Highlights
-
•
We evaluated the efficacy of zinc sulfate as an adjuvant in foot-and-mouth vaccine.
-
•
Zinc sulfate enhances cellular immune response by inducing IFNγ secretion.
-
•
Vaccine containing zinc sulfate induces humoral immune response in mice and pigs.
-
•
Vaccine containing zinc sulfate activated the TLR4 downstream signaling pathway.
Keywords: Foot-and-mouth disease, Zinc sulfate, Vaccine adjuvant, Viral disease, Cellular and humoral immunity
Abstract
Foot-and-mouth disease (FMD) is a rapidly propagating infectious disease of cloven-hoofed animals, especially cattle and pigs, affecting the productivity and profitability of the livestock industry. Presently, FMD is controlled and prevented using vaccines; however, conventional FMD vaccines have several disadvantages, including short vaccine efficacy, low antibody titers, and safety issues in pigs, indicating the need for further studies. Here, we evaluated the efficacy of a novel bivalent vaccine containing zinc sulfate as an immunostimulant and FMD type O and A antigens (O PA2 and A YC, respectively) against FMD virus in mice and pigs. Zinc sulfate induced cellular immunity in murine peritoneal exudate cells (PECs) and porcine peripheral blood mononuclear cells (PBMCs) by increasing IFNγ secretion. Additionally, FMD vaccine containing O PA2 and A YC antigens and zinc sulfate induced early, mid-, and long-term immune responses in mice and pigs, and enhanced cellular and humoral immunity by regulating the expression of pathogen recognition receptors (PRRs), transcription factors, co-stimulatory molecules, and cytokines in porcine PBMCs from vaccinated pigs. Overall, these results indicated that the novel immunostimulant zinc sulfate induced potent cellular and humoral immune responses by stimulating antigen-presenting cells (APCs) and T and B cells, and enhanced long-term immunity by promoting the expression of co-stimulatory molecules. These outcomes suggest that zinc sulfate could be used as a novel vaccine immunostimulant for difficult-to-control viral diseases, such as African swine fever (ASF) or COVID-19.
1. Introduction
Foot-and-mouth disease (FMD) is an acute and infectious viral disease that occurs in ungulates, such as cloven-hoofed pigs, cattle, goats, and sheep, and is characterized by fever, followed by blisters on the lips, tongue, gums, and nose (Donaldson, 1987). Myocarditis arising from FMD can cause high mortality in young animals, especially calves, and high economic losses in adults due to low productivity (Alexandersen et al., 2003). The World Organization for Animal Health (WOAH) divided the outbreak area into seven zones, except for Asia 1, where each serotype is geographically restricted and indigenous to the region (Samuel and Knowles, 2001). WOAH recommends vaccination to control and eradicate FMD. Commercial FMD vaccines are prepared by inactivating the whole virus by treating it with aziridines, such as binary ethylenimine (BEI), and then mixing it with adjuvants, such as oil emulsion. In addition to inactivated vaccines, live attenuated, DNA, virus-like particle (VLP), and synthetic peptide vaccines have been developed for FMD control and eradication (Lu et al., 2022). Successful vaccination and prevention of FMD outbreaks depends on effective and safe adjuvants, suitable antigen-delivery systems, and routes of administration (Amanna and Slifka, 2020). Adjuvants currently used include, mineral oil-based emulsions (montanide ISA 50, ISA 201, ISA 206, and marcol 52), Al(OH)3 as an immunostimulant, toll-like receptor (TLR) ligands, saponin (Quil-A), cytokines, and antigen delivery systems, such as liposomes (Cao, 2014; Kamel et al., 2019). Oil emulsion adjuvants induce stronger immune responses in host animals compared with other adjuvants, allowing for slow release of the antigen to prolong immune response. However, the use of oil emulsion is associated with several side effects (local reaction), including swelling, hemolysis, and necrosis at the injection site (Wu et al., 2020). Additionally, montanide ISA 206, a widely used oil adjuvant, was reported to accelerate the degradation of immunogenic 146S particles in inactivated FMDV antigen at the interface between the oil and water layers (Harmsen et al., 2015). Al(OH)3 provokes a typical antibody-mediated type 2 helper (Th2) immunity rather than a cell-mediated type 1 helper (Th1) immunity (Jiang et al., 2018), stimulating high production of IgE, which can induce neurotoxicity, hypersensitivity, and excessive inflammatory responses at the vaccination site. Saponin is usually combined with Al(OH)3 to efficiently compensate for cellular immunodeficiency; however, its use is limited by its toxicity when used in large quantities (Dalsgaard, 1987). Recent vaccine development is targeted at inducing maximum stimulation of Th1 and Th2 responses by antigens using methods that simultaneously increase cellular and humoral immune responses (Carr et al., 2013; Guzman et al., 2010). Antigens are endocytosed by dendritic cells (DCs), processed, and presented on major histocompatibility complex (MHC) molecules, and several agents can be recognized by both CD4+ and CD8+ T cells, inducing DC maturation. Mature DCs are recognized by these T cells to induce both cellular and humoral immune responses (Lee et al., 2020). Particularly, TLRs activate the secretion of inflammatory cytokines in neutrophils, allowing DCs to mature, thereby triggering the expression of co-stimulatory molecules and enhancing their antigen-presenting capacity (Finlay and McFadden, 2006). TLR4 is a major ligand that activates DCs and induces Th1 cell activation, which mediates cellular and humoral immunity (Lavelle et al., 2006).
Zinc sulfate is widely used to treat and prevent zinc deficiency in humans and animals. Zinc is a vital trace element that plays a pivotal role in immune cell physiology, growth, and maintenance (Read et al., 2019). Zinc deficiency negatively affects immune cell development and function, including lymphocyte proliferation, interleukin (IL)−2 level, delayed-type hypersensitivity (DTH) response, and antibody response (Haase and Rink, 2009; Keen and Gershwin, 1990; Mitchell et al., 2006; Mocchegiani and Malavolta, 2004; Prasad, 2000). However, zinc supplementation can reverse immune system damage (Haase and Rink, 2009). Zinc sulfate has the advantage of being supplemented using various vaccination routes, such as mucosal (oral) administration, and intradermal and intramuscular vaccination (Afsharian et al., 2011).
Based on this background, we evaluated the immunostimulatory efficacy and adjuvanticity of zinc sulfate as an FMD vaccine adjuvant to overcome the limitations of currently available commercial FMD vaccines. Specifically, we prepared a bivalent vaccine by adding zinc sulfate to FMD type O and A antigens (O PA2 and A YC, respectively) and investigated whether cellular and humoral immune responses were simultaneously induced in mice and pigs.
2. Materials and methods
2.1. Cells, virus, and reagent
BHK-21, LFBK cells were cultured in DMEM (Lonza, Basel, Switzerland), and ZZ-R 127 cells were cultured in DMEM/F12 (Lonza) containing 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic (Gibco) at 37 °C in a 5% CO2 condition. Recombinant O PA2 was prepared by replacing the P1 region of O1 Manisa/Turkey/69 (Genbank No. AY593823) with the P1 region of O/PAK/44/2008 (GenBank No. GU384642) as previously described (Lee et al., 2017). A/Yeoncheon/SKR/2017 (A YC, GenBank No. KY766148) was isolated by the Animal and Plant Quarantine Agency (APQA, Gyeongsangbuk-do, Korea). Commercial zinc sulfate was purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Virus antigen purification
The FMDV O PA2 and A YC antigens were purified according to a previously described method (Lee et al., 2022). Briefly, the viruses were harvested after complete cytopathic effect (CPE) was observed in infected BHK-21 cells, followed by centrifugation at 12,000 rpm for 20 min to remove cell debris and obtain viral supernatants. Thereafter, the virus was inactivated using 0.003 mM of BEI (Sigma-Aldrich) for 24 h, and concentrated using 7.5% polyethylene glycol (PEG) 6000 and 2.3% NaCl, followed by stirring for 16 h at 4 °C. The concentrated pellet was resuspended in Tris-NaCl buffer, and purified by a 15–45% sucrose gradient at 30,000 rpm for 4 h at 4 °C using an SW41Ti rotor (Beckman Coulter, Brea, CA, USA). Afterward, the 146S antigen quantity was measured at 259 nm using a spectrophotometer. The inactivated virus was passaged at least three times in ZZ-R 127 and BHK-21 cells to confirm the absence of live virus before use in experiments.
