Abstract
Photoacoustic imaging (PAI), also referred to as optoacoustic imaging, has shown promise in early-stage clinical trials in a range of applications from inflammatory diseases to cancer. While the first PAI systems have recently received regulatory approvals, successful adoption of PAI technology into healthcare systems for clinical decision making must still overcome a range of barriers, from education and training to data acquisition and interpretation. The International Photoacoustic Standardisation Consortium (IPASC) undertook an community exercise in 2022 to identify and understand these barriers, then develop a roadmap of strategic plans to address them. Here, we outline the nature and scope of the barriers that were identified, along with short-, medium- and long-term community efforts required to overcome them, both within and beyond the IPASC group.
Keywords: Photoacoustic imaging, Optoacoustic tomography, Standardisation, Quality assurance, Phantoms, Clinical translation
1. Introduction
Photoacoustic imaging (PAI), also referred to as optoacoustic imaging, is an exciting biomedical imaging technology that combines laser excitation with acoustic detection to enable deep-tissue imaging of optical absorption [1], [2]. PAI provides images with strong contrast based on light absorption by chromophores, such as haemoglobin found in blood, or exogenous contrast agents [3], [4]. PAI is also sensitive to physiological parameters such as flow [5] and temperature [6], [7]. Scattering of sound waves in tissue is far lower than that of light, meaning PAI can maintain spatial resolution over much greater imaging depths than all-optical imaging (order of cm rather than mm). Many different embodiments of PAI technology exist, ranging from 2D imaging and 3D tomography systems, which achieve moderate spatial resolution with high imaging depth through diffuse illumination and ultrasound array detection schemes, down to microscopy or endoscopy systems, which typically achieve high resolution at more limited depth by optical or acoustic focusing schemes.
PAI has been shown to reveal changes in tissue oxygenation in response to disease progression in a range of mouse models [8], [9], [10], [11], [12], [13], [14] and some larger animal models [15]. More importantly, PAI is on the pathway for clinical translation into mainstream medicine [16], [17], with a variety of applications demonstrated in patients using the tomographic PAI geometry, with a substantial number of studies applying PAI for cancer visualisation, especially in breast cancer [18], [19], [20], [21]. Recently, a wide range of potential further clinical applications have emerged, such as evaluation of skin microvasculature [22], [23], functional and endoscopic assessment of the gastrointestinal tract [24], [25], surgical guidance [26], monitoring inflammation [27], [28], [29] and monitoring lymphadenopathies [30] / lymphedema [31]. These diverse applications demonstrate the future clinical potential for PAI underpinned by the versatile nature of the hardware implementations available. Given the more frequent reporting of macroscopic imaging and tomography approaches in PAI clinical applications to date, the commentary here will focus on these implementations of the technology primarily using endogenous haemoglobin contrast, though many of the conclusions drawn from the roadmapping exercise apply broadly across all PAI system configurations. Due to the different nature of the requirements for clinical translation of new exogenous contrast agents, these are beyond the scope of the present review.
Despite the substantial growth of the PAI research community, significant investment by industry and government agencies, and promise for improved patient care, PAI has yet to be widely adopted in healthcare systems. Commercial systems are available, with some currently marketed for investigational or research use. Two clinical-grade devices have received the European CE mark [32], [33], which is not designated to a particular application. One breast cancer imaging device recently received US FDA approval [34]. One general purpose device has obtained manufacturing and marketing approval by the Japanese PMDA [35]. Nonetheless, there are many well-recognised challenges that must be overcome in navigating the translation of new optical imaging devices into clinical practice [36]. Enabling the adoption of new devices into clinical research and practice requires: the availability of equipment for clinical practitioners to develop experience; evidence building to demonstrate the clinical benefit of the technology, for instance through both technical validation of imaging biomarker measurement performance and biological validation of a well-established association with underlying physiological or pathological processes [37]; and ultimately showing an impact on patient care, which may be achieved with available reimbursement codes for widespread clinical use.
To achieve such validation, widespread availability of standardized test objects and methods is required. Internationally recognised standards that establish tools, test methods, and best practices for objective, quantitative image quality assessment exist for routine medical imaging modalities such as MRI, CT, and ultrasound [38]. For PAI, no such standards exist due to its nascent status, placing a burden on researchers and device manufacturers to design their own test methods. This leads to duplication of effort, lack of community consensus, and delayed patient access to safe and effective devices. Learning from other medical imaging communities, the availability of well-validated, consensus-based performance test methods for PAI systems would greatly accelerate device design optimization, device inter-comparison, quality assurance/control testing, and regulatory evaluation.
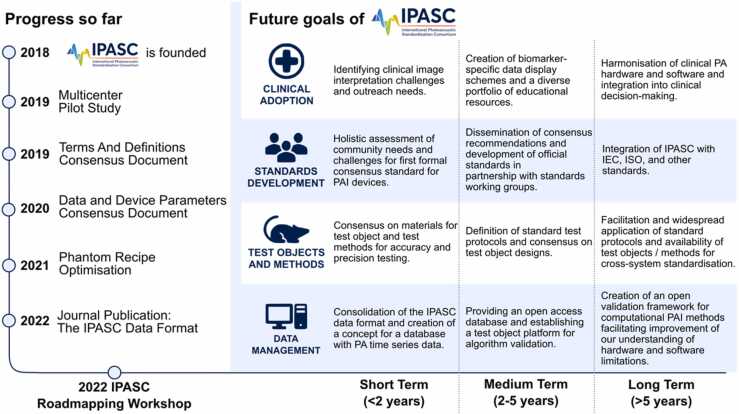
The International Photoacoustic Standardisation Consortium (IPASC) [39] was established in 2018 to unite the PAI community in achieving standardisation of test methods and data handling through consultation and consensus-finding. The IPASC membership currently includes representation from researchers, device developers, clinicians and government agencies, but does not encompass other stakeholders who will ultimately be important in the translational process, such as regulatory decision makers, policy makers or public or patient groups. In 2022, IPASC held a one-day roadmapping workshop to identify the barriers currently impeding clinical translation of PAI and to develop roadmaps that can guide the PAI community in overcoming those barriers, with the goal of enabling PAI to deliver on its potential through adoption into healthcare systems. The workshop involved 53 participants, including consortium members and those with a broader experience of standardisation activities in other imaging modalities. Attendees were drawn from a wide international horizon, representing 39 different organisations (including 8 PAI instrument vendors) from the UK, Ireland, the Netherlands, Germany, Switzerland, Italy, USA, Canada and Singapore. Here, we outline the nature and scope of the barriers that were identified, along with short-, medium- and long-term community efforts required to overcome them, both within and beyond the IPASC group.
2. Current barriers to clinical translation of PAI
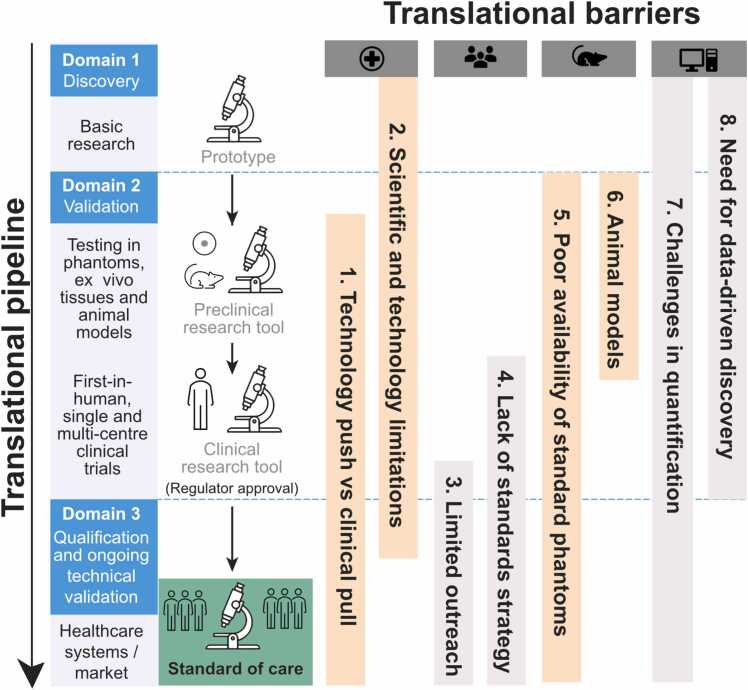
Prior to the roadmapping workshop, all attendees were asked to provide their views on the key barriers that they could foresee for clinical translation of PAI, according to the categories of: research, pre-clinical (animals or phantoms), clinical, regulatory, and other. They were also asked to define what resources, enablers or data they felt were necessary to overcome the barrier and what they believed the objectives for IPASC should be in this context. The raw data was compiled from the pre-work submitted by 26 of the workshop attendees, which yielded 133 responses for the question on key barriers. From these responses, the IPASC leadership team drew out eight broad groups of commonly identified barriers (illustrated in Fig. 1), which were expanded in scope during the workshop and then explored in detail to prepare summary recommendations and formulate related roadmaps. The key concerns that were grouped within each barrier are elaborated below.
Fig. 1.
Mapping the barriers identified, separated horizontally, onto their position in the clinical translational pipeline, shown in the vertical direction. The translational pipeline is divided according to phases of discovery, validation and qualification, as defined previously [36]. Each of the thematic groupings of barriers (left to right: clinical adoption, standards development, test objects and methods, and data management) are explained in more detail in the text and form the basis of the output roadmaps.
2.1. Barrier 1: mismatch between technology push and clinical pull
When translating a new technology into clinical use, co-development between technology innovators and clinicians is vital. Important clinical factors such as quality assurance or control (QA/QC), availability of operator or interpreter expertise, and patient acceptability are far from typical in the research and development landscape. Similarly, identifying clinical applications where greatest benefit can be derived is non-trivial; one must identify important clinical problems where PAI has the potential to either outperform the current standard-of-care, or be more cost effective. Such knowledge lies with clinicians and healthcare systems and is rarely captured and disseminated to innovators. Conversely, the capabilities and performance characteristics of PAI lie in the domain of the innovators and are rarely shared in an accessible manner in clinical educational programmes.
Unsurprisingly, many PAI clinical studies are of a small pilot scale “try it and see” nature with early enthusiastic clinical adopters, typically performed in centres with a strong PAI research team, or in close collaboration with vendors. While highly valuable to device development and optimization for clinical use, such studies are often not designed or intended to demonstrate statistically-powered evidence to support broader clinical uptake in the specific application. Furthermore, transparency of reporting according to published guidelines (e.g. STARD 15 [40]) is typically lacking and studies are not always registered as prospective trials, such as through clinicaltrials.gov, unless required by funding agencies or ethical review processes. Engagement of key opinion leaders is also important in expanding clinical reach.
For PAI in particular, additional challenges arise due to the wide range of available instrumentation geometries, image processing approaches and data display formats, especially for customized research-grade devices. Findings in one system and application do not necessarily extrapolate to another. In applications such as breast cancer imaging, where numerous clinical studies have been performed, the data are often not comparable between system or vendor because of the high variability in device design and data displays. Therefore, it is important to understand how best to train operators and interpreters to minimize reader variability. For example, depending on the healthcare system, ultrasound imaging may be performed by trained staff, such as radiographers, or by specialty physicians, such as radiologists or oncologists. If PAI follows a similar pathway to adoption, the need for specialist skills and training across diverse staff groups must be addressed. Consideration of these factors by the PAI community as a whole is currently rather limited.
