Abstract
Recently, digital health technologies (DHTs) and digital biomarkers have gained a lot of traction in clinical investigations, motivating sponsors, investigators, and regulators to discuss and implement integrated approaches for deploying DHTs. These new tools present new and unique challenges for optimal technology integration in clinical trial processes, including operational, ethical, and regulatory issues. In this paper, we gathered different perspectives to discuss challenges and perspectives from three different stakeholders: industry, US regulators, and a public‐private partnership consortium. The complexities of DHT implementation, which include regulatory definitions, defining the scope of validation experiments, and the need for partnerships between BioPharma and the technology sectors, are highlighted. Most of these challenges are related to translation of DHT‐derived measures into endpoints that are meaningful to clinicians and patients, participant safety, training, and retention and privacy of data. The example of the Wearable Assessments in the Clinic and Home in PD (WATCH‐PD) study is discussed as an example that demonstrated the advantages of pre‐competitive collaborations, which include early regulatory feedback, data sharing, and multistakeholder alignment. Future advances in DHTs are expected to spur device‐agnostic measured development and incorporate patient reported outcomes in drug development. More efforts are needed to define validation experiments for a defined context of use, incentivize data sharing and development of data standards. Multistakeholder collaborations via precompetitive consortia will help facilitate broad acceptance of DHT‐enabled measures in drug development.
INTRODUCTION
Digital health technology tools (DHTs) 1 are technologies such as apps, smartphones, and wearables that remotely acquire health‐related information from individuals. Advances across a range of DHTs, along with passive data collection applications in real‐world settings, have the potential to provide a more nuanced and comprehensive understanding of disease‐specific symptoms. Further, they have the advantages of objectivity and sensitivity of measurement, and richness of high‐frequency sensor data. DHTs, if integrated successfully, can spur restructuring, transformation, and disruption of the clinical trial landscape by providing innovative tools to address unmet medical needs, enable real‐time data collection for clinical endpoints, and accelerate drug development in addition to characterizing disease progression (Figure 1).
FIGURE 1.

Unique benefits of digital health technologies use in clinical investigations.
Although their promising potential has stimulated interest by sponsors, investigators, and regulators to discuss and implement integrated approaches for deploying DHTs in clinical studies, these new digital tools present new and unique challenges for optimal technology integration in clinical trial process, including operational, ethical, and regulatory issues. Therefore, wide DHT adoption requires careful thinking and sharing of thoughts and experiences to promote DHT‐enabled innovation in drug development. By bringing digital measures on par with more traditional drug development tools, such as biomarkers and electronic clinical outcome assessment (eCOA) instruments, there is tremendous potential in increasing the probability of success in approvals of novel medical products. 2 , 3
To streamline DHT applications and enable alignment across divisions, the US Food and Drug Administration (FDA) established a new Digital Health Center of Excellence to empower stakeholders in the digital health field. In 2021, the FDA issued a draft guidance aimed at facilitating DHT‐enabled remote data collection in clinical investigations to continue fostering innovation and patient centricity. 4 Wide adoption of DHT requires a dialogue among drug developers, technology providers, patient organizations, and regulators, which can be positioned for success via public‐private partnerships.
Parkinson's disease (PD) is considered a flagship disease for evaluating disease‐related symptoms using DHTs given many of the cardinal motor symptoms are amenable to detection by remote technologies. Such assessments enable long‐term monitoring of patients in naturalistic settings, such as the home—a key to understanding the early detection of onset as well as monitoring the dynamic nature of disease progression.
In this paper, we discuss the challenges and opportunities provided by DHTs and digital biomarkers in drug development. A digital biomarker is defined as a characteristic or set of characteristics, collected from digital health technologies, that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions, as defined by Vasudevan et al. 5 This topic was presented at a session during the 2022 ASCPT annual meeting. The aim of the session titled “Digital Technologies: Innovations That Transform the Face of Drug Development” was to bring together the three different stakeholder groups: industry (BioPharma and technology sectors), US regulators, and a public‐private partnership consortium. The application of DHTs in PD drug development was discussed as one promising example of the integration of these novel tools in drug development.
