results
From October-November 2022, 120 children with a median age of 2.8 years (IQR 1.6–4.0) were enrolled. The median duration since COVID-19 to vaccination was 4.4 months(IQR 3.8–5.4) in Group A and 7.9 months(7.0–8.5) in Group B. In Group A, the geometric means(GMs) of pVNT-BA.2.75 ID50 were 553 (95%CI 338–906) and 753(516–1098) after 1 and 2 doses, respectively, and the GMs of pVNT-BA.4/5 ID50 were 1936(1402–2673) and 1885(1414–2512), respectively. In Group B, the GMs of pVNT-BA.2.75 ID50 were 1383(1100–1742) and 1419 (1104–1823), and the GMs of pVNT-BA.4/5 ID50 were 2627(2048–3367) and 2056(1546–2735), respectively. Meanwhile in COVID-naïve group, the GMs of pVNT-BA.2.75 and pVNT-BA.4/5 ID50 were 158(98–255) and 59(31–114) after the 3rd dose, respectively. The geometric mean ratio(GMR) of pVNT-BA.2.75 ID50 after 1 dose in Group A and B compared with after 3 doses in COVID-naïve group were 3.50 (1.93–6.34) and 8.74 (4.79–15.95), respectively. The GMR of pVNT-BA.2.75 ID50 after 1 dose in Group B compared with Group A was 2.50 (1.45–4.31).
Conclusions
Children previously infected with SARS-CoV-2 Omicron variant, developed robust neutralizing antibody response against Omicron variant after single-dose BNT162b2. Children with an interval of > 6 months since COVID-19 infection developed higher neutralizing antibody response compared to those with a 3-to-6-month interval.
Introduction
The World Health Organization classified SARS-CoV-2 Omicron variant (B.1.1.529) as a “Variant of Concern” on November 26, 2021 [1]. Omicron variant spread rapidly and has become the dominant circulating strain worldwide since January 2022, including in Thailand [2]. The evolving changes of SARS-CoV-2 might affect the effectiveness of SARS-CoV-2 vaccines, which are based on the ancestral strain. In Thailand, since January 2022, the predominant sublineage was BA.1, followed by sublineages BA.2, BA.5 in July-October 2022, and BA.2.75 in November 2022 [3]. Meanwhile BQ.1 and XBB.1.5 predominated in Europe and the United States since November 2022 and January 2023, respectively [3]. Approximately 20% of children were reported to have Coronavirus Disease 2019 (COVID-19) in the United States, as of February 2023; although this number was likely underreported [4]. In February 2022, after the Omicron surge in the United States, the seroprevalence of infection-induced SARS-CoV-2 antibodies increased 35% in children aged 1 – 4 years, from 33% to 68% [5].
Three-dose regimen of BNT162b2 in children 6 months to under 5 years of age was demonstrated to be safe and efficacious [6], and has been recommended by the Advisory Committee on Immunization Practices (ACIP) in the United States, since June 2022 [7]. Phase 1 and 2 – 3 trials of BNT162b2 in children aged 6 months to under 5 years receiving 3 doses of 3-µg BNT162b2 at 21 days and at least 60 days apart demonstrated good immunogenicity and met the immunobridging criteria compared with 2 doses of 30-µg BNT162b2 in young adults aged 16 to 25 years [6]. This study reported a vaccine efficacy of 73.2% 1 week – 2 months after dose 3 in 2022, the Omicron-predominant period [6]. Following ACIP’s recommendation for the use of a 3-dose regimen of BNT162b2, 3 and 8 weeks apart, in June 2022, the vaccine effectiveness against symptomatic SARS-CoV-2 infection in children aged 3 to 4 years was 31% during 2 weeks – 4 months after dose 3 [8]. In Thailand, The Royal College of Pediatricians of Thailand recommended a three-dose regimen of 3-µg BNT162b2 at 4 (3 – 8) and 8 weeks apart [9]. However, there is no specific recommendation for previously infected children who may require fewer doses of the vaccine.
Previous SARS-CoV-2 Omicron variant infection provided some degree of protection against re-infection and may require fewer doses of the vaccine. Past Omicron infection demonstrated higher protection against Omicron re-infection than past infection with pre-Omicron variants, with the protection levels of 59 – 94% [10]. In the cohort of children aged 5 to 11 years, previous Omicron infection reduced the risk of Omicron re-infection with an effectiveness of 90.7% at 2 months and 62.9% at 4 months [11]. The effectiveness increased to 94.3% at 2 months and 79.4% at 4 months in vaccinated children with past Omicron infection [11]. In adults previously infected with SARS-CoV-2, a single-dose booster vaccination demonstrated protective effects against COVID-19 during the Omicron-variant dominant period over no vaccination, while 2- or 3-dose booster vaccinations provided no additional protection over a single-dose [12]. The systematic reviews showed that previous infection yielded protective effectiveness of 80.1% against hospitalization and severe disease at 6 months [13]. Vaccination in previously infected individuals, resulting in hybrid immunity, provided benefits in protection against hospitalization and severe disease [13].
We hypothesized that one or two doses of BNT162b2 induced robust immunologic responses in children with past COVID-19. This study aimed to compare immunogenicity against Omicron variant of 1- or 2-dose BNT162b2 in healthy children post COVID-19 with 3-dose BNT162b2 in COVID-naïve children 6 months to under 5 years of age in Thailand.