2.2. Barrier 2: scientific and technological limitations in the current state-of-the-art
PAI has reached a sufficient level of maturity to achieve the first regulatory approvals and is now expanding beyond single-centre demonstrations to multi-centre studies, for example, in assessment of inflammatory bowel disease [41]. Nonetheless, several scientific and technological developments are needed to improve clinical accessibility. Firstly, purchase of current clinically approved instruments by healthcare providers may require significant justification as the systems are typically high in cost, can have a large physical footprint compared to other localised imaging modalities, and include ultrasound imaging capability, which while advantageous for co-registration, may be perceived by purchasers as redundant with existing standalone ultrasound systems. Justification for acquisition could come either from strong evidence of benefit for a specific disease (also referred to as the “intended use”), or through application-agnostic system designs suitable for non-specialist clinics. The research and development community could thus prioritise refinement of the technology to address these concerns. For example, if the ultrasound component can achieve sufficiently high quality, the incorporation of the two technologies may make better financial sense and could be more practical in the clinical setting, by replacing existing ultrasound machines. Improved ultrasound hardware, for example, with ultrawide-band ultrasonic detection, is also important for quantitative imaging. Research into more portable systems with a range of add-on probes to suit different uses could add value; substantial research efforts are underway to develop new low-cost light sources, such as light emitting diodes or laser diodes [42], but limited reliability and imaging depth due to the low output energy and pulse-to-pulse variability of these sources must be addressed. Cost considerations are driving further innovations in data acquisition electronics, as well as other components, such as transducer elements. PAI may already have reached too high a ‘technology readiness level’ for such research in technology refinements to be funded in an academic setting, which could present a barrier to progress unless efforts can be pursued in laboratories with home-built instruments.
Secondly, PAI involves a trade-off between imaging depth and spatial resolution, which can limit potential applications. Research aimed at enhancing the signal-to-noise ratio at depth and pushing down the lower limits of chromophore detection could substantially expand the range of clinical applications. Underexplored avenues include using short wave infrared optical excitation and developing improved broadband ultrasound transducers.
Today’s PAI devices often use high-power lasers that carry risk of eye or skin injury; these risks may be mitigated through adhering to the various available laser safety standards such as ANSI Z136.1, ANSI Z136.3, and IEC 60825–1 [43], [44], [45]. These standards define maximum permissible exposure limits that apply to medical laser devices for eye and skin tissue but do not cover other tissue types, such as internal tissues that would be exposed to light during endoscopic and catheter-based PAI. The use of high-power pulsed LED systems is covered by a different safety standard, following the skin and eye exposure limits defined in IEC 62471 [46], which provides criteria for risk assessment and categorization. In terms of eye safety, both the retinal thermal hazard exposure limit (weak visual stimulus) and the infrared radiation eye safety limit must be considered according to local safety control measures. At present, there is community interest in improving image quality by operating PAI devices at levels exceeding the current limits, that may nonetheless be safe given the short exposure duration; the pathway to justify changing the exposure limits in this context remains unclear.
Finally, in addition to the hardware design limitations, there are data processing limitations in the ability to extract relevant quantitative information from PAI systems for clinical decision making (see also barriers 7 and 8). For example, current clinical PAI systems provide a qualitative display of imaging data from target chromophores, such as haemoglobin. Yet these simple qualitative outputs can be prone to a range of errors. PAI has the potential to provide quantitative images of the optical absorption coefficient, [47] which is proportional to the concentration of molecules, allowing calculation of functional parameters such as blood oxygen saturation, with great significance for medical diagnostics. To realise this potential requires the development of algorithms that can compensate for distortion of the optical fluence through the depth of tissue, as well as for mitigation of known imaging artifacts.
In addition, knowledge of repeatability and reproducibility of results is vital yet currently under-reported. It is also plausible that some PAI implementations could potentially suffer from racial bias effects similar to those reported in pulse oximetry [48] and other through-skin optical devices [49] where variable melanin content can affect results, yet the extent of this effect remains poorly understood. PAI also has the potential to provide complementary information beyond chromophore concentrations, such as thermometry based on the photoacoustic effect; integrating these complementary measures could add clinical value in areas such as photothermal therapy response monitoring, but at present, are not sufficiently well studied.
2.3. Barrier 3: limited community outreach and engagement
The research community contributing to innovation in PAI is international, diverse, and growing exponentially [50]. The current IPASC membership includes more than 150 individuals spanning 86 research or government laboratories and companies. Nonetheless, the clinical community engaging with PAI and IPASC remains limited. The diversity of representation among IPASC should also be improved to better reflect the geographical and demographic distribution of PAI specialists across the globe.
In order for IPASC to develop PAI standards, community engagement in consensus-based efforts is vital. Identifying the stakeholders that define our community is a key first step. Widespread engagement will help to ensure that the standards being developed can be accessed, deployed and adopted by as many groups as possible. For an evolving technology such as PAI, retaining flexibility in developed standards is also important; they should allow for continuous improvement and remain fit-for-purpose. Furthermore, the improved reporting and transparency of research findings are important to distinguish technical and biological sources of variability. These considerations are important when considering the dissemination of knowledge of PAI, for example in educational materials, as well as in documentation for experimental studies relating to associated standards.
2.4. Barrier 4: lack of strategy for standards or means to interface with existing standards
PAI is in a very early phase of standardisation. Even the name has yet to be standardised, with researchers and commercial products using both optoacoustic and photoacoustic imaging (OAI / PAI) terminology, along with optoacoustic tomography, photoacoustic tomography (PAT) and photoacoustic computed tomography (PACT), with many other terms applied for combined photoacoustic and ultrasonic systems. The existence of multiple terms is confusing for physicians and other clinical personnel. Prior activities by IPASC have established a standardised data format for raw photoacoustic data [51] and efforts are underway to integrate PAI images into the DICOM standard (currently in Public Comment phase [52]), which would enable storage in clinical picture archiving systems. The IPASC industry board has already initiated plans to enable vendor testing in the short-term and integrate DICOM compatibility with commercial devices long-term.
It is already clear that standards will be difficult to generalise given the wide range of instrument geometries and potential clinical applications. Existing standards for medical imaging equipment, such as those developed by ISO or IEC, have varied intent and scope. Some standards aim to define high-level basic safety and essential performance requirements for a given type of medical device (e.g., IEC 60601–2–37 for diagnostic ultrasound). Other standards describe consensus-based test methods that can be used to objectively and reproducibly characterise device safety and/or performance. Standardised test methods should include minimum acceptance criteria based on the level of performance needed for a particular clinical application. Specific recommendations for test methodology, test reports, and acceptance criteria require consensus definition by the PAI community.
The establishment of consensus standards can signal to stakeholders that a technology has reached a certain level of maturity and consistency. However, the current lack of available PAI standards places a burden on device manufacturers and early adopters to develop their own test methods, best practices, and recommended requirements, which could produce an environment rife with competing approaches that are not equivalent and cannot be directly compared. Such discrepancies can confuse potential users and other stakeholders, impeding clinical adoption of the technology and risking creation of an appearance that a technology has not yet matured [49]. The PAI community may be able to proactively address these challenges by leveraging available standards and insights from standardisation efforts for mature imaging modalities, including optics, ultrasound, X-ray CT, and MRI.
In the clinical research setting, there is also a high degree of regulatory uncertainty in moving a new technology from local studies by specialist PAI research groups to larger scale industry driven efforts. Regulations vary across jurisdictions and may change over time, highlighted by the recent changes in the European Medical Device Regulations (MDR), which now restricts the use of medical device prototypes to CE marked devices unless the device can be shown to be compliant with ISO 13485, which is rarely feasible for academic researchers who usually lack quality management systems. It is important that any PAI test methods are sufficiently general to provide a useful reference across the relevant range of regulations and can be readily adopted in standard hospital QA/QC workflows.
2.5. Barrier 5: poor availability and use of standard phantoms
At present, there are no standard phantoms recommended for use in PAI and much of the reported performance evaluation data is not comparable between instruments and research groups or companies. As a result, device vendors often rely on research collaborations to progress their own phantom developments. There is a growing literature on photoacoustic phantom materials, often inspired by materials used in the biomedical optics or ultrasound communities. While the PAI research community makes extensive use of physical phantoms, these are usually developed in-house and not readily available for widespread community distribution. Furthermore, methods for fabrication of PAI phantoms are often not well characterised or standardised in terms of temporal stability, repeatability and reproducibility, nor do available research publications provide sufficient methodological details to enable replication by other groups. PAI phantom materials require detailed characterization of their acoustic and optical properties, ideally also extending to their mechanical and thermoelastic properties. Accurate characterization of these material properties in itself can be challenging, impeded by a lack of consensus on appropriate characterization methods. For optical characterization, a study comparing the performance of eight different photon migration instruments revealed inter-system variation of 30–40 % for the absorption coefficient (μa) and reduced scattering coefficient (μs’) for a given phantom, highlighting the difficulty of achieving accurate characterization without standardized equipment. For the assessment of acoustic properties, guidance exists (IEC TS 63081:2019), but only covers some parameters (e.g., speed of sound and acoustic attenuation) and not others (e.g. acoustic backscattering coefficient).
Phantoms are usually designed towards a specific context of use, such as instrument-specific tests for quality assurance (such as assessment of precision, spatial resolution, contrast etc) or training and usability evaluation (e.g. in replicating a physiological process) [37]. Phantoms used for quality assurance testing require highly stable tissue-mimicking optical and acoustic properties but could employ simpler geometries. In such tests, phantom temporal and environmental stability, reproducibility, and uncertainty ranges need to be defined and agreed upon, requiring collaboration between many different research groups and imaging centres. For training purposes, phantoms should reflect the complexity of the tissue structures of interest; assembling realistic phantoms with anthropomorphic target structures can be very challenging, since they vary substantially by tissue type and hence require significant expertise to produce [53]. In addition, most current phantoms described for testing PAI oximetry performance require complex, custom blood flow circuits [54], [55], [56] that may not be widely accessible in different laboratories. Simpler phantom designs may be well suited for foundational performance testing and quality management systems [57], but may be less useful for studying complex, application-specific imaging scenarios. Phantoms that are prepared by research groups are therefore often unable to fulfil the general design criteria for performance assessment in biophotonics [37].
As alluded to in Barrier 4, standards (and the phantoms used in performing associated testing) need to account for a wide range of instrument geometries and imaging applications. The former requirement demands phantoms that can be tailored to different device geometries (e.g., linear or hemispherical array devices), while providing the same types of performance assessment in compliance with the defined standardized test method. The latter requirement is a challenge as the optical and acoustic properties of many biological tissues are both variable and not always well-characterised, which makes specification of phantom property requirements for a specific imaging application challenging. Achieving a stable supply of well-characterised standard phantoms also requires engagement with commercial phantom vendors, which may not be attractive until more widespread uptake of PAI and associated test methods provides a stable customer base.
IPASC has already taken initial steps towards developing standardised phantom test objects that can be easily fabricated and plans to reach consensus on a set of performance assessment characteristics for PAI devices. A multi-centre study, which should improve our understanding of the material fabrication and inter-site reproducibility is now underway using the proposed phantom base material.
2.6. Barrier 6: poor complementarity of animal models and studies with clinical testing
The availability of commercial pre-clinical animal PAI instruments together with the lower barrier to entry for use of a new imaging modality in small animal testing has led to a high level of initial testing and biological validation of PAI in animal models. Although animal studies are widely reported, standardisation efforts are lacking and reporting of the research protocols is often limited. Testing in small animals has predominantly been performed in mice and in a limited number of models. For example, the use of cancer models is almost entirely restricted to relatively simple cell-line derived subcutaneous xenografts, with the more advanced orthotopic, transgenic and patient-derived xenograft models favoured by the cancer biology research community rarely examined.