INDUSTRY PERSPECTIVE
Clinical trial measures derived from DHTs are relatively new to clinical investigations, for example, remote cardiac monitoring assessed by means of wearable electrocardiogram (ECG) patches 6 or remote data collection of motor function in patients with PD. 7 , 8 These measures share goals and purposes in drug development with more traditional biomarkers, like biomarker assays or imaging techniques. However, they differ substantially in terms of ascertaining technology suitability for use in clinical trials, which rely upon properly conducted validation experiments. 9 The definitions of DHT‐derived measures are important as they will have implications as to the level of evidence and types of experiments needed to determine whether an assessment, as well as a related endpoint, are fit for purpose. Some aspects of DHT‐based measures have features of biomarkers, and some resemble eCOAs and, thus, define the scope of validation experiments. As stated in the recent publication by the FDA: “Conflating the terms can hamper communication and evidence expectations between medical product developers and regulators”. 5 The matter is compounded by the fact that the complexity of DHT‐enabled measures requires a partnership between drug developers and technology service providers. In the case of more traditional biomarkers based on laboratory methods, development and validation of biomarker assays done in laboratories is an internal BioPharma activity as it is leveraging laboratory expertise, core to drug discovery and development. 9 However, DHTs and digital biomarkers are different in this regard. Successful deployment of DHT‐derived measures requires in‐depth knowledge of DHTs, a platform enabling data collection and integration, data analytics, and statistical analyses tailored to the specific nature of a measure of interest. This infrastructure takes place often in conjunction with data processing algorithms and development which traditionally reside outside of BioPharma R&D 6 , 10 (Table 1). Similar partnerships are already standard practice for biomarker companion diagnostic assays.
TABLE 1.
The industry challenges of developing DHT derived measures and potential solutions.
| Challenge | Example | Solutions |
|---|---|---|
| Digital technologies are not leveraging traditional Pharma expertise, such as laboratory assays | Successful deployment of a digitally enabled measure requires in‐depth knowledge of digital technologies, a platform enabling data collection and integration in conjunction with data analytics and algorithm development | Partnerships between drug developers and technology service providers |
| Definitional confusion of DHT‐ enabled measures: biomarkers or eCOA |
Gait and balance in patients with PD can be considered:
|
Continue updating FDA/NIH BEST glossary 1 Expand formal regulatory paths to advance DHTs beyond COA qualification |
| Digitally derived endpoints intended to support regulatory decisions are a complex and potentially lengthy undertaking | Experiments include verification of body‐worn sensors, analytical validation of data processing algorithms, relationship with conventional clinical outcomes, construct validity, sensitivity to change, test–retest reliability, usability, device safety, multisite operational tolerance, and face validity | Device agnostic measure development via precompetitive public‐private partnerships 8 |
Abbreviations: DHT, digital health technology; eCOA, electronic clinical outcome assessment; FDA, US Food and Drug Administration; NIH, National Institutes of Health; PD, Parkinson's disease.
Measuring gait and balance characteristics in patients with PD can serve as an illustrative example of validation experiment design and execution complexities. Gait and balance are two cardinal motor symptoms impaired in PD. 11 Certain features of gait and balance, such as walking distance, duration, speed, number of steps, and stride period, can be interrogated and quantified by body‐worn accelerometers. Moreover, gait and balance measures enabled by body‐worn sensors represent a good example of the issues that arise around terminology. On one hand, one can say that these measures are objective and quantifiable, representing physiology or a response to a therapeutic intervention. On the other hand, such measures represent assessments of how a patient functions and have the potential to serve as a proxy of the patient's ability to move and perform activities of daily living, making this measure a potential eCOA (Table 1).
