Abstract
This study investigated the appropriate way of dietary Acer truncatum leaves (ATL) addition, the effect of disease prevention and its mechanism of action. In experiment 1, 192 Arbor Acres broilers were assigned to 4 treatment groups, fed with basal diets containing 2% bran, replacing it with primary and fermented ATL, and additional 0.3% ATL extract to the basal diet for 42 d, respectively. In experiment 2, 144 broilers were assigned to 3 treatment groups for 21-d trial: (1) C-N group, basal diets, and injected with 0.9% (w/v) sterile saline; (2) C-L group, basal diets, and injected with lipopolysaccharide (LPS); (3) T-L group, ATL diets and injected with LPS. In experiment 1, ATL significantly decreased the index of abdominal fat at 42 d (P < 0.05). ATL extract had a better ability to improve antioxidant capacity and reduce inflammatory levels among all treatment groups, which significantly decreased the content of MDA in the liver and ileum mucosa at 21 d, and increased the expression of IL-10 and Occludin in jejunal mucosa at 42 d (P < 0.05). In experiment 2, ATL significantly increased the level of T-AOC in the liver, decreased the expression of NF-κB in the jejunal mucosa and ileum mucosa (P < 0.05), and restored LPS-induced the changed level of CAT in jejunal mucosa, the expression of IL-6, Claudin-1, and ZO-1 in jejunal mucosa and IL-1β in ileum mucosa (P < 0.05). Analysis of gut microbiota indicated that ATL enhanced the abundances of Bacteroidota and reduced the proportion of Firmicutes (P < 0.05), and the changed levels of T-AOC in body, IL-1β, IL-6, IL-10, and NF-κB in jejunum mucosa and propionic acid in cecal were associated with gut microbiota. Collectively, our data showed that the extract of ATL had a better antioxidant and anti-inflammatory effects than primality and fermented. Extraction of ATL modulated intestinal microbiota, and had a protective effect on oxidative stress, inflammation, and intestinal barrier function in broilers challenged with LPS.
Key words: Acer truncatum, antioxidant, lipopolysaccharide, inflammation, microbiota
BACKGROUND
The healthy and growth of broilers were challenged by multicomplex factors in intensive poultry farms, such as bad rearing equipment, excessive density, or uncomfortable temperature, which may induce the stress responses and inflammation, resulting in decreased performance and even death (Schulze Bernd et al., 2020; Li et al., 2021; Zhou et al., 2021). Oxidative stress processes are strongly interrelated with the immune system balance and inflammatory reaction. Antioxidants have the ability to neutralize the free radicals, protect cells from free radicals damage, and improve the immune responses of body (Bendich, 1993; Khadim and Al-Fartusie, 2021; Silvestre et al., 2022). Furthermore, the immune cell functions rely on reactive oxygen species (ROS) generation, but excessive reactive oxygen species can also cause damage to them, so the antioxidant have an important role in protecting immune cells against oxidative damages as well as maintaining their suitable function (Puertollano et al., 2011; Amir Aslani and Ghobadi, 2016). Researcher found that the immune system of broilers keeping constant development, especially before 34-day-old, which means birds cannot effectively adjust and maintain body homeostasis to face unpredictable stresses (Song et al., 2021). Therefore, exploitation and application of effective antioxidants is a potential way to prevent excessive immune response of poultry under unfavorable conditions (Mehrabadi et al., 2022).
Polyphenols are secondary metabolites of plants and the most abundant antioxidants in the diet. These compounds present different mechanisms to exert their antioxidant property, such as scavenging free radicals, reducing lipoperoxidation, and others (de Mello Andrade and Fasolo, 2014). Previous studies showed that polyphenols could alleviate oxidative damage and improve the antioxidant capacity (Raederstorff, 2009). Antioxidants such as polyphenols have been shown to modulate immune function (Carvalho et al., 2020; Wang et al., 2021). Polyphenols can modulate cytokines production and pro-inflammatory genes expression; it can also regulate inflammation by affect the NF-κB signaling pathway (Yahfoufi et al., 2018). Many studies have shown that polyphenols can alter gut microbiota composition, reduce the inflammatory response, and alleviate liver injury and colitis (Zhang et al., 2019; Hu et al., 2020; Sanjay et al., 2021; Zhao and Jiang, 2021).
Acer truncatum, a boreal deciduous tree in the Aceraceae family, which is rich in various organic compounds such as polyphenols and can use as a herbal medicine, is distributed mainly in northern China (Fan et al., 2022). Previous studies had found that Acer truncatum leaf (ATL) extracts have antioxidant, antitumor, and antibacterial activities (Zhao et al., 2006; Zhao et al., 2011; Yang et al., 2017; Fan et al., 2022). Dietary ATL addition improved the antioxidant indices of Taihang chicken (Wang et al., 2022). Fermented ATL is more beneficial to improve the growth performance, meat quality and the abundance of gut probiotics than unfermented leaves in finishing pigs (Zhang et al., 2018). Moreover, using ATL as a ruminant feed shows advantageous effect on the abundance of intestine beneficial bacteria and improves the resistance to diseases and survival rate of lamb (Liu et al., 2020). Lipopolysaccharide (LPS) is a component derived from the outer membrane of Gram-negative bacteria, it can induce a variety of pathophysiological effects on the host, such as immune response and tissue injury (Kong et al., 2022), and it is also the main cause of intestinal diseases (Tian et al., 2022). Based on these findings, this study aims to investigate the beneficial effect and antidisease ability of ATL and clarify its mechanism of action through broilers, and to explore the potential application value of ATL. In this experiment, the effects of dietary ATL addition and the optimal supplementation method in broilers were detected firstly, furthermore, we investigated the ability of ATL to alleviate the body inflammation caused by LPS and explored the role of microorganisms in this process.
METHODS
Ethics Statement
All of the animal studies we conducted were approved by the Institutional Animal Care and Use Committee of Northwest A&F University (Shaanxi, China). Permit Number: NWAFAC 1008.
Animal Management, Experimental Design, and Dietary Treatments
All the broilers were obtained from the Xianyang Dacheng Poultry Co., Ltd. (Xianyang, China). Chicks were reared in metal cages (70 cm-width × 95 cm-length × 40 cm-height), and the brooding temperature was maintained at 35°C for the first week and gradually decreased to 27°C in the third week. During the first week, the light cycle was 1-h of darkness, after that the cycle was 6 h in darkness. The feed formula and nutrient levels of the basic diet were shown in Table S1. Experimental diets in the control group were formulated according to the nutritional requirements of NRC (1994) for broilers. All birds had free access to feed and water.
Experiment 1
A total of 192 one-day-old Arbor Acres female commercial broilers were randomly assigned to 4 treatment groups, each group had 6 replicates and each replicate contained 8 birds. Broilers of 4 treatment groups were fed with corn–soybean meal basal diets with 2% bran (control, CON), 2% ATL of primary (PA), 2% ATL of fermented (FA), and added extra 0.3% ATL extract on the basis of control diet (EA), respectively. The nutrient composition of ATL is shown in Table S2. The experiment lasted for 42 d.
Experiment 2
The 144 female chicks were assigned to 3 treatment groups, randomly, and each group had 6 replicates and each replicate contained 8 birds. Broilers were treated with basal diets + 0.9% (w/v) sterile saline (C-N), basal diets + lipopolysaccharide (Escherichia coli) (C-L), and ATL extract addition diets + lipopolysaccharide (T-L), respectively. Intraperitoneal injections were performed at the age of 16 d, 18 d, and 20 d, and the dose of injection was 1 mg/kg body weight in broilers. The experiment lasted for 21 d.
Performance
In experiment 1, the body weight of broilers for each replicate were recorded at the age of 21 d and 42 d, and the feed consumption also be monitored. The performance variables including body weight (BW), average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR) were measured.
Blood and Tissue Sampling
On 21 and 42 d, one healthy bird with average body weight was selected from each replicate to collect blood and tissue sampling. Blood samples were collected from the wing vein into vacutainer tubes, clotted for approximately 2 h at 37°C, and then centrifuged at 3,600 × g for 10 min to obtain serums. Collected the liver sample and calculated the relative weight of the liver, spleen, thymus, bursa of Fabricius, and abdominal fat. The jejunum and ileum were separated and the intestinal contents were removed with precooling saline. Then the mucosa was scraped using a glass microscope slide. Parts of the liver and jejunum were taken for histomorphological examination, and other blood and tissue samples were stored at −80°C for further analysis.
Histomorphology
The collected liver and jejunum tissue of broilers were fixed in 10% neutral-buffered formalin for 24 h and then transferred to 70% ethanol. After dehydration and transparency, tissues were paraffin-embedded, then the paraffin blocks were cut into individual slides (5 μm / slide), and stained by hematoxylin and eosin. Slides were imaged using an Olympus BX 54/43 microscope with a DP80 Olympus camera (Olympus, Tokyo, Japan).