2.3. Experimental mice and pigs
Animal experiments were performed using C57BL/6 mice (female, 6–7 weeks old) and pigs (8–9 weeks old). All the mice and pigs were housed in animal biosafety level 3 (ABSL3) at the Animal and Plant Quarantine Agency (APQA), and acclimatized for one week prior to the experiment. The animal study was conducted in accordance with institutional guidelines and was approved by the APQA Ethics Committee (accreditation number: IACUC-2022–659).
2.4. Peritoneal exudate cell (PECs) isolation and culture
Briefly, naïve mice (n = 40) were sacrificed after CO2 anesthesia, and the peritoneal cavities were washed with 5 mL of cold HBSS (Gibco, Waltham, MA, USA) lacking Ca2+/Mg2+ and phenol-red. Thereafter, peritoneal lavage fluid was centrifuged at 400 g for 10 min at 4 °C to remove supernatants. After centrifugation, the cells were resuspended in the culture medium and purified PECs were subsequently incubated in RPMI 1640 (Gibco) containing 10% FBS and 1% antibiotic-antimycotic (Gibco) at 37 °C in a 5% CO2 atmosphere.
2.5. Peripheral blood mononuclear cell (PBMCs) isolation and culture
PBMCs were isolated from whole blood samples collected from pigs (n = 5–6/group) in heparin tubes, using Histopaque (Sigma-Aldrich). The remaining red blood cells (RBCs) were then removed by lysis using ACK lysing buffer (Gibco). Thereafter, PBMCs were resuspended in Ca2+/Mg2+ free DPBS (Gibco) and stored until use. Isolated PBMCs were incubated in RPMI 1640 (Gibco) containing 10% FBS and 1% antibiotic-antimycotic (Gibco) at 37 °C and in a 5% CO2 atmosphere.
2.6. Cell viability assay
BHK-21, LFBK, and ZZ-R 127 cells were plated into 96-well plates, 2 × 104 cells/well, and cultured in a growth medium. After 48 h of incubation, the growth medium was removed and replaced with a growth medium containing zinc sulfate (0–5 μg/mL), and incubated further at 37 °C in a 5% CO2 atmosphere. After incubation for 4 h, 20 μL MTS reagent (CellTiter 96® AQueous One solution Cell proliferation Assay, Promega, Madison, WI, USA) was added to each well, and the plates were incubated at 37 °C in a 5% CO2 atmosphere for 4 h. Following MTS incubation, the absorbance was measured at 490 nm using a Hidex 300 SL plate reader (Hidex, Turku, Finland). The viability of cells was calculated using the following equation:
2.7. ELISpot assay to measure IFNγ secretion in murine PECs and porcine PBMCs
The effect of zinc sulfate on IFNγ secretion by murine PECs and porcine PBMCs treated with FMDV O PA2 or A YC antigens was evaluated using ELISpot assay kits (mouse; Cat No. EL485 and porcine; Cat No. EL985, R&D Systems, Minneapolis, MN, USA), according to the manufacturer's protocol. Briefly, murine PECs or porcine PBMCs (5 × 105 cells/well) were cultured in an antibody-coated well for mouse or porcine IFNγ, and stimulated with 2 μg/mL (final concentration) of O PA2 antigen with or without zinc sulfate (0–5 μg/mL), and A YC antigen with or without zinc sulfate (0–5 μg/mL) for 18 h, and then incubated at 37 °C in a 5% CO2 atmosphere. Thereafter, the wells were washed four times and incubated with anti-mouse or anti-porcine IFNγ detection antibodies overnight at 2–8 °C, followed by washing four times and incubation with alkaline phosphate conjugated streptavidin at approximately 25 °C for 2 h. Finally, the wells were washed four times, developed with BCIP/NBT substrate at approximately 25 °C for 1 h, and analyzed using an AID ELISpot assay plate reader (Autoimmune Diagnostika GmbH, Strassberg, Germany). The data were calculated as number of spot forming cells (SFC).
2.8. Determination of zinc sulfate dosages for in vivo animal study
The dose of zinc sulfate used in animal studies was determined by deriving the Animal Equivalent Dose (AED) according to the guidelines of the US Food and Drug Administration (FDA) (Rockville et al., 2005; Nair and Jacob, 2016). The AED can be calculated based on body surface area by dividing or multiplying the human dose (mg/kg) by the Km ratio. The correction factor, Km is estimated by dividing the average body weight (kg) of the species by its body surface area (m2). Therefore, AED is determined by the following formula:
2.9. Evaluation of the adjuvanticity of zinc sulfate and early host defense in mice vaccinated with FMD vaccine
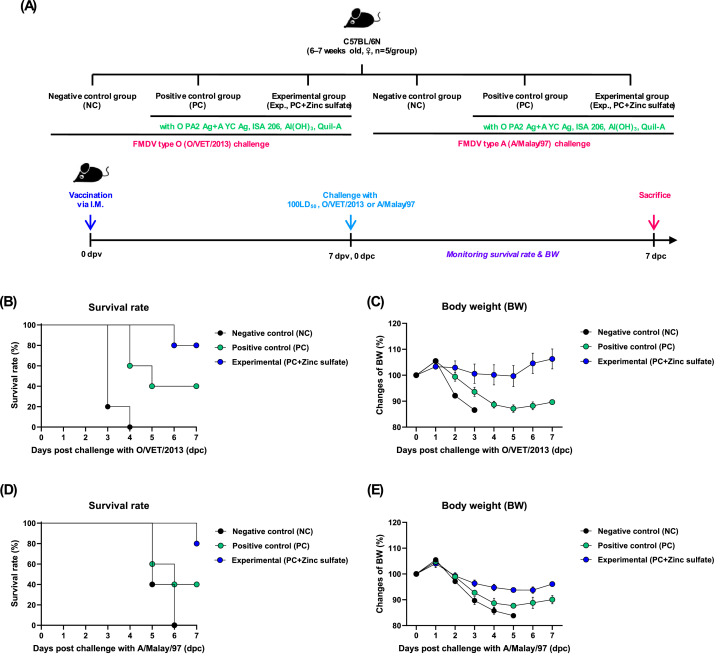
Animal experiments were conducted to evaluate the potential adjuvanticity of zinc sulfate and to validate the rapid immune protection of FMD vaccines containing zinc sulfate. Briefly, mice (n = 5/group) were administered with the experimental vaccine or control vaccine via intramuscular (IM) injection of the thigh, followed by inoculation with FMDV O/VET/2013 or A/Malay/97 virus at 100 LD50 dose via intraperitoneal (IP) injection at 7 days post-vaccination (dpv). The vaccine compositions for the positive control (PC) group were as follows: FMDV O PA2 and A YC antigens (15+15 μg/dose/mL; 1/40 of the dose for pigs), ISA 206 (50% w/w; Seppic, Paris, France), 10% Al(OH)3, and 15 μg/mouse Quil-A (InvivoGen, San Diego, CA, USA) in a total volume of 100 μL. Mice in the experimental (Exp) group received vaccines with the same composition, but with the addition of 100 μg zinc sulfate/dose/mouse, while those in the negative control (NC) group received an equal volume of PBS via the same route. To evaluate the short-term efficacy of the vaccines, survival rates and body weight changes were evaluated up to 7 days post-challenge (dpc) (Fig. 2A).
Fig. 2.
Zinc sulfate-containing FMD vaccine mediated host defense by inducing early immune response in mice. Experimental strategy (A); Survival rates of mice after challenge with O/VET/2013 (B) and A/Malay/97 (C); Body weight change after challenge with O/VET/2013 (D) and A/Malay/97 (E).