To ascertain whether these measures are fit for purpose, many researchers in the community use the V3 (Verification, analytical Validation, clinical Validation) framework developed by the Digital Medicine Society with multistakeholder feedback. 12 This framework is broadly consistent with the FDA draft guidance on DHT use for remote data collection in clinical investigations. 4 For example, gait and balance measures in PD can be successfully assessed by means of body‐worn accelerometers. Prior to testing these DHT‐derived measures consisting of a sensor and data processing algorithms in patients with disease, the verification of an accelerometer sensor is important to ascertain whether the accuracy of a given measure is suitable for a specific application. This is particularly important as different frequencies and accelerations are detected during, for example, a walk and hand tremor. 13 Testing different accelerometers and comparing accelerations acquired at different amplitudes and frequencies indicated that the context of use is extremely important: verification experiments should be completed regardless of the regulatory status of a device or a technology. Moreover, these experiments are feasible only for technologies where sample‐level data are accessible. We refer here to sample‐level data, also known as raw data, as data recorded directly by a sensor prior to being analyzed into processed data by means of data processing algorithms. The data are needed for verification experiments as the sensor output needs to be compared to an appropriate bench standard. For example, a shaker table is a standard benchmark used for verification of accelerometers. Moreover, a review of sample‐level data may be also needed for specific applications (e.g., an examination of an ECG waveform by a trained human reader). 14 It is important to note that sample‐level data may not be available from all devices regardless of whether a device of interest is a commercial technology or a medical device. The specifics of raw data requirements and associated challenges can be found elsewhere. 9
The second step entails establishing analytical validation of data processing algorithms. This step is usually done by comparing the results of the raw data processed by an algorithm with a gold standard benchmark, often trained human raters. In certain cases, it may require testing in healthy volunteers as multiple iterations for algorithms optimization may be required prior to testing in patients with disease. In other cases, testing is possible only in subjects with a medical condition, as disease features are not present in healthy subjects. Postural tremor, another feature of PD, is an example when testing is required in patients. 15 Any algorithm, proprietary or publicly available, should undergo the validation process to ensure the accuracy and repeatability of the output. Testing under different conditions, such as phone placement in a loose pocket or a bag, may provide valuable information about operational tolerance of algorithms under investigation. Clinical validation experiments ascertain whether a new measure is capturing the right concept of interest relevant to the disease state and may also include construct validity, 7 sensitivity to change, test–retest reliability, usability, safety, and data face validity. 16
There is recognition from both US and European regulators that digitally derived endpoints intended to support regulatory decisions are a complex and potentially lengthy undertaking that necessitates a multistakeholder approach. 17 , 18 Additionally, DHTs are rapidly evolving, making a device agnostic endpoint development strategy imperative. The emerging examples of technology validation indicate that this approach is feasible and can be achieved by selecting technologies with accessible sample‐level data and validating algorithms across data from multiple devices. 19 A similar approach was shown to be key in development, validation, and regulatory qualification of image analysis technologies for Alzheimer's disease. 20 Public‐private partnerships can de‐risk the evidence generation process by sharing knowledge and costs and advancing device agnostic strategies that hold promise to catalyze specific individual drug development programs 17 (Table 1).
REGULATORY PERSPECTIVE
Regulatory agencies, like the FDA, always place patients at the center of innovation in health care. Further, the need for regulating novel digital technologies utilized in drug development has been recognized as a priority by the FDA. This is fostered by the FDA Center for Devices and Radiological Health (CDRH) ensuring that in the United States, patients have access to high‐quality, safe, and effective medical devices. Patient‐generated health data (PGHD) collected by DHTs provides an opportunity to understand patient behavior in the context of daily life. The CDRH's Digital Health Center of Excellence was created to empower stakeholders to advance healthcare by fostering responsible and high‐quality digital health innovation. The center's aims are: (1) connect and build partnerships to accelerate digital health advancements; (2) share knowledge to increase awareness and understanding, advance best practices; and (3) innovate regulatory approaches to provide efficient and least burdensome oversight. 21
DHTs constitute a convergence of computing power, connectivity, sensors, and software used in healthcare. They can be used as a medical product, be incorporated into a medical product (including a pharmacological product), used to develop a medical product, used to study a medical product, or as a companion or adjunct to a medical product, including diagnostics and therapeutics. DHTs can be used in a variety of ways when evaluating medical products. Examples include remote data collection, patient recruitment and retention, and electronic informed consent (Figure 1).
The recent draft guidance issued by the FDA on DHT for remote data acquisition in clinical investigations 4 provides recommendations to facilitate the use of DHTs in clinical trials. It is designed to help accelerate efficient medical product development to bring innovations and technological advances to patients. The draft guidance builds on the launch of the Digital Health Center of Excellence to empower digital health stakeholders and provide regulatory clarity in collaborations with the FDA and was developed by cross‐agency division input.