PAI can also be considered for more widespread adoption in animal imaging, either in the veterinary clinical context or applied in animal models for fundamental studies on health and disease, which may not be possible in humans. The use of non-ionising radiation and relatively short procedure times open up the possibility for easy access to longitudinal imaging of biomarkers such as haemoglobin concentration and blood oxygen saturation, which would normally require specialized small-animal MRI equipment or display limited spatiotemporal resolution. That PAI is a relatively fast procedure is not only important from a scientific perspective but also in procedural refinement when considering the 3Rs (replacement, reduction and refinement) of animal use in research. Encouraging more widespread uptake of PAI in animal imaging facilities with associated support for image processing could transition PAI into a new status as a routine tool for biomedical research. Considering the clinical translational context, the benefit derived from small animal studies when using endogenous contrast may be questioned; larger animal models often provide better models of disease than mice or rats, albeit at higher cost. Applications of PAI to larger animals continue to be rather limited, in part due to the cost associated with undertaking such studies. Nonetheless, the use of larger animals, perhaps in a veterinary setting, is an avenue that could be fruitfully explored, both on its own merits and as a stepping-stone to establish clinical applications and reimbursement codes.
2.7. Barrier 7: difficulty in quantification of PAI data
One of the most important features of PAI is the potential for quantitative molecular imaging and in particular the quantitative imaging of blood oxygenation. Direct measurements of optical absorption coefficients and their relation to absolute chromophore concentrations would be hugely valuable for the clinical and research communities. Despite the attraction of quantitative PAI, there are significant hurdles to be overcome before PAI can deliver consistent and validated measurements, requiring improved understanding of: the propagation of light in tissue [58] and the associated effects of spectral colouring on measured signals [59]; system-specific hardware characteristics and uncertainties [60]; the effect of different approaches to image reconstruction (e.g. the use of priors and model assumptions) and processing; the quantity and quality (e.g. limited view challenges) of data that is required to achieve accurate biomarker evaluation; tools for benchmarking oximetry measurements in tissue-realistic environments with associated uncertainty budgets for test device properties; and methods to discriminate injected exogenous contrast agents from the background endogenous signals [61]. In current studies, most researchers use linear spectral unmixing algorithms to determine the contribution of different chromophores to the signals recorded using multiwavelength PAI, which use at best a crude fluence correction, and are therefore known to be inaccurate when applied in complex tissues [62].
Compensating for the spatially-dependent alterations in the spectral characteristics of the optical excitation requires knowledge of the wavelength-dependent internal light transport in tissues. Other modalities such as diffuse optics [63] or acousto-optics [64] can provide reference measurements as inputs to analytical or Monte Carlo models to form the basis of fluence correction factors, however, these modalities do not achieve the high spatial and temporal resolution of PAI for deep tissue imaging so such corrections are challenging to perform and outcomes are tissue- and model-dependent [65]. The computational complexity for 3D Monte Carlo models is also high, limiting its use in real-time imaging. Moreover, these simulations typically use simple tissue models due to the lack of available ground-truth for in vivo applications. Learning-based methods can show improved performance [66], but are trained for particular target applications [67] so generalisability can be limited. Instead, a simple mono-exponential decay is often assumed in studies where corrections are performed, however, these amplify both signal and noise, and do not account well for the local effects of fluence variation due to closely-spaced vessels. Where arterial vessels are identifiable, methods have been reported that use the known absorption coefficient of arterial blood to achieve fluence correction [68]. Validation of any correction method is challenging, given the aforementioned limitations of phantoms and the difficulty of making relevant reference measurements in vivo, both for endogenous and exogenous contrast agents, either in small animals or humans.
In addition to molecular imaging, high resolution PAI studies provide access to information on vessel architecture, which is important when assessing vascular function and disease status in a range of conditions [27], [69]. Quantification of these higher resolution images is rarely applied, and even more rarely validated, although a recent report shows that validation is possible [70].
2.8. Barrier 8: difficulty in enabling data-driven PAI analysis
Data-driven methods bring a wide range of potential benefits for PAI, however, the field is currently fragmented and limited by a scarcity of openly available high-quality data. Small-scale studies have shown that the application of artificial intelligence in PAI holds promise [71]. Two key challenges for clinical users of PAI instruments are image segmentation and interpretation; with appropriate annotated data libraries, artificial intelligence (AI) has the potential to solve these challenges by providing automated region of interest delineation and mapping the PAI signals onto an easily understandable colour bar or grading. AI could also assist with inter-modality image registration, from the new PAI + US systems (that are intrinsically co-registered), to existing US systems or to other modalities such as MRI in existing standard-of-care pathways [72]. At present, we often lack the scale of open-source annotated data with appropriate ethical approvals in place that would be needed to realise this potential.
Digital phantoms [73] are also starting to be established and in a translational context, with the potential to be expanded to perform in silico clinical trials [74]. Combining these with improved simulation pipelines, including both optical and acoustic sides of the PAI problem, could add value in future algorithm development. Such tools would also improve understanding of imaging artefacts and depth limitations seen in clinical imaging data from different applications, further aiding interpretation and operator training. At present, there remains a translational gap from available data and algorithms to specific clinical needs.
3. Development of roadmaps to overcome the barriers
Each of the highlighted barriers was investigated by discussions among two separate groups using a facilitated roadmapping template that considered the scope of the barrier, the successful end outcome if it were overcome and the steps needed to solve the identified challenges, along with any resources required to achieve the solutions. The results were compiled by combining adjacent barriers into four streamlined roadmaps.
3.1. Roadmap 1: clinical adoption
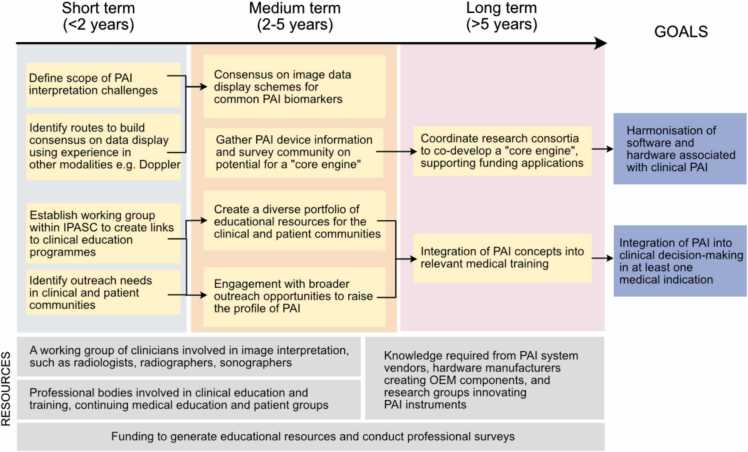
To promote adoption of PAI in the clinic, the PAI community needs to become more outward-facing and more comprehensively consider the needs of the clinical community. Clinicians should now be actively involved in co-developing the technology, for example, in instrument and clinical trials design. The roadmapping exercise indicated that improvements needed in this context can be summarised in three main areas: data acquisition hardware, image interpretation, and education (Fig. 2).
Fig. 2.
Roadmap for clinical adoption activities. OEM = original equipment manufacturer.
First, it is important to evaluate the size, complexity and upfront cost of PAI devices more critically in a clinical context. For example, the current dominant use of Class 4 lasers makes equipment large, often requiring a dedicated laser-safe room in a clinical environment, and expensive compared to a standard ultrasound system. Integrating high quality ultrasound imaging capability with a PAI device would offer the potential to replace an existing instrument, reducing the space demands by eliminating redundancy. Development of lower-cost, mobile systems with lighter handheld laser-diode or LED-based probes with a variety of shapes could open a wider and lower-threshold range of applications, for example, in primary care and low-resource settings, which is attractive with the increasing trends towards decentralisation of healthcare [75], [76]. Additional developments in methods that access longer excitation wavelengths for examination of absorption by molecules such as lipids, water or collagen, could also offer new application areas in future. A further path that could be considered would be the development of a PAI “core engine” akin to those used for plug-and-play ultrasound systems, where a common system design is developed, allowing different light source outputs and ultrasound probe inputs with tailored geometries, customised to different applications such as breast imaging, joint imaging etc. The result would be a multi-purpose system that could be used in a diverse range of application-specific settings, or situated in a centralised hospital radiology setting for referrals from different clinical specialties. It is vital that clinicians are involved in articulating functional requirements for new devices, or variants of existing ones, enabling technologists to translate these into technical specifications.
Second, following on from improved acquisition hardware is the challenge of image interpretation. There are relatively few expert interpreters of clinical PAI data at present. Even between the current commercial systems there is no agreement on colour schemes for presentation of PAI data, which is complicated by the inherently high-dimensionality of PA images (inherently 3D, 4D if wavelength is included, even 5D for high frame-rate systems). Akin to community convention ultrasound imaging for Colour Doppler mode, including the educational BART mnemonic (blue away, red towards) [77], IPASC should investigate ways to harmonize image visualization using PAI data from different vendors and researchers when displaying common information, for example, raw single wavelength data or spectrally processed haemoglobin-derived biomarkers. Linking closely with data management (see below), the development of methods for automated image segmentation and interpretation would overcome the high barrier to entry for new clinicians wanting to adopt the technology and would also help with distribution of the technology outside of expert centres.
A major need was also identified for education alongside further research and development to accelerate the process of clinical translation and more widespread adoption. Education of the PAI research community in the steps along the translational pathway would be beneficial. A training programme suited to early career researchers that highlights practical aspects associated with translational PAI research and provides high quality hands-on training for phantom design and data handling could add value. While the regulatory pathway will vary according to local requirements, guidance on the appropriate pre-clinical evidence base to underpin first-in-human studies could be valuable. Education of the clinical community beyond the current small set of key opinion leaders and early adopters could be improved through the development of relevant educational materials with clinical examples. IPASC could facilitate the process by building on educational materials already available within the broader community and by developing accredited Continuing Professional Development courses. As PAI becomes more established, these materials could be tailored to be suitable for inclusion into clinical training programmes, such as the Fellowship of the Royal College of Radiologists (FRCR) in the U.K. or the German Association for Ultrasound in Medicine in Germany. New technologies are often launched to existing practising clinicians through major international specialty conferences, such as those in radiology.
Once a device receives regulatory approval, further clinical study is generally needed to provide greater evidence of improved benefit vs. standard of care and thus justify broader clinical adoption. The availability of a larger volume of clinical evidence can then lead to guidelines and recommendations on the appropriate use of the technology from various professional societies. To promote clinical adoption, marketing studies can be undertaken to develop wider clinical experience with further registration studies conducted to ensure safe adoption of the technique. Added educational value would come from the availability of easy to use, open-access software tools to better understand and undertake high quality image reconstruction and subsequent analyses.
Finally, it is advantageous to incorporate patient and public involvement and engagement (PPIE) into the process. One could envisage reaching out to local PPIE groups associated with high profile PAI research centres where clinical trials are underway and also engaging with national advocacy groups for particular diseases where a strong evidence base is building, such as breast cancer or inflammatory diseases. The process of clinical and PPIE consultation is iterative but in the first instance, will need to build from clinical evidence and validation efforts with the help of early adopters and key opinion leaders.