2.10. Evaluation of early, mid-, and long-term immune response in mice
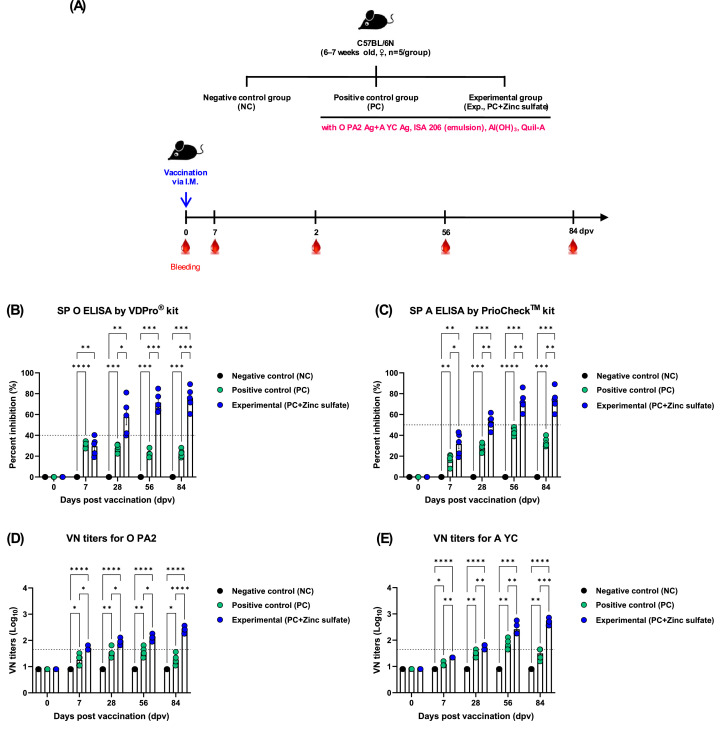
To evaluate the efficacy of zinc sulfate in eliciting early, mid-, and long-term immune responses as an FMD vaccine adjuvant, mice were administered FMD vaccine containing zinc sulfate as an adjuvant, following the protocol described in the previous section. Mice (n = 5/group) were vaccinated via IM injection, and blood samples were collected at 0, 7, 28, 56, and 84 dpv for serological assays (Fig. 3A).
Fig. 3.
Zinc sulfate-containing FMD vaccine elicited early, mid-, and long-term immune responses in mice. (A–E) Experimental strategies (A); Antibody titers by SP O ELISA (VDPro® kit) (B); Antibody titers by SP A ELISA (PrioCheck™ kit) (C); VN titers for O PA2 (D); VN titers for A YC (E). ⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001, and ⁎⁎⁎⁎p < 0.0001.
2.11. Evaluation of early, mid-, and long-term immune responses in pigs
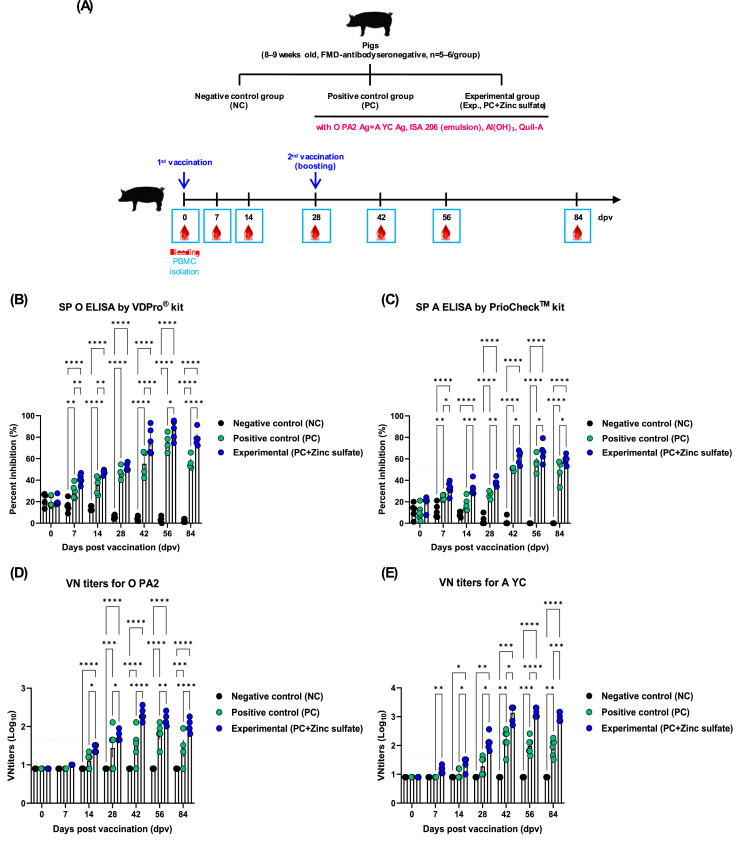
To evaluate the efficacy of zinc sulfate in eliciting early, mid-, and long-term immune responses, animal experiment was performed using pigs, according to a previously described method (Lee et al., 2019). Briefly, pigs were divided into NC, PC, and Exp groups (n = 5–6/group), and vaccinated with the respective FMD vaccines. The composition of the various vaccines was as follows: FMDV O PA2 and A YC antigens (15+15 μg/dose/pig/mL), without (PC group) or with (Exp group) zinc sulfate, with ISA 206 (Seppic), 10% Al(OH)3, and 150 μg/dose/pig Quil-A (InvivoGen). Pigs in the Exp group received a single dose of 1 mL of vaccine via IM at 28 d interval (0 and 28 dpv), while those in the NC group received an equal volume of PBS via the same route. Whole blood samples were collected during the experiment at 0, 7, 14, 28, 42, 56, and 84 dpv for serological assays (Fig. 4A).
Fig. 4.
Zinc sulfate-containing FMD vaccine effectively induced early, mid-, and long-term immune responses in pigs. (A–E) Experimental strategies (A); Percent inhibition of SP O ELISA (VDPro® kit)(B); Percent inhibition of SP A ELISA (PrioCheck™ kit) (C); VN titers for O PA2 (D); VN titers for A YC (E). ⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001, and ⁎⁎⁎⁎p < 0.0001.
2.12. Serological assays
2.12.1. ELISA for the detection of structural protein (SP) antibodies
Serum SP antibody levels were detected using VDPro® FMDV Type O Kit (Cat No. EM-FMD-05, Median Diagnostics, Gangwon-do, Korea) and PrioCheck™ FMDV Type A Kit (Cat No.7610850, Prionics AG, Schlieren, Switzerland), respectively, according to the manufacturer's protocol. The optical density (OD) values were converted to percent inhibition (PI) values. The animals were considered antibody positive, when the PI value was ≥ 40% for the VDPro® Type O Kit or ≥ 50% for the PrioCheck™ Type A Kit.
2.12.2. Virus neutralization (VN) test
Serum samples were heat-inactivated at 56 °C for 30 min, diluted 2-fold, and incubated with a 100 TCID50 (50% tissue culture infective dose) in 50 μL media of FMDV virus (O PA2 or A YC) at 37 °C for 1 h. Subsequently, 50 μL of LFBK cells (106 cells/mL) were added to each well, incubated at 37 °C in a 5% CO2 atmosphere for 3 d, and the wells were checked for CPE. Antibody titers were evaluated as the Log10 of the reciprocal antibody dilution required for neutralization of 100 TCID50 of viruses in 50% of the wells.
2.12.3. Isotype-specific antibodies immunoassay
The concentrations of isotype-specific antibodies, including IgG, IgA, and IgM, were determined using ELISA kits porcine (IgG; Cat. No. E101–104, IgA; Cat. No. E101–102, and IgE; Cat. No. E101–117, Bethyl Laboratories Inc., Montgomery, Texas, USA), according to the manufacturer's protocol. Briefly, 100 µL of serially diluted standards and serum samples were incubated in each well at approximately 25 °C for 1 h, followed by washing four times and incubation with 100 μL of Ig subtype antibodies at approximately 25 °C for 1 h. After washing four times, the samples were incubated with 100 μL of HRP solution at approximately 25 °C for 30 min. The wells were washed four times, and treated with 1 × TMB solution (100 μL/well) for 30 min at 25 °C to detect peroxidase activity, and the reaction was stopped by adding 2 N H2PO4 (100 μL). OD value was measured at 450 nm using a Hidex 300 SL plate reader (Hidex, Turku, Finland).