Method
Study design and participants
This is an open-label, controlled, phase 2 clinical trial, conducted at Center of Excellence for Pediatric Infectious Diseases and Vaccines, Chulalongkorn University, Bangkok, Thailand. Healthy children aged 6 months to under 5 years who had never received vaccination against SARS-CoV-2 were recruited and screened for eligibility. Children with a history of SARS-CoV-2 infection during the Omicron-predominant period in Thailand, from January 2022 onwards [2], were grouped by interval from SARS-CoV-2 infection to first vaccination: 3 to 6 months (Group A) or >6 months (Group B). The date of past SARS-CoV-2 infection was determined by interviewing the parents for the date of positive test for SARS-CoV-2, either by rapid antigen test or polymerase chain reaction, or the date of close contact with COVID-19 cases plus positive neutralizing antibodies against Omicron sublineage BA.4/5, as determined by surrogate virus neutralization test (sVNT-BA.4/5) of over 30% inhibition. Children without a history of SARS-CoV-2 infection and negative anti-nucleocapsid antibody were also recruited to serve as controls (COVID-naïve). Children with a history of known anaphylaxis to any of the vaccine components, receipt of immunoglobulins or blood products within 3 months, use of immunosuppressive medication, and any illness within 14 days were excluded. Parents of children enrolled signed informed consent forms to participate in this trial. This study was approved by the institutional review board of Faculty of Medicine, Chulalongkorn University (IRB no. 661/65), and registered in thaiclinicaltrials.org (TCTR20220927003).
Study procedure
Before the first vaccination, blood samples were collected from all participants to test for immunity against SARS-CoV-2 Omicron variant. For COVID-naïve children, anti-nucleocapsid IgG antibody, performed at Faculty of Medicine, Chulalongkorn University (by chemiluminescent microparticle immunoassay, SARS-CoV-2 IgG, Abbott Laboratories, Abbott Park, IL, USA), was also tested to exclude prior infection. All participants were vaccinated with 3-µg BNT162b2 in 0.2 ml, lot number GE0695 and GG3683, intramuscularly at the deltoid muscle in children aged 2 to under 5 years or anterolateral thigh in children aged < 2 years. The children with a history of SARS-CoV-2 infection (Group A and B) received 2 doses of BNT162b2, 4 weeks apart. The COVID-naïve children received 3 doses of BNT162b2, 4 and 8 weeks apart, respectively. Immunogenicity was assessed at 4 weeks after the first and second doses in Group A and B, and at 4 weeks after the second and third doses in the COVID-naïve group. Immunogenicity against Omicron variant was assessed by neutralizing antibody using pseudovirus neutralization test (pVNT) and surrogate virus neutralization test (sVNT); and anti-spike receptor binding domain (anti-S-RBD). Any history of COVID-19 diagnosis or close contact with COVID-19 patients was obtained at all visits.
Solicited reactogenicities, both local and systemic, were documented in a structured diary by the parents or caregivers for 7 days after each vaccination. Pain at injection site, swelling, and erythema were reported for local reactogenicities. Systemic reactogenicities included fever, vomiting, and diarrhea for all participants; decreased appetite, drowsiness, and irritability for participants aged 6 months to under 2 years; headache, fatigue, chills, myalgia, and arthralgia for participants aged 2 to under 5 years. Unsolicited adverse events were recorded by the study team until 1 month after final vaccination.
Immunogenicity outcomes
Immunogenicity was assessed by functional neutralizing antibody against SARS-CoV-2 Omicron sublineage BA.2.75, BA.4/5 by pseudovirus neutralization test (pVNT-BA.2.75 and pVNT-BA.4/5) and surrogate virus neutralization test (sVNT-BA.4/5); and IgG antibody against spike-receptor-binding domain of Omicron sublineage BA.4/5 (anti-S-RBD IgG-BA.4/5).
Pseudovirus neutralization test against SARS-CoV-2 Omicron sublineage BA.2.75 and BA.4/5 (pVNT-BA.2.75 and pVNT-BA.4/5)
Pseudovirus neutralization test (pVNT) against the Omicron variants was performed as described previously [14]. Two-fold serial dilutions of serum samples (starting 1:40 or 1:80) were incubated with pseudoviruses displaying the Omicron (BA.2.75 or BA.4/5) spikes in a 1:1 vol/vol ratio in a 96-well culture plate for 1 h at 37 °C. The pseudovirus input used was normalized to 1 × 105 RLU/well. Subsequently, suspensions of HEK293T-ACE-2 cells (2 × 104 cells/ml) were mixed with the serum-pseudovirus mixture and seeded into each well. At 48 h, the neutralizing antibodies were determined based on luciferase activity following entry of pseudovirus. Values were normalized against signals from no-serum controls. The ID50 values were calculated by determining the half-maximal inhibitory dilution. The limit of detection (LOD) for the pVNT assay is 40 (1:40 dilution). We used pVNT ID50 at 185 as a cutoff for correlation with 80% vaccine efficacy, which was derived from ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca) correlates of protection study [15].
Surrogate virus neutralization test against SARS-CoV-2 Omicron sublineage BA.4/5 (sVNT-BA.4/5)
The surrogate virus neutralization test was adjusted from Tan et al. (2020) [16] and performed as described previously [17], [18], utilizing the HRP-tagged recombinant SRBD from Omicron (BA.4/5) strain. The 96-well plates coated with 0.1 µg/well purified recombinant human ACE2 ectodomain were used to incubate with serum samples (at 1:10 dilution) - SRBD mixture. Then, ELISA was performed by incubating the mixture with the hACE2 coated plates for one hour and adding TMB substrate with ample washing in between. OD450 was measured. Purified Human IgG (50 µg/mL) was used as the negative sample. Two dilutions of “OM1” were used as the positive controls. “OM1”, collected from an individual infected with the Omicron strain of SARS-CoV-2 and tested > 40000 AU/mL by SARS-CoV-2 IgG II Quant (Abbott) and > 1000 ID50 by pVNT-BA.4/5, was designated the standard Omicron serum for both the quantitative anti-S-RBD IgG-BA.4/5 ELISA and sVNT-BA.4/5 assays. The percentage inhibition was calculated as follows:
Quantitative spike receptor binding domain IgG against SARS-CoV-2 Omicron sublineage BA.4/5 (anti-S-RBD IgG-BA.4/5) ELISA
The ELISA protocol was modified from Amanat et al. [19] and performed as described previously [17], [18]. Briefly, the ELISA plates were coated with purified recombinant Myc-His-tagged S-RBD, residues 319–541 from SARS-CoV-2 Omicron sublineage BA.4/5 (produced in-house from transfection in HEK 293T cells at the Virology and Cell Technology Lab, BIOTEC, Thailand). Participants’ sera were diluted 1:1000 in PBS-Tween 20 buffer containing 2.5% skim milk and used as primary antibodies. HRP-conjugated human IgG was used as a secondary antibody (Anti-human IgG-HRP cat. no. A8667, Sigma). After addition of TMB substrate, OD450 was measured for each sample and converted into arbitrary units (AU/mL) of anti-S-RBD IgG-BA.4/5, using the standard curve prepared from dilutions of the standard Omicron serum (OM1, positive serum from an individual infected with the Omicron strain). The dilutions used to construct the standard curve (500–15.6 AU/mL) span the linear range of OD450 measurements similar to those in anti-S-RBD IgG wild-type ELISA using the WHO international standard (NIBSC 20/136). The positive cutoff for anti-S-RBD IgG-BA.4/5 ELISA is 92 AU/mL. The cutoff value was based on the average and standard deviation of 121 negative samples collected before COVID-19 pandemic (values range from 2 to 127 AU/mL).