DHT use provides a potential for more efficient conduct of clinical trials, such as decentralized clinical trials, improved study accessibility for participants, patient recruitment, and retention, in addition to transmitting data directly from participants to study staff. From a scientific standpoint, it can generate real‐world data, and PGHD, enable continuous or more frequent data collection as compared to traditional methods as well as longitudinal monitoring of a patient's health status without requiring visits to study sites. Additionally, it provides the potential to inform digitally enabled novel endpoints. The potential benefits are provided in Figure 1.
Although some DHTs meet the definition of a medical device and others do not, a marketing authorization (clearance or approval) is not a requirement to use a DHT in a clinical trial. Devices intended only for use in clinical trials are typically exempt from many requirements applicable to devices—including premarket clearance for approval—as long as the investigation is compliant with applicable requirements on the 21 CRF part 812. 4 The appropriateness of a DHT that has marketing authorization is determined by whether it is fit for purpose: the level of validation associated with a biomarker or eCOA should be sufficient to support a proposed use (Figure 2). This includes defining a population of interest, a clinical event or characteristic of interest, the demonstrated ability of a DHT to measure an intended clinical event or characteristic of interest, and the DHT's physical properties. Additionally, clinical trial endpoints should reflect an outcome of interest. The endpoint is defined as a precisely defined variable intended to reflect an outcome of interest that is statistically analyzed to address a particular research question. 1 Verification and validation are important steps to help ensure a DHT is fit for purpose. Verification is a confirmation by examination and provision of objective evidence that the physical parameter of measurement is assessed accurately and precisely over time. Validation is a confirmation by examination and provision of objective evidence that a DHT appropriately assesses a clinical event or characteristic in the proposed patient population. Additional consideration for DHT use in remote data acquisition includes participant safety, participant and staff training, technical support, and retention and protection of data, as depicted in Figure 2. Digitally enabled endpoints should be treated like any other endpoint and should include a definition, a justification for use, determination of an endpoint type (e.g., safety for effectiveness), and positioning of an endpoint (e.g., primary, secondary, or exploratory).
FIGURE 2.
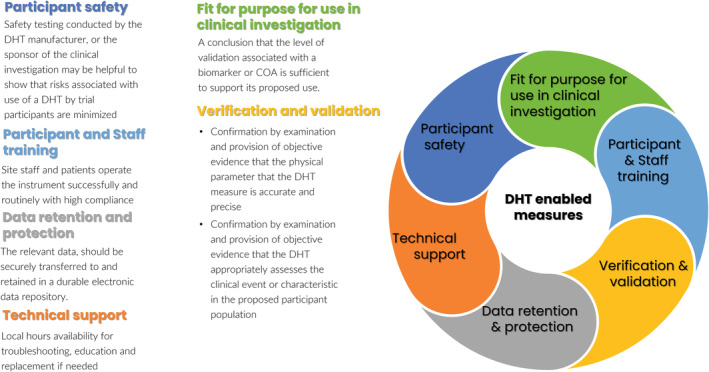
Regulatory considerations for DHT derived measures. 4 COA, clinical outcome assessment; DHT, digital health technology.
The regulatory science challenges include analytical validation of DHTs in different contexts of use; management of the data derived from DHTs including assessing data completeness; translation of DHT‐derived measures into endpoints meaningful to clinicians and patients; and development of methodological standards for data collection.
PUBLIC‐PRIVATE PARTNERSHIP CONSORTIUM PERSPECTIVE
One successful example of endorsing the application of digital technologies in drug development is the “Digital Drug Development Tools” (3DT) workstream of the Critical Path for Parkinson's Consortium (CPP). This project was initiated in 2019 to advance the use of DHTs in PD clinical research. CPP is one of many public‐private partnerships led by the Critical Path Institute; a nonprofit organization created to improve drug development processes. The 3DT collaborators include industry members, academic experts, clinicians, patient advocacy organizations, regulatory agencies, and people living with PD — all of whom work precompetitively to share data, knowledge, and costs to advance the regulatory maturity of DHTs.