2.13. RNA isolation, cDNA synthesis, and quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed to determine the effect of zinc sulfate-containing FMD vaccine on the expression of immune response-related genes. Porcine PBMCs were purified from the freshly whole blood of vaccinated pigs (n = 5–6/group) at 0, 7, 14, 28, 42, 56, and 84 dpv, according to a previously described PBMCs isolation method. Total RNA was isolated from purified porcine PBMCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and reverse-transcribed to synthesize cDNA using M-MLV RT (Promega, Madison, WI, USA). The cDNA products were amplified on CFX96™ touch Real-Time PCR (Bio-Rad, Hercules, CA, USA) using iQ SYBR Green Supermix (Bio-Rad). Quantitative gene expression levels were normalized to that of HPRT (endogenous housekeeping gene) and presented relative to the control values. The list of primers used in this study is in Table S1.
2.14. Statistical analysis
All quantitative data were presented as mean ± standard error of mean (SEM). Significant differences between groups were determined using two-way ANOVA, followed by Tukey's post-hoc test. Parametric tests were used to compare the different groups. Survival curves were generated using the Kaplan–Meier method, and differences were analyzed using the log-rank sum test. Statistical significance level is expressed as follows: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ⁎⁎⁎⁎p < 0.0001. All statistical analyses were conducted using GraphPad Prism 9.5.0 (GraphPad, San Diego, CA, USA).
3. Results
3.1. Zinc sulfate with or without inactivated FMDV antigen enhances cellular immune response by inducing IFNγ secretion
To evaluate the cytotoxic effects of zinc sulfate in the BHK-21, LFBK, and ZZ-R 127 cells, we measured the cell viability using MTS assay. As a result, cytotoxicity was not observed at 0–5 μg/μL in these cells (Supplementary Fig. 1A–C).
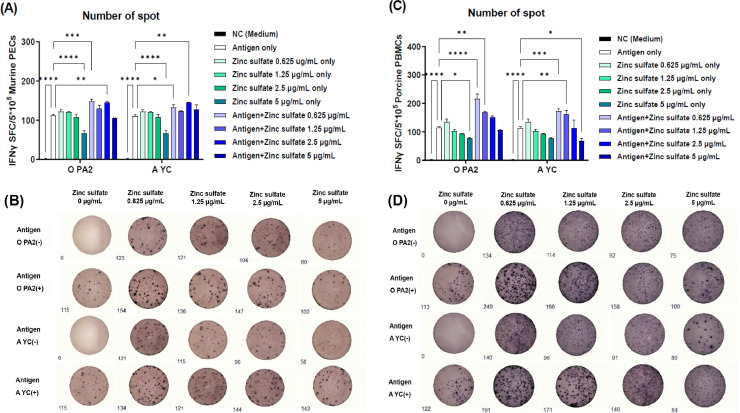
Murine PECs and porcine PBMCs were treated with an inactivated antigen (O PA2 or A YC) alone, zinc sulfate alone, or antigen with zinc sulfate to evaluate the efficacy of zinc sulfate on IFNγ secretion (cellular immune response) using an ELISpot assay. Treatment with low concentration (0.625 μg/mL) of zinc sulfate with O PA2 or A YC antigen significantly increased IFNγ secretion in both murine PECs and porcine PBMCs compared with treatment with antigen alone, indicating that zinc sulfate effectively induced a Th1-type cellular immune response (Fig. 1A–D).
Fig. 1.
Zinc sulfate and inactivated FMDV type O (O PA2) or A (A YC) antigen effectively induced cellular immune responses in murine peritoneal exudate cells (PECs) and porcine peripheral blood mononuclear cells (PBMCs). IFNγ-secreting cell spots in murine PECs (A); Images of IFNγ secretion in murine PECs (B); IFNγ-secreting cell spots in porcine PBMCs (C); Images of IFNγ secretion in porcine PBMCs (D). Data have been presented as spot-forming cells per number of cells in the well. ⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001, and ⁎⁎⁎⁎p < 0.0001.
3.2. FMD vaccine containing zinc sulfate as an immunostimulant elicits early host defense against viral infection in mice
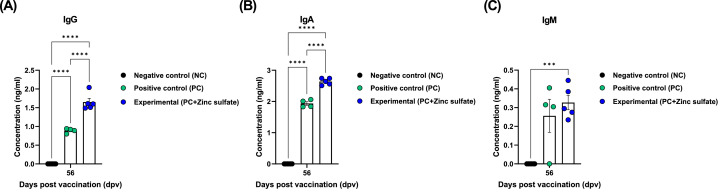
To determine whether zinc sulfate can induce early host defense against FMDV infection in vivo, mice were vaccinated with PC vaccine containing O PA2+A YC antigen or Exp vaccine containing zinc sulfate and O PA2+A YC antigen, and challenged with FMDV O/VET/2013 or A/Malay/97 at 7 dpv. The survival rate and body weight change of the mice were monitored for 7 dpc. Compared with the PC group, mice vaccinated with the Exp group had a higher survival rate, with no observable weight loss (Fig. 2B–E). FMD vaccine containing zinc sulfate as an immunostimulant induced early, mid-, and long-term immune responses in mice and pigs. To evaluate the effects of zinc sulfate on early (7 dpv), mid- (28 and 56 dpv), and long-term (84 dpv) immune responses, mice were vaccinated with the PC vaccine or Exp vaccine containing zinc sulfate. After vaccination, antibody titers were detected by SP O or A ELISA and VN titers were confirmed using VN test. Compared with the PC group, antibody titer and VN titers were significantly higher in the Exp group at 7, 28, 56, and 84 dpv (Fig. 3B–E). Additionally, pigs were injected with the PC vaccine or Exp group containing zinc sulfate to evaluate the effect of zinc sulfate on early, mid-, and long-term immune responses. Antibody titer, VN titers, and IgG, IgA, and IgM levels were determined at 0–84 dpv. Compared with the PC group, antibody titer was significantly higher in the Exp group after the first vaccination (7 dpv) and remained high at 84 dpv after booster shot (Fig. 4B–C). Additionally, VN titer assay showed that FMDV O PA2 and A YC neutralizing antibody titers were significantly higher in the Exp group at 14 dpv compared with the PC group, and was maintained up to 84 dpv (Fig. 4D–E). Moreover, IgG and IgA levels were significantly higher in the Exp group than in the PC group; however, there was no significant difference in IgM levels between the groups (Figs. 5A–C).
Fig. 5.
Zinc sulfate-containing FMD vaccine induced an increase in porcine serum immunoglobulin G (IgG), IgA and IgM levels. Serum IgG (A); IgA (B); and IgM (C) concentrations. ⁎⁎⁎p < 0.001 and ⁎⁎⁎⁎p < 0.0001.