3.2. Roadmap 2: standards development
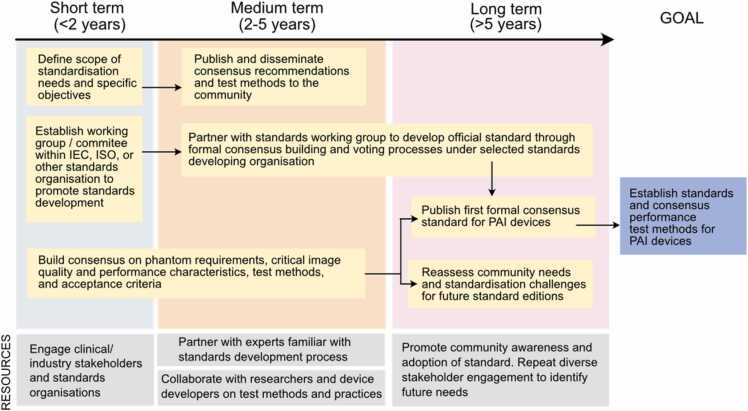
The first step towards developing a photoacoustic imaging standard is to define what, precisely, should be standardised, as well as defining priorities across the breadth of needs that are identified (Fig. 3). One short-term need is to standardise community language and term definitions, which will be critical for efficient consensus building and long-term harmonization with existing medical imaging standard language (for example, the IEC Electropedia). IPASC has already taken steps towards this goal (https://www.ipasc.science/ipasc.science/publications/). Another early need is to establish agreement as to the types and ranges of both device and phantom parameters relevant to clinical use applications. This includes defining the scope of potential device applications, phantom design requirements, critical image quality characteristics, quantitative imaging biomarkers such as oximetry measures, and other aspects of device performance. Another key consideration is whether standards will aim to establish any minimum performance acceptance criteria; such test criteria will need to be clearly linked to clinical needs. To begin consensus development, IPASC should leverage previous developments by reviewing scientific literature, medical imaging standards, and phantom use by companies in regulatory and other settings. Stakeholder engagement with clinicians, researchers, and patients should also be performed to support this effort.
Fig. 3.
Roadmap for standards activities.
Clearly, standardisation of quality assurance phantoms and associated test methods in the PAI community is imperative to ensure reproducible and comparable bench test results across devices and manufacturers, but several outstanding questions regarding scope remain to be addressed. Over the next 2–5 years, IPASC could facilitate this process by developing and publishing consensus-based recommendations and proposed standardised test methods through white papers, joint papers, conference presentations, and workshops. As part of this effort, standard phantom measurement and characterization techniques should be established to ensure phantoms are repeatable and reproducible. Notably, the authors are unaware of a standardised optical characterization technique for turbid media; IPASC should thus engage with world-leading experts in optical property measurement techniques to identify recommended methods and best practices. A further outstanding question is whether phantoms should be designed to have generalized, biologically relevant properties for training purposes or should be highly tailored to a given application and target tissue type; the answer may be application-dependent. To these points, initial standardisation efforts could consider fairly simple, general phantom approaches for quality assurance before moving on to consider the more complex needs in a training setting. After achieving these early deliverables, the challenges of tissue-specific or clinical application-specific phantoms and test methods could be addressed.
Another key challenge to address over the next three years is how the developed standards should address safety issues specific to photoacoustic imaging, including acoustic and optical exposures. Current laser safety standards from ANSI and IEC for lasers and non-laser sources prescribe maximum permissible exposures, but given their purpose, these limits only apply to skin and eye tissue. PAI clinical applications may include exposure of other tissues in endoscopy or surgical guidance and the community has expressed interest in identifying safety limits for these other tissues as well as investigating whether higher exposures can be safely used for applications involving skin exposure. Scientific evidence would generally be needed to support such recommendations; IPASC could facilitate this process by a survey of available safety standards and supporting literature in order to summarize current safety limits and evaluation methods as well as the level of existing evidence to justify any proposed changes in safety limits for photoacoustic imaging devices. IPASC should also use this information to develop recommended best practices and test methods for collecting data to establish tissue damage or perturbation thresholds, and set appropriate standardised safety and operational limits.
As a long-term goal, IPASC members should lead development and publication of a formal standard, for instance through IEC, ISO, or another official standards organization. In particular, participation of industry members will be key to ensuring the standard meets community needs and gains widespread community adoption. As formal standard development can be a long and arduous process, IPASC should liaise with existing standards groups like IEC, ISO or AAPM to establish a working group or subcommittee within an existing framework in the next two years. Consensus materials developed in the next 2–3 years can serve as the foundation for formal standard development process, addressing technical questions and also providing a high baseline of community-driven consensus to reduce risk of impasse or otherwise general failure to produce consensus. The standard should be designed from the outset to be useful for many phases of device development, including development, optimization, and regulatory purposes.
3.3. Roadmap 3: test objects and methods
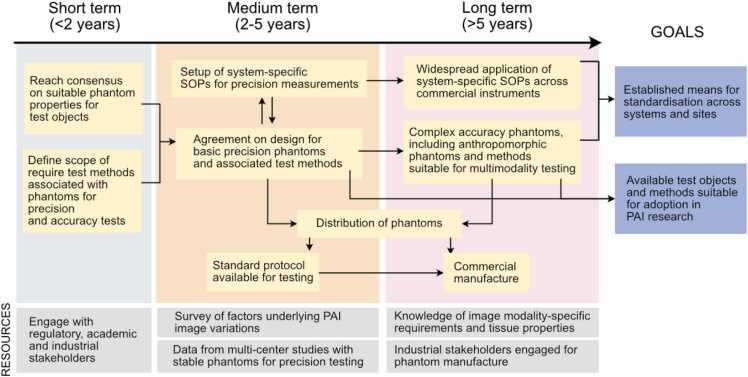
Three key steps were identified to overcome current barriers relating to test objects and methods within the field of PAI (Fig. 4).
Fig. 4.
Roadmap for the development of stable test objects and methods. SOP=Standard operating procedure.
First, consensus needs to be established on design of test objects and performance test methods for PAI systems. For this purpose, close interaction with all relevant stakeholders (industrial, academic and regulatory) is required. Preliminary evidence from ongoing single- and multi-centre studies is starting to outline the different sources of variation in phantom manufacture and PAI application, which can assist in future specification of test methods. For phantom properties, agreement needs to be reached on: target material properties; uncertainty tolerances; and choice of property characterisation techniques independent of photoacoustics. These are likely to vary according to phantom use-cases, for example, in quality assurance, such as image quality assessment or benchmarking, and training. IPASC are already in the process of reviewing a proposed consensus document defining some of these important characteristics, which will continue over the next 1–2 years.
Second, both phantoms and robust test methods need to be established in a way that can be distributed to the community, which may include open access publication or direct distribution of standard operating procedures (SOPs), for example by IPASC. A fabrication protocol has been established for testing to provide fast access to reference phantom materials. Preliminary phantom designs are basic, aiming towards quality assurance supporting device calibration. The resulting materials produced by different centres are tested centrally to confirm successful fabrication.
In the longer term, one can envisage that reference PAI quality assurance phantom manufacture and distribution would be taken over by a professional supplier supporting dissemination of test objects to customers who do not have access to the necessary equipment for phantom fabrication and characterization (e.g. clinical sites). IPASC could temporarily act as an entity to create and distribute phantoms “not-for-profit” in the pre-competitive space until commercial phantom vendors enter the market. IPASC should also consider submitting developed phantoms to FDA’s Medical Device Development Tool (MDDT) program, where FDA can qualify a tool as suitable for use in collecting data to support regulatory evaluation of a medical device. IPASC could also seek alignment with government testing laboratories such as NIST in the USA. A commercial supply of suitable phantoms would provide an ideal resource for the community. Additionally, the consortium could provide technical support and guidance for data acquisition, assisting with the development of system-specific SOPs. SOPs should not only cover phantom imaging, but also pre-clinical and clinical data acquisition. For optimization of such protocols, a detailed analysis of variation factors impacting in vivo imaging data should be conducted covering experimental factors (e.g., anaesthesia, acoustic coupling etc.) as well as model-specific factors (e.g., motion, skin tone, tissue composition etc.).
Finally, more complex phantoms for training and assessment of system accuracy should be developed, and SOPs for data acquisition should be actively developed and applied in both pre-clinical and clinical environments. Anthropomorphic phantom designs should be created mimicking different clinical models, tissue types and disease conditions, thereby enabling application of phantoms for specific use-cases, which will be important for clinical user training. Additionally, phantoms should be tailored towards multimodal applications supporting standardisation of hybrid ultrasound systems. For these purposes, a survey of requirements for phantoms in hybrid settings should be undertaken, as well as a more detailed survey of in vivo biological optical and acoustic properties of different disease and tissue types (either by validation of prior studies or by conducting new characterisation studies). Increased diversity and clinical relevance for pre-clinical models is desirable, along with an enhanced uptake of clinical studies. Expert training and improvement of imaging pipelines will help in boosting reproducibility and comparability across studies and sites.
Ultimately, standardisation across pre-clinical and clinical sites will be achieved by the wide-ranging application of robust, uniform data acquisition protocols and the availability of standard quantitative calibration phantoms along with variations of anthropomorphic and multimodal phantoms.
3.4. Roadmap 4: data management
A key conclusion of the discussion surrounding barriers relating to data was the need to build a database with high-quality annotated photoacoustic measurements for educational purposes, algorithm training, and methodological validation (Fig. 5).
Fig. 5.
Roadmap for the development of a comprehensive PA database that can function as a validation framework and aid in the understanding of technical and methodological limitations.
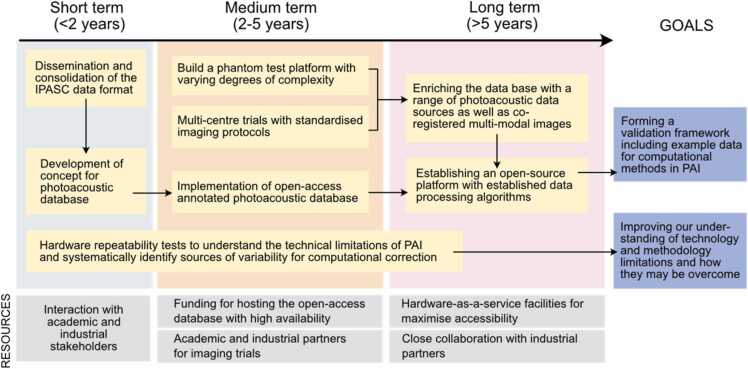
In the short term, IPASC should sustain efforts in disseminating the previously defined data format [51] to both academic research groups and industrial partners, a process that will enable the data format to be consolidated. In parallel, a concept for a sustainable database for high-quality annotated open-access photoacoustic measurements should be developed with well-defined inclusion and exclusion criteria that define if specific data are fit to be added. Furthermore, systematic hardware repeatability tests with well-characterised digital and physical phantoms should be conducted to better understand the limits of detection, current hardware limitations and uncertainty budgets, and model errors of image reconstruction algorithms. Uncertainty budgets should be considered in terms of what is practicable and clinically necessary for a given application.
In the medium term, sustainable funding must be acquired to put the concept of the database into practice and to begin the process of populating the database. To this end, a close link with roadmap 3 is vital since the most valuable data for inclusion will be that acquired using standardised imaging protocols and independent phantom validation, ideally across multiple centres. Datasets acquired from a diverse but reproducible suite of phantoms with imaging targets of varying complexities and known optical and acoustic properties should be included. The phantom suite should include both static and dynamic models (e.g. with variable oxygenation), as well as models specific to certain organs or pathologies. The phantom dataset should be supplemented by digital twins, while data from small animal and human in vivo imaging should be accompanied by expert annotations.
In the long term, the collected data sets should be used to establish a validation framework for computational methods developed in the field with the capabilities of objectively benchmarking new algorithms to identify their respective strengths and shortcomings. IPASC could lead the consensus-finding effort for this purpose. The open-access database should be enriched with co-registered multi-modal images of test objects. Furthermore, a platform should be established that disseminates open-source implementations of established image reconstruction and processing algorithms that are running “out-of-the-box” with the database.
3.5. Summary roadmap
A wide range of activities have been identified in the 4 roadmaps outlined above. While some of these require a broader research and development process, several are within the scope of IPASC. The roadmap of activities planned by IPASC in response to the roadmapping exercise are summarised in Fig. 6, along with a proposed timeline for implementation. Readers of this paper are encouraged to engage with IPASC and contribute to the process of consensus-finding in these important thematic areas.
Fig. 6.
Summary of the goals of IPASC emerging from the roadmapping workshop.