Determination of Antioxidant Capacity
The antioxidant indexes of the samples (serum, liver, jejunal mucosa, and ileum mucosa) which obtained from experiment 1 and experiment 2 both were measured. The total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), malondialdehyde (MDA), and catalase (CAT) were determined by biochemical assay kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All determination procedures and calculation formulas were carried out according to the manufacturer's instructions.
Gene Expression
Total RNA was extracted from the liver, jejunum mucosa and ileum mucosa using the TRIZOL reagent kit (Accurate Biology, Changsha, China). Then cDNA synthesis from 500 ng RNA was performed based on Evo M-MLV RT Premix for qPCR (Accurate Biology). Gene expression of inflammatory cytokines and tight junction protein were quantified by RT-PCR. The assay was carried out using SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biology) on a Roche-LightCycler 96 instrument (Basel, Switzerland). Genes expression was standardized by β-actin, and the method of 2–△△Ct was used to calculate the relative expression of each gene. Primer sequences used in the current study are listed in Table S3.
Cecal Microbiome
Cecal microbiome was detected in experiment 2. Microbial DNA was extracted from cecal digesta using the E.Z.N.A. Stool DNA Kit (Omega Bio-tek, Norcross, GA) according to the manufacturer's protocols. The V4-V5 region of the bacteria 16S ribosomal RNA gene was amplified by PCR using primers 515F 5′-barcode-GTGCCAGCMGCCGCGG)-3′ and 907R 5′-CCGTCAATTCMTTTRAGTTT-3′, where the barcode is an 8-base sequence unique to each sample. Sequences were clustered into operational taxonomic units (OTUs) at 100% similarity (identical) using the Deblur denoising algorithm, which removes noise due to sequencing error (Cole et al., 2009). Clusters of identical sequences allowed us to detect microbial changes at fine-scale resolution. The phylogenetic affiliation of each 16S rRNA gene sequence was analyzed by the uclust algorithm (http://www.drive5.com/usearch/manual/uclust_algo.html) against the silva (SSU138.1) 16S rRNA database using the confidence threshold of 80% (Caporaso et al., 2010). The principal coordinates analysis (PCoA) was employed to evaluate pairwise distances among samples and to establish β-diversity. Linear discriminant analysis (LDA) combined effect size measurements (LEfSe) and Kruskal–Wallis rank sum test were used to detect bacterial differences among groups. Functional contents of gut metagenome were predicted using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved State 2 (PICRUSt2). Spearman's correlation analysis was performed for the correlations between gut microbiota and other parameters.
Cecal Short-Chain Fatty Acids
GC-MS spectrometry was used to detect cecal short-chain fatty acid (SCFA) concentrations at experiment 2. First, 0.2 g cecal contents were homogenized in cold normal saline and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was obtained and mixed with metaphosphoric acid. After 4 h quiescence at 4°C, the mixture was centrifuged at 10,000 × g at 4°C for 15 min and crotonic acid was added to the supernatant. The supernatant is filtered through a 50 μm filter membrane into a meteorological bottle for GC-MS analysis. Parameters were set according to the reported method (Song et al., 2020). The acetic acid, propionic acid, isobutyric acid, butyric acid, and valeric acid peaks were measured and their concentrations were calculated using the ratio of the peak area with the standard solution.
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) procedure and differences were examined using Duncan's Multiple Range Test using SPSS 27.0 statistical software. Data were presented as mean with their pool standard error of the mean (SEM) and statistical significance was defined as a P value < 0.05.
RESULTS
Effect of Different Process ATL in Growth Performance and Antioxidant Capacity
In experiment 1 (Table 1), compared with CON, there were significantly decreased the ADG and BW of broilers during the experimental period in PA group (P < 0.05), and the ADG of broilers also decreased in FA group at 21 d (P < 0.05). The BW of broilers in EA group was statistically lower than CON group at 42 d (P < 0.05). Meanwhile, there were no significant differences in ADFI and FCR among groups throughout the trial period (P > 0.05). Furthermore, the organ indices and intestinal morphology were detected. The abdominal fat percentage was obviously decreased (P < 0.05) in treatment groups compared with CON at 42 d (Table S4). The jejunal villus height (VH), crypt depth (CD), and VH/CD ratio have no significant differences (Table S5, P > 0.05).
Table 1.
Effects of dietary Acer truncatum leaves adding on growth performance of broilers.1
| Items | CON | PA | FA | EA | SEM | P-value |
|---|---|---|---|---|---|---|
| 0–21 d | ||||||
| ADG (g) | 31.87a | 27.50c | 29.89b | 31.01ab | 0.46 | <0.001 |
| BW (g) | 701.16a | 602.09b | 659.40a | 682.00a | 10.49 | <0.001 |
| ADFI (g) | 51.88 | 46.56 | 47.31 | 50.72 | 0.81 | 0.052 |
| FCR | 1.62 | 1.69 | 1.58 | 1.64 | 0.02 | 0.358 |
| 0–42 d | ||||||
| ADG (g) | 61.36a | 53.05b | 57.64a | 57.65a | 0.87 | 0.004 |
| BW (kg) | 2.55a | 2.18c | 2.39ab | 2.33bc | 0.03 | 0.006 |
| ADFI (g) | 114.63 | 112.33 | 102.92 | 105.19 | 3.50 | 0.507 |
| FCR | 1.99 | 2.23 | 1.89 | 1.98 | 0.06 | 0.122 |
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; ATL, Acer truncatum leaves; BW, body weight; FCR, feed conversion ratio; SEM, pooled standard error.
Means in the same row without common superscript differ significantly (P < 0.05).
Treatments: CON, control; PA, primary ATL feed; FA, fermented ATL feed; EA, dietary ATL extractive added.
For antioxidant capacity, in serum (Figures 1A and 1E), the content of MDA was significantly increased in PA and FA groups at 42 d (P < 0.05). In the liver (Figures 1B and 1F), the level of MDA significantly decreased in FA and EA group than CON at 21d (P < 0.05). In jejunal mucosa (Figures 1C and 1G), the contents of T-AOC significantly increased in EA group at 21 d (P < 0.05). All ATL treatment groups significantly decreased the level of MDA than CON at 42 d (P < 0.05). In ileum mucosa (Figures 1D and 1H), the content of MDA was significantly decreased in PA and EA groups than CON at 21 d (P < 0.05). The contents of T-AOC and MDA in FA group were higher than PA group (P < 0.05), but it had no significant difference (P > 0.05) than CON at 21 d. These results indicated that the EA group had a better antioxidant level among 3 treatment groups.
Figure 1.
Effects of different Acer truncatum leaves treatments dietary adding on the antioxidant capacity in broilers. Effects of Acer truncatum treatments on the antioxidant capacity of serum, liver, jejunal mucosa, and ileal mucosa, including T-AOC (A–D) and MDA (E–H), respectively. Column charts not sharing a common lowercase letter differ significantly (P < 0.05). Treatments: CON, control; PA, primary ATL feed; FA, fermented ATL feed; EA, dietary ATL extractive added. Abbreviations: ATL, Acer truncatum leaves; SEM, pooled standard error.