3.3. FMD vaccines containing zinc sulfate elicits cellular and humoral immune responses by inducing PRR, transcription factor, co-stimulatory molecule, and cytokine expression
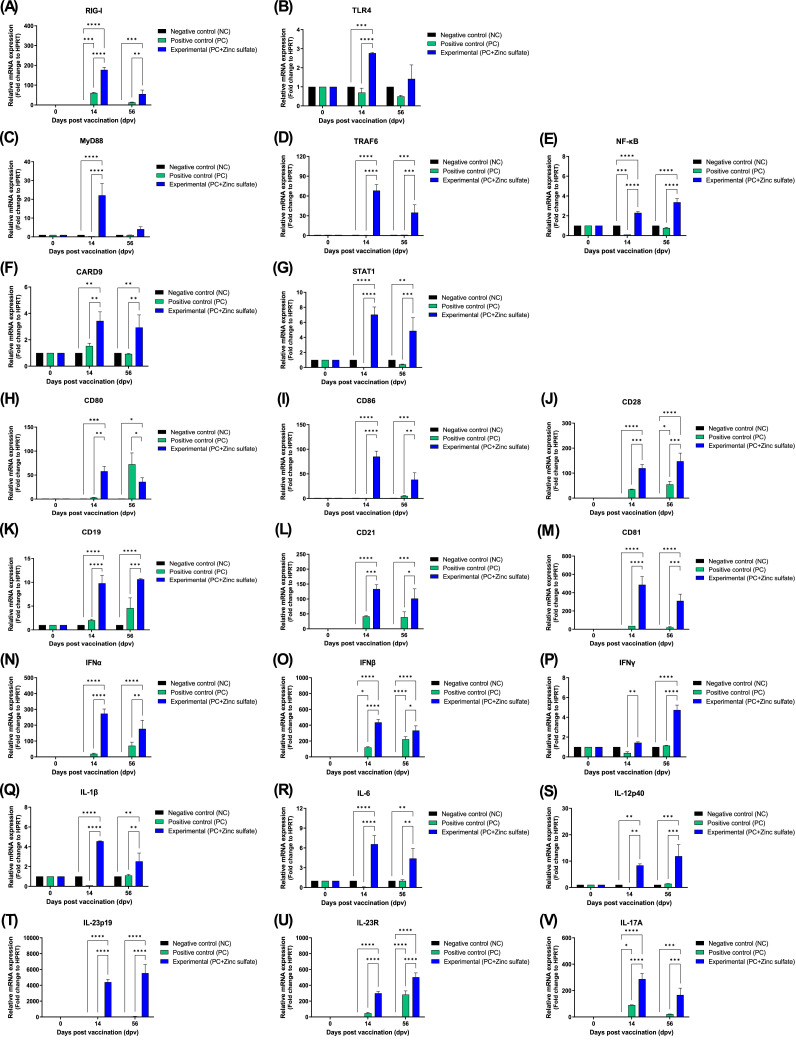
The mechanism through which FMD vaccine containing zinc sulfate as immunostimulant induces cellular and humoral immune responses was investigated. PBMCs were isolated from whole blood at 14 and 56 dpv after vaccination and used for qRT-PCR (Fig. 6A–V). The expression levels of PRRs [retinoic acid-inducible gene (RIG)-I, and Toll-like receptor (TLR)4], transcription factors [myeloid differentiation primary response (MyD)88, tumor necrosis factor (TNF) receptor associated factor (TRAF)6, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and signal transducer and activator of transcription (STAT1)], co-stimulatory molecules [cluster of differentiation (CD)21, CD19, CD81, CD28, CD80, and CD86], and cytokines [interferon (IFN)α, IFNβ, IFNγ, IL-1β, IL-6, IL-12p40, IL-23p19, IL-23R, and IL-17A] were compared between the groups. Compared with the PC group, the Exp group had significantly higher expression levels of RIG-I (p < 0.0001), TLR4 (p < 0.0001), and MyD88 (p < 0.0001) genes at 14 dpv (Figs. 6A–C). Additionally, the Exp group had significantly higher expression levels of transcription factors, including TRAF6 (p < 0.0001), NF-κB (p < 0.0001), CARD9 (p < 0.01), and STAT1 (p < 0.0001) (Figs. 6D–G), and co-stimulatory molecules, including CD80 (p < 0.01), CD86 (p < 0.0001), CD28 (p < 0.001), CD19 (p < 0.0001), CD21 (p < 0.001), and CD81 (p < 0.0001) than that of the PC group at 14 dpv (Fig. 6H–M). Compared with the PC group, the Exp group had significantly higher expression levels of IFNα (p < 0.0001), IFNβ (p < 0.0001), IFNγ (p < 0.01), IL-1β (p < 0.0001), IL-6 (p < 0.0001), IL-12p40 (p < 0.01), IL-23p19 (p < 0.0001), IL-23R (p < 0.0001), and IL-17A (p < 0.0001) at 14 dpv (Fig. 6N–V).
Fig. 6.
Zinc sulfate-containing FMD vaccine effectively elicit cellular and humoral immune responses in porcine PBMCs by inducing the expression of PRRs, transcription factors, co-stimulatory molecules, and cytokines. (A–V) Gene expression levels of RIG-I (A); TLR4 (B); MyD88 (C); TRAF6 (D); NF-κB (E); CARD9 (F); STAT1 (G); CD80 (H); CD86 (I); CD28 (J); CD19 (K); CD21 (L); CD81 (M); IFNα (N); IFNβ (O); IFNγ (P); IL-1β (Q); IL-6 (R); IL-12p40 (S); IL-23p19 (T); IL-23R (U); and IL-17A (V). ⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001, and ⁎⁎⁎⁎p < 0.0001.
4. Discussion
Presently, FMD vaccines are produced as monovalent or multivalent inactivated vaccines containing gel or oil emulsion as adjuvant and FMDV type O, A antigens, or a combination of both antigens (Sáiz et al., 2002). Gel vaccines were used only in cattle because of the short immunization period in pigs; however, vaccines with improved efficacy have been commercially prepared using oil emulsion as adjuvant (Doel, 1996). However, vaccines containing oil emulsions take long time to reach antibody titer levels necessary to induce host defense, limiting their ability to induce rapid protection. Therefore, studies are currently ongoing to develop novel vaccine adjuvants, including immunostimulants, capable of initiating early host defense by simultaneously inducing cellular and humoral immune responses (Bonam et al., 2017; Di Pasquale et al., 2015). However, little is known about the mechanism of the host response to vaccine adjuvant, especially immunostimulants; therefore, further research is needed. Adjuvants can reduce the antigenic content of vaccines, trigger cellular immune responses, and overcome weak immunity, and an ideal adjuvant should be stable, safe, and easy to produce and store. Zinc sulfate is used as a supplement to treat zinc deficiency-related immune system abnormalities (Prasad et al., 1988; Shankar and Prasad, 1998). Several studies have reported that zinc has antiviral activity against rhinovirus, equine arteritis virus (EVA), respiratory syncytial virus (RSV), infectious gastroenteritis virus, and severe acute respiratory syndrome coronavirus (SARS-CoV) (Kaushik et al., 2018; Korant et al., 1974; Suara and Crowe Jr, 2004; Te Velthuis et al., 2010; Wei et al., 2012). In the early 1974, zinc was found to have an inhibitory effect on Piconavirus polyprotein processing; additionally, zinc has been confirmed to inhibit FMDV protease activity (Polatnick and Bachrach, 1978). Moreover, studies have shown that treatment with appropriate concentrations of zinc ionophores, such as hydroxycycloquine, suppressed viral activity in respiratory diseases, such as COVID-19 (Jayawardena et al., 2020).
A previous study showed that immune response was enhanced using PRRs ligands, such as Mincle and STING, as adjuvants for FMD vaccine (Lee et al., 2019). TLRs are a subgroup of PRRs that link innate and acquired immunity, and are expressed in DCs and macrophages (MФs) (Roßmann et al., 2021). Adjuvant such as TLRs agonist, and Al(OH)3, can induce the maturation of DCs, thus enhancing vaccine response by stimulating and modulating innate immune response and inducing adaptive immune response (Jo et al., 2021; Ostuni et al., 2010). For instance, the binding of lipopolysaccharide (LPS) to TLR4 induces the recruitment of the adapter protein MyD88, activating NF-κB in the early stages, resulting in the secretion of IFNβ and IL-6 (Huang et al., 2004). TLR4 regulates the expression of inflammatory cytokine genes by activating TRAF6 through MyD88 and inducing the activation of NF-κB. This signaling pathway activates genes encoding subunits of IL-12 and IL-23 (Grumont et al., 2001; Lien and Golenbock, 2003; Yoshida et al., 2008). IL-23, a complex of IL-23p19 and IL-12p40, is mainly secreted by activated intestinal APCs, such as DCs and monocytes (Langrish et al., 2005). IL-23 stimulates γδ T and Th17 cells to produce IL-17, and induces T cell proliferation (Beadling and Slifka, 2006; Lin et al., 2009). Notably, γδ T cell, a major source of IL-17A, plays an important role in host defense by recruiting neutrophils from mucosal and non-mucosal tissues to the site of infection in the early stages of viral infection, forming neutrophil extracellular traps (NETs), and removing pathogens via NETosis (Ma et al., 2019). TLR4-mediated signaling pathway also plays a key role in stimulating and activating APCs, generating Th1 and cytotoxic T lymphocyte (CTL) responses and regulating B cell responses during viral infections (Wang et al., 2011). RIG-I recognizes viral RNA and activates CARD9, and TRAF6 induces transcription factors, such as IFNβ (Poeck et al., 2010).
In the present study, treatment with zinc sulfate alone or in combination with the antigens effectively induced cellular immune response by increasing IFNγ secretion in murine PECs and porcine PBMCs, indicating that zinc sulfate can be used as a novel adjuvant for FMD vaccine.