Recently, attention has turned to the role of DHTs to complement the traditional measures used in the evaluation of PD therapeutics. DHTs are unique in reaching people affected by PD in real‐world settings and are ideally suited to identify the heterogeneous range of symptoms that change dynamically over the course of the disease. A rich and promising list of drug candidates is currently in development for the treatment of PD 22 with many therapies targeting the very early stages of the disease, a time that holds the most promise for slowing disease progression. However, the functional impairment of PD in early stages is not readily measured with existing clinical outcome measures. DHTs hold promise in identifying and quantifying early signs and symptoms that impact people in their daily lives.
The consortium members agreed to focus on a case study, Wearable Assessments in the Clinic and Home in Parkinson's Disease (WATCH‐PD), and engage with regulators. WATCH‐PD is a 12‐month multicenter observational study that evaluates multiple digital devices in individuals with early, untreated PD. The study enrolled 82 individuals with PD and 50 control participants without PD. In the clinic, participants wear inertial sensors (APDM Mobility Lab), an Apple Watch, and an iPhone while performing motor tasks (e.g., Movement Disorder Society‐Sponsored Revision of the Unified Parkinson Disease Rating Scale [MDS‐UPDRS] Part III). At home, participants use the Apple Watch and iPhone to complete motor and cognitive tasks every 2 weeks. Participants also complete quality‐of‐life/activities of daily living questionnaires at each visit. Specifics of device use and data collection can be found elsewhere. 8 , 23
Because early and frequent interactions with health authorities are necessary to address regulatory challenges in the fast‐changing field of DHTs, the 3DT requested a Critical Path Innovation Meeting with the FDA and an Innovations Task Force meeting with the European Medicines Agency (EMA) to discuss the protocol and design of the WATCH‐PD study. The regulators provided feedback on security issues involving DHT use, interoperability of platforms, the need for transparency of data analytics and algorithms, metadata collection, sources of variability, patient engagement in trials that deploy DHTs, and the use of normative data. The 3DT members responded to the regulatory feedback by funding an additional cohort of age‐ and gender‐matched control subjects for the WATCH‐PD study, establishing working groups to address issues resulting in manuscripts and recommendations. Additional funding by the members was provided to support a WATCH‐PD qualitative study to capture experiences of patients with PD daily living related to DHT‐derived measures in an observational longitudinal study of de novo patients with PD, capturing both motor and non‐motor features of the disease.
CPP's 3DT initiative has made significant progress on the goal of reaching a shared understanding of the open regulatory and scientific issues related to DHT use for endpoints in PD clinical trials, using the WATCH‐PD study as a case example. By seeking regulatory agency feedback on this case study, multiple sponsors have been informed on issues related to the optimal use of DHT use in future clinical trials. Future 3DT strategies are underway to respond to regulatory feedback and advance multistakeholder engagement, clinical validity, data sharing, and regulatory endorsement (Figure 3).
FIGURE 3.

Public‐private partnerships consortia role in advancing digital health technology enabled measures.
DISCUSSION AND CONCLUSIONS
There is a unanimous agreement across multiple stakeholders that DHT use in clinical trials holds potential to transform drug development by conducting studies and collecting data in a way that is convenient for patients, getting novel insights by means of objective and quantifiable measures, and capturing PGHD under real‐world conditions. Multiple efforts have been initiated to shape future directions which are strategically aligned and, undoubtedly, will help advancethe field. Technology and translational challenges are related to the need to validate and determine whether DHT‐enabled measures are fit for purpose as tools for drug development which will be best accomplished on the public‐private precompetitive collaborations.
Additional communication is needed with regulatory agencies to overcome challenges related to handling missing data, deriving meaningful insights from the DHT‐generated data to clinicians and patients, and developing DHT data standards. The power of collaborative efforts to advance the use of DHT in PD, along with the benefits of early regulatory engagements, was one of the successful case examples in the application of digital technologies in clinical trials. Current challenges include the issue of the rapid rate of evolving technologies, harmonization required to advance device agnostic measure development, a lack of consensus data standards, and the need for open data sharing to inform the next steps.