Statistical analysis
The sample size calculation was based on a non-inferiority criterion of pVNT against SARS-CoV-2 Omicron sublineage BA.4/5 geometric mean ratio (GMR) at 4 weeks after the 2nd dose of BNT162b2 vaccination in children with previous SARS-CoV-2 infection for 3 to 6 months or >6 months, compared with at 4 weeks after the 3rd dose in children without previous SARS-CoV-2 infection. At 0.67 non-inferiority margin, 90% power, 0.85 GMR, 0.32 standard deviation on a logarithmic scale, and a ratio of 1:1 for both comparing groups, the minimum participants per group was 31. Considering the 30% drop-out rate, a total of 40 participants per group was required.
Demographic characteristics were described. Continuous variables were expressed as median with interquartile range (IQR), and as frequency with percentage for categorical variables. Differences in continuous variables between two or three groups were assessed using a Wilcoxon rank sum test or Kruskal Wallis test. Differences in proportion between groups were assessed using a Chi-square test.
The primary outcomes were GMRs of pVNT against Omicron sublineage BA.2.75 and BA.4/5 at 4 weeks after the 2nd dose of BNT162b2 vaccination in children with previous SARS-CoV-2 infection, compared with at 4 weeks after the 3rd dose in children without previous SARS-CoV-2 infection. The secondary outcomes were anti-S-RBD against Omicron sublineage BA.4/5, neutralizing antibodies against Omicron sublineage BA.2.75 and BA.4/5 by sVNT and pVNT. The calculation of geometric means (GMs) and GMRs with 95% confidence interval (95% CI) was done by two-independent sample t-test. Non-inferiority criterion was the lower bound of the 95% CI did not exceed 0.67. Spearman's rank correlation coefficient was used to determine the correlation of anti-S-RBD IgG-BA.4/5 and pVNT-BA.4/5. Reactogenicity was reported as a percentage. All reported P-values were two-sided. Statistical significance was defined as P < 0.05. Stata version 15.1 (Stata Corp., College Station, Texas) was used for the analysis.
Results
Baseline characteristics
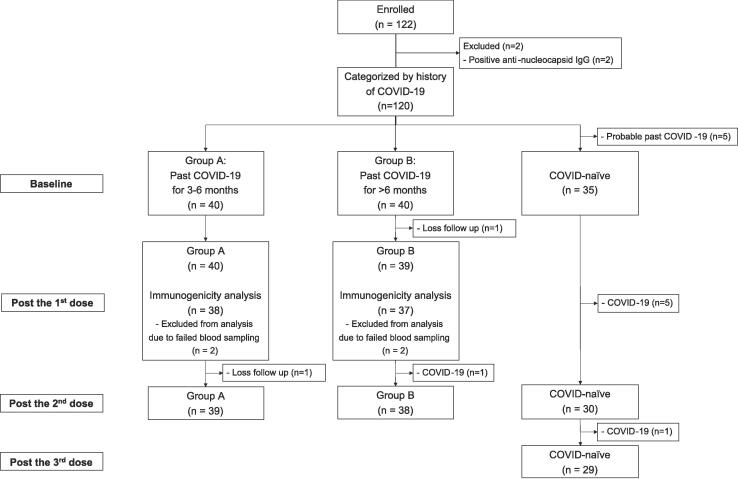
From October to November 2022, 120 children were enrolled, as shown in Fig. 1. Five participants were excluded from the COVID-naïve group due to probable past COVID-19 according to high baseline antibody against Omicron variant (sVNT-BA.4/5 of >30% inhibition) and history of close contact to COVID-19 cases. The median age (IQR) was 2.8 (1.3 – 4.1), 3.0 (2.3 – 4.2), and 2.8 (1.3 – 3.8) years for Group A, B, and control, respectively, as shown in Table 1. The numbers of participants in each age group (6 months to under 2 years of age and 2 to under 5 years of age) and of male participants were not statistically different between groups. The dates of prior COVID-19 were determined by the date of positive test for SARS-CoV-2, either by rapid antigen test or polymerase chain reaction in 38 participants of Group A and B. Two participants from each group had baseline sVNT-BA.4/5 of >30% inhibition and history of close contact to COVID-19 cases, which the dates were used to determine the time of past infection. The median interval between COVID-19 to first vaccination (IQR) was 4.4 (3.8–5.4) months and 7.9 (7.0–8.5) months in Group A and B, respectively. During the study period, 7 participants acquired COVID-19, 1 from Group B and 6 from COVID-naïve group and were excluded from immunogenicity analyses. From 6 COVID-naïve children acquiring COVID-19, 4 got infected after vaccinated with 1 dose of BNT162b2 and 2 got infected after 2 doses. All experienced upper respiratory symptoms. Four participants were hospitalized due to COVID-19 causing high-grade fever.
Fig. 1.
Flow diagram of study participants included in immunogenicity analyses.
Table 1.