4. Conclusion
There is significant enthusiasm to support clinical translation of PAI, yet several translational barriers remain. We identified a series of community activities that could help to overcome these barriers, in terms of clinical adoption, standards development, test objects and methods, and data management. The outlined roadmaps illustrate how the community, facilitated by IPASC, can deliver these activities and identify the enabling resources needed. We hope that by providing these roadmaps to the wider PAI community we can stimulate further excitement for clinical translation of PAI and engagement with IPASC consensus-finding activities to support this process. These roadmaps are designed to deliver on our goal to unlock the potential of PAI in alleviating disease burden across the world.
CRediT authorship contribution statement
SEB, JG, LH, JJ, LL, and WCV organised the roadmapping workshop. SEB, JG, LH, and WCV prepared the first draft of the manuscript. All other authors participated in the roadmapping workshop process and contributed to the preparation of the final manuscript. The author list is given in alphabetical order, except for SEB, who chaired the workshop and is last and corresponding author of the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The promotions and salary of Kurinchi Gurusamy are dependent upon journal publications. Ferdinand Knieling is co-inventor with iThera Medical GmbH, Germany on an EU patent (EP 19 163 304.9) relating to a device and a method for analysing optoacoustic data, an optoacoustic system and a computer program. He received travel support by iThera Medical GmbH, Germany, lecture fees from Sanofi Genzyme and Siemens Healthcare GmbH outside the submitted work. Given his role as Section Editor of this journal, Ferdinand Knieling had no involvement in the peer-review of articles for which he was an author and had no access to information regarding their peer-review. AAO is an employee and stock holder of TomoWave and Seno Medical, which develop and sell optoacoustic tomography systems. Given his role as the Editor in Chief of this Journal, AAO had no involvement in the peer-review process or the decisions regarding acceptance of manuscripts for which he is an author. Given her role as an Editorial Board member of this journal, Sarah Bohndiek had no involvement in the peer-review of articles for which she was an author and had no access to information regarding their peer-review. Full responsibility for the peer-review process for this article was delegated to another Editor. Martin Leahy is the inventor of WIPO patent (WO2015110349A1) relating to photoacoustic tomography method and system which uses known intrinsic absorbers for fluence correction. Lihong Wang has a financial interest in Microphotoacoustics, Inc., CalPACT, LLC, and Union Photoacoustic Technologies, Ltd., which, however, did not support this work. Ledia Lilaj is an employee of iThera Medical GmbH, a vendor of optoacoustic imaging instruments. Mithun Kuniyil Ajith Singh is an employee of CYBERDYNE Inc, a vendor of photoacoustic imaging instruments. The other authors do not declare any conflicts of interest.
Acknowledgements
We would like to thank Duncan Hurlstone and Nicky Athanassopoulou from the Institute for Manufacturing in Cambridge for facilitating the roadmapping event. This work was funded by UKRI grant EP/V027069/1. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services. This article reflects the views of the authors and should not be construed to represent FDA views or policies. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising.
Biographies
 Hisham Assi received his Ph.D. in Electrical Engineering in collaboration with Biomedical Engineering from the University of Toronto, where he studied theoretical and numerical wave propagation in fluids, solids, and metamaterials applied to ultrasound medical imaging. His current research focuses on quantitative ultrasound and photoacoustic imaging, image-guided therapies, and intraoperative imaging.
Hisham Assi received his Ph.D. in Electrical Engineering in collaboration with Biomedical Engineering from the University of Toronto, where he studied theoretical and numerical wave propagation in fluids, solids, and metamaterials applied to ultrasound medical imaging. His current research focuses on quantitative ultrasound and photoacoustic imaging, image-guided therapies, and intraoperative imaging.
 Rui Cao is currently a postdoc with Caltech Optical Imaging Laboratory at California Institute of Technology, Pasadena, CA, USA. He received his Bachelor's degree in optoelectronics and Master’s degree from Nankai University in 2010 and 2013, respectively. He earned his Ph.D. degree in Biomedical Engineering from the University of Virginia in 2018. His current research focuses on optical-resolution photoacoustic microscopy and functional photoacoustic computed tomography.
Rui Cao is currently a postdoc with Caltech Optical Imaging Laboratory at California Institute of Technology, Pasadena, CA, USA. He received his Bachelor's degree in optoelectronics and Master’s degree from Nankai University in 2010 and 2013, respectively. He earned his Ph.D. degree in Biomedical Engineering from the University of Virginia in 2018. His current research focuses on optical-resolution photoacoustic microscopy and functional photoacoustic computed tomography.
 Dr. Madhura Castelino is a Consultant Rheumatologist in adult Rheumatology at University College London and an Honorary Clinical Lecturer at University College London. She leads Clinical Trials for the Rheumatology Department and is the Divisional Audit Lead for Medical Specialties at UCLH. She has professional interest in the pathogenesis and management of inflammatory arthritis especially Spondyloarthritis and musculoskeletal ultrasonography. She is the principal investigator in several clinical studies including commercial and non-commercial trials at UCLH. Her research interest includes the role of the microbiome in inflammatory arthritis especially Spondyloarthritis, patient related outcomes in chronic disease and imaging in inflammatory arthritis. She has established the UCL Rheumatology Patient Partners a patient public involvement and engagement initiative to support patient focussed research. She is the Deputy Clinical Academic Lead for UCLH for UK Musculoskeletal Translational Research Collaboration. Dr Castelino completed her initial medical training at Manipal University, India (2002) and clinical training in rheumatology in Manchester, U.K. (2008–2018). She also holds an MSc in Clinical Rheumatology (2011) and a PhD in Rheumatology (2016) from the University of Manchester.
Dr. Madhura Castelino is a Consultant Rheumatologist in adult Rheumatology at University College London and an Honorary Clinical Lecturer at University College London. She leads Clinical Trials for the Rheumatology Department and is the Divisional Audit Lead for Medical Specialties at UCLH. She has professional interest in the pathogenesis and management of inflammatory arthritis especially Spondyloarthritis and musculoskeletal ultrasonography. She is the principal investigator in several clinical studies including commercial and non-commercial trials at UCLH. Her research interest includes the role of the microbiome in inflammatory arthritis especially Spondyloarthritis, patient related outcomes in chronic disease and imaging in inflammatory arthritis. She has established the UCL Rheumatology Patient Partners a patient public involvement and engagement initiative to support patient focussed research. She is the Deputy Clinical Academic Lead for UCLH for UK Musculoskeletal Translational Research Collaboration. Dr Castelino completed her initial medical training at Manipal University, India (2002) and clinical training in rheumatology in Manchester, U.K. (2008–2018). She also holds an MSc in Clinical Rheumatology (2011) and a PhD in Rheumatology (2016) from the University of Manchester.
 Fiona Gilbert is Professor of Radiology and Head of Department at the University of Cambridge. Her clinical work and research is focused on imaging breast cancer using multimodal functional imaging such as MRI and PET to study the tumour environment and evaluating different modalities for early detection. Professor Gilbert has over 250 peer reviewed publications, 5 book chapters and numerous international conference abstracts.She was awarded Honorary membership of Radiological Society of North America, Honorary fellowship of the American College of Radiologists, the Royal Society of Edinburgh and the Academy of Medical Sciences and the Gold Medal from the European Society of Radiology. She is immediate past President of the European Society of Breast Imaging.
Fiona Gilbert is Professor of Radiology and Head of Department at the University of Cambridge. Her clinical work and research is focused on imaging breast cancer using multimodal functional imaging such as MRI and PET to study the tumour environment and evaluating different modalities for early detection. Professor Gilbert has over 250 peer reviewed publications, 5 book chapters and numerous international conference abstracts.She was awarded Honorary membership of Radiological Society of North America, Honorary fellowship of the American College of Radiologists, the Royal Society of Edinburgh and the Academy of Medical Sciences and the Gold Medal from the European Society of Radiology. She is immediate past President of the European Society of Breast Imaging.
 Dr. Janek Gröhl does research computational biophotonics focusing on data-driven quantitative photoacoustic imaging. He received his masters degree in medical computer science from the University of Heidelberg and Heilbronn University of Applied Sciences in 2016. He received his PhD degree from the medical faculty of the University of Heidelberg in April 2021 and did his PhD research under Prof. Lena Maier-Hein at the German Cancer Research Center (DKFZ) and since December 2020 he is working as a postdoctoral fellow with Prof. Sarah Bohndiek funded by the Walter Benjamin Programme of the German Research Foundation (DFG) at Cancer Research UK, Cambridge Institute (CRUK CI).
Dr. Janek Gröhl does research computational biophotonics focusing on data-driven quantitative photoacoustic imaging. He received his masters degree in medical computer science from the University of Heidelberg and Heilbronn University of Applied Sciences in 2016. He received his PhD degree from the medical faculty of the University of Heidelberg in April 2021 and did his PhD research under Prof. Lena Maier-Hein at the German Cancer Research Center (DKFZ) and since December 2020 he is working as a postdoctoral fellow with Prof. Sarah Bohndiek funded by the Walter Benjamin Programme of the German Research Foundation (DFG) at Cancer Research UK, Cambridge Institute (CRUK CI).
 Kurinchi Gurusamy Kuruinchi is currently a Professor of Evidence-based Medicine and Surgery, Head of Research at Division of Surgery and Interventional Science, and Lead of Surgical and Interventional Group at Comprehensive Clinical Trials Unit at University College London (UCL). A surgeon by background, he is currently focused on research and teaching with an aim to achieve high quality healthcare for all and address inequalities in the society. He is one of the top 2 % of the scientists who have published their research in Medicine since 1960, based on the standardized information on citations, h-index, co authorship-adjusted hm-index, citations to papers indifferent authorship positions and a composite indicator (https://doi.org/10.17632/btchxktzyw.4). More than 50 of research publications have been used in formulating clinical practice guidelines (https://sites.google.com/view/kgurusamy-guidelinescitations/home).
Kurinchi Gurusamy Kuruinchi is currently a Professor of Evidence-based Medicine and Surgery, Head of Research at Division of Surgery and Interventional Science, and Lead of Surgical and Interventional Group at Comprehensive Clinical Trials Unit at University College London (UCL). A surgeon by background, he is currently focused on research and teaching with an aim to achieve high quality healthcare for all and address inequalities in the society. He is one of the top 2 % of the scientists who have published their research in Medicine since 1960, based on the standardized information on citations, h-index, co authorship-adjusted hm-index, citations to papers indifferent authorship positions and a composite indicator (https://doi.org/10.17632/btchxktzyw.4). More than 50 of research publications have been used in formulating clinical practice guidelines (https://sites.google.com/view/kgurusamy-guidelinescitations/home).
 Lina Hacker is a Junior Research Fellow at the Department of Oncology at the University of Oxford, UK. Her research is focused on the medical and technical validation of novel approaches for cancer imaging, specifically relating to tumour hypoxia. She received her PhD degree in Medical Sciences at the University of Cambridge, UK, and holds a Master’s and Bachelor’s degree in Biomedical Engineering and Molecular Medicine, respectively.
Lina Hacker is a Junior Research Fellow at the Department of Oncology at the University of Oxford, UK. Her research is focused on the medical and technical validation of novel approaches for cancer imaging, specifically relating to tumour hypoxia. She received her PhD degree in Medical Sciences at the University of Cambridge, UK, and holds a Master’s and Bachelor’s degree in Biomedical Engineering and Molecular Medicine, respectively.
 Aoife M. Ivory received her B.A. (Mod) degree in Physics with Astrophysics, M.Sc. degree in Physical Sciences in Medicine specialising in diagnostic imaging, and her Ph.D. degree in Ultrasound Physics from Trinity College Dublin, Dublin, Ireland, in 2013, 2014, and 2018, respectively, focusing on the characterization of the ultrasound contrast agents and the development of subharmonic imaging techniques for a clinical ultrasound system using novel phantom devices. She is currently a Senior Scientist with the Ultrasound and Underwater Acoustics Group, National Physical Laboratory, Teddington, U.K. Her research focuses on photoacoustic imaging, ultrasonic characterization, and the development of tissue mimicking materials (TMMs) and phantoms.