Effect of Different Process ATL in the mRNA Expression of Inflammation Factor and Tight Junction Proteins
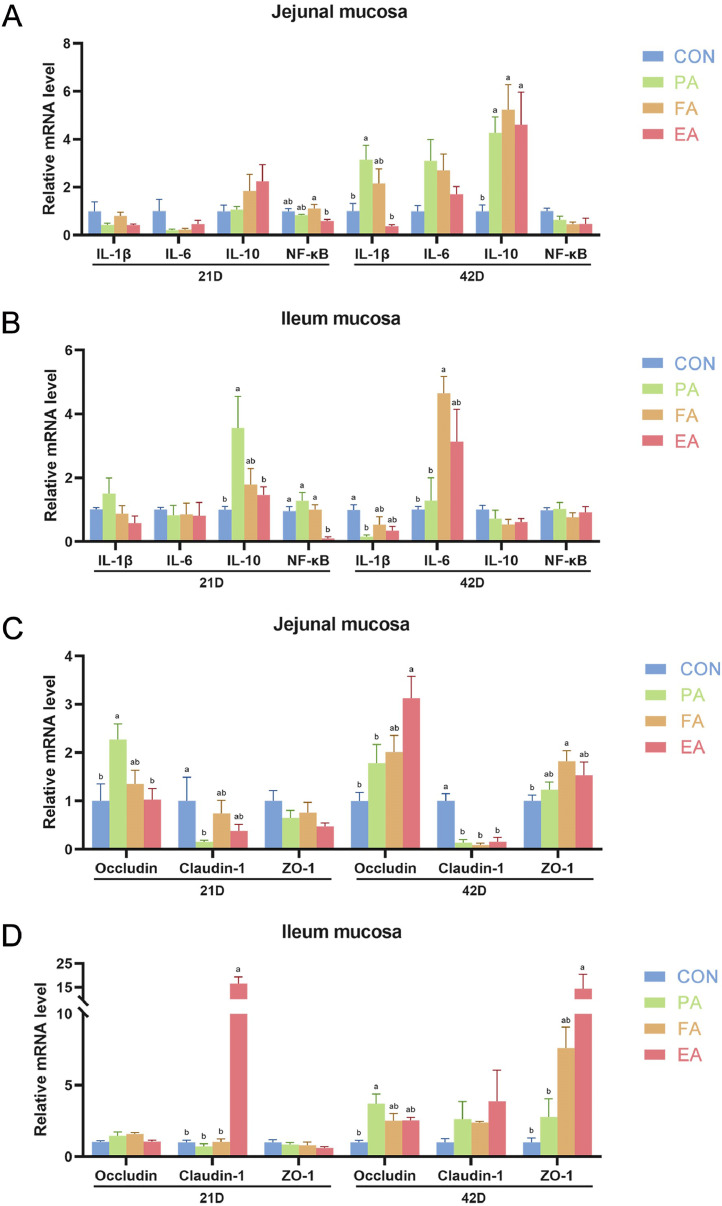
In experiment 1, in jejunum mucosa (Figure 2A), dietary EA addition has a tendency to decrease (P > 0.05) the relative expression of NF-κB at 21 d, and the relative expression of IL-1β was significantly upregulated (P < 0.05) in PA group at 42 d compared with CON. The relative expression of IL-10 was significantly upregulated (P < 0.05) in 3 ATL treatment groups at 42 d. In ileum mucosa (Figure 2B), compared with CON, dietary PA upregulated the relative expression of IL-10 at 21 d, and reduced IL-1β at 42 d, significantly (P < 0.05). Dietary FA addition significantly upregulated the level of IL-6 at 42 d. Dietary EA significantly decreased the expression of NF-κB than other groups at 21 d (P < 0.05). Thus, EA group had a better anti-inflammatory ability among treatment groups.
Figure 2.
Effects of different Acer truncatum leaves treatments dietary adding on the relative expression of inflammatory factors and tight junction proteins in broilers. The expression of inflammatory factors in jejunal mucosa (A) and ileum mucosa (B) in 21 d (left) and 42 d (right). The expression of tight junction proteins in jejunal mucosa (C) and ileum mucosa (D) in 21 d (left) and 42 d (right). Column charts not sharing a common lowercase letter differ significantly (P < 0.05). Treatments: CON, control; PA, primary ATL feed; FA, fermented ATL feed; EA, dietary ATL extractive added. Abbreviations: ATL, Acer truncatum leaves; SEM, pooled standard error.
Furthermore, the effects of different ATL treatments on intestinal barrier function were further compared. In jejunum mucosa (Figure 2C), compared with CON, dietary PA significantly upregulated the relative expression of Occludin and reduced the level of Claudin-1 at 21 d (P < 0.05). At 42 d, the level of Occludin was markedly increased in EA group and ZO-1 in FA group than CON (P < 0.05), and the relative expression of Claudin-1 significantly reduced in ATL treatment groups (P < 0.05). In ileum mucosa (Figure 2D), compared with CON, the level of Occludin was increased in PA group at 42 d, and the relative expression of Claudin-1 at 21 d and ZO-1 at 42 d were upregulated in EA group (P < 0.05). In conclusion, ATL extract produced the best prebiotic effects in broilers among treatment groups in experiment 1.
Effect of ATL on the Organ Damage and Intestinal Damage Caused by LPS
In experiment 2, the effects of ATL on oxidative stress caused by LPS were detected. The index of the spleen was significantly increased in 2 LPS-induced groups of broilers (P < 0.05), and ATL had a tendency (P > 0.05) to restore the decreased index of the bursa of Fabricius by LPS (Table S6). The antioxidant capacity indices in serum were no significant among treatment groups (Table 2, P > 0.05). For the liver, the assessment of liver morphology indicated that ATL alleviated the injury by LPS. The number of inflammatory cells reduced in the liver of T-L group, but eosinophilic particles were still abundant (Figure S1). ATL significantly raised the level of T-AOC in broilers of the liver (Table 2, P < 0.05). The results of inflammatory factors in the liver of broilers showed that LPS significantly increased the relative expression of IL-1β and IL-10 (Figure 3A, P < 0.05), and ATL has a tendency to recover the IL-10 expression but it not significantly (P > 0.05).
Table 2.
Effect of dietary Acer truncatum leaves addition on antioxidant capacity in lipopolysaccharide-challenge broilers.1
| Items | C-N | C-L | T-L | SEM | P-value |
|---|---|---|---|---|---|
| Serum | |||||
| T-AOC (mM) | 0.93 | 0.94 | 0.77 | 0.05 | 0.295 |
| CAT (U/mL) | 8.10 | 5.87 | 5.99 | 0.46 | 0.068 |
| MDA (nmol/mL) | 2.12 | 2.85 | 2.10 | 0.17 | 0.122 |
| Liver | |||||
| T-AOC (mM) | 0.68b | 0.69b | 0.98a | 0.04 | <0.001 |
| CAT (U/mgport) | 0.89 | 0.88 | 0.85 | 0.03 | 0.815 |
| MDA (nmol/mgport) | 0.86 | 1.03 | 0.82 | 0.05 | 0.277 |
| Jejunal mucosa | |||||
| T-AOC (mM) | 0.43 | 0.59 | 0.54 | 0.05 | 0.327 |
| CAT (U/mgport) | 13.05ab | 7.75b | 17.26a | 1.49 | 0.013 |
| T-SOD (nmol/mgport) | 41.53b | 70.20a | 61.47a | 3.33 | 0.009 |
| MDA (nmol/mgport) | 2.02 | 2.47 | 2.28 | 0.15 | 0.495 |
| Ileum mucosa | |||||
| T-AOC (mM) | 0.65 | 0.68 | 0.71 | 0.05 | 0.887 |
| CAT (U/mgport) | 24.52 | 28.90 | 31.58 | 3.15 | 0.689 |
| T-SOD (nmol/mgport) | 95.04 | 71.49 | 98.22 | 6.42 | 0.205 |
| MDA (nmol/mgport) | 5.76 | 7.37 | 5.30 | 0.51 | 0.259 |
Abbreviations: ATL, Acer truncatum leaves; CAT, catalase; LPS, lipopolysaccharide; MDA, malondialdehyde; SEM, pooled standard error; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase.
Means in the same row without common superscript differ significantly (P < 0.05).
Treatments: C-N, basal diet and intraperitoneally injected with saline; C-L, basal diet and intraperitoneally injected with LPS; T-L, ATL addition diet and intraperitoneally injected with LPS.
Figure 3.
The expression of inflammatory factors and tight junction protein in the liver, jejunum mucosa and ileum mucosa in broilers challenged with lipopolysaccharide. Effects of LPS and ATL on the expression of inflammatory in the liver (A), jejunal mucosa (B), and ileum mucosa (C). Effects of LPS and ATL on the expression of tight junction protein in jejunal mucosa (D) and ileum mucosa (E). a,bValues at the same index with no common superscripts differ significantly (P < 0.05). Treatments: C-N, basal diet and intraperitoneally injected with saline; C-L, basal diet and intraperitoneally injected with LPS; T-L, ATL addition diet and intraperitoneally injected with LPS. Abbreviations: ATL, Acer truncatum leaves; LPS, lipopolysaccharide.
For the intestine, the VH, CD, and VH/CD ratio in jejunum villi have no significant differences among treatments of broilers (Table S7, P > 0.05). ATL restored LPS-induced the changed level of CAT in jejunal mucosa (Table 2, P < 0.05), and T-SOD and MDA in ileum mucosa also had a similar tend (P > 0.05). The expression of IL-6 in jejunum mucosa and IL-1β in ileum mucosa significantly increased in the C-L group of broilers but alleviated in the T-L group (Figure 3B, C, P < 0.05). The expression of NF-κB in the jejunum mucosa and ileum mucosa decreased in T-L group of broilers significantly (Figures 3B and 3C, P < 0.05). For tight junction proteins expression, Figure 3D showed that ATL restored the changed expression of Claudin-1 and ZO-1 by LPS-induced (P < 0.05), and alleviated the decreased expression of Occludin in jejunal mucosa (P > 0.05). There was no significant difference in ileum mucosa among all treatment groups (Figure 3E, P > 0.05).