Demographic characteristics of participants in this study.
| Group A (n = 40) |
Group B (n = 40) |
COVID-naïve (n = 35) |
P-value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 2.8 (1.3 – 4.1) | 3.0 (2.3 – 4.2) | 2.8 (1.3 – 3.8) | 0.21† |
| Age group | 0.19‡ | |||
| − 6 months to under 2 years, n (%) | 13 (32.5) | 7 (17.5) | 12 (34.3) | |
| − 2 to under 5 years, n (%) | 27 (67.5) | 33 (82.5) | 23 (65.7) | |
| Male sex, n (%) | 23 (57.5) | 22 (55.0) | 19 (54.3) | 0.96‡ |
| Interval between COVID-19 to first vaccination (months), median (IQR) | 4.4 (3.8–5.4) | 7.9 (7.0–8.5) | – | <0.001§* |
Group A: Past COVID-19 for 3–6 months; Group B: Past COVID-19 for > 6 months.
*p < 0.05.
Kruskal–Wallis test.
Chi-square.
Wilcoxon rank sum test.
Immunogenicity after BNT162b2 vaccination
Neutralizing antibody assessed by pseudovirus neutralization test (pVNT) against SARS-CoV-2 Omicron sublineage BA.2.75 and BA.4/5
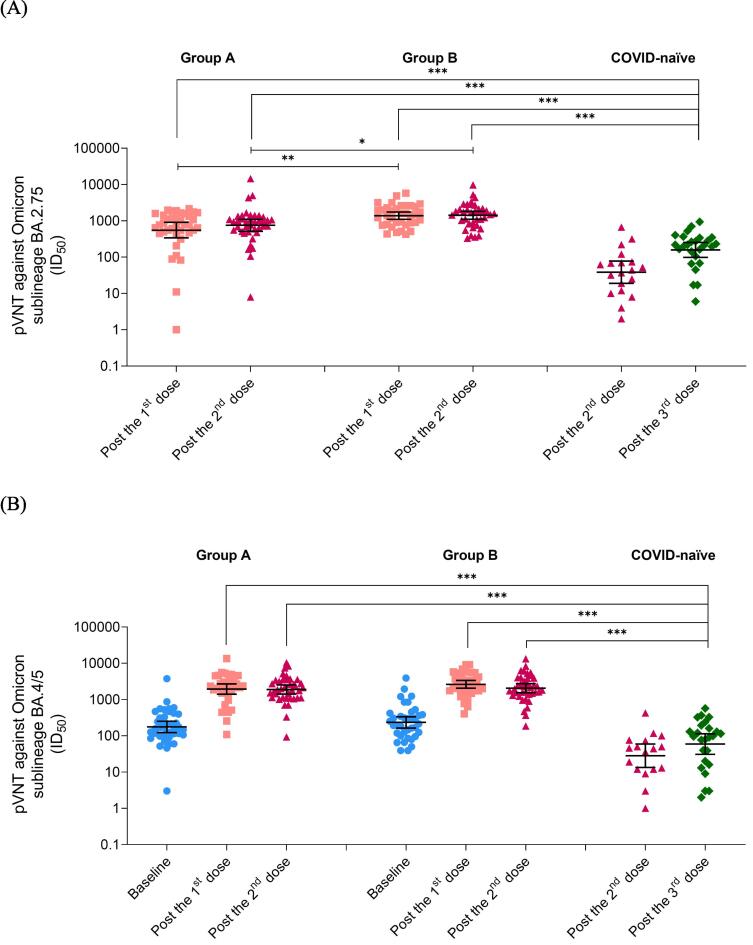
After vaccination, pVNT-BA.2.75 was evaluated in all participants, as shown in Table 2 and Fig. 2A. The GMs of pVNT-BA.2.75 were high after the first dose of BNT162b2 in Group A and B, and comparable to the GMs after the second dose. The GMs after 1- and 2-dose BNT162b2 in children with history of COVID-19 were significantly higher than after 3-dose BNT162b2 in COVID-naïve children, with the GMRs of 3.50 (95% CI 1.93–6.34) for Group A, 8.74 (95% CI 4.79–15.95) for Group B after 1 dose, 4.76 (95% CI 2.82–8.02) for Group A, and 8.97 (95% CI 5.31–15.15) for Group B after 2 doses. The > 6-month interval since previous COVID-19 (Group B) had elevated GMs of pVNT-BA.2.75 than 3-to-6-month interval group (Group A), with the GMRs of 2.50 (95% CI 1.45–4.31) after the first dose and 1.88 (95% CI 1.15–3.09) after the second dose.
Table 2.
Immunogenicity responses after BNT162b2 vaccination in healthy children 6 months to under 5 years of age with history of COVID-19 during Omicron-predominant period and in children without history of COVID-19.
| Immunogenicity outcomes | Group A | Group B | COVID-naïve |
|---|---|---|---|
| pVNT-BA.2.75 (ID50) | |||
| - Post the 1st dose, GM (95% CI) | 553 (338–906) |
1383 (1100–1742) |
– |
| - Post the 2nd dose, GM (95% CI) | 753 (516–1098) |
1419 (1104–1823) |
38 (19–78) |
| - Post the 3rd dose, GM (95% CI) | – | – | 158 (98–255) |
| pVNT-BA.4/5 (ID50) | |||
| - Baseline, GM (95% CI) | 176 (122–252) |
235 (165–336) |
– |
| - Post the 1st dose, GM (95% CI) | 1936 (1402–2673) |
2627 (2048–3367) |
– |
| - Post the 2nd dose, GM (95% CI) | 1885 (1414–2512) |
2056 (1546–2735) |
28 (13–60) |
| - Post the 3rd dose, GM (95% CI) | – | – | 59 (31–114) |
| sVNT-BA.4/5 (%inhibition) | |||
| - Baseline, GM (95% CI) | 40.0 (30.4–52.7) |
37.9 (26.7–53.9) |
0.6 (0.2–1.7) |
| - Post the 1st dose, GM (95% CI) | 97.1 (94.3–99.9) |
98.7 (97.1–100.3) |
– |
| - Post the 2nd dose, GM (95% CI) | 89.9 (80.2–100.8) |
97.4 (95.5––99.3) |
13.2 (7.2–24.4) |
| - Post the 3rd dose, GM (95% CI) | – | – | 32.9 (23.6–45.9) |
| Anti-S-RBD IgG-BA.4/5 (AU/ml) | |||
| - Baseline, GM (95% CI) | 46 (38–55) |
53 (42–69) |
29 (24–34) |
| - Post the 1st dose, GM (95% CI) | 252 (203–312) |
312 (252–385) |
– |
| - Post the 2nd dose, GM (95% CI) | 248 (204–302) |
283 (232–346) |
45 (37–56) |
| - Post the 3rd dose, GM (95% CI) | – | – | 135 (112–162) |
Anti-S-RBD IgG-BA.4/5: Anti-spike-receptor-binding-domain of SARS-CoV-2 Omicron sublineage BA.4/5 immunoglobulin G; AU: arbitrary unit; GM: Geometric mean; Group A: Past COVID-19 for 3–6 months; Group B: Past COVID-19 for > 6 months; ID50: Neutralization dilution for 50% pseudovirus inhibition; pVNT-BA.2.75: Pseudovirus neutralization test against SARS-CoV-2 Omicron sublineage BA.2.75; pVNT-BA.4/5: Pseudovirus neutralization test against SARS-CoV-2 Omicron sublineage BA.4/5; sVNT-BA.4/5: Surrogate virus neutralization test against SARS-CoV-2 Omicron sublineage BA.4/5.