Aoife M. Ivory received her B.A. (Mod) degree in Physics with Astrophysics, M.Sc. degree in Physical Sciences in Medicine specialising in diagnostic imaging, and her Ph.D. degree in Ultrasound Physics from Trinity College Dublin, Dublin, Ireland, in 2013, 2014, and 2018, respectively, focusing on the characterization of the ultrasound contrast agents and the development of subharmonic imaging techniques for a clinical ultrasound system using novel phantom devices. She is currently a Senior Scientist with the Ultrasound and Underwater Acoustics Group, National Physical Laboratory, Teddington, U.K. Her research focuses on photoacoustic imaging, ultrasonic characterization, and the development of tissue mimicking materials (TMMs) and phantoms.
 James Joseph obtained his Bachelor’s degree in Electronic and Computer Engineering from the University of Nottingham. He obtained his Master's degree and PhD from the School of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore. Further, he carried out his postdoctoral research for six years at Cancer Research UK Cambridge Institute and the Department of Physics, University of Cambridge. Since July 2020, he has been working as a Lecturer in Biomedical Engineering at the University of Dundee. His research is focused on translational optical and acoustic technologies. Specifically, his research interests are aligned towards developing innovative imaging instrumentation schemes, medical devices and establishing novel diagnostic and treatment approaches for oncological applications. He also leads and directs the phantom development theme for International Photoacoustic Standardisation Consortium (IPASC).
James Joseph obtained his Bachelor’s degree in Electronic and Computer Engineering from the University of Nottingham. He obtained his Master's degree and PhD from the School of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore. Further, he carried out his postdoctoral research for six years at Cancer Research UK Cambridge Institute and the Department of Physics, University of Cambridge. Since July 2020, he has been working as a Lecturer in Biomedical Engineering at the University of Dundee. His research is focused on translational optical and acoustic technologies. Specifically, his research interests are aligned towards developing innovative imaging instrumentation schemes, medical devices and establishing novel diagnostic and treatment approaches for oncological applications. He also leads and directs the phantom development theme for International Photoacoustic Standardisation Consortium (IPASC).
 Ferdinand Knieling is a specialist in pediatrics and works as a senior physician at the Department of Pediatrics and Adolescent Medicine at the University Hospital Erlangen, Germany. He leads the Translational Pediatrics research group, which focuses on the discovery of biological insights through novel imaging techniques and their translation into clinical applications. His group is particularly interested in the clinical application of opto-/photoacoustic imaging and its validation in advanced animal models. Dr. Knieling is a section editor of the journal PHOTOACOUSTICS and a member of the International Photoacoustic Standardisation Consortium (IPASC).
Ferdinand Knieling is a specialist in pediatrics and works as a senior physician at the Department of Pediatrics and Adolescent Medicine at the University Hospital Erlangen, Germany. He leads the Translational Pediatrics research group, which focuses on the discovery of biological insights through novel imaging techniques and their translation into clinical applications. His group is particularly interested in the clinical application of opto-/photoacoustic imaging and its validation in advanced animal models. Dr. Knieling is a section editor of the journal PHOTOACOUSTICS and a member of the International Photoacoustic Standardisation Consortium (IPASC).
 Martin Leahy is Chair of Applied Physics at NUI Galway where he leads the Tissue Optics and Microcirculation Imaging Group. His group invented the Heart Rate App, correlation mapping OCT, nanosensitive OCT and depth encoded superresolution microscopy. He chairs the biannual international Biophotonics and Imaging Graduate Summer School in Ireland and is a JBO Letters Editor. He leads H2020 STARSTEM on gold nanostars for optoacoustic stem cell tracking and is a partner in H2020 IMCUSTOMEYE to develop an instrument to measure the biomechanics of the eye.
Martin Leahy is Chair of Applied Physics at NUI Galway where he leads the Tissue Optics and Microcirculation Imaging Group. His group invented the Heart Rate App, correlation mapping OCT, nanosensitive OCT and depth encoded superresolution microscopy. He chairs the biannual international Biophotonics and Imaging Graduate Summer School in Ireland and is a JBO Letters Editor. He leads H2020 STARSTEM on gold nanostars for optoacoustic stem cell tracking and is a partner in H2020 IMCUSTOMEYE to develop an instrument to measure the biomechanics of the eye.
 Ledia Lilaj is a Senior Research and Development Engineer at iThera Medical, where she is responsible for designing and developing performance quantification test methods, test objects, and calibration tools for the new generation of optoacoustic devices. She also serves as a member of the leadership team for the International Photoacoustic Standardization Consortium, where she contributes to the development of test objects and methods. Dr. Lilaj holds an M.Sc. in Biomedical Engineering from Politecnico di Milano in Italy, as well as a Ph.D. with a thesis in Magnetic Resonance Elastography from Charité Universitätsmedizin Berlin in Germany.
Ledia Lilaj is a Senior Research and Development Engineer at iThera Medical, where she is responsible for designing and developing performance quantification test methods, test objects, and calibration tools for the new generation of optoacoustic devices. She also serves as a member of the leadership team for the International Photoacoustic Standardization Consortium, where she contributes to the development of test objects and methods. Dr. Lilaj holds an M.Sc. in Biomedical Engineering from Politecnico di Milano in Italy, as well as a Ph.D. with a thesis in Magnetic Resonance Elastography from Charité Universitätsmedizin Berlin in Germany.
 Srirang Manohar is Professor and Chair, Multi-Modality Medical Imaging (M3I), at the University of Twente. He works in photoacoustic imaging, ultrasound imaging and of late in fluorescence imaging. Prof. Manohar’s research spans technology development to early clinical assessment. The intended applications of the technologies span the range of ex vivo tissue imaging, open surgical imaging, minimally-invasive imaging to non-invasive imaging. He investigates the feasibility of photoacoustic imaging hybrid with ultrasound imaging in breast cancer diagnosis, image guidance of Radiofrequency Ablation, and imaging of thyroid nodules. He has also done investigations into synthesis and characterization of gold nanoparticles, and in vitro and in vivo application of these and iron oxide nanoparticles in the context of providing contrast for photoacoustics.
Srirang Manohar is Professor and Chair, Multi-Modality Medical Imaging (M3I), at the University of Twente. He works in photoacoustic imaging, ultrasound imaging and of late in fluorescence imaging. Prof. Manohar’s research spans technology development to early clinical assessment. The intended applications of the technologies span the range of ex vivo tissue imaging, open surgical imaging, minimally-invasive imaging to non-invasive imaging. He investigates the feasibility of photoacoustic imaging hybrid with ultrasound imaging in breast cancer diagnosis, image guidance of Radiofrequency Ablation, and imaging of thyroid nodules. He has also done investigations into synthesis and characterization of gold nanoparticles, and in vitro and in vivo application of these and iron oxide nanoparticles in the context of providing contrast for photoacoustics.
 Igor Meglinski is a Professor in Biomedical Engineering and Biophotonics at Aston University (UK). He completed PhD studies at the interface between Saratov State University (Russia) and University of Pennsylvania (USA) in 1997. His current research interest lies with advanced biomedical imaging, polarization of light, shaped light with orbital angular momentum, light-tissue interaction, dynamic light scattering and waves propagation in random tissue-like scattering medium. He is author of over 400 publications in peer-reviewed scientific journals, proceedings of conferences, 14 book chapters, 5 books and several patents. He is Chartered Physicist (CPhys), Chartered Engineer (CEng), Fellow of the Institute of Physics, Fellow of Royal Microscopical Society (FRMS), Fellow of SPIE and Fellow of OPTICA (formerly OSA).
Igor Meglinski is a Professor in Biomedical Engineering and Biophotonics at Aston University (UK). He completed PhD studies at the interface between Saratov State University (Russia) and University of Pennsylvania (USA) in 1997. His current research interest lies with advanced biomedical imaging, polarization of light, shaped light with orbital angular momentum, light-tissue interaction, dynamic light scattering and waves propagation in random tissue-like scattering medium. He is author of over 400 publications in peer-reviewed scientific journals, proceedings of conferences, 14 book chapters, 5 books and several patents. He is Chartered Physicist (CPhys), Chartered Engineer (CEng), Fellow of the Institute of Physics, Fellow of Royal Microscopical Society (FRMS), Fellow of SPIE and Fellow of OPTICA (formerly OSA).
 Professor Carmel Moran is Chair of Translational Ultrasound at University of Edinburgh. Her research interests span high frequency ultrasound, contrast agent development and phantom development for assessment of the performance of ultrasound scanners.
Professor Carmel Moran is Chair of Translational Ultrasound at University of Edinburgh. Her research interests span high frequency ultrasound, contrast agent development and phantom development for assessment of the performance of ultrasound scanners.
 Andrea Murray is a Senior Lecturer at the University of Manchester. She is based at Salford Royal Hospital where she oversees a cross-disciplinary research programme. Her special interest is development and application of non-invasive imaging to elucidate the pathogenesis, progression and response to treatment of Raynaud’s phenomenon and systemic sclerosis.
Andrea Murray is a Senior Lecturer at the University of Manchester. She is based at Salford Royal Hospital where she oversees a cross-disciplinary research programme. Her special interest is development and application of non-invasive imaging to elucidate the pathogenesis, progression and response to treatment of Raynaud’s phenomenon and systemic sclerosis.
Alexander A Oraevsky is the inventor of 21 patents, has published 10 book chapters and over 200 scientific papers dealing with novel laser technologies applicable in biology and medicine. Dr. Oraevsky is the recipient of multiple honors and awards, including Berthold Leibinger Innovations Prize.
 Dr. Mark “Marty” Pagel is a Professor in the Departments of Cancer Systems Imaging and Imaging Physics at the University of Texas MD Anderson Cancer Center. His research in academia and industry has evolved from chemistry to biomedical engineering to molecular imaging. His current research employs a variety of imaging modalities to interrogate biomarkers of the tumor microenvironment. He has developed these methods for pre-clinical and clinical imaging, especially to evaluate cancer therapies. Dr. Pagel has held many leadership positions in the molecular imaging research community, including roles in scientific societies, many grant review panels, and journals focused on molecular imaging.
Dr. Mark “Marty” Pagel is a Professor in the Departments of Cancer Systems Imaging and Imaging Physics at the University of Texas MD Anderson Cancer Center. His research in academia and industry has evolved from chemistry to biomedical engineering to molecular imaging. His current research employs a variety of imaging modalities to interrogate biomarkers of the tumor microenvironment. He has developed these methods for pre-clinical and clinical imaging, especially to evaluate cancer therapies. Dr. Pagel has held many leadership positions in the molecular imaging research community, including roles in scientific societies, many grant review panels, and journals focused on molecular imaging.
 Manojit Pramanik is currently Northrop Grumman Associate Professor in the Department of Electrical and Computer Engineering, and a Faculty of Biomedical Engineering at Iowa State University, Ames, Iowa, USA. Earlier he was at Nanyang Technological University (NTU), Singapore from 2014 to 2022. He received his PhD in biomedical engineering from Washington University in St. Louis, Missouri. His research interests include the development of photoacoustic/thermoacoustic imaging systems; image reconstruction, machine learning, medical image processing, contrast agents and molecular imaging, and Monte Carlo simulation of light-tissue interaction.
Manojit Pramanik is currently Northrop Grumman Associate Professor in the Department of Electrical and Computer Engineering, and a Faculty of Biomedical Engineering at Iowa State University, Ames, Iowa, USA. Earlier he was at Nanyang Technological University (NTU), Singapore from 2014 to 2022. He received his PhD in biomedical engineering from Washington University in St. Louis, Missouri. His research interests include the development of photoacoustic/thermoacoustic imaging systems; image reconstruction, machine learning, medical image processing, contrast agents and molecular imaging, and Monte Carlo simulation of light-tissue interaction.