One of the key questions is whether remotely collected data by means of DHTs is going to supplement or supplant data collected during clinical visits. This question is best answered by collecting and sharing data to understand the relationship between the two types of measures. At this time, the remotely collected data is intended to augment assessments done by physicians.
The other area of interest is the validation scope pertinent to a specific context of use. The example discussed was related to DHT use in pediatric indications. A lot of data can be leveraged from studies in adults if assessments are similar between the two populations; in this case, the validation scope may be limited to addressing differences. For example, verification experiments can be leveraged if intended to measure the same concept. However, analytical validation or usability may need to be addressed separately as pediatric and adult populations require different data processing algorithms (e.g., sleep studies using actigraphy). 24 The creation of normative databases can be extremely useful in addressing this challenge. The harmonization efforts across regulatory agencies will be also important for future broad DHT adoption. Although regulators may have different approaches to qualifying drug development tools, many of the questions could potentially be addressed via International Conference on Harmonization (ICH) and International Medical Device Regulators Forum (IMDRF) programs.
Public‐private partnerships have a proven record of success to drive clinical trial innovation and acceptance of drug development tools regardless of a specific regulatory pathway (e.g., biomarkers, eCOA, or efforts driven by computational modeling [model‐informed drug development]).
The future challenges to be addressed include data sharing across multiple stakeholders and avoiding DHT‐derived drug development in single institution silos, defining the scope of validation experiments in the specific context of use, acceptance of DHTs as tools for drug development on par with more traditional technologies, such as laboratory assays and imaging techniques, incorporating the voice of patients to inform DHT development and implementation, and avoiding creating a digital divide among clinical study participants.
CONFLICT OF INTEREST STATEMENT
E.S.I. is an employee of Koneksa Health and may own company stock. H.H. is an employee of Regeneron Inc. and may own company stock. All other authors declared no competing interests for this work.
DISCLAIMERS
The findings, conclusions, and opinions expressed in this article have not been formally disseminated by the US Food and Drug Administration and should not be construed to represent any Agency determination or policy.
ACKNOWLEDGMENTS
The authors acknowledge Erin Lowry Critical Path Institute for her assistance with finalizing the manuscript. The CPP 3DT consortium represents a collaboration with University of Rochester WATCH‐PD study principal investigators Jamie Adams and Ray Dorsey and is funded by industry partners, Parkinson's UK and The Michael J Fox Foundation.
Izmailova ES, AbuAsal B, Hassan HE, Saha A, Stephenson D. Digital technologies: Innovations that transform the face of drug development. Clin Transl Sci. 2023;16:1323‐1330. doi: 10.1111/cts.13533
REFERENCES
- 1. BEST . (Biomarkers, EndpointS, and other Tools) Resource. Accessed March 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK338448/
- 2. Lathia CD, Amakye D, Dai W, et al. The value, qualification, and regulatory use of surrogate end points in drug development. Clin Pharmacol Ther. 2009;86:32‐43. doi: 10.1038/clpt.2009.69 [DOI] [PubMed] [Google Scholar]
- 3. Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20:273‐286. doi: 10.1093/biostatistics/kxx069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. FDA . Digital Health Technologies for Remote Data Acquisition in Clinical Investigations. Draft Guidance for Industry, Investigators, and Other Stakeholders. Accessed March 30, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/digital‐health‐technologies‐remote‐data‐acquisition‐clinical‐investigations