IFNγ is a cytokine of the type II class of interferons (Tau and Rothman, 1999), which are primarily secreted by activated lymphocytes, such as natural killer (NK), γδ T, CD4+ Th1, and CD8+ cytotoxic T cells. The expression of type II IFN is up- or down-regulated by mitogens and cytokines, such as IL-12, IL-15, IL-18, and type I IFN (Gray and Goeddel, 1982). Type II IFN can also activate signaling pathways in cells, such as MФs, B cells, and CD8+ cytotoxic T cells, to promote antiviral activity, inflammation, or cell proliferation and differentiation (Castro et al., 2018). In the present study, ELISpot assay showed that zinc sulfate-containing vaccines induced significant IFNγ secretion, and that even small amount of zinc sulfate (0.625 µg/mL) induced IFNγ secretion and effectively induce a cellular immune response (Fig. 1).
Accordingly, an Exp vaccine was prepared using zinc sulfate as an adjuvant (specifically as an immunostimulant) and FMDV type O and type A antigens, and its ability to induce early host defense against FMDV was investigated. Mice administered the novel FMD vaccine containing zinc sulfate exhibited 100% survival rate, indicating that zinc sulfate is an effective FMD vaccine adjuvant (Fig. 2).
An evaluation of the effect of the bivalent vaccine containing zinc sulfate on early, mid- and long-term immune responses of mice and pigs (0–84 dpv) showed that antibody and VN titers were significantly higher in the Exp group than in the NC and PC groups, indicating that zinc sulfate can be used as an adjuvant with promising results (Figs. 3, 4). Additionally, serum samples from pigs vaccinated with the Exp vaccine containing zinc sulfate had significantly higher antibody titers compared with the PC group, as confirmed by SP O and A ELISA. Moreover, VN titers against the O PA2 and A YC viruses were significantly higher in the Exp group, and these immune responses were maintained after booster shot. Overall, these results showed that the addition of zinc sulfate to FMD vaccine as an immunostimulant effectively induced cellular and humoral immune responses in pigs, improving early, mid-, and long-term immune responses (Fig. 4).
In the present study, animals were considered seropositive for O PA2 and A YC antigens at PI ≥ 40% and ≥ 50%, respectively. FMD vaccines have been reported to improve host defense at VN titers > 1.74 (Log10) (Black et al., 1984), and VN titer > 1.65 (Log10) is regarded to induce sufficient host defense, according to the FMD vaccine evaluation standard of Republic of Korea (Shin et al., 2022). In the present study, VN titer was maintained at > 1.74 (Log10) up to 84 dpv after booster shot in the Exp group compared with that of the PC group, indicating that zinc sulfate may play an important role in inducing early immune response that is maintained in the long-term and provide cross-protection against FMDV type O and A infections. Additionally, isotype ELISA showed that there were significant differences in IgG and IgA levels between the Exp and PC groups at 56 dpv (Fig. 5). Overall, the results of these serological assays indicated that zinc sulfate can increase and maintain long-term antibody titers, VN titers, and total IgG and IgA levels, suggesting that the vaccine can achieve robust and long-lasting humoral immune responses.
To identify the mechanism of zinc sulfate-containing vaccines in the host immune response, the expression of PRRs, transcription factors, co-stimulatory molecules, and cytokines in porcine PBMCs isolated from whole blood at 14 and 56 dpv was examined using qRT-PCR. Compared with the PC group, administration of the zinc sulfate-containing Exp vaccine significantly increased the expression of immune response-related genes. An increase in RIG-I, a PRR, induces the expression of type I IFNs (IFNα and IFNβ), which activates NF-κB via TRAF6. Type I IFNs are cytokines that play critical roles in immune regulation, tumor cell recognition, inflammation, and T cell responses (Razaghi et al., 2021). Almost every cell in the host body can secrete IFNα and IFNβ, which usually occurs in response to stimulation of receptors known as PRRs. The RNA helicases RIG-I and melanoma differentiation-associated gene 5 (MDA5) are the major cytosolic receptors that are responsible for the recognition of viral RNA (Goubau et al., 2013).
In addition to these cytoplasmic receptors, several TLRs stimulate pathways involved in IFNα and IFNβ secretion. TLR4, which recognizes LPS, is the most potent type I IFN inducer and signals through the adapter protein TIR domain-containing adapter protein-induced IFNβ (TRIF) (Moynagh, 2005). NF-κB also serves as a cofactor in IFNα/β production (Honda et al., 2006). TRAF6 is an important factor for the activation of NF-κB and the production of type I IFN, and activates IKK (IκB kinase) in response to proinflammatory cytokines (Konno et al., 2009).
Additionally, the expression of co-stimulatory molecules CD80/CD86 in APCs activates T cells by binding to CD28 on Th1 cells, and the expression of IL-12 and STAT1 activates Th1 cells.
Signaling occurs when a T cell meets an APC, forming an immune synapse. For successful signal transduction, T cell receptors must bind to MHC, and co-stimulatory molecules, such as CD28 to CD80/CD86 (Pentcheva-Hoang et al., 2004). CD86 has been shown to enhance the interaction between DCs and T cells, as higher force reductions were founded after blocking CD86 alone (Lim et al., 2012). IL-12 is an interleukin naturally expressed by DCs, neutrophils, MФs, and human B lymphoblastoid cells (NC-37) in response to antigenic stimulation (Dale et al., 1989). STAT1 is a key mediator of cellular responses to IFN, and IFN expression and STAT1 activation are crucial steps in the initial immune response to viral infection (Parham et al., 2002). CD19 on the B cell surface forms a complex with CD21 and CD81 to induce B cell activity. B cells express the CD21 receptor on their surface, allowing the complement system to induce B cell activation and maturation. CD21 interacts with CD19 to form a complex with CD81 in mature B cells, which is called the B cell co-stimulatory complex (Abbas, 2003). Overall, it could be concluded that the administration of FMD vaccine containing zinc sulfate as an immunostimulant simultaneously induced cellular and humoral immune responses in pigs via the above signal transduction processes.
Our present study demonstrates that zinc sulfate induces NF-κB activation in an APCs-dependent manner through PRRs, cytokines, co-stimulatory molecules, and transcription factors. The mechanism of zinc sulfate in host immune response could be described as follows: zinc sulfate stimulated the TLR4 signaling pathway, promoting TRAF6 expression in APCs via MyD88 and inducing NF-κB mediated activation of proinflammatory cytokines, including IFNγ, IL-6, and IL-1β. Additionally, the increased expression of STAT1, IL-12, IL-17A, IL-23R, CD28, CD80, and CD86 activated T cells and induced B cell activity, with increased expression of CD19, CD21, and CD81, resulting in an immune-enhancing effect (Fig. 6 and Fig. 7).
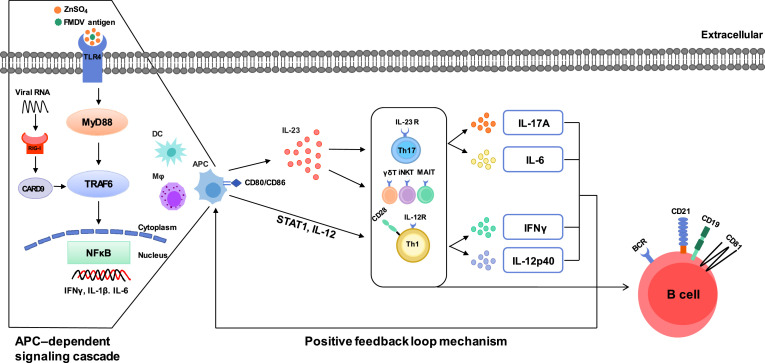
Fig. 7.
Schematic illustration of the potential mechanisms of zinc sulfate-containing FMD vaccine.