Effects of ATL on Cecum Microbiota Diversity and Function
In experiment 2, we analyzed the effect of LPS and ATL on the composition and abundance of the intestinal microbiota. The Shannon index which was used to assess the cecal microbiota α-diversity of broilers was significantly decreased in T-L than C-N group (Figure 4A, P < 0.05). The results of PCoA in β-diversity analysis (Figure 4B) indicated that there were structural differences in the intestinal microbiota among 3 groups. At the level of the bacterial phylum, we found that the abundance of Firmicutes was reduced and Bacteroidota was increased in the T-L group of broilers significantly (Figures 4C and 4D, P < 0.05). The results of LEfSe analysis in the cecal microbiota of broilers showed that 14 taxa were enriched in the C-N group, 12 taxa were enriched in the T-L group, and only 4 taxa were enriched in the C-L group (Figure 4E).
Figure 4.
Microbial diversity analysis of broilers dietary Acer truncatum supplementation and challenged with lipopolysaccharide. (A) α-diversity of the intestinal microbiota; (B) principal coordinate analysis (PCoA) diagram showing the β-diversity of intestinal microbiota among the 3 groups; (C) relative abundance of intestinal microbiota constituents at the phylum level; (D) relative abundance of Bacteroidota and Firmicutes among treatment groups; and (E) analysis of the differences in the intestinal microbiota by LEfSe (LDA > 3, P < 0.05). a,bValues at the same index with no common superscripts differ significantly (P < 0.05). Treatments: C-N, basal diet and intraperitoneally injected with saline; C-L, basal diet and intraperitoneally injected with LPS; T-L, ATL addition diet and intraperitoneally injected with LPS. Abbreviations: ATL, Acer truncatum leaves; LPS, lipopolysaccharide.
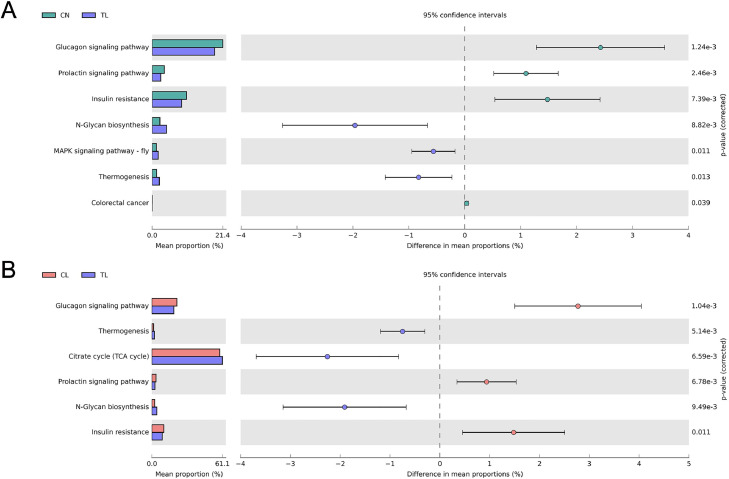
Based on KEGG database, PICRUSt2 revealed that the function of colonic microbiota was changed significantly after supplementing the ATL. The results of STAMP analysis showed that in KEGG level 3, the cecal microbiota of broilers increased the numbers of functional genes including N-Glycan biosynthesis, MAPK signaling pathway - fly, thermogenesis and citrate cycle (TCA cycle), and decreased the functional genes of glucagon signaling pathway, prolactin signaling pathway, insulin resistance and colorectal cancer in T-L group (Figure 5). In all KEGG levels, the results of function analysis by bar chart constitute, PCA and multiple comparisons were shown as Figure S2.
Figure 5.
Comparison of predicted metabolic pathway abundances among the groups by statistical analysis of taxonomic and functional profiles (STAMP) at level 3. (A) C-N vs. T-L; (B) C-L vs. T-L. Treatments: C-N, basal diet and intraperitoneally injected with saline; C-L, basal diet and intraperitoneally injected with LPS; T-L, ATL addition diet and intraperitoneally injected with LPS. Abbreviations: ATL, Acer truncatum leaves; LPS, lipopolysaccharide.
Correlations Between Intestinal Microbiota and Phenotypes
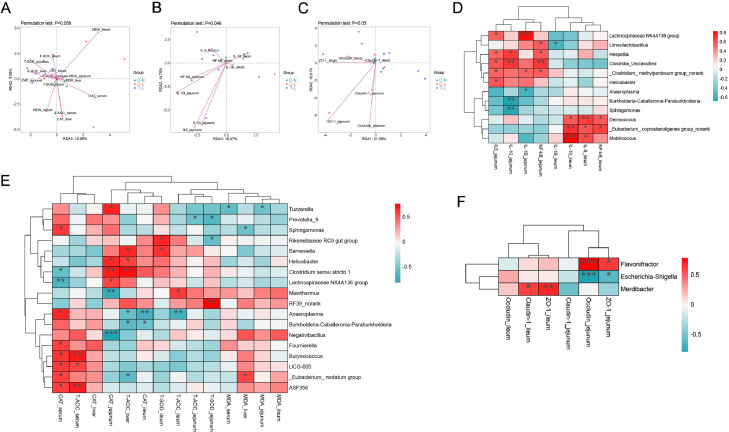
The correlations between the intestinal microbiota and phenotypes were analyzed to evaluate the role of microbiota generation. The result of redundancy analysis showed that the microbiota of broilers was positively related to indicators of T-AOC in T-L group, and was positively related to MDA in C-L group (Figure 6A, P = 0.058). The expression of IL-1β, IL-6, IL-10, and NF-κB in jejunum mucosa of broilers were positively related to the microbiota in T-L group, and the expression of IL-10, Occludin, and ZO-1 in ileum mucosa were positively related to the microbiota in C-L group (Figures 6B and 6C, P = 0.05). The associations among the intestinal microbiota and antioxidant capacity, inflammatory factors and tight junction proteins were further analyzed at the genus level. Heatmap showed that the levels of antioxidant capacity were positively linked to the genera Barnesiella, Helicobater, Clostridium sensu stricto 1, RF39_norank, Butyricicoccus, UCG_005 and ASF356, and negatively correlated with Tuzzerella, Prevotella_9, Meiothermus, Anaeroplasma, Burkholderia–Caballeronia–Paraburkholderia, and Negativibacillus (Figure 6D). The levels of inflammation were positively linked to the genera Hespellia, Clostridia_Unclassified, Anaeroplasma, Deinococcus, [Eubacterium] coprostanoligenes group_norank, and Mobilicoccus, but they were negatively correlated with Limosilactobacillus, Burkholderia–Caballeronia–Paraburkholderia, and Sphingomonas (Figure 6E). The levels of tight junction proteins were positively linked to the genera Flavonifractor and Merdibacter, but they were negatively correlated with Escherichia–Shigella (Figure 6F).
Figure 6.
Correlation analysis between the abundances of intestinal microbiota and phenotypes. The correlation by redundancy analysis between samples distribution and antioxidant capacity (A), inflammatory factors (B), and tight junction proteins (C) at the genus level. Spearman's correlation analysis of the relationship of the intestinal microbiota with antioxidant capacity (D), inflammatory factors (E), and tight junction proteins (F). a,bValues at the same index with no common superscripts differ significantly (P < 0.05). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Treatments: C-N, basal diet and intraperitoneally injected with saline; C-L, basal diet and intraperitoneally injected with LPS; T-L, ATL addition diet and intraperitoneally injected with LPS. Abbreviations: ATL, Acer truncatum leaves; LPS, lipopolysaccharide.
Cecum SCFAs Profiles
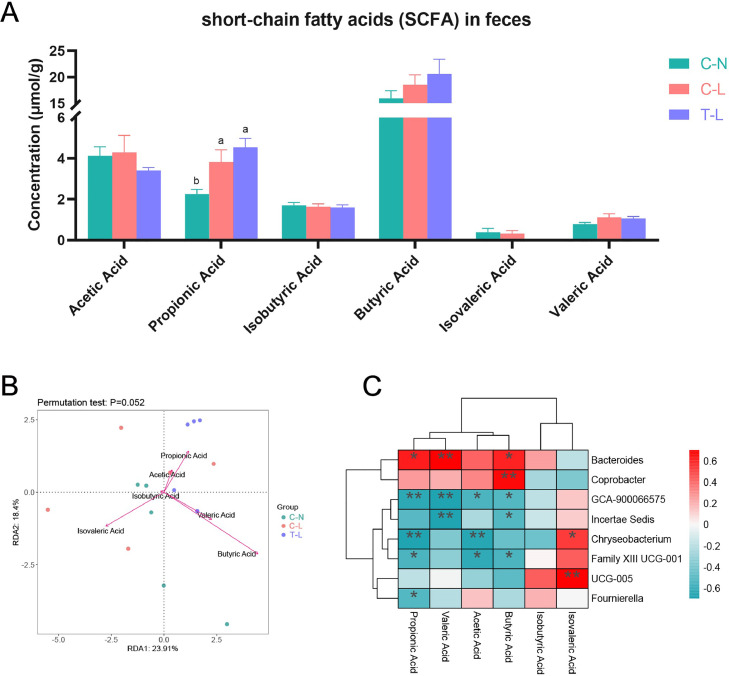
Figure 7A showed that the concentration of propionic acid was significantly increased in broilers of LPS treatment groups than C-N group (P < 0.05). However, there was no difference in the concentrations of acetic acid, isobutyric acid, butyric acid, and valeric acid (P > 0.05). The result of redundancy analysis showed that the concentrations of cecal butyric acid, propionic acid, valeric acid, and acetic acid were positively related to the microbiota related by ATL in broilers (Figure 7B, P = 0.052). The relationship heatmap between cecal SCFAs and microbiota at genus level showed that the levels were positively linked to the genera Bacteroides and Coprobacter, but they were negatively correlated with GCA-900066575, Incertae Sedis, Chryseobacterium, and Family XIII UCG-001 (Figure 7C).