 Jason Raymond is a Lecturer in the Department of Engineering Science at the University of Oxford. He holds a B.S. in Engineering Acoustics and M.S. in Mechanical Engineering from Boston University, and a Ph.D. in Biomedical Engineering from the University of Cincinnati. His research interests include the use of light and ultrasound for medical and biological applications.
Jason Raymond is a Lecturer in the Department of Engineering Science at the University of Oxford. He holds a B.S. in Engineering Acoustics and M.S. in Mechanical Engineering from Boston University, and a Ph.D. in Biomedical Engineering from the University of Cincinnati. His research interests include the use of light and ultrasound for medical and biological applications.
 Dr. Mithun Kuniyil Ajith Singh is an engineering scientist with vast experience in preclinical and clinical photoacoustic and ultrasound imaging. He is currently the Research and Business Development Manager at CYBERDYNE, INC, the Netherlands, where he initiates and coordinates scientific projects with globally renowned research groups. His focus is on translating LED-based photoacoustic imaging technology to clinical settings. Mithun received his PhD from the University of Twente, the Netherlands in 2016 under Professor Wiendelt Steenbergen’ s guidance. He has published multiple international journal articles and book chapters aimed at accelerating the translation of photoacoustic imaging from bench to bedside. Mithun was the editor of the first book on LED-based photoacoustic imaging, published by Springer Nature in 2020.
Dr. Mithun Kuniyil Ajith Singh is an engineering scientist with vast experience in preclinical and clinical photoacoustic and ultrasound imaging. He is currently the Research and Business Development Manager at CYBERDYNE, INC, the Netherlands, where he initiates and coordinates scientific projects with globally renowned research groups. His focus is on translating LED-based photoacoustic imaging technology to clinical settings. Mithun received his PhD from the University of Twente, the Netherlands in 2016 under Professor Wiendelt Steenbergen’ s guidance. He has published multiple international journal articles and book chapters aimed at accelerating the translation of photoacoustic imaging from bench to bedside. Mithun was the editor of the first book on LED-based photoacoustic imaging, published by Springer Nature in 2020.
 William C. Vogt received his BS degree in mechanical engineering from the University of Massachusetts Amherst in 2009 and his PhD in biomedical engineering from Virginia Polytechnic Institute and State University in 2013. Since joining FDA in 2013, he has been developing regulatory science tools and test methods for evaluating the safety and effectiveness of photoacoustic imaging devices. His research interests include photoacoustic imaging, tissue phantoms, nanoparticles, standardization, and biophotonic medical device characterization and evaluation.
William C. Vogt received his BS degree in mechanical engineering from the University of Massachusetts Amherst in 2009 and his PhD in biomedical engineering from Virginia Polytechnic Institute and State University in 2013. Since joining FDA in 2013, he has been developing regulatory science tools and test methods for evaluating the safety and effectiveness of photoacoustic imaging devices. His research interests include photoacoustic imaging, tissue phantoms, nanoparticles, standardization, and biophotonic medical device characterization and evaluation.
 Lihong Wang is Bren Professor of Medical and Electrical Engineering at Caltech. Published 580 journal articles (h-index = 152, citations = 100,000). Delivered 580 keynote/plenary/invited talks. Published the first functional photoacoustic CT, 3D photoacoustic microscopy, and compressed ultrafast photography (world’s fastest camera). Served as Editor-in-Chief of the Journal of Biomedical Optics. Received the Goodman Book Award, NIH Outstanding Investigator and Director’s Pioneer Awards, OSA Mees Medal, IEEE Technical Achievement and Biomedical Engineering Awards, SPIE Chance Award, IPPA Senior Prize, OSA Feld Biophotonics Award, and an honorary doctorate from Lund University, Sweden. Inducted into the National Academy of Engineering.
Lihong Wang is Bren Professor of Medical and Electrical Engineering at Caltech. Published 580 journal articles (h-index = 152, citations = 100,000). Delivered 580 keynote/plenary/invited talks. Published the first functional photoacoustic CT, 3D photoacoustic microscopy, and compressed ultrafast photography (world’s fastest camera). Served as Editor-in-Chief of the Journal of Biomedical Optics. Received the Goodman Book Award, NIH Outstanding Investigator and Director’s Pioneer Awards, OSA Mees Medal, IEEE Technical Achievement and Biomedical Engineering Awards, SPIE Chance Award, IPPA Senior Prize, OSA Feld Biophotonics Award, and an honorary doctorate from Lund University, Sweden. Inducted into the National Academy of Engineering.
 Dr. Shufan Yang is an associate professor in computational intelligence at the School of Computing, Engineering & the Built Environment and honorary associate professor in Medical Technologies in Faculty of Medical Science, University College London. Dr Yang has conducted multidisciplinary research work, most notably through a collaboration with biomedical engineering, veterinarians, physicists. Her work falls under the categories of artificial intelligence algorithms and edge acceleration, where she will lead the work on deep learning algorithms and optimisation algorithm development. Currently she holds a Royal Academy of Engineering Industry Fellowship to develop an software-hardware ecosystem for edge AI/ML devices.
Dr. Shufan Yang is an associate professor in computational intelligence at the School of Computing, Engineering & the Built Environment and honorary associate professor in Medical Technologies in Faculty of Medical Science, University College London. Dr Yang has conducted multidisciplinary research work, most notably through a collaboration with biomedical engineering, veterinarians, physicists. Her work falls under the categories of artificial intelligence algorithms and edge acceleration, where she will lead the work on deep learning algorithms and optimisation algorithm development. Currently she holds a Royal Academy of Engineering Industry Fellowship to develop an software-hardware ecosystem for edge AI/ML devices.
 Sarah Bohndiek completed her PhD in Radiation Physics at University College London in 2008 and then worked in both the UK (at Cambridge) and the USA (at Stanford) as a postdoctoral fellow in molecular imaging. Since 2013, Sarah has been a Group Leader at the University of Cambridge, where she is jointly appointed in the Department of Physics and the Cancer Research UK Cambridge Institute. She was appointed as Full Professor of Biomedical Physics and elected as a Fellow of SPIE in 2020. Sarah was recently awarded the CRUK Future Leaders in Cancer Research Prize and SPIE Early Career Achievement Award in recognition of her innovation in biomedical optics.
Sarah Bohndiek completed her PhD in Radiation Physics at University College London in 2008 and then worked in both the UK (at Cambridge) and the USA (at Stanford) as a postdoctoral fellow in molecular imaging. Since 2013, Sarah has been a Group Leader at the University of Cambridge, where she is jointly appointed in the Department of Physics and the Cancer Research UK Cambridge Institute. She was appointed as Full Professor of Biomedical Physics and elected as a Fellow of SPIE in 2020. Sarah was recently awarded the CRUK Future Leaders in Cancer Research Prize and SPIE Early Career Achievement Award in recognition of her innovation in biomedical optics.
Data Availability
No data was used for the research described in the article.
References
- 1.Wang, L. v & Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. 〈https://www.science.org〉. [DOI] [PMC free article] [PubMed]
- 2.Oraevsky A.A., Karabutov A.A. In: Biomedical Photonics Handbook. Vo-Dinh T., editor. CRC Press; 2003. Optoacoustic tomography. [Google Scholar]
- 3.Lemaster J.E., Jokerst J. v. What is new in nanoparticle-based photoacoustic imaging? Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnology. 2017;9 doi: 10.1002/wnan.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber J., Beard P.C., Bohndiek S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods. 2016;13:639–650. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y., Olick-Gibson J., Khadria A., Wang L.V. Photoacoustic vector tomography for deep hemodynamic imaging. Nat. Biomed. Eng. Press. 2023 doi: 10.1038/s41551-023-01148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah J., et al. Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J. Biomed. Opt. 2008;13 doi: 10.1117/1.294036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pramanik M., Wang L.V. Thermoacoustic and photoacoustic sensing of temperature. J. Biomed. Opt. 2009;14 doi: 10.1117/1.3247155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirg S., Turner K.L., Chen H., Drew P.J., Kothapalli S.R. Photoacoustic imaging for microcirculation. Microcirculation. 2022;29 doi: 10.1111/micc.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gargiulo S., Albanese S., Mancini M. State-of-the-art preclinical photoacoustic imaging in oncology: recent advances in cancer theranostics. Contrast Media Mol. Imaging. 2019;vol. 2019 doi: 10.1155/2019/5080267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence D.J., et al. Spectral photoacoustic imaging to estimate in vivo placental oxygenation during preeclampsia. Sci. Rep. 2019;9 doi: 10.1038/s41598-018-37310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quiros-Gonzalez I., et al. Photoacoustic tomography detects response and resistance to bevacizumab in breast cancer mouse models. Cancer Res. 2022;82:1658–1668. doi: 10.1158/0008-5472.CAN-21-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu X., et al. Real-time whole-brain imaging of hemodynamics and oxygenation at micro-vessel resolution with ultrafast wide-field photoacoustic microscopy. Light Sci. Appl. 2022;11 doi: 10.1038/s41377-022-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hysi E., et al. Photoacoustic signal characterization of cancer treatment response: correlation with changes in tumor oxygenation. Photoacoustics. 2017;5:25–35. doi: 10.1016/j.pacs.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv J., Xu Y., Xu L., Nie L. Quantitative functional evaluation of liver fibrosis in mice with dynamic contrast-enhanced photoacoustic imaging. Radiology. 2021;300:89–97. doi: 10.1148/radiol.2021204134. [DOI] [PubMed] [Google Scholar]
- 15.Petrova I.Y., et al. Noninvasive monitoring of cerebral blood oxygenation in ovine superior sagittal sinus with novel multi-wavelength optoacoustic system. Opt. Express. 2009;17:7285–7294. doi: 10.1364/oe.17.007285. [DOI] [PubMed] [Google Scholar]
- 16.Zackrisson S., van de Ven S.M.W.Y., Gambhir S.S. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res. 2014;74:979–1004. doi: 10.1158/0008-5472.CAN-13-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attia A.B.E., et al. A review of clinical photoacoustic imaging: current and future trends. Photoacoustics. 2019;16 doi: 10.1016/j.pacs.2019.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menezes G.L.G., et al. Downgrading of breast masses suspicious for cancer by using optoacoustic breast imaging. Radiology. 2018;288:355–365. doi: 10.1148/radiol.2018170500. [DOI] [PubMed] [Google Scholar]
- 19.Neuschler E.I., et al. A pivotal study of optoacoustic imaging to diagnose benign and malignant breast masses: a new evaluation tool for radiologists. Radiology. 2018;287:398–412. doi: 10.1148/radiol.2017172228. [DOI] [PubMed] [Google Scholar]
- 20.Manohar S., Dantuma M. Current and future trends in photoacoustic breast imaging. Photoacoustics. 2019;16 doi: 10.1016/j.pacs.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oraevsky A.A., et al. Clinical optoacoustic imaging combined with ultrasound for coregistered functional and anatomical mapping of breast tumors. Photoacoustics. 2018;12:30–45. doi: 10.1016/j.pacs.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D., Zhao M., Tao C., Qian X., Liu X. High-resolution in vivo imaging of human nailbed microvasculature by using photoacoustic microscopy. J. Biophotonics. 2023 doi: 10.1002/jbio.202300058. [DOI] [PubMed] [Google Scholar]
- 23.Nau T., et al. Raster-scanning optoacoustic mesoscopy biomarkers for atopic dermatitis skin lesions. Photoacoustics. 2023;31 doi: 10.1016/j.pacs.2023.100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He H., Englert L., Ntziachristos V. Optoacoustic endoscopy of the gastrointestinal tract. ACS Photonics. 2022 doi: 10.1021/acsphotonics.2c01264. [DOI] [Google Scholar]
- 25.Paulus L.P., et al. Contrast-enhanced multispectral optoacoustic tomography for functional assessment of the gastrointestinal tract. Adv. Sci. 2023 doi: 10.1002/advs.202302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lediju Bell M.A., Shubert J. Photoacoustic-based visual servoing of a needle tip. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regensburger A.P., Brown E., Krönke G., Waldner M.J., Knieling F. Optoacoustic imaging in inflammation. Biomedicines. 2021;9 doi: 10.3390/biomedicines9050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fasoula N.A., et al. Non-invasive multispectral optoacoustic tomography resolves intrahepatic lipids in patients with hepatic steatosis. Photoacoustics. 2023;29 doi: 10.1016/j.pacs.2023.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng X., et al. Longitudinal volumetric assessment of inflammatory arthritis via photoacoustic imaging and Doppler ultrasound imaging. Photoacoustics. 2023;31 doi: 10.1016/j.pacs.2023.100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Placke J.M., et al. Multispectral optoacoustic tomography to differentiate between lymph node metastases and coronavirus-19 vaccine-associated lymphadenopathy. J. Eur. Acad. Dermatol. Venereol. 2023 doi: 10.1111/jdv.18847. [DOI] [PubMed] [Google Scholar]
- 31.Van Heumen S., Riksen J.J.M., Singh M.K.A., Van Soest G., Vasilic D. LED-based photoacoustic imaging for preoperative visualization of lymphatic vessels in patients with secondary limb lymphedema. Photoacoustics. 2023;29 doi: 10.1016/j.pacs.2022.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seno Medical Seno Med. Instrum. Receiv. CE Mark. 2014 〈https://senomedical.com/newsroom/press-releases/newsroom-press-releases-2014-seno-medical-instruments-receives-ce-mark-for-imagio-breast-imaging-system〉 [Google Scholar]
- 33.iThera Medical. CE mark awarded to iThera Medical. 〈https://ithera-medical.com/ce-mark-awarded-to-ithera-medicals-msot-acuity-optoacoustic-imaging-system/〉 (2021).