The stimulation of TLR4 by zinc sulfate and antigen induces the recruitment of the adaptor protein MyD88 via downstream TRAF6, resulting in the activation of NF-κB and the mRNA expression of proinflammatory cytokines, such as IFNγ, IL-1β and IL-6 in APCs such as DCs and MФs. This is APC-dependent signaling cascade. CD80 and CD86 are then stimulated on the APC surface, which induced STAT1 and produced the proinflammatory cytokines IL-12 and IL-23. IL-23 produces IL-17A and IL-6 by binding to IL-23R on the surface of Th17 and unconventional T cells such as γδ T cells,invariant natural killer T (iNKT) cells, and mucosal-associated invariant T (MAIT) cells. IL-12 binds to IL-12R on the surface of Th1 cells and produces IFNγ and IL-12p40. Activated Th1 cell express CD28, restimulate APC through a positive feedback loop, while activating B cells and B cell core-receptor such as CD19, CD21, and CD81, resulting in immune-enhancing effect. Collectively, zinc sulfate contributes to cellular and humoral immunity by stimulating TLR4 and RIG-I and promoting the release of various transcription factors, co-stimulatory molecules, and cytokines.
Overall, these results suggest that the novel immunostimulant zinc sulfate induced a robust immune response by stimulating APCs, T, and B cells through stimulation of PRRs, and contributed to long-term immunity, including cellular and humoral immune responses by promoting the production of proinflammatory cytokines, transcription factors, and co-stimulatory molecules.
In summary, zinc sulfate was effective as an immunostimulant and adjuvant in the novel FMD type O and A bivalent vaccine for FMD prevention. However, further studies are necessary to examine the effect of administration routes, such as oral, on the efficacy of the vaccine. Based on these results, zinc sulfate could be used as a novel vaccine immunostimulant for viral diseases, such as African swine fever (ASF), avian influenza (AI), or COVID-19, which are difficult to control and prevention. Particularly, the vaccine is expected to have a wide range of applications, such as use as bait vaccine in the case of wildlife-borne disease such as ASF and AI.
CRediT authorship contribution statement
Mi-Kyeong Ko: Software, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Hyeong Won Kim: Software, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. So Hui Park: Investigation. Jong-Hyeon Park: Resources. Su-Mi Kim: Resources. Min Ja Lee: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no know competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This project was supported by grants from the APQA (B-1543386-2022-24).
Acknowledgments
This project was conducted independently using the APQA. We thank the staff and researchers of the Center for FMD Vaccine Research at APQA.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199189.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Afsharian M., Vaziri S., Janbakhsh A.R., Sayad B., Mansouri F., Nourbakhsh J., Qadiri K., Najafi F., Shirvanii M. The effect of zinc sulfate on immunologic response to recombinant hepatitis B vaccine in elderly: zinc sulfate and immunologic response to recombinant hepatitis B vaccine. Hepat. Mon. 2011;11(1):32–35. [PMC free article] [PubMed] [Google Scholar]
- Abbas A.K., Lichtman A.H. 5th ed. PA Saunders Co; Philadelphia: 2003. Cellular and Molecular Immunology. [Google Scholar]
- Alexandersen S., Zhang Z., Donaldson A.I., Garland A.J. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 2003;129(1):1–36. doi: 10.1016/s0021-9975(03)00041-0. [DOI] [PubMed] [Google Scholar]
- Amanna, I.J., Slifka, M.K., 2020. Successful vaccines. Vaccination Strategies Against Highly Variable Pathogens, 1–30.
- Beadling C., Slifka M.K. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch. Immunol. Ther. Exp. (Warsz.) 2006;54(1):15–24. doi: 10.1007/s00005-006-0002-6. [DOI] [PubMed] [Google Scholar]
- Black L., Francis M.J., Rweyemamu M.M., Umebara O., Boge A. The relationship between serum antibody titres and protection from foot and mouth disease in pigs after oil emulsion vaccination. J. Biol. Stand. 1984;12(4):379–389. doi: 10.1016/s0092-1157(84)80062-1. [DOI] [PubMed] [Google Scholar]
- Bonam S.R., Partidos C.D., Halmuthur S.K.M., Muller S. An overview of novel adjuvants designed for improving vaccine efficacy. Trends Pharmacol. Sci. 2017;38(9):771–793. doi: 10.1016/j.tips.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Cao Y. Adjuvants for foot-and-mouth disease virus vaccines: recent progress. Expert Rev. Vaccines. 2014;13(11):1377–1385. doi: 10.1586/14760584.2014.963562. [DOI] [PubMed] [Google Scholar]
- Carr B.V., Lefevre E.A., Windsor M.A., Inghese C., Gubbins S., Prentice H., Juleff N.D., Charleston B. CD4+ T-cell responses to foot-and-mouth disease virus in vaccinated cattle. J. Gen. Virol. 2013;94:97–107. doi: 10.1099/vir.0.045732-0. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro F., Cardoso A.P., Gonçalves R.M., Serre K., Oliveira M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale T.C., Imam A., Kerr I.M., Stark G.R. Rapid activation by interferon alpha of a latent DNA-binding protein present in the cytoplasm of untreated cells. P NAS. 1989;86(4):1203–1207. doi: 10.1073/pnas.86.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard K. Adjuvants. Vet. Immunol. Immunopathol. 1987;17(1–4):145–152. doi: 10.1016/0165-2427(87)90135-8. [DOI] [PubMed] [Google Scholar]
- Di Pasquale A., Preiss S., Tavares Da Silva F., Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines (Basel) 2015;3(2):320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doel T. Natural and vaccine-induced immunity to foot and mouth disease: the prospects for improved vaccines. Rev. Off. Int. Epizoot. 1996;15(3):883–911. doi: 10.20506/rst.15.3.955. [DOI] [PubMed] [Google Scholar]
- Donaldson A.I. Foot-and-mouth disease: the principal features. Ir. Vet. J. 1987;(41):325–327. [Google Scholar]
- Finlay B.B., McFadden G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006;124(4):767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Goubau D., Deddouche S., e Sousa C.R. Cytosolic sensing of viruses. Immunity. 2013;38(5):855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P.W., Goeddel D.V. Structure of the human immune interferon gene. Nature. 1982;298(5877):859–863. doi: 10.1038/298859a0. [DOI] [PubMed] [Google Scholar]
- Grumont R., Hochrein H., O'Keeffe M., Gugasyan R., White C., Caminschi I., Cook W., Gerondakis S. c-Rel regulates interleukin 12 p70 expression in CD8+ dendritic cells by specifically inducing p35 gene transcription. J. Exp. Med. 2001;194(8):1021–1032. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman E., Taylor G., Charleston B., Ellis S.A. Induction of a cross-reactive CD8+ T cell response following foot-and-mouth disease virus vaccination. J. Virol. 2010;84(23):12375–12384. doi: 10.1128/JVI.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H., Rink L. The immune system and the impact of zinc during aging. Immun. Ageing. 2009;6(1):1–17. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen M.M., Fijten H.P., Westra D.F., Dekker A. Stabilizing effects of excipients on dissociation of intact (146S) foot-and-mouth disease virions into 12S particles during storage as oil-emulsion vaccine. Vaccine. 2015;33(21):2477–2484. doi: 10.1016/j.vaccine.2015.03.066. [DOI] [PubMed] [Google Scholar]
- Honda K., Takaoka A., Taniguchi T. Type I inteferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25(3):349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Huang Q., Yang J., Lin Y., Walker C., Cheng J., Liu Z.-g., Su B. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat. Immunol. 2004;5(1):98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab. Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wang Q., Li L., Zeng Q., Li H., Gong T., Zhang Z., Sun X. Turning the old adjuvant from gel to nanoparticles to amplify CD8(+) T cell responses. Adv. Sci. (Weinh) 2018;5(1) doi: 10.1002/advs.201700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H., Kim B.Y., Park S.H., Kim H.M., Shin S.H., Hwang S.Y., Kim S.M., Kim B., Park J.H., Lee M.J. The HSP70-fused foot-and-mouth disease epitope elicits cellular and humoral immunity and drives broad-spectrum protective efficacy. NPJ Vaccines. 2021;6(1):42. doi: 10.1038/s41541-021-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel M., El-Sayed A., Castañeda Vazquez H. Foot-and-mouth disease vaccines: recent updates and future perspectives. Arch. Virol. 2019;164(6):1501–1513. doi: 10.1007/s00705-019-04216-x. [DOI] [PubMed] [Google Scholar]