Figure 7.
Cecal SCFAs content and correlation analysis with intestinal microbiota of broilers dietary Acer truncatum leaves supplementation and challenged with lipopolysaccharide. (A) The content of SCFAs in feces. (B) The correlation by redundancy analysis between samples distribution and SCFAs at the genus level. (C) Spearman's correlation analysis of the relationship of the intestinal microbiota with SCFAs. a,bValues at the same index with no common superscripts differ significantly (P < 0.05). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Treatments: C-N, basal diet and intraperitoneally injected with saline; C-L, basal diet and intraperitoneally injected with LPS; T-L, ATL addition diet and intraperitoneally injected with LPS. Abbreviations: ATL, Acer truncatum leaves; LPS, lipopolysaccharide.
DISCUSSION
ATL are rich in polyphenols and have a strong antioxidant capacity (Yang et al., 2017). Studies have shown that dietary ATL addition can improve the plasma antioxidant capacity, reduce the feed-to-egg ratio and alleviate liver damage in Taihang chicken (Wang et al., 2022). Fermented ATL can enhance the growth performance, and improve the serum biochemical indicators and the immune capacity of piglets (Liu, 2021). Broiler chickens are susceptible to the inflammation and oxidative stress caused by the environment because of the imperfect immune system and the weak antioxidant capacity during the growth and development (Song et al., 2021). Our research showed that dietary ATL increased broilers’ antioxidant capacity, which is consistent with previous studies. In experiment 1, ATL has the ability to promote antioxidants at 21 d but the effect receded at 42 d, which may be due to the development of the gastrointestinal tract and maturation of the intestinal mucosa affecting the function of ATL to broilers (Ravindran and Abdollahi, 2021). Dietary ATL addition may improve the antioxidant capacity by making up for the lack during the development of immune organs.
The different processing methods of ATL can affect the growth performance and antioxidant capacity of broilers. There is plenty of evidence that fermentation can increase the content of protein and amino acid; reduce the content of fiber, mycotoxin, and antinutritional factor; enhance the nutritional value and feed digestibility; and then improve the growth performance (Sugiharto and Ranjitkar, 2019; Xu et al., 2019). Our findings suggest that adding fermentation ATL in the diets had a tendency to improve growth performance of broilers. The extract of ATL are rich in tannin, chlorogenic acid and flavonoids, and have a splendid antioxidant capacity (Zhao et al., 2006; Yang et al., 2017; Yang et al., 2018). We found that dietary ATL extract addition had the highest antioxidant capacity of broilers compared with other treatment groups. Pellet size and feed form can also affect the growth performance of broilers, with crushed feed resulting in lower body weight and higher feed ratios compared to pellet feed (Chewning et al., 2012). In the study, based on the amount of feed are too small to be pelleted, so the crushed feed was used to feed the broilers throughout the experiment. Therefore, we hypothesize that the lower body weight and higher feed ratio of broilers in this experiment may be due to the feeding of crushed feed. Previous studies have shown that the antinutritional factors contained in leaf meals have a dual effect on the growth performance of broilers, which can promote health at the appropriate dose but have a negative effect on the use of nutrients when it is at high levels (Sugiharto et al., 2019). In the experiment, the growth performance of broilers in the EA group decreased at 42 d, which may be a cumulative result of the higher concentration of antinutritional factors, so a lower dose should be considered when using ATL extract.
Lipopolysaccharide can induce acute bursal atrophy in broilers (Ansari et al., 2017). Present study found that ATL extract alleviated the decreased tend of the bursal index by LPS challenge. Ajuwon et al. (2014) showed that polyphenols can recover acute liver injury caused by LPS. Previous study showed that LPS challenge led to liver damage and decreased the organ indices in chickens (Ye et al., 2022). In this study, HE staining showed that ATL extract recovered the liver injured by LPS induced, but the organ index was no significant difference among all groups, which similar to the outcomes from previous finding (Yang et al., 2021). Numerous studies have demonstrated that LPS causes hypertrophied spleen, it may have occurred because the LPS-induced immune response increased pro-inflammatory cytokine production and simultaneously recruited inflammatory cells to the spleen (Ahiwe et al., 2019; Yang et al., 2019; Bi et al., 2022). But in this study, ATL extract did not have a significant alleviating effect on LPS-induced spleen hyperplasia. Further testing is needed to assess spleen damage.
Previous studies revealed that ATL extract have antiobesity ability, and can inhibit fatty acid synthase activity and reduce the number of fat cells (Zhao et al., 2006, 2011). In our study, we found that different ways of ATL treatment all significantly reduced the 42 d abdominal fat rate of broilers, but there were no significant differences at 21 d. The growth of abdominal fat in broilers presents an S-shaped curve, which is slower in the early stage and faster in the later stage, and the relative growth coefficient of abdominal fat weight is the lowest in 14 to 21 d (Niu et al., 2021). Based on previous research, we thought that it was possibly related to the growth and development stage of broilers, and early lower fat accumulation rate led to no significant changes among groups.
Polyphenols, which are abundant in plants, can scavenge ROS and increase antioxidant capacity. It has been well documented that dietary addition polyphenols can improve antioxidant capacity and immunity levels in broilers, such as pterostilbene, mountain celery, grape seed extract, oridonin, and others which are rich in polyphenol (Zheng et al., 2016; Farahat et al., 2017; Ahmadipour et al., 2018; Chen et al., 2020). ATL are rich in polyphenols and have highly antioxidant capacity (Fan et al., 2022). Our study demonstrated that dietary ATL extract supplementation improved the antioxidant capacity at 21 d and partially restored the LPS-induced the decrease of antioxidant levels in broilers. This indicates that ATL extract may have the application value of preventing the disease of broilers.
Expression of inflammatory cytokines plays an important role in the immune response. IL-1β can exacerbate damage during chronic disease and acute tissue injuries, IL-6 has a pleiotropic effect on inflammation and immune response, IL-10 is a cytokine with potent anti-inflammatory properties (Lopez-Castejon and Brough, 2011; Iyer and Cheng, 2012; Tanaka et al., 2014). The transcription factor NF-κB induces the expression of various pro-inflammatory genes and serves as a pivotal mediator of inflammatory responses (Yahfoufi et al., 2018). The results of experiment 1 showed that ATL reduced the expression of IL-1β and NF-κB, and increased the level of IL-10 in the intestine mucosa of broilers, which indicates that it is feasible to alleviate the inflammatory reaction of broilers by adding ATL to the diet. LPS induces immune and oxidative stress by stimulating the secretion of several cytokines. Liu et al. (2017) and Candelli et al. (2021) shown that LPS can activate NF-κB signaling, induce IL-1β expression and initiate different inflammatory responses, and can activate the pro-inflammatory response of NF-κB pathway in macrophages and increase the levels of IL-1β and IL-6 in blood and tissues. Blocking NF-κB can reduce the inflammatory response (Wang et al., 2018; Hu et al., 2021; Shen et al., 2021). In this study, dietary ATL extract supplementation decreased the NF-κB level, and recovered the upregulated IL-1β and IL-6 expression by LPS-induced in the intestinal mucosa of broilers. ATL are rich in polyphenols with main quercetin and gallic acid, and the contents were reported to be 8.9 mg/g and 30.6 mg/g of primal leaves, respectively (Yang, 2019; Fan et al., 2022). Quercetin can scavenge free radicals as an antioxidant in vitro, and it also can reduce the early inflammatory response by reducing the levels of IL-1β, IL-6 and the expression of NF-κB in the gastrointestinal tract (Slimestad et al., 2007; Carullo et al., 2017). Gallic acid refrained the expression of pro-inflammatory cytokines IL-1/6, TGF-β and TNF-α, as well stimulated the release of anti-inflammatory cytokines IL-4/10 by inhibiting the IκB/NF-κB pathway, and then relieved enteritis (Bai et al., 2021). Thus, we inferred that the inhibitory effect of ATL extract on inflammatory response may be caused by the inhibition of NF-κB signaling pathway by polyphenols such as quercetin and gallic acid.