- 34.US Food and Drug Administration. Imagio Breast Imaging System - P200003. 〈https://www.fda.gov/medical-devices/recently-approved-devices/imagior-breast-imaging-system-p200003〉 (2021).
- 35.Luxonus Notice of acquisition of pharmaceutical approval. 2023 〈https://www.luxonus.jp/%e8%96%ac%e4%ba%8b%e6%89%bf%e8%aa%8d%e5%8f%96%e5%be%97%e3%81%ab%e9%96%a2%e3%81%99%e3%82%8b%e3%81%8a%e7%9f%a5%e3%82%89%e3%81%9b/〉 [Google Scholar]
- 36.Waterhouse D.J., Fitzpatrick C.R.M., Pogue B.W., O’Connor J.P.B., Bohndiek S.E. A roadmap for the clinical implementation of optical-imaging biomarkers. Nat. Biomed. Eng. 2019;3:339–353. doi: 10.1038/s41551-019-0392-5. [DOI] [PubMed] [Google Scholar]
- 37.Hacker L., et al. Criteria for the design of tissue-mimicking phantoms for the standardization of biophotonic instrumentation. Nat. Biomed. Eng. 2022;6:541–558. doi: 10.1038/s41551-022-00890-6. [DOI] [PubMed] [Google Scholar]
- 38.Pfefer J., Agrawal A. Vol. 8215. SPIE; 2012. A review of consensus test methods for established medical imaging modalities and their implications for optical coherence tomography; p. 82150D. (Design and Quality for Biomedical Technologies V). [Google Scholar]
- 39.IPASC. The International Photoacoustic Standardisation Consortium. 〈www.ipasc.science〉 (2022).
- 40.Equator Network. STARD An Updated List of Essential Items for Reporting Diagnostic Accuracy STudies. 2015 〈https://www.equator-network.org/reporting-guidelines/stard/〉 [Google Scholar]
- 41.Horizon 2020. EUPHORIA. 〈https://euphoria2020.eu/challenge/project/〉 (2022).
- 42.Erfanzadeh M., Zhu Q. Photoacoustic imaging with low-cost sources; a review. Photoacoustics. 2019;14:1–11. doi: 10.1016/j.pacs.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.〈https://webstore.iec.ch/publication/3587〉. Safety of laser products - Part 1: Equipment classification and requirements. IEC 60825–1:2014 Preprint at (2014).
- 44.〈https://webstore.ansi.org/standards/lia/ansiz1362014〉. American National Standard for Safe Use of Lasers. ANSI Z136.1:2022 Preprint at (2022).
- 45.〈https://webstore.ansi.org/standards/lia/ansiz1362018〉. Safe Use of Lasers in Health Care. ANSI Z136.3:2018 Preprint at (2018).
- 46.〈https://webstore.iec.ch/publication/7076〉. Photobiological safety of lamps and lamp systems. IEC 62471:2006 Preprint at (2006).
- 47.Park S., et al. Normalization of optical fluence distribution for three-dimensional functional optoacoustic tomography of the breast. J. Biomed. Opt. 2022;27 doi: 10.1117/1.JBO.27.3.036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjoding M.W., Dickson R.P., Iwashyna T.J., Gay S.E., Valley T.S. Racial bias in pulse oximetry measurement. N. Engl. J. Med. 2020;383:2477–2478. doi: 10.1056/NEJMc2029240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Afshari A., et al. Evaluation of the robustness of cerebral oximetry to variations in skin pigmentation using a tissue-simulating phantom. Biomed. Opt. Express. 2022;13:2909. doi: 10.1364/BOE.454020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beard P., Anastasio M., Oraevsky A.A., Wang L. v. Special section guest editorial: celebrating the exponential growth of optoacoustic/photoacoustic imaging. J. Biomed. Opt. 2019;24:1. doi: 10.1117/1.JBO.24.12.121901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gröhl J., et al. The IPASC data format: a consensus data format for photoacoustic imaging. Photoacoustics. 2022;26 doi: 10.1016/j.pacs.2022.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DICOM. DICOM Standard Comment. 〈https://www.dicomstandard.org/comment〉 (2022).
- 53.Dantuma M., van Dommelen R., Manohar S. Semi-anthropomorphic photoacoustic breast phantom. Biomed. Opt. Express. 2019;10:5921. doi: 10.1364/BOE.10.005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gehrung M., Bohndiek S.E., Brunker J. Development of a blood oxygenation phantom for photoacoustic tomography combined with online pO2 detection and flow spectrometry. J. Biomed. Opt. 2019;24:1. doi: 10.1117/1.JBO.24.12.121908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vogt W.C., et al. Photoacoustic oximetry imaging performance evaluation using dynamic blood flow phantoms with tunable oxygen saturation. Biomed. Opt. Express. 2019;10:449. doi: 10.1364/BOE.10.000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dantuma M., Kruitwagen S., Ortega-Julia J., Pompe van Meerdervoort R.P., Manohar S. Tunable blood oxygenation in the vascular anatomy of a semi-anthropomorphic photoacoustic breast phantom. J. Biomed. Opt. 2021;26 doi: 10.1117/1.JBO.26.3.036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dantuma M., Kruitwagen S.C., Weggemans M.J., Op’t Root T.J.P.M., Manohar S. Suite of 3D test objects for performance assessment of hybrid photoacoustic-ultrasound breast imaging systems. J. Biomed. Opt. 2021;27 doi: 10.1117/1.JBO.27.7.074709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L., Jacques S.L., Zheng L. MCML-Monte Carlo modeling of light transport in multi-layered tissues. Comput. Methods Prog. Biomed. 1995;47:131–146. doi: 10.1016/0169-2607(95)01640-f. [DOI] [PubMed] [Google Scholar]
- 59.Hochuli R., An L., Beard P.C., Cox B.T. Estimating blood oxygenation from photoacoustic images: can a simple linear spectroscopic inversion ever work? J. Biomed. Opt. 2019;24:1. doi: 10.1117/1.JBO.24.12.121914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahlstrom T., Pulkkinen A., Tick J., Leskinen J., Tarvainen T. Modeling of errors due to uncertainties in ultrasound sensor locations in photoacoustic tomography. IEEE Trans. Med Imaging. 2020;39:2140–2150. doi: 10.1109/TMI.2020.2966297. [DOI] [PubMed] [Google Scholar]
- 61.Cox B., Laufer J.G., Arridge S.R., Beard P.C. Quantitative spectroscopic photoacoustic imaging: a review. J. Biomed. Opt. 2012;17 doi: 10.1117/1.JBO.17.6.061202. [DOI] [PubMed] [Google Scholar]
- 62.Tzoumas S., Ntziachristos V. Spectral unmixing techniques for optoacoustic imaging of tissue pathophysiology. Philos. Trans. R. Soc. A: Math., Phys. Eng. Sci. 2017;vol. 375 doi: 10.1098/rsta.2017.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bauer A.Q., Nothdurft R.E., Erpelding T.N., Wang L. v, Culver J.P. Quantitative photoacoustic imaging: correcting for heterogeneous light fluence distributions using diffuse optical tomography. J. Biomed. Opt. 2011;16 doi: 10.1117/1.3626212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussain A., Hondebrink E., Staley J., Steenbergen W. Photoacoustic and acousto-optic tomography for quantitative and functional imaging. Optica. 2018;5:1579. [Google Scholar]
- 65.Zhou X., et al. Evaluation of fluence correction algorithms in multispectral photoacoustic imaging. Photoacoustics. 2020;19 doi: 10.1016/j.pacs.2020.100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gröhl J., et al. Learned spectral decoloring enables photoacoustic oximetry. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-83405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park S., et al. Normalization of optical fluence distribution for three-dimensional functional optoacoustic tomography of the breast. J. Biomed. Opt. 2022;27 doi: 10.1117/1.JBO.27.3.036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin J.Leahy. Photoacoust. Tomogr. Method Syst. 2015 [Google Scholar]
- 69.Deán-Ben X.L., Razansky D. Optoacoustic imaging of the skin. Exp. Dermatol. 2021;vol. 30:1598–1609. doi: 10.1111/exd.14386. [DOI] [PubMed] [Google Scholar]
- 70.Brown E.L., et al. Quantification of vascular networks in photoacoustic mesoscopy. Photoacoustics. 2022;26 doi: 10.1016/j.pacs.2022.100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gröhl J., Schellenberg M., Dreher K., Maier-Hein L. Deep learning for biomedical photoacoustic imaging: a review. Photoacoustics. 2021;22 doi: 10.1016/j.pacs.2021.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu Y., et al. Deep learning in medical image registration: a review. Phys. Med. Biol. 2020;65 doi: 10.1088/1361-6560/ab843e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lou Y., Zhou W., Matthews T.P., Appleton C.M., Anastasio M.A. Generation of anatomically realistic numerical phantoms for photoacoustic and ultrasonic breast imaging. J. Biomed. Opt. 2017;22 doi: 10.1117/1.JBO.22.4.041015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Badano A., et al. The stochastic digital human is now enrolling for in silico imaging trials -- Methods and tools for generating digital cohorts. 2023 ArXiv2301.08719. ArXiv2301.08719. [Google Scholar]
- 75.Gambhir S.S., Ge T.J., Vermesh O., Spitler R. Toward achieving precision health. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aao3612. 〈https://www.science.org〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smuck M., Odonkor C.A., Wilt J.K., Schmidt N., Swiernik M.A. The emerging clinical role of wearables: factors for successful implementation in healthcare. NPJ Digit. Med. 2021;4 doi: 10.1038/s41746-021-00418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shung K.K. Diagnostic ultrasound: past, present, and future. J. Med Biol. Eng. 2011;31:371–374. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.