- 5. Vasudevan S, Saha A, Tarver ME, Patel B. Digital biomarkers: convergence of digital health technologies and biomarkers. NPJ Digit Med. 2022;5:36. doi: 10.1038/s41746-022-00583-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Izmailova ES, Wood WA, Liu Q, et al. Remote cardiac safety monitoring through the lens of the FDA biomarker qualification evidentiary criteria framework: a case study analysis. Digit Biomark. 2021;5:103‐113. doi: 10.1159/000515110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viceconti M, Hernandez Penna S, Dartee W, et al. Toward a regulatory qualification of real‐world mobility performance biomarkers in Parkinson's patients using digital mobility outcomes. Sensors (Basel). 2020;20:5920. doi: 10.3390/s20205920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stephenson D, Alexander R, Aggarwal V, et al. Precompetitive consensus building to facilitate the use of digital health technologies to support Parkinson disease drug development through regulatory science. Digit Biomark. 2020;4:28‐49. doi: 10.1159/000512500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Godfrey A, Vandendriessche B, Bakker JP, et al. Fit‐for‐purpose biometric monitoring technologies: leveraging the laboratory biomarker experience. Clin Transl Sci. 2021;14:62‐74. doi: 10.1111/cts.12865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vandendriessche B, Godfrey A, Izmailova ES. Multimodal biometric monitoring technologies drive the development of clinical assessments in the home environment. Maturitas. 2021;151:41‐47. doi: 10.1016/j.maturitas.2021.06.009 [DOI] [PubMed] [Google Scholar]
- 11. Grabli D, Karachi C, Welter ML, et al. Normal and pathological gait: what we learn from Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83:979‐985. doi: 10.1136/jnnp-2012-302263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldsack JC, Coravos A, Bakker JP, et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit‐for‐purpose for biometric monitoring technologies (BioMeTs). NPJ Digit Med. 2020;3:55. doi: 10.1038/s41746-020-0260-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calzetti S, Baratti M, Gresty M, Findley L. Frequency/amplitude characteristics of postural tremor of the hands in a population of patients with bilateral essential tremor: implications for the classification and mechanism of essential tremor. J Neurol Neurosurg Psychiatry. 1987;50:561‐567. doi: 10.1136/jnnp.50.5.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izmailova ES, McLean I, Hather G, et al. Continuous monitoring using a wearable device detects activity‐induced heart rate changes after Administration of Amphetamine. Clin Transl Sci. 2019;12:677‐686. doi: 10.1111/cts.12673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipsmeier F, Taylor KI, Postuma RB, et al. Reliability and validity of the Roche PD Mobile application for remote monitoring of early Parkinson's disease. Sci Rep. 2022;12:12081. doi: 10.1038/s41598-022-15874-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Izmailova ES, McLean IL, Bhatia G, et al. Evaluation of wearable digital devices in a phase I clinical trial. Clin Transl Sci. 2019;12:247‐256. doi: 10.1111/cts.12602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Developing Digitally Derived Endpoints that Measure what Matters to Patients: The Case for Collaboration. Accessed June 1, 2022. https://globalforum.diaglobal.org/issue/december‐2021/developing‐digitally‐derived‐endpoints‐that‐measure‐what‐matters‐to‐patients‐the‐case‐for‐collaboration/
- 18. European Medicines Agency . Questions and answers: Qualification of digital technology‐based methodologies to support approval of medicinal products. Accessed April 27, 2022. https://www.ema.europa.eu/en/documents/other/questions‐answers‐qualification‐digital‐technology‐based‐methodologies‐support‐approval‐medicinal_en.pdf
- 19. Ellis R, Kelly P, Huang C, Pearlmutter A, Izmailova ES. Sensor verification and analytical validation of algorithms to measure gait and balance and pronation/supination in healthy volunteers. Sensors (Basel). 2022;22:6275. doi: 10.3390/s22166275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Izmailova ES, Maguire RP, McCarthy TJ, Müller MLTM, Murphy P, Stephenson D. Empowering drug development: leveraging insights from imaging technologies to enable the advancement of digital health technologies. Clin Transl Sci. 2022;16:383‐397. doi: 10.1111/cts.13461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. FDA . Digital health Center of Excellence. Accessed November 23, 2022. https://www.fda.gov/medical‐devices/digital‐health‐center‐excellence
- 22. McFarthing K, Prakash N, Simuni T. Clinical trial highlights – an update on previously reviewed trials. J Parkinsons Dis. 2022;12:523‐536. doi: 10.3233/JPD-229001 [DOI] [Google Scholar]
- 23. Enabling efficient use of digital health technologies to support Parkinson's disease drug development through precompetitive collaboration. Accessed February 14, 2023. https://c‐path.org/wp‐content/uploads/2019/09/cpp_mds_2019_final_revised.pdf
- 24. Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113‐124. doi: 10.1053/smrv.2001.0182 [DOI] [PubMed] [Google Scholar]