In the intestinal epithelial cells, tight junction proteins play an important role in the barrier function of intestinal mucosa, and the damage of these proteins will lead to an increase in cell-to-cell permeability (Chasiotis et al., 2012). Carboxy-termini of Occludin or Claudin, which are localized in the cytoplasm, are associated with ZO-1 and are required to maintain tight junction functions (Morita et al., 2004). According to the results of experiment 1, dietary ATL supplemented especially increased the expression of Occludin and ZO-1 in jejunum and ileum mucosa at 42 d, which implies an improvement in the intestinal mucosal barrier function. Previous studies have shown that intraperitoneal injection of LPS can damage intestinal barrier and reduce the expression of Occludin, Claudin-1, and ZO-1 in intestinal epithelial cells (Xie et al., 2021; Tian et al., 2022). In this study, we found that dietary ATL extract supplementation restored the level of tight junction proteins changed by LPS, suggested it contributes to the maintenance of intestinal barrier function in broilers. Similar to our data, Xu et al. (2020) reported that polyphenols restore the reduced level of tight junction proteins induced by LPS.
The gut microbiota, the largest symbiotic ecosystem with the host, can affect most physiological functions by altering the immune and metabolic levels, and plays an important role in maintaining homeostasis (Shreiner et al., 2015; Shi et al., 2017). In the present study of experiment 2, LPS injection caused the change of structure, and decreased alpha diversity in cecal microbiome, which is consistent with the results of previous studies. Polyphenols have poor bioavailability and only a small fraction (5%–10%) can be directly absorbed by the body, most polyphenols (90%–95%) are degraded by microorganisms after entering the colon to production of biological activity by simpler phenolic compounds (Rowland et al., 2018; Rodríguez-Daza et al., 2021). Previous studies suggested that polyphenols can alter the composition and diversity of gut microbiota by its prebiotic activities, enhance beneficial bacteria, and inhibit pathogenic ones (Li et al., 2020; Yang et al., 2020). In the present study, dietary ATL extract changed gut microbial structure; the results of RDA showed that the microbiome of broilers in T-L group had a strong positive correlation with the levels of antioxidant and inflammatory factors, which demonstrated that the effects may produce mainly through the gut microbiota.
The community of bacteria inhabiting the animal gastrointestinal tract provide unique metabolic functions to the host and are fundamentally important in health and disease (Valdes et al., 2018). Studies had demonstrated that the abundance of Barnesiella in the colon correlate with several immunoregulatory cells and enhance cognate anticancer immune response (Presley et al., 2010; Daillère et al., 2016). Lachnospiraceae NK4A136 group has been found that it enriched in the resveratrol-induced gut microbiota of mice, which reduces the obesity induced by high-fat diet (Wang et al., 2020). In experiment 2, Barnesiella and Lachnospiraceae NK4A136 group were significantly enriched in broilers of the T-L group, its abdominal fat rate tended to decrease, and it positively correlated with liver T-AOC and ileum T-SOD levels. Prevotella_9 has been shown to be associated with the production of inflammatory cytokines, and it negatively affects the intestinal health of piglets (Hu et al., 2020). Female mice fed a diet deficient in n-3 PUFA during gestation resulted in higher abundances of Anaeroplasma with a reduced content of cecal SCFAs production in their offspring (Robertson et al., 2017). The present study was shown that Prevotella_9 and Anaeroplasma were all enriched in broilers of the C-N group and correlated with indicators which impair body and intestinal health. These results may support that ATL can inhibit the colonization of harmful bacteria in the gut of broilers and then promote health.
Short-chain fatty acids are the primary end-products of fermentation of nondigestible carbohydrates (NDC) that become available to the gut microbiota (Morrison and Preston, 2016), and play an important role in the maintenance of health and the development of disease (Tan et al., 2014). SCFAs can exert complex interactions with colonic epithelium and immune cells to protect intestinal health (Sivaprakasam et al., 2016). The present study showed that LPS increased the contents of propionic acid and butyric acid in the cecum, which may be produced by the process of alleviating intestinal inflammation by the host and microbial. The highest cecal SCFAs levels were observed in broilers of the T-L group, and it increased the abundance of SCFA-producing microbe. This result is consistent with the previous research that polyphenols can promote the colonization of probiotics and the production of SCFAs, and then alleviate intestinal inflammation (Yang et al., 2020; Zhao and Jiang, 2021).
The shifts of gut microbiota, especially those with special functions, will inevitably change the functional profiles of the whole gut microbiota (Modi et al., 2014). Polyphenols can block TNF-α release by modulating MAPK pathway at different levels of the signaling pathway (Yahfoufi et al., 2018). Green tea polyphenols can enhance energy conversion by boost gut-microbiota-dependent mitochondrial TCA and urea cycles in rats (Zhou et al., 2020). N-glycosylation has many biological functions such as protein folding, trafficking, and signal transduction, and is involved in numerous physiological and pathological processes (Hirata and Kizuka, 2021). In this study, dietary ATL extract treatment but not LPS resulted in significant changes in the function of gut microbiota. It enhanced the relative abundance of MAPK signaling pathway, TCA cycle and N-glycosylation, and inhibited disease-related genes in broilers. A variety of metabolites produced by the interaction between polyphenols and gut microbes can protect the gastrointestinal tract from diseases (Chiu et al., 2021). Combined with these studies, we indicated that dietary ATL extract has biological activity, which can improve the structure of intestinal microbiota and promote body health of broilers. Therefore, dietary ATL addition was able to increase early antioxidant levels and alleviate LPS-induced inflammatory responses in broilers, and the production of these effects may be related to changes in the intestinal microbiota.
CONCLUSION
In conclusion, this study demonstrates that dietary supplement with ATL fermented products or extracts improved antioxidant levels and reduced intestinal inflammation, and extractive had the better effect at 21 d. Dietary ATL extract supplementation changed the structure and function of gut microbiota, and ameliorated immunological stress and tissue injury in broilers by suppressing inflammation and oxidative stress. ATL upregulated the abundance of Bacteroidota and downregulated the abundance of Firmicutes, promote the function of transport and catabolism pathways, and reduced the gene related to disease.
ACKNOWLEDGMENTS
This research was supported by the Program for Shaanxi Science and Technology (2019ZDXM3-02), and the Construction Project of Shaanxi Feed Forage Industry Technology System (NYKJ-2022-YL(XN)33).
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102951.