Fig. 2.
Geometric means (95% CI) of pVNT (ID50) after BNT162b2 vaccination in healthy children 6 months to under 5 years of age with history of COVID-19 during Omicron-predominant period and in children without history of COVID-19, (A) pVNT-BA.2.75 and (B) pVNT-BA.4/5. *p < 0.05; **p < 0.01; ***p < 0.001 by two-independent sample t-test.Group A: Past COVID-19 for 3–6 months; Group B: Past COVID-19 for > 6 months; ID50: Neutralization dilution for 50% pseudovirus inhibition; pVNT-BA.2.75: Pseudovirus neutralization test against SARS-CoV-2 Omicron sublineage BA.2.75; pVNT-BA.4/5: Pseudovirus neutralization test against SARS-CoV-2 Omicron sublineage BA.4/5.
Before vaccination, pVNT-BA.4/5 was assessed in Group A and B, as shown in Table 2 and Fig. 2B. After BNT162b2 vaccination, the GMs of pVNT-BA.4/5 remained elevated after the first and the second dose of BNT162b2 in both Group A and B. The GMs after 1- and 2-dose BNT162b2 in children with past SARS-CoV-2 infection were significantly higher than after 3-dose BNT162b2 in children without past SARS-CoV-2 infection, with the GMRs of 32.68 (95% CI 18.68–57.18) for Group A, 44.35 (95% CI 25.27–77.84) for Group B after 1 dose, 31.82 (95% CI 18.25–55.49) for Group A, and 34.72 (95% CI 19.9–60.54) for Group B after 2 doses. The different intervals since previous SARS-CoV-2 infection groups had similar pVNT-BA.4/5 at baseline (p 0.25), after the first dose (p 0.13), and after the second dose (p 0.71).
Neutralizing antibody levels by surrogate virus neutralization test (sVNT) against SARS-CoV-2 Omicron sublineage BA.4/5
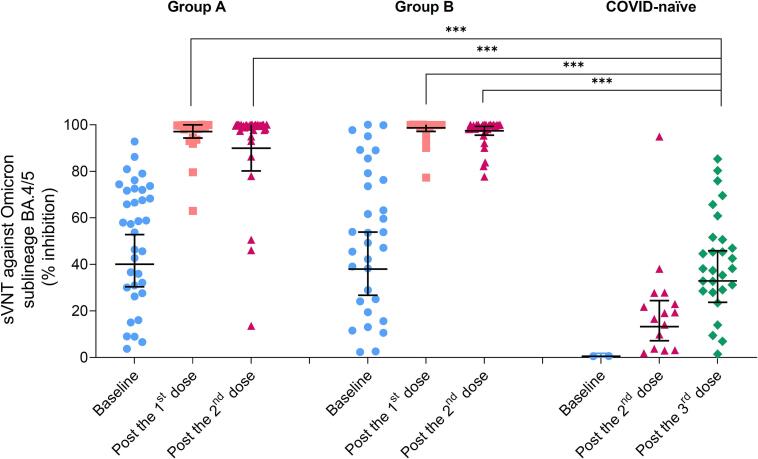
The results of sVNT-BA.4/5 showed the same trends as pVNT results, as shown in Table 2 and Fig. 3. At baseline, children with past SARS-CoV-2 infection had higher sVNT-BA.4/5 GMs than children without past infection, with GMRs of 73.0 (95% CI 20.3–262.7) for Group A, and 69.2 (95% CI 19.2–249.8) for Group B. For Group A and Group B, the GMs of sVNT-BA.4/5 increased after the first BNT162b2 and remained high after the second dose. The GMs after 1- and 2-dose BNT162b2 in children with previous SARS-CoV-2 infection were significantly higher than after 3-dose BNT162b2 in COVID-naïve children, with the GMRs of 2.95 (95% CI 2.35–3.71) for Group A, 3.00 (95% CI 2.39–3.77) for Group B after 1 dose, 2.73 (95% CI 2.15–3.48) for Group A, and 2.96 (95% CI 2.32–3.77) for Group B after 2 doses. The 3–6-month or > 6-month interval since previous SARS-CoV-2 infection groups had similar sVNT-BA.4/5 at baseline (p 0.80), after the first dose (p 0.32), and after the second dose (p 0.48).
Fig. 3.
Geometric means (95% CI) of sVNT-BA.4/5 (%inhibition) after BNT162b2 vaccination in healthy children 6 months to under 5 years of age with history of COVID-19 during Omicron-predominant period and in children without history of COVID-19. ***p < 0.001 by two-independent sample t-test. Group A: Past COVID-19 for 3–6 months; Group B: Past COVID-19 for > 6 months; sVNT-BA.4/5: Surrogate virus neutralization test against SARS-CoV-2 Omicron sublineage BA.4/5.