- Kaushik N., Anang S., Ganti K.P., Surjit M. Zinc: a potential antiviral against hepatitis E virus infection? DNA Cell Biol. 2018;37(7):593–599. doi: 10.1089/dna.2018.4175. [DOI] [PubMed] [Google Scholar]
- Keen C.L., Gershwin M.E. Zinc deficiency and immune function. Annu. Rev. Nutr. 1990;10(1):415–431. doi: 10.1146/annurev.nu.10.070190.002215. [DOI] [PubMed] [Google Scholar]
- Konno H., Yamamoto T., Yamazaki K., Gohda J., Akiyama T., Semba K., Goto H., Kato A., Yujiri T., Imai T. TRAF6 establishes innate immune responses by activating NF-κB and IRF7 upon sensing cytosolic viral RNA and DNA. PLoS One. 2009;4(5):e5674. doi: 10.1371/journal.pone.0005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B., Kauer J., Butterworth B. Zinc ions inhibit replication of rhinoviruses. Nature. 1974;248(5449):588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle E.C., Leavy O., Mills K.H. Springer; 2006. Modified Bacterial Toxins, Vaccine Adjuvants; pp. 111–153. [Google Scholar]
- Lee M.J., Jo H., Park S.H., Ko M.K., Kim S.M., Kim B., Park J.H. Advanced foot-and-mouth disease vaccine platform for stimulation of simultaneous cellular and humoral immune responses. Vaccines (Basel) 2020;8(2):254. doi: 10.3390/vaccines8020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Jo H., Shin S.H., Kim S.M., Kim B., Shim H.S., Park J.H. Mincle and STING-stimulating adjuvants elicit robust cellular immunity and drive long-lasting memory responses in a foot-and-mouth disease vaccine. Front. Immunol. 2019;10:2509. doi: 10.3389/fimmu.2019.02509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Kim H.M., Shin S., Jo H., Park S.H., Kim S.M., Park J.H. The C3d-fused foot-and-mouth disease vaccine platform overcomes maternally-derived antibody interference by inducing a potent adaptive immunity. NPJ Vaccines. 2022;7(1):70. doi: 10.1038/s41541-022-00496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Lee Y.J., Kim R.H., Park J.N., Park M.E., Ko M.K., Choi J.H., Chu J.Q., Lee K.N., Kim S.M. Rapid engineering of foot-and-mouth disease vaccine and challenge viruses. J. Virol. 2017;91(16):e00155. doi: 10.1128/JVI.00155-17. –00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E., Golenbock D.T. Adjuvants and their signaling pathways: beyond TLRs. Nat. Immunol. 2003;4(12):1162–1164. doi: 10.1038/ni1203-1162. [DOI] [PubMed] [Google Scholar]
- Lim T.S., Goh J.K.H., Mortellaro A., Lim C.T., Hämmerling G.J., Ricciardi-Castagnoli P. CD80 and CD86 differentially regulate mechanical interactions of T-cells with antigen-presenting dendritic cells and B-cells. PLoS One. 2012;7(9):e45185. doi: 10.1371/journal.pone.0045185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Ritchea S., Logar A., Slight S., Messmer M., Rangel-Moreno J., Guglani L., Alcorn J.F., Strawbridge H., Park S.M. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Yu S., Wang W., Chen W., Wang X., Wu K., Li X., Fan S., Ding H., Yi L., Chen J. Development of foot-and-mouth disease vaccines in recent years. Vaccines (Basel) 2022;10(11):1817. doi: 10.3390/vaccines10111817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W.T., Yao X.T., Peng Q., Chen D.K. The protective and pathogenic roles of IL-17 in viral infections: friend or foe? Open Biol. 2019;9(7) doi: 10.1098/rsob.190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell W.A., Meng I., Nicholson S.A., Aspinall R. Thymic output, ageing and zinc. Biogerontology. 2006;7(5):461–470. doi: 10.1007/s10522-006-9061-7. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3(4):177–184. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- Moynagh P.N. TLR signalling and activation of IRFs: revisiting old friends from the NF-κB pathway. Trends Immunol. 2005;26(9):469–476. doi: 10.1016/j.it.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. JBCP. 2016;7(2):27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni R., Zanoni I., Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell. Mol. Life Sci. 2010;67(24):4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham C., Chirica M., Timans J., Vaisberg E., Travis M., Cheung J., Pflanz S., Zhang R., Singh K.P., Vega F. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002;168(11):5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- Pentcheva-Hoang T., Egen J.G., Wojnoonski K., Allison J.P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21(3):401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M., Hannesschläger N., Schlee M., Rothenfusser S., Barchet W. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 2010;11(1):63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- Polatnick J., Bachrach H.L. Effect of zinc and other chemical agents on foot-and-mouth disease virus replication. Antimicrob. Agents Chemother. 1978;13(5):731–734. doi: 10.1128/aac.13.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000;182(1):S62–S68. doi: 10.1086/315916. Supplement_. [DOI] [PubMed] [Google Scholar]
- Prasad A.S., Meftah S., Abdallah J., Kaplan J., Brewer G.J., Bach J., Dardenne M. Serum thymulin in human zinc deficiency. J. Clin. Investig. 1988;82(4):1202–1210. doi: 10.1172/JCI113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razaghi A., Brusselaers N., Björnstedt M., Durand-Dubief M. Copy number alteration of the interferon gene cluster in cancer: individual patient data meta-analysis prospects to personalized immunotherapy. Neoplasia. 2021;23(10):1059–1068. doi: 10.1016/j.neo.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roßmann L., Bagola K., Stephen T., Gerards A.L., Walber B., Ullrich A., Schülke S., Kamp C., Spreitzer I., Hasan M. Distinct single-component adjuvants steer human DC-mediated T-cell polarization via Toll-like receptor signaling toward a potent antiviral immune response. Proc. Natl. Acad. Sci. 2021;118(39) doi: 10.1073/pnas.2103651118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockville, M.D., US Food and Drug Administration. 2005. USFDA. Guidance for industry: estimating the maximum safe starting dose in adult healthy volunteer.
- Sáiz M., Núñez J.I., Jimenez-Clavero M.A., Baranowski E., Sobrino F. Foot-and-mouth disease virus: biology and prospects for disease control. Microbes Infect. 2002;4(11):1183–1192. doi: 10.1016/s1286-4579(02)01644-1. [DOI] [PubMed] [Google Scholar]
- Samuel A.R., Knowles N.J. Foot-and-mouth disease type O viruses exhibit genetically and geographically distinct evolutionary lineages (topotypes) J. Gen. Virol. 2001;82(3):609–621. doi: 10.1099/0022-1317-82-3-609. [DOI] [PubMed] [Google Scholar]
- Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. 1998;68(2):447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Shin S., Park S.H., Park J.H., Kim S.M., Lee M.J. Age-dependent dynamics of maternally derived antibodies (Mdas) and understanding mda-mediated immune tolerance in foot-and-Mouth disease-vaccinated pigs. Vaccines (Basel) 2022;10(5):677. doi: 10.3390/vaccines10050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suara R.O., Crowe Jr J.E. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2004;48(3):783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau G., Rothman P. Biologic functions of the IFN-γ receptors. Allergy. 1999;54(12):1233. doi: 10.1034/j.1398-9995.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chan C., Yang M., Deng J., Poon V.K., Leung V.H., Ko K.H., Zhou J., Yung Yuen K., Zheng B.J. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell. Mol. Immunol. 2011;8(6):462–468. doi: 10.1038/cmi.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Burwinkel M., Palissa C., Ephraim E., Schmidt M.F. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet. Microbiol. 2012;160(3–4):468–472. doi: 10.1016/j.vetmic.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Zhang Y., Yin X., He Y., Zhang Q., Chen C. Layered double hydroxide nanoparticles as asn adjuvant for inactivated foot-and-mouth disease vaccine in pigs. BMC Vet. Res. 2020;16:1–19. doi: 10.1186/s12917-020-02689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Takaesu G., Yoshida H., Okamoto F., Yoshioka T., Choi Y., Akira S., Kawai T., Yoshimura A., Kobayashi T. TRAF6 and MEKK1 play a pivotal role in the RIG-I-like helicase antiviral pathway. J. Biol. Chem. 2008;283(52):36211–36220. doi: 10.1074/jbc.M806576200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.