Appendix. Supplementary materials
REFERENCES
- Ahiwe E.U., Abdallh M.E., Chang'a E.P., Al-Qahtani M., Omede A.A., Graham H., Iji P.A. Influence of autolyzed whole yeast and yeast components on broiler chickens challenged with Salmonella lipopolysaccharide. Poult. Sci. 2019;98:7129–7138. doi: 10.3382/ps/pez452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadipour B., Hassanpour H., Khajali F. Evaluation of hepatic lipogenesis and antioxidant status of broiler chickens fed mountain celery. BMC Vet. Res. 2018;14:234. doi: 10.1186/s12917-018-1561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajuwon O.R., Oguntibeju O.O., Marnewick J.L. Amelioration of lipopolysaccharide-induced liver injury by aqueous rooibos (Aspalathus linearis) extract via inhibition of pro-inflammatory cytokines and oxidative stress. BMC Complement. Altern. Med. 2014;14:392. doi: 10.1186/1472-6882-14-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir Aslani B., Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163–173. doi: 10.1016/j.lfs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Ansari A.R., Li N.Y., Sun Z.J., Huang H.B., Zhao X., Cui L., Hu Y.F., Zhong J.M., Karrow N.A., Liu H.Z. Lipopolysaccharide induces acute bursal atrophy in broiler chicks by activating TLR4-MAPK-NF-κB/AP-1 signaling. Oncotarget. 2017;8:108375–108391. doi: 10.18632/oncotarget.19964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Zhang Y., Tang C., Hou Y., Ai X., Chen X., Zhang Y., Wang X., Meng X. Gallic acid: pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110985. [DOI] [PubMed] [Google Scholar]
- Bendich A. Physiological role of antioxidants in the immune system. J. Dairy Sci. 1993;76:2789–2794. doi: 10.3168/jds.S0022-0302(93)77617-1. [DOI] [PubMed] [Google Scholar]
- Bi W., Zhang J., Zeng Z., Zhou R., Zhao J., Yan W., Wang L., Li X., Zhu L. Ubiquitin‑specific protease 8 ameliorates lipopolysaccharide‑induced spleen injury via suppression of NF‑κB and MAPK signaling pathways. Mol. Med. Rep. 2022;26:370. doi: 10.3892/mmr.2022.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelli M., Franza L., Pignataro G., Ojetti V., Covino M., Piccioni A., Gasbarrini A., Franceschi F. Interaction between lipopolysaccharide and gut microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 2021;22:6242. doi: 10.3390/ijms22126242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carullo G., Cappello A.R., Frattaruolo L., Badolato M., Armentano B., Aiello F. Quercetin and derivatives: useful tools in inflammation and pain management. Future Med. Chem. 2017;9:79–93. doi: 10.4155/fmc-2016-0186. [DOI] [PubMed] [Google Scholar]
- Carvalho J.T.G., Da Silva Baldivia D., Castro D.T.H., Santos H.F., Santos C.M., Oliveira A.S., Alfredo T.M., Vilharva K.N., de Picoli Souza K., dos Santos E.L. The immunoregulatory function of polyphenols: implications in cancer immunity. J. Nutr. Biochem. 2020;85 doi: 10.1016/j.jnutbio.2020.108428. [DOI] [PubMed] [Google Scholar]
- Chasiotis H., Kolosov D., Bui P., Kelly S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: a review. Respir. Physiol. Neurobiol. 2012;184:269–281. doi: 10.1016/j.resp.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen Y., Zhang H., Wang T. Pterostilbene as a protective antioxidant attenuates diquat-induced liver injury and oxidative stress in 21-day-old broiler chickens. Poult. Sci. 2020;99:3158–3167. doi: 10.1016/j.psj.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chewning C.G., Stark C.R., Brake J. Effects of particle size and feed form on broiler performance. J. Appl. Poult. Res. 2012;21:830–837. [Google Scholar]
- Chiu H.-F., Venkatakrishnan K., Golovinskaia O., Wang C.-K. Gastroprotective effects of polyphenols against various gastro-intestinal disorders: a mini-review with special focus on clinical evidence. Molecules. 2021;26 doi: 10.3390/molecules26072090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.R., Wang Q., Cardenas E., Fish J., Chai B., Farris R.J., Kulam-Syed-Mohideen A.S., McGarrell D.M., Marsh T., Garrity G.M., Tiedje J.M. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daillère R., Vétizou M., Waldschmitt N., Yamazaki T., Isnard C., Poirier-Colame V., Duong C.P.M., Flament C., Lepage P., Roberti M.P., Routy B., Jacquelot N., Apetoh L., Becharef S., Rusakiewicz S., Langella P., Sokol H., Kroemer G., Enot D., Roux A., Eggermont A., Tartour E., Johannes L., Woerther P.-L., Chachaty E., Soria J.-C., Golden E., Formenti S., Plebanski M., Madondo M., Rosenstiel P., Raoult D., Cattoir V., Boneca I.G., Chamaillard M., Zitvogel L. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- de Mello Andrade J.M., Fasolo D. In: Polyphenols in Human Health and Disease. Watson R.R., Preedy V.R., Zibadi S., editors. Academic Press; Washington, DC: 2014. Chapter 20: Polyphenol antioxidants from natural sources and contribution to health promotion; pp. 253–265. [Google Scholar]
- Fan Y., Lin F., Zhang R., Wang M., Gu R., Long C. Acer truncatum Bunge: a comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2022;282 doi: 10.1016/j.jep.2021.114572. [DOI] [PubMed] [Google Scholar]
- Farahat M.H., Abdallah F.M., Ali H.A., Hernandez-Santana A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal. 2017;11:771–777. doi: 10.1017/S1751731116002251. [DOI] [PubMed] [Google Scholar]
- Hirata T., Kizuka Y. N-glycosylation. Adv. Exp. Med. Biol. 2021;1325:3–24. doi: 10.1007/978-3-030-70115-4_1. [DOI] [PubMed] [Google Scholar]
- Hu R., He Z., Liu M., Tan J., Zhang H., Hou D.-X., He J., Wu S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020;11:92. doi: 10.1186/s40104-020-00492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Lin H., Wang M., Zhao Y., Liu H., Min Y., Yang X., Gao Y., Yang M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021;12:25. doi: 10.1186/s40104-020-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S.S., Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadim R.M., Al-Fartusie F.S. Antioxidant vitamins and their effect on immune system. J. Phys. Conf. Ser. 2021;1853 [Google Scholar]
- Kong L., Wang Z., Xiao C., Zhu Q., Song Z. Glycerol monolaurate attenuated immunological stress and intestinal mucosal injury by regulating the gut microbiota and activating AMPK/Nrf2 signaling pathway in lipopolysaccharide-challenged broilers. Anim. Nutr. 2022;10:347–359. doi: 10.1016/j.aninu.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Zhao Y., Purswell J.L., Magee C. Effects of feeder space on broiler feeding behaviors. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Christman L.M., Li R., Gu L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020;11:4878–4891. doi: 10.1039/d0fo00713g. [DOI] [PubMed] [Google Scholar]
- Liu J. Northwest A&F University]; 2021. The Effect of Acer Truncatum on Growth Performance and Antioxidant Capacity Intestinal Flora of Weaned Piglets [Master Thesis] [Google Scholar]
- Liu F., Sun P., Zhang Q., Wu Y., Zhu X., Zhao S. Effects of leaves of Acer truncatum on growth and immunity of Saanen dairy goats based on intestinal microbiome. Prog. Vet. Med. 2020;41:15–22. [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G., Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabadi M.H.F., Ghalyanchilangeroudi A., Tehrani F., Hajloo S.A., Bashashati M., Bahonar A.R., Pourjafar H., Ansari F. Assessing the economic burden of multi-causal respiratory diseases in broiler farms in Iran. Trop. Anim. Health Prod. 2022;54:117. doi: 10.1007/s11250-022-03110-0. [DOI] [PubMed] [Google Scholar]
- Modi S.R., Collins J.J., Relman D.A. Antibiotics and the gut microbiota. J. Clin. Invest. 2014;124:4212–4218. doi: 10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Tsukita S., Miyachi Y. Tight junction-associated proteins (occludin, ZO-1, claudin-1, claudin-4) in squamous cell carcinoma and Bowen's disease. Br. J. Dermatol. 2004;151:328–334. doi: 10.1111/j.1365-2133.2004.06029.x. [DOI] [PubMed] [Google Scholar]
- Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut. Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. [Google Scholar]
- Niu J.L., Wei L.Q., Luo Y.Q., Yang W.T., Lu Q.C., Zheng X.X., Niu Y.J., Sheng W., Cheng H., Zhang W.J., Nie C.X. Fermented cottonseed meal improves production performance and reduces fat deposition in broiler chickens. Anim. Biosci. 2021;34:680–691. doi: 10.5713/ajas.20.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley L.L., Wei B., Braun J., Borneman J. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 2010;76:936–941. doi: 10.1128/AEM.01561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano M.A., Puertollano E., de Cienfuegos G., de Pablo M.A. Dietary antioxidants: immunity and host defense. Curr. Top Med. Chem. 2011;11:1752–1766. doi: 10.2174/156802611796235107. [DOI] [PubMed] [Google Scholar]
- Raederstorff D. Antioxidant activity of olive polyphenols in humans: a review. Int. J. Vitam. Nutr. Res. 2009;79:152–165. doi: 10.1024/0300-9831.79.3.152. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Abdollahi M.R. Nutrition and digestive physiology of the broiler chick: state of the art and outlook. Animals. 2021;11:2795. doi: 10.3390/ani11102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R.C., Seira Oriach C., Murphy K., Moloney G.M., Cryan J.F., Dinan T.G., Ross R.P., Stanton C. Deficiency of essential dietary n-3 PUFA disrupts the caecal microbiome and metabolome in mice. Br. J. Nutr. 2017;118:959–970. doi: 10.1017/S0007114517002999. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Daza M.C., Pulido-Mateos E.C., Lupien-Meilleur J., Guyonnet D., Desjardins Y., Roy D. Polyphenol-mediated gut microbiota modulation: toward prebiotics and further. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.689456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I., Gibson G., Heinken A., Scott K., Swann J., Thiele I., Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur. J. Nutr. 2018;57:1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjay S., Girish C., Toi P.C., Bobby Z. Gallic acid attenuates isoniazid and rifampicin-induced liver injury by improving hepatic redox homeostasis through influence on Nrf2 and NF-κB signalling cascades in Wistar Rats. J. Pharm. Pharmacol. 2021;73:473–486. doi: 10.1093/jpp/rgaa048. [DOI] [PubMed] [Google Scholar]