Anti-spike receptor binding domain IgG (Anti-S-RBD IgG) against SARS-CoV-2 Omicron sublineage BA.4/5
At baseline, children with history of COVID-19 had higher anti-S-RBD IgG-BA.4/5 than COVID-naïve children, with GMRs of 1.59 (95% CI 1.19–2.12) for Group A, and 1.78 (95% CI 1.34–2.38) for Group B, as shown in Table 2. At 4 weeks after the first BNT162b2 vaccination, anti-S-RBD IgG-BA.4/5 of Group A and B increased and persisted after the second vaccination. The antibody levels after 1- and 2-dose BNT162b2 in children with past COVID-19 were significantly higher than after 3-dose BNT162b2 in children without history of COVID-19, with GMRs of 2.55 (95% CI 1.94–3.35) for Group A, 2.80 (95% CI 2.13–3.69) for Group B after 1 dose, 1.84 (95% CI 1.39–2.44) for Group A, and 2.11 (95% CI 1.58–2.79) for Group B after 2 doses. The interval since previous SARS-CoV-2 infection, 3 to 6 months or > 6 months, yielded similar anti-S-RBD IgG-BA.4/5 at baseline (p 0.29), after the first dose (p 0.15), and after the second dose (p 0.34). The results of anti-S-RBD IgG-BA.4/5 and pVNT-BA.4/5 were highly correlated, with the Spearman's rank correlation coefficient of 0.84.
Reactogenicity after BNT162b2 vaccination
Local and systemic reactogenicities during the first 7 days post BNT162b2 vaccination was graded according to the age of the participants: 6 to 23 months and 2 to under 5 years, as shown in Supplementary Table 1. Most reactogenicities were mild-to-moderate severity, except grade 3 fever in one child 2 to under 5 years of age following the second BNT162b2. Children 6 to 23 months of age tended to have fever after the first vaccination (9.1%) rather than after the second (3.2%) and third doses (0%). In children 2 to under 5 years of age, the most common reactogenicities following the first and second dose were pain at injection site (10.9 – 11.1%) and fatigue (6.2 – 7.3%).
Discussion
Single dose of BNT162b2 yielded higher neutralizing antibody levels against BA.2.75 and BA.4/5 sublineages of Omicron variant in children aged 6 months to under 5 years, previously infected with SARS-CoV-2 during Omicron-predominant period, compared to the three-dose regimen in COVID-naïve children. The longer duration since previous infection (>6 months), tended to have higher neutralizing antibody levels compared to those with a 3-to-6-month interval period. Reactogenicities after 3-µg BNT162b2 vaccination were mostly mild-to-moderate severity.
For the evolving SARS-CoV-2, a single-dose seasonal booster would be reasonable in prior infected children and might increase vaccine uptake. The immunity conferred from previous SARS-CoV-2 infection declined over time, thus vaccination should be considered. Considering Omicron variant as the dominant circulating strain, estimated protection from previous infection with any strains was 36.1% against re-infection and 88.9% against severe disease at 40 weeks [10]. Vaccination after previous infection contributed to improved protection against re-infection and hospitalization or severe disease [13]. The period of acquiring COVID-19 of children in this study was during the circulation by BA.1, BA.2, and BA.5 sublineages [3], which correlated with our results of stronger immune response against BA.4/5 than BA.2.75. After a single dose of vaccine, the levels of neutralizing antibody against BA.2.75 were similar to after 2 doses in prior infected children, and higher than after completion of the primary series in COVID-naïve children. Shrestha NK et al. [12] also reported the protective effect of single-dose vaccine in previously infected adults against Omicron variant infection, and no greater protection after >1 dose of vaccine. The COVID-19 vaccine uptake rate was low in children 6 months to under 5 years of age with a rate of 3.4% in Thailand [20] and 12% in the United States [21], for at least 1 dose of vaccine. Misinformation regarding vaccine safety in parents was the important factor associated with vaccine hesitancy in their children [22], [23]. After the reports of myocarditis following the administration of SARS-CoV-2 mRNA vaccine in adolescents, especially after the second dose, some people might link the vaccine-related adverse events with subsequent vaccine doses. The recommendation of fewer vaccine doses or a single dose in children might mitigate their concern on vaccine safety and lead to increased vaccine uptake.
The optimal interval from COVID-19 to vaccination might be > 6 months. The spike-specific B cells of previously infected adults within 6 months had muted response to stimulation and vaccination, unlike those of uninfected adults and adults with an interval from previous infection of > 6 months [24]. Furthermore, booster vaccination was shown to be associated with a reduced incidence of COVID-19 in individuals previously infected > 6 months [12]. The results from our study showed consistent findings with higher neutralizing antibody response after BNT162b2 vaccination in children previously infected for > 6 months compared to for 3 – 6 months.
In previously Omicron-infected children, ancestral strain-based vaccine could induce robust immune response against circulating sublineages of Omicron variant. Therefore, it could be used to vaccinate these children, especially during a time of limited access to bivalent vaccine. In this study, children primed with immunity against Omicron variant through infection, developed high neutralizing antibody levels against both BA.2.75 and BA.4/5 after a single dose of BNT162b2, the ancestral strain-based vaccine. Similarly, adults with previous SARS-CoV-2 infection vaccinated with BNT162b2 monovalent vaccine developed comparable neutralizing antibody levels to previously infected adults vaccinated with bivalent vaccine, containing mRNA encoding ancestral strain and BA.4/5 spike [25].
Omicron-based vaccine might be used to vaccinate COVID-naïve children, to improve the immune response to Omicron variant. The pivotal study of BNT162b2 in children of the same age group [6] reported that the neutralizing antibody titers against the Omicron BA.1 sublineage at 1 month after dose 3 of children 6 months to under 2 years and 2 to under 5 years of age were similar to that of adults 18 to 50 years of age, with the similar interval between dose 2 and dose 3 of approximately 3 months. Our study showed that, after completion of 3-dose BNT162b2 primary series, the GM of sVNT-BA.4/5 was 32.9% inhibition in COVID-naïve group, which might be similar to that of adults if vaccinated with the same interval. During our study period, 6 of 35 COVID-naïve children developed COVID-19 despite receiving 1 – 2 doses of vaccine. Two bivalent vaccines targeting Omicron variant were authorized under emergency use by the Food and Drug Administration in the United States: Moderna COVID-19 vaccine, bivalent (original and Omicron BA.4/BA.5) and Pfizer-BioNTech COVID-19 vaccine, bivalent (original and Omicron BA.4/BA.5), to use in primary vaccination series in children aged 6 months to under 5 years [26], [27]. Vaccination with BNT162b2 bivalent vaccine (Pfizer–BioNTech), containing 15 µg of mRNA encoding BA.4/5 and ancestral spike of SARS-CoV-2 each, was demonstrated to improve neutralizing antibody levels against circulating sublineages of Omicron variant compared to the monovalent vaccine in adults aged over 55 years without previous SARS-CoV-2 infection [25]. Bivalent vaccine boosters were shown to provide additional protection against symptomatic SARS-CoV-2 infection, with relative vaccine effectiveness of 28–56% in adults aged ≥ 18 years, compared to ≥ 2 doses of monovalent vaccine [28].
Our study had several strengths. We assessed immunogenicity of BNT162b2 against BA.2.75 and BA.4/5 sublineages of Omicron variant in children cohort, which was rarely reported, especially in children 6 months to under 5 years of age. Our limitations included the lack of cellular immunity assessment, data on immunity against XBB sublineages of Omicron variant, baseline pVNT-BA.2.75, and baseline pVNT-BA.4/5 in COVID-naïve group. The immunogenicity was assessed by pVNT, not microneutralization assay, however, these assays had been performed by our laboratory team [29], [30] and showed similar trends. Only healthy children were included in this study, so the results were not generalizable to other populations e.g., children with comorbidities. For real-world application, identifying COVID-naïve children might be difficult in asymptomatic or mildly symptomatic SARS-CoV-2 infection without the use of serologic tests. Due to short follow-up time after vaccination, the persistence of immunity was not evaluated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Thank you to all study teams for their assistance with the study.
- National Vaccine Institutes: Dr. Nakorn Premsri, Dr. Sunate Chuenkitmongkol, and Dr. Wisit Tangkeangsirisin for protocol scientific advice.
- Department of Diseases Control, Ministry of Public Health: Dr. Piyada Angsuwatcharakon, Piyanart Chuanark.
- Virology and Cell Technology Research Team, National Center for Genetic Engineering and Biotechnology (BIOTEC): Anan Jongkaewwattana, PhD, Jaraspim Narkpuk, Thorntun Deangphare, Kanjana Srisutthisamphan.
- Monoclonal Antibody Production and Application Research Team, National Center for Genetic Engineering and Biotechnology (BIOTEC): Kirana Yoohat, Channarong Seepiban.
- Center of Excellence for Pediatric Infectious Diseases and Vaccines, Department of Pediatrics, Faculty of Medicine, Chulalongkorn University: Tuangtip Theerawit, Thutsanun Meepuksom, Jitthiwa Athipunjapong, Pornpavee Nuncharoen, Rachaneekorn Nadsasarn, Jintana Intasan, Peeriya Prueksakaew, Watchara Sakares, Lucksanapon Pitikawinwong.
- The HIV Netherlands Australia Thailand Research Collaboration (HIV-NAT), The Thai Red Cross AIDS Research Centre: Suwat Wongmueng, Palida Pingthaisong, Thatri Iampornsin, Sukanya Janseeha, Dutmanee Thoungchomphunut, Thitiporn Somjit, Channuwat Baukor, Nutthida Phongam.
- Chula Clinical Research Center, Faculty of Medicine, Chulalongkorn University: Supapit Horpratum, Watchara Sakares, Ashara Semsri.
- English Editing: Dr. Sateesh Ganguli.
Funding
The study was funded by National Vaccine Institute, Thailand (2566.1/1). This research is supported by Ratchadapisek Somphot Fund for Postdoctoral Fellowship, Chulalongkorn University. Vaccines used in this study were supported by the Department of Diseases Control, Ministry of Public Health, Thailand.
Thai Clinical Trials Registry (thaiclinicaltrials.org): TCTR20220927003.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2023.100367.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern 2021 [Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed date February 26, 2023.
- 2.GISAID. Tracking of hCoV-19 Variants [Available from: https://gisaid.org/hcov19-variants/. Accessed date February 26, 2023.
- 3.Hodcroft EB. CoVariants: SARS-CoV-2 Mutations and Variants of Interest 2023 [Available from: https://covariants.org/. Accessed date February 26, 2023.
- 4.American Academy of Pediatrics and the Children’s Hospital Association. Children and COVID-19: State-Level Data Report 2023 [Available from: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/. Accessed date 5 March, 2023.
- 5.Clarke K.E.N., Kim Y., Jones J., Lee A., Deng Y., Nycz E., et al. Pediatric Infection-Induced SARS-CoV-2 Seroprevalence Increases and Seroprevalence by Type of Clinical Care September 2021 to February 2022. J Infect Dis. 2023;227:364–370. doi: 10.1093/infdis/jiac423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz F.M., Sher L.D., Sabharwal C., Gurtman A., Xu X., Kitchin N., et al. Evaluation of BNT162b2 Covid-19 vaccine in children younger than 5 years of age. N Engl J Med. 2023;388:621–634. doi: 10.1056/NEJMoa2211031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming-Dutra K.E., Wallace M., Moulia D.L., Twentyman E., Roper L.E., Hall E., et al. Interim Recommendations of the Advisory Committee on Immunization Practices for Use of Moderna and Pfizer-BioNTech COVID-19 Vaccines in Children Aged 6 Months-5 Years - United States, June 2022. MMWR Morb Mortal Wkly Rep. 2022;71:859–868. doi: 10.15585/mmwr.mm7126e2. [DOI] [PubMed] [Google Scholar]
- 8.Fleming-Dutra K.E., Ciesla A.A., Roper L.E., Smith Z.R., Miller J.D., Accorsi E.K., et al. Preliminary Estimates of Effectiveness of Monovalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection Among Children Aged 3–5 Years - Increasing Community Access to Testing Program, United States, July 2022-February 2023. MMWR Morb Mortal Wkly Rep. 2023;72:177–182. doi: 10.15585/mmwr.mm7207a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Royal College of Pediatricians of Thailand. Recommendation for COVID-19 Vaccines in Children and Adolescents, 12 September 2022 [Available from: https://www.thaipediatrics.org/?p=1761. Accessed date 14 March, 2023.