- Schulze Bernd K., Wilms-Schulze Kump A., Rohn K., Reich F., Kehrenberg C. Management factors influencing the occurrence of cellulitis in broiler chickens. Prev. Vet. Med. 2020;183 doi: 10.1016/j.prevetmed.2020.105146. [DOI] [PubMed] [Google Scholar]
- Shen L., Zhou Y., Wu X., Sun Y., Xiao T., Gao Y., Wang J. TREM1 blockade ameliorates lipopolysaccharide-induced acute intestinal dysfunction through inhibiting intestinal apoptosis and inflammation response. Biomed. Res. Int. 2021;2021 doi: 10.1155/2021/6635452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi N., Li N., Duan X., Niu H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017;4:14. doi: 10.1186/s40779-017-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiner A.B., Kao J.Y., Young V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015;31 doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre G.F.G., de Lucena R.P., da Silva Alves H. Cucurbitacins and the immune system: update in research on anti-inflammatory, antioxidant, and immunomodulatory mechanisms. Curr. Med. Chem. 2022;29:3774–3789. doi: 10.2174/0929867329666220107153253. [DOI] [PubMed] [Google Scholar]
- Sivaprakasam S., Prasad P.D., Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016;164:144–151. doi: 10.1016/j.pharmthera.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimestad R., Fossen T., Vågen I.M. Onions: a source of unique dietary flavonoids. J. Agric. Food Chem. 2007;55:10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- Song B., Tang D., Yan S., Fan H., Li G., Shahid M.S., Mahmood T., Guo Y. Effects of age on immune function in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:42. doi: 10.1186/s40104-021-00559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Li Q., Everaert N., Liu R., Zheng M., Zhao G., Wen J. Dietary inulin supplementation modulates short-chain fatty acid levels and cecum microbiota composition and function in chickens infected with Salmonella. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.584380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto S., Ranjitkar S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: a review. Anim. Nutr. 2019;5:1–10. doi: 10.1016/j.aninu.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Wahyuni H., Sartono T. Recent advances in the incorporation of leaf meals in broiler diets. Livestock Res. Rural Dev. 2019;31:2019. [Google Scholar]
- Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. In: Pages 91–119 in Advances in Immunology (121) Alt F.W., editor. Academic Press; 2014. Chapter 3: The role of short-chain fatty acids in health and disease. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Wang J., Gao R., Wang J., Zhu W. Early-life galacto-oligosaccharides supplementation alleviates the small intestinal oxidative stress and dysfunction of lipopolysaccharide-challenged suckling piglets. J. Anim. Sci. Biotechnol. 2022;13:70. doi: 10.1186/s40104-022-00711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Li D., Ke W., Liang D., Hu X., Chen F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020;44:213–225. doi: 10.1038/s41366-019-0332-1. [DOI] [PubMed] [Google Scholar]
- Wang Q., Jin L., Wang H., Tai S., Liu H., Zhang D. AWRK6, a synthetic cationic peptide derived from antimicrobial peptide dybowskin-2CDYa, inhibits lipopolysaccharide-induced inflammatory response. Int. J. Mol. Sci. 2018;19:600. doi: 10.3390/ijms19020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Huang Y., Llu X., Zeng N., Chen Y., Guanhua F.U., Bin W. Effects of Acer truncatum leaves on performance, egg quality and antioxidant indices of Taihang chicken. China Feed. 2022;697:137–142. [Google Scholar]
- Wang S., Li Z., Ma Y., Liu Y., Lin C.-C., Li S., Zhan J., Ho C.-T. Immunomodulatory effects of green tea polyphenols. Molecules. 2021;26:3755. doi: 10.3390/molecules26123755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Wen M., Zhao H., Liu G., Chen X., Tian G., Cai J., Jia G. Effect of zinc supplementation on growth performance, intestinal development, and intestinal barrier function in Pekin ducks with lipopolysaccharide challenge. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Zhu L., Fu J., Li Z., Wang Y., Jin M. Overall assessment of fermented feed for pigs: a series of meta-analyses. J. Anim. Sci. 2019;97:4810–4821. doi: 10.1093/jas/skz350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Hua H., Wang L., He P., Zhang L., Qin Q., Yu C., Wang X., Zhang G., Liu Y. Holly polyphenols alleviate intestinal inflammation and alter microbiota composition in lipopolysaccharide-challenged pigs. Br. J. Nutr. 2020;123:881–891. doi: 10.1017/S0007114520000082. [DOI] [PubMed] [Google Scholar]
- Yahfoufi N., Alsadi N., Jambi M., Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10 doi: 10.3390/nu10111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Beijing Forestry University]; 2019. Evaluation of Phenolic Compounds in Acer Truncatum Leaves and Characterizations of Its Flavonoids Biosynthesis Pathway [Doctor Dissertation] [Google Scholar]
- Yang L., Liu G., Zhu X., Luo Y., Shang Y., Gu X.-L. The anti-inflammatory and antioxidant effects of leonurine hydrochloride after lipopolysaccharide challenge in broiler chicks. Poult. Sci. 2019;98:1648–1657. doi: 10.3382/ps/pey532. [DOI] [PubMed] [Google Scholar]
- Yang L., Yin P., Fan H., Xue Q., Li K., Li X., Sun L., Liu Y. Response surface methodology optimization of ultrasonic-assisted extraction of Acer truncatum leaves for maximal phenolic yield and antioxidant activity. Molecules. 2017;22:232. doi: 10.3390/molecules22020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yin P., Ho C.T., Yu M., Sun L., Liu Y. Effects of thermal treatments on 10 major phenolics and their antioxidant contributions in Acer truncatum leaves and flowers. R. Soc. Open. Sci. 2018;5 doi: 10.1098/rsos.180364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Liang Q., Balakrishnan B., Belobrajdic D.P., Feng Q.-J., Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12:381. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhang J., Jiang Y., Xu Y.Q., Jin X., Yan S.M., Shi B.l. Effects of Artemisia argyi flavonoids on growth performance and immune function in broilers challenged with lipopolysaccharide. Anim. Biosci. 2021;34:1169–1180. doi: 10.5713/ab.20.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Zhang C., Fan Q., Lin X., Wang Y., Azzam M., Alhotan R., Alqhtani A., Jiang S. Antrodia cinnamomea polysaccharide improves liver antioxidant, anti-inflammatory capacity, and cecal flora structure of slow-growing broiler breeds challenged with lipopolysaccharide. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.994782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Chen Y., Chen Y., Li Y., Jia P., Ji S., Zhou Y., Wang T. Dietary pterostilbene supplementation attenuates intestinal damage and immunological stress of broiler chickens challenged with lipopolysaccharide. J. Anim. Sci. 2019;98 doi: 10.1093/jas/skz373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wu Y., Wang G., Cao F., Li D., Wang W. Effect of fermented Acer truncatum leaves on performance, meat quality and intestinal microflora of finishing pigs. Chin. J. Anim. Nutr. 2018;30:246–254. [Google Scholar]
- Zhao W.H., Gao L.F., Gao W., Yuan Y.S., Gao C.C., Cao L.G., Hu Z.Z., Guo J.Q., Zhang Y.X. Weight-reducing effect of Acer truncatum Bunge may be related to the inhibition of fatty acid synthase. Nat. Prod. Res. 2011;25:422–431. doi: 10.1080/14786419.2010.488625. [DOI] [PubMed] [Google Scholar]
- Zhao W.H., Zhang J.F., Zhe-Wang, Zhang Y.X., Tian W.X. The extract of leaves of Acer truncatum Bunge: A natural inhibitor of fatty acid synthase with antitumor activity. J. Enzyme. Inhib. Med. Chem. 2006;21:589–596. doi: 10.1080/14756360600774579. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Jiang Q. Roles of the polyphenol–gut microbiota interaction in alleviating colitis and preventing colitis-associated colorectal cancer. Adv. Nutr. 2021;12:546–565. doi: 10.1093/advances/nmaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.C., Wu Q.J., Song Z.H., Zhang H., Zhang J.F., Zhang L.L., Zhang T.Y., Wang C., Wang T. Effects of Oridonin on growth performance and oxidative stress in broilers challenged with lipopolysaccharide. Poult. Sci. 2016;95:2281–2289. doi: 10.3382/ps/pew161. [DOI] [PubMed] [Google Scholar]
- Zhou H.J., Kong L.L., Zhu L.X., Hu X.Y., Busye J., Song Z.G. Effects of cold stress on growth performance, serum biochemistry, intestinal barrier molecules, and adenosine monophosphate-activated protein kinase in broilers. Animal. 2021;15 doi: 10.1016/j.animal.2020.100138. [DOI] [PubMed] [Google Scholar]
- Zhou J., Tang L., Shen C.-L., Wang J.-S. Green tea polyphenols boost gut-microbiota-dependent mitochondrial TCA and urea cycles in Sprague–Dawley rats. J. Nutr. Biochem. 2020;81 doi: 10.1016/j.jnutbio.2020.108395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.