- 10.Stein C., Nassereldine H., Sorensen R.J.D., Amlag J.O., Bisignano C., Byrne S., et al. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401:833–842. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin D.Y., Gu Y., Xu Y., Zeng D., Wheeler B., Young H., et al. Effects of Vaccination and Previous Infection on Omicron Infections in Children. N Engl J Med. 2022 doi: 10.1056/NEJMc2209371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha N.K., Shrestha P., Burke P.C., Nowacki A.S., Terpeluk P., Gordon S.M. Coronavirus Disease 2019 Vaccine Boosting in Previously Infected or Vaccinated Individuals. Clin Infect Dis. 2022;75:2169–2177. doi: 10.1093/cid/ciac327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobrovitz N., Ware H., Ma X., Li Z., Hosseini R., Cao C., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023 doi: 10.1016/S1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaewborisuth C, Wanitchang A, Koonpaew S, Srisutthisamphan K, Saenboonrueng J, Im-Erbsin R, et al. Chimeric Virus-like Particle-Based COVID-19 Vaccine Confers Strong Protection against SARS-CoV-2 Viremia in K18-hACE2 Mice. Vaccines (Basel) 2022;10. https://doi.org/10.3390/vaccines10050786. [DOI] [PMC free article] [PubMed]
- 15.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 17.Nanthapisal S., Puthanakit T., Jaru-Ampornpan P., Nantanee R., Sodsai P., Himananto O., et al. A randomized clinical trial of a booster dose with low versus standard dose of AZD1222 in adult after 2 doses of inactivated vaccines. Vaccine. 2022;40:2551–2560. doi: 10.1016/j.vaccine.2022.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nantanee R., Aikphaibul P., Jaru-Ampornpan P., Sodsai P., Himananto O., Theerawit T., et al. Immunogenicity and reactogenicity after booster dose with AZD1222 via intradermal route among adult who had received CoronaVac. Vaccine. 2022;40:3320–3329. doi: 10.1016/j.vaccine.2022.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Disease Control (Ministry of Public Health Thailand). COVID-19 Vaccination Report on March 14, 2023 (Data as of March 10, 2023) [Available from: https://ddc.moph.go.th/vaccine-covid19/getFiles/10/1678949903705.pdf. Accessed date 6 April, 2023.
- 21.American Academy of Pediatrics. Children and COVID-19 Vaccinations Trends, AAP Analysis of Data Posted by the Centers for Disease Control and Prevention (as of March 1, 2023) [Available from: https://downloads.aap.org/AAP/PDF/Child%20Vaccinations%20Report%20US%20Cumulative%20and%20Weekly%203.1.2023.pdf?_ga=2.33362942.1554617421.1680770441-1531278721.1674300626. Accessed date 6 April, 2023.
- 22.Kitro A., Sirikul W., Dilokkhamaruk E., Sumitmoh G., Pasirayut S., Wongcharoen A., et al. COVID-19 vaccine hesitancy and influential factors among Thai parents and guardians to vaccinate their children. Vaccine X. 2022;11 doi: 10.1016/j.jvacx.2022.100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romer D., Winneg K.M., Jamieson P.E., Brensinger C., Jamieson K.H. Misinformation about vaccine safety and uptake of COVID-19 vaccines among adults and 5–11-year-olds in the United States. Vaccine. 2022;40:6463–6470. doi: 10.1016/j.vaccine.2022.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckner C.M., Kardava L., El Merhebi O., Narpala S.R., Serebryannyy L., Lin B.C., et al. Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses. Cell. 2022;185(4333–46):e14. doi: 10.1016/j.cell.2022.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou J., Kurhade C., Patel S., Kitchin N., Tompkins K., Cutler M., et al. Neutralization of BA.4-BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent Vaccine. N Engl J Med. 2023;388:854–857. doi: 10.1056/NEJMc2214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine Letter of Authorization (reissued) on April 28, 2023 [Available from: https://www.fda.gov/media/150386/download. Accessed date 13 July, 2023.
- 27.U.S. Food and Drug Administration. Moderna COVID-19 Vaccine Letter of Authorization (reissued) on April 18, 2023 [Available from: https://www.fda.gov/media/144636/download. Accessed date 13 July, 2023.
- 28.Link-Gelles R., Ciesla A.A., Fleming-Dutra K.E., Smith Z.R., Britton A., Wiegand R.E., et al. Effectiveness of Bivalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection - Increasing Community Access to Testing Program, United States, September-November 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1526–1530. doi: 10.15585/mmwr.mm7148e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muangnoicharoen S., Wiangcharoen R., Nanthapisal S., Kamolratakul S., Lawpoolsri S., Jongkaewwattana A., et al. Single Ad26.COV2.S booster dose following two doses of BBIBP-CorV vaccine against SARS-CoV-2 infection in adults: Day 28 results of a phase 1/2 open-label trial. Vaccine. 2023;41:4648–4657. doi: 10.1016/j.vaccine.2023.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niyomnaitham S., Jongkaewwattana A., Meesing A., Pinpathomrat N., Nanthapisal S., Hirankarn N., et al. Immunogenicity of a fractional or full third dose of AZD1222 vaccine or BNT162b2 messenger RNA vaccine after two doses of CoronaVac vaccines against the Delta and Omicron variants. Int J Infect Dis. 2023;129:19–31. doi: 10.1016/j.ijid.2023.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.